Introduction
Esophageal cancer (EC) is the eighth-ranked cancer
of incidence worldwide and the sixth leading cause of
cancer-related deaths in 2012 (1,2).
China is the country with the highest incidence and mortality rates
from EC globally, with a 5-year overall survival rate <30%
(3). Most patients with EC are in
advanced stages of the disease upon first diagnosis and outcomes
for surgical treatment are correspondingly very poor. Therefore,
concurrent chemoradiotherapy has become the standard treatment for
advanced localized EC, but treatment outcomes still remain poor
(4). In recent years, there has
been a rapid development in diagnosis and treatment of EC, and
although the therapeutic outcomes are better than before, certain
EC patients still have local recurrences of tumors and metastasis
after treatment (5,6). Such cases exemplify why radiotherapy
resistance is considered one of the major predictive factors for
poor prognosis and poor outcomes for EC patients (7). Therefore, it is necessary to find new
markers that help to accurately predict radiotherapy resistance, so
that novel detection targets and treatment methods for EC can be
developed and tested.
Long non-coding RNAs (lncRNAs) play an important
role in the development of various cancers, and have become a hot
topic for tumor research due to tumor tissue specificity and the
potential application in tumor diagnosis and treatment outcome
prediction (8,9). lncRNAs are a type of RNA that is
>200 bp in length, lacks or has only a minor portion of an open
reading frame, which does not encode a protein, is usually composed
of multiple exons spliced together, is transcribed by RNA
polymerase II, and has a histone modifier similar to the encoded
protein (10,11). An increasing number of lncRNAs have
been found to be closely associated with the development of tumors
in recent years (10,11), and studies have indicated that
lncRNAs can be used as promoters of development and progression for
certain types of tumors (12,13).
Colon cancer-associated transcript 2 (CCAT2), a
lncRNA, received its name when it was first discovered in
colorectal cancer (14).
Thereafter, CCAT2 was confirmed to be abnormally expressed in
various other tumor types, including breast cancer, ovarian cancer,
gastric cancer, non-small cell lung cancer, cervical cancer and
esophageal squamous cell carcinoma (15). Additional previous studies have
indicated that CCAT2 is highly expressed in tumor tissues of
esophageal squamous cell carcinoma and is associated with poor
patient prognosis (16,17). Although few studies on the
relationship between CCAT2 and radiotherapy resistance exist,
previous research has confirmed that CCAT2 can inhibit the
expression of miR-145 in cancer cells (18), and confirmed that the miR-145/P53
pathway is associated with radiotherapy resistance in cancer cells
(19). In addition, previous
studies have found that P70 ribosomal protein S6 kinase 1
(p70S6K1), an important effector protein downstream of the
apoptosis-associates Akt/ERK signaling pathway, is a target gene of
miR-145 (20). Therefore, our
study was designed to investigate effects of CCAT2 expression on
radiotherapy treatments for EC cells and to examine whether CCAT2
promoted radiotherapy resistance in EC cells via the
miR-145/p70S6K1 and the p53 signaling pathways.
Materials and methods
Tissue and ethics statement
A total of 60 EC tissue and 21 healthy esophageal
mucosa were sampled for biopsy between June 2016 and June 2018 at
Cangzhou Central Hospital Hospital. The EC tissues included 38 male
and 22 female patients with a mean age at diagnosis of 60.46±9.24
years and the healthy esophageal mucosa samples included 12 male
and 9 female patients with a mean age of 59.62±4.72 years.
Inclusion criteria were based on the following: i) No anticancer
treatment before tissue acquisition; ii) no other malignant tumors
or chronic infectious diseases present; iii) normal tissue samples
were confirmed by lack of any lesions in tissues; and iv)
completion of clinical information, such as age, gender, medical
history and a treatment plan. Exclusion criteria included: i)
Failure to complete 3 years of follow-up; ii) death due to other
diseases or accidents; iii) pregnancy, lactating and drug abusers;
and iv) withdrawal from other types of medical treatments, such as
participate in and withdraw from other clinical trials for drugs or
biotherapies). All EC patients received radiation therapy after
surgery. According to the patient's specific conditions, the
prescription dose was 50–66 Gy. The divided dose was 2 Gy/day, once
a day, 5 times a week, 6–7 weeks in total. Patients were treated
with the Varian 23EX and 120-leaf MLC 600C/D linear accelerators.
In addition, all patients or their representatives signed informed
consent documents. The Ethics Committee of the Cangzhou Central
Hospital reviewed, approved and supervised all aspects of the study
design and experiments.
Reagents
Lipofectamine 2000 (1668019), Lab-Tek Chambered
Coverglass (LOT1228622), the TUNEL assay kit (C10625), DAPI
(D21490), DMEM (61870044) and fetal bovine serum (FBS; 10437028)
were purchased from Thermo Fisher Scientific, Inc. We used the
PrimeScript RT reagent Kit with gDNA Eraser (RR047B; Takara Bio,
Inc.), GoTaq® qPCR Master Mix (A6002; Promega
Corporation), and the BCA Protein Assay kit (ab102536) and all
antibodies were purchased from Abcam.
Cell culture, treatment and
transfection
HEEC, TE-1, TE-3, ECA109, KYSE410, and KYSE520 cell
lines were purchased from The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences. TIE-3 cells overexpressing
CCAT2 were established by Genomeditech Co., Ltd. All cells were
cultured in DMEM plus 10% FBS at 37°C with 5% CO2. For
the X-ray treatment, 0.6–0.8×106 cells were seeded in
Lab-Tek Chambered Coverglass or 1.5–2.0×106 cells were
seeded in 6-well plates and cultured for 24 h. Then, cells were
exposed to 4 Gy irradiation for 24 h after transfection. We used
LncBASE v.2 (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex)
to analyze the sequences of CCAT2 and miR-145.
miR-negative control (NC;
5′-AGAUCGACCCAGGCCGUAUAUGU-3′), miR-145-mimic
(5′-GUCCAGUUUUCCCAGGAAUCCCU-3′) and miR-145-inhibitor
(5′-CAGGUCAAAAGGGUCCUUAGGGA-3′), as well as small interfering RNA
(si)-NC (forward, 5′-ACAUCAUAGUCGAACUUUATT-3′ and reverse,
5′GAAAAGGACACUAUGCGGCTT-3′), si-P53 (forward,
5′-UGGAUUUGUACCAUUCUUCUG-3′ and reverse,
5′-GAAGAAUGGUACAAAUCCAAG-3′) and si-CCAT2 (forward,
5′-ACUCAUUGGUUCCUUUAAGGG-3′ and reverse
5′-CUUAAAGGAACCAAUGAGUCC-3′) at 50 nmol/l were transfected into
2.5×106 TE-3 and ECA-109 using Lipofectamine 2000
according to the manufacturer's protocols. For wild type or mutated
versions of the 3′-UTR of MALAT1 were cloned into pisCHECK2 (cat.
no. 97157; Addgene, Inc.), and then began transfection into cells
as miRNA. After 72 h, expression was determined by reverse
transcription-quantitative (RT-q) PCR or western blot.
