Introduction
Irinotecan (CPT-11), a semi-synthetic camptothecin
topoisomerase I inhibitor, is mainly used in the treatment of
patients with advanced colorectal cancer (1). However, CPT-11-induced intestinal
mucosal injury, such as late-onset diarrhea, has significantly
impeded cancer chemotherapy treatment. The overall incidence of
clinically significant diarrhea due to CPT-11 treatment is up to
87%, with the incidence of grade 3 or 4 diarrhea ranging between
30-40% (2). With the long duration
or the improper treatment of diarrhea, patients may develop
excessive dehydration, electrolyte loss, acid-base balance
disorders, shock and even death (3).
The pathogenesis of CPT-11-induced intestinal
mucosal injury is complex. It is generally accepted that CPT-11 and
its active metabolite, SN-38, can damage intestinal mucosal cells
directly (4). Moreover, CPT-11 can
activate a variety of signaling pathways, such as the nuclear
factor (NF)-κB pathway, increase the expression of
inflammation-related factors, such as cyclooxygenase-2 (COX-2) and
prostaglandin E2 (PGE2) and promote the levels of the
inflammatory factors, tumor necrosis factor (TNF)-α and interleukin
(IL)-1β in succession, which eventually leads to intestinal
inflammation-related damage (5).
In addition, polymorphisms in the UGT1A1 gene (6) the expression of tight junction
proteins (7) and bacterial
translocation (8) are the probable
mechanisms responsible for CPT-11-induced intestinal mucosal
injury.
Curcumin is a polyphenolic compound derived from
dried turmeric rhizome of a plant in the ginger family. A number of
experimental studies have demonstrated that curcumin exerts
anti-inflammatory, antioxidant and anti-tumor effects (9-11).
According to a previous study, curcumin was shown to protect
intestinal mucosal barrier function in MTX-induced enteritis in rat
by suppressing NF-κB activation and inhibiting the activation of
p38 mitogen-activated protein kinase (MAPK) (12). Similarly, Arafa et al
(13) suggested that curcumin
exerted protective effects against ulcerative colitis induced by
dextran sulfate sodium (DSS) in rats by downregulating
malondialdehyde (MDA), and upregulating superoxide dismutase (SOD),
glutathione-S-transferase (GST) and GSH. In addition, our previous
study also demonstrated that curcumin enhanced the CPT-11-induced
apoptosis of LoVo colorectal cancer cells. We then selected 5
proteins of interest [glutathione S-transferase Mu 5
(GSTM5), peroxiredoxin 4 (PRDX4), prolyl 4-hydroxylase
subunit beta (P4HB), calpain small subunit 1 (CAPNS1) and signal
sequence receptor subunit 4 (SSR4)] out of 54 differential
expressed proteins identified by mass spectroscopy. P4HB and PRDX4
are oxidative stress- and endoplasmic reticulum (ER) stress-related
proteins, respectively (14). We
suspected that these two proteins may be involved in the protective
effects of curcumin on CPT-11-induced intestinal mucosal
injury.
The current study aimed to investigate the
protective effects of curcumin on CPT-11-induced intestinal mucosal
injury and to elucidate the associated mechanisms via in
vivo and in vitro experimentation.
Materials and methods
Chemicals
Curcumin, dimethylsulfoxide (DMSO),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and sodium carboxymethyl cellulose (CMC-Na) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Curcumin was purchased from
Sigma-Aldrich [assay≥98% (HPLC)] and dissolved in DMSO at a
concentration of 40 mg/ml, preserved at -80°C and protected from
light. Irinotecan (CPT-11) was purchased from Hengrui Medicine Co.
Ltd. (Jiangsu, China). SOD and MDA were purchased from Jiancheng
Biotech Ltd. (Nanjing, China). The Annexin-V-FITC apoptosis
detection kit was obtained from KeyGen (Nanjin, China). Finally,
the JC-1 mitochondrial membrane potential assay kit was purchased
from the Beyotime Institute of Biotechnology (Nantong, China).
Animals
Eighteen BALB/c nude mice (6 weeks old, male,
weighing 18±2 g) were purchased from Sun Yat-sen University Animal
Center Inc. (Guangzhou, China) and raised in a SPF environment. The
Laboratory Animal Use Certificate number for these animals is
SCXK(YUE)2011-0029. All the mice were housed in a clean laminar
flow rack in the SPF environment with a constant temperature
(20-26°C) and constant humidity (50-56%) and were exposed to a 12-h
light/dark cycle, and allowed free access to standard laboratory
sterile food and water. The cages, bedding materials, food and
drinking water of the nude mice were sterilized by high-pressure
steam and replaced timely under aseptic conditions. The 18
BALB/c-nu mice were randomly assigned to 3 groups as follows: The
normal control group (CON), the diarrhea model group (CPT-11) and
curcumin therapy group (CPT-11 + CUR) (n=6 mice per group). Mice
were intraperitoneally injected with CPT-11 (75 mg/kg) for 4 days
to establish a model of late-onset diarrhea. Mice in the curcumin
therapeutic group were intraperitoneally injected with CPT-11 (75
mg/kg) for 4 days, and treated with curcumin (100 mg/kg) by
intragastric administration for 8 days at the same time. Mice in
the normal control group and diarrhea model group received the
vehicle only (0.5% CMC-Na). This study was approved by the
Institutional Animal Care and Use Committee of Southern Medical
University (Foshan, China).
Histological examination
The animals were sacrificed by cervical dislocation
on the 9th day. Intestinal samples obtained and fixed in 4%
paraformaldehyde for 24 h. After rinsing with running water, the
samples were dehydrated with an increasing ethanol series, washed
in xylene and embedded in paraffin. Paraffin blocks were cut
4-µm-thick, deparaffinized in xylene and hydrated using an
serial dilution of ethanol. The intestinal tissue sections were
stained with hematoxylin and eosin (H&E) solution (Solarbio
Science & Technology Co., Ltd., Beijing, China) and observed
with an optical microscope (Olympus Corp., Tokyo, Japan).
Immunohistochemical analysis
The tissue sections were conventionally dewaxed in
water and then blocked with 1% BSA for 30 min at room temperature.
The sections were incubated with primary antibodies to P4HB (1:100;
cat. no. ab2792; Abcam, Cambridge, MA, USA) and PRDX4 (1:100; cat.
no. BS6787; BioWorld, Visalia, CA, USA) in a wet box at 4°C
overnight. The sections were subsequently incubated with
biotinylated goat anti-rabbit IgG for 30 min and
avidin-biotin-peroxidase complex (cat. no. SP-9000;
Biotin-Streptavidin HRP Detection Systems; ZSGB-BIO, Beijing,
China) for a further 30 min. The sections were then stained with
DAB solution. After counterstaining with hematoxylin, the sections
were dehydrated, cleared, mounted and examined under a microscope.
Images (x400 magnification) were then captured using a light
microscope (Olympus BX41-32P02-FLB3; Olympus Corp.). Using
Image-Pro Plus 6 software to analyze, the average optical density
values obtained (IOD) represents the expression level of the
proteins.
Cells and cell culture conditions
The normal rat small intestine epithelial cell line,
IEC-6, was obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA). This cell line was cultured in DMEM
(Gibco/Thermo Fisher Scientific, Waltham, MA, USA) with 1%
penicillin-streptomycin and 10% fetal bovine serum (Gibco/Thermo
Fisher Scientific) in a humidified 5% CO2 atmosphere at
37°C.
Evaluation of cell viability via MTT
assay
To investigate the viability of the IEC-6 cells
treated with curcumin or CPT-11, the cells were seeded in 96-well
plates at a density of 1×105/ml in 100 µl of
medium. The cells were then exposed to various concentrations of
curcumin (0.6, 1.2, 2.5, 5, 10, 20 and 40 µg/ml) or CPT-11
(2.5, 5, 10, 20, 40, 50, 80 and 100 µg/ml) for 24 h. The
medium was then removed and MTT solution (5 mg/ml) was added to the
cells at 150 µl in each well, followed by incubation for a
further 4 h at 37°C. The MTT solution was removed after 4 h and 150
µl of DMSO were added to each well for 10 min in the dark.
The absorbance was then measured at 490 nm on a microplate reader
(MD SpectraMax M5; Molecular Devices, LLC., San Jose, CA, USA).
To investigate the protective effects of curcumin on
CPT-11-induced damage to IEC-6 cells, the cells were co-treated
with curcumin (0.6, 1.2 and 2.5 µg/ml) and CPT-11 (20
µg/ml) for 24 h. Subsequently, 150 µl of MTT solution
(5 mg/ml) were added to each well followed by incubation for 4 h at
37°C. Following the removal of the MTT solution, the cells were
dissolved with DMSO and the absorbance was measured at 490 nm on a
microplate reader (as above).
