Introduction
The Runt-related transcription factor (RUNX) family
comprises context-dependent transcriptional regulators including
RUNX1, RUNX2 and RUNX3 in humans. The RUNX family has a highly
conserved Runt-homology domain that mediates site-specific DNA
binding and interactions with various proteins (1,2).
RUNX1 is essential for hematopoiesis; RUNX2 is involved in
osteogenesis during bone formation, and RUNX3 is expressed in
epithelial, neuronal and hematopoietic stem cells, and is involved
in the transforming growth factor (TGF)-β signaling pathway
(3), in which it functions to
control the lineage differentiation of hematopoietic and neuronal
progenitor cells (4). RUNX3 has
been documented to function as a tumor suppressor in numerous types
of tumors (5). Under hypoxic
conditions, RUNX3 is silenced by histone deacetylation and
methylation via histone deacetylase 1 and G9a histone
methyltransferase, respectively (6).
Blood vessel formation occurs via two distinct
mechanisms, namely vasculogenesis and angiogenesis; angiogenesis
comprises endothelial sprouting and intussusception from
pre-existing vessels, while vasculogenesis initiates from vascular
stem cells or hemangioblasts in the developing embryo or adult bone
marrow (BM) (7). Endothelial
progenitor cells (EPCs), mainly derived from BM hemangioblasts and
hematopoietic stem cells (HSCs), possess an ability to self-renew,
mobilize into the circulatory system, migrate to sites of
neovascularization and differentiate into mature endothelial cells
contributing to neonatal vasculogenesis as well as tumor
vasculature (8-10). Generally, EPCs can be obtained by
isolating a heterogeneous subpopulation of BM-derived mononuclear
cells (MNCs) that respond to stresses, such as tissue ischemia and
vascular-damaging signals (11,12).
In a previous study, an EPC colony-forming unit (CFU) assay was
used to discriminate the hierarchical lineage between primitive
(small colony) and definitive (large colony) CFUs in vitro
(13). These EPC CFUs take up
3,3′-dioctadecylindocarbocyanin-labeled acetylated low-density
lipoprotein (Dil-Ac-LDL), bind with isolectin B4, and express
vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) and
endothelial nitric oxide synthase (eNOS) independent of BM-MNC
isolation markers (13,14). Using a short-term protocol (4-7
days culture), it is possible to obtain cells with
myeloid/hematopoietic and typical endothelial cell markers that
function as therapeutics in ischemic diseases (15,16).
Cord blood-derived EPCs enhance tissue
neovascularization via physical incorporation into the ischemic
area and the perivascular paracrine effect (17). Hypoxia or ischemic stress is a
stimulator of EPC function through the activation of
hypoxia-induced signaling molecules, such as signal transducer and
activator of transcription 3 in the preconditioning to hypoxia
(18). A number of studies have
demonstrated that EPCs promote the angiogenic switch in solid
tumors, thereby increasing tumor growth and metastasis (10). Likewise, the genetic suppression of
EPC mobilization from BM inhibits tumor development and
colonization to remote organs (19). These findings indicate that EPCs
may be used as cell therapeutics in injured or regenerative
tissues, and also as therapeutic targets for the inhibition of
tumor growth or metastasis.
In a previous study, the present research team
identified a new function of RUNX3 that facilitates the degradation
of hypoxia-inducible factor (HIF)-1α by promoting the interaction
of proline hydroxylases (PHDs) with HIF-1α, thus inhibiting
hypoxia-induced VEGF secretion and angiogenesis in vitro and
in vivo (20). Hypoxia is a
critical microenvironment for the differentiation and maintenance
of hematopoietic stem cells (18,21)
and leukemia development (22).
However, the role of Runx3 in the differentiation and function in
EPCs has not yet been investigated.
In the present study, using a Runx3
heterozygous deletion mutant mouse model, it was demonstrated that
the mouse homolog Runx3 inhibits vasculogenesis via the suppression
of EPC differentiation and function. The mobilization of EPCs into
peripheral blood (23), their
differentiation into neovessels and homing to an ischemic limb
region was significantly increased in Runx3 heterozygous
mice. Signaling pathways involved in the maintenance or function of
EPCs, as well as HIF-1α were also significantly upregulated by
Runx3 heterozygous deletion. These results suggest that
Runx3 suppresses EPC function and differentiation from BM-derived
MNCs by the suppression of HIF-1α.
Materials and methods
Mice
The animal experiments were approved according to
the guidelines for care and use of laboratory animals by the
Kyungpook National University Institutional Animal Care and Use
Committee (Daegu, Korea). FVB/N male mice (n=10, 4-6 weeks old,
10-12 g; Japan SLC, Inc., Hamamatsu, Japan) were maintained under
specific pathogen-free conditions with a constant humidity and
tempera ture at 26°C, filtered air and a 12/12-h light/dark cycle.
Mice were treated with CO2 by inhalation in a chamber
for anesthesia, immediately prior to sacrifice. Runx3
heterozygous mice (Rx3+/−) were generated as previously
reported (24).
Isolation of mouse MNCs from BM and PB,
and hypoxic conditions
MNCs were isolated from the BM or PB of wild-type
(WT) and Rx3+/− mice using a Histopaque®-1083
density gradient centrifugation method (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) according to the manufacturer's instructions.
Briefly, BM or PB cells were centrifuged at 400 × g for 30 min at
room temperature following the addition of Histopaque-1083 and
MNC-containing layers were isolated. The freshly isolated MNCs were
washed with 5 mM EDTA in PBS and suspended in 4 ml of 0.8% ammonium
chloride (StemCell Technologies, Inc., Vancouver, BC, Canada) for 5
min at 4°C. The MNCs were then resuspended in Endothelial Cell
Growth Medium (EGM)-2 (Lonza Group, Ltd., Basel, Switzerland)
supplemented with 5% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), basic fibroblast growth
factor (bFGF; R&D Systems, Wiesbaden, Germany), VEGF
(PeproTech, Inc., Rocky Hill, CT, USA), insulin-like growth
factor-1 (R&D Systems), epidermal growth factor (EGF;
PeproTech, Inc.), ascorbic acid and heparin (Sigma-Aldrich; Merck
KGaA), and then seeded onto 60-mm dishes coated with 2% gelatin
(2×107 cells/dish). After culture for 3 days at 37°C,
the non-adherent cells were removed by washing with PBS, and the
attached cells were cultured for a further 4 days. These cells are
designated 'early EPCs' (15,16).
For exposure of the early EPCs to hypoxic conditions, hypoxic
chambers (Thermo Fisher Scientific, Inc., Waltham, MA, USA and
Astec Co., Ltd., Kasuya, Japan) were used for maintaining low
oxygen tension (1% O2 and 5% CO2, balanced
with N2).
EPC colony-forming assay
The isolation of
c-kit+/Sca-1−/Lin− (KSL) cells
from mouse BM-MNCs was performed as previously described (14,25,26).
The numbers of small and large colonies, i.e., primitive
endothelial progenitor cell colonies with clusters of homogeneous
small immature cells and definitive endothelial progenitor cell
colonies with clusters of large spindle-shaped differentiated cells
were counted following the culture of KSL cells (500 cells/35-mm
dish) for 10 days in a methyl cellulose-containing medium (M3236;
(StemCell Technologies, Inc.) with 20 ng/ml stem cell factor
(Sigma-Aldrich; Merck KGaA), 50 ng/ml VEGF, 20 ng/ml interleukin-3
(R&D Systems, Inc., Minneapolis, MN, USA), 50 ng/ml bFGF, 50
ng/ml EGF, 2 U/ml heparin, 30% FBS and antibiotics (100 U/ml
penicillin and 100 mg/ml streptomycin; Thermo Fisher Scientific,
Inc.). The CFUs were counted by blinded investigators 12 days after
seeding by visual inspection with an inverted microscope at x40
magnification. The expression of additional endothelial marker
genes, such as cluster of differentiation (CD)31, VEGFR2 [also
known as fetal liver kinase 1 (Flk1)], von Willebrand factor,
vascular endothelial cadherin and eNOS in small and large CFUs was
conducted as previously described (14).
Cell counting kit-8 (CCK-8) assay
EPCs (3×103) were cultured on 96-well
plates. Following 24 or 36 h incubation in a hypoxic chamber, CCK-8
solution (Dojindo Molecular Technologies, Inc., Rockville, MD, USA)
was added to each well and incubated for another 2 h at 37°C.
