Introduction
Gastric cancer (GC) is one of the most common
cancers worldwide and results in the second greatest rate
cancer-associated mortality globally (1). Although surgery is an effective
therapeutic strategy for patients with resectable tumors, the
majority of patients with GC, particularly in China, are diagnosed
at an advanced and are thus unresectable at that clinical stage
(2). For those patients,
chemotherapy becomes the preferred treatment method. However,
multidrug resistance (MDR) often develops during chemotherapy,
resulting in the failure of treatment (2-4).
Therefore, strategies that can reverse MDR of GC cells have a
notable impact on the success of chemotherapy. To generate an
efficient strategy for reversing MDR, it is important to discover
novel cancer-associated key molecules and MDR-associated mechanisms
in GC cells.
Sorcin is a soluble resistance-associated
calcium-binding protein, which is expressed in many human tissues,
with especially high levels in the bones, heart, kidneys and skin
(5,6). However, it was also demonstrated that
sorcin was overexpressed in many types of cancer, including
leukemia, breast cancer and GC (7-9).
Sorcin gene is positioned in the same amplicon of other genes
associated with the resistance to chemotherapeutic agents in cancer
cells. Therefore, in multidrug-resistant cancer cells, sorcin was
recognized as 'resistance-associated' (10). For instance, a high sorcin
expression level in leukemia patients indicated poor
chemotherapeutic response and poor prognosis (5). Previous studies have demonstrated
that sorcin overexpression is closely associated with the MDR of GC
(11-13). Silencing sorcin by shRNA, Xu et
al (14) demonstrated that the
drug chemosensitivity in myeloma KM3/DDP and U266/ADM cell lines
was enhanced. In MDA-MB-231 breast cancer cells, Hu et al
(15) demonstrated that sorcin
depletion by RNA interference inhibited epithelial-to-mesenchymal
transition and suppressed breast cancer metastasis in vivo.
It has been suggested that suppressing sorcin may have the
potential to improve the sensitivity of GC cells to drugs.
MicroRNAs (miRNAs or miRs), small non-coding RNA
molecules with ~22 nucleotides, control gene expression levels by
degrading mRNA at the posttranscriptional level (16). The binding sites of miRNAs are
usually the 3′-untranslated region (UTR) sequences of mRNAs. Upon
the hybridization of miRNAs and mRNA, the target gene is
downregulated (17). During GC
development, progression and therapeutic processes, >200 miRNAs
have been demonstrated to be involved (18). Furthermore, the MDR of various
types of cancer is also regulated by miRNAs (18). Among those miRNAs, miR-1 is
decreased in various human cancers, which indicated that it may be
a potential therapeutic target for cancer treatment (18). Conversely, the prediction of
Targetscan and previous studies indicated that miR-1 may directly
target sorcin. It was also reported that the expression level of
miR-1 was associated with the chemoresistance of GC and lung cancer
(19,20). Therefore, the interaction between
miR-1 and sorcin in the development of MDR of GC is of interest for
the diagnosis and treatment of MDR GC.
It has previously been reported that miR-1 is
significantly downregulated in GC cells (19). However, the mechanism of miR-1 in
regulating the MDR of GC cells remains unclear. In the present
study, the role of miR-1 and sorcin in MDR GC cells was assessed
using SGC7901/adriamycin (ADM) and SGC7901/vincristine (VCR)
resistant cell lines, which have been widely used as the model of
MDR GC (21,22). Doxorubicin, also known as ADM, is
an anthracycline-based anti-tumor agent and is an effective
FDA-approved drug for GCs (23).
Vincristine is also a common drug for cancer thermotherapy for many
types of cancer, such as acute myeloid leukemia, small cell lung
cancer and neuroblastoma (24-26).
The aim of the present study was to provide an insight into the
molecular mechanism of MDR in GC cells. It was demonstrated that
miR-1 was significantly downregulated in all MDR GC cells lines. By
overexpressing miR-1 in those MDR GC cell lines, the apoptosis and
drug accumulation rates were increased. In addition, the results
demonstrated that sorcin was one of the targets of miR-1 in GC.
Overexpression of miR-1 in GC cells inhibited sorcin and then
promoted the cell apoptosis and drug accumulation. Therefore, miR-1
may be used as a small therapeutic molecule and potential target
for new drug discovery in GC.
Materials and methods
Cell culture and treatment
The GC cell lines, SGC7901, SGC7901/VCR and
SGC7901/ADM, were obtained from the Shanghai Institutes for
Biological Sciences Cell Resource Center (Shanghai, China). The
cells were cultured with RPMI-1640 medium (Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.), penicillin and streptomycin at 37°C in a humid
atmosphere (5% CO2, 95% air). For drug treatment, the
SGC7901, SGC7901/ADM and SGC7901/VCR cells were cultured in the
same medium with the addition of 0, 0.5, 1, 2, 4, 8, 16 or 32
µg/ml ADM, or 0, 0.01, 0.1, 1, 5, 10 or 20 µg/ml VCR
and MTT assay was performed 24 h later. The half maximal inhibitory
concentration (IC50) was determined by plotting the
relationship between the concentrations of drugs and the cell
viabilities.
Cell transfection
miR-1 mimics, scramble miRNA negative control
(miR-NC) and miR-1 inhibitors were synthesized by Shanghai
GenePharma Co., Ltd.. The sequences were as follows: miR-1 mimic,
5′-UGGAAUGUAAAGAAGUAUG UAU-3′ (sense) and
5′-ACAUACUUCUUUACAUUCCAUU-3′ (antisense); miR-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′
(antisense); miR-1 inhibitor, 5′-UUCAGUUAUCACAGUACU GUA-3′;
inhibitor NC, 5′-CAGUACUUUUGUGUAGUA CAA-3′. SGC7901, SGC7901/ADM
and SGC7901/VCR cells were transfected with 100 nM miR-1 mimics or
miR-1 inhibitor for miR-1 overexpression and knockdown,
respectively. miR-NC (100 nM) and inhibitor NC (100 nM) were used
as negative controls. Transfection was conducted using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Six hours
post-transfection, the medium was changed with fresh medium. At 48
h post-transfection, the cells were used in subsequent
experiments.
MTT assay
In 96-well plates, the GC cells (2,000 cells/well)
were cultured in RPMI-1640 medium for 24 h following the different
treatments. Then, 10 µl MTT was added into the culture
medium for 60 min at 37°C, followed by the addition of 100
µl dimethyl sulfoxide in each well to dissolve the resulting
crystals by shaking for 10 min at room temperature. Then, the
optical absorbance at 490 nm was measured using a microplate
reader.
Apoptosis analysis by flow cytometry
For the apoptosis analysis, the cells were
transfected with miR-NC, miR-1 mimics and miR-1 inhibitors. Then,
the Annexin V-FITC/PI Apoptosis Detection kit (BD Biosciences) was
used to stain each sample, which were then analyzed by flow
cytometry. The quantitative analysis of the percentage of the
apoptosis cells was performed using FlowJo v10 (Tree Star,
Inc.,).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA from the GC cells were collected using
TRIzol reagent (Life Technologies; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Then, the reverse
transcription of cDNA from the RNAs was performed with TaqMan
Reverse Transcription reagents (Life Technologies; Thermo Fisher
Scientific, Inc.). The analysis of expression levels of miR-1, B
cell lymphoma (BCL)-2, BCL-2-associated X protein (Bax), c-fos,
c-jun and sorcin were performed using TaqMan microRNA assay kits
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The primer
sequences used for miR-1 were as follows: Forward,
5′-GCGGCTGGAATGTAAAGAAGT-3′; and reverse,
5′-CGGCCCAGTGTTCAGACTAC-3′. The sequences for BAX were as follows:
Forward, 5′-CTGACGGCAACTTCAACTGGG-3′; and reverse,
5′-CAACCACCCTGGTCTTGGATC-3′. The sequences for c-fos were as
follows: Forward, 5′-CTTACTACCACTCACCCGCAG-3′; and reverse,
5′-GCCTCCTGTCATGGTC TTCAC-3′. The sequences for c-jun were as
follows: Sense, 5′-GTGCCGAAAAAGGAAGCTGG-3′; and reverse, 5′-CTG
CGTTAGCATGAGTTGGC-3′. The sequences for BCL-2 were as follows:
Forward, 5′-GGATCCAGGATAACGGA GGC′; and reverse,
5′-GATAGGCACCCAGGGTGATG-3′. The sequences for sorcin were as
follows: Forward, 5′-GGAGA CTTGCCGGCTTATGG-3′; and reverse,
5′-CACAGCCTGGGGACTCAACC-3′. The sequences for GAPDH were as
follows: Forward, 5′-GTCAACGGATTTGGTCTGTATT-3′; and reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′. The sequences for U6 were as follows:
Forward, 5′-TGCGTTCCCT TTGTCATCCT-3′; and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The primers were synthesized by Sangon
Biotech Co., Ltd. The thermocycling conditions were: 95°C for 10
min, then 45 cycles of 94°C for 15 sec, 60°C for 40 sec, and 72°C
for 45 sec. The 2−ΔΔCq method was used to calculate the
relative fold change in expression compared with a reference sample
(27).
