Introduction
Acute myeloid leukemia (AML) is a malignant
hematopoietic stem cell disorder with markedly heterogeneous
cytogenetic and genomic alterations (1,2). In
2018, 60,300 new AML cases were reported in the United States
(3). Despite the development and
clinical application of novel targeted chemotherapeutic agents,
improved radiation techniques, hematopoietic stem cell
transplantation and other treatment protocols, the overall survival
rates have increased only minimally over the last two decades
(4). Therefore, more effective and
non-toxic therapeutics are urgently needed in leukemia
treatment.
Several therapeutic and chemopreventive drugs
eliminate cancerous cells by inducing apoptosis. Cancer cells
undergoing apoptosis display a number of characteristics, such as
plasma membrane blebbing, cell shrinkage, mitochondrial
depolarization, chromatin condensation and DNA fragmentation
(5,6). Caspases are vital for the execution
of apoptosis triggered by apoptotic stimuli (7). Caspase activities are regulated by
the expression of B-cell lymphoma-2 (Bcl-2) and inhibitor of
apoptosis protein (IAP) families (8,9)
Mounting evidence has demonstrated the modulation of Bcl-2 and IAP
expression by anticancer agents in several leukemia cells (10-13).
The pro-virus integration site for Moloney murine leukemia virus
(PIM) kinases, including PIM-1, -2 and -3, are active
serine/threonine kinases with overlapping functions and substrate
specificities (14). Previous
studies demonstrated the overexpression of PIM kinases in a number
of hematological malignancies and solid tumors (15-19).
Moreover, growth suppression and/or apoptosis induction due to
silencing and/or pharmacological inhibition of PIM kinases were
reported in several blood and solid cancer cells (20-22).
These results strongly support the oncogenic role of PIM
kinases.
Meridianin C is one of the natural indole alkaloids
(meridianin A-G) isolated from the marine sponge Aplidium
meridianum (23,24). The inhibitory and/or
antiproliferative effects of meridianin C, D or G
analogues/derivatives on hematological and solid cancer cells were
previously investigated (25-27).
Meridianin C and several meridianin C derivatives (compounds 7a-j)
were previously synthesized, and their PIM kinase inhibitory and
antiproliferative activities in human leukemia cells were reported
(27). In addition, the strong
anti-survival, but not pro-apoptotic, effects of meridianin C on
YD-10B human tongue cancer cells through macropinocytosis and
downregulation of Dickkopf-related protein-3 (DKK-3) were recently
reported (28). However, the
anti-leukemic effects and underlying mechanisms of action of
meridianin C and its derivatives remain largely unknown. The aim of
the present study was to investigate these anti-leukemic effects
and mechanisms of action of meridianin C and its derivatives using
MV4-11 human AML cells.
Materials and methods
Chemicals and antibodies
Meridianin C and its derivatives (compounds 7a-j)
were synthesized as previously described (27) and prepared as 10 mM stock solutions
in DMSO. AZD1208 (cat. no. S7104) was purchased from Selleck
Chemicals. RPMI-1640 (cat. no. LM011-01), fetal bovine serum (FBS;
S001-01), and penicillin/streptomycin cocktail (cat. no. LS202-02)
were purchased from Welgene, Inc. Anti-procaspase-9 antibody (cat.
no. ADI-AAM-139) was purchased from Enzo Life Sciences, Inc.
Anti-death receptor (DR)-5 antibody (cat. no. NBP1-45951) was
purchased from Novus Biologicals. Anti-poly(ADP-ribose) polymerase
(PARP) antibody (cat. no. 11835238001) was purchased from Roche
Diagnostics; anti-β-actin antibody (cat. no. A5441) was obtained
from Sigma-Aldrich; Merck KGaA; anti-human X-linked inhibitor of
apoptosis protein (XIAP) antibody (cat. no. AF221) was purchased
from R&D Systems, Inc. Anti-phosphorylated (p)-eukaryotic
initiation factor (eIF)-2α (S51) antibody (cat. no. ab32157) was
purchased from Abcam. Anti-p-S6 (S235/236; cat. no. 2211), anti-S6
(cat. no. 2317), anti-eIF-2α (cat. no. 9722), anti-p-extracellular
signal-regulated kinase (ERK)-1/2 (T202/Y204; cat. no. 9101), and
anti-ERK-1/2 (cat. no. 9102) antibodies were purchased from Cell
Signaling Technology, Inc. Anti-DR-4 (cat. no. sc-8411), anti-PIM-1
(cat. no. sc-13513), anti-PIM-2 (cat. no. sc-271844), anti-PIM-3
(cat. no. sc-293237), anti-p-Bcl-2-associated death promoter (BAD)
(Ser112) (cat. no. sc-7998), anti-BAD (cat. no. sc-8044),
anti-glucose-regulated protein (GRP)-78 (cat. no. sc-13968),
anti-Bcl-2 (cat. no. sc-509), anti-myeloid cell leukemia (Mcl)-1
(cat. no. sc-819), secondary goat anti-rabbit (cat. no. sc-2004),
and goat anti-mouse IgG (cat. no. sc-2005) antibodies were
purchased from Santa Cruz Biotechnology, Inc. z-VAD-fmk (627610)
and protease inhibitor cocktail (PIC, 100X) (539134) were purchased
from EMD Millipore. Super Signal™ West Pico PLUS Enhanced
chemiluminescence (ECL; cat. no. 34080) was purchased from Thermo
Fisher Scientific, Inc. Well plates (6- and 24-wells) were obtained
from SPL Life Sciences.
Cell culture
MV4-11 human AML cells [CRL-9591™, American Type
Culture Collection (ATCC)] and K562 human chronic myeloid leukemia
(CML) cells (CCL-243™, ATCC) were grown in RPMI-1640 supplemented
with 10% heat-inactivated FBS and 1% penicillin/streptomycin at
37°C in humidified air (95% air and 5% CO2). MV4-11 and
K562 cells carried a Fms-like tyrosine kinase 3 internal tandem
duplication and a breakpoint cluster region-Abelson mutation,
respectively (29,30).
Cell count analysis
MV4-11 or K562 cells were treated with vehicle
control (DMSO; 0.1%), meridianin C, meridianin C derivatives and/or
AZD1208 at indicated concentrations (1, 5 and 10 µM) for
different durations (24 and 48 h). The number of surviving cells
was counted with the trypan blue exclusion method, which based on
the principle that live cells have intact cell membranes and cannot
be stained. Briefly, equal amounts (15 µl) of 0.4% trypan
blue dye (cat. no. 15250-061, Gibco; Thermo Fisher Scientific,
Inc.) were added to the cell suspension and mixed by pipetting up
and down. The mixture was incubated for 1 min at room temperature
(25-27°C), after which time 10 µl of the mixture was added
to the hemocytometer and cells were counted under a phase-contrast
microscope. Approximately 50 cells were counted in each
evaluation.
Cell viability assay
MV4-11 cells were treated with vehicle control
(DMSO; 0.1%), meridianin C, meridianin C derivatives and/or AZD1208
at indicated concentrations (1, 5 and 10 µM) for different
durations (24 and 48 h). At the end of treatment, 20 µl of
MTS solution was added to each well, and the plates were incubated
at 37°C for 1 h. The absorbance of each well was measured at 490 nm
using a microplate reader (SPECTRA max 340PC; Molecular Devices,
LLC).
Measurement of DNA fragmentation
Measurement of DNA fragmentation was conducted as
previously described (21).
Briefly, MV4-11 cells were seeded into 6-well plates at a density
of 2×105 cells/ml with a volume of 2 ml in each well on
the day prior to treatment. Cells were treated with vehicle control
(DMSO) or compound 7a (1, 5 and 10 µM) and/or z-VAD-fmk (50
µM) at the indicated concentrations for 24 and 48 h. MV4-11
cells were then harvested, washed and lysed in a lysis buffer [50
mM Tris (pH 8.0), 0.5% sarkosyl, 0.5 mg/ml proteinase K and 1 mM
EDTA] at 55°C for 3 h. Subsequently, RNase A (0.5 µg/ml) was
added and the lysate was further incubated at 55°C for 18 h.
Finally, the lysate was centrifuged at 10,000 × g at 4°C for 20
min. Genomic DNA was extracted and analyzed via electrophoresis at
100 V on a 1.8% agarose gel for 20 min. The DNA was visualized and
photographed under UV illumination after staining with ethidium
bromide (0.1 µg/ml; Sigma-Aldrich; Merck KGaA) using a gel
documentation system (Gel Doc-XR; Bio-Rad Laboratories, Inc.).
Preparation of whole-cell lysates
MV4-11 cells were grown in 6-well plates at a
density of 0.25×106 cells/ml on the day prior to
treatment. After treatment and at each time point, the cells were
washed twice with PBS, and proteins were extracted using modified
RIPA buffer [50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 0.1% sodium
dodecyl sulfate, 0.25% sodium deoxycho-late, 1% Triton X-100, 1%
Nonidet P-40, 1 mM EDTA, 1 mM EGTA and PIC (1X)]. Cell lysates were
collected and centrifuged at 12,074 × g for 20 min at 4°C. Protein
concentration in the supernatant was determined by using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.).