RT-qPCR
Levels of CCAT2, miR-145 and p70S6K1 mRNA were
detected by RT-qPCR as previously described (21). Briefly, TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract
total RNA from tissues or cells and RT was performed under the
following conditions: 37°C for 15 min, 85°C for 5 sec and hold at
4°C. qPCR was performed using the following protocol: 95°C for 30
sec, followed by 40 cycles of 90°C for 5 sec and 65°C for 30 sec.
PCR primer sets were as follows: CCAT2 forward (F),
5′-CCCTGGTCAAATTGCTTAACCT-3′ and reverse (R),
5′-TTATTCGTCCCTCTGTTTTATGGAT-3′; miR-145-F,
5′-CCTTGTCCTCACGGTCCAGT-3′ and miR-145-R,
5′-AACCATGACCTCAAGAACAGTATTT-3′; p70S6K1-F,
5′-GGGGCTATGGAAAGGTTTTTCA-3′ and p70S6K12-R,
5′-CGTGTCCTTAGCATTCCTCACT-3′; U6-F, 5′-CTCGCTTCGGCAGCACA-3′ and
U6-R, 5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH-F,
5′-CTCGCCTAGAGTGAGCTCC-3′ and GAPDH-R,
5′-AACTGCTGCGTTGACGGGTATG-3′. The 2−ΔΔCq method
(22) was used to calculate
relative expression levels of genes. GAPDH as a reference gene and
in addition we used U6 as a reference gene in miR
quantification.
Fluorescence in situ hybridization
A fluorescent probe for binding to the human version
of the CCAT2 gene was synthesized by Genomeditech Co., Ltd.
Fluorescence levels of in situ hybridization of CCAT2 in
different EC cells was determined as described previously (18). We analyzed samples using
fluorescence microscopy (magnification, x20) and LAS AF Lite 4.0
image analysis software (Leica Microsystems, Inc.).
Dual-luciferase reporter assay
The Dual-Lucy Assay kit (cat. no. D00100; Beijing
Solarbio Science & Technology Co., Ltd.) was used to detect
luciferase activity following the manufacturer's protocol. Briefly,
cells were collected at 48 h after transfection and lysed for 5 min
on ice before centrifugation (12,000 × g for 1 min at room
temperature) to collect the cell supernatant. Five volumes of
firefly luciferase reaction solution or Renilla luciferase
reaction solution were added to the cell lysate and the enzyme
activity was detected. Firefly luciferase activity was normalized
to Renilla luciferase activity.
TUNEL assays
We seeded 0.6–0.8×106 cells in Lab-Tek
Chambered Coverglass and cultured for 24 h. Then, we added DAPI (5
μg/ml) to the culture medium for 5 min at 37°C, then removed
the medium, washed samples twice with PBS and applied 4%
paraformaldehyde to fix samples at room temperature for 20 min.
Cell membranes were penetrated by applying 0.3% Triton X-100 in PBS
for 1 h at room temperature. Next, TUNEL test solution (50
μl) was added and samples were incubated in the dark for 60
min at 37°C. Products were washed twice with PBS and analyzed using
fluorescence microscopy (magnification, ×20). We have observed ≥10
fields of view per sample and LAS AF Lite 4.0 image analysis
software was used to evaluate the data.
Western blotting
The EpiQuik Whole Cell Extraction Kit (cat. no.
OP-0003; Amyjet Scientific) to exact the total protein from cells.
Levels of Bax (cat. no. ab32503; 1:1,000), Bcl2 (cat. no. ab32124;
1:2,000), caspase 3 (cat. no. ab13847; 1:2,000), active-caspase 3
(cat. no. ab2302; 1:500), p70S6K1 (cat. no. ab9366; 1:1,000),
phosphorylated (P)-p70S6K1 (cat. no. ab60948; 1:500), P-ERK (cat.
no. ab184699; 1:1,000), ERK (cat. no. ab201015; 1:2,000), P-Akt
(cat. no. ab81283; 1:2,000), Akt (cat. no. ab64148; 1:2,000), p53
(cat. no. ab131442; 1:1,000), P21 (cat. no. ab159520; 1:3,000),
c-Myc (cat. no. ab32072; 1:1,000) and GAPDH (cat. no. ab181602;
1:5,000; all from Abcam) protein were detected by western blot as
previously described (21). We
used TBST diluted with 5% skim milk as a blocking solution at room
temperature for 1 h. Primary and secondary antibodies (goat
anti-rabbit HRP-conjugated IgG; cat. no. ab6721; 1:1,000 or goat
anti-mouse HRP-conjugated IgG; cat. no. ab205719; 1:2,000; from
Abcam) were diluted with blocking solution and were incubated for 2
or 1 h at room temperature, respectively. ImageJ (version 1.37;
National Institutes of Health) was used to analyze gray values of
protein bands and GAPDH was used as the control.