Absorbance of the plate was measured with a microplate reader
(Infinite M200 Pro; Tecan Group, Ltd., Mannedorf, Switzerland) at
540 nm. Three replicate wells were used for each analysis.
Migration assay of early EPCs
A migration assay of early EPCs was performed as
previously reported (27).
Briefly, using a modified Boyden chamber, 1 day-starved early EPCs
(1×104) were seeded onto a 24-well Transwell membrane
and treated with VEGF (10 ng/ml) or stromal cell-derived factor
(SDF)-1α (100 ng/ml) in the bottom chamber for 6 h. Migrated EPCs
were stained with hematoxylin and eosin, and then counted using a
CKX41SF microscope (Olympus Corporation, Tokyo, Japan).
Matrigel tube formation assay
Early EPCs (1:1; 1×104 cells/100
µl 5% FBS/EGM-2) were incubated with 0.4 µg/ml
DiI-Ac-LDL (Biomedical Technologies Inc., Stoughton, MA, USA) for 4
h at 37°C and then cocultured with human umbilical vein endothelial
cells (HUVECs; BD Biosciences, San Jose, CA, USA) on a 96-well
culture plate coated with Matrigel® (BD Biosciences) for
30 min at 37°C. Plates were examined for tube formation following
incubation for 8 h. The tube lengths and the number of incorporated
DiI-expressing EPCs per HPF were counted (n=3/group) using a
microscope (Olympus Corporation) at x200 magnification.
Enzyme-linked immunosorbent assay
(ELISA)
The amount of VEGF protein secreted by EPCs into the
medium was determined using a VEGF ELISA kit (cat. no. MWV00;
R&D Systems). The cells were cultured in 6-well plates until
they reached 80-90% confluence and the amount of VEGF was
quantified using a microplate reader (Infinite M200 Pro; Tecan
Group, Ltd.)
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and semi-quantitative PCR
Total RNA was isolated from mouse early EPCs using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and RT-qPCR was
performed as previously reported (28). The semi-quantitative PCR was
performed using AccuPower PCR premix (Bioneer Corporation, Daejeon,
Korea). The PCR conditions were as follows: 28 cycles of
denaturation (94°C/1 min), annealing (60°C/1 min), extension
(72°C/1 min) and final extension (72°C/10 min). The primer
sequences for the genes tested are shown in Table I. PCR products were separated on
1.5% agarose gels in 1X TAE buffer, stained with ethidium bromide
and visualized under UV light.
 | Table IPrimer sequences for polymerase chain
reaction. |
Table I
Primer sequences for polymerase chain
reaction.
| Gene | Direction | Primer
sequence |
| SDF-1α | Forward |
5′-CTGTAGCCTGACGGACCAAT-3′ |
| Reverse |
5′-CCATTCTACAGGAGGCCAAA-3′ |
| CXCR4 | Forward |
5′-AGCCTCTGCTCATGGAGTTG-3′ |
| Reverse |
5′-GCCAAGTTCAAAAGCTCTGC-3′ |
| VEGF | Forward |
5′-GGGCAGAGCTGAGTGTTAGC-3′ |
| Reverse |
5′-TCTCCCAGATCGGTGACAGT-3′ |
| Flk1 | Forward |
5′-TTCCCCCCTGGAAATCCT-3′ |
| Reverse |
5′-ACAGACCCGGCCAAACAA-3′ |
| eNOS | Forward |
5′-CGGCATCACCAGGAAGAAGA-3′ |
| Reverse |
5′-CATGAGCGAGGCGGAGAT-3′ |
| β-actin | Forward |
5′-AAGTCCCTCACCCTCCCAAAAG-3′ |
| Reverse |
5′-AAGCAATGCTGTCACCTTCCC-3′ |
Western blot analysis
EPCs were lysed with ProPrep protein extraction
solution (Intron Biotechnology, Inc., Seoul, Korea), and the
protein concentrations of the lysates were measured using a Pierce
BCA protein assay kit (Thermo Fisher Scientific, Inc.). Proteins
(50 µg/lane) were separated by 10% SDS-PAGE and transferred
to a nitrocellulose membrane (Whatman; GE Healthcare Life
Sciences). The membrane was blocked with 5% non-fat skimmed milk in
Tris-buffered saline containing 0.1% Tween-20, for 30 min at room
temperature. The membrane was incubated with primary antibodies at
4°C overnight, and then with secondary antibodies conjugated with
horseradish peroxidase for 1 h at room temperature. Finally, the
membrane was visualized using the West Pico Chemiluminescent
Substrate (Pierce; Thermo Fisher Scientific, Inc.). The
quantification was performed using ImageJ software (version 1.52a;
National Institutes of Health, Bethesda, MD, USA). Primary
antibodies used for western blot analysis were as follows: VEGF
(1:500; cat. no. sc-152; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), VEGFR2 (1:1,000; cat. no. 2479), phospho (P)-VEGFR2
(1:500; cat. no. 2478), extracellular signal-regulated kinase (ERK;
1:1,000; cat. no. 9102), P-ERK (1:1,000; cat. no. 9101), AKT
(1:1,000; cat. no. 4691), P-AKT (1:1,000; cat. no. 9271), Jun
N-terminal kinase (JNK: 1:1,000; cat. no. 9252), P-JNK (1:1,000;
cat. no. 9251), eNOS (1:1,000; cat. no. 9586), P-eNOS (1:1,000;
cat. no. 9571) (all from Cell Signaling Technology, Inc., Danvers,
MA, USA), HIF-1α (1:500; cat. no. 610958; BD Biosciences), RUNX3
(1:500; cat. no. 40278; Abcam, Cambridge, UK) and α-tubulin
(1:1,000; cat. no. 3873; Cell Signaling Technology, Inc.).
HRP-linked secondary antibodies were purchased from Cell Signaling
Technology Inc. (1:5000; cat. nos. 7076 and 7074).
Flow cytometric analysis
To count the number of circulating EPCs, MNCs
isolated from PB were stained with fluorescein isothiocyanate
(FITC)-conjugated anti-CD34 monoclonal antibody (1:100; cat. no.
GTX20218; GeneTex, Inc., Irvine, CA, USA) and
phycoerythrin-conjugated anti-VEGFR2 monoclonal antibody (1:100;
cat. no. 555308; BD Biosciences) for 1 h at room temperature. The
cells were washed with PBS three times. A FACS flow cytometer
(FACSAria flow cytometer; BD Biosciences) was used to detect the
double-positive fluorescent cells using FACSexpress Software
(version 4; De Novo Software, Glendale, CA, USA).
Mouse hindlimb ischemia model
A unilateral mouse hindlimb ischemia model was
established as previously described (29). Briefly, WT and Rx3+/−
mice were anesthetized with 2% Avertin (250 mg/kg) by
intraperitoneal injection (n=3 mice/group). The proximal and distal
portions of the femoral artery and the distal portion of the
saphenous artery were ligated. The arteries and all side branches
were dissected free and excised. A laser Doppler perfusion imaging
system (PeriScan PIM II; Perimed AB, Järfälla, Sweden) was used to
measure hindlimb blood perfusion immediately following surgery and
at the end of the study on day 14. To avoid the influence of
ambient light and temperature, the results were expressed as the
ratio between perfusion in the left (ischemic) versus right
(non-ischemic) limbs.
In vivo tumor allograft experiment
Lewis lung carcinoma (LLC; American Type Culture
Collection, Manassas, VA, USA) cells (2×105) were
injected subcutaneously into the right and left flanks of 6-week
old WT or Rx3+/− mice on day 0 (n=4 mice/group). Tumor
growth was measured with a caliper every other day from day 9, and
the tumor volume was calculated using the following formula: Volume
(cm3) = height x length x depth (cm). Tumor tissues were
removed at day 14 and fixed with 4% paraformaldehyde.
Immunohistochemistry
LLC-derived tumor tissues from the allograft
experiment and hindlimb muscle tissues from the mice in the
ischemia experiment were removed at day 14 and fixed with 4%
paraformaldehyde. Frozen blocks were made at -26°C and cut into
10-µm sections, blocked with 5% goat serum (Abcam) in PBS
containing 0.03% Triton X-100 for 1 h, and then incubated for 3 h
at room temperature with anti-CD34 (cat. no. 553731), anti-CD31
(cat. no. 553370) (both from BD Pharmingen; BD Biosciences) and
anti-VEGFR2 (cat. no. 2479; Cell Signaling Technology, Inc.)
primary antibodies, followed by Alexa Fluor 488 anti-rabbit IgG and
Alexa Fluor 594 anti-rat IgG (Thermo Fisher Scientific, Inc.)
secondary antibodies for 1 h at room temperature with protection
from light. After washing with PBS, the samples were mounted with
mounting medium containing DAPI (Vector Laboratories, Inc.,
Burlingame, CA, USA), and observed under a confocal microscope
(Leica Microsystems, Inc., Buffalo Grove, IL, USA). The
quantitation was acquired using ImageJ software.
