Introduction
Gastric cancer is a leading cause of mortality
worldwide according to the World Health Organization, accounting
for 754,000 mortalities in 2015 (1). According to the 2017 annual report by
the Ministry of Health and Welfare in Taiwan, gastric cancer is the
7th leading cause of cancer-associated mortality. The mortality
rate of gastric cancer was 9.8 per 100,000 of the population
(2). The major risk factors of
gastric cancer are Helicobacter pylori infection, and
dietary and environmental factors (3,4). The
overall 5-year relative survival rate of patients with gastric
cancer in the United States is ~31% (5). Paclitaxel, carboplatin, cisplatin,
5-fluorouracil, capecitabine and leucovorin are recognized as the
most effective agents against gastric cancer (6,7).
Apart from surgery, no satisfactory chemotherapeutic strategies are
currently available for gastric cancer, and novel effective
therapies are required to improve gastric anticancer treatment.
Metformin, a biguanide drug, is the first line
clinical agent for type 2 diabetes mellitus (T2D) treatment
(8,9). The pharmacological mechanism of
metformin is to downregulate blood glucose levels to enhance
insulin sensitivity in the liver and peripheral tissues
(stimulating glucose uptake into muscles and/or increasing fatty
acid oxidation in adipose tissue) by activation of adenosine
monophosphate (AMP)-activated protein kinase (AMPK) signaling
(10,11). In addition, the effectiveness of
metformin involves reduced hepatic gluconeogenesis (11,12).
The epidemiological studies have suggested that the use of
metformin is associated with a decreased incidence of cancer, and
improved prognosis and cancer-associated mortality in patients with
T2D (13,14). The anticancer effects of metformin
have been reported in breast (15,16),
colorectal (17), liver (18), cervical (19), endometrial (20), gastric (21), lung (22), ovarian (23), prostate (24), pancreatic (25) and renal (26) cancer. Various studies have
demonstrated that the anticancer mechanisms of metformin are
mediated via the AMPK/mammalian target of rapamycin (mTOR) cascade,
and the signaling is dependent on AMPK activation leading to
inhibition of mTOR that represses protein synthesis, cell
proliferation, cell cycle progression and apoptotic cell death
(27-29). A previous study demonstrated that
metformin inhibits the proliferation and metastasis of SGC-7901 and
BGC-823 gastric cancer cells by suppressing hypoxia-inducible
factor 1α/pyruvate kinase M1/2 signaling (30). Apoptosis (type I programmed cell
death) is a tightly regulated biological process (31,32).
Anticancer agents that trigger the apoptotic pathway in cancer
cells may be of potential clinical use (33). Metformin has been reported to
inhibit cell proliferation in human gastric cancer cell lines,
including MKN45, MKN47, MKN-28, SGC-7901 and BGC-823, and cancer
stem cells (34,35). Additionally, metformin reduces
metastasis of human gastric cancer AGS cells by inhibiting
epithelial-mesenchymal transition (EMT) in a glucose-independent
manner (36). Although the
mechanism responsible for the anti-metastatic action of metformin
has been investigated, its role of AMPK-mediated apoptotic
machinery in gastric cancer cells remains unclear. In the current
study, the anti-proliferation effect of metformin cells and
underlying apoptotic mechanism was investigated using human gastric
cancer AGS cells in vitro.
Materials and methods
Chemicals and materials
Metformin hydrochloride, thiazolyl blue tetrazolium
bromide (MTT), In Situ Cell Death Detection kit
(fluorescein), compound C, carbobenzoxyvalyl-alanyl-aspartyl
fluoromethyl ketone (z-VAD-fmk), and all other chemicals and
reagents were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany), unless otherwise stated. All primary antibodies,
anti-mouse and anti-rabbit immunoglobulin (Ig)G horseradish
peroxidase (HRP)-linked secondary antibodies were obtained from
GeneTex International Corporation (Hsinchu, Taiwan). Muse
Caspase-3/7 Assay Kit was obtained from Merck KGaA.
2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) and
3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] were obtained from Molecular Probes
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Ham’s Nutrient
Mixture F12 medium, minimum essential medium, fetal bovine serum
(FBS), L-glutamine, penicillin/streptomycin and trypsin-EDTA were
purchased from HyClone (GE Healthcare Life Sciences, Logan, UT,
USA). Mitochondria/Cytosol Fractionation Kit was bought from
BioVision, Inc. (Milpitas, CA, USA).
Cell culture
The human AGS gastric adenocarcinoma cell line was
purchased from the Bioresource Collection and Research Center
(Hsinchu, Taiwan) and cultured in Ham’s Nutrient Mixture F12 medium
supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin,
and 100 µg/ml streptomycin. The normal human colon CCD 841
CoN cells (CRL-1790) and embryonic lung fibroblast HEL 299 cells
(CCL-137) were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and cultured in minimum essential medium
containing 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin. Normal 293 cells (CRL-1573) were purchased from the
ATCC and maintained in minimum essential medium supplemented with
10% FBS, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate,
100 U/ml penicillin and 100 µg/ml streptomycin. All of the
cells were maintained at 37°C in a humidified atmosphere incubator
with 5% CO2.
Cytotoxicity assay
The cytotoxic effect of metformin was detected in an
MTT assay, as described previously (37). In brief, AGS, CCD 841 CoN, HEL 299
and 293 cells (1×104 cells/well) were cultured in
96-well plates and exposed to various concentrations (10, 20, 30,
40 and 50 mM) of metformin for 12, 24 or 48 h after pretreatment
with or without 10 µM compound C (an AMPK inhibitor), or 10
µM z-VAD-fmk (a pan-caspase inhibitor) for 2 h. Following
treatments, 10 µl MTT solution (5 mg/ml) was added per well,
and the cells were cultured for an additional 3 h. The medium was
then removed, and the formation of formazan was solubilized using
100 µl dimethyl sulfoxide. The absorbance was detected using
an ELISA plate reader at 570 nm in a spectrophotometer, as
previously described (38,39).
Morphological observation
AGS cells (1×105 cells/well) were plated
onto 12-well plates and then treated with or without 10, 20, 30, 40
and 50 mM metformin for 12, 24 and 48 h. The cells were
subsequently observed and images using a phase-contrast microscope
at a magnification of ×200.
Apoptosis analysis by flow cytometry
AGS cells (1×105 cells/ml) were cultured
with or without 10, 20, 30 and 40 mM metformin for 48 h. The cells
were subsequently washed with PBS and harvested. To detect
apoptosis by flow cytometry (BD FACSCalibur Flow Cytometer; BD
Biosciences; Becton-Dickinson Co., Franklin Lakes, NJ, USA), the
cells were then stained with the In Situ Cell Death
Detection Kit, Fluorescein (Sigma-Aldrich; Merck KGaA), following
the manufacturer’s instructions. The terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL)-positive cells
were quantified using the BD CellQuest Pro Software version 5.1 (BD
Biosciences; Becton-Dickinson and Company), as previously described
(38).
Caspase-3/7 activity
AGS cells (5×106 cells/75T flask) were
incubated with or without 10, 20, 30 and 40 mM metformin for 48 h.
The cells were collected by centrifugation at 400 × g prior to
incubation with the working solution provided in the Muse
Caspase-3/7 Assay Kit (Merck KGaA), according to the manufacturer’s
protocol.
Western blotting
AGS cells (5×106 cells per 75T flask)
were incubated with 0, 10, 20 and 30 mM metformin for the indicated
period of time (12 or 48 h) following pretreatment with or without
10 µM compound C for 2 h. At the end of the exposure period,
the cells were lysed using Trident radioimmunoprecipitation assay
lysis buffer (GeneTex International Corporation) to extract total
protein. The cytosolic and mitochondrial fractions were prepared
via the Mitochondria/Cytosol Fractionation Kit (BioVision, Inc.)
according to the manufacturer’s instructions. The protein
concentration was determined using the Pierce bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). A protein
sample (40 µg) was loaded in each well of a 10-12%
polyacrylamide gel, separated by SDS-PAGE and transferred to the
Immobilon-P Transfer membrane (Merck KGaA) for 1 h, as previously
described (40). The membrane was
blocked with 5% skim milk in Tris-buffered saline with 0.1%
Tween-20 (TBST) and incubated with the following primary antibodies
(GeneTex International Corporation): Phospho (p)-AMPK (cat no.
GTX52341), AMPK (cat no. GTX112998), p-protein kinase B (AKT; cat.
no. GTX28932), AKT (cat. no. GTX121937), p-mTOR (cat. no.
GTX50258), mTOR (cat. no. GTX101557), p-ribosomal protein S6 kinase
B1 (p70S6K; cat. no. GTX50304), p70S6K (cat. no. GTX103174),
p-extracellular signal regulated kinase (ERK; cat. no. GTX59568),
ERK (cat. no. GTX59618), p-c-Jun N-terminal kinase (JNK; cat. no.
GTX52326), JNK (cat. no. GTX52360), p-p38 (cat. no. GTX48614), p38
(cat. no. GTX110720), p-Bcl-2-associated agonist of cell death
(BAD; Ser136; cat. no. GTX50136), BAD (cat. no. GTX130108), B cell
lymphoma-2 (Bcl-2; cat. no. GTX100064), cytochrome c (cat.
no. GTX108585), apoptotic protease-activating factor-1 (Apaf-1;
cat. no. GTX22000), caspase-9 (cat. no. GTX112888), caspase-3 (cat.
no. GTX110543), caspase-7 (cat. no. GTX22301; all 1:1,000
dilution), β-actin (cat. no. GTX109639; 1:5,000 dilution), GAPDH
(cat. no. GTX100118; 1:5,000 dilution), and cytochrome c
oxidase subunit IV isoform 1 (COX IV; cat. no. GTX114330; 1:2,000
dilution) at 4°C overnight. The next day, the membrane was washed
with TBST and incubated with the appropriate anti-rabbit (cat. no.
