Introduction
Pancreatic cancer was the seventh leading cause of
cancer-related death worldwide in 2012 (1), with a 5-year overall survival rate of
<8% (2). Disease progression
and metastasis are major barriers in the survival of patients with
pancreatic cancer (3). Despite the
improvement in diagnosis and treatment, surgical resection is the
only effective therapy for pancreatic cancer. However, because of
local invasion and/or metastasis, only 15-20% of patients with
pancreatic cancer qualify for surgical intervention (4). Understanding the potential molecular
mechanisms contributing to metastasis could therefore be of great
value for effective pancreatic cancer therapy.
Long non-coding RNAs (lncRNAs) have been implicated
in the regulation of disease progression in numerous types of
cancer by functioning as tumor suppressors or promoters (5,6).
Increasing evidence indicates that lncRNA cancer susceptibility
candidate 2 (CASC2) exhibits tumor-suppressive functions by acting
as a competing endogenous RNA (ceRNA) for microRNAs (miRNAs/miRs)
(7-10). CASC2 was shown to be downregulated
and suppress tumor progression in pancreatic cancer (11). miR-24 has been reported to be
upregulated in pancreatic cancer cells (12,13).
While CASC2 was demonstrated to act as a sponge for miR-24 and
regulated tumorigenesis of hepatocellular carcinoma (8,14),
little is known about the interaction between CASC2 and miR-24 in
pancreatic cancer.
Mucins (MUCs) are a group of heavily glycosylated
proteins, which are involved in the regulation of proliferation,
migration and invasion of pancreatic cancer (15,16).
MUC6, a high molecular weight secretory polymeric MUC, has been
shown to be downregulated in pancreatic cancer (17,18),
and exerts tumor-suppressive effects by interfering with
cell-matrix adhesion in the local tumor microenvironment (19). Bioinformatics analysis using
Targetscan 7.2 predicted that MUC6 is a direct target of miR-24.
However, to the best of our knowledge, no studies have been
conducted that focus on the association between MUC6 and miR-24 in
pancreatic cancer. Integrin β4 (ITGB4), one of the main mediators
of cell-matrix adhesion of epithelial cells, is actively involved
in regulating progression of pancreatic cancer through the focal
adhesion kinase (FAK) signaling pathway (20). Moreover, MUC5AC, a MUC subtype,
regulates cell migration through the ITGB4/FAK signaling pathway in
lung cancer (21). It remains
unknown if a similar crosstalk exists between MUC6 and the
ITGB4/FAK pathway in pancreatic cancer.
The present study demonstrated that CASC2 was
downregulated in pancreatic cancer tissues and cell lines, and
served crucial roles in pancreatic cancer growth and progression.
Conversely, miR-24 was upregulated and had a pro-metastatic role in
pancreatic cancer. Furthermore, to the best of our knowledge, this
study is the first to demonstrate that CASC2 may function as a
ceRNA for miR-24 and may exert tumor-suppressive effects in part
through the MUC6/ITGB4/FAK signaling pathway. These findings may
provide promising therapeutic targets for pancreatic cancer.
Materials and methods
Clinical samples
A total of 20 pancreatic cancer tissues and adjacent
normal tissues were collected from patients (age, 45-70 years; 12
female and 8 male patients) that underwent surgical resection
between June 2016 and June 2018 at the Zhongda Hospital Affiliated
with Southeast University (Nanjing, China). Histologic diagnosis
was confirmed for each specimen. This study was approved by the
Ethical Committee of Zhongda Hospital Affiliated with Southeast
University and written informed consent was obtained from all
patients. Samples were frozen immediately after being surgically
resected and were stored in liquid nitrogen.
Cell culture
The human pancreatic normal epithelial cell line
(hTERT-HPNE; cat. no. ATCC® CRL-4023™), which is
comprised of intermediary cells formed during acinar-to-ductal
metaplasia, and the human pancreatic cancer cell lines AsPC-1 (cat.
no. ATCC® CRL-1682™) and PANC-1 (cat. no.
ATCC® CRL-1469™) were purchased from the American Type
Culture Collection. The human pancreatic cancer cell line PATU8988T
was provided by Cell Biology Research Center of Central South
University. Cells were cultured in DMEM supplemented with 10% fetal
bovine serum (FBS; both Gibco; Thermo Fisher Scientific, Inc.) in
the presence of 100 U/ml penicillin and streptomycin (Beyotime
Institute of Biotechnology) at 37°C with a humidified atmosphere
(5% CO2).
Cell transfection and transduction
For in vitro assays, the human CASC2 sequence
was cloned into the pIRES2-EGFP vector (cat. no. GV146; Shanghai
GeneChem Co., Ltd.) to create the CASC2 overexpression vector. The
empty pIRES2-EGFP vector served as a negative control (NC). miR-24
mimics, miR-24 inhibitor and their negative controls (NCs) were
purchased from Shanghai GenePharma Co., Ltd. AsPC-1 or PANC-1 cells
(5×105/well) were cultured in 6-well plates for 24 h and
were then transfected with 5 µg/ml pIRES2-EGFP-CASC2
overexpression vector, pIRES2-EGFP empty vector, 10 nM miR-24
mimics, miR-24 inhibitor or their NCs at 40-60% confluence using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The transfected cells were harvested after 48 h
at 37°C. Morphological changes of pancreatic cancer cells were
assessed by light microscopy. The sequences were as follows: miR-24
mimics, sense 5′-UGG CUC AGU UCA GCA GGA ACA G-3′, antisense 5′-GUU
CCU GCU GAA CUG AGC CAU U-3′; mimics NC, sense 5′-UUC UCC GAA CGU
GUC ACG UTT-3′, antisense 5′-ACG UGA CAC GUU CGG AGA ATT-3′; miR-24
inhibitor, 5′-CUG UUC CUG CUG AAC UGA GCC A-3′; inhibitor NC,
5′-CAG UAC UUU UGU GUA GUA CAA-3′.
For in vivo studies, AsPC-1 cells were
transduced with lentivirus (LV)-CASC2 (LV5-EF1a-GFP/Puro vector;
Shanghai GenePharma Co., Ltd.) and LV-miR-24 (LV3-pGLV-h1-GFP-puro
vector; Shanghai GenePharma Co., Ltd.), or LV-NC vectors
(LV-CASC2-NC and LV-miR-24 NC; Shanghai GenePharma Co., Ltd.) as
previously described (22).
Briefly, AsPC-1 cells (5×105 per well) were plated in
6-well plates for 24 h; the medium was then replaced with fresh
medium containing 8 µg/ml polybrene. The lentiviral vectors
were transduced into AsPC-1 cells for 24 h at 37°C at a
multiplicity of infection of 50, followed by puromycin (5
µg/ml) antibiotic selection for 3 days.
Immunohistochemistry
Tumor tissues were fixed with 3.7% paraformaldehyde
at room temperature for 12 h, and were then embedded in paraffin,
cut into 5-µm sections and washed with PBS. After blocking
with 5% FBS and 0.3% Triton X-100 in PBS for 1 h at room
temperature, MUC6 antibody (cat. no. sc-33668; Santa Cruz
Biotechnology, Inc.) was applied at a dilution of 1:200 and the
sections were incubated at 4°C overnight. After incubation with the
secondary antibody (anti-mouse biotinylated; 1:1,000; cat. no.
14709; Cell Signaling Technology, Inc.) for 1 h at room
temperature, the avidinbiotin peroxidase method (avidin peroxidase
conjugate, 1:2,000, cat. no. ab59653; Abcam) was adopted for 1 h at
room temperature to determine the location and relative expression
level of the target protein. Finally, the visualization signal was
developed with 3,3′-diaminobenzidine tetrahydrochloride, and the
slides were counterstained in hematoxylin diluted at 1:5 in
H2O for 1 min at room temperature and observed by light
microscopy at ×100 magnification.
Dual-luciferase reporter assay
TargetScan 7.2 software (http://www.targetscan.org/vert_72/) was applied to
predict the miR-24 binding sites on MUC6. RNA Interactome Database
(http://www.rna-society.org/raid/home.html) was applied
to predict the miR-24 binding sites on CASC2. The wild type and
mutant human MUC6 3′-untranslated regions (UTRs) and the CASC2
sequence containing putative binding sites for miR-24 were designed
and synthesized by Shanghai GeneChem Co., Ltd. The wild type and
mutant constructs were cloned into the pGL3-promoter vector
(Promega Corporation). For the dual-luciferase reporter assay,
AsPC-1 and PANC-1 cells (2×105/well) in 24-well plates
were harvested at 24 h and transfected with 5 µg/ml
pGL3-promoter vectors and 10 nM miR-24 mimics or miR-24 inhibitor,
or their NCs using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h, cells were harvested
and lysed, and luciferase activity was detected using the Dual
Luciferase Reporter Assay kit (Promega Corporation), according to
the manufacturer’s protocol. Firefly luciferase activities were
normalized to Renilla luciferase activities.
MTT assay
AsPC-1 and PANC-1 (1×104 cells/well) were
seeded in 96-well plates and grown overnight. After trans-fection
for 1, 2, 3 or 4 days, the medium was replaced with DMEM
supplemented with 10% FBS. Subsequently, 20 µl MTT solution
(5 mg/ml; Abcam) was added to each well and samples were incubated
for 4 h at 37°C. DMSO (100 µl) was added and samples were
agitated in the dark for 30 min at room temperature to dissolve
formazan crystals. The absorbance was measured at 490 nm using a
microplate reader; a 630 nm reference was subtracted.