Statistical analysis
SPSS software was used for all statistical analyses.
Differences among experimental groups were compared using one-way
analysis of variance with the Newman-Keuls post hoc test. P<0.05
was considered to indicate a significant difference. Results are
presented as the mean± standard deviation.
Results
Runx3 knockout enhances the
differentiation of EPCs
Since Runx3 homozygote knockout
(Rx3−/−) mice die soon after birth (24), Rx3+/− mice were used for
the isolation of MNCs from adult BM. To identify the role of Runx3
in EPC differentiation, the BM-derived MNCs isolated from
Rx3+/− mice were differentiated into EPCs. Western
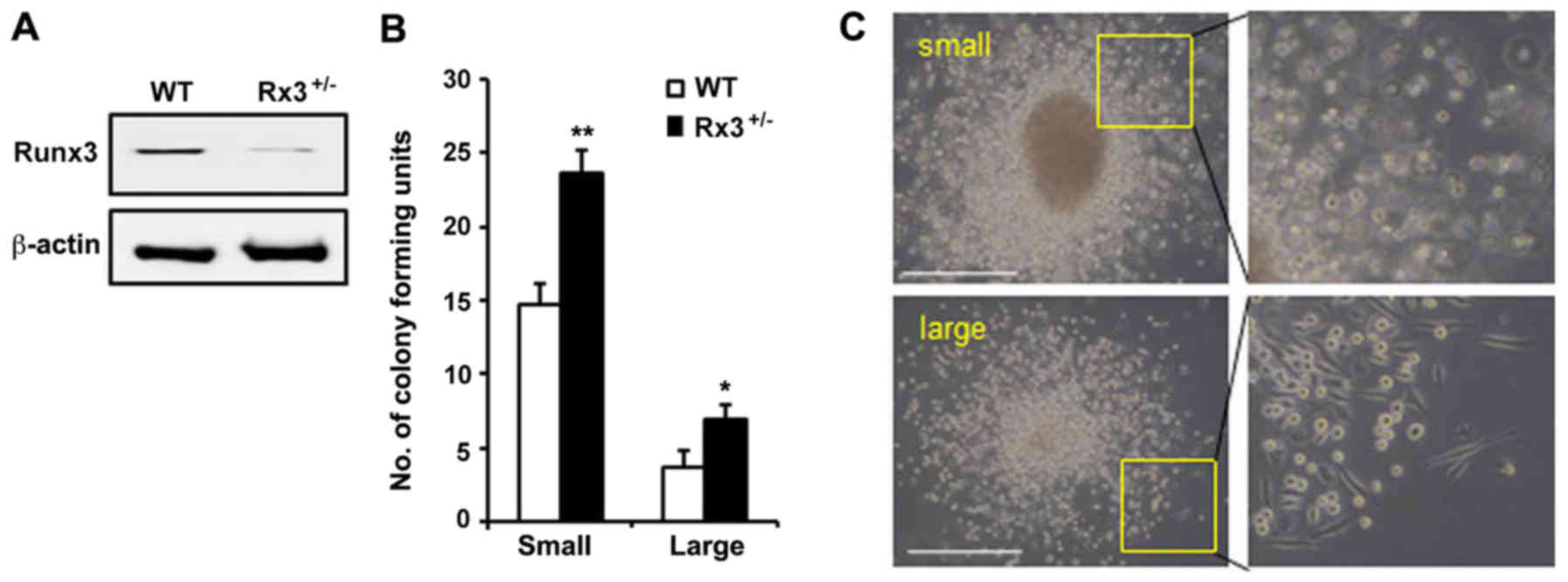
blotting results confirmed that Runx3 was expressed in the early
EPCs from WT mice but repressed in those from Rx3+/−
mice (Fig. 1A). Using the EPC
colony-forming assay, the self-renewal and differentiation
abilities of EPCs were investigated via the detection of two
differently differentiated EPCs in terms of EPC hierarchy. Colonies
formed from relatively small and rounded cells are known as small
EPC CFUs, which exhibit the characteristics of primitive EPCs,
including high proliferative activity and immature properties, and
colonies formed from spindle-shaped cells are known as large EPC
CFUs, which exhibit late or mature EPC characteristics, including
as improved tube formation ability and neovascularization (13). The numbers of small and large EPC
CFUs were observed to be significantly increased in the
Rx3+/− mice compared with the WT mice (Fig. 1B and C). These results suggest that
the deletion of Runx3 is associated with the increased self-renewal
and differentiation capacity of EPCs.
In vitro vasculogenic activity is
increased in Rx3+/− EPCs
Adherent MNC cells that were cultured for 7 days,
with the removal of non-adherent BM-MNCs after 3 days, changed
their morphology from round to spindle-like but did not form
colonies in the presence of endothelial cell growth supplements.
These cells were characterized as early EPCs (15,16)
and exhibited Dil-Ac-LDL uptake, isolectin B4 binding, expression
of CD34 (a HSC marker) and VEGFR2 (Flk1, an endothelial cell
marker) as in a previous study (21).
The growth rate of early EPCs derived from
Rx3+/− MNCs was significantly increased compared with
that of early EPCs derived from WT MNCs under normoxia and hypoxia
(Fig. 2A). As VEGF and SDF-1α
serve important roles in the regulation of various cellular
functions of EPCs, such as migration and homing to injured tissues
(16,30), a Boyden chamber migration assay
using early EPCs in the presence of VEGF or SDF-1α was performed in
the present study. VEGF or SDF-1α treatment induced the migration
of EPCs, and the number of migrated cells was significantly
increased in Rx3+/− EPCs compared with WT EPCs (Fig. 2B). To evaluate the ability of EPCs
to differentiate into ECs and form tubes, a tube formation assay
was performed on a Matrigel matrix using a 1:1 mixture of
DiI-Ac-LDL-labeled EPCs and HUVECs. The tube formation ability was
monitored using tube length measurements. As shown in Fig. 2C, the capillary networks developed
by Rx3+/− EPCs and HUVECs were more pronounced than
those formed by WT EPCs and HUVECs. The total tube length and
number of EPCs incorporated into the tubes were significantly
increased in the Rx3+/− group compared with the WT
group. These results suggest that the absence of Runx3 increases
the neovessel-forming differentiation ability of EPCs.
