Osteosarcoma is the most common human primary
malignant bone tumor affecting children and young adults (1), and is usually located in the distal
femur, the proximal tibia, or the proximal humerus (2). The tumor is evolved from mesenchymal
cells and is characterized by spindle cells and aberrant osteoid
formation pathologically (3). The
survival of patients with localized disease is increased with
aggressive, multi-agent, neo-adjuvant chemotherapy and limb-salvage
surgery. However, despite aggressive therapies, the long-term
survival rate of patients with metastatic or recurrent disease
however is <20% (2,4,5).
Although several genes have been reported to be involved in
osteosarcoma tumorigenesis and therapeutic resistance (6-11),
the precise molecular mechanisms involved in these processes remain
unclear. The identification of clinically relevant diagnostic and
prognostic biomarkers, such as circulating or cellular/tissue
biomarkers, is, therefore, urgently required.
Exosomes are lipid bilayer membrane bound,
nano-sized extracellular vesicles that are 40-100 nm in diameter
(12-14). The exosomes are formed by pinching
off of multivesicular endosomes (MVEs) on the membrane in the form
of small intraluminal vesicles within the MVE and packing with
cytoplasmic contents, including proteins, mRNAs and microRNAs
(miRNAs or miRs) (15,16). The secretion of exosomes occurs
when MVEs fuse with the plasma membrane, and thus release the
contents in exosomes into the extracellular environment. According
to the exosome content database, ExoCarta, 9,769 proteins, 1,116
lipids, 3,408 mRNAs and 2,838 miRNAs have been identified in
exosomes of different cell types in multiple organisms (17,18).
Exosomes were first described as vesicles released
from reticulocyte MVEs for the removal of obsolete transferrin
receptors (19,20). However, in recent years, exosomes
have been considered to be important mediators of cellular
communication in both normal physiological processes, and in the
development and progression of diseases, such as cancer. Exosomes
have been identified in the majority of bodily fluids, including
the serum, urine, amniotic fluid, saliva, breastmilk, cerebrospinal
fluid and nasal secretions (21,22).
Importantly, cancer cells secrete a greater number of exosomes
compared to healthy cells (23),
indicating their potential for use as diagnostic biomarkers.
miRNAs are a class of small non-coding endogenous
RNAs (18-24 nucleotides in length). miRNAs can act as
post-transcriptional gene regulators by pairing with complementary
sequences in the 3′ untranslated region (3′ UTR) of target mRNAs,
leading to mRNA degradation or translational repression (24,25).
miRNAs regulate a variety of cellular processes associated with
carcinogenesis, such as cell proliferation, cell cycle, apoptosis,
angiogenesis, invasion and metastasis (26-31).
The expression of miRNAs is altered in a variety of
cancer types and is associated with the disease stage in some
cases. Some specific miRNAs may contribute to tumor growth,
progression, metastasis and drug resistance (32-35).
Among these, miR-21 is most notable, since it has been extensively
studied in various types of cancer. The majority of miRNAs
detectable in serum and saliva is concentrated within exosomes
(23). The level of miRNAs is
similar in both circulating exosomes from cancer patients and tumor
cells (22,36). In a number of studies, the high
expression of circulating miR-21 has been used to differentiate
cancer patients from healthy individuals and predict disease
outcomes (37 and refs. therein), suggesting that circulating
exosome miRNAs, such as miR-21 can be utilized as for liquid biopsy
miRNA profiling.
hsa-mir-21 or miR-21 is an abundantly expressed
miRNA in different types of mammalian cells (38-40),
indicating its importance among miRNAs. miR-21 regulates biological
processes, such as osteoclastogenesis, osteoclast differentiation,
etc. Thus, miR-21 plays an essential role in the development of
diseases, such as cancer, cardiovascular diseases and inflammation
(41-43) The hsa-miR-21 gene is located
on chromosome 17q23.2. Pri-miR-21 (primary transcript containing
miR-21) is located within the intronic region of the tmem49
gene. Even though pri-miR-21 and tmem49 genes overlap in the
same direction of transcription, pri-miR-21 is transcribed by its
own promoter and is terminated with its own poly(A) tail. The
pri-miR-21 transcript is subsequently processed into mature miR-21
(44,45).
The expression of miR-21 has been found to be
increased in the majority of cancer types analyzed, rendering it an
established oncogenic miRNA (44-54).
Functional analyses in epithelial-, hepatocyte- and glial
cell-derived cell lines support the regulatory role of miR-21 in
cell growth, migration and invasion (46,47,55-57).
Moreover, miR-21 and its associated pathways play a critical role
in the pathogenesis of osteosarcoma and act as a therapeutic target
for this tumor type (58). miR-21
exhibits a significantly higher expression in osteosarcoma tissues
compared to adjacent normal tissues (59-61).
miRNA expression has been shown to be positively associated with
Enneking clinical staging and lung metastasis (62). Moreover, serum miR-21 has been
reported to be a biomarker for chemosensitivity and the prognosis
of human osteosarcoma (63).
Additionally, circulating miR-21 levels are higher in patients with
osteosarcoma than in healthy individuals. Studies have demonstrated
that the detection of plasma miR-21 together with miR-143 and
miR-199a-3p in patients with osteosarcoma can discriminate between
the presence or absence of this tumor (62,64).
The evaluation of tumor metastasis and histopathological subtype
from tumors of patients with osteosarcoma has revealed a higher
level of miR-21 in patients with metastatic compared to
non-metastatic disease (62).
The tumor microenvironment (TME) differs from that
of normal tissues. The TME is composed of cellular and
extracellular components. The cellular components of the TME
involve cancer-associated fibroblasts (CAFs), myofibroblasts,
adipocytes, endothelial cells, epithelial cells and immune
inflammatory cells, such as T lymphocytes, B lymphocytes, natural
killer cells and natural killer T-cells, and tumor-associated
macrophages (65). Cells in the
TME are in constant autocrine and paracrine communication, which
contributes to tumor development, progression, drug resistance and
metastasis (66-70).
