Introduction
With a 5-year survival rate of <25%, ovarian
cancer represents one of the most aggressive and challenging types
of cancer as regards treatment. Ovarian cancer is the leading cause
of cancer-associated mortality among all gynecological tumors, and
the majority of patients with this type of cancer are diagnosed at
advanced and metastatic stages (1,2).
Despite advances in systemic chemotherapies, including cisplatin
and carboplatin, which are widely used at present, the prognosis of
patients with ovarian cancer remains remarkably poor due to high
incidences of chemoresistance (1,3). To
date, multiple molecular mechanisms involved in ovarian
tumorigenesis and chemoresistance have been proposed; however, no
reliable biomarkers have been identified as predictors of an
individual's chemotherapeutic response (1,4).
Thus, the identification of leading factors mediating
chemoresistance and the elucidation of the underlying mechanisms is
of utmost importance, in order to develop novel and effective
therapeutic strategies for preventing or reversing therapeutic
resistance.
Ubiquitin-specific peptidase 39 (USP39) encodes a 65
kDa SR-associated protein, which has been demonstrated to be
involved in RNA splicing as a component of the U4/U6·U5 tri-small
nuclear ribonucleoprotein (snRNP) (5,6).
Zebrafish USP39 displays the ability to recruit the tri-snRNPs to
the pre-spliceosome and target retinoblastoma (rb1) and E2F
transcription factor 4 (e2f4) expression to regulate embryonic
pituitary homeostasis (7). In
accordance with its previous reported role in messenger RNA (mRNA)
processing, USP39 has also been implicated as a key factor in
regulating the splicing of Aurora B and other mRNAs to maintain
mitotic spindle checkpoint integrity (8). Notably, despite being classified as a
member of the deubiquitinating enzymes (DUBs), USP39 is fully
deprived of protease activity (9).
Recent evidence suggests that USP39 plays a vital role in
regulating the malignant phenotypes of various cancer types
(1,5). For instance, the overexpression of
USP39 promotes tumorigenesis in prostate cancer; mechanistic
investigations have demonstrated that the silencing of USP39
downregulates epidermal growth factor receptor (EGFR) expression by
inducing a splicing defect via the retention of intron 2 between
exons 2 and 3, thus affecting the expression of the 3′ end of EGFR
(10). The silencing of USP39 has
been shown to inhibit cell growth and colony formation of breast
cancer in vitro (11).
Additionally, USP39 has been identified as an indispensable gene
for the survival of KRAS-dependent lung cancer and colorectal
carcinoma (8). However, little is
known about the biological functions and the role of USP39 in the
chemosensitivity of human ovarian cancer.
The present study thus aimed to investigate the
roles of USP39 in ovarian cancer. USP39 was found to be highly
expressed in carboplatin-resistant ovarian cancer tissues compared
with carboplatin-sensitive tissues. Functional assays demonstrated
that USP39 plays a crucial role in regulating malignant phenotypes
and chemoresistance in ovarian cancer cells. In addition, USP39
confers ovarian cancer cell chemo-resistance via the
AKT/extracellular signal-regulated kinase (ERK) signaling pathway.
Therefore, the findings of this study suggest that USP39 may be a
potential molecular target for ovarian cancer therapy, and may
provide novel insight into the progression and chemotherapeutic
resistance of ovarian cancer.
Materials and methods
Clinical specimens
In the present study, a total of 119 clinical
specimens from patients with ovarian cancer who underwent initial
surgical treatment at the Department of Gynecologic Oncology of the
Chinese Academy of Medical Sciences Cancer Hospital (Beijing,
China) were collected from May, 2007 to January, 2013. All patients
provided informed consent to participate in the study. This study
was approved by the Ethics Committee of the Chinese Academy of
Medical Sciences Cancer Hospital.
Immunohistochemistry (IHC)
IHC was performed as previously described (12). USP39 expression was assessed using
a rabbit monoclonal antibody (ab131244; Abcam) at a 1:50 dilution
at 4°C overnight, according to the manufacturer's instructions. As
negative controls, sections incubated with rabbit immunoglobulin G
(1:1000; ab6721; Abcam) instead of primary antibodies were used.
Following incubation with horseradish peroxidase (HRP)-conjugated
secondary antibodies (OriGene Technologies, Inc.) at room
temperature for 1 h, the positive signals were visualized with
3,3′-diaminobenzidine (OriGene Technologies, Inc.) as a substrate.
All slides were scanned with an Aperio scanning system (Aperio
Group, LLC) and Aperio Image Scope software (version 10.2.2.2317,
Aperio Technologies) was employed for the quantitative analysis of
USP39 protein expression. For analysis, 4-6 different areas of the
slide were randomly selected. The intensity score was graded with a
score ranging from 0 to 3 according to the percentage of positively
stained tumor cells. When 0-10% of tumor cells were stained, a
score of 0 was given; when 10-25% of tumor cells were stained, a
score of 1 was given; when 25-50% of tumor cells were stained, a
score of 2 was given; and when 50-100% of tumor cells were stained,
a score of 3 was given. Scores of 0 and 1 were considered to
represent a low expression, while scores of 2 and 3 were considered
to represent a high expression. The staining results of USP39 were
evaluated according to the scoring criterion reported in a previous
study (13).
Cell culture
The human ovarian cancer cell lines ES2, SKOV3 and
the 293T cells were purchased from the American Tissue Culture
Collection. The human ovarian cancer cell lines were cultured in
RPMI-1640 (Gibco, Thermo Fisher Scientific) supplemented with 10%
fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific), 100
U/ml ampicillin (Life Technologies, Gibco) and 100 μg/ml
streptomycin (Life Technologies, Gibco). The 293T cells were
cultured in Dulbecco's modified Eagle medium (DMEM) supplemented
with 10% FBS, 100 U/ml ampicillin and 100 μg/ml
streptomycin. All cells were cultured at 37°C in 5%
CO2.
Construction of plasmids, lentivirus
packaging and transfection
For the stable knockdown of USP39, two small hairpin
RNA (shRNA) sequences (shUSP39#1 and shUSP39#2) targeting USP39
were respectively inserted into pSIH1-H1-Puro (Invitrogen, Thermo
Fisher Scientific, Inc.), and sh-green fluorescence protein (GFP)
(Invitrogen, Thermo Fisher Scientific, Inc.) was used as control.
The two USP39 shRNA sequences were as follows:
5′-GTACTTTCAAGGCCGGGGT-3′ (shUSP39#1) and 5′-ACAAGCAGTACACAAGAAT-3′
(shUSP39#2). shRNA plasmids were transfected into the 293T cells
together with 3 packaging plasmids (3 μg PLP1, 3 μg
VSVG and 3 μg PLP2) (Invitrogen, Thermo Fisher Scientific,
Inc.) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) with OPTI-MEM (Thermo Fisher Scientific, Inc.).
The virus-containing media were pooled at 48 h after transfection,
centrifuged under 800 × g for 10 min at 4°C and filtered by passing
through a 0.45-μm filtration device (EMD Millipore). Viral
supernatant was added in a 1:3 dilution to previously seeded cell
lines, supplemented with 5 μg/ml of polybrene (EMD
Millipore). Stably transduced cells were selected with puromycin at
a final concentration of 1 μg/ml. For the stable
overexpression of USP39, full-length complementary DNA of human
USP39, which was generated by polymerase chain reaction (PCR)
amplification, was cloned between the XhoI and XbaI
sites of pLVX-IRES-Neo (Invitrogen, Thermo Fisher Scientific, Inc.)
to prepare a constitutive lentiviral vector. A total of
8×106 293T cells were seeded in 100-cm2
plates. pLVX-IRES-Neo or pLVX-IRES-Neo-USP39 lentiviral vectors
(7.5 μg) and the corresponding packaging plasmid (6.5
μg pCMV ∆8.91, 3.5 μg VSVG and 2.5 μg PLP2)
were added to a mixture of Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) with OPTI-MEM (Thermo Fisher Scientific,
Inc.). Following a similar transfection procedure as the one
described above, stably transduced cells were selected with G418
(Sigma-Aldrich) at a final concentration of 400 μg/ml. At 48
h following transfection, knockdown and overexpression efficiencies
were evaluated by reverse transcription-quantitative PCR (RT-qPCR)
and western blot analysis.
Cell proliferation assay
To quantify cell proliferation, 100 μl stably
transfected cells at a density of 1×104 cells/ml (n=6)
were seeded in 96-well plates. A Cell Counting kit-8 (CCK-8) assay
kit was used to detect cell viability at 5 time points. The cells
were cultured in 100 μl/well fresh medium mixed with CCK-8
solution (10:1) (Dojindo Molecular Technologies, Inc.) and
incubated for 1 h at 37°C. The optical density values were then
measured at 450 nm using a spectrophotometer (Bio-Rad).
Apoptosis assay
The ES2 and SKOV3 cells were analyzed for apoptosis
using an Annexin V-fluorescein isothio-cyanate (FITC)/propidium
iodide (PI) kit [Multisciences (Lianke) Biotech Co., Ltd.].
Briefly, the cells were seeded at 5×105 cells/well in
6-well plates in triplicate. Following 24 h of incubation with
carboplatin, the cells were harvested through trypsinization and
washed twice with cold PBS. The cells were centrifuged at 3,000 × g
at room temperature for 5 min. The supernatant was then discarded
and the pellet was resuspended in 500 μl 1X binding buffer.
