Introduction
Neurofibromatosis type 1 (NF1) is an
autosomal-dominant inherited tumor susceptibility syndrome with an
incidence of approximately 1 per 3,000-4,000 individuals worldwide
(1). From a clinical perspective,
patients with NF1 present with café-au-lait macules, Lisch nodules,
gliomas of the optic tract, specific osseous lesions and
neurofibromas (2). As the main
manifestation of patients with NF1, neurofibromas are classified
based on the anatomical region, such as cutaneous, subdermal,
plexiform and intraneural neurofibromas. Plexiform neurofibromas
indicate a malignant tendency, whereas cutaneous neurofibromas
(cNFs) are considered the hallmark of NF1 (2). These tumors are undetectable at birth
and appear during adolescence (2).
Although malignant progression is a rare event in cNF, the
psychosocial management of this disease should not be ignored.
Surgery and laser therapy exhibit limited effectiveness in the
treatment of cNF. However, they are the only type of treatment for
large-sized cNF tumors. The lack of standard therapeutic modalities
has led to the continuous search for novel treatment options.
cNFs usually appear at puberty and plexiform
neurofibromas are capable of aggressive growth during puberty
(3,4). The contributions from the genetic
background of each patient (gene mutations) and from the
environmental conditions (trauma and altered hormone levels) may
act as 'triggers' for tumorigenesis, enlargement, or malignant
progression (5). Steroid hormones
contribute to the development of a number of tumors, such as
breast, and prostate cancer, renal cell carcinoma and benign
meningioma. Nevertheless, a limited number of studies have been
performed to investigate the role of steroid hormones in
neurofibromas. Previous studies have examined the expression levels
of estrogen receptor (ER) and progesterone receptor (PR) on
cultured Schwann cells (6-8). The proliferative response to
estradiol and progesterone was further detected in these cells
(6-8). Moreover, no difference was noted in
the progression of neurofibromas during puberty between the two
sexes. Rare cross-reactivity or binding to non-native receptors has
been noted following steroid binding (9). Considering the elevated androgen
levels in adolescent male patients with NF1, particular attention
has been paid on the function of the androgen receptor (AR) as
regards the progression of neurofibroma.
Neurofibroma is a highly vascularized tumor
(10) that can increase
substantially in size without avascular necrosis. Previous studies
have shown that the activation of platelet-derived growth factor
receptor (PDGFR) and vascular endothelial growth factor receptor
(VEGFR) can occur in the neurofibroma microenvironment (11,12).
Furthermore, the application of specific inhibitors for VEGFR and
PDGFR has been shown to reduce growth and induce regression of
neurofibroma in a mouse model (13). The malignant transformation and
progression of several tumors is linked to angiogenesis and is
dependent on the induction of this process (14-16).
The 'angiogenic switch' is usually triggered following two possible
processes: The reduction in the angiogenesis inhibitors and/or the
activation of the angiogenesis inducers (17). Among the inducers, VEGF plays a
pivotal role in tumor angiogenesis, due to the prominent, although
not exclusive, expression of VEGFR on endothelial cells. In
addition, the ligand, vascular endothelial growth factor (VEGF), is
usually secreted by tumor cells and the stroma (18). Moreover, AR signaling plays
critical roles in mediating angiogenesis in various tumors.
In the present study, we demonstrated that activated
AR signaling promotes neurofibroma angiogenesis by modulating VEGFA
expression and secretion. Specific inhibitors targeting AR
signaling may thus suppress cNF progression and improve its
treatment.
Materials and methods
Chemicals and reagents
MDV3100 (enzalutamide) was purchased from Selleck
Chemicals and dihydrotestosterone (DHT) from Sigma-Aldrich. X-treme
GENE HP DNA transfection reagent was obtained from Roche and the
simpleChIP® enzymatic chromatin IP kit (Magnetic Beads)
from Cell Signaling Technology, Inc. All the reagents were stored
and used according to the protocol provided by the manufacturer.
The primary antibodies for neurofibromin (ab17963) and VEGFA
(ab46154) were purchased from Abcam. The primary antibody for AR
(#5153) was purchased from Cell Signaling Technology, Inc.
Cell culture and RNA interference
Murine SW10 Schwann cells and human skin fibroblasts
were propagated in DMEM/F12 with 10% fetal bovine serum (FBS). The
cells were maintained in a humid atmosphere with 5% CO2
at 37°C. Recombinant replication-defective lentiviruses harboring
Nf1-specific short hairpin RNA (shRNA) or control shRNA were
employed to transfect the cells, which were then named shNf1 or
shNC cells, respectively. The efficiency of shRNA transfection was
detected by western blot analysis. The siRNAs targeting AR were
purchased from RiboBio. The cells were infected by lentiviruses at
a confluence of 70-80% or transfected with siRNA using X-treme GENE
siRNA Transfection Reagent (Roche) at a confluence of 30-50%.
Patients and tissue samples
Paraffin-embedded tissues from 29 patients with NF1
were collected from the first Affiliated Hospital of Xi'an Jiaotong
University and used for the immunohistochemical (IHC) analysis of
AR, CD31 and VEGFA expression. The tissue procurement protocol was
approved by the Institutional Review Board and informed consent was
provided from each patient. The sections with a thickness of 5
µm were derived from all the paraffin-embedded tissues and
were accordingly prepared. The staining for AR (1:200), VEGFA
(1:200) and CD31 (1:150) was performed using the DAKO Autostainer
Plus system. One pathologist analyzed the sections under a
high-power field (x400 magnification) in a double-blind protocol
setup. Microvessels were defined as CD31+ endothelial
cells or a cell cluster detached from any microvessel structures.
The average number of microvessels from 10 random fields was
defined as the microvessel density (MVD). The AR staining score was
calculated by both intensity and percentage. The tendency score was
estimated as follows: 0, 1, 2 and 3 indicated no staining, weak
positive staining, moderate positive staining and strong positive
staining, respectively. The percentage score was estimated as
follows: 0, 0%; 1, ≤25%; 2, 25-50%; 3, 50-75%; and 4, ≥75%. The
total score was calculated by the multiplication of the intensity
score with the percentage score. The clinicopathological
characteristics of the patients are presented in Table I.
 | Table IClinicopathologic characteristics of
the patients with neurofibromatosis type 1. |
Table I
Clinicopathologic characteristics of
the patients with neurofibromatosis type 1.
| Characteristic | No. (%) |
|---|
| Age (years) | |
| ≤20 | 14 (48.3) |
| >20, ≤40 | 10 (34.5) |
| >40 | 5 (17.2) |
| Sex | |
| Female | 0 |
| Male | 29 |
RNA extraction and reverse
transcription-quantitative PCR analysis (RT-qPCR)
A total RNA extraction kit (Fastagen Biotech) was
used to extract the total RNA, which was subsequently subjected to
reverse transcription by SuperScript III Transcriptase (Thermo
Fisher Scientific). qPCR was performed using the Bio-Rad CFX96
system with SYBR-Green for analysis of the mRNA levels of each
specific gene. Human GAPDH cDNA was used as the internal
control. The primer sequences are listed in Table SI. The PCR thermocycling
conditions were as follows: Step 1: 95°C, 30 sec, 1×; step 2: 95°C,
0 sec, and 60°C, 30 sec, 39×; step 3: 4°C, +∞, 1×.
Western blot analysis
RIPA lysis buffer containing protease inhibitor was
used for total protein extraction. The Bradford assay was used to
detect the protein concentration. Briefly, 12% SDS-polyacrylamide
gels were used to separate 30 µg of protein prior to
blotting onto the nitrocellulose filter membranes. Non-fat-milk
(5%) in Tris-buffered saline and Tween-20 were used to block the
non-specific bindings sites of the membranes. The membranes were
incubated with specific primary antibodies overnight at 4°C and the
following day with secondary antibodies for 1 h at room
temperature. Molecular Imager ChemiDoc XRS System (Bio-Rad
Laboratories, Inc.) was used to visualize the protein bands.