GTX213110-01) and anti-mouse (cat. no. GTX213111-01) IgG HRP-linked
antibodies (1:10,000 dilution) for 1 h at room temperature. An
enhanced chemiluminescence kit (Immobilon Western Chemiluminescent
HRP substrate; Merck KGaA) was used to visualize protein bands, and
protein band densitometry was performed using ImageJ software
(version 1.47; National Institutes of Health, Bethesda, MD,
USA).
Measuring reactive oxygen species (ROS)
and the mitochondrial membrane potential (ΔΨm) via flow
cytometry
AGS cells (2×105 cells/ml) seeded in
12-well plates were exposed to 0, 10, 20, 30 and 40 mM metformin
for 48 h. Subsequently, the cells were harvested and centrifuged at
400 × g for 5 min, and the cell pellet was suspended in 500
µl H2DCFDA (an ROS indicator dye, 10 µM)
or DiOC6(3) (a ΔΨm
probe, 50 nM) staining solution at 37°C for 30 min. The cells were
then analyzed using flow cytometry (BD FACSCalibur Flow Cytometer;
BD Biosciences; Becton-Dickinson Co.), as previously described
(40,41).
Statistical analysis
All results are presented as the mean ± standard
deviation of triplicates. The data were statistically analyzed by
one-way analysis of variance followed by Dunnett’s test using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Metformin is cytotoxic to human gastric
cancer AGS cells
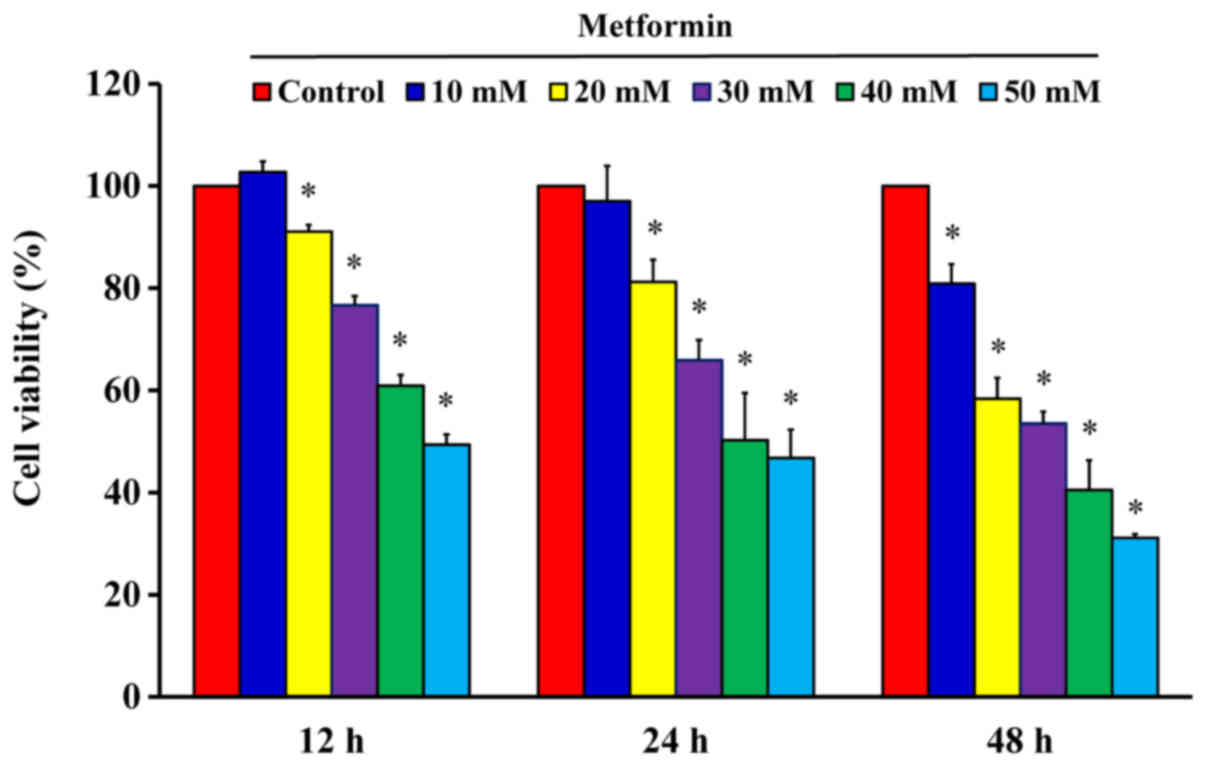
After cells were treated with 10, 20, 30, 40 and 50
mM metformin for 12, 24 and 48 h, the MTT assay was used to analyze
cell viability. The results demonstrated that metformin
significantly reduced cell viability after incubation with 20 mM
metformin for 12 h; furthermore, the reductions of viability were
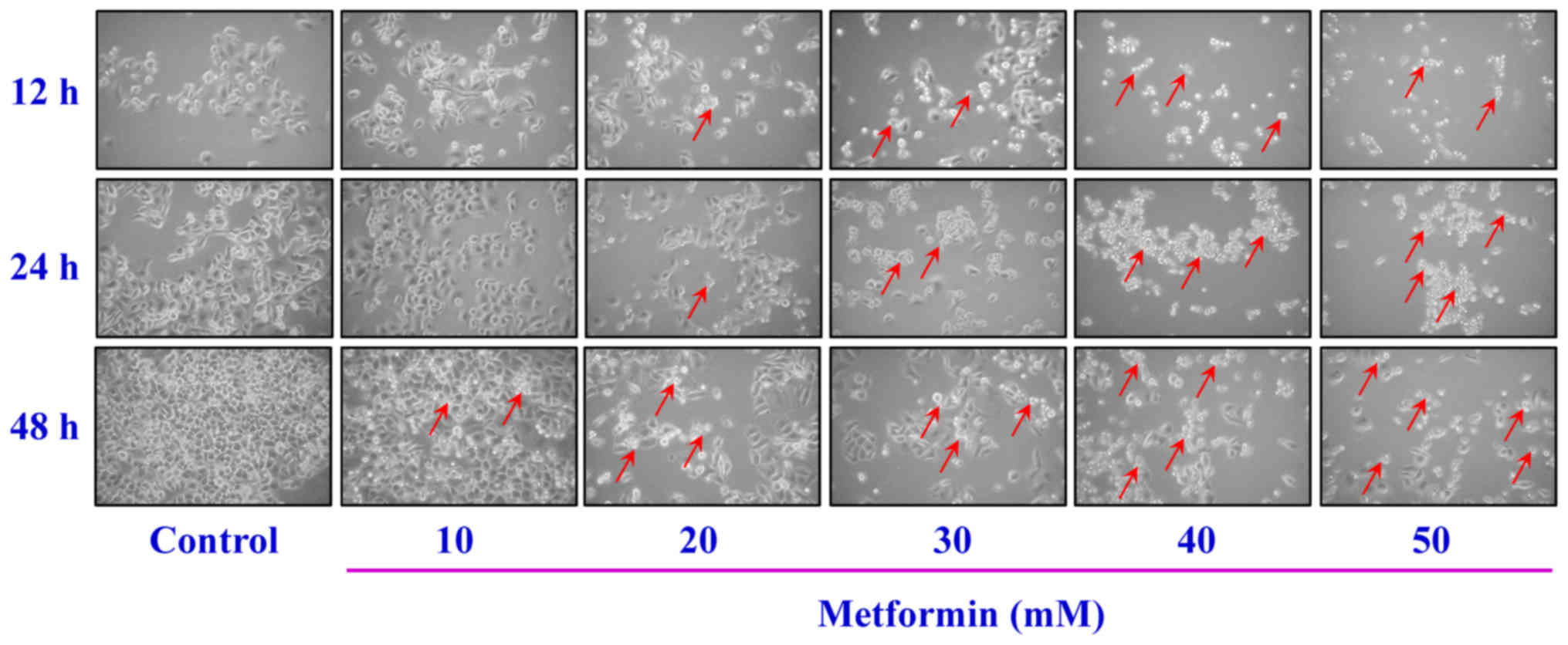
time- and concentration-dependent (Fig. 1). The cells were treated with
metformin prior to morphological characterization. The marked
morphologic alterations (such as cell shrinkage, nuclear
condensation, membrane blebbing and rounding) were present in a
time- and concentration-dependent manner in AGS cells (Fig. 2). Thus, metformin suppressed AGS
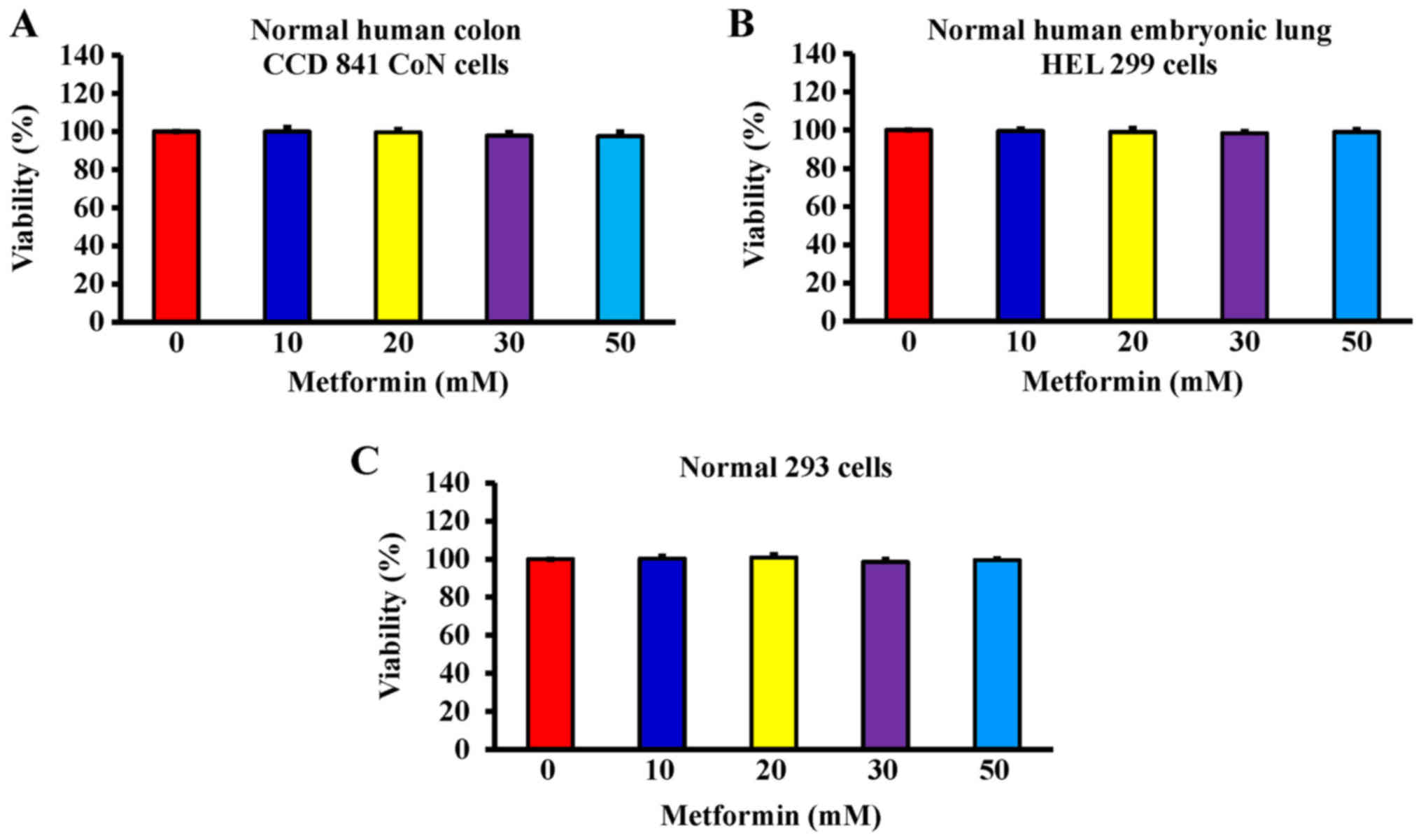
cell growth via induction of apoptotic death. Additionally, the
data demonstrated that metformin (0, 10, 20, 30 and 50 mM) after
exposure for 48 h had no significant effect of the viability of
normal colon CCD 841 CoN cells (Fig.