Colony formation assay
Transfected cells were seeded in 6-well plates at
200 cells/well and cultured for 2 weeks. Colonies were washed twice
with PBS, fixed with 100% methanol at 4°C for 15 min and stained
with 1% crystal violet at room temparature for 60 min. Images of
the number of colonies were captured with a camera. The colonies
were counted by three researchers who were blinded to the
experimental condition.
Apoptosis assay
Apoptotic cells were identified using the Annexin
V-APC/PI Apoptosis Detection kit (Nanjing KeyGen Biotech Co.,
Ltd.), according to the manufacturer’s protocol. Transfected cells
(2×105/well) in 24-well plates were collected, washed
twice with cold PBS and resuspended in 1X binding buffer.
Subsequently, cells were stained with 5 µl Annexin V-APC for
15 min and 5 µl PI for 10 min in the dark at room
temperature. Cells were examined using the FACSCanto II flow
cytometer (BD Biosciences). Analysis of flow cytometry data was
performed using FlowJo version X.10.0.7-1 (FlowJo, LLC).
Wound healing and Transwell assays
For cell migration, transfected cells were seeded in
6-well plates (5×105/well) and cultured overnight to
>95% confluence. Wounds were inflicted with the sterile
200-µl pipette tips and loose cells were washed off with
culture medium. Cells were then maintained in DMEM without FBS and
images were captured at 0 and 24 h using a light microscope at ×40
magnification. ImageJ version 1.52a (National Institutes of Health)
was used to analyze the wound width. The migration distance (%) was
determined by the following formula: (W0 h−W24
h)/W0 h ×100. W, refers to width.
For cell invasion, cells (2×104/well)
were cultured in 24-well Transwell chambers with the upper chambers
coated with Matrigel (BD Bioscience). Cells in the upper chamber
were incubated in 200 µl DMEM containing 10% FBS; as a
chemoattractant, the bottom chamber contained DMEM with 20% FBS.
After 24 h at 37°C, cells remaining on the upper membrane surface
were removed with a cotton swab and cells adhering to the lower
membrane surface were fixed with 4% paraformaldehyde at 4°C
overnight and stained with 1% crystal violet at room temparature
for 60 min. The number of invaded cells in five random fields was
counted under a light microscope at ×100 magnification.
Tumor xenograft using nude mice
A total of 20 male BALB/c nude (nu/nu) mice (age, 6
weeks; weight, 16-20 g) were purchased from the Model Animal
Research Center of Nanjing University. Animals were acclimated
under specific pathogen-free conditions. Mice were kept in cages
(n=5 mice/cage), and were housed in a sterile room under a 12-h
light/dark cycle at ~23°C and 50% humidity, with ad libitum
access to food and water. Animals were maintained on a balanced
diet for rodents and given free access to water and food. All of
the animal studies were conducted in accordance with the
Institutional Animal Care and Use Committee and were approved by
the Medical Ethics Committee of Southeast University (Nanjing,
China).
AsPC-1 cells were stably transduced with lentiviral
vectors, according to the indicated groups (n=5 mice/group).
Transduced AsPC-1 cells (1×106) were suspended in 100
µl PBS and subcutaneously injected into the flank of the
mice under anesthesia. The xenograft tumor size was measured every
5 days with a vernier caliper, and the tumor volumes were
calculated using the equation: 0.5 × length x width2.
After 30 days, mice were euthanized by cervical dislocation and the
tumor tissues were harvested. The tumor take rate was ~95%.
Pathological and molecular expression analyses of tumor tissues
were performed.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA from tumor tissues and transfected cells
was extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized using the PrimeScript RT
reagent kit (Takara Biotechnology Co., Ltd.) at 25°C for 5 min,
37°C for 30 min and 85°C for 5 sec. qPCR analysis was performed
using the StepOnePlus Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with SYBR Premix EX Taq kit (Takara
Biotechnology Co., Ltd.). The thermocycling conditions were as
follows: Initial denaturation, 95°C for 5 sec; followed by 35
cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 25
sec and extension at 70°C for 30 sec. Primers for CASC2, miR-24 and
MUC6 were purchased from Sangon Biotech Co., Ltd. GAPDH and U6 were
used as internal reference genes for mRNA and miRNA, respectively.
Relative expression was calculated using the 2−ΔΔCq
method (23). The primer sequences
were as follows: CASC2, forward 5′-AGC TCA TGT GGT TGC AAG GT-3′,
reverse 5′-CTG CCT GAA ACT AGG CGG AA-3′; miR-24, forward 5′-GCG
TGG CTC AGT TCA GCA G-3′, reverse 5′-AGT GCA GGG TCC GAG GTA TT-3′;
MUC6, forward 5′-CGT CTG TGG TGT CAA CGA CT-3′, reverse 5′-CCG GTG
ACC GTG TAG TTT CT-3′; U6 (internal control for miRNA), forward
5′-CTC GCT TCG GCA GCA CA-3′, reverse 5′-AAC GCT TCA CGA ATT TGC
GT-3′; and GAPDH (internal control for mRNA), forward, 5′-CCA CAG
TCC ATG CCA TCA C-3′ and reverse 5′-GCT TCA CCA CCT TCT TGA
TG-3′.
Western blotting
Total proteins were extracted from tissues and
cultured cells by RIPA buffer (Beyotime Institute of Biotechnology)
with protease inhibitor cocktail (Roche Diagnostics). Bicinchoninic
acid assays were used to detect protein concentration. Equal
amounts of protein (30 µg) were separated by SDS-PAGE on 10%
gels and were then transferred to polyvinylidene fluoride
membranes. Membranes were blocked with 5% non-fat milk for 1 h at
room temperature, followed by incubation with primary antibodies at
4°C overnight. The primary antibodies used were: Anti-INTB4
(1:1,000; cat. no. 4707; Cell Signaling Technology, Inc.),
anti-phosphorylated (p)-FAK (1:1,000; cat. no. 3284; Cell Signaling
Technology, Inc.), anti-FAK (1:2,000; cat. no. 3285; Cell Signaling
Technology, Inc.), anti-E-cadherin (1:5,000; cat. no. 3195; Cell
Signaling Technology, Inc.), anti-N-cadherin (1:1,000; cat. no.
4061; Cell Signaling Technology, Inc.), anti-vimentin (1:1,000;
cat. no. 5741; Cell Signaling Technology, Inc.), anti-matrix
metalloproteinase (MMP)-2 (1:500; cat. no. 4022; Cell Signaling
Technology, Inc.), anti-MMP-9 (1:1,000; cat. no. 3852; Cell
Signaling Technology, Inc.), anti-Snail (1:2,000; cat. no. 3879;
Cell Signaling Technology, Inc.), anti-fibronectin (1:1,000; cat.
no. F3648; Sigma-Aldrich; Merck KGaA), anti-MUC6 (1:1,000; cat. no.
ab192318; Abcam) and anti-GAPDH (1:3,000; cat. no. 60004-1-1g;
ProteinTech Group, Inc.). Membranes were then incubated with goat
anti-rabbit (cat. no. 7074) and a goat anti-mouse (cat. no. 7076)
horseradish peroxidase-conjugated secondary antibodies (1:3,000;
Cell Signaling Technology, Inc.) for 1 h at room temperature. Bands
were developed using chemiluminescence substance (Thermo Fisher
Scientific, Inc.). The proteins were semi-quantified using Quantity
One version 4.2.1 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated ≥3 times and
representative results are presented. Data were analyzed with Prism
6.0 (GraphPad Software, Inc.) and are expressed as the mean ±
standard deviation. Comparisons between two groups were performed
using Student’s t-test (both paired and unpaired tests).
Comparisons among ≥3 groups were conducted using one-way ANOVA
followed by Tukey’s test. P<0.05 was considered to indicate a
statistically significant difference.
Results
CASC2 and MUC6 are downregulated, and
miR-24 is upregulated in pancreatic cancer tissues and cell
lines
To investigate the clinical relevance of CASC2,
miR-24 and MUC6, 20 pancreatic tumor samples and paired adjacent
normal tissues were collected. RT-qPCR analysis revealed that CASC2
and MUC6 expression levels were significantly decreased, whereas
miR-24 levels were significantly increased in tumor samples
compared with normal tissues (Fig.
1A). Immunohistochemistry and western blot analyses
demonstrated that MUC6 protein expression levels were lower in
pancreatic cancer samples than in normal pancreatic tissues
(Fig. 1B-D). Subsequently, the
expression levels of CASC2, miR-24 and MUC6 were detected in three
pancreatic cancer cell lines, namely AsPC-1, PANC-1 and PATU8988T,
and in the human pancreatic normal epithelial cell line hTERT-HPNE.
The results revealed that CASC2 and MUC6 expression levels were
significantly lower, and miR-24 expression was significantly higher
in the pancreatic cancer cell lines compared with the hTERT-HPNE
cells (Fig. 1F). Western blotting
further confirmed that MUC6 protein expression was significantly
decreased in pancreatic cancer cell lines compared with the control
cells (Fig. 1E). As AsPC-1 and
PANC-1 cells had low CASC2 and MUC6 and high miR-24 expression
compared with PATU8988T cells, these two cell lines were used for
subsequent loss- and gain-of-function experiments. Collectively,
these data suggested the involvement of CASC2, miR-24 and MUC6 in
pancreatic cancer.