Western blotting
Western blotting was performed as previously
described (10,21). Briefly, equal amounts of protein
(50 µg) were separated via 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (EMD Millipore) by
electroplating. The membranes were washed with Tris-buffered saline
(TBS; 10 mM Tris, 150 mM NaCl, pH 7.5) supplemented with 0.05%
(v/v) Tween-20 (TBS-T), followed by blocking with TBS-T containing
5% (w/v) non-fat dried milk. The membranes were probed overnight
using antibodies against PIM-1 (1:2,000), PIM-2 (1:2,000), PIM-3
(1:2,000), p-BAD (1:2,000), T-BAD (1:2,000), procaspase-9
(1:2,000), procaspase-3 (1:2,000), DR-4 (1:2000), DR-5 (1:2,000),
PARP (1:2,000), XIAP (1:1,000), Bcl-2 (1:1,000), Mcl-1 (1:1,000),
p-eIF-2α (1:2,000), T-eIF-2α (1:2,000), GRP-78 (1:1,000), p-S6
(1:3,000), S6 (1:3,000), p-ERK-1/2 (1:2,000), T-ERK-1/2 (1:2,000)
or β-actin (1:10,000) at 4°C, followed by incubation with secondary
antibodies conjugated to horseradish peroxidase at room temperature
for 2 h. The membranes were washed, and immune reactivities were
detected by Super Signal™ West Pico PLUS ECL (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Equal protein loading was assessed via β-actin expression
levels.
Reverse-transcription polymerase chain
reaction (RT-PCR) analysis
Total cellular RNA from conditioned MV4-11 cells was
isolated using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RT-PCR
was performed as previously described (10,21).
Briefly, equal amounts of total RNA (5 µg) were
reverse-transcribed in a 40-µl reaction mixture containing 8
µl Molony Murine Leukemia Virus Reverse Transcriptase (M-MLV
RT) 5X buffer, 3 µl 10 mM dNTPs, 0.45 µl 40
U/µl RNase inhibitor, 0.3 µl 200 U/µl M-MLV RT
(Promega Corporation) and 3.75 µl 20 µM oligo dT
(Bioneer Corporation). Single-stranded cDNA was amplified by PCR
using 4 µl 5X Green Go-Taq® Flexi reaction
buffer, 0.4 µM 10 mM dNTPs, 0.1 µl 5 U/µl Taq
polymerase, 1.2 µl 25 mM MgCl2 (Promega
Corporation), and 0.4 µl primer (20 pM/µl). The
following primer pairs were used: DR-4 sense,
5′-CTGAGCAACGCAGACTCGCTGTCCAC-3′ and antisense,
5′-AAGGACACGGCAGAGCCTGTGCCAT-3′; DR-5 sense,
5′-AGCCGCTCATGAGGAAGTTGG-3′ and antisense,
5′-GGCAAGTCTCTCTCCCAGCGTCTC-3′ Mcl-1 sense,
5′-ATCTCTCGGTACCTTCGGGAG-3′ and antisense,
5′-ACCAGCTCCTACTCCAGCAAC-3′; Bcl-2 sense,
5′-GTGGAGGAGCTCTTCAGGGA-3′ and antisense,
5′-AGGCACCCAGGGTGATGCAA-3′; XIAP sense, 5′-CGTCGATTTTGTGCTCGTCAG-3′
and antisense, 5′-GAAGCATTTATCAGGGTTATTGTCTCATG-3′; and actin
sense, 5′-TCAAGATCATTGCTCCTCCTG-3′ and antisense, 5′-CTG
CTTGCTGATCCACATCTG-3′. The PCR conditions were as follows: For DR-4
and DR-5: 35 cycles of denaturation at 95°C for 30 sec, annealing
at 63°C for 30 sec and extension at 72°C for 30 sec; for Mcl-1: 25
cycles of denaturation at 95°C for 45 sec, annealing at 56°C for 45
sec and extension at 72°C for 45 sec; for Bcl-2: 30 cycles of
denaturation at 95°C for 45 sec, annealing at 56°C for 45 sec and
extension at 72°C for 45 sec; for XIAP: 30 cycles of denaturation
at 95°C for 35 sec, annealing at 54°C for 40 sec and extension at
72°C for 30 sec; and for β-actin: 25 cycles of denaturation at 95°C
for 30 sec, annealing at 56°C for 30 sec and extension at 72°C for
30 sec. β-actin was used as an internal control to evaluate the
relative expressions of DR-4, DR-5, Mcl-1, Bcl-2 and XIAP.
Statistical analyses
Cell count analysis and MTS assay were performed in
triplicate and repeated three times. All other data were obtained
via at least three independent measurements. Data are expressed as
mean ± standard error of the mean. One-way ANOVA followed by
Dunnett's post hoc test was performed using SPSS 11.5 software
(SPSS, Inc). P<0.05 was considered to indicate statistically
significant differences.
Results
Compound 7a strongly reduces the survival
and viability of MV4-11 cells, and inhibits PIM kinase
activity
We first examined the effects of meridianin C and
its derivatives (compounds 7a-j) at a concentration of 10 µM
for 24 and 48 h on the survival and viability of MV4-11 cells. The
parent compound meridianin C did not markedly affect cell growth.
By contrast, some of its derivatives, such as compounds 7a, 7g and
7j, strongly reduced MV4-11 survival and viability after treatment
for 24 and 48 h (Fig. 1A and B).
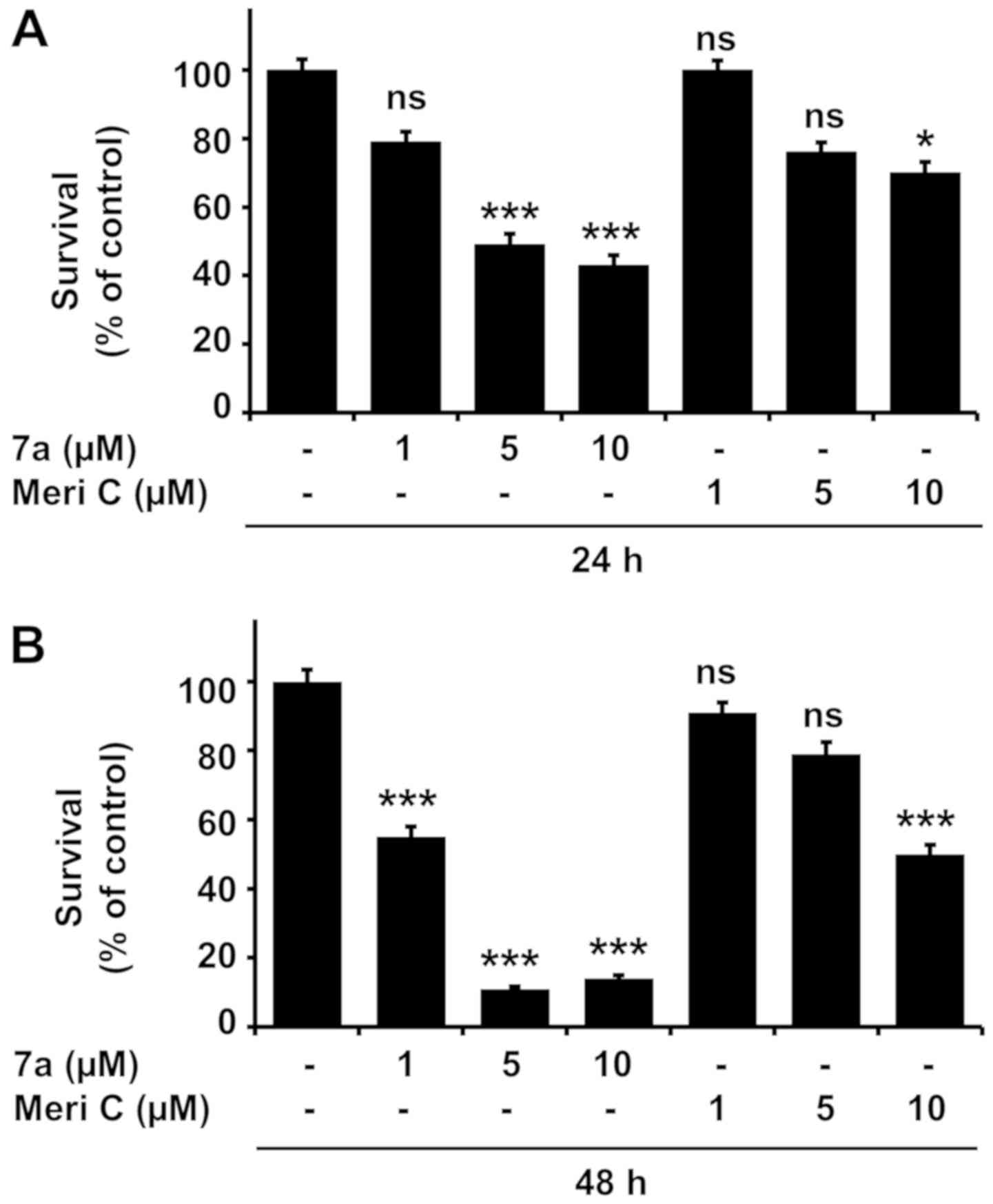
We next investigated the effects of compounds 7a, 7g and 7j on
MV4-11 cell survival and viability at different concentrations (1,
5 and 10 µM) and treatment durations (24 or 48 h) (Fig. 1C and D). The highest reduction in
MV4-11 cell survival and viability was achieved by compound 7a in a
concentration-dependent manner. The effects of compounds 7g and 7j
were substantial as well. Compared with the other compounds,
meridianin C exerted no or a very weak inhibitory effect on MV4-11
survival and viability within the tested concentration and for the
specified treatment durations. Taking into consideration the PIM
kinase inhibitory activities of meridianin C derivatives (27), we next investigated whether MV4-11
cells expressed PIM kinases and whether compound 7a affected PIM
kinase expression and activity. PIM kinase activity was assessed by
measuring the levels of phosphorylated BAD (Ser112), a downstream
target of PIM kinases (31). High
and low levels of PIM-3 and PIM-1/PIM-2, respectively, were
detected in control MV4-11 cells (Fig.
