Introduction
There were an estimated ~34,000 new cases of oral
cancer and 7,000 oral cancer-related deaths in the United States in
2018 (1). Oral squamous cell
carcinoma (OSCC), the most common malignancy of the oral cavity,
accounts for >90% of all diag-nosed oral cancers (2). Although the 5-year survival rate of
OSCC has increased from 56 to 62% due to improvements in treatment,
including surgery, radiation and chemotherapy (3), the development of new therapeutic
strategies is important.
Baicalein, a bioactive flavonoid present in the dry
root of Scutellariae Radix (Huang Qin), has been reported to
have effects on various malignancies, including lung, breast,
hepatocellular, pancreatic and gastric cancer (4-8). In
addition, baicalein induces various biological molecular activities
by blocking tumor-associated signaling pathways (9). For example, baicalein suppresses the
expression of superoxide dismutase and hypoxia-inducible factor-1α,
and inhibits lung carcinoma cell proliferation and metastasis
(10). Baicalein was previously
found to induce apoptosis of pancreatic cancer cells via a myeloid
cell leukemia 1-dependent pathway (11). Baicalein also induces apoptosis and
autophagy of breast cancer cells by suppressing the
phosphatidylinositol 3 kinase/protein kinase B pathway in
vivo and in vitro (12). Importantly, baicalein does not
appear to cause mutagenesis in normal cells, which is the major
side effect of conventional anticancer drugs (13,14).
Previous studies have revealed that baicalein is an effective
molecular anticarcinogenic agent against oral cancer (15,16),
and that it induces autophagy of oral cancer cells by promoting
reactive oxygen species-dependent signaling pathways and arresting
the proliferation of oral cancer cells in the G0/G1 phase by
enhancing the degradation of cyclin D1 and activating aryl
hydrocarbon receptor to decrease retinoblastoma (Rb)
phosphorylation (15,16).
Specificity protein 1 (Sp1) is a zinc finger-type
transcription factor with a guanine-cytosine-rich binding sequence
in the gene promoter (17). Sp1 is
involved in multiple aspects of tumor cell behavior, including
growth, survival, angiogenesis and apoptosis (18-20).
Sp1 expression and activation are considered to be associated with
human cancer development and progression (21). Lines of evidence have indicated
that targeting Sp1 and its downstream target proteins may be a
potential treatment strategy for oral cancer (22). Our previous study suggested that
baicalein inhibits the expression of Sp1 and the downstream protein
Epstein-Barr virus nuclear antigen 1 (EBNA1) and induces apoptosis
of nasopharyngeal carcinoma (NPC) (23).
The present study examined the effects of baicalein
on the proliferation, apoptosis and cell cycle progression of OSCC
cells and xenograft tumors in vivo, and investigated the
underlying mechanism.
Materials and methods
Cell lines and reagents
SCC25, CAL27 and HSC3 cells were kindly provided by
Professor Bin Shi (University of Wuhan, China). All cell lines were
cultured in RPMI-1640 media (HyClone; GE Healthcare Life Sciences)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C and humidified atmosphere of 5% CO2. Baicalein
(Sigma-Aldrich; Merck KGaA) was dissolved in DMSO at 100 mM as a
stock solution, and diluted to a working concentration (1 mM) with
PBS prior to use. For the in vivo xenograft studies,
baicalein was dissolved in a solution containing 80% PBS and 20%
DMSO.
Plasmid
For the construction of the Sp1 expression plasmid,
the full-length cDNA was amplified and inserted into the
EcoRI/XhoI sites of the pcDNA3.1 vector (Invitrogen;
Thermo Fisher Scientific, Inc.). The primers for Sp1 were as
follows: Forward, 5'-CCA AAA TGC GAT CGC ATG AGC GAC CAA GAT CAC-3'
and reverse, 5'-GAA TCA AGT TTA AAC TCA GAA GCC ATT GCC ACT-3'. The
NF-κB activity plasmid was a gift from Professor H. Shu (University
of Wuhan). The PRL-TK plasmid was a gift from Professor D. Guo.
Cell viability assay
SCC25, CAL27 and HSC3 cells (1x104
cells/well) were seeded in 96-well plates and treated with DMSO
control (0.01%) or increasing concentrations of baicalein (30, 60
and 120 µM) at 37°C. After incubation for 24, 48 and 72 h, the
cells were treated with 10 µl Cell Counting Kit-8 reagent (Dojindo
Molecular Technologies, Inc.) and the plates were incubated at 37°C
for 1 h in the dark. The optical density was measured at an
absorbance of 450 nm using an ELx800 microimmunoanalyser (BioTek
Instruments, Inc.).
Cell cycle analysis
SCC25, CAL27 and HSC3 cells were seeded in 6-well
plates and treated with DMSO (0.01%) or baicalein (60 µM) at 37°C
for 24 h. The process was performed as described previously
(24). Briefly, cells were
digested and washed twice with cold PBS solution, and then
resuspended in cold 75% ethanol. After fixation at -20°C for 24 h,
cells were collected and resuspended in 0.5 ml cold PBS. Cells were
mixed with reagent A [Multisciences (Lianke) Biotech Co., Ltd.] and
incubated at 4°C for 30 min in the dark. Cell cycle analysis was
performed using a Beckman Coulter system (EPICS Altra II; Beckman
Coulter, Inc.).
Cell apoptosis analysis
Apoptosis of OSCC cells was detected using the
Annexin V-FITC/propidium iodide (PI) apoptosis detection kit
[Multisciences (Lianke) Biotech Co., Ltd.], as instructed by the
manufacturer. Briefly, following treatment with baicalein (15, 30
and 60 µM) at 37°C for 24 h, cells were digested. After three
washes with PBS, 2x105 cells were resuspended in 500 µl
1X binding buffer, followed by addition of 5 µl Annexin V-FITC and
10 µl PI, and incubated for 30 min at 20-28°C in the dark. Cell
apoptosis was immediately analyzed using a Beckman Coulter system
(EPICS Altra II; Beckman Coulter, Inc.).
Western blot analysis
At 4 h post-transfection, SCC25, CAL27 and HSC3
cells were washed with PBS and treated with DMSO (0.01%) or
baicalein (30 or 60 µM). After 48 h of incubation, cells were
harvested and dissolved in RIPA lysis buffer (Beyotime Institute of
Biotechnology) with 0.5% cocktail protease inhibitor (Roche
Diagnostics). Following incubation on ice for 10 min, cell lysates
were collected and sonicated for 20 sec. Protein concentration was
determined using a BCA assay (BioRad Laboratories, Inc.).
Quantified proteins were mixed with 5X loading buffer [250 nM
Tris-Hcl (pH 6.8), 0.5% bromophenol blue, 50% glycerol, 10% SDS and
5% β-mercaptoethanol] and boiled for 5 min. The lysates were
separated by SDS-PAGE on 10% gels, then subjected to immunoblot
analyses. The primary antibodies used were as follows: GAPDH (cat.
no. 10494-1-AP; 1:5,000; ProteinTech Group, Inc.); cleaved
caspase-3 (cat. no. 9664; 1:1,000; Cell Signaling Technology,
Inc.); caspase-9 polyclonal antibody (cat. no. A2636; 1:1,000;
ABclonal Biotech Co., Ltd.); cleaved poly(ADP-ribose) polymerase
(PARP-1; cat. no. sc-56196; 1:500; Santa Cruz Biotechnology, Inc.);
Sp1 (cat. no. 9389; 1:1,000; Cell Signaling Technology, Inc.); p65
(cat. no. ab16502; 1:1,500; Abcam); p-p65 (cat. no. ab86299;
1:1,000; Abcam); and p50 (cat. no. 3035; 1:1,000; Cell Signaling
Technology, Inc.). Western blot gray values were determined by
ImageJ software (National Institutes of Health).
