Introduction
Bladder cancer (BC) was the fifth most common type
of cancer and the eighth most common cause of cancer-related
mortality among 40 countries investigated in 2012 (1). In fact, 429,800 new cases of BC were
diagnosed, and 165,000 patients succumbed to the disease worldwide
in 2012 (2). The 5-year survival
rate of patients with BC has improved by only a small percentage
over the past 30 years according to Surveillance, Epidemiology and
End Results (SEER) program of the National Cancer Institute.
Patients with metastasis are generally treated with cisplatin-based
combination chemotherapy, although its efficacy is limited
(3). Standard chemotherapy as a
first-line therapy has shifted from the methotrexate, vinblastine,
doxorubicin and cisplatin (MVAC) regimen to the gemcitabine and
cisplatin (GC) regimen over the past several years due to the lower
toxicity of GC. However, GC therapy has not conferred an overall
survival benefit to patients with BC compared with MVAC therapy
(4). Therefore, the 5-year
survival rate of patients with lymph node or distant metastasis
remains <20% (5). A main reason
behind the fact that the 5-year survival rate of patients with BC
has not markedly improved is that the mechanisms of recurrence and
metastasis in BC have not yet been sufficiently elucidated.
MicroRNAs (miRNAs or miRs) are endogenous small
non-coding RNAs (19-22 nucleotides in length) that negatively
regulate protein-coding genes by binding to the 3′-untranslated
region (UTR) of the target mRNA and inhibiting transcriptional or
post-transcriptional expression (6). A single miRNA is able to regulate
thousands of target transcripts, and >60% of protein-coding
genes may be regulated by miRNAs (7,8). It
has previously been demonstrated that miRNAs are aberrantly
expressed in various human malignancies and play significant roles
in oncogenesis and metastasis (9).
Therefore, the detection of aberrantly expressed miRNAs and their
target genes is an important step for elucidating miRNA-regulated
oncogenic pathways.
Our previous study on BC miRNA profiles revealed
significantly downregulated miRNAs in BC tissues compared with
normal bladder tissues (10).
According to the profile, we have previously demonstrated that
several miRNAs function as tumor suppressors by targeting numerous
oncogenes in BC cells (11-16).
In the current study, we focused on miRNA-223
(miR-223), which was also listed as one of the top 50
downregulated miRNAs in the profile. miR-223 has been
reported to function not only as a tumor suppressor, but also as an
oncogenic miRNA in different types of cancer cells. In BC, only two
reports to date have demonstrated the tumor-suppressive functions
of miR-223 (17,18), at least to the best of our
knowledge. However, the functional roles of miR-223 have not
been sufficiently elucidated in BC. Thus, the aim of this study was
to investigate further the functional significance of
miR-223 and to identify the novel molecular targets of this
miRNA in BC. The identification of novel molecular targets of
miR-223 could provide important information on BC
oncogenesis and may thus lead to the development of novel
therapeutic strategies for BC.
Material and methods
Clinical specimens and cell culture
Clinical specimens were collected from patients with
BC (n=32) who had undergone cystectomy (n=6) or transurethral
resection of their bladder tumors (n=26) at the Kagoshima
University Hospital (Kagoshima, Japan) from 2004 to 2013. normal
bladder epithelial (NBE; n=12) specimens were derived from patients
with non-cancerous diseases. The specimens were staged according to
the American Joint Committee on Cancer (AJCC)/Union Internationale
Contre le Cancer (UICC) tumor-node-metastasis classification system
and were histologically graded. This study was approved by the
Bioethics Committee of Kagoshima University; the study numbers were
H27-104 and H27-105. Written informed consent and approval were
obtained from all patients prior to obtaining the samples. The
clinicopathological characteristics of the patients are presented
in Table I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Bladder cancer
(BC) | No. |
Range/percentage |
|---|
| Total | 32 | |
| Median age (range),
years | 71.5 | (43-93) |
| Sex | | |
| Male | 22 | 68.8% |
| Female | 10 | 31.2% |
| Tumor grade | | |
| Low grade | 14 | 43.7% |
| High grade | 16 | 50.0% |
| Unknown | 2 | 6.3% |
| T stage | | |
| Tis | 1 | 3.1% |
| Ta | 5 | 15.1% |
| T1 | 7 | 21.9% |
| T2 | 11 | 34.4% |
| T3 | 3 | 9.4% |
| T4 | 2 | 6.3% |
| Unknown | 3 | 9.4% |
| N stage | | |
| N0 | 22 | 68.8% |
| N1 | 3 | 9.4% |
| Unknown | 7 | 21.9% |
| M stage | | |
| M0 | 28 | 87.5% |
| M1 | 2 | 6.3% |
| Unknown | 2 | 6.3% |
| Surgical
method | | |
| TURBT | 26 | 81.3% |
| Cystectomy | 6 | 18.8% |
| Normal bladder
epithelium | | |
| Total number | 12 | |
| Median age
(range), years | 63.5 | (51-75) |
In addition, we used two human BC cell lines:
Invasive T24 cells, which were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA: cat. no. HTB-4) and
BOY cells, which were established in our laboratory from an Asian
male patient, 66 years of age, given a diagnosis of stage III BC
with lung metastasis. These cell lines were maintained in the
recommended media containing 10% fetal bovine serum (FBS) in a
humidified atmosphere of 95% air at 37°C. Eagle’s minimum essential
medium (MEM) was used for the culture medium for the BOY cells.
These cell lines were subjected to the experiments at passage
numbers between 10 and 20. Regularly, mycoplasma testing was
carried out for the cell lines and no infection was confirmed. We
found no information regarding misidentified or cross-contaminated
cell lines the all cell lines used were cross-checked from the
International Cell Line Authentication Committee (http://iclac.org/databases/cross-contaminations/)
and ExPASy Cellosaurus databases (https://web.expasy.org/cellosaurus/).
Tissue collection and RNA extraction
Tissues were treated with RNAlater (Thermo Fisher
Scientific, Waltham, MA, USA) and stored at -20°C until RNA
extraction. Total RNA, including miRNAs, was extracted from the
tissues and the cells using the mirVana miRNA Isolation kit (Thermo
Fisher Scientific) and Isogen (Nippon Gene, Tokyo, Japan),
respectively following the manufacturer’s protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Stem-loop RT-PCR (TaqMan MicroRNA Assay for
miR-223; P/N 002295; Applied Biosystems, Foster City, CA,
USA) was used for RT-qPCR to evaluate miRNA expression levels
according to previously published methods (11). We used human RNU48 (P/N
001006; Applied Biosystems) as an internal control and employed the
ΔΔCq method to calculate the fold changes in expression (12). For WDR62 and
glucuronidase beta (GUSB) expression, we applied a
SYBR-Green qPCR system using the following primers: WDR62
forward, 5′-GCCTTCTCACCCAA TATGAAGC-3′ and reverse,
5′-GCCTTCTCACCCAATATGA AGC-3′; and GUSB forward,
5′-CGTCCCACCTAGAATCT GCT-3′ and reverse,
5′-TTGCTCACAAAGGTCACAGG-3′. We also performed RT-qPCR analyses of
the 11 genes that were significantly upregulated in the TCGA cohort
of BC. The primers for detecting expressions of those genes are
described in Table III. For the
reverse transcription step, 500 ng total RNA was reverse
transcribed into cDNA using the High Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.) under the
incubation conditions of 25°C for 10 min, 37°C for 120 min and 85°C
for 5 min. qPCR was performed using a Power SYBR-Green Master Mix
(cat. no. 4367659) on a 7300 Real-time PCR System (both Applied
Biosystems; Thermo Fisher Scientific, Inc.). Furthermore, the
initial step time for activation was 10 min at 95°C, followed by 40
cycles between a denaturation step for 15 sec at 95°C and an
annealing/extension step for 1 min at 60°C.
 | Table IIISequences of the primers used in the
present study. |
Table III
Sequences of the primers used in the
present study.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| GUSB |
CGTCCCACCTAGAATCTGCT |
TTGCTCACAAAGGTCACAGG |
| ITGA3 |
TCAACCTGGATACCCGATTCC |
GCTCTGTCTGCCGATGGAG |
| WDR62 |
GCCTTCTCACCCAATATGAAGC |
GCCTTCTCACCCAATATGAAGC |
| ECT2 |
ACTACTGGGAGGACTAGCTTG |
CACTCTTGTTTCAATCTGAGGCA |
| DNAJB13 |
ATGGGCCAGGATTATTACTCTGT |
GCTCATTTGACTTCAACGGGTG |
| CDKN3 |
TCCGGGGCAATACAGACCAT |
GCAGCTAATTTGTCCCGAAACTC |
| CSPG5 |
GCTGACTTACCCATTTCAGGG |
AGGGTGGTTCTCTGAGGTTCC |
| CENPN |
TGAACTGACAACAATCCTGAAGG |
CTTGCACGCTTTTCCTCACAC |
| CENPM |
GCGGACTCGATGCTCAAAGA |
TTCTGGAGACTGTATTTGCTGTG |
| CEP72 |
CTCTCGCGCAACTCCTTGG |
GTGGAGCCGAAACACTTCTG |
| SLC24A2 |
GTCTGGTAGCCATTAGCACTG |
TGGGCGTGATCTGTACTATTCTC |
| SOX11 |
AGCAAGAAATGCGGCAAGC |
ATCCAGAAACACGCACTTGAC |
The specificity of amplification was monitored
according to the dissociation curve of the amplified product. All
expression values were normalized to those for GUSB, and the ΔΔCq
method was employed to calculate the fold change values (12). All experiments were performed in
triplicate.
Transfection of miRNAs, siRNAs and
anti-miRNAs
As previously described (11), the T24 and BOY cells were
transfected with 10 nM mature miRNA, siRNA or anti-miRNA using
Lipofectamine RNAiMAX transfection reagent and Opti-MEM (both from
Thermo Fisher Scientific). Mature miRNA molecules, Pre-miR™ miRNA
precursors (miR-223, hsa-miR-223-3p, P/N PM12301;
Thermo Fisher Scientific) and negative control miRNA (P/N AM17111;
Thermo Fisher Scientific) were used for the gain-of-function
experiments, whereas two different WDR62 siRNA (P/N
HSS138565 and HSS138567; Thermo Fisher Scientific), negative
control siRNA (P/N D-001810-10; Thermo Fisher Scientific),
mirVana® miRNA inhibitor (anti-miR-223,
hsa-miR-223-3p, P/N MH12301; Thermo Fisher Scientific) and
negative control miRNA inhibitor (P/N AM17010; Thermo Fisher
Scientific) were used for the loss-of-function experiments. For
co-transfection, the BC cells were simultaneously transfected with
10 nM miR-223 and 10 nM anti-miR-223 using
Lipofectamine RNAiMAX and Opti-MEM (both from Thermo Fisher
Scientific).
