Introduction
Bladder cancer (BC) is one of most common
malignancies in urinary tract worldwide (1). A total of ~80% of patients with BC
are diagnosed with non-muscle invasive BC (NMIBC) (2). The standard treatment for NMIBC is
transurethral resection (TUR) (3).
Recurrence of NMIBC following TUR is 60-80% (4). Recurrence is attributed to incomplete
resection, growth of microscopic tumors, reimplantation of tumor
cells or new tumor formation (5).
Intravesical therapy with Bacillus Calmette-Geurin (BCG) vaccine or
other chemotherapeutic agents, including mitomycin C, are used to
prevent or delay recurrence following TUR (6). However, 20-40% of patients respond
poorly to these treatments (7) and
new therapeutic modalities to prevent high recurrence rates are in
a demand.
Benzyl isothiocyanate (BITC) is of the ITC family,
which exerts anticancer activity by apoptosis induction in BC cells
and inhibiting chemical-induced cancer in animal models (8). A recent study has revealed that BITC
induces autophagic cell death in breast cancer (9). In addition, BITC treatment induces
apoptosis and autophagy via inhibiting the mammalian target of
rapamycin (mTOR) signaling pathway in prostate cancer cells
(10). BITC has been reported to
inhibit growth of pancreatic cancer cells through manipulating
microRNA (miRNA or miR) expression. It is of interest to elucidate
the mechanism detailing the anticancer effect of BITC in BC.
miRNAs are small noncoding RNAs (~20-24 nucleotides)
that regulate target gene expression through translational blockage
or mRNA degradation (11).
Increasing studies have reported that miRNAs serve important roles
in regulating tumor formation and progression (12). Numerous anticancer agents have been
suggested to exert cell toxicity through manipulating miRNA
expression (13). A recent study
has reported that in patients with BC, miR-99a-5p is downregulated
in cancerous tissues (14). The
miR-99 family is known to be involved in the mTOR signaling pathway
of other cancers (15,16).
The present study focused on the association of
miRNA with BITC-induced inhibitory effects in BC. It was
hypothesized that BITC inhibited BC cell growth through altering
the expression of certain miRNAs. miRNA expression profiles were
explored in response to BITC treatment using a miRNA microarray
approach. The target genes of miR-99a and downstream effectors were
further investigated.
Materials and methods
Chemicals
All chemicals, unless otherwise stated, were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). BITC
(purity, ~98%) was prepared as described previously (10).
Cell culture and transfection
Human BC cell lines RT4 (cat. no. HTB-2), 5637 (cat.
no. HTB-9), HT1376 (cat. no. CRL-1472), HT1197 (cat. no. CRL-1473),
T24 (cat. no. HTB-4) and human-ureter-sumian-virus-40 transformed
immortalized epithelial cell line SV-HUC-1 (cat. no. CRL-9520) were
obtained from the American Type Culture Collection (Manassas, VA,
USA). Cells were routinely checked for mycoplasma contamination
using a polymerase chain reaction (PCR)-based method as described
previously (17). RT4 cells were
cultured in McCoy’s 5A medium, HT1376 and HT1397 cells were
maintained in Minimum essential medium, 5637 and T24 cells were
cultured in RPMI-1640 and SV-HUC-1 cells were cultured in F12
medium; all media (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) were supplemented with 10% fetal bovine serum and essential
supplements (Thermo Fisher Scientific, Inc.).
Cells at 70-80% confluence were transfected with
below described plasmids in 6-well plates or 10-cm dishes for 24 h
prior to treatment with BITC (20 µM) for 24 h. Transfection
of 1 µg/ml plasmid was performed using a polymer-based
transfection reagent (Ultra293; GeneDireX, Inc., Taipei, Taiwan)
according to the manufacturer’s instructions and transfection
efficiency was evaluated by reverse transcription-quantitative
(RT-q) PCR.
Profiling of miRNAs expression using a BC
miRNA RT-qPCR array
T24 bladder cancer cells were incubated with BITC
(20 µM) for 24 h. The miProfile™ Human BC miRNA qPCR array
(cat. no. QM-018; GeneCopoeia, Inc., Rockville, MD, USA), which is
able to profile 79 aberrantly expressed miRNAs most relevant to BC,
was used to identify miRNA responses to BITC treatment of BC cells.
Total RNA from control or BITC-treated 5637 cells was isolated
using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to
the manufacturer’s instructions, and the quality and concentration
of RNA was determined using a NanoDrop2000 (Thermo Fisher
Scientific, Inc.). miRNAs were reverse-transcribed (37°C; 60 min)
from 2.5 µg of total RNA using poly-A polymerase with an
oligo(dT) adaptor from the All-in-One™ miRNA First Strand cDNA
Synthesis kit provided with the miRNA qPCR array (GeneCopoeia,
Inc.). The qPCR array was performed in 20 µl reactions
containing 1 µl RT product using the SYBR-Green (cat. no.
KK4600; Kapa Biosystems; Roche Diagnostics, Indianapolis, IN, USA)
detection on a StepOne Plus instrument (Thermo Fisher Scientific,
Inc.). Primers for the miProfile™ human bladder cancer miRNA qPCR
arrays were provided with the kit. The following protocol was used:
95°C for 3 min; followed by 40 cycles of 95°C for 3 sec and 60°C
for 20 sec; melting curves were recorded between 60-95°C with using
0.1°C/sec as a heat ramp and storage at 4°C. Data was analyzed
using All-in-One™ qPCR Primer Array Data Analysis software provided
by GeneCopoeia, Inc. and the 2−ΔΔCq method was applied
for quantification (18). Small
nucleolar RNA U43 (SNORD43) was used as control.
Detection of miRNAs expression by
stem-loop RT-qPCR
5637 bladder cancer cells were incubated with BITC
(10 µM) for 24 h. Total RNA was extracted and miR-99a-5p,
miR-133b-5p, miR-30a-3p, miR-30a-5p, miR-125b-5p and miR-195-5p
expression was determined by stem-loop RT-qPCR according to a
previously published protocol (19). Stem-loop RT primers, universal
reverse primer and miRNA specific forward primers are listed in
Table I. miRNA was reverse
transcribed into cDNA using the miRNA stem loop-RT primers and
TaqMan™ MicroRNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.). miRNAs quantification was performed using the
StepOne Plus instrument (Thermo Fisher Scientific, Inc.), with
universal reverse primer and miRNA specific forward primers. The
Universal ProbeLibrary probe #21 (UPL21) hydrolysis probe had the
following sequence: 5′-T+G+G+C+T+C+TG-3′, where ‘+’ identifies a
unique nucleotide chemistry (Locked Nucleic Acid). SNORD43 was used
as loading control. The following protocol was used: 95°C for 5
min; followed by 40 cycles of 95°C for 5 sec, 60°°C for 10 sec and
72°C for 1 sec with 0.2°C/sec heating and storage at 4°C.
 | Table IOligonucleotides and probe used for
stem-loop RT-qPCR analysis. |
Table I
Oligonucleotides and probe used for
stem-loop RT-qPCR analysis.