Reverse transcription-quantitative
PCR (RT-qPCR). SCC25, CAL27 and HSC3 cells
(1x105) were placed in 24-well plates. After attaching
to the wells, the cells were washed with PBS and treated with DMSO
(0.01%) or baicalein (30 or 60 µM). After incubation at 37°C for 24
h, total RNA was extracted by using TRIzol reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Subsequently, cDNA was synthesized from total RNAs (1 µg) using an
RT kit (Takara Bio, Inc.) according to the manufacturer's
instructions. The mRNA expression levels of Sp1, p65 and p50 were
quantified using the CFX96 Real-Time PCR Detection System with an
SYBR Premix Ex Taq kit (Takara Bio, Inc.). The primers were as
follows: p65, forward 5'-CGG GAT GGC TTC TAT GAG G-3' and reverse
5'-CTC CAG GTC CCG CTT CTT-3'; p50, forward 5'-ACC CTG ACC TTG CCT
ATT TG-3' and reverse 5'-AGC TCT TTT TCC CGA TCT CC-3'; Sp1,
forward 5'-ATG GGG GCA ATG GTA ATG GTG G-3' and reverse 5'-TCA GAA
CTT GCT GGT TCT GTA AG-3'; GAPDH, forward 5'-GGT GGC TTC TGA CTT
CAA CA-3' and reverse 5'-GTT GCT GTA GCC AAA TTC GTT GT-3'. The
mRNA levels were normalized to GAPDH as the reference gene.
Cell transfection
A specific short hairpin RNA (shRNA) targeting Sp1
(5'-GCA TAT TTG CCA CAT CCA AGG-3', Sp1-Homo-1828; GenePharma, Co.,
Ltd.) or a non-specific control (NC; 5'-TTC TCC GAA CGT GTC ACG
T-3'; Shanghai GenePharma Co., Ltd.) were transfected to cells
using X-treme GENE HP DNA Transfection Reagent (Roche Diagnostics;
40 pmol for each shRNA) according to the manufacturer's
instructions. PcDNA3.1-Sp1 or pcDNA3.1 (vehicle control) was
transfected to cells using X-treme GENE HP DNA Transfection
Reagent. At 4 h post-transfection, cells (SCC25) were washed with
PBS and treated with DMSO (0.01%) or baicalein (60 µM). After 48 h,
the cells were harvested for western blot analysis. For RT-qPCR,
total RNA was collected after 12 or 24 h of incubation. For the
dual luciferase reporter assay, cells were harvested after 24 or 48
h of incubation.
Dual luciferase reporter assay
Cells (1x105) were placed in 24-well
plates and incubated at 37°C overnight prior to transfection.
Plasmids were co-transfected into the cells for 24 h. After
treatment with baicalein or control for 24 h, cells were collected
and analyzed using the Dual Luciferase Reporter Assay system
(Promega Corporation) as previously described (25).
Animal studies
The Medical Ethics Committee of Wuhan University
approved the animal experimental protocols in the present study
(G201725). A total of 10 BALB/c nude mice were obtained from the
Animal Biosafety Level-III Laboratory of Wuhan University and
housed in a specific pathogen-free environment. SCC25 cells
(5x106/100 µl) were subcutaneously injected into the
flanks of the mice when they were 6-7 weeks old. When the tumors
became macroscopically visible (~7 days), the mice were randomly
divided into two groups (n=5/group). The control group mice were
injected with PBS (0.01% DMSO) and baicalein group mice were
injected with baicalein (30 mg/kg, three times a week). The mice
were sacrificed after 21 days of treatment. The mouse weight and
tumor size were measured. Tumor volume was calculated as 0.5 x
length x width2. Tissue samples were fixed in 10%
neutral buffered formalin, embedded in paraffin and cut into 3-µm
sections).
Immunohistochemical analysis
Immunohistochemistry was performed using antibodies
against Sp1 (cat. no. 9389; 1:1,000; Cell Signaling Technology,
Inc.), p65 (cat. no. ab16502; 1:1,500; Abcam) and cleaved caspase-3
(cat. no. 9664; 1:1,000; Cell Signaling Technology, Inc.) according
to the manufacturer's instructions. The process was performed as
described previously (24).
Briefly, after deparaffinization, the tissue sections were boiled
in citric acid (pH 6.0) for 20 min and immersed in 3%
H2O2 for 10 min to quench the endogenous
peroxidase activity. After blocking in goat serum for 1 h at
20-28°C, the tissue sections were incubated with primary antibodies
overnight at 4°C. After washing, the tissue sections were incubated
with secondary antibody (MaxVision™ Kits; MaxVision Biosciences,
Inc.) conjugated to horseradish peroxidase. Subsequently, the
tissue sections were incubated with diaminobenzidine for 1 min and
lightly counterstained with hematoxylin.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. For the comparison of two groups,
Student's t-test was selected. For the comparison of multiple
groups, one-way ANOVA followed by the Newman-Keuls post hoc test
was carried out. All statistical data were analyzed by using
GraphPad Prism for Windows, version 5.0 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baicalein effectively suppresses the
proliferation of OSCC cells
To determine whether baicalein decreases the
viability of OSCC cells, SCC25, CAL27 and HSC3 cells were treated
with varying doses of baicalein and for different durations. The
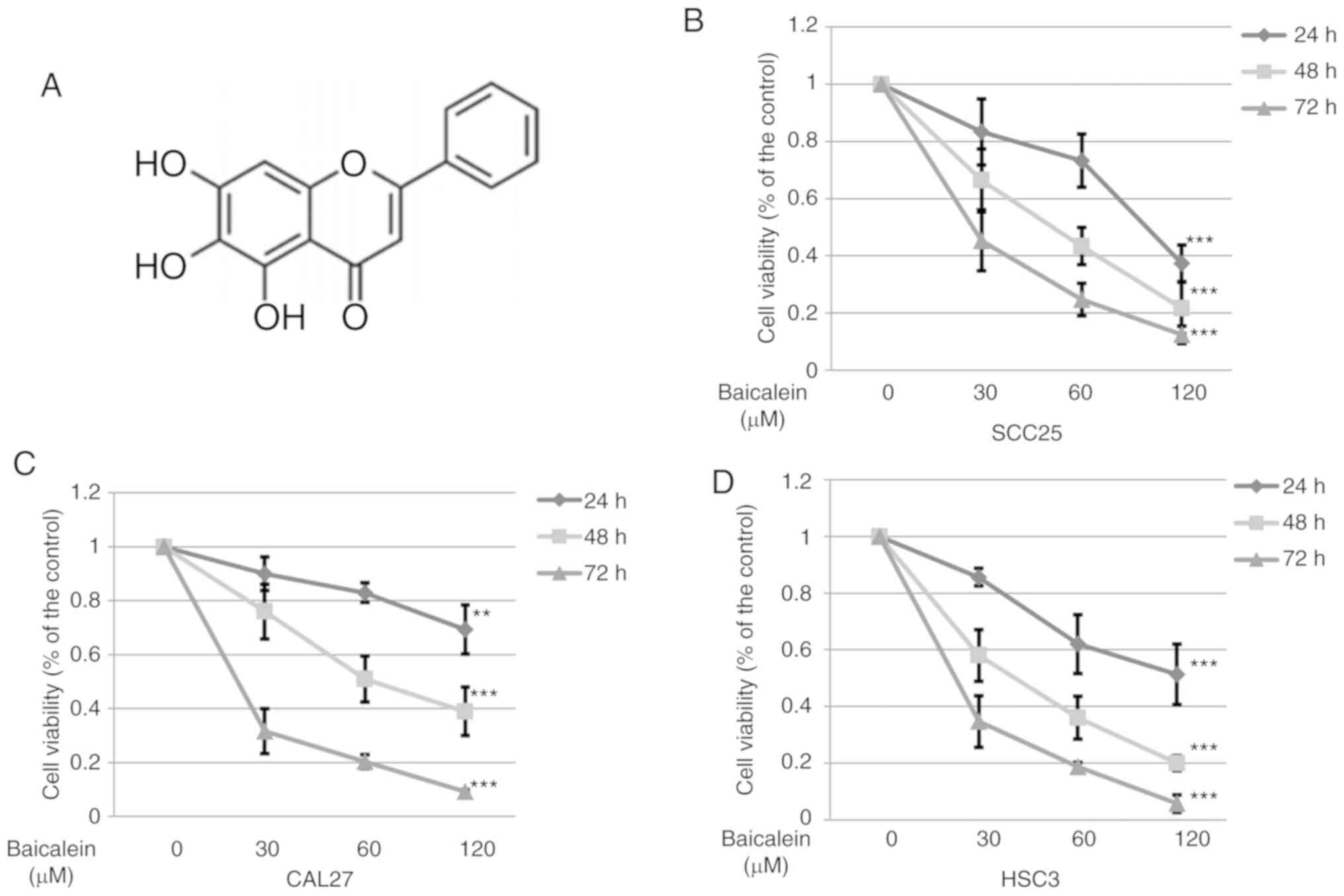
chemical structure of baicalein (5,6,7-trihydroxyflavone) is shown
in Fig. 1A. Baicalein
significantly (P<0.05) reduced the viability of SCC25, CAL27 and
HSC3 cells compared with cells treated with DMSO control (Fig. 1B-D). These results suggest that
baicalein effectively inhibits the proliferation of different OSCC
cell lines.