Cell viability, migration and invasion
assays
In order to investigate the functional significance
of miR-223 and WDR62, we performed cell viability,
migration and invasion assays using the T24 and BOY cells
transfected with 10 nM miRNA or siRNA by reverse transfection. The
cells were seeded in 96-well plates at 3×103/well for
XTT assays. After 72 h, cell viability was determined using the
Cell Proliferation kit II (Roche Diagnostics GmbH, Mannheim,
Germany) as previously described (11). The cell migration ability was
evaluated using wound healing assays. Cells were plated in 6-well
plates at 2×105/well, and after 48 h of transfection,
the cell monolayer was scratched using a P-20 micropipette tip. The
initial gap length (0 h) and residual gap length at 24 h after
wounding were calculated from photomicrographs by using an OLYMPUS
CK2 microscope (Olympus Optical Corp., Tokyo Japan) as previously
described (13). Cell invasion
assays were assessed by modified Boyden chambers consisting of
Transwell pre-coated Matrigel membrane filter inserts with
8-micrometer pores in 24-well tissue culture plates (BD
Biosciences, Bedford, MA, USA). At 72 h following transfection, the
cells were seeded in the upper chamber of 24-well plates at
1×105/well with serum-free Eagle’s MEM. After 24 h, 37°C
incubation. MEM containing 10% FBS in the lower chamber served as
the chemoattractant, as previously described (11). The cells were stained by Diff-Quick
(a modified Giemsa stain) (Richard Allan Scientific, San Diego, CA,
USA). Briefly, the cells were fixed with pure methanol for 2 min,
followed-by stained by the dye I and dye II each for 2 min. The
number of the cells on the surface of the chamber was counted by
using OLYMPUS BX41 (Olympus Optical Corp.). All experiments were
performed in triplicate.
Colony formation assay
For colony formation assays to examine effects of
WDR62 knockdown, the cells transfected with 10 nM siRNA were
seeded into 10-cm dish at a density of 1,000 cells/well. Following
7 days of incubation in the MEM containing 10% FBS in a humidified
atmosphere of 95% air at 37°C, the resulting colonies were fixed
with 4% paraformaldehyde phosphate buffer solution (Nacalai Tesque,
Kyoto, Japan) and stained with 0.04% crystal violet (Nacalai
Tesque) for 10 min at room temperature. The numbers of colonies
were counted. The average colony density was calculated and
expressed as the relative percentage of the mock group (only with
opti-MEM Lipofectamine RNAiMAX).
Apoptosis assays
The BC cell lines were transfected with reagent only
(mock), miR-control, miR-223, siRNA-control or
si-WDR62 at the concentration of 10 nM in 6-well tissue
culture plates, as described previously (14). The cells were harvested by
trypsinization at 72 h following transfection and washed in cold
phosphate-buffered saline. For the apoptosis assays, double
staining with FITC-Annexin V and propidium iodide was performed
using the FITC Annexin V Apoptosis Detection kit (BD Biosciences)
according to the manufacturer’s recommendations and analyzed within
1 h by flow cytometry (CytoFLEX analyzer; Beckman Coulter, Brea,
CA, USA). Cells were identified as viable, dead, early apoptotic or
apoptotic cells using CytExpert™ 1.2 software (Beckman Coulter),
and the percentages of early apoptotic and apoptotic cells in each
experiment were then compared.
Caspase-3/7 activity assays
Caspase-3/7 activity was measured using CellEvent™
caspase-3/7 Green Detection Reagent (Invitrogen/Thermo Fisher
Scientific). BC cell lines in a 96-well plate were transfected with
mature miRNAs and siRNAs as described above. After 72 h, 5
µM CellEvent™ caspase-3/7 Green Detection Reagent were added
to each well and incubated at 37°C for 30 min. The fluorescence was
then measured in each well. For densitometric analysis, the total
fluorescence was quantitated using the BZ-II Analyzer (Keyence,
Osaka, Japan). Experiments were performed in triplicate.
Cell cycle assays
For cell cycle analyses, the cells were fixed in 70%
aqueous ethanol and stained with propidium iodide at 4°C for 30 min
following treatment with RNase A as previously described (15). The data were analyzed using the
CytoFLEX analyzer (Beckman Coulter). The percentages of cells in
the G0/G1, S and G2/M phases were determined and compared.
Experiments were performed in triplicate. We used 1 µg/ml
nocodazole (P/N ab120630; Abcam, Cambridge, UK) to induce G2/M
arrest in each cell line as previously described (16). Experiments were done in
triplicate.
Western blot analysis
Following 3 days of transfection, total protein
lysate was prepared with a radioimmunoprecipitation assay (RIPA)
buffer (Thermo Fisher Scientific, Inc.) containing protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA). The protein
concentrations were determined using the Bradford assay (17). Protein lysate (20 µg) per
lane was loaded in NuPAGE on 4-12% bis-tris gel (Invitrogen/Thermo
Fisher Scientific) and transferred into a polyvinylidene fluoride
membrane. Following transfer, the membranes were blocked in washing
buffer (0.35 M NaCl, 10 mM Tris-HCl, pH 8.0, and 0.05% Tween-20)
containing 3% skim milk for 2 h at room temperature, followed by an
overnight incubation at 4°C with rabbit anti-WDR62
antibodies (1:500, P/N GTX119724; GeneTex, San Antonio, TX, USA),
anti-cleaved poly (ADP-ribose) polymerase (PARP) antibodies
(1:1,000, P/N 5625), anti-cleaved caspase-3 antibodies (1:1,000,
P/N 9664) (both from Cell Signaling Technology, Danvers, MA, USA)
and anti-β-actin antibodies (1:1,000, P/N bs-0061R; Bioss, Woburn,
MA, USA). The secondary antibodies were peroxidase-labelled
anti-rabbit IgG (1 h at 25°C; 1:5,000; cat. no. 7074S; Cell
Signaling Technology, Inc.). Specific complexes were visualized
using an echochemiluminescence detection system (GE Healthcare,
Little Chalfont, UK) as previously described (13).
Immunohistochemistry (IHC)
A tissue microarray containing 78 urothelial cancers
and 20 normal bladder tissues was obtained from US Biomax, Inc.
(product ID: BL 1002; Rockville, MD, USA). Detailed information on
all tumor specimens can be found at http://www.biomax.us/index.php. The tissue microarray
was immunostained following the manufacturer’s protocol with an
Ultra Vision Detection System (Thermo Fisher Scientific). Primary
rabbit polyclonal antibodies against WDR62 (P/N GTX119724; GeneTex)
were diluted 1:1,000. Immunostaining was evaluated according to the
scoring method described previously (13). Each case was scored on the basis of
the intensity and area of staining. The intensity of staining was
graded on the following scale: 0, no staining; 1+, mild staining;
2+, moderate staining; and 3+, intense staining. The area of
staining was evaluated as follows: 0, no staining of cells in any
microscopic field; 1+, <30% of cells stained positive; 2+,
30-60% stained positive; 3+, >60% stained positive. The
immunostaining scores (intensity + extent) were combined and
analyzed. All samples were independently scored by two of the
authors (T.S. and K.M.), who were blinded to the patient
status.
In silico analysis to identify the genes
regulated by miR-223
In silico analysis was performed to identify
target genes of miR-223 using the TargetScan database
Release 7.1 (http://www.targetscan.org/vert_71/). Additionally, the
Gene Expression Omnibus (accession nos. GSE11783 and GSE31684) and
The Cancer Genome Atlas (TCGA) databases were used to identify
upregulated genes in BC specimens. We merged these datasets and
selected possible miR-223 target genes.
Plasmid construction and dual-luciferase
reporter assays
Partial sequences of the wild-type WDR62
3′-UTR or the WDR62 3′-UTR with deletion of the
miR-223 target site (position 62-69 of the WDR62
3′-UTR) and the miR-223 target site sequence (position 62-69
of the WDR62 3′-UTR) were inserted between the XhoI
and PmeI restriction sites within the 3′-UTR of the
hRluc gene in the psiCHECK-2 vector (P/N C8021; Promega,
Madison, WI, USA). The T24 and BOY cells were transfected with 50
ng vector and 10 nM miR-223 using Lipofectamine 2000 (Thermo
Fisher Scientific) and Opti-MEM (Thermo Fisher Scientific). The
activities of Firefly and Renilla luciferases in the cell
lysates were determined using a dual luciferase assay system
according to the manufacturer′s protocol (P/N E1960; Promega).
Normalized data were presented as Renilla/Firefly luciferase
activity ratios. Experiments were performed in triplicate.
Analysis of TCGA BC datasets
BC samples (n=407) and normal bladder samples (n=19)
from the TCGA database were used to analyze the WDR62 mRNA
expression levels in BC compared with normal and BC tissues. BC
samples from the TCGA database were used to determine the
associations between the expression of miR-223 or
WDR62 and clinicopathological factors. Gene and miRNA
quantification were performed using RNA-seq expression data
(normalized RSEM) and miRNA-seq data (reads per million mapped
reads) (18). Whole-exome
sequencing data were available for the 407 BC samples and 19 normal
samples. Full sequencing information and clinical information were
acquired using the cBio Portal (http://www.cbioportal.org/public-portal/) and TCGA
(https://tcga-data.nci.nih.gov/).
Statistical analysis
The differences between 2 groups were analyzed using
Mann-Whitney U tests. The differences between 3 variables and
numerical values were analyzed using Bonferroni-adjusted
Mann-Whitney U tests. The overall survival of patients with BC from
the TCGA cohort was evaluated by the Kaplan-Meier method. Patients
were divided into 2 groups according to the median miR-223
or WDR62 expression level, and differences between the 2
groups were evaluated by log-rank tests. All analyses were
performed using Expert StatView software, version 5.0 (SAS
Institute Inc., Cary, NC, USA). All data are expressed as the means
± standard deviation. P-values <0.05 were considered to indicate
statistically significant differences.