Western blot analysis
Total cellular proteins were extracted from cells
using cell lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd.) and the amount of protein was determined by
bicinchoninic acid assay. Then, the proteins (50 µg) were
separated by 10% SDS-PAGE and then transferred to polyvinylidene
difluoride membranes (EMD Millipore). Subsequently, 5% milk was
used to block the membrane for 1 h at room temperature followed by
incubation overnight at 4°C with primary antibodies against MDR
protein 1/P-glycoprotein (MDR1/P-gp; cat. no. 13342; 1:1,000),
MDR-associated protein 1 (MRP-1; cat. no. 72202; 1:600), Bax (cat.
no. 2774; 1:800), c-fos (cat. no. 4384; 1:1,000), c-jun (cat. no.
9165; 1:800) and BCL-2 (cat. no. 4223; 1:600) from Cell Signaling
Technology, Inc., and against sorcin (cat. no. A6751, 1:1,000) from
ABclonal. Membranes were then incubated at room temperature for 2 h
with horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit; cat. no. ZB-2301; 1:5,000; and goat anti-mouse; cat.
no. ZDR5307; 1:1,500; OriGene Technologies, Inc.). An enhanced
chemiluminescence detecting system (iBright CL1000 imaging system;
Thermo Fisher Scientific, Inc.) was used to quantify the expression
levels. The bands were quantified using Image Lab™ software
(Bio-Rad Laboratories, Inc.). β-actin (cat. no. AC004; 1:1500;
ABclonal) was used as loading control.
Dual luciferase activity assay
The dual luciferase activity assay was used to
confirm the target of mir-1. The potential target site of miR-1 on
the sorcin gene was predicted using the miRDB database (http://mirdb.org/). Fragments containing the 3′UTR
target sites of sorcin were amplified and then cloned into psiCHECK
(Invitrogen; Thermo Fisher Scientific, Inc.). Forward and reverse
PCR primers carried a 5′ overhang that contained SpeI and
HindIII recognition sites, respectively. Then, the sorcin
3′-UTR fragment was cloned into the dual luciferase reporter vector
psiCHECK and the recombinant plasmid was named psiCHECK-wt. The
miR-1 targeting sequence in the above sorcin 3′UTR reporter plasmid
was also mutated and named psiCHECK-mut. Then, the SGC7901 cells
were transfected with the psiCHECK-wt, psiCHECK-mut, miR-NC and
miR-1 mimic using Lipofectamine 3000 for 48 h (Thermo Fisher
Scientific, Inc.). The Dual-Luciferase assay kit (Promega
Corporation) was used to measure the luciferase activities in
transfected cells following to the manufacturer's information. The
luciferase activity, normalized against protein concentration, was
expressed as a ratio of firefly luciferase to Renilla
luciferase units.
Drug accumulation assay
The treated GC cells (2×106 cells/well in
a 6-well plate) were collected and incubated with 0.3 µM
Rhodanmin 123 (Rho123; cat. no. R8004; Sigma-Aldrich; Merck KGaA)
in RPMI-1640 medium for 90 min at 37°C. Then, the cells were washed
with ice-cold PBS twice and finally suspended in 500 µl
ice-cold PBS. Beckman CytoFLEX flow cytometry (CytExpert 2.0;
Beckman Coulter, Inc.) was used to quantify the Rho123 accumulation
in cells with excitation at 488 nm and emission at 530 nm.
Statistical analysis
Data are presented as the means ± standard deviation
of at least three separate experiments. The difference between two
groups was analyzed by unpaired two-tailed Student's t-test.
One-way analysis of variance was used for comparison among multiple
groups and multiple comparisons were further performed using post
hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-1 and sorcin in GC
cells
In order to discover the roles of miR-1 and sorcin
in GC cells, the expression levels of miR-1 and sorcin were
examined by RT-qPCR and western blotting in GC cell lines,
including SGC7901, SGC7901/ADM and SGC7901/VCR. The half maximal
inhibitory concentration (IC50) of VCR and ADM was
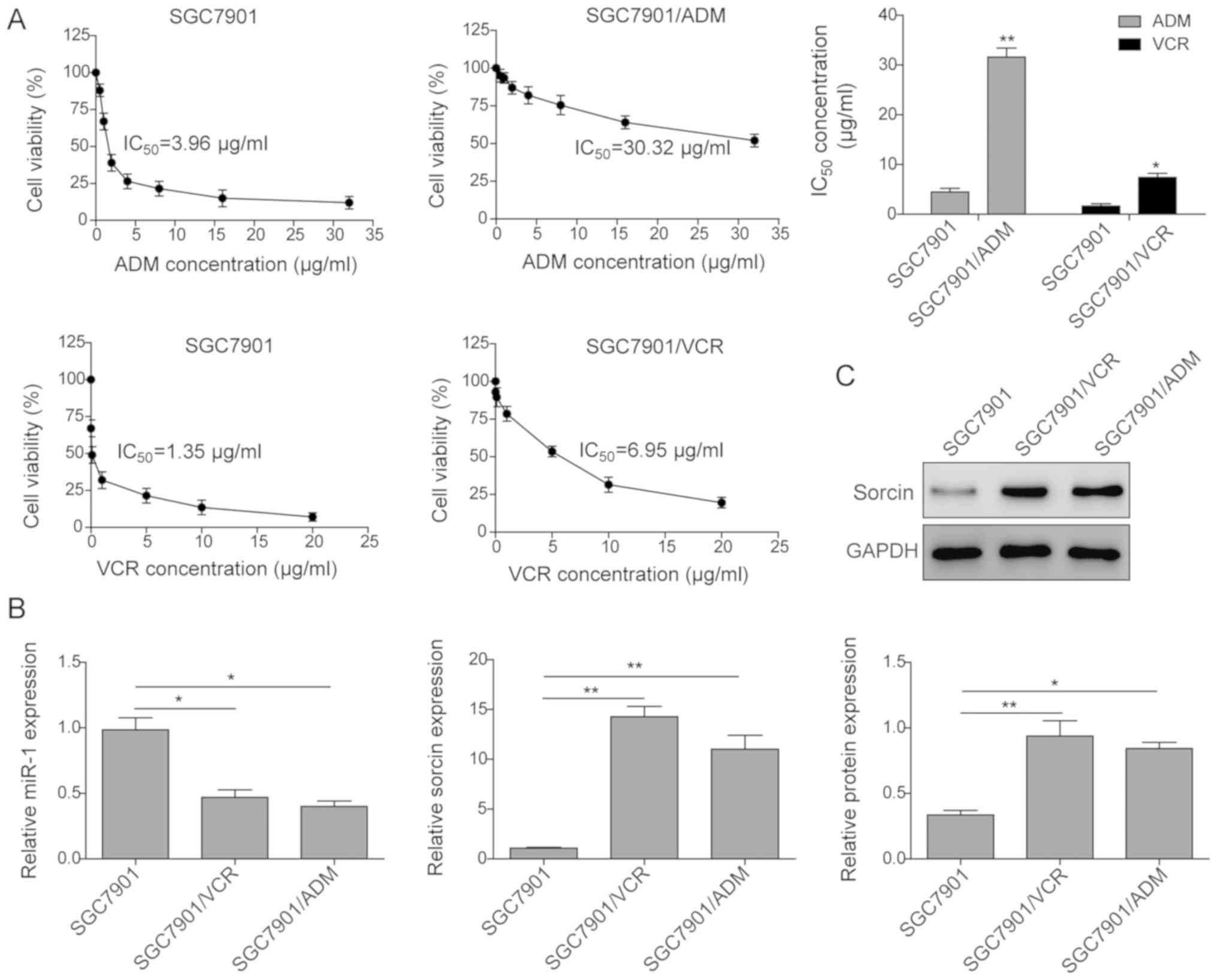
investigated in different gastric cell lines. As presented in
Fig. 1A, SGC7901/VCR and
SGC7901/ADM exhibited significant resistance to VCR and ADM
treatments, respectively. IC50 of ADM to SGC7901/ADM
increased 7.65-fold compared with the SGC7901 cells.
IC50 of VCR to SGC7901/VCR increased 5.94 times compared
with the SGC7901 cells. Furthermore, the expression level of miR-1
and mRNA level of sorcin were determined in these three GC cell
lines. As presented in Fig. 1B,
miR-1 was significantly downregulated in the drug resistant cell
lines, which were downregulated to 0.47 and 0.40 in SGC7901/ADM and
SGC7901/VCR, respectively, compared with the SGC7901 cells.
