Introduction
Colon cancer is the third most common cancer and a
leading cause of cancer-associated death in the world (1). Despite the availability of effective
screening, chemopreventive and lifestyle strategies that have
resulted in a decline in the mortality rate, about one-fifth of
patients with colon cancer present with metastatic disease at
diagnosis, and one-fifth develop metastasis during the course of
treatment (2). Therefore, new
colon cancer therapies are urgently needed.
Tumor necrosis factor (TNF)-related apoptosis
inducing ligand (TRAIL) is considered to be a potential target in
anticancer therapy due to its specific ability to induce apoptosis
of cancer cells, but not normal human cells (3). TRAIL interacts with the extracellular
domain of death receptors (DRs), DR4 and DR5, which in turn
activate intracellular apoptotic signaling (3). Mutations or decreased levels of DR4
and DR5 induce cancer cell resistance to TRAIL, whereas
upregulation of DR4 and DR5 expression using co-treatment with
subtoxic doses of chemotherapeutic drugs is an effective strategy
to overcome TRAIL resistance (4,5).
TRAIL recombinant protein and agonistic antibodies for DR4 and DR5
have been developed as potential treatments for cancer (6,7).
However, advances in pharmacotherapy may limit the effectiveness of
agonistic antibodies by monotherapy, and it is imperative to
identify molecules that promote TRAIL-induced cell death or
sensitize resistant cancer cells to TRAIL.
TRAIL induces cell death via two main apoptotic
pathways: Death receptor-mediated extrinsic pathway and
mitochondria-mediated intrinsic apoptotic pathways (8). In the extrinsic pathway, TRAIL binds
with death TRAIL receptor (DR4 or DR5) to stimulate the recruitment
of Fas-associated death domain (FADD) and pro-caspase-8, known as
the death-inducing signaling complex (DISC). Subsequently,
pro-caspase-8 is cleaved to caspase-8, which directly activates
caspase-3 (3). In the intrinsic
pathway, caspase-8 cleaves BH3 interacting-domain death agonist
(Bid), and truncated Bid (tBid) is translocated to the mitochondria
to initiate mitochondrial apoptosis; mitochondrial disruption leads
to the release of cytochrome c from the mitochondria into
the cytoplasm, which binds with apoptotic peptidase-activating
factor 1 to form an apoptosome and activates caspase-9, as well as
downstream caspases (9).
In addition, reactive oxygen species (ROS) are
upstream signaling molecules that induce DR expression in various
cancer cells and are involved in the regulation of TRAIL signaling
(10). High ROS activity in cancer
cells compared with normal cells is known to induce apoptosis by
damaging the DNA, proteins and lipid membranes (11). ROS induces apoptosis through the
regulation of pro- and anti-apoptotic proteins by mitogen-activated
protein kinase (MAPK) phosphorylation (12). Based on this, ROS are highly
dependent on the sustained MAPK signaling activity (12). Thus, TRAIL and its receptor DR4 and
DR5 agonists targeting the apoptotic pathway have been actively
studied as potential therapeutic methods for inhibiting the
proliferation of various types of cancer cells (6,7).
Icariin (ICA), a prenylated flavonol glycoside
derived from the Chinese herb Epimedium sagittatum, exhibits
a variety of pharmacological properties including antioxidant
(13), anti-tumor (14,15)
and estrogen-like (16,17) activities. In particular, ICA
exhibits a broad spectrum of anticancer effects, such as tumor
growth inhibition (14),
suppression of tumor cell invasion and migration (18) and induction of the S-phase in cell
cycle arrest and apoptosis (19).
Based on the effects of ICA reported in these previous studies, it
was hypothesized that ICA may activate TRAIL-induced apoptosis
through the induction of DR expression. Therefore, this study
investigated whether ICA can sensitize colon cancer cells to
TRAIL-induced apoptosis and which mechanism is involved in this
pathway.
Materials and methods
Reagents
ICA was prepared by Professor Ki Yong Lee (College
of Pharmacy, Korea University). Dulbecco's modified Eagle's medium
(DMEM), RPMI-1640 medium, Antibiotic-antimycotic and fetal bovine
serum (FBS) were purchased from Gibco; Thermo Fisher Scientific,
Inc. 2′,7′-dichlorofluorescein diacetate (DCFH-DA),
Lipofectamine® 2000, TRIzol® reagent and kits
for the LIVE/DEAD assay were purchased from Invitrogen; Thermo
Fisher Scientific, Inc. AccuPower Rocketscript cycle RT premix and
AccuPower PCR PreMix were purchased from Bioneer Corporation.
Soluble recombinant human TRAIL/Apo2L (10 mg/ml; cat. no. 310-04)
was purchased from PeproTech, Inc. Antibodies against CCAAT
enhancer-binding protein homologous protein (CHOP; 1:1,000; cat.
no. 2895), Bcl-xL (1:1,000; cat. no. 2764), cIAP-1 (1:1,000; cat.
no. 4952), poly (ADP-ribose) polymerase (PARP; 1:1,000; cat. no.
9542), caspase-3 (1:1,000; cat. no. 9662), caspase-9 (1:1,000; cat.
no. 9502), cleaved PARP (1:1,000; cat. no. 5625), cleaved caspase-3
(1:1,000; cat. no. 9661), cleaved caspapse-8 (1:1,000; cat. no.
9496), cleaved caspapse-9 (1:1,000; cat. no. 7237), ERK (1:1,000;
cat. no. 9102), phospho-ERK (1:1,000; cat. no. 9101), p38 (1:1,000;
cat. no. 9212), phospho-p38 (1:1,000; cat. no. 9211), phospho-JNK
(1:1,000; cat. no. 9255), survivin (1:1,000; cat. no. 2808), JNK
(1:1,000; cat. no. 9252), X-linked inhibitor or apoptosis proteins
(XIAP; 1:1,000; cat. no. 2042), cytochrome c (1:1,000; cat.
no. 11940), DR5 (1:1,000; cat. no. 3696) and β-actin (1:1,000; cat.
no. 3700) antibodies, and anti-rabbit (1:5,000; cat. no. 7074) and
anti-mouse (1:5,000; cat. no. 7076) secondary antibodies were
obtained from Cell Signaling Technology, Inc. Bcl-2 associated X
protein (BAX; 1:1,000; cat. no. sc-493), Bcl-2 (1:1,000; cat. no.
sc-492) and DR4 (1:1,000; cat. no. sc-7863) were obtained from
Santa Cruz Biotechnology, Inc. N-acetyl-L-cysteine (NAC) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
were purchased from Sigma-Aldrich; Merck KGaA. RNAiMAX transfection
reagent was purchased from Invitrogen; Thermo Fisher Scientific,
Inc. The control small interfering (si)RNA (scRNA) and CHOP siRNA
were obtained from Santa Cruz Biotechnology, Inc. The PD98059,
SB202190, SP600125 and ERK1/2-MAPK siRNA were obtained from Cell
Signaling Technology, Inc.
Cell culture
Cell lines were obtained from ATCC. The human cell
lines HCT-116 (colon adenocarcinoma), MDA-MB-231 (breast
adenocarcinoma), HPAC, PANC-1 and BxPC3 (pancreatic adenocarcinoma)
were cultured in DMEM with 10% FBS and 1% penicillin-streptomycin.
The human cell line MCF-7 (breast adenocarcinoma) was cultured in
RPMI-1640 medium with 10% FBS and 1% penicillin-streptomycin. The
human colon cancer cell line HT-29 was cultured in RPMI-1640 medium
containing 25 mM HEPES, 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin. Cells were maintained at 37°C in an
atmosphere of 5% CO2.
Cytotoxicity assay
MTT assay was used to test the effects of ICA on the
cytotoxic potential of TRAIL. To investigate the synergy between
ICA and TRAIL, HCT116 and HT-29 cells (5×103) were
treated with ICA alone (5, 10, 20, 40 and 80 µM), TRAIL
alone (5, 10, 20,40 and 80 ng/ml) and ICA (5, 10, 20, 40 and 80
µM) in combination with 20 ng/ml TRAIL for 24 h at 37°C.
Subsequently, 10 µl MTT solution (5 mg/ml) was added to each
well and cultured for 4 h at 37°C. The medium was removed, formazan
was dissolved in DMSO, and cell viability was measured at 560 nm
using a microplate reader (Tecan Group, Ltd.). The results were
described as the relative percentage compared with untreated
cells.
LIVE/DEAD assay
The LIVE/DEAD assay, which is a two-color
fluorescence assay that determines numbers of live and dead cells,
was used to measure apoptosis. Briefly, 1×106 HCT116
cells were incubated with 10 µM ICA, 20 ng/ml TRAIL or 10
µM of ICA in combination with 20 ng/ml TRAIL for 24 h at
37°C. Cells were stained with the LIVE/DEAD reagent (5 µM
ethidium homodimer and 5 µM calcein-AM) and incubated at
37°C for 30 min. Cells were analyzed under a fluorescence
microscope (×200 magnification; Nikon Corporation). The percentage
value was derived by calculating the number of red and green
cells.