3A), embryonic lung HEL 299 cells (Fig. 3B) and 293 cells (Fig. 3C). This suggested that metformin
may have lower toxicity in normal cells (CCD 841 CoN, HEL 299 and
293 cells) compared with cancer cells.
Metformin promotes apoptosis of AGS
cells
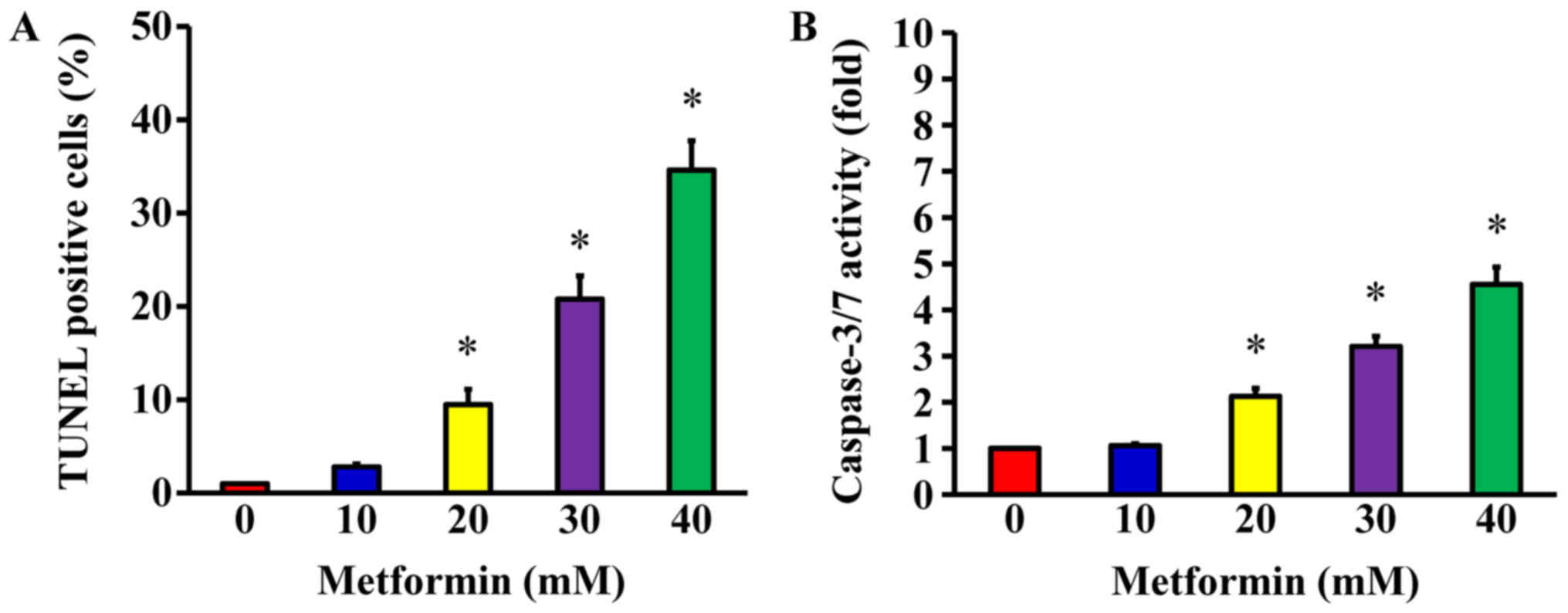
Following treatment of AGS cells with 10, 20, 30 and
40 mM metformin for 48 h, a TUNEL assay was used to detect DNA
breaks, which are a direct apoptotic response. The results
demonstrated that metformin at 20, 30 and 40 mM
concentration-dependently produced double-stranded DNA
fragmentation (a unique biochemical hallmark of apoptosis) and
enhanced the number of TUNEL-positive cells (Fig. 4A), indicating that metformin
induces AGS cell apoptosis. To determine whether caspase-3/7 are
involved in the metformin-induced apoptosis, caspase-3/7 activity
was analyzed using a Muse Caspase-3/7 Assay kit. The data indicated
that metformin (20, 30, and 40 mM) significantly enhanced the
activity of caspase-3/7 in a concentration-dependent manner
(Fig. 4B). These findings
demonstrate that the ability of metformin to trigger apoptosis of
AGS cell may be caspase-3/7-dependent.
AMPK pathway contributes to
metformin-induced cytotoxicity and apoptosis in AGS cells
AMPK and AKT/mTOR signaling are usually involved in
the regulation of cell proliferation and apoptosis (42). AGS cells were treated with 10, 20
and 30 mM metformin for 12 h, or pretreated with or without 10
µM compound C (an AMPK inhibitor) for 2 h prior to metformin
exposure. The findings indicated that metformin stimulated
phosphorylation of AMPK at Thr172, but there was no alteration in
AMPK expression in AGS cells (Fig.
5A). To confirm whether the AMPK pathway has a key molecular
role in metformin-treated AGS cells, an AMPK inhibitor, compound C,
was applied, and the level of p-AMPK and cell viability were
analyzed by western blotting and an MTT assay, respectively. The
data demonstrated that compound C suppressed phosphorylation of
AMPK (Fig. 5B) and significantly
reversed the effect of metformin on cell viability compared with
metformin treatment only (Fig.
5C). Thus, metformin-induced apoptosis is mediated via
modulated AMPK signaling in AGS cells. To further clarify the
downstream signaling involved, cells were treated with metformin
and harvested for western blot analysis to detect the
phosphorylation of AKT (p-AKT), mTOR (p-mTOR), and p70S6K
(p-p70S6K). The results demonstrated that metformin decreased the
phosphorylation of AKT, mTOR, and p70S6K, whereas metformin did not
affect the protein expression in AGS cells (Fig. 5D). These data indicate that
metformin enhances apoptosis potentially by targeting AMPK and
AKT/mTOR pathway in AGS cells.