Finally, the cells were incubated with 5 μl FITC-conjugated
Annexin V and 10 μl of PI for 5 min at room temperature in
the dark. The samples were analyzed by a FACSCalibur flow cytometer
(BD Biosciences).
Analysis of the cell cycle
For cell cycle analysis, the cells were harvested
and fixed with 70% ethanol for 18 h at -20°C upon digestion with
0.05% trypsin. Upon washing with PBS and collecting the fixed cells
by 800 × g centrifugation at 4°C for 5 min, samples were
resuspended with 500 μl DNA staining solution and then
incubated in 37°C for 30 min. Analysis was performed on a
FACSCalibur flow cytometer (BD Biosciences).
RT-qPCR analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The cDNA was
synthesized by the FastQuant RT kit [TIANGEN Biotech (Beijing) Co.
Ltd.] using 100 ng of total RNA. Quantitative analysis was
performed using diluted cDNA combined with the SYBR-Green PCR
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.),
forward and reverse primers, and RNase-free water. The primer
sequences were as follows: USP39 forward,
5′-GGTTTGAAGTCTCACGCCTAC-3′ and reverse,
5′GGCAGTAAAACTTGAGGGTGT-3′; and β-actin forward,
5′-CCGTTCCGAAAGTTGC CTTTT-3′ and reverse,
5′GAGGCGTACAGGGATAGCAC-3′. The RT-qPCR reactions were conducted
using the following parameters: 95°C for 10 min, 40 cycles at 95°C
for 10 sec, at 60°C for 20 sec and at 72°C for 30 sec. The relative
mRNA expression in each sample was calculated using the
2−ΔΔCq method (14).
Western blot analysis
Total protein was extracted from whole cells using
ice-cold cell lysis buffer (1% Triton X-100; 10 mM Tris-HCl, pH
7.4; 150 mM NaCl; 0.25% sodium deoxycholate; 5 mM EDTA, pH 7.4) and
assayed for protein concentration by a bicinchoninic acid assay
(Pierce). Samples containing 15 μg of protein were separated
by 8-12% SDS-PAGE and transferred to polyvinylidene difluoride
membranes (Merck KGaA). After blocking with 5% skimmed milk powder
for 1 h at room temperature, the membranes were incubated with
antibodies against USP39 (1:1,000; ab131244; Abcam), caspase-3
(1:1,000; #9661; Cell Signaling Technology, Inc.), poly-ADP ribose
polymerase (PARP) (1:1,000; #9542; Cell Signaling Technology, Inc.)
and β-actin (1:5,000; A5316; Sigma-Aldrich; Merck KGaA) overnight
at 4°C, followed by incubation with a appropriate HRP-linked
secondary antibody (1:2,000; #7074/#7076; Cell Signaling
Technology, Inc.) at room temperature for 1 h. Samples were
detected with an enhanced chemiluminescence detection system
(Pierce; Thermo Fisher Scientific, Inc.).
Colony formation assay
For the colony formation assay, lentivirus-infected
cells were seeded evenly in 6-well plates at an initial density of
2,000 cells/well and cultured in a 5% CO2 incubator for
10 days at 37°C. The cells were washed with ice-cold PBS, fixed
with 4% paraformaldehyde (PFA), stained with crystal violet at room
temperature for 30 min and rinsed 3 times with double distilled
H2O.
Wound healing assay
The cells were plated until they reached 90%
confluence in 6-well plates. Cell monolayers were scratched with
200-μl pipette tips. Upon forming wound gaps, the cells were
washed with PBS twice to remove floating cells and cultured in
serum-free medium. Cell migration into the wound area was observed
under an inverted microscope (Leica Microsystems) at different time
points. The speed of wound closure was analyzed by measuring the
distance of the migrating cells from different areas for each
wound.
Cell migration and invasion assays
For the cell migration assay, cells
(2×104), which has been resuspended in 100 μl
serum-free medium, were added to the upper chambers of a Transwell
plate (Corning Inc.). The lower chambers were filled with 600
μl RPMI-1640 complete medium containing 10% FBS. Following
24 h of incubation, the cells were fixed with 4% PFA for 30 min and
stained with 0.1% crystal violet at room temperature for 1 min.
After washing gently with water, the cells were imaged and counted
under a microscope (Leica Microsystems). For the cell invasion
assay, Matrigel (Sigma-Aldrich; Merck KGaA) was diluted from 5
mg/ml to 1 mg/ml by serum free-cold RPMI-1640. The upper chambers
of the Transwell plates were pre-coated with 100 μl of the
diluted Matrigel. The subsequent steps of the procedure were the
same as those for the migration assay.
Subcutaneous xenograft models
Pathogen-free female BALB/c nude mice (age, 5-6
weeks; weight, 18-22 g, 6 mice per group) were provided standard
laboratory chow and allowed free access to water, unless otherwise
stated, in a climate-controlled room with a 12-h light/12-h dark
cycle at the Animal Experiment Center of Cancer Hospital, Chinese
Academy of Medical Sciences. Animal studies were approved by the
Animal Care and Use Committee of Cancer Hospital, Chinese Academy
of Medical Sciences. All procedures involving animals were
conducted according to the guidelines for the care and use of
laboratory animals. A total of 5×105 stably transfected
ES2 cells were resuspended in 100 μl normal saline and
injected subcutaneously into the flank of each mouse with a 28G
syringe. At 3 days post-inoculation, carboplatin was administered
to the mice by intraperitoneal injection at a dose of 50 mg/kg
every 3 days for 3 consecutive times as described previously
(15). Tumors were measured using
an electronic caliper twice per week, and tumor volume was
calculated using the formula: Volume = (length x
width2)/2. All mice were sacrificed by cervical
dislocation at 21 days post-injection. Tumor samples were fixed in
formalin for paraffin embedding and sectioned into 4 μm
slides for IHC analyses. The sections were incubated with
antibodies against USP39 (1:50; ab131244; Abcam) and cleaved
caspase-3 (1:50; #9579; Cell Signaling Technology, Inc.) to measure
the expression of USP39 and cleaved caspase-3.
Intraperitoneal tumor xenograft
models
Luciferase-expressing control or
USP39-overexpressing ES2 cells were cultured, harvested and
suspended in RPMI-1640. Subsequently, 5×105 cells were
injected intraperitoneally into the female BALB/c nude mice (3 mice
per group). At 3 days post-inoculation, carboplatin was
administered to the mice by intraperitoneal injection at a dose of
50 mg/kg. Mice were housed for 10 days and injected with 200
μl 15 mg/ml D-luciferin PBS solution 10 min prior to
imaging. The whole animals were imaged using an IVIS Lumina XRMS
In Vivo Imaging System (PerkinElmer, Inc.).
Statistical analysis
Statistical analyses were conducted using Prism
software 6 (GraphPad Software, Inc.). The associations between
USP39 expression and the patient clinicopathological data were
assessed using a Chi-square test. All data are presented as the
means ± standard error of the mean of ≥3 independent experiments.
