Introduction
Colorectal cancer (CRC) is a very common malignancy
associated with a high mortality rate, and it has become a major
health problem worldwide (1). Due
to advances in screening techniques and surgical management, the
mortality rate of CRC has reduced considerably in developed
countries (2). However, as is the
case in other organ malignancies, recurrence and metastasis in CRC
counteracts these improvements in prognosis following resection.
The 5-year overall survival rate of patients with CRC is nearly 90%
when the CRC is localized, but it reduces to <70% once distant
metastases occurs (3). It is well
known that the development of CRC metastasis is an extremely
complex process with multiple stages and various molecular
mechanisms. Therefore, it is urgently necessary to obtain a greater
understanding of the factors involved in invasion and migration,
and to confirm novel prognostic biomarkers to improve the survival
rate in CRC.
Chicken ovalbumin upstream promoter-transcription
factor II (COUP-TFII), also known as nuclear receptor subfamily 2
group F member 1, is a nuclear orphan receptor that possesses two
highly conserved motifs (a DNA-binding domain and a putative
ligand-binding domain), and it belongs to the steroid/thyroid
hormone receptor super-family (4).
Biochemical studies have confirmed that COUP-TFII commonly exists
in its dimeric form, has a high affinity to down-regulator of
transcription 1, and competes for other nuclear receptors activated
by hormone responses, which results in the suppression of a large
number of genes (5,6). In addition, COUP-TFII forms
DNA-binding heterodimers with retinoid X receptor, competes for
various nuclear receptors, and consequently reduces hormone
responsiveness (7). Apart from
acting as a repressor, COUP-TFII also activates the promoters of a
vast number of genes by interacting with known co-activators such
as p300 or other transcription factors (8). However, no data published thus far
has shown whether COUP-TFII regulates the expression of non-coding
RNA, including long non-coding RNA and microRNA (miR/miRNA).
COUP-TFII is mainly expressed in early embryonic tissues and serves
an important role in regulating various developmental processes
such as peripheral and central nervous system developments
(9).
Epithelial-mesenchymal transition (EMT) is
considered the main process by which various cancers progress,
including CRC, which gains metastatic features as tumor cells
transition from having an epithelial morphology to an elongated,
fibroblast-like morphology with depolarization and cell-cell
disconnection. In addition, EMT promotes the development of drug
resistance and stemness, which are significant inhibitors of the
successful treatment of cancer (10). COUP-TFII expression is often
downregulated shortly after birth, but several recent reports have
shown that COUP-TFII expression is significantly increased in
various cancer tissues when compared with corresponding
non-cancerous tissues and is correlated with cancer development
(11,12). Qin et al (13,14)
demonstrated that by regulating two major angiogenic signaling
pathways, vascular endothelial growth factor (VEGF)/VEGF receptor
(VEGFR)-2 and angiopoietin (Ang)-1/tyrosine kinase with
immunoglobulin and epidermal growth factor homology domains 2
(Tie2), ectopic COUP-TFII expression serves a crucial role in
promoting angiogenesis in xenograft mouse models. Furthermore,
COUP-TFII was reported to suppress the expression of several tumor
suppressors such as BRCA1 to promote tumor cell proliferation and
inhibit apoptosis (15). Although
COUP-TFII is detected in the mesenchyme and associated with
mesenchymal differentiation to epithelium (16), previous results have suggested that
the paradoxical effect of this receptor may regulate the EMT
process in cancer. Bao et al (17) demonstrated that upregulation of
COUP-TFII expression is associated with the overexpression of Snail
family transcription repressor 1 (Snail1), an important enhancer of
EMT. This finding was corroborated by Zhang et al (18) who demonstrated that miRNA-382
against COUP-TFII led to the inhibition of Snail1 expression.
Conversely, a high nuclear receptor subfamily 2 group F member 2
transcript level was revealed to be negatively associated with the
transforming growth factor (TGF)-β signaling pathway and EMT in
breast cancer (19).
miRNAs are non-coding RNAs of 18-22 nucleotides in
length that negatively regulate gene expression at the
post-transcriptional level by directly binding with the
3'-untranslated regions of target mRNAs to induce mRNA degradation
or suppress mRNA translation (20). miRNAs serve a key role in cell
growth and metastasis in colorectal cancer (21). miR-34a is a known tumor suppressor
that takes part in the proliferation, migration and metastasis of
tumor cells. It has also been reported that miR-34a could inhibit
cell migration and invasion in various cancer cell types, such as
breast cancer, laryngeal carcinoma and human glioma (22-24).
Therefore, the aim of the present study was to
further understand the role of COUP-TFII in CRC migration and the
mechanism underlying the EMT process to prevent the invasion and
migration of CRC by inhibiting miR-34a expression.
Materials and methods
Cell lines and antibodies
Three human CRC cell lines (HCT116, HT29 and LOVO)
were purchased from Cell Bank of Type Culture Collection of Chinese
Academy of Sciences, Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in 1640
complete medium containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
streptomycin and penicillin in a humidified incubator with 5%
CO2 at 37°C. Primary antibodies against COUP-TFII (cat.
no. ab50487, Abcam, Cambridge, MA, USA; dilution 1:1,000), GAPDH
(cat. no. 5174), E-cadherin (cat. no. 14472; dilution 1:1,000) and
Vimentin (cat. no. 5741; dilution 1:1,000), and goat anti-rabbit
horseradish peroxidase (HRP)-conjugated (cat. no. 7074; dilution
1:2,000) and goat anti-Mouse HRP-conjugated secondary antibodies
(cat. no. 7076; dilution 1:2,000) (Cell Signaling Technology, Inc.,
Danvers, MA, USA) were utilized in the present study.