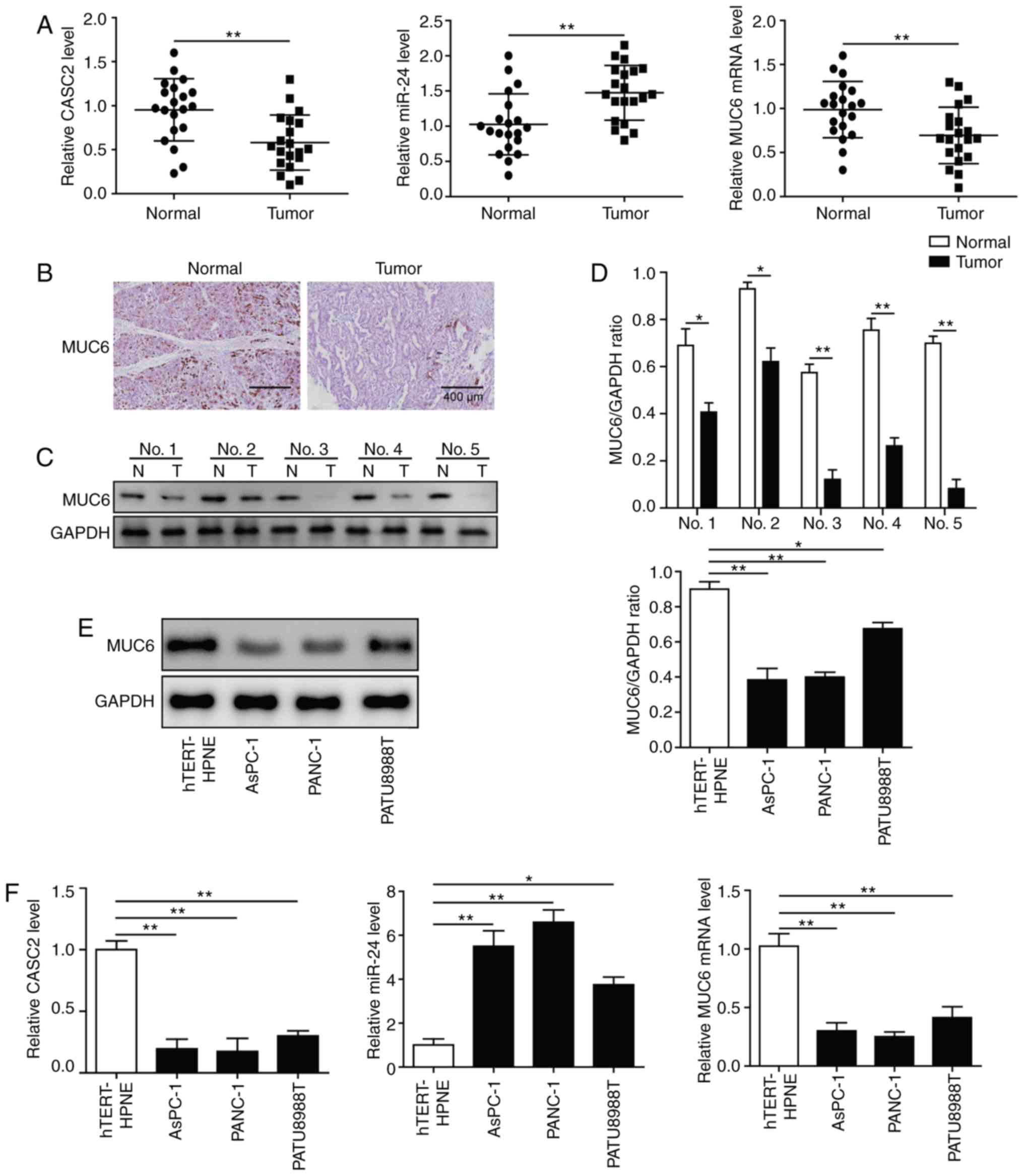 | Figure 1CASC2 and MUC6 are downregulated, and
miR-24 is upregulated in pancreatic cancer tissues and cell lines.
(A) RT-qPCR analysis of CASC2, miR-24 and MUC6 expression in 20
paired pancreatic cancer and adjacent normal tissues. (B)
Immunohistochemistry (scale bar, 400 µm) and (C and D)
western blot analysis of MUC6 protein expression levels in
pancreatic cancer and adjacent normal tissues. (E) Western blot
analysis of MUC6 protein expression levels in AsPC-1, PANC-1 and
PATU8988T pancreatic cancer cell lines, and the human pancreatic
normal epithelial cell line hTERT-HPNE. (F) RT-qPCR analysis of
CASC2, miR-24 and MUC6 expression levels in pancreatic cancer cell
lines and hTERT-HPNE cells. *P<0.05 and
**P<0.01. CASC2, cancer susceptibility candidate 2;
miR, microRNA; MUC6, mucin 6; N, adjacent normal tissues; RT-qPCR,
reverse transcription-quantitative PCR; T, pancreatic cancer
tissues. |
Overexpression of CASC2 inhibits
pancreatic cancer cell growth and progression partially by altering
cell-cell adhesion
This study explored the role of CASC2 in regulating
pancreatic cancer cell proliferation and colony formation.
Overexpression of CASC2 significantly reduced cell proliferation
and colony formation of AsPC-1 and PANC-1 cells (Fig. 2A and B). This study also assessed
whether CASC2 affected other aspects of pancreatic cancer
progression. To this end, the effects of CASC2 overexpression on
pancreatic cancer cell migration, invasion and apoptosis were
analyzed. The results revealed that overexpression of CASC2
significantly inhibited the migration and invasion of pancreatic
cancer cells (Fig. 2C and D), and
induced cell apoptosis (Fig. 2E).
On the basis of the results that CASC2 overexpression suppressed
pancreatic cell development and progression, the expression of pro-
and anti-metastatic proteins was analyzed (Fig. 2F). Previous studies have reported
that FAK is a key downstream signaling molecule triggered by
integrin activation (24) and that
FAK activity is required for the formation of cell-cell adhesion
(25). Additionally, it has been
shown that Snail induces the expression of MMPs that degrade the
basement membrane, thereby favoring invasion (26). Consistent with these reports, upon
CASC2 overexpression, the expression of ITGB4 and phosphorylation
of FAK were decreased. Additionally, N-cadherin and fibronectin
expression was decreased, and E-cadherin expression was increased
in CASC2-overexpressing cells. CASC2 overexpression further
downregulated Snail, while attenuating MMP-2 and MMP-9 expression
(Fig. 2F). The effect of CASC2 on
cell adhesion was further confirmed by light microscopy. AsPC-1 and
PANC-1 cells appeared round and had shortened synapses following
transfection with CASC2 overexpression vector compared with the
spindle shape under normal condition (Fig. 2G). Also, the number of cells was
decreased, which may be due to increased cell apoptosis in
CASC2-overexpressing cells. These observations demonstrated that
CASC2 suppressed development and progression of pancreatic cancer
cells, potentially via negative regulation of the ITGB4/FAK
signaling pathway.
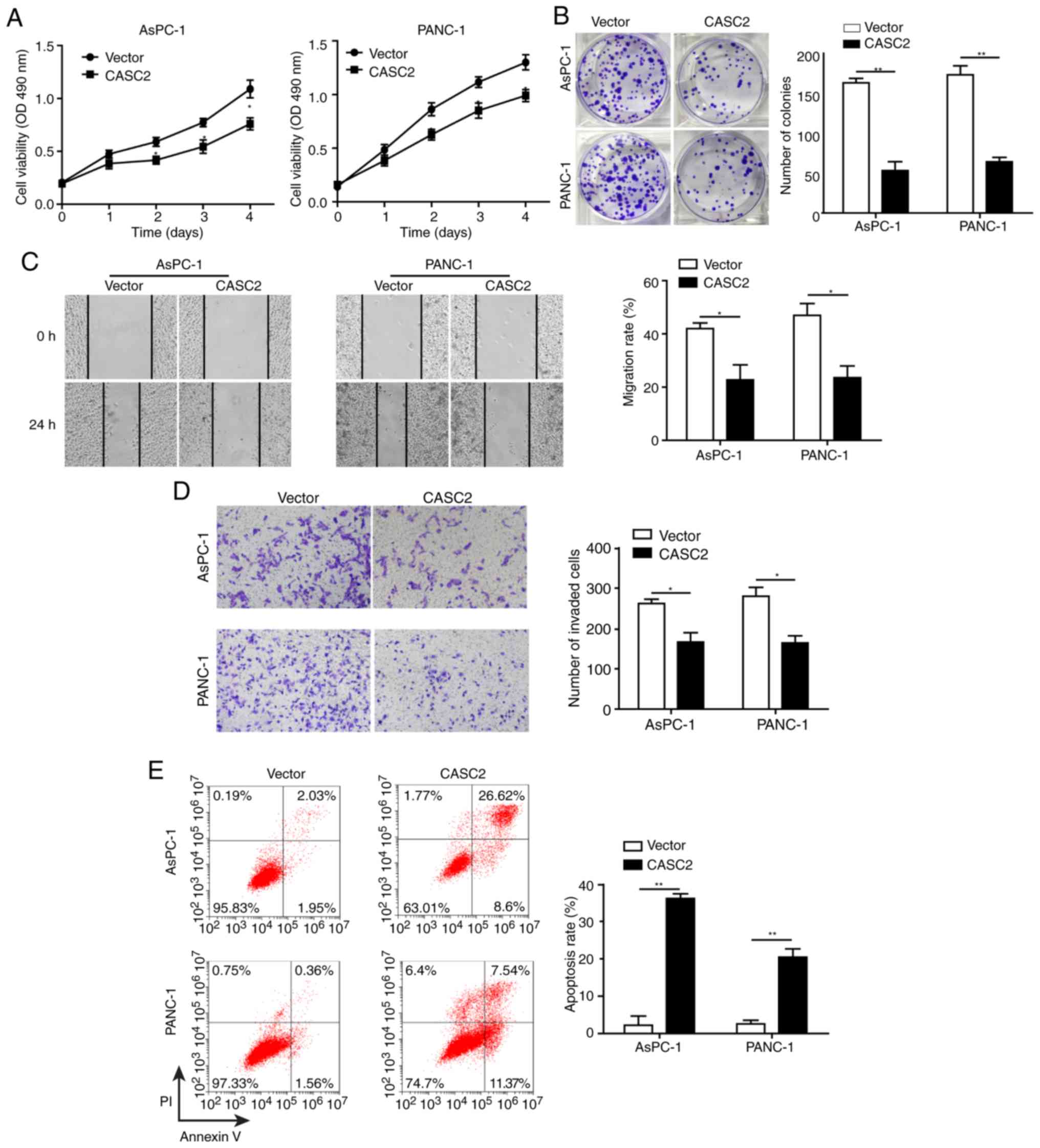 | Figure 2Overexpression of CASC2 inhibits
pancreatic cancer cell growth and progression partially by altering
cell-cell adhesion. Overexpression of CASC2 suppresses (A)
proliferation of AsPC-1 and PANC-1 cells, as determined by MTT
assay, (B) cell colony formation of AsPC-1 and PANC-1 cells, as
determined by colony formation assay, (C) migration of AsPC-1 and
PANC-1 cells, as determined by wound healing assay (×40
magnification), and (D) invasion of AsPC-1 and PANC-1 cells, as
determined by Transwell assay (×100 magnification). (E)
Overexpression of CASC2 promotes apoptosis of AsPC-1 and PANC-1
cells, as determined by flow cytometry. (F) Western blot analysis
of indicated proteins in AsPC-1 and PANC-1 cells overexpressing
CASC2. (G) Morphology of AsPC-1 and PANC-1 cells transfected with
CASC2 overexpression vector; scale bar, 50 µm.
