Introduction
Temperature serves an important role in regulating
biological reactions. The human body has the remarkable ability to
maintain its core temperature between 36.5 and 37.5˚C.
Occasionally, the body raises its temperature to support the immune
system, making the environment less favorable for replicating
viruses and bacteria. Scientists have been interested in the
profound effects of heat on cells for a long time, and have
utilized it in various types of therapies. The most popular, called
hyperthermia (HT) therapy, is used in the treatment of cancer.
Multiple attempts have been made to uncover the biological effects
of HT on tumors in recent years (1-3).
Although the treatment of cancer with HT has been explored in
previous studies (4-6), research on the effect of HT alone as
a cancer treatment is limited.
Previous results indicated that a direct cancer cell
killing effect could occur when cells were heated to >42°C for
≥1 h (1). This made HT less
feasible in clinical treatment, since damage to the central nervous
system occurs within a few minutes of exposure to 42°C (7). Therefore, a second line of HT
research, which focused on its use in combination with chemotherapy
or radiotherapy, quickly emerged (8). Previous clinical trials or in
vitro studies showed that HT improves the effect of anticancer
drugs and radiation (9-11). For example, Schaaf et al
(8) demonstrated that HT
synergizes with cisplatin or doxorubicin by inhibiting
poly(ADP)-ribose polymerase (PARP)1-dependent DNA replication
arrest. Mild HT also improves drug delivery by breaking the stromal
barrier in pancreatic cancer xenograft mouse models and sensitizes
cancer cells to PARP1 inhibition (9,12).
These studies suggested that HT could be an adjuvant method to
cancer chemotherapy. However, little attention has been paid to
discussing the optimal treatment temperature and time sequences
that provide the maximum potentiating effects and the minimum
unwanted cell damage. Mild HT could have limited potentiating
effects, while too high temperatures may cause unwanted cell
damage. In fact, previous in vitro studies revealed that HT
is not tumor selective and could also damage normal tissue cells
(7,13). Therefore, it is of utmost
importance to select the correct temperature and duration so that
the combination of HT and chemotherapeutic drugs can provide an
optimal anticancer effect while minimizing the unwanted cell damage
caused by HT. Furthermore, the drugs used in HT combination
therapies are conventional chemotherapy drugs, which may also cause
serious side effects. There is currently an emerging area of
research on cancer prevention and cure focused on natural
compounds, particularly dietary products, due to their low toxicity
and potent efficacy. The present study focused on the effects of
propolis, which is a resinous substance produced by honeybees. It
has historically been used to treat or alleviate several maladies
in traditional medicine (14-16),
and it has been the focus of numerous studies due to its
anticancer, anti‑inflammatory and antioxidant activities (17‑19).
Frión-Herrera et al (20)
reported that Brazilian propolis induced apoptosis in human lung
cancer A549 cells through the mitochondria-mediated pathway. Demir
et al (21) also
demonstrated the antiproliferative and proapoptotic activity of
propolis on human lung cancer cells. Therefore, the objective of
the present study was to investigate the synergistic anticancer
effect of thermal cycle (TC)-HT and propolis.
The present study reports a refined method of
cycling high and low temperatures to achieve a synergistic
anticancer effect with natural compounds while minimizing the
damage caused by HT. In this strategy, high temperatures markedly
enhance the anticancer effect of the natural compounds while the
cooling process prevents cell damage caused by an excessive thermal
dosage. Various time-temperature combinations were examined to
achieve the most marked synergistic cell-killing effect when
combined with propolis. Notably, our TC-HT parameters alone did not
damage the cells, which makes thermal therapy safer and more
feasible.
In the present study, the results demonstrated for
the first time that TC-HT has a synergistic cytotoxic effect with a
natural compound, propolis, on the human pancreatic cancer cell
line PANC-1. The results indicated that TC-HT augmented
propolis-induced apoptotic cell-killing and cell inhibition through
the mitochondria-dependent apoptosis pathway and G2/M phase arrest.
The TC methodology was introduced as an efficient manner to avoid
unwanted cell damage in HT therapy. These findings indicated that
combining TC‑HT with propolis is a promising thermal therapy
strategy, which sheds light on novel anticancer treatments
combining TC-HT with other natural compounds.
Materials and methods
Cell culture and treatment
PANC-1 and AsPC-1 pancreatic cancer cells, and the
normal human embryonic skin cell line Detroit 551, were obtained
from the Bioresource Collection and Research Center. Normal human
pancreatic duct H6c7 cells were obtained from Kerafast, Inc.
PANC-1, AsPC-1 and Detroit cells were maintained in DMEM (PANC-1),
RPMI-1640 medium (AsPC-1) or EMEM (Detroit 551) (all from HyClone;
GE Healthcare Life Sciences) supplemented with 10% fetal bovine
serum (HyClone; GE Healthcare Life Sciences) and 1%
penicillin-streptomycin. H6c7 cells were maintained in
keratinocyte-serum free medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with human recombinant epidermal
growth factor, bovine pituitary extract (Invitrogen; Thermo Fisher
Scientific, Inc.), and 1% (v/v) penicillin and streptomycin. All
cells were maintained in a humidified incubator with 5%
CO2 and 95% air at 37°C. Liquid bee propolis was
purchased from Grandhealth™. The Thermal Cycler (model 2720) was
purchased from Applied Biosystems; Thermo Fisher Scientific, Inc.
Cells were plated in 24-well plates or 3-cm culture dishes 24 h
before treatment with or without TC-HT and/or propolis. Propolis
was added for 1 h before TC-HT treatment. In the present study,
TC-HT was performed using a modified PCR system. The cells were
heated to the desired high temperature followed by a cooling
period, and this protocol was repeated for different numbers of
cycles. The actual temperatures sensed by the cancer cells were
measured by a needle thermocouple. During the TC-HT treatment (~30
min), the control and treated groups were in ambient conditions at
room temperature (RT). Upon treatment, the cells were maintained in
the cell culture incubator for an additional 72 h.
Cell viability assay
Cells were seeded at a density of 2×104
cells/well in 24-well plates. After treatment, cell viability was
determined by MTT assay. In brief, the medium was replaced with MTT
solution (0.5 mg/ml in DMEM) and incubated at 37°C for 4 h. The
supernatants were discarded, and dimethyl sulfoxide was added to
dissolve the formazan crystals. The optical density in each well
was then evaluated by the measurement of absorbance at 570 nm using
a FLUOstar OPTIMA microplate reader (BMG Labtech, Ltd.). The cell
viability was calculated based on the intensity of the formazan,
and was expressed as a percentage of the untreated controls, which
were set at 100%.
Cellular growth assay
The long-term cell killing effect of the combination
treatment was assessed by colony formation area. PANC-1 cells were
seeded at a density of 2×104 cells/dish in 3-cm culture
dishes 24 h before being treated with or without TC-HT and/or 0.2%
propolis. Upon treatment, the cells were continuously cultured for
10 days. Cells were stained with 0.5% crystal violet in methanol
for 5 min at RT, washed with PBS, and images were captured. The
colony area was measured using the 'ColonyArea' plugin in ImageJ
software (version 1.49j; National Institutes of Health) (22). The sums of the pixel depth over the
region of interest were calculated and are represented as arbitrary
units. The actual units of colony formation area were
cm2.
Flow cytometric detection of apoptotic
cells
Apoptotic cells were analyzed by flow cytometry with
an Annexin V‑FITC and propidium iodide (PI) double-staining kit (BD
Biosciences). Cells used for flow cytometry were collected by
trypsinization and resuspended in 100 µl 1X binding buffer
containing Annexin V and PI. Cells were stained for 15 min at 25°C
in the dark before being analyzed by flow cytometry. All the
cytometry data in the present study were acquired with a BD
FACSVerse flow cytometer and analyzed using FlowJo software
(version 10.0.7; Tree Star, Inc.).
Immunofluorescence microscopy
Cells were seeded on 20 mm coverslips in 6-well
plates at a density of 2×104 cells/well for 24 h and
then treated with TC-HT and/or propolis. Next, cells were washed
with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck
KGaA) in PBS for 20 min at RT. Fixed cells were then permeabilized
with 0.1% Triton X-100 in PBS for 20 min. Nonspecific protein
binding was blocked with 2% BSA (BioShop Canada, Inc.) in PBS for
30 min at RT. The cells were then incubated with anti-β-tubulin
(cat. no. ab21057; 1:1,500 dilution; Abcam) and anti-active
caspase-3 (cat. no. 9661; 1:800 dilution; Cell Signaling
Technology, Inc.) primary antibodies overnight at 4°C. After
washing three times in PBS, the cells were incubated with Alexa
Fluor 488-conjugated donkey anti-goat (cat. no. 705-545-003) and
Alexa Fluor 647-conjugated donkey anti-rabbit (cat. no.
711-605-152) secondary antibodies (both 1:500 dilution; Jackson
ImmunoResearch Laboratories, Inc.) for 1 h at 37°C in the dark. The
coverslips were mounted to slides using mounting medium with DAPI
(Abcam). The mounted samples were examined with an inverted laser
scanning confocal microscope with a x20 objective (Zeiss LSM 880;
Zeiss AG). Images of randomly selected areas were captured for each
sample. The integrated fluorescence density was calculated using
the ImageJ software. The sums of the pixel density over the region
of interest were calculated and are represented as arbitrary units.