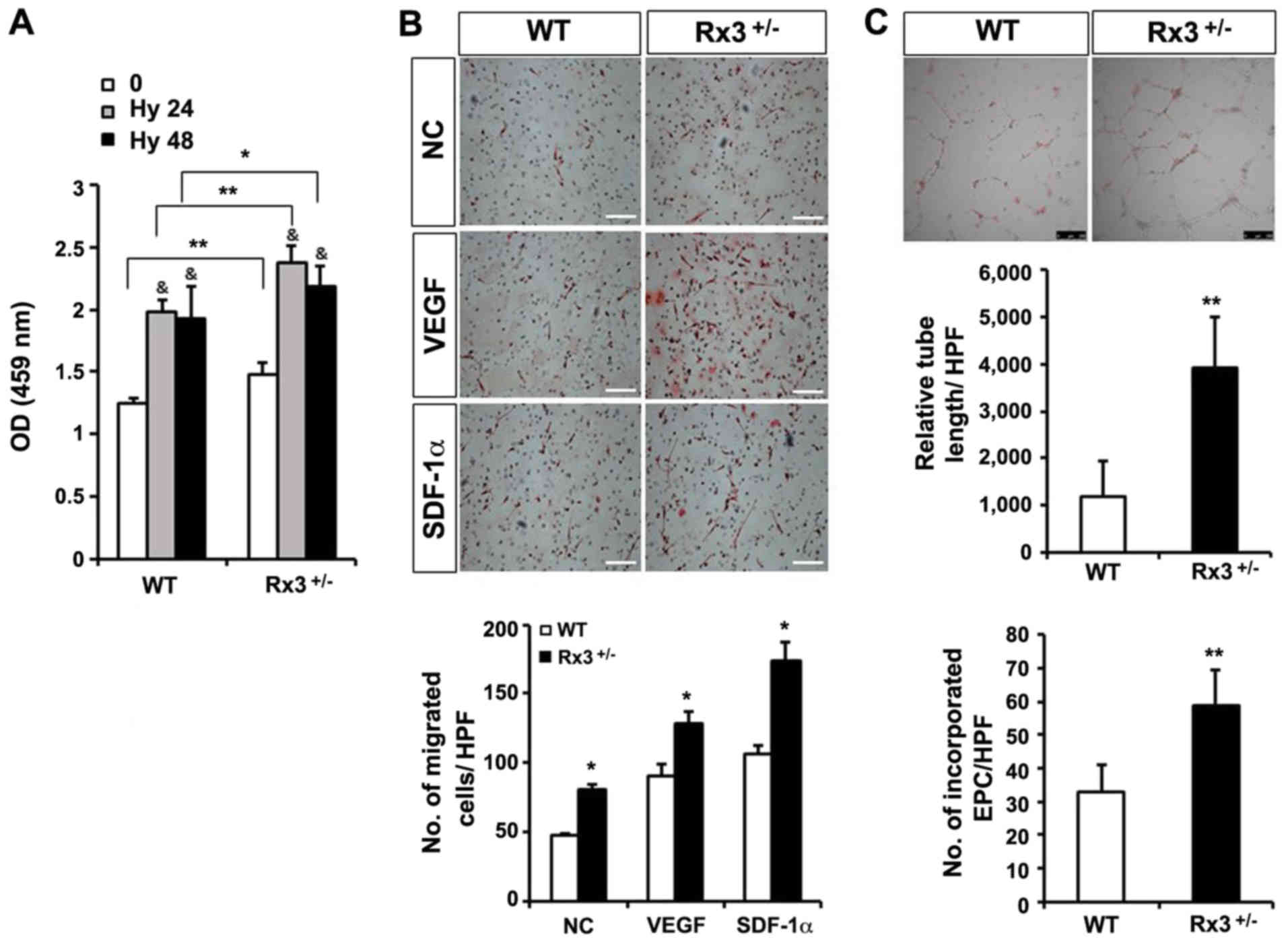 | Figure 2Enhanced function of EPCs by
heterozygous deletion of Runx3 in response to hypoxia and
angiogenic factors. (A) The cell proliferation ability of early
EPCs under hypoxic conditions for 24 or 48 h was measured by CCK-8
assay (n=4/group; three independent experiments).)
&P<0.01 vs. normoxic control;
*P<0.05, **P<0.01 as indicated. (B)
Migration assay with early EPCs treated with VEGF (10 ng/ml) or
SDF-1α (100 ng/ml) was performed. *P<0.05 vs. WT
control. (n=4/group; three independent experiments). Scale bar, 250
µm. (C) Early EPCs stained with 3,3’-DiI-labeled acetylated
low-density lipoprotein were co-cultured with human umbilical vein
endothelial cells on a Matrigel matrix for 8 h. The tube length was
measured and the number of incorporated DiI-expressing EPCs per HPF
was counted (n=3/group; three independent experiments).
Representative images are shown in the upper panel.
**P<0.01 vs. WT control. (n=4/group; three
independent experiments). Scale bar, 250 µm. EPC,
endothelial progenitor cell; Runx3, runt-related transcription
factor 3; Rx3+/−, Runx3 heterozygous; CCK-8, Cell
counting kit-8; DiI, dioctadecylindocarbocyanin; WT, wild-type; NC,
negative control; VEGF, vascular endothelial growth factor; SDF,
stromal cell-derived factor; 0, normoxia; Hy 24, hypoxia 24 h; Hy
48, hypoxia 48 h; OD, optical density; HPF, high-power field. |
Expression of growth factors and their
receptors involved in EPC functions is upregulated in
Rx3+/− EPCs
To investigate whether the increased vessel
formation ability of EPCs with Runx3 deletion is due to increased
vasculogenic gene expression patterns in EPCs, the mRNA expression
levels of growth factors and their receptors involved in
vasculogenesis were analyzed. RT-qPCR revealed that the mRNA
expression levels of VEGF, SDF-1α, C-X-C chemokine
receptor type 4 (CXCR4) and eNOS were significantly
upregulated in Rx3+/− EPCs compared with WT EPCs
(Fig. 3A), while a significant
increase in VEGFR2 (Flk1) mRNA expression was
detected. To confirm these results, the phosphoinositide-3 kinase
(PI3K) and mitogen-activated protein kinase (MAPK) signaling
pathways that are activated by VEGFR2 were investigated.
VEGFR2 mRNA expression was not altered with Runx3 deletion,
but the phosphorylation of VEGFR2 was observed to be significantly
increased in Rx3+/− EPCs compared with WT EPCs (Fig. 3B). As PI3K and MAPKs, including JNK
and ERK1/2, are downstream molecules for CXCR4/SDF-1α (31,32)
as well as VEGF/VEGFR2 signaling pathways (33), the activation of Akt, JNK and
ERK1/2 in Rx3+/− EPCs was examined. The phosphorylation
levels of Akt, JNK and ERK1/2 were significantly increased in
Rx3+/− EPCs compared with WT EPCs (Fig. 3C). The activation of eNOS is
positively controlled by Akt through the phosphorylation of Ser1178
(34) and results in the
generation of NO that serves a crucial role in vessel vasodilation
and vasculogenesis (35). Thus,
the amount of the activated form of eNOS was evaluated. The
activated form of eNOS was observed to be significantly increased
in Rx3+/− EPCs compared with WT EPCs (Fig. 3C). Furthermore, the secretion of
VEGF from Rx3+/− EPCs was confirmed to be significantly
increased compared with that from WT EPCs (Fig. 3D). These results suggest that Runx3
knockout enhances vasculogenic signaling via the activation of
VEGF/VEGFR2 and CXCR-4/SDF-1α pathways.
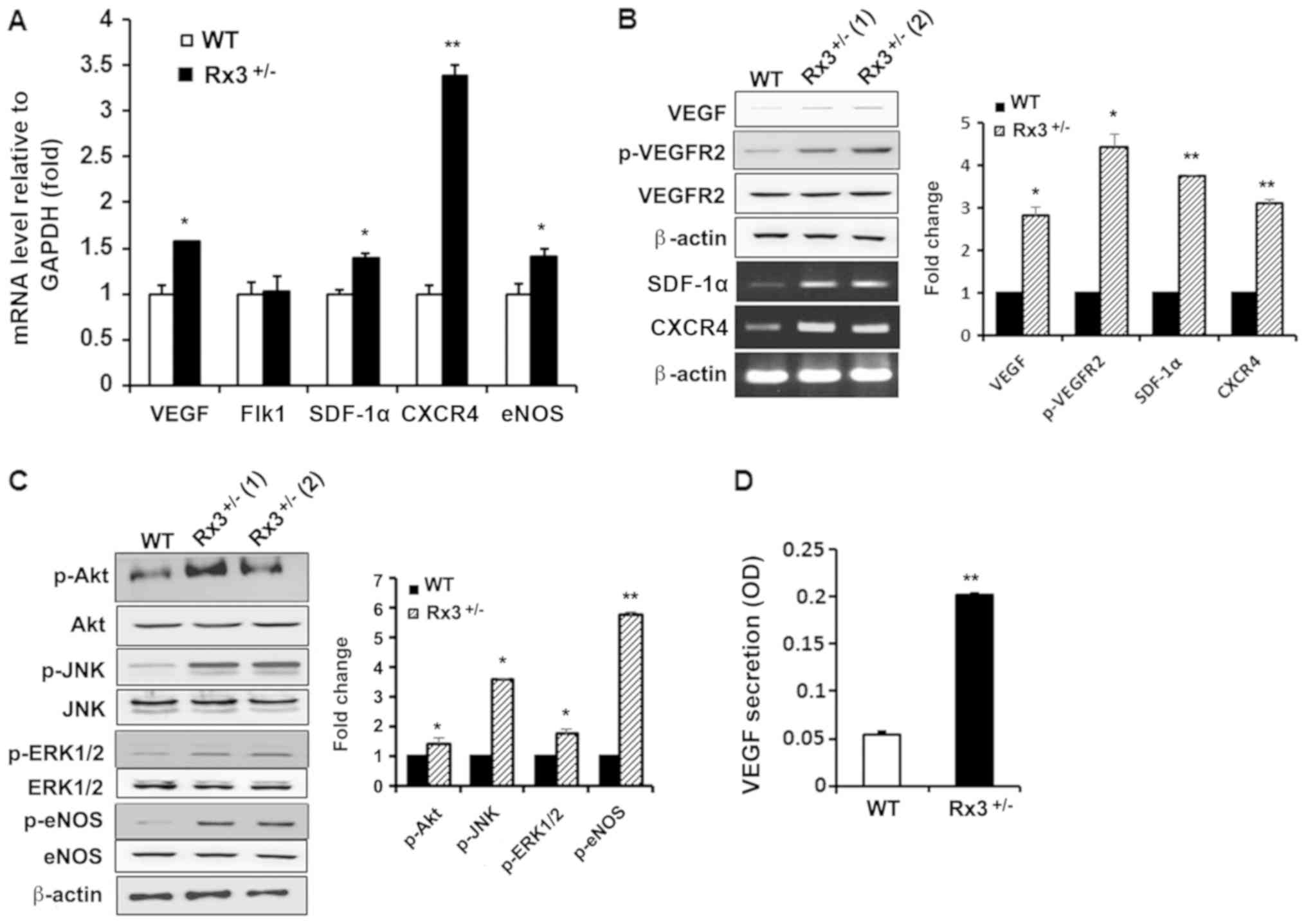 | Figure 3Heterozygous deletion of Runx3
activates VEGF and SDF-1α signaling pathways. (A) Detection of
total RNA extracted from early EPCs using RT-qPCR. Data are the
mean ± standard deviation of three independent experiments. (B)
Western blot analysis of VEGF, p-VEGFR2 and VEGFR2, and analysis of
SDF-1α and CXCR4 mRNA levels using semi-quantitative
RT-PCR analysis. VEGF, SDF-1α and CXCR4 are
normalized to β-actin while p-VEGFR2 and VEGFR2 are normalized to
β-actin and presented as the p-VEGFR2/VEGFR2 ratio. (C) Activation
of Akt, JNK, ERK1/2 and eNOS in early EPCs from WT and
Rx3+/− mice was determined using western blot analysis.