*P<0.05 and **P<0.01. cad, cadherin;
CASC2, cancer susceptibility candidate 2; FAK, focal adhesion
kinase; ITGB4, Integrin β4; MMP, matrix metalloproteinase; NC,
negative control; OD, optical density; p-, phosphorylated. |
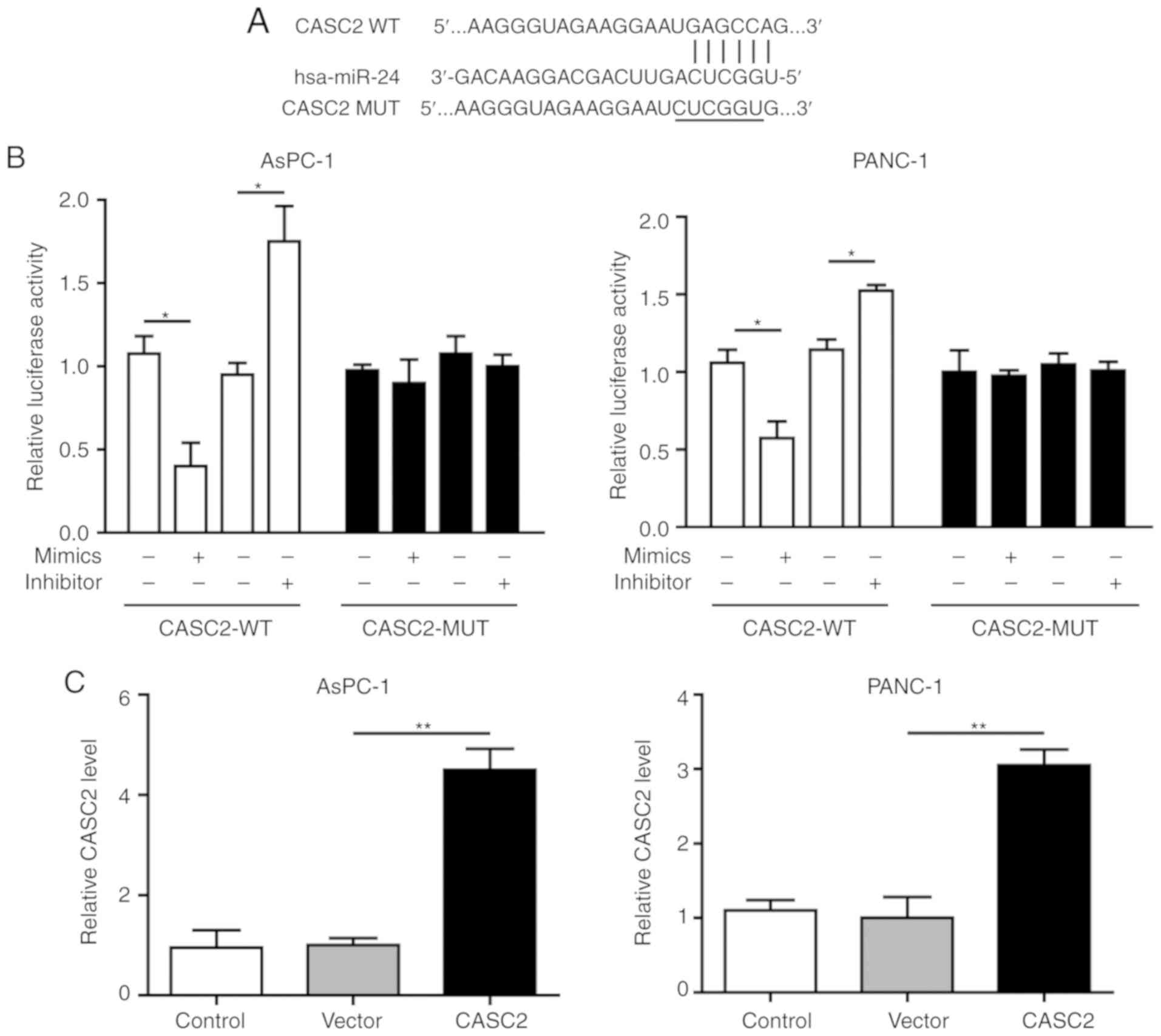
CASC2 sponges miR-24 in pancreatic
cancer
To explore the potential regulatory associations
between CASC2 and miR-24 in pancreatic cancer cells, the CASC2
promoter sequence was analyzed and a binding site for miR-24 was
identified (Fig. 3A). The present
results revealed that miR-24 knockdown in pancreatic cancer cells
promoted CASC2 promoter-driven luciferase activity, and miR-24
overexpression decreased luciferase activity; in both cases,
luciferase activity was not affected when the miR-24 binding site
was mutated (Fig. 3B). This
interaction was further assessed by overexpression of CASC2 and
inhibition of miR-24 in AsPC-1 and PANC-1 cells; the findings
confirmed that upregulation of CASC2 significantly inhibited miR-24
expression (Fig. 3C and D). In
addition, downregulation of miR-24 had no significant effect on
CASC2 expression (Fig. 3E and F).
Collectively, these results demonstrated that CASC2 sponged miR-24
in pancreatic cancer cells.
Knockdown of miR-24 inhibits pancreatic
cancer cell growth and progression partially by altering cell-cell
adhesion
To determine the effects of miR-24 on pancreatic
cancer cell development and progression, AsPC-1 and PANC-1 cells
were transfected with miR-24 inhibitor and miR-24 NC. Knockdown of
miR-24 (transfection success is shown in Fig. 3F) significantly reduced cell
proliferation, colony formation, migration and invasion, and
promoted apoptosis of AsPC-1 and PANC-1 cells (Fig. 4A-E). In agreement with the results
in CASC2-overexpressing pancreatic cancer cells, decreased cell
migration and invasion in the miR-24 knockdown cells were
accompanied by significantly decreased levels of ITGB4, p-FAK,
N-cadherin, fibronectin, Snail, MMP-2 and MMP-9, and significantly
increased levels of E-cadherin (Fig.
4F). AsPC-1 and PANC-1 cells transfected with miR-24 inhibitor
changed from spindle-shaped to rounded cells with shortened
synapses, similar to observations in CASC2-overexpressing cells
(Fig. 4G). In addition, the number
of cells was decreased, potentially due to increased cell apoptosis
in miR-24 inhibitor treatment group. These results demonstrated
that miR-24 may act as a tumor promoter in pancreatic cancer cells,
potentially via positive regulation of the ITGB4/FAK signaling
pathway.
