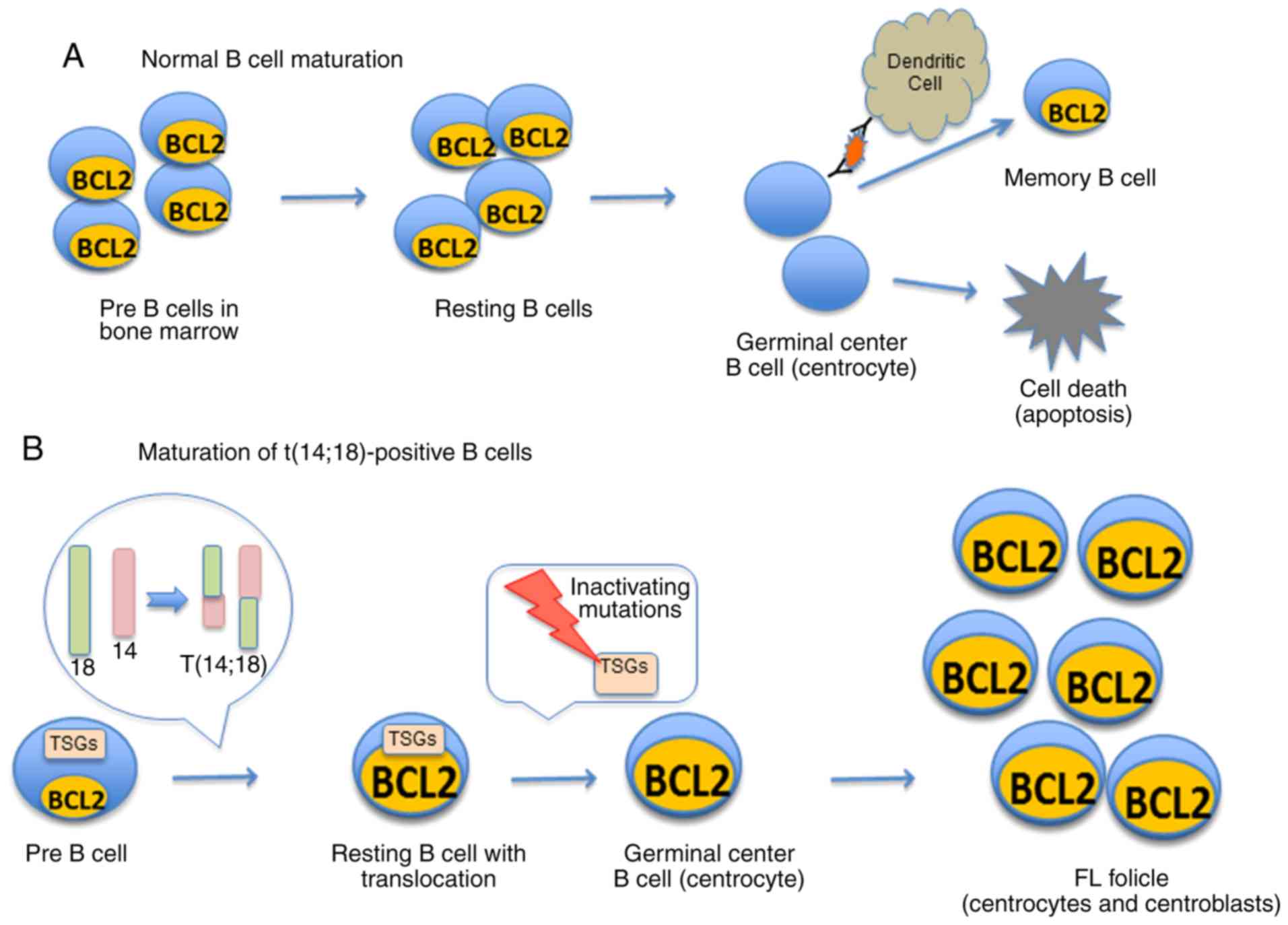
Follicular lymphoma (FL) is one of the most common
hematological neoplasms. It is an indolent type of non-Hodgkin
lymphoma that typically grows and spreads at a slow rate. In normal
B cell development, resting follicular B cells are exposed to
antigens and may eventually develop into memory B cells (Fig. 1A); in FL, neoplastic germinal
center B cells accumulate to form neoplastic follicles (Fig. 1B). These follicles are usually
composed of a mixture of cleaved centrocytes and noncleaved
centroblasts (1,2). FL is the second most common form of
nodal lymphoma, following diffuse large B-cell lymphoma (DLBCL)
(3). It is diagnosed at a median
age of 60 years, with life expectancy ranging from 6 to 10 years
following initial diagnosis (3-5).
However, in 20-60% of patients, histological transformation to
DLBCL [transformed FL (tFL)] may occur, which reduces life
expectancy to 1.2 years (4,6,7).
The genetic hallmark of FL is the t(14;18)(q32;q21)
chromosomal aberration, identifiable in 80-90% of FL cases
(8,9). This translocation juxtaposes the
BCL2 gene on chromosome 18 to the enhancer sequences of the
immunoglobulin heavy chain gene (IGH) promoter region on
chromosome 14 (9-11), leading to the overexpression of
BCL2 in B cells (12) (Fig. 1B). BCL2 normally inhibits
apoptosis. BCL2 overexpression resulting from t(14;18)
translocation contributes to the development of FL by preventing
the apoptosis of neoplastic cells (2,13-16)
However, the t(14;18) translocation can also be detected at a low
level in the blood of healthy individuals (17), indicating that the translocation
alone is insufficient for the development of FL. Numerous genetic
alterations (Table I), many
affecting tumor suppressor genes (TSGs), are additional
factors in the pathogenesis of FL (Fig. 1B) (18) Chromatin modifiers, such as MLL2 and
the transcription factor, interferon regulatory factor 4 (IRF4),
can be mutated or deleted, affecting the molecular structure and
transcription of DNA. Alterations in cell cycle regulators, such as
CDKN2A, which activates the p53 and pRb pathways, can also occur.
Signal transduction processes can also be affected by mutations in
genes such as FAS, which also plays a role in apoptosis
(18).
While the majority of FL cases are
t(14;18)-positive, 10-20% of FL cases lack the translocation
(19,20). Several recent studies have focused
on the biology and clinical features of t(14;18)-negative FL. Apart
from BCL2, the role of other transcription factors or regulators,
such as BCL6 expression, has been investigated (21,22).
This review summarizes the current knowledge of the molecular
characteristics of t(14;18)-negative FL. The review also describes
the diagnosis and grading of t(14;18)-negative FL and summarizes
the clinical features of the various subtypes.