| A, Stem-Loop
RT |
|---|
|
|---|
| Name | Sequence
(5′-3′) |
|---|
|
hsa-miR-99a-5p_RT |
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACCACAAG |
|
hsa-miR-133b-5p_RT |
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTAGCTG |
|
hsa-miR-30a-3p_RT |
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACGCTGCA |
|
hsa-miR-30a-5p_RT |
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACCTTCCA |
|
hsa-miR-125b-5p_RT |
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTGACAA |
|
hsa-miR-195-5p_RT |
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACGCCAAT |
| SNORD43_RT |
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACAATCAG |
|
| B, qPCR |
|
| Name | Sequence
(5′-3′) |
|
|
hsa-miR-99a-5p_F |
GTGAACCCGTAGATCCGAT |
|
hsa-miR-133b-5p_F |
GGGTTTGGTCCCCTTCAAC |
|
hsa-miR-30a-3p_F |
GTGCTTTCAGTCGGATGTT |
|
hsa-miR-30a-5p_F |
GGGTGTAAACATCCTCGAC |
|
hsa-miR-125b-5p_F |
GTGTCCCTGAGACCCTAAC |
|
hsa-miR-195-5p_F |
GGGGTAGCAGCACAGAAAT |
| SNORD43_F |
GTGAACTTATTGACGGGCG |
| Universal reverse
primer |
GTGCAGGGTCCGAGGT |
Construction of miR expression and
reporter vectors
The miR-99a-5p expression vector (pSM-99a-5p) was
constructed by annealing a paired oligonucleotides consisting of
the mature miR-99a sequence (oligonucleotide 1, 5′-TGCTGAACCCGTA
GATCCGATCTTGTGGTTTTGGCCACTGACTGACCACA AGATGATCTACGGGTT-3′; and
oligonucleotide 2, 5′-CCTGAACCCGTAGATCATCTTGTGGTCAGTCAGTGGCCAA
AACCACAAGATCGGATCTACGGGTTC-3′) and cloned into a small-RNA
expression vector (pSM; cat. no. 19170; Addgene, Inc., Cambridge,
MA, USA) as previously described (20). The concept of a miRNA sponge
targeting miRNA and attenuating its function has been well
established (21,22). Following a previous study
describing the establishment of a let-7 sponge (23), the synthesized double strand
oligonucleotides containing 3 repeats of matured miR-99a-5p
antisense sequences were inserted to pmiR-GLO (Promega Corporation,
Madison, WI, USA) generating a positive reporter construct
(pmiR-GLO-99a-5p-PTS). 5637 and T24 cells (5×104
cells/well) at 7-80% confluence were seeded into 24-well plates and
transfected with pmiR-GLO-99a-5p-PTS or empty control (pmiR-GLO; 1
µg/ml) using a polymer-based transfection reagent (Ultra293;
GeneDireX, Inc.) according to the manufacturer’s instructions.
Cells were treated with BITC (20 µM) for 24 h
post-transfection. Following further 24 h, the activities of
Firefly and Renilla luciferase were detected using
Dual-luciferase kit (Promega Corporation). Relative protein levels
were expressed as Firefly/Renilla luciferase.
Detection of IGF1R, FGFR3 and mTOR
expression
T24 and 5637 bladder cancer cells seeded in 6-well
plates (3×105 cells/well) were transfected with pSM-99a-5p,
pmiR-GLO-99a-5p-PTS or control vectors (1 µg/ml) for 24 h
using a polymer-based transfection reagent (Ultra293; GeneDireX,
Inc.). Transfection efficiency was evaluated by RT-qPCR and
luciferase activity assay as previously described (24). At 24 h post-transfection,
transfected cells were incubated with BITC (20 µM) for 24 h.
Cells were harvested and lysed by radioimmunoprecipitation assay
lysis buffer (Sigma-Aldrich; Merck KGaA). Total proteins from
BITC-treated cells transfected with pSM-99a-5p, pmiR-GLO-99a-5p-PTS
or control vectors were collected from the lysate and subjected to
the detection of insulin-like growth factor 1 receptor (1GF1R),
fibroblast growth factor receptor 3 (FGFR3) and mTOR by western
blot as described previously (10).
Cell viability assays and detection of
apoptosis
Cell viability was determined in bladder cancer
cells transfected with pSM-99a-5p, pmiR-GLO-99a-5p-PTS or control
vectors treated with BITC (20 µM) for 24 h using WST-1
reagent (Roche Diagnostics GmbH, Mannheim, Germany) as previously
described (25). The induction of
apoptosis in BITC-treated cells was determined by assessment of
