Introduction
Human colorectal cancer (CRC) is the third most
commonly diagnosed malignancy worldwide, with >1.8 million new
cases and 881,000 deaths reported in 2018 (1). The global burden of CRC is expected
to increase to >2.2 million new cases and 1.1 million deaths by
2030 (2). CRC is caused by a
series of genetic changes in key oncogenes, tumor suppressor genes
and signaling pathways, among which the epidermal growth factor
receptor (EGFR) pathway and its components have been found to be
crucial. EGFR overexpression has been observed in several cancers,
including CRC, and EGFR expression has been reported to be
associated with the survival of CRC patients (3,4).
Monoclonal antibodies targeting EGFR, such as cetuximab and
panitumumab, have been used in the clinical treatment of metastatic
CRC. However, the lack of response in a significant proportion of
patients, high cost and side effects compromise the efficacy of
these drugs in CRC treatment (5-8).
Hence, there is an urgent need for novel anticancer agents against
EGFR signaling that exhibit high efficiency and low toxicity.
Chemoprevention by natural dietary phytochemicals or
plant-derived compounds appears to be an appealing approach to
cancer treatment (9). Ginger
(Zingiber officinale Roscoe) is widely used as a spice in
foods and as an ingredient in traditional herbal medicine (10). Due to its antioxidant and
anti-inflammatory properties, ginger has been used to treat various
diseases, such as arthritis, rheumatism, indigestion, hypertension,
infectious diseases, helminthiasis and cancer (11,12).
Ginger contains >400 compounds, with 6-gingerol, 8-gingerol,
10-gingerol and 6-shogaol being the major constituents (11); these constituents belong to the
pungent compounds of ginger and contain 3-methoxy-4-hydroxyphenyl
functional groups (13). Among
these compounds, 6-gingerol and 6-shogaol are currently the most
extensively investigated in cancer. Previous studies have reported
that 6-gingerol exerts suppressive effects on cell proliferation,
angiogenesis, or metastasis in various cancers, such as lung,
liver, oral, cervical, gastrointestinal and colon cancers (10,14-18).
The anticancer effect of 6-gingerol is mainly attributed to its
ability to modulate several signaling pathways, including the
nuclear factor-κB, AKT, extracellular-signal-regulated kinase
(ERK)1/2, c-Jun N-terminal kinase and p53 pathways (10,19).
In addition, numerous studies have revealed the antitumor activity
of 6-shogaol in colon cancer, head and neck squamous cell
carcinoma, pancreatic, breast and lung cancer (20-24).
10-Gingerol has been reported to suppress cell proliferation and
migration in breast and colon cancer through manipulating the
mitogen-activated protein kinase pathway (25-29).
Similar to 6-gingerol, 10-gingerol and 6-shogaol, 8-gingerol has
antioxidant and anti-inflammatory properties (30); however, whether 8-gingerol has
antitumor properties remains largely unknown.
The aim of the present study was to investigate the
anti-tumor activity and mechanisms of action of 8-gingerol in CRC
cells, and determine whether 8-gingerol can inhibit CRC cell
proliferation, migration and invasion. The mechanism underlying the
inhibitory effect of 8-gingerol on CRC cell proliferation and the
involvement of the EGFR/signal transducer and activator of
transcription (STAT)3/ERK cascades were also investigated.
Materials and methods
Chemicals, cell lines, antibodies and
reagents
The compound 8-gingerol (99% purity, verified by
high-performance liquid chromatography) was obtained from Shanghai
Yuanye Bio-Technology Company. 5-Fluorouracil (5-FU; cat. no.
F6627) was purchased from Sigma-Aldrich; Merck KGaA, and gefitinib
(ZD1839) was purchased from Selleck Chemicals. The human colon
cancer cell lines HCT116 and DLD1 were obtained from the American
Type Culture Collection. HCT116 cells were maintained in McCoy's 5A
medium (Gibco; Thermo Fisher Scientific, Inc.) and DLD1 cells were
maintained in RPMI-1640 at 37°C in a humidified incubator with an
atmosphere of 5% CO2. All media were supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 μg/ml streptomycin. Primary
antibodies (1:1,000) against caspase-3 (cat. no. 9662), cleaved
caspase-3 (cat. no. 9661), cleaved caspase-8 (cat. no. 9496), EGFR
(cat. no. 4267), p-EGFR (cat. no. 3777), STAT3 (cat. no. 9139),
p-STAT3 (cat. no. 9145), ERK (cat. no. 9107), p-ERK1/2 (cat. no.
4377), and GAPDH (cat. no. 2118) were purchased from Cell Signaling
Technology, Inc. Antibodies (1:500) against cyclin D1 (cat. no.
Sc-20044), CDK4 (cat. no. Sc-23896), CDK6 (Sc-7961), caspase-8
(cat. no. Sc-56070) and Bcl-2 (cat. no. Sc-509) were purchased from
Santa Cruz Biotechnology, Inc. Anti-Myc (1:1,000, cat. no. R95025)
and anti-matrix metallopeptidase (MMP)2 (1:1,000, cat. no.
10373-2-AP) antibodies were purchased from Invitrogen; Thermo
Fisher Scientific, Inc. and ProteinTech Group, Inc., respectively.
Secondary antibodies (anti-rabbit, cat. no. SA00001-1, 1:2,000; and
anti-mouse, cat. no. SA00001-2, 1:2,000) were purchased from
ProteinTech Group, Inc. Recombinant human EGF protein was purchased
from R&D Systems, Inc.
Cell proliferation assay
The effect of 8-gingerol on CRC cell viability was
determined by a Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc.). Briefly, cells (5×103
cells/well) were seeded in 96-well plates and treated with
different concentrations of 8-gingerol. After incubation for 24, 48
or 72 h, 10 µl of CCK-8 solution was added to each well.
After incubation for another 1 h, the absorbance was measured at
450 nm using a spectrophotometric plate reader (Sunrise; TECAN,
Inc.). Results were calculated as percentages of vehicle
(DMSO)-treated cells. The cell viability assay of 5-FU and the
combination of 5-FU and 8-gingerol was performed using the same
protocol as mentioned above, except that cells were treated with
5-FU or 5-FU and 8-gingerol combination for 48 h. The
IC50 values are expressed as the means ± standard
deviation from triplicate experiments.
Colony formation assay
Cells (500 cells/well) were plated in 6-well plates
and treated with different concentrations of 8-gingerol (0, 10, 30,
50 and 70 µM). After treatment for 10-14 days, cells were
fixed with methanol for 15 min at room temperature. Subsequently,
the cells were stained with 0.5% crystal violet solution for 10 min
at room temperature and washed with PBS three times. The cells were
then photographed (GS-800, ×1 magnification; Bio-Rad Laboratorie,
Inc.) and the colonies were counted.
Cell cycle analysis
Cell cycle analysis was performed using flow
cytometry. Cells in 6-well plates were treated with different
concentrations of 8-gingerol for 48 h. Subsequently, the cells were
harvested and washed with PBS. After centrifugation at 300 × g for
5 min at room temperature, the cell suspension was fixed with cold
100% ethanol for 30 min at 4°C. After washing with PBS, cells were
stained using a Cell Cycle Staining kit (Multisciences Biotech)
according to the manufacturer's instructions. The cell cycle
distribution was determined using a Gallios™ flow cytometer
(Beckman Coulter, Inc.).
Apoptosis analysis
Apoptosis of CRC cells was analyzed by flow
cytometry using an Annexin V-FITC/PI Apoptosis kit (Multisciences
Biotech) according to the manufacturer's instructions. Briefly,
cells were seeded (2×105 cells/well) in 6-well plates
and treated with different concentrations of 8-gingerol for 48 h,
harvested, washed with PBS, and resuspended in 1X binding buffer.