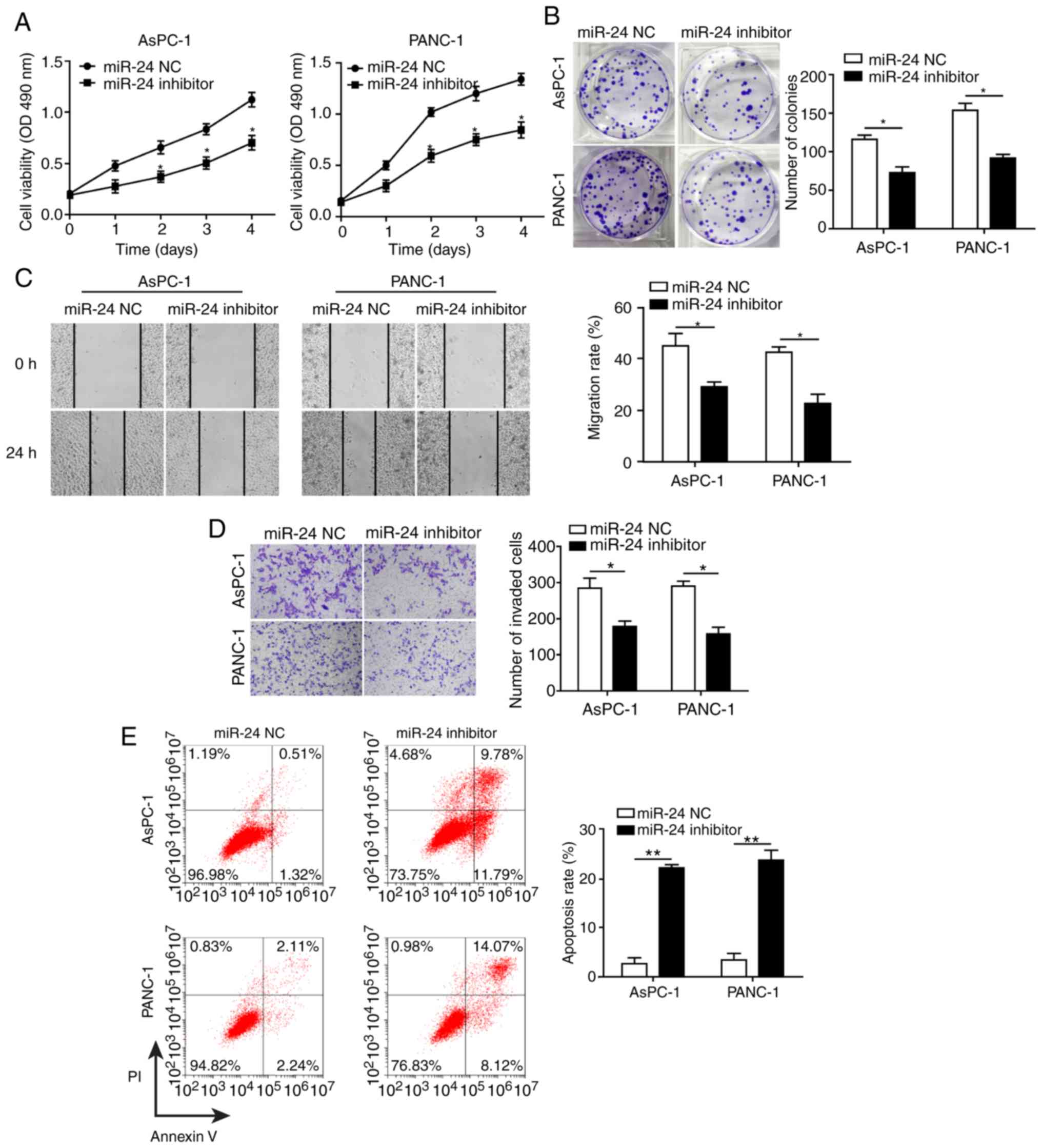 | Figure 4Knockdown of miR-24 inhibits
pancreatic cancer cell growth and progression partially by altering
cell-cell adhesion. Knockdown of miR-24 suppressed (A)
proliferation, as measured by MTT assay, (B) colony formation, (C)
migration, as measured by wound healing assay (×40 magnification),
and (D) invasion, as measured by Transwell assay (×100
magnification) in AsPC-1 and PANC-1 cells transfected with miR-24
inhibitor. (E) Knockdown of miR-24 promoted apoptosis of AsPC-1 and
PANC-1 cells transfected with miR-24 inhibitor, as measured by flow
cytometry. (F) Western blot analysis of indicated proteins in
AsPC-1 and PANC-1 cells transfected with miR-24 inhibitor or
negative control. (G) Morphology of AsPC-1 and PANC-1 cells
transfected with miR-24 inhibitor; scale bar, 50 µm.
*P<0.05 and **P<0.01. cad, cadherin;
CASC2, cancer susceptibility candidate 2; FAK, focal adhesion
kinase; ITGB4, Integrin β4; MMP, matrix metalloproteinase; NC,
negative control; OD, optical density; p-, phosphorylated. |
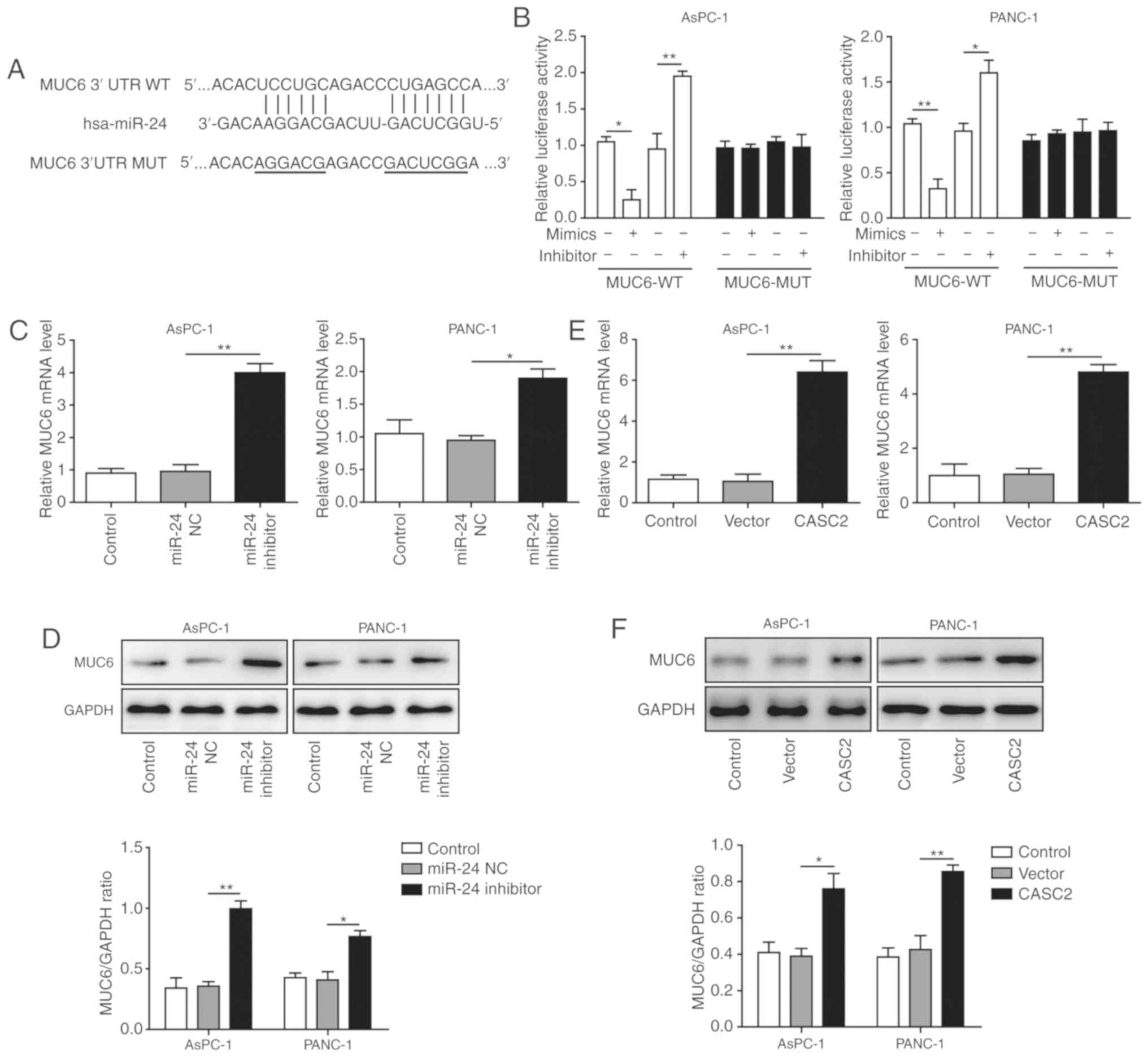
MUC6 is a target of miR-24 and is
positively regulated by CASC2 in pancreatic cancer cells
Bioinformatics analysis predicted miR-24 binding
sites on the human MUC6 3’-UTR (Fig.
5A). Upon transfection, miR-24 overexpression significantly
reduced the wild-type reporter activity, whereas miR-24 inhibition
exhibited an opposite, promoting effect (Fig. 5B). No significant effects were
observed in the mutated MUC6 group. These findings indicated that
miR-24 directly targeted MUC6. To further assess the impact of
miR-24 on MUC6 level, AsPC-1 and PANC-1 cells were transfected with
miR-24 inhibitor. It was revealed that the mRNA and protein
expression levels of MUC6 were significantly increased upon miR-24
knockdown (Fig. 5C and D),
suggesting that miR-24 functioned as an antagonist to MUC6. Based
on the aforementioned results that CASC2 exerted tumor-suppressive
functions, the association between CASC2 and MUC6 was analyzed. To
this end, MUC6 mRNA and protein expression levels in
CASC2-overexpressing pancreatic cancer cells were detected. MUC6
was upregulated and accumulated in the transfected cells (Fig. 5E and F). Therefore, it was
hypothesized that MUC6 was a direct target of miR-24 and was
positively modulated by CASC2 in pancreatic cancer cells.