Statistical analysis
Data were analyzed using SPSS 20.0 (IBM Corp.). Data
are presented as the mean ± SD of at least 3 independent
experiments. Two treatment groups were compared using unpaired
Student's t-test or Chi-square test. Multiple group (>2)
comparisons were made using one-way ANOVA followed by Duncan's
test. Kaplan-Meier analysis was used to generate survival plots and
significance was assessed by log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
CCAT2 is associated with radiotherapy
efficacy in EC patients
A total of 60 EC tissues and 21 normal esophageal
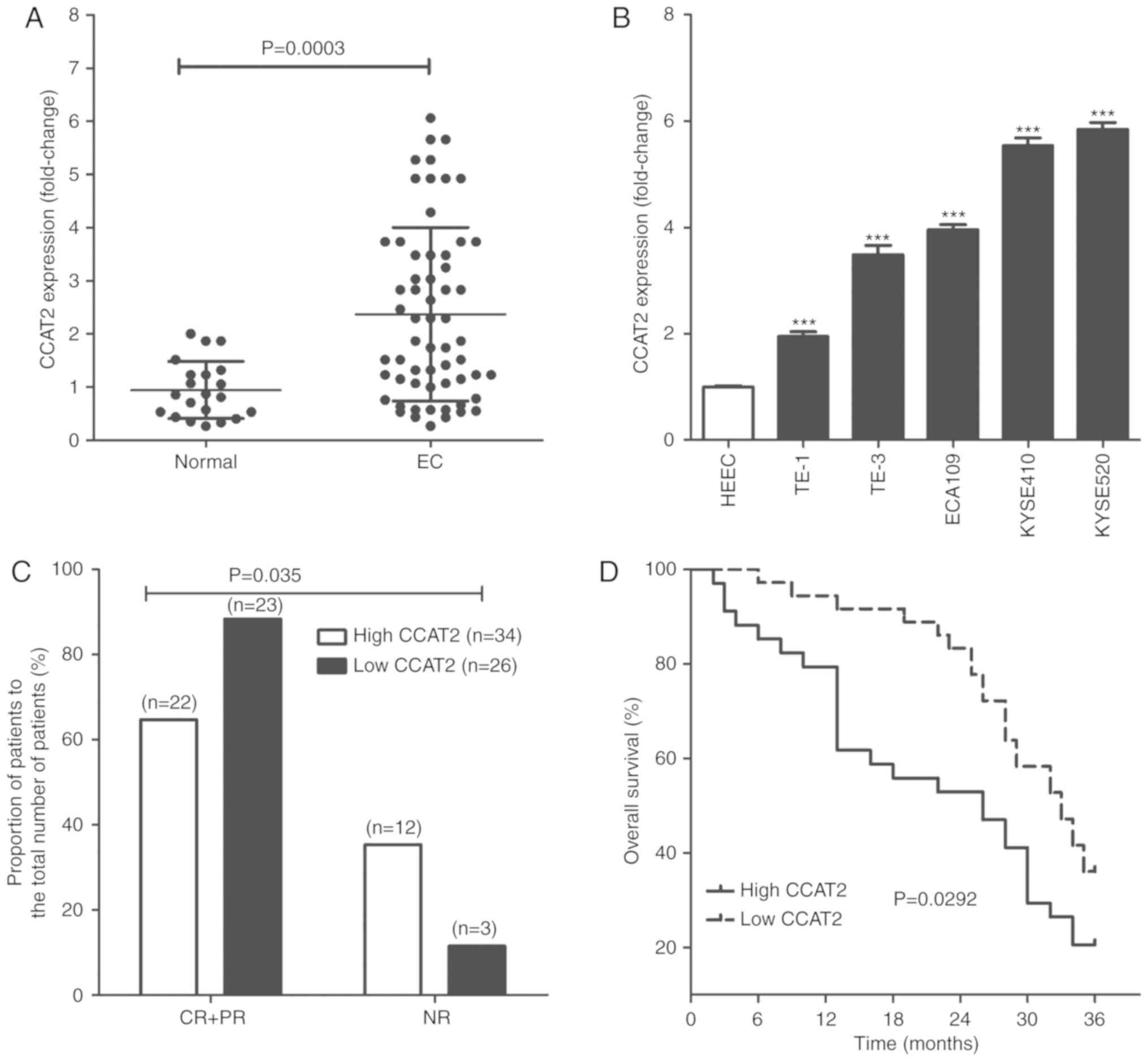
mucosa were obtained and CCAT2 levels were determined. As seen in
Fig. 1A, CCAT2 levels were
significantly higher in EC tissues compared with normal esophageal
mucosa (P<0.05). The same result was observed in vitro
for various cell lines, where CCAT2 was significantly increased in
EC cells, including TE-1, TE-3, ECA109, KYSE410 and KYSE520
compared with normal HEECs (P<0.05; Fig. 1B).
EC patients were assigned into two treatment groups
based on CCAT2 expression level; namely a high and an low CCAT2
group. Upon conclusion of radiotherapy treatments, patients were
evaluated for resultant effects according to Response Evaluation
Criteria In Solid Tumor in 2000 (23) and we identified 22 cases of
complete response (CR) + partial response (PR) and 12 cases of no
response (NR) in the high CCAT2 group. In the low CCAT2 group, we
identified 23 cases of CR+PR and 3 cases of NR (Fig. 1C). The 3-year overall survival of
EC patients in the low CCAT2 group was significantly longer than
for patients with EC in the high CCAT2 group (Fig. 1D).
Knockdown of CCAT2 induces enhanced
radiosensitivity of EC cells
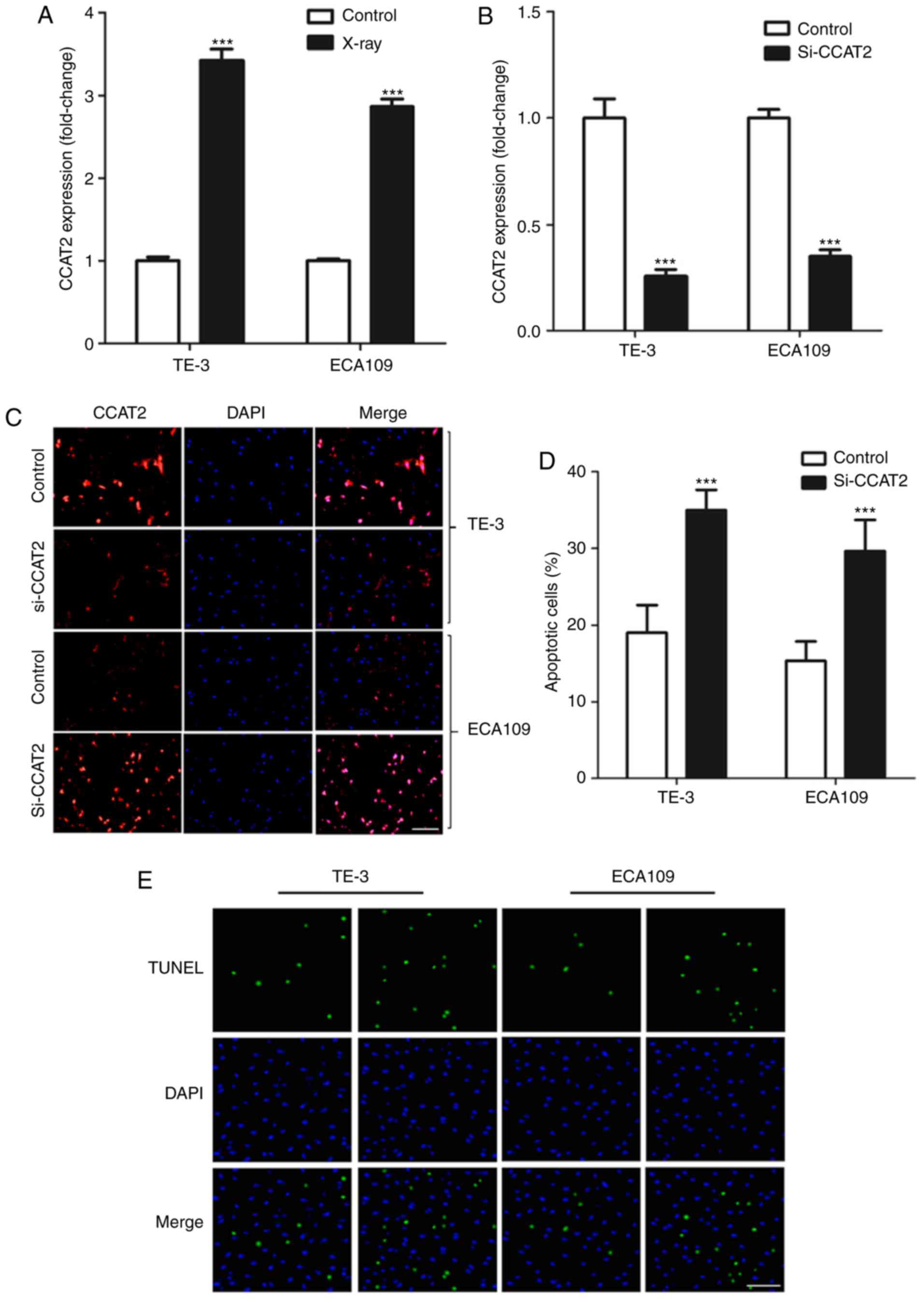
Firstly, we found that X-ray treatment significantly