Subsequently, the cells were incubated with 5 µl Annexin
V-FITC and 10 µl PI at room temperature for 10 min in the
dark. The samples were analyzed using the Gallios™ flow
cytometer.
Transwell migration and invasion
assays
Transwell migration assays were performed using
Corning chambers with 8.0-µm-pore polycarbonate membranes
(Corning, Inc.). Cells cultured in 6-well plates were treated with
different concentrations of 8-gingerol for 48 h. Subsequently,
1×105 cells in 100 µl of serum-free medium were
seeded in the upper chambers, and 600 µl of basic medium
supplemented with 10% FBS was added to the lower chambers. After
incubation for 24 h, cells on the upper surface of the membrane
were removed with a cotton swab, and the cells that had migrated to
the lower surface of the membrane were fixed with methanol for 15
min and stained with 0.5% crystal violet solution for 10 min at
room temperature. Cells in five randomly selected fields of the
membrane were counted under an inverted microscope (×100
magnification; Leica DMI4000B; Leica, Inc.). Transwell invasion
assays were performed using the same protocol as the Transwell
migration assays, except that the Transwell membranes were coated
with Matrigel (BD Biosciences) for 1 h at 37°C prior to cell
seeding.
Western blot analysis
Cells were harvested and lysed in lysis buffer [50
mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.1% SDS, 1% NP-40] supplemented
with protease/phosphatase inhibitors. After incubation on ice for
20 min, the cells were centrifuged at 12,000 × g for 15 min at 4°C.
Protein concentration was determined using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Protein samples (50 µg per
lane) were separated by 10% SDS-PAGE and transferred to PVDF
membranes. The membranes were blocked in TBST [50 mM Tris-HCl, 150
mM NaCl, 0.1% Tween-20 (pH 7.6)] containing 5% non-fat milk.
Subsequently, the membranes were incubated with the aforementioned
primary antibodies against caspase-3, cleaved caspase-3, cleaved
caspase-8, EGFR, p-EGFR, STAT3, p-STAT3, ERK, p-ERK1/2, GAPDH,
cyclin D1, CDK4, CDK6, caspase-8, Bcl-2, anti-Myc and MMP2
overnight, followed by horseradish peroxidase (HRP)-conjugated
secondary antibodies for 1 h at room temperature. Protein bands
were detected using Immobilon™ Western Chemiluminescent HRP
Substrate (ECL; EMD Millipore).
Statistical analysis
Statistical analysis was performed using SPSS
version 22.0 (IBM Corp.). Data are expressed as the means ±
standard deviation. Statistical comparisons were performed using
one-way ANOVA. The Bonferroni post hoc test was used for multiple
comparisons between groups (Fig.
5), and Dunnett's t-test was used for comparison with the
control group. P<0.05 was considered to indicate statistically
significant differences.
Results
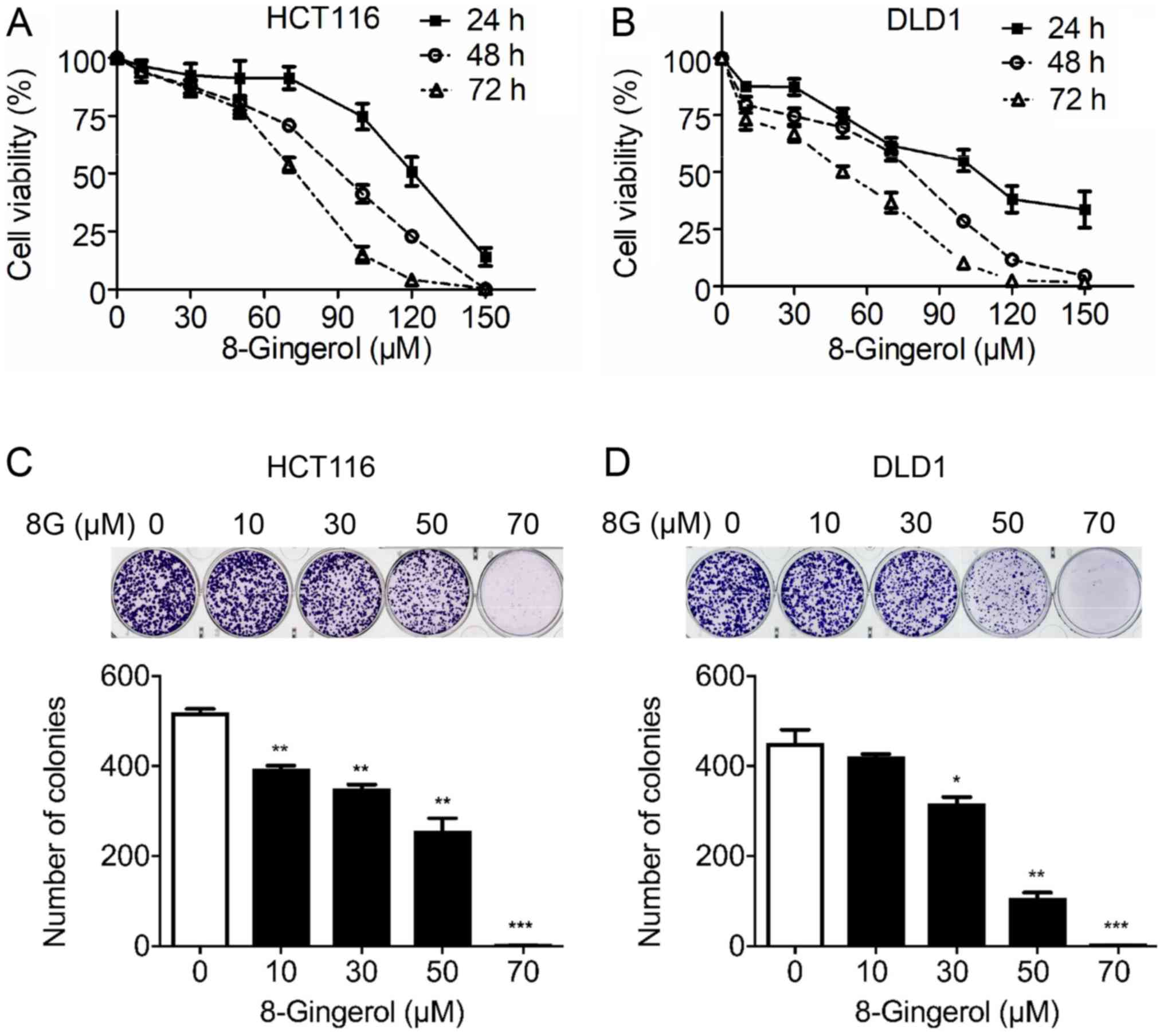
8-Gingerol suppresses CRC cell
proliferation
To gain insight into the role of 8-gingerol in CRC,
the effects of increasing concentrations of 8-gingerol on the
viability of the CRC cell lines HCT116 and DLD1 for 24, 48 and 72 h
were determined via a CCK-8 assay. The results demonstrated that
8-gingerol decreased the viability of HCT116 and DLD1 cells in a
time- and dose-dependent manner (Fig.
1A and B). The IC50 value (50% inhibition) of
8-gingerol for HCT116 cells was 118.2±7.37 µM at 24 h,
77.4±4.70 µM at 48 h, and 61.8±3.57 µM at 72 h. The
IC50 value of 8-gingerol for DLD1 cells was 100.3±6.32
µM at 24 h, 53.7±2.24 µM at 48 h, and 34.5±2.33
µM at 72 h. Consistent with the CCK-8 assay results, the
colony formation assay results also revealed that 8-gingerol
dose-dependently inhibited the clonogenic activity of both the
HCT116 and DLD1 cell lines (Fig. 1C
and D).