Baicalein induces apoptosis in OSCC
cells
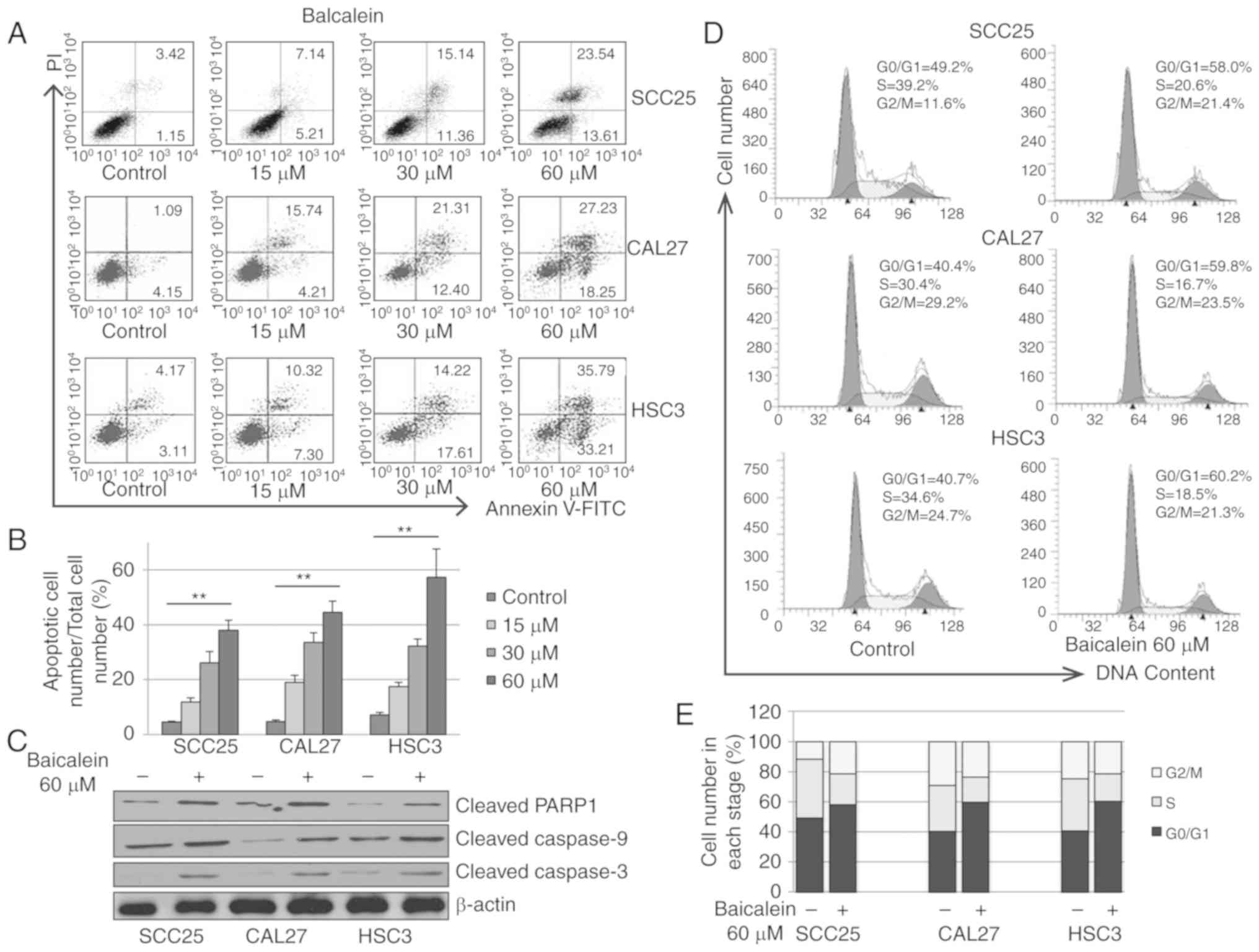
To examine the apoptotic effect exerted by baicalein
on OSCC cells, SCC25, CAL27 and HSC3 cells were treated with
increasing doses of baicalein for 24 h. As shown in Fig. 2A and B, baicalein significantly
induced apoptosis of SCC25, CAL27 and HSC3 cells. To further assess
stimulation of the apoptotic pathway, expression of several
apoptosis-associated proteins was detected by western blot
analysis. Baicalein increased the protein levels of cleaved
caspase-9, cleaved caspase-3 and cleaved PARP-1 in SCC25, CAL27 and
HSC3 cells (Fig. 2C), suggesting
that baicalein induces OSCC cell apoptosis via the mitochondrial
apoptotic pathway.
Baicalein arrests the cell cycle in the
G0/G1 phase
To determine whether baicalein affects the cell
cycle, SCC25, CAL27 and HSC3 cells were treated with vehicle
control (0.01% DMSO) or baicalein (60 µM) for 24 h. The
distribution of cells in different cell cycle phases was analyzed
by flow cytometry. As shown in Fig. 2D
and E, baicalein treatment induced cell cycle arrest in the
G0/G1 phase. The fraction of SCC25, CAL27 and HSC3 cells in the
G0/G1 phase was increased by 18.8, 29.4 and 19.5%, respectively,
when treated with baicalein (60 µM). These results demonstrated
that baicalein reduces the proliferation of OSCC cells by causing
G0/G1 cell cycle arrest.
Baicalein decreases the expression of
Sp1, p65 and p50
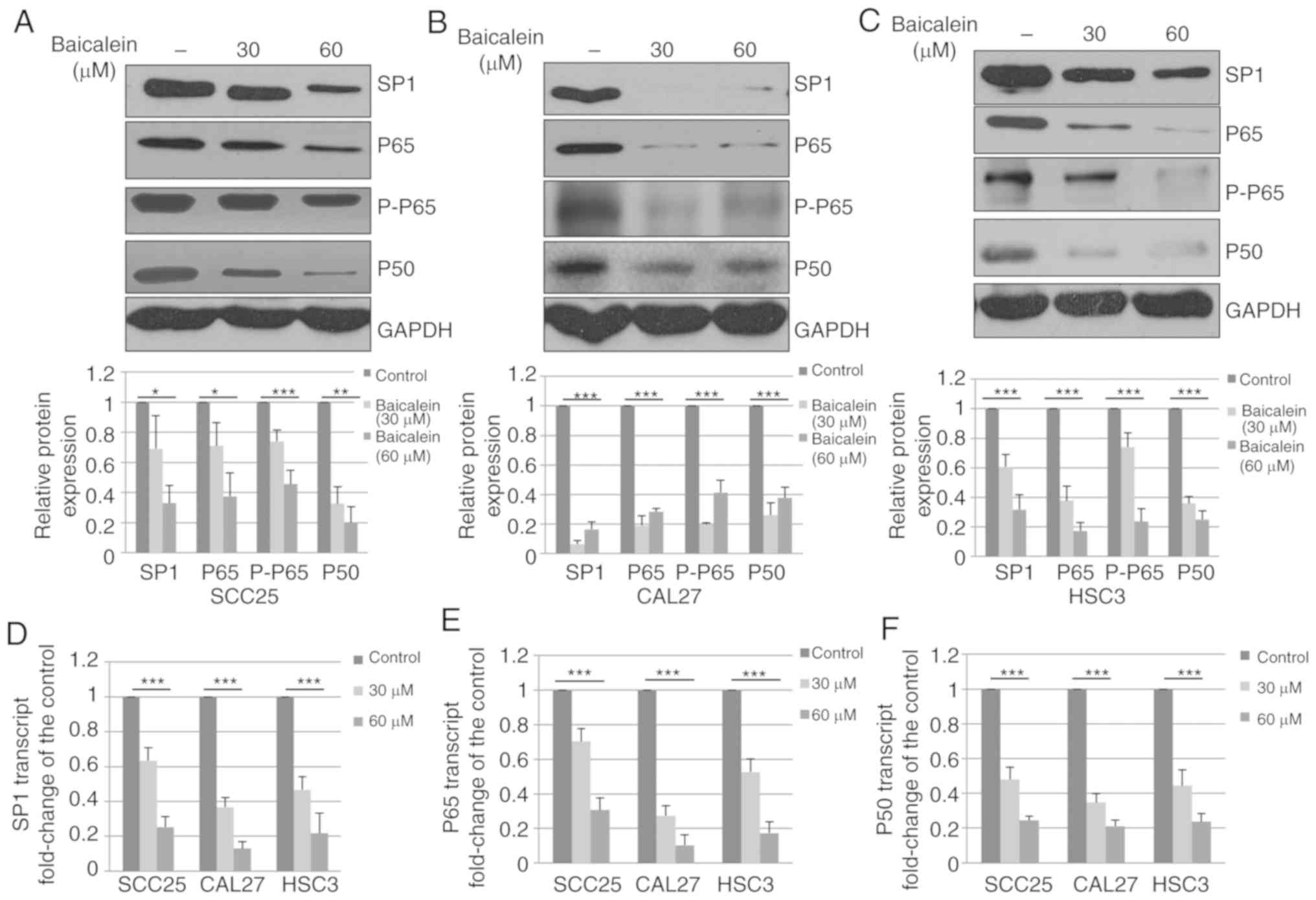
To determine whether baicalein alters the expression
of Sp1 in OSCC cells, three different OSCC cell lines (SCC25, CAL27
and HSC3) were treated with DMSO control or baicalein. As shown in
Fig. 3A–C, baicalein significantly
(P<0.05) decreased Sp1 expression in SCC25, CAL27 and HSC3
cells. Sp1 has been previously demonstrated to be a transcription
factor that regulates the expression of p65 and p50 (26). Therefore, the expression of p65 and
p50 was measured, and the levels of p65, p-p65 and p50 were found
to be decreased following treatment with baicalein (Fig. 3A-C).