cleaved (c-) poly ADP-ribose polymerase (PARP) and c-caspase-3 by
western blotting.
Western blot analysis
Protein levels of cells treated with 10 or 20
µM BITC for 24 h were examined using western blot analysis
as described for the immunoblotting for IGF1R, FGFR3 and mTOR3.
Antibodies against IGF1R (ab39675), FGFR3 (ab133644), mTOR
(ab87540) were purchased from Abcam (Cambridge, UK). c-PARP
(#9532), c-caspase-3 (#9661) and β-actin (#4967) antibodies were
purchased from Cell Signaling Technology (Danvers, MA, USA).
Resolved proteins were transferred to polyvinyl difluoride
membranes. Blots were blocked with 5% nonfat milk for 1 h at room
temperature followed by incubation with primary antibodies
(1:1,000) for 1 h at room temperature. Following washes with TBST
(3×), blots were incubated with horseradish peroxidase-conjugated
goat anti-rabbit (1:1,000; cat. no. GTX213110-01) or anti-mouse
secondary antibody (1:1,000; cat. no. GTX213111-01) (both from
GeneTex, Inc., Irvine, CA, USA) for 1 h at room temperature
followed by TBST washes (3×). Blots were visualized using an
enhanced chemiluminescence detection system (Amersham; GE
Healthcare, Chicago, IL, USA) according to the manufacturer’s
instruction. Densitometry was performed using ImageJ software 1.49v
(National Institutes of Health, Bethesda, MD, USA). β-actin was
used as internal control. Results are expressed as the mean ±
standard deviation (SD) of three independent experiments.
Statistical analysis
All experiments were performed ≥3 times, each in
triplicate and data are presented as the mean ± SD. Statistical
analysis between two samples were performed using Student’s t-test.
Multiple group comparisons were performed using one-way analysis of
variance with Bonferroni’s post-hoc tests. P<0.05 was considered
to indicate a statistically significant difference.
Results
BITC upregulates miR-99a-5p expression in
BC
Dysregulation of miRNA has been reported in BC
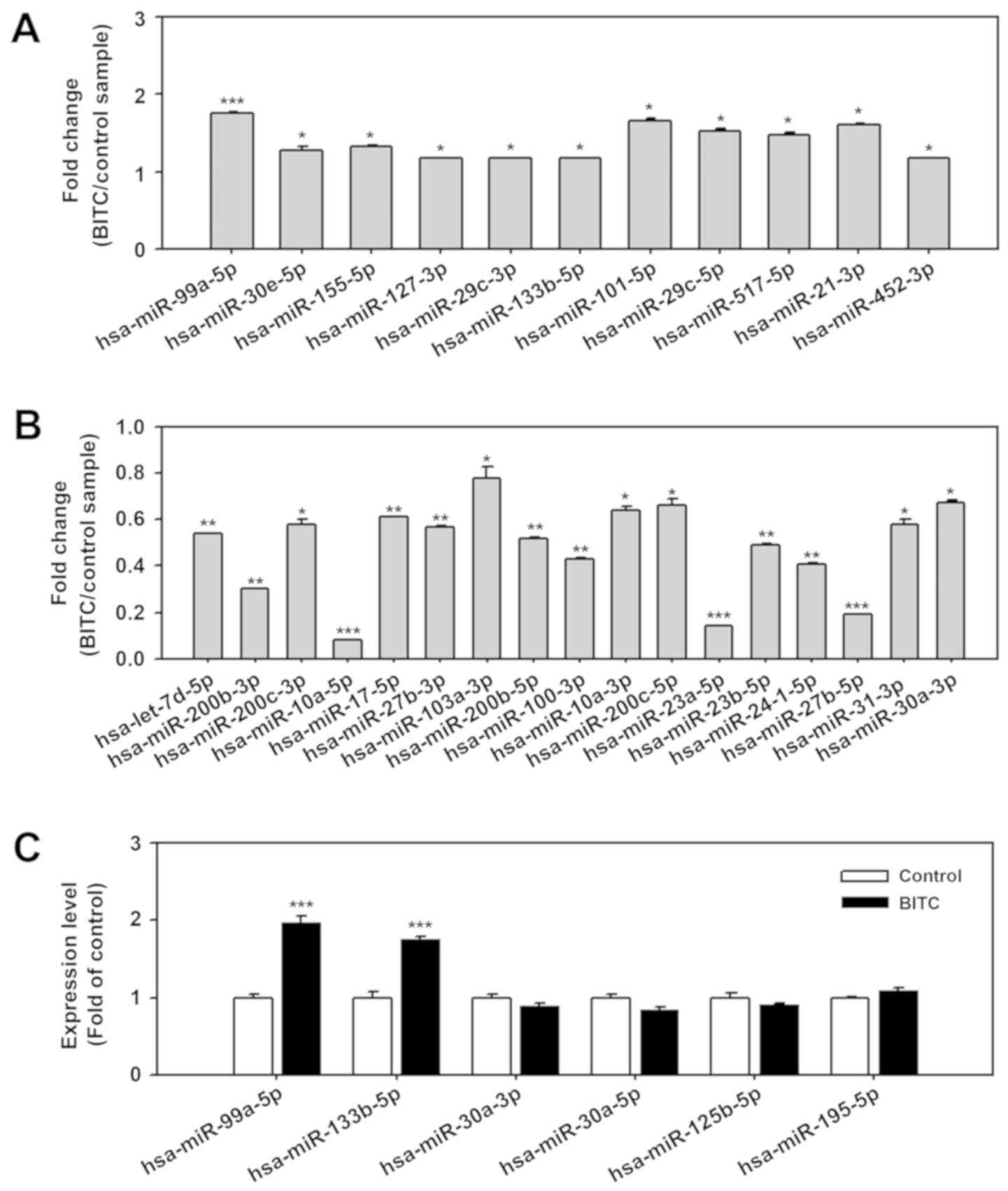
tissues, with 19 up- and 11 downregulated miRNAs (14). To investigate the effect on BITC
treatment on miRNA expression in BC cells, expression profiles were
determined using a miRNA qPCR array containing 79 aberrantly
expressed miRNAs in BC. The results suggested that 11 miRNAs were
significantly upregulated in BITC-treated BC cells compared with
untreated control cells, including miR-30e-5p, miR-155-5p,
miR-127-3p, miR-29c-3p, miR-133b-5p, miR-101-5p, miR-29c-5p,
miR-517-5p, miR-21-3p, miR-452-3p (all P<0.05) and miR-99a-5p
(P<0.001; Fig. 1A). A total of
17 miRNAs were significantly downregulated in BITC-treated 5637
cells compared with untreated control cells including miR-200c-3p,
miR-103a-3p, miR-10a-3p, miR-200c-5p, miR-31-3p, miR-30a-3p (all
P<0.05), let-7d-5p, miR-200b-3p, miR-17-5p, miR-27b-3p,
miR-200b-5p, miR-100-3p, miR-23b-5p, miR-24-1-5p (all P<0.01),
miR-10a-5p, miR-23a-5p and miR-27b-5p (all P<0.001; Fig. 1B). BITC has been proposed to induce
autophagy in breast cancer and prostate cancer cells (9,10).
To explore the correlation between dysregulated miRNAs and
autophagy regulation, miRNAs reported to regulate the autophagy
pathway, including miR-99a-5p (24), miR-133b-5p (26), miR-30a-3p (27), miR-30a-5p (28), miR-125b-5p (29) and miR-195-5p (30) were further validated by miRNA
stem-loop RT-qPCR. Following validation by stem-loop RT-qPCR, data
confirmed that miR-99a-5p and miR-133b-5p expression was
significantly upregulated in 5637 cells treated with BITC compared
with the normal control (P<0.001; Fig. 1C). However, expression of
miR-30a-3p, miR-30a-5p, miR-125b-5p and miR-195-5p were not
significantly affected by exposure to BITC.
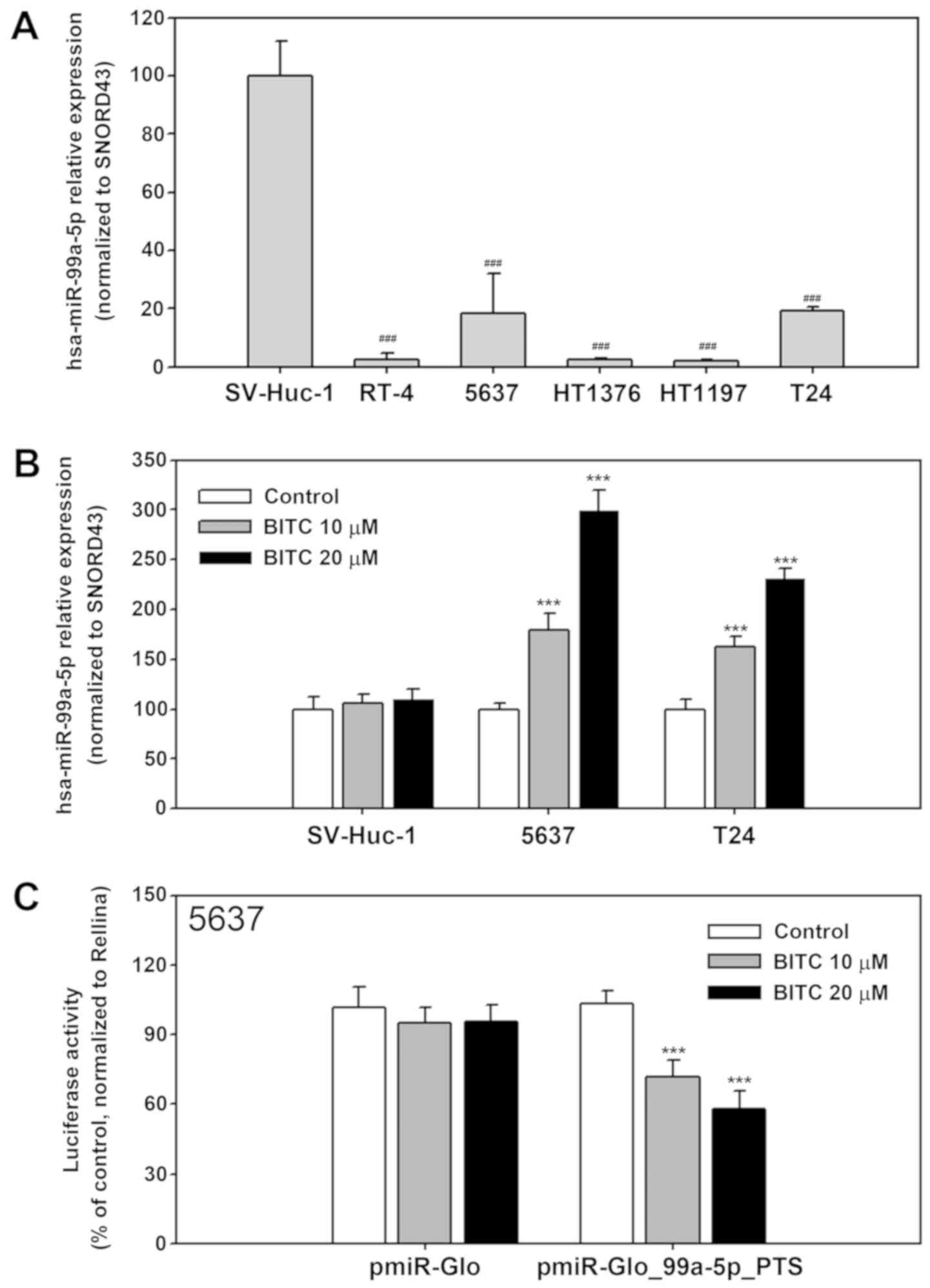
To confirm that BITC induced miR-99a-5p expression,
miR-99a-5p expression in untreated SV-Huc-1, RT4, 5637, HT1376,
HT1197 and T24 cells was determined using stem-loop miRNA RT-qPCR.
