1. Introduction
Osteosarcoma (OS) is the most frequent primary
malignant bone tumor that predominantly occurs in children and
adolescents, and accounts for ~15% of all bone malignancies
(1,2). Its predilection sites include distal
femur (43%), proximal tibia (23%) and humerus (10%). Since
chemotherapy was introduced in the 1970s, the 5-year survival rate
for OS has markedly improved from <20 to 70% (3). Doxorubicin, cisplatin and
methotrexate are the most commonly used chemotherapy drugs in the
treatment of OS (4). Despite great
advances in chemotherapy for OS, survival rates have reached a
plateau and remained unsatisfactory during the past three decades
(5). Drug resistance is one of the
main reasons contributing to this (6); 35-45% of OS patients are not
sensitive to chemotherapy drugs, with their 5-year survival rate at
only 5-20% (7,8). Chemoresistance often leads to
treatment failure and poor prognosis. It has become a major
obstacle to improving OS treatment. Therefore, elucidating the
underlying molecular mechanisms implicated in OS chemoresistance is
urgently required.
Autophagy, initially discovered by Ohsumi in 1992
(who received the 2016 Nobel Prize in Physiology or Medicine for
his outstanding contributions to the field), is a catabolic process
via which cells eliminate and recycle their own damaged proteins
and organelles to provide energy (9). There are three types of autophagy,
including microautophagy, macroautophagy and chaperone-mediated
autophagy (10). The difference
between these autophagic processes is the substrates delivered to
the lysosomes (11).
Microautophagy refers to the direct engulfment of cytoplasmic
material by lysosomes for degradation. It can be activated by
signaling molecules on the surface of damaged organelles, such as
mitochondria or peroxisomes, leading to the fusion of lysosomes
with these organelles (12).
Macroautophagy is the process during which damaged organelles are
first enclosed in double-membrane vesicles (also known as
autophagosomes) and then fused with lysosomes to become
autophagolysosomes (12).
Chaperone-mediated autophagy is selective for specific substrate
proteins containing a pentapeptide amino acid sequence, which can
be recognized by molecular chaperone and then carried into
lysosomes for degradation (10,12).
This review focused on macroautophagy (hereafter referred to as
autophagy).
The autophagic process can be mainly divided into 4
steps: i) Formation of the phagophore to wrap the damaged material;
ii) elongation and closure of the phagophore followed by
autophagosome generation; iii) fusion of autophagosomes and
lysosomes to form autolysosomes; and iv) content degradation and
recycling (9). Autophagy can be
triggered under stressful conditions, such as starvation, hypoxia
and cytotoxicity, to maintain cell survival by providing energy
(11). To date, >30
autophagy-related proteins (ATGs) have been found to participate in
autophagy regulation (9).
Autophagy is initiated by the UNC-51-like kinase (ULK1) complex
comprising ULK1/2, ATG13, ATG101 and focal adhesion kinase family
interacting protein of 200 kDa (FIP200), and the class III
phosphoinositide 3-kinase (PI3K) complex consisting of Beclin-1,
vacuolar protein sorting 34, p150, ultraviolet irradiation
resistance-associated gene, BAX-interacting factor-1, ATG14-like
protein and Run domain Beclin-1-interacting and cysteine-rich
domain-containing protein (9,10,13).
The ULK1 complex is negatively regulated by mammalian target of
rapamycin (mTOR) in nutrient-rich conditions; conversely, in
nutrient-deprived conditions, mTOR is inhibited and the ULK1
complex is then activated to induce autophagy (9). Autophagosome formation is controlled
by the ATG12 and LC3 conjugation systems. In the first system,
ATG12 and ATG5 are conjugated in the presence of ATG7 and ATG10.
ATG12-ATG5 conjugation then binds to ATG16 to form the
ATG12-ATG5-ATG16 complex (9). In
the second system, the protease ATG4 cleaves microtubule-associated
protein 1-light chain 3 (LC3; also known as ATG8) to LC3-I, which
is then conjugated with phosphatidylethanolamine and converted into
LC3-II (11). In this process,
LC3-II is translocated from the cytoplasm to the autophagosome
membrane. For that reason, LC3-II is considered an important marker
for autophagosomes. ATG2, ATG9 and ATG18 are involved in
autolysosome formation, and p62 and neighbor of BRCA1 protein in
degradation and recycling regulation (Fig. 1) (10).
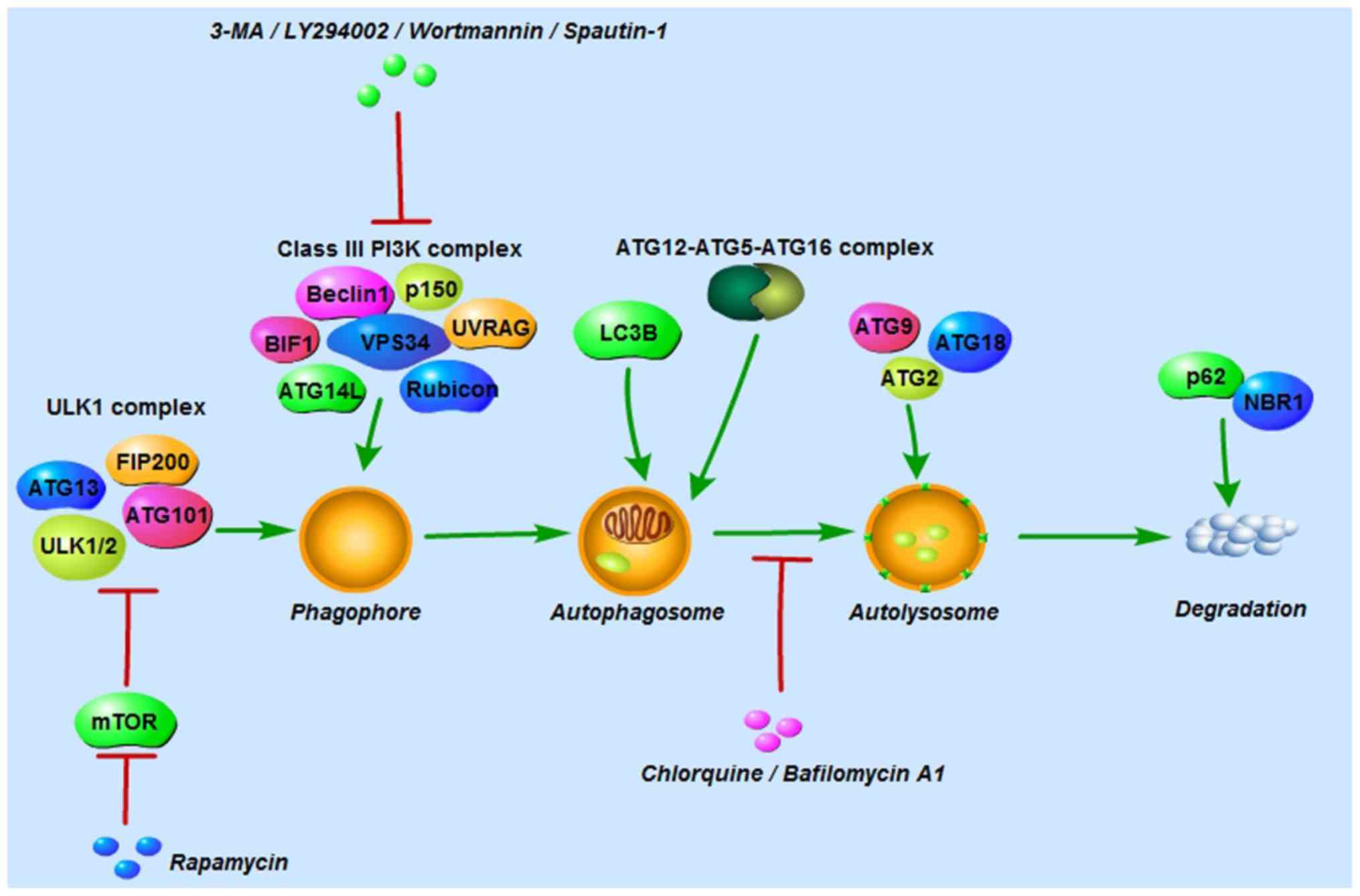 | Figure 1Autophagy-related proteins, and
autophagy inducers and inhibitors involved in the autophagic
process. Autophagy is initiated by the ULK1 complex and the class
III PI3K complex. The former is composed of ULK1/2, ATG13, ATG101
and FIP200, and the latter of Beclin-1, VPS34, p150, UVRAG, BIF1,
ATG14L and rubicon. Autophagosome formation is controlled by the
ATG12 and LC3 conjugation systems. ATG2, ATG9 and ATG18 are
involved in autolyso-some formation, and p62 and NBR1 in the
regulation of degradation and recycling. Rapamycin activates
autophagy by inhibiting mTOR, a negative regulator of the ULK1
complex. 3-MA, LY294002, wortmannin and spautin-1 suppress early
autophagy by inhibiting the class III PI3K complex. Chloroquine and
bafilomycin A1 inhibit late autophagy by blocking the fusion of
autophagosomes and lysosomes. ULK1, UNC-51-like kinase; ATG,
autophagy-related protein; FIP200, focal adhesion kinase family
interacting protein of 200; VPS34, vacuolar protein sorting 34;
UVRAG, ultraviolet irradiation resistance-associated gene; BIF1,
BAX-interacting factor-1; ATG14L, ATG14-like protein; rubicon, Run
domain Beclin-1-interacting and cysteine-rich domain-containing
protein; mTOR, mammalian target of rapamycin; 3-MA,
3-methyladenine; NBR1, neighbor of BRCA1 protein. |
2. Methods for detecting autophagy
As autophagy is a dynamic multi-stage process, it is
necessary to identify whether autophagy occurs in stressful
conditions induced by chemotherapeutic agents, such as starvation,
hypoxia, and cytotoxicity, and which steps of autophagy, if any,
are affected. Given that LC3-II is the only protein marker for
autophagosomes, one of the key characteristics of autophagy, LC3-II
detection has been widely used in autophagy-related research.