Small interfering (si)-RNA
transfection
LOVO, HCT116, and HT29 cells (1×105) in
the logarithmic phase of growth were suspended in 2 ml of 1640
complete medium and subsequently plated in a 6-well plate for 24 h
at 37°C prior to transfection. Then, 50 nM siRNAs for target genes
or a scramble control (Shanghai GenePharma Co., Ltd., Shanghai,
China) were transfected into the cell monolayers with 20-30%
confluence using the Lipofectamine® 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. The sequences of siRNAs were as
follows: COUP-TFII-homo-2445 sense, 5′-GGCCGUAUAUGGCAAUUCATT-3′ and
antisense, 5′-UGAAUUGCCAUAUAC GGCCTT-3′; COUP-TFII-homo-1971 sense,
5′-GCGAGCUGUUUGUGUUGAATT-3′ and antisense,
5′-UUCAACACAAACAGCUCGCTT-3′; COUP-TFII-homo-2100 sense, 5′-GG
AUCUUCCAAGAGCAAGUTT-3′ and antisense, 5′-ACUUGCUCUUGGAAGAUCCTT-3′;
scramble control sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3'. Subsequent experiments were performed
6 h post-transfection.
RNA oligoribonucleotides and
transfection
The miR-34a mimic, inhibitor (5 nM) and negative
control siRNA (as aforementioned) were synthesized by Shanghai
GenePharma Co., Ltd. Transfection was conducted using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's instructions.
Subsequent experiments were performed following 6 h. The sequences
were as follows: miR-34a mimic sense, 5′-UGGCAGUGUCUUAGCUGGUUGU-3′
and antisense, 5′-AACCAGCUAAGACACUGCCAUU-3′; miR-34a inhibitor,
5′-ACAACCAGCUAAGACACUGCCA-3′.
Cell migration and invasion assays
Migration and invasion assays were performed using
the Transwell system (24-well insert; 8.0-µm pores). For
this, 5×104 CRC cells transfected with siRNAs were
suspended in 200 µl 1640 complete medium without FBS and
plated in the upper chamber. For invasion assays, this chamber was
coated with Matrigel. The bottom chamber was filled with 500
µl 1640 complete medium supplemented with 10% FBS, in order
to drive cell translocation. Following incubation at 37°C for 24,
48 and 72 h, the cells on the upper surface of the chamber were
scraped off using cotton swabs, while cells in the lower chamber
were fixed with 95% methanol for 20 min at room temperature then
stained with 0.4% crystal violet at room temperature for 5 min. The
number of invaded and migrated cells were then counted under a
light microscope (magnification, ×100; Olympus Corporation, Tokyo,
Japan).
Wound scratch assay
CRC cells (1×105) transfected with siRNAs
in the logarithmic phase of growth were suspended in 2 ml 1640
complete medium without FBS and added to 6-well plates. The CRC
monolayers with 80-90% confluence were scratched with 100 µl
pipettes. The distance to which the cells migrated to was recorded
at 0 and 48 h following scratching.
Colony forming assay
A total of 600 cells in the log phase were suspended
in 2 ml 1640 complete medium supplemented with 10% FBS and added to
6-well plates in a humidified incubator with 5% CO2 at
37°C. Following 1 week, the colonies on the plates were fixed with
95% methanol for 10 min at room temperature and then stained with
0.4% crystal violet at room temperature for 5 min. The number of
colonies was then counted under a light microscope (Olympus
Corporation).
Western blotting
Protein was extracted from CRC cells using
Radioimmunoprecipitation Assay buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) supplemented with
protease/phosphatase inhibitors (Cell Signaling Technology, Inc.).
Total protein concentration was determined using the bicinchoninic
acid assay, and the protein sample was then denatured by boiling at
95°C for 10 min. Equal amounts (40 µg) of protein were
subjected to 10% SDS-PAGE and then transferred to 0.45 µm
polyvinylidene fluoride membranes (EMD Millipore, Bedford, MA, USA)
by electroblotting at 350 mA for 90 min. Following membrane
blocking with 5% nonfat milk at 37°C for 2 h, they were immersed in
10 ml of TBS/0.1% Tween-20 containing 0.1% of the aforementioned
primary antibodies and 5% FBS (Gibco; Thermo Fisher Scientific,
Inc.) overnight at 4°C. The membranes were washed three times with
TBS/0.1% Tween-20 and then incubated with the aforementioned
secondary antibodies for 1 h at room temperature. Protein
expression was analyzed using SuperSignal West Pico
Chemiluminescent Substrate (Pierce; Thermo Fisher Scientific,
Inc.). The bands were quantified by densitometry using ImageLab 5.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). GADPH was used as
the internal control.
Statistical analysis
Data were presented as the mean ± standard deviation
of triplicate experiments and analyzed using one-way analysis of
variance followed by Tukey's post hoc test. Data were analyzed
using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). TargetScan
7.2 (www.targetscan.org) software was used to
predict miRNAs associated with COUP-TFII. P<0.05 was considered
to indicate a statistically significant difference.
