Introduction
Oral squamous cell carcinoma (OSCC) is a common form
of cancer, with an estimated 447,751 new cases and 228,389 deaths
reported worldwide in 2018, which represents ~2.5 and ~2.4% of all
cancer incidence and mortality, respectively (1). In the USA, 53,000 new cases and
10,860 deaths from OSCC are estimated for 2019 (2). OSCC also exhibits high frequency in
various other countries, including India, Sri Lanka, Pakistan,
Afghanistan and Papua New Guinea; indeed, OSCC is the leading cause
of cancer-related death among males in India and Sri Lanka
(1). The incidence of OSCC, and
lip and pharyngeal cancers is expected to increase in the future,
reaching 855,900 new cases per year in 2035 due to changes in
demographics (3). The overall
5-year survival rates of OSCC have not changed in the past 30
years, and remain <50% (4).
Therefore, early detection and treatment, and the clarification of
the molecular details of OSCC remain essential to address this
increasing health problem.
Considerable attention has been paid to the role of
mast cells (MCs) in the tumor microenvironment (TME) (5). Human MCs are a rich source of the
serine proteases tryptase and chymase; MCs that only contain
tryptase are classified as MCT, whereas those that
contain both tryptase and chymase are classified as MCTC
(5,6). Chymase acts indirectly to evoke
angiogenesis through the activation of vascular endothelial growth
factor (VEGF)-A, via the conversion of angiotensin I (Ang I) to Ang
II and cleavage of matrix metalloproteinase-9 (MMP-9) (5). An increase in MCT count is
closely associated with angiogenesis, cellular proliferation and
poor prognosis in various types of cancer (5,6).
MCT also promote lymphangio-genesis by releasing VEGF-C
and VEGF-D (7,8), as well as overall immunosuppression
(5,9). However, previous studies focusing on
the relationship between MCTC and tumors have been
inconclusive. The accumulation of MCTC appears to be
significantly associated with angiogenesis, nodal metastasis and/or
poor prognosis in lung cancer (10-13),
and T grade, clinical stage, angiogenesis and poor prognosis in
gastric cancer (14). In contrast,
low MCTC density is closely related to poor prognosis in
melanoma (15) and colon cancer
(16), and a more favorable
immunophenotype in breast carcinoma (17). A strong negative correlation has
also observed between melanoma proliferation and MC infiltration
(6,18). Therefore, the detailed roles of
MCTC and chymase in various malignancies remain
controversial.
Members of the melanoma inhibitory activity
(MIA) gene family include MIA, MIA2,
transport and Golgi organization protein 1 (TANGO or
MIA3), and otoraplin. These secreted proteins share 34-45%
amino acid and 47-59% cDNA sequence homology, and also possess a
highly conserved Src homology 3-like domain (4,19).
Our previous studies reported that MIA gene family members
act as oncogenes in OSCC by acting on the TME (4,20-24).
One of the major functions of MIA and MIA2 in OSCC is the induction
of angiogenesis and lymphangiogenesis through the activation of
VEGF-A, VEGF-C and VEGF-D, as well as the general suppression of
tumor immunity (4,20-22,24).
TANGO is also associated with microvessel density (MVD) and lymph
vessel density (LVD) through the activation of platelet-derived
growth factor β polypeptide (PDGFB) (4,23).
MCTC in the TME may enhance the vasculogenic and
immunosuppressive activity of the MIA gene family in OSCC.
Thus, in the present study, the roles of MCTC and
MIA gene family activation in OSCC were investigated.
Materials and methods
Tumor samples
Tissues were fixed over 96 h with formalin at 4˚C.
Formalin-fixed, paraffin-embedded (FFPE) specimens of 93 primary
OSCC (43 males and 50 females; age range, 45-89 years; mean,
66.8±10.7 years) without preoperative therapy were used in the
present study. All specimens were selected at random from the Nara
Medical University Hospital. Written informed consent was obtained
from all individuals for the use of their tissue specimens. Tumor
staging and the histological grade of OSCC were based on the Union
for International Cancer Control (UICC) TNM classification system
(8th edition) (25) and the World
Health Organization criteria (26), respectively. Medical records and
prognostic follow-up data were obtained from the patient database
managed by the hospital. The present study was conducted following
a protocol approved by the Medical Ethical Committee of the Nara
Medical University (approval no. 719). The study protocol using
human samples was performed according to the ethical standards
stated in the Declaration of Helsinki.