Exosomes provide a unique method of information
transfer both locally and globally by releasing their contents into
the target cell, e.g., miRNAs. By releasing exosomes, tumor cells
reprogram their surroundings in the TME into a tumor-permissive or
tumor-promoting environment (71-74).
mir-21 present in osteosarcoma cell-derived exosomes may affect the
TME (75,76), mediating the crosstalk between
cancer cells, endothelial cells, immune cells, and fibroblasts in
the TME to promote osteosarcoma development by i) the stimulation
of tumor angiogenesis; ii) the inhibition of the immune response by
acting directly on effector cells; iii) interfering with the
regulation of stromal cell activation; and iv) the promotion of
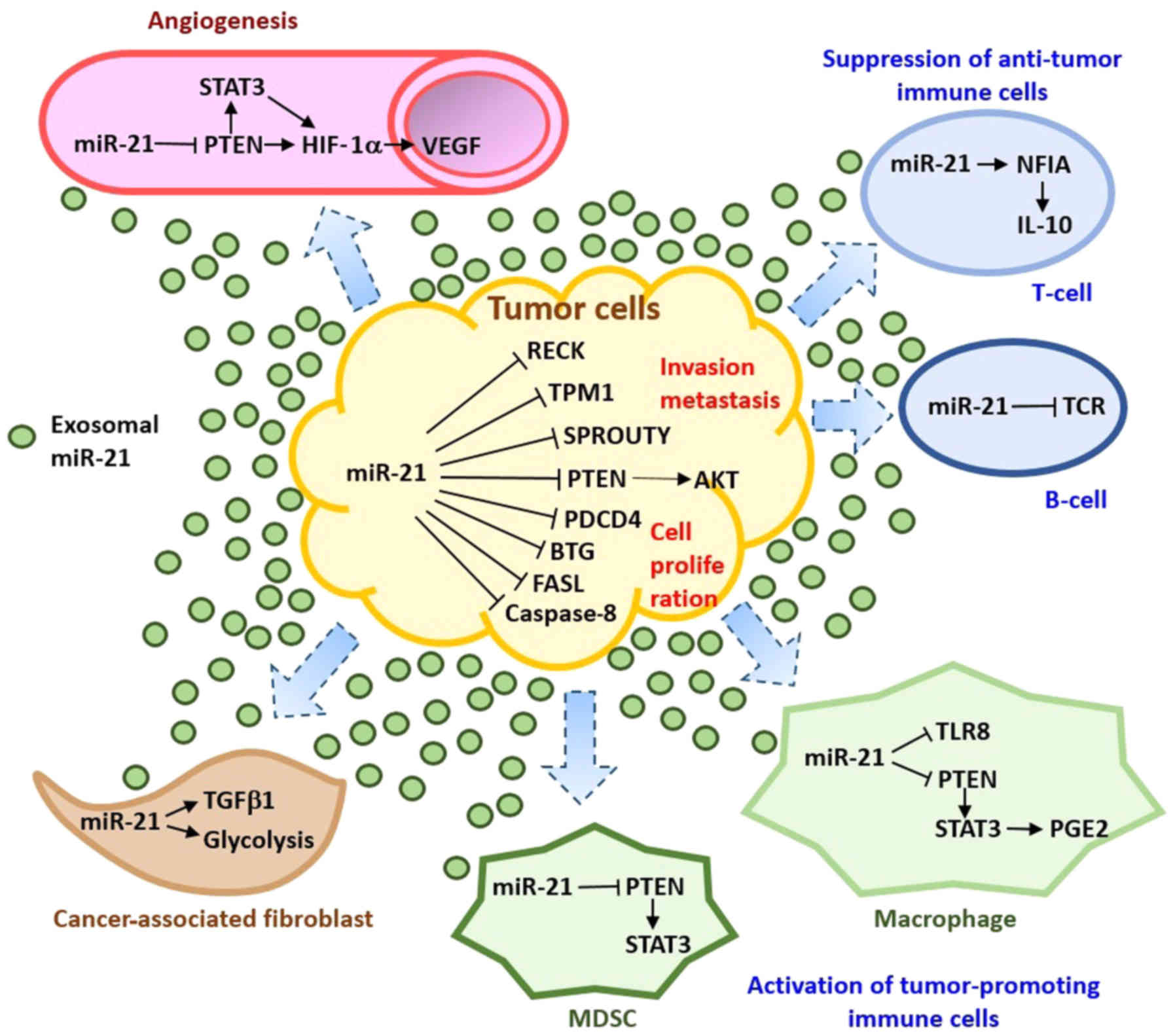
cancer progression by preparing the metastatic niche (Fig. 1).
Cancer is a highly heterogeneous disease. A single
tumor is composed of groups of genetically clonal cells that have
different growth rates, metastatic potential, invasion potential
and sensitivities to chemotherapy and radiotherapy. Individual
malignant tumor cells can affect the invasion and migration of
surrounding tumor cells by releasing exosomal miRNAs. In contrast
to exosomes released from healthy cells, exosomes derived from
patients with osteosarcoma have been shown to significantly
increase the adhesion, migration and viability of MG63 human
osteosarcoma cells (77).
Synthetic miR-143 introduced into osteosarcoma cells is released
via exosomes. The delivery of exosome-contained miR-143
significantly reduces the migration of osteosarcoma cells,
suggesting that miRNAs in exosomes regulate the function of cancer
cells (78).
miR-21 is suggested to function as an oncogene,
since it promotes the growth and development of osteosarcoma and
other cancer types. In MG-63 cells, the downregulation of miR-21
results in decreased proliferation and invasion. By contrast, the
elevation of miR-21 by using a mimic increases cell proliferation
and invasion (79). The
phosphoinositide 3-kinase (PI3K)/ R AC-α ser ine/th reon
ine-protein k inase (AKT)/mammalian target of rapamycin (mTOR)
signaling pathway is one of the important pathways dysregulated in
osteosarcoma (80-82). miR-21 may regulate the expression
of the tumor suppressor phosphatase and tensin homolog (PTEN). The
downregulation or deletion of PTEN activates the PI3K/AKT
(phosphoinositide 3-kinase/RAC-α serine/threonine protein kinase)
signaling pathway, leading to cancer development, invasion and
metastasis (83,84).
In addition to PTEN, miR-21 is suggested to target
the TGF-β1 signaling pathway to promote cell proliferation in
osteosarcoma. miR-21 knockdown inhibits the proliferation of
osteosarcoma and promotes the expression of PTEN and TGF-β1
proteins in MG63 and U2OS human osteosarcoma cell lines (85). Moreover, it has been demonstrated
that treatment with a TGF-β1 inhibitor countered the inhibitory
effects of miR-21 knockdown on osteosarcoma cell proliferation
(86).
