Introduction
In Japan, urothelial carcinoma of the bladder (UCB)
is the eighth most common malignancy in men (1). Worldwide, UCB is the second most
frequent malignancy of the urogenital tract and the fourth most
common cancer among men (2). In
total, ~20-40% of cases of UCB present as or develop to muscle
invasive bladder cancer (MIBC) (3). Radical cystectomy is the gold
standard treatment for MIBC and the administration of neoadjuvant
or adjuvant cisplatin-based chemotherapy has become common due to
improvements of prognosis for patients treated by this method
(4-6). Cisplatin-based chemotherapy is also a
first-line therapy for patients with advanced UCB, including
locally advanced and metastatic disease (7). Chemotherapy-induced myelosuppression
is often a problem for patients treated with chemotherapy,
resulting in febrile neutropenia (FN) and sometimes leading to
infection-associated mortality. The aim of clinical management for
advanced UCB is to continue chemotherapy safely while controlling
adverse effects like FN, resulting in prolonged survival time.
The administration of granulocyte colony-stimulating
factor (G-CSF) is recommended for patients who are treated with
strong chemotherapies to reduce the rate of chemotherapy-induced
mortality (8,9). In addition, the administration of
G-CSF can result in a shorter duration of grade IV neutropenia,
antibiotic treatment and hospital stay (10). The effects of administration of
granulocyte-macrophage CSF (GM-CSF) and macrophage CSF (M-CSF) on
chemotherapy-induced myelosuppression and FN have also studied. The
duration of neutrophil recovery and hospital stay was reduced by
GM-CSF administration, and the incidence rate and duration of FN
was reduced by the administration of M-CSF (11,12).
G-CSF, GM-CSF and M-CSF serve roles in regulating
the hematopoiesis of blood cells, modulating the functional
response and maintaining immune response (13). G-CSF and M-CSF serve roles in the
proliferation and differentiation of macrophage and neutrophils,
respectively, at stages of lineage commitment. In addition, GM-CSF
regulates the expansion and maturation of primitive hematopoietic
progenitors at earlier stages of lineage commitment, induces
activation status of macrophages and mediates differentiation to
other cells, including dendritic cells, that participate in immune
responses (13). G-CSF was
initially purified from the human bladder cancer cell line 5637 in
1985 and has been identified to serve a role in promoting the
growth of bladder cancer cells, which express the G-CSF receptor
(G-CSFR) (14,15). To the best of our knowledge, at
present, the association between CSFs and tumor progression remains
unclear. Although G-CSF is most frequently used for patients with
chemotherapy-induced myelosuppression and FN, G-CSF has also been
demonstrated to promote tumor growth, progression and metastasis
through the enhancement of tumor angiogenesis, proliferation and
migration, and functions in the maintenance of myeloid derived
suppressor cells (MDSCs), which promote cancer progression by
immune suppression, resulting in poor prognosis (16-18).
GM-CSF and M-CSF also affect cancer progression through the
modulation of anti-tumor immunity in the tumor microenvironment
(19-22). However, the associations between
CSFs and tumor progression in patients with UCB remain unclear and
changes in the molecular profile in the tumor microenvironment have
not been fully understood. The present study used xenograft tumors
generated by subcutaneously inoculating mice with human bladder
cancer cell lines, MGH-U3 (low-grade) and UM-UC-3 (high-grade)
(23). The aim of the present
study was to evaluate the pro- and anti-tumor effects of G-CSF,
GM-CSF and M-CSF, and the changes in the molecular profiles.
Materials and methods
Cell lines
Two human urothelial carcinoma cell lines, MGH-U3
and UM-UC-3, and two mouse urothelial carcinoma cell lines, MB49
and MBT2, were used in the current study. UM-UC-3 and MBT2 were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and the Japanese Collection of Research Bioresources Cell Bank
(Osaka, Japan), respectively. MGH-U3 and MB49 cells were kindly
provided by Dr Helene LaRue (Laval University Cancer Research
Centre, Quebec, Canada) and Dr Tatsuya Nakatani (Osaka City
University, Osaka, Japan), respectively. Cancer cell lines were
maintained in RPMI-1640 (Nacalai Tesque, Inc., Kyoto, Japan) or
Dulbecco’s modified Eagle’s medium (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA) and 1% penicillin/streptomycin (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in a standard humidified
incubator at 37°C in an atmosphere of 5% CO2. Human
umbilical vascular endothelial cells (HUVECs; Lonza, Tokyo, Japan)
were also used in the present study. HUVECs were cultured in EBM-2
basal media supplemented with the EGM-2 MV kit (Lonza) containing
2% FBS in a standard humidified incubator at 37°C in an atmosphere
of 5% CO2. HUVECs were used at passages 4-6.
Animals
Animal care was in compliance with the
recommendations of the Guide for Care and Use of Laboratory Animals
(National Research Council) and the study was approved by the
Animal Facility Committee at Nara Medical University (ID: 11883;
Nara, Japan). A total of 42 male athymic BALB/c nu/nu mice
(six-week-old; 22 g) were purchased from Oriental Bio Service, Ltd.
(Kyoto, Japan). All mice were maintained under pathogen-free
conditions and provided with sterile food and water. The mice were
kept in a temperature- and humidity-controlled room, with a
12-h/12-h light/dark cycle and food was provided ad
libitum.
Reagents
To treat tumor-bearing mice, the following
recombinant human and mouse CSFs were purchased from Miltenyi
Biotec (Auburn, CA, USA); human G-CSF, GM-CSF and M-CSF, and mouse
G-CSF, GM-CSF and M-CSF. All reagents were diluted in sterile PBS
solution according to the manufacturer’s protocol. Sterile PBS was
used as a treatment control.
Xenograft model and intraperitoneal
administration
Fig. 1A presents a
schematic representation of the present study. After allowing mice
to acclimate to the facility for 1 week, MGH-U3 and UM-UC-3
urothelial cancer cells (5×105/tumor) in 50 µl
RPMI-1640 medium and 50 µl growth factor-reduced Matrigel
(Corning Inc., Corning, NY, USA) were injected into the flank of
each mouse. A total of 2 weeks after cell inoculation, when the
tumors reached 5 mm in diameter, the mice were randomly divided
into four groups: Control (PBS, n=6), G-CSF (1.25 µg/kg,
n=12), GM-CSF (4.5 µg/kg, n=12) and M-CSF (145,000 U/kg,
n=12). The prescribed dose of each drug was calculated based on the
dosage for humans in a clinical setting of neutropenia (8-12).
Intraperitoneal administration was initiated from the third week
and was performed thrice a week for two weeks. Tumor diameters were
measured thrice a week using electronic calipers and tumor volumes
were calculated using the following formula: (width2 ×
length) / 2. Subsequently, 1 week after the last treatment, all
mice were euthanized by exsanguination under anesthesia with 2-3%
isoflurane and tissues (tumor and whole blood by cardiac puncture)
were harvested for the subsequent experiments. Tumors were examined
by hematoxylin and eosin (H&E) staining and immunohistochemical
(IHC) staining analysis. Treatment-associated changes in pro- and
anti-tumoral cytokines and chemokines in serum were evaluated by
enzyme-linked immunosorbent assay (ELISA)-based assays. The maximum
tumor diameter observed in the present study was 19.9 mm.
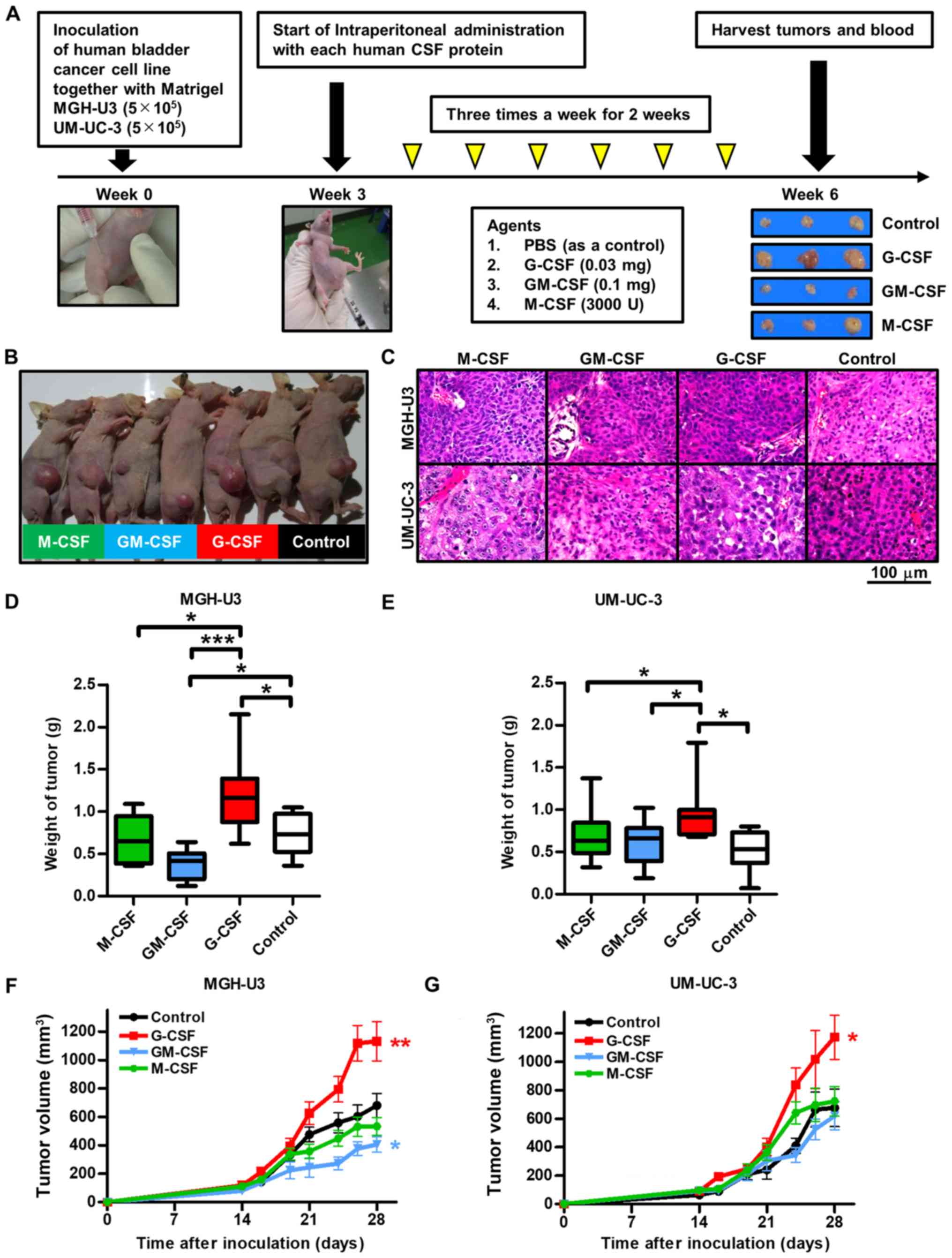 | Figure 1Study treatment scheme and inoculated
xenografts. (A) Schematic diagram illustrating the study workflow.