1E). BAD phosphorylation was also observed. Treatment with
compound 7a for 24 and 48 h inhibited PIM kinase activity in MV4-11
cells, as evidenced by reduced PIM kinase expression and decreased
BAD phosphorylation levels. Densitometry data (PIM-3/actin or
p-BAD/T-BAD ratio) were obtained by analyzing Fig. 1E using ImageJ software (version
1.8.0; National Institutes of Health) (Fig. 1F). The 2D structures of meridianin
C and compound 7a are shown in Fig.
1G. Due to the stronger inhibitory effect of compound 7a on
MV4-11 cell growth, we only focused on this particular meridianin C
derivative in the following analyses.
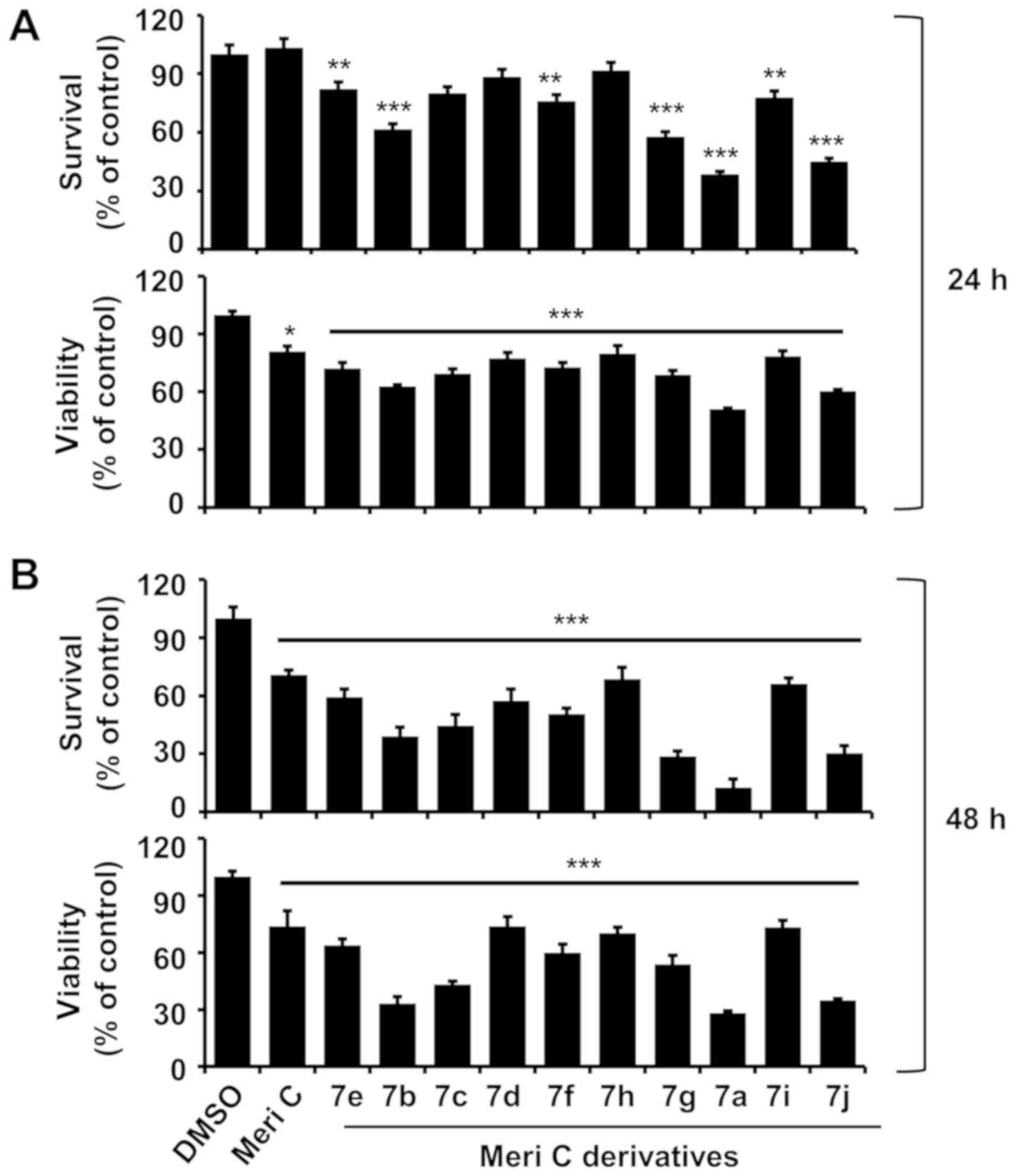 | Figure 1Effects of meridianin C and its
derivatives on the growth and/or the expression and activity of PIM
kinases in MV4-11 cells. (A and B) MV4-11 cells were treated with
vehicle control (DMSO; 0.1%), meridianin C and meridianin C
derivative (compounds 7a-j) at a final concentration of 10
µM for 24 and 48 h. The survival percentage or viable cells
was measured by cell counting or by using the MTS assay.
Experiments were performed in triplicate. Data are the means ±
standard error of the mean of three independent experiments.
*P<0.05, **P<0.01,
***P<0.001 compared with the value of vehicle control
at the indicated times. (C and D) MV4-11 cells were treated with
vehicle control and compounds 7a, 7g or 7j at the indicated
concentrations for 24 and 48 h. The survival percentage or viable
cells were measured by cell counting or by using the MTS assay.
Experiments were performed in triplicate. Data are the means ±
standard error of the mean of three independent experiments.
*P<0.05, **P<0.01,
***P<0.001 compared with the value of vehicle control
at the indicated times. (E) MV4-11 cells were treated with vehicle
control or compound 7a at the indicated concentrations for 24 and
48 h. Whole-cell lysates were prepared and analyzed by western
blotting. Each image is representative of three independent
experiments. (F) Densitometry data of (E). *P<0.05,
**P<0.01, ***P<0.001 compared with the
value of vehicle control at 24 h. @@@P<0.001 compared
with the value of vehicle control at 48 h. (G) 2D structures of
compound 7a and meridianin C. ns, non-significant; Meri C,
meridianin C; p-BAD, phosphorylated BAD; T-BAD, total BAD; PIM,
pro-viral integration site for Moloney murine leukemia virus
kinase; BAD, Bcl-2-associated death promoter. |
Compound 7a induces nuclear DNA
fragmentation, caspase-9 and -3 activation and PARP cleavage in
MV4-11 cells
Whether treatment with compound 7a induced apoptosis
in MV4-11 cells was next determined by measuring the level of
nuclear DNA fragmentation, a hallmark of apoptosis. Treatment with
5 and 10 µM compound 7a for 24 or 48 h resulted in marked
accumulation of nuclear DNA fragments in MV4-11 cells (Fig. 2A). The expression and activation
levels of caspases in MV4-11 cells treated with compound 7a were
next determined in order to investigate a possible association
between compound 7a-induced apoptosis and caspase activation.
Treatment with 5 or 10 µM compound 7a increased and
decreased the levels of upstream caspase-9 (proteolytically cleaved
active caspase form) and downstream effector caspase-3 (inactive
caspase proform) in MV4-11 cells, respectively (Fig. 2B). Caspase activation often leads
to the proteolytic cleavage of several target proteins, such as
PARP. PARP cleavage was observed in MV4-11 cells following
treatment with compound 7a for 24 and 48 h. The densitometry data
of Fig. 2B are shown in Fig. 2C-E. Finally, DR-4 and -5 expression
levels were measured in MV4-11 cells treated with compound 7a for
24 h in order to assess whether compound 7a-induced apoptosis
occurred via the extrinsic pathway. The protein and mRNA expression
levels of DR-4 and -5 were unaffected in MV4-11 cells treated with
1, 5 and 10 µM of compound 7a (Fig. 2F and G). Actin expression levels
(control) also remained constant.