increased in CCAT2 expression in EC cells in vitro (Fig. 2A). We knocked down CCAT2 expression
using si-CCAT2 (Fig. 2B and C) to
study the effect of CCAT2 expression on radiosensitivity of EC
cells and found that knockdown of CCAT2 significantly enhanced
apoptosis of EC cells after X-ray treatment (Fig. 2D and E). Moreover, we found that
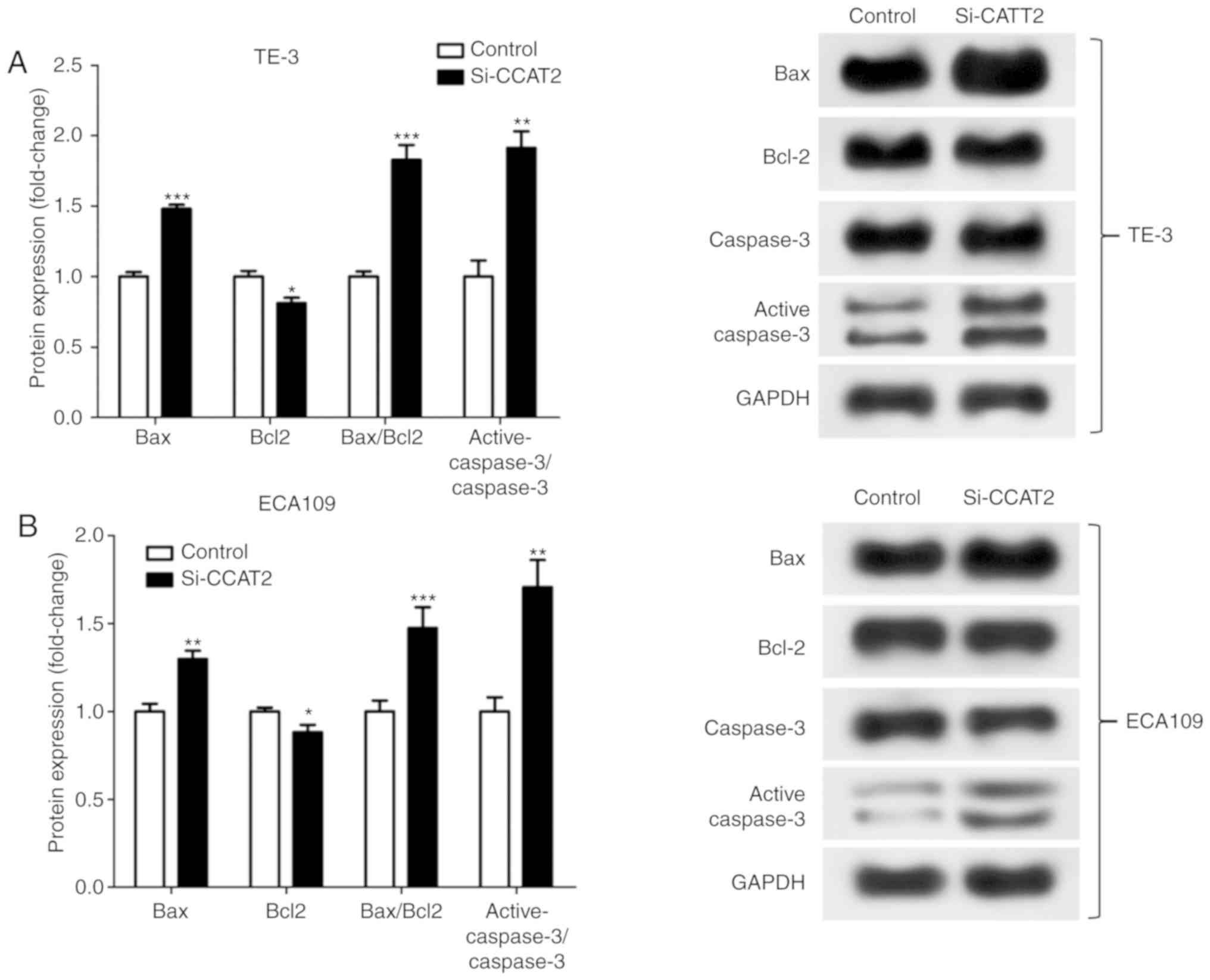
knockdown of CCAT2 significantly increased Bax/Bcl2 protein
expression and significantly increased the active-caspase 3/caspase
3 ratio in EC cells after X-ray treatment (Fig. 3).
CCAT2 negatively regulates the
miR-145/p70S6K1 and the Akt/ERK/p70S6K1 signaling pathways
As presented in Fig.
4A, si-CCAT2 was significantly reduced CCAT2 expression and
significantly increased miR-145 expression. Furthermore, we showed
that transfection with Up-CCAT2 significantly increased CCAT2
expression and significantly decreased miR-145 expression.
Additionally, miR-145-mimics significantly increased in miR-145
expression and significantly decreased CCAT2 and p72S6K1 mRNA
expression (Fig. 4B).
miR-145-inhibitor transfection resulted in a significant decrease
in miR-145 levels and significant increases CCAT2 and p72S6K1 mRNA
expression. Results from the dual luciferase reporter assay
suggested that in the WT, miR-145-mimic transfection significantly
decreased luciferase activity and miR-145-inhibitor transfection
significantly increased luciferase activity compared with the
miR-145-NC transfected cells (Fig.
4C). However, miR-145-mimic and miR-145-inhibitor did not
affect the luciferase activity in the MUT group. As seen in
Fig. 4D, transfection with
miR-145-mimic significantly decreased p70S6K1 protein expression
and miR-145-inhibitor transfection significantly increased p70S6K1
protein expression compared with the miR-145-NC transfected
cells.