Flow cytometric detection of apoptotic
cells by propidium iodide staining
The apoptosis of the IEC-6 cells was determined
using the Annexin V-FITC apoptosis detection method. According the
result of the protective effect of curcumin, IEC-6 cells were
treated with or without 2.5 µg/ml curcumin in the presence
of 20 µg/ml CPT-11 for 24 h. Following treatment for 24 h,
the cells were harvested with 0.25% trypsin without EDTA, washed
with cold PBS twice, and then resuspended in 400 µl binding
buffer at density of 1×106 cells/ml. Separately, 5
µl of Annexin V-FITC and 5 µl of PI were added to the
cell suspension on ice for 10 min. The apoptosis of the IEC-6 cells
was assayed using a BD FACSCalibur flow cytometer (FACSCalibur
cytometer, BD Biosciences, San Jose, CA, USA) for 1 h.
Approximately 10,00 cells were analyzed per sample.
Flow cytometric detection of
mitochondrial membrane potential
The mitochondrial membrane potential was analyzed
using JC-1 dye. IEC-6 cells were treated with or without 2.5
µg/ml curcumin in the presence of 20 µg/ml CPT-11 for
24 h. Following treatment for 24 h, both floating and adherent
IEC-6 cells were harvested by 0.25% trypsin without EDTA and washed
twice with cold PBS. The cells were then resuspended in JC-1
staining solution and incubated at 37°C for 25 min in the dark. The
cells were then washed with PBS twice, resuspended in 0.5 ml PBS
and analyzed by a BD FACSCalibur flow cytometer for 1 h.
Approximately 10,000 cells were analyzed per sample.
Flow cytometric detection of reactive
oxygen species (ROS)
IEC-6 cells were treated with or without 2.5
µg/ml curcumin in the presence of 20 µg/ml CPT-11 for
24 h. Following treatment for 24 h, the IEC-6 cells were harvested
using 0.25% trypsin without EDTA and washed with cold PBS twice,
followed by incubation with 10 µmol/l DCHF-DA for 25 min in
the dark for at 37°C. Follwoing incubation with DCHF-DA, the cells
were washed with cold PBS twice, resuspended in 0.5 ml PBS and
analyzed by a BD FACSCalibur flow cytometer for 1 h. Approximately
10,000 cells were analyzed for per sample.
Immunofluorescence staining
IEC-6 cells were treated with or without 2.5
µg/ml curcumin in the presence of 20 µg/ml CPT-11 for
24 h. Following treatment for 24 h, the cells were washed with PBS
and fixed with 4% paraformaldehyde for 30 min. The cells were then
permeabilized with 0.1% Triton X-100 for 30 min and then blocked
with 0.5% bovine serum albumin for another 30 min at room
temperature. After washing with PBS, the cells were incubated at
4°C overnight with specific primary antibodies to NF-κB (1:100;
cat. no. sc-71675; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Afterwards, the cells were washed with PBS again and incubated in
DyLight 488 affiniPure goat anti-rabbit IgG (1:500; cat. no.
E032220; EarthOx Life Sciences, Millbrae, CA, USA) for 1 h in the
dark at room temperature. Finally, the resulting cells were stained
with DAPI (Beyotime Institute of Biotechnology). The images were
visualized and captured at (x400 magnification) with an Olympus
microscope.
Western blot analysis
RIPA lysis buffer was added to the intestinal
tissues or IEC-6 cells for protein extraction. Following high-speed
centrifugation (12,000 rpm for 15 min at 4°C), the supernatant was
collected and the protein concentration was determined using a BCA
protein assay kit (Beyotime Institute of Biotechnology). The
protein suspension (30 µg) mixed with 5X loading buffer
(Beyotime Institute of Biotechnology), loaded onto a sodium dodecyl
sulfate-polyacrylamidegel (SDS-PAGE, 8-12%) for electrophoresis and
then transferred onto 0.22 µm PVDF membranes (Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat
milk at room temperature for 1 h. The membranes were then incubated
overnight at 4°C with the following primary antibodies:
Glucose-regulated protein, 78 kDa (GRP78; 1:1,000; cat. no.
1587-1-AP; Proteintech, Rosemont, IL, USA), P4HB (1:1,000), PRDX4
(1:1,000), CHOP (1:1,000; cat. no. 15204-1-AP; Proteintech),
cleaved caspase-3 (1:1,000; cat. no. 9664; Cell Signaling
Technology, Danvers, MA, USA), β-actin (1:1,000; cat. no. BS1002;
BioWorld). After washing with TBST, the membranes were incubated
with goat anti-rabbit IgG-HRP (1:1,000; cat. no. BS13278; BioWorld)
at room temperature for 2 h. After washing with TBST again, the
membranes with proteins were visualized using ECL Plus (Millipore)
and exposed by Kodak In-vivo Imaging System FX Pro (Kodak, Tokyo,
Japan). The expression levels of the proteins were analyzed using
ImageJ 1.48 software (National Institutes of Health, Bethesda, MD,
USA). β-actin served as an internal loading control. The results
are representative of 3 independent experiments.
Statistical analysis
Statistical analyses were conducted using SPSS 19
software (IBM, New York, NY, USA). Data are expressed as the means
± standard deviation (SD). Differences between groups were
evaluated by two-way ANOVA followed by Fisher’s least significant
difference (LSD) test. A P-value <0.05 was considered to be a
statistically significant difference.
Results
Conditions of and delayed-onset diarrhea
in nude mice
The food intake and activity of the mice were
significantly reduced in the diarrhea model group; symptoms of
fatigue, malaise and anorexia were also apparent. The general
condition of the mice in the curcumin therapeutic group had
improved energy, food intake and activity and were in a better
condition than the mice in the model group. The mice were weighed
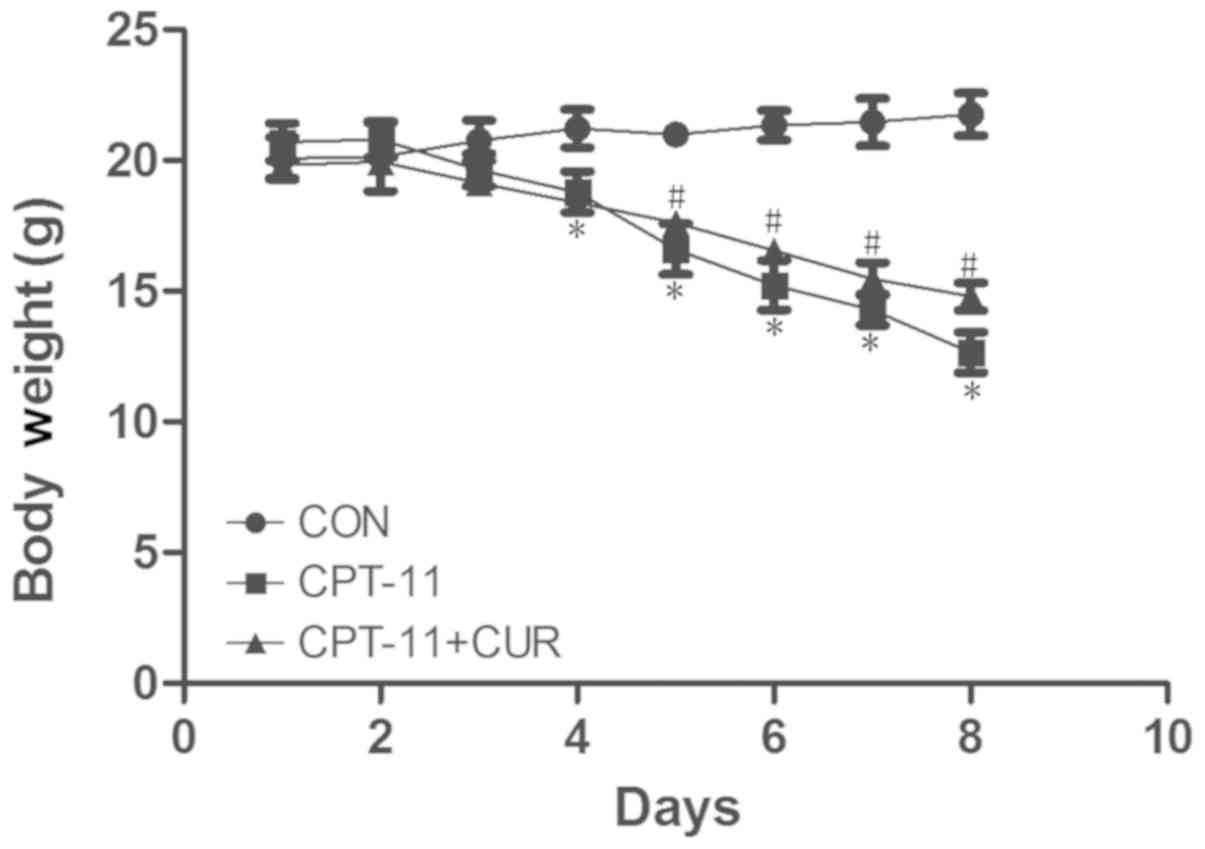
daily and the results of all the groups were compared. As shown in
Fig. 1, the weight of the mice in
the diarrhea model group was significantly lower than that of the
control group (P<0.05). However, the weight of the mice in the
curcumin therapeutic group was significantly increased compared to
that of the mice in the diarrhea model group (P<0.05). All mice
in the model group had delayed-onset diarrhea, a considerably
increased stool frequency, wetter stool loose in shape, and some
mice had watery stool. Stool was slightly squishy in the curcumin
therapeutic group; some stool were wetter without shape; however,
the frequency of diarrhea was less than that in the model group. As
shown in Table I, the diarrhea
scores of the curcumin therapeutic group were lower than those of
the model group (P<0.05).