The actual units of the integrated fluorescence density were
candela.
Measurement of mitochondrial membrane
potential (MMP)
The loss of MMP was determined using the lipophilic
cationic fluorescent dye 3,3′‑dihexyloxacarbocyanine iodide
[DiOC6(3); Enzo Life
Sciences, Inc.] (23).
Depolarization of MMP results in the loss of
DiOC6(3) from the
mitochondria and a decrease in intracellular fluorescence. Cells
were harvested and suspended at a density of 1×106
cells/ml in dye working solution (1 µM dye in culture
medium) in the dark. After 15 min of culture at 37°C, the
supernatant was removed by centrifugation, and the cells were
gently resuspended in pre-warmed (37°C) culture medium. Cells
labeled with DiOC6(3)
were detected by flow cytometer with the FL1 channel.
Cell cycle analysis
Upon treatment, the cells were collected by
trypsinization and fixed in 70% ethanol overnight at 4°C. Prior to
the analysis, the cells (1×106 cells/ml) were washed
with cold PBS and treated with RNase A (0.1 mg/ml) for 20 min at
37°C. Subsequently, the cells were stained with PI (0.2 mg/ml) for
30 min at RT. The distribution of cell cycle stages was then
determined by flow cytometry.
Western blot analysis
The protein expression levels of PANC-1 cells were
investigated by western blot analysis. Cells were scraped off from
culture dishes in RIPA lysis buffer (EMD Millipore). After
centrifugation, the supernatants were collected and the protein
concentrations were determined by Bradford protein assay (BioShop,
Inc.). Equal amount of proteins (30 µg) were resolved on 10%
SDS-PAGE and then transferred onto polyvinylidene fluoride
membranes. For the detection of cytochrome c release, the
cytosolic fractions were collected via the REAP method (24). Nonspecific antibody binding sites
were blocked in 5% nonfat dry milk in TBS with Tween-20 (TBST; 20
mM Tris-base, pH 7.6; 0.15 M NaCl; and 0.1% Tween-20) for 1 h at
RT. The blocked membranes were probed with anti-cell division cycle
protein 2 (cdc2; cat. no. GTX108120; 1:1,000), anti-actin (cat. no.
GTX109639; 1:10,000) (both from GeneTex, Inc.), anti-Bcl-2 (cat.
no. 2872; 1:1,000), anti-Bax (cat. no. 2772; 1:1,000),
anti-cytochrome c (cat. no. 4272; 1:1,000) (all from Cell
Signaling Technology, Inc.) and anti-GAPDH (cat. no. GTX100118;
1:10,000; GeneTex, Inc.) antibodies overnight at 4°C. The membranes
were washed three times with TBST solution and then incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies (cat. no. 111-035-003; 1:5,000; Jackson ImmunoResearch
Laboratories, Inc.) in a blocking solution. Immunoreactivity was
visualized with an enhanced chemiluminescence substrate (Advansta,
Inc.) and detected with an imaging system (Amersham Imager 600; GE
Healthcare Life Sciences). The images were analyzed with Image Lab
software (version 6.0.1; Bio-Rad Laboratories, Inc.)
Statistical analysis
The results are expressed as the mean ± standard
deviation, and each data point represents the average from three
independent experiments. Analyses were performed using OriginPro
2015 software (OriginLab). Differences in statistical significance
were determined by one-way analysis of variance followed by Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
In vitro-applied TC
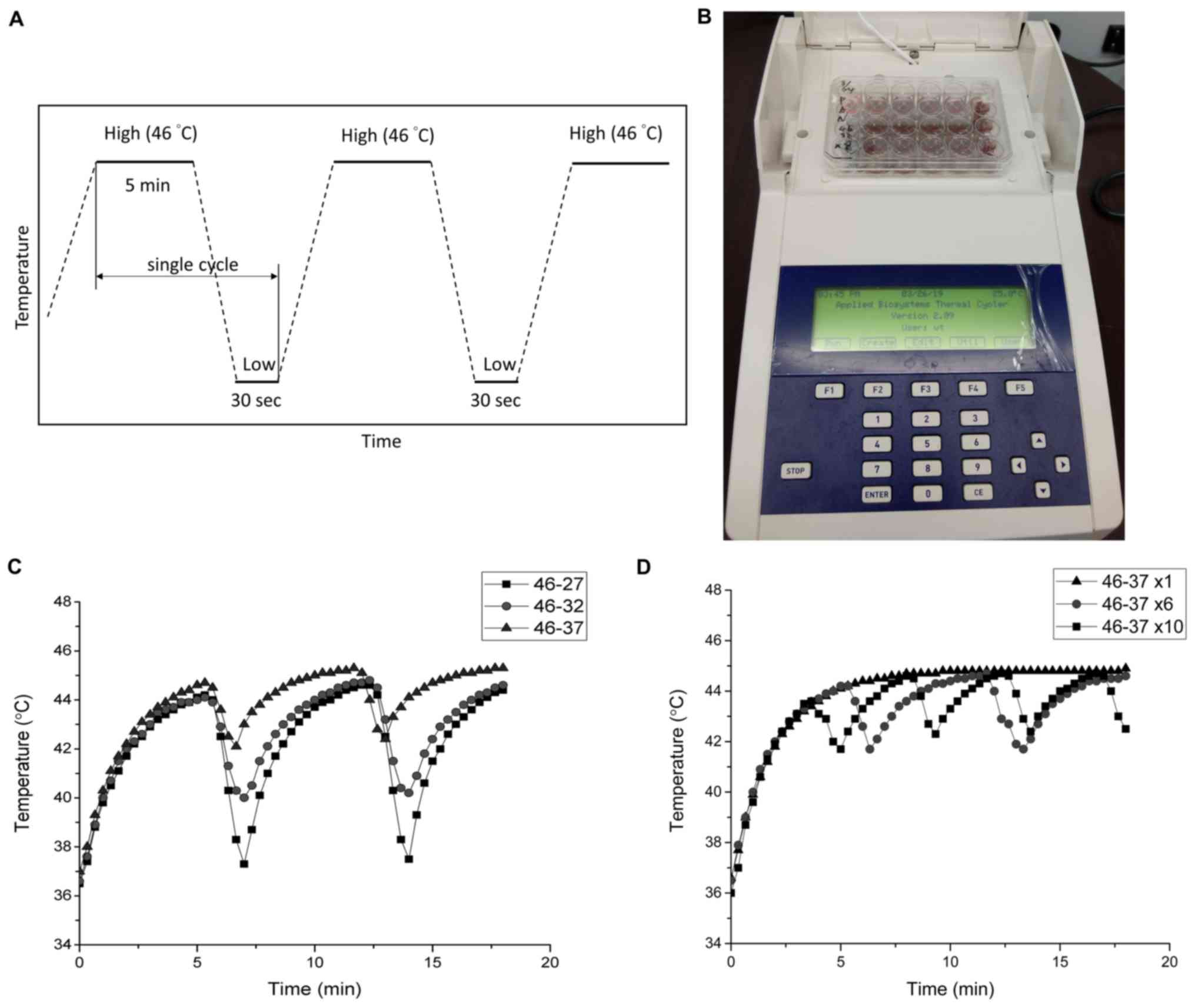
To apply a TC with a rapid temperature change,
modified PCR equipment was used as the TC controller (Fig. 1B) in the following in vitro
experiments. In this design, some protruding parts of the PCR
machine and plastic well were cut off so that the bottom of the
well could touch the heat sink. The schematic TC settings are shown
in Fig. 1A, where the temperature
was elevated to the desired HT temperature followed by a cooling
period. The actual temperatures sensed by the cancer cells were
measured with a needle thermocouple. Fig. 1C and D represent the tumor cell
temperature by TC between 46°C and three different cooling
temperatures and cycle numbers; the temperature was measured every
20 sec. As shown in Fig. 1C, the
temperature in the tumor cells could be raised from 37 to 44°C
within 5 min and returned to a relatively safe low temperature
(~42°C) rapidly. The 46-37°C parameter setting was selected to
mimic the passive cooling process in the human body. The actual
cycling temperature of the cancer cells measured by the needle
thermocouple was 44-42°C. In practice, the heating device can be
switched off in the cooling process to achieve similar results. For
other cycling parameters that require a higher thermal dissipation
rate, active cooling devices could be used as pre-cooling, such as
liquid cooling blankets containing circulating water with
antifreeze (25).