Colony formation assays
Colony formation assays can be used to analyze
colony formation in vitro and determine cell viability. For
the anchorage-dependent colony formation assay, HCT-116 cells
(5×104) were seeded on top of 1 ml of 0.9% agar
containing 10 ml each of 10 µM ICA, 20 ng/ml TRAIL and 10
µM ICA in combination with 20 ng/ml TRAIL. The plates were
incubated in complete DMEM for 14 days. Images of the stained
colonies were acquired using a digital camera (Canon, Inc.), and
the number of colonies was counted using ImageJ bundled with 64-bit
Java 1.6.0_20 software (National Institutes of Health).
Measurement of intracellular ROS
Intracellular ROS levels were measured using the
fluorescent probe 2,7-dichloro-fluorescein diacetate (DCFH-DA).
HCT116 cells (5×105) were pre-exposed to NAC for 1 h,
treated with ICA for 24 h, washed and labeled with 25 µM
DCFH-DA. Following incubation for 30 min at 37°C in a 5%
CO2 incubator, the cells were washed twice with PBS, and
intracellular ROS was detected under fluorescence microscopy (×200
magnification; Nikon Corporation) at an excitation of 488 nm and
emission of 525 nm. Image-Pro Plus 4.5 software (Media Cybernetics,
Inc.) was used for analysis. The mean fluorescence intensity of the
images was assessed and normalized to obtain relative ratios that
were compared between the experimental groups.
MAPK inhibitor treatment
HCT116 cells (1×106) were pretreated with
MAPK inhibitors, such as ERK1/2-specific inhibitor PD98059 (20
µM), JNK-specific inhibitor SP600125 (20 µM) and
p38-specific inhibitor SB202190 (10 µM). After 30 min at
37°C, the cells were treated with ICA (10 µM) for 24 h.
Western blot analysis
The protein extracts from cells and xenograft tumors
were subjected to western blot analysis as previously described
(20). Cells and tissues were
harvested and lysed with RIPA buffer, and the protein samples were
quantified using a Bicinchoninic Acid Protein Assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amount of protein extracts
was denatured by boiling at 100°C for 5 min in sample buffer (5X
SDS-PAGE buffer). The proteins (30 µg) were separated by
8-12% SDS-PAGE and transferred to a PVDF membrane (Roche
Diagnostics GmbH). The membranes were blocked with 5% skimmed milk
in Tris-buffered saline with Tween-20 (TBS-T; 10 mM Tris, 150 mM
NaCl, pH 7.5; 0.1% Tween-20) for 1 h at room temperature. The
membranes were washed three times for 10 min with TBS-T and
incubated with primary antibodies at 4°C. After three washes of 10
min each in TBS-T, the membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse secondary
antibodies for 2 h and washed. The membranes were incubated with
SuperSignal Pico Chemiluminescent substrate or Dura-Luminol
substrate (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instruction and visualized with Imagequant™ LAS 4000
(Fujifilm Life Science, Tokyo, Japan).
RNA analysis and reverse
transcription-polymerase chain reaction (RT-PCR)
mRNA levels were measured using RT-PCR as described
in our previous study (20). The
following primers were used: DR4 forward, 5′-AAG TCC CTG CAC CAC
GAC-3′ and reverse, 5′-CCA CAA CCT GAG CCG ATG-3′; DR5 forward,
5′-AAG ACC CTT GTG CTC GTT GT-3′ and reverse, 5′-GAC ACA TTC GAT
GTC ACT CCA G-3′; and GAPDH forward, 5′-CAG CCT CAA GAT CAT CAG
CA-3′ and reverse, 5′-GTC TTC TGG GTG GCA GTG AT-3′. The amplified
products were analyzed by electrophoresis using a 1.5% agarose gel
stained with ethidium bromide and photographed with ImageQuant LAS
4000 (Fujifilm Corporation).
Transfection with siRNA
HCT116 cells (5×105) were plated in
6-well plates and allowed to adhere for 24 h. On the day of
transfection, 9 µl Lipofectamine RNAiMAX transfection
reagent was added to 10 µM CHOP siRNA, ERK1/2 siRNA and
scRNA in 150 µl Opti-MEM. After 24 h of transfection, the
cells were treated with ICA alone or ICA in combination with TRAIL.
CHOP siRNA (cat. no. sc-35437; Santa Cruz Biotechnology, Inc.)
contained three unique 21mer siRNAs as follows: 35437A, sense,
5′-GAA GGC UUG GAG UAG ACA ATT-3′, antisense 3′-UUG UCU ACU CCA AGC
CUU CTT-5′; 35437B, sense 5′-GGA AAG GUC UCA GCU UGU ATT-3′,
antisense 3′UAC AAG CUG AGA CCU UUC CTT-5′; 35437C, sense 5′-GUC
UCA GCU UGU AUA UAG ATT-3′, antisense 3′-UCU AUA UAC AAG CUG AGA
CTT-5′. ERK1/2 siRNA (cat. no. 6560; Cell Signaling Technology,
Inc.) sequences were as follows: Sense 5′-CCU CCA ACC UGC UCA UCA
A-3′, antisense 3′-UUG AUG AGC AGG UUG GAG G-5′. The negative
control scRNA (cat. no. sc-37007; Santa Cruz Biotechnology, Inc.),
which did not target any endogenous transcript, was used as a
control.
Animals
Male BALB/c (nu/nu) mice (5 weeks old) were
purchased from Orient Bio, Inc. All protocols were approved by the
Institutional Animal Care and Use Committee of the Keimyung
University (Daegu, South Korea; approval no. KM_2018-010; 1 August
2018) and were performed in accordance with the criteria outlined
in the Institutional Guidelines for Animal Research. The mice were
maintained in a room with no airborne pathogen under controlled
illumination (12 h light/day) with free access to food and water.
Humane endpoints were based on activity assessments such as
hunching, lack of activity, poor grooming and ruffling or a 20%
reduction in the overall body weight of the mice. No animals were
sacrificed due to meeting these endpoints.
In vivo xenograft tumor model and
treatment
HCT-116 cells (5×105/100 µl) were
suspended in DMEM with 100 µl Matrigel and inoculated
subcutaneously into the left flank of each mouse. When tumor masses
were established and palpable (tumor volume >150
mm3), mice were randomly divided into 4 groups (5
mice/group) for intraperitoneal injection as follows: i) Vehicle
group, 0.9% sodium chloride + 1% DMSO; ii) ICA group, 10 mg/kg ICA
dissolved in vehicle; iii) TRAIL group, 100 µg/kg TRAIN
dissolved in vehicle; and iv) ICA and TRAIL group, ICA and TRAIL in
combination three times per week for 3 weeks. Tumor volumes and
body weights were measured three times per week. Tumor growth was
monitored twice a week by measuring two axes of the tumor (L,
longest axis; W, shortest axis) with a digital caliper during the
treatment. Tumor volume was calculated as V=LxW2/2. All
mice were sacrificed by carbon dioxide 19 days after the first day
of treatment, and cancer tissues were collected. Mice were
euthanized by 100% carbon dioxide at 20-30% volume/min. for 5-6
min. Death was confirmed when no spontaneous breathing or blinking
reflex was observed for 2-3 min.
Statistical analysis
Data are presented as the mean ± SEM of least three
independent experiments. The statistical analyses were performed
using GraphPad Prism 6 software package (GraphPad Software, Inc.).
Statistical analyses were performed using one-way ANOVA and
Bonferroni's post hoc test to identify significant differences in
MTT assay, western blot analysis, colony formation assay, RT-PCR
and in vivo measurements. The Bonferroni's post hoc test was
used for comparison with the control group. P<0.05 was
considered to indicate a statistically significant difference.