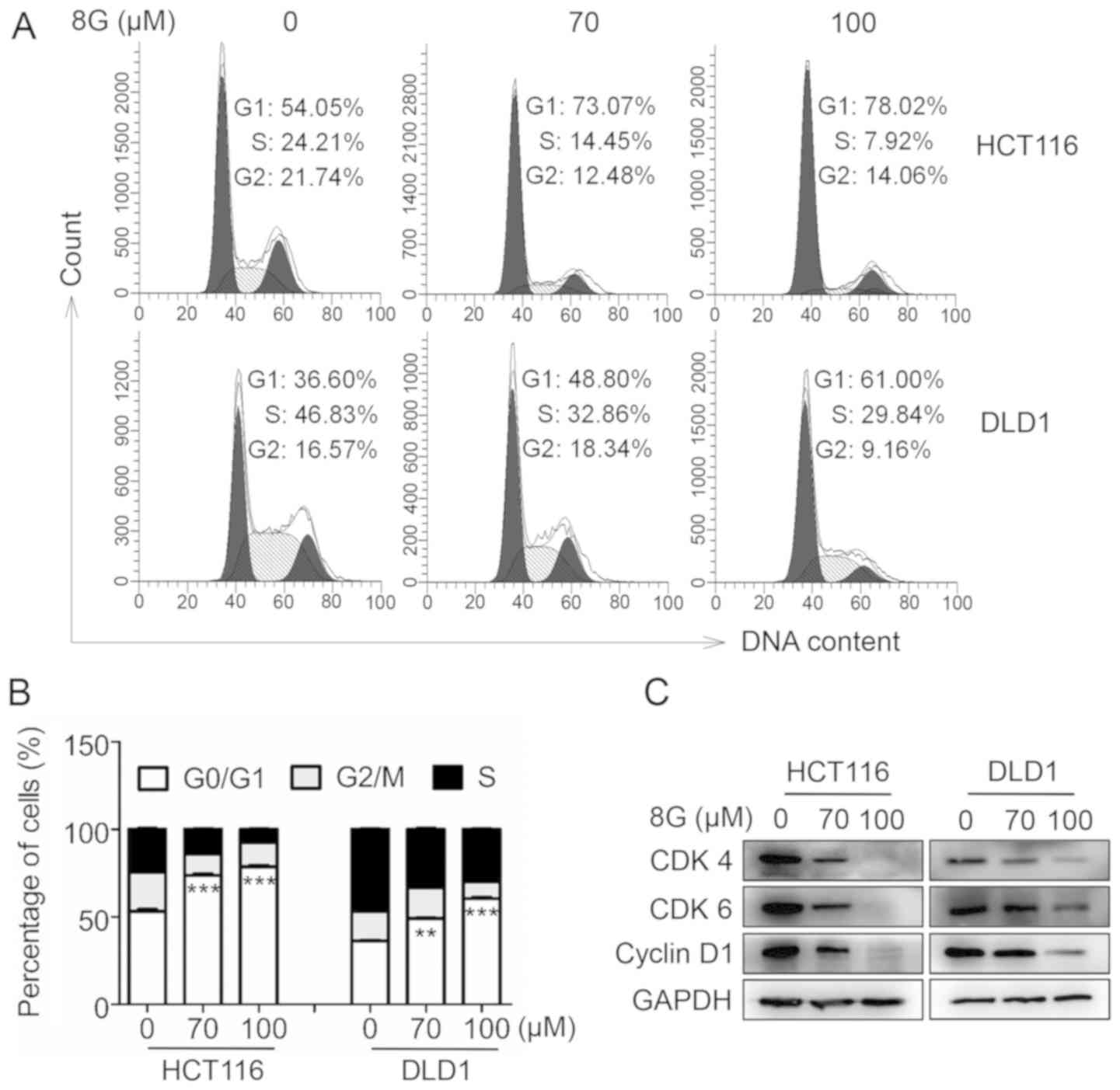
8-Gingerol induces G0/G1 cell cycle
arrest in CRC cells
To elucidate the mechanism underlying the inhibitory
effect of 8-gingerol on cell proliferation, the cell cycle
distribution of CRC cells following 8-gingerol treatment was first
investigated by flow cytometry. It was observed that 8-gingerol
treatment markedly induced G0/G1 phase cell cycle arrest in both
HCT116 and DLD1 cells (Fig. 2A and
B). In agreement with the cell cycle arrest pattern, the levels
of CDK4, CDK6 and cyclin D1, the key regulators of the G0/G1 phase
transition, were markedly decreased in both the HCT116 and DLD1
cell lines following 8-gingerol exposure (Fig. 2C). Taken together, these results
suggest that 8-gingerol induces G0/G1 cell cycle arrest in CRC
cells.
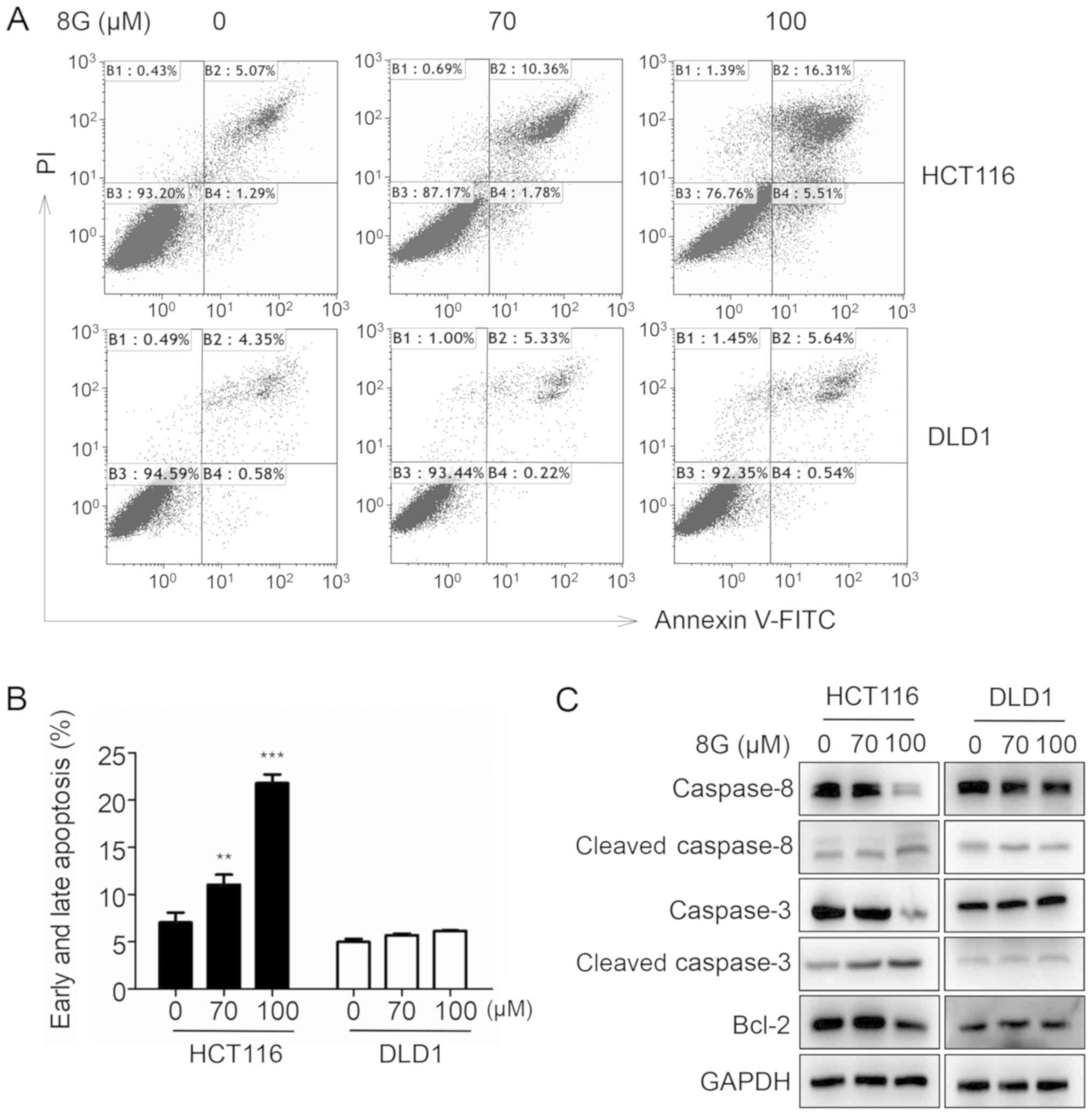
8-Gingerol enhances apoptosis in HCT116
cells
Apoptosis is another important cause of cell growth
inhibition. Next, we investigated whether 8-gingerol was also able
to induce apoptosis in CRC cells. Indeed, flow cytometric analysis
revealed that treatment with 8-gingerol increased the apoptosis
rates of HCT116 cells in a dose-dependent manner; however, this
phenomenon was not observed in DLD1 cells (Fig. 3A and B). Consistent with these
results, the expression levels of the apoptosis markers cleaved
caspase-3 and cleaved caspase 8 were significantly increased, and
the expression level of the antiapoptotic regulator Bcl-2 was
decreased in HCT116 cells following 8-gingerol exposure (Fig. 3C). Taken together, these data
suggest that 8-gingerol induces apoptosis in HCT116 cells.