TC-HT enhances the anticancer effect of
propolis via the apoptosis pathway in PANC-1 cells
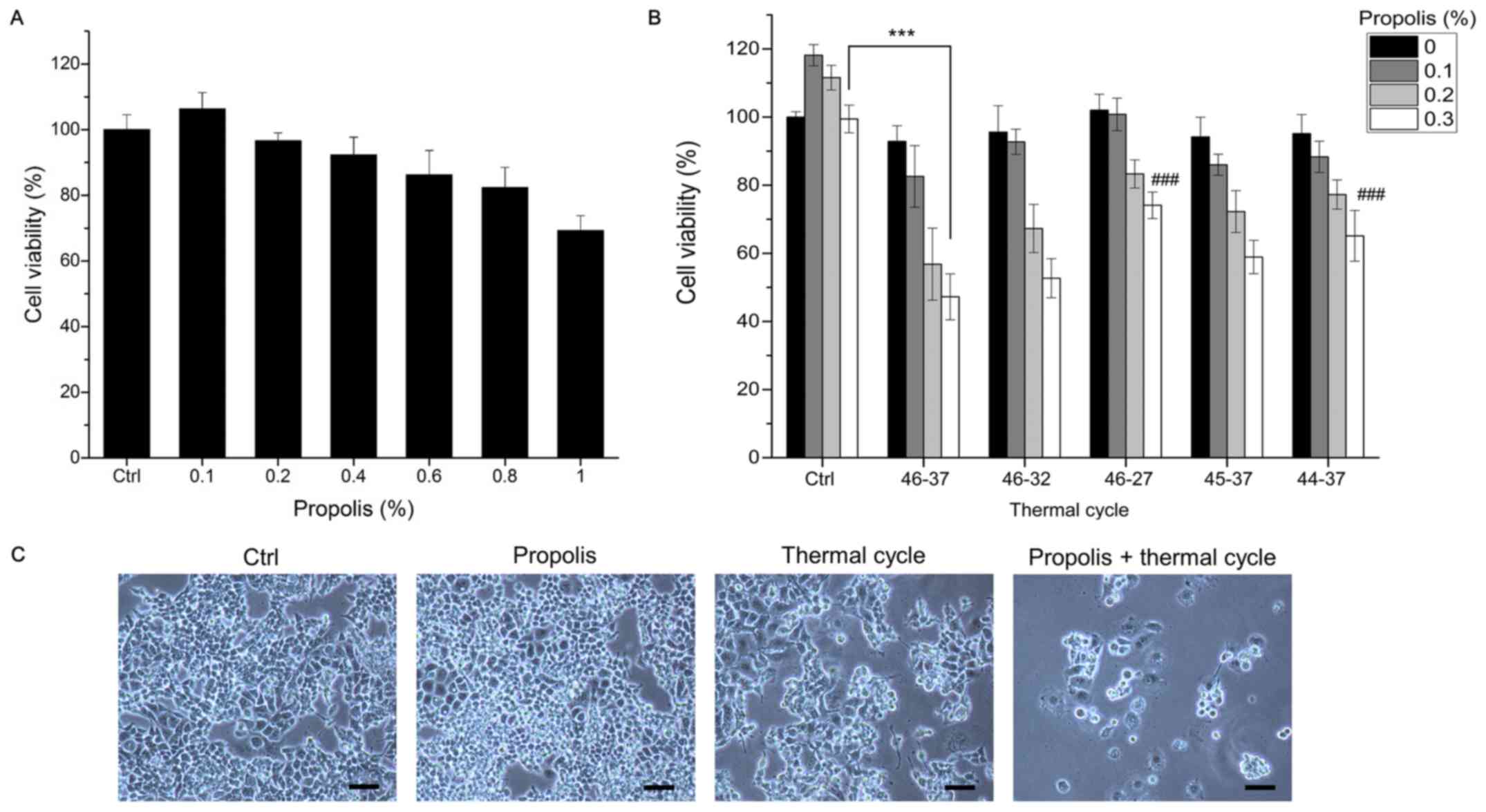
To examine whether TC treatment could enhance the
anticancer effect of honeybee propolis, PANC-1 cancer cells were
treated with increasing doses of propolis with or without TC-HT
treatment. In the TC-HT groups, 5 different TC parameters for 6
cycles were applied 1 h after propolis administration. The
viability of the cancer cells was examined by MTT assay 72 h after
treatment. As shown in Fig. 2A,
the viability of PANC-1 pancreas cancer cells decreased in a
dose-dependent manner, except at markedly low doses. As shown in
Fig. 2B, treatment with TC-HT
alone or low doses of propolis had little effect on cell viability
compared with control cells. However, when TC-HT treatment was
combined with a low dosage of propolis, the viability of PANC-1
cells was significantly reduced. The most effective parameter was
the 46-37°C cycling, showing a >50% enhancement in cell killing
compared with the single treatment. Light microscopy images also
revealed a marked inhibitory effect in cells subjected to the
combination of TC-HT and 0.2% propolis after 72 h of treatment
(Fig. 2C). To elucidate the effect
of different TC parameters on cell viability, different high
temperatures and low temperatures were used in the experiments and
the results are shown in Fig. 2B.
The results revealed that 46-37°C (which led to an actual cycling
temperature in the cancer cells of 44-42°C, as measured by needle
thermocouple) was the best temperature cycling parameter against
PANC-1 cancer cells. For other cancer cell lines, one could
modulate the temperature cycle parameters to achieve the desire
therapeutic effect. The present study also compared the effect of
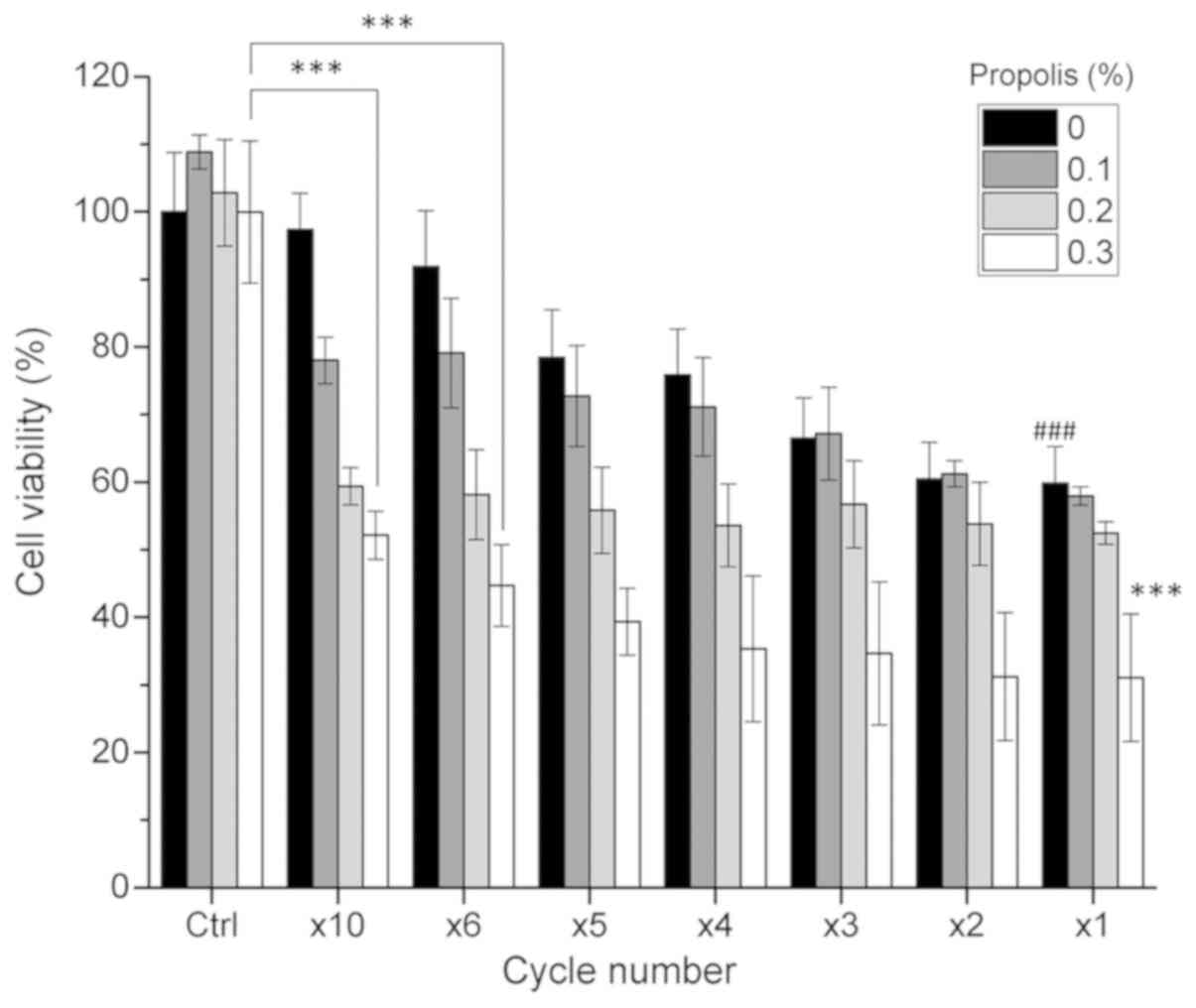
different cycle numbers in combination with propolis on the
viability of PANC-1 cells (Fig.
3), in which the total thermal dosage was divided into
different cycles. For example, 6 cycles (x6) means that the high
temperature of 46°C was sustained for 5 min (with the actual PANC-1
cell temperature being 44°C), followed by a cooling period, and
this process was repeated six times. In the x1 cycle group (or the
HT group), a high temperature of 46°C was sustained for 30 min
uninterruptedly. The results revealed that the viability of PANC-1
cells was cycle-dependent and decreased as the cycle number
decreased. Although HT (x1 cycle) in combination with propolis
induced the maximum cell death, heat alone exerted marked
cytotoxicity towards the cells. In the x10 and x6 groups, the cell
viability was >90% when the cells were treated with TC-HT alone,
while TC-HT in combination with propolis also caused a notable
decrease in PANC-1 cell viability (Fig. 3). It was found that the x10 TC-HT
combined with propolis caused a >45% decrease in PANC-1 cell
viability.