However, it may yield opposite results only by analyzing the LC3-II
expression. For example, an increased LC3-II expression can either
represent increased autophagosome formation or reduction of
autophagosome degradation (14).
More and more methods of monitoring autophagy are being identified.
Transmission electron microscopy (TEM), first used to detect
autophagy in the 1950s, is now considered the golden standard for
autophagy detection, as it is the only tool to morphologically
observe the ultrastructure of autophagosomes in the nm range
(14). Two autophagic vacuoles,
initial autophagic vacuole (AVi) and degradative autophagic vacuole
(AVd), can be observed in a TEM image. The defining structure of
AVi, also referred to as autophagosomes, is that intact organelles
are sequestrated by a special double-membrane structure (14). And the defining structure of AVd,
also referred to as autolysosomes, is that degraded organelles are
sequestrated by only one limiting membrane (14). However, the limitation of TEM is
that it can only statically observe a certain stage of autophagy
rather than the entire process. Therefore, greater attention has
been paid to autophagic flux monitoring. The utilization of tandem
monomeric red fluorescent protein (mRFP)-green fluorescent protein
(GFP)-LC3 via confocal microscopy is one of the most utilized
approaches in autophagic flux monitoring (14). This method is based on the
principle that GFP signal is quenched in the acid environment of
lysosomes, whereas RFP is stable. To be specific, when
autophagosomes have not yet been fused with lysosomes, GFP and RFP
fluorescence are colocalized in autophagosomes displaying yellow
dots. When autophagosomes fuse with lysosomes to form
autolysosomes, only RFP fluorescence is localized in autolysosomes
(14). In order to improve
understanding of autophagy, experts in autophagy from all over the
world published the 3rd edition of the guidelines for monitoring
autophagy in 2016 (14). In
addition to the methods mentioned above, several other assays were
introduced in this edition. They strongly recommend that multiple
assays should be used to monitor autophagy instead of one (14).
3. Autophagy inducers and inhibitors
Autophagy inducers and inhibitors are indispensable
in the regulation of autophagy. The most commonly used inducers are
rapamycin and its analogs, including temsirolimus, everolimus and
deforolimus, which activate autophagy by inhibiting mTOR, a
negative regulator of autophagy (11). As autophagy can be blocked at
different stages, a large number of inhibitors have been used in
different mechanisms. At an early stage, 3-methyladenine (3-MA),
LY294002 and wortmannin can suppress autophagy by inhibiting class
III PI3K (15). Another novel PI3K
inhibitor, spautin-1 has been shown to degrade the class III PI3K
complex via Beclin-1 (15). It was
demonstrated by Schott et al (16) that pre-treatment with spautin-1
enhanced the canine OS cell inhibition induced by doxorubicin. At a
later stage, chloroquine and its derivatives (such as
hydroxychloroquine), which were originally used as anti-malarial
drugs, are capable of preventing lysosomal acidification and
blocking the fusion of autophagosomes and lysosomes (10). Bafilomycin A1, an inhibitor of
vacuolar-type H+-ATPase, also prevents lysosome
acidification (Fig. 1) (15).
4. Dual role of autophagy in OS
chemoresistance
As autophagy can be triggered by chemotherapy drugs,
a growing number of studies have focused on the association between
autophagy and chemoresistance in tumor cells (11,16).
Of note, autophagy has been shown to play a dual role in cancer;
either tumor-promoting or tumor-suppressing. On the one hand,
autophagy helps tumor cells survive in the presence of chemotherapy
drugs by eliminating its own damaged organelles and proteins
(17). On the other hand,
excessive autophagy ultimately leads to cell death (17). This double-edged sword effect of
autophagy was observed by O'Farrill and Gordon (11), who found that autophagy inhibition
resulted in increased sensitivity of LM7 metastatic human OS cells
to gemcitabine, but decreased sensitivity in K7M3 metastatic murine
OS cells. Consistent with the above findings, Hollomon et al
(18) revealed that autophagy
inhibition via ATG5 knockdown reduced camptothecin-induced cell
death in DLM8 metastatic murine OS cells but increased it in K7M3
cells. These contradictory outcomes largely depend on the stage and
type of tumor (10).
In OS, accumulating evidence has indicated that
autophagy plays a crucial role in chemoresistance, either by
promoting drug resistance or increasing drug sensitivity. Various
oncogenic and tumor-suppressing genes have been confirmed to
regulate OS chemoresistance via autophagy activation or inhibition.
In autophagy-related OS chemoresistance, autophagy can act as
either a cytoprotective process or autophagic cell death (Fig. 2).