Immunohistochemistry
Consecutive 3-µm sections were cut from each block,
and immunohistochemistry was performed. An immunoperoxidase
technique was performed following antigen retrieval with microwave
treatment (95˚C) in citrate buffer (pH 6.0) for 45 min. Sections
were pretreated with 3% H2O2-methanol to
block endogenous peroxidase activity at room temperature, and
specimens were incubated in 10% skim milk solution (Morinaga Milk
Industry Co., Ltd.) for 20 min at room temperature to avoid
false-positive antibody reactions. Antibodies (all diluted to 0.5
µg/ml) specific for MC chymase (cat. no. ab2377; Abcam), CD34 (cat.
no. M7165; Dako; Agilent Technologies, Inc.), used as a marker of
endothelial cells, and the lymphatic vessel endothelial hyaluronan
receptor 1 (LYVE1) antibody (cat. no. ab10278; Abcam), a marker for
lymphatic endothelial cells, were used. After incubation at room
temperature for 2 h, sections were incubated with polyclonal
anti-goat/mouse/rabbit Multi-Link secondary antibody (1:200; cat.
no. E0453; Dako; Agilent Technologies, Inc.) for 30 min at room
temperature. Specimens were visualized by exposure to
diaminobenzidine solution (Dako; Agilent Technologies, Inc.), and
counter-stained with Meyer's hematoxylin (Sigma-Aldrich; Merck
KGaA) at room temperature for 10 min.
Evaluation of immunohistochemistry
Verification of histological diagnoses and grading
of immunohistochemistry were performed by two pathologists. To
quantify the MCTC density, MVD and LVD, five strongly
immunoreactive areas surrounding tumor cells were selected and
examined using a light microscope (magnification, x200; BX53;
Olympus Corporation), and densities were averaged. To determine the
association between MCTC density and disease-free
survival, the specimens were divided into two groups according to
the MCTC density based on the overall mean value
(23).
Laser capture microdissection (LCM)
Laser capture micro-dissection was performed to
specifically select OSCC cells for the preparation of small RNAs.
Tissue sections (7 µm) were prepared from each paraffin block, and
stained using hematoxylin and eosin at room temperature for 10 min.
A PixCell II LCM microscope (Arcturus) was used to capture and
transfer cells for microdissection according to the manufacturer's
instructions; ~5,000 tumor cells were acquired from each tissue
sample.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of tissues and cultured cells was
extracted using an RNeasy FFPE Kit (Qiagen, Inc.), and 1 ng of
total RNA was converted to cDNA using a ReverTra Ace qRT kit
(Toyobo Life Science) at 37˚C for 15 min and 95˚C for 5 min. qPCR
was performed on a StepOnePlus Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with TaqMan Fast
Universal PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reactions were pre-incubated at 95˚C for 20
sec, followed by 40 cycles of denaturation at 95˚C for 1 sec and
annealing/extension at 60˚C for 20 sec. Results were analyzed using
the 2-∆∆Cq method (27). GAPDH mRNA was used as the
internal control. The TaqMan Gene Expression Assays for MIA
(cat. no. Hs00197954_m1), MIA2 (cat. no. Hs00365015_m1),
TANGO/MIA3 (cat. no. Hs00412706_m1), and GAPDH
(cat. no. Hs03929097_g1) were purchased from Applied Biosystems
(Thermo Fisher Scientific, Inc.). All PCR reactions were performed
in triplicate.
Cell culture and reagents
Our previous studies reported that OSCC-derived HSC3
cells overexpress MIA (21,22),
MIA2 (20) and TANGO
(23). Therefore, HSC3 cells were
used in the present study and maintained in Dulbecco's modified
Eagle's medium (Wako Pure Chemical Industries, Ltd.) supplemented
with 10% fetal bovine serum (Nichirei Biosciences, Inc.) and 10,000
U/ml penicillin/10,000 µg/ml streptomycin (Wako Pure Chemical
Industries, Ltd.) under 5% CO2 and 95% air at 37˚C.
Cells were treated with various concentrations (0, 5 or 10 nM) of
recombinant human chymase (R&D Systems, Inc.) for 48 h at
37˚C.
Primary human umbilical vein endothelial cells
(HUVECs) and primary human dermal lymphatic microvascular
endothelial cells (HDLMVECs) were purchased from Cell Applications,
Inc. HUVECs were cultured in endothelial cell media (Cell
Applications, Inc.), and HDLMVECs were cultured in microvascular
endothelial cell media (Cell Applications, Inc.), both with 5%
CO2 at 37˚C.
Small interfering RNA (siRNA)
Stealth Select RNAi siRNAs for MIA (cat. no.