Results
ICA enhances TRAIL-induced apoptosis in
HCT-116 Cells
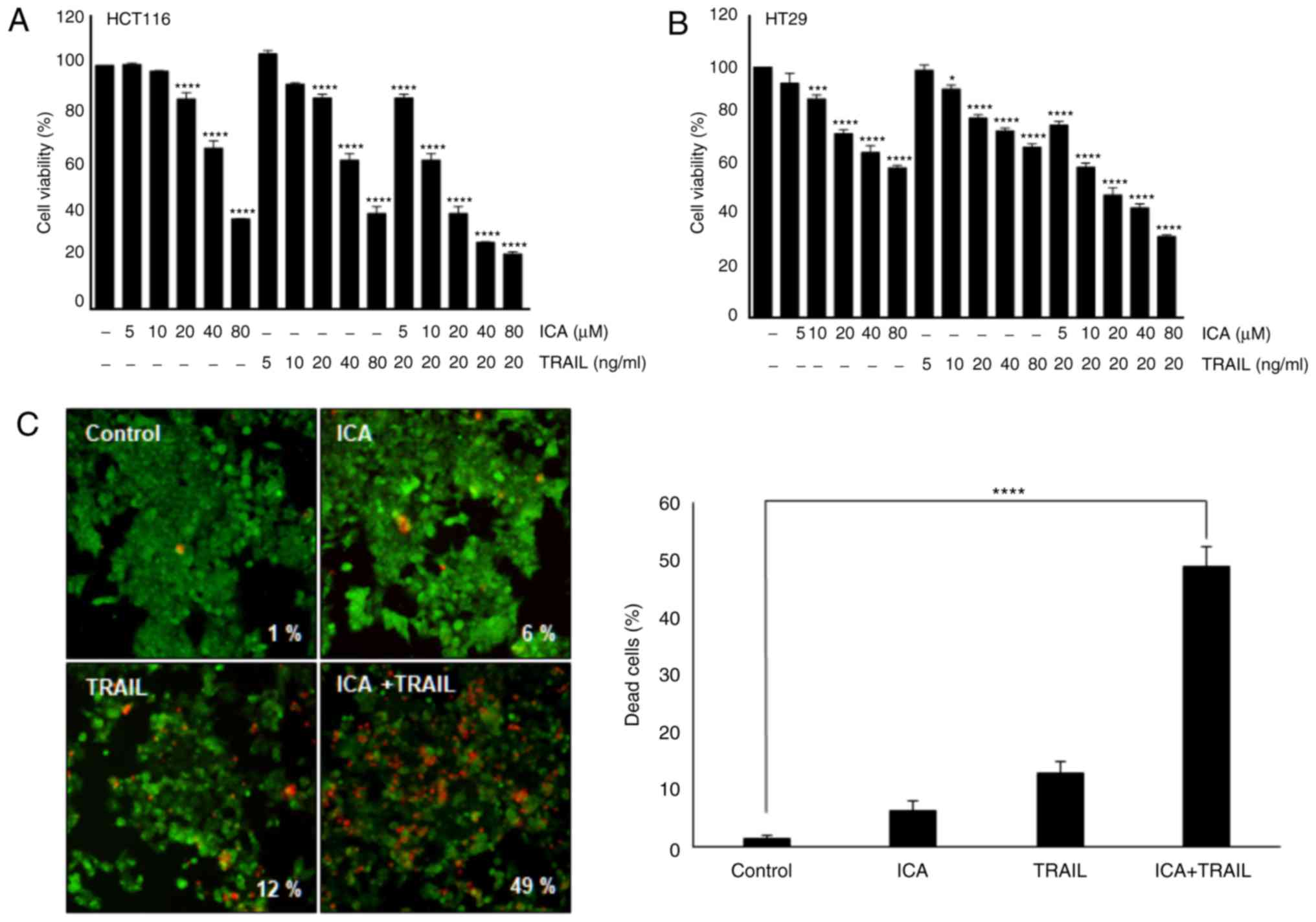
The present study investigated the effects of ICA on
TRAIL-induced cytotoxicity by MTT assay. The viability of HCT-116
and HT-29 cells was significantly reduced by ICA and TRAIL
co-treatment compared with the control cells (Fig. 1A and B). To determine the effect of
ICA on TRAIL-induced apoptosis in HCT116 cells, apoptotic cell
death was measured using the LIVE/DEAD assay. The results indicated
that TRAIL alone exhibited a minimal effect (12%) on apoptosis, and
ICA alone induced apoptosis in 6% of HCT-116 cells. However,
co-treatment with TRAIL and ICA synergistically induced apoptosis
in 49% of HCT-116 cells (Fig. 1C).
Next, long-term colony formation assay was used to determine
whether ICA enhanced the effect of TRAIL. The results demonstrated
that ICA or TRAIL alone exhibited minimal effects on colony
formation of HCT-116 cells, whereas the combination treatment
completely suppressed the colony-forming ability of these cells
compared with the control cells (Fig.
1D). The present study also investigated whether ICA enhanced
TRAIL-induced activation of apoptosis markers caspase-9 and
caspase-3, as well as consequent PARP cleavage. The results
demonstrated that that ICA enhanced TRAIL-induced activation of
caspases compared with the control group, leading to increased PARP
cleavage (Fig. 1E). These results
indicated that ICA enhanced TRAIL-induced apoptosis in HCT-116
cells.
ICA induces the expression of DR4 and DR5
in HCT-116 and HT-29 cells
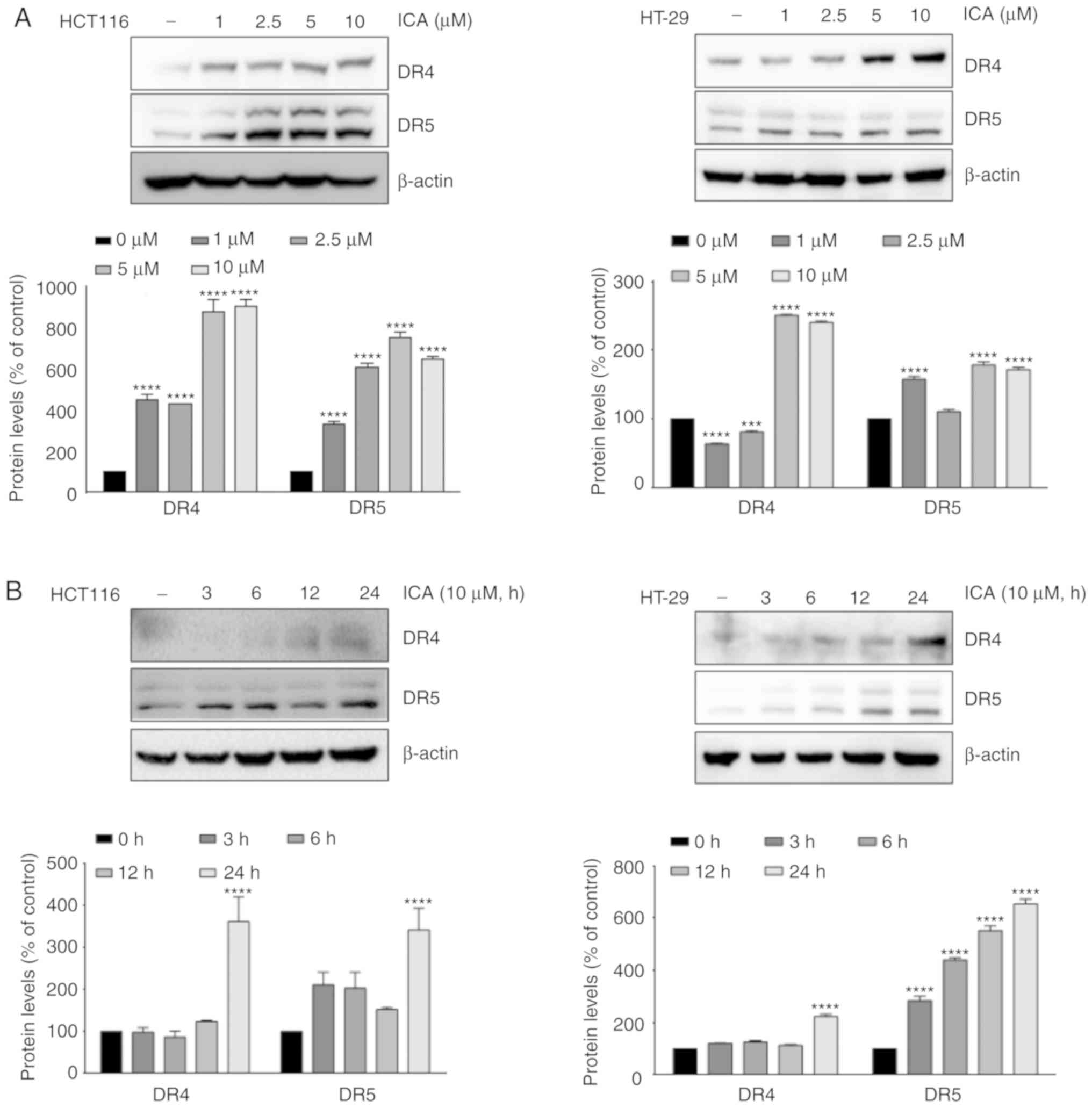
To explore the mechanism underlying the increased
levels of TRAIL-induced apoptosis following exposure to ICA, the