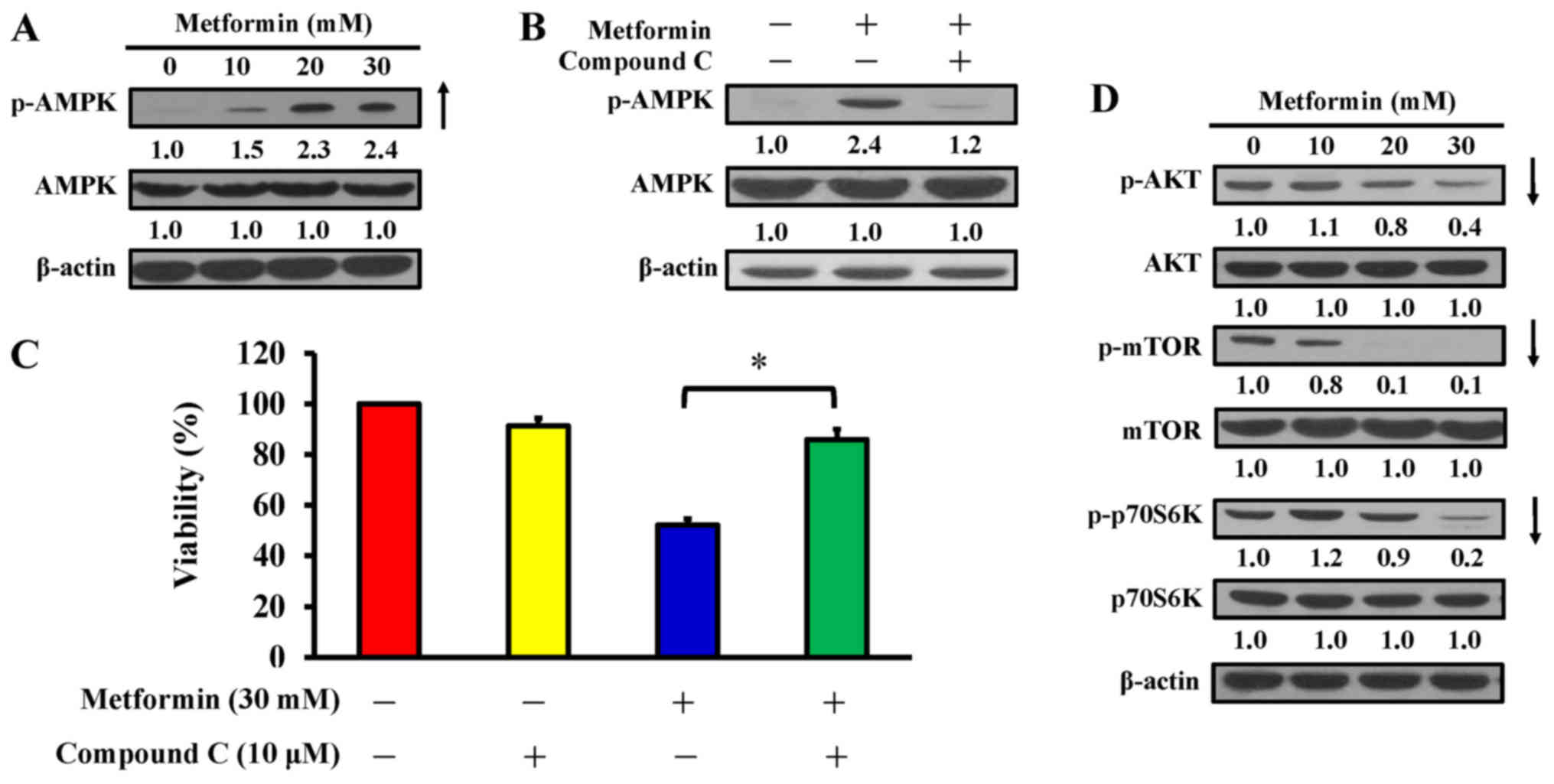 | Figure 5Effect(s) of metformin on AMPK
signaling and its downstream molecules of AGS cells. (A) Cells were
exposed to 0, 10, 20 and 30 mM metformin for 12 h and protein
levels of p-AMPK and AMPK were detected. (B) Cells were cultured
without or with 10, 20 and 30 mM metformin for 12 h following
pre-incubation with or without 10 µM compound C (an AMPK
inhibitor) for 2 h and protein levels of p-AMPK and AMPK were
detected. (C) Cells were treated without or with 30 mM metformin
for 48 h after pre-incubation with or without 10 µM compound
C for 2 h. Cell viability was estimated by the MTT assay. The
values are presented as the mean ± standard deviation of
triplicates. *P<0.05 vs. metformin-treated only. (D)
Cells were treated without or with 10, 20 and 30 mM of metformin
for 12 h and protein levels of p-AKT, AKT, p-mTOR, mTOR, p-p70S6K
and p70S6K were determined by immunoblot analysis. β-actin was an
internal loading control. p-, phospho; AMPK, adenosine
monophosphate-activated protein kinase; AKT, protein kinase B;
mTOR, mammalian target of rapamycin; p70S6K, ribosomal protein S6
kinase B1. |
Metformin inhibits mitogen-activated
protein kinase (MAPK) signaling in AGS cells
To assess whether MAPKs (ERK, JNK and p38)
contribute to metformin-induced apoptosis, the cells were exposed
to metformin and MAPK proteins were detected via western blot
analysis. MAPK signals are essential for induction of apoptosis
(43,44). Treating AGS cells with metformin
markedly attenuated the phosphorylation of ERK, JNK and p38
(Fig. 6), with no obvious
alterations in ERK, JNK and p38 MAPK protein expression. The
results demonstrate that the apoptotic mechanism of metformin may
involve ERK, JNK, and p38 MAPK-regulated pathways in AGS cells.
 | Figure 6Effect(s) of metformin on ERK, JNK
and p38 pathways of AGS cells. The cells were incubated with 0, 10,
20 and 30 mM of metformin for 12 h, and whole-cell lysates were
then collected. Cell fractions were individually probed with
anti-p-ERK, anti-ERK, anti-p-JNK, anti-JNK, anti-p-p38 and anti-p38
by western blotting analysis. β-Actin was an internal loading
control. p-, phospho; ERK, extracellular signal-regulated kinase;
JNK, c-Jun N-terminal kinase. |
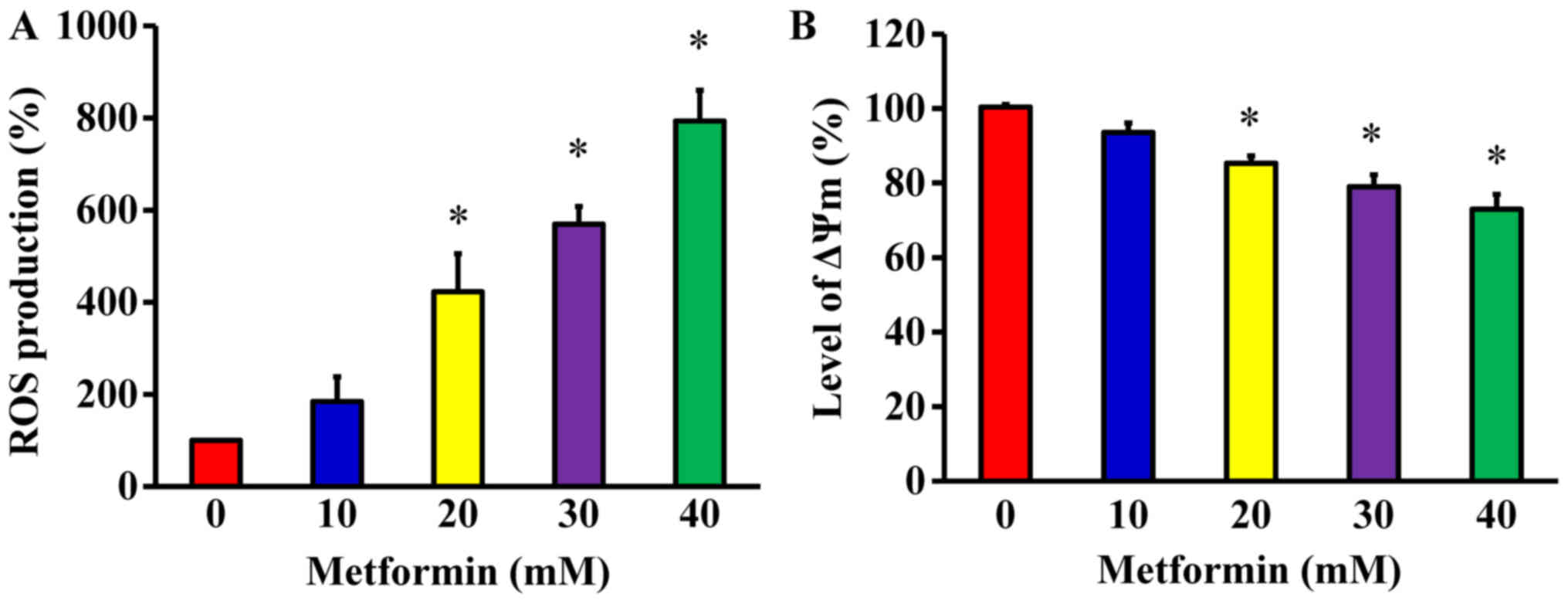
Metformin promotes ROS production and ΔΨm
in AGS cells
To determine whether metformin-induced apoptosis is
mitochondria-dependent, ROS production and the ΔΨm were measured in
AGS cells. The cells were treated with metformin at various
concentrations (10, 20, 30 and 40 mM) for 48 h. The levels of ROS
production and ΔΨm were measured using the specific fluorochromes
H2DCFDA and DiOC6(3), respectively, via flow cytometry. The
results revealed that metformin increased the production of ROS
(Fig. 7A) and decreased the ΔΨm
(Fig. 7B) in AGS cells.
Furthermore, these effects were concentration-dependent.
Metformin induces apoptosis via the
intrinsic signaling pathway in AGS cells
To determine the effect of metformin on apoptosis,
the expression of Bcl-2 family proteins and mitochondria-mediated
proteins were analyzed in metformin-treated AGS cells. Western blot
analysis indicated that metformin treatment reduced the
phosphorylation of BAD and expression of Bcl-2, but metformin
induced total BAD expression in AGS cells (Fig. 8A). Furthermore, metformin increased
the protein expression of Apaf-1 (Fig.
8B) and reduced the expression of pro-caspase-9, pro-caspase-3
and pro-caspase-7 expression (Fig.