Results
Expression levels of miR-223 in clinical
BC specimens and cell lines
First, we evaluated the expression levels of
miR-223 in BC samples (n=32) and NBE samples (n=12) in our
facility by RT-qPCR. Patient details and clinicopathological
characteristics are summarized in Table I. The expression level of
miR-223 was significantly lower in the BC tissues than in
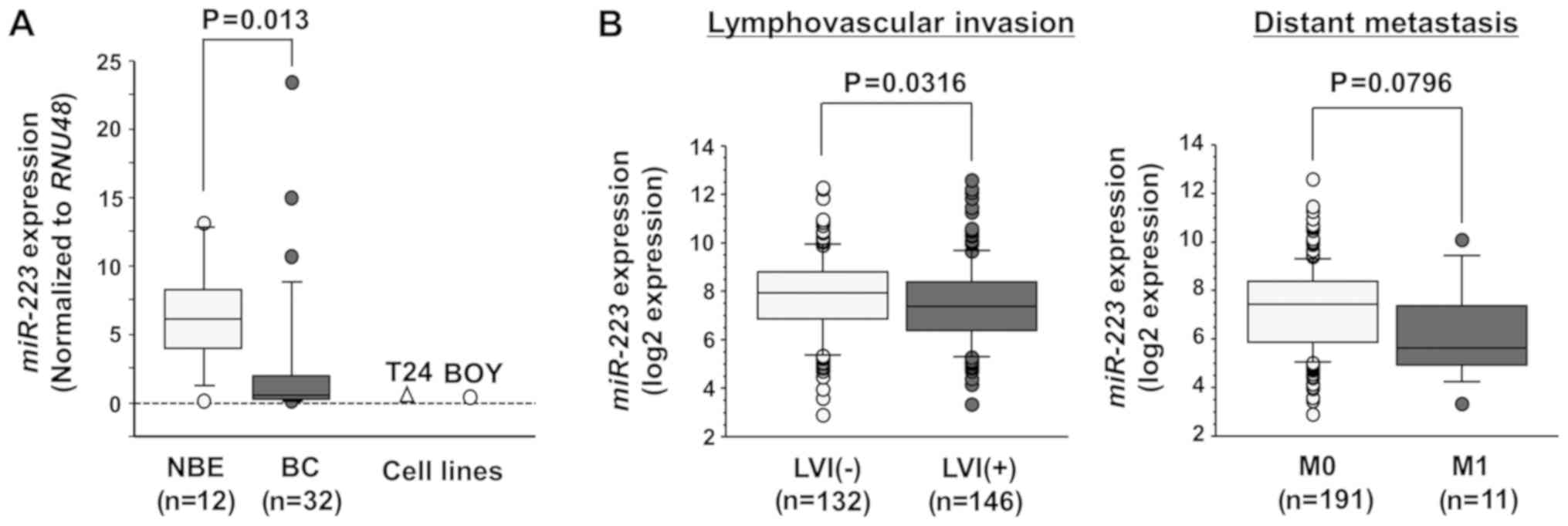
the NBE tissues (P=0.013; Fig.
1A). miR-223 expression was also decreased in the T24
and BOY BC cell lines compared with the NBE samples.
Associations between miR-223 expression
and clinicopathological parameters in the TCGA datasets
We evaluated the association between miR-223
expression and the patient clinicopathological parameters or
overall survival. Among the BC cohort of TCGA, we evaluated 407
patients with available miR-223 expression,
clinicopathological and survival data. Among these, data of
lymphovascular invasion and distant metastasis were available for
278 and 202 patients, respectively. The expression level of this
miRNA was significantly lower in patients with BC with
lymphovascular invasion (LVI) (P=0.0316; Fig. 1B, left panel) and had a tendency to
be lower in patients with BC with distant metastasis compared with
their counterparts (P=0.0796; Fig.
1B, right panel). Next, the cohort was divided into 2 groups
according to the median miR-223 expression level.
Kaplan-Meier analysis revealed no differences in the overall
survival rate between patients with a high (n=203) and patients
with a low miR-223 expression (n=203) (P=0.2866) (data not
shown).
Effects of miR-223 restoration on the
viability, migration and invasion of BC cell lines
To investigate the functional roles of
miR-223, we performed gain-of-function analyses using
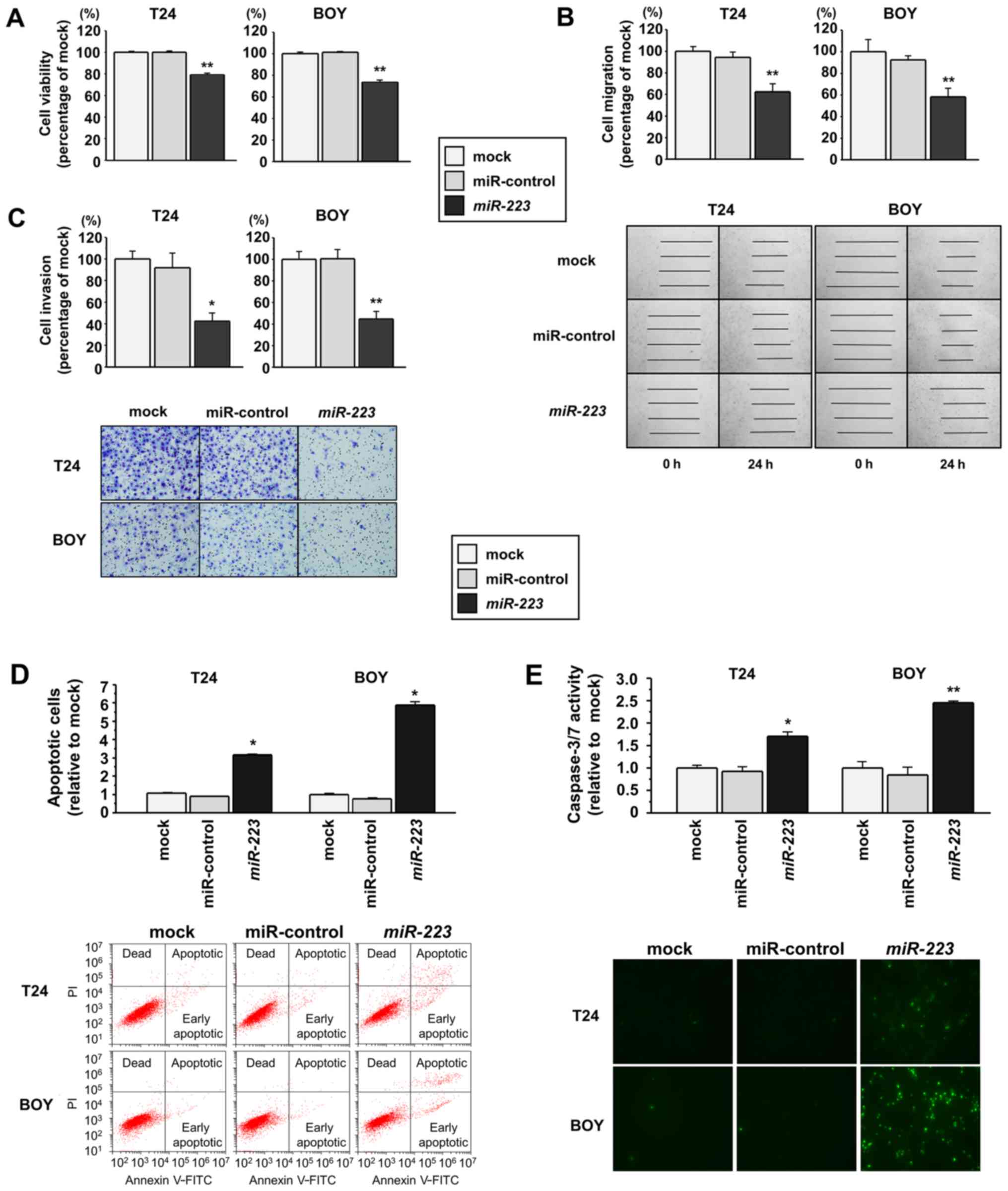
miRNA-transfected T24 and BOY BC cell lines. XTT assays revealed a
significant inhibition of the viability of the T24 and BOY cells
transfected with miR-223 in comparison with the mock or
miR-control transfectants (each P<0.0001; Fig. 2A). Wound healing assays
demonstrated that the migration activity was significantly
suppressed in the miR-223 transfectants compared with the
mock or miR-control transfectants (each P<0.0001; Fig. 2B). Matrigel invasion assays also
demonstrated that the number of invading cells was significantly
decreased in the miR-223 transfectants compared with the
mock or miR-control transfectants (T24 cells: P=0.0002, BOY cells:
P<0.0001; Fig. 2C).
Effects of miR-223 restoration on the
apoptosis of BC cell lines
As it has been previously reported that
miR-223 promotes the apoptosis of acute myeloid leukemia
cell lines (22), in this study,
we performed flow cytometric analyses to determine the number of
apoptotic cells following the restoration of this miRNA into BC
cell lines. The numbers of apoptotic cells (apoptotic and early
apoptotic cells) were significantly higher in the miR-223
transfectants than in the mock or miR-control transfectants (each
P<0.0001; Fig. 2D). Moreover,
in a caspase-3/7 activity assay, the fluorescence intensity was
significantly increased in the miR-223 transfectants
compared with the mock or miR-control transfectants (T24 cells:
P=0.0013, BOY cells: P=0.0002; Fig.
2E).
Identification of possible target genes
regulated by miR-223 in BC
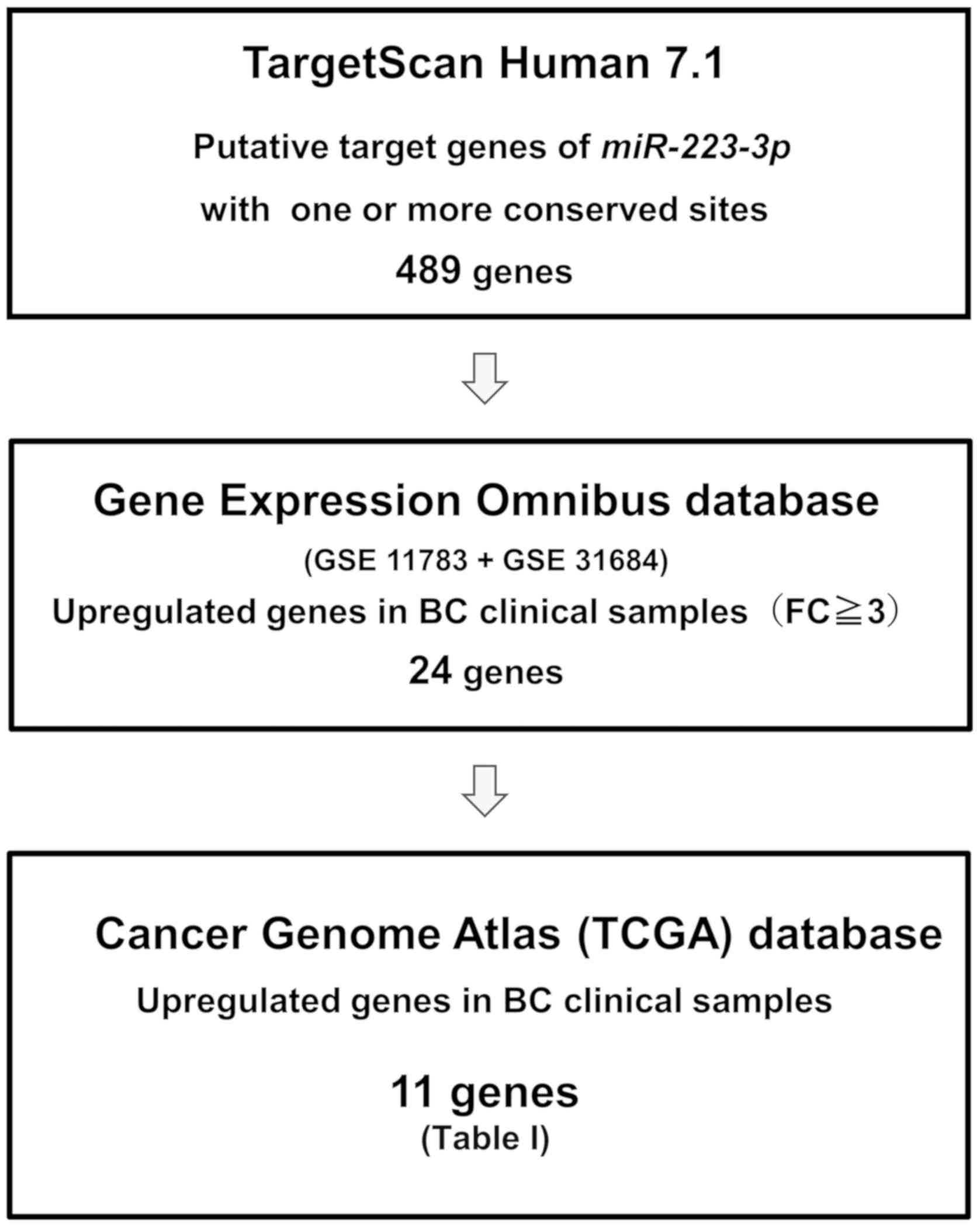
To identify the target genes of miR-223, we
performed in silico analysis. Fig. 3 shows our strategy used to narrow
down possible target genes of miR-223. Candidate targets of
miR-223 with one or more conserved miR-223 binding
sites (489 genes) were identified using the TargetScan database,
release 7.1 (http://www.targetscan.org). Among these candidate
genes, 24 genes were upregulated in the GEO database (accession
nos. GSE11783 and GSE31684). Moreover, the TCGA database was used
to identify genes with a higher expression in the BC samples than
in the normal bladder samples. The analysis of the TCGA database
revealed that the mRNA expression levels of 11 genes were
significantly upregulated in BC. Subsequently, we performed RT-qPCR
analyses of these 11 genes to confirm which genes have a lower mRNA
expression in the miR-223 transfectants compared with the
mock transfectants (Table II).