Mice were injected with UM-UC-3 cells (5×105/tumor) or
MGH-U3 cells (5×105/tumor), together with growth
factor-reduced Matrigel. A total of 2 weeks after inoculation, mice
were randomly divided into four treatment groups [PBS (control),
recombinant human G-CSF, recombinant human GM-CSF and recombinant
human M-CSF]. Subsequently, mice were treated thrice a week for two
weeks. A week after the last treatment, mice were euthanized, and
xenografts and blood were harvested. Anti-Ki-67, CD31, LYVE-1,
VEGF, CD204 and E-cadherin antibodies were used to evaluate cell
proliferation, angiogenesis, lymphangiogenesis, M2 macrophages and
epithelial-mesenchymal transition. Serum was used to perform ELISA.
(B) Representative tumor sizes of each group. Xenografts of mice
treated with G-CSF were the largest and those of mice treated with
GM-CSF were the smallest in the four treatment groups. (C)
Representative images of hematoxylin and eosin-stained xenografts
from each group. Viable cancer cells were observed in all resected
xenografts. (D) Weight of xenografts following inoculation with
MGH-U3 cells. Weight of xenografts in mice treated with G-CSF
significantly increased compared with the other groups. Weight of
xenografts in mice treated with GM-CSF significantly decreased
compared with the control. *P<0.05,
***P<0.0001. (E) Weight of xenografts of inoculated
UM-UC-3 cells. Weight of xenografts in mice treated with G-CSF
significantly increased compared with the other groups.
*P<0.05. (F) Tumor growth rate during treatment was
significantly higher in mice treated with G-CSF and lower in mice
treated with GM-CSF compared with the control in xenografts
inoculated with MGH-U3 cells. *P<0.05,
*P<0.01 vs. Control. (G) Tumor growth rate during
treatment was significantly higher in mice treated with G-CSF
compared with the control in xenografts inoculated with UM-UC-3
cells. *P<0.01 vs. Control. G-CSF, granulocyte
colony-stimulating factor; GM-CSF, granulocyte-macrophage
colony-stimulating factor; M-CSF, macrophage colony-stimulating
factor; CSF, colony-stimulating factor; LYVE-1, lymphatic vessel
endothelial hyaluronan receptor 1; VEGF, vascular endothelial
growth factor. |
IHC staining analysis and
quantification
Tumors were examined by IHC staining analysis as
previously described (24).
Briefly, tumors were fixed in 10% neutral buffered formalin for 48
h at room temperature, embedded in paraffin and then the sections
(3-µm-thick) were subjected to IHC staining for various
markers involved in cell proliferation, angiogenesis,
lymphangiogenesis, anti-tumor immunity and epithelial-mesenchymal
transition (EMT): Ki-67 (proliferation), CD31 (angiogenesis),
lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1;
lymphangiogenesis), vascular endothelial growth factor (VEGF;
angiogenesis), CD204 (as a marker of M2 macrophages; anti-tumor
immunity) and E-cadherin (EMT). IHC staining was performed using
the Histofine ABC kit (cat. no. 424043; ready-to-use; Nichirei
Biosciences, Tokyo, Japan), according to the manufacturer’s
protocol. Slides were incubated overnight at 4°C with antibodies
against Ki-67 (clone MIB-1, ready-to-use; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA), CD31 (cat. no. M0823;
1:1,000; Dako; Agilent Technologies, Inc.), LYVE-1 (cat. no.
ab14917; 1:100; Abcam, Cambridge, MA, USA), VEGF (cat. no. sc-152;
1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), CD204 (cat.
no. KT022; 1:2,000; Trans Genic, Kobe, Japan) and E-cadherin (cat.
no. 3195; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA). In each case, an evaluation was performed by two
investigators independently and blindly, without the information
pertaining the treatment. Positive cells from each specimen were
counted from a minimum of four randomly selected fields per high
power field (HPF; magnification, ×400; 0.0625
µm2) using a light microscope for Ki-67, VEGF and
CD204. The number of stained microvessels was counted from a
minimum of four randomly selected fields per HPF for CD31 and
LYVE-1, as a marker for angiogenesis and lymphangiogenesis,
respectively. E-cadherin was evaluated according to intensity level
(0, none; 1, low; 2, intermediate; 3, high) (25).
Quantification of serum cytokines
Serum cytokines involved in angiogenesis,
recruitment of M2 macrophages and EMT were measured by ELISA. Serum
was collected and centrifuged at 10,000 × g for 15 min at 4°C, and
the supernatant was stored at -80°C in cryotubes. The profiles of
eight cytokines in serum were determined using an ELISA array (cat.
no. EA-1021; Signosis, Inc., Hilden, Germany), according to the
manufacturer’s protocol. Serum samples were thawed, mixed and then
incubated in 96-well microplates coated with anti-mouse primary
antibodies against eight cytokines involved in angiogenesis: Tumor
necrosis factor (TNF)-α, insulin-like growth factor-1 (IGF-1),
VEGF, interleukin (IL)-6, fibroblast growth factor (FGF)-b,
interferon (IFN)-γ, epidermal growth factor and leptin. Samples
were developed with horseradish peroxidase-conjugated secondary
antibodies. After adding the substrate and stop solution, a Tecan
microplate reader (Tecan Group, Ltd., Mannedorf, Switzerland) was
used to measure absorbance at 450 nm. IL-6 and VEGF were measured
in serum samples of each mouse using the ELISA method (860.020.048
and SEA143Mu, respectively; Diaclone, Besancon Cedex, France and
Cloud-Clone, Houston, TX, USA, respectively). In addition,
transforming growth factor (TGF)-β1 and TGF-β2 were also measured
in the serum samples of each mouse by ELISA (cat. nos. EA100357 and
EA100629, respectively; OriGene Technologies, Rockville, MD, USA),
according to the manufacturer’s protocol. A Tecan microplate reader
(Tecan Group, Ltd.) was used to measure absorbance at 450 nm.
Cell viability assays
To investigate the effect of human and mouse
recombinant CSFs on proliferation ability, the cell viability assay
was performed. GH-U3, UM-UC-3, MB49, and MBT2 cells were seeded in
a 96-well plate at a density of 2,000 cells/well in serum-free
RPMI-1640 (Nacalai Tesque, Inc.), incubated for 24 h and treated
with three different concentrations of each CSF (0, 2 or 10 ng/ml)
for 48 h. Cell Counting Kit-8 (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) was used to measure viability, according to
the manufacturer’s protocol. The viability index was expressed
relative to the untreated cells.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Semi-quantitative RT-PCR was performed to measure
the RNA expression levels of G-CSFR, granulocyte macrophage colony
stimulating factor receptor (GM-CSFR) and macrophage colony
stimulating factor receptor (M-CSFR) in each cell line (MGH-U3,
UM-UC-3 and HUVEC), as previously described (26). Briefly, cells were seeded in 6-well
plates at a density of 1×105 cells/well in RPMI-1640
(Nacalai Tesque, Inc.) or Dulbecco’s modified Eagle’s medium
(Sigma-Aldrich; Merck KGaA) and incubated for 24 h. RNA was
extracted from these cells using the RNeasy mini kit (Qiagen, Inc.,
Valencia, CA, USA), according to the manufacturer’s protocol.
Conversion to cDNA was achieved using the High Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.).
Semi-quantitative PCR was performed with cDNA, 0.2 µM each
probe mix and 10 µl AmpliTaq Gold® PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) under the
following conditions: Denaturation at 95°C for 10 min, 25-30 cycles
of denaturation at 95°C for 15 sec, and an annealing and final
extension step at 60°C for 1 min. PCR products were then
electrophoresed in a 1.5% agarose gel and visualized using ethidium
bromide with a transilluminator. GAPDH was used as an internal
control.