As presented in Fig. 2A,
miR-99a-5p expression was significantly downregulated in all BC
cells compared with the SV-Huc-1 normal cells (P<0.001). 5637
and T24 cells were used in following based on higher transfection
efficiencies compared with the other cell lines. BITC treatment
increased miR-99a-5p expression in 5637 and T24 cells compared with
the untreated cells (P<0.001); no significant changes were
observed for SV-Huc-1 cells (P>0.05; Fig. 2B). To further evaluate the effect
of BITC treatment on miR-99a-5p expression, luciferase assays were
performed using pmiR-Glo-99a-5p-PTS, which is able to bind
miR-99a-5p. The reporter construct was transfected into 5637 cells
and cells were treated with BITC for 24 h post transfection. The
results demonstrated a significant dose-dependent decrease in
luciferase activity upon BITC treatment compared with the untreated
control, indicating miR-99a-5p upregulation in BITC-treated cells
(P<0.001; Fig. 2C).
miR-99a-5p overexpression and BITC
treatment decrease IGF1R, FGFR3 and mTOR expression in BC
cells
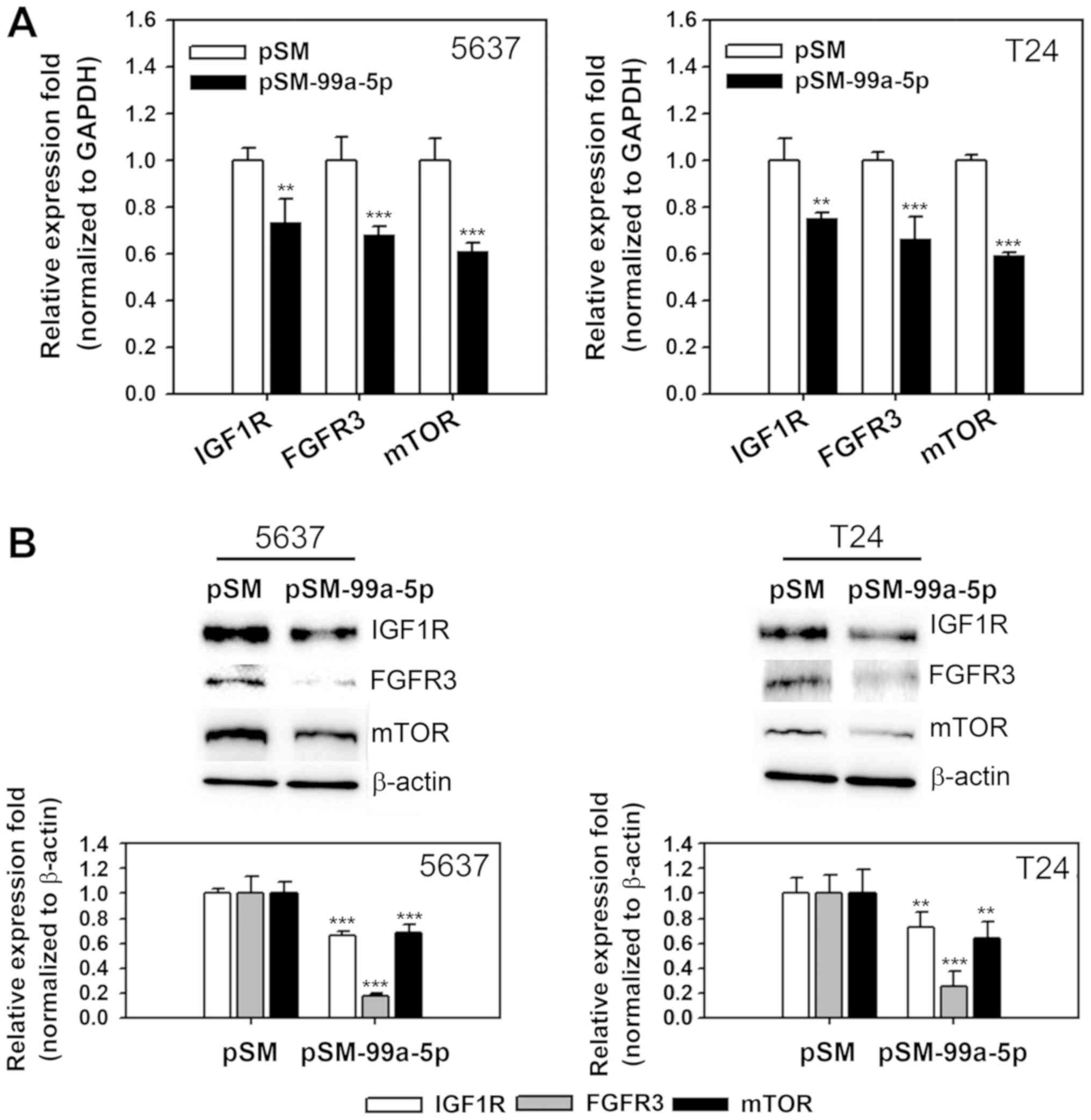
Changes in the expression of target genes of
miR-99a-5p were evaluated following miR-9a-5p overexpression and
BITC treatment of BC cells. IGF1R, mTOR and FGFR3 expression was
determined in 5637 and T24 cells transfected with a miR-99a-5p
overexpressing vector. IGF1R, mTOR and FGFR3 mRNA was significantly
decreased in pSM-99a-5p transfected 5637 and T24 cells compared
with the empty vector control (P<0.01, P<0.001 and
P<0.001, respectively; Fig.
3A). Protein expression levels were also significantly
decreased in the miR-99a-5p overexpression samples compared with
the control (P<0.001 for 5637 cells and P<0.01, P<0.001
and P<0.01 for IGF1R, mTOR and FGFR3 in T24, respectively;
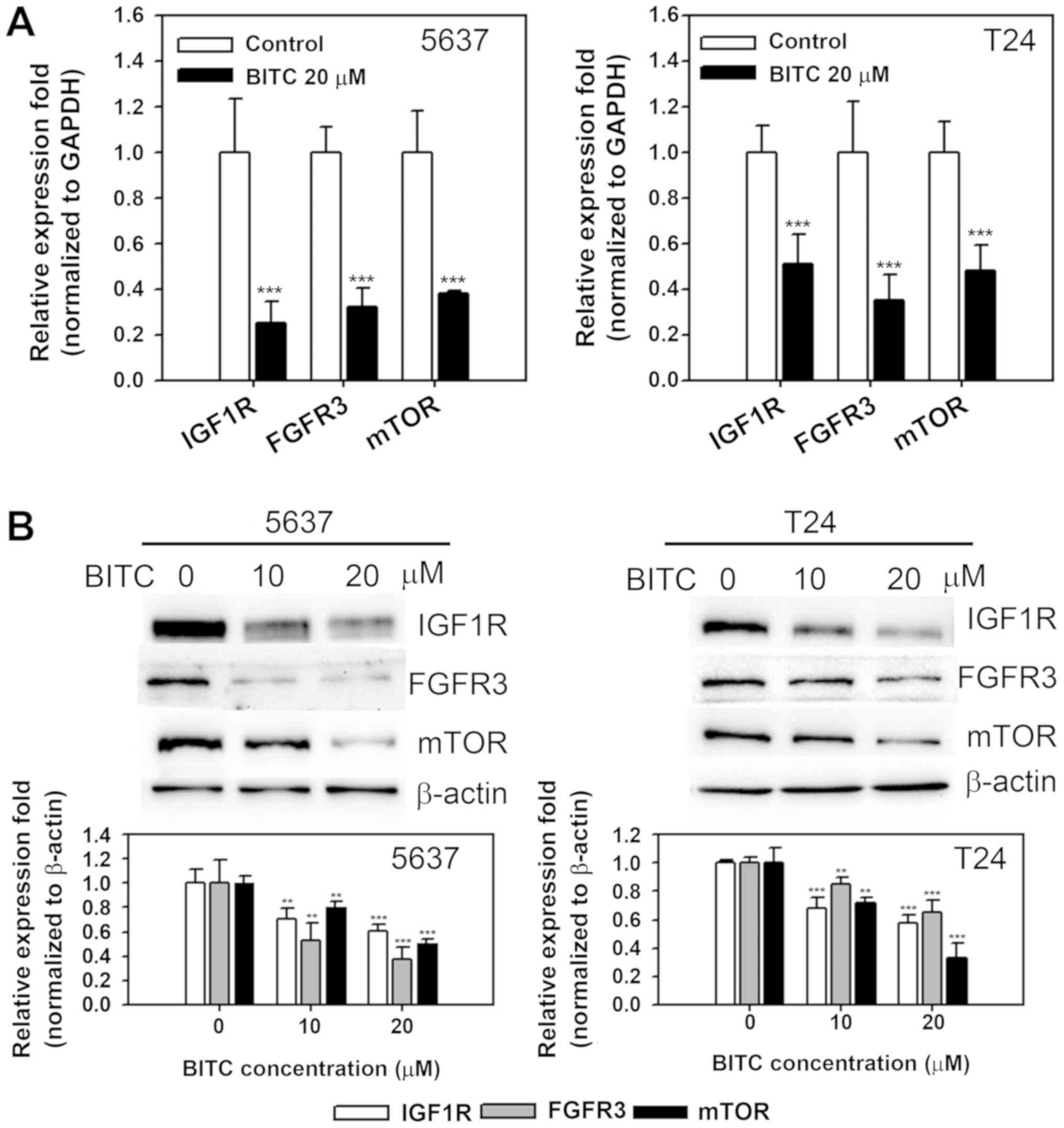
Fig. 3B). To investigate the
expression of IGF1R, mTOR and FGFR3 in BITC-treated cells, mRNA and
protein expression was determined in 5637 and T24 cells treated
with BITC. As presented in Fig.