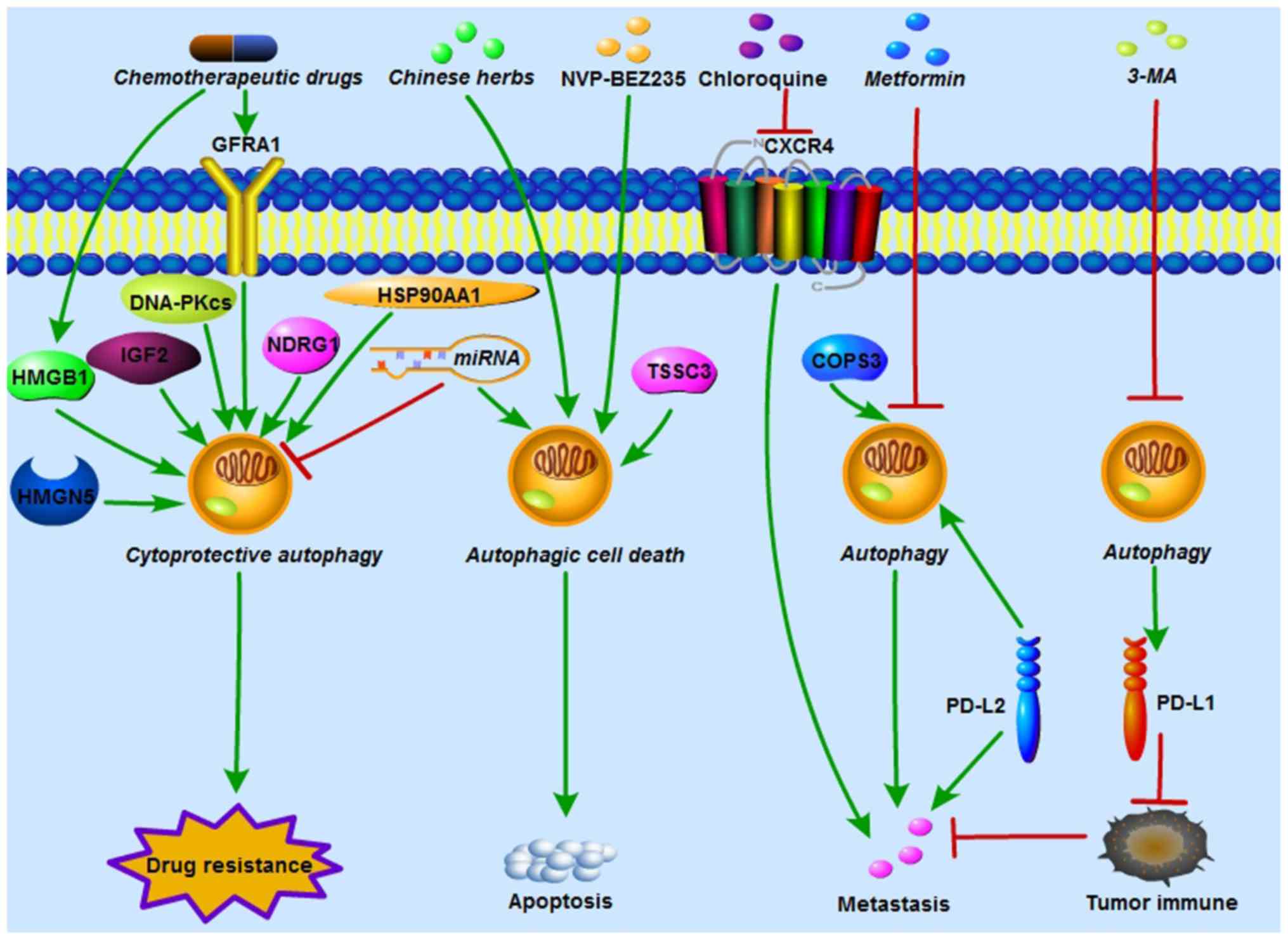 | Figure 2Autophagy regulates OS
chemoresistance, metastasis and tumor immunity. HMGB1, GFRA1,
HMGN5, IGF2, DNA-PKcs, NDRG1 and HSP90AA1 induced by
chemotherapeutic drugs activate cytoprotective autophagy and
contribute to chemoresistance in OS. In addition, miRNAs increase
OS chemosensitivity by either inhibiting cytoprotective autophagy
or inducing autophagic cell death. NVP-BEZ235 (a PI3K/mTOR
inhibitor), TSSC3 and certain Chinese herbs enhance
chemosensitivity in OS by increasing apoptosis which is dependent
of autophagic cell death. COPS3 knockdown and metformin reduce
autophagy-mediated metastasis in OS. Polymeric chloroquine
decreased CXCR4-mediated OS metastasis, and this effect was
autophagy-independent. PD-L1 suppression by 3-MA and PD-L2
knockdown enhanced immunological response and inhibited OS
metastasis. HMGB1, High mobility group box 1; GFRA1, GDNF receptor
α1; HMGN5, high-mobility group nucleosome-binding domain 5; IGF2,
insulin growth factor 2; DNA-PKcs, DNA-dependent protein kinase
catalytic subunit; miRNA, microRNA; NDRG1, N-myc
downstream-regulated gene 1; HSP90AA1, heat shock protein 90AA1;
OS, osteosarcoma; TSSC3, tumor-suppressing STF cDNA 3; COPS3, COP9
signalosome subunit 3; CXCR4, chemokine receptor 4; PD-L,
programmed death ligand; 3-MA, 3-methyladenine. |
Autophagy acts as a cytoprotective
process contributing to OS chemoresistance
Directly targeting autophagy with either ATG
silencing or autophagy modulators is a commonly used method to
determine autophagy-mediated OS chemoresistance. Silencing of
ATG14, also termed Beclin-1-associated autophagy-related key
regulator, increased cisplatin-induced apoptosis in SaOS-2 cells
(19). Beclin-1 inhibition
enhanced the sensitivity of both MG63 and cisplatin-resistant MG63
cells to cisplatin in vitro and in vivo (20). Autophagy inhibition with
chloroquine triggered apoptotic cell death in SaOS-2 cells which
were resistant to cisplatin (21).
Inhibition of autophagy via either ATG7 small interfering (si)RNA
or 3-MA enhanced doxorubicin cytotoxicity in U2OS and SaOS-2 cells
(22). It was reported by Zhou
et al (23) that celecoxib,
a selective cyclo-oxygenase-2 inhibitor, exerted an antitumor
effect on 143B and U2OS cells. ATG5 silencing, and autophagy
inhibitors chloroquine or SAR405 further enhanced cell
proliferation inhibition and celecoxib-induced apoptosis. Guo et
al (24) observed that
rapamycin, an autophagy inducer, decreased paclitaxel-induced
apoptosis in MG63. On the contrary, pretreatment with 3-MA, an
autophagy inhibitor, increased MG63 apoptosis induced by
paclitaxel. It was first revealed by Liu et al (25) that apatinib, a highly selective
inhibitor of vascular endothelial growth factor receptor-2, induced
OS cells apoptosis and autophagy. In addition, autophagy inhibition
via 3-MA markedly enhanced apatinib-induced apoptosis in KHOS
cells.
In addition to directly modulating autophagy as
mentioned above, several upstream target genes and signaling
pathways have been demonstrated to regulate autophagy-mediated OS
chemoresistance (Table I).
 | Table IAutophagy acts as a cytoprotective
process contributing to OS chemoresistance. |
Table I
Autophagy acts as a cytoprotective
process contributing to OS chemoresistance.
| First author,
year | Target
gene/signaling pathway | Autophagy | Alteration | OS cell lines | Chemotherapeutic
agents | Resistance | Reference |
|---|
| Huang, 2012 and
2012 | HMGB1 |
Beclin-1-PI3KC3 | | | | | |
|
ULK1-Matg13-FIP200 | ↑ | MG-63, SaOS-2,
U-2OS | DOX, CDDP and
MTX | ↑ | (26,27) |
| Kim, 2017 and
2018 | GFRA1 | Beclin-1,
LC3-II | ↑ | MG-63 | CDDP | ↑ | (29,30) |
| Meng, 2016 | miR-140-5p | LC3-II | ↑ | SaOS-2 | DOX, CDDP | ↑ | (33) |
| Wei, 2017 | miR-140-5p | Beclin-1, ATG5,
LC3-II | ↓ | HOS, U-2OS
MG-63 | DOX, CDDP and
MTX | ↓ | (34) |
| Chen, 2014 | miR-155 | LC3-II, ATG5 | ↑ | SaOS-2 | DOX, CDDP | ↑ | (35) |
| Xu, 2016 | miR-30a | Beclin-1,
LC3-II | ↓ | MG-63/Dox-resistant
cells | DOX, CDDP and
MTX | ↓ | (31) |
| Chen, 2017 | miR-410 | LC3-II,
ATG16L1 | ↓ | U-2OS, MG-63 | DOX, CDDP and
RAPA | ↓ | (32) |
| Guo, 2014 | miR-22 | LC3-II, ATG7 | ↓ | MG-63 | DOX, CDDP | ↓ | (37) |
| Li, 2014 | miR-22 | LC3-II, ATG7 | ↓ | U-2OS, MG-63 | DOX, CDDP | ↓ | (38) |
| Wang, 2019 | miR-22 | Beclin-1, LC3-II,
ATG5 | ↓ | MG-63 | CDDP | ↓ | (39) |
| Chang, 2014 | miR-101 | LC3-II, ATG5 | ↓ | U-2OS | DOX | ↓ | (40) |
| Zhou, 2015 | miR-143 | LC3-II, ATG2B | ↓ |
SaOS-2/Dox-resistant cells,
U-2OS/Dox-resistant cells | DOX | ↓ | (41) |
| Li, 2016 | miR-199a-5p | Beclin-1,
LC3-II | ↓ | MG-63 | CDDP | ↓ | (42) |
| Yang, 2014 | HMGN5 | Beclin-1,
LC3-II | ↑ | U-2OS, MG-63 | DOX, CDDP and
MTX | ↑ | (47) |
| Shimizu, 2014 | IGF2 | LC3-II, ATG7 | ↑ | SaOS-2, U-2OS | DOX, CDDP | ↑ | (48) |
| Zhen, 2016 | DNA-PKcs | Beclin-1,
LC3-II | ↑ | U-2OS, MG-63 | Salinomycin | ↑ | (49) |
| Wang, 2017 | NDRG1 | LC3-II | ↑ | MG-63.2 | CA-4 | ↑ | (50) |
| Xiao, 2018 | HSP90AA1 | LC3-II | ↑ | MG-63 | DOX, CDDP | ↑ | (51) |
| Tao, 2017 | Wnt/β-catenin | Beclin-1 | ↓ | MG-63 | Gemcitabine | ↓ | (52) |
| Mukherjee,
2017 | JNK | LC3-II, ATG5,
ATG12 | ↓ | HOS | CDDP | ↓ | (53) |
| Zhang, 2017 | JNK | ATG5 | ↑ | MG-63 | Curcumin | ↑ | (54) |
| Guan, 2016 | Caveolin-1,
PI3K-Akt-JNK | Beclin-1, LC3-II,
ATG5, ATG7 | ↓ |
SaOS-2/Taxol-resistant cells,
U-2OS/Taxol-resistant cells | Taxol | ↓ | (55) |
High mobility group box 1 (HMGB1)
HMGB1, a chromatin-binding nuclear protein with 215
amino acid residues, is composed of three different domains: An A
box, B box and C-terminal acidic tail (26,27).