For 2-group comparison, analyses were performed with a Student's
unpaired t-test (two-tailed). For multiple comparisons, analyses
were performed with one-way ANOVA with Tukey's post hoc test. All
experiments other than histological examinations were repeated ≥2
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
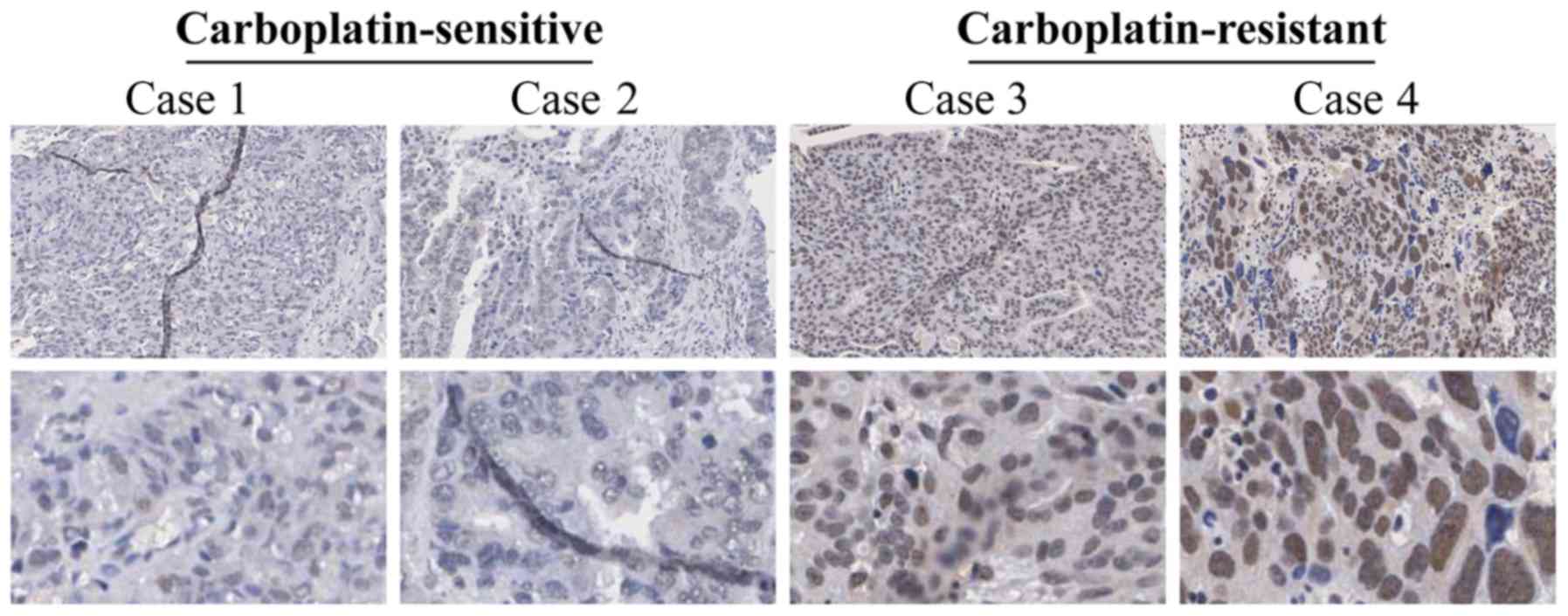
USP39 is overexpressed in ovarian cancer
tissues and is associated with carboplatin resistance
USP39 expression was analyzed in
carboplatin-sensitive and carboplatin-resistant human ovarian
cancer tissues by IHC. The results revealed that USP39 was
primarily expressed in the nucleus, but rarely in the cytoplasm
(Fig. 1). In the
carboplatin-resistant group, 60.71% of the specimens presented a
strong positive expression vs. 41.27% in the carboplatin-sensitive
group (P= 0.034), determined by a Chi-square test (Table I). Subsequently, we analyzed
whether the expression of USP39 can influence the
clinicopathological characteristics of patients with ovarian
cancer. As shown in Table I, USP39
expression was not associated with age, residual tumor size, level
of cancer antigen 125 in primary tumors, International Federation
of Gynecology and Obstetrics stage or tumor grade. These results
indicate that high expression levels of USP39 in ovarian cancer
tissues may be closely associated with the development of
carboplatin resistance.
 | Table IAssociation between the expression of
USP39 and the patient clinicopathological features in the ovarian
cancer tissue arrays. |
Table I
Association between the expression of
USP39 and the patient clinicopathological features in the ovarian
cancer tissue arrays.
| Clinical
variable | USP39 expression
[no. (%)]
|
|---|
| Patients | Low | High | P-value |
|---|
| All cases | 119 | 59 (49.58) | 60 (50.42) | |
| Age at diagnosis
(years) | ≥60 | 15 (42.86) | 20 (57.14) | 0.344 |
| <60 | 44 (52.38) | 40 (47.62) | |
| Residual tumor size
(cm) | ≥2 | 8 (44.44) | 10 (55.56) | 0.636 |
| <2 | 51 (50.50) | 50 (49.50) | |
| Level of CA125
in | ≥500 | 46 (50.00) | 46 (50.00) | 0.866 |
| primary tumors
(U/ml) | <500 | 13 (48.15) | 14 (51.85) | |
| Recurrent type |
Carboplatin-sensitive | 37 (58.73) | 26 (41.27) | 0.034 |
|
Carboplatin-resistant | 22 (39.29) | 34 (60.71) | |
| FIGO stage | III | 45 (48.91) | 47 (51.09) | 0.788 |
| IV | 14 (51.85) | 13 (48.15) | |
| Tumor grade | G1/G2 | 8 (47.06) | 9 (52.94) | 0.881 |
| G3 | 50 (49.02) | 52 (50.98) | |
USP39 promotes cell proliferation and
colony formation by inducing cell cycle phase arrest in ovarian
cancer cells
To verify the role of USP39 in ovarian cancer cells,
the ES2 and SKOV3 cell lines were transduced with shUSP39s or shGFP
lentiviruses. The inhibitory effects of shUSP39s on its endogenous
expression in the ES2 and SKOV3 cells were determined by RT-qPCR
and western blot analysis (Fig. 2A and
B). To examine the effects of USP39 on cell proliferation, a
CCK-8 assay was performed. As shown in Fig. 2C, the proliferative rate of the ES2
and SKOV3 cells was significantly lower in shUSP39s-transduced
cells compared with the shGFP control cells. To further
characterize the effects of USP39 on the cell proliferative
capability, a colony formation assay was employed. As depicted in
Fig. 2D, upon the knockdown of
USP39, the capacity of colony formation of the ES2 and SKOV3 cells
was substantially reduced. To elucidate the mechanisms through
which USP39 modulates cell proliferation and colony formation, cell
cycle distribution analysis was employed. The cell percentage in
the G2/M phase was increased by USP39 knockdown in the SKOV3 cells,
suggesting that cell growth suppression may be associated with
increased G2/M phase arrest (Fig.
2E). Cell cycle-associated protein analysis also revealed that
the expression of cyclin B1 was suppressed following USP39
knockdown (Fig. 2F). The
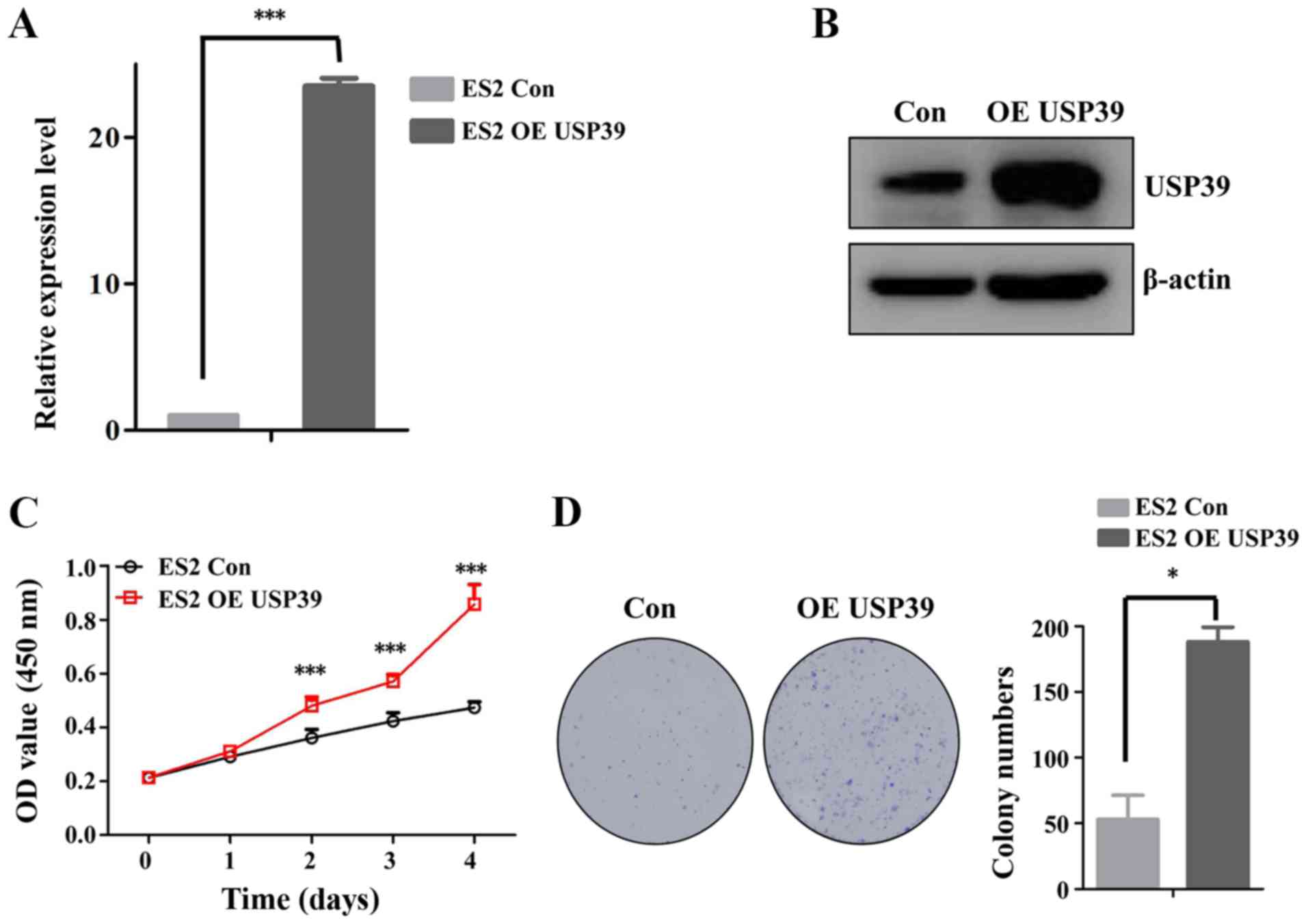
overexpression of USP39 in the ES2 cells indicated that USP39
significantly increased the cell proliferation and colony formation
capacity of ES2 cells (Fig. 3).
Taken together, these data support the important role of USP39 in
regulating the proliferation of ovarian cancer cells.