Table II shows that the 11 genes
were candidates which could possibility be targets of
miR-223 in clinical BC. Finally, we focused on WDR62
as, to date, to the best of our knowledge, there have been no
reports on this gene as a target of miR-223, while the
direct regulation of integrin alpha 3 (ITGA3) by
miR-223 has been previously reported in prostate cancer
(23).
 | Table IIPossible candidates of target genes
by in silico analyses. |
Table II
Possible candidates of target genes
by in silico analyses.
| Entrez gene ID | Gene symbol | Description | Genomic
location | TargetScan omnibus
(GEO)
| Gene expression
| The Cancer Genome
Atlas (TCGA)
| Knock down
efficiency in miR-223 transfectants
|
|---|
| Conserved
sites | Poorly conserved
sites | Expression | Fold change | Expression | P-value | T24 cells | BOY cells | Average |
|---|
| 1 | 3675 | ITGA3 | Integrin alpha
3 | 17q21.33 | 1 | 0 | Up | 3.042 | Up | 0.0058 | 0.510 | 0.122 | 0.316 |
| 2 | 284403 | WDR62 | WD repeat domain
62 | 19q13.12 | 1 | 0 | Up | 3.294 | Up | <0.0001 | 0.599 | 0.505 | 0.552 |
| 3 | 1894 | ECT2 | Ect2 oncogene | 3q26.1-q26.2 | 1 | 1 | Up | 11.070 | Up | <0.0001 | 0.712 | 0.860 | 0.786 |
| 4 | 374407 | DNAJB13 | DnaJ (Hsp40)
related, subfamily B, member 13 | 11q13.4 | 1 | 0 | Up | 5.137 | Up | 0.002 | 1.377 | 0.204 | 0.790 |
| 5 | 1033 | CDKN3 | Cyclin-dependent
kinase inhibitor 3 | 14q22 | 2 | 0 | Up | 10.685 | Up | <0.0001 | 0.655 | 1.210 | 0.932 |
| 6 | 10675 | CSPG5 | Chondroitin sulfate
proteoglycan 5 | 3p21.3 | 1 | 0 | Up | 3.329 | Up | 0.0056 | 0.673 | 1.257 | 0.965 |
| 7 | 55839 | CENPN | Centromere protein
N | 16q23.2 | 1 | 0 | Up | 4.295 | Up | <0.0001 | 0.879 | 1.105 | 0.992 |
| 8 | 79019 | CENPM | Centromere protein
M | 22q13.2 | 1 | 0 | Up | 29.162 | Up | <0.0001 | 0.696 | 1.490 | 1.093 |
| 9 | 55722 | CEP72 | Centrosomal protein
72 | 5p15.33 | 1 | 2 | Up | 3.187 | Up | <0.0001 | 0.896 | 1.313 | 1.104 |
| 10 | 25769 | SLC24A2 | Solute carrier
family 24 (sodium/potassium/calcium exchanger), member 2 | 9p22.1 | 1 | 1 | Up | 3.610 | Up | <0.0001 | 0.884 | 6.311 | 3.597 |
| 11 | 6664 | SOX11 | SRY (sex
determining region Y)-box 11 | 2p25 | 1 | 0 | Up | 5.056 | Up | < 0.0001 | 0.460 | 26.297 | 13.378 |
WDR62 is a direct target of miR-223 in BC
cells
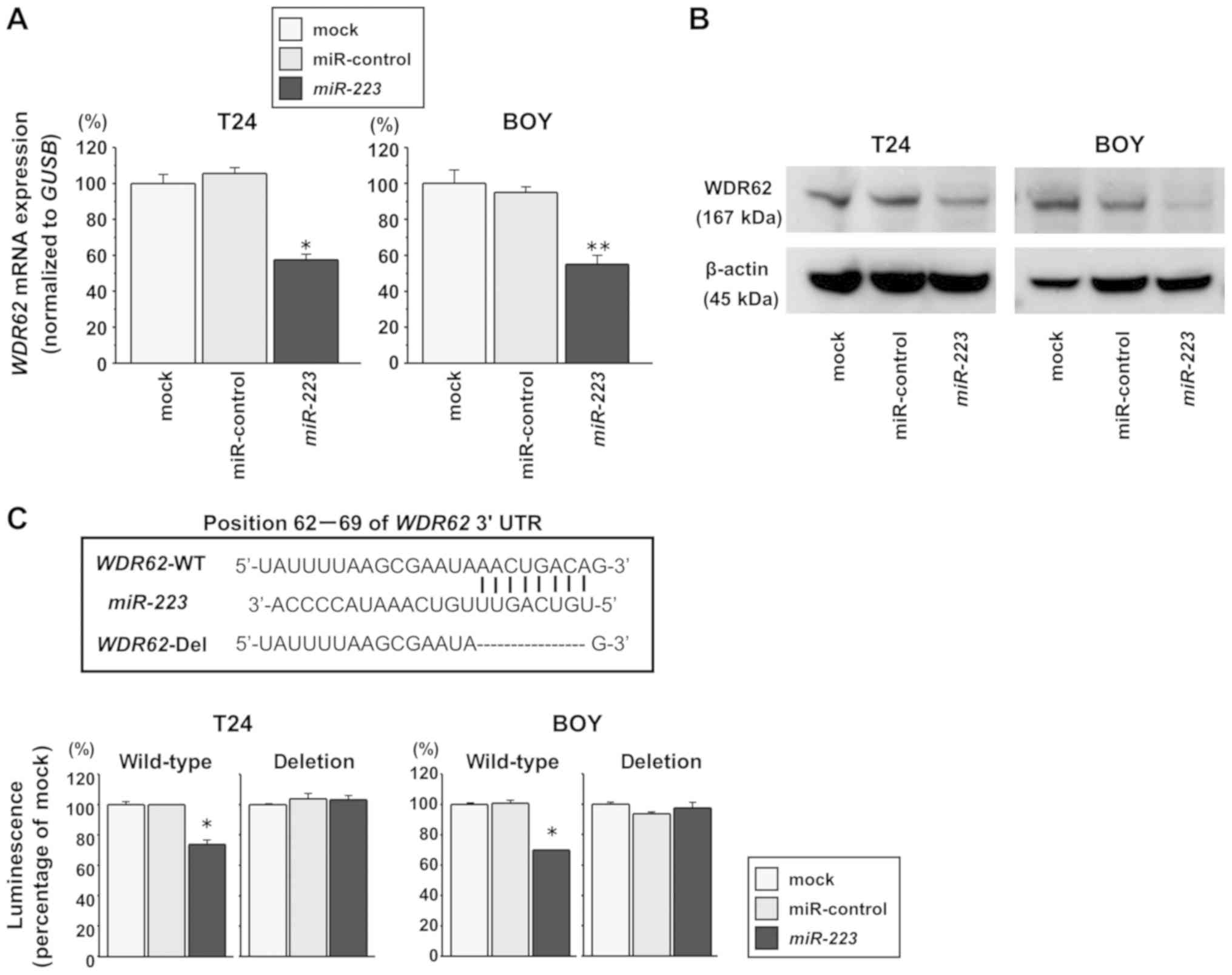
We performed RT-qPCR and western blot analyses to
investigate whether the restoration of miR-223 expression
downregulates WDR62 expression in BC cells. The WDR62
mRNA levels were significantly decreased in the miR-223
transfectants compared with the mock or miR-control transfectants
(T24 cells: P=0.0002, BOY cells: P=0.0012; Fig. 4A). The WDR62 protein levels
were also decreased in the miR-223 transfectants compared
with the mock or miR-control transfectants (Fig. 4B). In addition, we performed
dual-luciferase reporter assays in the BC cells to determine
whether WDR62 was regulated by miR-223 directly. As
the TargetScan database predicts a binding site for miR-223
in the 3′-UTR of WDR62 mRNA (position 62-69), we constructed
vectors encoding a partial wild-type WDR62 mRNA sequence
including the miR-223 binding site in the 3′-UTR. We found a
significantly reduced luminescence intensity following the
co-transfection of miR-223 and the vector carrying the
wild-type sequences at position 62-69 of the WDR62 3′-UTR
(each P<0.0001), whereas no reduction in luminescence was
observed following transfection with a vector in which the binding
site had been deleted (Fig. 4C).
These data suggest that miR-223 binds directly to the
specific site of the 3′-UTR of WDR62 mRNA.