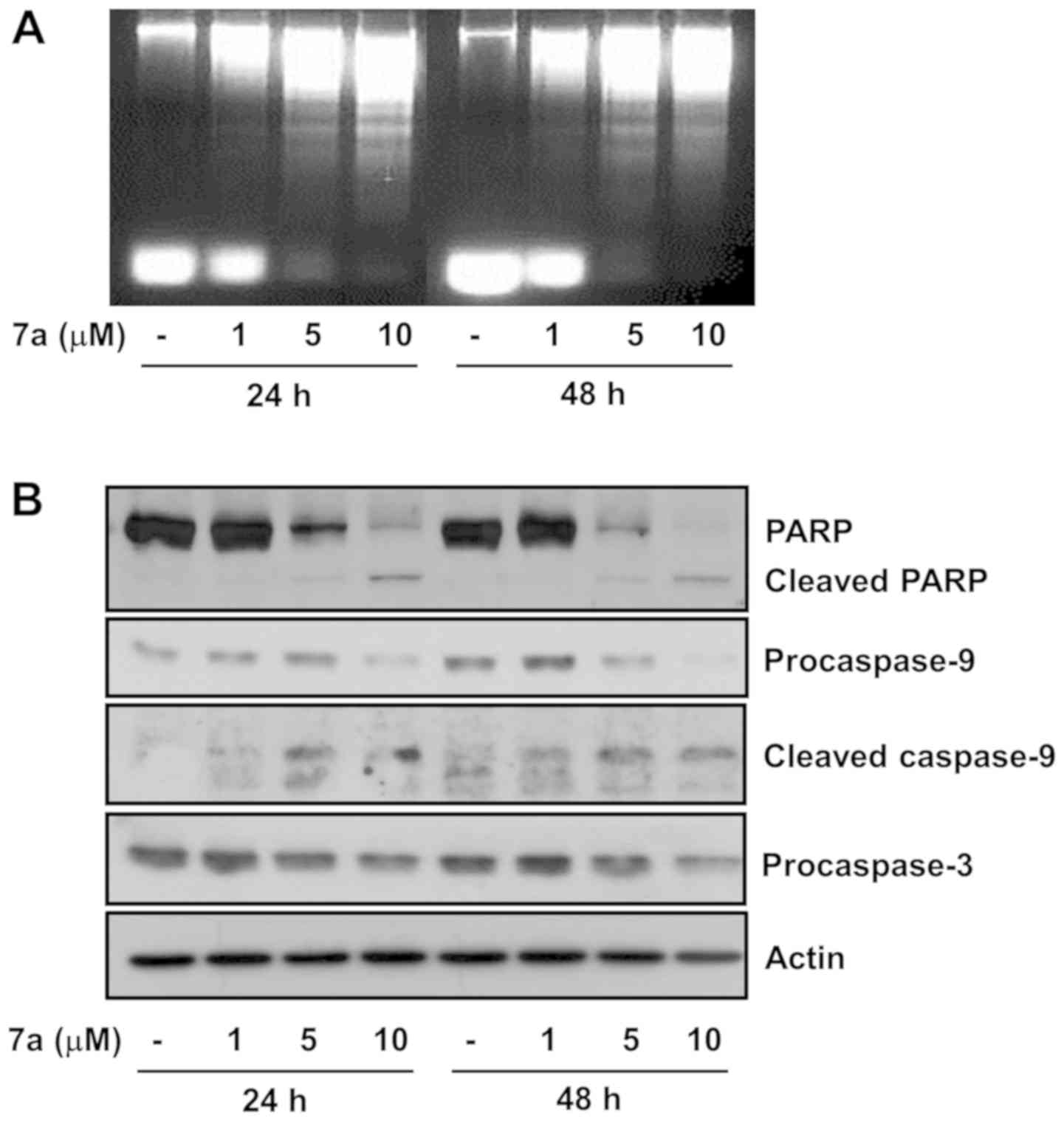 | Figure 2Effects of compound 7a on DNA
fragmentation and expression of procaspase-9 and -3, PARP, and DR-4
and-5 in MV4-11 cells. (A) MV4-11 cells were treated with vehicle
control (DMSO) or compound 7a at the indicated concentrations for
24 or 48 h. At each time point, extranuclear fragmented DNA was
extracted and analyzed on a 1.8% agarose gel. (B) MV4-11 cells were
treated with vehicle control (DMSO) or compound 7a at the indicated
concentrations for 24 or 48 h. At each time point, whole-cell
lysates were prepared and analyzed by western blotting. (C-E)
Densitometry analysis of (B). ns, non-significant;
*P<0.05, **P<0.01,
***P<0.001 compared to the value of vehicle control
at 24 h. @P<0.01, @@@P<0.001 compared
to the value of vehicle control at 48 h. (F and G) MV4-11 cells
were treated with vehicle control (DMSO) or compound 7a at the
indicated concentrations for 24 h. Whole cells lysates and total
cellular RNA were prepared and analyzed by western blotting (F) and
reverse transcription-polymerase chain reaction analysis,
respectively (G). The image is representative of three independent
experiments. DR, death receptor. |
Comparison of the effects of compound 7a,
meridianin C and AZD1208 on MV4-11 cell survival, PIM kinase
expression/activity and PARP cleavage
AZD1208 is a highly selective pan-PIM kinase
inhibitor (32). We herein
evaluated a possible association between the anti-survival and
pro-apoptotic effects of compound 7a and PIM kinase inhibition. For
this purpose, MV4-11 cells were treated with compound 7a,
meridianin C or AZD1208 at different concentrations (1, 5 and 10
µM), and cell survival, PARP cleavage and PIM kinase
expression and activity levels were measured in the conditioned
cells. As expected, compound 7a reduced the survival of MV4-11
cells in a concentration-dependent manner (Fig. 3A), and inhibited the expression and
activity of PIM kinases (Fig. 3B).
There was also slight and marked accumulation of cleaved PARP in
MV4-11 cells treated with 5 µM and 10 µM compound 7a,
respectively. However, AZD1208 and meridianin C exerted weaker
inhibitory effects on MV4-11 survival compared with compound 7a
within the tested concentration range. AZD1208 did not affect the
expression of PIM kinases, but weakly inhibited BAD phosphorylation
in MV4-11 cells. Moreover, PARP cleavage was not observed in MV4-11
cells treated with AZD1208. Meridianin C exerted little or no
effect on PARP cleavage in MV4-11 cells, although it strongly
inhibited the expression and activity of PIM kinases. Densitometry
data of Fig. 3B are shown in
Fig. 3C-E. Actin expression
(control) was unchanged. Additionally, the viability of MV4-11
cells treated with combinations of compound 7a and AZD1208 was
determined at different concentrations (1, 5 and 10 µM) for
24 h, and it was observed that the combination of AZD1208 and
compound 7a did not markedly affect the viability of MV4-11 cells
(Fig. 3F). These results indicate
that AZD1208 does not affect the activity of compound 7a and the
activity of compound 7a is not associated with any effects on PIM
kinases.