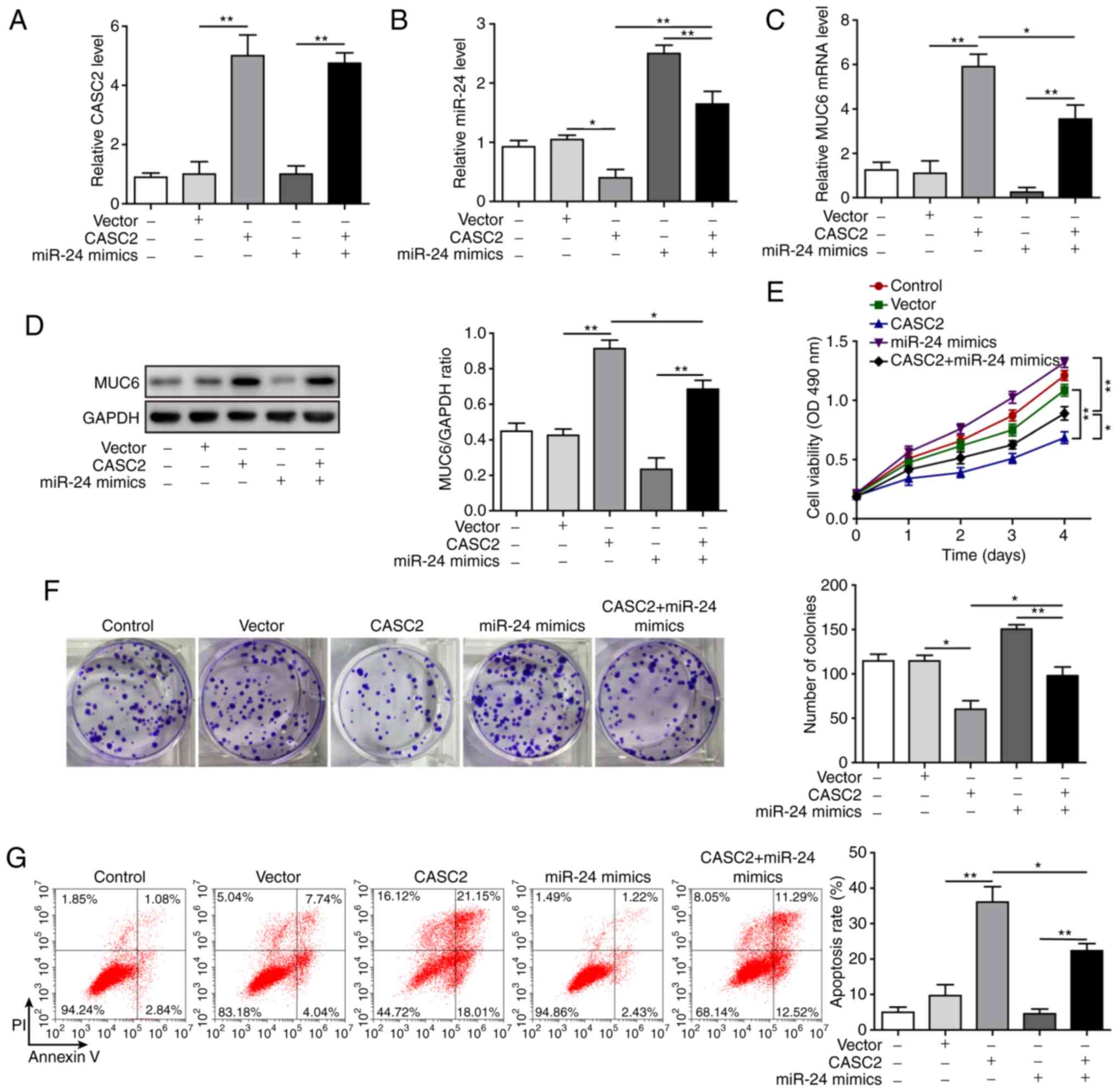
miR-24 mediates suppressive effects of
CASC2 in tumorigenesis of pancreatic cancer cells
After confirming the molecular mechanism by which
CASC2 functioned as a ceRNA for miR-24 and upregulated its
downstream target MUC6 in tumorigenesis of pancreatic cancer cells,
this study investigated the functional implication of miR-24 in
CASC2 overexpression-induced tumor suppression in AsPC-1 cells. The
study initially analyzed whether miR-24 mimics reversed alterations
in miR-24 and MUC6 levels induced by CASC2 overexpression. The
results revealed that overexpression of miR-24 significantly
restored miR-24 and reduced MUC6 expression in CASC2-overexpressing
cells (Fig. 6A-D). Furthermore, it
was investigated as to whether overexpression of miR-24 reversed
the effects of CASC2 overexpression on cell functions. The results
confirmed that miR-24 overexpression promoted cell proliferation,
colony formation, migration and invasion, and decreased apoptosis
of AsPC-1 cells induced by CASC2 overexpression (Figs. 6E-G, 7A and B). In addition, miR-24
overexpression resulted in partial restoration of ITGB4, p-FAK,
epithelial-mesenchymal transition (EMT) and cell adhesion marker
protein levels (Fig. 7C).
Collectively, these observations suggested that CASC2 exerted its
inhibitory effects on tumorigenesis partially via the miR-24/MUC6
signaling pathway.
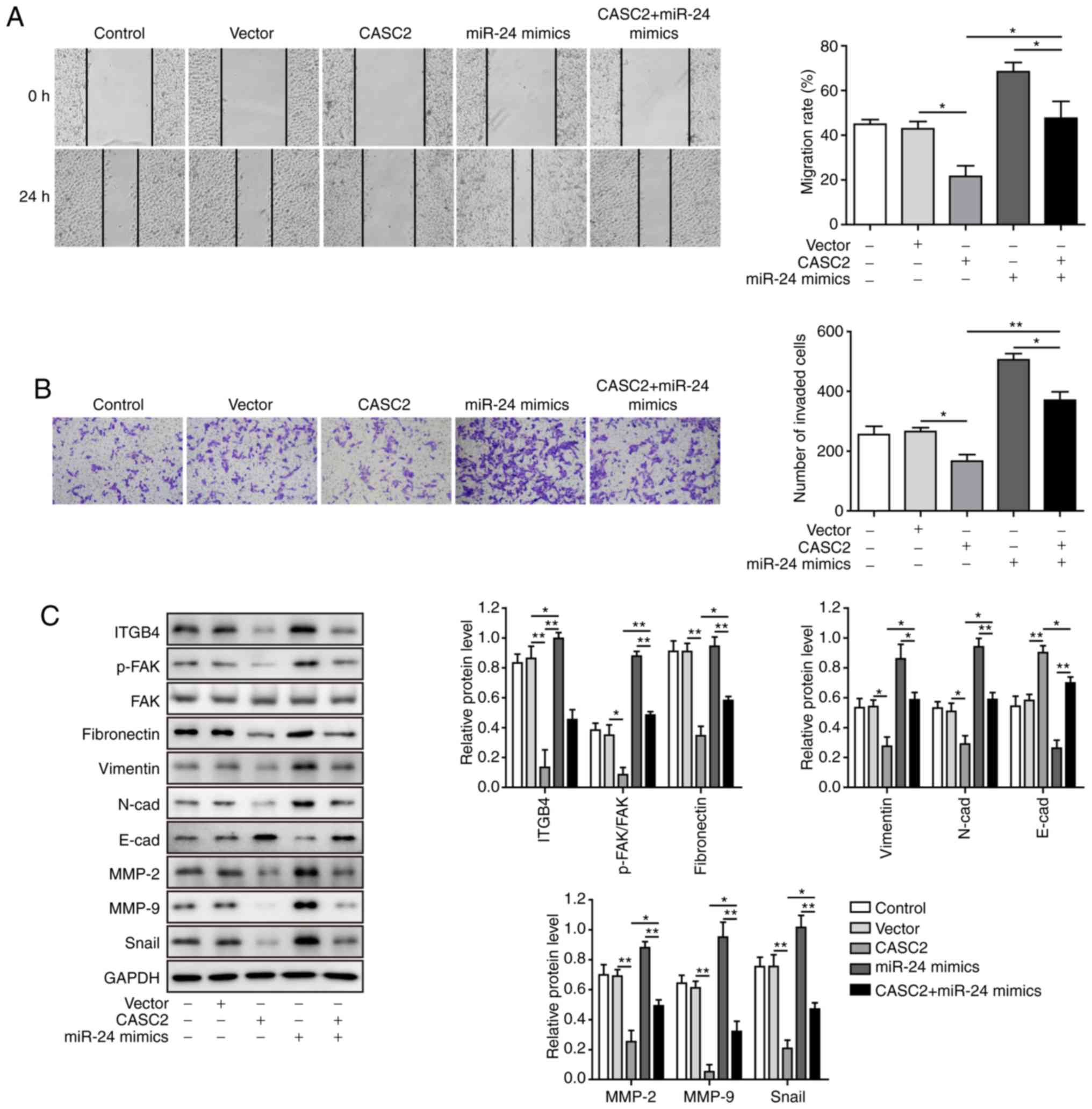 | Figure 7miR-24 mediates suppressive effects
of CASC2 on migration and invasion of pancreatic cancer cells. (A)
Migration, as determined by wound healing assay (×40
magnification), (B) invasion, as determined by Transwell assay
(×100 magnification), and (C) western blot analysis of indicated
proteins in AsPC-1 cells transfected with the indicated targets.
*P<0.05 and **P<0.01. cad, cadherin;
CASC2, cancer susceptibility candidate 2; FAK, focal adhesion
kinase; ITGB4, Integrin β4; miR, microRNA; MMP, matrix
metalloproteinase; p-, phosphorylated. |
CASC2 inhibits and miR-24 promotes
tumorigenesis of pancreatic cancer cells in vivo
To further explore whether CASC2 and miR-24 affected
tumorigenesis in vivo, AsPC-1 cells were transduced with
LV-CASC2, LV-miR-24 or LV-NC vectors (LV-CASC2-NC and LV-miR-24
NC), and these cells were injected subcutaneously into nude mice.