Results
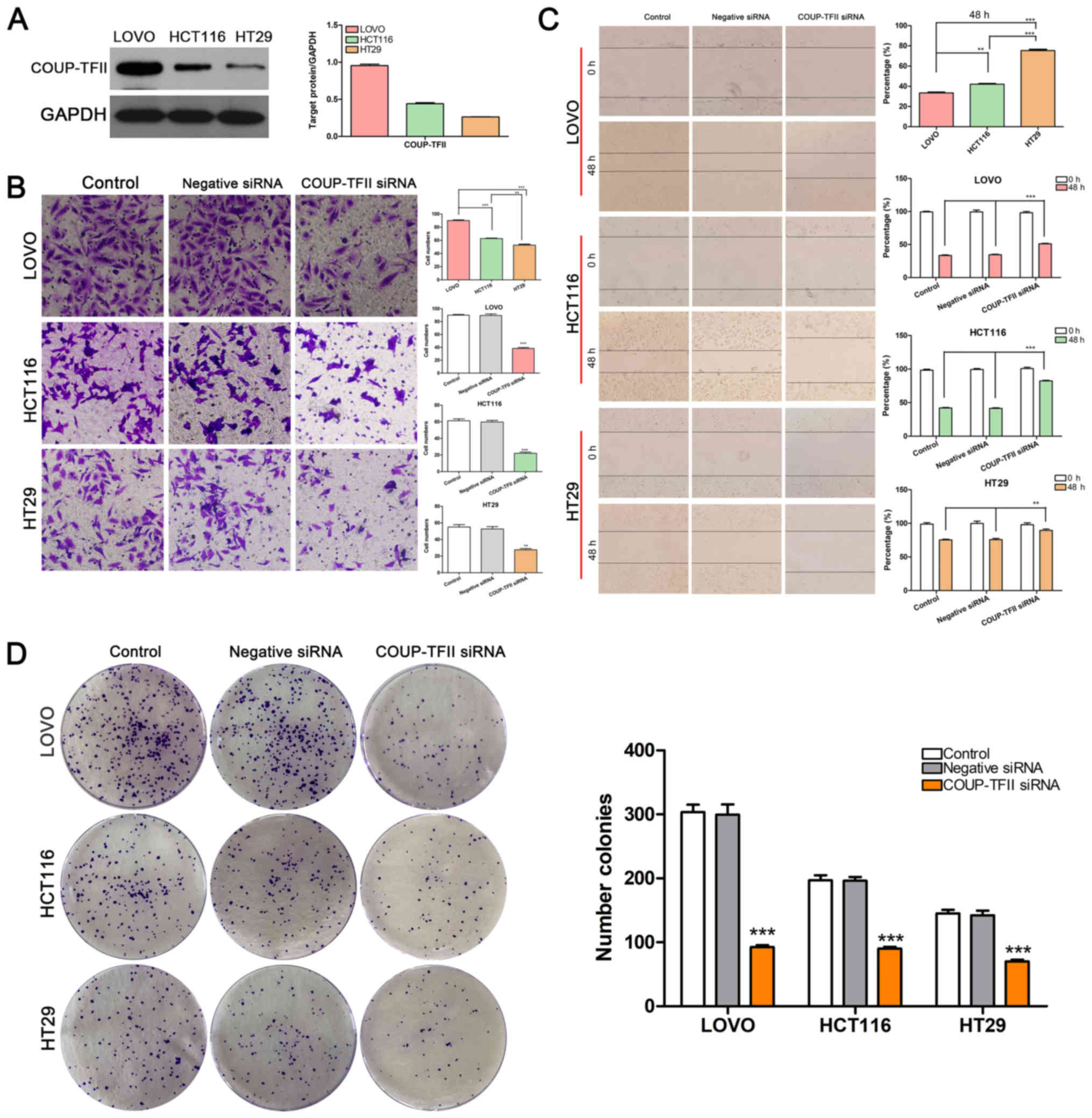
COUP-TFII knockdown suppresses the
migration and invasion of CRC cell lines
The present study performed western blotting to
determine the level of COUP-TFII expression in the three CRC cell
lines and revealed that this receptor was strongly expressed in
LOVO cells, moderately expressed in HCT116 cells, and weakly
expressed in HT29 cells (Fig. 1A).
Notably, the cells' ability to migrate and invade exhibited a
similar trend as COUP-TFII expression (P<0.01 and P<0.001 vs.
Control; Fig. 1B and C). Once
COUP-TFII expression was knocked down using siRNA, the number of