It can localize in the nucleus, cytoplasm and cell surface, and it
can be released extracellularly. Different forms of HMGB1 exhibit
different functions. For example, nuclear HMGB1 regulates DNA
replication, recombination, transcription and repair, and sustains
genomic stability (28).
Cytoplasmic HMGB1 contributes to cell motility and autophagy. Cell
surface HMGB1 is associated with neurite outgrowth and platelet
activation (28). Extracellular
HMGB1 is implicated in cancer cell activation, inflammation
progression and apoptosis of monocyte-lineage and immune cells
(28). When it comes to
HMGB1-mediated autophagy in OS, it was first reported by Huang
et al (26,27) that HMGB1 overexpres-sion induced
autophagy by regulating Beclin-1-PI3K catalytic subunit 3 and
ULK1-mATG13-FIP200 complex formation, and increased the drug
resistance of MG-63, SaOS-2 and U-2OS cells to doxorubicin,
cisplatin and methotrexate. Conversely, the suppression of HMGB1 by
short hairpin (sh) RNA inhibited autophagy and enhanced sensitivity
to these chemotherapeutic agents.
Glial cell line-derived neurotrophic
factor (GDNF) receptor α1 (GFRA1)
The GDNF family, consisting of GDNF, neurturin,
artemin and persephin, plays a crucial role in the development and
maintenance of the nervous system (29,30).
GFRA1 is the receptor of GDNF, and the binding of GFRA1 with GDNF
promotes neuronal cell survival and differentiation (29,30).
Of note, it was found by Kim et al (29,30)
that GFRA1-mediated autophagy was also implicated in OS cisplatin
resistance. They demonstrated that GFRA1 was significantly
upregulated in the presence of cisplatin, but not doxorubicin and
methotrexate in two OS cell lines (MG-63 and U-2OS). In addition,
GFRA1 induced autophagy in MG-63 cells by activating SRC-AMPK
signaling following cisplatin treatment.
microRNAs (miRNAs/miRs)
miRNAs are a class of small non-coding RNAs (18-25
nucleotides) that can negatively regulate gene expression by
binding to the 3′-untranslated region (3′-UTR) of their target
mRNAs, and modulate mRNA and protein expression at the
post-transcriptional level (31).
The dysregulation of miRNAs has been identified in the
carcinogenesis of various malignancies, including OS (32). Recently, they have emerged as key
regulators of OS chemosensitivity or chemotherapy resistance by
targeting autophagy; notably, certain miRNAs have been shown to
lead to contradictory outcomes due to their dual role in OS
chemo-resistance. For example, miR-140-5p functioned as a tumor
promoter and was clearly upregulated in SaOS-2 and MG-63 cells
following doxorubicin and cisplatin treatment (33). miR-140-5p overexpression induced
autophagy, as confirmed by increased GFP-LC3 puncta and LC3-II, and
decreased p62, which contributed to OS chemoresistance. Conversely,
it was revealed by another study (34) that miR-140-5p serves as a tumor
suppressor and is downregulated in 40 clinical OS tissues and three
OS cell lines (HOS, U-2OS and MG63). Overexpression of miR-140-5p
increased the sensitivity of OS cells to doxorubicin, cisplatin and
methotrexate by inhibiting autophagy, as detected by TEM, confocal
microscopy and western blotting. In addition, certain miRNAs
contribute to OS chemoresistance not only via autophagy activation,
but also by inhibiting autophagy. One study demonstrated that
miR-155 promoted OS chemoresistance by inducing autophagy (35). Conversely, miR-155 inhibited
autophagy by regulating the PTEN-PI3K/AKT/mTOR pathway, and
enhanced resistance of MG63 cells to doxorubicin in another study
(36).
The majority of miRNAs that function as tumor
suppressors increase OS chemosensitivity by negatively regulating
autophagy. Xu et al (31)
found that miR-30a was down-regulated, while ATGs Beclin-1 and
LC3-II were increased in doxorubicin-resistant MG-63 cells.
Furthermore, miR-30a overexpression enhanced OS chemosensitivity by
suppressing Beclin-1-mediated autophagy, which could be partly
reversed by rapamycin, an autophagy activator. Chen et al
(32) observed that miR-410
sensitized U-2OS and MG-63 cells to doxorubicin and cisplatin via
ATG16L1 inhibition. Certain studies have shown that miR-22
increases OS chemosensitivity by inhibiting HMGB1-mediated
autophagy (37,38). Consistent with these findings,
miR-22 can also sensitize MG-63 cells to cisplatin via
metadherin-mediated autophagy (39). miR-101 blocks doxorubicin-induced
autophagy and enhance U-2OS cell chemosensitivity (40). miR-143 was found to reverse
chemoresistance in SaOS-2 and U-2OS doxorubicin-resistant cells
through the inhibition of autophagy (41). miR-199a-5p was reported to reduce
the resistance of MG-63 cells to cispl-atin by inhibiting
autophagy, as indicated by the decreased expression of LC3-II and
Beclin-1 (42). Long non-coding
RNA (LncRNA) small nucleolar RNA host gene 15 was found to increase
proliferation, invasion, migration and autophagy in MG-63 cells by
negatively regulating miR-141 (43). LncRNA CTA was reported to reduce
doxorubicin resistance in SaOS-2, MG-63 and doxorubicin-resistant
MG-63 cells by suppressing miR-210 and autophagy (44).
In contrast with the aforementioned studies, Yu
et al (45) revealed that
miR-100 and Beclin-1 expression levels were markedly reduced in
cisplatin-resistant MG-63 cells, compared with their sensitive
counterparts, and miR-100 upregulation enhanced cisplatin-induced
apoptosis via mTOR inhibition and autophagy activation. Similar to
their findings, it was confirmed by Wu et al (46) that miR-145-3p overexpression
promoted apoptosis and autophagy in U-2OS and MG-63 cells by
negatively regulating histone deacetylase 4.
Certain other genes are also implicated in OS
chemoresistance via autophagy. High-mobility group
nucleosome-binding domain 5 was required for OS chemoresistance by
upregulating autophagy (47).
Insulin growth factor 2 was shown to maintain OS cell survival in
the presence of chemotherapeutic drugs by activating autophagy.
Blocking autophagy with chloroquine or bafilomycin A restored
chemosensitivity (48). Zhen et
al (49) demonstrated that
DNA-dependent protein kinase catalytic subunit (DNA-PKcs) was
involved in autophagy-mediated sali-nomycin resistance in OS cells.