(D) Secreted VEGF was determined by ELISA in the serum-free media
collected from WT and Rx3+/− early EPCs.
*P<0.05, **P<0.01 vs. WT. Runx3,
runt-related transcription factor 3; Rx3+/−, Runx3
heterozygous; WT, wild-type; VEGF, vascular endothelial growth
factor; Flk1, fetal liver kinase 1, also known as VEGFR2; VEGFR2,
VEGF receptor 2; SDF, stromal cell-derived factor; CXCR4, C-X-C
chemokine receptor type 4; eNOS, endothelial nitric oxide synthase;
EPC, endothelial progenitor cell; RT-(q)PCR, reverse
transcription-(quantitative) polymerase chain reaction; p, phospho;
JNK, Jun N-terminal kinase; ERK1/2, extracellular signal-regulated
kinase1/2; OD, optical density. |
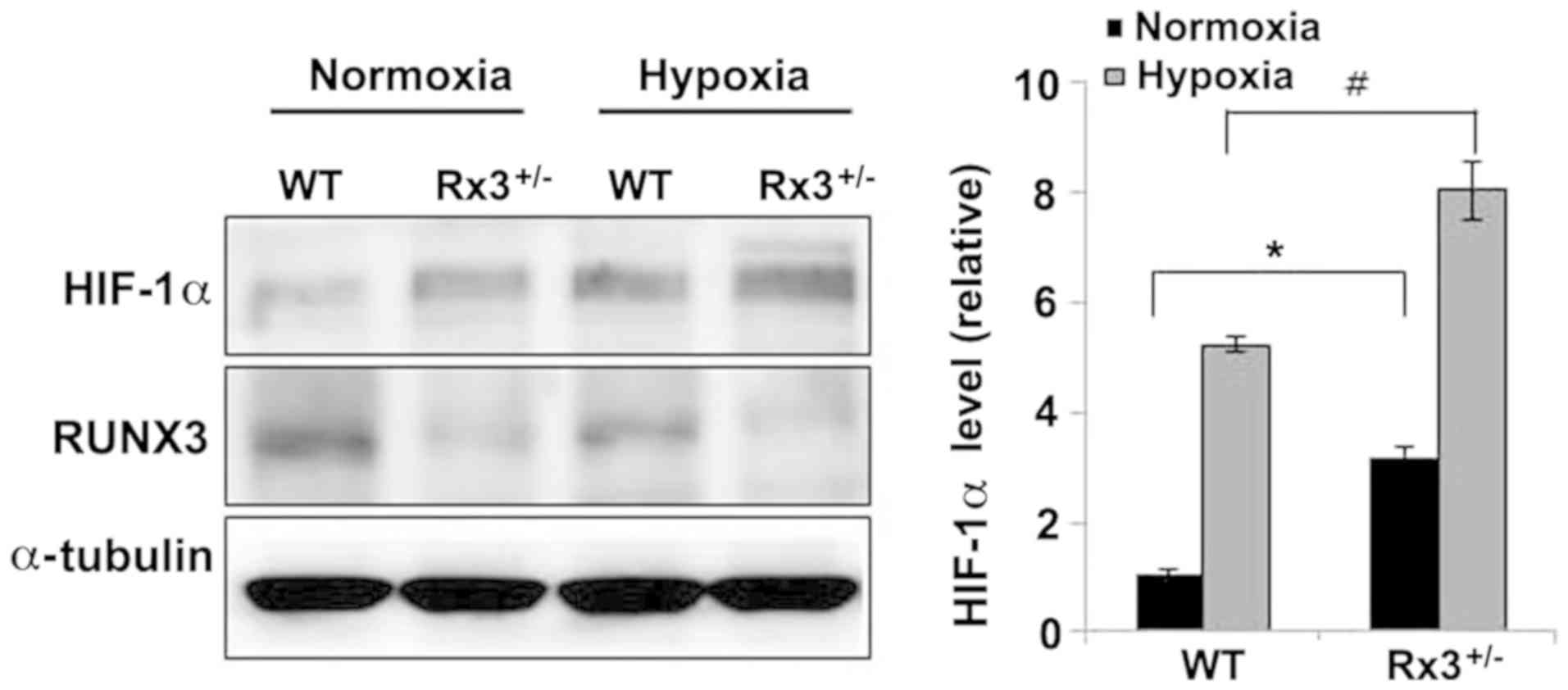
HIF-1α is stabilized under normoxic
conditions in Rx3+/− EPCs
As VEGF, VEGFR (36), SDF-1α and CXCR4 (37) are downstream targets of HIF-1α, the
HIF-1α protein levels in Rx3+/− EPCs were measured under
normoxic and hypoxic conditions. Compared with WT EPCs, HIF-1α
expression was increased 3-fold in Rx3+/− EPCs under
normoxic conditions (Fig. 4, lanes
1 and 2). Under hypoxic conditions, HIF-1α was highly stabilized by
5-fold in WT EPCs (Fig. 4, lane 1
and lane 3), whereas in Rx3+/− EPCs, HIF-1α was more
increased by 2.7-fold (Fig. 4,
lanes 2 and 4), suggesting that Runx3 destabilizes HIF-1α
protein.
Mobilization into PB circulation is
reduced in Rx3+/− EPCs
The mobilization of EPCs from the BM into the
peripheral circulation, resulting in circulating EPCs (CEPCs), is a
critical step during the process of neovascularization at ischemic
sites (35). Therefore, the
percentage and number of CEPCs in the PB were investigated via the
flow cytometric detection of the EPC markers CD34 and VEGFR2. The
percentage of EPCs in PB-MNCs was found to be significantly
increased in Rx3+/− mice (1.1±0.12%) compared with WT
mice (0.5±0.08%; Fig. 5A and B).
Furthermore, the number of EPCs in the PB of Rx3+/− mice
(1,603.2±38.12 EPCs/µl) was 3-fold higher than that of WT
mice (497.1±32.74 EPCs/µl; Fig.
5C). The results indicate that, consistent with the expression
patterns of vascu-logenic signaling molecules, the mobilization of
EPCs into the PB is also stimulated by the knockdown of Runx3.