4A, BITC treatment (20 µM) resulted in decreased mRNA
expression of IGF1R, mTOR and FGFR3 compared with the untreated
control (P<0.001 for 5637 and P<0.001, P<0.01 and
P<0.001 for IGF1R, mTOR, and FGFR3 in T24, respectively).
Protein expression was significantly decreased in BITC-treated
cells in a dose-dependent manner compared with the untreated
control (P<0.01 and P<0.001 for 10 and 20 µM BITC,
respectively; Fig. 4B). The
results indicated that miR-99a-5p overexpression and BITC treatment
inhibited the expression of IGF1R, mTOR and FGFR3 prosurvival
proteins in BC cells.
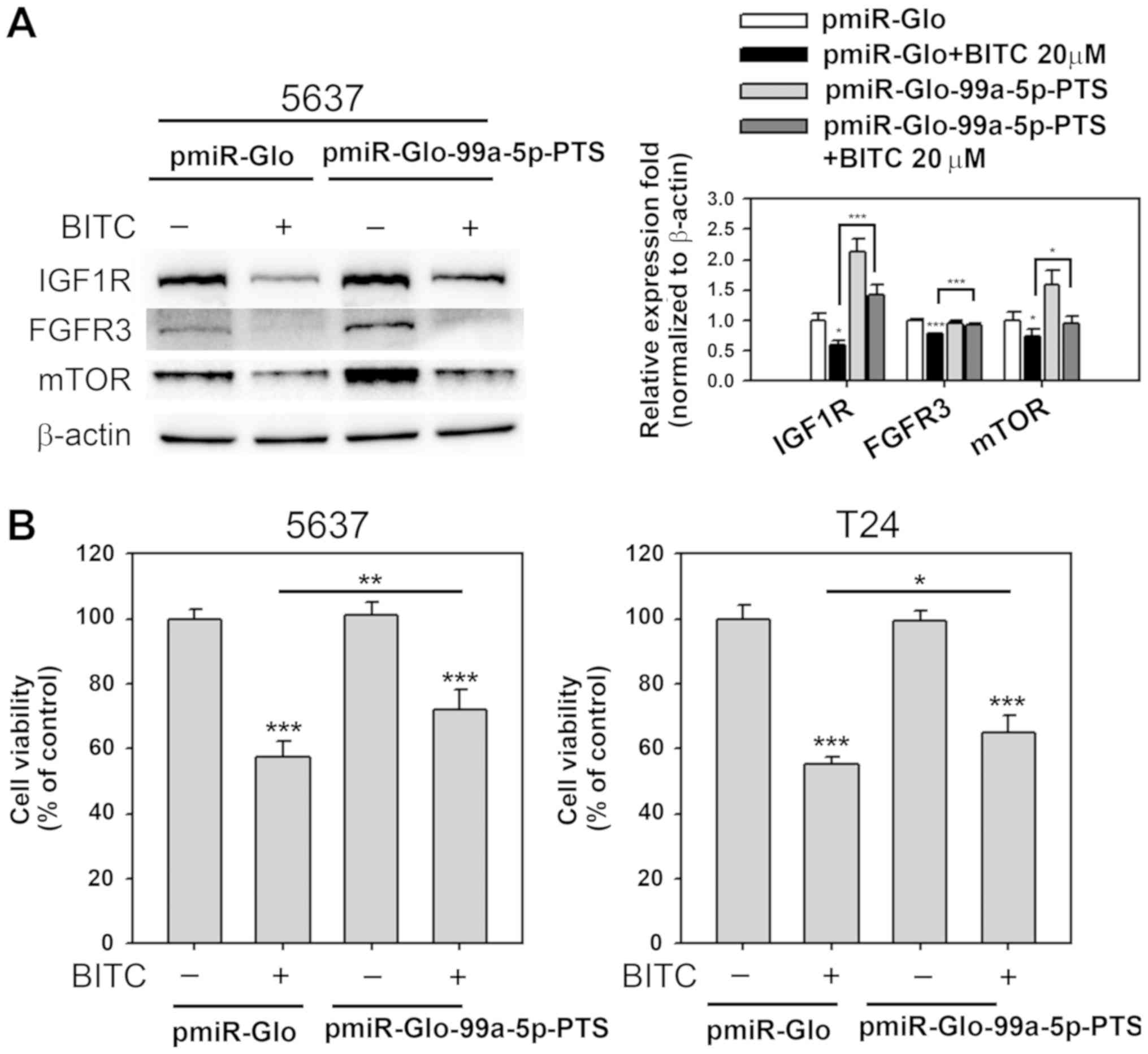
miR-99a-5p inhibition attenuates
BITC-induced IGF1R, FGFR3 and mTOR downregulation and decreased
cell viability
A previous study by the authors focused on
pmiR-Glo-99a-5p-PTS, which expressed antisense miR-99a-5p and
exhibited inhibitory effects on miR-99a-5p function (24). To confirm that IGF1R, mTOR and
FGFR3 inhibition was mediated by miR-99a-5p upregulation through
BITC treatment, experiments using pmiR-Glo-99a-5p-PTS acting as
competitors to BITC-induced miR-99a-5p expression were performed.
IGF1R, mTOR and FGFR3 expression was detected in transfected cells
that received 24 h BITC treatment (20 µM). As presented in
Fig. 5A, overexpression of
miR-99a-5p induced IGF1R and mTOR expression in 5637 cell
(P<0.001 and P<0.01, respectively). Furthermore, IGF1R, mTOR
and FGFR3 protein expression downregulation in BITC-treated cells
was significantly reversed by antisense miR-99a-5p expressing,
BITC-treated cells (P<0.001, P<0.05 and P<0.001,
respectively). Effects of miR-99a-5p inhibition on the viability of
BITC-treated cells were further evaluated. Cell viability was
significantly decreased in BITC-treated cells compared with the
untreated controls (P<0.001; Fig.
5B). Inhibition of miR-99a-5p significantly reversed the
BITC-induced viability decrease in 5637 and T24 cells (P<0.01
and P<0.05, respectively; Fig.
5B). The results suggested that BITC treatment suppressed
IGF1R, mTOR and FGFR3 expression by upregulating miR-99a-5p levels.
However, there may be further effectors, in addition to miR-99a-5p,
that contributed to BITC-induced cytotoxicity in BC cells.