The observed decrease in protein expression may be
due to reduced mRNA levels; therefore, the effects of baicalein on
Sp1 were also analyzed at the transcriptional level. SCC25, CAL27
and HSC3 were treated with DMSO control or baicalein, and total RNA
was collected and subjected to RT-qPCR analysis. The mRNA
expression of Sp1 in SCC25, CAL27 and HSC3 cells was found to be
decreased by 75.7, 87.0 and 78.8%, respectively, compared with
controls at 48 h (Fig. 3D).
Additionally, the mRNA levels of p65 and p50 were analyzed. As
shown in Fig. 3E and F, the mRNA
levels of p65 and p50 were significantly decreased following
treatment with baicalein. These results suggest that baicalein
decreases the expression of Sp1, p65 and p50 in OSCC cell lines by
reducing the mRNA expression.
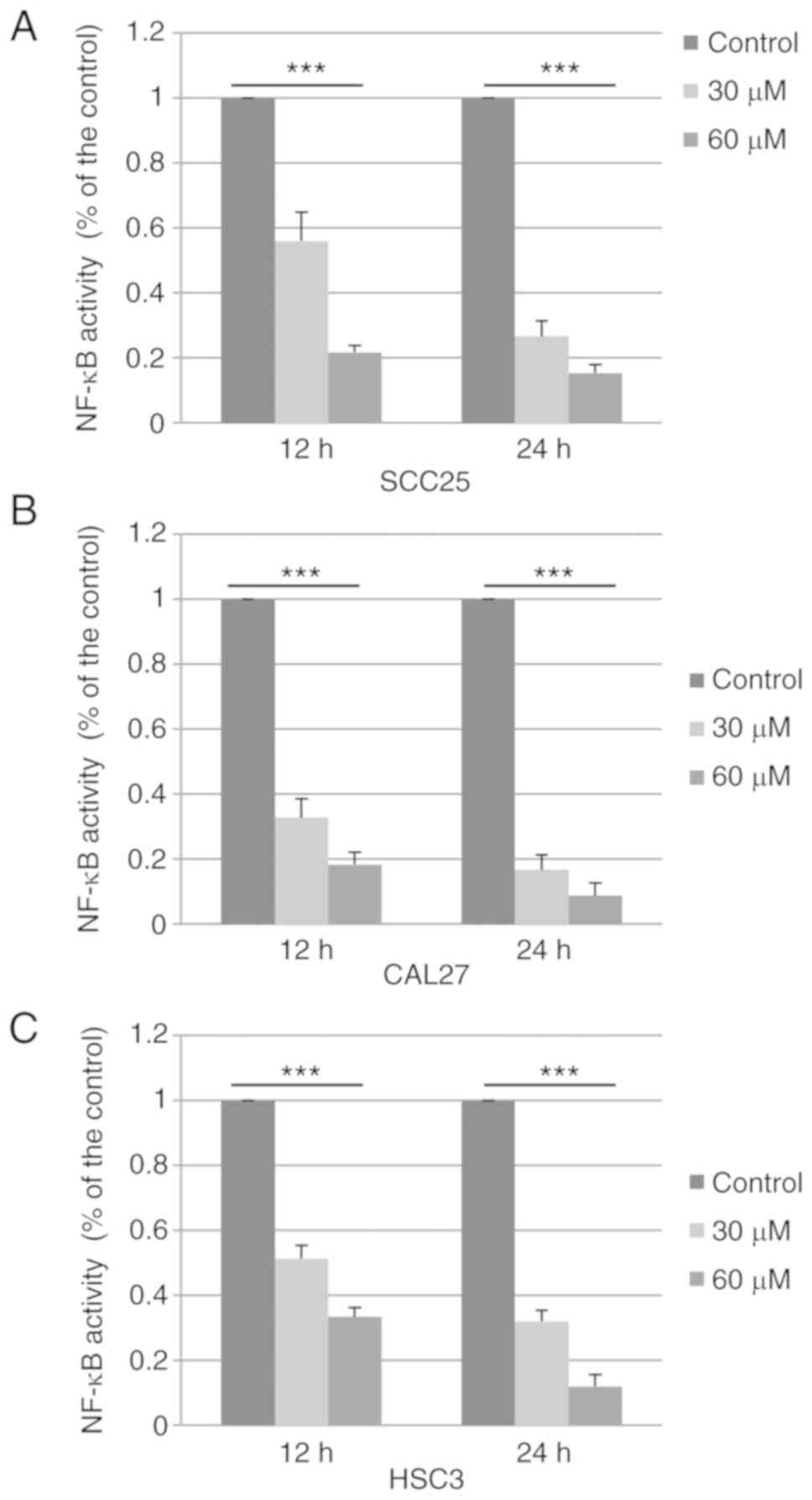
Baicalein decreases NF-κB activity in
OSCC cells
The reduced expression of p65 and p50 may lead to
suppression of the NF-κB pathway. To confirm this hypothesis,
SCC25, CAL27 and HSC3 cells were treated with DMSO control or
baicalein, and the activity of NF-κB was determined using a Dual
Luciferase Reporter Assay system. As shown in Fig. 4A-C, the activity of the NF-κB
pathway was downregulated to 15.3% (SCC25), 8.6% (CAL27) and 12.0%
(HSC3) following treatment with baicalein (60 µM) for 24 h. These
results suggest that baicalein markedly inhibits the activation of
NF-κB signaling in OSCC cells.