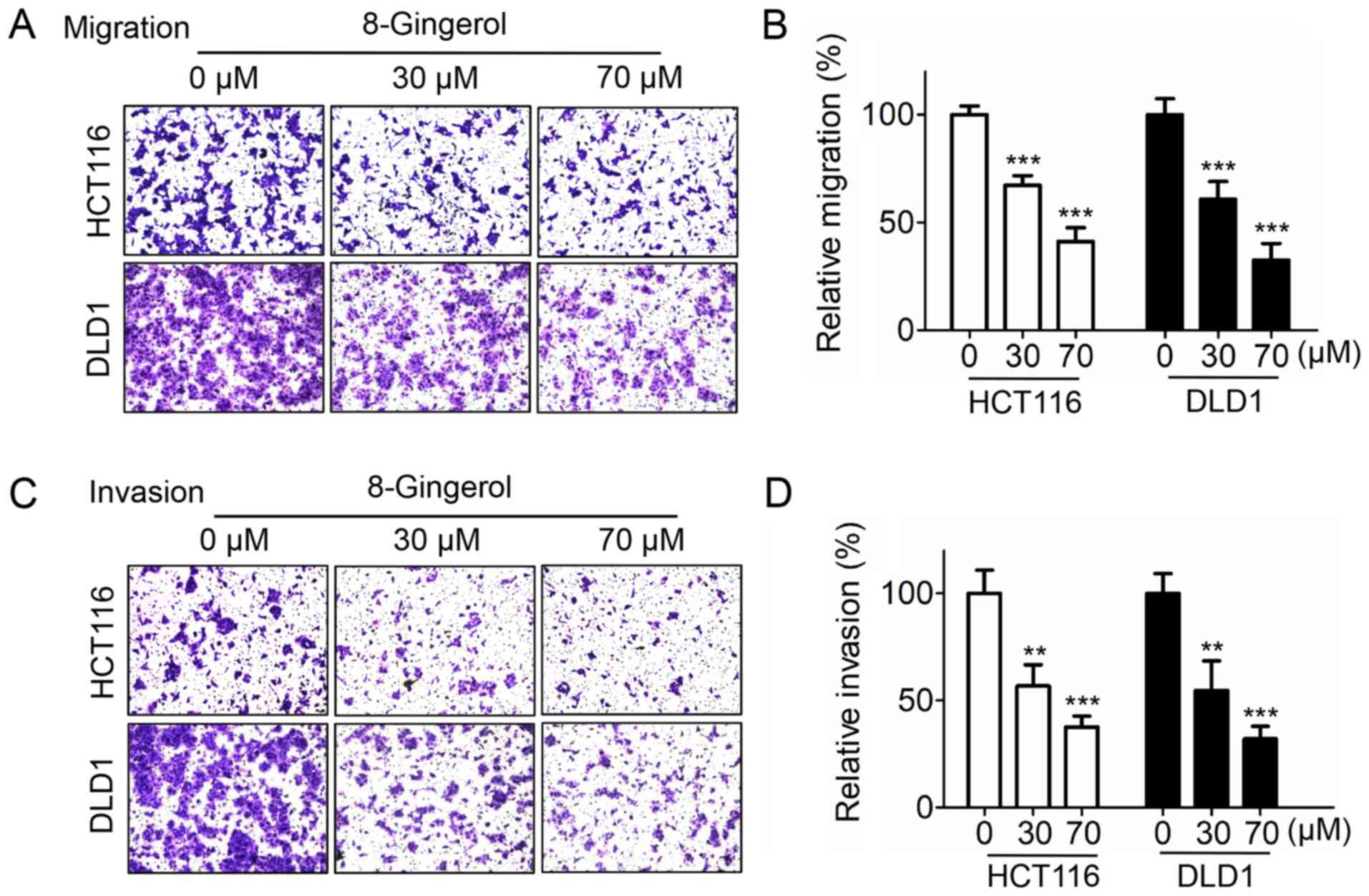
8-Gingerol inhibits CRC cell migration
and invasion
It was next investigated whether 8-gingerol affects
CRC cell migration and invasion. The Transwell migration assay
results revealed that 8-gingerol dose-dependently decreased the
migration of both HCT116 and DLD1 cells (Fig. 4A and B). Similarly, 8-gingerol
treatment markedly reduced the invasion ability of HCT116 and DLD1
cells, as demonstrated by the Transwell invasion assay (Fig. 4C and D). Collectively, these
results suggest that 8-gingerol exerts an inhibitory effect on CRC
cell migration and invasion.