Capillary tube formation assays
Each well of 96-well plates was coated with 50
µl Matrigel and allowed to solidify for 30 min at 37°C.
HUVECs were seeded on top of Matrigel in triplicate at a density of
2×104 cells/well in Dulbecco’s modified Eagle’s medium
(Sigma-Aldrich; Merck KGaA) and incubated for 6 h at 37°C in an
atmosphere of 5% CO2. Similarly, HUVECs were resuspended
in EBM-2 basal media with or without each human recombinant CSF (10
ng/ml) prior to seeding on top of Matrigel and incubated for 6 h at
37°C in an atmosphere of 5% CO2. Formed tubes were
stained with calcein AM (PromoCell GmbH, Heidelberg, Germany) for
15 min at room temperature and the cells were immediately examined
under a fluorescence microscope (magnification, ×200; EVOS™ FL Auto
Imaging System; Thermo Fisher Scientific, Inc.). The total length
of tube-like structures in at least four viewed fields per well was
measured using ImageJ 1.47 software (National Institute of Health,
Bethesda, MD, USA).
Dual-immunofluorescence staining of human
bladder cancer tissue
To investigate whether CSFRs are present at tumoral
areas and cancer-free areas, human bladder cancer tissues were
evaluated by dual-immunofluorescence staining as previously
described (27). The protocol for
the research project was approved by the Institutional Review Board
for Clinical Studies (Medical Ethics Committee ID: NMU-1630; Nara
Medical University, Kashihara, Japan) and all participants provided
written informed consent. Two patients with newly diagnosed
non-muscle invasive bladder cancer undergoing transurethral
resection of bladder tumor in May 2018 at Nara Medical University
were enrolled. Tissue samples were obtained at the initial
treatment and each specimen of the tumor site and normal site were
evaluated. The two patients were randomly selected based on being
the same sex (male), a similar age (69 and 66 years old), and
presenting with the same stage and grade (T1 high-grade).
Dual-immunofluorescence staining was performed with antibodies
specific to either CD31/G-CSFR (mouse monoclonal/goat polyclonal),
CD31/GM-CSFR (mouse monoclonal/rabbit polyclonal) or CD31/M-CSFR
(mouse monoclonal/rabbit polyclonal). Sections were frozen at
-80°C, cut into 6-µm sections mounted on Superfrost Plus
slides (Thermo Fisher Scientific, Inc.) and fixed in 10% neutral
buffered formalin for 10 min at room temperature and then 3%
hydrogen peroxide methanol for 10 min at room temperature, followed
by blocking in donkey serum (cat. no. 017-000-001; Jackson
ImmunoResearch Inc., West Grove, PA, USA) for 1 h at room
temperature. The sections were incubated with either anti-CD31
(cat. no. M0823; 1:50; Dako; Agilent Technologies, Inc.) and G-CSFR
(cat. no. sc-323898; 1:100; Santa Cruz Biotechnology, Inc.),
GM-CSFR (cat. no. GTX51383; 1:100; GeneTex, Irvine, CA, USA) or
M-CSFR (cat no. bs-3074R; 1:100, BIOSS, Beijing, China) overnight
at 4°C. The sections were then incubated with Alexa Fluor 488
anti-mouse IgG (cat. no. 715-545-150), Alexa Fluor 594 anti-rabbit
IgG (cat. no. 711-585-152) and Alexa Fluor 594 anti-goat IgG (cat.
no. 705-585-147) secondary antibodies (1:250; Jackson
Immunoresearch Laboratories, Inc.) for 30 min at room temperature,
as appropriate, and mounted with mounting medium with DAPI (Vector
Laboratories, Burlingame, CA, USA). The sections were immediately
examined under a fluorescence microscope (magnification, ×400;
EVOS™ FL Auto Imaging System; Thermo Fisher Scientific, Inc.).
Statistical analysis
Statistical analyses and figure plotting were
performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). Data are presented by bar charts or box plots and
expressed as the mean ± standard error of the mean. All analyses
were performed from three independent experiments. Kruskal-Wallis
test followed by Dunn’s multiple comparison test were applied for
statistical analysis, as appropriate. Survival curves were
generated using the Kaplan-Meier method and compared with a
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Treatment with G-CSF enhances tumor
growth
Treatments were well tolerated with no body weight
loss (data not shown). Fig. 1B
presents representative images of mice with xenografts in each
treatment group. Mice treated with G-CSF presented with the largest
tumors, whereas mice treated with GM-CSF presented with the
smallest tumors among the four groups. Fig. 1C presents representative H&E
stained images from each treatment group, which confirmed that all
mice contained tumor cells. The maximum tumor size observed in the
present study was 20 mm in diameter in the control group. Fig. 1D and E presents the resected tumor
weights following inoculation with MGHU-3 and UM-UC-3 cells,
respectively. Significant tumor weight gain was observed in the
G-CSF treatment group compared with the control in both human
bladder cancer cell lines (P<0.0001 and P=0.014, respectively).
With MGH-U3 cells, significant tumor weight loss was observed in
the GM-CSF treatment group compared with the control group, which
suggests that treatment with GM-CSF may exhibit an anti-tumor
effect for low-grade tumor and not exhibit a tumor growth effect
for high-grade tumor compared with the treatment with G-CSF
(P<0.05; Fig. 1D). Significant
tumor weight loss was also observed in the GM-CSF and M-CSF
treatment groups compared with the G-CSF treatment group in MGH-U3
(P<0.0001 and P<0.05, respectively; Fig. 1D) and UM-UC-3 (P<0.05 and
P<0.05, respectively; Fig. 1E)
human bladder cancer cell lines. The rate of tumor growth during
the treatment was significantly higher in mice treated with G-CSF
compared with the control mice for MGH-U3 (P=0.003; Fig. 1F) and UM-UC-3 (P=0.019; Fig. 1G) human bladder cancer cell
lines.
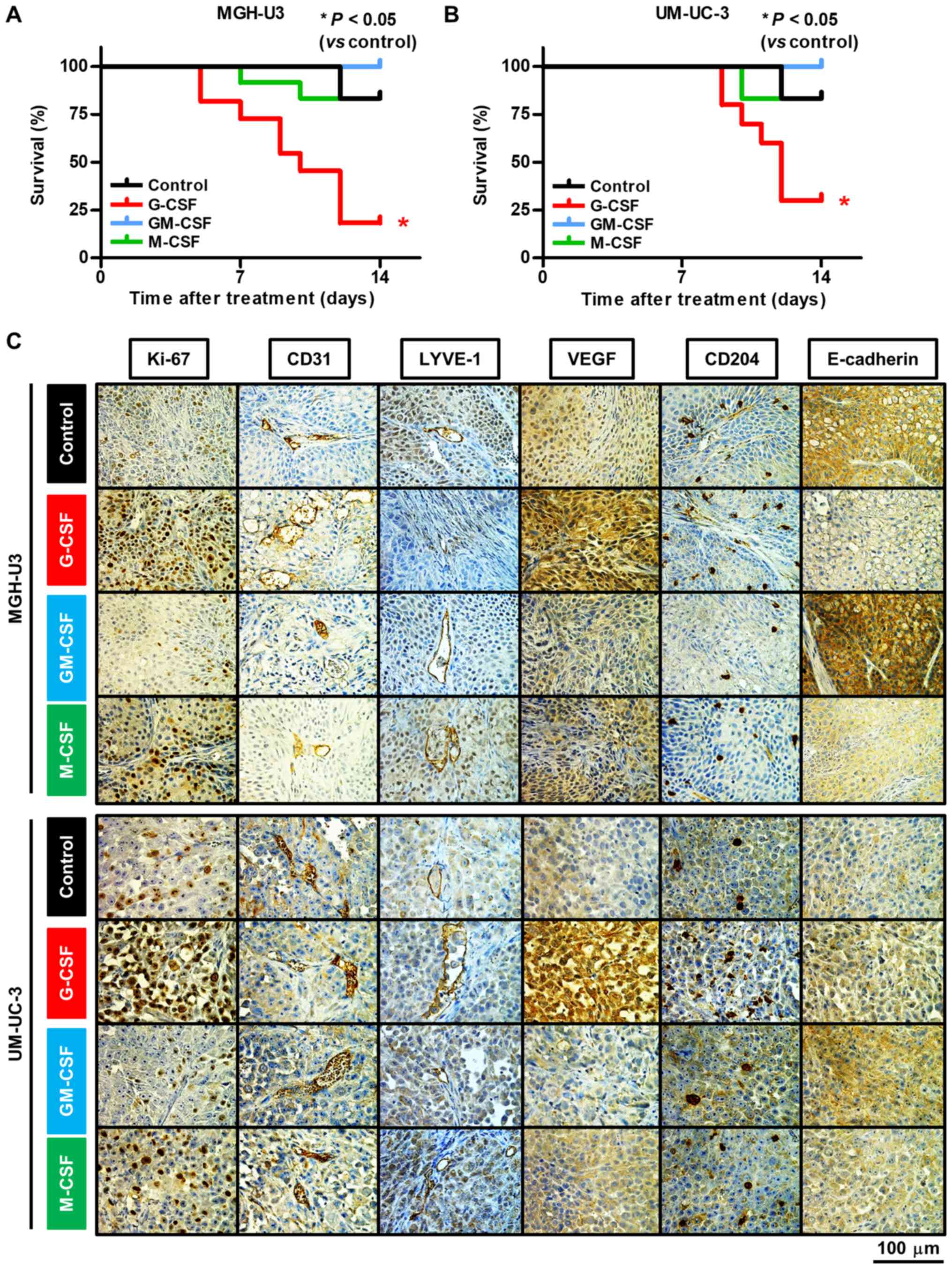
In addition, a tumor size of ≥15 mm in diameter was
described as an event and a tumor survival curve was generated by
the Kaplan-Meier method (28).