effect of ICA on the expression of DRs was analyzed. When HCT-116
and HT-29 cells were treated with ICA, the expression levels of DR4
and DR5 were increased at 5 and 10 µM (Fig. 2A). In addition, ICA induced DR4 and
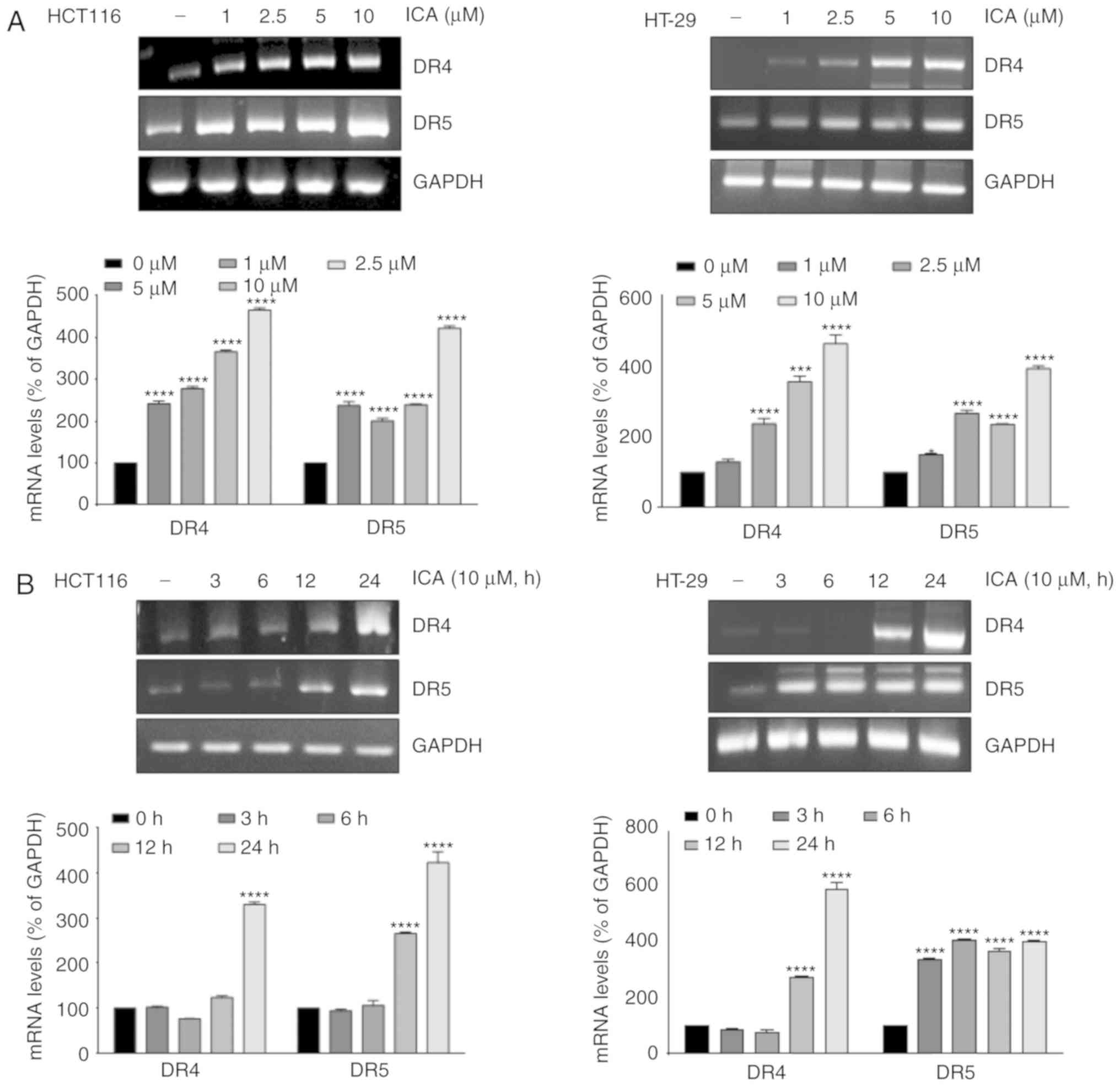
DR5 at 24 h (Fig. 2B). RT-PCR
results also demonstrated that ICA substantially upregulated DR4
and DR5 mRNA expression levels at 10 µM (Fig. 3A) and 24 h (Fig. 3B). ICA-induced expression of DR4
and DR5 was also examined in various cancer cell types; ICA induced
the expression of DR4 and DR5 in pancreatic adenocarcinoma (HPAC,
PANC-1 and BxPC3) and breast adenocarcinoma (MDA-MB-231 and MCF7)
cells compared with untreated cells (Fig. 3C). These results suggested that ICA
induced the upregulation of DR4 and DR5 in various types of cancer
cells.
Co-treatment with TRAIL and ICA modulates
the expression of apoptotic proteins
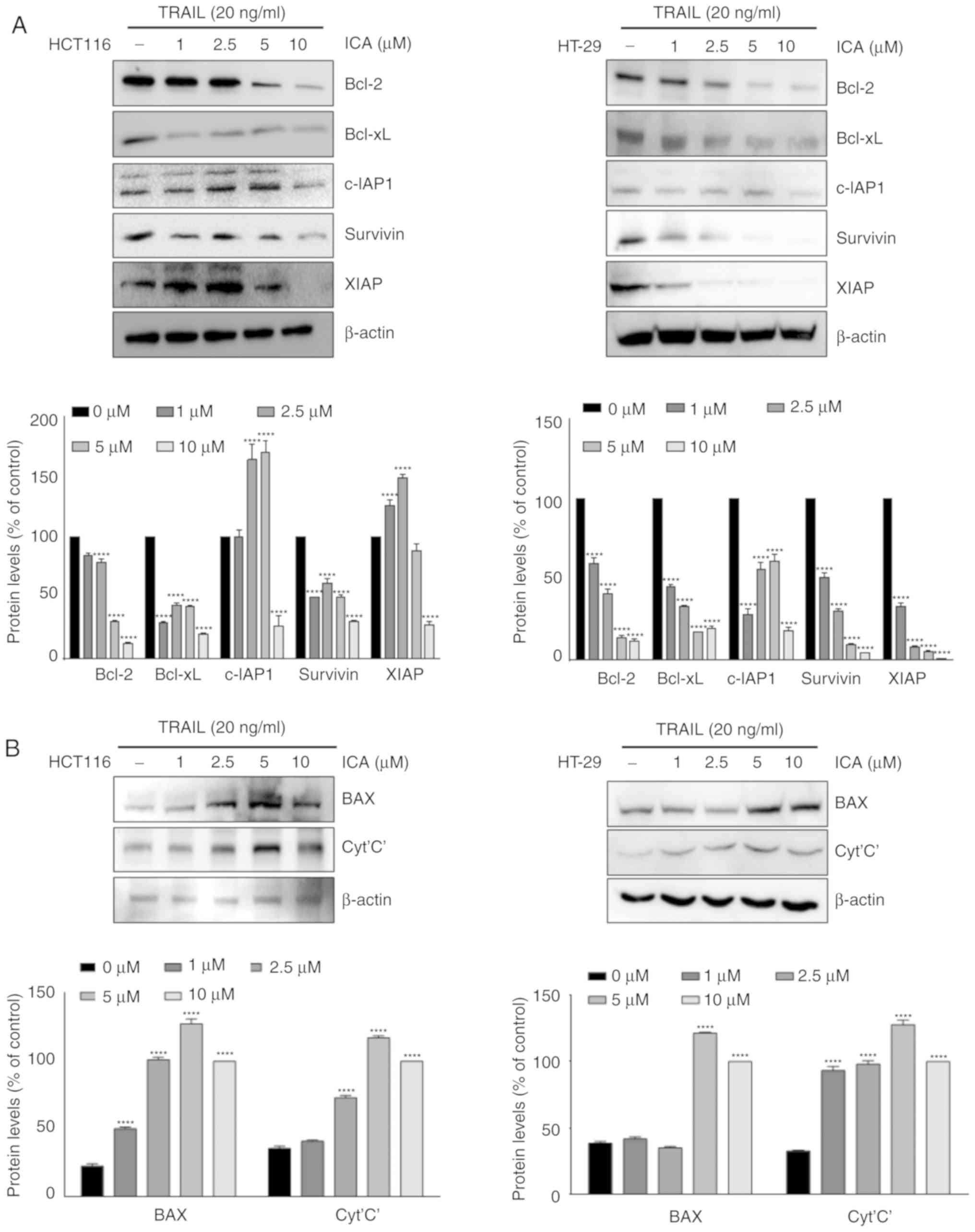
Whether ICA modulated the expression of proteins
involved in apoptosis of colon cancer cells upon co-treatment with
20 ng/ml TRAIL was then examined. In HCT-116 and HT-29 cells, the
expression of antiapoptotic proteins Bcl-2, Bcl-xL, c-IAP-1,
survivin and XIAP was reduced by 10 µM ICA (Fig. 4A). In addition, the results
presented in Fig. 4B indicated
that the expression levels of the pro-apoptotic proteins BAX and
cytochrome c were significantly increased following co-treatment
with ICA and TRAIL compared with those in the control group. These
results suggested that ICA sensitized TRAIL-induced apoptosis by
modulating the expression of apoptotic proteins.
ICA-induced DR5 upregulation is mediated
by the induction of CHOP
Several studies have reported that the induction of
DRs by various agents is mediated by the activation of CHOP
(10,21-23).