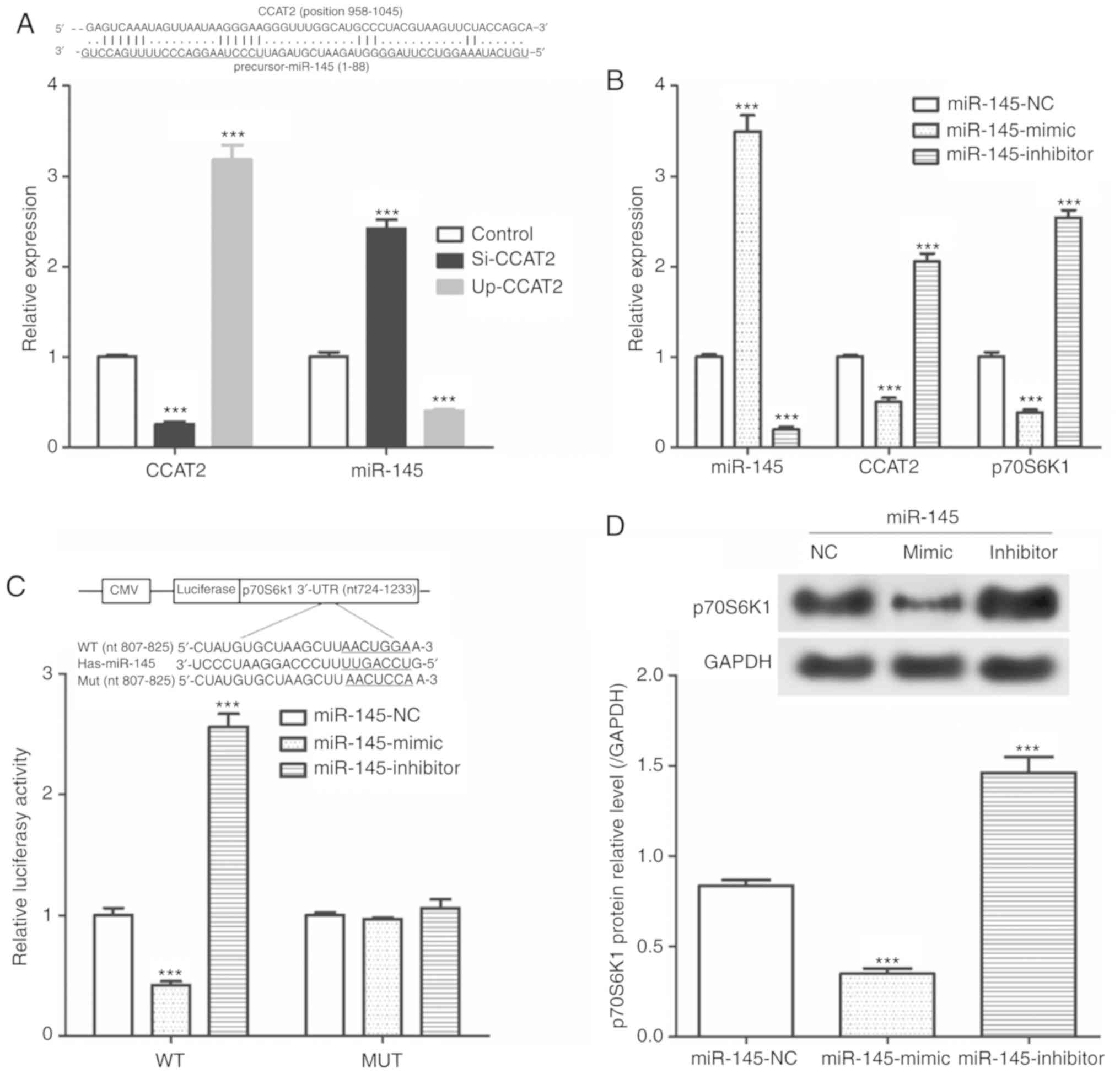 | Figure 4CCAT2 expression effects the
miR-145/p70S6K1 signaling pathway in TE-3 cells. (A) Alignment of
CCAT2 and miR-145 sequences and expression of CCAT2 and miR-145
following transfection with Si-CCAT2 and Up-CCAT2.
***P<0.001 vs. Control. (B) Expression of miR-145,
CCAT2 and p70S6K1 following transfection with miR-145 mimic,
inhibitor or NC. (C) Sequence alignment of miR-145 and p70S6K1 and
luciferase activity in WT and MUT groups transfected with miR-145
mimic, inhibitor or NC. (D) p70S6K1 protein expression in cells
transfected with miR-145 mimic, inhibitor or NC.
***P<0.001 vs. miR-145-NC. CCAT2, long non-coding RNA
colon cancer-associated transcript 2; Si, small interfering RNA;
Up, overexpression vector; NC, negative control; WT, wild type;
MUT, mutant; p70S6K1, P70 ribosomal protein S6 kinase 1. |
In addition, in TE-3 cells exposed to X-ray
irradiation, we found that knockdown of CCAT2 significantly
decreased levels of p70S6K1, P-p70S6K1, P-ERK and P-Akt and CCAT2
overexpression significantly increased levels of p70S6K1,
P-p70S6K1, P-ERK and P-Akt (Fig.
5). Results further indicated that knockdown of CCAT2
significantly decreased in phosphorylation of p70S6K1, ERK and Akt,
and overexpression significantly increased activation of these
proteins.
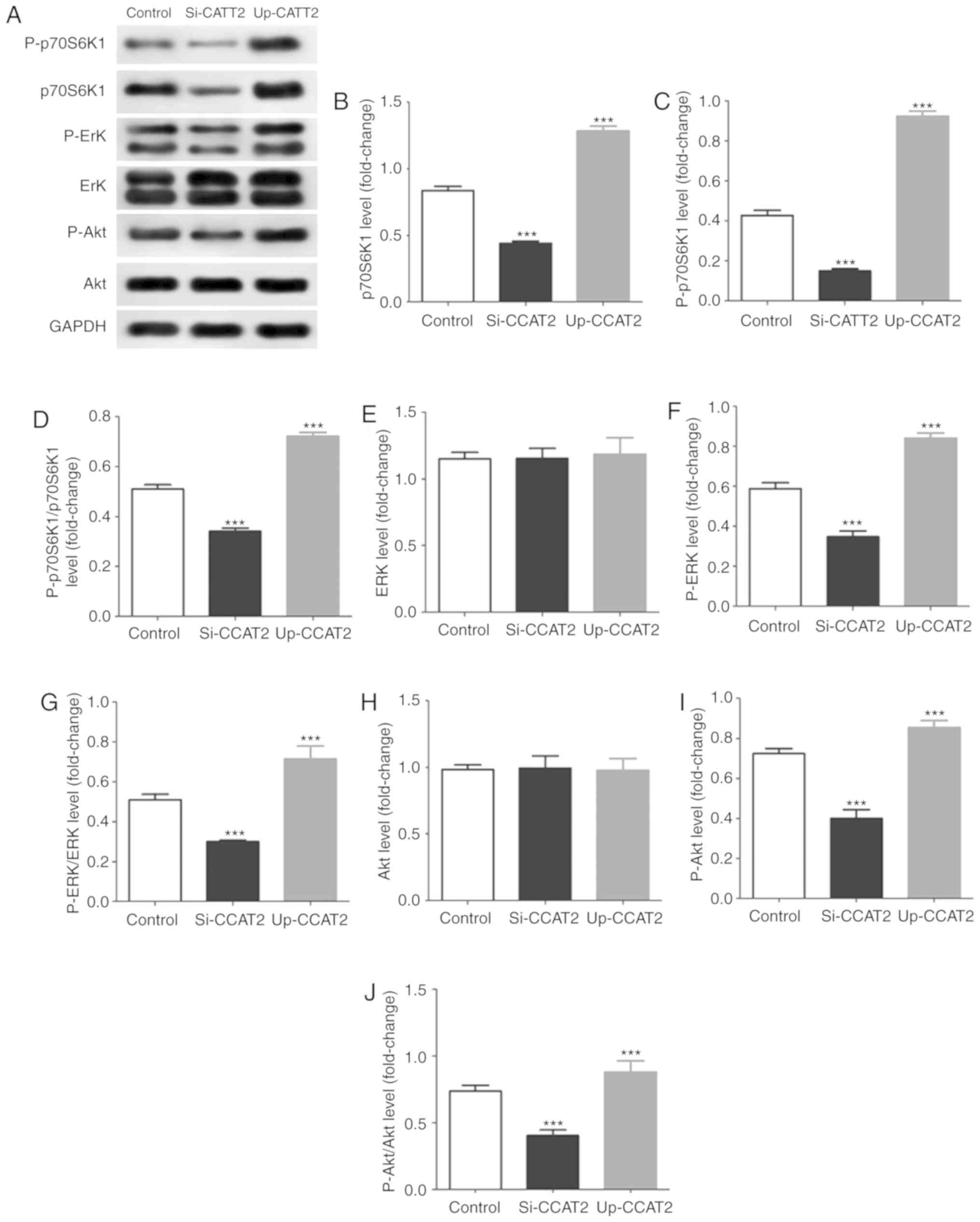 | Figure 5CCAT2 expression affects the
Akt/ERK/p70S6K1 signaling pathway in TE-3 cells after X-ray