Immunoblotting of GAPDH was used as the internal control. The
primary antibodies for neurofibromin (ab17963, 1:1,500) and VEGFA
(ab46154, 1:1,500) were purchased from Abcam. Primary antibody for
AR (#5153, 1:1,000) and horseradish peroxidase-conjugated secondary
antibodies (#7074 for anti-rabbit IgG and #7076 for anti-mouse IgG,
1:200) were purchased from Cell Signaling Technology, Inc.
Conditioned medium collection and
ELISA
A total of 5×105 shNf1-SW10 cells or
shNf1 fibroblasts were seeded in a 60-mm culture dish. Following 24
h of incubation, the cells were washed with serum-free medium (SFM)
3 times, and an additional 5 ml of SFM were added. The cells were
then cultured for an additional 24 h at 37°C. The supernatants were
centrifuged (1,000 × g, 5 min) to remove the cell debris and the
conditioned medium (CM) was stored at -80°C. The RayBio®
Human VEGF ELISA kit (RayBiotech Inc.) was used to examine the
concentration of VEGF in CM. The VEGF concentration was modified by
the addition of neutralizing VEGF antibody (MAB293) or the addition
of recombinant human VEGF (AF-293-NA) (both from R&D
Systems).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability and growth rate were analyzed by MTT
assay. Briefly, 4×103 cells were seeded in 96-well
culture plates. After 24 h, the cells were treated with DHT (5 nM)
or DMSO for the indicated time periods (0, 24, 48 and 72 h). The
cells were then washed and incubated with MTT solution (M2128;
Sigma-Aldrich) (0.5 mg/ml) at 37°C. Following 4 h of incubation,
the medium was carefully removed and 150 µl of DMSO was used
to solubilize the formazan crystals. The absorbance of each well
was detected by a microplate automatic reader (Bio-Tek Instruments,
Inc.) at 490 nm.
Androgen-AR mediated Schwann cell growth
in vivo
A total of 12 4-week-old nude mice were randomly
allocated to 2 groups with random digits. All mice were injected
with 2×106 shNf1-SW10 cells subcutaneously. One week
later, the mice in the 2 groups were treated daily with 100
µl of saline and with 0.6 mg/kg of DHT, respectively. The
body weight was measured every 3 days. Following 4 weeks of
xenograft tumor growth, the mice were sacrificed and the tumors
were excised surgically and measured. A solution with 4%
paraformaldehyde was used to fix the tumors. The samples were
embedded in paraffin, and IHC staining of VEGFA expression was
performed. The animal experiments were approved by the
Institutional Review Board of the First Affiliated Hospital of
Xi'an Jiaotong University.
Colony formation assay
A total of 1,000 shNf1-SW10 cells or shNf1
fibroblasts were seeded into 1 well of a 6-well plate. The cells
were treated with DHT (5 nM) or saline for 2 weeks. Subsequently,
the cells were washed, fixed with 4% paraformaldehyde and stained
with 0.1% crystal violet (C6158; Sigma-Aldrich) solutions for 15
min at room temperature. The cells were then washed with PBS 3
times at room temperature. Visible cell colonies in each well were
counted and the average colony number of each treatment was
calculated.
Tube formation assay
The human umbilical vascular endothelial cell
(HUVEC) cell line was purchased from the American Type Culture
Collection (ATCC). SFM or SFM added with CM (1:1) were prepared for
HUVEC (1×105) culture. A 24-well plate with
Matrigel-coated wells was used for the tube formation assay.
Imaging was conducted using an optical microscope (IX50-S8F2;
Olympus) at the 6-h time period. A tube was identified as 2
branching points that were perfectly connected.
Cell cycle analysis
The cells were passaged at a confluence of 60-80%
with Trypsin/EDTA. Subsequently, the cells were washed with cold
PBS resuspended in ice-cold 70% ethanol and stored for >24 h at
−20°C. On the day of the analysis, the ethanol was removed and the
cells were washed 2 times with cold PBS. RNAse A (0.5 µg/ml)
and propidium iodide (50 µg/ml) were added to the cells that
were incubated at room temperature for 30 min in the dark prior to
flow cytometric analysis (BD FACSCalibur™ Flow Cytometer; BD
Biosciences).
HUVEC migration assay
Transwell migration analysis was conducted by
8-µm-pore Transwell inserts (Millipore Corp.). HUVECs
(3×105 cells/ml) were mixed with 300 µl of SFM
and subsequently seeded into the upper chamber of the Transwell. In
the lower chamber, 1 ml of CM or neurofibroma cells treated with
reagents was added. Following 16 h of culture, the cells that
migrated to the lower surface of the inserts were fixed with 4%
paraformaldehyde and stained using 0.1% crystal violet for 15 min
at room temperature. The visible cells were counted in 5 random
fields (×200 magnification) for each insert with an optical
microscope (IX50-S8F2; Olympus).
Dual luciferase activity assay
The promoter region of VEGFA (-1618 to +100) was
amplified and inserted into the pGL3-basic plasmids (GenePharma),
denoted as pGL3-VEGFA. Nf1-ablated SW10 cells and fibroblasts were
transfected with pGL3-basic or pGL3-VEGFA by X-tremeGENE HP DNA
transfection reagent (Roche). A control sample was prepared by the
addition of the DNA transfection reagent without any plasmids
(pGL3-control). A dual luciferase assay kit (Promega) was used for
the luciferase assay following the instructions of the
manufacturer. The luciferase activity of each well was normalized
by comparison with Renilla luciferase activity. The data
from 3 wells were collected and the average value was used for
analysis of luciferase activity of each sample. The primers used
for PCR amplification were the following: forward
(5′-ATTCCCATTCTCAGTCCATG-3′) and reverse
(5′-CTGACCGGTCCACCTAACCG-3′).
Chromatin immunoprecipitation assay
The SimpleChIP® enzymatic chromatin IP
kit was used for ChIP assay in Nf1-ablated SW10 cells and
fibroblasts following the indicated protocol. The precipitation of
the protein/DNA complex was achieved by antibodies against the AR
(#5153, 1:50; Cell Signaling Technology, Inc.) or against normal
rabbit IgG (from the kit). The DNA of interest was detected with
genetic region-specific primers (Table SII) and amplified by PCR.
Oligonucleotides pull-down assay
Biotin was added to the oligonucleotides of the
specific sets of the VEGFA promoter
(biotin-5′-CTTCCCCTGCCCCCTTCAATATTCCTAGCAAA GAGGGAACGGCTCT-3′,
synthesized by GENEWIZ). The lysis buffer contained NaCl (150 mM),
Tris HCl (50 mM, pH 7.4), EDTA (1 mM) and Triton X-100 (1%).
Nf1-ablated SW10 cells and fibroblasts were treated with 10
ng/ml of DHT for the indicated time periods (0, 12, 24 and 36 h).
The cells were lysed in lysis buffer with protease and phosphatase
inhibitors. Following centrifugation (15,000 × g, 4°C, 15 min), 20
µl of ImmunoPure streptavidin-agarose beads were added to
each cell extract at 4°C. Following 1 h of incubation at 4°C, the
bead-bound cell extracts were centrifuged at 5,000 × g for 1 min at
4°C. The supernatant was collected and incubated with 100 pmol of
biotinylated oligonucleotides at 4°C for 24 h. The immobilized
streptavidin-agarose beads (30 µl) were used to precipitate
DNA-bound proteins at 4°C. Following 1 h of incubation,
centrifugation (5,000 × g, 4°C, 1.5 min) was performed and the
supernatant was carefully discarded. The precipitate was washed 3
times with lysis buffer prior to western blot analysis.