Caspase-8 is a direct target of miR-21; miRNA
negatively regulates the expression of caspase-8. The
overexpression of miR-21 has been shown to enhance cell viability
and survival, whereas it suppresses the apoptosis of the human
osteosarcoma cell line, SAOS-2. In a subsequent study, miR-21
suppression was shown to increase caspase-8 expression and decrease
apoptosis (60).
miR-21 is a regulator of drug resistance in
osteosarcoma. The suppression of miR-21 activity has been shown to
enhance the resistance of U2OS cells to cisplatin, while the
ectopic expression of miR-21 in MG-63 cells reduces the resistance.
Elevated miR-21 levels suppress the expression of Sprouty2. On the
other hand, the ectopic expression of Sprouty2 has been shown to
rescue the miR-21-mediated suppression of resistance to cisplatin,
but not doxorubicin or methotrexate in osteosarcoma cells (87).
The process of metastasis is considered to be
initiated in the majority, if not all, by an
epithelial-to-mesenchymal transition (EMT) of the tumor cells. This
allows them to migrate to and enter the vascular or lymphatic
vessels, resulting in either local or distant metastasis (88,89).
miR-21 has been revealed to be an important miRNA associated with
cancer invasion and metastasis. The study by Yan et al
(90) demonstrated that miR-21
enhanced the invasion and migration of human breast cancer MCF7 and
MDA-MB-231 cells, whereas cancer cell invasion was inhibited by the
knockdown of miR-21. The mechanism of metastasis regulation by
miR-21 is highly complex. miR-21 targets PDCD4, tropomyosin and
PTEN that are known to modulate cancer cell invasion and metastasis
(91-94). Moreover, miR-21 can inhibit PTEN
expression and activate AKT signaling, inducing EMT, and promoting
the invasion and migration of cancer cells (95-97).
Furthermore, miR-21 can trigger the interleukin (IL)-6/signal
transducer and activator of transcription (STAT)3/nuclear factor
(NF)-κB-mediated signaling loop for the maintenance of EMT in
cancer cells (98).
Tumor angiogenesis refers to the ability of tumor
cells to recruit their own vasculature, which is critical for
cancer progression. Several studies have demonstrated that
exosome-encapsulated miRNAs secreted from tumor cells are able to
induce angiogenesis in different cancer types (99-102). Exosomal miR-21 released by
transformed lung cancer cells has been shown to induce vascular
endothelial growth factor (VEGF) production and angiogenesis in
nearby normal bronchial cells in a STAT3-dependent manner (99). In another study, the overexpression
of miR-21 in the human prostate cancer cell line, DU145, was shown
to increase the expression of Hypoxia-inducible factor (HIF)-1α and
VEGF, and induce tumor angiogenesis. The miR-21-mediated activation
of the AKT and extracellular signal-regulated kinase (ERK)1/2
signaling pathways enhances HIF-1α and VEGF expression (103).
CAFs become 'activated' during the neoplastic
process. CAFs are capable of accelerating the growth and promoting
the invasion of tumor cells (104-109). Cancer exosomes trigger the
transformation of fibroblasts through the transforming growth
factor (TGF) β/Smad pathway and elicit unique effects from soluble
TGFβ. The depletion of miR-21 blocks TGF-β1-induced CAF formation,
whereas the overexpression of miR-21 promotes CAF induction,
independent of TGF-β1. These findings clearly demonstrate that
miR-21 is a critical regulator of TGF-β1 signaling induction of CAF
formation (110). The conditioned
medium from human lung cancer A549 cells has been shown to increase
miR-21 expression and thus, through the TGF-β pathway, induce
migration, CAF-like morphology and CAF markers [periostin, α-smooth
muscle actin (α-SMA) and podoplanin] in human lung fibroblast MRC-5
and IMR-90 cells (111).
Exosomes from the multiple myeloma cell line, OPM2,
have been showon to contain high levels of miR-21. The Co-culture
of OPM2 exosomes with CAFs has also been shown to significantly
increase the proliferation of and induce the CAF transformation of
mesenchymal stem cells, demonstrated by the increased expression
levels of fibroblast-activated protein (FAP), α-SMA and
stromal-derived factor 1 (SDF-1), and the secretion of IL-6
(112). In another study, miR-21
was demonstrated to induce the metabolic alteration of CAFs and to
affect the development of the human pancreatic cancer cell line,
BxPC-3. Compared to normal fibroblasts, CAFs exhibited an increased
miR-21 expression, glucose uptake and lactic acid production.
Treatment with a miR-21 inhibitor reduced glycolysis in CAFs. The
co-culture of BxPC-3 cells with CAFs treated with the miR-21
inhibitor reduced oxidative phosphorylation and the invasion of
BxPC-3 cells (113).
Exosomes secreted by tumor cells are able to inhibit
the immune system by acting directly on effector cells or
indirectly through their regulation (114-120). Tumor cells secrete miR-21
together with miR-29a to communicate with macrophages, eliciting a
pro-inflammatory pro-metastatic response. miR-21 is a paracrine
agonist of Toll-like receptor-8 (TLR-8). The activation of TLR-8 in
immune cells triggers a pro-inflammatory response, that may lead to
tumor growth and metastasis (121). miR-21 induces tumor-associated
macrophage reprogramming through STAT3, and thereby facilitates
growth, intravasation and the spread of tumor cells (122,123).
Myeloid-derived suppressor cells (MDSCs) are the
major myeloid cells responsible for the immune evasion of cancer.
They are composed of a heterogeneous population of immature myeloid
cell progenitors, macrophage precursors, granulocytes and dendritic
cells. MDSCs can promote tumor growth by the promotion of
angiogenesis or the suppression of innate and adaptive immune
responses. miR-21 has been shown to be upregulated in bone
marrow-derived and splenic MDSCs, and increases MDSC survival and
proliferation by targeting PTEN and thus, STAT3 activation
(112,124).
Certain miRNAs are secreted selectively into
exosomes. For example, the let-7 miRNA family in certain metastatic
gastric cancer cell lines is secreted selectively to extracellular
environment via exosomes (130).
Breast cancer cell lines release the majority of miR-451 and
miR-1246 selectively via exosomes, whereas both miRNAs are retained
inside the non-malignant mammary epithelial cells and normal
fibroblasts (131). Additionally,
the TME may influence exosome release and uptake. The acidic
microenvironment of tumors increases the rate of exosomal release
and uptake by cancer cells (132). Furthermore, the aberrant exosome
biogenesis and secretion in cancer cells, as compared with normal
cells, may cause the differences in receptor recycling/degradation,
plasma membrane remodeling, and the ability of endosomes to
function as a signaling entity (133). In a number of types of cancer,
aberrant p53 activity may result in the overexpression of the tumor
suppressor-activated pathway 6 (TSAP6), through which it increases
exosome production (134,135). Furthermore, heparanase, an enzyme
upregulated in numerous cancer cell lines, has been shown to
regulate exosome secretion in cancer cells (136).