 | Table IEffects of curcumin on the diarrhea
score in mice with CPT-11-induced delayed-onset diarrhea. |
Table I
Effects of curcumin on the diarrhea
score in mice with CPT-11-induced delayed-onset diarrhea.
| Group | Diarrhea scores
|
|---|
| Day 6 | Day 7 | Day 8 | Average |
|---|
| Control group | 0 | 0 | 0 | 0 |
| Model group | 2.33±0.51a | 2.33±0.51a | 2.50±0.55a | 2.39±0.50a |
| Curcumin
therapeutic group | 1.33±0.52b | 1.67±0.84b | 1.33±0.52b | 1.44±0.62b |
Effects of curcumin on CPT-11-induced
damage to the small intestinal mucosa
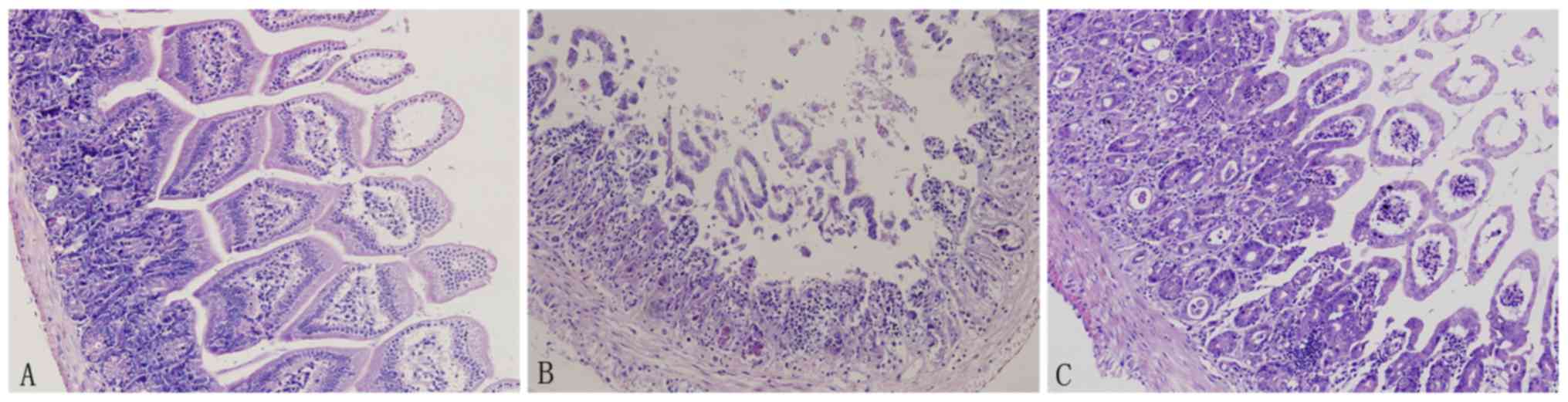
H&E staining revealed a normal small intestinal
mucosa structure in the control group. However, the mucosal
structure was severely damaged in the CPT-11 group. Crypt
structures were damaged, glands were irregularly arranged and villi
were shortened or ‘dropped out’ with inflammatory cell
infiltration. Notably, curcumin treatment significantly improve the
histological structure of the intestinal mucosa (Fig. 2).
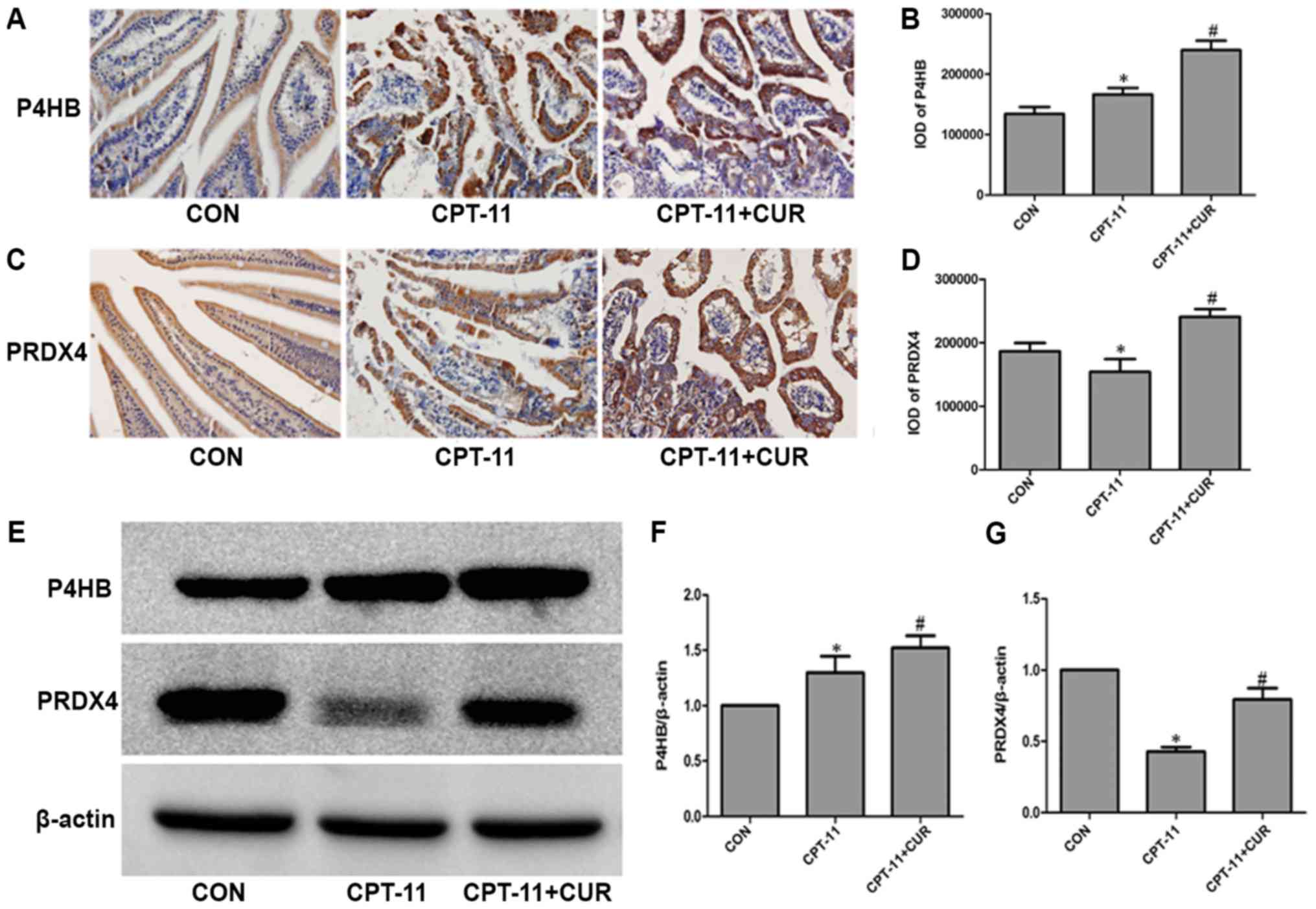
Effect of curcumin on the expression of
P4HB and PRDX4 in small intestinal tissue
The molecular chaperones, P4HB and PRDX4, were
detected by immunohistochemistry in the small intestinal tissue.
P4HB (Fig. 3A) and PRDX4 (Fig. 3C) were mainly expressed in the
cytoplasm of the small intestinal epithelial cells. As shown in
Fig. 3B, the expression of P4HB in
the model group was higher than that in the normal control group
(P<0.05), and curcumin further upregulated the expression of
P4HB compared with the model group (P<0.05). In general, as
shown in Fig. 3D, the suppression
of PRDX4 expression was observed following injury by CPT-11
(P<0.05). The CPT-11 induced suppression of PRDX4 expression was
significantly reversed by treatment with curcumin (P<0.05).
These two proteins were also examined by western blot analysis, and
the results were in agreement with those obtained by
immunohistochemistry (Fig.
3E-G).