However, the mRNA expression level of sorcin in these two
drug-resistant cell lines was significantly enhanced. The sorcin
protein expression level measured by western blot assay also
demonstrated the enhanced expression of sorcin in the
drug-resistant cell lines (Fig.
1C).
miR-1 reverses the MDR in GC cells
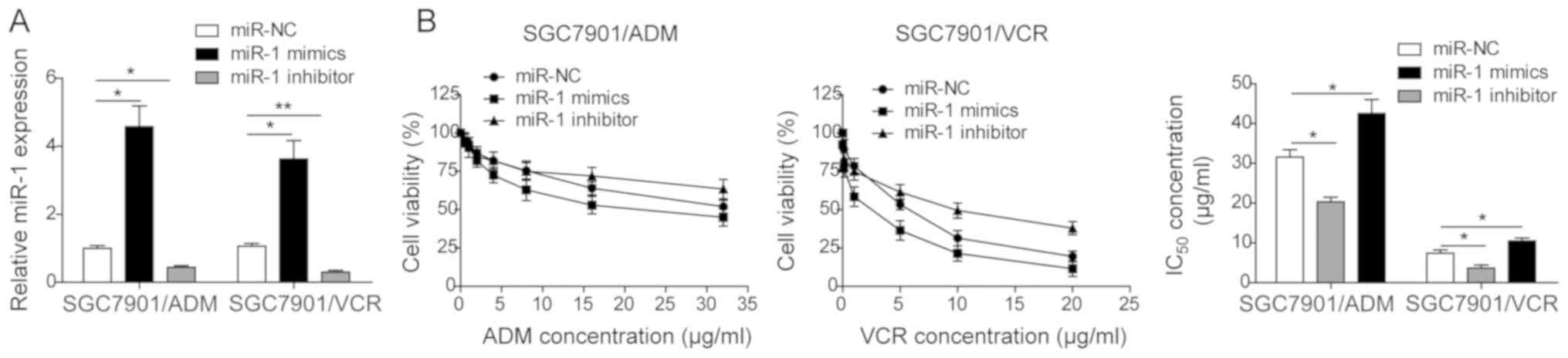
The effect of miR-1 on the MDR in GC cells was
investigated. The miR-NC, miR-1 mimics and miR-1 inhibitor were
transfected into two drug-resistant GC cells, SGC7901/VCR and
SGC7901/ADM. As presented in Fig.
2A, the miR-1 expression was significantly enhanced when miR-1
mimics were transfected in both cell lines. In contrast, the
transfection of miR-1 inhibitor downregulated the expression of
miR-1 in both cell lines. Furthermore, the IC50 of these
two drug-resistant cell lines following transfection was
determined. As presented in Fig.
2B, the IC50 of both SGC7901/VCR and SGC7901/ADM
treated with miR-1 mimics was significantly decreased and the
transfection of miR-1 inhibitor increased the IC50 in
both cell lines. The results indicated that miR-1 reversed the
multi-drug resistance in GC cell lines.
miR-1 induces GC cell apoptosis
To elucidate how miR-1 reversed the MDR, the effect
of miR-1 on the apoptosis of GC cell lines was investigated. As
presented in Fig. 3A, SGC7901/ADM
and SGC7901/VCR cells were transfected with miR-NC, miR-1 mimics
and miR-1 inhibitor and the apoptosis rates were measured by flow
cytometry without the treatment of drugs. The results demonstrated
that the transfection of miR-1 mimics significantly promoted the
apoptosis rates in both SGC7901/ADM and SGC7901/VCR cell lines, but
miR-1 inhibitor suppressed cell apoptosis. Furthermore, the
transfection of miR-1 mimics significantly enhanced the
drug-resistant GC cell apoptosis rates under the treatment of the
drugs (Fig. 3B). The apoptosis
rate of SGC7901/ADM cells following transfection of miR-1 mimics
and treatment with 10 µM ADM was ~1.9 times higher than
control groups. Similarly, the apoptosis rate of SGC7901/VCR cells
following transfection of miR-1 mimics and treatment with 5
µM VCR was ~2.1 times higher than the control groups.
Meanwhile, the transfection of miR-1 inhibitor significantly
decreased the apoptosis rate compared with the control groups when
the ADM or VCR drugs were present.
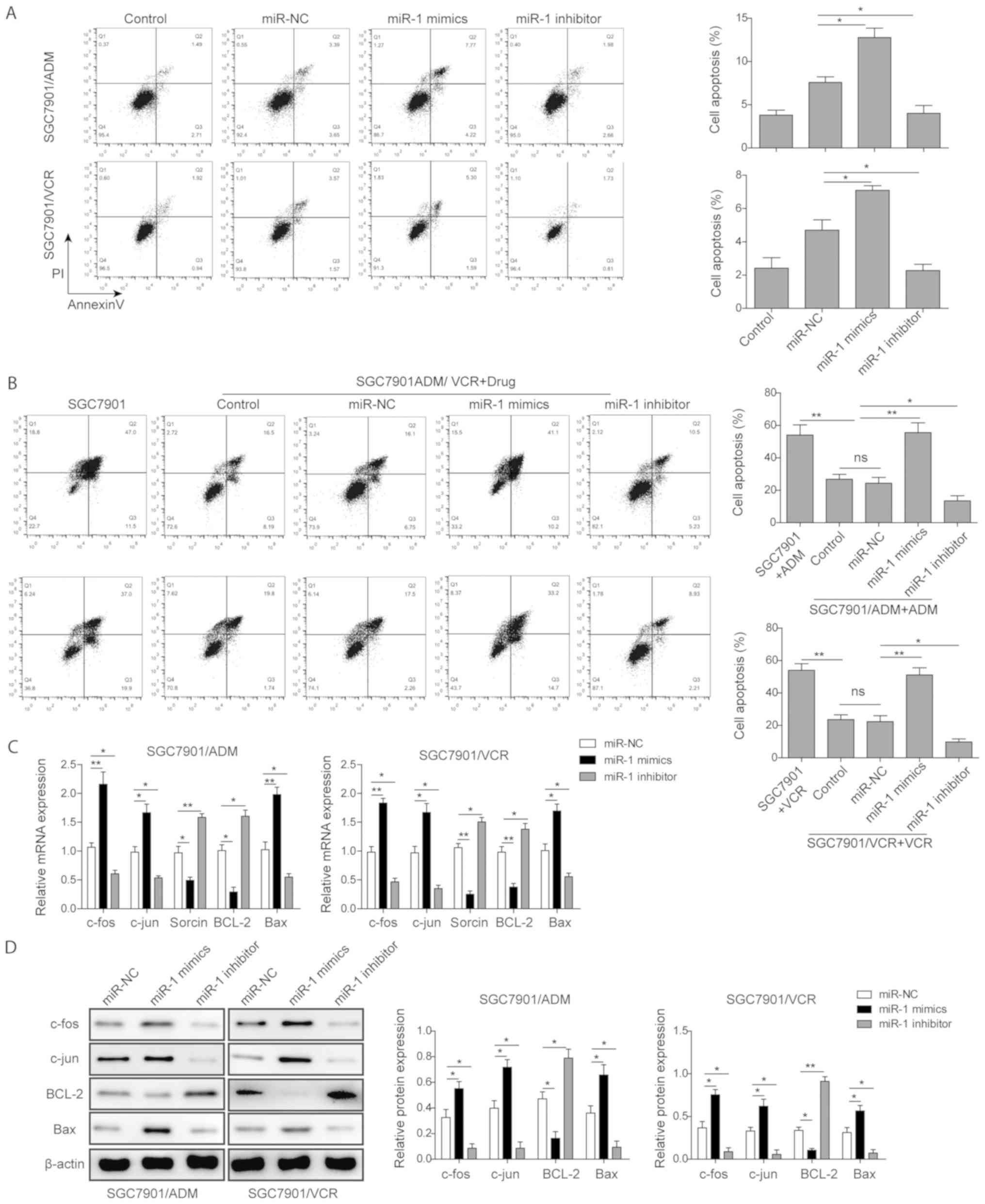 | Figure 3miR-1 induced gastric cancer cell
apoptosis. (A) The flow cytometric analysis of apoptosis rates of
SGC7901/ADM and SGC7901/VCR with transfection of miR-NC, miR-1
mimics and miR-1 inhibitor. (B) The flow cytometric analysis of
apoptosis rates of SGC7901/ADM and SGC7901/VCR cells with
transfection of miR-NC, miR-1 mimics and miR-1 inhibitor followed
by treatment with ADM and VCR, respectively. The (C) mRNA and (D)
protein expression levels of c-fos, c-jun, sorcin, BCL-2 and Bax in
SGC7901/ADM and SGC7901/VCR with transfection of miR-NC, miR-1
mimics and miR-1 inhibitor. Data are presented as the mean ±
standard deviation of three repeated experiments.