Notably, the combination of x6 TC-HT and propolis
further caused >50% inhibitory effect on PANC-1 cells.
Therefore, the x6 cycle was selected for subsequent experiments. A
dose of 0.2% propolis was selected for subsequent analyses because
excessive cell loss would adversely affect the subsequent
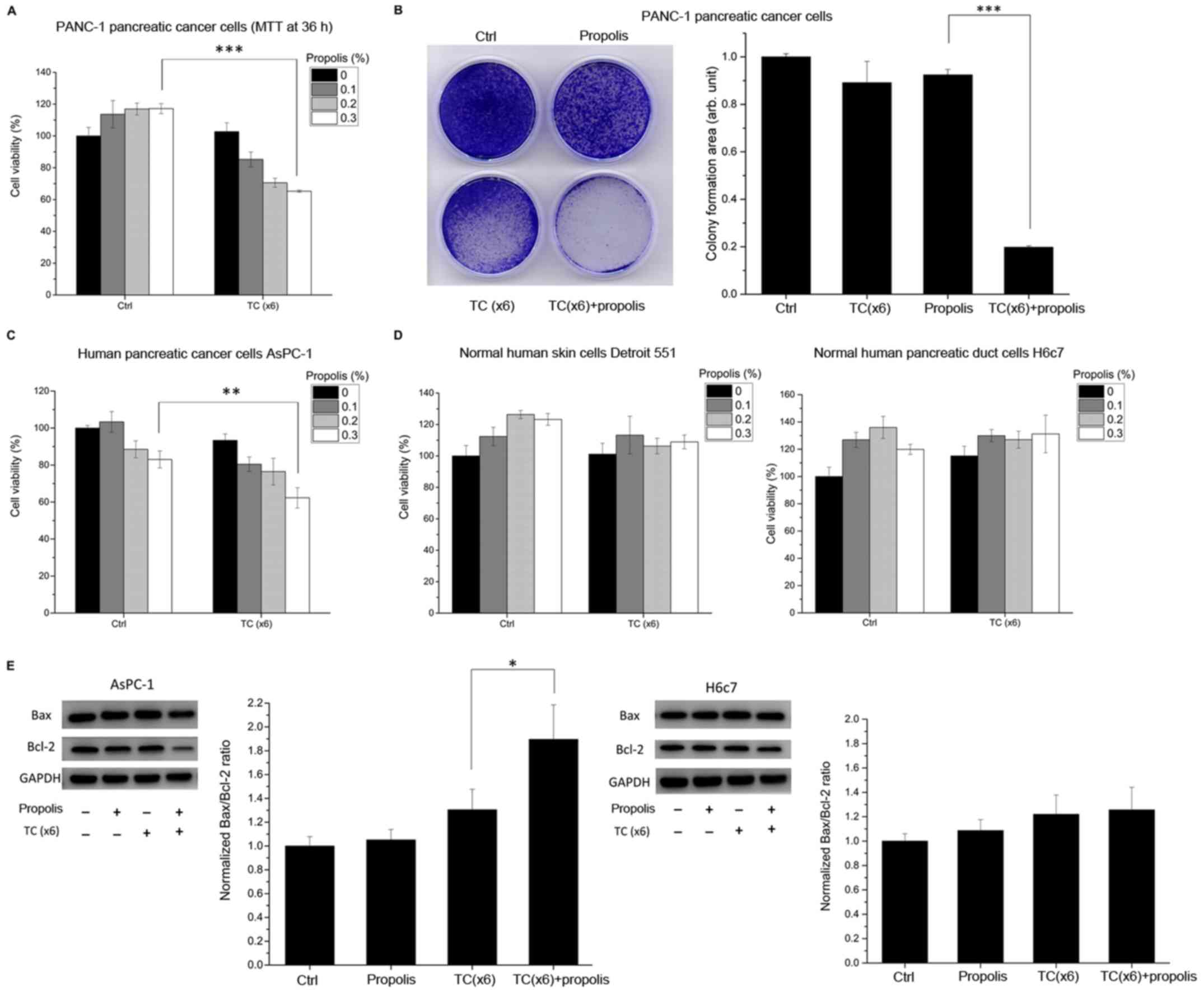
experiments. To compare the short-term (compared to 72 h) and
long-term effects of the combination treatment, an MTT assay at 36
h and a cellular growth assay at 10 days after the combination
treatment were performed. The results revealed that the combination
treatment suppressed the growth of PANC-1 cancer cells (Fig. 4A), and apoptosis proceeded after 36
h (Fig. 4B). To further
demonstrate the specificity of the combination treatment, another
human pancreatic cancer cell line, namely AsPC-1, normal human
embryonic skin Detroit 551 cells and normal human pancreatic duct
H6c7 cells were selected for comparison with the pancreatic cancer
cell line PANC-1. The results revealed that the combination
treatment also had inhibitory effects on AsPC-1 cancer cells
(Fig. 4C) but was unharmful to the
normal human cell lines Detroit 551 and H6c7 (Fig. 4D). It is worth noting that the
effect of the TC parameters used in the present study was less
pronounced in AsPC-1 cells than in PANC-1 cells. In order to
examine the apoptotic signaling, the Bax/Bcl-2 ratio was also
analyzed in AsPC-1 and H6c7 pancreatic cells (Fig. 4E). Notably, the apoptotic effect on
AsPC-1 was less pronounced than that on PANC-1 cells, which is in
accordance with the MTT results. Therefore, it was hypothesized
that TC parameters are tissue-specific and should be optimized in
different cell types. On the contrary, for normal human pancreatic
duct H6c7 cells, the results showed that the Bax/Bcl-2 ratio was
only slightly increased in the combined treatment group.
TC-HT enhances propolis-induced apoptosis
in PANC-1 cells
To confirm whether TC and propolis treatments
decreased cell viability via the induction of apoptosis, PANC-1
cells were cultured with propolis with or without TC-HT treatment
and then assessed by flow cytometry. Cells were stained with
Annexin V-FITC and PI for detecting apoptotic cells. The cycling
parameters were 46-37°C for 6 cycles (x6). To elucidate the
influence of the duration of high temperature within each cycle on
the synergistic anticancer effect, the high temperature duration
was doubled to 10 min and the cycle number was halved to 3 cycles
(x3), so that the total high temperature duration was the same. As
shown in Fig. 5, propolis alone
did not cause apoptosis, which is consistent with the results of
the MTT assay. When propolis was combined with TC (x6 or x3), it
resulted in >70% Annexin V-positive apoptotic cells compared
with 5.7% in the control group (upper right and lower right
quadrants). Notably, the x3 TC protocol resulted in 40.2% apoptotic
cells, while the x6 protocol did not notably harm the cells.