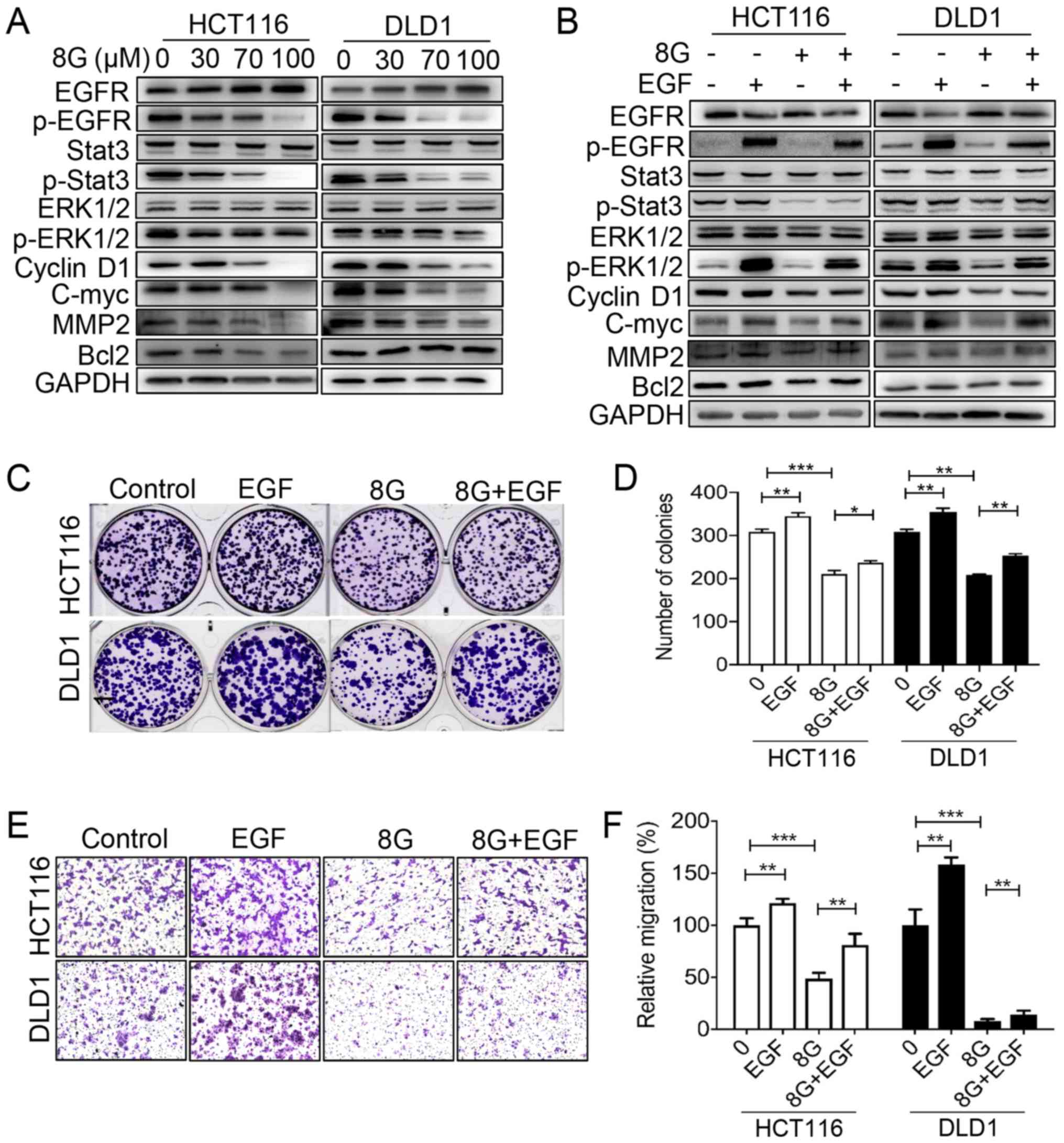
8-Gingerol affects CRC cell proliferation
and migration via EGFR/STAT/ERK cascades
EGFR signaling plays a key role in CRC development
and progression (3). Therefore,
whether this pathway is involved in the effects of 8-gingerol in
CRC was next examined. Western blot analysis revealed that
8-gingerol significantly decreased the level of phosphorylated EGFR
and, accordingly, the phosphorylation levels of its downstream
effectors, STAT3 and ERK, leading to down-regulated expression of
the target genes cyclin D1, c-Myc and MMP2, in both the HCT116 and
DLD1 cell lines; however, a decrease in Bcl-2 protein expression
was only observed in HCT116 cells, whereas Bcl-2 protein expression
was unchanged in DLD1 cells (Fig.
5A). By contrast, addition of EGF restored the phosphorylation
of EGFR, STAT3 and ERK and the expression of cyclin D1, c-Myc,
Bcl-2 and MMP2 (Fig. 5B).
Moreover, in the colony formation assay, administration of EGF
partially restored the proliferation of HCT116 and DLD1 cells
suppressed by 8-gingerol (Fig. 5C and
D). Similarly, in the Transwell migration assay, administration
of EGF partially restored the migration of HCT116 and DLD1 cells
inhibited by 8-gingerol (Fig. 5E and
F). These results suggest that EGFR/STAT/ERK signaling
contributes to the inhibitory effects of 8-gingerol on CRC cell
proliferation and migration.
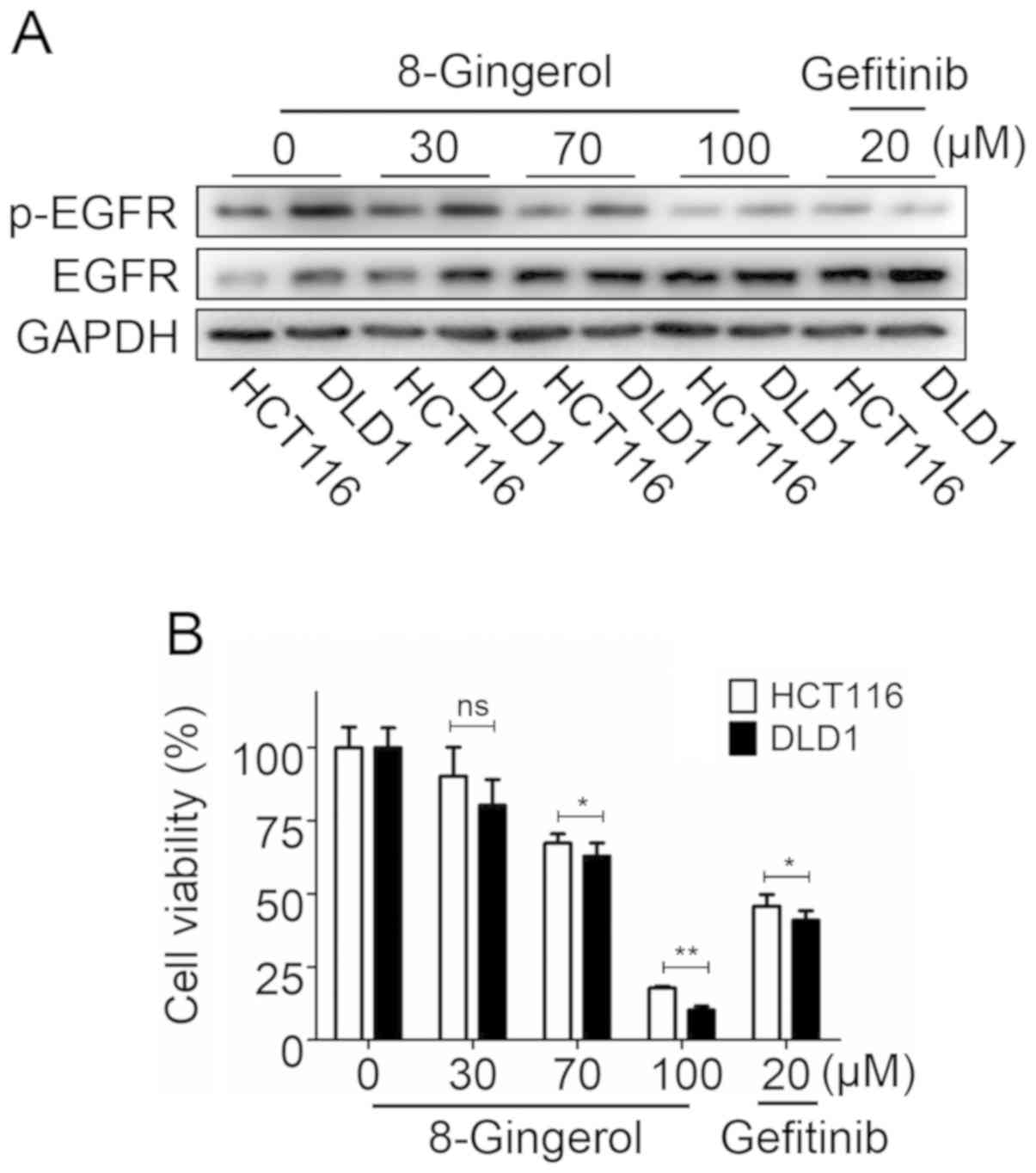
The chemotherapeutic effects of
8-gingerol are dependent on EGFR protein expression
To investigate whether the efficacy of 8-gingerol
depends on EGFR protein expression levels in CRC cells, the levels
of EGFR and EGFR phosphorylation were examined in HCT116 and DLD1
cell lines following exposure to 8-gingerol or the positive drug
control gefitinib. The results demonstrated that the endogenous
EGFR level of DLD1 cells was higher compared with that of HCT116
cells. Following treatment with 8-gingerol or gefitinib, the EGFR
levels decreased in both HCT116 and DLD1 cells, but the degree of
EGFR reduction in DLD1 cells was greater compared with that in
HCT116 cells (Fig. 6A).