Statistical analysis
Statistical analyses were performed by GraphPad
Prism (version 5.0; GraphPad Software, Inc.) software, and the
Student's t-test was used for 2-group comparisons. For comparisons
of >2 groups, we used one-way ANOVA and Fisher's Least
Significant Difference test (LSD test) with statistical software
SPSS for Windows 10.0. For the correlation analysis, we employed
the Spearman's correlation test with SPSS. A P-value <0.05
(P<0.05) was considered to indicate statistically significant
differences.
Results
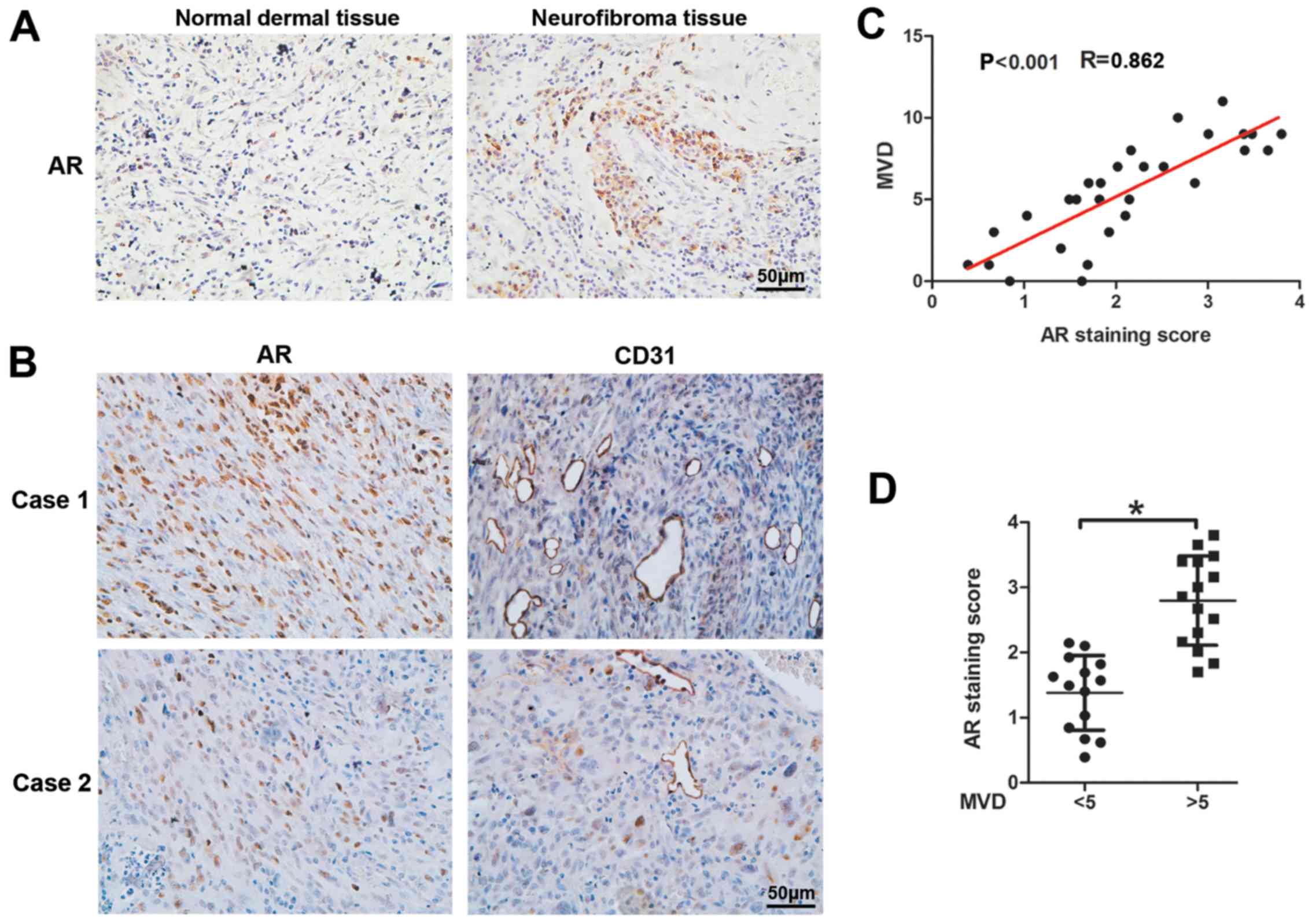
AR expression is positively associated
with cNF angiogenesis
To explore the potential association of AR
expression and cNF angiogenesis, we performed IHC assays with
antibodies against AR and CD31 (marker of vascular endothelial
cells) of 29 cNF and 29 adjacent normal dermal tissues from male
patients. The results revealed significantly elevated AR expression
levels in cNF tissues compared with those noted in adjacent tissues
(Fig. 1A). CD31 staining of the
cNF tissues indicated an increased MVD (Fig. 1B) and an enhanced AR expression
that was associated with MVD (Fig.
1D). Moreover, a positive linear correlation was noted between
MVD and AR expression in the human cNF tissues (r=0.862,
P<0.001, Fig. 1C). Taken
collectively, the results indicated that AR expression was
positively associated with angiogenesis in human cNF samples
(Fig. 1).
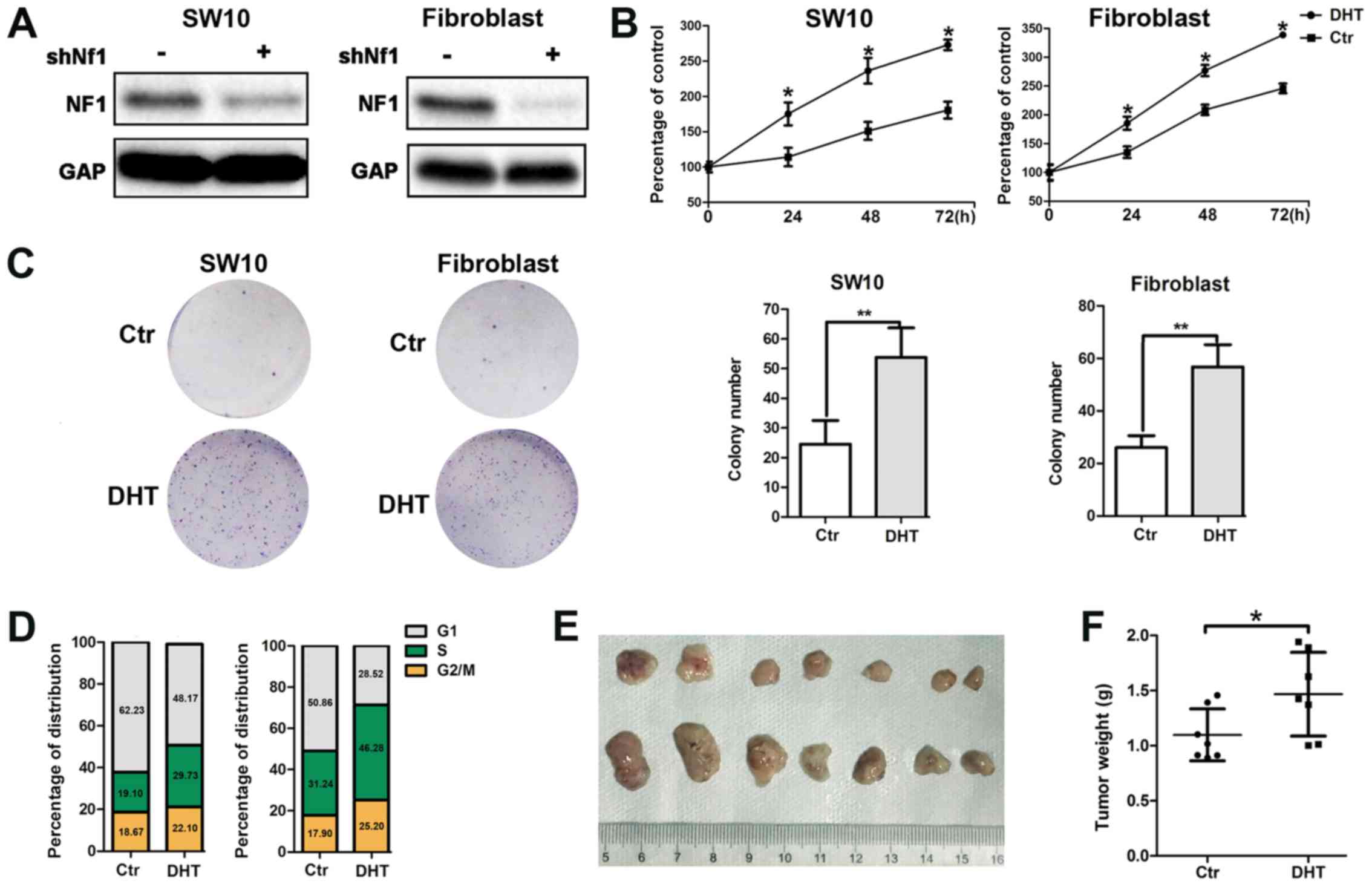
Activated AR promotes neurofibroma the
proliferation of shNf1-SW10 cells and shNf1 fibroblasts in vitro
and in shNf1-SW10 xenografts in vivo
The loss-of-function mutation of the Nf1 gene
is considered a major genetic change responsible for the
development of the NF1 type disease. Therefore, we constructed
Nf1 knockout (k/o) SW10 cells and Nf1 k/o fibroblasts
using lentivirus-delivered specific shRNA (Fig. 2A). To further identify the role of
AR signaling in neurofibroma, we monitored the activation of the AR
with DHT binding and examined the enhanced cellular proliferation
by MTT assay (Fig. 2B). In
addition, we performed s colony formation assay and noted an
increased colony number in the aforementioned groups (Fig. 2C). Moreover, DHT treatment resulted
in an increased number of cells entering the S phase, as
demonstrated by flow cytometric assay (Fig. 2D).