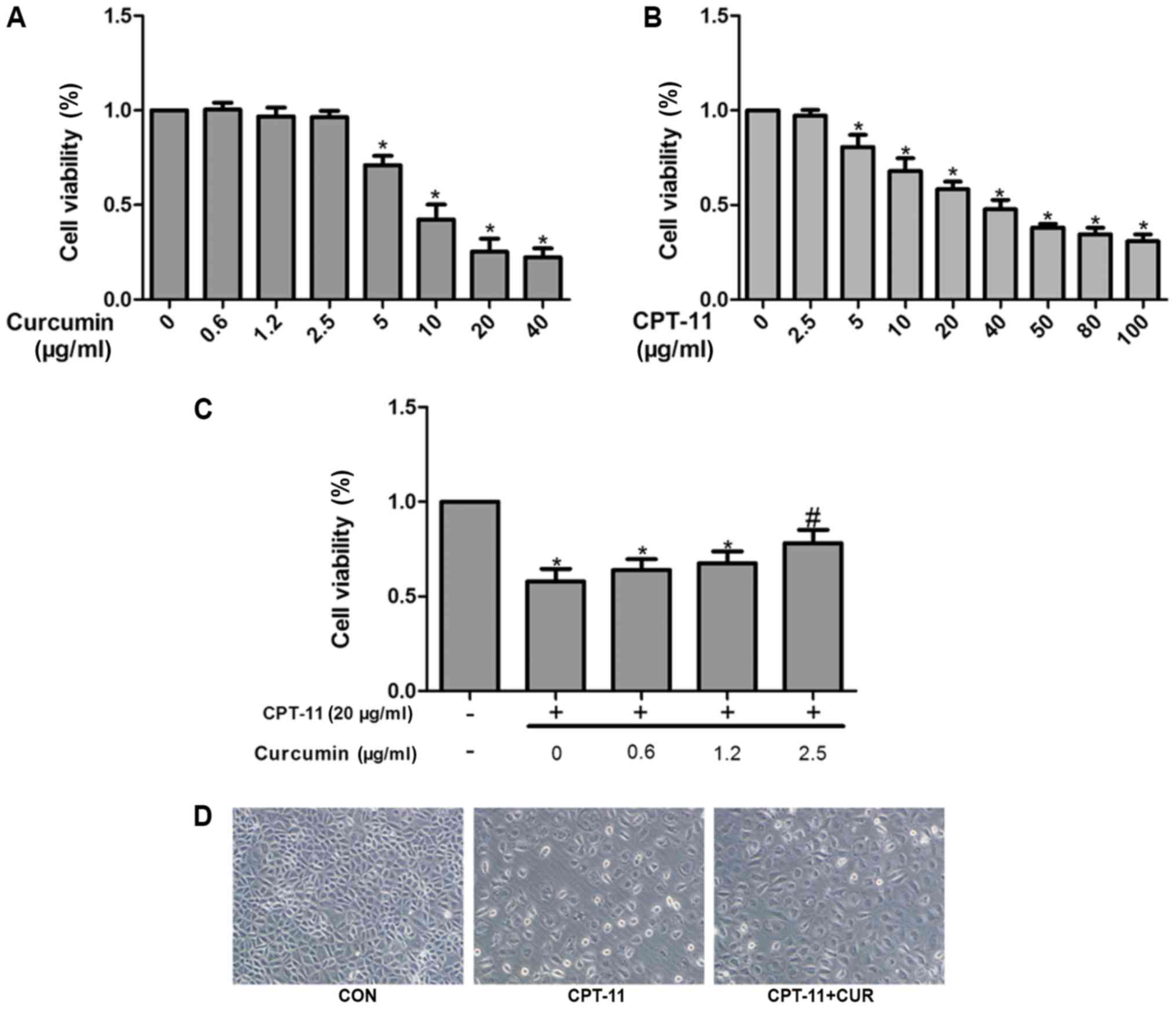
Effect of curcumin and CPT-11 on IEC-6
cell viability
The effects of curcumin and CPT-11 on the viability
IEC-6 cells was evaluated by MTT assay. As shown in Fig. 4A, curcumin treatment at
concentrations of 0.6, 1.2 and 2.5 µg/ml exhibited no
obvious cytotoxic effect on IEC-6 cells (P>0.05). However, at
concentrations >2.5 µg/ml curcumin, dose-dependent cell
death was observed (P<0.05). Compared with the control group,
the viability of the IEC-6 cells decreased gradually with
increasing concentrations of CPT-11 (P<0.05; Fig. 4B). IEC-6 cell viability was
approximately 60% following incubation with 20 µg/ml CPT-11
for 24 h. The IEC-6 cells were also co-treated with various
concentrations of curcumin (0.6, 1.2 and 2.5 µg/ml) and 20
µg/ml CPT-11 for 24 h; cell viability was increased as
compared with the CPT-11 group (Fig.
4C).
Protective effects of curcumin against
CPT-11-induced cytotoxicity and morphological changes
The morphological changes of the IEC-6 cells treated
with or without curcumin (2.5µg/ml) in the presence of 20
µg/ml CPT-11 for 24 h were examined. Compared with the
control group, the cells in the CPT-11 group were shrunken, and the
size and morphology of the cells was clearly altered from a
fusiform to polygonal shape. As observed in the curcumin treatment
group, curcumin maintained the normal morphology of the IEC-6 cells
to a certain extent (Fig. 4D).
Curcumin protects the IEC-6 cells from
CPT-11-induced apoptosis
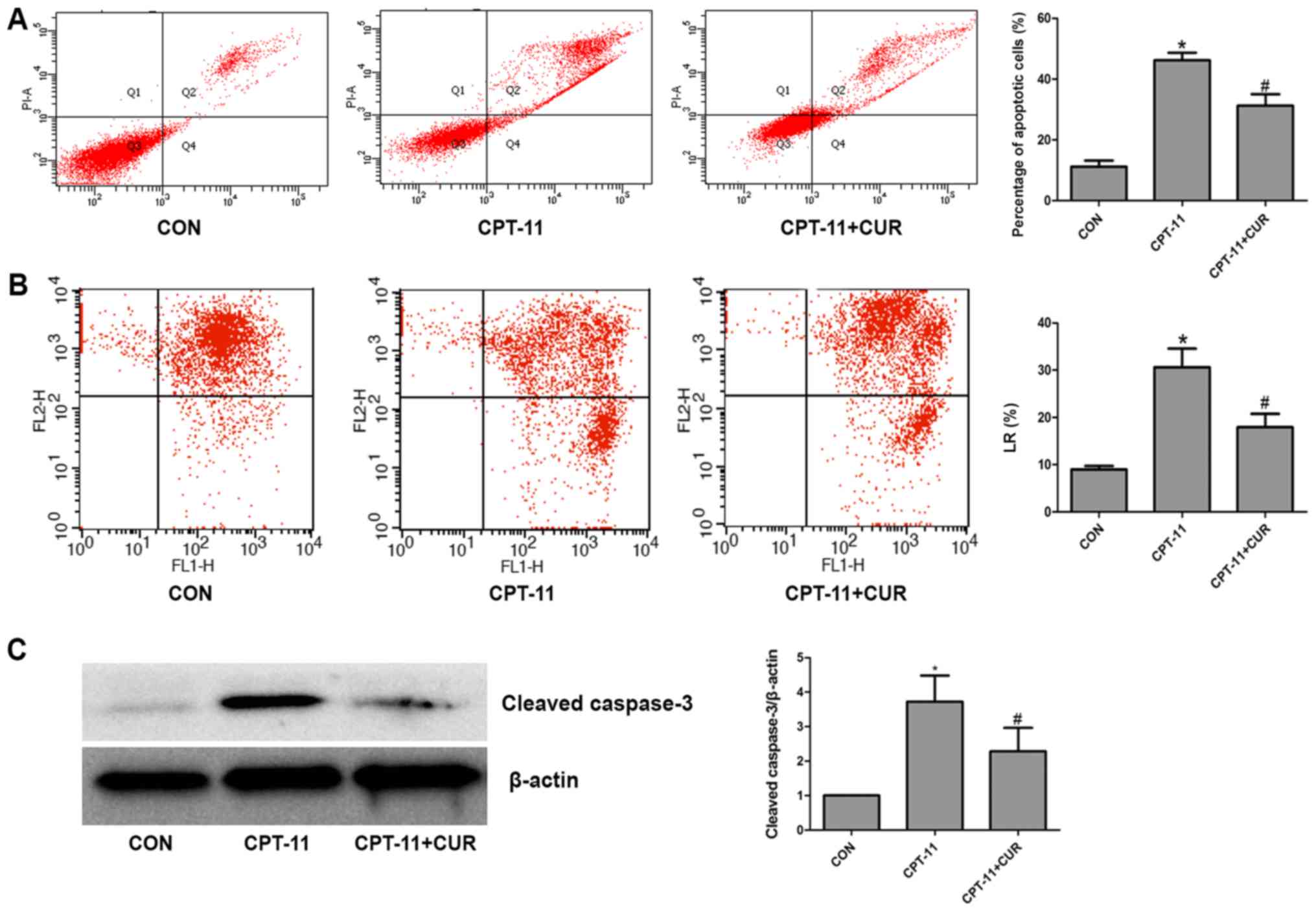
Annexin V/PI staining and flow cytometry were used
to detect the apoptotic rate of the IEC-6 cells. As shown in
Fig. 5A, the cell apoptotic rate
was significantly increased following exposure to the indicated
concentration (20 µg/ml) of CPT-11 for 24 h (P<0.05).