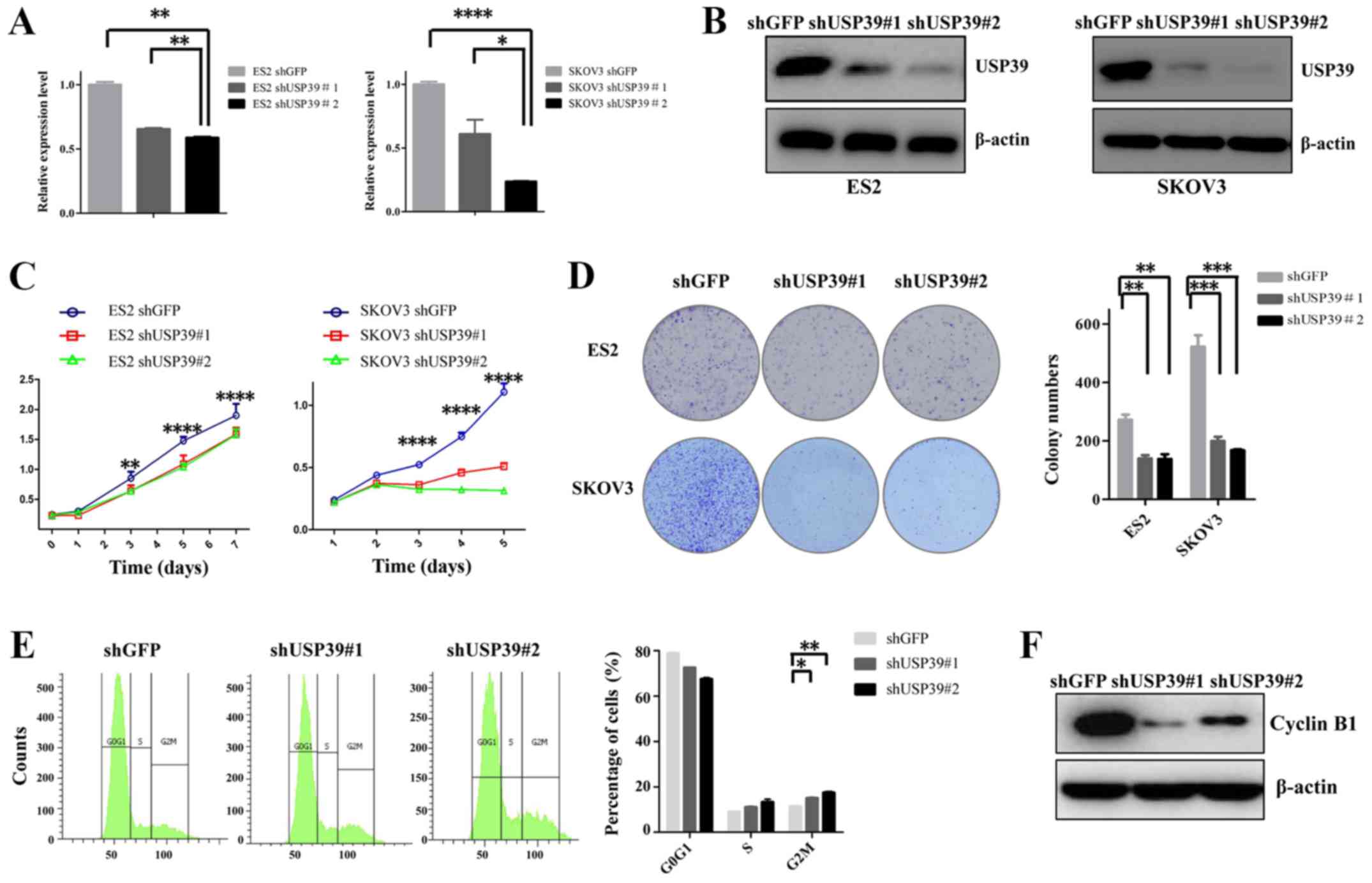 | Figure 2Effect of USP39 knockdown on the
growth of ES2 and SKOV3 cell lines. (A) Reverse
transcription-quantitative polymerase chain reaction analysis of
the messenger RNA levels of USP39 in shGFP and shUSP39s-transduced
ES2 and SKOV3 cell lines. β-actin was used as a reference gene. (B)
Identification of knockdown efficiency in shGFP and
shUSP39s-transduced ES2 and SKOV3 cell lines by western blot
analysis. β-actin was used as a loading control. (C) The cell
proliferative capability was obviously suppressed upon USP39
silencing in ES2 and SKOV3 cells, as measured by Cell Counting
kit-8 assay. (D) The number of colonies was significantly decreased
following USP39 knockdown in ES2 and SKOV3 cells. (E) USP39
knockdown induced G2/M phase arrest in SKOV3 cells. (F) Western
blot analysis of changes in the expression of cell cycle-associated
proteins upon knockdown of USP39 in SKOV3 cells. Data represent the
means ± standard error of the mean of 3 independent experiments.
shGFP, cells infected with shGFP; shUSP39, cells infected with
USP39 shRNA. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001, compared with
the shGFP group. USP39, ubiquitin-specific protease 39; sh, small
hairpin; GFP, green fluorescent protein. |
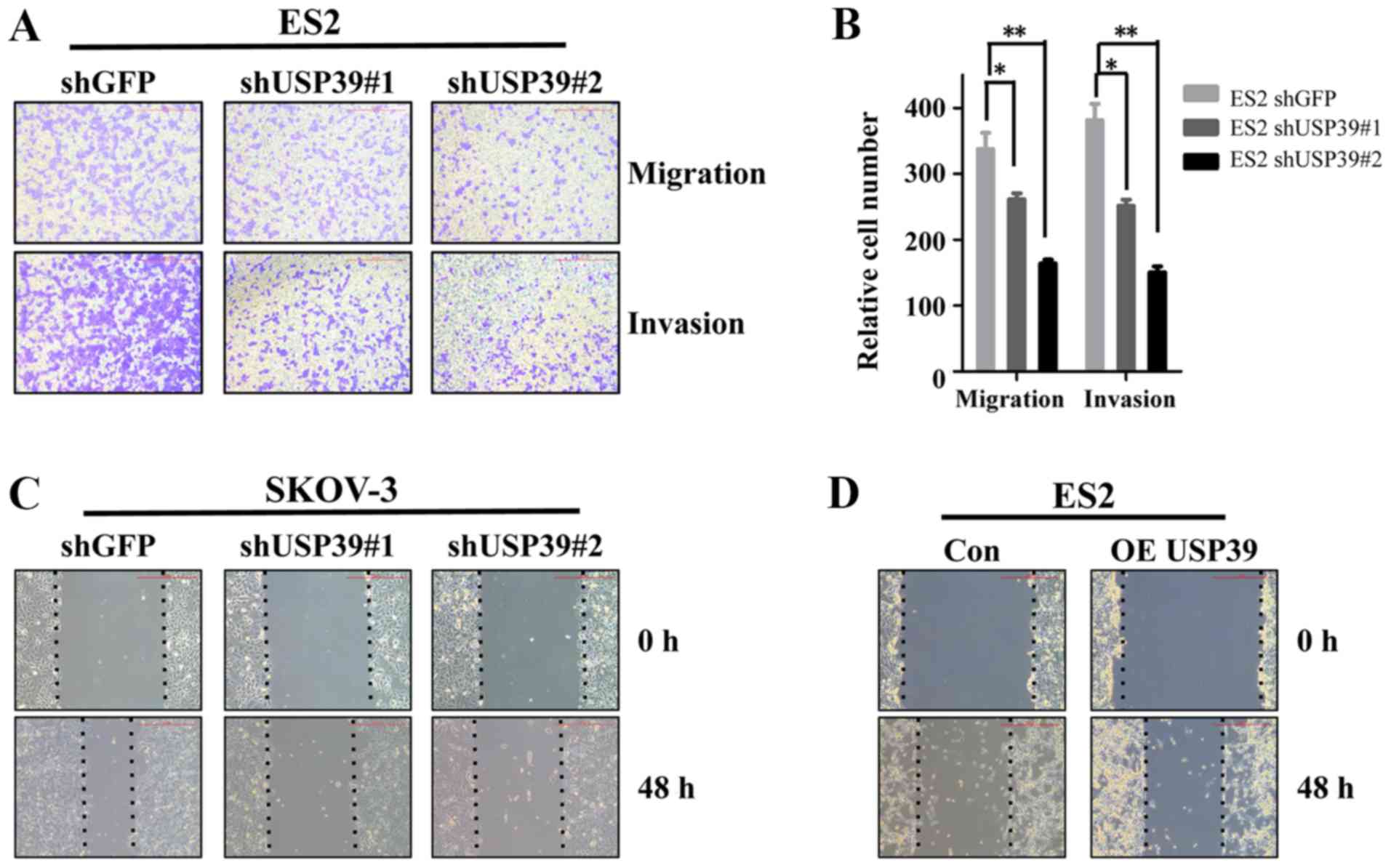
Knockdown of USP39 suppresses ovarian
cancer cell migration and invasion
Previous studies have reported that USP39 is a
potential target for several types of cancer (5,6). To
explore the possibility that USP39 activity may be involved in the
metastatic behaviors of ovarian cancer cells, migration and
invasion assays were performed in vitro. USP39 knockdown
significantly suppressed the migration and invasion of ES2 cells
(Fig. 4A and B). Similarly, the
capacity of wound healing was markedly decreased in the SKOV3 cells
in which USP39 was silenced and increased in the
USP39-overexpressing ES2 cells compared with their respective
control cells (Fig. 4C and D).