Furthermore, the subcutaneous tumorigenesis
potential of the shNf1-SW10 cells was significantly increased
following DHT intraperitoneal injection. Following 4 weeks of
xenograft growth, tumor weight was considerably higher in the
DHT-treated nude mice compared with that noted in the control
animals (1.468±0.1434 mg, n=7 vs. 1.098±0.08905 mg, n=7) (Fig. 2E).
Taken collectively, the results demonstrated that
active AR signaling promoted neurofibroma cell viability (Fig. 2), which is consistent with the
clinical observations regarding the increased number and size of
neurofibroma during puberty.
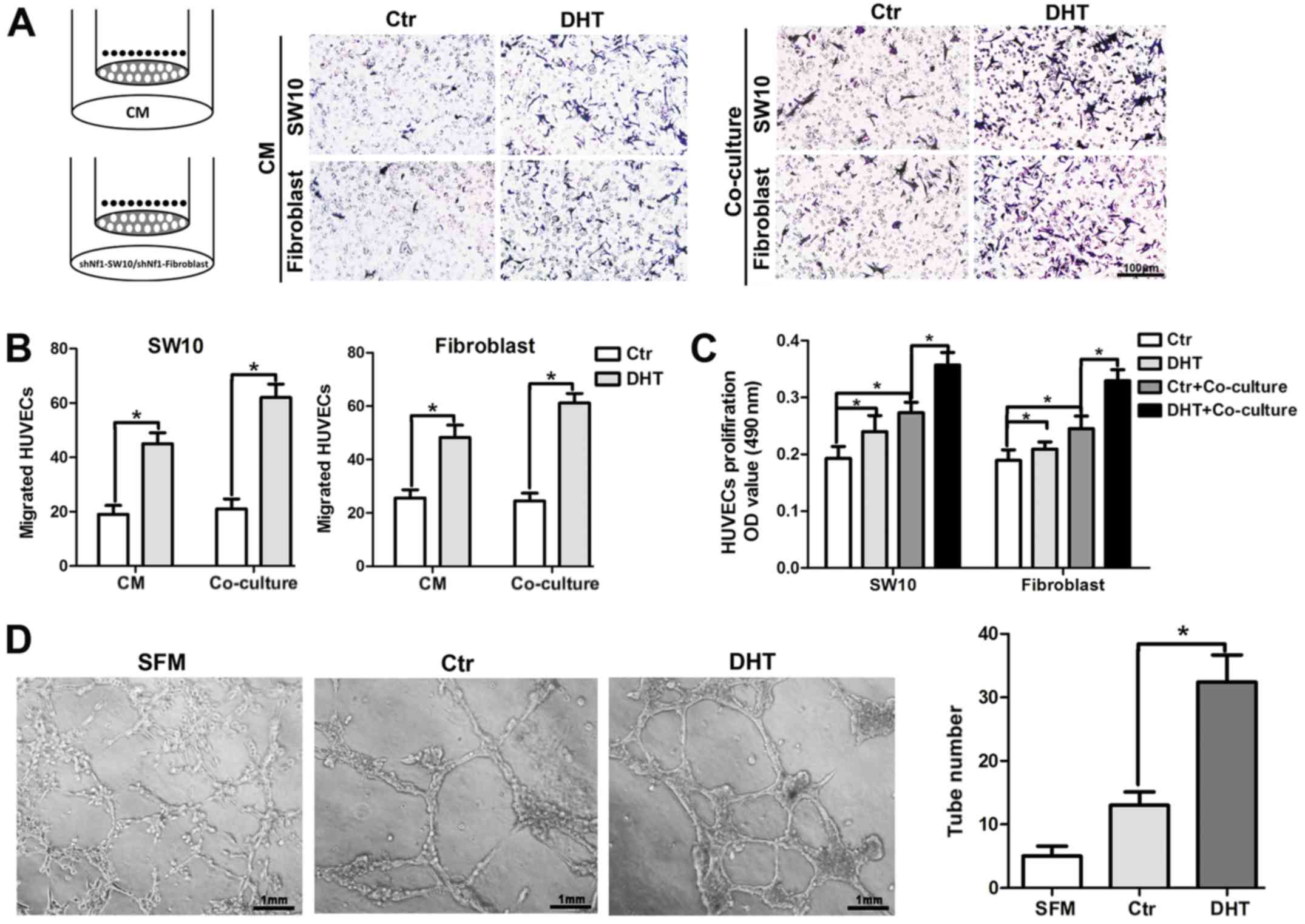
AR signaling promotes the angiogenesis of
neurofibroma
Since AR expression was increased with MVD in human
cNF tissues, we employed HUVEC recruitment assays to further
confirm the effects of AR signaling on angiogenesis. Briefly, DHT-
or saline-treated shNf1-SW10 cells and shNf1 fibroblasts were
seeded to the lower chamber or CM from Nf1 ablated cNF cells with
or without treatment with DHT were added to the lower chamber of
the Transwell and the HUVECs were seeded in the upper chamber of
the Transwell. Following incubation at 37°C for 16 h, the CM from
DHT-treated cells attracted additional HUVECs to the lower surface
of the upper chamber compared with those of the control group
(Fig. 3A and B, quantitative
data). As regards the co-culture system, neurofibroma cells were
seeded in the upper chamber and HUVECs were added to the lower
chamber. We detected multiple viable cells in the HUVECs
co-cultured with neurofibroma cells and in HUVECs treated with DHT
(Fig. 3C). Moreover, the capillary
tube formation on Matrigel was significantly higher in the
DHT-treated shNf1-SW10 cells compared with that of the control
cells (Fig. 3D).
On the whole, the results shown in Fig. 3 suggest that the activated AR
signaling may modulate the paracrine function of neurofibroma cells
and may alter the interaction of neurofibroma cells with HUVECs.
These processes can promote angiogenesis.