The knockdown of DNA-PKcs by its inhibitors, shRNA and miR-101,
reduced salinomycin resistance by inhibiting autophagy. Wang et
al (50) found that N-myc
downstream-regulated gene 1 (NDRG1) was associated with OS
chemoresistance. Combretastatin A-4 (CA-4), a
tubulin-depoly-merizing agent with antitumor effects, activated
cytoprotective autophagy in OS. A synergistic cytotoxic effect was
observed when CA-4 was combined with chloroquine. Furthermore,
NDRG1 inhibition by siRNA enhanced the sensitivity of OS cells to
CA-4 by suppressing autophagosome-lysosome fusion (50). Heat shock protein 90AA1 (HSP90AA1)
was confirmed to regulate OS drug resistance via autophagy
(51). The overexpression of
HSP90AA1 promoted autophagy and led to increased resistance. This
pro-survival effect of HSP90AA1 could be reversed by 3-MA.
Conversely, the suppression of HSP90AA1 enhanced chemosensitivity
by inhibiting autophagy (51).
Signaling pathways
Accumulating evidence has indicated that the
regulation of autophagy-related signaling pathways is implicated in
OS chemoresistance. Wnt/β-catenin signaling pathway activation
enhanced sensitivity of MG-63 cells to gemcitabine by attenuating
Beclin-1-mediated autophagy (52).
Mukherjee et al (53)
observed that a negative feedback loop between the Jun N-terminal
kinase (JNK) pathway and autophagy, and the inhibition of both, led
to maximal cisplatin sensitivity in HOS cells. Similar to the
results of the present study, it was revealed by Zhang et al
(54) that the inhibition of
autophagy with 3-MA enhanced the apoptosis of MG63 cells induced by
curcumin, a chemotherapeutic drug derived from the rhizome of the
East Indian plant Curcuma longa. It was further confirmed
that this cell apoptosis promoted by 3-MA was dependent on the JNK
pathway. In order to investigate the association between caveolin-1
and taxol resistance, Guan et al (55) established taxol-resistant SaOS-2
and U-2OS cells by gradually increasing taxol concentration for 6
months. Reduced caveolin-1 expression and enhanced autophagy
activity were identified in taxol-resistant cells compared with
their parental cells. In addition, caveolin-1 overexpression
reduced taxol resistance by attenuating PI3K-Akt-JNK-dependent
autophagy.
Autophagy acts as autophagic cell death
reversing OS chemo-resistance
For a long time, autophagy has been considered to
have a crucial pro-survival effect on OS chemoresistance, as it can
maintain tumor cell growth in response to chemotherapeutic drugs by
eliminating and recycling its own damaged proteins and organelles
to provide energy (23,25). However, an increasing number of
studies have focused on the other primary outcome of autophagy:
Autophagic cell death characterized by excessive autophagy, one of
the three main forms of programmed cell death (PCD) (56,57).
The other two forms of PCD are apoptosis and programmed necrosis
(56,57). An intricate cross-talk between
apoptosis and autophagy is most widely discussed in OS
chemoresistance-related studies (56,57).
Autophagic cell death, different from cytoprotective autophagy, can
be increased by autophagy activation or decreased by autophagy
inhibition.
Autophagy induced by rapamycin inhibits the
proliferation of SaOS-2 and U-2OS in vitro and tumor growth
in mice xenograft models in vivo (58). Tumor-suppressing STF cDNA 3-induced
autophagy was found to be indispensable for the suppression of OS
tumorigenesis and metastasis in vitro and in vivo
(59). NVP-BEZ235, a PI3K/mTOR
inhibitor, increases cisplatin-induced apoptosis in U-2OS and
SaOS-2 cells by turning cytoprotective autophagy into pro-death
autophagy (60). Voacamine, a
bisindolic alkaloid extracted from Peschiera fuchsiaefolia,
enhances the chemosensitivity of doxorubicin-resistant U-2OS cells
by inducing autophagic cell death rather than apoptosis (61).
Recently, several Chinese herbs have been reported
to exert their antitumor effects on OS via autophagic cell death.
For example, Huang et al (62) indicated that honokiol, extracted
from Magnolia trees, inhibited HOS and U-2OS cell
proliferation by inducing both apoptosis and autophagy. They
further discovered that the honokiol-induced cell death was largely
dependent on autophagic cell death, as shown by the results that
honokiol-induced cell death was more clearly reversed by 3-MA
compared with Z-VAD-FMK, a widely used caspase inhibitor. Autophagy
induced by tanshinone IIA, isolated from the herb Salvia
miltiorrhiza, was reported to be cytotoxic to 143B cells
(63). Brazilin, purified from
Biancaea sappan wood, induces autophagic cell death in MG-63
cells (64). Liu et al
(65) suggested that
andrographolide reduced MG-63 and U-2OS cell viability by inducing
autophagy, but not apoptosis. In addition, the inhibition of
autophagy via 3-MA and Beclin-1 silencing could rescue the
cytotoxic effects of andrographolide, indicating that autophagic
cell death contributed to the tumor-suppressing effect of
andrographolide. Furthermore, marrubenol, escin and chamaejasmine
can also inhibit OS by inducing autophagic cell death (66-69).
Surprisingly, different active ingredients from the same herb can
induce opposing autophagy functions in the same OS cell line by
activating the same pathway. Cytoprotective autophagy and
autophagic cell death were induced by curcumin and curcumol,
respectively, in MG-63 cells via the JNK pathway (Table II) (54,70).
 | Table IIAutophagy acts as autophagic cell
death, reversing OS chemoresistance. |
Table II
Autophagy acts as autophagic cell
death, reversing OS chemoresistance.
| First author,
year | Autophagy
inducers/Chinese herbs | Autophagy | Alteration | OS cell lines | Chemotherapeutic
agents |
Sensitivity/autophagic cell death | Reference |
|---|
| Zhao, 2015 | Rapamycin | LC3-II | ↑ | SaOS-2, U-2OS | / | ↑ | (58) |
| Zhao, 2018 | TSSC3 | ATG5, LC3-II | ↑ | SaOS-2 | / | ↑ | (59) |
| Huang, 2018 | NVP-BEZ235 | LC3-II | ↑ | U-2OS, SaOS-2 | CDDP | ↑ | (60) |
| Meschini, 2007 | Voacamine |
Autophagosomes, | ↑ | U-2OS-R | DOX | ↑ | (61) |
| Huang, 2018 | Honokiol | ATG5, LC3-II | ↑ | HOS, U-2OS | / | ↑ | (62) |
| Yen, 2018 | Tanshinone IIA | LC3-II | ↑ | 143B | / | ↑ | (63) |
| Kang, 2018 | Brazilin | LC3-II, ATG5, ATG7,
ATG10, ULK1 | ↑ | MG-63 | / | ↑ | (64) |
| Liu, 2017 |
Andrographolide | ATG5, Beclin-1,
LC3-II | ↑ | MG-63, U-2OS | / | ↑ | (65) |
| Zhang, 2018 | Marrubenol | Beclin-1,
LC3-II | ↑ | SaOS-2 | / | ↑ | (66) |
| Liu, 2017; Zhu,
2017 | Escin | Beclin-1, LC3-II,
ATG5, ATG12 | ↑ | U-2OS, HOS,
SaOS-2 | / | ↑ | (67,68) |
| Yang, 2019 | Chamaejasmine | Beclin-1, LC3-II,
ATG7 | ↑ | MG-63 | / | ↑ | (69) |
| Zhang, 2017 | Curcumol | LC3-II | ↑ | MG-63 | / | ↑ | (70) |
5. Autophagy and metastasis
Metastasis (particularly lung metastasis), detected
in 13-27% of patients with OS at diagnosis and 40% at progressive
stage, is one of the main reasons contributing to unfavorable
prognosis (71). It is estimated
that ~30-40% of patients with OS show poor response to chemotherapy
due to metastasis (72). It has
been revealed by certain studies that autophagy is also implicated
in OS metastasis. Zhang et al (73) reported that COP9 signalosome
subunit 3 knockdown reduced OS metastasis by inhibiting Beclin-1.