Therefore, the present examined whether ICA induced the expression
of CHOP. Cells were pretreated with the indicated concentrations of
ICA for 24 h or 10 µM ICA for the indicated times, and the
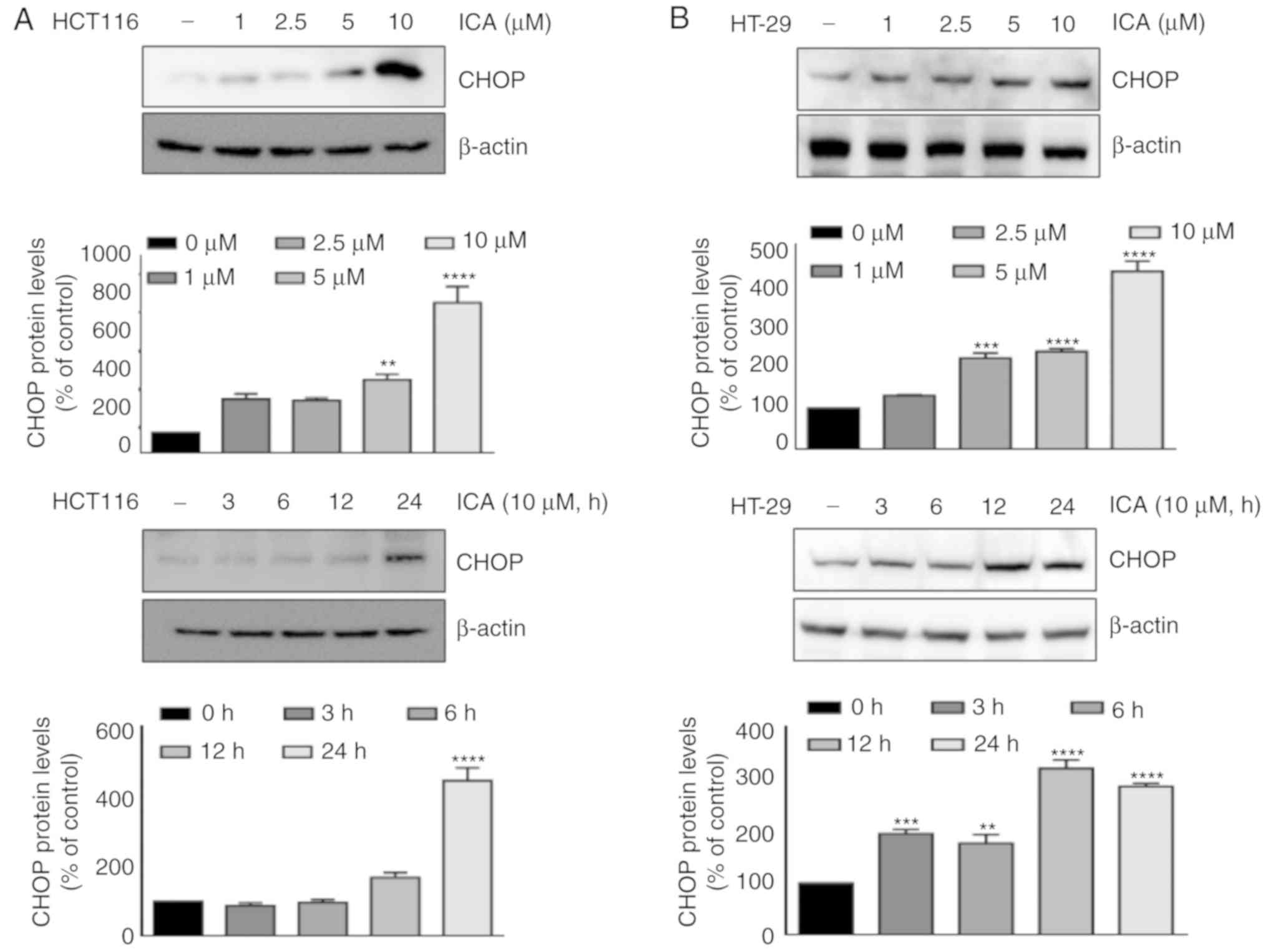
expression of CHOP was measured. The results demonstrated that ICA
induced CHOP expression in HCT-116 and HT-29 cells in a
dose-(Fig. 5A) and time-dependent
manner (Fig. 5B). To elucidate the
functional role of CHOP in ICA-induced upregulation of DR4 and DR5,
CHOP siRNA was transfected into HCT-116 cells. Transfection with
CHOP siRNA significantly abrogated the ICA-mediated upregulation of
DR5 compared with the control group, whereas DR4 and DR5 expression
levels were increased by ICA in non-transfected and
scrRNA-transfected cells. CHOP siRNA did not exhibit notable
effects on ICA-induced DR4 expression. Knockdown of CHOP by siRNA
also reduced PARP cleavage in HCT-116 cells co-treated with ICA and
TRAIL compared with the control (Fig.
5C).
ROS are required for the upregulation of
DR4 and DR5 by ICA
The role of ICA in the induction of CHOP and DRs was
further investigated. Several studies have reported that ROS serve
a role in the induction of DR4 and DR5 (24-26).
Therefore, the present study investigated whether ROS mediated
ICA-induced upregulation of DRs. Whether ICA triggered ROS
generation in HCT-116 cells was first examined; as demonstrated in
Fig. 6A, treatment with ICA
increased the ROS generation compared with the control cells, which
was reversed by pretreatment with the ROS scavenger NAC. To examine
whether ROS production was required for the expression of DRs by
ICA, HCT-116 cells were pretreated with the indicated
concentrations of NAC and subsequently supplemented with ICA. The
results demonstrated that ICA induced DR4 and DR5 expression
compared with the control group, which was suppressed by NAC in a
concentration-dependent manner (Fig.
6B).
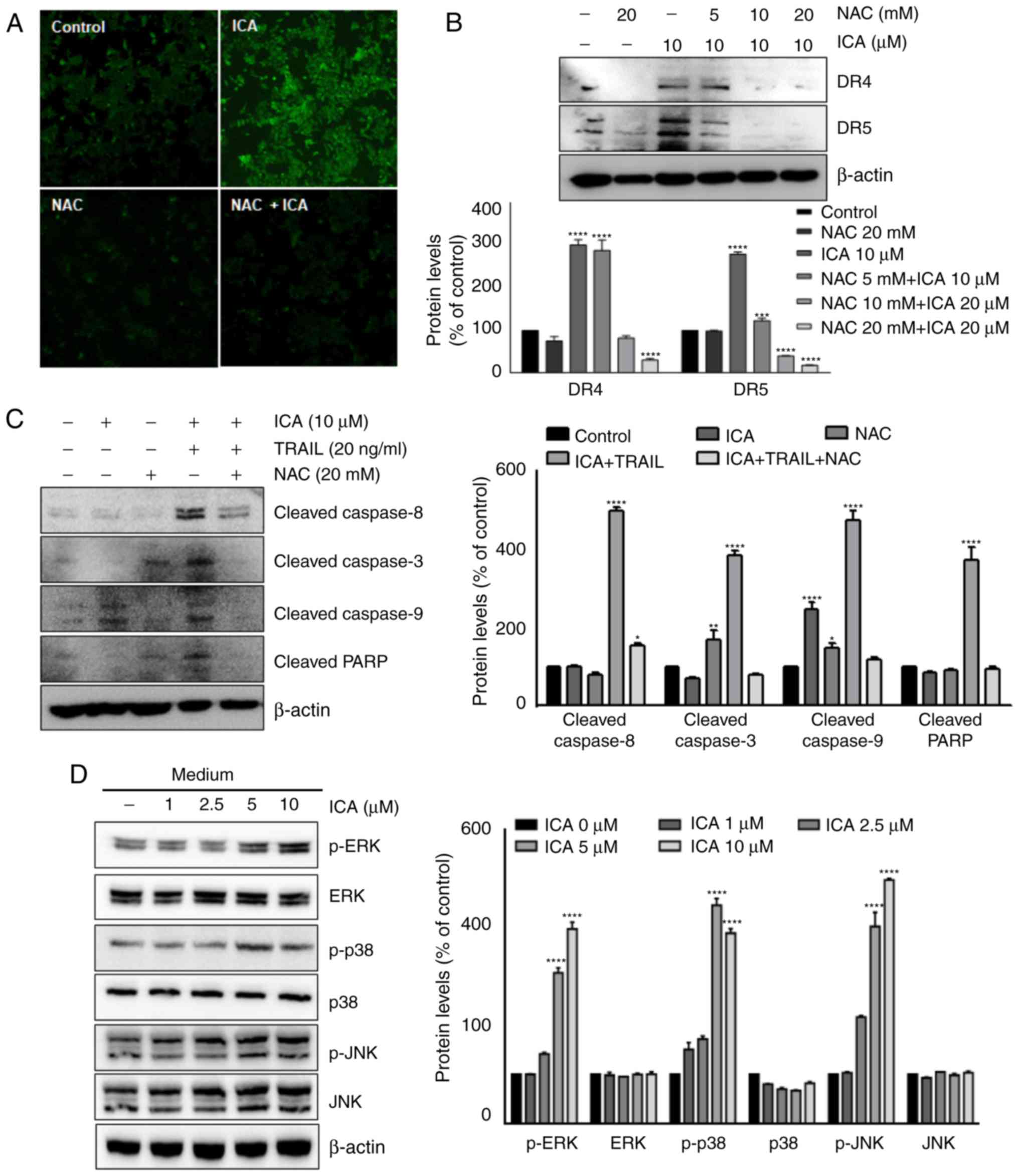 | Figure 6ROS are required for ICA-induced
upregulation of DR4 and DR5. (A) HCT-116 cells were pre-exposed to
NAC for 1 h, treated with ICA for 24 h, washed and labeled with
DCFH-DA. The levels of ROS were observed by fluorescence
microscopy. (B) HCT-116 cells were treated with the indicated
concentrations of NAC for 1 h and 10 µM ICA for 24 h. The
whole cell extracts were analyzed by western blotting using DR5 and
DR4 antibodies. (C) Cells were pretreated with NAC for 1 h, washed,
treated with 10 µM ICA and 20 ng/ml TRAIL for 24 h. Whole
cell extracts were analyzed by western blotting using the indicated
antibodies. (D) HCT116 cells were treated with the indicated
concentrations of ICA for 24 h. Whole cell extracts were analyzed
by western blotting using the indicated antibodies. (E) HCT116
cells were pretreated with NAC for 1 h and exposed to the indicated
concentrations of ICA for 24 h. Whole cell extracts were analyzed
by western blotting using the indicated antibodies. The results are
expressed as mean percentage of control ± SEM and represent three
independent experiments. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001 vs. control. ICA, icariin; TRAIL, tumor
necrosis factor-related apoptosis inducing ligand; DR, death
receptor; NAC, N-acetyl-L-cysteine; PARP, poly (ADP-ribose)
polymerase; p, phosphorylated. |
Whether NAC pretreatment affected the sensitization
of TRAIL-induced apoptosis by ICA was also determined. ICA enhanced
TRAIL-induced the cleavage of caspase-8, caspase-3, caspase-9 and
PARP compared with the control cells, whereas NAC abrogated this
increase (Fig. 6C).