Consistently with the EGFR protein expression levels, the CCK-8
results revealed that the effect of 8-gingerol and gefitinib on
DLD1 cells was more prominent compared with that on HCT116 cells
(Fig. 6B). Moreover, the
inhibitory effects of 8-gingerol (100 µM) on HCT116 and DLD1
cells were stronger compared with those of gefitinib (20
µM). These data indicate that the effects of 8-gingerol on
CRC cells depends on the expression level of EGFR in the two cell
lines.
Effects of treatment with 5-FU and
8-gingerol on the CRC cell lines HCT116 and DLD1
To determine the potential use of 8-gingerol in
future drug combination therapy, cell proliferation experiments
were conducted with of 5-FU and 8-gingerol treatment. The results
of the CCK-8 assay revealed that the presence of 8-gingerol reduced
the IC50 value of 5-FU from 12.2±2.42 to 9.1±0.75
µM in HCT116 cells, and from 11.2±0.85 to 3.6±0.37 µM
in DLD1 cells; however, the IC50 value of 8-gingerol
alone was only 77.4±4.70 µM in HCT116 cells and 53.7±2.24
µM in DLD1 cells (Table I).
These results indicate that 8-gingerol may reduce the effective
concentration of 5-FU, thereby decreasing the toxicity of 5-FU in
drug combination therapy.
 | Table IEffects of treatment with 5-FU and
8-gingerol on the colorectal cancer cell lines HCT116 and DLD1. |
Table I
Effects of treatment with 5-FU and
8-gingerol on the colorectal cancer cell lines HCT116 and DLD1.
| Compound | IC50
(µM)48 h
|
|---|
| HCT116 | DLD1 |
|---|
| 8-Gingerol | 77.4±4.70 | 53.7±2.24 |
| 5-FU | 12.2±2.42 | 11.2±0.85 |
| 5-FU +
8-Gingerola | 9.1±0.75 | 3.6±0.37 |
Discussion
Chemotherapy is one of the most common types of
treatment for metastatic CRC (33); however, resistance and serious side
effects are major obstacles to effective treatment (31). Novel strategies to enhance
chemotherapeutic effectiveness and reduce resistance and side
effects are urgently needed. Natural products are a good source of
novel anticancer drugs. Ginger is a major ingredient in traditional
herbal medicine used for treating a number of diseases, including
cancer (10). 8-Gingerol is one of
the major non-volatile components of ginger (11). Apart from its antioxidant and
anti-inflammatory properties, the activity of 8-gingerol against
cancer is unclear. To the best of our knowledge, the present study
is the first to report the suppressive effects of 8-gingerol on CRC
cells in vitro.
Cell cycle arrest and apoptosis are the main causes
of the cell growth inhibition induced by chemopreventive agents. It
has been reported that 6-gingerol and 10-gingerol inhibit cell
growth by inducing cell cycle arrest and apoptosis in several
cancers, including CRC (25,28,32,33).
Consistently with these reports, we herein demonstrated that
8-gingerol induced G0/G1 cell cycle arrest in both the HCT116 and
DLD1 cell lines. Notably, 8-gingerol induced apoptosis in HCT116
cells, which express wild-type p53, but not in DLD1 cells, which
express mutant p53, consistently with the concept that p53 mutation
in cancers usually confers resistance to apoptosis-inducing
chemotherapeutic agents (34,35).
Although 8-gingerol did not affect the apoptosis of DLD1 cells, it
exerted an even greater antiproliferative effect on DLD1 cells
compared with HCT116 cells, indicating that this compound may be a
promising agent targeting cancer cells, particularly those with
mutant p53.
EGFR signaling is crucial for driving the transition
from healthy colonic epithelium to malignant tumors, and also for
controlling tumor metastasis (36). EGFR is a member of the ERBB family
of cell surface receptor tyrosine kinases (37). Upon binding to its ligands, such as
EGF and transforming growth factor-α, EGFR is autophosphorylated
and activates downstream signaling to promote cell proliferation
and metastasis (38).
Overexpression of EGFR is frequently observed in CRC tumor tissues;
hence, targeting EGFR signaling appears to be a promising strategy
for CRC treatment (3). Monoclonal
anti-EGFR antibodies, such as cetuximab, have induced a good
response in patients with metastatic CRC; however, resistance to
these EGFR-targeted therapies eventually develops (39). Moreover, cetuximab is expensive and
is associated with a number of side effects (5). Therefore, great efforts are still
needed to develop novel chemopreventive agents targeting EGFR
signaling. In the present study, 8-gingerol was identified as a
novel inhibitor of EGFR signaling. Treatment with 8-gingerol
significantly decreased EGFR phosphorylation, and the therapeutic
effects of 8-gingerol largely depended on the EGFR expression in
CRC cells. The STAT and ERK pathways are two downstream effector
pathways of EGFR (40,41). Correspondingly, upon 8-gingerol
exposure, the levels of phosphorylated STAT3 and ERK1/2 were
significantly decreased, leading to decreased expression of their
downstream target genes, such as cyclin D1, c-Myc and MMP2
(42,43), in turn leading to the suppression
of cell proliferation and migration. By contrast, addition of EGF
partially restricted the inhibitory effect induced by 8-gingerol.
However, downregulation of Bcl-2 protein expression only occurred
in HCT116 cells, and not in DLD1 cells. Bcl-2 is an antiapoptotic
regulator that enhances cell survival and inhibits apoptosis
triggered by several different apoptotic pathways (44,45).
Englert et al demonstrated that suppression of EGFR could
promote cell apoptosis (46). The
effect of apoptosis induced by EGFR withdrawal may counteract the
protective effect of Bcl-2 on cell growth, which ultimately
resulted in the unchanged Bcl-2 expression seen in DLD1 cells.
Taken together, these data suggest that 8-gingerol inhibits CRC
cell proliferation and migration by targeting the EGFR/STAT3/ERK
pathway (Fig. 7).