8C) in AGS cells. Furthermore, metformin caused an increase in
cytochrome c in cytoplasmic extracts (Fig. 8D); however, mitochondrial
cytochrome c levels were decreased in AGS cells (Fig. 8D). Notably, z-VAD-fmk, a
pan-caspase inhibitor, significantly abrogated the effect of
metformin on viability compared with metformin-treated cells
(Fig. 8E), suggesting that
mitochondria-mediated caspase-dependent apoptosis may be required
for the cytotoxic effect of metformin on human gastric
adenocarcinoma AGS cells (Fig.
9).
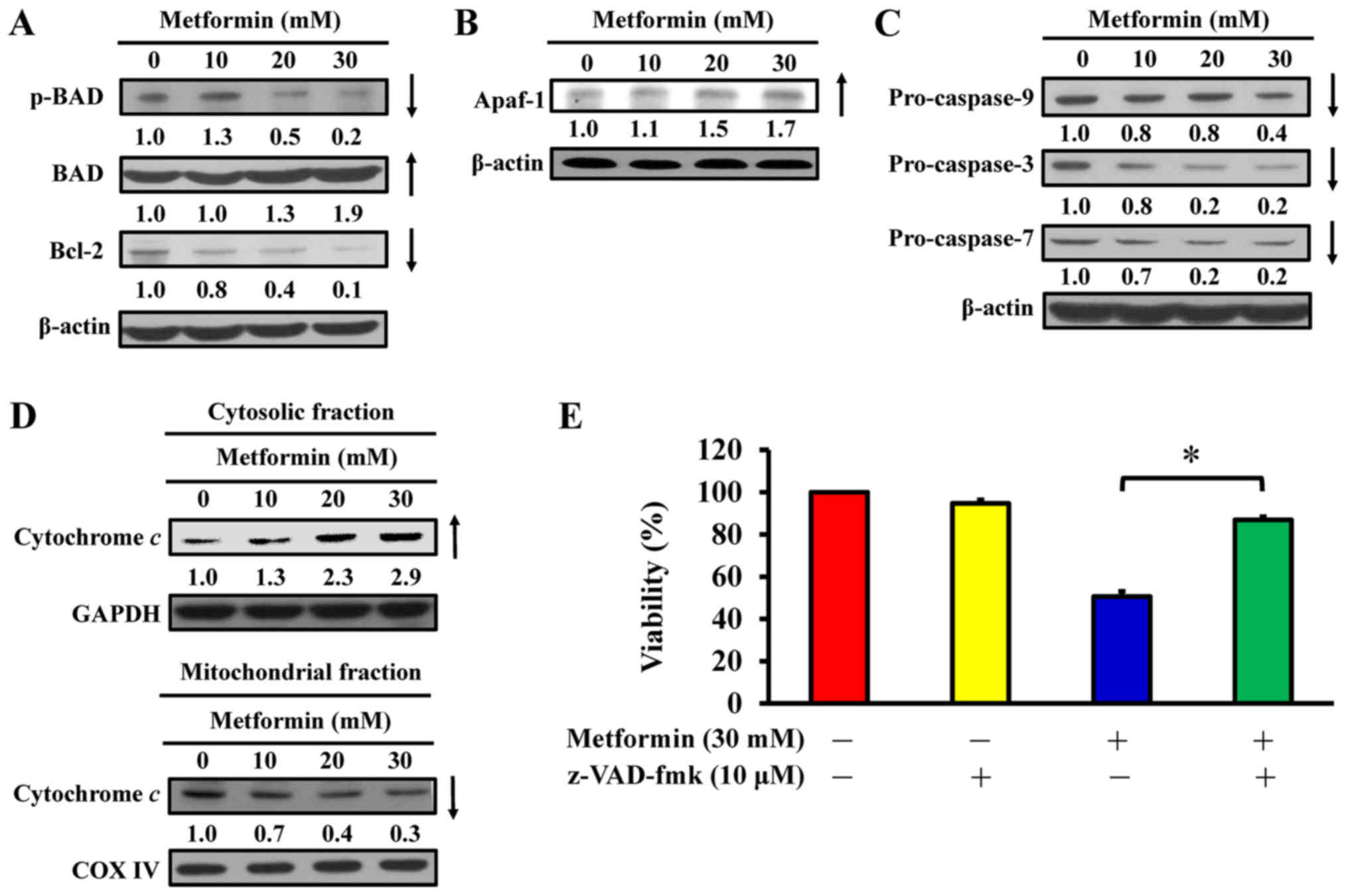 | Figure 8Effect(s) of metformin on
mitochondria-mediated caspase-dependent apoptotic signaling of AGS
cells. The cells were treated with 0, 10, 20 and 30 mM of metformin
for 48 h, and whole-cell lysates and mitochondrial and cytosolic
fractions were then harvested. Protein levels of (A) p-BAD, BAD and
Bcl-2, (B) Apaf-1 and (C) caspase-9, caspase-3 and caspase-7
signals were determined by western blot analysis. β-actin was an
internal loading control. (D) The cytosolic (top) and mitochondrial
(bottom) fractions were used to determine for cytochrome c
translocation by western blot analysis. GAPDH and COX IV were
internal loading controls. (E) Following pre-incubation with or
without 10 µM z-VAD-fmk (a pan-caspase inhibitor) for 2 h,
the cells were exposed to 30 mM metformin for 48 h. Cell viability
was assessed using the MTT assay. The values are presented as the
mean ± standard deviation of triplicates. *P<0.05 vs.
metformin-treated only. p-, phospho; BAD, Bcl-2-associated agonist
of cell death; Bcl-2, B-cell lymphoma-2; Apaf-1, apoptotic
protease-activating factor-1; z-VAD-fmk,
carbobenzoxyvalyl-alanyl-aspartyl fluoromethyl ketone. |
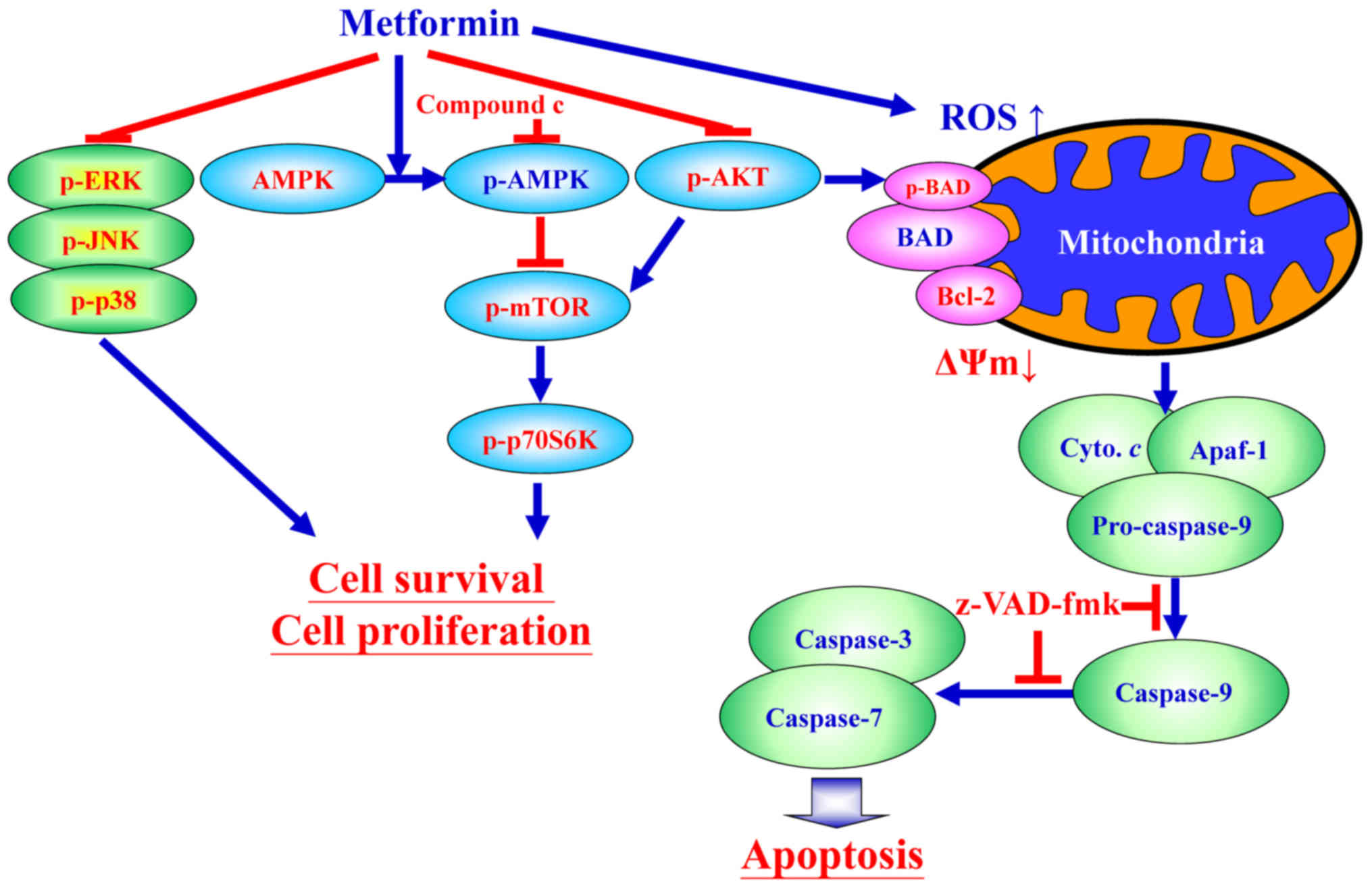 | Figure 9Schematic diagram of an integrated
circuit regarding that AMPK, AKT/mTOR, and apoptosis-related
molecular machinery caused by metformin in human gastric
adenocarcinoma AGS cells. p-, phospho; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; AMPK,
adenosine monophosphate-activated protein kinase; mTOR, mammalian
target of rapamycin; p70S6K, ribosomal protein S6 kinase B1; AKT,
protein kinase B; ROS, reactive oxygen species; BAD,
Bcl-2-associated agonist of cell death; Bcl-2, B-cell lymphoma-2;
ΔΨm, mitochondrial membrane potential; Cyto. c, cytochrome
c; Apaf-1, apoptotic protease-activating factor-1;
z-VAD-fmk, carbobenzoxyvalyl-alanyl-aspartyl fluoromethyl
ketone. |
Discussion
Metformin, an oral biguanide agent that was
FDA-approved in 1957, has been used as a safe and cost-efficient
treatment for T2D worldwide (45,46).