In addition, both 3-MA and Beclin-1 silencing induced
anti-metastasis effects. It was reported by Bao et al
(72) that metformin, mainly used
in the treatment of type II diabetes, inhibited OS metastasis via
miR-570-3p-mediated suppression of lung cancer metastasis-related
protein 1 and ATG12. miR-506-3p reversed epithelial-to-mesenchymal
transition, which is closely associated with cancer metastasis, by
suppressing autophagy in OS cells (74). It has already been reported that
chemokine receptor 4 (CXCR4) is crucial for the regulation of OS
metastasis; in our previous study, it was found that CXCR4
inhibition with AMD3100 significantly reduces OS survival and
metastasis (75). Yu et al
(76) discovered that polymeric
chloroquine decreased CXCR4-mediated U-2OS cell metastasis by
promoting the internalization of surface CXCR4 receptors, which
made CXCR4 inaccessible for binding with its ligand, chemokine 12.
However, no change in LC3 expression was observed when cells were
treated with polymeric chloroquine, indicating that this
anti-metastasis effect was independent of autophagy. Whether CXCR4
influences autophagy in the regulation of OS chemoresistance and
metastasis remains largely unknown; this will be the focus of
future studies (Fig. 2).
6. Autophagy and immunotherapy
Recently, immunotherapy has emerged as a novel
therapeutic method for OS; due to their immune function, T-cells
can help kill cancer cells. The binding of programmed death
ligand-1 (PD-L1) to PCD protein-1 (PD-1) attenuates the anti-tumor
effects of T-cells, ultimately leading to tumor immune escape,
chemoresistance and metastasis (77,78).
Yu et al (77) indicated
that PD-L1 suppression via photodynamic therapy combined with the
autophagy inhibitor 3-MA enhanced the immune response, and
inhibited OS growth and metastasis in vitro and in
vivo. Similarly, Ren et al (78) revealed the pro-metastatic function
of PD-L2, another ligand of PD-1, in OS. In addition, PD-L2
knockdown was found to decrease OS migration and invasion by
inhibiting Beclin-1 expression (Fig.
2).
7. Autophagy as a prognostic marker in
OS
It is noteworthy that certain clinical studies have
explored whether autophagy could be used to predict treatment
response and survival rate in OS. Livingston et al (79) detected LC3B and HSP27 expression in
394 tumor samples, including pre-treatment, post-treatment and
metastatic samples from 260 OS patients via immunohistochemistry.
It was revealed that the percentage of LC3B-positive samples in the
pre-treatment, post-treatment and metastatic groups were 34, 50 and
67%, respectively. Furthermore, patients with positive LC3B and
negative HSP27 expression exhibited the highest 10-year survival
rate (75%), whereas those with negative LC3B and positive HSP27
expression the worst (25%), indicating that LC3B and HSP27 were
associated with favorable and poor outcomes in OS, respectively. Lu
et al (80) demonstrated
that p62 was detected in 54/70 OS samples (77.1%), and that its
overexpression was associated with tumor size, metastasis, clinical
stage and poor prognosis. Conversely, Ma et al (81) discovered that the 5-year survival
rate of patients with OS with low p62 expression was lower than
that of patients with high p62 expression, suggesting that
decreased p62 expression was associated with higher metastasis and
chemotherapy resistance rates in OS.
8. Conclusion
Chemoresistance is one of the most important factors
contributing to treatment failure and poor prognosis in OS.
Autophagy, a catabolic process via which cells eliminate and
recycle their own damaged proteins and organelles to provide
energy, can be activated by chemotherapeutic drugs. Accumulating
evidence indicates that autophagy serves a dual role in the
regulation of OS chemoresistance, by either exerting cytoprotection
or causing autophagic cell death. Therefore, both the elimination
of cytoprotective autophagy and the stimulation of autophagic cell
death could enhance OS chemosensitivity. In addition, autophagy is
also implicated in OS metastasis, immunotherapy and clinical
prognosis. It is anticipated that targeting autophagy may be a
promising therapeutic strategy for OS.
Abbreviations:
|
OS
|
osteosarcoma
|
|
ATGs
|
autophagy-related proteins
|
|
ULK1
|
UNC-51-like kinase
|
|
FIP200
|
focal adhesion kinase family
interacting protein of 200 kDa
|
|
mTOR
|
mammalian target of rapamycin
|
|
LC3
|
light chain 3
|
|
TEM
|
transmission electron microscopy
|
|
AVi
|
initial autophagic vacuole
|
|
AVd
|
degradative autophagic vacuole
|
|
3-MA
|
3-methyladenine
|
|
PI3K
|
class III phosphoinositide
3-kinase
|
|
HMGB1
|
high mobility group box 1
|
|
GDNF
|
glial cell line-derived neurotrophic
factor
|
|
GFRA1
|
GDNF receptor α1
|
|
miRNAs/miRs
|
microRNAs
|
|
DNA-PKcs
|
DNA-dependent protein kinase catalytic
subunit
|
|
NDRG1
|
N-myc downstream-regulated gene 1
|
|
CA-4
|
combretastatin A-4
|
|
HSP90AA1
|
heat shock proteins 90AA1
|
|
JNK
|
Jun N-terminal kinase
|
|
PCD
|
programmed cell death
|
|
CXCR4
|
chemokine receptor 4
|
|
PD-L1
|
programmed death ligand-1
|
|
PD-1
|
programmed cell death protein-1
|
|
HSP27
|
heat shock protein 27
|
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YXL was involved in designing the study, literature
review and drafting of the manuscript. YXL was also responsible for
designing the figures. HYY, JYL, YRC and FL participated in
acquisition and analysis of data, and discussion of the manuscript.
ZMH and SSH designed the study and revised the manuscript
critically. All of the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morrow JJ, Bayles I, Funnell APW, Miller
TE, Saiakhova A, Lizardo MM, Bartels CF, Kapteijn MY, Hung S,
Mendoza A, et al: Positively selected enhancer elements endow
osteosarcoma cells with metastatic competence. Nat Med. 24:176–185.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu J, Wang H, Hu Y, Zhang YS, Wen L, Yin
F, Wang Z, Zhang Y, Li S, Miao Y, et al: Inhibition of CaMKIIα
activity enhances antitumor effect of fullerene C60 nanocrystals by
suppression of autophagic degradation. Adv Sci (Weinh).
6:18012332019. View Article : Google Scholar
|
|
6
|
Chen R, Wang G, Zheng Y, Hua Y and Cai Z:
Drug resistance-related microRNAs in osteosarcoma: Translating
basic evidence into therapeutic strategies. J Cell Mol Med.
23:2280–2292. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Duan G and Feng S: MicroRNA-301a
modulates doxorubicin resistance in osteosarcoma cells by targeting
AMP-activated protein kinase alpha 1. Biochem Biophys Res Commun.
459:367–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment - where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar
|
|
9
|
Camuzard O, Santucci-Darmanin S, Carle GF
and Pierrefite-Carle V: Role of autophagy in osteosarcoma. J Bone
Oncol. 16:1002352019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar A, Singh UK and Chaudhary A:
Targeting autophagy to overcome drug resistance in cancer therapy.
Future Med Chem. 7:1535–1542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Farrill JS and Gordon N: Autophagy in
osteosarcoma. Adv Exp Med Biol. 804:147–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagelkerke A, Sweep FC, Geurts-Moespot A,
Bussink J and Span PN: Therapeutic targeting of autophagy in
cancer. Part I: Molecular pathways controlling autophagy. Semin
Cancer Biol. 31:89–98. 2015. View Article : Google Scholar
|
|
14
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagelkerke A, Bussink J, Geurts-Moespot A,
Sweep FC and Span PN: Therapeutic targeting of autophagy in cancer.
Part II: Pharmacological modulation of treatment-induced autophagy.
Semin Cancer Biol. 31:99–105. 2015. View Article : Google Scholar
|
|
16
|
Schott CR, Ludwig L, Mutsaers AJ, Foster
RA and Wood GA: The autophagy inhibitor spautin-1, either alone or
combined with doxorubicin, decreases cell survival and colony
formation in canine appendicular osteosarcoma cells. PLoS One.
13:e02064272018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hollomon MG, Gordon N, Santiago-O'Farrill
JM and Kleinerman ES: Knockdown of autophagy-related protein 5,
ATG5, decreases oxidative stress and has an opposing effect on
camptothecin-induced cytotoxicity in osteosarcoma cells. BMC
Cancer. 13:5002013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Z, Tao L, Shen C, Liu B, Yang Z and
Tao H: Silencing of Barkor/ATG14 sensitizes osteosarcoma cells to
cisplatin induced apoptosis. Int J Mol Med. 33:271–276. 2014.