Cases of FL are generally classified into 1 of 3
grades based on the guidelines of the World Health Organization
(WHO) (23). The grading of FL is
based on the average number of centroblasts in 10 randomly selected
microscopic x40 high power fields (HPFs). In grade 1 FL, up to 5
centroblasts per HPF are present; grade 2 FL has 6 to 15
centroblasts per HPF, and grade 3 FL has >15 centroblasts per
HPF. Grade 3 is further subdivided into 3A, in which some
centrocytes are present, and 3B with no centrocytes (24). Approximately 25% of FL patients are
in grade 1, 25-50% in grade 2, and ≥50% in grade 3 (25). FL grades 1 and 2 are considered low
grade and affected patients do not necessarily require
chemotherapy. For grade 3 FL, chemotherapy is often prescribed
(24). Approximately 10% of cases
of low-grade FL lack t(14;18). However, approximately 30-40 and
70-85% of grades 3A and 3B FL, respectively, are t(14;18)-negative
(26,27).
Unlike grade 3A FL, grade 3B FL is less often found
to be BCL2-positive by immunostaining and is associated with an
increased p53 and MUM1/IRF expression (28). Grade 3B FL is positive for CD10,
also known as common acute lymphocytic leukemia antigen (CALLA), in
approximately 50% of cases (29),
and is associated with translocations in the 3q27 locus, which
affects BCL6 expression (30). In
these respects, grade 3B FL more closely resembles DLBCL than the
other grades of FL (3,26,27,30,31).
The t(14;18) translocation leads to the
overexpression of BCL2. However, in t(14;18)-negative FL, BCL2
overexpression is more variable (22,32,34-37).
Leich et al (34,35) studied nodal FL grades 1, 2 and 3A,
and found that t(14;18)-positive and t(14;18)-negative FL were
morphologically indistinguishable. A small subset of
t(14;18)-negative FL samples exhibit evidence of BCL2
overexpression, indicating that factors other than the t(14;18)
translocation regulate its expression. The majority of
t(14;18)-negative FL cases in this previous study (34) expressed BCL6, CD10, and IRF8. In
the study by Horsman et al (21) 49 t(14;18)-negative FL cases were
analyzed by immunohistochemistry. These researchers found that BCL2
was expressed in 33% of cases. In t(14;18)-negative FLs without
BCL2 expression, Leich et al (35) performed microRNA expression
analysis and found a decreased expression of T cell
leukemia/lymphoma 1A (TCL1A), supporting the hypothesis that
this subtype of FL has a late germinal center B cell phenotype.
Through the use of next-generation sequencing
techniques in recent years, researchers have concluded that
epigenetic mechanisms of gene regulation play an important role in
the development of FL (48). Among
the most important and consistent results from these
next-generation sequencing studies has been the identification of
an important role for histone methyltransferases KMT2D and EZH2 in
epigenetic mutations (Table I)
(48,49). Next-generation sequencing has also
been used to identify the important role of TNFRSF14, which
is abnormally expressed in about 40% of FL patients (50). TNFRSF14 encodes a gene
termed herpes virus entry mediator, which acts to limit T-cell
activation (Table I) (50).
Various treatment regimens exist for FL, and the
appropriate treatment strategy depends upon the grade and clinical
characteristics of the disease. A clinical staging system for
lymphomas was published by the Committee on Hodgkin's Disease
Staging Classification (51). In
this system, stage I indicates the involvement of a single lymph
node region or a single extranodal site in the cancer. Stage II
indicates the involvement of two or more separate lymph node
regions on the same side of the diaphragm. Stage III indicates the
involvement of lymph node regions on both sides of the diaphragm.
Stage IV indicates diffuse or disseminated involvement of one or
more extralymphatic organs. Stage IV also includes localized
involvement of liver, bone marrow, or nodular involvement of the
lungs.
Unless the disease can be cured by surgery or local
irradiation, FL is generally managed with radiation or
chemotherapy. For those projected for treatment with radiation or
chemotherapy, clinical monitoring using a 'watch and wait' approach
is likely to be recommended until the patient requires treatment
(1). For patients who have stage I
or II low-grade FL, radiation is usually recommended as a first
treatment approach. For patients with low-grade FL with a high
tumor burden, chemotherapy is often followed by involved field
irradiation therapy (IFRT) (22).
In stage III or IV FL, patients typically receive chemotherapy
regimens, such as alkylating agents for inhibition of cell growth,
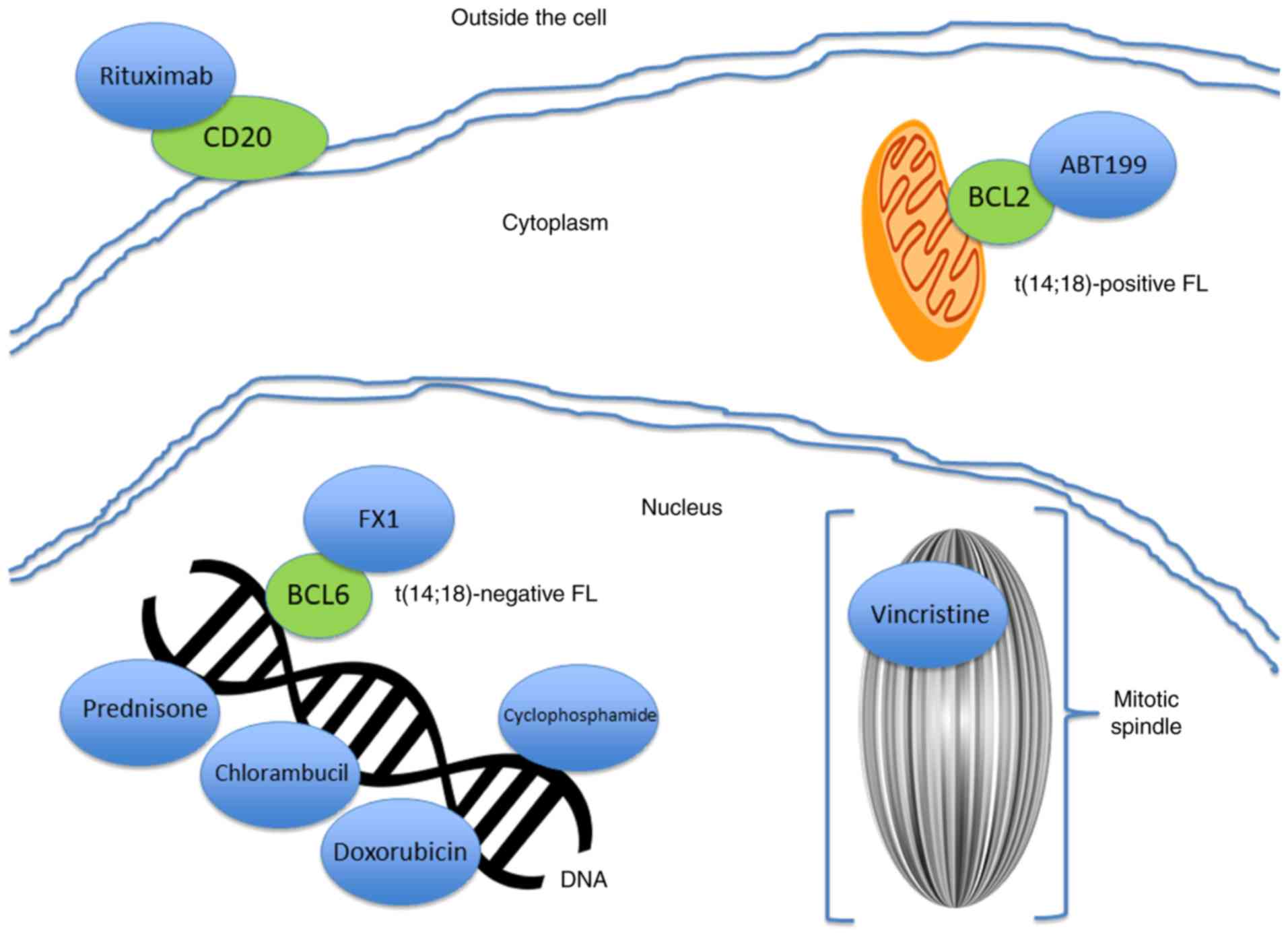
purine analogs and anthracycline-based agents (22). Monochemotherapy with chlorambucil
or cyclophosphamide is a common approach (22). Treatment with chemotherapy plus