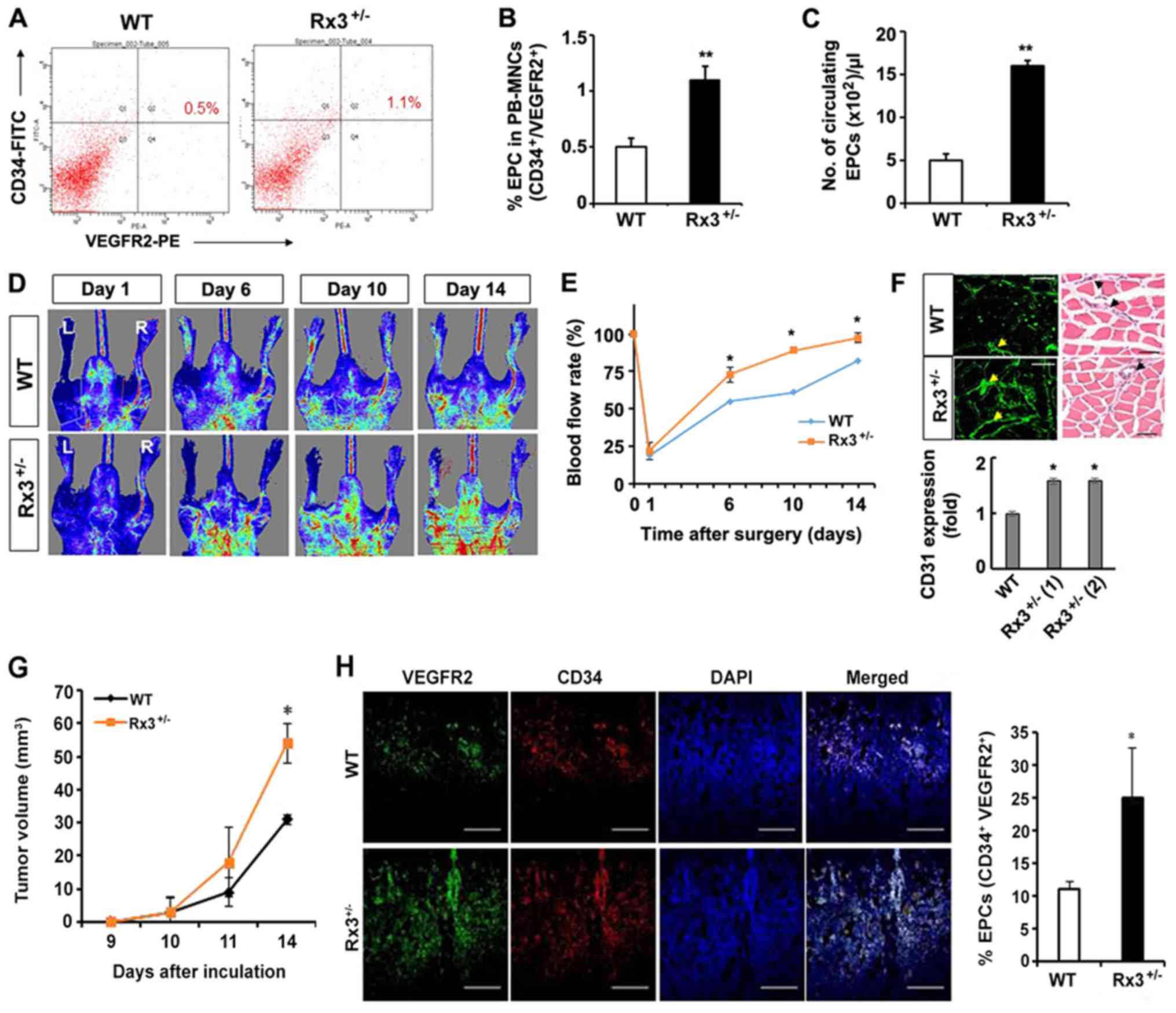 | Figure 5Heterozygous deletion of Runx3
increases the mobilization of EPCs from the bone marrow to the
peripheral circulation and ischemic hindlimb. (A) Mononuclear cells
were isolated using the density gradient method and stained with a
FITC-conjugated anti-CD34 antibody and PE-conjugated anti-VEGFR2
antibody. Flow cytometric analysis was performed to detect
CD34+/VEGFR2+ circulating EPCs. (B)
Percentage and (C) total numbers of circulating EPCs were counted
in 10,000 peripheral blood cells. **P<0.01 vs. WT.
(D) Representative images of the ischemic (L) and non-ischemic (R)
hindlimbs immediately and 6, 10 and 14 days after surgery. In the
color-coded images, red indicates normal perfusion, and blue
indicates a marked reduction in blood flow in the ischemic
hindlimb. (E) Blood flow rate was recovered in Rx3+/−
mice compared with WT mice (n=3/group). *P<0.05 vs.
WT. (F) CD31+ regions (green fluorescence) in ischemic
muscle detected using immunohistochemistry (left panels) were
quantified, and representative images of hematoxylin and eosin
stained tissue are shown (right panels). Arrow heads indicate the
vessels. Scale bar, 50 µm. (G) LLC cells (2×105)
were inoculated into bilateral flanks of WT or Runx3 heterozygous
knockout mice (day 0) and the tumor size were measured at various
time points. *P<0.05 vs. WT (n=4). (H) Sectioned (10
µm) tumor tissue from LLC-injected WT or heterozygous
Runx3-deleted mice was immunostained with anti-VEGFR2 and anti-CD34
antibodies, which exhibit green and red fluorescence, respectively.
Infiltrated EPCs (CD34+/VEGFR+ cells) were
counted. *P<0.05 vs. WT. Scale bar, 200 µm.
Runx3, runt-related transcription factor 3; Rx3+/−,
Runx3 heterozygous; WT, wild-type; EPC, endothelial progenitor
cell; FITC, fluorescein isothiocyanate; PE, phycoerythrin; CD,
cluster of differentiation; VEGFR2, vascular endothelial growth
factor receptor 2; L, left; R, right; LLC, Lewis lung
carcinoma. |
Repair of blood flow in hindlimb-ischemia
and tumor tissue infiltration of EPCs of Rx3+/−
mice
As the aforementioned results reveal that the
haploinsufficient deletion of Runx3 increased EPC function
(Figs. 1-4), and a previous study demonstrated that
RUNX3 inhibits the stability of HIF-1α (20), a main transcription factor of
angiogenesis and vasculogenesis in hypoxia, angiogenesis in
Rx3+/− mice was investigated in vivo using a
hindlimb-ischemia model. Compared with WT mice, blood flow was
significantly increased in Rx3+/− mice from day 6 after
ligation of the hindlimb artery and vein. At 10 and 14 days
post-ligation, the blood flow rate in the hindlimb and foot was
completely recovered in Rx3+/− mice (Fig. 5D and E). When the hindlimb muscle
was analyzed by immunohistochemistry using an anti-CD31 antibody,
the microvessel density was observed to be markedly increased in
the Rx3+/− mice compared with the WT mice (Fig. 5F). Furthermore, in an LLC allograft
model, the tumor volume in Rx3+/− mice was significantly
larger than that in WT mice (Fig.
5G) and the EPC population (CD34+/VEGFR2+
cells) in the tumor mass was ~2.5-fold greater in the
Rx3+/− mice compared with the WT mice (Fig. 5H). These results suggest that the
mobilization of EPCs into the tumor vessels is increased in
Rx3+/− mice.
Discussion
The present study suggests that Runx3 may be an
inhibitor of EPC function and the differentiation of BM-MNCs into
EPCs. EPCs, first identified in human PB (38), are a small population of
circulating or BM-derived MNCs, which are incorporated into sites
of neovascularization, and contribute to vascular repair in
ischemic tissues by increasing the expression of cytokines, growth
factors and/or hormones through autocrine, paracrine and/or
endocrine systems (39); this is
the process of postnatal vasculogenesis. Thus, EPCs are considered
to have potential value in therapeutic vasculogenesis for various
ischemic diseases (40). With the
same properties as EPCs, they could also be incorporated into
hypoxic tumor regions for neovessel formation. Thus, they may be a
target for the molecular inhibition of tumor vasculature.
RUNX3, a tumor suppressor, activates the TGF-β
signaling pathway and inhibits angiogenesis via the suppression of
VEGF transcription (41) and by
reducing HIF-1α stability (20).
Since VEGF and HIF-1α are crucial factors for vasculogenesis, the
present study investigated whether RUNX3 influences the
differentiation of EPCs and vasculogenesis. Similar to RUNX3, RUNX1
is also known to function as a tumor suppressor because its
mutation results in the development of leukemia (23). As RUNX1 is required for
hematopoiesis, a RUNX1 null mutation results in defective
hematopoiesis, which leads to the impairment of angiogenesis in the
developing embryo (42),
suggesting that RUNX1 is required for embryonic vasculogenesis via
the modulation of hematopoiesis. However, a transcriptional
activity assay in a previous study revealed that VEGF gene
expression is inhibited by RUNX1 but increased by an acute myeloid
leukemia (AML)/RUNX1 fusion protein (AML/ETO) (43). Therefore, the function of RUNX1 in
vessel formation is different between embryogenesis and leukemia
development. As RUNX3 and RUNX1 have similar properties in a number
of biological responses, the observation that loss of Runx1
function in zebrafish embryos leads to defects in hematopoiesis and
vasculogenesis (44) suggests that
Runx3 deletion may affect hematopoiesis and/or vasculogenesis.