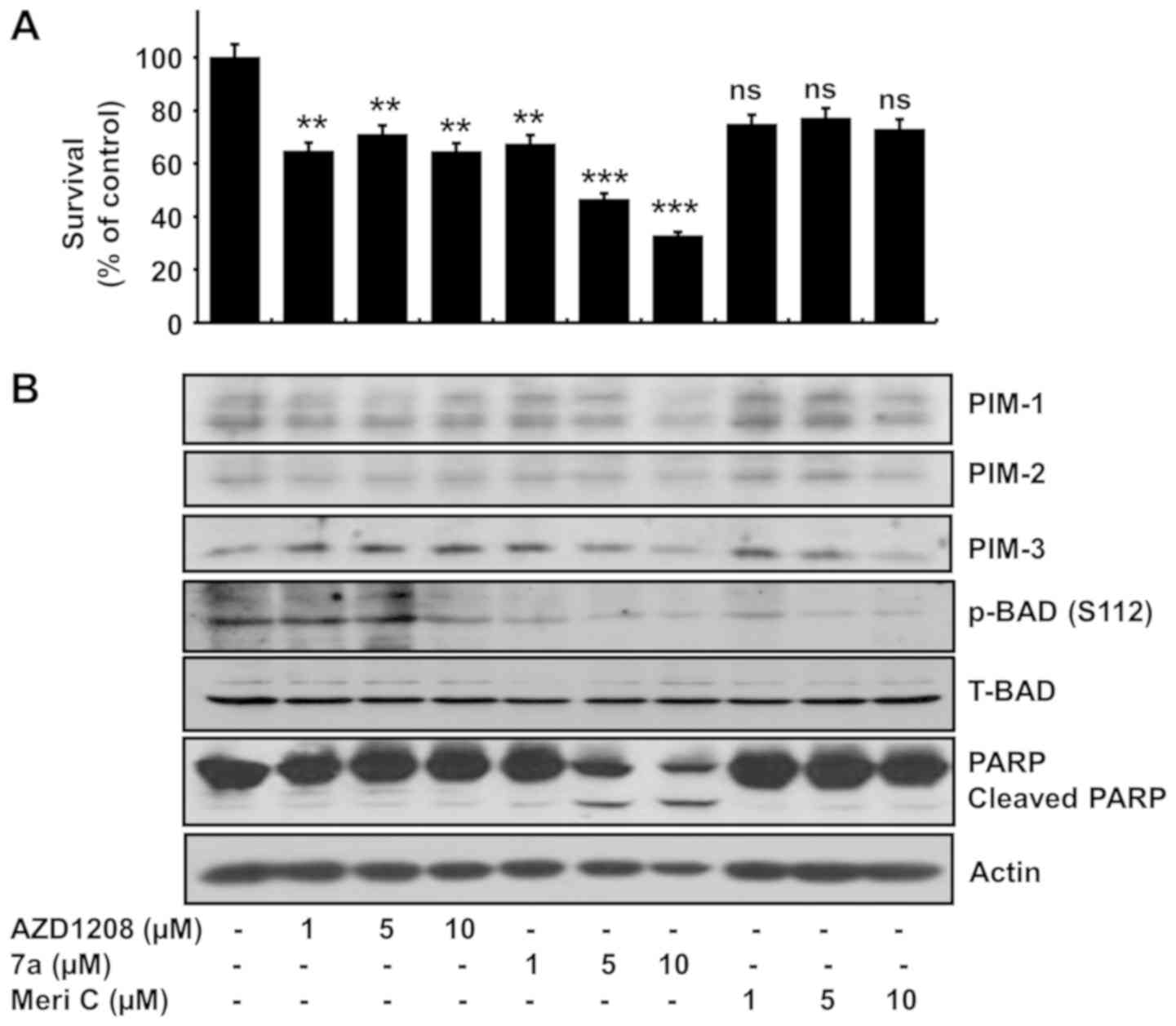 | Figure 3Effects of the pan-PIM inhibitor
AZD1208, compound 7a and meridianin C on growth and expression
and/or phosphorylation of PIM kinases, BAD and PARP in MV4-11
cells. (A) MV4-11 cells were treated with vehicle control (DMSO;
0.1%), compound 7a and meridianin C at the indicated concentrations
(1, 5 and 10 µM) for 24 h. The numbers of surviving cells
were measured by using a cell counting assay. The cell counting
assay was performed in triplicate. Data are the means ± standard
error of the mean of three independent experiments.
**P<0.01, ***P<0.001 compared to the
value of AZD1208, compound 7a or meridianin C-free control at the
indicated times. (B) MV4-11 cells were treated with vehicle control
(DMSO; 0.1%), compound 7a and meridianin C at the indicated
concentrations (1, 5 and 10 µM) for 24 h. Whole-cell lysates
were prepared and analyzed by western blotting. Each picture is
representative of three independent experiments. (C-E) Densitometry
data of (B). *P<0.05, **P<0.01,
***P<0.001 compared to the value of vehicle control
at 24 h. (F) MV4-11 cells were treated with vehicle control (DMSO;
0.1%), compound 7a and/or AZD1208 at the indicated concentrations
(1, 5 and 10 µM) for 24 h. The percentage of viable cells
was measured using an MTS assay. Experiments were performed in
triplicate. Data are the means ± standard error of the mean of
three independent experiments. ***P<0.001 compared
with the value of vehicle control at the indicated times. ns,
non-significant; p-BAD, phosphorylated BAD; T-BAD, total BAD; Meri
C; meridianin C; PIM, pro-viral integration site for Moloney murine
leukemia virus kinase; BAD, Bcl-2-associated death promoter; PARP,
poly(ADP-ribose) polymerase. |
Compound 7a alters the expression and/or
phosphorylation of caspase-9 and -3, PARP, Mcl-1, XIAP, eIF-2α and
S6 in MV4-11 cells
The molecular signaling mechanisms underlying the
growth-suppressive and apoptosis-inducing effects of compound 7a
were investigated. For this purpose, MV4-11 cells were cultured
with or without 5 µM compound 7a for 2, 4, 8 or 24 h, and
the expression and phosphorylation levels of growth and
apoptosis-related factors were measured. Compound 7a treatment led
to early activation of caspase-9 and -3 in MV4-11 cells, as
evidenced by increased and decreased expression levels of active
caspase-9 and procaspase-3, respectively (Fig. 4A). Compound 7a treatment for 4 h
also led to a marked decrease in PARP protein levels. Moreover, the
expressions of Mcl-1 and XIAP proteins were strongly downregulated
after 2 h of treatment with compound 7a in MV4-11 cells, followed
by a further decline in levels of these proteins. eIF-2α
phosphorylation increased, whereas S6 phosphorylation decreased in
MV4-11 cells after 2 h of treatment with compound 7a. The
expression of GRP-78, an endoplasmic reticulum (ER) stress marker,
and the phosphorylation of ERK-1/2, a member of the MAPK family
involved in cancer cell growth, remained unchanged in MV4-11 cells
upon compound 7a treatment. Total eIF-2α, S6 and ERK-1/2 expression
levels remained constant throughout the experiment, except for the
significant decrease in the total eIF-2α and S6 expression levels
at 24 h of compound 7a treatment. Treatment with compound 7a for 2,
4 or 8 h did not significantly affect Mcl-1 mRNA expression in
MV4-11 cells; however, Mcl-1 transcript levels substantially
decreased with 24 h of treatment (Fig.
4B). By contrast, the XIAP, DR-4 and DR-5 mRNA levels remained
unchanged in compound 7a-treated MV4-11 cells for all treatment
durations. Actin mRNA expression (control) also remained
constant.
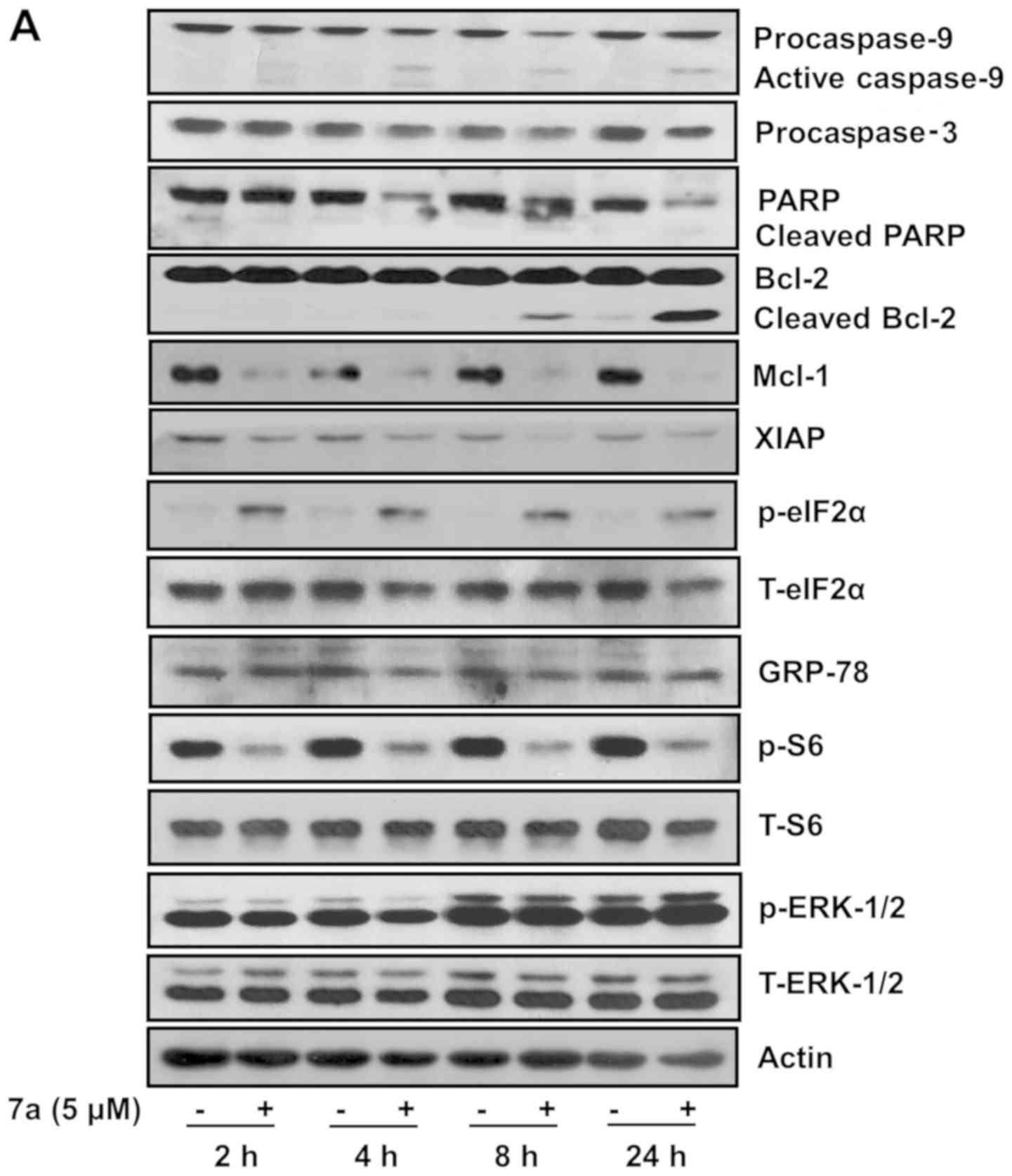 | Figure 4Effects of compound 7a on expression
and/or phosphorylation of procaspase-9 and -3, PARP, Bcl-2, Mcl-1,
XIAP, eIF-2α, GRP-78, S6, ERK-1/2 and DR-4 and -5 in MV4-11 cells.