These results indicate that USP39 plays a potential role in the
induction of cell migration and invasion in ovarian cancer.
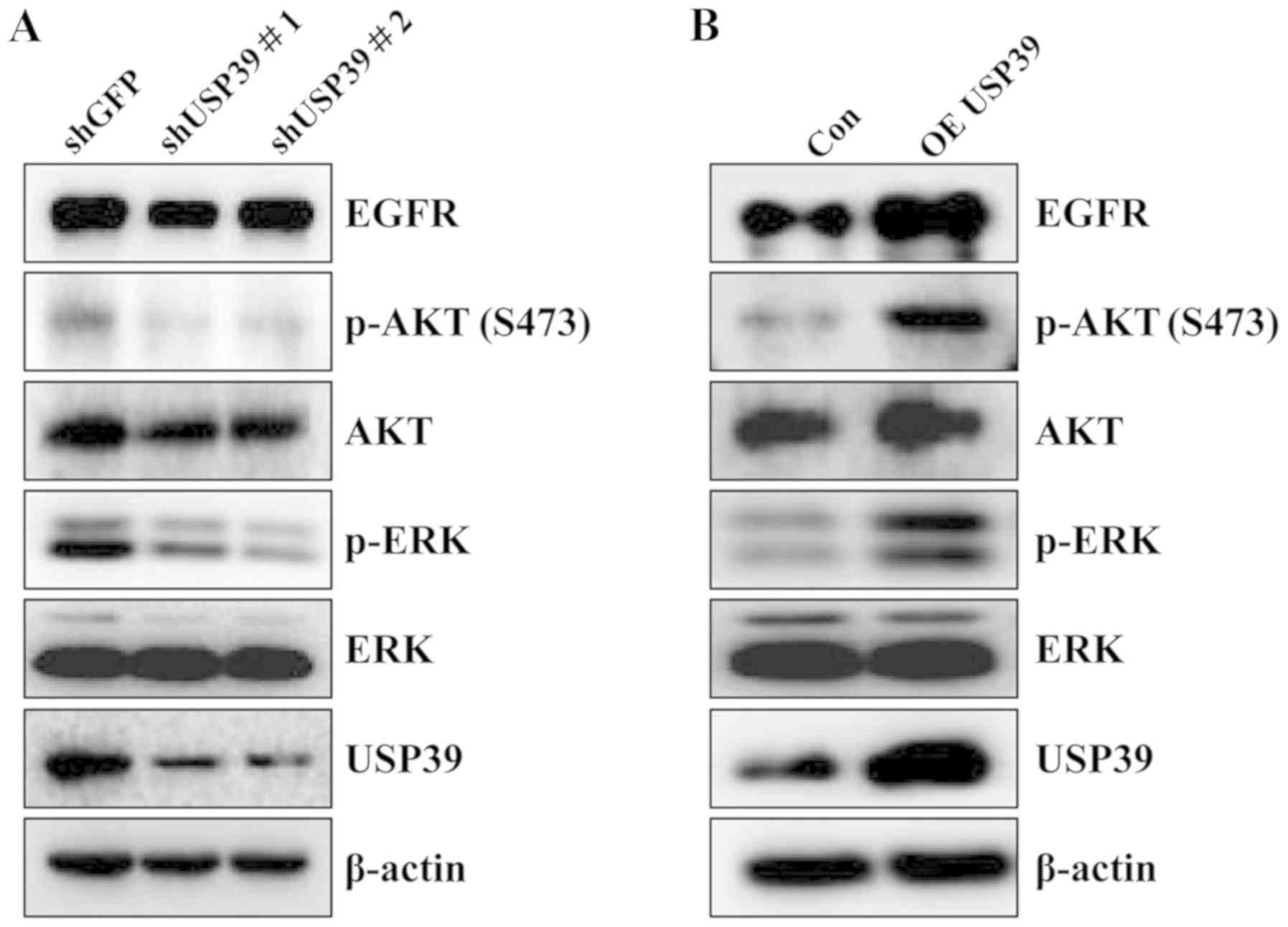
USP39 is involved in the regulation of
AKT and ERK signaling pathways
To ascertain the regulatory mechanisms of action of
USP39 as regards the tumorigenesis of ovarian cancer, multiple
signaling pathways were analyzed in the ES2 cells upon altering
USP39 expression. As shown in Fig.
5, USP39 knockdown markedly decreased the expression of EGFR,
phosphorylated (p)-ERK and p-AKT. On the contrary, the expression
levels of EGFR, p-ERK and p-AKT were increased upon the
overexpression of USP39.
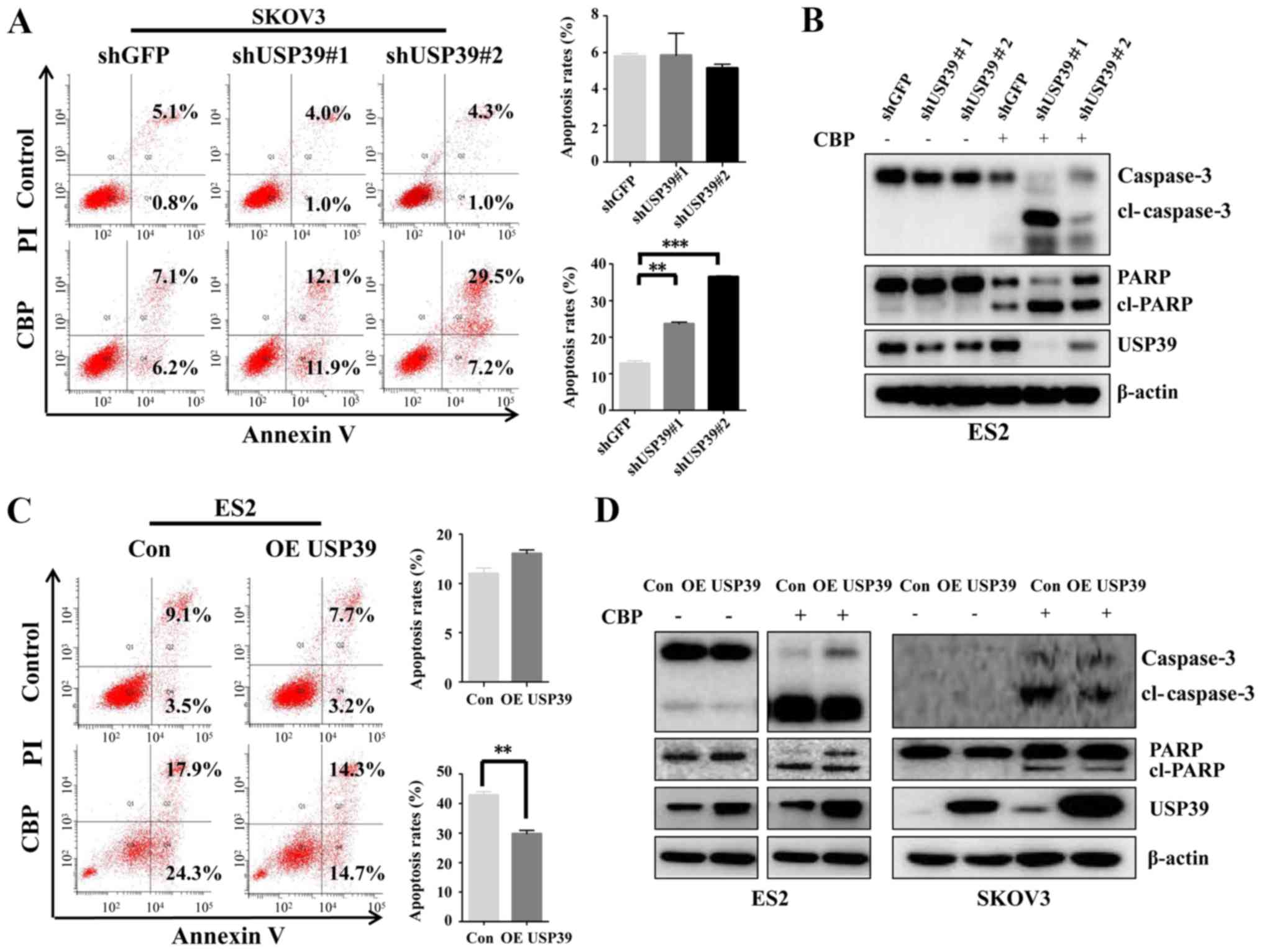
Reduced USP39 levels sensitize ovarian
cancer cells to carboplatin treatment
Based on the aforementioned histological and
clinical analyses, USP39 may play a role in the regulation of
patient's response to carboplatin treatment. Thus, to clarify the
possible mechanisms involved in the expression level of USP39 in
regulating the apoptosis induced by carboplatin in ovarian cancer
cells, flow cytometry was performed and the expression of
apoptosis-associated proteins was examined. USP39 deficiency
significantly potentiated the apoptotic rate induced by carboplatin
in the SKOV3 cells, whereas USP39 overexpression markedly inhibited
the apoptotic rate of the ES2 cells (Fig. 6A and C). Consistent with the
effects observed on the cell apoptotic rate exerted by USP39,
higher levels of cleaved PARP and cleaved caspase-3 were observed
in the ES2 cells in which USP39 was silenced than in the control
cells (Fig. 6B). By contrast,
USP39 overexpression in the SKOV3 and ES2 cells significantly
suppressed carboplatin-induced apoptosis, as demonstrated by the
marked decrease in cleaved PARP and cleaved caspase-3 levels
(Fig. 6D). These data indicate
that USP39 plays an important role in mediating the sensitivity of
ovarian cancer cells to carboplatin.