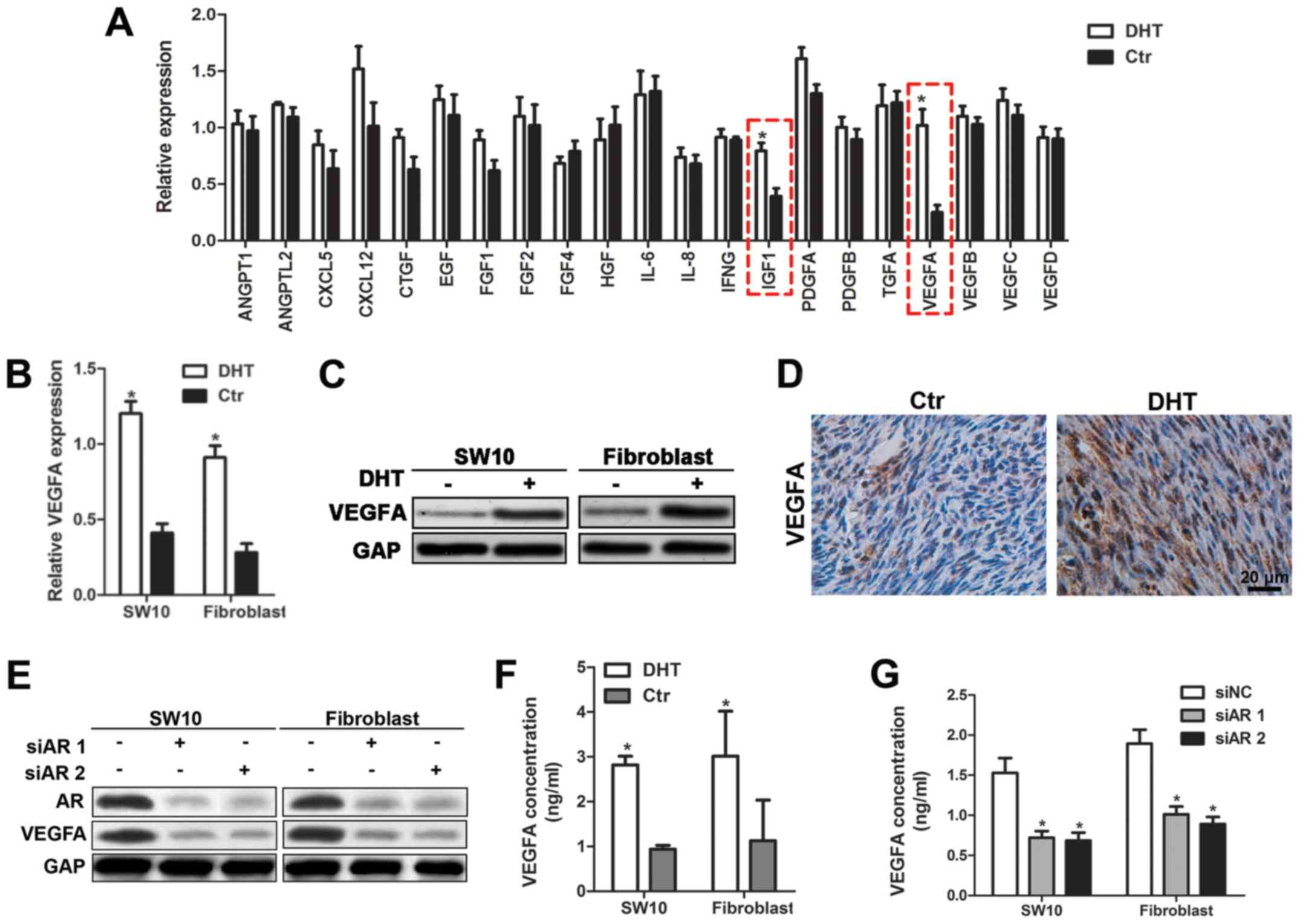
AR regulates VEGFA expression and
mediates angiogenesis in neurofibroma
Since AR binds to DNA and promotes the transcription
of target genes, we hypothesized that the activation of AR
signaling may cause the release of angiogenic factors and promote
angiogenesis in neurofibroma. Therefore, we performed RT-qPCR assay
and detected the expression levels of angiogenic cytokines in
shNf1-SW10 cells with or without DHT treatment. Statistical
analysis indicated that VEGFA and IGF-1 were key players involved
in angiogenesis of neurofibroma cells (Fig. 4A). Considering the predominant
roles of VEGFA in tumor angiogenesis, we focused on the regulation
of VEGFA by AR. The mRNA and protein expression levels of
VEGFA were significantly increased following DHT treatment
(Fig. 4B and C). In addition, the
expression levels of VEGFA were monitored in DHT-treated tumors
(Fig. 4D). The transfection of
shNf1-SW10 cells and shNf1 fibroblasts with AR-specific siRNA
effectively impaired AR expression and reduced VEGFA expression
(Fig. 4E). Furthermore, ELISA was
performed to detect the reduction in VEGFA secretion caused by siAR
transfection and the increase in VEGFA expression following DHT
treatment in the medium (Fig. 4F and
G). The data indicated that the inhibition of the AR signaling
could be considered a novel strategy to abolish HUVEC recruitment.
Taken together, these data demonstrated that AR signaling regulated
neurofibroma angiogenesis by promoting VEGFA expression.
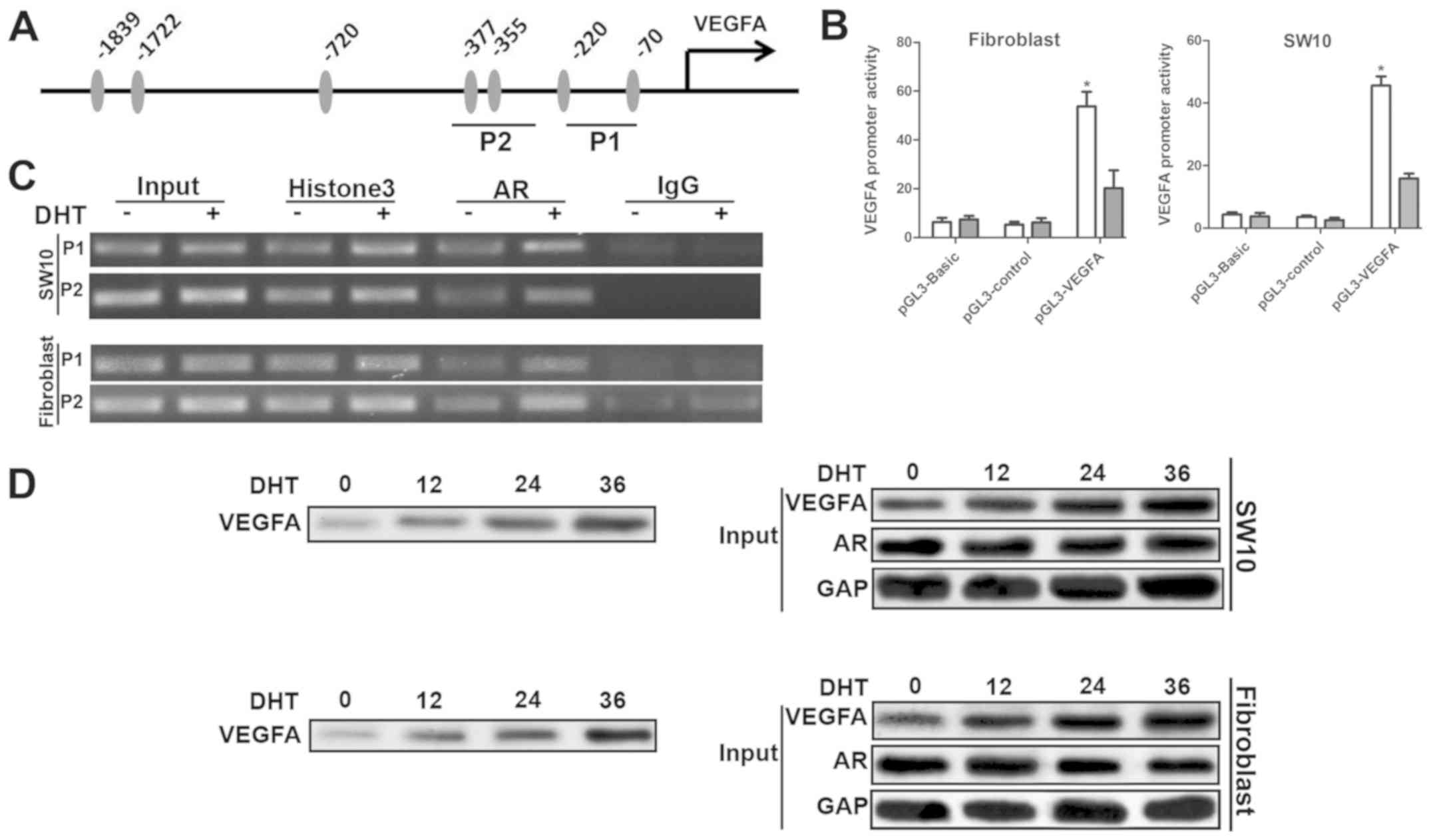
AR enhanced VEGFA transcription by direct
interaction with the VEGFA promoter
AR has been shown to bind to the promoters of target
genes at the androgen receptor binding element (ARE). We found 7
putative AREs approximately 1,700 bp from the transcription
initiation site (Fig. 5A).