Co-treatment of the IEC-6 cells with 20 µg/ml CPT-11 and 2.5
µg/ml curcumin markedly reduced the rate of apoptosis
(P<0.05). It is understood that the loss of mitochondrial
membrane potential is an important event in early apoptosis. The
percentage of cells in the lower right (LR) quadrant of the flow
cytometric scatter plot represent the rate of early apoptosis. The
analysis of mitochondrial membrane potential revealed that compared
with the control group, the percentage of cells in the LR quadrant
was significantly increased in the CPT-11 group (P<0.05).
However, the curcumin treatment group had a lower percentage of
cells in the LR quadrant as compared to the CPT-11 group
(P<0.05; Fig. 5B). The data
from western blot analysis revealed that following exposure to 20
µg/ml CPT-11 for 24 h, cleaved caspase-3 expression
significantly increased compared to the control group (P<0.05).
Co-treatment with 2.5 µg/ml curcumin resulted in a
significant decrease in CPT-11-induced cleaved caspase-3 expression
(P<0.05; Fig. 5C). These
results indicated that curcumin inhibited the apoptosis of the
IEC-6 cells induced by CPT-11.
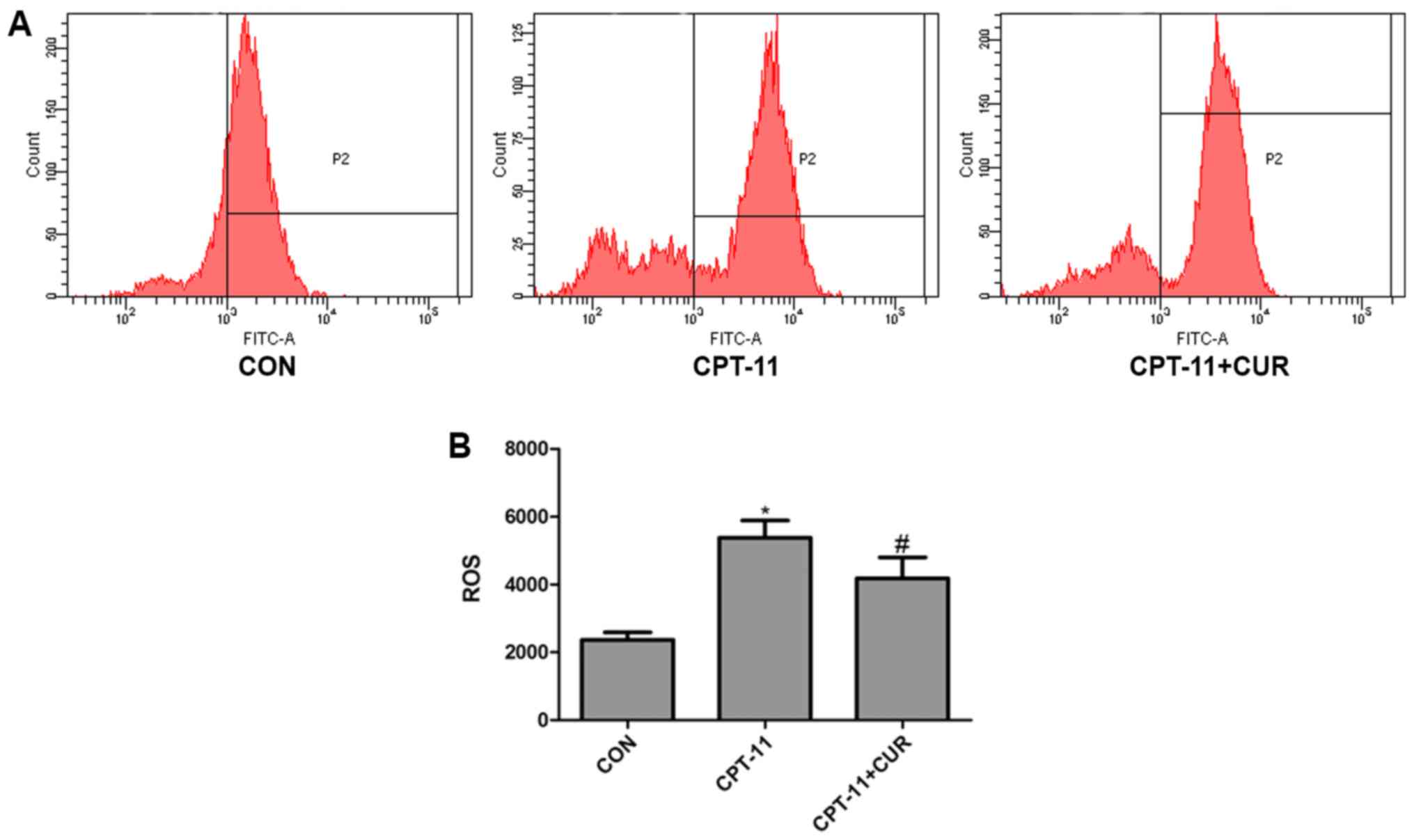
Protective effects of curcumin on
CPT-11-induced ROS generation
As shown in Fig. 6,
20 µg/ml CPT-11 caused ROS levels to increase compared to
the control group (P<0.05). However, treatment with 2.5
µg/ml curcumin suppressed the generation of ROS induced by
CPT-11 (P<0.05). These findings suggest that curcumin reduces
ROS levels which are indcued by CPT-11.
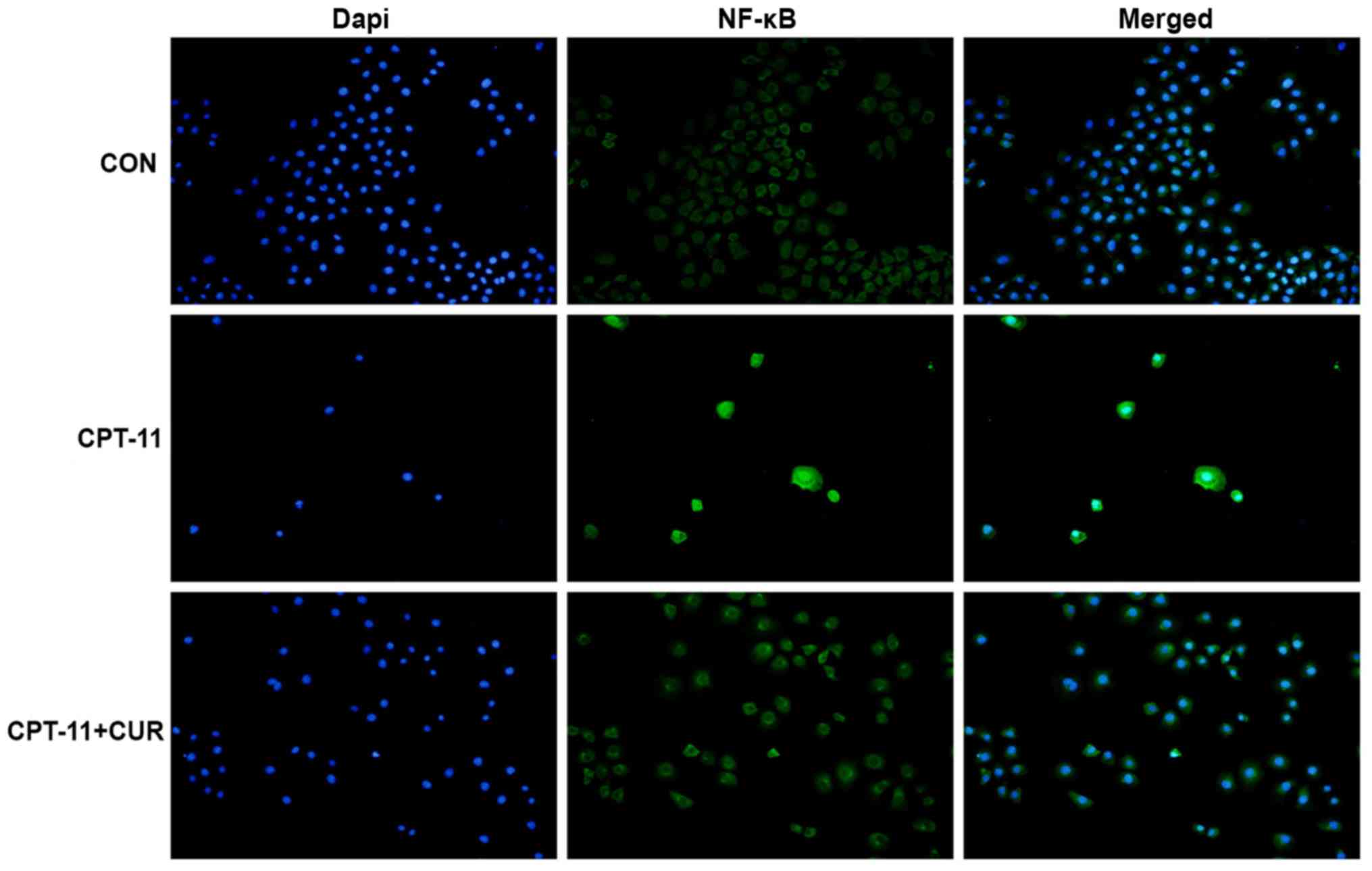
Curcumin inhibits the CPT-11-induced
nuclear translocation of NF-κB
Normally, NF-κB is a dimer mainly composed of NF-κB
p65 and NF-κB p50 subunits and is maintained in an inactive state
in the cytoplasm. When cells are stimulated by internal or external
factors, such as lipopolysaccharide (LPS), TNF-α or CPT-11, NF-κB
p65 is released from the NF-κB transcription complex and
translocates to the nucleus in order to activate a series of gene
transcription (12,15-17).