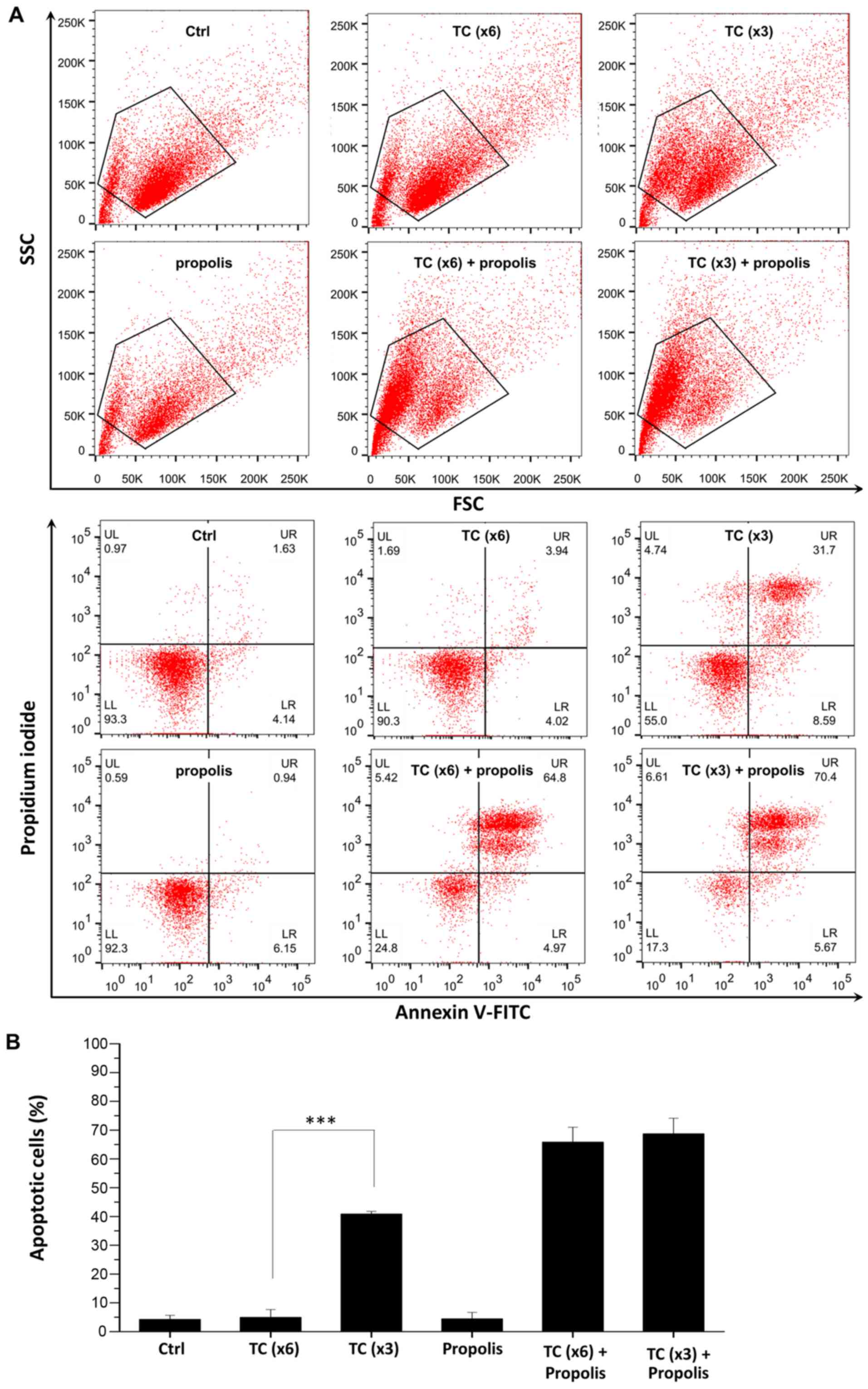 | Figure 5In vitro assessment of
apoptosis via Annexin V-FITC/PI double staining. (A) PANC-1 cells
were treated with 0.2% propolis and x3 or x6 TC-HT alone or in
combination for 72 h, and then assessed by flow cytometric analysis
of Annexin V-FITC and PI staining. Cells were gated by FSC/SSC plot
to exclude cell debris. (B) Quantification of apoptotic cells. The
cells in the LR and UR quadrants indicate Annexin V-positive
apoptotic cells. Data represent the mean ± standard deviation
(n=3). ***P<0.001. PI, propidium iodide; TC-HT,
thermal cycle-hyperthermia; Ctrl, control; SSC, side scatter; FSC,
forward scatter; LR, lower right; UR, upper right; LL, lower left;
UL, upper left. |
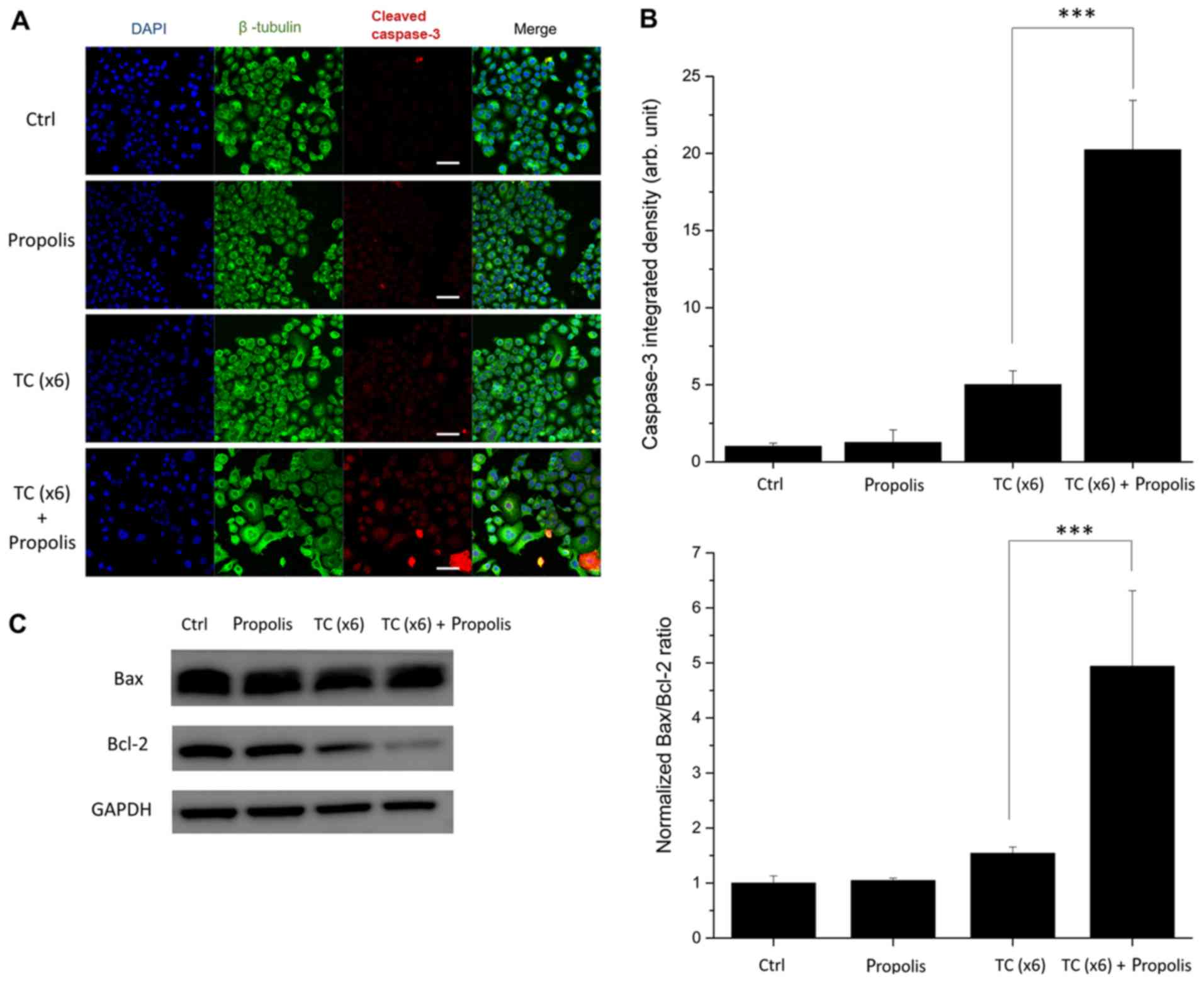
TC-HT enhances propolis-induced
apoptosis
To further examine the mechanism of TC and
propolis-induced cell death, the present study investigated the
expression of cleaved caspase-3, Bax and Bcl-2 in PANC-1 cells by
confocal microscopy and western blotting. Since caspase-3 serves as
a convergent downstream of apoptotic events in cells, it is a
useful indicator in apoptosis assays. In the present study, PANC-1
cells were stained with anti-cleaved caspase-3 antibody to identify
the apoptotic cells, and the cell shape was outlined with a
β-tubulin marker, while the nuclei of the cells were visualized
with DAPI. As seen in Fig. 6A and
B, the combined treatment of x6 TC-HT and 0.2% propolis
resulted in a marked increase in cleaved caspase-3 immunostaining.
By contrast, there was only a negligible increase in the cleaved
caspase-3 signal with TC-HT or propolis treatment alone. The Bcl-2
family is an important regulator of the mitochondria-dependent
apoptosis pathway. The Bax/Bcl-2 ratio can be used to assess the
upregulation of the apoptotic signaling pathway. The results of the
western blotting (Fig. 6C)
indicated that the Bax/Bcl-2 ratio was significantly increased in
the combined treatment group, suggesting that TC-HT and propolis
could trigger the mitochondria-dependent apoptosis pathway.