The level of exosomal miR-21 in serum is higher in
patients with osteosarcoma than in healthy individuals, which is
also reflected by the high levels of miR-21 found in tumor tissue
(61-64). The molecular mechanisms of exosome
sorting and release have been studied extensively recently.
However, the differences in miRNA content between cancer- and
healthy cell-derived exosomes, the differences in exosome
biogenesis between cancer and healthy cells, and the reasons why
cancer cells release more exosomes with distinct content, such as
miR-21, remain unclear. Additionally, whether the release of miR-21
in exosomes occurs through a distinct secretary pathway remains to
be elucidated. Further investigation into the distinct exosome
cargo loading mechanisms for miR-21 and other miRNAs in
osteosarcoma and other cancers may aid in the identification of
specific therapeutic targets related to exosome biogenesis. Further
research may facilitate the use of exosomes as delivery vehicles
for drugs, antigens, etc., for cancer treatment in the future.
Exosomal miR-21 is detected in human serum and
plasma. Differences in miR-21 expression have been found in serum
or plasma from patients with osteosarcoma and in healthy controls,
supporting the role of miR-21 as a biomarker for osteosar-coma
(61-64). The advantages of using exosomal
miR-21 as a non-invasive biomarker are that miRNAs in blood are
persistent and are highly stable against destruction by
ribo-nucleases (137,138). The current methods used for
exosome isolation include ultracentrifugation, size exclusion or
precipitating with reagents (139). Ultracentrifugation is the
commonly used method for exosome isolation. However, it is a
lengthy process, which requires a large volume of samples and
costly reagents and equipment. Thus, the method is impractical for
clinical diagnosis. The size exclusion method uses columns to
separate extracellular vesicles based on size and requires only a
small amount of samples. However, the exosomes collected have
heterogeneous sizes, curtailing the use for cancer diagnosis. The
precipitation of exosomes with reagents not only pulls down miRNAs,
but also protein aggregates that complicate the analysis for
disease-specific markers.
In order to implement miR-21 as a biomarker for
osteo-sarcoma, along with more effective methods for the isolation
of cancer exosomes and exosomal miR-21, additional clinical trials
are required to validate its use in the diagnosis of osteosarcoma
and other types of cancer. Most importantly, miR-21 needs to be
profiled together with other miRNAs or biomarkers specific to
osteosarcoma to improve the reliability and specificity of miR-21
as a biomarker.
Exosomal miR-21 is detected in human serum and
plasma. miR-21 expression in serum or plasma from patients with
osteosarcoma differs from that in healthy controls, supporting the
use of miR-21 as a biomarker for osteosarcoma. Exosomal miR-21
promotes cancer progression and development by mediating the
crosstalk among cells in the TME. Larger-scale studies are
warranted to further validate the sensitivity, specificity and
applicability of circulating miR-21 as a biomarker for osteosarcoma
in the future. Furthermore, extensive efforts need to be made to
identify the specific cargo-loading mechanisms for exosomal miR-21.
These studies will be critical for targeting miR-21 in cancer
therapy to improve early-stage exosome- and miRNA-based
therapeutics.
This study was supported by the National Natural
Science Foundation of China (grant nos. 81672176, 81871783 and
81702582).
Not applicable.
SW and TL wrote the manuscript. SW performed the
literature search for this review article. FM and YF revised and
corrected the manuscript. FM and YF assisted in the literature
search for this review article and also contributed to the
conception and design of the study. FM contributed to processing of
the figure. TL and SH conceived and designed the study. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors confirm they have competing
interests.
Not applicable.
|
1
|
Meyers PA and Gorlick R: Osteosarcoma.
Pediatr Clin North Am. 44:973–989. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Gill J, Ahluwalia MK, Geller D and Gorlick
R: New targets and approaches in osteosarcoma. Pharmacol Ther.
137:89–99. 2013. View Article : Google Scholar
|
|
4
|
Ostenfeld MS, Jeppesen DK, Laurberg JR,
Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P,
Dagnaes-Hansen F, Rasmussen MH, et al: Cellular disposal of miR23b
by RAB27-dependent exosome release is linked to acquisition of
metastatic properties. Cancer Res. 74:5758–5771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: Where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sadikovic B, Yoshimoto M, Chilton-MacNeill
S, Thorner P, Squire JA and Zielenska M: Identification of
interactive networks of gene expression associated with
osteosarcoma oncogenesis by integrated molecular profiling. Hum Mol
Genet. 18:1962–1975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Dou P, Liu T and He S: Application
of Long Noncoding RNAs in Osteosarcoma: Biomarkers and Therapeutic
Targets. Cell Physiol Biochem. 42:1407–1419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Liu T and Wang W: Prognostic
significance of matrix metalloproteinase 9 expression in
osteosarcoma: A meta-analysis of 16 studies. Medicine (Baltimore).
97:e130512018. View Article : Google Scholar
|
|
9
|
Liu T, Yan Z, Liu Y, Choy E, Hornicek FJ,
Mankin H and Duan Z: CRISPR-Cas9-Mediated Silencing of CD44 in
Human Highly Metastatic Osteosarcoma Cells. Cell Physiol Biochem.
46:1218–1230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng L, Liu T, Zhang B, Wu H, Zhao J and
Chen J: Forkhead box C1 is targeted by microRNA-133b and promotes
cell proliferation and migration in osteosarcoma. Exp Ther Med.
14:2823–2830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu T, Li Z, Zhang Q, De Amorim Bernstein
K, Lozano-Calderon S, Choy E, Hornicek FJ and Duan Z: Targeting
ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the
CRISPR-Cas9 system to reverse drug resistance. Oncotarget.
7:83502–83513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simons M and Raposo G: Exosomes -
vesicular carriers for inter-cellular communication. Curr Opin Cell
Biol. 21:575–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee TH, D'Asti E, Magnus N, Al-Nedawi K,
Meehan B and Rak J: Microvesicles as mediators of intercellular
communication in cancer--the emerging science of cellular 'debris'.