*P<0.05, **P<0.01. miR, microRNA; VCR,
vincristine; ADM, adriamycin; miR-NC, scramble control; BCL-2, B
cell lymphoma-2; Bax, Bcl-2-associated X protein. |
Furthermore, the expression levels of several
apoptosis-associated proteins in these transfected drug-resistant
GC cell lines were investigated. It was observed that miR-1
upregulated pro-apoptotic proteins including Bax, c-fos and c-jun,
but inhibited anti-apoptotic protein BCL-2 and sorcin expression in
both SGC7901/ADM and SGC7901/VCR cells. In contrast, miR-1
inhibitor promoted the expression of BCL-2 and sorcin while
inhibiting the expression levels of Bax, c-fos and c-jun (Fig. 3C). Similarly, the protein
expression levels of these components measured by western blotting
assay exhibited the same trends in these drug-resistant GC cell
lines (Fig. 3D).
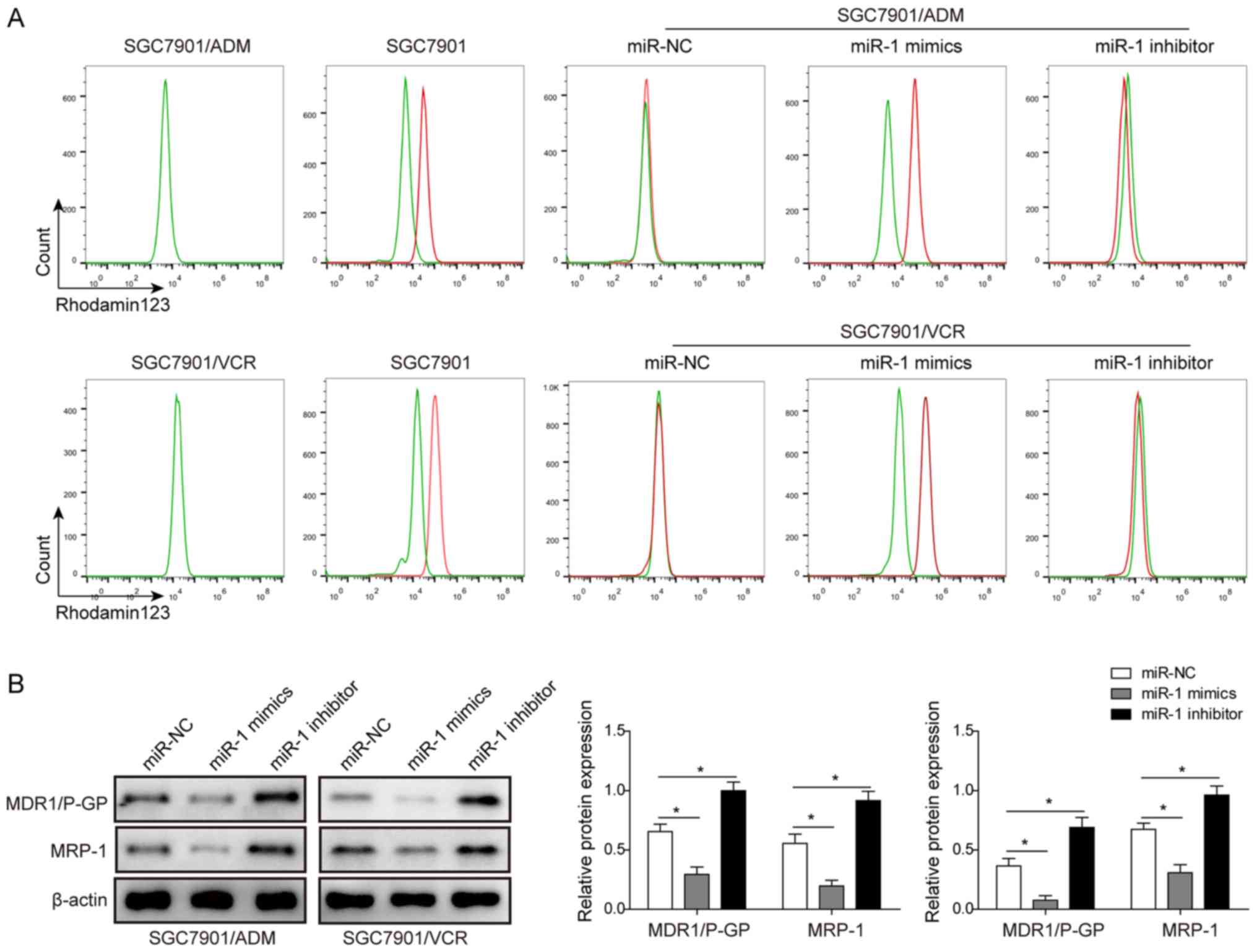
miR-1 promotes the drug accumulation in
multidrug resistant GC cells
The effect of miR-1 on the drug accumulation in
multidrug resistant GC cells was also investigated. Two cell lines,
SGC7901/ADM and SGC7901/VCR, were transfected with miR-NC, miR-1
mimics and miR-1 inhibitor. Then, the drug accumulation was
measured by flow cytometry with Rho123 following the treatment of
10 µM ADM and 5 µM VCR in SGC7901/ADM and
SGC7901/VCR, respectively. The results in Fig. 4A demonstrated that the transfection
of miR-1 mimics markedly enhanced the drug accumulation in both
SGC7901/ADM and SGC7901/VCR cells. In contrast, the transfection of
miR-1 inhibitor inhibited the drug accumulation in these two cell
lines. The expression levels of MDR1/P-gp and MRP-1 were determined
using western blot analysis following the transfection of miR-1
mimics and miR-1 inhibitor. The results demonstrated that both
MDR1/P-gp and MRP-1 were significantly inhibited in SGC7901/ADM and
SGC7901/VCR cells transfected with miR-1 mimics. The transfection
of miR-1 inhibitor upregulated the MDR1/P-gp and MRP-1 expression
levels in SGC7901/ADM and SGC7901/VCR cell lines (Fig. 4B). All these results indicated that
the drug accumulation in these two MDR GC cell lines was
significantly enhanced by overexpression of miR-1.
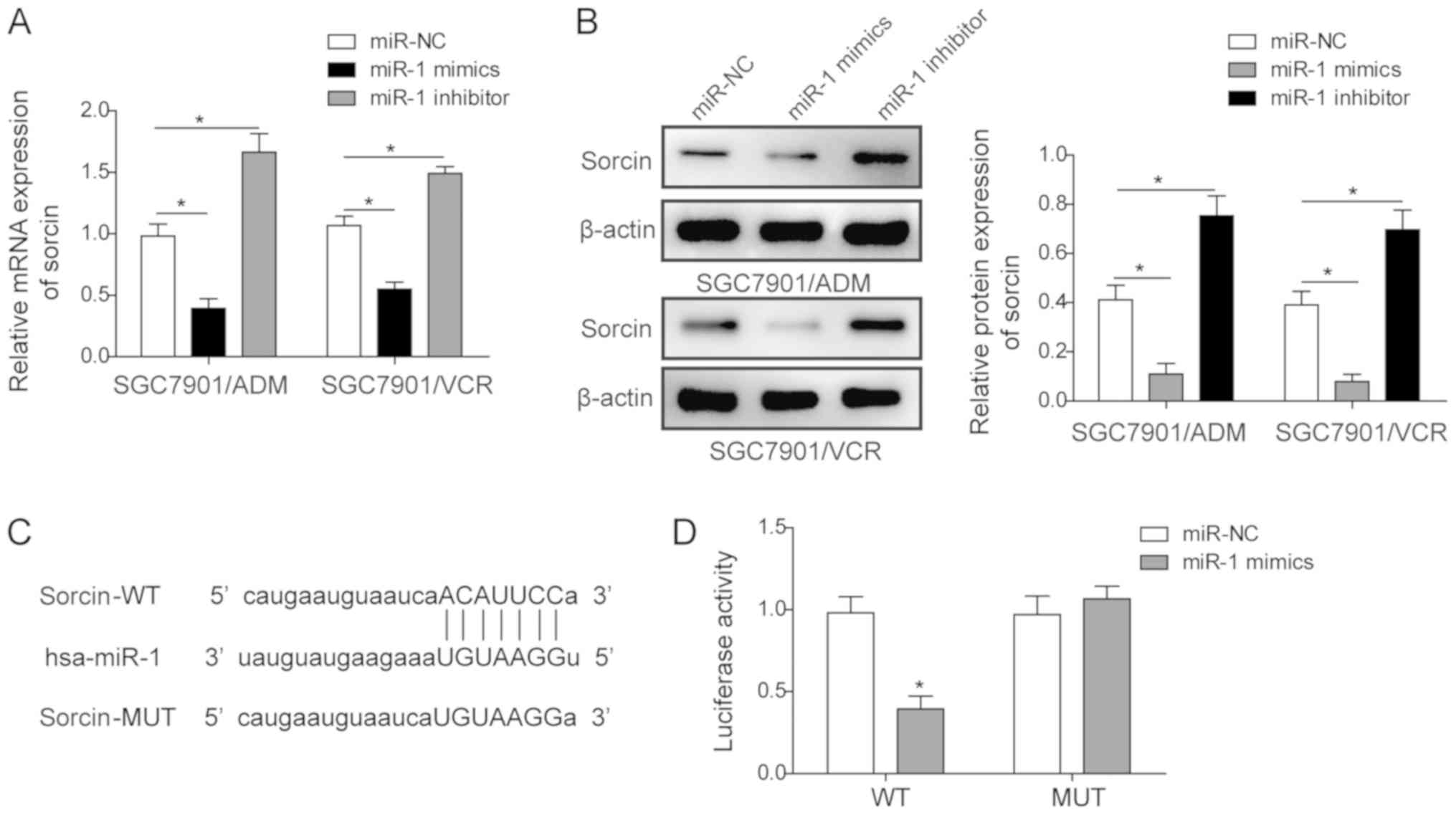
Sorcin is a target of miR-1
In order to elucidate the mechanism of miR-1 in the
drug-resistant GC cell lines, the interaction between miR-1 and
sorcin in these cell lines was investigated. The association
between the miR-1 expression level and the sorcin expression level
in the MDR GC cell lines was initially investigated. It was
demonstrated that the mRNA expression level and protein level of
sorcin in both SGC7901/ADM and SGC7901/VCR cell lines were
significantly inhibited when the cells were transfected with miR-1
mimics (Fig. 5A and B). In
contrast, the transfection of miR-1 inhibitor in these two cell
lines significantly upregulated the sorcin mRNA and protein levels.