rituximab is the accepted standard for most patients and this
method is associated with increases in the overall survival of
patients (2). Among
anthracycline-based regimens, one of the most common treatments is
R-CHOP, a combination of 5 drugs: Rituximab, cyclophosphamide
(Cytoxan), doxorubicin (Adriamycin), vincristine and prednisone
(57). Another commonly used
combination chemotherapy is R-CVP, which includes rituximab,
cyclophosphamide, vincristine and prednisone (58). CHOP-RIT, the administration of
tositumomab/iodine-131 radioimmunotherapy (targets CD20)
immediately following CHOP, is also common (59). Another commonly used approach is
bendamustine (an alkylating agent) plus rituximab (60). However, rituximab, a monoclonal
anti-CD20 antibody, is ineffective in variants of FL that are
negative for CD20 (61,62). A recently developed approach is
ABT199, a BH3 mimetic investigational drug (venetoclax) that
targets the BCL2 pathway (63).
However, as the majority of cases of t(14;18)-negative FL lack BCL2
overexpression, this approach would be contraindicated for these
patients. A better understanding of the underlying genetics of each
variation on the disease may lead to more precisely targeted
treatment regimens.
Although the exact mechanisms of rituximab, the most
common drug treatment for FL, are not yet fully known (64), it is considered that rituximab
achieves its clinical effect by altering intracellular signaling
pathways (64). Evidence indicates
that rituximab operates by reducing activity of the p38MAPK
pathway. This reduction in activity results in the inhibition of
the interleukin-10 paracrine cytokine auto-regulatory loop, which
in turn inhibits signal transducer and activator of transcription 3
(STAT-3) activity. This inhibition of STAT-3 activity leads to a
downregulation of BCL2 expression, and subsequent
chemosensitization (65).
Additionally, rituximab upregulates expression of Raf-1 kinase
inhibitor protein (RKIP) (64).
RKIP acts to reduce the activity of the extracellular
signal-related kinase (ERK) 1/2 and the NF-κB pathways, which in
turn inhibits transcription of AP-1 and NF-κB. The downregulation
of both of these pathways leads to the subsequent reduction of BCL2
transcription and expression and susceptibility to apoptosis
(64). A better understanding of
the effects of drugs on molecular pathways will allow better
targeted treatments.
Studies have examined the clinical differences
between patients with and without BCL2 and BCL6
rearrangements. Although the majority of results underscore a
similarity in clinical presentation between patients with and
without BCL2 and BCL6 rearrangements, some
differences have been noted. A total of 98 patients with FL and 93
DLBCL were examined by in the study by Watanabe et al
(68) with the aim of determining
the frequencies of BCL6 and BCL2 translocations in
the two patient groups. BCL2 translocations were detected in
77 patients with FL and 22 patients with DLBCL. BCL6
translocations were detected in 14 patients with FL and 14 patients
with DLBCL. The patients were followed-up for 29 months after
undergoing R-CHOP therapy. For patients with FL and DLBCL, the
presence of BCL2 or BCL6 translocation was
uncorrelated with clinical outcomes after treatment with R-CHOP
therapy. Furthermore, neither overall survival nor progression free
survival differed between the patient groups (68).
Studies have identified uncommon forms of
t(14;18)-negative FL, some with diffuse, extranodal expression, and
others with expression limited to the skin. One study demonstrated
that a subtype of CD10−MUM1+ FL exhibited
distinctive biological and clinical features compared with the more
typical CD10+MUM1− FL (42). Twenty-two mostly t(14;18)-negative
FL patients who were negative for expression of CD10 and positive
for expression of MUM1 were generally older than typical FL
patients (mean of 67 vs. 58.7 years old).
CD10-MUM1+ FL patients also exhibited diffuse
FL proliferation and were generally of a higher disease grade,
either FL 3A or 3B, having a relatively poor prognosis.
In another study, a different subtype of
t(14;18)-negative nodal follicular non-Hodgkin lymphoma exhibited a
predominantly diffuse pattern and was characterized by deletions in
the chromosomal region 1p36. These patients were more frequently
diagnosed as low-grade (stage I and II) FL and generally presented
with isolated large tumors (median size of 5 cm) that were
frequently localized to the inguinal region. Although a complete
remission could be achieved by treatment with radiotherapy alone or
chemotherapy in combination with radiotherapy, disease progression
generally followed an indolent course (5). This diffuse form of t(14;18)-negative
FL represents a previously undocumented form of FL with distinct
molecular and clinical features (5).