Upon transduction, CASC2 expression was increased by ~5-fold and
miR-24 expression by ~3.5-fold compared with the respective NC
(Fig. 8A and B). Notably, the size
and weight of tumors in the CASC2-overexpressing group were
significantly smaller than those in the LV-NC group (Fig. 8C-E). However, miR-24 overexpression
enhanced the size and weight of tumors compared with the miR-NC
injected animals (Fig. 8C-E). This
study further confirmed the upregulation of CASC2 and MUC6, and
downregulation of miR-24, in the tumors tissues of
CASC2-overexpressing animals compared with the respective control.
In addition, overexpression of miR-24 suppressed mRNA and protein
expression levels of MUC6 (Fig.
8F-H). These results indicated that CASC2 inhibited and miR-24
promoted tumor growth in vivo.
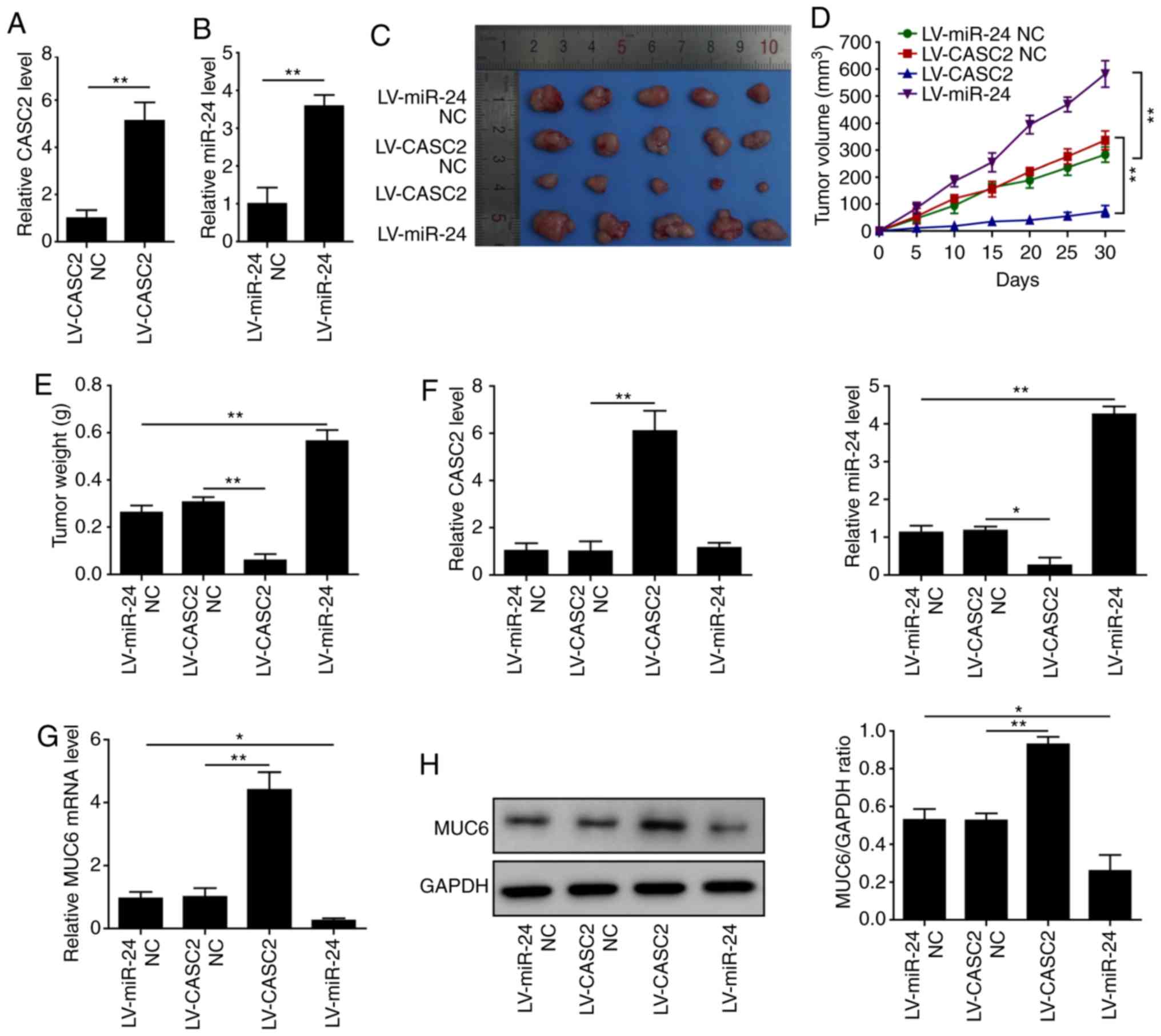 | Figure 8CASC2 inhibits and miR-24 promotes
tumorigenesis of pancreatic cancer in vivo. (A) Relative
expression of CASC2 in AsPC-1 cells stably expressing LV-CASC2 or
LV-CASC2-NC, as assessed by RT-qPCR. (B) Relative expression of
miR-24 in AsPC-1 cells stably expressing LV-miR-24 or LV-miR-24-NC,
as assessed by RT-qPCR. (C) Images, (D) volume and (E) weights of
tumors from mice injected with LV-CASC2 and LV-miR-24 cells
(n=5/group). Expression levels of (F) CASC2 and miR-24, and (G)
MUC6 in tumors. (H) Protein expression levels of MUC6 in tumors.
*P<0.05 and **P<0.01. CASC2, cancer
susceptibility candidate 2; LV, lentivirus; miR, microRNA; MUC6,
mucin 6; NC, negative control; RT-qPCR, reverse
transcription-quantitative PCR. |
Discussion
This study identified CASC2 as a suppressor of cell
growth and progression in pancreatic cancer. These findings
resulted in the drawing of several important conclusions. Firstly,
CASC2 and MUC6 were downregulated, and miR-24 was upregulated in
pancreatic cancer tissues and cell lines. Secondly, CASC2
overexpression or miR-24 knockdown suppressed pancreatic cancer
cell growth and progression partially by altering cell-cell
adhesion. Finally, CASC2 acted as a ceRNA for miR-24 and
upregulated its downstream target MUC6, in order to suppress
pancreatic cancer growth and progression in vitro and in
vivo. Therefore, this study suggested a novel mechanism for the
progression of pancreatic cancer modulated by CASC2, and proposed
the clinical implication of CASC2 as a potential biomarker or
therapeutic target in pancreatic cancer.
Aggressiveness and recurrence of pancreatic cancer
are closely associated with cancer cell migration and invasion
(3), and increasing numbers of
lncRNAs have been implicated in the regulation of these processes
in pancreatic cancer (27-29). In this study, CASC2 was
downregulated in pancreatic cancer tissues and cell lines, and
downregulated proliferation, migration and invasion, and promoted
the apoptotic abilities of pancreatic cancer cells. Furthermore,
CASC2 altered cell-cell adhesion, as evidenced by the decrease in
the levels of ITGB4 and p-FAK, together with attenuation of
N-cadherin and MMP expression, enhancement of E-cadherin
expression, and morphological alterations. These findings were
consistent with previous reports in which CASC2 functioned as a
tumor suppressor in numerous types of human cancer, including
colorectal cancer, hepatocellular cancer, osteosarcoma and
pancreatic cancer (7-11). To the best of our knowledge, this
study was the first to indicate that CASC2 exerted its
tumor-suppressive effects through altering cell-cell adhesion in
pancreatic cancer.
lncRNAs predominantly serve the role of miRNA
sponges that reduce the availability of the target miRNA, which in
turn prevents miRNAs from binding and negatively regulating
downstream target genes (30).
Available evidence suggested that CASC2 acts as a tumor suppressor
gene via interactions with several networks, including miRNAs and
other elements (7-10). miR-24 has been recognized as a
tumor-associated miRNA that regulates cancer-associated processes,
including adhesion, migration, invasion and metastasis in
colorectal, pancreatic and lung cancer (31-33).
In this study, miR-24 expression levels were increased and
negatively associated with CASC2 levels in pancreatic cancer
tissues and cell lines. The results from loss- and gain-of-function
experiments confirmed that miR-24 promoted migration and invasion,
and regulated the ITGB4/FAK pathway and EMT progression of
pancreatic cancer cells. Furthermore, bioinformatics analysis and
luciferase reporter assay identified CASC2 sponged miR-24 in
pancreatic cancer cells. A previous study reported that miR-24
functions as a tumor-promoting target that leads to increased
pancreatic cancer cell migration and invasion (32). The present results demonstrated
that CASC2 exerted its tumor-suppressive effects on pancreatic
cancer cells via interacting with miR-24. The rescue experiments
demonstrated that overexpression of miR-24 partially reversed the
inhibitory effects of CASC2 on tumor cell growth and progression.
Other reports have revealed that CASC2 serves as a sponge of miR-24
to suppress tumorigenesis of hepatocellular carcinoma (8,14).
To the best of our knowledge, this study is the first to elaborate
on the interaction between CASC2 and miR-24 in pancreatic
cancer.
Bioinformatics analysis was used to identify
potential downstream targets of miR-24 and identified MUC6. To the
best of our knowledge, this is the first study that explored the
associations between MUC6 and miR-24 in pancreatic cancer. Several
other MUCs, such as MUC1 and MUC4, have been confirmed as targets
of miR-200c/141 and miR-219-1 in pancreatic cancer (34,35).
Previous reports also identified decreases in MUC6 expression
during pancreatic cancer progression (17,36).