Semin Immunopathol. 33:455–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kahlert C, Melo SA, Protopopov A, Tang J,
Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, et al:
Identification of double-stranded genomic DNA spanning all
chromosomes with mutated KRAS and p53 DNA in the serum exosomes of
patients with pancreatic cancer. J Biol Chem. 289:3869–3875. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Villarroya-Beltri C, Baixauli F,
Gutiérrez-Vázquez C, Sánchez-Madrid F and Mittelbrunn M: Sorting it
out: Regulation of exosome loading. Semin Cancer Biol. 28:3–13.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mathivanan S, Fahner CJ, Reid GE and
Simpson RJ: ExoCarta 2012: Database of exosomal proteins, RNA and
lipids. Nucleic Acids Res. 40(D1): D1241–D1244. 2012. View Article : Google Scholar :
|
|
18
|
Mathivanan S and Simpson RJ: ExoCarta: A
compendium of exosomal proteins and RNA. Proteomics. 9:4997–5000.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan BT, Teng K, Wu C, Adam M and Johnstone
RM: Electron microscopic evidence for externalization of the
transferrin receptor in vesicular form in sheep reticulocytes. J
Cell Biol. 101:942–948. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harding C, Heuser J and Stahl P:
Receptor-mediated endocytosis of transferrin and recycling of the
transferrin receptor in rat reticulocytes. J Cell Biol. 97:329–339.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keller S, Rupp C, Stoeck A, Runz S, Fogel
M, Lugert S, Hager HD, Abdel-Bakky MS, Gutwein P and Altevogt P:
CD24 is a marker of exosomes secreted into urine and amniotic
fluid. Kidney Int. 72:1095–1102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallo A, Tandon M, Alevizos I and Illei
GG: The majority of microRNAs detectable in serum and saliva is
concentrated in exosomes. PLoS One. 7:e306792012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bartel DP and Chen CZ: Micromanagers of
gene expression: The potentially widespread influence of metazoan
microRNAs. Nat Rev Genet. 5:396–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen LT, Xu SD, Xu H, Zhang JF, Ning JF
and Wang SF: MicroRNA-378 is associated with non-small cell lung
cancer brain metastasis by promoting cell migration, invasion and
tumor angiogenesis. Med Oncol. 29:1673–1680. 2012. View Article : Google Scholar
|
|
27
|
Ivanovska I, Ball AS, Diaz RL, Magnus JF,
Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson
AL, et al: MicroRNAs in the miR-106b family regulate p21/CDKN1A and
promote cell cycle progression. Mol Cell Biol. 28:2167–2174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu Y, Thomson JM, Wong HY, Hammond SM and
Hogan BL: Transgenic over-expression of the microRNA miR-17-92
cluster promotes proliferation and inhibits differentiation of lung
epithelial progenitor cells. Dev Biol. 310:442–453. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang
KM and De W: Prognostic significance of serum miRNA-21 expression
in human non-small cell lung cancer. J Surg Oncol. 104:847–851.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Ling C, Bai Y and Zhao J:
MicroRNA-206 is associated with invasion and metastasis of lung
cancer. Anat Rec (Hoboken). 294:88–92. 2011. View Article : Google Scholar
|
|
32
|
Ferracin M, Veronese A and Negrini M:
Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev
Mol Diagn. 10:297–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nana-Sinkam P and Croce CM: MicroRNAs in
diagnosis and prognosis in cancer: What does the future hold?
Pharmacogenomics. 11:667–669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fan H, Lu S, Wang S and Zhang S:
Identification of critical genes associated with human osteosarcoma
metastasis based on integrated gene expression profiling. Mol Med
Rep. 20:915–930. 2019.PubMed/NCBI
|
|
35
|
Gulino R, Forte S, Parenti R, Memeo L and
Gulisano M: MicroRNA and pediatric tumors: Future perspectives.
Acta Histochem. 117:339–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Gao X, Wei F, Zhang X, Yu J, Zhao
H, Sun Q, Yan F, Yan C, Li H, et al: Diagnostic and prognostic
value of circulating miR-21 for cancer: A systematic review and
meta-analysis. Gene. 533:389–397. 2014. View Article : Google Scholar
|
|
38
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kumarswamy R, Volkmann I and Thum T:
Regulation and function of miRNA-21 in health and disease. RNA
Biol. 8:706–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu CH, Sui BD, Du FY, Shuai Y, Zheng CX,
Zhao P, Yu XR and Jin Y: miR-21 deficiency inhibits osteoclast
function and prevents bone loss in mice. Sci Rep. 7:431912017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li X, Guo L, Liu Y, Su Y, Xie Y, Du J,
Zhou J, Ding G, Wang H, Bai Y, et al: MicroRNA-21 promotes
osteogenesis of bone marrow mesenchymal stem cells via the
Smad7-Smad1/5/8-Runx2 pathway. Biochem Biophys Res Commun.
493:928–933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Krichevsky AM and Gabriely G: miR-21: A
small multi-faceted RNA. J Cell Mol Med. 13:39–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan X, Wang ZX and Wang R: MicroRNA-21: A
novel therapeutic target in human cancer. Cancer Biol Ther.
10:1224–1232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar
|
|
47
|
Frankel LB, Christoffersen NR, Jacobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar
|
|
48
|
Li T, Li D, Sha J, Sun P and Huang Y:
MicroRNA-21 directly targets MARCKS and promotes apoptosis
resistance and invasion in prostate cancer cells. Biochem Biophys
Res Commun. 383:280–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu Z, Liu M, Stribinskis V, Klinge CM,
Ramos KS, Colburn NH and Li Y: MicroRNA-21 promotes cell
transformation by targeting the programmed cell death 4 gene.
Oncogene. 27:4373–4379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol
Chem. 282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zheng J, Xue H, Wang T, Jiang Y, Liu B, Li
J, Liu Y, Wang W, Zhang B and Sun M: miR-21 downregulates the tumor
suppressor P12 CDK2AP1 and stimulates cell proliferation and
invasion. J Cell Biochem. 112:872–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu J, Wu C, Che X, Wang L, Yu D, Zhang T,
Huang L, Li H, Tan W, Wang C, et al: Circulating microRNAs, miR-21,
miR-122, and miR-223, in patients with hepatocellular carcinoma or
chronic hepatitis. Mol Carcinog. 50:136–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schramedei K, Mörbt N, Pfeifer G, Läuter
J, Rosolowski M, Tomm JM, von Bergen M, Horn F and Brocke-Heidrich
K: MicroRNA-21 targets tumor suppressor genes ANP32A and SMARCA4.