treatment. (A) Western blot images and quantified levels of (B)
p70S6K1, (C) P-p70S6K1, (D) P-ERK/ERK, (E) P-p70S6K1/p70S6K1, (F)
ERK, (G) P-ERK, (H) Akt, (I) P-Akt and (J) P-Akt/Akt in cells
transfected with Si-CCAT2, Up-CCAT2 or a control.
***P<0.001 vs. Control. CCAT2, long non-coding RNA
colon cancer-associated transcript 2; Si, small interfering RNA;
Up, overexpression vector; p70S6K1, P70 ribosomal protein S6 kinase
1; P-, phosphorylated. |
CCAT2 negatively regulates the p53
signaling pathway
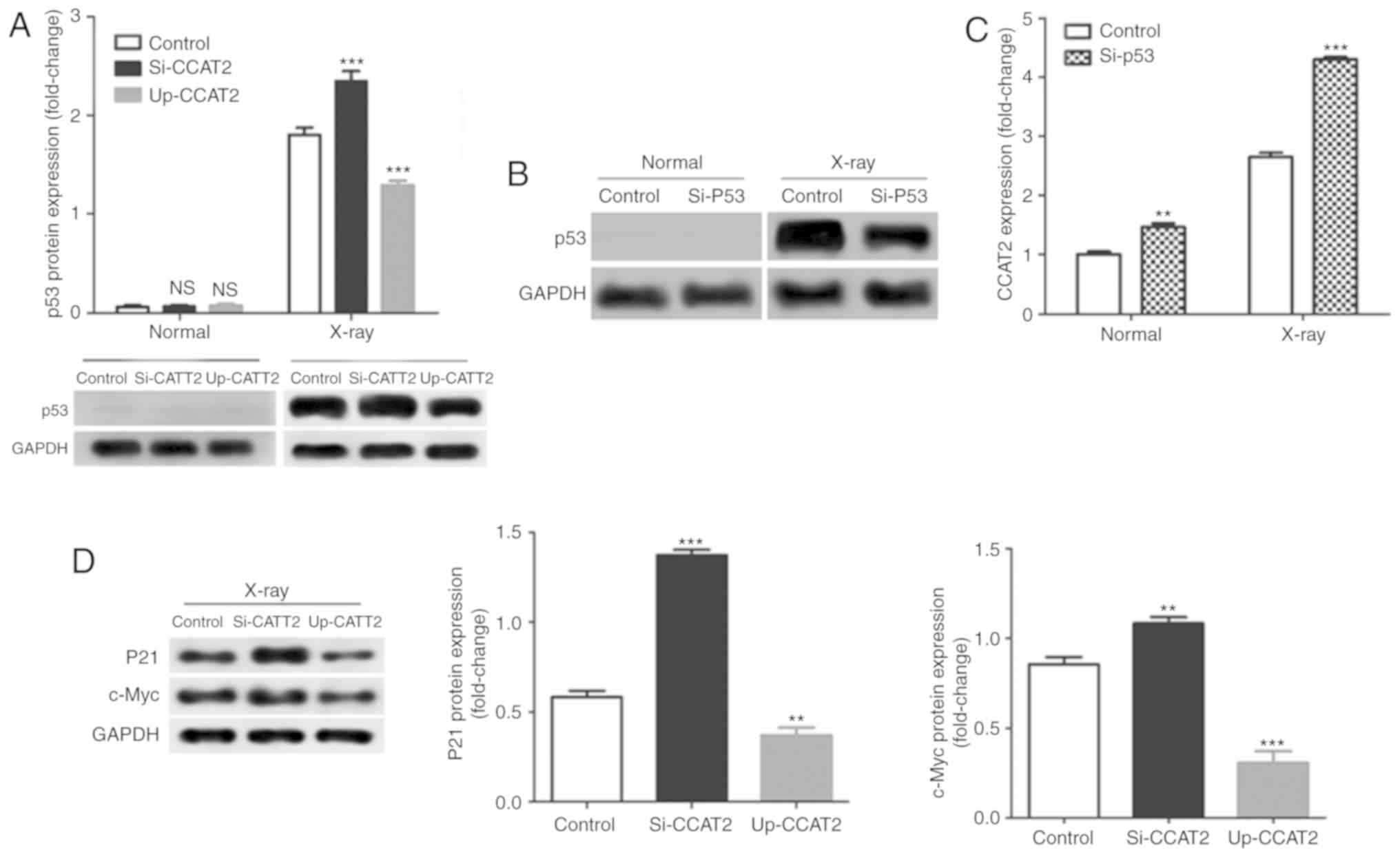
Levels of CCAT2 expression did not significantly
affect p53 expression in TE-3 cells without X-ray treatment;
however, knockdown of CCAT2 significantly increased and
overexpression significantly decreased p53 protein expression in
TE-3 cells after X-ray treatment compared with the control
(Fig. 6A). Next, we knocked down
p53 expression by transfection with si-p53; a decrease in protein
expression is only visible in the X-ray treated cells due to the
absence of protein expression in the non-X-ray exposed cells
(Fig. 6B). Knockdown of p53
significantly increased in CCAT2 expression in TE-3 cells with or
without X-ray treatment compared with the control (Fig. 6C). In addition, knockdown of CCAT2
significantly increased and overexpression of CCAT2 significantly
decreased levels of p21 and c-Myc protein expression in TE-3 cells
after X-ray treatment compared with the control (Fig. 6D).