Subsequently, we cloned the DNA fragment, which was inserted into
the pGL3-basic luciferase reporter plasmid to construct the
pGL3-VEGFA plasmid. The dual luciferase assay in shNf1-SW10 cells
and shNf1 fibroblasts indicated that DHT significantly enhanced the
activity of pGL3-VEGFA plasmids (Fig.
5B), which confirmed the increase in VEGFA mRNA levels
in DHT-treated cells. Four primer pairs specific for amplifying
different regions of the VEGFA promoter were employed in the ChIP
assay. A total of 2 out of 4 regions maintained a higher binding
affinity with the AR (Fig. 5C). To
further clarify whether AR binds directly to the −400/−50 region,
which is close to the transcription initiation site of the VEGFA
promoter, oligonucleotides with the same sequences were synthesized
with biotin bound at the 5' terminus to pull-down the AR proteins.
The cells that had been treated with DHT indicated the
amplification of AR binding to the VEGFA promoter, which explained
the upregulation in the expression of VEGFA following DHT treatment
(Fig. 5D). Taken collectively,
these data suggested that AR acted as a transcription factor bound
directly to the VEGFA promoter that could initiate its
transcription (Fig. 5).
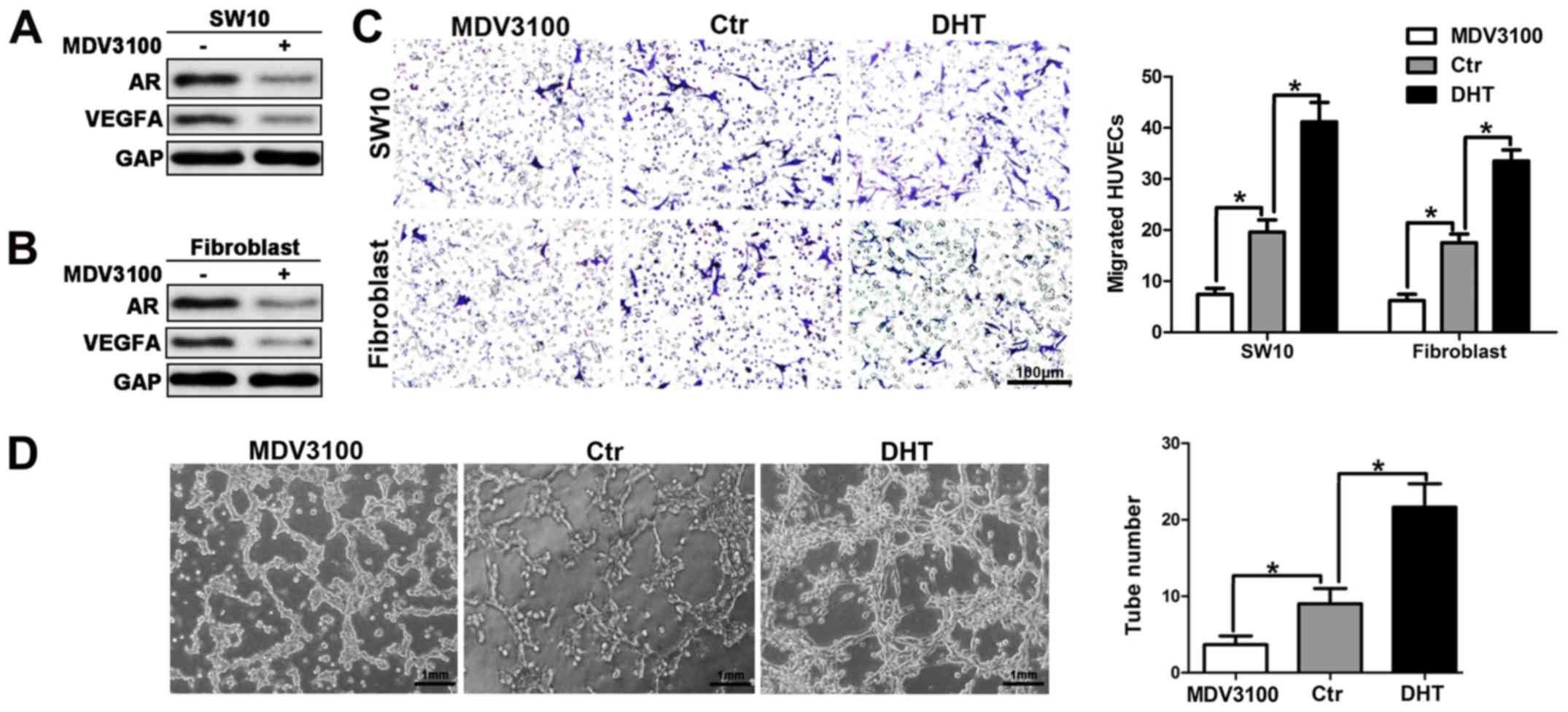
Inhibition of AR signaling suppresses
angiogenesis in neurofibroma
The activation of the AR signaling can increase
VEGFA expression and secretion, by promoting neurofibroma
angiogenesis. Therefore, we further explored the latent therapeutic
action of AR targeting in neurofibroma. Enzalutamide or MDV3100 is
an androgen receptor antagonist that was selected to reduce the
activation of the AR signaling. MDV3100 reduced AR and VEGFA
protein levels, which was consistent with the results obtained from
the cells transfected with siRNA for AR (Fig. 6A and B). To examine the effects of
MDV3100 on HUVEC recruitment, CM from shNf1-SW10 cells and shNf1
fibroblasts was used to attract HUVECs. The results indicated a
weakened HUVEC migration in the aforementioned groups (Fig. 6C). In addition, diminished tube
formation was noted in the presence of CM from cells with MDV3100
treatment. The activation of the AR signaling following DHT
treatment promoted HUVEC recruitment and tube formation, which was
consistent with the results noted in Fig. 3.
The results shown in Fig. 6 demonstrated that the inhibition of
AR signaling suppressed HUVEC recruitment and impaired tube
formation of these cells, which further suggested that the
inhibition of AR signaling could partially disrupt the angiogenesis
of neurofibroma.
Discussion
Cutaneous neurofibromas are usually associated with
morbidity and exhibit resistance to conventional chemotherapy. To
date, several promising treatments have failed to show therapeutic
efficacy in clinical trails (19-22),
which highlights the importance of identifying molecular mechanisms
and novel targets for these neoplasms. In the present study, we
identified several potential AR binding sites in the promoter of
VEGFA and demonstrated that activated AR significantly enhanced
VEGFA transcription. The aforementioned findings were similar with
the regulation of the VEGFA transcription by the AR noted in
bladder cancer (23). Moreover, an
elevated AR expression and MVD were found in human cNF samples,
whereas AR protein levels were associated with MVD (Fig. 1), which further confirmed the
regulatory role of AR in regulating VEGFA expression in cNF.