cells that passed through the Matrigel membrane and the percentage
of wound healing were significantly reduced in CRC cells when
compared with control cells (Fig. 1B
and C). These results demonstrated that COUP-TFII expression
was associated with CRC cell invasion and migration. Furthermore,
COUP-TFII knockdown impaired the colony-forming ability of HCT116,
HT29 and LOVO cells (P<0.001 vs. Control; Fig. 1D).
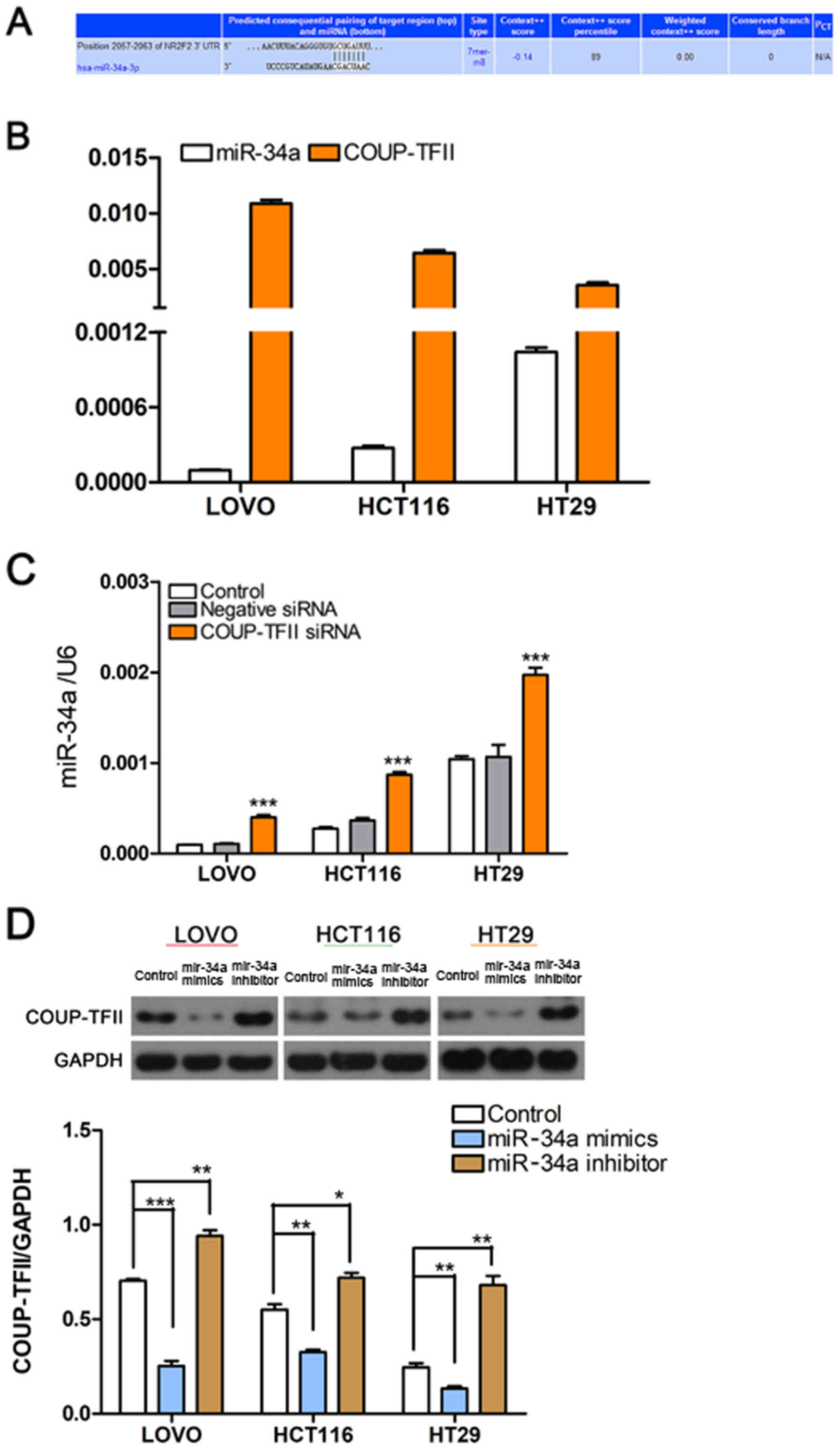
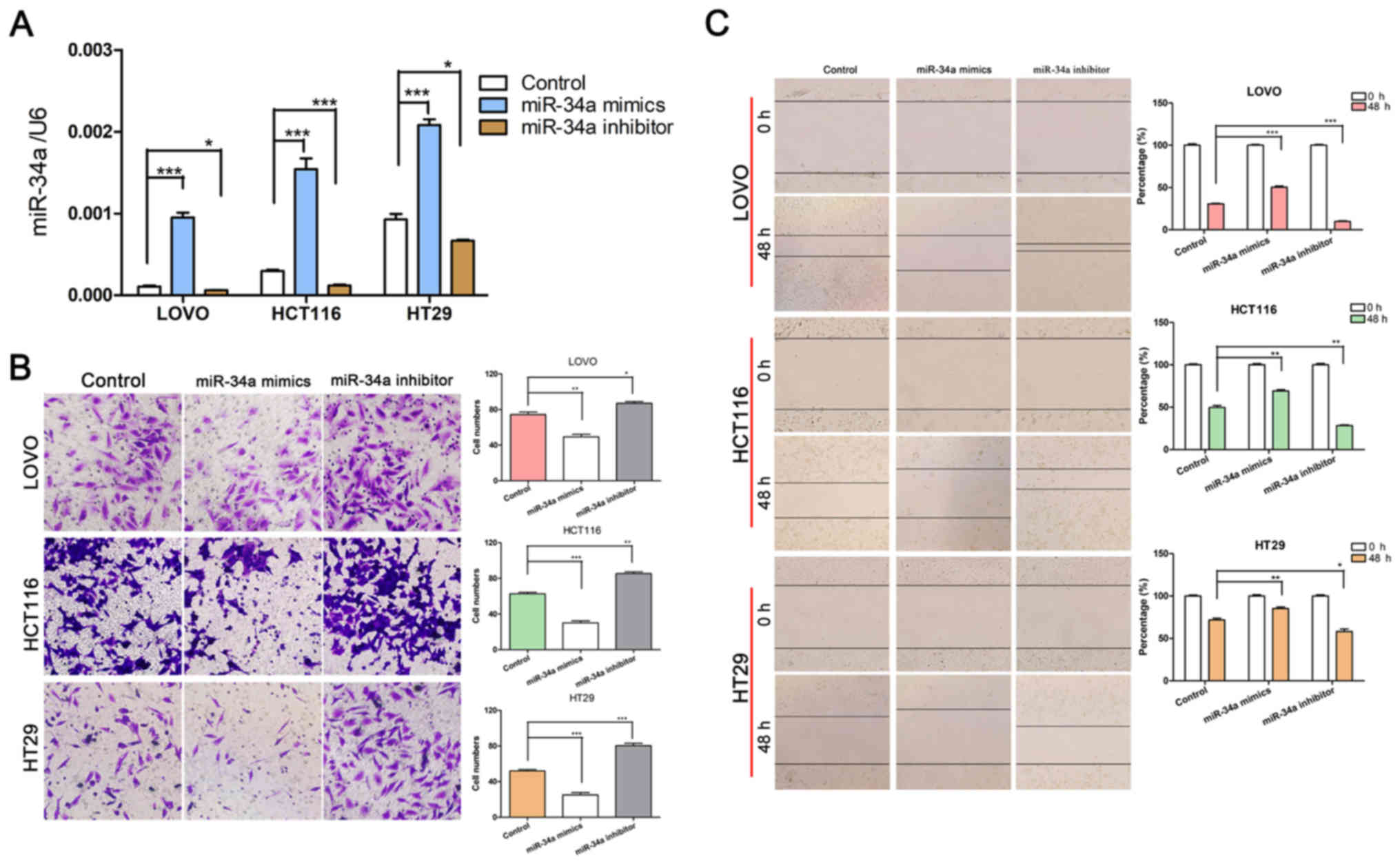
miR-34a suppresses CRC invasion and
migration via a process mediated by COUP-TFII
TargetScan (www.targetscan.org) software was used to predict
miRNAs associated with COUP-TFII (Fig.