Discussion
In the present study, we found that CCAT2 was highly
expressed in EC tissues and cells, which was consistent with
available literature (16,17). On further analysis of the
association between CCAT2 expression and radiotherapy efficacy in
patients with EC, we found that EC patients with low levels of
CCAT2 had better efficacy and outcomes from radiotherapy, and
longer overall survival times. Tumor staging and other treatment
options for EC patients may have additional effects on the efficacy
of radiotherapy and overall survival times and require further
analysis in the future. However, our results suggested that CCAT2
may be involved in the dynamics of sensitivity of EC patients to
radiotherapy.
Using in vitro experiments, we found that
X-ray treatment increased levels of CCAT2 in EC cells and knockdown
of CCAT2 promoted X-ray-induced apoptosis of EC cells. Although no
prior research has reported associations between CCAT2 expression
and EC cell apoptosis, it was reported that overexpression of CCAT2
inhibits apoptosis of cervical cancer (24) and hepatocellular carcinoma cells
(25). At present, neoadjuvant
chemoradiotherapy or chemotherapy alone have become the standards
for treatment of localized developing and advanced EC (26,27).
Accordingly, radiotherapy has played a key role in the treatment of
EC patients and has been rapidly developed as a course for
treatment in recent decades (28).
As radiotherapy is an important treatment for EC patients,
radiotherapy resistance has recently attracted more attention.
Previous studies indicated that radiotherapy can induce tumor cell
apoptosis and that sensitivity of tumors to radiotherapy is
consistent with sensitivities of tumor cells to apoptosis (29,30).
Our results suggested that CCAT2 enhanced the resistance of EC
cells to radiotherapy by inhibiting apoptosis.
Although studies that established a link between
CCAT2 expression and tumor cell radiotherapy sensitivity are rare,
potential molecular mechanisms can be hypothesized. Firstly,
previous studies indicated CCAT2 inhibits expression of miR-145 in
tumor cells (18) and miR-145
enhances radiosensitivity of cervical cancer (31), prostate cancer (32) and hepatocellular carcinoma cells
(33). Our results also indicated
that CCAT2 and miR-145 inhibited each other in EC cells. Moreover,
our results indicated that miR-145 inhibited p70S6K1 protein
expression. Previous studies indicated that the PI3K/Akt signaling
pathway and downstream kinase p70S6K1 are important in mediating
cell proliferation, differentiation and apoptosis (34,35).
Activation of PI3K is a primary signal for inhibition of apoptosis,
and PI3K induces Akt activation, which in turn activates p70S6K1
ultimately resulting in the inhibition of cell apoptosis (34,35).
Our results indicated that CCAT2 decreased phosphorylation of Akt,
ERK and p70S6K1 in EC cells. Therefore, indicating that CCAT2
enhanced the resistance of EC cells to radiotherapy by inhibiting
apoptosis via inhibiting the miR-145/p70S6K1 signaling pathway and
by activating the Akt/ERK/p70S6K1 signaling pathway.
Radiotherapy is based on the energy transmitted by
radiation destroying the chromosome of the cell causing DNA damage
in tumor cells, stopping cell growth by inducing apoptosis and
halting proliferation of tumor cells (36,37).
The P53 signaling pathway plays an important role in some of the
aspects related to cellular DNA damage, repair, and apoptosis
(38,39). We found that CCAT2 downregulated
expression of p53, p21 and c-Myc proteins. When DNA damage occurs
in cells, elevated levels of p53 induce a variety of downstream
events, including cell cycle arrest, DNA repair or differentiation,
and apoptosis (38,39). p53 activates the expression of p21,
which induces cell cycle inhibition and repairs of damaged DNA.
Further, if DNA damaged cannot be repaired smoothly, p53 regulates
apoptosis (40,41). In the p53-dependent apoptotic
signaling pathway, p21 is indirectly involved in apoptosis through
cell cycle arrest, but is not essential for apoptosis (40,41).
In addition, studies have indicated that c-Myc regulates the
function and activity levels of p21 in association with apoptosis
(42,43). Furthermore, it was found that c-Myc
does not affect binding of p53 to p21, but that p53 selectively
inhibits the activation of p21, thereby regulating p21 expression
beneficial for p53-mediated apoptosis (42,43).
In conclusion, we found that CCAT2 was highly
expressed in EC tissues and that it promoted the resistance of
radiotherapy treatment in patients with EC and EC cells by
decreasing apoptosis via inhibiting the miR-145/p70S6K1 signaling
pathway. CCAT2 further inhibited the p53 signaling pathway and
induced activation of the Akt/ERK/p70S6K1 signaling pathway. Our
findings provided novel information to help further methodological
approaches for the management of patients with EC.
Funding
This study was supported by The Excellent Going
Abroad Experts' Training Program in Hebei Province.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MW and LW conceived and designed the study. XH, JZ,
ZZ, MZ and XL performed the experiments and analyzed the data. MW
prepared and revised the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was performed with the approval of
the Ethics Committee of Cangzhou Central Hospital. All aspects of
the study complied with the Declaration of Helsinki. All
participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng H, Ren W, Pan X, Zhang Q, Liu B, Liu
S, He J and Zhou Z: Role of intravoxel incoherent motion MRI in
early assessment of the response of esophageal squamous cell
carcinoma to chemo-radiotherapy: A pilot study. J Magn Reson
Imaging. 48:349–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smyth EC, Lagergren J, Fitzgerald RC,
Lordick F, Shah MA, Lagergren P and Cunningham D: Oesophageal
cancer. Nat Rev Dis Primers. 3:170482017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burki TK: Definitions of oesophageal
cancer. Lancet Oncol. 18:e712017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakajima M and Kato H: Treatment options
for esophageal squamous cell carcinoma. Expert Opin Pharmacother.
14:1345–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clark MB, Johnston RL, Inostroza-Ponta M,
Fox AH, Fortini E, Moscato P, Dinger ME and Mattick JS: Genome-wide
analysis of long noncoding RNA stability. Genome Res. 22:885–898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tani H, Mizutani R, Salam KA, Tano K,
Ijiri K, Ai W, Isogai T, Suzuki Y and Akimitsu N: Genome-wide
determination of RNA stability reveals hundreds of short-lived
noncoding transcripts in mammals. Genome Res. 22:947–956. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:39652017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hon CC, Ramilowski JA, Harshbarger J,
Bertin N, Rackham OJ, Gough J, Denisenko E, Schmeier S, Poulsen TM,
Severin J, et al: An atlas of human long non-coding RNAs with
accurate 5′ ends. Nature. 543:199–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rao AKDM, Rajkumar T and Mani S:
Perspectives of long non-coding RNAs in cancer. Mol Biol Rep.