Numerous studies have indicated that long-term administration of
metformin reduces the risk of various types of cancer, including
breast, colon and endometrial cancer, and glioma (13-17,20,47).
Recently, Li et al (48)
demonstrated that metformin can increase the survival rate of
diabetic patients with gastric cancer. Previous studies have
demonstrated that metformin inhibits cell proliferation and induces
cell death in various types of cancer cells, including HepG2
hepatoma cells (49), SKOV3, A2780
and ES2 ovarian cancer cells (50,51),
paclitaxel-resistant A2780-PR and cisplatin-resistant ACRP cells
(52), B16F10 melanoma cells
(53), Dami and MEG-01
megakaryoblastic cancer cells (54), and CAL 27, CAL 33, and UMSCC47 head
and neck carcinoma cells (55).
Furthermore, metformin also suppresses the cell metastasis of MG63
and U-2 OS osteosarcoma cells (56), SiHa and HeLa cervical cancer cells
(57), and EC109 esophageal
squamous cells carcinoma cells (58). In addition, synergistic
interactions with metformin enhance antitumor activities; for
example, sirolimus in colorectal cancer cells (59), chrysin in breast cancer (60), quercetin in prostate cancer cells
(24), rapamycin in pancreatic
cancer cells (61), vincristine in
leukemia cancer cells (62),
curcumin in hepatocellular carcinoma cells (63), cisplatin in gallbladder cancer
cells (64). Metformin at 10-100
mM has been reported to dose- and time-dependently inhibit cell
proliferation in AGS cells in low-and high-glucose conditioned
media (36). In the current study,
the results revealed that treatment with 50 mM metformin
significantly inhibited the viability of AGS cells (Video S1). These results are in
accordance with those from a study by Valaee et al (36), indicating that metformin suppresses
the proliferation and viability of AGS cells. An in vivo
study also demonstrated that metformin did not cause apparent
toxicity in nude mice bearing with hepatocellular carcinoma tumors
(65). The findings also revealed
that metformin has no effect on viability in normal cells (human
colon CCD 841 CoN, embryonic lung HEL 299 and 293 cells).
AMPK is a serine/threonine protein kinase (10,11).
AMPK signaling is a cellular energy and nutrient sensor, and also
has an essential role in metabolic pathways (27,28).
AMPK activation inhibits protein synthesis and cell proliferation
(11,28). Furthermore, activation of the AMPK
signaling inhibits tumor growth (27,28).
Metformin suppresses the respiratory complex I, which increases the
adenosine diphosphate/adenosine triphosphate (ATP) and AMP/ATP
ratios, and attenuates of ATP production and oxidative
phosphorylation, resulting reduced cellular ATP and activation of
AMPK (10,12). Zakikhani et al (66) demonstrated that metformin
attenuates the proliferation of breast cancer cells through the
activation of AMPK, causing the inhibition of mTOR signaling.
Metformin activates the expression of AMPK and inhibits
phosphorylation of mTOR, downstream p70S6K, and eIF4E-binding
proteins (67). The present study
demonstrated that metformin-induced apoptosis was accompanied by
upregulation AMPK Thr172 phosphorylation, and downregulation of AKT
(Ser473), mTOR (Ser2448) and p70S6K (Ser424) phosphorylation. The
data also demonstrated that attenuation of AMPK signaling using an
AMPK inhibitor (compound C) abrogated the effects of metformin on
the viability of AGS cells.
MAPKs include three main molecules, ERK, JNK and
p38, which have various biological functions, including apoptotic
mechanisms, cell cycle regulation and cell survival (43,44).
Activation of AMPK signaling and the attenuation of ERK signaling
contribute to the antitumor effects of metformin in MCF-7 breast
cancer cells (68). Furthermore,
the inhibitory effect of metformin on MAPK activity is involved in
protection against atherosclerosis (69). Lu and Xu (70) demonstrated that ERK1/2 activation
can inhibit cell apoptosis via modulation of tumor necrosis factor,
Fas ligand, radiation stress, hypoxia and response to
chemotherapeutic agents. Potapova et al (71) indicated that inhibition of JNK2
activity can also suppress tumorigenesis via promotion of cell
apoptosis. Subramanian and Shaha (72) suggested that an estrogen-induced
increase in Ca2+ leads to ERK phosphorylation and,
consequently, phosphorylation of cAMP responsive element binding
protein 1, resulting in an increase in the expression of
anti-apoptotic Bcl-2 protein. Furthermore, p38 has a role in cell
survival and promotes increased levels of Bcl-2 and Bcl-xL in
response to DNA damage and stress (73,74).
The current study demonstrated that metformin-induced apoptosis may
be mediated via downregulation of ERK, JNK and p38 phosphorylation,
and Bcl-2 expression in AGS cells. Phosphorylation of MAPKs may be
involved in Bcl-2 modulation in metformin-induced apoptosis of AGS
cells. Additionally, metformin was previously reported to inhibit
the invasion of human hepatocellular carcinoma cells via
downregulation of ERK/JNK-mediated nuclear factor-κB-dependent
signaling (75). The findings of
the current study are in accordance with previous reports, and
suggesting that metformin-suppressed cell growth is associated with
AMPK-modulated AKT/mTOR and MAPK signaling pathways.
Wang et al (76) and Gao et al (77) have reported that metformin induces
mitochondria-dependent apoptosis in human lung adenocarcinoma A549
cells and in human MDA-MB-231 and MDA-MB-435 breast cancer cells.
Energy disruptors and AMPK activation lead to
mitochondria-dependent apoptosis. Metformin is an energy disruptor
and activator of AMPK (76,77).
The current study investigated apoptosis induction and
mitochondria-dependent pathway by ROS production, and the protein
expression levels of pro- and anti-apoptotic proteins in
metformin-treated AGS cells. The results suggest that metformin
promotes caspase-dependent mitochondria-derived apoptosis in AGS
cells and are in agreement with the previous study by Xiong et
al (49).
Metformin has been established to exhibit clinical
efficacy in conditions characterized by hyperinsulinemia, including
polycystic ovarian syndrome, gestational diabetes, non-alcoholic
steatohepatitis and pre-diabetes (78). Anticancer effects of metformin have
been reported in various cancer types. In non-small cell lung
cancer, metformin monotreatment or combined treatment resulted in
decreased cell proliferation and increased apoptotic death
(79). In colorectal cancer (CRC),
metformin was demonstrated to interfere with the EMT process
(80). Patients with T2D treated
with metformin exhibited a lower rate of CRC than non-metformin
users, with a statistically significant cumulative tumor-free
survival (81). In breast cancer,
cell growth was reduced by targeting the AMPK signaling pathway
(82). The results of the present
study suggested that metformin may be a promising therapy for human
gastric adenocarcinoma and useful as an adjunct to other
chemotherapies. There are two molecular actions of metformin can be
implicated in anticancer actions (29): i) By decreasing insulinemia and
glycemia action, metformin can block the PI3K/MAPKs signaling
pathway, which are implicated in cancer cell growth (81); and ii) metformin can directly act
on cancer cells by targeting various processes, including tumor
cell metabolism, inflammation, angiogenesis and cancer stem cells,
via the activation of the AMPK pathway (36,81).
Metformin may become an alternative cancer adjuvant therapy,
providing a novel approach for cancer prevention and treatment.
In conclusion, the findings of the current study
provide an understanding of the mechanisms of metformin that can
induce apoptosis of AGS cells through AMPK/AKT/mTOR signaling
(Fig. 9). It is probable complete
underlying mechanisms involved and the inhibitory effect of
metformin on human gastric adenocarcinoma AGS cells have not been
fully elucidated. The present study supports further research on
the therapeutic use of metformin in treating human gastric cancer
should be performed in the near future.
Supplementary Materials
Funding
This study was supported by the project (grant no.
TCRD107-55) from the Hualien Tzu Chi Hospital (Hualien, Taiwan) and
in part by the China Medical University Hospital (Taichung, Taiwan;
grant no. DMR-107-123).
Availability of data and materials
The data sets generated during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
CL, JY and HC conceived and designed the
experiments. JC, YH and YJ performed the experiments. CL, FT and JY
analyzed the data. CL, JY and HC wrote and modified the paper. All
authors read and approved the final manuscript.
Ethics approval and consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We wish to acknowledge the work of Mr. Chang-Wei Li
(AllBio Science Incorporated, Taichung, Taiwan) for the excellent
technique. We also thank Mr. Meng-Jou Liao and Mr. Chin-Chen Lin
(Tekon Scientific Corp., Taipei, Taiwan) for their assistance and
equipment support on this study.
References
|
1
|
Newell M, Baker K, Postovit LM and Field
CJ: A critical review on the effect of docosahexaenoic acid (DHA)
on cancer cell cycle progression. Int J Mol Sci. 18:182017.
View Article : Google Scholar
|
|
2
|
Ministry of Health and Welfare: Republic
of China (Taiwan). https://goo.gl/K1mgSD.
2018
|
|
3
|
Lee YY and Derakhshan MH: Environmental
and lifestyle risk factors of gastric cancer. Arch Iran Med.