In this study, the nuclear translocation of NF-κB p65 in the IEC-6
cells was analyzed by immunofluorescence staining. The results
revealed that NF-κB p65 was expressed in the cytoplasm of the
untreated IEC-6 cells, whereas CPT-11 induced the nuclear
translocation of NF-κB p65. Curcumin significantly reduced the
NF-κB p65 nuclear translocation induced by CPT-11 in the IEC-6
cells (Fig. 7).
Effect of curcumin on ER
stress-associated proteins
To determine whether the CPT-11-induced apoptosis of
IEC-6 cells occurs through ER stress the effect of curcumin on ER
stress-induced cell death, the expression levels of the ER
stress-associated proteins, GRP78, P4HB, PRDX4 and CHOP, were
examined by western blot analysis. As expected, the level of GRP78
in the CPT-11 group was upregulated compared to the control group
(P<0.05), and compared with the CPT-11 group, curcumin caused a
robust enhancement of GRP78 expression (P<0.05). In addition,
the group exposed to CPT-11 exhibited a significant reduction in
the expression levels of P4HB and PRDX4 (P<0.05) compared with
the control group. Curcumin suppressed the upregulation of these
two proteins induced by CPT-11 (P<0.05). Furthermore, the
expression of CHOP was also significantly increased compared with
the control group (P<0.05), while curcumin markedly decreased
CHOP expression (P<0.05; Fig.
8). Thus, it was demonstrated that CPT-11 induces injury to and
the apoptosis of IEC-6 cells through the activation of ER stress,
and ER stress is significantly suppressed by curcumin
treatment.
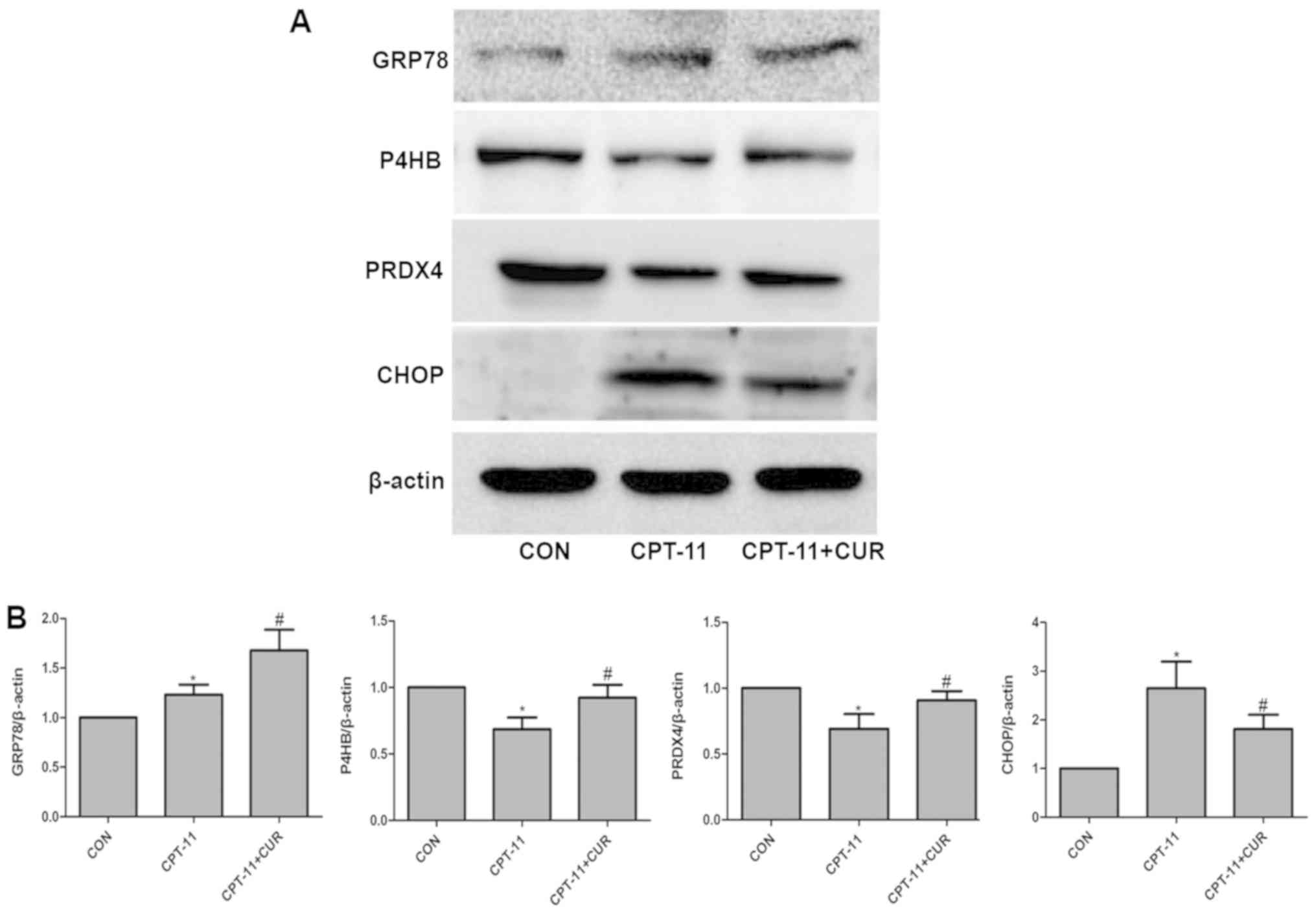 | Figure 8Effects of curcumin on endoplasmic
reticulum (ER) stress-induced changes in GRP78, P4HB, PRDX4 and
CHOP expression. (A) IEC-6 cells were treated with or without 2.5
µg/ml curcumin in the presence of 20 µg/ml CPT-11 for
24 h and the expression of GRP78, P4HB, PRDX4 and CHOP was
determined by western blot analysis. (B) Quantitative analysis of
the relative protein levels of GRP78, P4HB, PRDX4 and CHOP. Data
are presented as the means ± SD, n=3 experiments.
*P<0.05 vs. the control group, #P<0.05
vs. the CPT-11 group. GRP78, glucose-regulated protein, 78 kDa;
P4HB, prolyl 4-hydroxylase subunit beta; PRDX4, peroxiredoxin
4. |
Discussion
Curcumin has poor systemic availability due to its
low aqueous solubility, efficient first-pass effect and a certain
degree of intestinal metabolism when administered via the oral
route. However, in this study, we demonstrated that curcumin
exerted a protective effect on CPT-11 induced intestinal mucosal
injury both in vivo and in vitro. In vivo, we
found that the general and diarrheal conditions of the nude mice in
the curcumin therapeutic group were better than those of the mice
in the model group. With light microscopy we also observed that
curcumin improved intestinal mucosal injury caused by CPT-11.
Moreover, in vitro, treatment with 2.5 µg/ml curcumin
exhibited no cytotoxicity and effectively improved the viability of
the IEC-6 cells treated with CPT-11 at 20 µg/ml for 24 h. In
addition, the data from IEC-6 cell morphological observation, cell
apoptosis assay, mitochondrial membrane potential assay and
apoptosis protein cleaved caspase-3 detection demonstrated that
curcumin reduced CPT-11-induced apoptosis, which indicates that
this molecule may exert a protective effect against certain
chemotherapeutic drugs.
Currently, CPT-11-induced intestinal mucosal injury
mainly manifests as late-onset diarrhea; however, the mechanisms of
the pathogenesis of late-onset diarrhea remain unclear. It is
accepted that following absorption into the body, CPT-11 is
metabolized by enzymes, such as carboxylesterase (CES), cytochrome
P450 (CYP) and intestinal β glucose polygalacturonase
(β-glucuronidase) and is changed into the active metabolite, SN-38,
which directly damages intestinal mucosa cells (4). In addition, CPT-11-induced intestinal
mucosal injury can cause a variety of signals, such as the
activation of NF-κB pathway, the upregulated expression of the
inflammation related factors, COX-2, PGE2 and TAX2, and the
promotion of the inflammatory factors, TNF-α and IL-1β, resulting
in inflammation within the intestines and damage to the intestinal
mucosal barrier function (5).