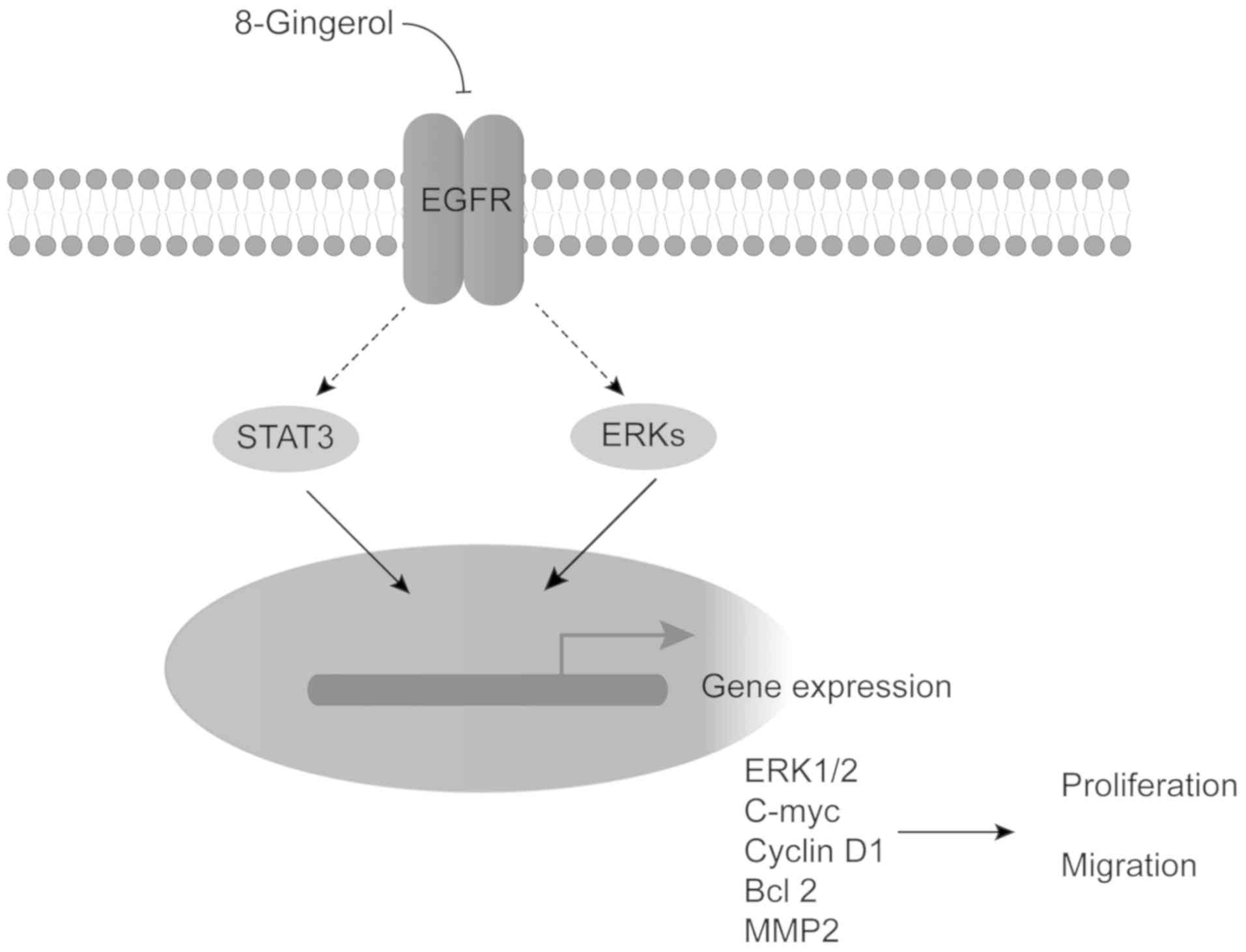 | Figure 7A proposed action mechanism of
8-gingerol in CRC cells. 8-Gingerol decreases the EGFR
phosphorylation level, resulting in inhibition of STAT3 and ERK
signaling pathway activity, leading to blockade of cyclin D1,
c-Myc, Bcl-2 and MMP2 expression, thus suppressing cell
proliferation and migration. CRC, colorectal cancer; EGFR,
epidermal growth factor receptor; STAT, signal transducer and
activator of transcription; ERK, extracellular signal-regulated
kinase; MMP, matrix metallopeptidase; Bcl-2, B-cell lymphoma 2. |
In the present study, the antitumor effects of the
natural product 8-gingerol on CRC cells were first verified.
Further experiments indicated that 8-gingerol inhibits CRC cell
proliferation and migration by targeting EGFR signaling. However,
these results require validation in an in vivo animal model
and confirmation by clinical evidence in the future.
In summary, 8-gingerol was shown to have antitumor
activity against CRC cell proliferation, migration and invasion.
Additionally, 8-gingerol was found to be a novel inhibitor of EGFR
signaling in CRC cells, and its effects depended on the EGFR
expression in the two CRC cell types. In addition, the addition of
8-gingerol to 5-FU therapy may reduce the effective concentration
of 5-FU, thereby decreasing the toxicity of 5-FU in drug
combination therapy. These data suggest that 8-gingerol may be a
promising candidate for the development of antitumor agents against
CRC.
Funding
The present study was supported in part by the
Science and Technology Program of Guangzhou (grant no.
201803010027), the 111 Project (grant no. B12003), and the Science
and Technology Planning Project of Guangdong Province (grant nos.
2015A020210048 and 2017A020215170).
Availability of materials and data
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XWZ initiated and designed the study, revised the
manuscript, and participated in data interpretation. SMH and XHY
performed the experiments, analyzed the data and wrote the
manuscript. YHH contributed to data analysis. AHP was responsible
for data interpretation. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Wanqin Liao and
Suli Zhu of Sun Yat-sen University, China, for their kind help.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
STAT
|
signal transducer and activator of
transcription
|
|
ERK
|
extracellular-signal-regulated
kinase
|
|
5-FU
|
5-fluorouracil
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar
|
|
3
|
Spano JP, Lagorce C, Atlan D, Milano G,
Domont J, Benamouzig R, Attar A, Benichou J, Martin A, Morere JF,
et al: Impact of EGFR expression on colorectal cancer patient
prognosis and survival. Ann Oncol. 16:102–108. 2005. View Article : Google Scholar
|
|
4
|
Vignot S and Spano JP: Prognostic value of
EGFR in colorectal cancer. Bull Cancer. 92:S13–S16. 2005.In
French.
|
|
5
|
Emani MK and Zaiden RA Jr: Aseptic
meningitis: A rare side effect of cetuximab therapy. J Oncol Pharm
Pract. 19:178–180. 2013. View Article : Google Scholar
|
|
6
|
Huxley N, Crathorne L, Varley-Campbell J,
Tikhonova I, Snowsill T, Briscoe S, Peters J, Bond M, Napier M and
Hoyle M: The clinical effectiveness and cost-effectiveness of
cetuximab (review of technology appraisal no. 176) and panitumumab
(partial review of technology appraisal no. 240) for previously
untreated metastatic colorectal cancer: A systematic review and
economic evaluation. Health Technol Assess. 21:1–294. 2017.
View Article : Google Scholar
|
|
7
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stremitzer S, Sebio A, Stintzing S and
Lenz HJ: Panitumumab safety for treating colorectal cancer. Expert
Opin Drug Saf. 13:843–851. 2014.PubMed/NCBI
|
|
9
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prasad S and Tyagi AK: Ginger and its
constituents: Role in prevention and treatment of gastrointestinal
cancer. Gastroenterol Res Pract. 2015:1429792015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali BH, Blunden G, Tanira MO and Nemmar A:
Some phytochemical, pharmacological and toxicological properties of
ginger (Zingiber officinale Roscoe): A review of recent research.
Food Chem Toxicol. 46:409–420. 2008. View Article : Google Scholar
|
|
12
|
Kaur IP, Deol PK, Kondepudi KK and Bishnoi
M: Anticancer Potential of Ginger: Mechanistic and Pharmaceutical
Aspects. Curr Pharm Des. 22:4160–4172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao WZ, Zhang RX, Yu ZP, Wang XK, Li JR
and Liu JB: Research process in ginger chemical composition and
biological activity. Sci Techn Food Ind. 11:383–389. 2016.In
Chinese.
|
|
14
|
Eren D and Betul YM: Revealing the effect
of 6-gingerol, 6-shogaol and curcumin on mPGES-1, GSK-3β and
β-catenin pathway in A549 cell line. Chem Biol Interact.