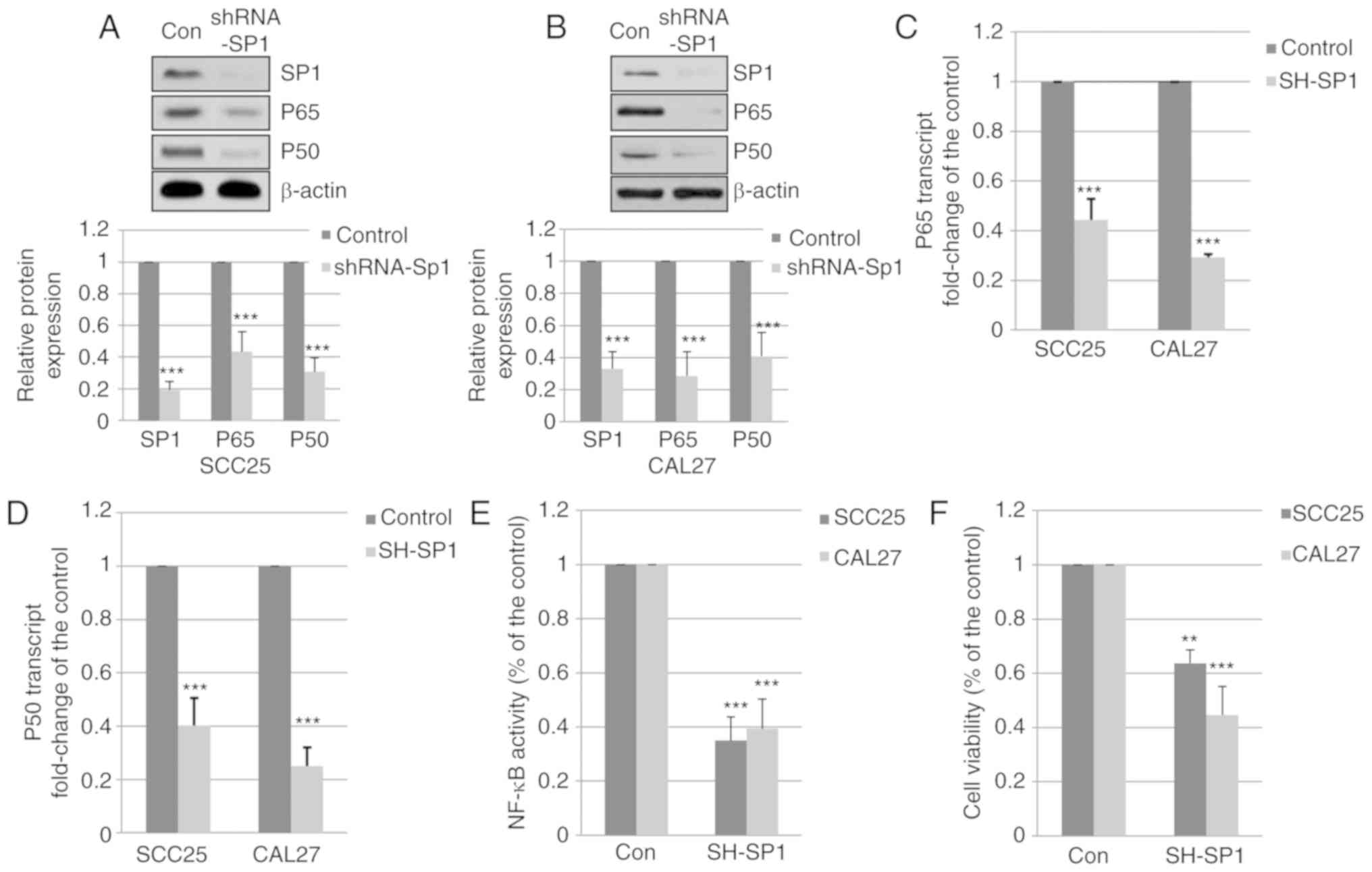
Reduced expression of Sp1 decreases the
expression of p65 and p50
To determine whether the decrease in p65 and p50
expression was associated with the expression of Sp1 in OSCC cells,
SCC25 and CAL27 cells were transfected with a specific shRNA
targeting Sp1. Total proteins were harvested and subjected to
western blot analysis. As shown in Fig. 5A and B, silencing of Sp1
significantly reduced the expression of p65 and p50. Additionally,
the mRNA levels of p65 were decreased to 44.3 and 29.3% following
silencing of Sp1 in SCC25 and CAL27 cells, respectively (Fig. 5C). The mRNA levels of p50 in SCC25
and CAL27 cells with silenced Sp1 expression were decreased to 40.3
and 25.0%, respectively (Fig. 5D).
These results indicate that downregulated expression of Sp1 reduces
the expression of p65 and p50 in OSCC cells.
Downregulated expression of Sp1 inhibits
NF-κB activity and the viability of OSCC cells
To determine the effect of Sp1 silencing on NF-κB
activity and cell viability, SCC25 and CAL27 cells were transfected
with Sp1-specific shRNA. The activity of NF-κB was determined using
the Dual Luciferase Reporter Assay system. As shown in Fig. 5E, the activity of the NF-κB pathway
was reduced to 35 and 39.3% by Sp1-shRNA in SCC25 and CAL27 cells,
respectively. The viability of SCC25 and CAL27 cells was decreased
to 63.7 and 44.7%, respectively, by specific Sp1-shRNA (Fig. 5F). These results indicate that
silencing of Sp1 expression inhibits NF-κB activity and reduces the
viability of OSCC cells.
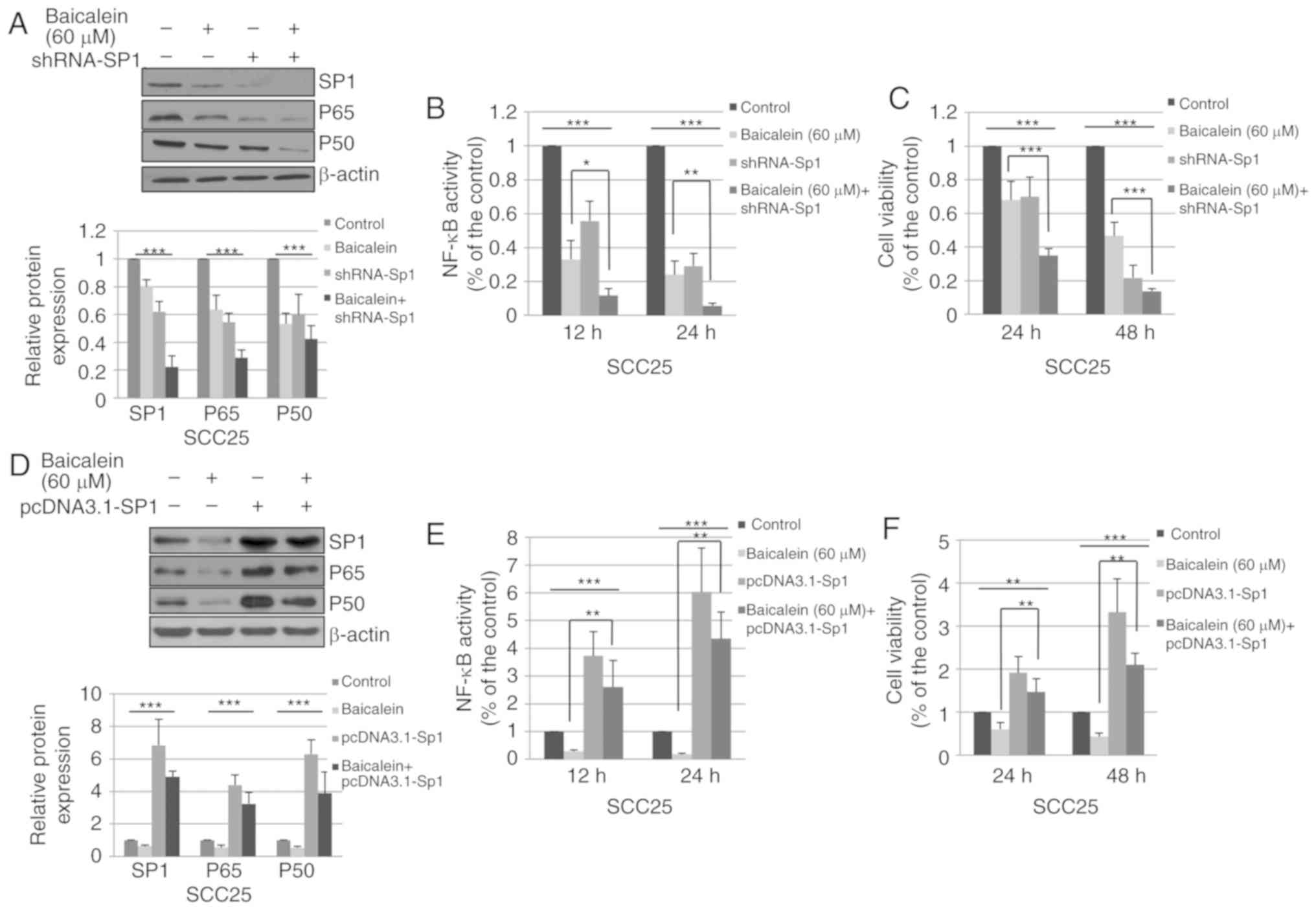
shRNA-Sp1 enhances the effect of
baicalein
To determine whether the effects of baicalein on
NF-κB activity and cell viability are dependent on Sp1, SCC25 cells
were transfected with shRNA-Sp1 or homo-NC, and treated with
baicalein or DMSO control. As shown in Fig. 6A, combination with shRNA-Sp1
significantly enhanced the baicalein-induced suppression of Sp1,
p65 and p50 expression. In addition, knockdown of Sp1 significantly
contributed to the inhibitory effects of baicalein on NF-κB
activity (Fig. 6B) and cell
viability (Fig. 6C).