Moreover, the UGT1A1 gene polymorphism (6), intestinal epithelial cell tight
junction protein destruction (7)
and bacteria group translocation (8) might be possible mechanisms of CPT-11
induced diarrhea. In this study, following CPT-11 treatment at 20
µg/ml for 24 h, NF-κB in the IEC-6 cells was localized to
the nucleus from the cytoplasm. In addition, the level of ROS was
significantly increased, and the results of western blot analysis
revealed that CPT-11 upregulated the expression of the ER
stress-related marker proteins, GRP78 and CHOP. Thus, the
mechanisms of CPT-11-induced intestinal mucosa injury are not only
related with NF-κB activation, but also with oxidative and ER
stress.
As a traditional Chinese medicine, curcumin has many
characteristics, such as an abundant source, low toxicity, multiple
molecular targets and is well-tolerated. It also has a wide range
of pharmacological effects, including anti-inflammatory,
antioxidant, anti-fibrotic and anti-tumor activity (9-11).
NF-κB is a nuclear transcription factor, involved in various
inflammatory responses, cell stress, cell transformation, tumor
invasion and drug resistance (15). Normally, NF-κB is a dimer mainly
composed of NF-κB p65 and NF-κB p50 subunits and is maintained in
an inactive state in the cytoplasm. When the cells are stimulated
by internal and external factors, such as LPS, TNF-α or CPT-11,
NF-κB p65 is released from the NF-κB transcription complex and
translocates to the nucleus in order to activate a series of gene
transcription and expression including IL-2, IL-6, ICAM-1,
E-selectin among others (16,17).
This is the classical NF-κB activation pathway.
A large number of studies have proven that curcumin
inhibits the NF-κB activation. As demonstrated by Song et al
(12), curcumin exerted a
protective effect on MTX-induced rat enteritis, and the underlying
mechanism involved the inhibition of the activation of NF-κB in
intestinal mucosal cells and the regulation of the production of
inflammatory factors and cytokines by curcumin, probably via the
NF-κB signaling pathway. In human articular cartilage cells, has
been shown to curcumin inhibit the activation of NF-κB induced by
IL-1β (18). Jian et al
(19) also found that curcumin
inhibited NF-κB activation in a colitis model induced by
trinitrobenzene sulfonic acid. In this study, CPT-11 induced the
nuclear translocation of NF-κB p65 in IEC-6 cells, and curcumin
significantly reduced NF-κB p65 nuclear translocation induced by
CPT-11 in IEC-6 cells. It was thus suggested that the inhibitory
effects of curcumin against the production of inflammatory factors
and cytokines probably occurred via the NF-κB signaling
pathway.
In addition, some studies have indicated that an
increase in ROS generation is an important factor in intestinal
mucosal injury. As an important factor, SOD can effectively
scavenge oxygen free radical ROS and can inhibit lipid
peroxidation, so as to reduce the level of MDA, thus playing a role
as an antioxidant (20). In
vitro, wheat peptide administration may be an effective tool
for protecting small intestinal tissue against non-steroidal
anti-inflammatory drug (NSAID)-induced small intestinal damage and
oxidative stress by suppressing NF-κB activation and regulating the
expression levels of SOD, glutathione peroxidase (GPx) and MDA
(21).
DNA damage is the one cell killing mechanisms of
CPT-11. Kang et al (22)
indicated that DNA damage induces ROS generation. Therefore, we
hypothesized that CPT-11 may induce DNA damage in IEC-6 cells and
result in increasing intracellular ROS levels and NF-κB activation,
disrupting the intestinal redox balance, and causing intestinal
mucosal injury, as well as inducing cell apoptosis. Antioxidant
activity, the scavenging oxygen free radicals, is an important
pharmacological effect of curcumin. In a diabetic rat model of
gastric light paralysis, curcumin was shown to improve gastric
emptying symptoms, and this mechanism may block the generation of
oxidative stress, and inhibit the signal transduction of NF-κB
(23). Arafa et al
(13) demonstrated that curcumin
increased intestinal SOD, GSH and GST, and downregulated MDA levels
in a model of ulcerative colitis models induced by DSS. In
vitro, in this study, curcumin inhibited the upregulation of
ROS induced by CPT-11 in IEC-6 cells. Thus, curcumin can
effectively scavenge oxygen free radicals and can counteract
oxidative damage in the intestinal mucosa induced by CPT-11.
Moreover, we found that ER stress participated in
the pathogenesis of CPT-11-induced intestinal mucosal injury. The
ER is the main site of protein synthesis, lipid production and
Ca2+ storage in eukaryotic cells. When the cells are in
a low sugar, hypoxic and acidosis microenvironment, or if there is
a calcium ion, or redox imbalance, unfolded or misfolded proteins
will gather in the endoplasmic reticulum causing endoplasmic
reticulum stress and activation of the unfolded protein response
(UPR) (24).
The unfolded protein response is mainly mediated by
the activation of three transmembrane proteins, inositol-requiring
enzyme 1 (IRE1), protein kinase R-like endoplasmic reticulum kinase
(PERK) and activating transcription factor 6 (ATF6). Under normal
resting conditions, these transmembrane proteins are bound to the
ER stress marker protein, GRP78, in an inactive form. When ER
stress occurs, GRP78 dissociates from the three membrane proteins
and plays the role of a molecular chaperone, inducing the synthesis
of other molecular chaperones such as P4HB and PRDX4, and promoting
the correct refolding of protein in order to maintain the
endoplasmic reticulum homeostasis (24-26).
However, when the pressure of ER stress is too strong or prolonged,
it leads to insufficient endoplasmic reticulum homeostasis,
CHOP/GADD153 expression, JNK activation and caspase-12 expression
will be induced, eventually leading to cell apoptosis (27). In addition, Bcl-2 and Bax are two
classic proteins in the apoptotic pathway. They are mutually
validated and have a clear persuasion in terms of apoptosis. and
these two proteins will be the direction of our future research.
From what has been mentioned above, the inhibition of ER stress can
play a protective role in cells and tissues.
Kim et al (28) indicated that NELL2 upregulated
GRP78 and downregulated CHOP expression by the regulation of the
ERK signaling pathway, thereby inhibiting the apoptosis of COS-7
cells induced by ER stress. Similarly, Xu et al (29) demonstrated that GSP upregulated
GRP78 and downregulated the expression of CHOP, which alleviates
endoplasmic reticulum stress caused by hepatic ischemia reperfusion
in rats. It has also been reported that curcumin potently inhibits
ER stress. Afrin et al (30) found that streptozotocin (STZ)
induced liver injury in diabetic rats via ER stress and that
curcumin alleviated liver ER stress by regulating the expression of
GRP78, ASK, IRE1 and CHOP proteins. Curcumin has also been shown to
suppress ER stress and oxidative stress in mouse hippocampal cells
induced by CoCl2 (31).
In this study, following treatment with CPT-11 for
24 h, the expression of GRP78 was markedly increased, resulting in
ER stress in IEC-6 cells. The expression levels of GRP78, P4HB and
PRDX4 were increased further in the curcumin protection group
compared to the CPT-11 group. As molecular chaperones, these
proteins exert a certain protective effect by helping to correctly
fold unfolded or misfolded proteins and maintaining ER homeostasis.
CHOP, as a specific protein in the ER stress pathway, mediates
apoptosis by regulating the mitochondrial apoptosis pathway or the
expression of death receptors (24,25,27).
Therefore, it follows that the CHOP expression level directly
reflects the strength of ER stress. In this study, the expression
of CHOP in CPT-11 group was also increased significantly compared
to the control group. It is therefore clear that curcumin markedly
reduces the relative amounts of CHOP. These results indicate that
curcumin can protect intestinal mucosa cells by improving or
mitigating ER stress induced by CPT-11.
In conclusion, the findings of this study
demonstrate that curcumin exets a protective effect against
CPT-11-induced intestinal mucosal injury mediated by the inhibition
of NF-κB activation, oxidative stress and ER stress, induced by
CPT-11. Recent studies have demonstrated that NF-κB activation,
oxidative stress and ER stress are interrelated biological process
that commonly regulate signal transduction pathways in cells, and
participate in the development of human physiological changes and
diseases. However, the association among these three has not yet
been clarified (32,33). It was thus concluded that the
protective effects of curcumin is mediated by suppressing the NF-κB
activation, oxidative stress and ER stress. However, how to improve
the bioavailability of curcumin, and the specific signaling
pathways where curcumin plays a role and the association between
these three physiological processes in this manuscript will be the
focus of our research in the future.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
XY, ZL and MO conceived and designed the
experiments. ZL and WZ performed the experiments and acquired data.