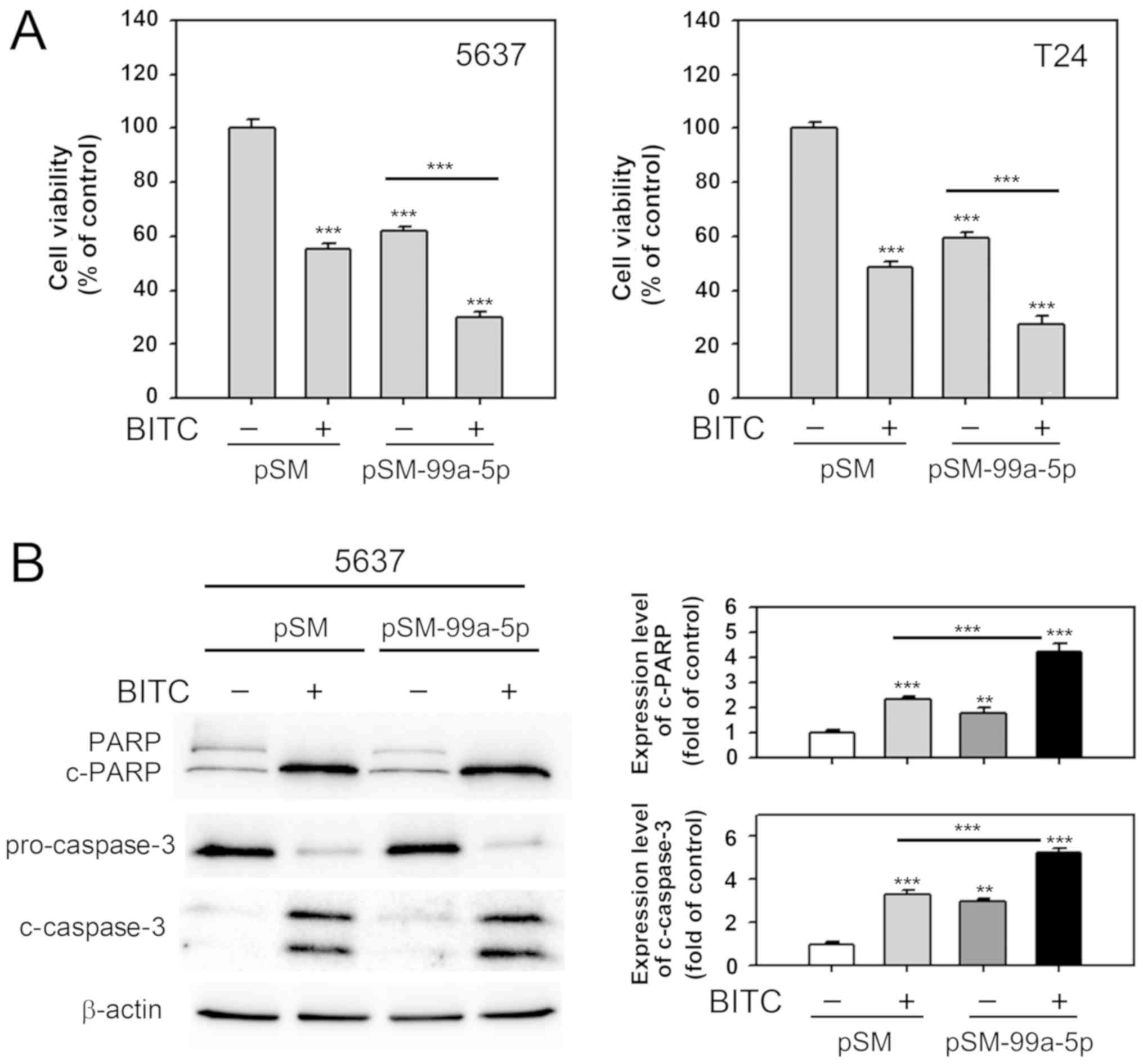
Effects of miR-99a-5p overexpression are
enhanced by BITC treatment in BC cells
It was evaluated if combination of miR-99a-5p
overexpression and BITC treatment enhanced cell death in BC cells
compared with the single treatments. As presented in Fig. 6A, BITC treatment (10 µM) and
miR-99a-5p overexpression alone significantly decreased cell
viability in 5637 and T24 cells compared with the untreated cells
(P<0.001). Cell viability was further significantly decreased
when combining the two treatments in 6537 and T24 cells compared
with miR-99a-5p overexpression alone (P<0.001). Apoptosis
induction was evaluated by determining the cleavage of PARP and
caspase-3. Cleaved protein levels were significantly increased
miR-99a-5p overexpressing or BITC-treated cells compared with the
untreated cells (P<0.01 and P<0.001, respectively; Fig. 6B). The combination of miR-99a-5p
overexpression and BITC treatment further significantly increased
the c-PARP and c-caspase-3 levels compared with the BITC treatment
group (P<0.001). The results suggested that miR-99a-5p
overexpression enhances the effects of BITC in BC cells.
Discussion
BC is one of the leading causes of cancer-associated
mortality worldwide (31). Novel
therapeutic approaches preventing the recurrence or improving the
survival of patients with BC are desirable. In the current study,
it was described that BITC inhibited IGF1R, mTOR and FGFR3
expression through upregulation of the tumor suppressing
miR-99a-5p. The results further demonstrated that elevation of
miR-99a-5p levels enhanced BITC-induced cytotoxicity in BC cells.
Increasing evidence has highlighted anti-cancer activities of BITC
in a variety of tumor cell lines and in rodent animal models
(32). BITC is suggested to
inhibit growth, induce apoptosis and G2/M phase cell cycle arrest
in BC cells (33). The current
study suggested that BITC decreased 5637 and T24 cell viability by
induction of apoptosis.
miRNAs are important regulatory components in
tumorigenesis and several miRNAs are considered as therapeutic
targets in BC (34). Tumor
suppressing activities of miR-99a-5p have been investigated in
various types of cancer; miR-99a-5p exerts anti-metastasis
abilities in human non-small cell lung cancer cells by inhibiting
protein kinase B1 and in oral cancer by inhibiting
myotubularin-related protein 3 expression (35,36).
In mammary gland cells miR-99a-5p modulates transforming growth
factor-β induced epithelial to mesenchymal plasticity (37). mTOR targeting and inhibition of
cell proliferation or induction of apoptosis have been demonstrated
in anaplastic thyroid (15),
breast (16) and cervical cancer
(38). miR-99a-5p controls IGF1R
and mTOR expression in human hepatocellular carcinoma (39-41)
and has been reported to be downregulated in human BC, leading to
the upregulation of FGFR3 (14,42).
IGF1R, FGFR3 and mTOR are known anti-apoptotic regulators (39-41).
The results of the current study indicated antitumor effects of
miR-99a-5p by inducing apoptosis in BC. A previous study reported
that miR-99a-5p is downregulated in bladder urothelial carcinoma
tissue compared with normal tissue (14). Furthermore, expression levels and
prognostic roles of IGF-1R, mTOR and FGFR3 have previously been
reported for BC (43-45). The small number of patients with BC
that participated in the current study describes a limitation and
IGF1R, mTOR and FGFR3 expression will be investigated further in
future experiments.
miR-99a-5p exhibits anticancer activity in various
cancer types and BITC has been reported to induce apoptosis in BC
cells (46). The current study
demonstrated that BITC induced miR-99a-5p expression in BC cells
but not normal human urothelial cells. Furthermore, it was
suggested that miR-99a-5p may be involved in the regulation of
IGF1R, mTOR and FGFR3 in BITC-treated BC cells. A previous review
has reported the application of a miRNA sponge, containing multiple
miRNA binding sites, in miRNA inhibition (21,22).
The results of the current study confirmed that overexpression of
miR-99a-5p sponge reversed IGF1R, mTOR and FGFR3 protein expression
downregulation in BITC-treated cells. A previous review has
addressed the antitumor mechanisms exerted by various ITCs
(47). The report proposed that
production of reactive oxygen species (ROS) is the common link of
ITCs in apoptosis induction. In addition, normal cells exhibit
increasing resistance to ROS production and apoptosis induced by
ITCs, suggesting that the induction of miR-99a-5p by BITC treatment
in BC cells may contribute to ROS production. These suggestions
require to be verified in future experiments.