Moreover, a subtype of generally t(14;18)-negative
FL which arises primarily in the skin has also been documented
(69). Each of the evaluated 47
patients with FL was in clinical stage I. Compared with
t(14;18)-positive cases, these FL cases were less likely to express
BCL2 or CD10. These cases also had a higher proportion of
extranodal FL sites, and had a significantly higher overall and
disease specific five-year survival rate. This subtype of FL has a
generally more favorable clinical outcome when compared to
t(14;18)-positive FL (19,69).
Another report of atypical clinical cases with
difficult diagnoses was made by Nybakken et al (71). These researchers reported on 7
patients (5 females ranging in age from 51 to 61 years, and 2 males
aged 6 and 61 years). The patients presented with isolated atypical
follicles that were composed of cytologically atypical centroblasts
located within otherwise normal lymph nodes. The isolated follicles
had unusual morphologic features that could be mistaken for
lymphoma. The cases were generally positive for CD20 and BCL6, and
were negative for BCL2 and CD3. The sites of involvement included
peripheral and internal sites, such as a periaortic lymph node and
rectal mucosa-associated lymphoid tissue. The authors considered a
diagnosis of partial involvement by FL. The cytological
characteristics of involved areas were consistent with FL grade 3B
(enlarged with vesicular chromatin and prominent nucleoli, and
nuclear membrane irregularity inconsistent with the morphology of
the surrounding lymph node). However, the pattern of single
scattered follicles was not consistent with partial FL.
Furthermore, none of the patients had evidence of systematic
lymphoma at the time of diagnosis or clinical follow-up visits up
to 44 months after diagnosis, and the patients did not require
aggressive clinical management. Nybakken et al (71) noted that it is of high importance
to differentiate these cases from aggressive FL or a similar
disease to avoid unnecessarily aggressive treatment of patients
with indolent conditions.
Finally, a surgical treatment for a patient with
t(14;18)-negative FL has also been reported (72). The patient was a 74-year-old
Japanese female who presented with a complaint of swelling in the
submandibular region. The patient was diagnosed with stage I FL. A
surgery was performed and the lymph node was removed successfully.
Flow cytometric analysis revealed that the lymphoma cells were
positive for CD19, CD20, CD23, CD10 and BCL6. The cells were
negative for BCL2 and MUM1. There was no recurrence of FL upon
follow-up a year post-surgery. This case suggests a distinct type
of surgical treatment for certain types of BCL2-negative FL
(72).
The t(14;18) translocation is absent in
approximately 10-20% of FL, a condition less common in FL grades 1
and 2 and more common in FL grades 3A and 3B. t(14;18)-negative FL
is often associated with a t(3;14) translocation or the
overexpression of BCL6, which is associated with morphological
effects that closely resemble those associated with t(14;18).
t(14;18)-negative FL patients are generally indistinguishable from
t(14;18)-positive patients from a clinical perspective. However,
diffuse and pediatric forms of FL, which often lack t(14;18), have
a better clinical prognosis than t(14;18)-positive FL patients. In
the future, the development of novel methods for targeting pathways
other than the BCL2 pathway to effectively manage t(14;18)-negative
FL may prove to be useful. For example, the recent study by
Cardenas et al (73)
detailed the development of specific BCL6 inhibitors, such as FX1,
which could be effective in controlling t(14;18)-negative FL
(Fig. 2).
Not applicable.
This study was supported in part by grants from
Colleges and Universities Key Scientific Research Project Plan
Basic Research Special of Henan Province (grant no. 19zx009),
Science and Technology Project for Tackling Key Problems of Henan
Province (grant no. 182102311172), Basic and Frontier Technology
Research Program of Henan Province (grant no. 132300410448), and
Science and Technology Innovation Talent Program of Henan Province
(grant no. 154100510019).
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
WR, ST, DZ and PL contributed to the conception and
design of this study. WR, ST, ZuZ, TL, XZ and ZhZ were involved in
the acquisition of the data and the study design. WR, ST, ZuZ, TL,
XZ, ZhZ, DZ and PL were involved in the writing of the article. WR,
ST critically revised the manuscript. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Follicular Lymphoma, Pamphlet. Lymphoma
Association; 2017
|
|
2
|
Abd El-Ghaffar HA, Shamaa S, Attwan N, et
al: Stratification of Patients with Follicular Lymphoma.
IntechOpen; Rijeka: 2012, https://www.intechopen.com/books/hematology-science-and-practice/stratification-of-patients-with-follicular-lymphoma.
Accessed March 2, 2012.
|
|
3
|
Kridel R, Sehn LH and Gascoyne RD:
Pathogenesis of follicular lymphoma. J Clin Invest. 122:3424–3431.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bisikirska B, Bansal M, Shen Y,
Teruya-Feldstein J, Chaganti R and Califano A: Elucidation and
pharmacological targeting of novel molecular drivers of follicular
lymphoma progression. Cancer Res. 76:664–674. 2016. View Article : Google Scholar :
|
|
5
|
Katzenberger T, Kalla J, Leich E,
Stöcklein H, Hartmann E, Barnickel S, Wessendorf S, Ott MM,
Müller-Hermelink HK, Rosenwald A and Ott G: A distinctive subtype
of t(14;18)-negative nodal follicular non-Hodgkin lymphoma
characterized by a predominantly diffuse growth pattern and
deletions in the chromosomal region 1p-36. Blood. 113:1053–1061.
2009. View Article : Google Scholar
|
|
6
|
Casulo C, Burack WR and Friedberg JW:
Transformed follicular non-Hodgkin lymphoma. Blood. 125:40–47.
2015. View Article : Google Scholar
|
|
7
|
Christie L, Kernohan N, Levison D, Sales
M, Cunningham J, Gillespie K, Batstone P, Meiklejohn D and Goodlad
J: C-MYC translocation in t(14;18) positive follicular lymphoma at
presentation: An adverse prognostic indicator? Leuk Lymphoma.
49:470–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bakhski A, Jensen JP, Goldman P, Wright
JJ, McBride OW, Epstein AL and Korsmeyer SJ: Cloning the
chromosomal break-point of t(14;18) human lymphomas: Clustering
around Jh on chromosome 14 and near a transcriptional unit on 18.
Cell. 41:899–906. 1985. View Article : Google Scholar
|
|
9
|
Tsujimoto Y, Gorham J, Cossman J, Jaffe E
and Croce CM: The t(14;18) chromosome translocations involved in
B-cell neoplasms result from mistakes in VDJ joining. Science.