However, RUNX1 cross-regulates the expression of RUNX3 in lymphoid
cells (45), further suggesting
that the role of Runx3 in the differentiation of BM-HSCs might be
distinct from that of Runx1. A genomic study demonstrating
different gene expression patterns between early EPCs (small EPCs
in the present study) and outgrowth ECs (OECs having similar
properties as large EPCs in the present study) revealed that Runx1
is expressed in early EPCs (46).
Early EPCs (small EPCs) exhibit more premature and undifferentiated
properties in a differentiation hierarchy relative to large EPCs.
The present study demonstrated that in small and large EPC CFUs,
Runx3 knockout cells formed a greater number of CFUs than did WT
cells, suggesting that Runx3 has an inhibitory role in the early
and late stages of EPC differentiation from BM stem cells. Runx3
regulates the differentiation of BM-derived dendritic cells by the
TGF-β signaling pathway (47).
Dendritic cell maturation is essential for the immune response;
however, the differentiation process of EPCs may differ from those
of other myeloid and lymphoid cells. Runx3 is required for the
silencing of CD4 during T-cell lineage decisions (48,49).
Human CD34+ HSCs and several normal and malignant
hematopoietic cell lines express RUNX3, suggesting that it plays a
role in EPCs (50,51). As the differentiation of EPCs from
BM-MNCs and their mobilization or homing to injured or ischemic
regions are dependent on VEGFR2 and CXCR4-SDF1α signaling pathways
(52,53), Runx3 knockout may cause increased
EPC differentiation, probably through VEGFR2 and CXCR4-SDF1α
pathways. As Runx3 destabilizes HIF-1α and VEGF/VEGFR2 expression
is induced by HIF-1α, it may be speculated that the heterozygous
knockout of Runx3 increases HIF-1α accumulation and thus
VEGF/VEGFR2 expression.
The observation that CXCR4 and SDF1α are also
increased by HIF-1α indicates a causative role in the increased EPC
function induced by the heterozygous deletion of Runx3. The
increase of EPC differentiation and function in heterozygous
deletion of Runx3 may be explained by the tumor suppressive
function of Runx3. Our results suggest that the stabilization of
HIF-1α in Rx3+/− EPCs increases VEGF/VEGFR2 and
SDF-1α/CXCR4 expression and their signaling pathways to enhance the
differentiation of BM stem cells into EPCs. In a previous study, it
was found that RUNX3 facilitates the degradation of HIF-1α
(54) by enhancing the interaction
of PHDs with HIF-1α, which is a key inhibitor of hypoxia-induced
VEGF secretion and angiogenesis in vitro and in vivo
(20). Hypoxia downregulates
RUNX3 expression and function (6), and is a strong inducer of EPC
mobilization and homing via the enhanced expression of various
cytokines, including eNOS and SDF1α. The present study indicates
that Runx3 downregulation in ischemic regions results in
hypoxia-induced cytokine expression and secretion which induces the
recruitment of EPCs. Taken together, these results suggest that
Runx3 functions to inhibit the differentiation of EPCs from
BM-MNCs. Furthermore, Runx3 inhibits EPC functions, including
self-renewal, migration, tube formation, as well as mobilization
into the PB and homing to injured tissues. Therefore, Runx3 may
totally inhibit vasculogenesis via the suppression of EPC
differentiation and recruitment to ischemic tissues during the
development of new vessels from BM. Therefore, it may be concluded
that the inhibition of EPC differentiation and function by RUNX3
recovery or overexpression is a potential therapeutic strategy for
the treatment of tumor progression and metastasis, but may inhibit
tissue regeneration in ischemic diseases.
Funding
This study was supported by National Research Foundation grants
from the Korean government (MSIP; NRF-2012R1A4A1028835,
NRF-2013R1A2A2A01068868 and NRF-2017R1A2B3002227).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SYC and SHY performed EPC isolation and analysis,
cell culture, molecular analyses and in vivo experiments.
DJL and SHL performed the isolation of EPCs and western blot
analysis. KL, IHK and WL performed in vivo tumor allograft
and IHC experiments with tumor tissue. SCB provided the
Runx3+/− mice and analyzed the data. YML initiated the
study, supervised the experiments, analysed data and wrote the
manuscript.
Ethics approval and consent to
participate
The animal experiments were by the Kyungpook
National University Institutional Animal Care and Use
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Sarala Manandhar
(Kyungpook National University, Daegu, Korea) for reading and
editing the manuscript.
References
|
1
|
Ito Y: Oncogenic potential of the RUNX
gene family: 'overview'. Oncogene. 23:4198–4208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chuang LS, Ito K and Ito Y: RUNX family:
Regulation and diversification of roles through interacting
proteins. Int J Cancer. 132:1260–1271. 2013. View Article : Google Scholar
|
|
3
|
Miyazono K, Maeda S and Imamura T:
Coordinate regulation of cell growth and differentiation by
TGF-beta superfamily and Runx proteins. Oncogene. 23:4232–4237.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stifani S and Ma Q: 'Runxs and
regulations' of sensory and motor neuron subtype differentiation:
Implications for hematopoietic development. Blood Cells Mol Dis.
43:20–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chuang LS and Ito Y: RUNX3 is
multifunctional in carcinogenesis of multiple solid tumors.
Oncogene. 29:2605–2615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SH, Kim J, Kim WH and Lee YM: Hypoxic
silencing of tumor suppressor RUNX3 by histone modification in
gastric cancer cells. Oncogene. 28:184–194. 2009. View Article : Google Scholar
|
|
7
|
Patan S: Vasculogenesis and angiogenesis.
Cancer Treat Res. 117:3–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asahara T and Kawamoto A: Endothelial
progenitor cells for postnatal vasculogenesis. Am J Physiol Cell
Physiol. 287:C572–C579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moschetta M, Mishima Y, Sahin I, Manier S,
Glavey S, Vacca A, Roccaro AM and Ghobrial IM: Role of endothelial
progenitor cells in cancer progression. Biochim Biophys Acta.
1846:26–39. 2014.PubMed/NCBI
|
|
11
|
Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi
JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA,
Benezra R, et al: Bone marrow-derived endothelial progenitor cells
are a major determinant of nascent tumor neovascularization. Genes
Dev. 21:1546–1558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Obi S, Yamamoto K, Shimizu N, Kumagaya S,
Masumura T, Sokabe T, Asahara T and Ando J: Fluid shear stress
induces arterial differentiation of endothelial progenitor cells. J
Appl Physiol (1985). 106:203–211. 2009. View Article : Google Scholar
|
|
13
|
Masuda H, Alev C, Akimaru H, Ito R,
Shizuno T, Kobori M, Horii M, Ishihara T, Isobe K, Isozaki M, et
al: Methodological development of a clonogenic assay to determine
endothelial progenitor cell potential. Circ Res. 109:20–37. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwon SM, Lee YK, Yokoyama A, Jung SY,
Masuda H, Kawamoto A, Lee YM and Asahara T: Differential activity
of bone marrow hematopoietic stem cell subpopulations for EPC
development and ischemic neovascularization. J Mol Cell Cardiol.
51:308–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hur J, Yang HM, Yoon CH, Lee CS, Park KW,
Kim JH, Kim TY, Kim JY, Kang HJ, Chae IH, et al: Identification of
a novel role of T cells in postnatal vasculogenesis:
Characterization of endothelial progenitor cell colonies.
Circulation. 116:1671–1682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwarz TM, Leicht SF, Radic T,
Rodriguez-Arabaolaza I, Hermann PC, Berger F, Saif J, Böcker W,
Ellwart JW, Aicher A, et al: Vascular incorporation of endothelial
colony-forming cells is essential for functional recovery of murine
ischemic tissue following cell therapy. Arterioscler Thromb Vasc
Biol. 32:e13–e21. 2012. View Article : Google Scholar
|
|
18
|
Lee SH, Lee JH, Han YS, Ryu JM, Yoon YM
and Han HJ: Hypoxia accelerates vascular repair of endothelial
colony-forming cells on ischemic injury via STAT3-BCL3 axis. Stem
Cell Res Ther. 6:1392015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moccia F, Zuccolo E, Poletto V, Cinelli M,
Bonetti E, Guerra G and Rosti V: Endothelial progenitor cells
support tumour growth and metastatisation: Implications for the
resistance to anti-angiogenic therapy. Tumour Biol. 36:6603–6614.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SH, Bae SC, Kim KW and Lee YM: RUNX3
inhibits hypoxia-inducible factor-1α protein stability by
interacting with prolyl hydroxylases in gastric cancer cells.