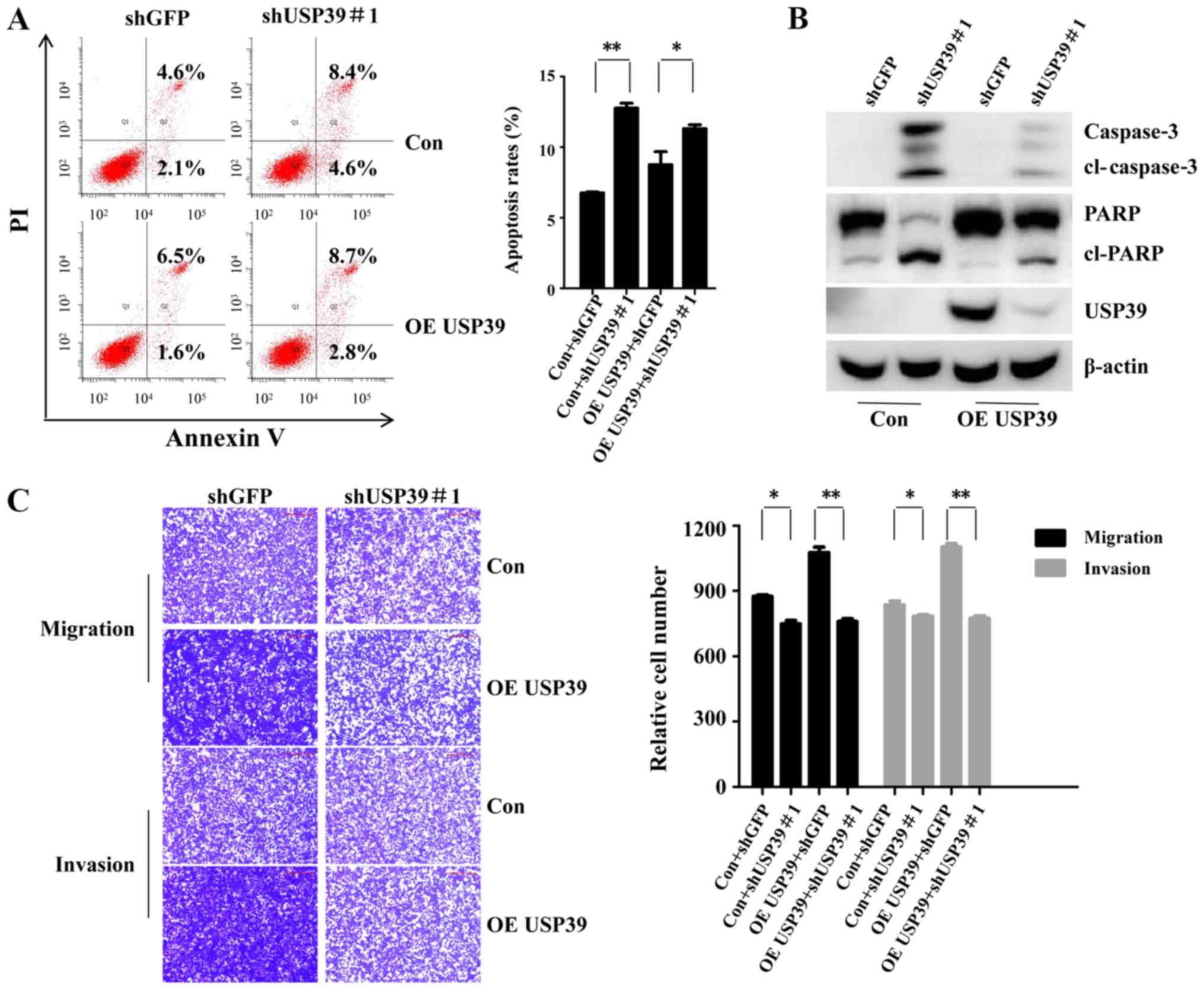
USP39 knockdown reverses the promoting
effects of USP39 on the chemosensitivity of the ovarian cancer
cells to carboplatin, and on migration and invasion
Rescue assays were conducted to prove the function
of USP39 in the chemosensitivity of ES2 cells to carboplatin, and
its effects on migration and invasion. As shown in Fig. 7A and B, shUSP39#1 partly attenuated
the promoting effects of USP39 on the chemosensitivity of the cells
to carboplatin, as evidenced by the increased cell apoptotic rate,
and the increased expression of cleaved PARP and cleaved caspase-3
in the shUSP39#1-transduced cells. Moreover, cell migration and
invasion promoted by USP39 was partially recovered by shUSP39#1
(Fig. 7C). Taken together, these
findings demonstrated USP39 promoted the chemoresistance of ovarian
cancer to carboplatin, as well as migration and invasion.
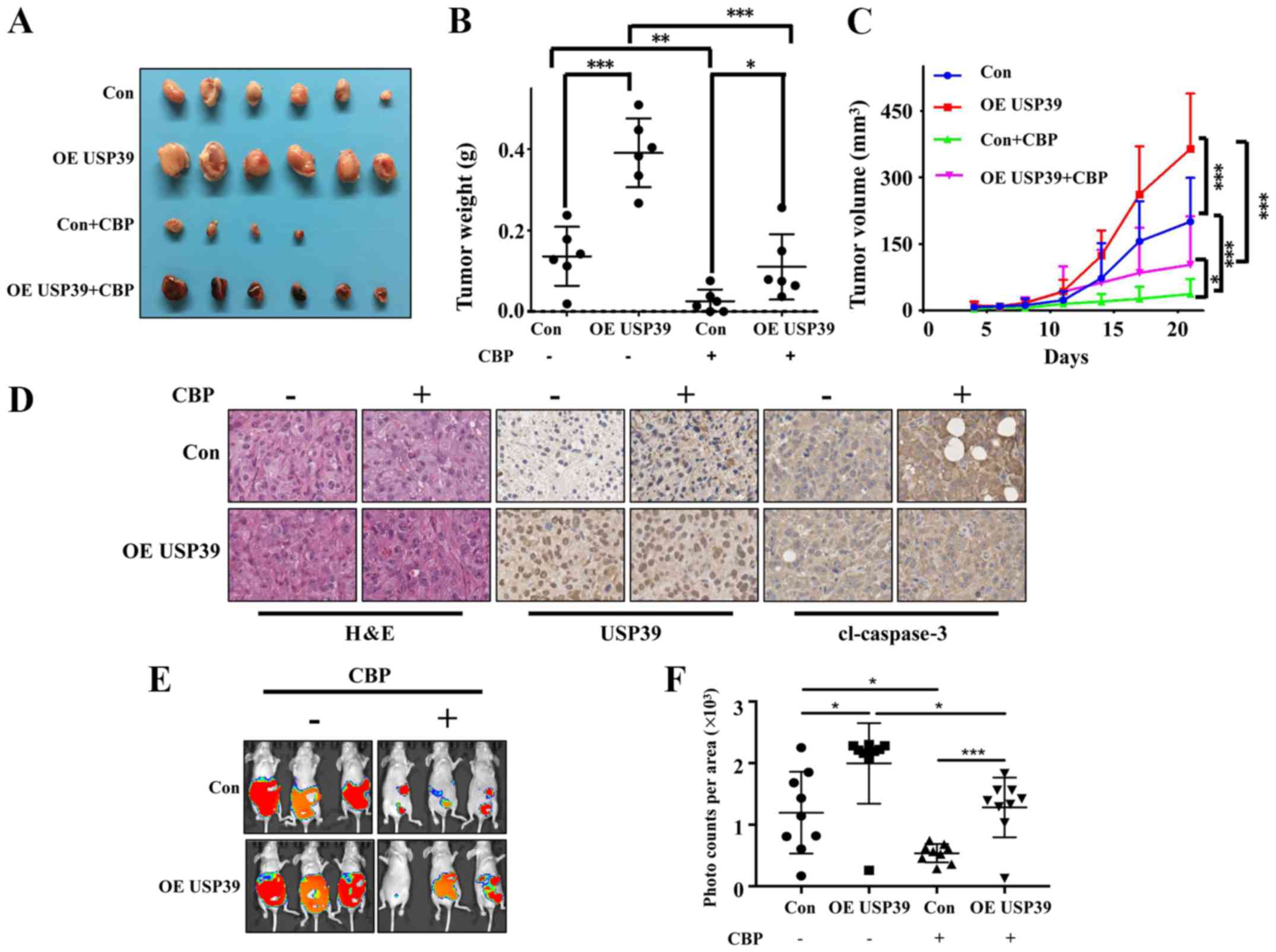
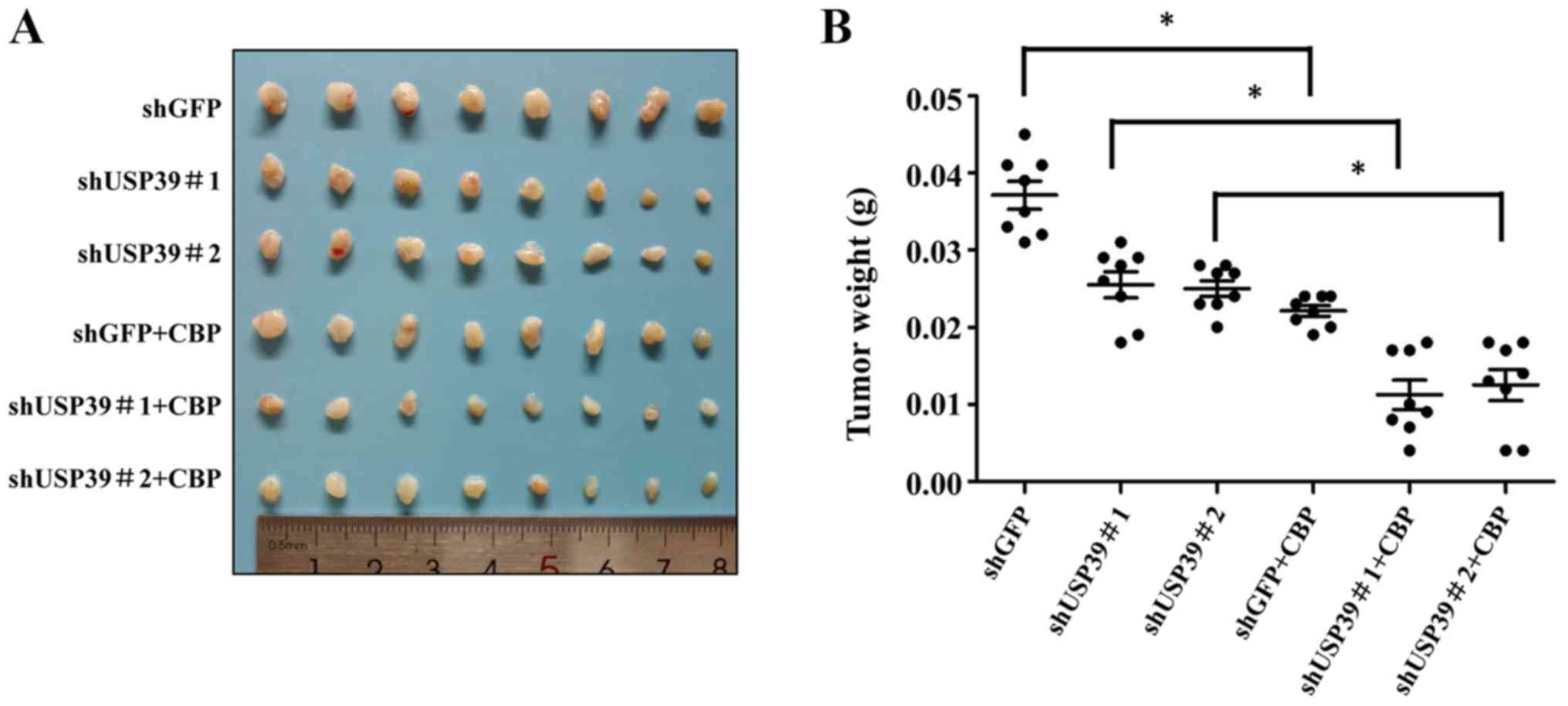
Expression level of USP39 influences the
chemosensitivity of ES2 and SKOV3 cells to carboplatin in vivo
To further validate the in vitro results, the
present study examined the effects of USP39 on the chemosensitivity
of ES2 and SKOV3 cells in vivo. Mice were randomly divided
into 4 groups (n=6/group). The results revealed that the ectopic
expression of USP39 significantly increased tumor size. Carboplatin
treatment markedly suppressed the growth of ES2 cells in the animal
model. The overexpression of USP39 led to a more rapid growth rate
with carboplatin treatment (Fig.
8A-C). Mice were randomly divided into 6 groups (n=8/group).
The results revealed that the knockdown of USP39 reduced tumor
burden and carboplatin treatment markedly suppressed the growth of
SKOV3 cells in the animal model (Fig.
9). IHC revealed a lower level of cleaved caspase-3 in the
USP39-overexpressing cells than in the control cells treated with
carboplatin
(Fig. 8D). These
results suggested that USP39 attenuated the chemosensitivity to
carboplatin administration. To further investigate the role of
USP39 in ovarian cancer metastasis, an experimental murine
peritoneal metastasis model was used. The overexpression of USP39
in the ES2 cells promoted the formation of peritoneal metastases
(Fig. 8E and F). Taken together,
these results demonstrate that USP39 promotes the malignant
phenotype of ovarian cancer cells.
Discussion
As one of the most common gynecological
malignancies, ovarian cancer is a major contributor to the high
rate of mortality associated with cancer among women due to the
absence of methods for early diagnosis of this disease (16,17).
Thus far, platinum-based chemotherapy is the most common strategy
for ovarian cancer post-operative chemotherapy (18). To the best of our knowledge,
therapeutic resistance is one of the major obstacles to the
successful treatment of ovarian cancer (19,20).
Although remarkable advances have been made in the understanding of
the basic mechanisms of cancer over the past decades, the
mechanisms of tumor progression and therapy resistance have not yet
been entirely determined (21).
Thus, there are numerous candidate biomarkers under development to
evaluate their efficacy in the treatment of ovarian cancer
(22).
The dysregulation of splicing factors has been
reported to be associated with cancer development. For example, RNA
binding motif protein 4 (RBM4) has been shown to be markedly
decreased in patients with cancer (23,24),