Furthermore, a previous study suggested that MUC6 inhibits the
invasion of pancreatic cancer cells by affecting basement membrane
organization (19). MUC5AC, a
similar secretory MUC, regulates the ITGB4/FAK signaling pathway in
lung cancer (21). In line with
these studies, the present findings demonstrated that miR-24
regulated the tumorigenesis of pancreatic cancer cells via
targeting MUC6 and modulating the ITGB4/FAK signaling pathway. In
addition, CASC2 positively regulated the expression of MUC6 via
targeting miR-24 in pancreatic cancer cells, and the
tumor-suppressive role of CASC2 was mediated via miR-24. Our
findings demonstrated that overexpression of CASC2 inhibited
miR-24, which in turn, activated MUC6 to regulate cell-cell
adhesion and suppress pancreatic cancer growth and progression.
In conclusion, the present study demonstrated that
CASC2 was downregulated in pancreatic cancer tissues and cell
lines, and inhibited cell growth and progression. To the best of
our knowledge, this study is the first to show that CASC2
suppressed progression of pancreatic cancer cells potentially via
the miR-24/MUC6 axis. Given that low CASC2 expression is closely
correlated with clinical progression and unfavorable prognosis in
patients with pancreatic cancer (37), future studies on the diagnostic
power of CASC2, including specificity and sensitivity, may
facilitate investigations into CASC2 functions and its use as a
potential biomarker to monitor or screen high-risk patients with
pancreatic cancer. These findings further provided insight into the
molecular mechanisms associated with the tumorigenesis of
pancreatic cancer and supplied potential therapeutic targets for
pancreatic cancer treatment.
Funding
This project was supported by a grant from National
Natural Science Foundation Of China (grant no. 81572408) and
Natural Science Foundation of Jiangsu Province (grant no.
BE2015712).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
DFX, LSW and JHZ conceived the study. DFX and LSW
collected and analyzed the data. DFX and LSW performed the
experiments. DFX and JHZ provided the resources and supervised the
study. DFX and LSW wrote the original draft. JHZ reviewed and
edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The assessment of patient-derived samples in this
study was approved by the Ethical Committee of Zhongda Hospital
Affiliated with Southeast University and written informed consent
was obtained from all patients. All of the animal studies were
conducted in accordance with the Institutional Animal Care and Use
Committee and were approved by the Medical Ethics Committee of
Southeast University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amundadottir L, Kraft P,
Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA,
Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, et al:
Genome-wide association study identifies variants in the ABO locus
associated with susceptibility to pancreatic cancer. Nat Genet.
41:986–990. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martens-Uzunova ES, Bottcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in
prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang G, Wu X, Li S, Xu X, Zhu H and Chen
X: The long noncoding RNA CASC2 functions as a competing endogenous
RNA by sponging miR-18a in colorectal cancer. Sci Rep. 6:265242016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan JC, Zeng F, Le YG and Xin L: LncRNA
CASC2 inhibited the viability and induced the apoptosis of
hepatocellular carcinoma cells through regulating miR-24-3p. J Cell
Biochem. 119:6391–6397. 2018. View Article : Google Scholar
|
|
9
|
Wang Y, Liu Z, Yao B, Li Q, Wang L, Wang
C, Dou C, Xu M, Liu Q and Tu K: Long non-coding RNA CASC2
suppresses epithelial-mesenchymal transition of hepatocellular
carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol Cancer.
16:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ba Z, Gu L, Hao S, Wang X, Cheng Z and Nie
G: Downregulation of lncRNA CASC2 facilitates osteosarcoma growth
and invasion through miR-181a. Cell Prolif. 51:pp. e124092018,
View Article : Google Scholar
|
|
11
|
Yu Y, Liang S, Zhou Y, Li S, Li Y and Liao
W: HNF1A/CASC2 regulates pancreatic cancer cell proliferation
through PTEN/Akt signaling. J Cell Biochem. 120:2816–2827. 2019.
View Article : Google Scholar
|
|
12
|
Liu R, Chen X, Du Y, Yao W, Shen L, Wang
C, Hu Z, Zhuang R, Ning G, Zhang C, et al: Serum microRNA
expression profile as a biomarker in the diagnosis and prognosis of
pancreatic cancer. Clin Chem. 58:610–618. 2012. View Article : Google Scholar
|
|
13
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin X, Cai L, Wang C, Deng X, Yi S, Lei Z,
Xiao Q, Xu H, Luo H and Sun J: CASC2/miR-24/miR-221 modulates the
TRAIL resistance of hepatocellular carcinoma cell through
caspase-8/caspase-3. Cell Death Dis. 9:3182018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ringel J and Löhr M: The MUC gene family:
Their role in diagnosis and early detection of pancreatic cancer.
Mol Cancer. 2:92003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suh H, Pillai K and Morris DL: Mucins in
pancreatic cancer: Biological role, implications in carcinogenesis
and applications in diagnosis and therapy. Am J Cancer Res.
7:1372–1383. 2017.PubMed/NCBI
|
|
17
|
Sierzega M, Mlynarski D, Tomaszewska R and
Kulig J: Semiquantitative immunohistochemistry for mucin (MUC1,
MUC2, MUC3, MUC4, MUC5AC, and MUC6) profiling of pancreatic ductal
cell adenocarcinoma improves diagnostic and prognostic performance.
Histopathology. 69:582–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jonckheere N, Skrypek N and Van Seuningen
I: Mucins and pancreatic cancer. Cancers (Basel). 2:1794–1812.
2010. View Article : Google Scholar
|
|
19
|
Leir SH and Harris A: MUC6 mucin
expression inhibits tumor cell invasion. Exp Cell Res.
317:2408–2419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baril P, Gangeswaran R, Mahon PC, Caulee
K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and
Lemoine NR: Periostin promotes invasiveness and resistance of
pancreatic cancer cells to hypoxia-induced cell death: Role of the
beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007.
View Article : Google Scholar
|
|
21
|
Lakshmanan I, Rachagani S, Hauke R, Krishn
SR, Paknikar S, Seshacharyulu P, Karmakar S, Nimmakayala RK,
Kaushik G, Johansson SL, et al: MUC5AC interactions with integrin
β4 enhances the migration of lung cancer cells through FAK
signaling. Oncogene. 35:4112–4121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou M, Huang W, Jiang W, Wu Y and Chen Q:
Role of Cav-1 in HIV-1 Tat-induced dysfunction of tight junctions
and Aβ-transferring proteins. Oxid Med Cell Longev.
2019:34032062019. View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK,
Schaefer E, Damsky CH and Schlaepfer DD: FAK integrates
growth-factor and integrin signals to promote cell migration. Nat
Cell Biol. 2:249–256. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yano H, Mazaki Y, Kurokawa K, Hanks SK,
Matsuda M and Sabe H: Roles played by a subset of integrin
signaling molecules in cadherin-based cell-cell adhesion. J Cell
Biol. 166:283–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu P, Yang H, Zhang J, Peng X, Lu Z, Tong
W and Chen J: The lncRNA MALAT1 acts as a competing endogenous RNA
to regulate KRAS expression by sponging miR-217 in pancreatic
ductal adenocarcinoma. Sci Rep. 7:51862017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deng SJ, Chen HY, Ye Z, Deng SC, Zhu S,
Zeng Z, He C, Liu ML, Huang K, Zhong JX, et al: Hypoxia-induced
LncRNA-BX111 promotes metastasis and progression of pancreatic
cancer through regulating ZEB1 transcription. Oncogene.
37:5811–5828. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giulietti M, Righetti A, Principato G and
Piva F: LncRNA co-expression network analysis reveals novel
biomarkers for pancreatic cancer. Carcinogenesis. 39:1016–1025.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kerimis D, Kontos CK, Christodoulou S,
Papadopoulos IN and Scorilas A: Elevated expression of miR-24-3p is
a potentially adverse prognostic factor in colorectal
adenocarcinoma. Clin Biochem. 50:285–292. 2017. View Article : Google Scholar
|
|
32
|
Listing H, Mardin WA, Wohlfromm S, Mees ST
and Haier J: miR-23a/-24-induced gene silencing results in
mesothelial cell integration of pancreatic cancer. Br J Cancer.
112:131–139. 2015. View Article : Google Scholar :
|
|
33
|
Jing P, Zhao N, Xie N, Ye M, Zhang Y,
Zhang Z, Li M, Lai X, Zhang J and Gu Z: miR-24-3p/FGFR3 signaling
as a novel axis is involved in epithelial-mesenchymal transition
and regulates lung adenocarcinoma progression. J Immunol Res.
2018:28341092018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mohr AM, Bailey JM, Lewallen ME, Liu X,
Radhakrishnan P, Yu F, Tapprich W and Hollingsworth MA: MUC1
regulates expression of multiple microRNAs involved in pancreatic
tumor progression, including the miR-200c/141 cluster. PLoS One.
8:e733062013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lahdaoui F, Delpu Y, Vincent A, Renaud F,
Messager M, Duchêne B, Leteurtre E, Mariette C, Torrisani J,
Jonckheere N and Van Seuningen I: miR-219-1-3p is a negative
regulator of the mucin MUC4 expression and is a tumor suppressor in
pancreatic cancer. Oncogene. 34:780–788. 2015. View Article : Google Scholar
|
|
36
|
Kim GE, Bae HI, Park HU, Kuan SF, Crawley
SC, Ho JJ and Kim YS: Aberrant expression of MUC5AC and MUC6
gastric mucins and sialyl Tn antigen in intraepithelial neoplasms
of the pancreas. Gastroenterology. 123:1052–1060. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu L, Chen S, Bao H, Zhang W, Liao M,
Liang Q and Cheng X: The role of lncRNA CASC2 on prognosis of
malignant tumors: A meta-analysis and bioinformatics. Onco Targets
Ther. 11:4355–4365. 2018. View Article : Google Scholar : PubMed/NCBI
|