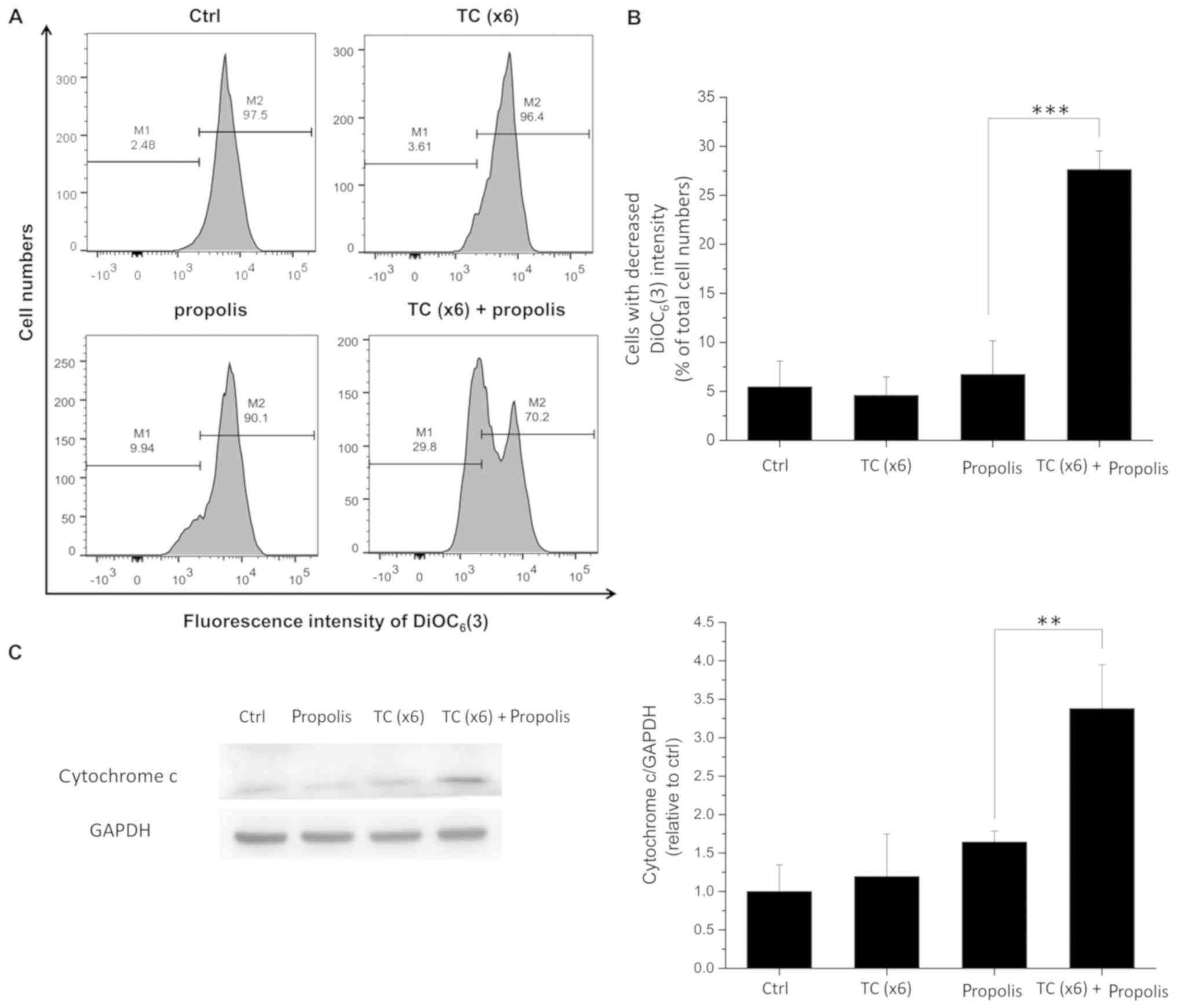
Effects of TC-HT and propolis on MMP in
PANC-1 cells
During apoptosis, the MMP decreases, causing the
release of cytochrome c and other apoptotic factors
(26). To address the possibility
that the synergistic anticancer effect of TC-HT in combination with
propolis could be associated with the mitochondria-dependent
apoptosis pathway, the MMP was assessed by flow cytometry and the
cytochrome c release was detected by western blotting. Cells
were pre-treated with propolis and/or TC-HT and then collected for
further analyses. The MMP was detected using a
mitochondria-specific probe, namely DiOC6(3). As shown in Fig. 7A and B, treatment with x6 TC-HT or
0.2% propolis alone did not affect the MMP. However, the combined
treatment of TC-HT and 0.2% propolis markedly increased the number
of cells exhibiting a loss in MMP, as indicated by a lower
DiOC6(3) intensity. The
collapse of the MMP leads to the opening of the mitochondrial
permeability transition pores, and the subsequent release of
cytochrome c in the cytosol. The results of the western
blotting showed that the cytosolic cytochrome c level was
significantly increased in the combined treatment group (Fig. 7C).
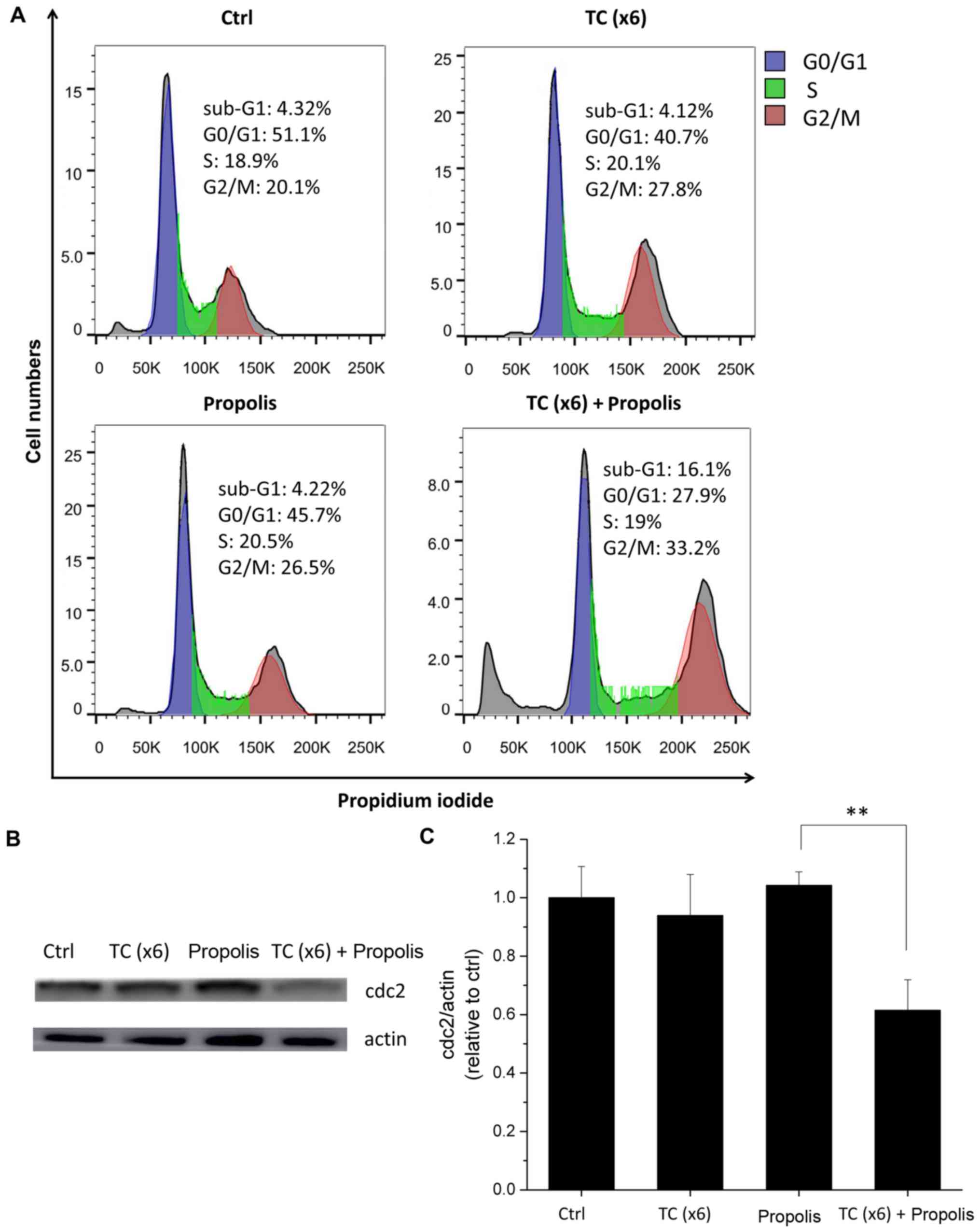
Combination of TC-HT and propolis causes
PANC-1 cell arrest at the G2/M phase
Cell cycle arrest is an important target in cancer
therapy, since it is critical in the growth and development of
tumors. In order to determine whether the cause of cell growth
inhibition observed in the viability assay was associated with cell
cycle arrest, the DNA content of the cells was analyzed by PI
staining followed by flow cytometry. After 72 h of treatment with
TC and/or propolis, the cell cycle phase was determined by flow
cytometry. As shown in Fig. 8A,
the combination of TC-HT and propolis induced an accumulation of
cells in the G2/M phase, changing from 20.1% in the control group
to 33.2% in the group subjected to the x6 protocol, with a
concurrent decline in the number of cells at the G0/G1 phase from
51.1 to 27.9%. In addition, an increase in the fraction of the
sub-G1 population, which is an indication of dying cells, was
observed in the TC and propolis co-treated group. To further
examine the proteins that regulate cell cycle progression, the
effects of TC and propolis on cyclin-dependent kinase 1, also known
as cdc2, were subsequently investigated. It is known that the
primary participant in the G2/M phase transition is cdc2 protein
(27). In the present study
(Fig. 8B), cdc2 protein expression
was markedly reduced in the combined treatment with x6 TC-HT and
0.2% propolis group, while either treatment alone did not
significantly affect cdc2 expression.
Discussion
The focus of the present study was to investigate
the synergistic anticancer effect of TC-HT and propolis on PANC-1
cells. The anticancer effects of propolis were strongly potentiated
by TC administration. This novel TC method was able to enhance the
cytotoxicity of propolis by >10-fold, while being unharmful to
normal cells and efficient as a combination therapy. In fact, in
the present results, >50% of cancer cells were inhibited or
killed by <1 h of combination treatment, while the TC alone
hardly harmed the cells.
HT is a promising strategy in combination with
conventional therapies to halt tumor growth. It has been proposed
that HT could enhance the sensitivity of cancer cells to drug
treatment, thereby exhibiting a synergistic anticancer effect
(28). The advantage of such
combination treatments is the possibility of using minimal doses of
chemotherapy and radiation, leading to a maximum curative effect
with less unwanted cell damage. Numerous studies have demonstrated
the beneficial effect of HT in chemotherapy (8,11,29).
However, the unwanted damage caused by HT cannot be effectively
controlled and avoided. One major concern is that the thermal
dosage applied to tumor cells may also harm normal tissue cells.
Previous studies revealed that a sustained temperature >42°C
will cause necrosis of living cells (30,31).