View Article : Google Scholar
|
|
20
|
Wu W, Li W, Zhou Y and Zhang C: Inhibition
of beclin1 affects the chemotherapeutic sensitivity of
osteosarcoma. Int J Clin Exp Pathol. 7:7114–7122. 2014.PubMed/NCBI
|
|
21
|
Shen C, Wang W, Tao L, Liu B, Yang Z and
Tao H: Chloroquine blocks the autophagic process in
cisplatin-resistant osteosarcoma cells by regulating the expression
of p62/SQSTM1. Int J Mol Med. 32:448–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu ZM, Li CB, Liu QL, Li P and Yang H:
Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through
the inhibition of autophagy and endoplasmic reticulum stress in
mice. Int J Mol Sci. 19:192018. View Article : Google Scholar
|
|
23
|
Zhou P, Li Y, Li B, Zhang M, Xu C, Liu F,
Bian L, Liu Y, Yao Y and Li D: Autophagy inhibition enhances
celecoxib-induced apoptosis in osteosarcoma. Cell Cycle.
17:997–1006. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Y, Huang C, Li G, Chen T, Li J and
Huang Z: Paxilitaxel induces apoptosis accompanied by protective
autophagy in osteosarcoma cells through hypoxia-inducible factor-1α
pathway. Mol Med Rep. 12:3681–3687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012. View Article : Google Scholar
|
|
27
|
Huang J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L, Tang D and Ni J: Targeting HMGB1-mediated
autophagy as a novel therapeutic strategy for osteosarcoma.
Autophagy. 8:275–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pistoia V and Pezzolo A: Involvement of
HMGB1 in resistance to tumor vessel-targeted, monoclonal
antibody-based immunotherapy. J Immunol Res. 2016:31423652016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim M, Jung JY, Choi S, Lee H, Morales LD,
Koh JT, Kim SH, Choi YD, Choi C, Slaga TJ, et al: GFRA1 promotes
cisplatin-induced chemoresistance in osteosarcoma by inducing
autophagy. Autophagy. 13:149–168. 2017. View Article : Google Scholar :
|
|
30
|
Kim M and Kim DJ: GFRA1: A novel molecular
target for the prevention of osteosarcoma chemoresistance. Int J
Mol Sci. 19:192018.
|
|
31
|
Xu R, Liu S, Chen H and Lao L:
MicroRNA-30a downregulation contributes to chemoresistance of
osteosarcoma cells through activating Beclin-1-mediated autophagy.
Oncol Rep. 35:1757–1763. 2016. View Article : Google Scholar
|
|
32
|
Chen R, Li X, He B and Hu W: MicroRNA-410
regulates autophagy-related gene ATG16L1 expression and enhances
chemosensitivity via autophagy inhibition in osteosarcoma. Mol Med
Rep. 15:1326–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei R, Cao G, Deng Z, Su J and Cai L:
miR-140-5p attenuates chemotherapeutic drug-induced cell death by
regulating autophagy through inositol 1,4,5-trisphosphate kinase 2
(IP3k2) in human osteosarcoma cells. Biosci Rep. 36:362016.
View Article : Google Scholar
|
|
34
|
Meng Y, Gao R, Ma J, Zhao J, Xu E, Wang C
and Zhou X: MicroRNA-140-5p regulates osteosarcoma chemoresistance
by targeting HMGN5 and autophagy. Sci Rep. 7:4162017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen L, Jiang K, Jiang H and Wei P:
miR-155 mediates drug resistance in osteosarcoma cells via inducing
autophagy. Exp Ther Med. 8:527–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Tang B, Han H, Mao D, Chen J, Zeng
Y and Xiong M: miR-155 affects osteosarcoma MG-63 cell autophagy
induced by adriamycin through regulating PTEN-PI3K/AKT/mTOR
signaling pathway. Cancer Biother Radiopharm. 33:32–38. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang
Y, Wang Y, Zhao W and Wang W: miR-22 inhibits osteosarcoma cell
proliferation and migration by targeting HMGB1 and inhibiting
HMGB1-mediated autophagy. Tumour Biol. 35:7025–7034. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Wang S, Chen Y, Liu G and Yang X:
miR-22 targets the 3′UTR of HMGB1 and inhibits the HMGB1-associated
autophagy in osteosarcoma cells during chemotherapy. Tumour Biol.
35:6021–6028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang P, Zhao ZQ, Guo SB, Yang TY, Chang
ZQ, Li DH, Zhao W, Wang YX, Sun C, Wang Y, et al: Roles of
microRNA-22 in suppressing proliferation and promoting sensitivity
of osteo-sarcoma cells via metadherin-mediated autophagy. Orthop
Surg. 11:285–293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang Z, Huo L, Li K, Wu Y and Hu Z:
Blocked autophagy by miR-101 enhances osteosarcoma cell
chemosensitivity in vitro. ScientificWorldJournal. 2014:7947562014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou J, Wu S, Chen Y, Zhao J, Zhang K,
Wang J and Chen S: microRNA-143 is associated with the survival of
ALDH1+CD133+ osteosarcoma cells and the
chemoresistance of osteosarcoma. Exp Biol Med (Maywood).
240:867–875. 2015. View Article : Google Scholar
|
|
42
|
Li Y, Jiang W, Hu Y, Da Z, Zeng C, Tu M,
Deng Z and Xiao W: MicroRNA-199a-5p inhibits cisplatin-induced drug
resistance via inhibition of autophagy in osteosarcoma cells. Oncol
Lett. 12:4203–4208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu K, Hou Y, Liu Y and Zheng J: LncRNA
SNHG15 contributes to proliferation, invasion and autophagy in
osteosarcoma cells by sponging miR-141. J Biomed Sci. 24:462017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Z, Liu Z and Wu S: Long non-coding
RNA CTA sensitizes osteosarcoma cells to doxorubicin through
inhibition of autophagy. Oncotarget. 8:31465–31477. 2017.PubMed/NCBI
|
|
45
|
Yu Z, Li N, Jiang K, Zhang N and Yao LL:
MiR-100 up-regulation enhanced cell autophagy and apoptosis induced
by cisplatin in osteosarcoma by targeting mTOR. Eur Rev Med
Pharmacol Sci. 22:5867–5873. 2018.PubMed/NCBI
|
|
46
|
Wu G, Yu W, Zhang M, Yin R, Wu Y and Liu
Q: MicroRNA-145-3p suppresses proliferation and promotes apotosis
and autophagy of osteosarcoma cell by targeting HDAC4. Artif Cells
Nanomed Biotechnol. 46(Suppl 2): 579–586. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang C, Gao R, Wang J, Yuan W, Wang C and
Zhou X: High-mobility group nucleosome-binding domain 5 increases
drug resistance in osteosarcoma through upregulating autophagy.
Tumour Biol. 35:6357–6363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shimizu T, Sugihara E, Yamaguchi-Iwai S,
Tamaki S, Koyama Y, Kamel W, Ueki A, Ishikawa T, Chiyoda T, Osuka
S, et al: IGF2 preserves osteosarcoma cell survival by creating an
autophagic state of dormancy that protects cells against
chemotherapeutic stress. Cancer Res. 74:6531–6541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhen YF, Li ST, Zhu YR, Wang XD, Zhou XZ
and Zhu LQ: Identification of DNA-PKcs as a primary resistance
factor of salinomycin in osteosarcoma cells. Oncotarget.
7:79417–79427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang H, Li W, Xu J, Zhang T, Zuo D, Zhou
Z, Lin B, Wang G, Wang Z, Sun W, et al: NDRG1 inhibition sensitizes
osteosarcoma cells to combretastatin A-4 through targeting
autophagy. Cell Death Dis. 8:e30482017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xiao X, Wang W, Li Y, Yang D, Li X, Shen
C, Liu Y, Ke X, Guo S and Guo Z: HSP90AA1-mediated autophagy
promotes drug resistance in osteosarcoma. J Exp Clin Cancer Res.