Associations between WDR62 expression and
patient clinicopathological parameters in the TCGA datasets
In a cohort of BC samples (n=407) and normal bladder
samples (n=19) from the TCGA database, the mRNA expression level of
WDR62 was significantly higher in the BC samples than in the
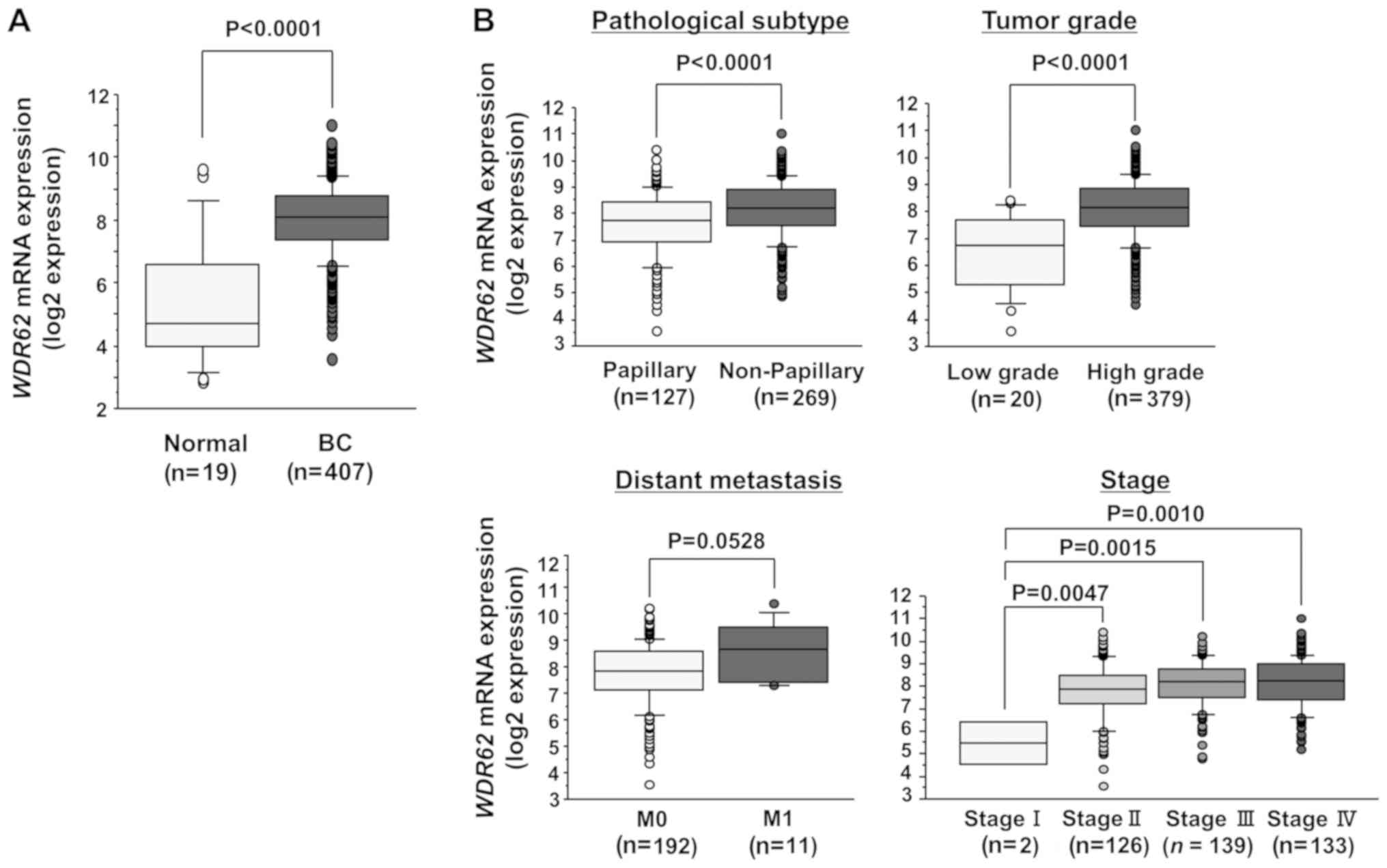
normal bladder samples (P<0.0001; Fig. 5A). In addition, we evaluated the
associations between WDR62 expression and the patient
clinicopathological parameters or overall survival. Among the BC
cohort of TCGA, we evaluated 403 patients with available
WDR62 expression, clinicopathological and survival data.
Among these, data of pathological subtype, tumor grade, distant
metastasis and clinical were available for 396, 399, 203 and 400
patients, respectively. The mRNA expression level of WDR62
was significantly higher in the non-papillary BC (P<0.0001),
high-grade tumors (P<0.0001) and high-stage tumors (P<0.01;
Fig. 5B). The higher expression
level exhibited a trend towards significance in patients with BC
with distant metastasis compared with their counterparts (P=0.0528;
Fig. 5B). Subsequently, the cohort
was divided into 2 groups according to the median WDR62
expression level. Kaplan-Meier analysis revealed no difference in
the overall survival rate between patients with a high (n=201) and
those with a low WDR62 expression (n=202) (P=0.5194) (data
not shown). In the BC samples (n=32) and normal bladder samples
(n=12) from our facility, WDR62 mRNA expression was
significantly higher in the BC samples than in the normal bladder
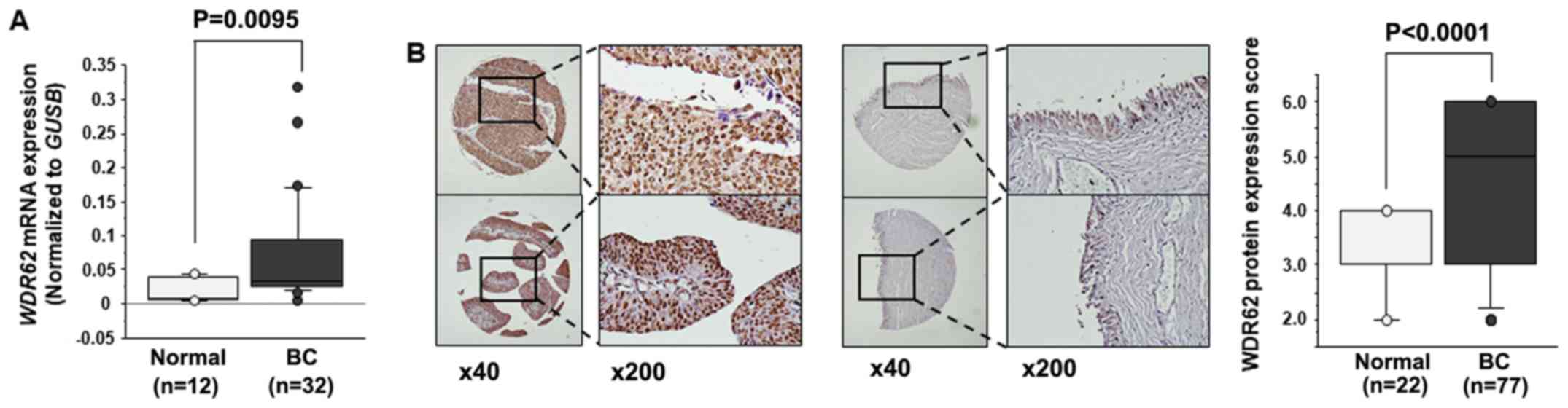
samples (P<0.0095; Fig. 6A).
However, there was no significant association between WDR62
expression and the patient clinicopathological parameters, such as
non-papillary BC, high-grade tumors and high-stage tumors in
clinical specimens from our facility (data not shown). Our cohort
was too small to evaluate the precise statistical value.
IHC analysis of WDR62 in a tissue
microarray
We examined the expression level of WDR62 in
BC specimens by IHC staining. WDR62 was strongly expressed
in several tumor lesions (Fig. 6B,
left panel), whereas a low expression was observed in the normal
tissue (Fig. 6B, middle panel).
Tissue microarray analysis revealed that the IHC score of the tumor
tissues was significantly higher than that of the normal tissues
(P<0.0001; Fig. 6B, right
panel).
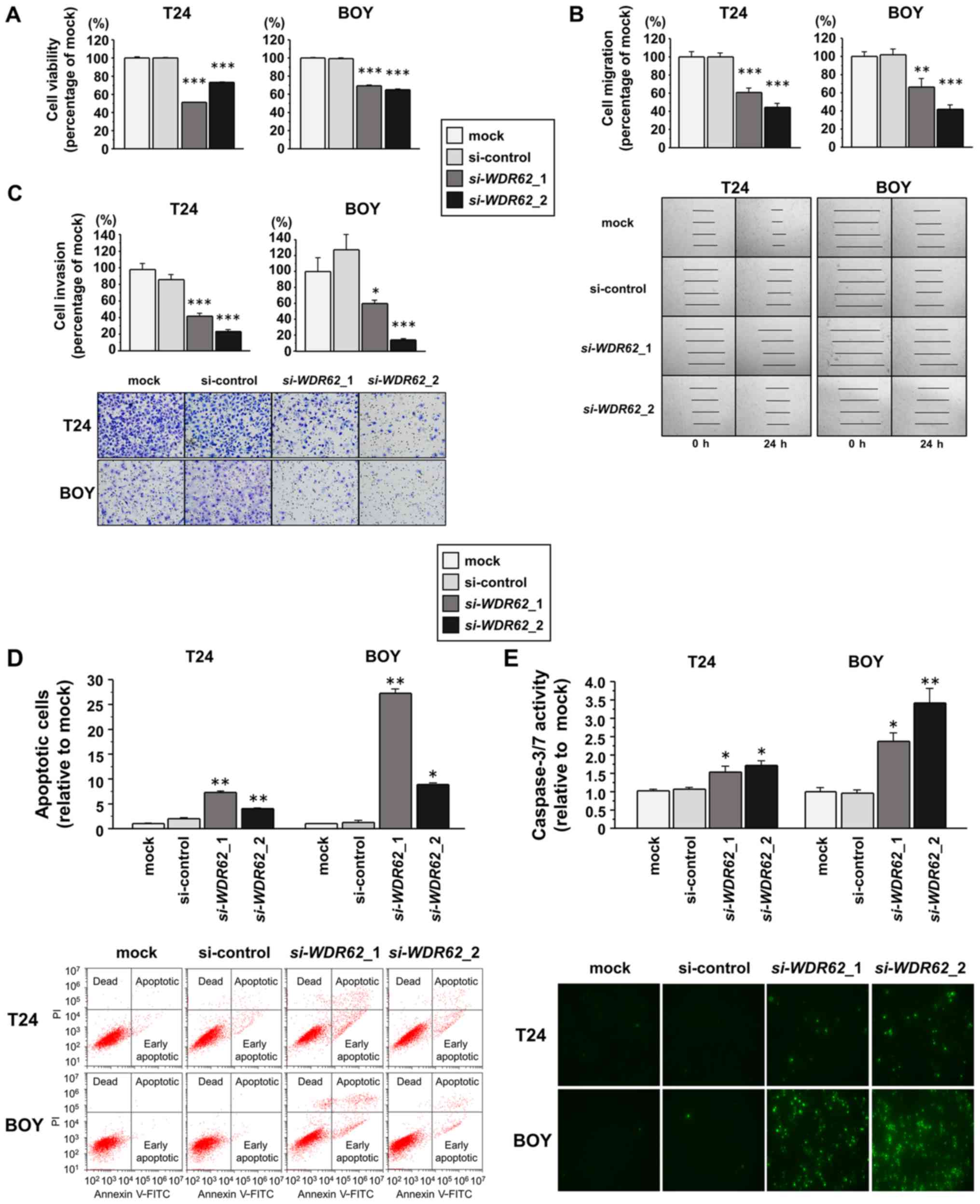
Effects of WDR62 knockdown on the
viability, migration and invasion of BC cell lines
To investigate the functional role of WDR62