Oncogene. 33:1458–1467. 2014. View Article : Google Scholar
|
|
21
|
Choi JH, Nguyen MP, Lee D, Oh GT and Lee
YM: Hypoxia-induced endothelial progenitor cell function is blunted
in angiotensinogen knockout mice. Mol Cells. 37:487–496. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benito J, Zeng Z, Konopleva M and Wilson
WR: Targeting hypoxia in the leukemia microenvironment. Int J
Hematol Oncol. 2:279–288. 2013. View Article : Google Scholar
|
|
23
|
Gaidzik VI, Teleanu V, Papaemmanuil E,
Weber D, Paschka P, Hahn J, Wallrabenstein T, Kolbinger B, Köhne
CH, Horst HA, et al: RUNX1 mutations in acute myeloid leukemia are
associated with distinct clinicopathologic and genetic features.
Leukemia. 30:2160–2168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li QL, Ito K, Sakakura C, Fukamachi H,
Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, et al: Causal
relationship between the loss of RUNX3 expression and gastric
cancer. Cell. 109:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon SM, Eguchi M, Wada M, Iwami Y, Hozumi
K, Iwaguro H, Masuda H, Kawamoto A and Asahara T: Specific Jagged-1
signal from bone marrow microenvironment is required for
endothelial progenitor cell development for neovascularization.
Circulation. 118:157–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka R, Wada M, Kwon SM, Masuda H, Carr
J, Ito R, Miyasaka M, Warren SM, Asahara T and Tepper OM: The
effects of flap ischemia on normal and diabetic progenitor cell
function. Plast Reconstr Surg. 121:1929–1942. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi JH, Nguyen MP, Jung SY, Kwon SM, Jee
JG, Bae JS, Lee S, Lee MY and Lee YM: Inhibitory effect of
glyceollins on vasculogenesis through suppression of endothelial
progenitor cell function. Mol Nutr Food Res. 57:1762–1771.
2013.PubMed/NCBI
|
|
28
|
Nguyen MP, Lee D, Lee SH, Lee HE, Lee HY
and Lee YM: Deguelin inhibits vasculogenic function of endothelial
progenitor cells in tumor progression and metastasis via
suppression of focal adhesion. Oncotarget. 6:16588–16600. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Biscetti F, Straface G, Arena V, Stigliano
E, Pecorini G, Rizzo P, De Angelis G, Iuliano L, Ghirlanda G and
Flex A: Pioglitazone enhances collateral blood flow in ischemic
hindlimb of diabetic mice through an Akt-dependent VEGF-mediated
mechanism, regardless of PPARgamma stimulation. Cardiovasc
Diabetol. 8:492009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamaguchi J, Kusano KF, Masuo O, Kawamoto
A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner
JM, et al: Stromal cell-derived factor-1 effects on ex vivo
expanded endothelial progenitor cell recruitment for ischemic
neovascularization. Circulation. 107:1322–1328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kucia M, Jankowski K, Reca R, Wysoczynski
M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J and Ratajczak MZ:
CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol
Histol. 35:233–245. 2004. View Article : Google Scholar
|
|
32
|
Okabe S, Fukuda S, Kim YJ, Niki M, Pelus
LM, Ohyashiki K, Pandolfi PP and Broxmeyer HE: Stromal cell-derived
factor-1alpha/CXCL12-induced chemotaxis of T cells involves
activation of the RasGAP-associated docking protein p62Dok-1.
Blood. 105:474–480. 2005. View Article : Google Scholar
|
|
33
|
Matsumoto T and Claesson-Welsh L: VEGF
receptor signal transduction. Sci STKE. 2001:re212001.PubMed/NCBI
|
|
34
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal
transduction pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Llevadot J and Asahara T: Effects of
statins on angiogenesis and vasculogenesis. Rev Esp Cardiol.
55:838–844. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ramakrishnan S, Anand V and Roy S:
Vascular endothelial growth factor signaling in hypoxia and
inflammation. J Neuroimmune Pharmacol. 9:142–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Y, Jin M, Xu H, Shimin Z, He S, Wang L
and Zhang Y: Clinicopathologic significance of HIF-1α, CXCR4, and
VEGF expression in colon cancer. Clin Dev Immunol. 2010:5375312010.
View Article : Google Scholar
|
|
38
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Iwami Y, Masuda H and Asahara T:
Endothelial progenitor cells: Past, state of the art, and future. J
Cell Mol Med. 8:488–497. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Asahara T, Kawamoto A and Masuda H:
Concise review: Circulating endothelial progenitor cells for
vascular medicine. Stem Cells. 29:1650–1655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peng Z, Wei D, Wang L, Tang H, Zhang J, Le
X, Jia Z, Li Q and Xie K: RUNX3 inhibits the expression of vascular
endothelial growth factor and reduces the angiogenesis, growth, and
metastasis of human gastric cancer. Clin Cancer Res. 12:6386–6394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takakura N, Watanabe T, Suenobu S, Yamada
Y, Noda T, Ito Y, Satake M and Suda T: A role for hematopoietic
stem cells in promoting angiogenesis. Cell. 102:199–209. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ter Elst A, Ma B, Scherpen FJ, de Jonge
HJ, Douwes J, Wierenga AT, Schuringa JJ, Kamps WA and de Bont ES:
Repression of vascular endothelial growth factor expression by the
runt-related transcription factor 1 in acute myeloid leukemia.
Cancer Res. 71:2761–2771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kalev-Zylinska ML, Horsfield JA, Flores
MV, Postlethwait JH, Vitas MR, Baas AM, Crosier PS and Crosier KE:
Runx1 is required for zebrafish blood and vessel development and
expression of a human RUNX1-CBF2T1 transgene advances a model for
studies of leukemogenesis. Development. 129:2015–2030.
2002.PubMed/NCBI
|
|
45
|
Spender LC, Whiteman HJ, Karstegl CE and
Farrell PJ: Transcriptional cross-regulation of RUNX1 by RUNX3 in
human B cells. Oncogene. 24:1873–1881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Medina RJ, O'Neill CL, Sweeney M,
Guduric-Fuchs J, Gardiner TA, Simpson DA and Stitt AW: Molecular
analysis of endothelial progenitor cell (EPC) subtypes reveals two
distinct cell populations with different identities. BMC Med
Genomics. 3:182010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fainaru O, Shseyov D, Hantisteanu S and
Groner Y: Accelerated chemokine receptor 7-mediated dendritic cell
migration in Runx3 knockout mice and the spontaneous development of
asthma-like disease. Proc Natl Acad Sci USA. 102:10598–10603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ehlers M, Laule-Kilian K, Petter M,
Aldrian CJ, Grueter B, Würch A, Yoshida N, Watanabe T, Satake M and
Steimle V: Morpholino antisense oligonucleotide-mediated gene
knockdown during thymocyte development reveals role for Runx3
transcription factor in CD4 silencing during development of
CD4-/CD8+ thymocytes. J Immunol. 171:3594–3604. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Taniuchi I, Osato M, Egawa T, Sunshine MJ,
Bae SC, Komori T, Ito Y and Littman DR: Differential requirements
for Runx proteins in CD4 repression and epigenetic silencing during
T lymphocyte development. Cell. 111:621–633. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Le XF, Groner Y, Kornblau SM, Gu Y,
Hittelman WN, Levanon D, Mehta K, Arlinghaus RB and Chang KS:
Regulation of AML2/CBFA3 in hematopoietic cells through the
retinoic acid receptor alpha-dependent signaling pathway. J Biol
Chem. 274:21651–21658. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gomes I, Sharma TT, Edassery S, Fulton N,
Mar BG and Westbrook CA: Novel transcription factors in human CD34
antigen-positive hematopoietic cells. Blood. 100:107–119. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Leone AM, Valgimigli M, Giannico MB,
Zaccone V, Perfetti M, D'Amario D, Rebuzzi AG and Crea F: From bone
marrow to the arterial wall: The ongoing tale of endothelial
progenitor cells. Eur Heart J. 30:890–899. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|