Oncogene. 30:2975–2985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gabriely G, Wurdinger T, Kesari S, Esau
CC, Burchard J, Linsley PS and Krichevsky AM: MicroRNA 21 promotes
glioma invasion by targeting matrix metalloproteinase regulators.
Mol Cell Biol. 28:5369–5380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar
|
|
58
|
Sekar D, Mani P, Biruntha M,
Sivagurunathan P and Karthigeyan M: Dissecting the functional role
of microRNA 21 in osteosarcoma. Cancer Gene Ther. 26:179–182. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hua Y, Jin Z, Zhou F, Zhang YQ and Zhuang
Y: The expression significance of serum MiR-21 in patients with
osteosarcoma and its relationship with chemosensitivity. Eur Rev
Med Pharmacol Sci. 21:2989–2994. 2017.PubMed/NCBI
|
|
60
|
Xu B, Xia H, Cao J, Wang Z, Yang Y and Lin
Y: MicroRNA-21 Inhibits the Apoptosis of Osteosarcoma Cell Line
SAOS-2 via Targeting Caspase 8. Oncol Res. 25:1161–1168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ren X, Shen Y, Zheng S, Liu J and Jiang X:
miR-21 predicts poor prognosis in patients with osteosarcoma. Br J
Biomed Sci. 73:158–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao H, Yan P, Wang J, Zhang Y, Zhang M,
Wang Z, Fu Q and Liang W: Clinical significance of tumor miR-21,
miR-221, miR-143, and miR-106a as biomarkers in patients with
osteo-sarcoma. Int J Biol Markers. 34:184–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yuan J, Chen L, Chen X, Sun W and Zhou X:
Identification of serum microRNA-21 as a biomarker for
chemosensitivity and prognosis in human osteosarcoma. J Int Med
Res. 40:2090–2097. 2012. View Article : Google Scholar
|
|
64
|
Ouyang L, Liu P, Yang S, Ye S, Xu W and
Liu X: A three-plasma miRNA signature serves as novel biomarkers
for osteosarcoma. Med Oncol. 30:3402013. View Article : Google Scholar
|
|
65
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cretu A, Brooks PC. Impact of the
non-cellular tumor micro-environment on metastasis: Potential
therapeutic and imaging opportunities. J Cell Physiol. 213:391–402.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hartmann S, Bhola NE and Grandis JR:
HGF/Met Signaling in Head and Neck Cancer: Impact on the Tumor
Microenvironment. Clin Cancer Res. 22:4005–4013. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gu F, Hu C, Tai Z, Yao C, Tian J, Zhang L,
Xia Q, Gong C, Gao Y and Gao S: Tumour microenvironment-responsive
lipoic acid nanoparticles for targeted delivery of docetaxel to
lung cancer. Sci Rep. 6:362812016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Friedl P and Alexander S: Cancer invasion
and the microenvi-ronment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu T, Hong Y, Jia L, Wu J, Xia J, Wang J,
Hu Q and Cheng B: Modulation of IL-1β reprogrammes the tumor
microenvironment to interrupt oral carcinogenesis. Sci Rep.
6:202082016. View Article : Google Scholar
|
|
71
|
Maia J, Caja S, Strano Moraes MC, Couto N
and Costa-Silva B: Exosome-Based Cell-Cell Communication in the
Tumor Microenvironment. Front Cell Dev Biol. 6:182018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hu C, Chen M, Jiang R, Guo Y, Wu M and
Zhang X: Exosome-related tumor microenvironment. J Cancer.
9:3084–3092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang Z, Chen JQ, Liu JL and Tian L:
Exosomes in tumor microenvironment: Novel transporters and
biomarkers. J Transl Med. 14:2972016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvi-ronment. J Control Release. 219:278–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Raimondi L, De Luca A, Gallo A, Costa V,
Russelli G, Cuscino N, Manno M, Raccosta S, Carina V, Bellavia D,
et al: Osteosarcoma cell-derived exosomes affect tumor
microenvironment by specific packaging of microRNAs.
Carcinogenesis. Jul 10–2019.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jerez S, Araya H, Hevia D, Irarrázaval CE,
Thaler R, van Wijnen AJ and Galindo M: Extracellular vesicles from
osteo-sarcoma cell lines contain miRNAs associated with cell
adhesion and apoptosis. Gene. 710:246–257. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shen RK, Zhu X, Yi H, Wu CY, Chen F, Dai
LQ and Lin JH: Proteomic identification of osteosarcoma-derived
exosomes and their activation o f pentose phosphate pathway. Int J
Clin Exp Pathol. 9:4140–4148. 2016.
|
|
78
|
Shimbo K, Miyaki S, Ishitobi H, Kato Y,
Kubo T, Shimose S and Ochi M: Exosome-formed synthetic microRNA-143
is transferred to osteosarcoma cells and inhibits their migration.
Biochem Biophys Res Commun. 445:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lv C, Hao Y and Tu G: MicroRNA-21 promotes
proliferation, invasion and suppresses apoptosis in human
osteosarcoma line MG63 through PTEN/Akt pathway. Tumour Biol.
37:9333–9342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Graziano AC, Cardile V, Avola R, Vicario
N, Parenti C, Salvatorelli L, Magro G and Parenti R: Wilms' tumor
gene 1 silencing inhibits proliferation of human osteosarcoma MG-63
cell line by cell cycle arrest and apoptosis activation.
Oncotarget. 8:13917–13931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang L, Tang B, Han H, Mao D, Chen J, Zeng
Y and Xiong M: miR-155 Affects Osteosarcoma MG-63 Cell Autophagy
Induced by Adriamycin Through Regulating PTEN-PI3K/AKT/mTOR
Signaling Pathway. Cancer Biother Radiopharm. 33:32–38. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wu YR, Qi HJ, Deng DF, Luo YY and Yang SL:
MicroRNA-21 promotes cell proliferation, migration, and resistance
to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal
cancer. Tumour Biol. 37:12061–12070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xue R, Lei S, Xia ZY, Wu Y, Meng Q, Zhan
L, Su W, Liu H, Xu J, Liu Z, et al: Selective inhibition of PTEN
preserves ischaemic post-conditioning cardioprotection in
STZ-induced Type 1 diabetic rats: Role of the PI3K/Akt and
JAK2/STAT3 pathways. Clin Sci (Lond). 130:377–392. 2016. View Article : Google Scholar
|
|
85
|
Li C, Xu B, Miu X, Deng Z, Liao H and Hao
L: Inhibition of miRNA-21 attenuates the proliferation and
metastasis of human osteosarcoma by upregulating PTEN. Exp Ther
Med. 15:1036–1040. 2018.PubMed/NCBI
|
|
86
|
Hu X, Li L, Lu Y, Yu X, Chen H, Yin Q and
Zhang Y: miRNA-21 inhibition inhibits osteosarcoma cell
proliferation by targeting PTEN and regulating the TGF-β1 signaling
pathway. Oncol Lett. 16:4337–4342. 2018.PubMed/NCBI
|
|
87
|
Vanas V, Haigl B, Stockhammer V and
Sutterlüty-Fall H: MicroRNA-21 Increases Proliferation and
Cisplatin Sensitivity of Osteosarcoma-Derived Cells. PLoS One.