in BC cells, we performed loss-of-function experients in the T24
and BOY cell lines transfected with 2 siRNA constructs targeting
WDR62 (si-WDR62_1 and si-WDR62_2). The results
of RT-qPCR and western blot analyses revealed that both siRNAs
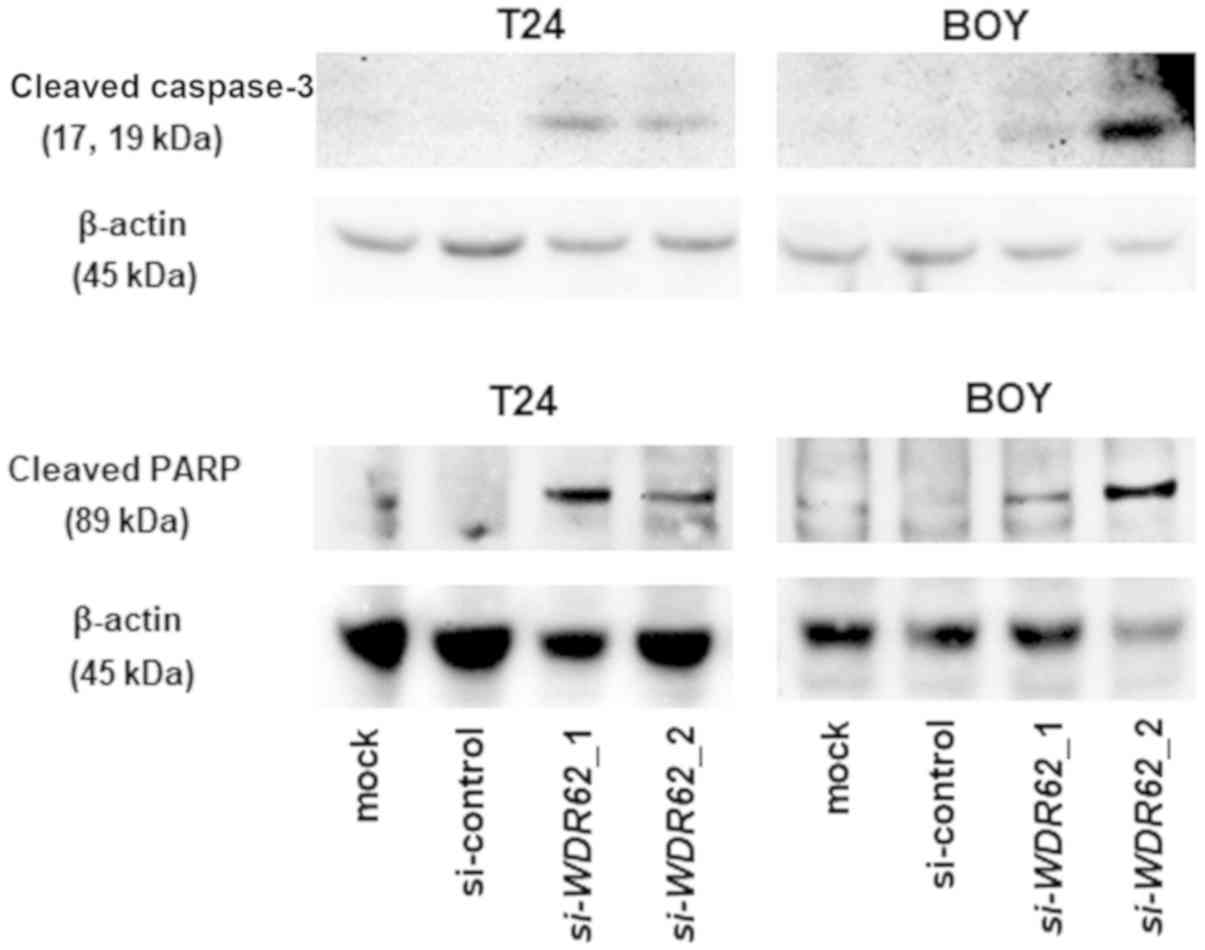
effectively decreased WDR62 expression in both cell lines (Fig. S1). XTT assays then revealed a
significant inhibition of viability in the si-WDR62
transfectants compared with the mock or siRNA-control transfectants
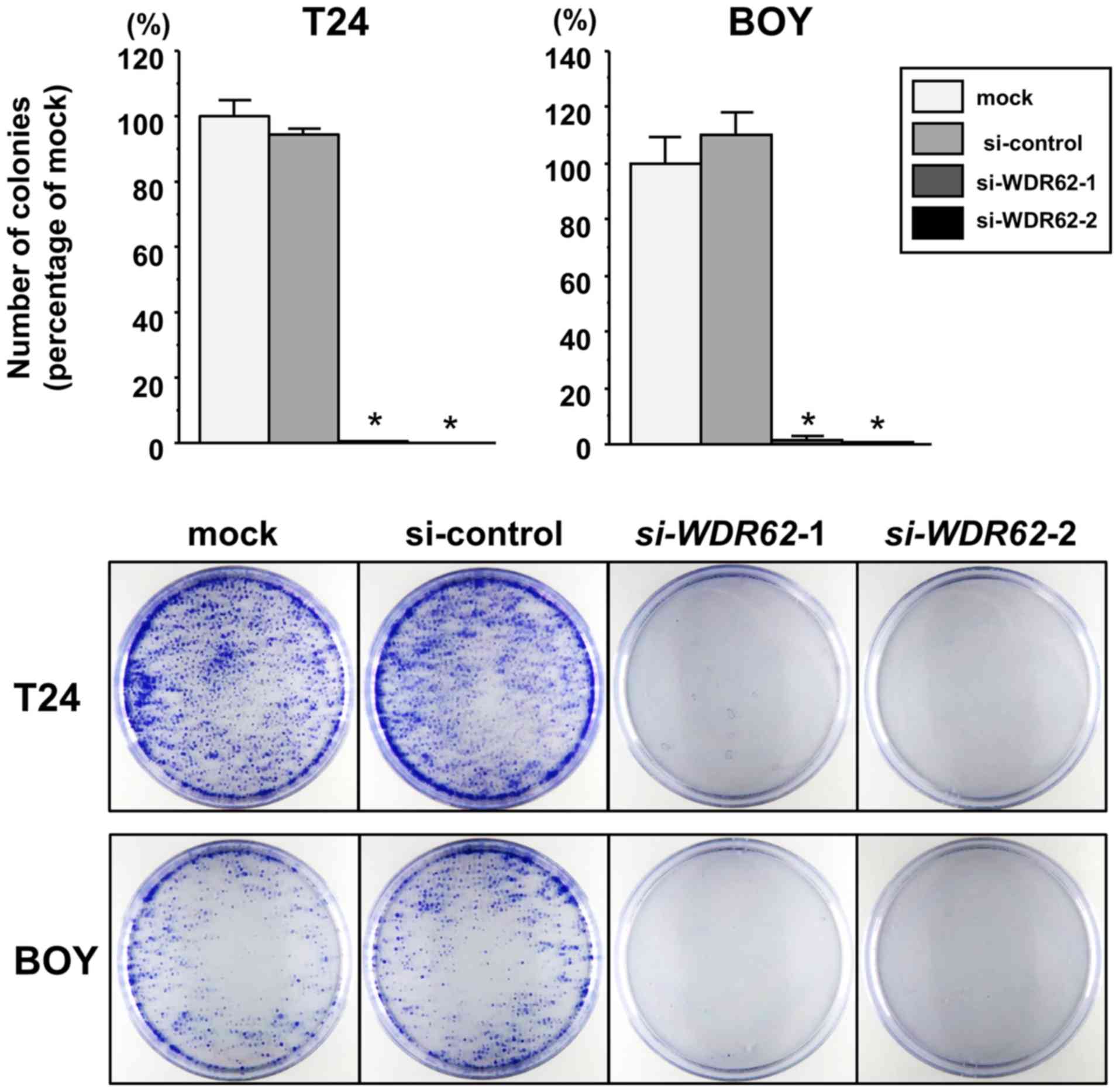
(each P<0.0001; Fig. 7A). In
addition, colony formation assay confirmed that there was a
significantly lower number of surviving BC cell colonies among the
si-WDR62 transfectants than among the mock or siRNA-control
transfectants (each P<0.0001; Fig.
8). Wound healing assays also demonstrated that the cell
migratory activity was significantly suppressed in the
si-WDR62 transfectants compared with the mock or
siRNA-control transfectants (T24 cells: each P<0.0001; BOY
cells: P=0.0005 and P<0.0001, respectively; Fig. 7B). Matrigel invasion assays
demonstrated a significantly decreased number of invading cells
among the si-WDR62 transfectants compared with the mock or
siRNA-control transfectants (T24 cells: each P<0.0001; BOY
cells: P=0.0277 and P<0.0001, respectively; Fig. 7C).
Effects of WDR62 knockdown on the
apoptosis of BC cell lines
We performed flow cytometric analyses to determine
the number of apoptotic cells following the siRNA-mediated
knockdown of WDR62 expression. There was a significantly
greater number of apoptotic cells among the si-WDR62
transfectants than among the mock or siRNA-control transfectants
(T24 cells: each P<0.0001; BOY cells: P<0.0001 and P=0.0147,
respectively; Fig. 7D). In
addition, a caspase-3/7 activity assay revealed that the
fluorescence intensity was significantly increased in the
si-WDR62 transfectants than in mock or siRNA-control
transfectants (T24 cells: P=0.0095 and P=0.0017; BOY cells:
P=0.0045 and P=0.0001, respectively; Fig. 7E). Moreover, the results of western
blot analysis confirmed that cleaved caspase-3 and cleaved PARP
expression increased in the si-WDR62 transfectants in
comparison with the mock or siRNA-control transfectants (Fig. 9).
Effects of miR-223 restoration or WDR62
knockdown on the cell cycle of BC cell lines
To investigate the cell cycle effects, we performed
flow cytometric analyses using miR-223 or si-WDR62
transfectants. Cell cycle assays revealed no fixed tendency in
either transfectant (data not shown).