Furthermore, a dual-luciferase assay was conducted to investigate
the interaction between miR-1 and sorcin. The potential target site
of miR-1 for sorcin was predicted by the miRDB database (Fig. 5C). Then, the sorcin 3′-UTR fragment
was cloned into the dual luciferase reporter vector psiCHECK and
the recombinant plasmid was named psiCHECK-wt. The miR-1 targeting
sequence in the above sorcin 3′UTR reporter plasmid was also
mutated and named psiCHECK-mut. Dual-luciferase reporters
containing either psiCHECK-wt or psiCHECK-mut were co-transfected
into SGC7901 cells with either miR-NC or miR-1. The group with
co-transfection of psiCHECK-wt and miR-1 demonstrated a significant
luminescence intensity decrease, suggesting that sorcin may be a
target of miR-1 (Fig. 5D).
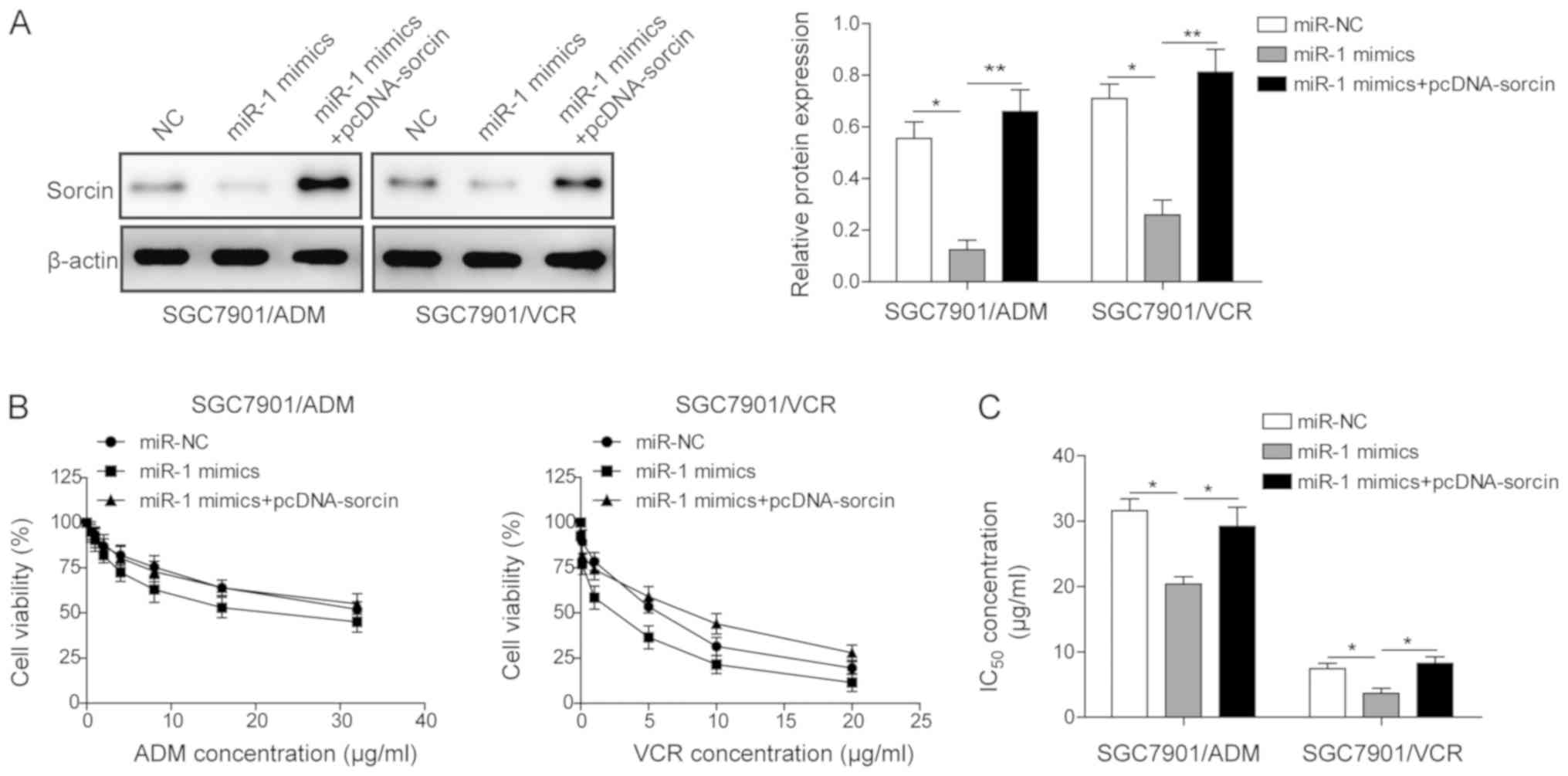
Overexpression of sorcin in both
SGC7901/ADM and SGC7901/VCR cell lines partially reverses the
impact of miR-1 on drug resistance
In order to elucidate whether miR-1 regulates the
chemosensitivity of MDR GC cell lines through targeting sorcin,
sorcin was overexpressed following upregulating miR-1 in
SGC7901/ADM and SGC7901/VCR and their impacts on IC50,
apoptosis rates and drug accumulation were investigated.
SGC7901/ADM and SGC7901/VCR were transfected with miR-NC, miR-1
mimics and miR-1 mimics with pcDNA-sorcin. The sorcin expression
levels in different groups were measured by western blot assay
(Fig. 6A). The transfection of
miR-1 alone significantly downregulated the expression of sorcin in
both cell lines. However, co-transfection of miR-1 mimics and
pcDNA-sorcin partially recovered the expression of sorcin in both
cell lines (Fig. 6A). The MTT
assay was used to measure IC50 of ADM and VCR in
different groups. The results demonstrated that the transfection of
miR-1 mimics in both cell lines decreased the IC50.
However, the co-transfection of miR-1 mimics and pcDNA-sorcin
partially restored the IC50 from 20.4 to 29.2, and 3.7
to 8.3 in SGC7901/ADM and SGC7901/VCR cells, respectively. These
results demonstrated that the effect of miR-1 on drug resistance
was partially reversed by overexpressing sorcin in MDR GC cells
(Fig. 6B and C).
Secondly, SGC7901/ADM and SGC7901/VCR were
co-transfected with miR-1 mimics and pcDNA-sorcin and then treated
with 10 µM ADM and 5 µM VCR, respectively. The flow
cytometry results indicated that co-transfection of pcDNA-sorcin
with miR-1 mimics partially reduced the apoptosis rates compared
with the groups that were only transfected with miR-1 mimics
(Fig. 7A). From the western blot
assay, it was demonstrated that by overexpressing sorcin in
SGC7901/ADM and SGC7901/VCR cells, the upregulation of the
apoptosis-associated proteins, including Bax, c-fos and c-jun, and
downregulation of BCL-2 induced by transfection of miR-1 mimics,
were both partially reversed (Fig.