229:1390–1393. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cleary ML and Sklar J: Nucleotide sequence
of a t(14;18) chromosomal breakpoint in follicular lymphoma and
demonstration of a breakpoint-cluster region near a
transcriptionally active locus on chromosome 18. Proc Natl Acad Sci
USA. 82:7439–7443. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsujimoto Y, Finger LR, Yunis J, Nowell PC
and Croce CM: Cloning of the chromosome breakpoint of neoplastic B
cells with the t(14;18) chromosome translocation. Science.
226:1097–1099. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Graninger WB, Seto M, Boutain B, Goldman P
and Korsmeyer SJ: Expression of Bcl-2 and Bcl-2-Ig fusion
transcripts in normal and neoplastic cells. J Clin Invest.
80:1512–1515. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Godon A, Moreau A, Talmant P,
Baranger-Papot L, Geneviéve F, Milpied N, Zandecki M and
Avet-Loiseau H: Is t(14;18)(q32;q21) a constant finding in
follicular lymphoma? An interphase FISH study on 63 patients
Leukemia. 17:255–259. 2003.
|
|
14
|
Husson H, Carideo EG, Neuberg D, Schultze
J, Munoz O, Marks PW, Donovan JW, Chillemi AC, O'Connell P and
Freedman AS: Gene expression profiling of follicular lymphoma and
normal germinal center B cells using cDNA arrays. Blood.
99:282–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masir N, Campbell LJ, Goff LK, Jones M,
Marafioti T, Cordell J, Clear AJ, Lister TA, Mason DY and Lee AM:
BCL2 protein expression in follicular lymphomas with t(14;18)
chromosomal translocations. Br J Haematol. 144:716–725. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staudt LM: A closer look at follicular
lymphoma. N Engl J Med. 356:741–742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roulland S, Kelly RS, Morgado E, Sungalee
S, Solal-Celigny P, Colombat P, Jouve N, Palli D, Pala V, Tumino R,
et al: t(14;18) Translocation: A predictive blood biomarker for
follicular lymphoma. J Clin Oncol. 32:1347–1355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graham C and LeBrun DP: Tumor suppressors
in follicular lymphoma. Leuk Lymphoma. 56:1981–1988. 2015.
View Article : Google Scholar
|
|
19
|
Gu K, Fu K, Jain S, Liu Z, Iqbal J, Li M,
Sanger WG, Weisenburger DD, Greiner TC, Aoun P, et al:
t(14;18)-negative follicular lymphomas are associated with a high
frequency of BCL6 rearrangement at the alternative breakpoint
region. Mod Pathol. 22:1251–1257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Küppers R and Dalla-Favera R: Mechanisms
of chromosomal translocations in B cell lymphomas. Oncogene.
20:5580–5594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horsman DE, Okamoto I, Ludkovski O, Le N,
Harder L, Gesk S, Siebert R, Chhanabhai M, Sehn L, Connors JM and
Gascoyne RD: Follicular lymphoma lacking the t(14;18)(q32;q21):
Identification of two disease subtypes. Br J Haematol. 120:424–433.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vitolo U, Ferreri AJ and Montoto S:
Follicular lymphomas. Crit Rev Oncol Hematol. 66:248–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of theWorld Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samsi S, Lozanski G, Shana'ah A,
Krishanmurthy AK and Gurcan MN: Detection of follicles from
IHC-stained slides of follicular lymphoma using iterative
watershed. IEEE Trans Biomed Eng. 57:2609–2612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paik PK, Johnson ML, D'Angelo SP, Sima CS,
Ang D, Dogan S, Miller VA, Ladanyi M, Kris MG and Riely GJ: Driver
mutations determine survival in smokers and never-smokers with
stage IIIB/IV lung adenocarcinomas. Cancer. 118:5840–5847. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katzenberger T, Ott G, Klein T, Kalla J,
Müller-Hermelink HK and Ott MM: Cytogenetic alterations affecting
BCL6 are predominantly found in follicular lymphomas grade 3B with
a diffuse large B-cell component. Am J Pathol. 165:481–490. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ott G, Katzenberger T, Lohr A,
Kindelberger S, Rüdiger T, Wilhelm M, Kalla J, Rosenwald A, Müller
JG, Ott MM and Müller-Hermelink HK: Cytomorphologic,
immunohistochemical, and cytogenetic profiles of follicular
lymphoma: 2 types of follicular lymphoma grade 3. Blood.
99:3806–3812. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horn H, Schmelter C, Leich E, Salaverria
I, Katzenberger T, Ott MM, Kalla J, Romero M, Siebert R, Rosenwald
A and Ott G: Follicular lymphoma grade 3B is a distinct neoplasm
according to cytogenetic and immunohistochemical profiles.
Haematologica. 96:1327–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salaverria I and Siebert R: Follicular
lymphoma grade 3B. Best Pract Res Clin Haematol. 24:111–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bosga-Bouwer AG, van Imhoff GW, Boonstra
R, van der Veen A, Haralambieva E, van den Berg A, Jong B, Krause
V, Palmer MC, Coupland R, et al: Follicular lymphoma grade 3B
includes 3 cytogenetically defined subgroups with primary t(14;18),
3q27, or other translocations: t(14;18) and 3q27 are mutually
exclusive. Blood. 101:1149–1154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shustik J, Han G, Farinha P, Johnson NA,
Ben Neriah S, Connors JM, Sehn LH, Horsman DE, Gascoyne RD and
Steidl C: Correlations between BCL6 rearrangement and outcome in
patients with diffuse large B-cell lymphoma treated with CHOP or
R-CHOP. Haematologica. 95:96–101. 2010. View Article : Google Scholar :
|
|
32
|
Vaandrager JW, Schuuring E, Raap T,
Philippo K, Kleiverda K and Kluin P: Interphase FISH detection of
BCL2 rearrangement in follicular lymphoma using breakpoint-flanking
probes. Genes Chromosomes Cancer. 27:85–94. 2000. View Article : Google Scholar
|
|
33
|
Weinberg OK, Ai WZ, Mariappan MR, Shum C,
Levy R and Arber DA: 'Minor' BCL2 breakpoints in follicular
lymphoma: Frequency and correlation with grade and disease
presentation in 236 cases. J Mol Diagn. 9:530–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leich E, Salaverria I, Bea S, Zettl A,
Wright G, Moreno V, Gascoyne RD, Chan WC, Braziel RM, Rimsza LM, et
al: Follicular lymphomas with and without translocation t(14;18)
differ in gene expression profiles and genetic alterations. Blood.
114:826–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leich E, Zamo A, Horn H, Haralambieva E,
Puppe B, Gascoyne RD, Chan WC, Braziel RM, Rimsza LM, Weisenburger
DD, et al: MicroRNA profiles of t(14;18)-negative follicular
lymphoma support a late germinal center B-cell phenotype. Blood.