Effects of miR-223 inhibition by
anti-miR-223 in BC cell lines
To confirm the roles of miR-223, we performed
functional analyses using BC cells co-transfected with
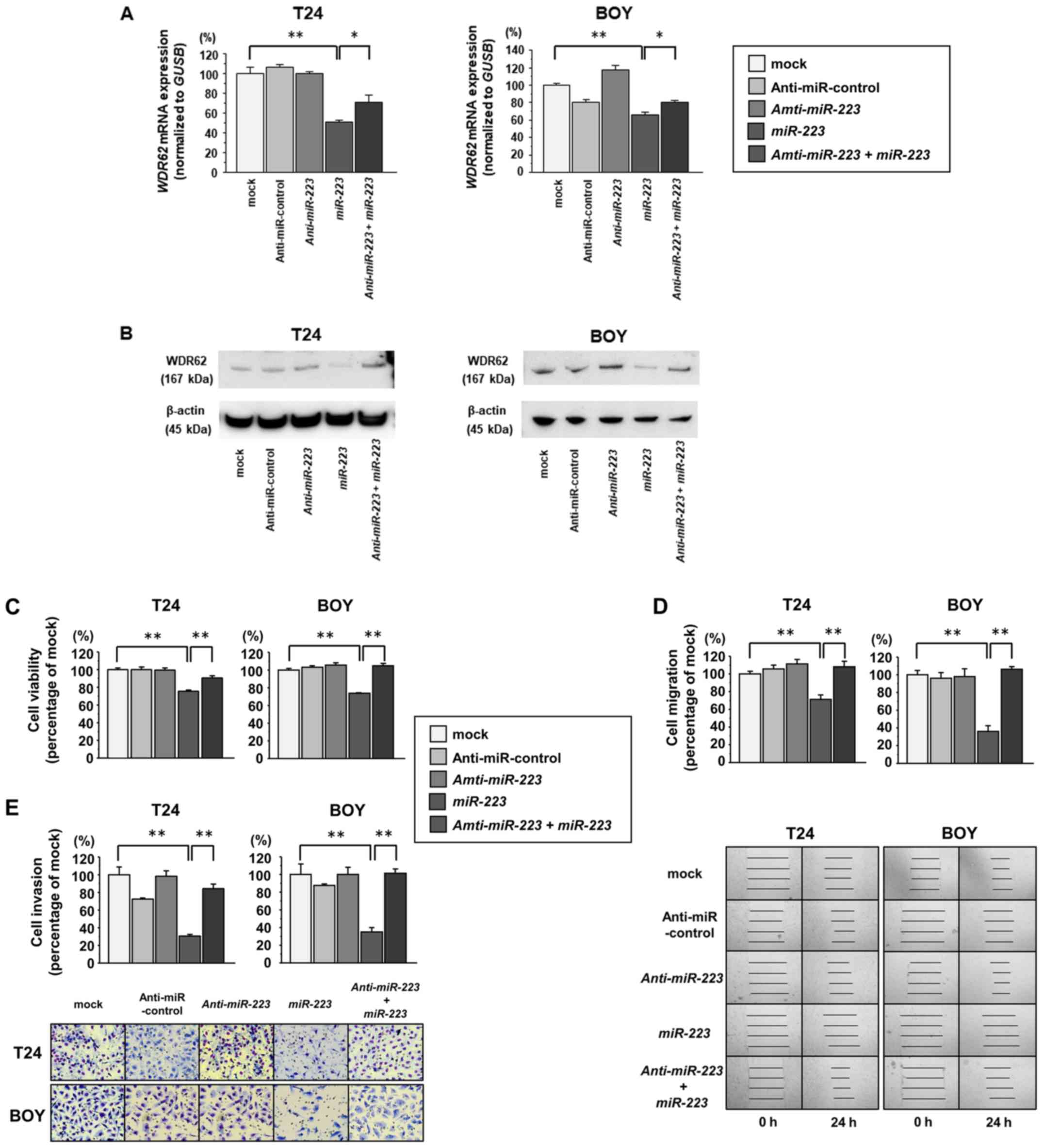
miR-223 and anti-miR-223. The results of RT-qPCR and
western blot analyses revealed that co-transfection with
anti-miR-223 and miR-223 recovered the WDR62 mRNA and
protein expression levels that were suppressed by transfection
miR-223 with alone (Fig. 10A
and B, respectively). XTT assays revealed that cell viability
was restored in the cells co-transfected with anti-miR-223
and miR-223 in comparison with that of the cells transfected
with miR-223 alone (each P<0.0001; Fig. 10C). Wound healing and Matrigel
invasion assays also demonstrated that the migratory and invasive
activity was significantly restored in the cells co-transfected
with anti-miR-223 and miR-223 in comparison with the
cells transfected with miR-223 alone (each P<0.0001;
Fig. 10D and E, respectively).
These findings suggest that miR-223 suppresses cell
viability, migration and invasion through WDR62 knockdown in
BC.
Discussion
The aberrant expression of miRNAs has been found in
a number of types of human cancer and plays significant roles in
oncogenesis and metastasis (9).
Therefore, the detection of aberrantly expressed miRNAs and their
target genes is important for the elucidation of miRNA-regulated
oncogenic pathways. Many genome-wide miRNA expression analyses,
including deep sequencing, have been applied for the evaluation of
several types of cancer. In our previous studies, the
miR-1/133a cluster, miR-23b/27b cluster,
miR-135a, miR-138, miR-143/145 cluster,
miR-200 family (miR-200a/b/c, miR-141 and
miR-429), miR-218, miR-1285 and
miR-1291 were found to be frequently decreased in renal cell
carcinoma, and these miRNAs have been identified as tumor
suppressors by targeting several oncogenic genes (20-28).
Our previous deep sequencing analysis in BC
revealed that miR-223 expression was decreased in BC tissues
compared with normal bladder tissues (10). Zhi et al and Guo et
al demonstrated that miR-223 inhibited the migration and
invasion of BC cell lines (29,30).
These findings suggest that miR-223 may function as a tumor
suppressor in BC. However, the analysis of the functional role of
miR-223 has yielded controversial results in previous
studies. In studies on gastric cancer, colorectal cancer and vulvar
carcinoma (31-34), miR-223 has been reported to
be an oncogenic miRNA. On the other hand, miR-223 has been
reported to function as a tumor suppressor miRNA in prostate
cancer, breast cancer, cervical cancer and acute myeloid leukemia
(19,35-37).
In support of its tumor-suppressive role, Kurozumi et al
demonstrated that miR-223 inhibited the migration and
invasion of prostatic cancer cells (37), and Xiao et al demonstrated
that miR-223 inhibited the proliferation and enhanced the
apoptosis of acute myeloid leukemia cell lines (19). The present study revealed that
restoration of miR-223 markedly suppressed the viability,
migration and invasion, and induced the apoptosis of BC cells,
suggesting that miR-223 may function as a tumor suppressor
miRNA in BC.
According to our miR-223 target analyses, we
focused on the WDR62 gene. WDR62 is a recently identified
centrosome-associated protein that plays important roles in DNA
replication and cell cycle progression (38). WDR62 was originally reported as a
scaffold protein associated with the JNK pathway. WDR62 consists of
13 repeats of the WD40 domain in its N-terminal half and MKK7/JNK
binding domains and 6 potential JNK phosphorylation sites in its
C-terminal half, and it mediates cell signaling, transcriptional,
mitotic and apoptotic functions (39-41).
Of note, WDR62 expression was observed in the spindle poles
of dividing cells, but not in the nucleus during mitosis,
suggesting a critical role of WDR62 in cell proliferation
(42). Previous studies have
revealed that WDR62 plays important roles in cerebral
cortical development, and mutations in its gene have been
associated with microcephaly (42-45).
However, to the best of our knowledge, the functional roles of
WDR62 in human cancer remain unclear, as there are only 3
reports available regarding the functional roles of WDR62 in
human cancer (38,46-47).
Zeng et al demonstrated that suppression of WDR62
induced G2/M cell cycle arrest and apoptosis, and its expression
was associated with chemoresistance and a poor prognosis in gastric
cancer (46). Zhang et al
demonstrated that WDR62 overexpression may be related to
centrosome amplification in ovarian cancer (47). Shinmura et al demonstrated
that WDR62 overexpression was associated with a poor
prognosis and centrosome amplification in lung adenocarcinoma
(38). In nerve cells, the
upregulation of WDR62 has been shown to induce cell apoptosis via
the JNK pathway (41). Conversely,
in this study, the downregulation of WDR62 induced the apoptosis
and inhibited the viability of BC cells. The functional roles of
WDR62 may differ between benign and cancer tissues. Therefore,
there seems to be no association between WDR62 with the JNK pathway
in BC. Further studies are warranted to identify other pathways
related to WDR62 in BC.
In addition, we found that WDR62 knockdown
suppressed cell invasion and migration, which has not been reported
previously. Notably, two of the three previous reports indicated
that WDR62 overexpression was associated with centrosome
amplification in distinct types of cancer. Centrosome amplification
is an abnormal cellular process in which cells acquire three or
more centrosomes, and it results in multipolar mitosis, the
consequences of which may include mitotic catastrophe or delayed
mitotic progression (48,49). In addition, aberrant mitoses may
result in the acquisition of aneuploidy and chromosome instability
(50,51). Centrosome amplification is well
known as a common feature of many types of cancer; however, its
effect on cancer is not yet fully understood. Raff et al
demonstrated that centrosome amplification initiated tumorigenesis
in a mouse model (52). Godinho
et al suggested that centrosome amplification was involved
in cellular invasions (53).
Centrosome amplification may be an important mechanism of the
oncogenic role of WDR62. Further studies are warranted to
clarify this hypothesis.
In this study, the analyses of the TCGA database
indicated that WDR62 expression, as well as miR-223
expression, may be associated with distant metastasis. Moreover,
WDR62 knockdown, as well as miR-223 restoration,
inhibited the migration and invasion of BC cells. However, the
analyses of the TCGA database revealed that miR-223 or
WDR62 expression was not related to the survival rate. The
upregulation of oncogenic WDR62 due to the suppression of
miR-223 may be an important mechanism for cell invasion and
metastasis in BC cells. However, it is not a critical factor that
affects disease survival in BC. In addition, no significant inverse
correlation was detected between miR-223 and WDR62
mRNA expression in the TCGA database (data not shown). A number of
miRNAs, including miR-223 target WDR62, implying that miR-223 alone
does not suppress WDR62 expression with statistical significance.
Further studies are warranted to elucidate other regulatory factors
of WDR62 expression.
In conclusion, in this study, miR-223 was
shown to function as a tumor suppressor in BC cells. To the best of
our knowledge, this is the first report demonstrating that the
tumor suppressor miR-223 directly regulates WDR62 in
BC cells. The identification of novel molecular pathways and
targets regulated by the miR-223/WDR62 axis may
provide important insight into the potential mechanisms of BC
progression.
Supplementary Materials
Funding
This study was supported by the KAKENHI (KIBAN-B)
16H05464 and 17H04332, KAKENHI (KIBAN-C) 16K11015, KAKENHI
(WAKATE-B) 17K16799.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article or are available from the
corresponding author on reasonable request.
Authors’ contributions
SS, HY, MY, HE and MN conceived of the study and
designed the experiments. SS, HY, KM, RM and TS performed the
experiments. TI and ST contributed to the interpretation of the
data. SS, TI, ST and HE drafted the manuscript. All authors have
reviewed the manuscript and approved the final version.
Ethics approval and consent to
participate
This study was approved by the Bioethics Committee
of Kagoshima University; the study approval numbers were H27-104
and H27-105. Written informed consent and approval were obtained
from all patients prior to obtaining the samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Ms. Mutsumi
Miyazaki for her excellent laboratory assistance.
References
|
1
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vale C; Advanced Bladder Cancer
Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive
bladder cancer: A systematic review and meta-analysis. Lancet.