Besides, heat tolerance will be dissimilar in different tissues
(32). Therefore, it is important
to select the optimal thermal dosage, as different temperatures and
durations may be necessary to achieve the desired outcomes.
The present study provides an efficient way of
controlling the applied thermal dosage to cells. The heat-and-cool
cycling used in the present study has advantages when combined with
anticancer compounds or chemotherapy drugs. During the heating
process, the temperature was elevated to a certain threshold and
maintained for a specific period, which can synergize with
anticancer drugs. In the cooling process, the tissue cell
temperature was lowered to prevent excessive thermal dosage
accumulation and subsequent cytotoxic cell damage. The
heat-and-cool process can be repeated numerous times to achieve the
desired anticancer effect. As shown in Fig. 2B, three different maximum thermal
cycling temperatures for 6 cycles were applied upon administration
of propolis. The most effective parameter setting was 46-37°C
cycling (notably, the actual cycling temperature of the cancer
cells measured with the needle thermocouple was 44-42°C), which
mimics the passive cooling process in the human body. The results
indicated that there was a specific threshold temperature for
maximizing the cytotoxic anticancer effect of propolis.
Furthermore, as shown in Fig. 2B,
an excessively low temperature in the cooling process will diminish
the synergistic effect, since the total thermal dosage is
insufficient to sensitize PANC-1 cells in the cycling procedure.
Therefore, it is important to determine the appropriate cycling
parameters when combining TC-HT with different anticancer drugs.
The results of the present study showed the advantages of TC in
preventing cytotoxic damage when the total thermal dosage was
divided into different cycles. In the x1 cycle group, a high
temperature of 46°C was sustained for 30 min continuously. Although
HT acts synergistically with propolis in killing cancer cells, heat
alone also causes severe cytotoxicity. The aim of combination
therapy is to avoid the latter. An earlier study analyzed the
time-dependent modifications of cancer cells during exposure to HT,
and revealed that the survival rate decreased with increasing
exposure time (33). Namely, the
short exposure of cancer cells to HT may induce cellular stress
without affecting cell viability, while prolonged exposure may lead
to cell death. The present study demonstrated that the viability of
PANC-1 cells was >90% in the x6 group when treated with TC-HT
alone. Notably, the combination of x6 TC-HT and propolis caused a
>50% inhibitory effect on PANC-1 cell viability. Moreover, the
same combination treatment exerted low cytotoxicity on the normal
human cell lines Detroit 551 and H6c7. These data indicated that
TC-HT and propolis are promising candidates for anti-pancreatic
cancer treatment, with low toxicity towards normal cells.
The molecular mechanisms responsible for this
potentiation were investigated by determining the MMP and cell
cycle progression. The expression of downstream proteins of the
apoptotic pathway and cell cycle regulatory proteins was also
evaluated. Previous in vitro and in vivo studies
demonstrated that propolis exerts cytotoxic properties against
cancer cells through the mitochondria-mediated apoptosis pathway
and cell growth arrest (21).
Mitochondria are associated with cell stress responses, including
oxidative stress and cell death. During apoptosis, the MMP
decreases, causing the release of cytochrome c into the
cytosol and the activation of the subsequent caspase cascades
(26). In the present study, the
combined treatment of TC-HT and propolis greatly suppressed MMP,
which is an important index for damage and dysfunction of the
mitochondria. In addition, the western blotting results showed that
the expression level of cytosolic cytochrome c was
significantly higher in the combined treatment group. Moreover, it
was found that caspase-3 expression was promoted by the combined
treatment. These data indicated that the enhancement of apoptosis
mediated by the combined treatment was induced by a
mitochondria-dependent apoptosis pathway.
Cancer is characterized by uncontrolled cell
division, which is linked to the aberrant activity of various cell
cycle regulators. Therefore, cell cycle regulatory proteins are
considered attractive targets in cancer therapy. In this study, it
was found that the combined treatment of TC-HT and propolis
resulted in G2/M phase arrest. Cell cycle analysis by flow
cytometry also demonstrated an increase in the fraction of dying
cells, as indicated by the sub-G1 population in the combined
treatment group. The main participant in the G2-M transition is
CDK1, also known as cdc2. According to the western blotting
results, cdc2 expression was downregulated in the combined
treatment with propolis and TC group, while neither TC or propolis
alone interfered with cdc2 expression. These results indicated that
the thermal enhancement of propolis cytotoxicity was mediated in
part by inhibition of the kinase activity of cdc2, which prevents
cell progression into mitosis. Although there is evidence that
TC-HT and propolis result in G2/M phase arrest, it is not possible
exclude the possibility that G0/G1-phase cells are more susceptible
to treatment. Further studies are required to elucidate the
detailed mechanism underlying the increased G2/M population.
Pancreatic cancer has the highest mortality rate of
all cancer types, due in part to the lack of diagnostic tools for
early detection. Treatment options are limited and mostly rely on
chemotherapy or radiation. In pancreatic cancer, the effects of
chemotherapy combined with HT using several heating methods and
technologies have been investigated in clinical settings, such as
whole body HT and HT intraperitoneal chemotherapy (34-37).
Previous studies have demonstrated the benefits of HT, which can
enhance the cytotoxicity of chemotherapy drugs towards pancreatic
cancer (38-40). The present study provides a novel
methodology in HT therapy, namely TC-HT therapy, which is safer and
has more feasible administration when combined with anticancer
compounds. The present results directly confirmed that, by
thermally cycling the pancreatic cancer cells and administering
propolis, significant cytotoxic and inhibitory effects were
observed. The present study used PCR equipment to demonstrate TC-HT
in vitro. For in vivo or clinical experiments, other
heating strategies must be used and tested, such as high-intensity
focused ultrasound (HIFU) (41).
HIFU has been widely used as a hyperthermal technique. It can be
used for thermal ablation as well as for producing mild HT in
cancer (42). The thermal
parameters may be finely tuned by modulating the heating power and
the size of the heated volume to meet the specific requirement for
the application of mild HT therapy in vivo (43). It was hypothesized that TC-HT will
be a promising strategy that could be applied to pancreatic cancer,
and thereby may be able to improve the quality of life of the
majority of patients with pancreatic cancer.
In summary, the present study demonstrated a novel
methodology in HT therapy, namely the TC-HT technique. The
heat-and-cool cycling can create a therapeutic window with a
maximum synergistic effect when combined with natural compounds,
with minimal unwanted cell damage. This would allow for repeated
and long-term treatments without the limitations associated with
the accumulation of toxic cell damage. The present results
confirmed that TC and propolis could synergistically inhibit PANC-1
cancer cell growth through the mitochondria-dependent apoptosis
pathway and cell cycle arrest. It is thought that this strategy
could be extended to other HT therapies in the fight against
cancer. Further studies are required to examine the association
between specific TC parameters and different anticancer drugs to
optimize the curative effects.
Funding
The present study was supported by grants from the
Ministry of Science and Technology (grant no.
105-2112-M-002-006-MY3 awarded to CYC) and the Ministry of
Education (grant no. MOE 106R880708 to CYC) of Taiwan, R.O.C.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CYC initiated the study, conceived the experiments
and managed the project. WTC and CYC wrote the manuscript. WTC,
YKS, CHL and CYC performed experiments and analyzed the data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to acknowledge the service
provided by Mrs. J. Y. Tsai at the Research Core Facilities 3
Laboratory of the Department of Medical Research at National Taiwan
University Hospital.
References
|
1
|
van den Tempel N, Horsman MR and Kanaar R:
Improving efficacy of hyperthermia in oncology by exploiting
biological mechanisms. Int J Hyperthermia. 32:446–454. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantso T, Goussetis G, Franco R, Botaitis
S, Pappa A and Panayiotidis M: Effects of hyperthermia as a
mitigation strategy in DNA damage-based cancer therapies. Semin
Cancer Biol. 37-38:96–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu KF and Dupuy DE: Thermal ablation of
tumours: Biological mechanisms and advances in therapy. Nat Rev
Cancer. 14:199–208. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paulson JR, Kresch AK and Mesner PW:
Moderate hyperthermia induces apoptosis in metaphase-arrested cells
but not in interphase Hela cells. Adv Biol Chem. 6:126–139. 2016.
View Article : Google Scholar
|
|
5
|
Lim CU, Zhang Y and Fox MH: Cell cycle
dependent apoptosis and cell cycle blocks induced by hyperthermia
in HL-60 cells. Int J Hyperthermia. 22:77–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhowmick S, Coad JE, Swanlund DJ and
Bischof JC: In vitro thermal therapy of AT-1 Dunning prostate
tumours. Int J Hyperthermia. 20:73–92. 2004. View Article : Google Scholar
|
|
7
|
Sminia P, van der Zee J, Wondergem J and
Haveman J: Effect of hyperthermia on the central nervous system: A
review. Int J Hyperthermia. 10:1–30. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schaaf L, Schwab M, Ulmer C, Heine S,
Mürdter TE, Schmid JO, Sauer G, Aulitzky WE and van der Kuip H:
Hyperthermia synergizes with chemotherapy by inhibiting
PARP1-dependent DNA replication arrest. Cancer Res. 76:2868–2875.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyamoto R, Oda T, Hashimoto S, Kurokawa
T, Inagaki Y, Shimomura O, Ohara Y, Yamada K, Akashi Y, Enomoto T,
et al: Cetuximab delivery and antitumor effects are enhanced by
mild hyperthermia in a xenograft mouse model of pancreatic cancer.