Although the hyperproliferation of neural crest
tumors are considered hallmark features of NF1, specific disorders
that are often found in affected individuals include hypertension,
an increased probability of vascular diseases and congenital heart
disease that are not directly related to the neural system
(24-26). Neurofibromin is also expressed in
endothelial and smooth muscle cells (27), suggesting that an altered
neurofibromin function in these cells may be attributed to
vasculopathy in patients with NF1. cNF is widely accepted as a
vascularized type of solid tumor with complex Nf1+/−
cells comprising Schwann cells, fibroblasts, endothelial and
inflammatory cells. The role of endothelial cells in cNF
development has not been well defined. The results of this study
demonstrated an increased HUVEC proliferation and recruitment
accompanied by an enhanced tube formation ability upon interaction
of these cells with neurofibroma cells. This disposition of HUVECs
led to the accelerated angiogenesis noted in neurofibroma.
The angiogenic effects of the endothelial cells are
mainly driven by VEGF (28) and
preliminary studies have demonstrated an altered angiogenesis,
which is associated with the signaling of Nf1+/−. In
addition, Schwann cells express and secrete various types of
ligands (29-33), which are capable of regulating
multiple cell functions in the neurofibroma microenvironment. The
results demonstrated that the knockdown of the Nf1 gene
increased VEGFA secretion in Schwann cells and fibroblasts, which
were considered the principal cells encountered in neurofibroma
(pathognomonic tumor of NF1). In addition, Nf1-k/o HUVECs exhibited
optimal recruitment and an angiogenic phenotype. Therefore VEGFR in
HUVECs may participate in this process, which is possibly
associated with tumor progression. This finding is consistent with
the findings of previous studies (34-37)
reporting that neurofibroma-associated growth factors (e.g., PDGF
and VEGF) can alter the function of endothelial cells with
dysfunctional neurofibromin, which is considered a critical step in
angiogenesis.
It is widely accepted that the transformation of the
endothelial cells to the angiogenic phenotype plays a key role for
the increase in tumor size (38,39).
Angiogenesis may be critical for cNF enlargement, since cNF can be
increased to a substantial tumor mass (kg). Schwann cells with
neurofibromin loss have been shown to promote angiogenesis
(40,41) and increase VEGF secretion. Although
an increased vascular density is noted in malignant peripheral
nerve sheath tumor (MPNST), the mechanism of the angiogenic switch
requires further clarification (10). The association of steroid hormones
with cNF is due to the role of this tumor at puberty (43). An increase in numbers and size can
be observed during puberty and pregnancy (42), which suggests that the elevated
steroid hormones during puberty may contribute to the
'aggressiveness' of cNF progression. The present study indicated an
amplified MVD and increased AR expression in human neurofibroma
tissues. Moreover, the data indicated that activated AR signaling
can enhance tube formation in vitro and in vivo. Our
results suggested that activated AR signaling promoted vascular
formation that in turn contributed to neurofibroma growth. These
findings are in concordance with those of previous studies
(43-45).
AR expression is ubiquitously found in various types
of cells (46,47). Upon the binding of an androgen to
the AR, the receptor is activated and translocates to the nucleus.
The activated receptor binds to the specific DNA sequences in the
promoter of target genes, and modulates gene transcription
(48). In the present study, we
detected the expression levels of genes that are implicated in
angiogenesis and found that AR promoted angiogenesis by regulating
VEGF expression. We further explored the molecular mechanisms of AR
with regard to the upregulation of VEGFA by monitoring its direct
binding to the corresponding promoter region. The inhibition of
steroid hormones is often used as a clinical therapeutic strategy.
Enzalutamide (MDV3100) is an AR inhibitor approved by FDA for
prostate cancer treatment. The results of the present study
suggested that MDV3100 was capable of diminishing HUVEC
infiltration and suppressing tube formation, which indicated the
therapeutic potential of androgen-AR inhibition in neurofibroma
(Fig. 6).
In conclusion, the present study indicated that the
aberrant activation of androgen-AR signaling may increase VEGFA
expression and vascularization of neurofibroma in male patients
with cNF. Increased VEGFR contributes to HUVEC recruitment and
consequently in enhanced angiogenesis and neurofibroma progression.
Targeting the newly identified pathway of angiogenesis may open a
novel avenue for the effective treatment of cNF.
Supplementary Materials
Funding
The present study was supported by the general
project of major research plan for social development of the
Shaanxi province (grand no. 2018SF-250 to JJ).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MS designed the experiments, conducted the data
analyses and wrote the manuscript. HZ and WL performed the clinical
sample preparation and validated CD31 and AR expression in
neurofibroma tissues. HZ and JJ performed the cellular experiments
and HD performed the in vivo assays. All authors
participated in the discussion and revision of the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All procedures performed involving human
participants were in accordance with the ethical standards of the
Institutional Review Board of the First Affiliated Hospital of
Xi'an Jiaotong University. The study adhered to the guidelines of
the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. The animal experiments were approved
by the Institutional Review Board of the First Affiliated Hospital
of Xi'an Jiaotong University.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Huson SM, Compston DA, Clark P and Harper
PS: A genetic study of von Recklinghausen neurofibromatosis in
south east Wales. I. Prevalence, fitness, mutation rate, and effect
of parental transmission on severity. J Med Genet. 26:704–711.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gutmann DH, Ferner RE, Listernick RH, Korf
BR, Wolters PL and Johnson KJ: Neurofibromatosis type 1. Nat Rev
Dis Primers. 3:170042017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dugoff L and Sujansky E: Neurofibromatosis
type 1 and pregnancy. Am J Med Genet. 66:7–10. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McLaughlin ME and Jacks T:
Neurofibromatosis type 1. Methods Mol Biol. 222:223–237.
2003.PubMed/NCBI
|
|
5
|
Posma E, Aalbers R, Kurniawan YS, van
Essen AJ, Peeters PM and van Loon AJ: Neurofibromatosis type I and
pregnancy: A fatal attraction? Development of malignant schwannoma
during pregnancy in a patient with neurofibromatosis type I. BJOG.
110:530–532. 2003.PubMed/NCBI
|
|
6
|
Jung-Testas I, Schumacher M, Bugnard H and
Baulieu EE: Stimulation of rat Schwann cell proliferation by
estradiol: Synergism between the estrogen and cAMP. Brain Res Dev
Brain Res. 72:282–290. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung-Testas I, Schumacher M, Robel P and
Baulieu EE: Demonstration of progesterone receptors in rat Schwann
cells. J Steroid Biochem Mol Biol. 58:77–82. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pennanen P, Peltonen S, Kallionpää RA and
Peltonen J: The effect of estradiol, testosterone, and human
chorionic gonadotropin on the proliferation of Schwann cells with
NF1+/− or NF1−/− genotype derived from human
cutaneous neurofibromas. Mol Cell Biochem. 444:27–33. 2018.
View Article : Google Scholar
|
|
9
|
Gao W, Bohl CE and Dalton JT: Chemistry
and structural biology of androgen receptor. Chem Rev.
105:3352–3370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gesundheit B, Parkin P, Greenberg M,
Baruchel S, Senger C, Kapelushnik J, Smith C and Klement GL: The
role of angiogenesis in the transformation of plexiform
neurofibroma into malignant peripheral nerve sheath tumors in
children with neurofibromatosis type 1. J Pediatr Hematol Oncol.
32:548–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Staser K, Yang FC and Clapp DW:
Pathogenesis of plexiform neurofibroma: Tumor-stromal/hematopoietic
interactions in tumor progression. Annu Rev Pathol. 7:469–495.