DZ, YL and JW provided the methodology, analyzed and interpreted
the data. ZL and WZ drafted the manuscript. XY and MO directed the
writing of the manuscript. ZL revised the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee of Southern Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
All the authors would like to thank the public
experimental platform (Research Center of Clinical Medicine of
Nfanfang Hospital Affiliated to Southern Medical University,
Guangzhou, Guangdong, China) for providing the experimental
facilities.
References
|
1
|
Tanizawa A, Fujimori A, Fujimori Y and
Pommier Y: Comparison of topoisomerase I inhibition, DNA damage,
and cytotoxicity of camptothecin derivatives presently in clinical
trials. J Natl Cancer Inst. 86:836–842. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bleiberg H and Cvitkovic E:
Characterisation and clinical management of CPT-11
(irinotecan)-induced adverse events: The European perspective. Eur
J Cancer. 32A(Suppl 3): S18–S23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benson AB III, Ajani JA, Catalano RB,
Engelking C, Kornblau SM, Martenson JA Jr, McCallum R, Mitchell EP,
O’Dorisio TM, Vokes EE, et al: Recommended guidelines for the
treatment of cancer treatment-induced diarrhea. J Clin Oncol.
22:2918–2926. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanghani SP, Quinney SK, Fredenburg TB,
Davis WI, Murry DJ and Bosron WF: Hydrolysis of irinotecan and its
oxidative metabolites, 7-ethyl-10-[4-N-(5-aminopentanoic
acid)-1-piperidino] carbonyloxycamptothecin and
7-ethyl-10-[4-(1-piperidino)-1-a mino]-carbonyloxycamptothecin, by
human carboxylesterases CES1A1, CES2, and a newly expressed
carboxylesterase isoenzyme, CES3. Drug Metab Dispos. 32:505–511.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swami U, Goel S and Mani S: Therapeutic
targeting of CPT-11 induced diarrhea: A case for prophylaxis. Curr
Drug Targets. 14:777–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu JM, Wang Y, Ge FJ, Lin L, Liu ZY and
Sharma MR: Severe irinotecan-induced toxicity in a patient with
UGT1A1 28 and UGT1A1 6 polymorphisms. World J Gastroenterol.
19:3899–3903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakao T, Kurita N, Komatsu M, Yoshikawa K,
Iwata T, Utusnomiya T and Shimada M: Irinotecan injures tight
junction and causes bacterial translocation in rat. J Surg Res.
173:341–347. 2012. View Article : Google Scholar
|
|
8
|
Lin XB, Farhangfar A, Valcheva R, Sawyer
MB, Dieleman L, Schieber A, Gänzle MG and Baracos V: The role of
intestinal microbiota in development of irinotecan toxicity and in
toxicity reduction through dietary fibres in rats. PLoS One.
9:e836442014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal BB, Gupta SC and Sung B:
Curcumin: An orally bioavailable blocker of TNF and other
pro-inflammatory biomarkers. Br J Pharmacol. 169:1672–1692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Woo JM, Shin DY, Lee SJ, Joe Y, Zheng M,
Yim JH, Callaway Z and Chung HT: Curcumin protects retinal pigment
epithelial cells against oxidative stress via induction of heme
oxygenase-1 expression and reduction of reactive oxygen. Mol Vis.
18:901–908. 2012.PubMed/NCBI
|
|
11
|
Rahmani AH, Al Zohairy MA, Aly SM and Khan
MA: Curcumin: A potential candidate in prevention of cancer via
modulation of molecular pathways. BioMed Res Int. 2014:7616082014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song WB, Wang YY, Meng FS, Zhang QH, Zeng
JY, Xiao LP, Yu XP, Peng DD, Su L, Xiao B, et al: Curcumin protects
intestinal mucosal barrier function of rat enteritis via activation
of MKP-1 and attenuation of p38 and NF-κB activation. PLoS One.
5:e129692010. View Article : Google Scholar
|
|
13
|
Arafa HM, Hemeida RA, El-Bahrawy AI and
Hamada FM: Prophylactic role of curcumin in dextran sulfate sodium
(DSS)-induced ulcerative colitis murine model. Food Chem Toxicol.
47:1311–1317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu DJ, Chen XW, Wang JZ, Ju YL, Ou Yang
MZ and Zhang WJ: Proteomic analysis identifies proteins associated
with curcumin-enhancing efficacy of irinotecan-induced apoptosis of
colorectal cancer LOVO cell. Int J Clin Exp Pathol. 7:1–15.
2013.
|
|
15
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
16
|
Meng Z, Yan C, Deng Q, Gao DF and Niu XL:
Curcumin inhibits LPS-induced inflammation in rat vascular smooth
muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways.
Acta Pharmacol Sin. 34:901–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Logan RM, Gibson RJ, Bowen JM, Stringer
AM, Sonis ST and Keefe DM: Characterisation of mucosal changes in
the alimentary tract following administration of irinotecan:
Implications for the pathobiology of mucositis. Cancer Chemother
Pharmacol. 62:33–41. 2008. View Article : Google Scholar
|
|
18
|
Csaki C, Mobasheri A and Shakibaei M:
Synergistic chondroprotective effects of curcumin and resveratrol
in human articular chondrocytes: Inhibition of IL-1beta-induced
NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther.
11:R1652009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC
and Fang YX: Preventive and therapeutic effects of NF-kappaB
inhibitor curcumin in rats colitis induced by trinitrobenzene
sulfonic acid. World J Gastroenterol. 11:1747–1752. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Q, Ren X, Jiang Y, Jin H, Liu N and Li
J: Advanced glycation end products accelerate rat vascular
calcification through RAGE/oxidative stress. BMC Cardiovasc Disord.
13:132013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin H, Pan X, Song Z, Wang S, Yang L and
Sun G: Protective effect of wheat peptides against
indomethacin-induced oxidative stress in IEC-6 cells. Nutrients.
6:564–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang MA, So EY, Simons AL, Spitz DR and
Ouchi T: DNA damage induces reactive oxygen species generation
through the H2AX-Nox1/Rac1 pathway. Cell Death Dis. 3:e2492012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin QH, Shen HX, Wang H, Shou QY and Liu
Q: Curcumin improves expression of SCF/c-kit through attenuating
oxidative stress and NF-κB activation in gastric tissues of
diabetic gastro-paresis rats. Diabetol Metab Syndr. 5:122013.
View Article : Google Scholar
|
|
24
|
Latham KE: Endoplasmic reticulum stress
signaling in mammalian oocytes and embryos: Life in balance. Int
Rev Cell Mol Biol. 316:227–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schönthal AH: Endoplasmic reticulum
stress: Its role in disease and novel prospects for therapy.
Scientifica (Cairo). 2012:8575162012.
|
|
26
|
Bravo R, Parra V, Gatica D, Rodriguez AE,
Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E,
et al: Endoplasmic reticulum and the unfolded protein response:
Dynamics and metabolic integration. Int Rev Cell Mol Biol.
301:215–290. 2013. View Article : Google Scholar :
|
|
27
|
Liu MQ, Chen Z and Chen LX: Endoplasmic
reticulum stress: A novel mechanism and therapeutic target for
cardiovascular diseases. Acta Pharmacol Sin. 37:425–443. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim DY, Kim HR, Kim KK, Park JW and Lee
BJ: NELL2 function in the protection of cells against endoplasmic
reticulum stress. Mol Cells. 38:145–150. 2015.
|
|
29
|
Xu ZC, Yin J, Zhou B, Liu YT, Yu Y and Li
GQ: Grape seed proanthocyanidin protects liver against
ischemia/reperfusion injury by attenuating endoplasmic reticulum
stress. World J Gastroenterol. 21:7468–7477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Afrin R, Arumugam S, Soetikno V,
Thandavarayan RA, Pitchaimani V, Karuppagounder V, Sreedhar R,
Harima M, Suzuki H, Miyashita S, et al: Curcumin ameliorates
streptozotocin-induced liver damage through modulation of
endoplasmic reticulum stress-mediated apoptosis in diabetic rats.
Free Radic Res. 49:279–289. 2015. View Article : Google Scholar :
|
|
31
|
Chhunchha B, Fatma N, Kubo E, Rai P, Singh
SP and Singh DP: Curcumin abates hypoxia-induced oxidative stress
based-ER stress-mediated cell death in mouse hippocampal cells
(HT22) by controlling Prdx6 and NF-κB regulation. Am J Physiol Cell
Physiol. 304:C636–C655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhandary B, Marahatta A, Kim HR and Chae
HJ: An involvement of oxidative stress in endoplasmic reticulum
stress and its associated diseases. Int J Mol Sci. 14:434–456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaudhari N, Talwar P, Parimisetty A,
Lefebvre d’Hellencourt C and Ravanan P: A molecular web:
Endoplasmic reticulum stress, inflammation, and oxidative stress.
Front Cell Neurosci. 8:2132014. View Article : Google Scholar : PubMed/NCBI
|