37:2012018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tao H, Chen F, Liu H, Hu Y, Wang Y and Li
H: Wnt/β-catenin signaling pathway activation reverses gemcitabine
resistance by attenuating Beclin1-mediated autophagy in the MG63
human osteosarcoma cell line. Mol Med Rep. 16:1701–1706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mukherjee S, Dash S, Lohitesh K and
Chowdhury R: The dynamic role of autophagy and MAPK signaling in
determining cell fate under cisplatin stress in osteosarcoma cells.
PLoS One. 12:e01792032017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Y, Chen P, Hong H, Wang L, Zhou Y
and Lang Y: JNK pathway mediates curcumin-induced apoptosis and
autophagy in osteosarcoma MG63 cells. Exp Ther Med. 14:593–599.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guan J, Yuan Z, He J, Wu Z, Liu B, Lin X,
Mo L and Mo H: Overexpression of caveolin-1 reduces Taxol
resistance in human osteosarcoma cells by attenuating PI3K-Akt-JNK
dependent autophagy. Exp Ther Med. 12:2815–2822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li J, Yang Z, Li Y, Xia J, Li D, Li H, Ren
M, Liao Y, Yu S, Chen Y, et al: Cell apoptosis, autophagy and
necroptosis in osteosarcoma treatment. Oncotarget. 7:44763–44778.
2016.PubMed/NCBI
|
|
58
|
Zhao S, Lu N, Chai Y and Yu X: Rapamycin
inhibits tumor growth of human osteosarcomas. J BUON. 20:588–594.
2015.PubMed/NCBI
|
|
59
|
Zhao GS, Gao ZR, Zhang Q, Tang XF, Lv YF,
Zhang ZS, Zhang Y, Tan QL, Peng DB, Jiang DM, et al: TSSC3 promotes
autophagy via inactivating the Src-mediated PI3K/Akt/mTOR pathway
to suppress tumorigenesis and metastasis in osteosarcoma, and
predicts a favorable prognosis. J Exp Clin Cancer Res. 37:1882018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Huang JC, Cui ZF, Chen SM, Yang LJ, Lian
HK, Liu B, Su ZH, Liu JS, Wang M, Hu ZB, et al: NVP-BEZ235
synergizes cisplatin sensitivity in osteosarcoma. Oncotarget.
9:10483–10496. 2017.
|
|
61
|
Meschini S, Condello M, Marra M, Formisano
G, Federici E and Arancia G: Autophagy-mediated chemosensitizing
effect of the plant alkaloid voacamine on multidrug resistant
cells. Toxicol In Vitro. 21:197–203. 2007. View Article : Google Scholar
|
|
62
|
Huang K, Chen Y, Zhang R, Wu Y, Ma Y, Fang
X and Shen S: Honokiol induces apoptosis and autophagy via the
ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro
and in vivo. Cell Death Dis. 9:1572018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yen JH, Huang ST, Huang HS, Fong YC, Wu
YY, Chiang JH and Su YC: HGK-sestrin 2 signaling-mediated autophagy
contributes to antitumor efficacy of Tanshinone IIA in human
osteosarcoma cells. Cell Death Dis. 9:10032018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kang Y, He P, Wang H, Ye Y, Li X, Xie P
and Wu B: Brazilin induces FOXO3A-dependent autophagic cell death
by disturbing calcium homeostasis in osteosarcoma cells. Cancer
Chemother Pharmacol. 82:479–491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu Y, Zhang Y, Zou J, Yan L, Yu X, Lu P,
Wu X, Li Q, Gu R and Zhu D: Andrographolide induces autophagic cell
death and inhibits invasion and metastasis of human osteosarcoma
cells in an autophagy-dependent manner. Cell Physiol Biochem.
44:1396–1410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Y, Ma R, Cheng S and Gu G:
Marrubenol inhibits osteosarcoma cancer cell growth by inducing
autophagic cell death and inhibiting cancer cell migration and
invasion. J BUON. 23:729–734. 2018.PubMed/NCBI
|
|
67
|
Liu ZR, Sun LZ, Jia TH and Jia DF:
β-Aescin shows potent antiproliferative activity in osteosarcoma
cells by inducing autophagy, ROS generation and mitochondrial
membrane potential loss. J BUON. 22:1582–1586. 2017.
|
|
68
|
Zhu J, Yu W, Liu B, Wang Y, Shao J, Wang
J, Xia K, Liang C, Fang W, Zhou C, et al: Escin induces
caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK
signalling pathway in human osteosarcoma cells in vitro and in
vivo. Cell Death Dis. 8:e31132017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang D, Zhang H, Wu J, Ma R, Li Z, Wang K
and Yang F: The role of chamaejasmine in cellular apoptosis and
autophagy in MG-63 cells. Biosci Rep. 39:392019. View Article : Google Scholar
|
|
70
|
Zhang C and Wang LM: Inhibition of
autophagy attenuated curcumol-induced apoptosis in MG-63 human
osteosarcoma cells via Janus kinase signaling pathway. Oncol Lett.
14:6387–6394. 2017.PubMed/NCBI
|
|
71
|
Liao YX, Zhou CH, Zeng H, Zuo DQ, Wang ZY,
Yin F, Hua YQ and Cai ZD: The role of the CXCL12-CXCR4/CXCR7 axis
in the progression and metastasis of bone sarcomas (Review). Int J
Mol Med. 32:1239–1246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bao X, Zhao L, Guan H and Li F: Inhibition
of LCMR1 and ATG12 by demethylation-activated miR-570-3p is
involved in the anti-metastasis effects of metformin on human
osteosarcoma. Cell Death Dis. 9:6112018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang F, Yan T, Guo W, Sun K, Wang S, Bao
X, Liu K, Zheng B, Zhang H and Ren T: Novel oncogene COPS3
interacts with Beclin1 and Raf-1 to regulate metastasis of
osteosarcoma through autophagy. J Exp Clin Cancer Res. 37:1352018.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang D, Bao F, Teng Y, Li Q and Li J:
MicroRNA-506-3p initiates mesenchymal-to-epithelial transition and
suppresses autophagy in osteosarcoma cells by directly targeting
SPHK1. Biosci Biotechnol Biochem. 83:836–844. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liao YX, Fu ZZ, Zhou CH, Shan LC, Wang ZY,
Yin F, Zheng LP, Hua YQ and Cai ZD: AMD3100 reduces CXCR4-mediated
survival and metastasis of osteosarcoma by inhibiting JNK and Akt,
but not p38 or Erk1/2, pathways in in vitro and mouse experiments.
Oncol Rep. 34:33–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yu F, Li J, Xie Y, Sleightholm RL and
Oupicky D: Polymeric chloroquine as an inhibitor of cancer cell
migration and experimental lung metastasis. J Control Release.
244:347–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yu W, Wang Y, Zhu J, Jin L, Liu B, Xia K,
Wang J, Gao J, Liang C and Tao H: Autophagy inhibitor enhance
ZnPc/BSA nanoparticle induced photodynamic therapy by suppressing
PD-L1 expression in osteosarcoma immunotherapy. Biomaterials.
192:128–139. 2019. View Article : Google Scholar
|
|
78
|
Ren T, Zheng B, Huang Y, Wang S, Bao X,
Liu K and Guo W: Osteosarcoma cell intrinsic PD-L2 signals promote
invasion and metastasis via the RhoA-ROCK-LIMK2 and autophagy
pathways. Cell Death Dis. 10:2612019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Livingston JA, Wang WL, Tsai JW, Lazar AJ,
Leung CH, Lin H, Advani S, Daw N, Santiago-O'Farrill J, Hollomon M,
et al: Analysis of HSP27 and the autophagy marker LC3B+
puncta following preoperative chemotherapy identifies high-risk
osteo-sarcoma patients. Mol Cancer Ther. 17:1315–1323. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lu Y, Wang Q, Zhou Y, Sun L, Hu B, Xue H,
Li M, Zhang K, Ren C, Duan N, et al: Overexpression of p62 is
associated with poor prognosis and aggressive phenotypes in
osteosarcoma. Oncol Lett. 15:9889–9895. 2018.PubMed/NCBI
|
|
81
|
Ma H, Li X, Wang J, Hornicek FJ, Garbutt
CC, Chang X and Duan Z: Expression and clinical implication of
autophagy-associated protein p62 in osteosarcoma. Oncology.
95:52–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
