258:257–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geng S, Zheng Y, Meng M, Guo Z, Cao N, Ma
X, Du Z, Li J, Duan Y and Du G: Gingerol Reverses the
Cancer-Promoting Effect of Capsaicin by Increased TRPV1 Level in a
Urethane-Induced Lung Carcinogenic Model. J Agric Food Chem.
64:6203–6211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Abbasi FA, Alghamdi EA, Baghdadi MA,
Alamoudi AJ, El-Halawany AM, El-Bassossy HM, Aseeri AH and Al-Abd
AM: Gingerol Synergizes the Cytotoxic Effects of Doxorubicin
against Liver Cancer Cells and Protects from Its Vascular Toxicity.
Molecules. 21:212016. View Article : Google Scholar
|
|
17
|
Kapoor V, Aggarwal S and Das SN:
6-Gingerol Mediates its Anti Tumor Activities in Human Oral and
Cervical Cancer Cell Lines through Apoptosis and Cell Cycle Arrest.
Phytother Res. 30:588–595. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong CH, Bode AM, Pugliese A, Cho YY, Kim
HG, Shim JH, Jeon YJ, Li H, Jiang H and Dong Z: [6]-Gingerol
suppresses colon cancer growth by targeting leukotriene A4
hydrolase. Cancer Res. 69:5584–5591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Lima RMT, Dos Reis AC, de Menezes APM,
Santos JVO, Filho JWGO, Ferreira JRO, de Alencar MVOB, da Mata
AMOF, Khan IN, Islam A, et al: Protective and therapeutic potential
of ginger (Zingiber officinale) extract and [6]-gingerol in cancer:
A comprehensive review. Phytother Res. 32:1885–1907. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi LW, Zhang Z, Zhang CF, Anderson S, Liu
Q, Yuan CS and Wang CZ: Anti-Colon Cancer Effects of 6-Shogaol
Through G2/M Cell Cycle Arrest by p53/p21-cdc2/cdc25A Crosstalk. Am
J Chin Med. 43:743–756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kotowski U, Kadletz L, Schneider S, Foki
E, Schmid R, Seemann R, Thurnher D and Heiduschka G: 6-shogaol
induces apoptosis and enhances radiosensitivity in head and neck
squamous cell carcinoma cell lines. Phytother Res. 32:340–347.
2018. View
Article : Google Scholar
|
|
22
|
Zhou L, Qi L, Jiang L, Zhou P, Ma J, Xu X
and Li P: Antitumor activity of gemcitabine can be potentiated in
pancreatic cancer through modulation of TLR4/NF-κB signaling by
6-shogaol. AAPS J. 16:246–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan BS, Kang O, Mai CW, Tiong KH, Khoo AS,
Pichika MR, Bradshaw TD and Leong CO: 6-Shogaol inhibits breast and
colon cancer cell proliferation through activation of peroxisomal
proliferator activated receptor γ (PPARγ). Cancer Lett.
336:127–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim MO, Lee MH, Oi N, Kim SH, Bae KB,
Huang Z, Kim DJ, Reddy K, Lee SY, Park SJ, et al: [6]-shogaol
inhibits growth and induces apoptosis of non-small cell lung cancer
cells by directly regulating Akt1/2. Carcinogenesis. 35:683–691.
2014. View Article : Google Scholar
|
|
25
|
Bernard MM, McConnery JR and Hoskin DW:
[10]-Gingerol, a major phenolic constituent of ginger root, induces
cell cycle arrest and apoptosis in triple-negative breast cancer
cells. Exp Mol Pathol. 102:370–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin ACBM, Fuzer AM, Becceneri AB, da
Silva JA, Tomasin R, Denoyer D, Kim SH, McIntyre KA, Pearson HB,
Yeo B, et al: [10]-gingerol induces apoptosis and inhibits
metastatic dissemination of triple negative breast cancer in vivo.
Oncotarget. 8:72260–72271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joo JH, Hong SS, Cho YR and Seo DW:
10-Gingerol inhibits proliferation and invasion of MDA-MB-231
breast cancer cells through suppression of Akt and
p38MAPK activity. Oncol Rep. 35:779–784. 2016.
View Article : Google Scholar
|
|
28
|
Ryu MJ and Chung HS: [10]-Gingerol induces
mitochondrial apoptosis through activation of MAPK pathway in
HCT116 human colon cancer cells. In Vitro Cell Dev Biol Anim.
51:92–101. 2015. View Article : Google Scholar
|
|
29
|
Chen CY, Li YW and Kuo SY: Effect of
[10]-gingerol on [ca2+]i and cell death in human colorectal cancer
cells. Molecules. 14:959–969. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dugasani S, Pichika MR, Nadarajah VD,
Balijepalli MK, Tandra S and Korlakunta JN: Comparative antioxidant
and anti-inflammatory effects of [6]-gingerol, [8]-gingerol,
[10]-gingerol and [6]-shogaol. J Ethnopharmacol. 127:515–520. 2010.
View Article : Google Scholar
|
|
31
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
32
|
Lin CB, Lin CC and Tsay GJ: 6-Gingerol
Inhibits Growth of Colon Cancer Cell LoVo via Induction of G2/M
Arrest. Evid Based Complement Alternat Med. 2012:3260962012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee SH, Cekanova M and Baek SJ: Multiple
mechanisms are involved in 6-gingerol-induced cell growth arrest
and apoptosis in human colorectal cancer cells. Mol Carcinog.
47:197–208. 2008. View
Article : Google Scholar :
|
|
34
|
Arango D, Corner GA, Wadler S, Catalano PJ
and Augenlicht LH: c-myc/p53 interaction determines sensitivity of
human colon carcinoma cells to 5-fluorouracil in vitro and in vivo.
Cancer Res. 61:4910–4915. 2001.PubMed/NCBI
|
|
35
|
Iacopetta B: TP53 mutation in colorectal
cancer. Hum Mutat. 21:271–276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lowery FJ and Yu D: Growth factor
signaling in metastasis: Current understanding and future
opportunities. Cancer Metastasis Rev. 31:479–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cataldo VD, Gibbons DL, Pérez-Soler R and
Quintás-Cardama A: Treatment of non-small-cell lung cancer with
erlotinib or gefitinib. N Engl J Med. 364:947–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lurje G and Lenz HJ: EGFR signaling and
drug discovery. Oncology. 77:400–410. 2009. View Article : Google Scholar
|
|
39
|
Chong CR and Jänne PA: The quest to
overcome resistance to EGFR-targeted therapies in cancer. Nat Med.
19:1389–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park OK, Schaefer TS and Nathans D: In
vitro activation of Stat3 by epidermal growth factor receptor
kinase. Proc Natl Acad Sci USA. 93:13704–13708. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jackson NM and Ceresa BP: EGFR-mediated
apoptosis via STAT3. Exp Cell Res. 356:93–103. 2017.PubMed/NCBI
|
|
43
|
Sirkisoon SR, Carpenter RL, Rimkus T,
Miller L, Metheny-Barlow L and Lo HW: EGFR and HER2 signaling in
breast cancer brain metastasis. Front Biosci (Elite Ed). 8:245–263.
2016.
|
|
44
|
Radha G and Raghavan SC: BCL2: A promising
cancer therapeutic target. Biochim Biophys Acta Rev Cancer.
1868:309–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang E, Korsmeyer SJ and Korsmeyer EYaSJ:
Molecular Thanatopsis: A Discourse on the BCLZ Family and Cell
Death. Blood. 88:386–401. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Englert C, Hou X, Maheswaran S, Bennett P,
Ngwu C, Re GG, Garvin AJ, Rosner MR and Haber DA: WT1 suppresses
synthesis of the epidermal growth factor receptor and induces
apoptosis. EMBO J. 14:4662–4675. 1995. View Article : Google Scholar : PubMed/NCBI
|