2012. View Article : Google Scholar
|
|
12
|
Staser K, Yang FC and Clapp DW: Mast cells
and the neurofibroma microenvironment. Blood. 116:157–164. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Needle MN, Cnaan A, Dattilo J, Chatten J,
Phillips PC, Shochat S, Sutton LN, Vaughan SN, Zackai EH, Zhao H,
et al: Prognostic signs in the surgical management of plexiform
neurofibroma: The Children's Hospital of Philadelphia experience,
1974-1994. J Pediatr. 131:678–682. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Folkman J: New perspectives in clinical
oncology from angiogenesis research. Eur J Cancer. 32A:2534–2539.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Folkman J: Fighting cancer by attacking
its blood supply. Sci Am. 275:150–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukumura D, Xavier R, Sugiura T, Chen Y,
Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, et al: Tumor
induction of VEGF promoter activity in stromal cells. Cell.
94:715–725. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Babovic-Vuksanovic D, Ballman K, Michels
V, McGrann P, Lindor N, King B, Camp J, Micic V, Babovic N, Carrero
X, et al: Phase II trial of pirfenidone in adults with
neurofibromatosis type 1. Neurology. 67:1860–1862. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta A, Cohen BH, Ruggieri P, Packer RJ
and Phillips PC: Phase I study of thalidomide for the treatment of
plexiform neurofibroma in neurofibromatosis 1. Neurology.
60:130–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Widemann BC, Dombi E, Gillespie A, Wolters
PL, Belasco J, Goldman S, Korf BR, Solomon J, Martin S, Salzer W,
et al: Phase 2 randomized, flexible crossover, double-blinded,
placebo-controlled trial of the farnesyltransferase inhibitor
tipifarnib in children and young adults with neurofibromatosis type
1 and progressive plexiform neurofibromas. Neuro Oncol. 16:707–718.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Widemann BC, Salzer WL, Arceci RJ, Blaney
SM, Fox E, End D, Gillespie A, Whitcomb P, Palumbo JS, Pitney A, et
al: Phase I trial and pharmacokinetic study of the
farnesyltransferase inhibitor tipifarnib in children with
refractory solid tumors or neurofibromatosis type I and plexiform
neurofibromas. J Clin Oncol. 24:507–516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao Y, Wu K, Chen Y, Zhou J, Du C, Shi Q,
Xu S, Jia J, Tang X, Li F, et al: Beyond proliferation: KLF5
promotes angiogenesis of bladder cancer through directly regulating
VEGFA transcription. Oncotarget. 6:43791–43805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamilton SJ, Allard MF and Friedman JM:
Cardiac findings in an individual with neurofibromatosis 1 and
sudden death. Am J Med Genet. 100:95–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rasmussen SA, Yang Q and Friedman JM:
Mortality in neurofibromatosis 1: An analysis using U.S. death
certificates. Am J Hum Genet. 68:1110–1118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Friedman JM, Arbiser J, Epstein JA,
Gutmann DH, Huot SJ, Lin AE, McManus B and Korf BR: Cardiovascular
disease in neurofibromatosis 1: Report of the NF1 Cardiovascular
Task Force. Genet Med. 4:105–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamilton SJ and Friedman JM: Insights into
the pathogenesis of neurofibromatosis 1 vasculopathy. Clin Genet.
58:341–344. 2000. View Article : Google Scholar
|
|
28
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mashour GA, Ratner N, Khan GA, Wang HL,
Martuza RL and Kurtz A: The angiogenic factor midkine is aberrantly
expressed in NF1-deficient Schwann cells and is a mitogen for
neurofibroma-derived cells. Oncogene. 20:97–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirota S, Nomura S, Asada H, Ito A, Morii
E and Kitamura Y: Possible involvement of c-kit receptor and its
ligand in increase of mast cells in neurofibroma tissues. Arch
Pathol Lab Med. 117:996–999. 1993.PubMed/NCBI
|
|
31
|
Mashour GA, Driever PH, Hartmann M,
Drissel SN, Zhang T, Scharf B, Felderhoff - ser U, Sakuma S,
Friedrich RE, Martuza RL, et al: Circulating growth factor levels
are associated with tumorigenesis in neurofibromatosis type 1. Clin
Cancer Res. 10:5677–5683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ryan JJ, Klein KA, Neuberger TJ, Leftwich
JA, Westin EH, Kauma S, Fletcher JA, DeVries GH and Huff TF: Role
for the stem cell factor/KIT complex in Schwann cell neoplasia and
mast cell proliferation associated with neurofibromatosis. J
Neurosci Res. 37:415–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang FC, Ingram DA, Chen S, Hingtgen CM,
Ratner N, Monk KR, Clegg T, White H, Mead L, Wenning MJ, et al:
Neurofibromin-deficient Schwann cells secrete a potent migratory
stimulus for Nf1+/− mast cells. J Clin Invest.
112:1851–1861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Munchhof AM, Li F, White HA, Mead LE,
Krier TR, Fenoglio A, Li X, Yuan J, Yang FC and Ingram DA:
Neurofibroma-associated growth factors activate a distinct
signaling network to alter the function of neurofibromin-deficient
endothelial cells. Hum Mol Genet. 15:1858–1869. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawachi Y, Xu X, Ichikawa E, Imakado S and
Otsuka F: Expression of angiogenic factors in neurofibromas. Exp
Dermatol. 12:412–417. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kotsuji-Maruyama T, Imakado S, Kawachi Y
and Otsuka F: PDGF-BB induces MAP kinase phosphorylation and VEGF
expression in neurofibroma-derived cultured cells from patients
with neurofibromatosis 1. J Dermatol. 29:713–717. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawachi Y, Maruyama H, Ishitsuka Y,
Fujisawa Y, Furuta J, Nakamura Y, Ichikawa E, Furumura M and Otsuka
F: NF1 gene silencing induces upregulation of vascular endothelial
growth factor expression in both Schwann and non-Schwann cells. Exp
Dermatol. 22:262–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Folkman J: Seminars in Medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. N Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sheela S, Riccardi VM and Ratner N:
Angiogenic and invasive properties of neurofibroma Schwann cells. J
Cell Biol. 111:645–653. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim HA, Ling B and Ratner N: Nf1-deficient
mouse Schwann cells are angiogenic and invasive and can be induced
to hyper-proliferate: Reversion of some phenotypes by an inhibitor
of farnesyl protein transferase. Mol Cell Biol. 17:862–872. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McLaughlin ME and Jacks T: Progesterone
receptor expression in neurofibromas. Cancer Res. 63:752–755.
2003.PubMed/NCBI
|
|
43
|
Sieveking DP, Lim P, Chow RW, Dunn LL, Bao
S, McGrath KC, Heather AK, Handelsman DJ, Celermajer DS and Ng MK:
A sex-specific role for androgens in angiogenesis. J Exp Med.
207:345–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu M, Wallace MR and Muir D: Nf1
haploinsufficiency augments angiogenesis. Oncogene. 25:2297–2303.
2006. View Article : Google Scholar
|
|
45
|
Harigai R, Sakai S, Nobusue H, Hirose C,
Sampetrean O, Minami N, Hata Y, Kasama T, Hirose T, Takenouchi T,
et al: Tranilast inhibits the expression of genes related to
epithelial-mesenchymal transition and angiogenesis in
neurofibromin-deficient cells. Sci Rep. 8:60692018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin AL, McGill HC Jr and Shain SA: Hormone
receptors of the baboon cardiovascular system. Biochemical
characterization of myocardial cytoplasmic androgen receptors. Circ
Res. 49:1010–1016. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Horwitz KB and Horwitz LD: Canine vascular
tissues are targets for androgens, estrogens, progestins, and
glucocorticoids. J Clin Invest. 69:750–758. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mangelsdorf DJ, Thummel C, Beato M,
Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M,
Chambon P, et al: The nuclear receptor superfamily: The second
decade. Cell. 83:835–839. 1995. View Article : Google Scholar : PubMed/NCBI
|