118:5550–5558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schraders M, de Jong D, Kluin P, Groenen P
and van Krieken H: Lack of Bcl-2 expression in follicular lymphoma
may be caused by mutations in the BCL2 gene or by absence of the
t(14;18) translocation. J Pathol. 205:329–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Skinnider BF, Horsman DE, Dupuis B and
Gascoyne RD: Bcl-6 and Bcl-2 protein expression in diffuse large
B-cell lymphoma and follicular lymphoma: Correlation with 3q27 and
18q21 chromosomal abnormalities. Hum Pathol. 30:803–808. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo Y, Karube K, Kawano R, Suzumiya J,
Takeshita M, Kikuchi M, Huang GS, Li Q and Ohshima K: Bcl2-negative
follicular lymphomas frequently have Bcl6 translocation and/or Bcl6
or p53 expression. Pathol Int. 57:148–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jardin F, Gaulard P, Buchonnet G,
Contentin N, Lepretre S, Lenain P, Stamatoullas A, Picquenot JM,
Duval C, Parmentier F, et al: Follicular lymphoma without t(14;18)
and with BCL-6 rearrangement: A lymphoma subtype with distinct
pathological, molecular and clinical characteristics. Leukemia.
16:2309–2317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gollub W, Stassek B, Huckhagel T, Bernd
HW, Krokowski M, Merz H, Feller AC and Thorns C:
BCL6-translocations affect the phenotype of follicular lymphomas
only in the absence of t(14;18)IgH/BCL2. Anticancer Res.
29:4649–4655. 2009.PubMed/NCBI
|
|
41
|
Pan Y, Meng B, Sun B, Guan B, Liang Y,
Wang H, Hao X and Fu K: Frequencies of BCL2 and BCL6 translocations
in representative Chinese follicular lymphoma patients:
Morphologic, immunohistochemical, and FISH analyses. Diagn Mol
Pathol. 21:234–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karube K, Guo Y, Suzumiya J, Sugita Y,
Nomura Y, Yamamoto K, Shimizu K, Yoshida S, Komatani H, Takeshita
M, et al: CD10-MUM1 + follicular lymphoma lacks BCL2 gene
translocation and shows characteristic biologic and clinical
features. Blood. 109:3076–3079. 2007. View Article : Google Scholar
|
|
43
|
Gagyi E, Balogh Z, Bödör C, Timár B,
Reiniger L, Deák L, Csomor J, Csernus B, Szepesi A and Matolcsy A:
Somatic hyper-mutation of IGVH genes and aberrant somatic
hypermutation in follicular lymphoma without BCL-2 gene
rearrangement and expression. Haematologica. 93:1822–1828. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Q, Salaverria I, Pittaluga S, Jegalian
AG, Xi L, Siebert R, Raffeld M, Hewitt SM and Jaffe ES: Follicular
lymphomas in children and young adults: A comparison of the
pediatric variant with usual follicular lymphoma. Am J Surg Pathol.
37:333–343. 2013. View Article : Google Scholar :
|
|
45
|
Martin-Guerrero I, Salaverria I, Burkhardt
B, Szczepanowski M, Baudis M, Bens S, de Leval L, Garcia-Orad A,
Horn H, Lisfeld J, et al: Recurrent loss of heterozygosity in 1p36
associated with TNFRSF14 mutations in IRF4 translocation negative
pediatric follicular lymphomas. Haematologica. 98:1237–1241. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jaffe ES: The 2008 WHO classification of
lymphomas: Implications for clinical practice and translational
research. Hematol Am Soc Hematol Educ Program. 523–531. 2009.
View Article : Google Scholar
|
|
47
|
Louissaint A Jr, Ackerman AM,
Dias-Santagata D, Ferry JA, Hochberg EP, Huang MS, Iafrate AJ, Lara
DO, Pinkus GS, Salaverria I, et al: Pediatric-type nodal follicular
lymphoma: An indolent clonal proliferation in children and adults
with high proliferation index and no BCL2 rearrangement. Blood.
120:2395–2404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Araf S, Okosun J, Koniali L, Fitzgibbon J
and Heward J: Epigenetic dysregulation in follicular lymphoma.
Epigenomics. 8:77–84. 2016. View Article : Google Scholar :
|
|
49
|
Braggio E, Egan JB, Fonseca R and Stewart
AK: Lessons from next-generation sequencing analysis in
hematological malignancies. Blood Cancer J. 3:e1272013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kotsiou E, Okosun J, Besley C, Iqbal S,
Matthews J, Fitzgibbon J, Gribben JG and Davies JK: TNFRSF14
aberrations in follicular lymphoma increase clinically significant
allogeneic T-cell responses. Blood. 128:72–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
52
|
Armitage JO and Weisenburger DD: New
approach to classifying non-Hodgkin's lymphomas: Clinical features
of the major histo-logic subtypes. Non-Hodgkin's lymphoma
classification project. J Clin Oncol. 16:2780–2795. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Horning SJ and Rosenberg SA: The natural
history of initially untreated low-grade non-Hodgkin's lymphomas. N
Engl J Med. 311:1471–1475. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Takata K, Miyata-Takata T, Sato Y and
Yoshino T: Pathology of follicular lymphoma. J Clin Exp Hematop.
54:3–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nathwani BN: A promising new biologic
prognostic model in diffuse large B-cell lymphoma. Blood.
120:2161–2162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pezella F, Jones M, Ralfkiaer E, Ersbøll
J, Gatter KC and Mason DY: Evaluation of bcl-2 protein expression
and 14;18 translocation as prognostic markers in follicular
lymphoma. Br J Cancer. 65:87–89. 1992. View Article : Google Scholar
|
|
57
|
Tomita N, Takasaki H, Miyashita K,
Fujisawa S, Ogusa E, Matsuura S, Kishimoto K, Numata A, Fujita A,
Ohshima R, et al: R-CHOP therapy alone in limited stage diffuse
large B-cell lymphoma. Br J Haematol. 161:383–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Siddhartha G and Vijay P: R-CHOP versus
R-CVP in the treatment of follicular lymphoma: A meta-analysis and
critical appraisal of current literature. J Hematol Oncol.