Mice treated with G-CSF were at a significantly higher risk of
tumor growth compared with the control groups following inoculation
with MGH-U3 (P=0.011; Fig. 2A) and
UM-UC-3 (P=0.040; Fig. 2B) human
bladder cancer cell lines.
Treatment with G-CSF results in
angiogenesis, recruitment of M2 macrophages and EMT
To investigate the influence of the three CSFs on
angiogenesis, lymphangiogenesis, recruitment of M2 macrophages and
EMT, the present study performed IHC staining for six markers:
Ki-67, CD31, LYVE-1, VEGF, CD204, and E-cadherin. Representative
images of antibody-stained resected tumors are presented in
Fig. 2C and the results are
summarized in Table I. Treatment
with G-CSF and M-CSF significantly increased the expression level
of Ki-67 in MGH-U3 (P<0.0001 and P<0.05, respectively;
Fig. S1A) and UM-UC-3 (P<0.05
and P<0.05, respectively; Fig.
S1B) human bladder cancer cell lines compared with the control.
The number of cells with CD31 was significantly enhanced by the
treatment with G-CSF in MGH-U3 cells compared with the control
(P<0.05; Fig. S1C). With
UM-UC-3 cells, the number of cells with CD31 was significantly
enhanced by treatment with G-CSF and significantly suppressed by
treatment with GM-CSF (P<0.05 and P<0.05, respectively;
Fig. S1D). By contrast, the
number of cells with LYVE-1 was significantly inhibited by
treatment with GM-CSF in MGH-U3 (P<0.05; Fig. S1E). The number of M2 macrophages
was significantly increased by treatment with G-CSF and
significantly reduced by treatment with GM-CSF in MGH-U3 (P<0.05
and P<0.01, respectively; Fig.
S1I). In UM-UC-3, the number of M2 macrophages was
significantly induced by treatment with G-CSF and M-CSF, and
significantly reduced by treatment with GM-CSF (all P<0.05;
Fig. S1J). The intensity level of
E-cadherin was significantly reduced by treatment with G-CSF and
M-CSF in MGH-U3 (P<0.05 and P<0.01, respectively; Fig. S1K). These results suggest that
angiogenesis is induced by treatment with G-CSF, lymphangiogenesis
is suppressed by treatment with GM-CSF, M2 macrophages are induced
and reduced by treatment with G-CSF and GM-CSF, respectively, and
EMT is enhanced by treatment with G-CSF. In addition, the different
results in the different cell lines suggest that high-grade tumors
may be less affected by treatment with GM-CSF in angiogenesis,
lymphangiogenesis and EMT.
 | Table ISummary of immunohistochemical
staining analysis and ELISA assay of murine serum. |
Table I
Summary of immunohistochemical
staining analysis and ELISA assay of murine serum.
| Treatment | Immunohistochemical
staining analysis of the resected tumor
|
ELISA of
serum
|
|---|
| Ki-67 | CD31 | LYVE-1 | VEGF | CD204 | E-cadherin | TNF-α | IGF-1 | VEGF | IL-6 | FGF-b | INF-γ | EGF | Leptin | TGF-β1 | TGF-β2 |
|---|
| MGH-U3 cells | | | | | | | | | | | | | | | | |
| G-CSF | upa | upa | ns | upa | upa | downa | upa | upa | upa | upa | upa | upa | upa | upa | upa | upa |
| GM-CSF | ns | ns | downa | ns | downa | ns | upa | upa | upa | upa | upa | upa | upa | upa | upa | upa |
| M-CSF | upa | upa | ns | upa | downa | downa | upa | upa | upa | upa | upa | upa | ns | upa | upa | upa |
| UM-UC-3 cells | | | | | | | | | | | | | | | | |
| G-CSF | upa | upa | ns | upa | upa | ns | ns | ns | ns | upa | upa | ns | upa | upa | upa | upa |
| GM-CSF | ns | downa | ns | downa | downa | ns | ns | upa | ns | upa | upa | upa | upa | ns | upa | upa |
| M-CSF | upa | ns | ns | upa | upa | ns | ns | upa | ns | upa | upa | upa | upa | upa | ns | upa |
Systemic changes in serum cytokines
caused by treatment with CSFs
To investigate the association between treatment
with CSFs and induced cytokines involved in angiogenesis, the
current study analyzed the serum obtained from euthanized mice by
ELISA. The results are summarized in Table I. TNF-α, IGF-1, VEGF, IL-6, FGF-b,
IFN-γ and leptin were significantly increased in all treatment
groups compared with the control in MGH-U3-inoculated mice. In
UM-UC-3-inoculated mice, IL-6 and leptin were significantly
increased in all treatment groups compared with the control
(Tables I and SI). Subsequently, the present study
focused on IL-6 and VEGF, key cytokines involved in angiogenesis,
and TGF-β1 and TGF-β2, cytokines involved in EMT. The
concentrations of these cytokines were measured using all samples.
In mice inoculated with MGHU-3, IL-6 was significantly decreased by
treatment with GM-CSF (P<0.05; Fig.
3A) and VEGF was significantly increased by treatment with
G-CSF (P<0.05; Fig. 3B)
compared with the control. TGF-β1 was significantly increased by
treatment with G-CSF compared with the control (P<0.05; Fig. 3C). TGF-β2 was significantly
increased in all treatment groups compared with the control (all
P<0.05; Fig. 3D). In mice
inoculated with UM-UC-3 cells, IL-6 was significantly increased by
treatment with G-CSF compared with the control (P<0.05; Fig. 3E). TGF-β1 was significantly
increased by treatment with G-CSF and GM-CSF compared with the
control (all P<0.05; Fig. 3G).
TGF-β2 was significantly increased by treatment with G-CSF compared
with the control (P<0.05; Fig.
3H).
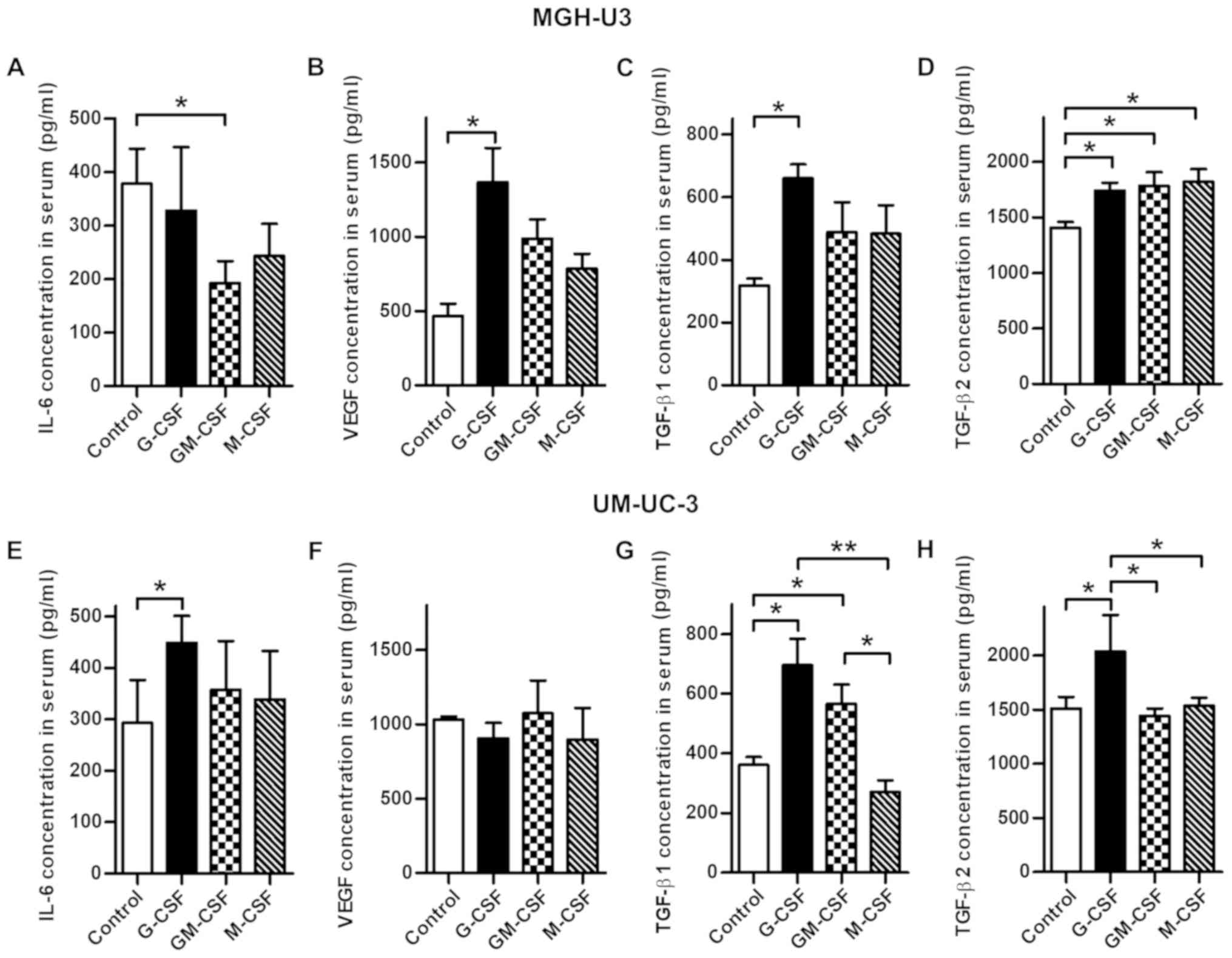 | Figure 3Comparison of each serum cytokine
concentration, including IL-6, VEGF, TGF-β1 and TGF-β2, in the
treatment groups and the control. (A) Treatment with GM-CSF
demonstrated a significantly lower concentration of IL-6 in mice
inoculated with MGH-U3 compared with the controls. (B) Treatment
with G-CSF resulted in a significantly higher concentration of VEGF
compared with the controls in mice inoculated with MGH-U3. (C)
Treatment with G-CSF resulted in a significantly higher
concentration of TGF-β1 compared with the controls in mice
inoculated with MGH-U3. (D) Treatment with all three CSFs resulted
in a significantly higher concentration of TGF-β2 compared with the
controls in mice inoculated with MGH-U3. (E) Treatment with G-CSF
demonstrated significantly higher concentration of IL-6 in mice
inoculated with UM-UC-3 cells compared with the controls. (F) There
was no significant difference in the concentration of VEGF
following treatment with each CSF in mice inoculated with UM-UC-3
cells. (G) Treatment with G-CSF and GM-CSF resulted in
significantly higher concentrations of TGF-β1 in mice inoculated
with UM-UC-3 cells compared with the controls. (H) Treatment with
G-CSF demonstrated a significantly higher concentration of TGF-β2
in mice inoculated with UM-UC-3 cells compared with the controls.