11:e01610232016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Karlsson MC, Gonzalez SF, Welin J and Fuxe
J: Epithelial-mesenchymal transition in cancer metastasis through
the lymphatic system. Mol Oncol. 11:781–791. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ,
Hou JH, Fu J, Zeng MS, Yun JP, Wu QL, et al: Knockdown of miR-21 in
human breast cancer cell lines inhibits proliferation, in vitro
migration and in vivo tumor growth. Breast Cancer Res. 13:R22011.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhao MY, Wang LM, Liu J, Huang X, Liu J
and Zhang YF: MiR-21 Suppresses Anoikis through Targeting PDCD4 and
PTEN in Human Esophageal Adenocarcinoma. Curr Med Sci. 38:245–251.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liao J, Liu R, Shi YJ, Yin LH and Pu YP:
Exosome-shuttling microRNA-21 promotes cell migration and
invasion-targeting PDCD4 in esophageal cancer. Int J Oncol.
48:2567–2579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Melnik BC: MiR-21: An environmental driver
of malignant melanoma? J Transl Med. 13:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jiang LH, Ge MH, Hou XX, Cao J, Hu SS, Lu
XX, Han J, Wu YC, Liu X, Zhu X, et al: miR-21 regulates tumor
progression through the miR-21-PDCD4-Stat3 pathway in human
salivary adenoid cystic carcinoma. Lab Invest. 95:1398–1408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bera A, Das F, Ghosh-Choudhury N, Kasinath
BS, Abboud HE and Choudhury GG: microRNA-21-induced dissociation of
PDCD4 from rictor contributes to Akt-IKKβ-mTORC1 axis to regulate
renal cancer cell invasion. Exp Cell Res. 328:99–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhao M, Ang L, Huang J and Wang J:
MicroRNAs regulate the epithelial-mesenchymal transition and
influence breast cancer invasion and metastasis. Tumour Biol.
39:10104283176916822017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Han M, Wang Y, Liu M, Bi X, Bao J, Zeng N,
Zhu Z, Mo Z, Wu C and Chen X: MiR-21 regulates
epithelial-mesenchymal transition phenotype and hypoxia-inducible
factor-1α expression in third-sphere forming breast cancer stem
cell-like cells. Cancer Sci. 103:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
De Mattos-Arruda L, Bottai G, Nuciforo PG,
Di Tommaso L, Giovannetti E, Peg V, Losurdo A, Pérez-Garcia J,
Masci G, Corsi F, et al: MicroRNA-21 links
epithelial-to-mesenchymal transition and inflammatory signals to
confer resistance to neoadjuvant trastuzumab and chemotherapy in
HER2-positive breast cancer patients. Oncotarget. 6:37269–37280.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu Y, Luo F, Wang B, Li H, Xu Y, Liu X,
Shi L, Lu X, Xu W, Lu L, et al: STAT3-regulated exosomal miR-21
promotes angio-genesis and is involved in neoplastic processes of
transformed human bronchial epithelial cells. Cancer Lett.
370:125–135. 2016. View Article : Google Scholar
|
|
100
|
Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin
L, Liu X and Wang N: Tumor-derived microRNA-494 promotes
angiogenesis in non-small cell lung cancer. Angiogenesis.
18:373–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Umezu T, Tadokoro H, Azuma K, Yoshizawa S,
Ohyashiki K and Ohyashiki JH: Exosomal miR-135b shed from hypoxic
multiple myeloma cells enhances angiogenesis by targeting
factor-inhibiting HIF-1. Blood. 124:3748–3757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y,
Zheng L and Zhuang SM: Hepatocellular Carcinoma Cell-Secreted
Exosomal MicroRNA-210 Promotes Angiogenesis In Vitro and In Vivo.
Mol Ther Nucleic Acids. 11:243–252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R,
Jiang Y, Kung HF, Lai L and Jiang BH: MiR-21 induced angiogenesis
through AKT and ERK activation and HIF-1α expression. PLoS One.
6:e191392011. View Article : Google Scholar
|
|
104
|
Brentnall TA: Arousal of cancer-associated
stromal fibroblasts: Palladin-activated fibroblasts promote tumor
invasion. Cell Adhes Migr. 6:488–494. 2012. View Article : Google Scholar
|
|
105
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Camps JL, Chang SM, Hsu TC, Freeman MR,
Hong SJ, Zhau HE, von Eschenbach AC and Chung LW:
Fibroblast-mediated acceleration of human epithelial tumor growth
in vivo. Proc Natl Acad Sci USA. 87:75–79. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gleave M, Hsieh JT, Gao CA, von Eschenbach
AC and Chung LW: Acceleration of human prostate cancer growth in
vivo by factors produced by prostate and bone fibroblasts. Cancer
Res. 51:3753–3761. 1991.PubMed/NCBI
|
|
108
|
Kanekura T, Chen X and Kanzaki T: Basigin
(CD147) is expressed on melanoma cells and induces tumor cell
invasion by stimulating production of matrix metalloproteinases by
fibroblasts. Int J Cancer. 99:520–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sameshima T, Nabeshima K, Toole BP,
Yokogami K, Okada Y, Goya T and Wakisaka S: Glioma cell
extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147)
stimulates production of membrane-type matrix metalloproteinases
and activated gelatinase A in co-cultures with brain-derived
fibroblasts. Cancer Lett. 157:177–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li Q, Zhang D, Wang Y, Sun P, Hou X,
Larner J, Xiong W and Mi J: MiR-21/Smad 7 signaling determines
TGF-β1-induced CAF formation. Sci Rep. 3:20382013. View Article : Google Scholar
|
|
111
|
Kunita A, Morita S, Irisa TU, Goto A, Niki
T, Takai D, Nakajima J and Fukayama M: MicroRNA-21 in
cancer-associated fibroblasts supports lung adenocarcinoma
progression. Sci Rep. 8:88382018. View Article : Google Scholar
|
|
112
|
Cheng Q, Li X and Liu J, Ye Q, Chen Y, Tan
S and Liu J: Multiple Myeloma-Derived Exosomes Regulate the
Functions of Mesenchymal Stem Cells Partially via Modulating miR-21
and miR-146a. Stem Cells Int. 2017:90121522017. View Article : Google Scholar
|
|
113
|
Mace TA, Collins AL, Wojcik SE, Croce CM,
Lesinski GB and Bloomston M: Hypoxia induces the overexpression of
microRNA-21 in pancreatic cancer cells. J Surg Res. 184:855–860.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Muller L, Mitsuhashi M, Simms P, Gooding
WE and Whiteside TL: Tumor-derived exosomes regulate expression of
immune function-related genes in human T cell subsets. Sci Rep.