2:142009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Press OW, Unger JM, Rimsza LM, Friedberg
JW, LeBlanc M, Czuczman MS, Kaminski M, Braziel RM, Spier C, Gopal
AK, et al: A comparative analysis of prognostic factor models for
follicular lymphoma based on a phase III trial of CHOP-rituximab
versus CHOP + 131iodine-tositumomab. Clin Cancer Res. 19:6624–6632.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kahl BS and Yang DT: Follicular lymphoma:
Evolving therapeutic strategies. Blood. 127:2055–2063. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Maloney DG, Grillo-López AJ, White CA,
Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles
TM, Dallaire BK, et al: IDEC-C2B8 (Rituximab) anti-CD20 monoclonal
antibody therapy in patients with relapsed low-grade non-Hodgkin's
lymphoma. Blood. 90:2188–2195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
McLaughlin P, Grillo-López AJ, Link BK,
Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I,
White CA, Cabanillas F, et al: Rituximab chimeric anti-CD20
monoclonal antibody therapy for relapsed indolent lymphoma: Half of
patients respond to a four-dose treatment program. J Clin Oncol.
16:2825–2833. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cang S, Iragavarapu C, Savooji J, Song Y
and Liu D: ABT-199 (venetoclax) and BCL-2 inhibitors in clinical
development. J Hematol Oncol. 8:1292015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jazirehi AR and Bonavida B: Cellular and
molecular signal transduction pathways modulated by rituximab
(rituxan, anti-CD20 mAb) in non-Hodgkin's lymphoma: Implications in
chemosensitization and therapeutic intervention. Oncogene.
24:2121–2143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Alas S, Emmanouilides C and Bonavida B:
Inhibition of interleukin 10 by rituximab results in
down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin's
lymphoma to apoptosis. Clin Cancer Res. 7:709–723. 2001.PubMed/NCBI
|
|
66
|
Leich E, Hoster E, Wartenberg M, Unterhalt
M, Siebert R, Koch K, Klapper W, Engelhard M, Puppe B, Horn H, et
al: Similar clinical features in follicular lymphomas with and
without breaks in the BCL2 locus. Leukemia. 30:854–860. 2016.
View Article : Google Scholar
|
|
67
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Watanabe R, Tomita N, Matsumoto C, Hattori
Y, Matsuura S, Takasaki H, Hashimoto C, Fujita H, Fujisawa S and
Ishigatsubo Y: The 3q27 and 18q21 translocations for follicular
lymphoma and diffuse large B-cell lymphoma in the rituximab era. J
Clin Exp Hematop. 53:107–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Goodlad JR, Batstone PJ, Hamilton DA,
Kernohan NM, Levison DA and White JM: BCL2 gene abnormalities
define distinct clinical subsets of follicular lymphoma.
Histopathology. 49:229–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wong YP, Faridah AR, Ahmad Toha S and
Noraidah M: A case of t(14;18)-negative follicular lymphoma with
unusual immunopheno-type: A diagnostic dilemma. Med Health. 6(1
Suppl): S2072011.
|
|
71
|
Nybakken GE, Bala R, Gratzinger D, Jones
CD, Zehnder JL, Bangs CD, Cherry A, Warnke RA and Natkunam Y:
Isolated follicles enriched for centroblasts and lacking
t(14;18)/BCL2 in lymphoid tissue: Diagnostic and clinical
implications. PLoS One. 11:e01517352016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kamatani T, Mishima K, Kutsuna T,
Yoshihama Y, Kondo S and Shintani S: A case of surgical treatment
for primary BCL2-'negative' follicular lymphoma without t(14;18) in
early stage of the submandibular lymph node. J Oral Maxillofac Surg
Med Pathol. 26:599–602. 2014. View Article : Google Scholar
|
|
73
|
Cardenas MG, Yu W, Beguelin W, Teater MR,
Geng H, Goldstein RL, Oswald E, Hatzi K, Yang SN, Cohen J, et al:
Rationally designed BCL6 inhibitors target activated B cell diffuse
large B cell lymphoma. J Clin Invest. 126:3351–3362. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ahearn IM, Haigis K, Bar-Sagi D and
Philips MR: Regulating the regulator: Post-translational
modification of RAS. Nat Rev Mol Cell Biol. 13:39–51. 2012.
View Article : Google Scholar
|
|
75
|
Bouska A, McKeithan TW, Deffenbacher KE,
Lachel C, Wright GW, Iqbal J, Smith LM, Zhang W, Kucuk C, Rinaldi
A, et al: Genome-wide copy-number analyses reveal genomic
abnormalities involved in transformation of follicular lymphoma.
Blood. 123:1681–1690. 2014. View Article : Google Scholar :
|
|
76
|
Li H, Kaminski MS, Li Y, Yildiz M,
Ouillette P, Jones S, Fox H, Jacobi K, Saiya-Cork K, Bixby D, et
al: Mutations in linker histone genes HIST1H1 B, C, D, and E; OCT2
(POU2F2); IRF8; and ARID1A underlying the pathogenesis of
follicular lymphoma. Blood. 123:1487–1498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Migliazza A, Martinotti S, Chen W, Fusco
C, Ye BH, Knowles DM, Offit K, Chaganti RS and Dalla-Favera R:
Frequent somatic hypermutation of the 5' noncoding region of the
BCL6 gene in B-cell lymphoma. Proc Natl Acad Sci USA.
92:12520–12524. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Alhejaily A, Day AG, Feilotter HE, Baetz T
and Lebrun DP: Inactivation of the CDKN2A tumor-suppressor gene by
deletion or methylation is common at diagnosis in follicular
lymphoma and associated with poor clinical outcome. Clin Cancer
Res. 20:1676–1686. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cheung KJ, Johnson NA, Affleck JG,
Severson T, Steidl C, Ben-Neriah S, Schein J, Morin RD, Moore R,
Shah SP, et al: Acquired TNFRSF14 mutations in follicular lymphoma
are associated with worse prognosis. Cancer Res. 70:9166–9174.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fitzgibbon J, Iqbal S, Davies A, O'Shea D,
Carlotti E, Chaplin T, Matthews J, Raghavan M, Norton A, Lister TA
and Young BD: Genome-wide detection of recurring sites of
uniparental disomy in follicular and transformed follicular
lymphoma. Leukemia. 21:1514–1520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Oricchio E, Ciriello G, Jiang M, Boice MH,
Schatz JH, Heguy A, Viale A, de Stanchina E, Teruya-Feldstein J,
Bouska A, et al: Frequent disruption of the RB pathway in indolent
follicular lymphoma suggests a new combination therapy. J Exp Med.
211:1379–1391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Pasqualucci L, Khiabanian H, Fangazio M,
Vasishtha M, Messina M, Holmes AB, Ouillette P, Trifonov V, Rossi
D, Tabbò F, et al: Genetics of follicular lymphoma transformation.