361:1927–1934. 2003. View Article : Google Scholar
|
|
4
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multi-center, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meeks JJ, Bellmunt J, Bochner BH, Clarke
NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T,
et al: A systematic review of neoadjuvant and adjuvant chemotherapy
for muscle-invasive bladder cancer. Eur Urol. 62:523–533. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carthew RW and Sontheimer EJ: Origins and
Mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar :
|
|
8
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Itesako T, Seki N, Yoshino H, Chiyomaru T,
Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa
M, et al: The microRNA expression signature of bladder cancer by
deep sequencing: The functional significance of the miR-195/497
cluster. PLoS One. 9:e843112014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ichimi T, Enokida H, Okuno Y, Kunimoto R,
Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama
K, et al: Identification of novel microRNA targets based on
microRNA signatures in bladder cancer. Int J Cancer. 125:345–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
13
|
Yoshino H, Chiyomaru T, Enokida H,
Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N and Nakagawa
M: The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsushita R, Seki N, Chiyomaru T,
Inoguchi S, Ishihara T, Goto Y, Nishikawa R, Mataki H, Tatarano S,
Itesako T, et al: Tumour-suppressive microRNA-144-5p directly
targets CCNE1/2 as potential prognostic markers in bladder cancer.
Br J Cancer. 113:282–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Block AL, Bauer KD, Williams TJ and
Seidenfeld J: Experimental parameters and a biological standard for
acridine orange detection of drug-induced alterations in chromatin
condensation. Cytometry. 8:163–169. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar A, Sahu SK, Mohanty S, Chakrabarti
S, Maji S, Reddy RR, Jha AK, Goswami C, Kundu CN, Rajasubramaniam
S, et al: Kaposi sarcoma herpes virus latency associated nuclear
antigen protein release the G2/M cell cycle blocks by modulating
ATM/ATR mediated checkpoint pathway. PLoS One. 9:e1002282014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B and Dewey CN: RSEM: Accurate
transcript quantification from RNA-Seq data with or without a
reference genome. BMC Bioinformatics. 12:3232011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao Y, Su C and Deng T: miR-223 decreases
cell proliferation and enhances cell apoptosis in acute myeloid
leukemia via targeting FBXW7. Oncol Lett. 12:3531–3536. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawakami K, Enokida H, Chiyomaru T,
Tatarano S, Yoshino H, Kagara I, Gotanda T, Tachiwada T, Nishiyama
K, Nohata N, et al: The functional significance of miR-1 and
miR-133a in renal cell carcinoma. Eur J Cancer. 48:827–836. 2012.
View Article : Google Scholar
|
|
21
|
Ishihara T, Seki N, Inoguchi S, Yoshino H,
Tatarano S, Yamada Y, Itesako T, Goto Y, Nishikawa R, Nakagawa M,
et al: Expression of the tumor suppressive miRNA-23b/27b cluster is
a good prognostic marker in clear cell renal cell carcinoma. J
Urol. 192:1822–1830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada Y, Hidaka H, Seki N, Yoshino H,
Yamasaki T, Itesako T, Nakagawa M and Enokida H: Tumor-suppressive
microRNA-135a inhibits cancer cell proliferation by targeting the
c-MYC oncogene in renal cell carcinoma. Cancer Sci. 104:304–312.
2013. View Article : Google Scholar
|
|
23
|
Yamasaki T, Seki N, Yamada Y, Yoshino H,
Hidaka H, Chiyomaru T, Nohata N, Kinoshita T, Nakagawa M and
Enokida H: Tumor suppressive microRNA 138 contributes to cell
migration and invasion through its targeting of vimentin in renal
cell carcinoma. Int J Oncol. 41:805–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshino H, Enokida H, Itesako T, Kojima S,
Kinoshita T, Tatarano S, Chiyomaru T, Nakagawa M and Seki N:
Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in
renal cell carcinoma. Cancer Sci. 104:1567–1574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshino H, Enokida H, Itesako T, Tatarano
S, Kinoshita T, Fuse M, Kojima S, Nakagawa M and Seki N:
Epithelial-mesenchymal transition-related microRNA-200s regulate
molecular targets and pathways in renal cell carcinoma. J Hum
Genet. 58:508–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamasaki T, Seki N, Yoshino H, Itesako T,
Hidaka H, Yamada Y, Tatarano S, Yonezawa T, Kinoshita T, Nakagawa
M, et al: MicroRNA-218 inhibits cell migration and invasion in
renal cell carcinoma through targeting caveolin-2 involved in focal
adhesion pathway. J Urol. 190:1059–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hidaka H, Seki N, Yoshino H, Yamasaki T,
Yamada Y, Nohata N, Fuse M, Nakagawa M and Enokida H: Tumor
suppressive microRNA-1285 regulates novel molecular targets:
Aberrant expression and functional significance in renal cell
carcinoma. Oncotarget. 3:44–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamasaki T, Seki N, Yoshino H, Itesako T,
Yamada Y, Tatarano S, Hidaka H, Yonezawa T, Nakagawa M and Enokida
H: Tumor-suppressive microRNA-1291 directly regulates glucose
transporter 1 in renal cell carcinoma. Cancer Sci. 104:1411–1419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhi Y, Pan J, Shen W, He P, Zheng J, Zhou
X, Lu G, Chen Z and Zhou Z: Ginkgolide B inhibits human bladder
cancer cell migration and invasion through microRNA-223-3p. Cell
Physiol Biochem. 39:1787–1794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo J, Cao R, Yu X, Xiao Z and Chen Z:
MicroRNA-223-3p inhibits human bladder cancer cell migration and
invasion. Tumour Biol. 39:10104283176916782017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Jin W, Jia H, Yan J and Zhang G:
MiR-223 promotes the cisplatin resistance of human gastric cancer
cells via regulating cell cycle by targeting FBXW7. J Exp Clin
Cancer Res. 34:282015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eto K, Iwatsuki M, Watanabe M, Ishimoto T,
Ida S, Imamura Y, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, et al:
The sensitivity of gastric cancer to trastuzumab is regulated by
the miR-223/FBXW7 pathway. Int J Cancer. 136:1537–1545. 2015.
View Article : Google Scholar
|
|
33
|
Li ZW, Yang YM, Du LT, Dong Z, Wang LL,
Zhang X, Zhou XJ, Zheng GX, Qu AL and Wang CX: Overexpression of
miR-223 correlates with tumor metastasis and poor prognosis in
patients with colorectal cancer. Med Oncol. 31:2562014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Melo Maia B, Rodrigues IS, Akagi EM,
Soares do Amaral N, Ling H, Monroig P, Soares FA, Calin GA and
Rocha RM: MiR-223-5p works as an oncomiR in vulvar carcinoma by
TP63 suppression. Oncotarget. 7:49217–49231. 2016.PubMed/NCBI
|
|
35
|
Sun X, Li Y, Zheng M, Zuo W and Zheng W:
MicroRNA-223 increases the sensitivity of triple-negative breast
cancer stem cells to TRAIL-induced apoptosis by targeting HAX-1.
PLoS One. 11:e01627542016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang Y, Wang Y, Chen Q, Qiu N, Zhao Y and
You X: MiR-223 inhibited cell metastasis of human cervical cancer
by modulating epithelial-mesenchymal transition. Int J Clin Exp
Pathol. 8:11224–11229. 2015.PubMed/NCBI
|
|
37
|
Kurozumi A, Goto Y, Matsushita R, Fukumoto
I, Kato M, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M, Ichikawa
T, et al: Tumor-suppressive microRNA-223 inhibits cancer cell
migration and invasion by targeting ITGA3/ITGB1 signaling in
prostate cancer. Cancer Sci. 107:84–94. 2016. View Article : Google Scholar
|
|
38
|
Shinmura K, Kato H, Kawanishi Y, Igarashi
H, Inoue Y, Yoshimura K, Nakamura S, Fujita H, Funai K, Tanahashi
M, et al: WDR62 overexpression is associated with a poor prognosis
in patients with lung adenocarcinoma. Mol Carcinog. 56:1984–1991.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wasserman T, Katsenelson K, Daniliuc S,
Hasin T, Choder M and Aronheim A: A novel c-Jun N-terminal kinase
(JNK)-binding protein WDR62 is recruited to stress granules and
mediates a nonclassical JNK activation. Mol Biol Cell. 21:117–130.
2010. View Article : Google Scholar :
|
|
40
|
Cohen-Katsenelson K, Wasserman T, Khateb
S, Whitmarsh AJ and Aronheim A: Docking interactions of the JNK
scaffold protein WDR62. Biochem J. 439:381–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuan CY, Yang DD, Samanta Roy DR, Davis
RJ, Rakic P and Flavell RA: The Jnk1 and Jnk2 protein kinases are
required for regional specific apoptosis during early brain
development. Neuron. 22:667–676. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nicholas AK, Khurshid M, Désir J, Carvalho
OP, Cox JJ, Thornton G, Kausar R, Ansar M, Ahmad W, Verloes A, et
al: WDR62 is associated with the spindle pole and is mutated in
human microcephaly. Nat Genet. 42:1010–1014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu TW, Mochida GH, Tischfield DJ, Sgaier
SK, Flores-Sarnat L, Sergi CM, Topçu M, McDonald MT, Barry BJ,
Felie JM, et al: Mutations in WDR62, encoding a
centrosome-associated protein, cause microcephaly with simplified
gyri and abnormal cortical architecture. Nat Genet. 42:1015–1020.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu D, Zhang F, Wang Y, Sun Y and Xu Z:
Microcephaly-associated protein WDR62 regulates neurogenesis
through JNK1 in the developing neocortex. Cell Rep. 6:104–116.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pervaiz N and Abbasi AA: Molecular
evolution of WDR62, a gene that regulates neocorticogenesis. Meta
Gene. 9:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zeng S, Tao Y, Huang J, Zhang S, Shen L,
Yang H, Pei H, Zhong M, Zhang G, Liu T, et al: WD40
repeat-containing 62 overexpression as a novel indicator of poor
prognosis for human gastric cancer. Eur J Cancer. 49:3752–3762.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Tian Y, Yu JJ, He J, Luo J, Zhang
S, Tang CE and Tao YM: Overexpression of WDR62 is associated with
centrosome amplification in human ovarian cancer. J Ovarian Res.
6:552013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fukasawa K: Oncogenes and tumour
suppressors take on centrosomes. Nat Rev Cancer. 7:911–924. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Godinho SA, Kwon M and Pellman D:
Centrosomes and cancer: How cancer cells divide with too many
centrosomes. Cancer Metastasis Rev. 28:85–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Anderhub SJ, Krämer A and Maier B:
Centrosome amplification in tumorigenesis. Cancer Lett. 322:8–17.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chan JY: A clinical overview of centrosome
amplification in human cancers. Int J Biol Sci. 7:1122–1144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Raff JW and Basto R: Centrosome
Amplification and Cancer: A Question of Sufficiency. Dev Cell.
40:217–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Godinho SA, Picone R, Burute M, Dagher R,
Su Y, Leung CT, Polyak K, Brugge JS, Théry M and Pellman D:
Oncogene-like induction of cellular invasion from centrosome
amplification. Nature. 510:167–171. 2014. View Article : Google Scholar : PubMed/NCBI
|