*P<0.05, **P<0.01. G-CSF, granulocyte
colony-stimulating factor; GM-CSF, granulocyte-macrophage
colony-stimulating factor; M-CSF, macrophage colony-stimulating
factor; IL-6, interleukin-6; VEGF, vascular endothelial growth
factor; TGF, transforming growth factor. |
Exogenous CSFs enhance the viability of
human and mouse bladder cancer cell lines
To investigate the effects of treatment with
exogenous CSFs on cell viability, a cell viability assay was
performed with human and mouse urothelial carcinoma cell lines
(human, MGH-U3 and UM-UC-3; mouse, MB49 and MBT2). With MGH-U3
cells, no significant effect of each CSF was identified (Fig. 4A). By contrast, treatment with 10
nm/ml G-CSF, GM-CSF and M-CSF significantly enhanced UM-UC-3 cell
viability (all P<0.05; Fig.
4B), which suggests that high-grade tumors are more affected by
treatment with CSFs directly. With regard to mouse urothelial
carcinoma cell lines, treatment with G-CSF and M-CSF enhanced
cancer cell viability (Fig. S2).
However, a dose-dependent mechanism was not observed.
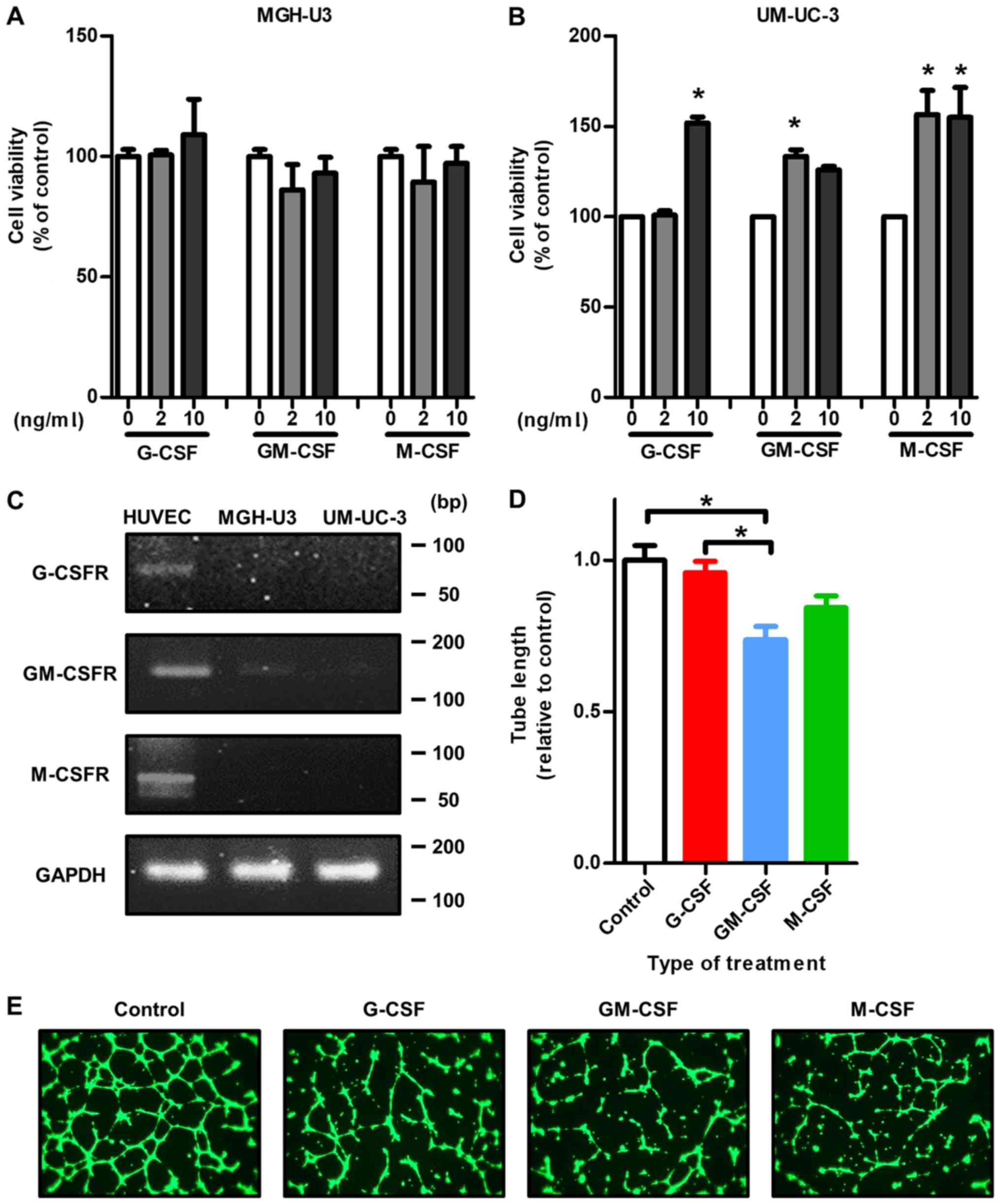 | Figure 4Treatment with each CSF indirectly
affects tumor growth through stromal cells in the tumor
microenvironment rather than affecting tumor growth directly. (A)
There was no effect on cell viability of MGH-U3 cells following
treatment with exogenous CSFs. (B) In the cell viability assay,
treatment with exogenous CSFs significantly increased the
proliferation ability of UM-UC-3 cells. *P<0.05 vs. 0
ng/ml. (C) HUVECs expressed G-CSFR, GM-CSFR and M-CSFR. By
contrast, MGH-U3 and UM-UC-3 cells did not express any CSFR. (D) In
the capillary tube formation assay, treatment with GM-CSF
significantly decreased the tube formation capacity of HUVECs
compared with the control and cells treated with G-CSF.
*P<0.05. (E) Representative images of the different
treatment groups stained with calcein AM in the tube formation
assay. Treatment with GM-CSF and M-CSF did not promote angiogenesis
to the same extent as the control and treatment with G-CSF.
Magnification, ×200. G-CSF, granulocyte colony-stimulating factor;
GM-CSF, granulocyte-macrophage colony-stimulating factor; M-CSF,
macrophage colony-stimulating factor; G-CSFR, granulocyte
colony-stimulating factor receptor; GM-CSFR, granulocyte-macrophage
colony-stimulating factor receptor; M-CSFR, macrophage
colony-stimulating factor receptor; HUVEC, human umbilical vascular
endothelial cell. |
Each CSFR is not expressed in human
bladder cancer cell lines
In the in vitro study, it was demonstrated by
semi-quantitative RT-PCR analysis that there were mostly
undetectable mRNA expression levels of G-CSFR, GM-CSFR or M-CSFR in
MGH-U3 and UM-UC-3 cells. Whereas, HUVECs expressed mRNA of all
three CSFRs examined (Fig.
4C).
Capillary tube formation capability is
suppressed by treatment with GM-CSF and M-CSF
To investigate the effect of treatment with
exogenous CSFs on angiogenesis, a capillary tube formation assay
was performed using HUVECs. Although treatment with G-CSF had no
significant effect on capillary tube formation capability compared
with the control, treatment with GM-CSF significantly suppressed
capillary tube formation capability compared with the control in
vitro (P=0.0087 and P=0.041, respectively; Fig. 4D). A significant difference was
also identified between treatment with G-CSF and with GM-CSF
(P=0.0085; Fig. 4D). Fig. 4E presents the representative images
of each treatment group.
G-CSFR is expressed in endothelial cells
but not tumor cells in the tumor microenvironment
To evaluate whether CSFRs are present in tumor and
endothelial cells around the tumoral area, dual-immunofluorescence
staining was performed with antibodies specific to CD31/G-CSFR,
CD31/GM-CSFR and CD31/M-CSFR using tissues obtained from patients
with UCB. The clinicopathological information of the patients is
presented in Table SII.