16:358–365. 2013.PubMed/NCBI
|
|
4
|
Khatoon J, Rai RP and Prasad KN: Role of
Helicobacter pylori in gastric cancer: Updates. World J
Gastrointest Oncol. 8:147–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American Cancer Society. https://goo.gl/QdHTvk.
2018
|
|
6
|
Tebbutt NC, Cummins MM, Sourjina T,
Strickland A, Van Hazel G, Ganju V, Gibbs D, Stockler M, Gebski V
and Zalcberg J; Australasian Gastro-Intestinal Trials Group:
Randomised, non-comparative phase II study of weekly docetaxel with
cisplatin and 5-fluorouracil or with capecitabine in
oesophagogastric cancer: The AGITG ATTAX trial. Br J Cancer.
102:475–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wöhrer SS, Raderer M and Hejna M:
Palliative chemotherapy for advanced gastric cancer. Ann Oncol.
15:1585–1595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou YC, Hu Q, Huang J, Fang JY and Xiong
H: Metformin therapy and the risk of colorectal adenoma in patients
with type 2 diabetes: A meta-analysis. Oncotarget. 8:8843–8853.
2017.
|
|
9
|
Castilla-Guerra L, Fernandez-Moreno MD,
Leon-Jimenez D and Carmona-Nimo E: Antidiabetic drugs and stroke
risk. Current evidence Eur J Intern Med. 48:1–5. 2018. View Article : Google Scholar
|
|
10
|
Coughlan KA, Valentine RJ, Ruderman NB and
Saha AK: AMPK activation: A therapeutic target for type 2 diabetes?
Diabetes Metab Syndr Obes. 7:241–253. 2014.PubMed/NCBI
|
|
11
|
Nyane NA, Tlaila TB, Malefane TG, Ndwandwe
DE and Owira PM: Metformin-like antidiabetic, cardio-protective and
non-glycemic effects of naringenin: Molecular and pharmacological
insights. Eur J Pharmacol. 803:103–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng J, Woo SL, Hu X, Botchlett R, Chen
L, Huo Y and Wu C: Metformin and metabolic diseases: A focus on
hepatic aspects. Front Med. 9:173–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mallik R and Chowdhury TA: Metformin in
cancer. Diabetes Res Clin Pract. 143:409–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bridgeman SC, Ellison GC, Melton PE,
Newsholme P and Mamotte CD: Epigenetic effects of metformin: From
molecular mechanisms to clinical implications. Diabetes Obes Metab.
20:1553–1562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan M, Wu A, Liao N, Liu M, Guo Q, Yi J,
Wang T, Huang Y, Qiu B and Zhou W: Inhibiting ROS-TFE3-dependent
autophagy enhances the therapeutic response to metformin in breast
cancer. Free Radic Res. 52:872–886. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amaral I, Silva C, Correia-Branco A and
Martel F: Effect of metformin on estrogen and progesterone
receptor-positive (MCF-7) and triple-negative (MDA-MB-231) breast
cancer cells. Biomed Pharmacother. 102:94–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fransgaard T, Thygesen LC and Gögenur I:
Association between metformin use after surgery for colorectal
cancer and oncological outcomes: A nationwide register-based study.
Int J Cancer. 143:63–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai X, Hu X, Cai B, Wang Q, Li Y, Tan X,
Hu H, Chen X, Huang J, Cheng J, et al: Metformin suppresses
hepatocellular carcinoma cell growth through induction of cell
cycle G1/G0 p hase arrest and p21CIP and p27KIP expression and
downregulation of cyclin D1 in vitro and in vivo. Oncol Rep.
30:2449–2457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia C, Liang S, He Z, Zhu X, Chen R and
Chen J: Metformin, a first-line drug for type 2 diabetes mellitus,
disrupts the MALAT1/miR-142-3p sponge to decrease invasion and
migration in cervical cancer cells. Eur J Pharmacol. 830:59–67.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai M, Yang L, Liao H, Liang X, Xie B,
Xiong J, Tao X, Chen X, Cheng Y, Chen X, et al: Metformin
sensitizes endometrial cancer cells to chemotherapy through
IDH1-induced Nrf2 expression via an epigenetic mechanism. Oncogene.
37:5666–5681. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kheirandish M, Mahboobi H, Yazdanparast M,
Kamal W and Kamal MA: Anticancer effects of metformin: Recent
evidences for its role in prevention and treatment of cancer. Curr
Drug Metab. 19:793–797. 2018. View Article : Google Scholar
|
|
22
|
Lacroix O, Couttenier A, Vaes E, Cardwell
CR, De Schutter H and Robert A: Impact of metformin on gastric
adenocarcinoma survival: A Belgian population based study. Cancer
Epidemiol. 53:149–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Gao WN, Xue YN, Zhang LC, Zhang JJ,
Lu SY, Yan XY, Yu HM, Su J and Sun LK: SIRT3 aggravates
metformin-induced energy stress and apoptosis in ovarian cancer
cells. Exp Cell Res. 367:137–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun S, Gong F, Liu P and Miao Q: Metformin
combined with quercetin synergistically repressed prostate cancer
cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene.
664:50–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu R, Yang J, Wei R, Ke J, Tian Q, Yu F,
Liu J, Zhang J and Hong T: Synergistic antitumor effects of
liraglutide with metformin on pancreatic cancer cells. PLoS One.
13:e01989382018. View Article : Google Scholar
|
|
26
|
Wei M, Mao S, Lu G, Li L, Lan X, Huang Z,
Chen Y, Zhao M, Zhao Y and Xia Q: Valproic acid sensitizes
metformin-resistant human renal cell carcinoma cells by
upregulating H3 acetylation and EMT reversal. BMC Cancer.
18:4342018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng M, Darko KO, Tao T, Huang Y, Su Q, He
C, Yin T, Liu Z and Yang X: Combination of metformin with
chemotherapeutic drugs via different molecular mechanisms. Cancer
Treat Rev. 54:24–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sośnicki S, Kapral M and Węglarz L:
Molecular targets of metformin antitumor action. Pharmacol Rep.
68:918–925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daugan M, Dufaÿ Wojcicki A, d’Hayer B and
Boudy V: Metformin: An anti-diabetic drug to fight cancer.
Pharmacol Res. 113:675–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen G, Feng W, Zhang S, Bian K, Yang Y,
Fang C, Chen M, Yang J and Zou X: Metformin inhibits gastric cancer
via the inhibition of HIF1α/PKM2 signaling. Am J Cancer Res.
5:1423–1434. 2015.
|
|
31
|
Dorn GW II: Molecular mechanisms that
differentiate apoptosis from programmed necrosis. Toxicol Pathol.
41:227–234. 2013. View Article : Google Scholar
|
|
32
|
Fulda S: The mechanism of necroptosis in
normal and cancer cells. Cancer Biol Ther. 14:999–1004. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baig S, Seevasant I, Mohamad J, Mukheem A,
Huri HZ and Kamarul T: Potential of apoptotic pathway-targeted
cancer therapeutic research: Where do we stand? Cell Death Dis.
7:e20582016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Courtois S, Durán RV, Giraud J, Sifré E,
Izotte J, Mégraud F, Lehours P, Varon C and Bessède E: Metformin
targets gastric cancer stem cells. Eur J Cancer. 84:193–201. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han G, Gong H, Wang Y, Guo S and Liu K:
AMPK/mTOR-mediated inhibition of survivin partly contributes to
metformin-induced apoptosis in human gastric cancer cell. Cancer
Biol Ther. 16:77–87. 2015. View Article : Google Scholar :
|
|
36
|
Valaee S, Yaghoobi MM and Shamsara M:
Metformin inhibits gastric cancer cells metastatic traits through
suppression of epithelial-mesenchymal transition in a
glucose-independent manner. PLoS One. 12:e01744862017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu CC, Huang BR, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840.2310–2320. 2014.
|
|
39
|
Chang HP, Lu CC, Chiang JH, Tsai FJ, Juan
YN, Tsao JW, Chiu HY and Yang JS: Pterostilbene modulates the
suppression of multidrug resistance protein 1 and triggers
autophagic and apoptotic mechanisms in cisplatin-resistant human
oral cancer CAR cells via AKT signaling. Int J Oncol. 52:1504–1514.
2018.
|
|
40
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG, et al: Antitumor effects
of emodin on LS1034 human colon cancer cells in vitro and in vivo:
Roles of apoptotic cell death and LS1034 tumor xenografts model.
Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lin JJ, Huang WW, Tsuzuki M, Lee TH and Chung JG: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Motoshima H, Goldstein BJ, Igata M and
Araki E: AMPK and cell proliferation - AMPK as a therapeutic target
for atherosclerosis and cancer. J Physiol. 574:63–71. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eblen ST: Extracellular-regulated kinases:
Signaling from Ras to ERK substrates to control biological
outcomes. Adv Cancer Res. 138:99–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Peluso I, Yarla NS, Ambra R, Pastore G and
Perry G: MAPK signalling pathway in cancers: Olive products as
cancer preventive and therapeutic agents. Semin Cancer Biol: Sep.
11:2017(Epub ahead of print). View Article : Google Scholar
|
|
45
|
Bailey CJ: Metformin: Historical overview.
Diabetologia. 60:1566–1576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kinaan M, Ding H and Triggle CR:
Metformin: An old drug for the treatment of diabetes but a new drug
for the protection of the endothelium. Med Princ Pract. 24:401–415.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Seliger C, Meyer AL, Renner K, Leidgens V,
Moeckel S, Jachnik B, Dettmer K, Tischler U, Gerthofer V, Rauer L,
et al: Metformin inhibits proliferation and migration of
glioblastoma cells independently of TGF-β2. Cell Cycle.