Cancer Sci. 107:514–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Man J, Shoemake JD, Ma T, Rizzo AE, Godley
AR, Wu Q, Mohammadi AM, Bao S, Rich JN and Yu JS: Hyperthermia
sensitizes glioma stem-like cells to radiation by inhibiting AKT
signaling. Cancer Res. 75:1760–1769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schaaf L, van der Kuip H, Zopf W, Winter
S, Münch M, Mürdter TE, Thon KP, Steurer W, Aulitzky WE and Ulmer
C: A temperature of 40°C appears to be a critical threshold for
potentiating cytotoxic chemotherapy in vitro and in peritoneal
carcinomatosis patients undergoing HIPEC. Ann Surg Oncol. 22(Suppl
3): S758–S765. 2015. View Article : Google Scholar
|
|
12
|
Krawczyk PM, Eppink B, Essers J, Stap J,
Rodermond H, Odijk H, Zelensky A, van Bree C, Stalpers LJ, Buist
MR, et al: Mild hyperthermia inhibits homologous recombination,
induces BRCA2 degradation, and sensitizes cancer cells to poly
(ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA.
108:9851–9856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dewey WC: Arrhenius relationships from the
molecule and cell to the clinic. Int J Hyperthermia. 10:457–483.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishiai S, Tahara W, Yamamoto E, Yamamoto R
and Nagai K: Histone deacetylase inhibitory effect of Brazilian
propolis and its association with the antitumor effect in Neuro2a
cells. Food Sci Nutr. 2:565–570. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taira N, Nguyen BCQ, Be Tu PT and Tawata
S: Effect of Okinawa propolis on PAK1 activity, Caenorhabditis
elegans longevity, melanogenesis, and growth of cancer cells. J
Agric Food Chem. 64:5484–5489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang S, Zhang C-P, Wang K, Li GQ and Hu
F-L: Recent advances in the chemical composition of propolis.
Molecules. 19:19610–19632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seydi E, Hosseini SA, Salimi A and
Pourahmad J: Propolis induce cytotoxicity on cancerous hepatocytes
isolated from rat model of hepatocellular carcinoma: Involvement of
ROS-mediated mitochondrial targeting. Pharma Nutrition. 4:143–150.
2016. View Article : Google Scholar
|
|
18
|
Seda Vatansever H, Sorkun K, Ismet
Deliloğlu Gurhan S, Ozdal-Kurt F, Turkoz E, Gencay O and Salih B:
Propolis from Turkey induces apoptosis through activating caspases
in human breast carcinoma cell lines. Acta Histochem. 112:546–556.
2010. View Article : Google Scholar
|
|
19
|
Kouidhi B, Zmantar T and Bakhrouf A:
Anti-cariogenic and anti-biofilms activity of Tunisian propolis
extract and its potential protective effect against cancer cells
proliferation. Anaerobe. 16:566–571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frión-Herrera Y, Díaz-García A,
Ruiz-Fuentes J, Rodríguez-Sánchez H and Sforcin JM: Brazilian green
propolis induced apoptosis in human lung cancer A549 cells through
mitochondrial-mediated pathway. J Pharm Pharmacol. 67:1448–1456.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demir S, Aliyazicioglu Y, Turan I, Misir
S, Mentese A, Yaman SO, Akbulut K, Kilinc K and Deger O:
Antiproliferative and proapoptotic activity of Turkish propolis on
human lung cancer cell line. Nutr Cancer. 68:165–172. 2016.
View Article : Google Scholar
|
|
22
|
Guzmán C, Bagga M, Kaur A, Westermarck J
and Abankwa D: ColonyArea: An ImageJ plugin to automatically
quantify colony formation in clonogenic assays. PLoS One.
9:e924442014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korchak HM, Rich AM, Wilkenfeld C,
Rutherford LE and Weissmann G: A carbocyanine dye,
DiOC6(3), acts as a mitochondrial probe in human
neutrophils. Biochem Biophys Res Commun. 108:1495–1501. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki K, Bose P, Leong-Quong RY, Fujita
DJ and Riabowol K: REAP: A two minute cell fractionation method.
BMC Res Notes. 3:294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaity C, Al-Subaie N and Cecconi M:
Cooling techniques for targeted temperature management post-cardiac
arrest. Crit Care. 19:1032015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai J, Yang J and Jones D: Mitochondrial
control of apoptosis: The role of cytochrome c. Biochimica et
Biophysica Acta (BBA). Bioenergetics. 1366:139–149. 1998.
View Article : Google Scholar
|
|
27
|
Park M, Chae H-D, Yun J, Jung M, Kim YS,
Kim SH, Han MH and Shin DY: Constitutive activation of cyclin
B1-associated cdc2 kinase overrides p53-mediated G2-M arrest.
Cancer Res. 60:542–545. 2000.PubMed/NCBI
|
|
28
|
Kampinga HH: Cell biological effects of
hyperthermia alone or combined with radiation or drugs: A short
introduction to newcomers in the field. Int J Hyperthermia.
22:191–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu X, Akutsu Y, Suganami A, Qin W, Hanari
N, Murakam K, Kano M, Usui A, Suito H, Takahashi M, et al: Low-dose
hyperthermia enhances the antitumor effects of chemotherapy in
squamous cell carcinoma. Dis Esophagus. 30:1–7. 2017. View Article : Google Scholar
|
|
30
|
Christophi C, Winkworth A, Muralihdaran V
and Evans P: The treatment of malignancy by hyperthermia. Surg
Oncol. 7:83–90. 1998. View Article : Google Scholar
|
|
31
|
Dewey WC: Arrhenius relationships from the
molecule and cell to the clinic. Int J Hyperthermia. 25:3–20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Overgaard K and Overgaard J:
Investigations on the possibility of a thermic tumour therapy. I.
Short-wave treatment of a transplanted isologous mouse mammary
carcinoma. Eur J Cancer. 8:65–78. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dewey WC, Hopwood LE, Sapareto SA and
Gerweck LE: Cellular responses to combinations of hyperthermia and
radiation. Radiology. 123:463–474. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishikawa T, Kokura S, Sakamoto N, Ando T,
Imamoto E, Hattori T, Oyamada H, Yoshinami N, Sakamoto M, Kitagawa
K, et al: Phase II trial of combined regional hyperthermia and
gemcitabine for locally advanced or metastatic pancreatic cancer.
Int J Hyperthermia. 28:597–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishii H, Okada S, Tokuuye K, Nose H,
Okusaka T, Yoshimori M, Nagahama H, Sumi M, Kagami Y and Ikeda H:
Protracted 5-fluorouracil infusion with concurrent radiotherapy as
a treatment for locally advanced pancreatic carcinoma. Cancer.
79:1516–1520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robins HI, Cohen JD, Schmitt CL, Tutsch
KD, Feierabend C, Arzoomanian RZ, Alberti D, d'Oleire F, Longo W
and Heiss C: Phase I clinical trial of carboplatin and 41.8 degrees
C whole-body hyperthermia in cancer patients. J Clin Oncol.
11:1787–1794. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stephens AD, Alderman R, Chang D, Edwards
GD, Esquivel J, Sebbag G, Steves MA and Sugarbaker PH: Morbidity
and mortality analysis of 200 treatments with cytoreductive surgery
and hyperthermic intraoperative intraperitoneal chemotherapy using
the coliseum technique. Ann Surg Oncol. 6:790–796. 1999. View Article : Google Scholar
|
|
38
|
Adachi S, Kokura S, Okayama T, Ishikawa T,
Takagi T, Handa O, Naito Y and Yoshikawa T: Effect of hyperthermia
combined with gemcitabine on apoptotic cell death in cultured human
pancreatic cancer cell lines. Int J Hyperthermia. 25:210–219. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Basel MT, Balivada S, Wang H, Shrestha TB,
Seo GM, Pyle M, Abayaweera G, Dani R, Koper OB, Tamura M, et al:
Cell-delivered magnetic nanoparticles caused hyperthermia-mediated
increased survival in a murine pancreatic cancer model. Int J
Nanomedicine. 7:297–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang L, Dong J, Ouyang W, Wang X and Tang
J: Anticancer effect and feasibility study of hyperthermia
treatment of pancreatic cancer using magnetic nanoparticles. Oncol
Rep. 27:719–726. 2012.
|
|
41
|
Partanen A, Tillander M, Yarmolenko PS,
Wood BJ, Dreher MR and Köhler MO: Reduction of peak acoustic
pressure and shaping of heated region by use of multifoci
sonications in MR-guided high-intensity focused ultrasound mediated
mild hyperthermia. Med Phys. 40:0133012013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frazier N, Payne A, de Bever J, Dillon C,
Panda A, Subrahmanyam N and Ghandehari H: High intensity focused
ultrasound hyperthermia for enhanced macromolecular delivery. J
Control Release. 241:186–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Partanen A, Yarmolenko PS, Viitala A,
Appanaboyina S, Haemmerich D, Ranjan A, Jacobs G, Woods D, Enholm
J, Wood BJ, et al: Mild hyperthermia with magnetic resonance-guided
high-intensity focused ultrasound for applications in drug
delivery. Int J Hyperthermia. 28:320–336. 2012. View Article : Google Scholar : PubMed/NCBI
|