Cell Rep. 6:130–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ross CW, Ouillette PD, Saddler CM, Shedden
KA and Malek SN: Comprehensive analysis of copy number and allele
status identifies multiple chromosome defects underlying follicular
lymphoma pathogenesis. Clin Cancer Res. 13:4777–4785. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Schwaenen C, Viardot A, Berger H, Barth
TF, Bentink S, Döhner H, Enz M, Feller AC, Hansmann ML, Hummel M,
et al: Microarray-based genomic profiling reveals novel genomic
aberrations in follicular lymphoma which associate with patient
survival and gene expression status. Genes Chromosomes Cancer.
48:39–54. 2009. View Article : Google Scholar
|
|
85
|
Ogura M, Ando K, Suzuki T, Ishizawa K, Oh
SY, Itoh K, Yamamoto K, Au WY, Tien HF, Matsuno Y, et al: A
multicentre phase II study of vorinostat in patients with relapsed
or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell
lymphoma. Br J Haematol. 165:768–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Okosun J, Bödör C, Wang J, Araf S, Yang
CY, Pan C, Boller S, Cittaro D, Bozek M, Iqbal S, et al: Integrated
genomic analysis identifies recurrent mutations and evolution
patterns driving the initiation and progression of follicular
lymphoma. Nat Genet. 46:176–181. 2014. View Article : Google Scholar :
|
|
87
|
Krajnovic M, Radojkovic M, Davidovic R,
Dimitrijevic B and Krtolica K: Prognostic significance of
epigenetic inactivation of p16, p15, MGMT and DAPK genes in
follicular lymphoma. Med Oncol. 30:4412013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Morin RD, Mendez-Lago M, Mungall AJ, Goya
R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field
M, et al: Frequent mutation of histone-modifying genes in
non-Hodgkin lymphoma. Nature. 476:298–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Morin RD, Johnson NA, Severson TM, Mungall
AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, et
al: Somatic mutations altering EZH2 (Tyr641) in follicular and
diffuse large B-cell lymphomas of germinal-center origin. Nat
Genet. 42:181–185. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
90
|
Grønbaek K, Straten PT, Ralfkiaer E,
Ahrenkiel V, Andersen MK, Hansen NE, Zeuthen J, Hou-Jensen K and
Guldberg P: Somatic Fas mutations in non-Hodgkin's lymphoma:
Association with extranodal disease and autoimmunity. Blood.
92:3018–3024. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xia Q, Wang G, Wang H, Hu Q and Ying Z:
Folliculin, a tumor suppressor associated with Birt-Hogg-Dube (BHD)
syndrome, is a novel modifier of TDP-43 cytoplasmic translocation
and aggregation. Hum Mol Genet. 25:83–96. 2016. View Article : Google Scholar
|
|
92
|
Limpens J, Stad R, Vos C, de Vlaam C, de
Jong D, van Ommen GJ, Schuuring E and Kluin PM: Lymphoma-associated
translocation t(14;18) in blood B cells of normal individuals.
Blood. 85:2528–2536. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhu D, McCarthy H, Ottensmeier CH, Johnson
P, Hamblin TJ and Stevenson FK: Acquisition of potential
N-glycosylation sites in the immunoglobulin variable region by
somatic mutation is a distinctive feature of follicular lymphoma.
Blood. 99:2562–2568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chim CS, Wong KY, Loong F and Srivastava
G: SOCS1 and SHP1 hypermethylation in mantle cell lymphoma and
follicular lymphoma: Implications for epigenetic activation of the
Jak/STAT pathway. Leukemia. 18:356–358. 2004. View Article : Google Scholar
|
|
95
|
Koyama M, Oka T, Ouchida M, Nakatani Y,
Nishiuchi R, Yoshino T, Hayashi K, Akagi T and Seino Y: Activated
proliferation of B-cell lymphomas/leukemias with the SHP1 gene
silencing by aberrant CpG methylation. Lab Invest. 83:1849–1858.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Oka T, Yoshino T, Hayashi K, Ohara N,
Nakanishi T, Yamaai Y, Hiraki A, Sogawa CA, Kondo E, Teramoto N, et
al: Reduction of hematopoietic cell-specific tyrosine phosphatase
SHP-1 gene expression in natural killer cell lymphoma and various
types of lymphomas/leukemias: Combination analysis with cDNA
expression array and tissue microarray. Am J Pathol. 159:1495–1505.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Honma K, Tsuzuki S, Nakagawa M, Tagawa H,
Nakamura S, Morishima Y and Seto M: TNFAIP3/A20 functions as a
novel tumor suppressor gene in several subtypes of non-Hodgkin
lymphomas. Blood. 114:2467–2475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kato M, Sanada M, Kato I, Sato Y, Takita
J, Takeuchi K, Niwa A, Chen Y, Nakazaki K, Nomoto J, et al:
Frequent inactivation of A20 in B-cell lymphomas. Nature.
459:712–716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mottok A, Rennè C, Seifert M, Oppermann E,
Bechstein W, Hansmann ML, Küppers R and Bräuninger A: Inactivating
SOCS1 mutations are caused by aberrant somatic hypermutation and
restricted to a subset of B-cell lymphoma entities. Blood.
114:4503–4506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cheung KJ, Delaney A, Ben-Neriah S, Schein
J, Lee T, Shah SP, Cheung D, Johnson NA, Mungall AJ, Telenius A, et
al: High resolution analysis of follicular lymphoma genomes reveals
somatic recurrent sites of copy-neutral loss of heterozygosity and
copy number alterations that target single genes. Genes Chromosomes
Cancer. 49:669–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Launay E, Pangault C, Bertrand P, Jardin
F, Lamy T, Tilly H, Tarte K, Bastard C and Fest T: High rate of
TNFRSF14 gene alterations related to 1p36 region in de novo
follicular lymphoma and impact on prognosis. Leukemia. 26:559–562.
2012. View Article : Google Scholar
|
|
102
|
O'Shea D, O'Riain C, Taylor C, Waters R,
Carlotti E, Macdougall F, Gribben J, Rosenwald A, Ott G, Rimsza LM,
et al: The presence of TP53 mutation at diagnosis of follicular
lymphoma identifies a high-risk group of patients with shortened
time to disease progression and poorer overall survival. Blood.
112:3126–3129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sander CA, Yano T, Clark HM, Harris C,
Longo DL, Jaffe ES and Raffeld M: p53 mutation is associated with
progression in follicular lymphoma. Blood. 82:1994–2004. 1993.
View Article : Google Scholar : PubMed/NCBI
|