Representative images from fluorescence microscopy demonstrated
that certain endothelial cells around the tumoral area expressed
G-CSFR, GM-CSFR and M-CSFR, and these receptors were not expressed
by tumor cells in human UCB tissues (Fig. 5A). By contrast, representative
images of the cancer-free area revealed that endothelial cells did
not express any CSFRs (Fig.
S3).
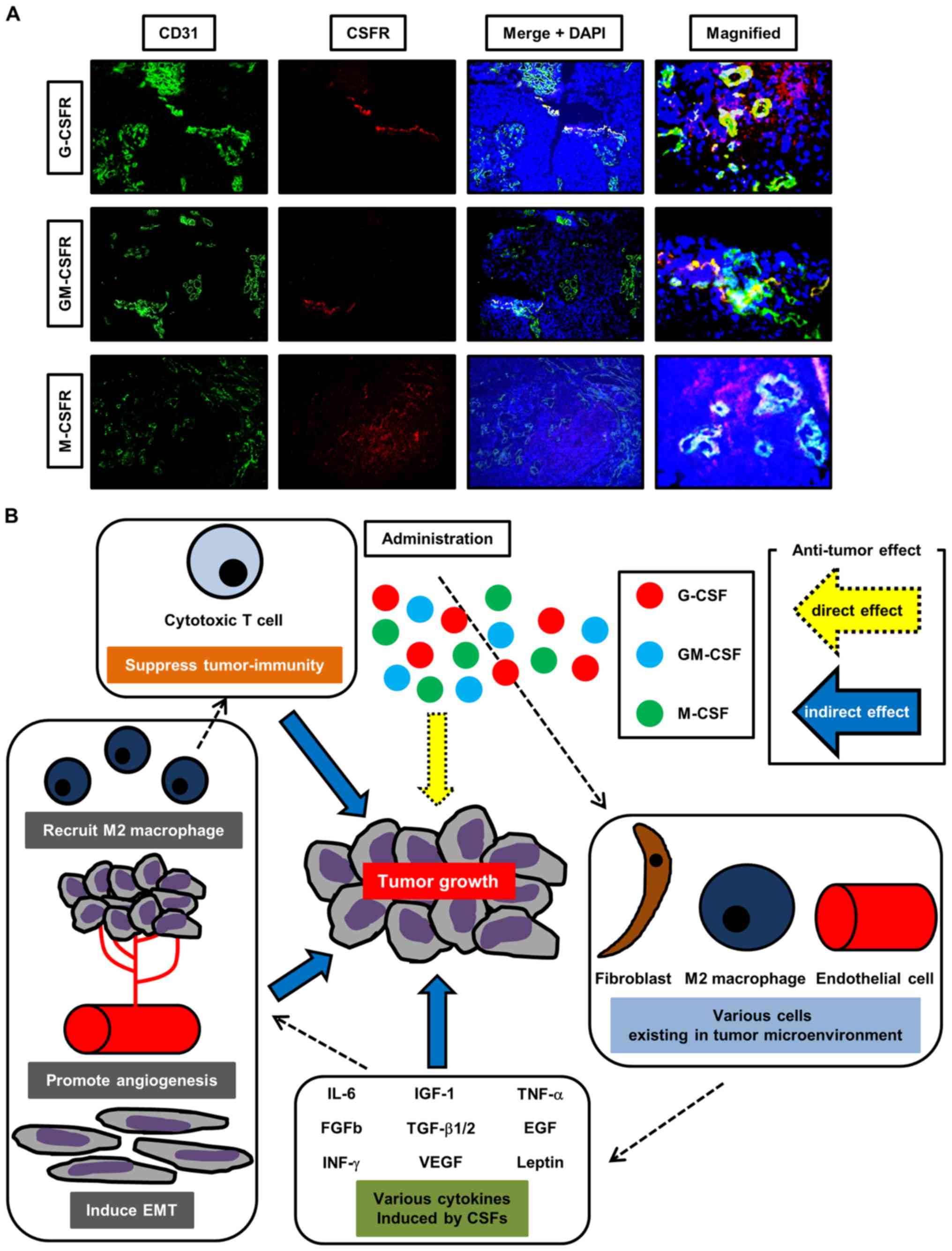 | Figure 5Dual-immunofluorescence staining
analysis and a schematic diagram of the proposed mechanism of the
roles of CSFs. (A) Representative dual-immu-nofluorescence staining
images of human bladder cancer tissues stained with antibodies
specific to CD31/G-CSFR, CD31/GM-CSFR or CD31/M-CSFR. At the
tumoral area, certain endothelial cells expressed each CSFR.
Magnification, ×400. (B) Treatment with CSFs affects the degree of
bladder cancer growth. Notably, the administration of G-CSF
promotes tumor growth through the enhancement of angiogenesis,
recruitment of M2 macrophages and induction of EMT in the tumor
microenvironment. Treatment with CSFs also induces various
cytokines involved in angiogenesis, recruitment of M2 macrophages
and EMT. These cytokines exhibit roles of enhancing tumor growth
via unknown mechanisms and activating cross-talk of stromal cells,
including fibroblasts, endothelial cells and immune-associated
cells, in the tumor microenvironment. Our hypothesis is that
treatment with CSFs could cause tumor progression not only directly
but also indirectly. Understanding of the pro- and anti-tumor
effects induced by the three CSFs could lead to an improved
prognosis for patients with advanced bladder cancer undergoing
cytotoxic chemotherapy. G-CSF, granulocyte colony-stimulating
factor; GM-CSF, granulocyte-macrophage colony-stimulating factor;
M-CSF, macrophage colony-stimulating factor; CSF,
colony-stimulating factor; EMT, epithelial-mesenchymal transition;
G-CSFR, granulocyte colony-stimulating factor receptor; GM-CSFR,
granulocyte-macrophage colony-stimulating factor receptor; M-CSFR,
macrophage colony-stimulating factor receptor; IL-6, interleukin-6;
IGF-1, insulin-like growth factor-1; FGF-b, fibroblast growth
factor-b; IFN, interferon; TGF-β1/2, transforming growth
factor-β1/2; VEGF, vascular endothelial growth factor; TNF, tumor
necrosis factor. |
Discussion
The present study demonstrated that no exogenous CSF
protein had the ability to promote proliferation directly in human
bladder cancer cell lines. This can be explained by the lack of
expression of their receptors, including G-CSFR, GM-CSFR and
M-CSFR, in the present in vitro study. However, in the in
vivo study, the administration of G-CSF and M-CSF promoted
tumor growth over time, whereas the administration of GM-CSF did
not promote tumor growth, which resulted in a significantly higher
cancer mortality rate in mice treated with G-CSF compared with the
control. Cancer mortality in mice treated with GM-CSF was almost
the same as the control. The administration of CSFs could affect
the acceleration or inhibition of tumor growth, angiogenesis,
lymphangiogenesis, recruitment of M2 macrophages and EMT. ELISA
using mouse serum samples suggested that various cytokines involved
in angiogenesis and EMT serve roles in enhancing tumor growth
through unknown and/or indirect mechanisms. Therefore, we
hypothesize that CSF administration indirectly affects tumor growth
via various mechanisms in the tumor microenvironment, resulting in
progression and metastasis in patients with UCB (Fig. 5B).
The use of CSFs, particularly G-CSF, is common for
patients with chemotherapy-induced myelosuppression. The use of
chemotherapy is becoming increasingly common for patients with UCB
due to the success of neoadjuvant or adjuvant-based chemotherapies
(4-6,8-10).
Therefore, understanding of the potential effects of CSFs against
cancer is important for the use of CSFs appropriately. Even though
in Japan the administration of G-CSF has been approved for patients
with chemotherapy-induced myelosuppression in UCB (9), the present study demonstrated that
G-CSF could promote tumor growth in UCB. Segawa et al
(29) also reported that the
intraperitoneal administration of G-CSF enhanced tumor growth in
animal models. In addition, although G-CSF had no effects on
accelerating angiogenesis directly, endothelial progenitor cells
(EPCs), which are involved in angiogenesis, were demonstrated to be
induced by G-CSF (30-32). MDSCs have also been associated with
tumor growth through induction of EPCs, resulting in angiogenesis.
G-CSF has been demonstrated to enhance MDSC survival and activation
by signal transducer and activator of transcription 3 (STAT3)
signaling (18,30). The present results suggest that
G-CSF could have a stimulating effect on stromal cells in the tumor
microenvironment, thereby indirectly enhancing tumor growth by
angiogenesis. These effects may be induced by the interaction of
cytokines and the cross-talk of cells. In a dual-immunofluorescence
staining analysis using human UCB tissues, G-CSFR was identified to
not be expressed at cancer-free areas; however, the expression
level of G-CSFR increased in tumoral areas. G-CSFR expression may
be induced by tumors and may create a favorable microenvironment
for tumor survival and progression. In addition, CSFs can be
ligands not only to CSFRs but also to unknown receptors (termed
orphan receptors) (33). Although
UM-UC-3 cells did not express any CSFRs in the present study, CSFs
could promote the viability of UM-UC-3 cells. Therefore, tumor
progression may be induced by unknown mechanisms including the
activation of CSFs and unknown receptors.