ICA activates MAPK signaling
As reported in previous studies, MAPK activation
serves an important role in DR upregulation (27,28).
Therefore, to further investigate the upstream signaling pathways
mediating CHOP and DR expression regulation by ICA, HCT-116 cells
were treated for 24 h with the indicated concentrations of ICA, and
the expression of the MAPK pathway proteins was analyzed by western
blotting. ICA markedly induced the phosphorylation of ERK and p38
MAPK in HCT-116 cells (Fig. 6D).
In addition, it has been reported that ROS are involved in the
activation of MAPK (29).
Therefore, the present study investigated whether the activation of
MAPK proteins by ICA was mediated by ROS production. Cells were
exposed to 20 mM NAC for 1 h, and treated with the indicated
concentrations of ICA. Pretreatment with NAC did not affect the
activation of p38 and JNK; however, NAC inhibited ICA-induced ERK
phosphorylation (Fig. 6E). These
results suggested that the activation of the ERK signaling pathway
induced by ICA was mediated by ROS synthesis.
Activation of ERK is required for
ICA-induced upregulation of DR4 and DR5
To elucidate the association between DR expression
and ERK activation, HCT-116 cells were pretreated with 20 µM
PD98059 (ERK inhibitor), 10 µM SB202190 (p38 inhibitor) and
20 µM SP600125 (JNK inhibitor) prior to treatment with 10
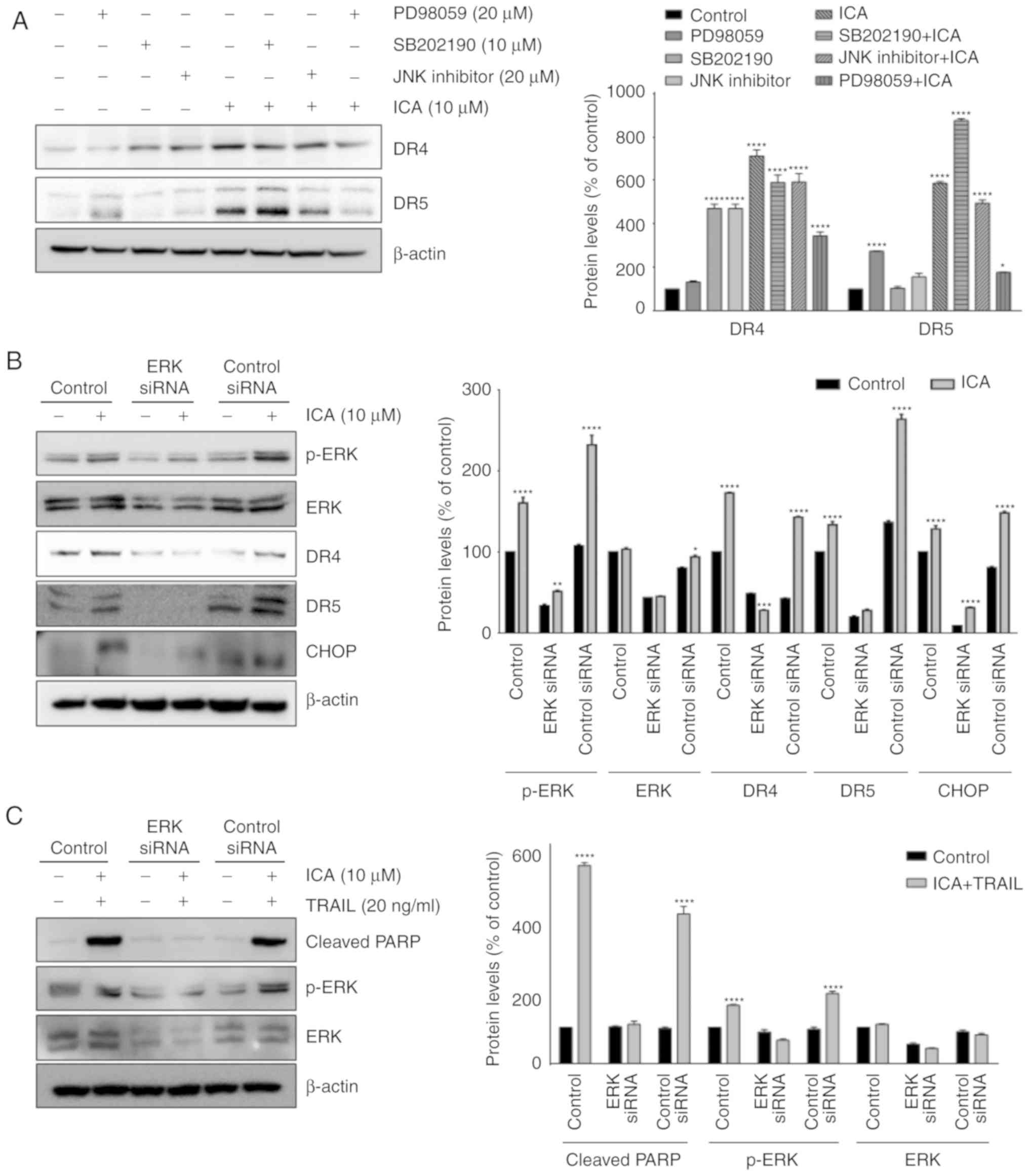
µM ICA. The results revealed that PD98059 markedly
suppressed ICA-induced expression of DR4 and DR5 (Fig. 7A). To demonstrate that ICA served
an important role in promoting TRAIL induced apoptosis by
increasing ERK phosphorylation and death receptor expression, ERK
siRNA was used to inhibit the activity of ERK. As presented in
Fig. 7B, the knockdown of ERK
notably inhibited ICA-induced DR4, DR5 and CHOP expression in
HCT-116 cells. In addition, ERK knockdown reduced the cleavage of
PARP induced by ICA and TRAIL co-treatment in HCT-116 cells
(Fig. 7C). These results indicated
that the activation of ERK by ICA was essential for the
upregulation of CHOP, DR4 and DR5 expression and sensitization of
TRAIL-induced apoptosis in colon cancer cells.