(A and B) MV4-11 cells were treated with vehicle control (DMSO) or
compound 7a (5 µM) for the designated times. (A) At each
time point, whole-cell lysates were prepared and analyzed by
western blotting. (B) At each time point, total cellular RNA was
extracted and analyzed by reverse transcription-polymerase chain
reaction analysis. Each picture in (A) or (B) is representative of
three independent experiments. p-eIF-2α, phosphorylated eIF-2α;
T-eIF-2α, total eIF-2α; p-S6, phosphorylated S6; T-S6, total S6;
p-ERK-1/2, phosphorylated ERK-1/2; T-ERK-1/2, total ERK-1/2. PARP,
poly(ADP-ribose) polymerase; Bcl-2, B-cell lymphoma 2; Mcl-1,
myeloid cell leukemia; XIAP, X-linked inhibitor of apoptosis; eIF,
eukaryotic initiation factor; GRP, glucose-regulated protein; ERK,
extracellular signal-regulated kinase; DR, death receptor. |
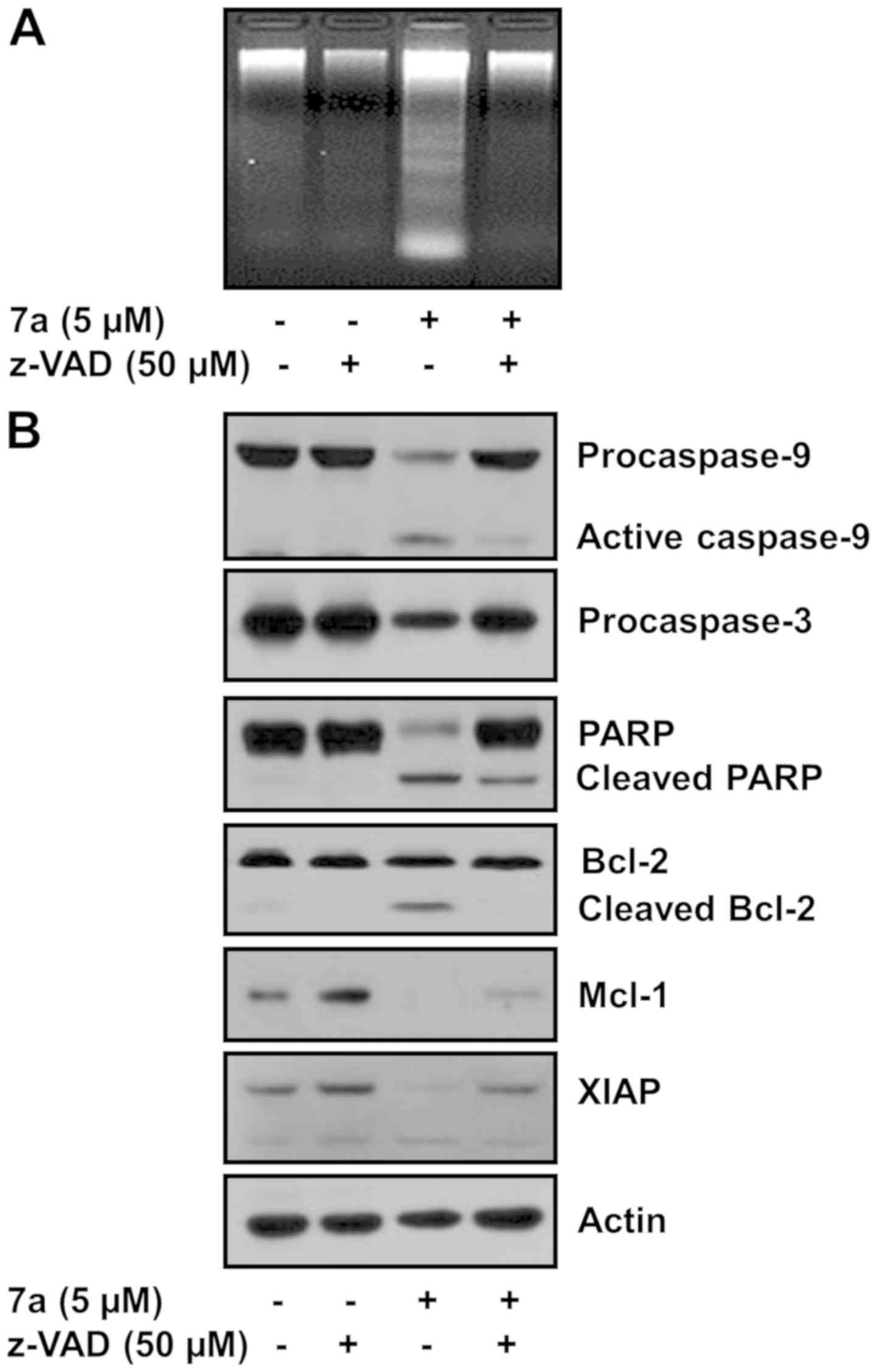
Pan-caspase inhibitor z-VAD-fmk strongly
inhibits compound 7a-induced apoptosis and alteration of PARP,
caspase-9 and -3, Bcl-2, Mcl-1 and XIAP expression in MV4-11
cells
The role of caspases in compound 7a-induced
apoptosis in MV4-11 cells was next investigated via treatment with
the pan-caspase inhibitor z-VAD-fmk. Treatment with 50 µM
z-VAD-fmk strongly inhibited compound 7a-induced DNA fragmentation
(5 µM, Fig. 5A), caspase-9
and -3 activation and PARP cleavage (Fig. 5B). Moreover, z-VAD-fmk abolished
the ability of compound 7a to induce cleaved Bcl-2 and Mcl-1 and
XIAP downregulation. Actin protein expression (control) remained
constant.
Compound 7a strongly reduces the survival
of K562 human CML cells
Finally, K562 CML cells were treated with compound
7a or meridianin C at different concentrations (1, 5 and 10
µM) for different durations (24 and 48 h), and K562 cell
survival was measured in order to determine whether the growth
inhibitory effect of compound 7a is limited to MV4-11 cells.
Meridianin C and compound 7a weakly and strongly reduced the
survival of K562 cells, respectively (Fig. 6A and B). In particular, treatment
with 5 µM compound 7a, rather than meridianin C, for 24 and
48 h resulted in a markedly higher reduction of K562 survival.
Discussion
Analogues/derivatives of meridianin C, D or G
inhibit the proliferation of hematological and solid tumor cells
(25-27). However, the regulation of leukemic
cancer cell growth by meridianin C and its derivatives remains
unclear. The present study demonstrated that a meridianin C
derivative, compound 7a, exerted strong anti-survival and
pro-apoptotic effects on MV4-11 human AML cells by regulating the
expression of caspase-9 and -3, Bcl-2, Mcl-1, XIAP, eIF-2α and S6
molecules.
Mounting evidence has demonstrated the
overexpression of PIM kinases and the oncogenic/pro-survival role
of this overexpression in a number of hematological malignancies
(15-17,20).
PIM kinase inhibitors exhibiting promising antitumor activities in
different AML cells, xenograft models and AML patients were
recently developed (15-21,32).
These results strongly support the use of PIM kinases as targets in
leukemia treatment. We previously demonstrated the
antiproliferative effects of meridianin C derivatives on human
leukemia cells, including MV4-11 cells (27), via an MTT-based cell proliferation
assay; however, the underlying mechanism remained elusive. In the
present study, while the parent compound meridianin C only weakly
affected the survival of MV4-11 cells, compound 7a markedly reduced
it. Although other meridianin C derivatives, such as compounds 7g
and 7j, also exerted growth inhibitory effects on MV4-11 cells,
their effects were notably weaker compared with that of compound
7a, particularly at a concentration of 5 µM. Meridianin C
derivatives, including compound 7a, were previously shown to exert
inhibitory effects against different PIM kinases with different
potencies in cell-free systems (27). In the present study, compound 7a
inhibited the expression and activity of PIM kinases in MV4-11
cells, as evidenced by the reduced PIM kinase expression and
decreased BAD Ser112 phosphorylation levels. Meridianin C also
suppressed PIM kinase expression and activity, and the pan-PIM
kinase inhibitor AZD1208 exerted a weak inhibitory effect on MV4-11
cell survival. Combined treatment with AZD1208 and compound 7a did
not significantly affect MV4-11 cell viability. Hence, PIM kinase
inhibition is unlikely a key mechanism in the anti-survival effect
of compound 7a on MV4-11 cells.