44:203–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CY and Xu HM: Novel perspectives of
long non-coding RNAs in esophageal carcinoma. Carcinogenesis.
36:1255–1262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Xie H, Gao Q, Zhan H, Xiao H, Zou
Y, Zhang F, Liu Y and Li J: Colon cancer associated transcripts in
human cancers. Biomed Pharmacother. 94:531–540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xin Y, Li Z, Zheng H, Chan MTV, Ka Kei and
Wu W: CCAT2: A novel oncogenic long non-coding RNA in human
cancers. Cell Prolif. 50:2017. View Article : Google Scholar
|
|
16
|
Zhang X, Xu Y, He C, Guo X, Zhang J, He C,
Zhang L, Kong M, Chen B and Zhu C: Elevated expression of CCAT2 is
associated with poor prognosis in esophageal squamous cell
carcinoma. J Surg Oncol. 111:834–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumour Biol. 36:5523–5528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Nangia-Makker P, Farhana L and
Majumdar APN: A novel mechanism of lncRNA and miRNA interaction:
CCAT2 regulates miR-145 expression by suppressing its maturation
process in colon cancer cells. Mol Cancer. 16:1552017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang P, Yang Y, An W, Xu J, Zhang G, Jie J
and Zhang Q: The long noncoding RNA-ROR promotes the resistance of
radiotherapy for human colorectal cancer cells by targeting the
p53/miR-145 pathway. J Gastroenterol Hepatol. 32:8372017.
View Article : Google Scholar
|
|
20
|
Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li
D, Lai L and Jiang BH: MiR-145 directly targets p70S6K1 in cancer
cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res.
40:761–774. 2012. View Article : Google Scholar :
|
|
21
|
Tao J, Zhang J, Ling Y, McCall CE and Liu
TF: Mitochondrial sirtuin 4 resolves immune tolerance in monocytes
by rebalancing glycolysis and glucose oxidation homeostasis. Front
Immunol. 9:4192018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Kusaba H and Saijo N: A summary report of
response evaluation criteria in solid tumors (RECIST criteria). Gan
To Kagaku Ryoho. 27:1–5. 2000.In Japanese. PubMed/NCBI
|
|
24
|
Wu L, Jin L, Zhang W and Zhang L: Roles of
long non-coding RNA CCAT2 in cervical cancer cell growth and
apoptosis. Med Sci Monit. 22:875–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou N, Si Z, Li T, Chen G, Zhang Z and Qi
H: Long non-coding RNA CCAT2 functions as an oncogene in
hepatocellular carcinoma, regulating cellular proliferation,
migration and apoptosis. Oncol Lett. 12:132–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Campbell NP and Villaflor VM: Neoadjuvant
treatment of esophageal cancer. World J Gastroenterol.
16:3793–3803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lordick F, Hölscher AH, Haustermans K and
Wittekind C: Multimodal treatment of esophageal cancer. Langenbecks
Arch Surg. 398:177–187. 2013. View Article : Google Scholar
|
|
28
|
Sihvo E, Anttonen A and Huuhtanen R:
Treatment of esophageal cancer. Duodecim. 130:565–572. 2014.In
Finnish.
|
|
29
|
Leszczynska KB, Foskolou IP, Abraham AG,
Anbalagan S, Tellier C, Haider S, Span PN, O'Neill EE, Buffa FM and
Hammond EM: Hypoxia-induced p53 modulates both apoptosis and
radiosensitivity via AKT. J Clin Invest. 125:2385–2398. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olive PL and Durand RE: Apoptosis: An
indicator of radiosensitivity in vitro? Int J Radiat Biol.
71:695–707. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye C, Sun NX, Ma Y, Zhao Q, Zhang Q, Xu C,
Wang SB, Sun SH, Wang F and Li W: MicroRNA-145 contributes to
enhancing radiosensitivity of cervical cancer cells. FEBS Lett.
589:702–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong P, Zhang T, He D and Hsieh JT:
MicroRNA-145 modulates tumor sensitivity to radiation in prostate
cancer. Radiat Res. 184:630–638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Shen Z, Zhi Y, Zhou H, Zhang K,
Wang T, Feng B, Chen Y, Song H, Wang R and Chu X: Long non-coding
RNA ROR promotes radioresistance in hepatocelluar carcinoma cells
by acting as a ceRNA for microRNA-145 to regulate RAD18 expression.
Arch Biochem Biophys. 645:117–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao P, Meng Q, Liu LZ, You YP, Liu N and
Jiang BH: Regulation of survivin by PI3K/Akt/p70S6K1 pathway.
Biochem Biophys Res Commun. 395:219–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chai X, Sun D, Han Q, Yi L, Wu Y and Liu
X: Hypoxia induces pulmonary arterial fibroblast proliferation,
migration, differentiation and vascular remodeling via the
PI3K/Akt/p70S6K signaling pathway. Int J Mol Med. 41:2461–2472.
2018.PubMed/NCBI
|
|
36
|
Weichselbaum RR, Liang H, Deng L and Fu
YX: Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev
Clin Oncol. 14:365–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krause M, Dubrovska A, Linge A and Baumann
M: Cancer stem cells: Radioresistance, prediction of radiotherapy
outcome and specific targets for combined treatments. Adv Drug
Deliv Rev. 109:63–73. 2017. View Article : Google Scholar
|
|
38
|
Ma J, Li Y, Wu M and Li X: Oxidative
stress-mediated p53/p21WAF1/CIP1 pathway may be involved
in microcystin-LR-induced cytotoxicity in HepG2 cells. Chemosphere.
194:773–783. 2018. View Article : Google Scholar
|
|
39
|
Ma J, Chen X, Xin G and Li X: Chronic
exposure to the ionic liquid [C8mim]Br induces
inflammation in silver carp spleen: Involvement of oxidative
stress-mediated p38MAPK/NF-κB signalling and microRNAs. Fish
Shellfish Immunol. 84:627–638. 2019. View Article : Google Scholar
|
|
40
|
Kim EM, Jung CH, Kim J, Hwang SG, Park J
and Um HD: The p53/p21 complex regulates cancer cell invasion and
apoptosis by targeting Bcl-2 family proteins. Cancer Res.
77:3092–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hernandez Borrero LJ, Sikder R, Lulla A,
Gokare P, Del Valle PR, Tian X, Zhang S, Abbosh PH and El-Deiry WS:
Bcl-2 protein targeting by the p53/p21 complex-letter. Cancer Res.
78:2770–2771. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Yu H, Cai H and Wang Y: Expression
of CD24, p21, p53, and c-myc in alpha-fetoprotein-producing gastric
cancer: Correlation with clinicopathologic characteristics and
survival. J Surg Oncol. 109:859–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hermeking H, Funk JO, Reichert M, Ellwart
JW and Eick D: Abrogation of p53-induced cell cycle arrest by
c-Myc: Evidence for an inhibitor of p21WAF1/CIP1/SDI1. Oncogene.
11:1409–1415. 1995.PubMed/NCBI
|