Exogenous Sp1 attenuates the effects of
baicalein
To determine whether the effects of baicalein on
cell viability are primarily caused by the reduced expression of
Sp1, SCC25 cells were transiently transfected with pcDNA3.1-Sp1 or
vehicle control (pcDNA3.1). The cells were subsequently treated
with DMSO or baicalein. As shown in Fig. 6D, pcDNA3.1-Sp1 significantly
increased the expression of Sp1, p65 and p50. Compared with Sp1
overexpression alone, combined treatment with baicalein and
pcDNA3.1-Sp1 transfection decreased the expression of Sp1, p65 and
p50. As shown in Fig. 6E, the
NF-κB activity was enhanced by 273.3 and 503.1% following
transfection with pcDNA3.1-Sp1 for 12 and 24 h, respectively.
Overexpression of Sp1 attenuated the effects of baicalein on NF-κB
activity. In addition, Sp1 overexpression increased the viability
of SCC25 cells and attenuated the inhibitory effect of baicalein on
cell viability (Fig. 6F). These
results suggest that baicalein reduces SCC25 cell viability and
NF-κB activity, which is mediated by the Sp1 pathway.
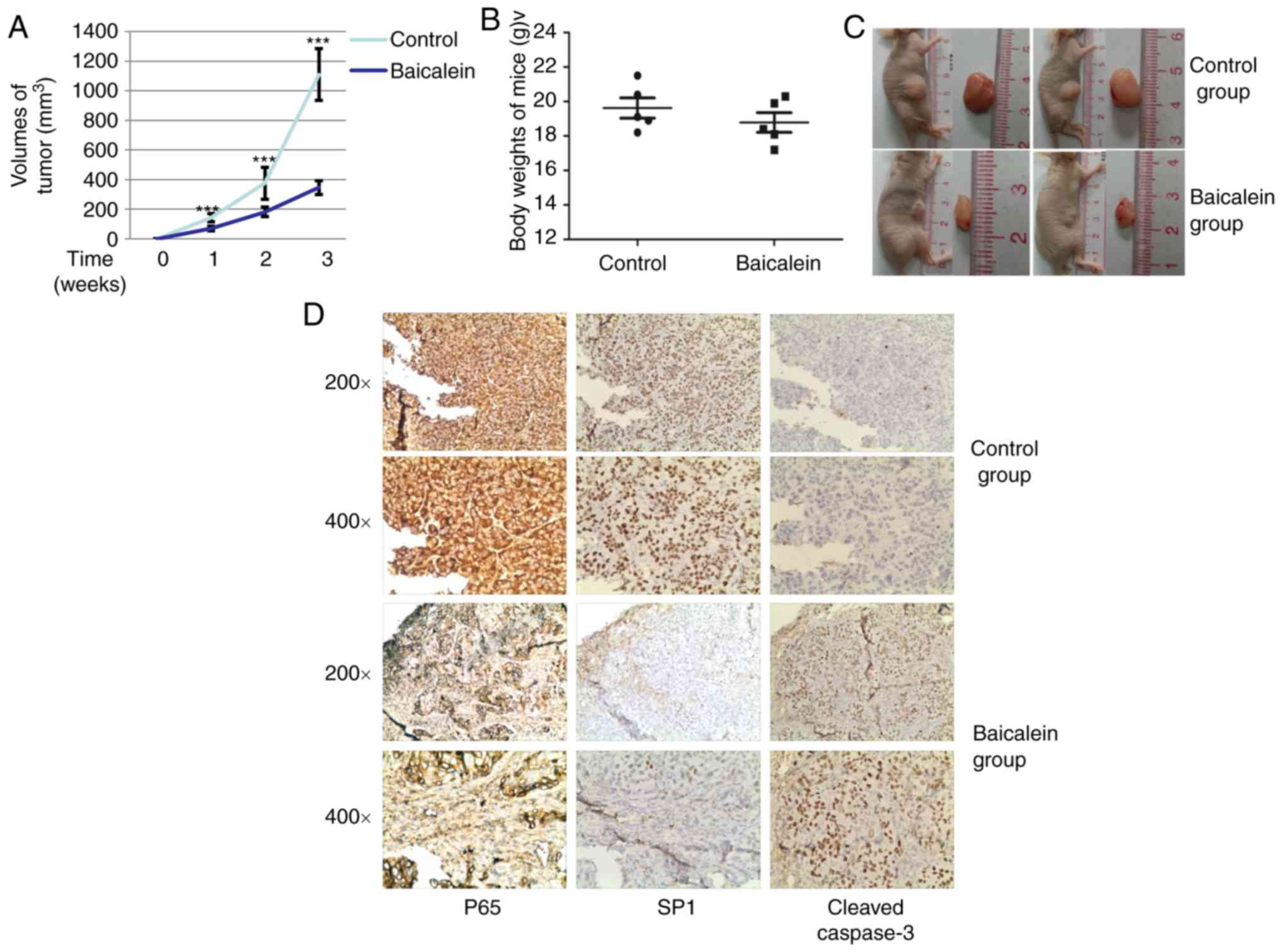
In vivo effects of baicalein on BALB/c
mice inoculated with SCC25 cells
To determine the effects of baicalein on OSCC in
vivo, SCC25 cells were used to establish subcutaneous xenograft
tumors in immune-deficient BALB/c nude mice. The mice were
administered baicalein or DMSO at 7 days after tumor cell
inoculation. After treatment with baicalein or DMSO for 21 days,
the mice were sacrificed and their tumors were measured. The weight
of the mice was also evaluated. As shown in Fig. 7A, tumor volumes were decreased by
treatment with baicalein. Additionally, there was no significant
difference in the weight of the mice between the two groups
(Fig. 7B). Representative images
of the tumors are shown in Fig.
7C. To further elucidate the mechanism underlying the effects
of baicalein on tumor growth, the expression and distribution of
p65, Sp1 and cleaved caspase-3 were determined by
immunohistochemical examination of the tumor tissues. As shown in
Fig. 7D, the expression p65 and
Sp1 was stronger in the sections from the control group compared
with the baicalein-treated group. Conversely, cleaved caspase-3
expression was weaker in the control group compared with that in
the baicalein-treated group. These results suggest that baicalein
reduces the growth of SCC25 OSCC cell xenografts in
vivo.
Discussion
Accumulating evidence suggests that targeting Sp1
may be a novel therapeutic strategy for cancer treatment, as Sp1 is
involved in tumor development, growth and metastasis (27,28).
Sp1 is overexpressed in a number of human cancer cells, including
oral cancer cells (29), which
suggests that Sp1 may be associated with cancer cell growth. Our
previous study revealed that baicalein inhibits cancer development
and expression of EBNA1 in Epstein-Barr virus-positive NPC cells
via an Sp1-dependent mechanism (23); thus, it would be of interest to
investigate the effects of baicalein in EBV-negative epithelial
cancer cells. In the present study, baicalein, a traditional
extract used in herbal medicine, effectively suppressed the
proliferation of OSCC cell lines. Baicalein induced apoptosis of
OSCC cells and arrested the cell cycle in the G0/G1 phase.
Baicalein significantly decreased the protein and mRNA expression
of Sp1, p65 and p50. Additionally, baicalein reduced NF-κB activity
in OSCC cells. Furthermore, knockdown of Sp1 reduced the expression
of p65 and p50, and Sp1 silencing enhanced the effects of
baicalein. By contrast, overexpression of Sp1 attenuated the
inhibitory effects of baicalein on NF-κB activity and cell
viability. Furthermore, baicalein reduced the growth of
SCC25-induced tumor xenografts in vivo.
Baicalein has been attracting increasing attention
due to its cytotoxic effects on cancer cells at a low dose
(30-33). Our previous study indicated that a
low-toxicity dose of baicalein exerted a strong antitumor effect on
NPC cells in vivo and in vitro (23). In this context, the effect of
baicalein on OSCC were investigated. Baicalein has been reported to
induce apoptosis via both the intrinsic and extrinsic apoptotic
pathways in cancer cells. For example, baicalein treatment induces
caspase-3 and caspase-9 activation, decreases the expression of
Bcl-2 and increases the level of Bax and p53 via the ERK/p38 MAPK
pathway in breast cancer (34). In
addition to its pro-apoptosis effects, baicalein also regulates the
cell cycle. In lung cancer cells, baicalein arrests the cell cycle
in the S phase by downregulating the expression of cyclin A
(35); however, baicalein induces
G0/G1 phase arrest in other cancer types, such as prostate cancer,
hepatocellular carcinoma and lung squamous cell carcinoma.