Treatment with G-CSF also induces lymphangiogenesis
in vitro (34). In the
current study, treatment with GM-CSF suppressed the expression
level of LYVE-1, suggesting an inhibition of lymphangiogenesis in
the tumor microenvironment. Fiorentini et al (35) suggested an association between the
induction of lymphangiogenesis and GM-CSF. However, to the best of
our knowledge, in cancer, the effect of GM-CSF on lymphangiogenesis
is not clear. Therefore, the present result is a proposal for
treatment with GM-CSF to suppress lymphangiogenesis in the tumor
microenvironment.
M2 macrophages (also termed tumor-associated
macrophages) serve multiple important roles in cancer progression
through the suppression of adaptive immunity and the induction of
angiogenesis (27). Van Overmeire
et al (22) reported that a
M-CSFR blockade resulted in the reduction of M2 macrophages in the
tumor microenvironment. Kitoh et al (36) demonstrated that GM-CSF and M-CSF
signals are associated with the immunosuppressive properties of M2
macrophages in vitro. However, the association between CSFs
and M2 macrophages is unclear. The present results suggest that
treatment with G-CSF has an effect on the recruitment of M2
macrophages and treatment with GM-CSF has an effect of the
reduction of M2 macrophages in the tumor microenvironment among
three CSFs, resulting in an alteration of immunosuppression and
cancer progression. Stromal cells that express CSFRs may have
positive or negative effects on tumor growth.
Although Elghonaimy et al (37) suggested that G-CSF is associated
with the enhancement of EMT in breast cancer, the associations
between CSFs and EMT in UCB remain unclear. Our results suggest
that treatment with G-CSF promotes EMT by the reduction of
E-cadherin expression and induction of TGF-β1, thus enhancing tumor
growth. Cui et al (38)
reported that G-CSF promoted tumor invasion by inducing EMT in
non-small-cell lung cancer when radiation therapy was performed.
This previous study suggested that G-CSF binds to G-CSFR, which
promotes the Janus kinase (JAK)/STAT3 signaling pathway, resulting
in the induction of EMT (38). In
addition, Yan et al (39)
suggested that infiltration of M2 macrophages was associated with
EMT through the TGF-β pathway. Therefore, treatment with G-CSF may
be able to induce TGF-β systemically and trigger tumor growth by
multiple pathways, including suppression of anti-tumor immunity and
EMT in the tumor microenvironment.
The present results revealed that various cytokines
involved in angiogenesis were elevated in mice treated with CSFs
compared with the control. However, the elevated cytokines were
similar among the three treatment groups. The current IHC and ELISA
results suggest that angiogenesis is predominantly induced by the
interactions between cytokines and cross-talk among cells compared
with the direct effects of these cytokines on angiogenesis. In
addition, TGF-β1/2 was increased by treatment with the three CSFs.
Therefore, recruitment of M2 macrophages and EMT are also induced
by TGF-β1/2 indirectly and various signal pathways, including the
JAK/STAT3 pathway, may be involved. Although further studies are
required to clarify the association between these cytokines and the
mechanism of tumor growth, the knowledge of profiling CSF-induced
cytokines could help to elucidate this in the near future.
The present study has a number of limitations.
First, a single dose was tested for each treatment; therefore,
dose-dependent effects were not evaluated in the current study.
Second, although CSFs are often used together with cytotoxic
chemotherapy (8-12), in the current study cytotoxic
chemotherapeutic agents were not administered in mice. Therefore,
the effects of cytotoxic chemotherapy and CSFs interaction on tumor
growth could not be evaluated. Treatment with G-CSF could enhance
not only the growth of neutrophils but also tumor cells; however,
the effect of G-CSF during chemotherapy needs to be further
investigated. In addition, to confirm whether a combination of
G-CSF and M-CSF demonstrates a similar anti-tumor effect, this
combined treatment should be compared with GM-CSF alone. Although
there are differences in roles, including activation of macrophages
and differentiation of dendritic cells, between GM-CSF and
G-CSF/M-CSF, the effect of a combination of G-CSF and M-CSF on
tumor growth should be investigated. Finally, the sample size of
the human bladder tissues used in the present study was small;
therefore, dual staining analyses using a larger sample size are
required to validate the results of the current study.
In conclusion, the present study demonstrated that
treatment with CSFs has an effect of tumor growth in an UCB animal
model. Notably, treatment with G-CSF exhibited the greatest effect
on tumor growth among the three CSFs. Treatment with G-CSF may
promote angiogenesis, recruitment of M2 macrophages and EMT in the
tumor microenvironment. In addition, CSFRs, particularly G-CSFR,
were demonstrated to not be expressed in cancer-free areas but were
expressed by tumor cells, particularly endothelial cells, and serve
a role in creating a favorable tumor microenvironment for the
survival of tumor cells. An improved understanding of the
association between UCB and CSFs could lead to improved outcomes
and fewer side effects in patients with advanced UCB. Further
studies, including clinical trials, are required to establish the
appropriate use of CSFs during cytotoxic chemotherapy, including
during a combination of gemcitabine and cisplatin for patients with
advanced UCB.
Supplementary Materials
Funding
The present study was supported by Japan Society for
the Promotion of Science KAKENHI (grant nos. 15K10605 and 16K20159)
and Fiscal Years 2015-2016 Nara Medical University Grant-in-Aid for
Collaborative Research Projects.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to hospital policy but
are available from the corresponding author on reasonable
request.
Authors’ contributions
SH, MM and KF contributed to the design of the study
and writing the manuscript. SH, MM, SO, YN, YT and YM performed the
animal experiments and molecular biology studies. SH, YM, KO, KI,
DG and YI assisted with the acquisition of patient data. MM, YN and
NT performed the statistical analysis. MM and KF assisted with
writing the manuscript. MM and YT reviewed the pathological
diagnosis of bladder tissues. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Committee on Animal Research of the Nara Medical
University (Kashihara, Japan) approved the animal study (reference
no. 11883). The Institutional Review Board of Nara Medical
University (Kashihara, Japan) approved the patient study (reference
no. 1630) and all participants provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
UCB
|
urothelial carcinoma of the
bladder
|
|
MIBC
|
muscle invasive bladder cancer
|
|
FN
|
febrile neutropenia
|
|
G-CSF
|
granulocyte colony-stimulating
factor
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
G-CSFR
|
granulocyte colony-stimulating factor
receptor
|
|
MDSC
|
myeloid-derived suppressor cell
|
|
HUVEC
|
human umbilical vascular endothelial
cell
|
|
FBS
|
fetal bovine serum
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
EMT
|
epithelial-mesenchymal transition
|
|
VEGF
|
vascular endothelial growth factor
|
|
HPF
|
high power field
|
|
TNF
|
tumor necrosis factor
|
|
IGF-1
|
insulin-like growth factor-1
|
|
IL
|
interleukin
|
|
FGF
|
fibroblast growth factor
|
|
IFN
|
interferon
|
|
TGF
|
transforming growth factor
|
|
GM-CSFR
|
granulocyte-macrophage
colony-stimulating factor receptor
|
|
M-CSFR
|
macrophage colony-stimulating factor
receptor
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
EPC
|
endothelial progenitor cell
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
JAK
|
Janus kinase
|
Acknowledgments
The authors wish to thank Mrs. Aya Asano (Department
of Pathology, Nara Medical University, Nara, Japan) for providing
substantial help with intraperitoneal treatment. This abstract was
presented at the 32nd Annual EAU Congress, 24-28 March 2017,
London, UK and is published as Abstract no. 965 in European Urology
Supplements (Volume 16, Issue 3, Pages e1681-e1682).
References
|
1
|
Hori M, Matsuda T, Shibata A, Katanoda K,
Sobue T and Nishimoto H; Japan Cancer Surveillance Research Group:
Cancer incidence and incidence rates in Japan in 2009: A study of
32 population-based cancer registries for the Monitoring of Cancer
Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 45:884–891.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishiyama H, Habuchi T, Watanabe J,
Teramukai S, Tada H, Ono Y, Ohshima S, Fujimoto K, Hirao Y,
Fukushima M, et al: Clinical outcome of a large-scale
multi-institutional retrospective study for locally advanced
bladder cancer: A survey including 1131 patients treated during
1990-2000 in Japan. Eur Urol. 45:176–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grossman HB, Natale RB, Tangen CM,
Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF,
Wood DP Jr, Raghavan D, et al: Neoadjuvant chemotherapy plus
cystectomy compared with cystectomy alone for locally advanced
bladder cancer. N Engl J Med. 349:859–866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vale CL; Advanced Bladder Cancer (ABC)
Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive
bladder cancer: Update of a systematic review and meta-analysis of
individual patient data advanced bladder cancer (ABC) meta-analysis
collaboration. Eur Urol. 48:202–205; discussion 205–206. 2005.
View Article : Google Scholar
|
|
6
|
Leow JJ, Martin-Doyle W, Rajagopal PS,
Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL,
Choueiri TK, et al: Adjuvant chemotherapy for invasive bladder
cancer: A 2013 updated systematic review and meta-analysis of
randomized trials. Eur Urol. 66:42–54. 2014. View Article : Google Scholar
|
|
7
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sternberg CN, de Mulder PH, Schornagel JH,
Théodore C, Fossa SD, van Oosterom AT, Witjes F, Spina M, van
Groeningen CJ, de Balincourt C, et al European Organization for
Research and Treatment of Cancer Genitourinary Tract Cancer
Cooperative Group: Randomized phase III trial of
high-dose-intensity methotrexate, vinblastine, doxorubicin, and
cisplatin (MVAC) chemotherapy and recombinant human granulocyte
colony-stimulating factor versus classic MVAC in advanced
urothelial tract tumors: European Organization for Research and
Treatment of Cancer Protocol no 30924. J Clin Oncol. 19:2638–2646.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kotake T, Usami M, Miki T, Togashi M,
Akaza H, Kubota Y and Matsumura Y: Effect of recombinant human
granulocyte colony stimulating factor (lenograstim) on chemotherapy
induced neutropenia in patients with urothelial cancer. Int J Urol.