ICA enhances the effects of TRAIL to
reduce tumor growth in vivo
Based on the aforementioned results, the present
study evaluated the therapeutic effect of ICA using an in
vivo xenograft mouse model. Since a previous study reported
that administration of ICA with 10 mg/kg to mice contributed to the
improvement of colitis (30), the
same dose was selected in the present study. Treatment was
initiated when tumor size reached 150 mm3, and mice
received intraperitoneal injections of 10 mg/kg ICA and 100
µg/kg TRAIL. Tumor volumes were calculated to evaluate the
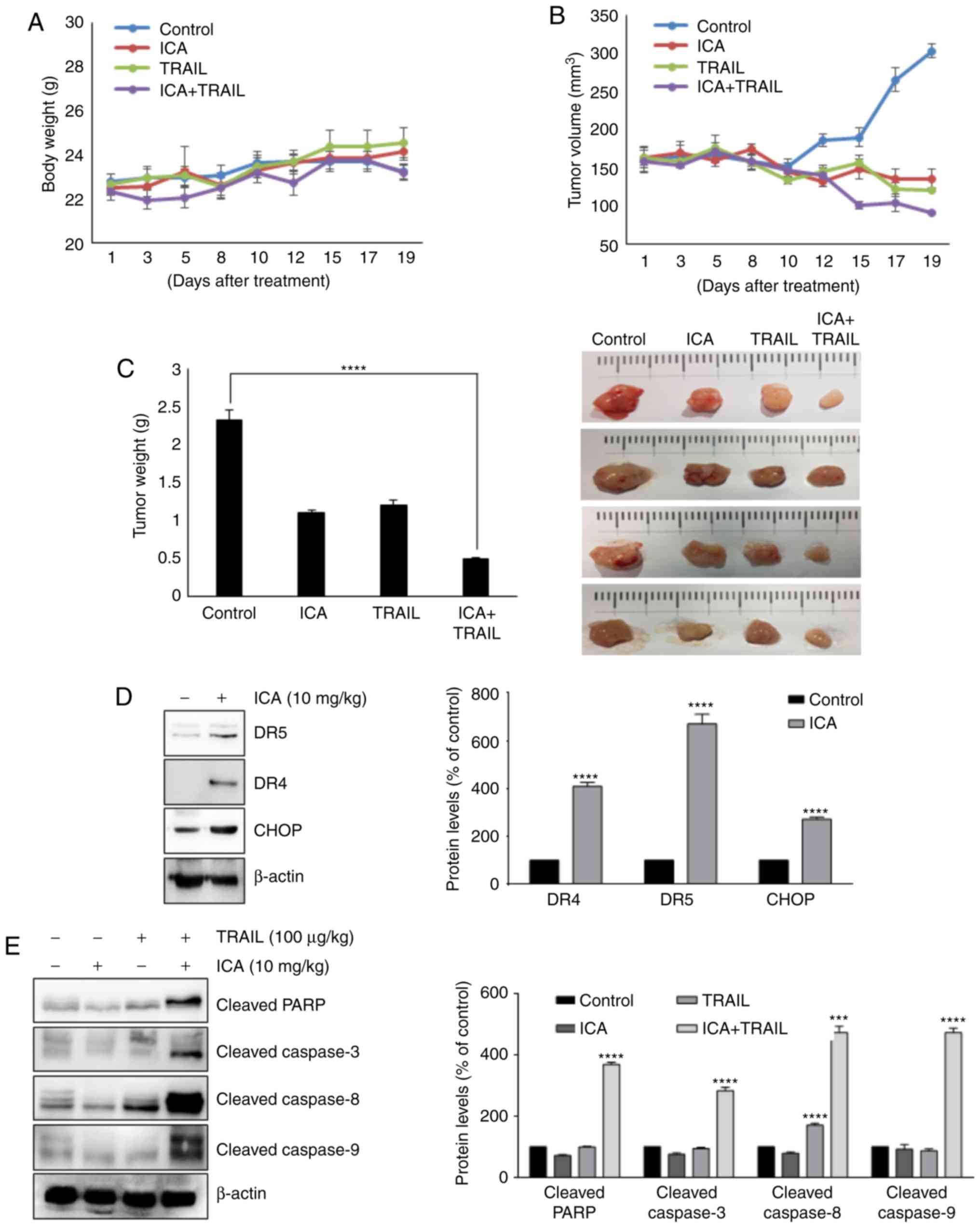
tumor suppression efficacy of the drugs. The largest volumes of the
subcutaneous tumors in the study were as follows: Control group,
303.5 mm3; ICA group, 135.5 mm3; TRAIL group,
121.6 mm3; and ICA + TRAIL group, 91.6 mm3.
As presented in Fig. 8B and C,
co-treatment with ICA and TRAIL significantly suppressed the
xenograft tumor growth compared with ICA or TRAIL treatment alone.
In addition, no apparent loss of body weight was detected in mice
during the experiment (Fig. 8A).
These results demonstrated that in vivo tumor co-treatment
with ICA and TRAIL inhibited tumor growth more efficiently compared
with either drug alone. This suggested that ICA may help treat
cancer with TRAIL resistance.
ICA augments TRAIL-induced apoptosis by
upregulating DRs and CHOP in vivo
The present study demonstrated that ICA enhanced
TRAIL-induced apoptosis by upregulating various molecules that are
involved in apoptosis in vitro. Western blot analysis of
mouse xenograft samples confirmed a strong induction of DR4, DR5
and CHOP protein expression by ICA compared with the control group
(Fig. 8D). In addition, the
cleaved forms of PARP, caspase-3, caspase-8 and caspase-9 were
markedly increased in the group co-treated with TRAIL and ICA
compared with the control group (Fig.
8E). In conclusion, these results suggested that co-treatment
with TRAIL and ICA significantly promoted apoptosis in vivo,
resulting in the suppression of colon cancer growth.
Discussion
To the best of our knowledge, among the various
apoptosis-inducing cytokines, TRAIL is currently the only protein
under active clinical investigation as a promising anticancer drug
(3). However, increasing TRAIL
resistance in various types of cancer is a major issue limiting its
therapeutic efficacy. Therefore, agents that can overcome TRAIL
resistance in tumor cells can be useful in effective cancer
treatment. The present study provided evidence that ICA sensitized
cancer cells to TRAIL-induced apoptosis by downregulating cell
survival proteins, upregulating cell death proteins and inducing
DR4 and DR5 expression through the activation of the ROS-ERK-CHOP
signal transduction pathway.
Overexpression of DRs in TRAIL-resistant cancer
cells has been reported to restore TRAIL sensitivity (24,31).
The results of the present study demonstrated that ICA
significantly increased the protein levels of DR4 and DR5 in
HCT-116 and HT-29 colon cancer cells, which led to the activation
of caspase-3, -9 and PARP in cells treated with TRAIL and ICA.
These results suggested that ICA promoted TRAIL-induced apoptosis
via caspase activation followed by DR4 and DR5 upregulation.
Overexpression of survival proteins such as survivin, XIAP, Bcl-2,
c-IAP-1 and Bcl-xL has also been reported as a cause of TRAIL
resistance in tumor cells (32).
The results of the present study revealed that ICA downregulated
the expression of these survival proteins when the apoptosis of
colon cancer cells was stimulated by TRAIL. Therefore, the
sensitivity of tumor cells to TRAIL may be attributed to the
downregulation of cell survival proteins by ICA. However, the
downregulation of these survival proteins by ICA is still unclear.
The majority of the antiapoptotic proteins described above are
regulated by NF-κB. Since ICA has been demonstrated to inhibit
NF-κB activation (20), it is
possible that the downregulation of these proteins is mediated by
the inhibition of NF-kB. The present results also indicated that
ICA significantly upregulated the expression of BAX and cytochrome
c, both of which have been identified to be critical for
TRAIL-induced apoptosis (33).
Thus, the upregulation of BAX and cytochrome c by ICA may
also contribute to TRAIL-induced apoptosis.
The results of the present study also revealed that
ICA induced TRAIL receptor expression through the induction of
CHOP. ICA treatment upregulated CHOP expression, whereas CHOP
silencing diminished the effects of ICA on DR5 upregulation and
TRAIL-induced apoptosis. In previous studies, a number of cancer
chemopreventive agents upregulated DRs through ROS generation
(27,34,35).
Consistent with these studies, the results of the present study
demonstrated that ICA induced ROS production, and the loss of ROS
by the antioxidant reagent NAC abrogated ICA-induced DR4 and DR5
upregulation, which attenuated TRAIL-induced apoptosis by ICA.
These results suggested that ROS was the most important upstream
regulator in TRAIL-induced apoptosis. Based on previous studies
reporting that MAPK signaling regulates CHOP, DR4 and DR5
expression (27,28,36),
the present study also investigated the involvement of MAPK
signaling during TRAIL-induced apoptosis; the results demonstrated
that ICA stimulated ERK activation, and a specific ERK inhibitor or
siRNA targeting ERK abolished the ability of ICA to increase CHOP
and DR expression and sensitize TRAIL-induced apoptosis in colon
cancer cells.
The present study also investigated whether these
results were applicable to in vivo conditions. In the tumor
xenograft model, the combination of ICA and TRAIL effectively
inhibited tumor growth. These results suggested that the
ICA-induced sensitivity to TRAIL was increased in vivo. ICA
increased DR4, DR5 and CHOP expression, as well as promoted
TRAIL-induced apoptosis via the activation of the caspase pathway
in vivo. Therefore, ICA may be useful to overcome TRAIL
resistance during cancer treatment. However, further animal study
with oral administration is urgently needed to verify these
results.
In conclusion, the present study provided evidence
that ICA increased DR5 and DR4 expression through ROS-, ERK-, and
CHOP-mediated pathways, suggesting that ICA may overcome TRAIL
resistance in tumor cells. ICA and TRAIL exhibited synergistic
anticancer effects in vitro and in vivo, suggesting
that ICA may be a potent therapeutic agent for chemotherapy.
Funding
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
NRF-2016R1A6A1A03011325) and the Keimyung University Research Grant
of 2018 to BP.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
BP and BK conceived the study. BK developed the
methodology, obtained and validated the data. BK and BP performed
the experiments. BP, KL and JS analyzed and interpreted the data.
BK and BP prepared the original draft. BP, KL and JS revised the
draft.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Ms. Jangmi Yun
(Keimyung University) for volunteered contributions to our research
efforts.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamilton SR: Characterizing the killer
colorectal carcinomas. Cancer Cell. 33:7–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lemke J, von Karstedt S, Zinngrebe J and
Walczak H: Getting TRAIL back on track for cancer therapy. Cell
Death Differ. 21:1350–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kischkel FC, Lawrence DA, Chuntharapai A,
Schow P, Kim KJ and Ashkenazi A: Apo2L/TRAIL-dependent recruitment
of endogenous FADD and caspase-8 to death receptors 4 and 5.