Baicalein upregulates the expression of Rb, p53, p21(Cip1) and
p27(Kip1), and decreases the expression of cyclin D1, cyclin E,
p-Rb and CDK4, which results in an increased percentage of
hepatocellular carcinoma cells in the G0/G1 phase (36,37).
Baicalein is also reported to reduce the growth of tumor
cell-induced xenografts in animal studies, including breast, colon
and pancreatic cancer xenografts (12,38,39).
In the present study, baicalein induced G0/G1 phase cell cycle
arrest, induced apoptosis, and inhibited the growth of OSCC cells
in vitro and in vivo.
Shin et al (29) reported that Sp1 is overexpressed in
OSCC tissues compared with normal oral mucosal tissues, suggesting
that Sp1 may be a valuable molecular target for the treatment of
oral cancer (29). Several
targeted drugs have exhibited strong cytotoxic effects against OSCC
cells, including mithramycin A, an Sp1-specific inhibitor (22,29,40).
Our previous study revealed that baicalein reduces the expression
of Sp1 in NPC cells (23); thus,
it was hypothesized that baicalein may also decrease the expression
of Sp1 in OSCC cells. As expected, the expression of Sp1 was
reduced by baicalein treatment. Sp1 has been reported to be crucial
for the transcription of the NF-κB subunits, p50 and p65, and
involved in regulating the activity of the NF-κB pathway. Silencing
of Sp1 was shown to reduce the expression of p50 and p65, resulting
in reduced activity of the NF-κB pathway in OSCC cells. Knockdown
of Sp1 was also found to be associated with reduced cell colony
formation, consistently with our current findings (41). In addition, the expression of p65
and p50 in OSCC cells was decreased by baicalein treatment in the
present study. Of note, decreased Sp1 expression was reduced at the
mRNA level, which suggested that baicalein targets another protein
in order to reduce Sp1 expression.
NF-κB is a transcription factor that targets
anti-apoptotic genes and can promote tumor development, cell
survival and malignant progression in a variety of cancer types,
including OSCC (42). Matrix
metalloprotease-9, which is synergistically upregulated by
pro-inflammatory cytokines and growth factors in an NF-κB-dependent
manner, has been reported to be associated with nodal metastasis
and reduced survival in oral cancer (43). Chemokine receptor 7, which is
associated with metastasis, is also upregulated by NF-κB activation
(44). Thus, it may be inferred
that the NF-κB pathway is a target for chemoprevention. The results
of the present study suggested that Sp1 regulates the activity of
NF-κB and the viability of OSCC cells, and attenuates the
inhibitory effects induced by baicalein, which indicates that the
mechanism of action of baicalein is Sp1/NF-κB-dependent. Of note, a
number of studies have reported that baicalein inhibits the
activity of NF-κB by reducing the expression of p65, with affects
in various diseases and disease models, including cervical cancer,
tubular-interstitial nephritis, hepatocellular carcinoma, vascular
endothelial injury and Parkinson's disease (45-49).
The findings of the present study demonstrated that baicalein
decreases the expression of p65 and inhibits the activity of NF-κB
via an Sp1-dependent mechanism.
In conclusion, the present study uncovered a novel
mechanism to explain how baicalein decreases the viability and the
activity of NF-κB in OSCC cells in vivo and in vitro.
Knockdown of Sp1 decreased the expression of p65 and p50 in OSCC
cells at the mRNA level, which resulted in reduced cell viability
and reduced activity of NF-κB. Baicalein also induced cell
apoptosis and arrested the cell cycle in the G0/G1 phase. Notably,
baicalein reduced the growth of OSCC cells by blocking an
Sp1/NF-κB-dependent pathway.
Acknowledgments
The authors would like to thank Professor Bin Shi
(University of Wuhan, China), Professor H. Shu (University of
Wuhan, China) and Professor D. Guo (University of Wuhan, China) for
supplying the cell lines and plasmids.
Funding
The present study was supported by the Nature
Science Foundation for Young Scholars (grant no. 43150086), without
commercial or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ designed the study; ZG performed the experiments;
YZ and JL contributed new reagents/analytic tools; ZG analyzed the
data; HZ and JL wrote the manuscript. All authors have read and
approved the final version of the manuscript for publication.
Ethics approval and consent to
participate
All animal experiments were approved by the ABSL-3
animal laboratory at Wuhan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinevici N and O'Sullivan J: Oral cancer:
Deregulated molecular events and their use as biomarkers. Oral
Oncol. 61:12–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Braakhuis BJ, Leemans CR and Visser O:
Incidence and survival trends of head and neck squamous cell
carcinoma in the Netherlands between 1989-2011. Oral Oncol.
50:670–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi EO, Park C, Hwang HJ, Hong SH, Kim
GY, Cho EJ, Kim WJ and Choi YH: Baicalein induces apoptosis via
ROS-dependent activation of caspases in human bladder cancer 5637
cells. Int J Oncol. 49:1009–1018. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du G, Han G, Zhang S, Lin H, Wu X, Wang M,
Ji L, Lu L, Yu L and Liang W: Baicalin suppresses lung carcinoma
and lung metastasis by SOD mimic and HIF-1alpha inhibition. Eur J
Pharmacol. 630:121–130. 2010. View Article : Google Scholar
|
|
6
|
Chen M, Lai L, Li X, Zhang X, He X, Liu W,
Li R, Ke X, Fu C, Huang Z and Duan C: Baicalein attenuates
neurological deficits and preserves blood-brain barrier integrity
in a rat model of intracerebral hemorrhage. Neurochem Res.
41:3095–3102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chao JI, Su WC and Liu HF: Baicalein
induces cancer cell death and proliferation retardation by the
inhibition of CDC2 kinase and survivin associated with opposite
role of p38 mitogen-activated protein kinase and AKT. Mol Cancer
Ther. 6:3039–3048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bie B, Sun J, Guo Y, Li J, Jiang W, Yang
J, Huang C and Li Z: Baicalein: A review of its anti-cancer effects
and mechanisms in Hepatocellular Carcinoma. Biomed Pharmacother.
93:1285–1291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su G, Chen H and Sun X: Baicalein
suppresses non small cell lung cancer cell proliferation, invasion
and Notch signaling pathway. Cancer Biomark. 22:13–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, et
al: Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta. 1813:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan W, Ma X, Zhao X and Zhang S: Baicalein
induces apoptosis and autophagy of breast cancer cells via
inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther.
12:3961–3972. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiu YW, Lin TH, Huang WS, Teng CY, Liou
YS, Kuo WH, Lin WL, Huang HI, Tung JN, Huang CY, et al: Baicalein
inhibits the migration and invasive properties of human hepatoma
cells. Toxicol Appl Pharmacol. 255:316–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M,
Dong Q, Liu Y and Xu H: The traditional chinese medicine baicalein
potently inhibits gastric cancer cells. J Cancer. 7:453–461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Lu M, Jiang XX, Pan MX, Mao JW and
Chen M: Inhibiting reactive oxygen species-dependent autophagy
enhanced baicalein-induced apoptosis in oral squamous cell
carcinoma. J Nat Med. 71:433–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng YH, Li LA, Lin P, Cheng LC, Hung CH,
Chang NW and Lin C: Baicalein induces G1 arrest in oral cancer
cells by enhancing the degradation of cyclin D1 and activating AhR
to decrease Rb phosphorylation. Toxicol Appl Pharmacol.
263:360–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suske G, Bruford E and Philipsen S:
Mammalian SP/KLF transcription factors: Bring in the family.
Genomics. 85:551–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi Q, Le X, Abbruzzese JL, Peng Z, Qian
CN, Tang H, Xiong Q, Wang B, Li XC and Xie K: Constitutive Sp1
activity is essential for differential constitutive expression of
vascular endothelial growth factor in human pancreatic
adenocarcinoma. Cancer Res. 61:4143–4154. 2001.PubMed/NCBI
|
|
19
|
Abdelrahim M, Smith R III, Burghardt R and
Safe S: Role of Sp proteins in regulation of vascular endothelial
growth factor expression and proliferation of pancreatic cancer
cells. Cancer Res. 64:6740–6749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan NY and Khachigian LM: Sp1
phosphorylation and its regulation of gene transcription. Mol Cell
Biol. 29:2483–2488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh MJ, Chen JC, Yang WE, Chien SY, Chen
MK, Lo YS, His YT, Chuang YC, Lin CC and Yang SF:
Dehydroandrographolide inhibits oral cancer cell migration and
invasion through NF-kB-, AP-1-, and SP-1-modulated matrix
metalloproteinase-2 inhibition. Biochem Pharmacol. 130:10–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Wang H, Liu Y, Wang C, Wang J,
Long C, Guo W and Sun X: Baicalein inhibits growth of Epstein-Barr
virus-positive nasopharyngeal carcinoma by repressing the activity
of EBNA1 Q-promoter. Biomed Pharmacother. 102:1003–1014. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou H, Liu Y, Wang C, Liu L, Wang H,
Zhang Y, Long C and Sun X: Triptolide inhibits Epstein-Barr nuclear
antigen 1 expression by increasing sensitivity of mitochondria
apoptosis of nasopharyngeal carcinoma cells. J Exp Clin Cancer Res.