while serine and arginine rich splicing factor 1 (SRSF1) has been
shown to be is highly upregulated in breast and colon cancer
(25,26). In this study, the analysis of
patients with ovarian cancer that exhibited an overexpression of
the spliceosome factor, USP39, suggested that USP39 was involved in
carboplatin treatment and USP39 was identified as a potential
biomarker of chemotherapeutic resistance in ovarian cancer.
To further confirm our clinical research and explore
the biological function of USP39, the effects of USP39 were
observed in SKOV3 and ES2 cells. The results helped to uncover the
mechanisms of action of USP39 that affect the phenotypes of ovarian
cancer cells. Chemotherapeutic treatment resistance could be
overcome by the knockdown of USP39. In this study, we demonstrated
that the shRNA-mediated downregulation of USP39 resulted in
attenuated cell proliferation and colony formation, whereas USP39
overexpression led to an enhanced cell proliferation and colony
formation. As stated in a previous study, USP39 participated in the
control of cell proliferation, colony formation and apoptosis
through PARP cleavage in U2OS cells (27). Previous studies have shown that the
induction of G2/M phase arrest contributes to alterations in the
expression of cell cycle regulatory proteins. The knockdown of
USP39 was previously identified to arrest progression of the cell
cycle at G2/M phase accompanied by upregulation of p21 (27). In addition, a similar effect was
detected in human SMMC-7721 cells, along with the inhibition of
Cdc2 activity (28). Similarly,
this study demonstrated that the knockdown of USP39 decreased the
expression of cyclin B1, which controls the entrance into mitosis,
thereby, accumulating the cell cycle in the quiescent G2/M phase.
Although the upregulation of USP39 has been identified to be an
oncogenic factor for tumor progression, its molecular mechanisms of
action remain to be fully determined. In recent years, the
mechanisms contributing to the dysregulation of oncogenes have been
investigated (29). It has been
reported that the phosphorylation of EGFR and its downstream
signaling molecules, such as AKT and ERK can be activated, thereby
promoting tumorigenesis and tumor progression (29). In this study, the results revealed
that the overexpression of USP39 markedly induced the
phosphorylation of EGFR, AKT and ERK. It has been demonstrated that
the depletion of USP39 induces U2OS cell apoptosis by PARP
cleavage, which is a reliable indicator of apoptosis (27). Notably, this study demonstrated
that the silencing of USP39 promoted carboplatin-mediated apoptosis
via PARP and caspase-3 cleavage. To validate the function of USP39
in ovarian cancer progression, rescue assays were carried out. The
results revealed that the knockdown of USP39 attenuated the
promoting effects of USP39 on cell chemosensitivity to carboplatin,
migration and invasion, indicating the role of USP39 in ovarian
cancer progression. In the in vivo experiments, the
overexpression of USP39 decreased the chemosensitivity of ES2 cells
to carboplatin in subcutaneous xenograft models. By contrast, the
knockdown of USP39 reduced the tumor burden. Due to the rapid and
early metastasis to the peritoneum in ovarian cancer, it was
concluded that USP39 was involved in metastasis in the peritoneal
metastasis model. Based on these data, we hypothesized that USP39
promotes ovarian cancer chemoresistance and may thus serve as a
therapeutic marker for ovarian cancer.