Various miRNAs that promote cancer cell death are
recognized as potential novel anti-cancer agents in various
cancers, including BC (34).
miR-34a is downregulated during cancer progression and considered a
novel target for treating various types of cancer (48). miRNAs rapidly degrade in
circulating blood, making an oral or intravenous administration
ideal for delivery (49). Bladder
instillation had been routinely performed in clinic using
chemotherapeutic agents, including BCG or mitomycin C to prevent
recurrence of BC (50).
Intravesical therapy by delivery of small non-coding RNA is an
alternative approach to overcome drug delivery system problems and
successfully deliver siRNAs in vivo (51). Therapeutic effects of miR-582-5p
and -3p (52) and miR-145
(53) administered intravesically
in a mouse orthotopic model suggest promising effects against BC.
N-acetylcysteine (NAC)-conjugated BITC is the major metabolite
detected in urine collected from human donors in different studies
(54). It remains to be
investigated whether NAC-BITC has the ability to induce miR-99a-5p
expression. The present study focused on the dysregulation of
miRNAs following BITC treatment in BC. In initial experiments,
miR-99a-5p demonstrated the strongest response to BITC treatment
and was selected for further investigation of its role in BC
progression. It was demonstrated that ectopic miR-99a-5p expression
in combination with BITC treatment decreased cell viability of BC
cells compared with either single treatment. The current study
suggested that miR-99a-5p may be a novel anticancer agent, alone or
combined with other chemotherapeutic agents, and has the potential
to inspire future experiments in a preclinical setting.
In this study, it was demonstrated for the first
time that BITC treatment inhibited expression of prosurvival
proteins IGF1R, mTOR and FGFR3 by upregulation of miR-99a-5p in
human BC cells. miR-99a-5p overexpression potentiated the
cytotoxicity of BITC in BC cells. Orthotopic animal models using
in vivo imaging system detection have been widely applied in
BC studies (55) and miRNA
replacement therapy provides strong preclinical evidence for
miRNA-based treatment of cancer (56,57).
These preclinical results may shape future experiments studying the
effects of miR-99a-5p in BC treatment.
Funding
This study was supported by Ministry of Science and
Technology, Taiwan (grant no. NSC102-2314-B-341-003-MY3) and Shin
Kong Wu Ho-Su Memorial Hospital (grant nos. SKH-8302-103-DR-13,
SKH-8302-103-NDR-06, SKH-8302-104-0201 and SKH-8302-104-0202).
Availability of data and material
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors’ contributions
JFL and TFT conceived and performed the experiments,
data interpretation and writing of the manuscript. JFL and TIH
designed the study. YCL, HEC and KYC provided the study materials
and participate in the interpretation of the experiment data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
BITC
|
benzyl isothiocyanate
|
|
BC
|
bladder cancer
|
|
miRNAs
|
microRNAs
|
|
IGF1R
|
insulin-like growth factor 1
receptor
|
|
mTOR
|
mammalian target of rapamycin
|
|
FGFR3
|
fibroblast growth factor receptor
3
|
Acknowledgments
Not applicable.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chavan S, Bray F, Lortet-Tieulent J,
Goodman M and Jemal A: International variations in bladder cancer
incidence and mortality. Eur Urol. 66:59–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gudjónsson S, Adell L, Merdasa F, Olsson
R, Larsson B, Davidsson T, Richthoff J, Hagberg G, Grabe M, Bendahl
PO, et al: Should all patients with non-muscle-invasive bladder
cancer receive early intravesical chemotherapy after transurethral
resection? The results of a prospective randomised multicentre
study. Eur Urol. 55:773–780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Metts MC, Metts JC, Milito SJ and Thomas
CR Jr: Bladder cancer: A review of diagnosis and management. J Natl
Med Assoc. 92:285–294. 2000.PubMed/NCBI
|
|
5
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brausi M, Witjes JA, Lamm D, Persad R,
Palou J, Colombel M, Buckley R, Soloway M, Akaza H and Böhle A: A
review of current guidelines and best practice recommendations for
the management of nonmuscle invasive bladder cancer by the
International Bladder Cancer Group. J Urol. 186:2158–2167. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brausi M, Oddens J, Sylvester R, Bono A,
van de Beek C, van Andel G, Gontero P, Turkeri L, Marreaud S,
Collette S, et al: Side effects of Bacillus Calmette-Guérin (BCG)
in the treatment of intermediate- and high-risk Ta, T1 papillary
carcinoma of the bladder: Results of the EORTC genito-urinary
cancers group randomised phase 3 study comparing one-third dose
with full dose and 1 year with 3 years of maintenance BCG. Eur
Urol. 65:69–76. 2014. View Article : Google Scholar
|
|
8
|
Smith TJ: Mechanisms of carcinogenesis
inhibition by isothiocyanates. Expert Opin Investig Drugs.
10:2167–2174. 2001. View Article : Google Scholar
|
|
9
|
Xiao D, Bommareddy A, Kim SH, Sehrawat A,
Hahm ER and Singh SV: Benzyl isothiocyanate causes FoxO1-mediated
autophagic death in human breast cancer cells. PLoS One.
7:e325972012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin JF, Tsai TF, Liao PC, Lin YH, Lin YC,
Chen HE, Chou KY and Hwang TI: Benzyl isothiocyanate induces
protective autophagy in human prostate cancer cells via inhibition
of mTOR signaling. Carcinogenesis. 34:406–414. 2013. View Article : Google Scholar
|
|
11
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iorio MV and Croce CM: Causes and
consequences of microRNA dysregulation. Cancer J. 18:215–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phuah NH and Nagoor NH: Regulation of
microRNAs by natural agents: New strategies in cancer therapies.
BioMed Res Int. 2014:8045102014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai TF, Lin YC, Chen HE, Chou KY, Lin JF
and Hwang TI: Involvement of the insulin-like growth factor I
receptor and its downstream antiapoptotic signaling pathway is
revealed by dysreg-ulated microRNAs in bladder carcinoma. Urol Sci.
25:58–64. 2014. View Article : Google Scholar
|
|
15
|
Huang HG, Luo X, Wu S and Jian B: MiR-99a
inhibits cell proliferation and tumorigenesis through targeting
mTOR in human anaplastic thyroid cancer. Asian Pac J Cancer Prev.
16:4937–4944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Z, Han Y, Cheng K, Zhang G and Wang
X: miR-99a directly targets the mTOR signalling pathway in breast
cancer side population cells. Cell Prolif. 47:587–595. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin JF, Lin YC, Lin YH, Tsai TF, Chou KY,
Chen HE and Hwang TI: Zoledronic acid induces autophagic cell death
in human prostate cancer cells. J Urol. 185:1490–1496. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Varkonyi-Gasic E, Wu R, Wood M, Walton EF
and Hellens RP: Protocol: A highly sensitive RT-PCR method for
detection and quantification of microRNAs. Plant Methods. 3:122007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CJ, Gong HY, Tseng HC, Wang WL and Wu
JL: miR-122 targets an anti-apoptotic gene, Bcl-w, in human
hepatocellular carcinoma cell lines. Biochem Biophys Res Commun.
375:315–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebert MS, Neilson JR and Sharp PA:
MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian
cells. Nat Methods. 4:721–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ebert MS and Sharp PA: MicroRNA sponges:
Progress and possibilities. RNA. 16:2043–2050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng L, Yang SB, Xu FF and Zhang JH: Long
noncoding RNA CCAT1 promotes hepatocellular carcinoma progression
by functioning as let-7 sponge. J Exp Clin Cancer Res. 34:182015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsai TF, Lin JF, Chou KY, Lin YC, Chen HE
and Hwang TI: miR-99a-5p acts as tumor suppressor via targeting to
mTOR and enhances RAD001-induced apoptosis in human urinary bladder
urothelial carcinoma cells. OncoTargets Ther. 11:239–252. 2018.