2A). RT-qPCR revealed that miR-34a expression in CRC cells was
inversely associated with COUP-TFII expression; miR-34a was highly
expressed in HT29 cells, moderately expressed in HCT116 cells, and
the lowest expression was observed in LOVO cells (Fig. 2B). In addition, a decrease in
COUP-TFII expression led to a significant increase in miR-34a
expression (P<0.001 vs. Control; Fig. 2C). Furthermore, miR-34a mimics
could decrease the expression of COUP-TFII and the miR-34a
inhibitor could increase COUP-TFII expression (Fig. 2D). These results indicated that
COUP-TFII and miR-34a may regulate each other (Fig. 2C and D). Since miR-34a is an
important suppressor in CRC (25),
and its role in CRC invasion and migration has also been explored,
miR-34a mimics and inhibitors were used to up- or downregulate its
expression (Fig. 3A). As expected,
transfection with the miR-34a inhibitor increased the invasion and
migration abilities of CRC cells, while miR-34a mimics had the
opposite effect (P<0.05, P<0.01 and P<0.001 vs. Control;
Fig. 3B and C).
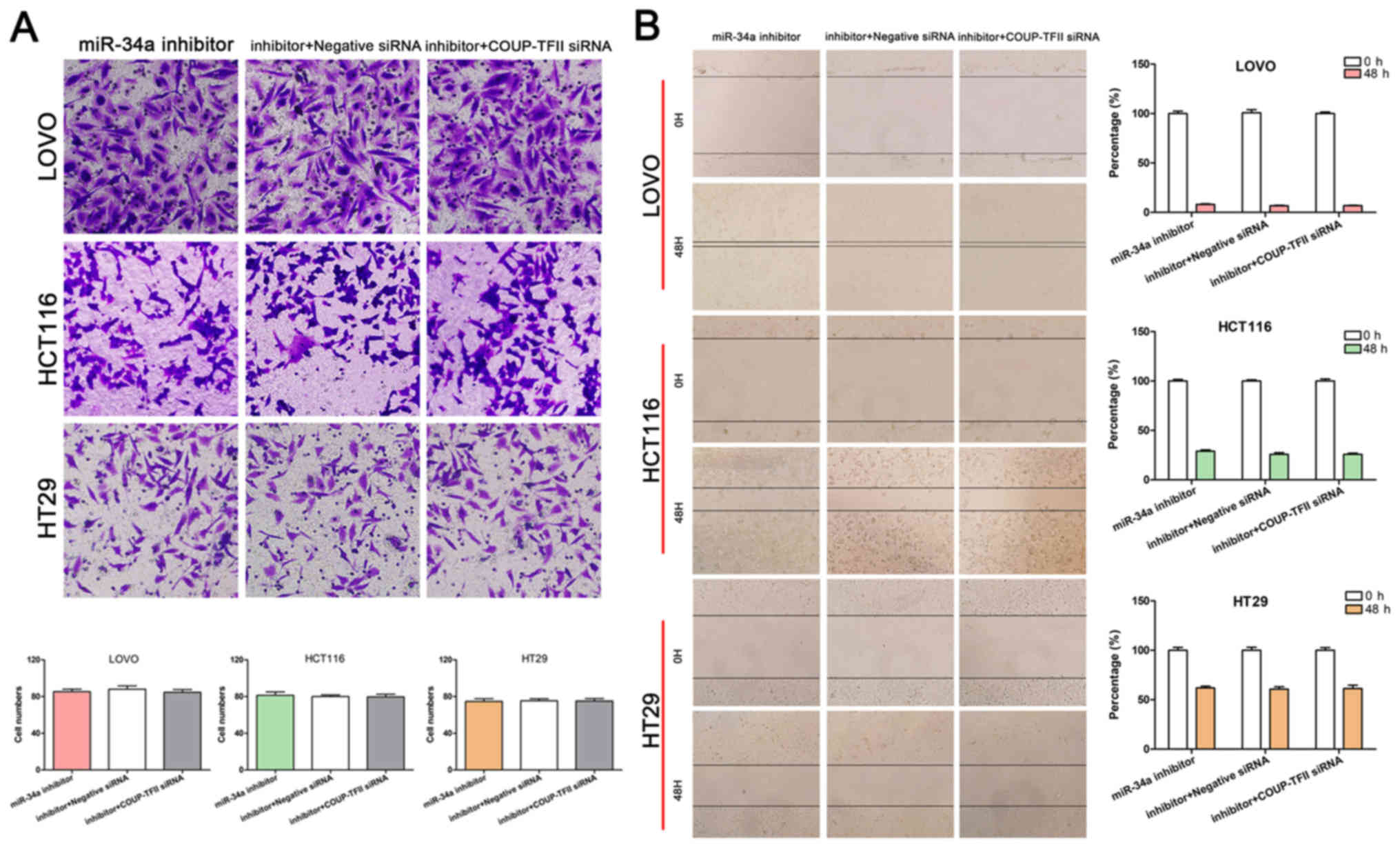
miR-34a reverses the effect of COUP-TFII
on CRC cells
To determine whether miR-34a is essential in
maintaining the effects of COUP-TFII on the promotion of CRC
invasion and migration, an miR-34a inhibitor and siRNA targeting
COUP-TFII were transfected into CRC cells, respectively. The
results revealed that the number of cells that passed through the
Matrigel membrane and the percentage of wound healing did not
differ between the miR-34a inhibitor group and the
COUP-TFII-miR-34a joint knockdown group (Fig. 4A and B). Thus, miR-34a reversed the
impairment of COUP-TFII knockdown on CRC cell invasion and
migration, and could be regulated by COUP-TFII.
COUP-TFII knockdown reduces EMT in CRC
cell lines
Furthermore, Vimentin expression was high in LOVO
cells, moderate in HCT116 cells and low in HT29 cells; however,
E-cadherin expression exhibited the opposite trend and had low
expression in LOVO cells, moderate expression in HCT116 cells, and
high expression in HT29 cells (Fig.