Immunity. 12:611–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mühlethaler-Mottet A, Bourloud KB,
Auderset K, Joseph JM and Gross N: Drug-mediated sensitization to
TRAIL-induced apoptosis in caspase-8-complemented neuroblastoma
cells proceeds via activation of intrinsic and extrinsic pathways
and caspase-dependent cleavage of XIAP, Bcl-xL and RIP. Oncogene.
23:5415–5425. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zinonos I, Labrinidis A, Lee M, Liapis V,
Hay S, Ponomarev V, Diamond P, Zannettino AC, Findlay DM and
Evdokiou A: Apomab, a fully human agonistic antibody to DR5,
exhibits potent antitumor activity against primary and metastatic
breast cancer. Mol Cancer Ther. 8:2969–2980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Byun HS, Zhou W, Park I, Kang K, Lee SR,
Piao X, Park JB, Kwon TK, Na M and Hur GM: C-27-carboxylated
oleanane triterpenoids up-regulate TRAIL DISC assembly via p38 MAPK
and CHOP-mediated DR5 expression in human glioblastoma cells.
Biochem Pharmacol. 158:243–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kimberley FC and Screaton GR: Following a
TRAIL: Update on a ligand and its five receptors. Cell Res.
14:359–372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yadav VR, Prasad S and Aggarwal BB:
Cardamonin sensitizes tumour cells to TRAIL through ROS- and
CHOP-mediated up-regulation of death receptors and down-regulation
of survival proteins. Br J Pharmacol. 165:741–753. 2012. View Article : Google Scholar :
|
|
11
|
Fruehauf JP and Meyskens FL Jr: Reactive
oxygen species: A breath of life or death? Clin Cancer Res.
13:789–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yip NC, Fombon IS, Liu P, Brown S,
Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL and Wang W:
Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast
cancer cells with cancer stem cell-like properties. Br J Cancer.
104:1564–1574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sze SC, Tong Y, Ng TB, Cheng CL and Cheung
HP: Herba Epimedii: Anti-oxidative properties and its medical
implications. Molecules. 15:7861–7870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li S, Dong P, Wang J, Zhang J, Gu J, Wu X,
Wu W, Fei X, Zhang Z, Wang Y, et al: Icariin, a natural flavonol
glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via
a ROS/JNK-dependent mitochondrial pathway. Cancer Lett.
298:222–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Wu J, Chen X, Fortenbery N,
Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY and Wei S: Icariin
and its derivative, ICT, exert anti-inflammatory, anti-tumor
effects, and modulate myeloid derived suppressive cells (MDSCs)
functions. Int Immunopharmacol. 11:890–898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Zhang X, Wang H, Qi L and Lou Y:
Neuroprotective effects of icaritin against beta amyloid-induced
neurotoxicity in primary cultured rat neuronal cells via
estrogen-dependent pathway. Neuroscience. 145:911–922. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Lu D, Guo J, Meng X, Zhang G and
Wang F: Icariin from epimedium brevicornum maxim promotes the
biosynthesis of estrogen by aromatase (CYP19). J Ethnopharmacol.
145:715–721. 2013. View Article : Google Scholar
|
|
18
|
Wang Y, Dong H, Zhu M, Ou Y, Zhang J, Luo
H, Luo R, Wu J, Mao M, Liu X, et al: Icariin exterts negative
effects on human gastric cancer cell invasion and migration by
vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur J
Pharmacol. 635:40–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Sun XH, Fan WJ, Jiang XM and Li AW:
Icariin induces S-phase arrest and apoptosis in medulloblastoma
cells. Cell Mol Biol (Noisy-le-grand). 62:123–129. 2016.
|
|
20
|
Kim B, Lee KY and Park B: Icariin
abrogates osteoclast formation through the regulation of the
RANKL-mediated TRAF6/NF-κB/ERK signaling pathway in Raw264.7 cells.
Phytomedicine. 51:181–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung KJ, Min KJ, Bae JH and Kwon TK:
Carnosic acid sensitized TRAIL-mediated apoptosis through
down-regulation of c-FLIP and Bcl-2 expression at the post
translational levels and CHOP-dependent up-regulation of DR5, Bim,
and PUMA expression in human carcinoma caki cells. Oncotarget.
6:1556–1568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sung B, Prasad S, Ravindran J, Yadav VR
and Aggarwal BB: Capsazepine, a TRPV1 antagonist, sensitizes
colorectal cancer cells to apoptosis by TRAIL through
ROS-JNK-CHOP-mediated upregulation of death receptors. Free Radic
Biol Med. 53:1977–1987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Xue Q, Wu L, Wang B and Liang H:
Dasatinib promotes TRAIL-mediated apoptosis by upregulating
CHOP-dependent death receptor 5 in gastric cancer. FEBS Open Bio.
8:732–742. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yi L, Zongyuan Y, Cheng G, Lingyun Z,
Guilian Y and Wei G: Quercetin enhances apoptotic effect of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) in
ovarian cancer cells through reactive oxygen species (ROS) mediated
CCAAT enhancer-binding protein homologous protein (CHOP)-death
receptor 5 pathway. Cancer Sci. 105:520–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jayasooriya RG, Choi YH, Hyun JW and Kim
GY: Camptothecin sensitizes human hepatoma Hep3B cells to
TRAIL-mediated apoptosis via ROS-dependent death receptor 5
upregulation with the involvement of MAPKs. Environ Toxicol
Pharmacol. 38:959–967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yodkeeree S, Sung B, Limtrakul P and
Aggarwal BB: Zerumbone enhances TRAIL-induced apoptosis through the
induction of death receptors in human colon cancer cells: Evidence
for an essential role of reactive oxygen species. Cancer Res.
69:6581–6589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Do MT, Na M, Kim HG, Khanal T, Choi JH,
Jin SW, Oh SH, Hwang IH, Chung YC, Kim HS, et al: Ilimaquinone
induces death receptor expression and sensitizes human colon cancer
cells to TRAIL-induced apoptosis through activation of ROS-ERK/p38
MAPK-CHOP signaling pathways. Food Chem Toxicol. 71:51–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta SC, Francis SK, Nair MS, Mo YY and
Aggarwal BB: Azadirone, a limonoid tetranortriterpene, induces
death receptors and sensitizes human cancer cells to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) through a p53
protein-independent mechanism: evidence for the role of the
ROS-ERK-CHOP-death receptor pathway. J Biol Chem. 288:32343–32356.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta SC, Hevia D, Patchva S, Park B, Koh
W and Aggarwal BB: Upsides and downsides of reactive oxygen species
for cancer: The roles of reactive oxygen species in tumorigenesis,
prevention, and therapy. Antioxid Redox Signal. 16:1295–1322. 2012.
View Article : Google Scholar :
|
|
30
|
Tao F, Qian C, Guo W, Luo Q, Xu Q and Sun
Y: Inhibition of Th1/Th17 responses via suppression of STAT1 and
STAT3 activation contributes to the amelioration of murine
experimental colitis by a natural flavonoid glucoside icariin.
Biochem Pharmacol. 85:798–807. 2013. View Article : Google Scholar
|
|
31
|
Reuss DE, Mucha J, Hagenlocher C, Ehemann
V, Kluwe L, Mautner V and von Deimling A: Sensitivity of malignant
peripheral nerve sheath tumor cells to TRAIL is augmented by loss
of NF1 through modulation of MYC/MAD and is potentiated by curcumin
through induction of ROS. PLoS One. 8:e571522013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian X, Ye J, Alonso-Basanta M, Hahn SM,
Koumenis C and Dorsey JF: Modulation of CCAAT/enhancer binding
protein homologous protein (CHOP)-dependent DR5 expression by
nelfinavir sensitizes glioblastoma multiforme cells to tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL). J Biol
Chem. 286:29408–29416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ravi R and Bedi A: Sensitization of tumor
cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of
casein kinase II. Cancer Res. 62:4180–4185. 2002.PubMed/NCBI
|
|
34
|
Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA,
Lim JH, Kwon TK and Choi KS: Monensin, a polyether ionophore
antibiotic, overcomes TRAIL resistance in glioma cells via
endoplasmic reticulum stress, DR5 upregulation and c-FLIP
downregulation. Carcinogenesis. 34:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park SK, Sanders BG and Kline K:
Tocotrienols induce apoptosis in breast cancer cell lines via an
endoplasmic reticulum stress-dependent increase in extrinsic death
receptor signaling. Breast Cancer Res Treat. 124:361–375. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stolfi C, Caruso R, Franzè E, Rizzo A,
Rotondi A, Monteleone I, Fantini MC, Pallone F and Monteleone G:
2-methoxy-5-amino-N-hydroxybenzamide sensitizes colon cancer cells
to TRAIL-induced apoptosis by regulating death receptor 5 and
survivin expression. Mol Cancer Ther. 10:1969–1981. 2011.
View Article : Google Scholar : PubMed/NCBI
|