Taken together, in this study, using in vitro
and in vivo approaches, the carcinogenic function of USP39
in ovarian cancer cells was observed for the first time, at least
to the best of our knowledge. USP39 knockdown enhanced the
sensitivity of ovarian cancer cells to carboplatin. Therefore,
USP39 may be used for predicting patients with ovarian cancer who
are at a high risk of developing resistance to carboplatin-based
chemotherapy. Developing strategies with which to target USP39 may
be useful for overcoming therapeutic resistance in patients with
ovarian cancer. Several studies have reported a pre-mRNA splicing
function for USP39 (7,10). Thus, genes involved in splicing and
which are regulated by USP39 should be screened using splicing
microarrays in future studies in order to elucidate the mechanisms
of action of USP39 in mediating chemoresistance.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81650015, 81130043 and
81472452), and by the CAMS Initiative for Innovative Medicine
(grant no. 2017-I2M-3-004).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
TC conceived the study. LW and TC designed and
conducted the experiments. XL, WY and YL conducted data acquisition
and data analysis. TC drafted the article and critically revised it
for important intellectual content. ZL, HC and ZC contributed to
refining the ideas, carrying out additional analyses, processing
figures, and finalizing this article. All authors discussed the
results, revised the manuscript and agree to be accountable for all
aspects of the work. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients provided informed consent to
participate in the study. The study was approved by the ethics
committee of the Chinese Academy of Medical Sciences Cancer
Hospital (Beijing, China). Animal studies were approved by the
Animal Care and Use Committee of Cancer Hospital, Chinese Academy
of Medical Sciences.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Cheng JQ, Jiang X, Fraser M, Li M, Dan HC,
Sun M and Tsang BK: Role of X-linked inhibitor of apoptosis protein
in chemoresistance in ovarian cancer: Possible involvement of the
phosphoinositide-3 kinase/Akt pathway. Drug Resist Updat.
5:131–146. 2002. View Article : Google Scholar
|
|
2
|
Russo A, Czarnecki AA, Dean M, Modi DA,
Lantvit DD, Hardy L, Baligod S, Davis DA, Wei JJ and Burdette JE:
PTEN loss in the fallopian tube induces hyperplasia and ovarian
tumor formation. Oncogene. 37:1976–1990. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Zyl B, Tang D and Bowden NA:
Biomarkers of platinum resistance in ovarian cancer: What can we
use to improve treatment. Endocr Relat Cancer. 25:R303–R318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ricciardelli C, Lokman NA, Ween MP and
Oehler MK: Women in cancer thematic review: Ovarian
cancer-peritoneal cell interactions promote extracellular matrix
processing. Endocr Relat Cancer. 23:T155–T168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li KY, Zhang J, Jiang LC, Zhang B, Xia CP,
Xu K, Chen HY, Yang QZ, Liu SW and Zhu H: Knockdown of USP39 by
lentivirus-mediated RNA interference suppresses the growth of oral
squamous cell carcinoma. Cancer Biomark. 16:137–144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Zhang B, Lei Y, Sun J, Zhang Y,
Yang S and Zhang X: Knockdown of USP39 induces cell cycle arrest
and apoptosis in melanoma. Tumour Biol. 37:13167–13176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ríos Y, Melmed S, Lin S and Liu NA:
Zebrafish usp39 mutation leads to rb1 mRNA splicing defect and
pituitary lineage expansion. PLoS Genet. 7:e10012712011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fraile JM, Manchado E, Lujambio A, Quesada
V, Campos-Iglesias D, Webb TR, Lowe SW, López-Otín C and Freije JM:
USP39 Deubiquitinase Is Essential for KRAS Oncogene-driven Cancer.
J Biol Chem. 292:4164–4175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
An Y, Yang S, Guo K, Ma B and Wang Y:
Reduced USP39 expression inhibits malignant proliferation of
medullary thyroid carcinoma in vitro. World J Surg Oncol.
13:2552015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Y, Pan XW, Li L, Chen L, Liu X, Lu
JL, Zhu XM, Huang H, Yang QW, Ye JQ, et al: Overexpression of USP39
predicts poor prognosis and promotes tumorigenesis of prostate
cancer via promoting EGFR mRNA maturation and transcription
elongation. Oncotarget. 7:22016–22030. 2016.PubMed/NCBI
|
|
11
|
Wang H, Ji X, Liu X, Yao R, Chi J, Liu S,
Wang Y, Cao W and Zhou Q: Lentivirus-mediated inhibition of USP39
suppresses the growth of breast cancer cells in vitro. Oncol Rep.
30:2871–2877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Kong Y, Zhou Z, Chen H, Wang Z,
Hsieh YC, Zhao D, Zhi X, Huang J, Zhang J, et al: The HECTD3 E3
ubiquitin ligase facilitates cancer cell survival by promoting
K63-linked polyu-biquitination of caspase-8. Cell Death Dis.
4:e9352013. View Article : Google Scholar
|
|
13
|
Shu T, Li Y, Wu X, Li B and Liu Z:
Down-regulation of HECTD3 by HER2 inhibition makes serous ovarian
cancer cells sensitive to platinum treatment. Cancer Lett.
411:65–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
15
|
Wu X, Luo Q, Zhao P, Chang W, Wang Y, Shu
T, Ding F, Li B and Liu Z: MGMT-activated DUB3 stabilizes MCL1 and
drives chemoresistance in ovarian cancer. Proc Natl Acad Sci USA.
116:2961–2966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueno M, Shiomi T, Mochizuki S, Chijiiwa M,
Shimoda M, Kanai Y, Kataoka F, Hirasawa A, Susumu N, Aoki D, et al:
ADAM9 is over-expressed in human ovarian clear cell carcinomas and
suppresses cisplatin-induced cell death. Cancer Sci. 109:471–482.
2018. View Article : Google Scholar :
|
|
17
|
Xu Y, Miao C, Jin C, Qiu C, Li Y, Sun X,
Gao M, Lu N and Kong B: SUSD2 promotes cancer metastasis and
confers cisplatin resistance in high grade serous ovarian cancer.
Exp Cell Res. 363:160–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Longoria TC and Tewari KS: Pharmacokinetic
drug evaluation of niraparib for the treatment of ovarian cancer.
Expert Opin Drug Metab Toxicol. 14:543–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ricciardelli C, Lokman NA, Sabit I,
Gunasegaran K, Bonner WM, Pyragius CE, Macpherson AM and Oehler MK:
Novel ex vivo ovarian cancer tissue explant assay for prediction of
chemosensitivity and response to novel therapeutics. Cancer Lett.
421:51–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei W, Giulia F, Luffer S, Kumar R, Wu B,
Tavallai M, Bekele RT and Birrer MJ: How can molecular
abnormalities influence our clinical approach. Ann Oncol.
28:viii16–viii24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Zhang P, Shi B, Zhou M, Jiang H,
Zhang H, Pan X, Gao H, Sun H and Li Z: Galectin-1 overexpression
promotes progression and chemoresistance to cisplatin in epithelial
ovarian cancer. Cell Death Dis. 5:e9912014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Birkbak NJ, Li Y, Pathania S,
Greene-Colozzi A, Dreze M, Bowman-Colin C, Sztupinszki Z,
Krzystanek M, Diossy M, Tung N, et al: Overexpression of BLM
promotes DNA damage and increased sensitivity to platinum salts in
triple-negative breast and serous ovarian cancers. Ann Oncol.
29:903–909. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Chen D, Qian H, Tsai YS, Shao S,
Liu Q, Dominguez D and Wang Z: The splicing factor RBM4 controls
apoptosis, proliferation, and migration to suppress tumor
progression. Cancer Cell. 26:374–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
No authors listed. RBM4-regulated
alternative splicing suppresses tumorigenesis. Cancer Discov.
4:12532014. View Article : Google Scholar
|
|
25
|
Chen L, Luo C, Shen L, Liu Y, Wang Q,
Zhang C, Guo R, Zhang Y, Xie Z, Wei N, et al: SRSF1 Prevents DNA
Damage and Promotes Tumorigenesis through Regulation of DBF4B
Pre-mRNA Splicing. Cell Rep. 21:3406–3413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anczuków O, Akerman M, Cléry A, Wu J, Shen
C, Shirole NH, Raimer A, Sun S, Jensen MA, Hua Y, et al:
SRSF1-regulated alternative splicing in breast cancer. Mol Cell.
60:105–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gan Z, Han K, Lin S, Hu H, Shen Z and Min
D: Knockdown of ubiquitin-specific peptidase 39 inhibited the
growth of osteo-sarcoma cells and induced apoptosis in vitro. Biol
Res. 50:152017. View Article : Google Scholar
|
|
28
|
Pan Z, Pan H, Zhang J, Yang Y, Liu H, Yang
Y, Huang G, Ni J, Huang J and Zhou W: Lentivirus mediated silencing
of ubiquitin specific peptidase 39 inhibits cell proliferation of
human hepato-cellular carcinoma cells in vitro. Biol Res.
48:182015. View Article : Google Scholar
|
|
29
|
Tuan Anh HL, Tran PT, Thao DT, Trang DT,
Dang NH, Van Cuong P, Kiem PV, Minh CV and Lee JH:
Degalactotigonin, a steroidal glycoside from solanum nigrum,
induces apoptosis and cell cycle arrest via inhibiting the EGFR
signaling pathways in pancreatic cancer cells. BioMed Res Int.
2018:31209722018. View Article : Google Scholar
|