7B). Finally, the drug accumulation in SGC7901/ADM and
SGC7901/VCR cells was tested following co-transfection of miR-1
mimics and pcDNA-sorcin. As presented in Fig. 7C, the flow cytometric analysis
indicated the accumulation of Rho123 in SGC7901/ADM and SGC7901/VCR
cells following the overexpression of sorcin was markedly decreased
compared with the groups which were only transfected with miR-1
mimics. The western blot analysis demonstrated that the expression
of MDR1/P-gp and MRP-1 in SGC7901/ADM and SGC7901/VCR cells were
partially recovered when the cells were overexpressed with sorcin
(Fig. 7D). Based on the results,
the impact of miR-1 on MDR GC cells could be partially reversed by
the overexpression of sorcin, which indicated that miR-1 regulated
the MDR in GC cells by inhibiting the expression of sorcin.
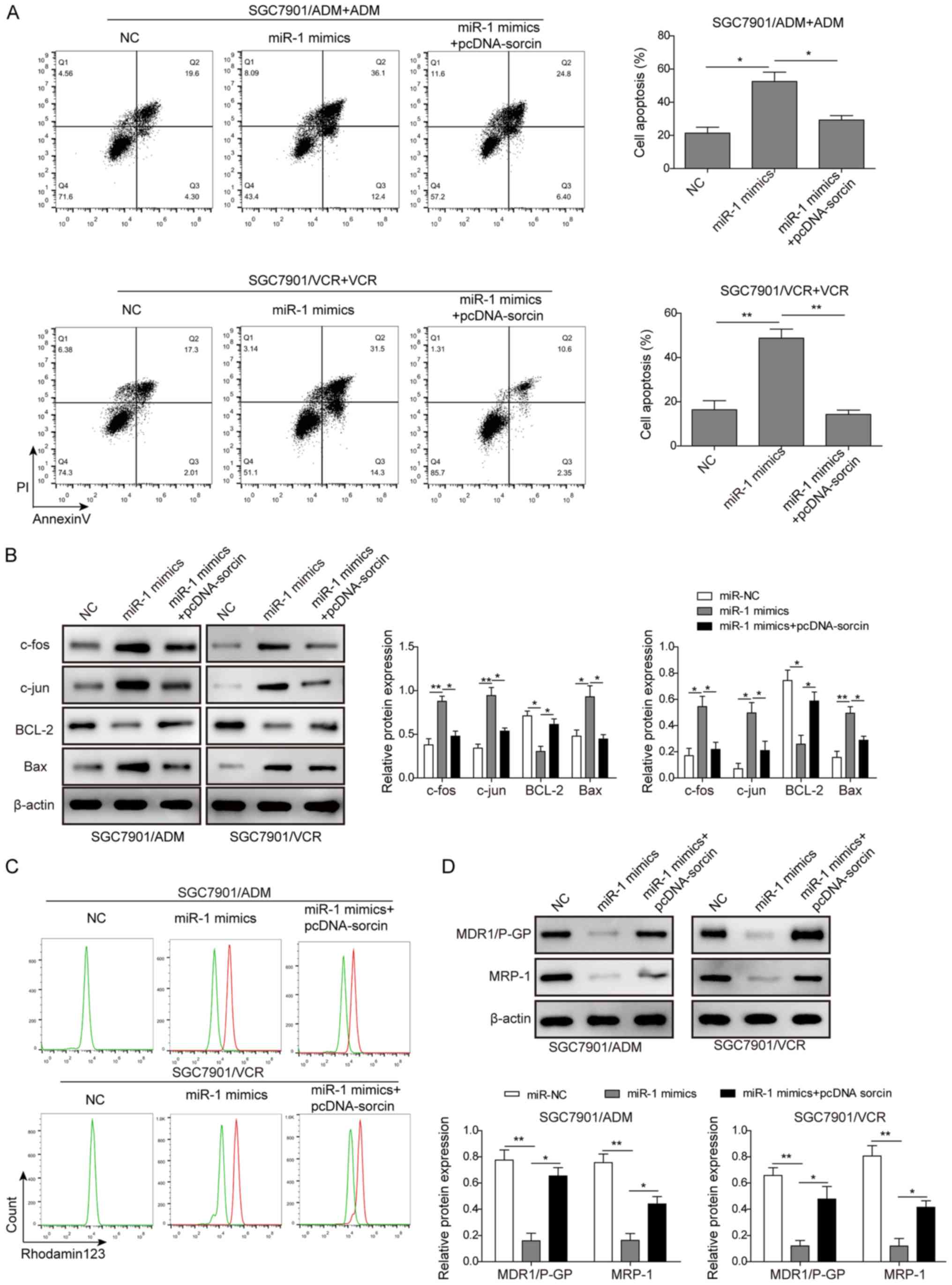 | Figure 7Overexpression of sorcin partially
recovered the effect of miR-1 on cell apoptosis and drug
accumulation. (A) The cells apoptosis rates of SGC7901/ADM and
SGC7901/VCR cells following transfection of miR-NC, miR-1 mimics
and miR-1 mimics with pcDNA-sorcin followed by the treatment of ADM
and VCR, respectively. The apoptosis rates were measured by flow
cytometric analysis. (B) The cell apoptosis associated proteins,
including c-fos, c-jun, BCL-2 and Bax were analyzed in SGC7901/ADM
and SGC7901/VCR cells following transfection of miR-NC, miR-1
mimics and miR-1 mimics with pcDNA-sorcin. (C) The drug
accumulation of Rhodamine 123 in SGC7901/ADM and SGC7901/VCR cells
following transfection of miR-NC, miR-1 mimics and miR-1 mimics
with pcDNA-sorcin were analyzed by flow cytometry. (D) The
expression levels of MDR1/P-gp and MRP-1 were analyzed in
SGC7901/ADM and SGC7901/VCR cells following transfection of miR-NC,
miR-1 mimics and miR-1mimics with pcDNA-sorcin. Data are presented
as the mean ± standard deviation of three repeated experiments.
*P<0.05, **P<0.01. miR, microRNA; VCR,
vincristine; ADM, adriamycin; miR-NC, scramble control; BCL-2, B
cell lymphoma-2; Bax, Bcl-2-associated X protein; MDR1/P-gp,
multidrug resistance protein 1/P-glycoprotein; MRP-1, multidrug
resistance-associated protein 1. |
Discussion
GC is a highly life-threating disease due to the
lack of efficient early diagnostic methods and treatment of tumor
metastases (1). Most patients with
GC are diagnosed at an advanced stage and lose the opportunity to
be treated by surgery. Alternatively, chemotherapy is the
preferable therapeutic option for those patients. However,
developed MDR in chemotherapy becomes a major obstacle leading to
the failure of treatment (3,4).
Therefore, it is of interest to elucidate the mechanism of MDR.
There have been several mechanisms reported, such as increased drug
efflux, dysfunction of pro-apoptotic proteins, mutation of target
genes and inactivation of detoxification enzymes (28). However, the mechanism of
chemoresistance remains to be elucidated. In the present study, it
was demonstrated that miR-1 was highly downregulated in SGC7901/ADM
and SGC7901/VCR cell lines. Overexpression of miR-1 in these MDR GC
cells decreased IC50 but increased cell apoptosis rates
and promoted drug accumulation in cancer cells. It was also
demonstrated that sorcin was the target of miR-1 in GC.
Furthermore, overexpression of sorcin could partially reverse the
effects of miR-1 on the MDR of GC cells. The role of miR-1 in MDR
GC cells makes it a potential therapeutic target for a successful
clinical outcome.
It has been reported that miRNA participated in the
development of drug resistance and metastasis in GC cells (29). miRNA microarray and bioinformatics
were widely used to examine the miRNAs levels and their correlation
with progression and prognosis of GC (30). For instance, Cao et al
(31) reported that miRNA-647
regulated drug resistance and metastasis of GC cells via inhibiting
ANK2. Yan et al (32)
demonstrated that the recurrence rate of GC could be discriminated
by the seven upregulated and five downregulated miRNAs. Therefore,
it is of importance to elucidate the mechanism of miRNAs on the
regulation of MDR in GC for improving the treatment efficiency and
discovering novel therapeutic targets. Among miRNAs, miR-1 was
demonstrated to be widely downregulated in various types of cancer,
including lung (33), prostate
(34) and colon (35) cancer and GC. In GC, Tsai et
al (36) demonstrated that
downregulation of miR-1 directly regulated endothelin-1 expression
to enhance the cell proliferation and metastasis, and finally
inhibited cell apoptosis. It was also reported that aberrant
expression of miR-1 impacted the chemoresistance in cancers. For
instance, overexpression of miR-1 in lung cancer cells enhanced
cells response rate to an anticancer drug (doxorubicin) (37). However, the status of miR-1 and its
underlining mechanism to regulate the MDR in GC cells are still
unclear. Therefore, the expression levels of miR-1 were
investigated in the MDR cell lines in the present study. It was
demonstrated that miR-1 was downregulated in the MDR gastric cell
lines, indicating that miR-1 might serve an important role in the
drug resistance of GC. Furthermore, when the MDR GC cells were
transfected to overexpress miR-1, the chemosensitivity of these MDR
GC cells significantly increased, indicating the regulation
function of miR-1 in the drug resistance in GC cells.