Compound 7a notably induced apoptosis in MV4-11
cells, as evidenced by increased nuclear DNA fragmentation and PARP
cleavage. Apoptosis is mainly mediated via intrinsic
(mitochondrial) and extrinsic (DR-mediated) pathways (33), where either mitochondria-mediated
caspase-9 or DR-dependent caspase-8 activation, respectively,
mediates the events. In the present study, compound 7a induced
caspase-9 and -3 activation without affecting DR-4 or -5 expression
in MV4-11 cells, and the pan-caspase inhibitor z-VAD-fmk greatly
attenuated compound 7a-induced apoptosis. Hence, the pro-apoptotic
effects of compound 7a on MV4-11 cells may be mainly associated
with the intrinsic apoptosis pathway.
A hallmark of cancer is apoptosis evasion, which is
often associated with the upregulation of anti-apoptotic proteins,
such as the Bcl-2 family proteins. The Bcl-2 family of proteins,
including Bcl-2 and Mcl-1, regulate apoptosis and caspase
activation by controlling the mitochondrial membrane integrity
(34). Mcl-1 is a critical
survival factor for multiple myeloma cells (35), as deduced from apoptosis induction
via downregulation of Mcl-1 by anti-sense RNA, and delay of caspase
activation via Mcl-1 overexpression. A role of Mcl-1 in the
survival of AML and chronic lymphocytic leukemia cells has also
been hypothesized (13,36). Moreover, down-regulation of Bcl-2
family proteins has been associated with the apoptotic effects of
certain anticancer agents (10-12).
Interestingly, compound 7a treatment in the present study led to
the progressive accumulation of cleaved Bcl-2 and the rapid
downregulation of Mcl-1 at the protein level in MV4-11 cells. It is
worth noting that caspases, particularly caspase-3, mediate the
cleavage of anti-apoptotic Bcl-2 (37,38).
Proteasome-dependent Mcl-1 protein degradation/downregulation in
AML cells upon treatment with anticancer agents was introduced
recently (39). AML cells were
also reported to express high Mcl-1 levels. shRNA-mediated Mcl-1
depletion induced apoptosis, whereas Mcl-1 overexpression promoted
cell survival (40). In the
present study, the pan-caspase inhibitor z-VAD-fmk strongly
inhibited compound 7a-induced accumulation of cleaved Bcl-2 and
Mcl-1 downregulation in MV4-11 cells. Thus, caspase activation is
likely responsible for compound 7a-induced Bcl-2 cleavage and Mcl-1
downregulation. Caspase-dependent Bcl-2 degradation and loss of
Mcl-1 may further contribute to the pro-apoptotic and/or
anti-survival effects of compound 7a. Human IAPs, including XIAP
and HIAP-1/2, are anti-apoptotic proteins (8). In particular, XIAP has been reported
to directly inhibit caspase family members, including caspase-3
(41) and caspase-9 (42). We herein demonstrated that compound
7a reduced XIAP protein expression in MV4-11 cells, and z-VAD-fmk
strongly blocked this process. These data suggest that caspase
activation was further responsible for the compound 7a-induced XIAP
downregulation in MV4-11 cells, and loss of XIAP is likely to favor
caspase activation and apoptosis induction in cells treated with
compound 7a.
ER stress induction is a common effect of several
anticancer agents (43). Cells
undergoing ER stress are characterized by the upregulation of
molecular chaperones (e.g., GRP-78) and phosphorylation of eIF-2α
along with inhibition of global translation (44). In the present study, compound 7a
rapidly induced eIF-2α phosphorylation, yet did not affect GRP-78
expression in MV4-11 cells, suggesting regulation of translation
rather than ER stress induction. S6 is a ribosomal protein involved
in protein synthesis (45). S6
hyperphosphorylation is associated with increased growth or
survival of cancer cells (46).
Compound 7a rapidly and sustainably inhibited S6 phosphorylation in
MV4-11 cells. Thus, the anti-survival and/or pro-apoptotic effects
of compound 7a on MV4-11 cells are partly due to the eIF-2α and
S6-dependent translation interference. As aforementioned, we
recently reported the strong anti-survival, but not pro-apoptotic,
effects of meridianin C on YD-10B cells through macropinocytosis
and DKK-3 downregulation (independently of the caspase pathway)
(28). Our results demonstrated
that, while compound 7a exerts strong anti-survival and
pro-apoptotic effects on MV4-11 cells via activation of the caspase
pathways, meridianin C does not exert such effects. The discrepancy
between these results from MV4-11 cells and those previously
obtained from YD-10B cells may be attributed to the differences
between the ability of each compound to induce activation of the
caspase pathways, and the use of different cell types (e.g.,
leukemia vs. solid cancer) with different characteristics (e.g.,
floating vs. adherent). It is also interesting to note how the
structural difference between compound 7a and the parent compound
meridianin C resulted in differences in biological activity. Unlike
meridianin C, a unique structural moiety within the compound 7a
structure may be associated with its strong anti-survival,
pro-apoptosis and caspase-activating effects on MV4-11 cells.
Compound 7a has an ethoxypyrazine substituent, which is bulkier and
more polar compared with the bromo substituent of meridianin C.
This structural feature may have contributed to the differences in
biological activity observed herein.
The effects of compound 7a and meridianin C on the
growth of K562 human CML cells were also examined, and compound 7a
was found to exert a significantly stronger growth inhibitory
effect on K562 cells compared with meridianin C (particularly at a
concentration of 5 µM). Hence, the anti-survival effects of
compound 7a were not limited to MV4-11 AML cells.
In summary, to the best of our knowledge, the
present study is the first to demonstrate the strong anti-survival
and pro-apoptotic effects of compound 7a, a meridianin C
derivative, on MV4-11 AML cells. These effects were mediated via
control of the expression and phosphorylation of caspase-9 and -3,
Mcl-1, Bcl-2, XIAP, eIF-2α and S6 molecules. Although important
issues remain to be further elucidated, such as the antitumor
effects of compound 7a on animal models, these findings suggest
that compound 7a may serve as a basis for the development of novel
anti-leukemic agents.
Funding
The present study was supported and funded by a
National Research Foundation of Korea (NRF) grant from the Korean
Government (MSIP) (no. 2014R1A5A2010008).
Availability of data and materials
All the data generated or analysed during the
present study are included in this published article.
Authors' contributions
HRC and AKY performed the experiments. YRD, MHH and
DBB analyzed the data. JL and BCJ designed and supervised the
study. AKY, DBB and BCJ wrote the paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent to publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Kornblau SM, Tibes R, Qiu YH, Chen W,
Kantarjian HM, Andreeff M, Coombes KR and Mills GB: Functional
proteomic profiling of AML predicts response and survival. Blood.
113:154–164. 2009. View Article : Google Scholar
|
|
2
|
Short NJ, Rytting ME and Cortes JE: Acute
myeloid leukaemia. Lancet. 392:593–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Cancer Institute: Cancer Stat
Facts: Leukemia. https://seer.cancer.gov/statfacts/html/leuks.html
Accessed March 2, 2019.
|
|
4
|
Ossenkoppele G and Löwenberg B: How I
treat the older patient with acute myeloid leukemia. Blood.
125:767–774. 2015. View Article : Google Scholar
|
|
5
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: The significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Q, Kang J and Fu C: The independence
of and associations among apoptosis, autophagy, and necrosis.
Signal Transduct Target Ther. 3:182018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deveraux QL and Reed JC: IAP family
proteins--suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: Changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang BC, Paik JH, Jeong HY, Oh HJ, Park
JW, Kwon TK, Song DK, Park JG, Kim SP, Bae JH, et al: Leptomycin
B-induced apoptosis is mediated through caspase activation and
down-regulation of Mcl-1 and XIAP expression, but not through the
generation of ROS in U937 leukemia cells. Biochem Pharmacol.