6:202542016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Greening DW, Gopal SK, Xu R, Simpson RJ
and Chen W: Exosomes and their roles in immune regulation and
cancer. Semin Cell Dev Biol. 40:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kurywchak P, Tavormina J and Kalluri R:
The emerging roles of exosomes in the modulation of immune
responses in cancer. Genome Med. 10:232018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Robbins PD and Morelli AE: Regulation of
immune responses by extracellular vesicles. Nat Rev Immunol.
14:195–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang L, He L, Zhang R, Liu X, Ren Y, Liu
Z, Zhang X, Cheng W and Hua ZC: Regulation of T lymphocyte
activation by microRNA-21. Mol Immunol. 59:163–171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Carissimi C, Carucci N, Colombo T,
Piconese S, Azzalin G, Cipolletta E, Citarella F, Barnaba V, Macino
G and Fulci V: miR-21 is a negative modulator of T-cell activation.
Biochimie. 107(Pt B): 319–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sheedy FJ: Turning 21: Induction of miR-21
as a Key Switch in the Inflammatory Response. Front Immunol.
6:192015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Fabbri M, Paone A, Calore F, Galli R,
Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, et al:
MicroRNAs bind to Toll-like receptors to induce prometastatic
inflammatory response. Proc Natl Acad Sci USA. 109:E2110–E2116.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang Z, Brandt S, Medeiros A, Wang S, Wu
H, Dent A and Serezani CH: MicroRNA 21 is a homeostatic regulator
of macrophage polarization and prevents prostaglandin E2-mediated
M2 generation. PLoS One. 10:e01158552015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Curtale G: MiRNAs at the Crossroads
between Innate Immunity and Cancer: Focus on Macrophages. Cells.
7:E122018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li L, Zhang J, Diao W, Wang D, Wei Y,
Zhang CY and Zen K: MicroRNA-155 and MicroRNA-21 promote the
expansion of functional myeloid-derived suppressor cells. J
Immunol. 192:1034–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Klebanoff CA, Gattinoni L and Restifo NP:
CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol
Rev. 211:214–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Cereghetti DM and Lee PP: Tumor-Derived
Exosomes Contain microRNAs with Immunological Function:
Implications for a Novel Immunosuppression Mechanism. MicroRNA.
2:194–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Miao BP, Zhang RS, Li M, Fu YT, Zhao M,
Liu ZG and Yang PC: Nasopharyngeal cancer-derived microRNA-21
promotes immune suppressive B cells. Cell Mol Immunol. 12:750–756.
2015. View Article : Google Scholar
|
|
128
|
Kosaka N, Iguchi H, Yoshioka Y, Takeshita
F, Matsuki Y and Ochiya T: Secretory mechanisms and intercellular
transfer of microRNAs in living cells. J Biol Chem.
285:17442–17452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Melo SA, Sugimoto H, O'Connell JT, Kato N,
Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et
al: Cancer exosomes perform cell-independent microRNA biogenesis
and promote tumorigenesis. Cancer Cell. 26:707–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ohshima K, Inoue K, Fujiwara A, Hatakeyama
K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi
K, et al: Let-7 microRNA family is selectively secreted into the
extracellular environment via exosomes in a metastatic gastric
cancer cell line. PLoS One. 5:e132472010. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Pigati L, Yaddanapudi SC, Iyengar R, Kim
DJ, Hearn SA, Danforth D, Hastings ML and Duelli DM: Selective
release of microRNA species from normal and malignant mammary
epithelial cells. PLoS One. 5:e135152010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Boussadia Z, Lamberti J, Mattei F, Pizzi
E, Puglisi R, Zanetti C, Pasquini L, Fratini F, Fantozzi L,
Felicetti F, et al: Acidic micro-environment plays a key role in
human melanoma progression through a sustained exosome mediated
transfer of clinically relevant metastatic molecules. J Exp Clin
Cancer Res. 37:2452018. View Article : Google Scholar
|
|
133
|
Hessvik NP and Llorente A: Current
knowledge on exosome biogenesis and release. Cell Mol Life Sci.
75:193–208. 2018. View Article : Google Scholar :
|
|
134
|
Lespagnol A, Duflaut D, Beekman C, Blanc
L, Fiucci G, Marine JC, Vidal M, Amson R and Telerman A: Exosome
secretion, including the DNA damage-induced p53-dependent secretory
pathway, is severely compromised in TSAP6/Steap3-null mice. Cell
Death Differ. 15:1723–1733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yu X, Harris SL and Levine AJ: The
regulation of exosome secretion: A novel function of the p53
protein. Cancer Res. 66:4795–4801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Thompson CA, Purushothaman A, Ramani VC,
Vlodavsky I and Sanderson RD: Heparanase regulates secretion,
composition, and function of tumor cell-derived exosomes. J Biol
Chem. 288:10093–10099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang K, Zhang S, Marzolf B, Troisch P,
Brightman A, Hu Z, Hood LE and Galas DJ: Circulating microRNAs,
potential biomarkers for drug-induced liver injury. Proc Natl Acad
Sci USA. 106:4402–4407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Greening DW, Xu R, Ji H, Tauro BJ and
Simpson RJ: A protocol for exosome isolation and characterization:
Evaluation of ultra-centrifugation, density-gradient separation,
and immunoaffinity capture methods. Methods Mol Biol. 1295:179–209.
2015. View Article : Google Scholar
|