5A). The results also demonstrated that miR-34a knockdown
reduced E-cadherin expression and increased Vimentin expression in
CRC cells (P<0.05, P<0.01 and P<0.001 vs. Control;
Fig. 5B). Conversely, COUP-TFII
inhibition significantly increased E-cadherin expression but
decreased Vimentin expression (P<0.05, P<0.01 and P<0.001
vs. Control; Fig. 5C). Successful
transfection was verified for miR-34a (Fig. 3A) and COUP-TFII (Fig. 5C) via RT-qPCR and western
blotting.
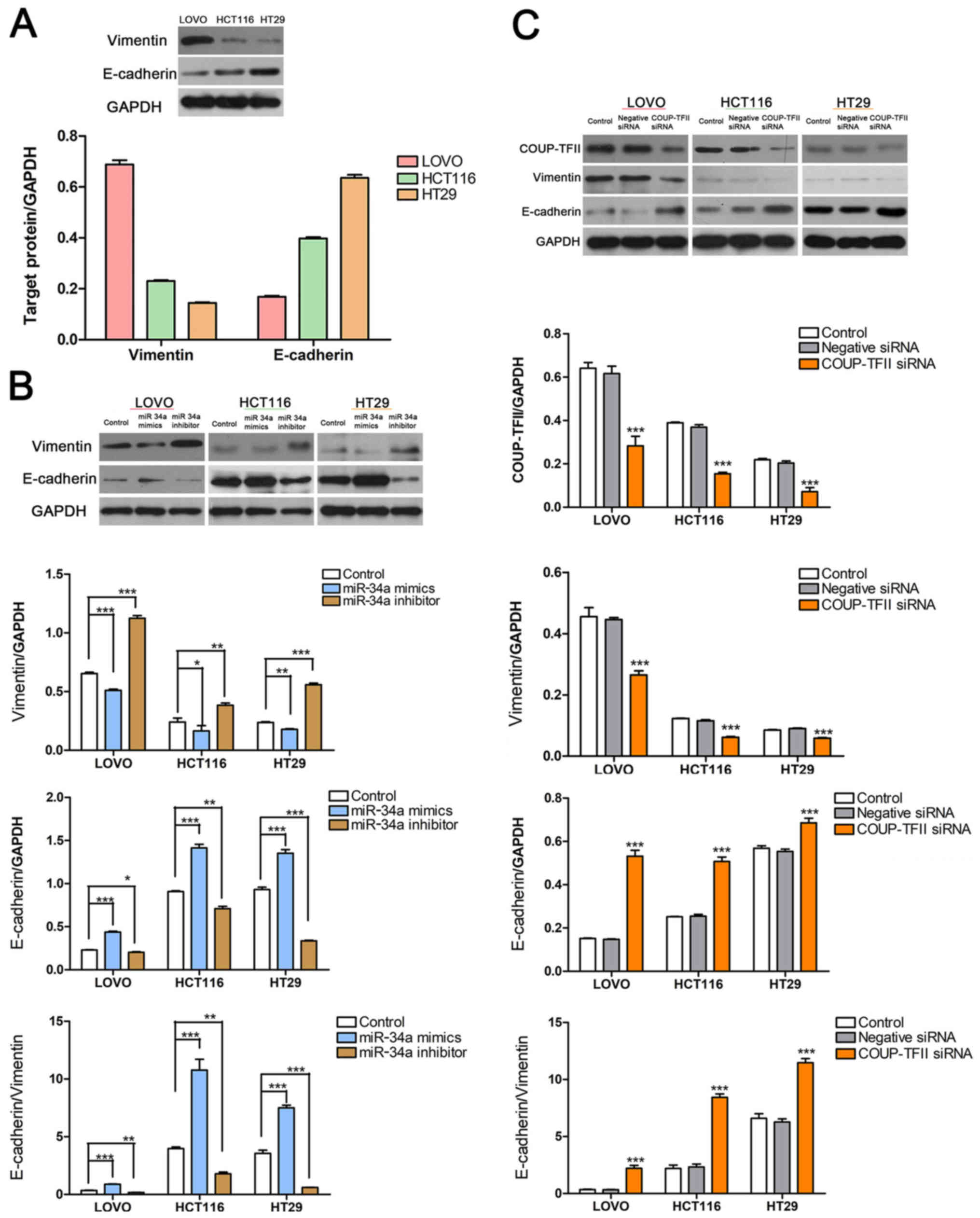 | Figure 5COUP-TFII knockdown reduces EMT in
CRC cell lines. (A) The level of EMT was high in LOVO cells,
moderate in HCT116 cells, and low in HT29 cells, as indicated by
the protein levels of Vimentin and E-cadherin. (B) miR-34a
knockdown decreased the expression of E-cadherin and increased the
expression of Vimentin, but miR-34a overexpression induced the
opposite effect. *P<0.05, **P<0.01 and
***P<0.001, as indicated. (C) Inhibited COUP-TFII
expression significantly increased E-cadherin expression but
decreased the expression of Vimentin. ***P<0.001 vs.
Control. COUP-TFII, chicken ovalbumin upstream
promoter-transcription factor II; siRNA, small interfering RNA;
EMT, epithelial-mesenchymal transition; CRC, colorectalcancer cell;
miR, microRNA. |
Discussion
Among factors such as age, race and metastasis,
which all negatively affect the prognosis of CRC patients, distant
metastasis causes the maximum reduction in survival rate (1). Aberrant activation of the EMT process
enables adherent epithelial carcinoma cells to become migratory,
contributing to the early-stage dissemination of tumor cells from
primary tumor tissues to novel organ sites through the blood
(26). It has been reported that
mesenchymal circulating tumor cells were significantly associated
with disease progression in the patients with breast cancer
(27). Furthermore, circulating
tumor cells have frequently exhibited a reversible shift between
the epithelial and mesenchymal phenotypes with therapy and disease
progression (28).
A previous study revealed that synthetic steroid
hormones could mediate miR-34a expression (29), and that steroid hormones and
COUP-TFII competitively regulate transcription factor function.