15:1755–1766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li P, Zhang C, Gao P, Chen X, Ma B, Yu D,
Song Y and Wang Z: Metformin use and its effect on gastric cancer
in patients with type 2 diabetes: A systematic review of
observational studies. Oncol Lett. 15:1191–1199. 2018.PubMed/NCBI
|
|
49
|
Xiong Y, Lu QJ, Zhao J and Wu GY:
Metformin inhibits growth of hepatocellular carcinoma cells by
inducing apoptosis via mitochondrion-mediated pathway. Asian Pac J
Cancer Prev. 13:3275–3279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang G, Guo J, Zhu Y, Huang Z, Liu T, Cai
J, Yu L and Wang Z: Metformin inhibits ovarian cancer via
decreasing H3K27 trimethylation. Int J Oncol. 52:1899–1911.
2018.PubMed/NCBI
|
|
51
|
Huo J, Bian XH, Huang Y, Miao ZC and Song
LH: Inhibitory effect and mechanism of metformin on human ovarian
cancer cells SKOV-3 and A2780. Eur Rev Med Pharmacol Sci.
21:484–489. 2017.PubMed/NCBI
|
|
52
|
Dos Santos, Guimarães I, Ladislau-Magescky
T, Tessarollo NG, Dos Santos DZ, Gimba ERP, Sternberg C, Silva IV
and Rangel LBA: Chemosensitizing effects of metformin on cisplatin-
and paclitaxel-resistant ovarian cancer cell lines. Pharmacol Rep.
70:409–417. 2018. View Article : Google Scholar
|
|
53
|
Tomic T, Botton T, Cerezo M, Robert G,
Luciano F, Puissant A, Gounon P, Allegra M, Bertolotto C, Bereder
JM, et al: Metformin inhibits melanoma development through
autophagy and apoptosis mechanisms. Cell Death Dis. 2:e1992011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liang X, Kong P, Wang J, Xu Y, Gao C and
Guo G: Effects of metformin on proliferation and apoptosis of human
megakaryoblastic Dami and MEG-01 cells. J Pharmacol Sci. 135:14–21.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Madera D, Vitale-Cross L, Martin D,
Schneider A, Molinolo AA, Gangane N, Carey TE, McHugh JB, Komarck
CM, Walline HM, et al: Prevention of tumor growth driven by PIK3CA
and HPV oncogenes by targeting mTOR signaling with metformin in
oral squamous carcinomas expressing OCT3. Cancer Prev Res (Phila).
8:197–207. 2015. View Article : Google Scholar
|
|
56
|
Li Z, Wang L, Luo N, Zhao Y, Li J, Chen Q
and Tian Y: Metformin inhibits the proliferation and metastasis of
osteo-sarcoma cells by suppressing the phosphorylation of Akt.
Oncol Lett. 15:7948–7954. 2018.PubMed/NCBI
|
|
57
|
Cheng K and Hao M: Metformin inhibits
TGF-β1-induced epithelial-to-mesenchymal transition via PKM2
relative-mTOR/p70s6k signaling pathway in cervical carcinoma cells.
Int J Mol Sci. 17:172016. View Article : Google Scholar
|
|
58
|
He Y, Tan X, Hu H, Wang Q, Hu X, Cai X,
Guan Y, Chen B and Jing X: Metformin inhibits the migration and
invasion of esophageal squamous cell carcinoma cells by
downregulating the protein kinase B signaling pathway. Oncol Lett.
15:2939–2945. 2018.PubMed/NCBI
|
|
59
|
Mussin N, Oh SC, Lee KW, Park MY, Seo S,
Yi NJ, Kim H, Yoon KC, Ahn SW, Kim HS, et al: Sirolimus and
metformin synergistically inhibits colon cancer in vitro and in
vivo. J Korean Med Sci. 32:1385–1395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rasouli S and Zarghami N: Synergistic
growth inhibitory effects of chrysin and metformin combination on
breast cancer cells through hTERT and cyclin D1 suppression. Asian
Pac J Cancer Prev. 19:977–982. 2018.PubMed/NCBI
|
|
61
|
Zhang JW, Zhao F and Sun Q: Metformin
synergizes with rapamycin to inhibit the growth of pancreatic
cancer in vitro and in vivo. Oncol Lett. 15:1811–1816.
2018.PubMed/NCBI
|
|
62
|
Yi Y, Gao L, Wu M, Ao J, Zhang C, Wang X,
Lin M, Bergholz J, Zhang Y and Xiao ZJ: Metformin sensitizes
leukemia cells to vincristine via activation of AMP-activated
protein kinase. J Cancer. 8:2636–2642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao
ZQ, Yu LG and Guo XL: Metformin incombination with curcumin
inhibits the growth, metastasis, and angiogenesis of hepatocellular
carcinoma in vitro and in vivo. Mol Carcinog. 57:44–56. 2018.
View Article : Google Scholar
|
|
64
|
Bi T, Zhu A, Yang X, Qiao H, Tang J, Liu Y
and Lv R: Metformin synergistically enhances antitumor activity of
cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway.
Cytotechnology. 70:439–448. 2018. View Article : Google Scholar :
|
|
65
|
Zhang Q, Kong J, Dong S, Xu W and Sun W:
Metformin exhibits the anti-proliferation and anti-invasion effects
in hepatocellular carcinoma cells after insufficient radiofrequency
ablation. Cancer Cell Int. 17:482017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jiang W, Zhu Z and Thompson HJ: Dietary
energy restriction modulates the activity of AMP-activated protein
kinase, Akt, and mammalian target of rapamycin in mammary
carcinomas, mammary gland, and liver. Cancer Res. 68:5492–5499.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Malki A and Youssef A: Antidiabetic drug
metformin induces apoptosis in human MCF breast cancer via
targeting ERK signaling. Oncol Res. 19:275–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Eriksson L and Nyström T: Activation of
AMP-activated protein kinase by metformin protects human coronary
artery endothelial cells against diabetic lipoapoptosis. Cardiovasc
Diabetol. 13:1522014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lu Z and Xu S: ERK1/2 MAP kinases in cell
survival and apoptosis. IUBMB Life. 58:621–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Potapova O, Anisimov SV, Gorospe M,
Dougherty RH, Gaarde WA, Boheler KR and Holbrook NJ: Targets of
c-Jun NH(2)-terminal kinase 2-mediated tumor growth regulation
revealed by serial analysis of gene expression. Cancer Res.
62:3257–3263. 2002.PubMed/NCBI
|
|
72
|
Subramanian M and Shaha C: Up-regulation
of Bcl-2 through ERK phosphorylation is associated with human
macrophage survival in an estrogen microenvironment. J Immunol.
179:2330–2338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Flacke JP, Kumar S, Kostin S, Reusch HP
and Ladilov Y: Acidic preconditioning protects endothelial cells
against apoptosis through p38- and Akt-dependent Bcl-xL
overexpression. Apoptosis. 14:90–96. 2009. View Article : Google Scholar :
|
|
74
|
Kim MJ, Choi SY, Park IC, Hwang SG, Kim C,
Choi YH, Kim H, Lee KH and Lee SJ: Opposing roles of c-Jun
NH2-terminal kinase and p38 mitogen-activated protein kinase in the
cellular response to ionizing radiation in human cervical cancer
cells. Mol Cancer Res. 6:1718–1731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hsieh SC, Tsai JP, Yang SF, Tang MJ and
Hsieh YH: Metformin inhibits the invasion of human hepatocellular
carcinoma cells and enhances the chemosensitivity to sorafenib
through a downregulation of the ERK JNK-mediated NF-κB-dependent
pathway that reduces uPA and MMP-9 expression. Amino Acids.
46:2809–2822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang J, Gao Q, Wang D, Wang Z and Hu C:
Metformin inhibits growth of lung adenocarcinoma cells by inducing
apoptosis via the mitochondria-mediated pathway. Oncol Lett.
10:1343–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gao ZY, Liu Z, Bi MH, Zhang JJ, Han ZQ,
Han X, Wang HY, Sun GP and Liu H: Metformin induces apoptosis via a
mitochondria-mediated pathway in human breast cancer cells in
vitro. Exp Ther Med. 11:1700–1706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Inzucchi SE, Bergenstal RM, Buse JB,
Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R and
Matthews DR: Management of hyperglycemia in type 2 diabetes, 2015:
A patient-centered approach: update to a position statement of the
American Diabetes Association and the European Association for the
Study of Diabetes. Diabetes Care. 38:140–149. 2015. View Article : Google Scholar
|
|
79
|
Yousef M and Tsiani E: Metformin in Lung
Cancer: Review of in vitro and in vivo animal studies. Cancers
(Basel). 9:92017. View Article : Google Scholar
|
|
80
|
Wang Y, Wu Z and Hu L:
Epithelial-mesenchymal transition phenotype, metformin, and
survival for colorectal cancer patients with diabetes mellitus II.
Gastroenterol Res Pract. 2017.2520581:2017.
|
|
81
|
Su T, Liao B, Dong Y, Peng Z, Zhou Q, Li
B, Peng S and Zhang N: Effect of metformin on colorectal carcinoma
in type 2 diabetes mellitus patients: A Markov model analysis.
Zhonghua Wei Chang Wai Ke Za Zhi. 20:689–693. 2017.In Chinese.
PubMed/NCBI
|
|
82
|
Zhang J, Li G, Chen Y, Fang L, Guan C, Bai
F, Ma M, Lyu J and Meng QH: Metformin inhibits tumorigenesis and
tumor growth of breast cancer cells by upregulating miR-200c but
downregulating AKT2 expression. J Cancer. 8:1849–1864. 2017.
View Article : Google Scholar : PubMed/NCBI
|