68:263–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao J, Xu DR, Zheng FM, Long ZJ, Huang SS,
Wu X, Zhou WH, Huang RW and Liu Q: Curcumin reduces expression of
Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute
myeloid leukemia cell lines and primary sorted CD34+ acute myeloid
leukemia cells. J Transl Med. 9:712011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitada S, Zapata JM, Andreeff M and Reed
JC: Protein kinase inhibitors flavopiridol and
7-hydroxy-staurosporine down-regulate antiapoptosis proteins in
B-cell chronic lymphocytic leukemia. Blood. 96:393–397. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luedtke DA, Niu X, Pan Y, Zhao J, Liu S,
Edwards H, Chen K, Lin H, Taub JW and Ge Y: Inhibition of Mcl-1
enhances cell death induced by the Bcl-2-selective inhibitor
ABT-199 in acute myeloid leukemia cells. Signal Transduct Target
Ther. 2:170122017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nawijn MC, Alendar A and Berns A: For
better or for worse: The role of Pim oncogenes in tumorigenesis.
Nat Rev Cancer. 11:23–34. 2011. View
Article : Google Scholar
|
|
15
|
Decker S, Finter J, Forde AJ, Kissel S,
Schwaller J, Mack TS, Kuhn A, Gray N, Follo M, Jumaa H, et al: PIM
kinases are essential for chronic lymphocytic leukemia cell
survival (PIM2/3) and CXCR4-mediated microenvironmental
interactions (PIM1). Mol Cancer Ther. 13:1231–1245. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Zavorotinskaya T, Dai Y, Niu XH,
Castillo J, Sim J, Yu J, Wang Y, Langowski JL, Holash J, et al:
Pim2 is required for maintaining multiple myeloma cell growth
through modulating TSC2 phosphorylation. Blood. 122:1610–1620.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gómez-Abad C, Pisonero H, Blanco-Aparicio
C, Roncador G, González-Menchén A, Martinez-Climent JA, Mata E,
Rodríguez ME, Muñoz-González G, Sánchez-Beato M, et al: PIM2
inhibition as a rational therapeutic approach in B-cell lymphoma.
Blood. 118:5517–5527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo S, Mao X, Chen J, Huang B, Jin C, Xu Z
and Qiu S: Overexpression of Pim-1 in bladder cancer. J Exp Clin
Cancer Res. 29:1612010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Anderson PD, Luo W, Gius D, Roh M
and Abdulkadir SA: Pim1 kinase is required to maintain
tumorigenicity in MYC-expressing prostate cancer cells. Oncogene.
31:1794–1803. 2012. View Article : Google Scholar
|
|
20
|
Keane NA, Reidy M, Natoni A, Raab MS and
O'Dwyer M: Targeting the Pim kinases in multiple myeloma. Blood
Cancer J. 5:e3252015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yadav AK, Kumar V, Bailey DB and Jang BC:
AZD1208, a Pan-Pim Kinase Inhibitor, Has Anti-Growth Effect on
93T449 Human Liposarcoma Cells via Control of the Expression and
Phosphorylation of Pim-3, mTOR, 4EBP-1, S6, STAT-3 and AMPK. Int J
Mol Sci. 20:22019. View Article : Google Scholar
|
|
22
|
Cervantes-Gomez F, Chen LS, Orlowski RZ
and Gandhi V: Biological effects of the Pim kinase inhibitor,
SGI-1776, in multiple myeloma. Clin Lymphoma Myeloma Leuk. 13(Suppl
2): S317–S329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gompel M, Leost M, De Kier Joffe EB,
Puricelli L, Franco LH, Palermo J and Meijer L: Meridianins, a new
family of protein kinase inhibitors isolated from the ascidian
Aplidium meridianum. Bioorg Med Chem Lett. 14:1703–1707. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bharate SB, Yadav RR, Battula S and
Vishwakarma RA: Meridianins: Marine-derived potent kinase
inhibitors. Mini Rev Med Chem. 12:618–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giraud F, Alves G, Debiton E, Nauton L,
Théry V, Durieu E, Ferandin Y, Lozach O, Meijer L, Anizon F, et al:
Synthesis, protein kinase inhibitory potencies, and in vitro
antiproliferative activities of meridianin derivatives. J Med Chem.
54:4474–4489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radwan MA and El-Sherbiny M: Synthesis and
antitumor activity of indolylpyrimidines: Marine natural product
meridianin D analogues. Bioorg Med Chem. 15:1206–1211. 2007.
View Article : Google Scholar
|
|
27
|
More KN, Jang HW, Hong VS and Lee J: Pim
kinase inhibitory and antiproliferative activity of a novel series
of meridianin C derivatives. Bioorg Med Chem Lett. 24:2424–2428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park NS, Park YK, Ramalingam M, Yadav AK,
Cho HR, Hong VS, More KN, Bae JH, Bishop-Bailey D, Kano J, et al:
Meridianin C inhibits the growth of YD-10B human tongue cancer
cells through macropinocytosis and the down-regulation of
Dickkopf-related protein-3. J Cell Mol Med. 22:5833–5846. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drexler HG, MacLeod RA and Uphoff CC:
Leukemia cell lines: In vitro models for the study of Philadelphia
chromosome-positive leukemia. Leuk Res. 23:207–215. 1999.PubMed/NCBI
|
|
30
|
Meshinchi S and Appelbaum FR: Structural
and functional alterations of FLT3 in acute myeloid leukemia. Clin
Cancer Res. 15:4263–4269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Macdonald A, Campbell DG, Toth R,
McLauchlan H, Hastie CJ and Arthur JS: Pim kinases phosphorylate
multiple sites on Bad and promote 14-3-3 binding and dissociation
from Bcl-XL. BMC Cell Biol. 7:12006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Keeton EK, McEachern K, Dillman KS,
Palakurthi S, Cao Y, Grondine MR, Kaur S, Wang S, Chen Y, Wu A, et
al: AZD1208, a potent and selective pan-Pim kinase inhibitor,
demonstrates efficacy in preclinical models of acute myeloid
leukemia. Blood. 123:905–913. 2014. View Article : Google Scholar :
|
|
33
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the Nomenclature Committee on Cell Death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Festjens N, van Gurp M, van Loo G, Saelens
X and Vandenabeele P: Bcl-2 family members as sentinels of cellular
integrity and role of mitochondrial intermembrane space proteins in
apoptotic cell death. Acta Haematol. 111:7–27. 2004. View Article : Google Scholar
|
|
35
|
Zhang B, Gojo I and Fenton RG: Myeloid
cell factor-1 is a critical survival factor for multiple myeloma.
Blood. 99:1885–1893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bose P and Grant S: Mcl-1 as a Therapeutic
Target in Acute Myelogenous Leukemia (AML). Leuk Res Rep. 2:12–14.
2013.PubMed/NCBI
|
|
37
|
Cheng EH, Kirsch DG, Clem RJ, Ravi R,
Kastan MB, Bedi A, Ueno K and Hardwick JM: Conversion of Bcl-2 to a
Bax-like death effector by caspases. Science. 278:1966–1968. 1997.
View Article : Google Scholar
|
|
38
|
Kirsch DG, Doseff A, Chau BN, Lim DS, de
Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA and Hardwick JM:
Caspase-3-dependent cleavage of Bcl-2 promotes release of
cytochrome c. J Biol Chem. 274:21155–21161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kapoor S, Natarajan K, Baldwin PR, Doshi
KA, Lapidus RG, Mathias TJ, Scarpa M, Trotta R, Davila E, Kraus M,
et al: Concurrent Inhibition of Pim and FLT3 Kinases Enhances
Apoptosis of FLT3-ITD Acute Myeloid Leukemia Cells through
Increased Mcl-1 Proteasomal Degradation. Clin Cancer Res.
24:234–247. 2018. View Article : Google Scholar
|
|
40
|
Kasper S, Breitenbuecher F, Heidel F,
Hoffarth S, Markova B, Schuler M and Fischer T: Targeting MCL-1
sensitizes FLT3-ITD-positive leukemias to cytotoxic therapies.
Blood Cancer J. 2. pp. e602012, View Article : Google Scholar
|
|
41
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell-death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ,
Li P, Srinivasula SM, Alnemri ES, Fairman R and Shi Y: Mechanism of
XIAP-mediated inhibition of caspase-9. Mol Cell. 11:519–527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han M, Gao H, Xie J, Yuan YP, Yuan Q, Gao
MQ, Liu KL, Chen XH, Han YT and Han ZW7: Hispidulin induces ER
stress-mediated apoptosis in human hepatocellular carcinoma cells
in vitro and in vivo by activating AMPK signaling pathway. Acta
Pharmacol Sin. 40:666–676. 2019. View Article : Google Scholar
|
|
44
|
de Haro C, Méndez R and Santoyo J: The
eIF-2alpha kinases and the control of protein synthesis. FASEB J.
10:1378–1387. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ruvinsky I and Meyuhas O: Ribosomal
protein S6 phos-phorylation: From protein synthesis to cell size.
Trends Biochem Sci. 31:342–348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen B, Tan Z, Gao J, Wu W, Liu L, Jin W,
Cao Y, Zhao S, Zhang W, Qiu Z, et al: Hyperphosphorylation of
ribosomal protein S6 predicts unfavorable clinical survival in
non-small cell lung cancer. J Exp Clin Cancer Res. 34:1262015.
View Article : Google Scholar : PubMed/NCBI
|