Therefore, the present study investigated the association between
miR-34a and COUP-TFII. The results revealed that miR-34a expression
exhibited a reverse trend to COUP-TFII expression in CRC cell
lines, and that COUP-TFII knockdown was associated with increased
miR-34a expression. miR-34a is a well-known suppressor of multiple
types of cancers and is considered a novel biomarker for diagnosis
and prognosis prediction as well as being a therapeutic target
(30,31). Transcription of miR-34a and
COUP-TFII was reported to be competitively regulated by some common
mechanisms. COUP-TFII was previously verified to be associated with
the invasion and migration of many types of tumors including CRC
(32), but its mechanism of action
was largely unclear. Several genes associated with cancer
development were also confirmed to be regulated by COUP-TFII
(9). For example, ectopic
COUP-TFII expression was revealed to promote angiogenesis in a
tumor model by enhancing angiopoietin-1 expression and repressing
VEGFR-1 expression (13,14). In the present study, miR-34a mimics
could inhibit the CRC cell migration and invasion abilities. In
addition, miR-34a siRNA transfection reversed the effect of
COUP-TFII knockdown on CRC cell migration and invasion.
An accumulating body of clinical evidence has
demonstrated that activation of EMT and overexpression of
EMT-associated transcription factors in CRC promotes the metastasis
of this type of cancer and limits long-term survival following
resection. Slug and Vimentin expression were also revealed to be
increased in CRC and these proteins were considered to be novel
predictive biomarkers for lymph node metastasis and poor prognosis
in CRC (33). Several previous
studies have reported that various molecular mechanisms are
involved in the EMT process. Wang et al (34) reported that in the TGF-β signaling
pathway, high COUP-TFII expression was associated with negative
mothers against decapentaplegic homolog 4 expression, while Zhang
et al (19) showed that
high COUP-TFII transcript levels inhibited TGF-β-dependent EMT. In
addition, COUP-TFII suppressed the cadherin-11 to cadherin-6
switch, leading to the inactivation of EMT during the development
of kidney cancer (35). To better
determine the role of COUP-TFII in the EMT process of CRC and the
associated mechanisms, the present study knocked down COUP-TFII
expression using siRNA and confirmed that COUP-TFII significantly
enhanced the migration and invasion abilities of CRC cells by
promoting EMT. Furthermore, the expression of E-cadherin was
increased and Vimentin expression was decreased following
transfection with miR-34a mimics compared with negative siRNA. By
contrast, inhibition of miR-34a decreased the expression of
E-cadherin and promoted the expression of Vimentin, thereby
increasing EMT in CRC cells. These results suggest that inhibition
of miR-34a expression may have been the main mechanism underlying
how COUP-TFII regulates EMT. In addition, miR-34a suppression could
be essential to COUP-TFII in regulating other malignant behaviors
as well.
In conclusion, the present study confirmed that
COUP-TFII knockdown was negatively associated with the migration
and invasiveness of CRC cells. High COUP-TFII expression
competitively inhibited miR-34a transcription, thereby promoting
the EMT process. The results suggest that control of EMT through
the inhibition of COUP-TFII or restoration of miR-34a may be a
novel therapeutic approach for CRC.
Funding
The present study was financially supported by
National Natural Science Foundation (grant no. 81870377) Zhejiang
Province Natural Science Foundation of China (grant nos.
LY16H160041, 2016C33192 and 2018C37187) and Huzhou Science and
Technology Project (grant nos. 2014GZ11, 2015GZ16, 2016GY24,
2016GY37 and 2017GYB09).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW and WC conceived the study. YB, YL and WF
performed the experiments. HY, HG, YT and QS analyzed the data. WC
wrote the manuscript. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ait Ouakrim D, Pizot C, Boniol M, Malvezzi
M, Boniol M, Negri E, Bota M, Jenkins MA, Bleiberg H and Autier P:
Trends in colorectal cancer mortality in Europe: Retrospective
analysis of the WHO mortality database. BMJ. 351:h49702015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang LH, Tsai SY, Cook RG, Beattie WG,
Tsai MJ and O'Malley BW: COUP transcription factor is a member of
the steroid receptor superfamily. Nature. 340:163–166. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tran P, Zhang XK, Salbert G, Hermann T,
Lehmann JM and Pfahl M: COUP orphan receptors are negative
regulators of retinoic acid response pathways. Mol Cell Biol.
12:4666–4676. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cooney AJ, Tsai SY, O'Malley BW and Tsai
MJ: Chicken ovalbumin upstream promoter transcription factor
(COUP-TF) dimers bind to different GGTCA response elements,
allowing COUP-TF to repress hormonal induction of the vitamin D3,
thyroid hormone, and retinoic acid receptors. Mol Cell Biol.
12:4153–4163. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kliewer SA, Umesono K, Heyman RA,
Mangelsdorf DJ, Dyck JA and Evans RM: Retinoid X receptor-COUP-TF
interactions modulate retinoic acid signaling. Proc Natl Acad Sci
USA. 89:1448–1452. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bailey P, Sartorelli V, Hamamori Y and
Muscat GE: The orphan nuclear receptor, COUP-TF II, inhibits
myogenesis by post-transcriptional regulation of MyoD function:
COUP-TF II directly interacts with p300 and myoD. Nucleic Acids
Res. 26:5501–5510. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pereira FA, Tsai MJ and Tsai SY: COUP-TF
orphan nuclear receptors in development and differentiation. Cell
Mol Life Sci. 57:1388–1398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heery R, Finn SP, Cuffe S and Gray SG:
Long Non-Coding RNAs: Key Regulators of Epithelial-Mesenchymal
Transition, Tumour Drug Resistance and Cancer Stem Cells. Cancers
(Basel). 9:92017. View Article : Google Scholar
|
|
11
|
Xu M, Qin J, Tsai SY and Tsai MJ: The role
of the orphan nuclear receptor COUP-TFII in tumorigenesis. Acta
Pharmacol Sin. 36:32–36. 2015. View Article : Google Scholar :
|
|
12
|
Feng Q, Wu X, Li F, Ning B, Lu X, Zhang Y,
Pan Y and Guan W: miR-27b inhibits gastric cancer metastasis by
targeting NR2F2. Protein Cell. 8:114–122. 2017. View Article : Google Scholar :
|
|
13
|
Qin J, Chen X, Xie X, Tsai MJ and Tsai SY:
COUP-TFII regulates tumor growth and metastasis by modulating tumor
angiogenesis. Proc Natl Acad Sci USA. 107:3687–3692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin J, Chen X, Yu-Lee LY, Tsai MJ and Tsai
SY: Nuclear receptor COUP-TFII controls pancreatic islet tumor
angio-genesis by regulating vascular endothelial growth
factor/vascular endothelial growth factor receptor-2 signaling.