View Article : Google Scholar
|
|
25
|
Lin YC, Lin JF, Wen SI, Yang SC, Tsai TF,
Chen HE, Chou KY and Hwang TI: Inhibition of high basal level of
autophagy induces apoptosis in human bladder cancer cells. J Urol.
195:1126–1135. 2016. View Article : Google Scholar
|
|
26
|
Sugiyama T, Taniguchi K, Matsuhashi N,
Tajirika T, Futamura M, Takai T, Akao Y and Yoshida K: MiR-133b
inhibits growth of human gastric cancer cells by silencing pyruvate
kinase muscle-splicer polypyrimidine tract-binding protein 1.
Cancer Sci. 107:1767–1775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Cheng R and Huang Y: MiR-30a
inhibits BECN1-mediated autophagy in diabetic cataract. Oncotarget.
8:77360–77368. 2017.PubMed/NCBI
|
|
28
|
Fu XT, Shi YH, Zhou J, Peng YF, Liu WR,
Shi GM, Gao Q, Wang XY, Song K, Fan J, et al: MicroRNA-30a
suppresses autophagy-mediated anoikis resistance and metastasis in
hepatocellular carcinoma. Cancer Lett. 412:108–117. 2018.
View Article : Google Scholar
|
|
29
|
Wang S, Wu J, Ren J, Vlantis AC, Li MY,
Liu SY, Ng EK, Chan AB, Luo DC, Liu Z, et al: MicroRNA-125b
Interacts with Foxp3 to Induce Autophagy in Thyroid Cancer. Mol
Ther. 26:2295–2303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z,
Ding J, Jia L and Yuan W: Increased miR-195 aggravates neuropathic
pain by inhibiting autophagy following peripheral nerve injury.
Glia. 61:504–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao CV: Benzyl isothiocyanate: Double
trouble for breast cancer cells. Cancer Prev Res (Phila).
6:760–763. 2013. View Article : Google Scholar
|
|
33
|
Tang L and Zhang Y: Dietary
isothiocyanates inhibit the growth of human bladder carcinoma
cells. J Nutr. 134:2004–2010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Catto JW, Alcaraz A, Bjartell AS, De Vere
White R, Evans CP, Fussel S, Hamdy FC, Kallioniemi O, Mengual L,
Schlomm T, et al: MicroRNA in prostate, bladder, and kidney cancer:
A systematic review. Eur Urol. 59:671–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu SH, Zhang CL, Dong FS and Zhang YM:
miR-99a suppresses the metastasis of human non-small cell lung
cancer cells by targeting AKT1 signaling pathway. J Cell Biochem.
116:268–276. 2015. View Article : Google Scholar
|
|
36
|
Kuo YZ, Tai YH, Lo HI, Chen YL, Cheng HC,
Fang WY, Lin SH, Yang CL, Tsai ST and Wu LW: MiR-99a exerts
anti-metastasis through inhibiting myotubularin-related protein 3
expression in oral cancer. Oral Dis. 20:e65–e75. 2014. View Article : Google Scholar
|
|
37
|
Turcatel G, Rubin N, El-Hashash A and
Warburton D: MIR-99a and MIR-99b modulate TGF-β induced epithelial
to mesenchymal plasticity in normal murine mammary gland cells.
PLoS One. 7:e310322012. View Article : Google Scholar
|
|
38
|
Wang L, Chang L, Li Z, Gao Q, Cai D, Tian
Y, Zeng L and Li M: miR-99a and -99b inhibit cervical cancer cell
proliferation and invasion by targeting mTOR signaling pathway. Med
Oncol. 31:9342014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peruzzi F, Prisco M, Dews M, Salomoni P,
Grassilli E, Romano G, Calabretta B and Baserga R: Multiple
signaling pathways of the insulin-like growth factor 1 receptor in
protection from apoptosis. Mol Cell Biol. 19:7203–7215. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Plowright EE, Li Z, Bergsagel PL, Chesi M,
Barber DL, Branch DR, Hawley RG and Stewart AK: Ectopic expression
of fibroblast growth factor receptor 3 promotes myeloma cell
proliferation and prevents apoptosis. Blood. 95:992–998.
2000.PubMed/NCBI
|
|
41
|
Castedo M, Ferri KF and Kroemer G:
Mammalian target of rapamycin (mTOR): Pro- and anti-apoptotic. Cell
Death Differ. 9:99–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Catto JW, Miah S, Owen HC, Bryant H, Myers
K, Dudziec E, Larré S, Milo M, Rehman I, Rosario DJ, et al:
Distinct microRNA alterations characterize high- and low-grade
bladder cancer. Cancer Res. 69:8472–8481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Li Q, Niu X, Wang G, Zheng S, Fu G
and Wang Z: miR-143 inhibits bladder cancer cell proliferation and
enhances their sensitivity to gemcitabine by repressing IGF-1R
signaling. Oncol Lett. 13:435–440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park SJ, Lee TJ and Chang IH: Role of the
mTOR pathway in the progression and recurrence of bladder cancer:
An immunohistochemical tissue microarray study. Korean J Urol.
52:466–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hammam O, Aboushousha T, El-Hindawi A,
Khairy H, Khalil H, Kamel A, Akl M, Abdel-Hady A, Magdy M, Badawy
M, et al: Expression of FGFR3 protein and gene amplification in
urinary bladder lesions in relation to schistosomiasis. Open Access
Maced J Med Sci. 5:160–166. 2017.PubMed/NCBI
|
|
46
|
Tang L and Zhang Y: Mitochondria are the
primary target in isothiocyanate-induced apoptosis in human bladder
cancer cells. Mol Cancer Ther. 4:1250–1259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh SV and Singh K: Cancer
chemoprevention with dietary isothiocyanates mature for clinical
translational research. Carcinogenesis. 33:1833–1842. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bader AG: miR-34 - a microRNA replacement
therapy is headed to the clinic. Front Genet. 3:1202012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Wang Z and Gemeinhart RA:
Progress in microRNA delivery. J Control Release. 172:962–974.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang SJ, Ye LY and Meng FH: Comparison of
intravesical bacillus Calmette-Guerin and mitomycin C
administration for non-muscle invasive bladder cancer: A
meta-analysis and systematic review. Oncol Lett. 11:2751–2756.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nogawa M, Yuasa T, Kimura S, Tanaka M,
Kuroda J, Sato K, Yokota A, Segawa H, Toda Y, Kageyama S, et al:
Intravesical administration of small interfering RNA targeting
PLK-1 successfully prevents the growth of bladder cancer. J Clin
Invest. 115:978–985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Uchino K, Takeshita F, Takahashi RU,
Kosaka N, Fujiwara K, Naruoka H, Sonoke S, Yano J, Sasaki H, Nozawa
S, et al: Therapeutic effects of microRNA-582-5p and -3p on the
inhibition of bladder cancer progression. Mol Ther. 21:610–619.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Inamoto T, Taniguchi K, Takahara K,
Iwatsuki A, Takai T, Komura K, Yoshikawa Y, Uchimoto T, Saito K,
Tanda N, et al: Intravesical administration of exogenous
microRNA-145 as a therapy for mouse orthotopic human bladder cancer
xenograft. Oncotarget. 6:21628–21635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lamy E, Scholtes C, Herz C and
Mersch-Sundermann V: Pharmacokinetics and pharmacodynamics of
isothiocyanates. Drug Metab Rev. 43:387–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hadaschik BA, Black PC, Sea JC, Metwalli
AR, Fazli L, Dinney CP, Gleave ME and So AI: A validated mouse
model for orthotopic bladder cancer using transurethral tumour
inoculation and bioluminescence imaging. BJU Int. 100:1377–1384.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kota J, Chivukula RR, O’Donnell KA,
Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P,
Torbenson M, Clark KR, et al: Therapeutic microRNA delivery
suppresses tumorigenesis in a murine liver cancer model. Cell.
137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Toffanin S, Villanueva A and Llovet JM:
miRNA delivery: Emerging therapy for hepatocellular carcinoma.
Gastroenterology. 138:1202–1204. 2010. View Article : Google Scholar : PubMed/NCBI
|