In order to uncover the mechanism of miR-1 for
reversing drug resistance properties of MDR GC cells, it was
demonstrated that the overexpression of miR-1 could upregulate the
pro-apoptotic proteins including Bax, c-fos and c-jun, but inhibit
the anti-apoptotic protein Bcl-2, which promoted the cell apoptosis
with the treatment of chemotherapeutic drugs. These findings are
consistent with a previous report, which demonstrated that ectopic
miR-1 expression could decrease cell viability in lung cancer cells
in response to the chemotherapeutic drug (37). Apoptosis has been proved to be a
major mechanism of programmed cell death and most of the
chemotherapeutic drugs induce apoptosis of cancer cells. For
instance, Ma et al (4)
demonstrated that the inhibition of cell apoptosis induced
chemoresistance in GC, which was regulated by overexpression of
hepatocyte nuclear factor-4α. It is well known that in the process
of chemotherapy-induced apoptosis, Bcl-2 is a critical survival
factor which inhibits apoptosis in various cell systems (38). In many cases, the resistance of
cancer cells to chemotherapeutic drugs may be caused by the
overexpression of Bcl-2 (39,40).
Also, Bcl-2/Bax was recognized to be critically involved in
regulating mitochondrial function, which ultimately modulates cell
apoptosis (39). A number of
studies demonstrated the high expression ratio of Bcl-2/Bax in
chemoresistant cancer cells (40,41).
Furthermore, the AP-1 proteins, composed of c-fos/c-jun, were
reported to work as tumor suppressors by inducing apoptosis of
cells (41).
On the other hand, drug efflux is also recognized as
an important pathway to generate drug resistance in chemotherapy of
many cancer types (42). P-gp and
MRP-1 are members of the ATP-binding cassette transporters, which
serve as drug efflux pumps that extrude chemotherapeutic agents
from MDR cancer cells, inducing drug resistance (43,44).
P-gp and MRP-1 are usually overexpressed in many types of MDR
cancer to increase drug efflux, which correlates with poor
prognosis and relapse in human cancers (42,45).
For instance, it was demonstrated that in human hepatocellular
carcinoma cells, MDR could be significantly reversed by inhibiting
P-gp and MRP1 expression using indomethacin and SC236 (45). Recently, Wang et al
(45) demonstrated that the
multidrug resistant leukemic cell proliferation was inhibited by
reducing P-gp expression via the knockdown of Wnt receptor
Frizzled-1. By overexpressing miR-1 mimics in MDR GC cells, the
present study demonstrated that the drug accumulation in those MDR
GC cells were significantly enhanced and the expression levels of
MDR1/P-gp and MRP-1 were lower than those of the control
groups.
All these data indicated that miR-1 overexpression
could reverse drug resistance through promoting cell apoptosis and
inhibiting drug efflux pumps. However, the mechanism of miR-1 to
promote the cell apoptosis and drug accumulation in MDR GC cells
remains unclear. The present results indicated that the MDR in GC
cells could be regulated by miR-1 via targeting sorcin. Sorcin is a
cytoplasmic protein with a molecular mass of 22 kDa and is part of
the penta-EF-hand protein family. In a number of drug-resistant
tumor cell lines, sorcin was reported to be overexpressed, which
was consistent with the results that sorcin was highly
overexpressed in the SGC7901/ADM and SGC7901/VCR cells. In
addition, more evidences indicated that sorcin was involved in MDR
in a wide number of tumor types, including colorectal cancer cells,
GC cells, leukemia and lung cancer cells (5,13,15,46).
It was reported that sorcin expression upregulated P-gp expression
via a cyclic adenosine monophosphate response element, which
induced the drug efflux in leukemia cells (9). In human breast cancer, Gong et
al (12) demonstrated that
sorcin was overexpressed in the human serum of the neoadjuvant
chemotherapy (NAC)-resistant patients as compared with that of
NAC-sensitive patients. Therefore, the present study systematically
investigated the interaction between miR-1 and sorcin in MDR GC
cells and demonstrated that overexpression of miR-1 could promote
the chemotherapeutic sensitivity, but overexpression of sorcin in
those miR-1 mimics transfected groups could partially reverse the
impact of miR-1 in MDR GC cells. The reason that the impact of
miR-1 in MDR GC cells was only partially but not totally reversed
by overexpression of sorcin may be that sorcin is not the only
protein that is regulated by miR-1. For instance, miR-1 was
reported to regulate the stromal cell-derived factor 1 and
angiogenesis-associated growth factors to regulate cell
chemoresistance in different types of cancer (19,20).
These results indicated that in MDR GC cells, miR-1 regulated the
expression level of sorcin, which controls the cell apoptosis and
drug accumulation.
In conclusion, the present findings demonstrated
that miR-1 was downregulated and sorcin was upregulated in
multidrug resistant GC cells. Furthermore, it was demonstrated that
miR-1 reversed the MDR in GC cells via inhibiting the expression of
sorcin, which promoted the accumulation of intracellular drugs and
enhanced the apoptosis of cells. Hence, by understanding the
involved mechanisms of miR-1 and sorcin in multidrug resistant GC
cells, miR-1 may be considered a valuable target for chemotherapy
of MDR GC. However, the present study only demonstrated the
function of miR-1 in MDR GC cells. A future in vivo
investigation should be conducted to further promote the usage of
miR-1 as a therapeutic target in MDR GC treatment, which is our
future direction.
Acknowledgments
Not applicable.
Funding
This study was supported by Hunan Provincial Natural
Science Foundation (grant no. 2016JJ3163).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LMD and TT conceived and designed the experiments.
HG performed the experiments. LMD, TYZ and XFX analyzed the data
and wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang D and Fan D: New insights into the
mechanisms of gastric cancer multidrug resistance and future
perspectives. Future Oncol. 6:527–537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Du X, Liu B, Luan X, Cui Q and Li L:
miR-30 decreases multidrug resistance in human gastric cancer cells
by modulating cell autophagy. Exp Ther Med. 15:599–605.
2018.PubMed/NCBI
|
|
3
|
Ozben T: Mechanisms and strategies to
overcome multiple drug resistance in cancer. FEBS Lett.
580:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma Y, Wei X and Wu Z: HNF-4α promotes
multidrug resistance of gastric cancer cells through the modulation
of cell apoptosis. Oncol Lett. 14:6477–6484. 2017.
|
|
5
|
Colotti G, Poser E, Fiorillo A, Genovese
I, Chiarini V and Ilari A: Sorcin, a calcium binding protein
involved in the multidrug resistance mechanisms in cancer cells.
Molecules. 19:13976–13989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ilari A, Johnson KA, Nastopoulos V,
Verzili D, Zamparelli C, Colotti G, Tsernoglou D and Chiancone E:
The crystal structure of the sorcin calcium binding domain provides
a model of Ca2+-dependent processes in the full-length
protein. J Mol Biol. 317:447–458. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Genovese I, Fiorillo A, Ilari A,
Masciarelli S, Fazi F and Colotti G: Binding of doxorubicin to
Sorcin impairs cell death and increases drug resistance in cancer
cells. Cell Death Dis. 8:e29502017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng L, Su T, Leng A, Zhang X, Xu M, Yan
L, Gu H and Zhang G: Upregulation of soluble resistance-related
calcium-binding protein (sorcin) in gastric cancer. Med Oncol.
27:1102–1108. 2010. View Article : Google Scholar
|
|
9
|
Yamagishi N, Nakao R, Kondo R, Nishitsuji
M, Saito Y, Kuga T, Hatayama T and Nakayama Y: Increased expression
of sorcin is associated with multidrug resistance in leukemia cells
via up-regulation of MDR1 expression through cAMP response
element-binding protein. Biochem Biophys Res Commun. 448:430–436.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van der Bliek AM, Meyers MB, Biedler JL,
Hes E and Borst P: A 22-kd protein (sorcin/V19) encoded by an
amplified gene in multidrug-resistant cells, is homologous to the
calcium-binding light chain of calpain. EMBO J. 5:3201–3208. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Q, Zhang G, Hou D, Leng A, Xu M, Peng J
and Liu T: Overexpression of sorcin results in multidrug resistance
in gastric cancer cells with up-regulation of P-gp. Oncol Rep.