37:1922018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou H, Guo W, Long C, Wang H, Wang J and
Sun X: Triptolide inhibits proliferation of Epstein-Barr
virus-positive B lymphocytes by down-regulating expression of a
viral protein LMP1. Biochem Biophys Res Commun. 456:815–820. 2015.
View Article : Google Scholar
|
|
26
|
Banerjee S, Sangwan V, McGinn O, Chugh R,
Dudeja V, Vickers SM and Saluja AK: Triptolide-induced cell death
in pancreatic cancer is mediated by O-GlcNAc modification of
transcription factor Sp1. J Biol Chem. 288:33927–33938. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao JC, Wang L, Wei D, Gong W, Hassan M,
Wu TT, Mansfield P, Ajani J and Xie K: Association between
expression of transcription factor Sp1 and increased vascular
endothelial growth factor expression, advanced stage, and poor
survival in patients with resected gastric cancer. Clin Cancer Res.
10:4109–4117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Enya K, Hayashi H, Takii T, Ohoka N,
Kanata S, Okamoto T and Onozaki K: The interaction with Sp1 and
reduction in the activity of histone deacetylase 1 are critical for
the constitu-tive gene expression of IL-1 alpha in human melanoma
cells. J Leukoc Biol. 83:190–199. 2008. View Article : Google Scholar
|
|
29
|
Shin JA, Kim JJ, Choi ES, Shim JH, Ryu MH,
Kwon KH, Park HM, Seo JY, Lee SY, Lim DW, et al: In vitro apoptotic
effects of methanol extracts of dianthus chinensis and acalypha
australis L. targeting specificity protein 1 in human oral cancer
cells. Head Neck. 35:992–998. 2013. View Article : Google Scholar
|
|
30
|
Fox JT, Sakamuru S, Huang R, Teneva N,
Simmons SO, Xia M, Tice RR, Austin CP and Myung K: High-throughput
genotoxicity assay identifies antioxidants as inducers of DNA
damage response and cell death. Proc Natl Acad Sci USA.
109:5423–5428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu J, Liu H, Lei J, Tan W, Hu X and Zou G:
Antitumor activity of chloroform fraction of Scutellaria barbata
and its active constituents. Phytother Res. 21:817–822. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong WY, Zhao ZX, Liu BJ, Lu LW and Dong
JC: Exploring the chemopreventive properties and perspectives of
baicalin and its aglycone baicalein in solid tumors. Eur J Med
Chem. 126:844–852. 2017. View Article : Google Scholar
|
|
33
|
Baumann S, Fas SC, Giaisi M, Müller WW,
Merling A, Gülow K, Edler L, Krammer PH and Li-Weber M: Wogonin
preferentially kills malignant lymphocytes and suppresses T-cell
tumor growth by inducing PLCgamma1- and Ca2+-dependent apoptosis.
Blood. 111:2354–2363. 2008. View Article : Google Scholar
|
|
34
|
Zhou QM, Wang S, Zhang H, Lu YY, Wang XF,
Motoo Y and Su SB: The combination of baicalin and baicalein
enhances apoptosis via the ERK/p38 MAPK pathway in human breast
cancer cells. Acta Pharmacol Sin. 30:1648–1658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao J, Morgan WA, Sanchez-Medina A and
Corcoran O: The ethanol extract of Scutellaria baicalensis and the
active compounds induce cell cycle arrest and apoptosis including
upregulation of p53 and Bax in human lung cancer cells. Toxicol
Appl Pharmacol. 254:221–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ling Y, Chen Y, Chen P, Hui H, Song X, Lu
Z, Li C, Lu N and Guo Q: Baicalein potently suppresses angiogenesis
induced by vascular endothelial growth factor through the p53/Rb
signaling pathway leading to G1/S cell cycle arrest. Exp Biol Med
(Maywood). 236:851–858. 2011. View Article : Google Scholar
|
|
37
|
Chen CH, Huang TS, Wong CH, Hong CL, Tsai
YH, Liang CC, Lu FJ and Chang WH: Synergistic anti-cancer effect of
baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem
Toxicol. 47:638–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dou J, Wang Z, Ma L, Peng B, Mao K, Li C,
Su M, Zhou C and Peng G: Baicalein and baicalin inhibit colon
cancer using two distinct fashions of apoptosis and senescence.
Oncotarget. 9:20089–20102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tian Y, Zhen L, Bai J, Mei Y, Li Z, Lin A
and Li X: Anticancer effects of baicalein in pancreatic
neuroendocrine tumors in vitro and in vivo. Pancreas. 46:1076–1081.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sachita K, Yu HJ, Yun JW, Lee JS and Cho
SD: YM155 induces apoptosis through downregulation of specificity
protein 1 and myeloid cell leukemia-1 in human oral cancer cell
lines. J Oral Pathol Med. 44:785–791. 2015. View Article : Google Scholar
|
|
41
|
Cho JH, Lee RH, Jeon YJ, Shin JC, Park SM,
Choi NJ, Seo KS, Yoon G, Cho SS, Kim KH, et al: Role of
transcription factor Sp1 in the 4-O-methylhonokiol-mediated
apoptotic effect on oral squamous cancer cells and xenograft. Int J
Biochem Cell Biol. 64:287–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Druzgal CH, Chen Z, Yeh NT, Thomas GR,
Ondrey FG, Duffey DC, Vilela RJ, Ende K, McCullagh L, Rudy SF, et
al: A pilot study of longitudinal serum cytokine and angiogenesis
factor levels as markers of therapeutic response and survival in
patients with head and neck squamous cell carcinoma. Head Neck.
27:771–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bond M, Fabunmi RP, Baker AH and Newby AC:
Synergistic upregulation of metalloproteinase-9 by growth factors
and inflammatory cytokines: An absolute requirement for
transcription factor NF-kappa. B FEBS Lett. 435:29–34. 1998.
View Article : Google Scholar
|
|
44
|
Mburu YK, Egloff AM, Walker WH, Wang L,
Seethala RR, van Waes C and Ferris RL: Chemokine receptor 7 (CCR7)
gene expression is regulated by NF-κB and activator protein 1 (AP1)
in metastatic squamous cell carcinoma of head and neck (SCCHN). J
Biol Chem. 287:3581–3590. 2012. View Article : Google Scholar
|
|
45
|
Lee E, Park HR, Ji ST, Lee Y and Lee J:
Baicalein attenuates astroglial activation in the
1-methyl-4-phenyl-1,2,3,4-tetrahydro-pyridine-induced Parkinson's
disease model by downregulating the activations of nuclear
factor-κB, ERK, and JNK. J Neurosci Res. 92:130–139. 2014.
View Article : Google Scholar
|
|
46
|
Sahu BD, Mahesh Kumar J and Sistla R:
Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney
injury by up-regulating antioxidant defenses and down-regulating
the MAPKs and NF-κB Pathways. PloS One. 10:e01341392015. View Article : Google Scholar
|
|
47
|
Wan CX, Xu M, Huang SH, Wu QQ, Yuan Y,
Deng W and Tang QZ: Baicalein protects against endothelial cell
injury by inhibiting the TLR4/NFkB signaling pathway. Mol Med Rep.
17:3085–3091. 2018.
|
|
48
|
Yu X, Liu Y, Wang Y, Mao X, Zhang Y and
Xia J: Baicalein induces cervical cancer apoptosis through the
NF-κB signaling pathway. Mol Med Rep. 17:5088–5094. 2018.PubMed/NCBI
|
|
49
|
Yu X, Tang W, Yang Y, Tang L, Dai R, Pu B,
Feng C and Xia J: Long noncoding RNA NKILA enhances the anti-cancer
effects of baicalein in hepatocellular carcinoma via the regulation
of NF-κB signaling. Chem Biol Interactions. 285:48–58. 2018.
View Article : Google Scholar
|