Cancer Res. 70:8812–8821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng J, Qin W, Jiao D, Ren J, Wei M, Shi
S, Xi W, Wang H, Yang AG, Huan Y, et al: Knockdown of COUP-TFII
inhibits cell proliferation and induces apoptosis through
upregulating BRCA1 in renal cell carcinoma cells. Int J Cancer.
139:1574–1585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pereira FA, Qiu Y, Tsai MJ and Tsai SY:
Chicken ovalbumin upstream promoter transcription factor (COUP-TF):
Expression during mouse embryogenesis. J Steroid Biochem Mol Biol.
53:503–508. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao Y, Gu D, Feng W, Sun X, Wang X, Zhang
X, Shi Q, Cui G, Yu H, Tang C, et al: COUP-TFII regulates
metastasis of colorectal adenocarcinoma cells by modulating Snail1.
Br J Cancer. 111:933–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Liu J, Qiu J, Fu X, Tang Q, Yang
F, Zhao Z and Wang H: MicroRNA-382 inhibits prostate cancer cell
proliferation and metastasis through targeting COUP-TFII. Oncol
Rep. 36:3707–3715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Han Y, Huang H, Qu L and Shou C:
High NR2F2 transcript level is associated with increased survival
and its expression inhibits TGF-β-dependent epithelial-mesenchymal
transition in breast cancer. Breast Cancer Res Treat. 147:265–281.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eulalio A, Huntzinger E and Izaurralde E:
Getting to the root of miRNA-mediated gene silencing. Cell.
132:9–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng B, Wang B, Fang J, Zhu X, Cao Z, Lin
Q, Zhou L and Sun X: MiRNA-203 suppresses cell proliferation,
migration and invasion in colorectal cancer via targeting of
EIF5A2. Sci Rep. 6:283012016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang JX, Zhang QJ, Pei SG and Yang BL:
Effect and mechanism of miR-34a on proliferation, apoptosis and
invasion of laryngeal carcinoma cells. Asian Pac J Trop Med.
9:494–498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue F, Liu Y, Zhang H, Wen Y, Yan L, Tang
Q, Xiao E and Zhang D: Let-7a enhances the sensitivity of
hepatocellular carcinoma cells to cetuximab by regulating STAT3
expression. OncoTargets Ther. 9:7253–7261. 2016. View Article : Google Scholar
|
|
24
|
Dong X, Jin Z, Chen Y, Xu H, Ma C, Hong X,
Li Y and Zhao G: Knockdown of long non-coding RNA ANRIL inhibits
proliferation, migration, and invasion but promotes apoptosis of
human glioma cells by upregulation of miR-34a. J Cell Biochem.
119:2708–2718. 2018. View Article : Google Scholar
|
|
25
|
Zhang D, Zhou J and Dong M: Dysregulation
of microRNA-34a expression in colorectal cancer inhibits the
phosphorylation of FAK via VEGF. Dig Dis Sci. 59:958–967. 2014.
View Article : Google Scholar
|
|
26
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng Z, Wang CX, Fang EH, Wang GB and Tong
Q: Role of epithelial-mesenchymal transition in gastric cancer
initiation and progression. World J Gastroenterol. 20:5403–5410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsu CY, Hsieh TH, Tsai CF, Chen HS, Liang
PI, Hsu YL and Tsai EM: Synthetic Steroid Hormones Regulated Cell
Proliferation Through MicroRNA-34a-5p in Human Ovarian
Endometrioma. Biol Reprod. 94:602016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beg MS, Brenner AJ, Sachdev J, Borad M,
Kang YK, Stoudemire J, Smith S, Bader AG, Kim S and Hong DS: Phase
I study of MRX34, a liposomal miR-34a mimic, administered twice
weekly in patients with advanced solid tumors. Invest New Drugs.
35:180–188. 2017. View Article : Google Scholar
|
|
31
|
Imani S, Wei C, Cheng J, Khan MA, Fu S,
Yang L, Tania M, Zhang X, Xiao X, Zhang X, et al: MicroRNA-34a
targets epithelial to mesenchymal transition-inducing transcription
factors (EMT-TFs) and inhibits breast cancer cell migration and
invasion. Oncotarget. 8:21362–21379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou B, Song J, Han T, Huang M, Jiang H,
Qiao H, Shi J and Wang Y: MiR-382 inhibits cell growth and invasion
by targeting NR2F2 in colorectal cancer. Mol Carcinog.
55:2260–2267. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang C, Zhou Y, Ruan R, Zheng M, Han W and
Liao L: High expression of COUP-TF II cooperated with negative
Smad4 expression predicts poor prognosis in patients with
colorectal cancer. Int J Clin Exp Pathol. 8:7112–7121.
2015.PubMed/NCBI
|
|
35
|
Bringuier PP, Schalken JA, Hervieu V and
Giroldi LA: Involvement of orphan nuclear receptor COUP-TFII in
cadherin-6 and cadherin-11 regulation: Implications in development
and cancer. Mech Dev. 136:64–72. 2015. View Article : Google Scholar : PubMed/NCBI
|