25:237–243. 2011.
|
|
12
|
Gong Z, Sun P, Chu H, Zhu H, Sun D and
Chen J: Overexpression of sorcin in multidrug-resistant human
breast cancer. Oncol Lett. 8:2393–2398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi H, Yang YX, Tang CE, Chen ZC, Zhang GY
and Xiao ZQ: Sorcin overexpression and multidrug resistance in
human gastric cancer cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
31:340–344. 3492006.In Chinese.
|
|
14
|
Xu P, Jiang YF and Wang JH: shRNA-mediated
silencing of sorcin increases drug chemosensitivity in myeloma
KM3/DDP and U266/ADM cell lines. Int J Clin Exp Pathol.
8:2300–2310. 2015.PubMed/NCBI
|
|
15
|
Hu Y, Li S, Yang M, Yan C, Fan D, Zhou Y,
Zhang Y, Yagüe E and Xiong D: Sorcin silencing inhibits
epithelial-to-mesenchymal transition and suppresses breast cancer
metastasis in vivo. Breast Cancer Res Treat. 143:287–299. 2014.
View Article : Google Scholar
|
|
16
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar
|
|
17
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han C, Yu Z, Duan Z and Kan Q: Role of
microRNA-1 in human cancer and its therapeutic potentials. BioMed
Res Int. 2014:4283712014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie M, Dart DA, Guo T, Xing XF, Cheng XJ,
Du H, Jiang WG, Wen XZ and Ji JF: MicroRNA-1 acts as a tumor
suppressor microRNA by inhibiting angiogenesis-related growth
factors in human gastric cancer. Gastric Cancer. 21:41–54. 2018.
View Article : Google Scholar :
|
|
20
|
Li J, Guan J, Long X, Wang Y and Xiang X:
mir-1-mediated paracrine effect of cancer-associated fibroblasts on
lung cancer cell proliferation and chemoresistance. Oncol Rep.
35:3523–3531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu M, Zheng S, Zhang X, Guo H, Shi X,
Kang X, Qu Y, Hu Z and Tian J: Cerenkov luminescence imaging on
evaluation of early response to chemotherapy of drug-resistant
gastric cancer. Nanomedicine. 14:205–213. 2018. View Article : Google Scholar
|
|
22
|
Wang Y, Wu K, Yang Z, Zhao Q and Fan D, Xu
P, Nie Y and Fan D: Multidrug-resistance related long non-coding
RNA expression profile analysis of gastric cancer. PLoS One.
10:e01354612015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu YC, Liu X, Li M, Li Y, Li CY, Lu Y,
Sanches J, Wang L, Du Y, Mao LM, et al: A novel mechanism of
doxorubicin resistance and tumorigenesis mediated by
microRNA-501-5p-suppressed BLID. Mol Ther Nucleic Acids.
12:578–590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koontz MZ, Horning SJ, Balise R, Greenberg
PL, Rosenberg SA, Hoppe RT and Advani RH: Risk of therapy-related
secondary leukemia in Hodgkin lymphoma: The Stanford University
experience over three generations of clinical trials. J Clin Oncol.
31:592–598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waqar SN and Morgensztern D: Treatment
advances in small cell lung cancer (SCLC). Pharmacol Ther.
180:16–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dower CM, Bhat N, Gebru MT, Chen L, Wills
CA, Miller BA and Wang HG: Targeted inhibition of ULK1 promotes
apoptosis and suppresses tumor growth and metastasis in
neuroblastoma. Mol Cancer Ther. 17:2365–2376. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Chen QN, Wei CC, Wang ZX and Sun M: Long
non-coding RNAs in anti-cancer drug resistance. Oncotarget.
8:1925–1936. 2017.
|
|
29
|
Sui H, Cai GX, Pan SF, Deng WL, Wang YW,
Chen ZS, Cai SJ, Zhu HR and Li Q: miR200c attenuates P-gp-mediated
MDR and metastasis by targeting JNK2/c-Jun signaling pathway in
colorectal cancer. Mol Cancer Ther. 13:3137–3151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie M, Dart DA, Owen S, Wen X, Ji J and
Jiang W: Insights into roles of the miR-1, -133 and -206 family in
gastric cancer (Review). Oncol Rep. 36:1191–1198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao W, Wei W, Zhan Z, Xie D, Xie Y and
Xiao Q: Regulation of drug resistance and metastasis of gastric
cancer cells via the microRNA647-ANK2 axis. Int J Mol Med.
41:1958–1966. 2018.PubMed/NCBI
|
|
32
|
Yan Z, Xiong Y, Xu W, Gao J, Cheng Y, Wang
Z, Chen F and Zheng G: Identification of hsa-miR-335 as a
prognostic signature in gastric cancer. PLoS One. 7:e400372012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mataki H, Enokida H, Chiyomaru T, Mizuno
K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T,
Nakagawa M, et al: Downregulation of the microRNA-1/133a cluster
enhances cancer cell migration and invasion in lung-squamous cell
carcinoma via regulation of Coronin1C. J Hum Genet. 60:53–61. 2015.
View Article : Google Scholar
|
|
34
|
Chang YS, Chen WY, Yin JJ,
Sheppard-Tillman H, Huang J and Liu YN: EGF receptor promotes
prostate cancer bone metastasis by downregulating miR-1 and
activating TWIST1. Cancer Res. 75:3077–3086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu L, Zhang Y, Wang H, Zhang G, Ding Y and
Zhao L: Tumor suppressor miR-1 restrains epithelial-mesenchymal
transition and metastasis of colorectal carcinoma via the MAPK and
PI3K/AKT pathway. J Transl Med. 12:2442014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsai KW, Hu LY, Chen TW, Li SC, Ho MR, Yu
SY, Tu YT, Chen WS and Lam HC: Emerging role of microRNAs in
modulating endothelin-1 expression in gastric cancer. Oncol Rep.
33:485–493. 2015. View Article : Google Scholar
|
|
37
|
Nasser MW, Datta J, Nuovo G, Kutay H,
Motiwala T, Majumder S, Wang B, Suster S, Jacob ST and Ghoshal K:
Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression
of tumorigenic property of lung cancer cells and their
sensitization to doxorubicin-induced apoptosis by miR-1. J Biol
Chem. 283:33394–33405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beale PJ, Rogers P, Boxall F, Sharp SY and
Kelland LR: BCL-2 family protein expression and platinum drug
resistance in ovarian carcinoma. Br J Cancer. 82:436–440. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen J, Wang L, Tang Y, Gong G, Liu L,
Chen M, Chen Z, Cui Y, Li C, Cheng X, et al: Maspin enhances
cisplatin chemosensitivity in bladder cancer T24 and 5637 cells and
correlates with prognosis of muscle-invasive bladder cancer
patients receiving cisplatin based neoadjuvant chemotherapy. J Exp
Clin Cancer Res. 35:22016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu Y, Cheng X, Li S, Zhou Y, Wang J, Cheng
T, Yang M and Xiong D: Inhibition of sorcin reverses multidrug
resistance of K562/A02 cells and MCF-7/A02 cells via regulating
apoptosis-related proteins. Cancer Chemother Pharmacol. 72:789–798.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tainton KM, Smyth MJ, Jackson JT, Tanner
JE, Cerruti L, Jane SM, Darcy PK and Johnstone RW: Mutational
analysis of P-glycoprotein: Suppression of caspase activation in
the absence of ATP-dependent drug efflux. Cell Death Differ.
11:1028–1037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Groot DJ, van der Deen M, Le TK,
Regeling A, de Jong S and de Vries EG: Indomethacin induces
apoptosis via a MRP1-dependent mechanism in doxorubicin-resistant
small-cell lung cancer cells overexpressing MRP1. Br J Cancer.
97:1077–1083. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ye CG, Wu WK, Yeung JH, Li HT, Li ZJ, Wong
CC, Ren SX, Zhang L, Fung KP and Cho CH: Indomethacin and SC236
enhance the cytotoxicity of doxorubicin in human hepatocellular
carcinoma cells via inhibiting P-glycoprotein and MRP1 expression.
Cancer Lett. 304:90–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang YH, Imai Y, Shiseki M, Tanaka J and
Motoji T: Knockdown of the Wnt receptor Frizzled-1 (FZD1) reduces
MDR1/P-glycoprotein expression in multidrug resistant leukemic
cells and inhibits leukemic cell proliferation. Leuk Res.
67:99–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tuo H, Shu F, She S, Yang M, Zou XQ, Huang
J, Hu HD, Hu P, Ren H, Peng SF, et al: Sorcin induces gastric
cancer cell migration and invasion contributing to STAT3
activation. Oncotarget. 8:104258–104271. 2017. View Article : Google Scholar : PubMed/NCBI
|