6:61–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
García-Carbonero R, Mayordomo JI,
Tornamira MV, López-Brea M, Rueda A, Guillem V, Arcediano A, Yubero
A, Ribera F, Gómez C, et al: Granulocyte colony-stimulating factor
in the treatment of high-risk febrile neutropenia: A multicenter
randomized trial. J Natl Cancer Inst. 93:31–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clark OA, Lyman GH, Castro AA, Clark LG
and Djulbegovic B: Colony-stimulating factors for
chemotherapy-induced febrile neutropenia: A meta-analysis of
randomized controlled trials. J Clin Oncol. 23:4198–4214. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohno R, Miyawaki S, Hatake K, Kuriyama K,
Saito K, Kanamaru A, Kobayashi T, Kodera Y, Nishikawa K, Matsuda S,
et al: Human urinary macrophage colony-stimulating factor reduces
the incidence and duration of febrile neutropenia and shortens the
period required to finish three courses of intensive consolidation
therapy in acute myeloid leukemia: A double-blind controlled study.
J Clin Oncol. 15:2954–2965. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barreda DR, Hanington PC and Belosevic M:
Regulation of myeloid development and function by colony
stimulating factors. Dev Comp Immunol. 28:509–554. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Welte K, Platzer E, Lu L, Gabrilove JL,
Levi E, Mertelsmann R and Moore MA: Purification and biochemical
characterization of human pluripotent hematopoietic
colony-stimulating factor. Proc Natl Acad Sci USA. 82:1526–1530.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chakraborty A and Guha S: Granulocyte
colony-stimulating factor/granulocyte colony-stimulating factor
receptor biological axis promotes survival and growth of bladder
cancer cells. Urology. 69:1210–1215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chakraborty A, White SM and Guha S:
Granulocyte colony-stimulating receptor promotes
beta1-integrin-mediated adhesion and invasion of bladder cancer
cells. Urology. 68:208–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yokoyama T, Hyodo M, Hosoya Y, Koinuma K,
Kurashina K, Saitoh S, Hirashima Y, Arai W, Zuiki T, Yasuda Y, et
al: Aggressive G-CSF-producing gastric cancer complicated by lung
and brain abscesses, mimicking metastases. Gastric Cancer.
8:198–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng
Q, Wang Y, Yuan W and Ma J: G-CSF is a key modulator of MDSC and
could be a potential therapeutic target in colitis-associated
colorectal cancers. Protein Cell. 7:130–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Demirci U, Coskun U, Sancak B, Ozturk B,
Bahar B, Benekli M and Buyukberber S: Serum granulocyte
macrophage-colony stimulating factor: A tumor marker in colorectal
carcinoma. Asian Pac J Cancer Prev. 10:1021–1024. 2009.
|
|
20
|
Urdinguio RG, Fernandez AF, Moncada-Pazos
A, Huidobro C, Rodriguez RM, Ferrero C, Martinez-Camblor P, Obaya
AJ, Bernal T, Parra-Blanco A, et al: Immune-dependent and
independent antitumor activity of GM-CSF aberrantly expressed by
mouse and human colorectal tumors. Cancer Res. 73:395–405. 2013.
View Article : Google Scholar
|
|
21
|
Wei XX, Chan S, Kwek S, Lewis J, Dao V,
Zhang L, Cooperberg MR, Ryan CJ, Lin AM, Friedlander TW, et al:
Systemic GM-CSF Recruits Effector T Cells into the Tumor
Microenvironment in Localized Prostate Cancer. Cancer Immunol Res.
4:948–958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Overmeire E, Stijlemans B, Heymann F,
Keirsse J, Morias Y, Elkrim Y, Brys L, Abels C, Lahmar Q, Ergen C,
et al: M-CSF and GM-CSF Receptor Signaling Differentially Regulate
Monocyte Maturation and Macrophage Polarization in the Tumor
Microenvironment. Cancer Res. 76:35–42. 2016. View Article : Google Scholar
|
|
23
|
Mugabe C, Matsui Y, So AI, Gleave ME,
Baker JH, Minchinton AI, Manisali I, Liggins R, Brooks DE and Burt
HM: In vivo evaluation of mucoadhesive nanoparticulate docetaxel
for intravesical treatment of non-muscle-invasive bladder cancer.
Clin Cancer Res. 17:2788–2798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hori S, Miyake M, Onishi S, Tatsumi Y,
Morizawa Y, Nakai Y, Anai S, Tanaka N and Fujimoto K: Clinical
significance of α and β Klotho in urothelial carcinoma of the
bladder. Oncol Rep. 36:2117–2125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hori S, Miyake M, Tatsumi Y, Morizawa Y,
Nakai Y, Onishi S, Onishi K, Iida K, Gotoh D, Tanaka N, et al:
Gamma-Klotho exhibits multiple roles in tumor growth of human
bladder cancer. Oncotarget. 9:19508–19524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyake M, Hori S, Morizawa Y, Tatsumi Y,
Nakai Y, Anai S, Torimoto K, Aoki K, Tanaka N, Shimada K, et al:
CXCL1-Mediated Interaction of Cancer Cells with Tumor-Associated
Macrophages and Cancer-Associated Fibroblasts Promotes Tumor
Progression in Human Bladder Cancer. Neoplasia. 18:636–646. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raj L, Ide T, Gurkar AU, Foley M, Schenone
M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al:
Selective killing of cancer cells by a small molecule targeting the
stress response to ROS. Nature. 475:231–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Segawa K, Ueno Y and Kataoka T: In vivo
tumor growth enhancement by granulocyte colony-stimulating factor.
Jpn J Cancer Res. 82:440–447. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okazaki T, Ebihara S, Asada M, Kanda A,
Sasaki H and Yamaya M: Granulocyte colony-stimulating factor
promotes tumor angiogenesis via increasing circulating endothelial
progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int
Immunol. 18:1–9. 2006. View Article : Google Scholar
|
|
31
|
Lyden D, Hattori K, Dias S, Costa C,
Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et
al: Impaired recruitment of bone-marrow-derived endothelial and
hemato-poietic precursor cells blocks tumor angiogenesis and
growth. Nat Med. 7:1194–1201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davidoff AM, Ng CY, Brown P, Leary MA,
Spurbeck WW, Zhou J, Horwitz E, Vanin EF and Nienhuis AW: Bone
marrow-derived cells contribute to tumor neovasculature and, when
modified to express an angiogenesis inhibitor, can restrict tumor
growth in mice. Clin Cancer Res. 7:2870–2879. 2001.PubMed/NCBI
|
|
33
|
Kelly ME, Mohan HM, Baird AW, Ryan EJ and
Winter DC: Orphan Nuclear Receptors in Colorectal Cancer. Pathol
Oncol Res. 24:815–819. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee AS, Kim D, Wagle SR, Lee JE, Jung YJ,
Kang KP, Lee S, Park SK and Kim W: Granulocyte colony-stimulating
factor induces in vitro lymphangiogenesis. Biochem Biophys Res
Commun. 436:565–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fiorentini S, Luganini A, Dell’Oste V,
Lorusso B, Cervi E, Caccuri F, Bonardelli S, Landolfo S, Caruso A
and Gribaudo G: Human cytomegalovirus productively infects
lymphatic endo-thelial cells and induces a secretome that promotes
angiogenesis and lymphangiogenesis through interleukin-6 and
granu-locyte-macrophage colony-stimulating factor. J Gen Virol.
92:650–660. 2011. View Article : Google Scholar
|
|
36
|
Kitoh Y, Saio M, Gotoh N, Umemura N,
Nonaka K, Bai J, Vizkeleti L, Torocsik D, Balazs M, Adany R, et al:
Combined GM-CSF treatment and M-CSF inhibition of tumor-associated
macrophages induces dendritic cell-like signaling in vitro. Int J
Oncol. 38:1409–1419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Elghonaimy EA, Ibrahim SA, Youns A,
Hussein Z, Nouh MA, El-Mamlouk T, El-Shinawi M and Mostafa Mohamed
M: Secretome of tumor-associated leukocytes augment
epithelial-mesenchymal transition in positive lymph node breast
cancer patients via activation of EGFR/Tyr845 and NF-κB/p65
signaling pathway. Tumour Biol. 37:12441–12453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cui YH, Suh Y, Lee HJ, Yoo KC, Uddin N,
Jeong YJ, Lee JS, Hwang SG, Nam SY, Kim MJ, et al: Radiation
promotes invasiveness of non-small-cell lung cancer cells through
granulocyte-colony-stimulating factor. Oncogene. 34:5372–5382.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yan Y, Zhang J, Li JH, Liu X, Wang JZ, Qu
HY, Wang JS and Duan XY: High tumor-associated macrophages
infiltration is associated with poor prognosis and may contribute
to the phenomenon of epithelial-mesenchymal transition in gastric
cancer. OncoTargets Ther. 9:3975–3983. 2016. View Article : Google Scholar
|