HSS144615), MIA2 (cat. no. HSS133246) and TANGO (cat.
no. HSS180081) were purchased from Thermo Fisher Scientific, Inc.
AllStars Negative Control siRNA was used as a control (cat. no.
SI03650318; Qiagen, Inc.). Cells were seeded (6,000 cells/well) in
24-well culture plates and cultured for 24 h. Cells were
transfected with 10 nM siRNA using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. Subsequent experiments were performed
after 48 h of transfection.
Immunoblotting
Whole cell lysates were obtained using M-PER™
Mammalian Protein Extraction Reagent (Thermo Fisher Scientific,
Inc.). Protein concentrations of the lysates were determined by
using a DC™ protein assay (Bio-Rad Laboratories, Inc.). Lysates (50
µg/lane) were subjected to 12.5% SDS-PAGE and immunoblotting by
electrotransfer to PVDF membranes (Thermo Fisher Scientific, Inc.).
Bullet Blocking One for Western Blotting (Nacalai Tesque, Inc.) was
used as a blocking reagent (ready to use) at room temperature for
10 min. The membranes were incubated with anti-MIA (1:10,000; cat.
no. sc-377375; Santa Cruz Biotechnology, Inc.), anti-MIA2
(1:10,000; cat. no. ab58973; Abcam) and anti-TANGO/MIA3 antibodies
(1:15,000; cat. no. LS-B4210; LifeSpan BioSciences, Inc.) for 12 h
at room temperature. Binding of the primary antibodies was detected
with peroxidase-conjugated anti-mouse (cat. no. sc-516102; Santa
Cruz Biotechnology, Inc.) or anti-rabbit antibody (1:5,000; cat.
no. sc-2357; Santa Cruz Biotechnology, Inc.) for 2 h at room
temerature. The immune complex was visualized with an ECL Western
Blotting Detection System (Amersham; GE Healthcare Life Sciences).
Anti-GAPDH antibody (1:10,000; cat. no. sc-20357; Santa Cruz
Biotechnology, Inc.) was used as an internal control.
ELISA for MIA family
Cell culture medium was collected and centrifuged at
270 x g at 4˚C for 10 min. Proteins in the supernatant were then
extracted using M-PER Mammalian Protein Extraction Reagent. ELISA
kits were used to analyze MIA (cat. no. 11976826001; Roche
Diagnostics), MIA2 (cat. no. LS-F16959; LifeSpan BioSciences,
Inc.), TANGO/MIA3 (cat. no. LS-F52248; LifeSpan BioSciences, Inc.),
VEGF-A (cat. no. RAB0508; Calbiochem; Merck KGaA), VEGF-C (cat. no.
DVEC00; R&D Systems, Inc.), VEGF-D (cat. no. DVED00; R&D
Systems, Inc.) and PDGFB (cat. no. DBB00; R&D Systems, Inc.).
The assays were performed according to the manufacturers'
instructions and in triplicate. The presented data are the mean of
three independent experiments.
Interaction assays of OSCCs and
endothelial cells
The reciprocal actions of OSCC cells and endothelial
cells were tested using a CytoSelect Tumor-Endothelium Adhesion
Assay (Cell Biolabs, Inc.) in conjunction with the CytoSelect Tumor
Transendothelial Migration Assay system (Cell Biolabs, Inc.)
according to the manufacturer's protocols. Cellular adhesion and
migration were measured using a Multiskan GO Microplate
Spectrophotometer (Thermo Fisher Scientific, Inc.) at 480 and 520
nm, respectively.
Statistical analysis
All statistical analyses were conducted using JMP13
(SAS Institute). Continuous data were presented as the mean ± SD.
The differences in MCTC density between were analyzed by
unpaired parametric t-test or one-way ANOVA, and multiple
comparisons were analyzed by Tukey test. Moreover, cell experiments
were analyzed using one-way ANOVA, and multiple comparisons were
made using Tukey's test. The correlations between MVD, LVD and
expression levels of MIA gene family and MCTC
density were analyzed with Pearson's correlation coefficient.
Disease-free survival was calculated using the Kaplan-Meier method,
and differences between groups were tested by means of a log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
MCTC density, MVD and LVD in
OSCC specimens
First, MCTC density, MVD and LVD were
evaluated in 93 patients with OSCC using immunohistochemistry. The
mean ± SD values of the MCTC density, MVD and LVD in
OSCC cases were 49.7±30.6, 50.6±34.5 and 25.6±13.4 cells or
vessels/field of view, respectively (Fig. 1A-F). No expression of chymase was
observed in OSCC cells.
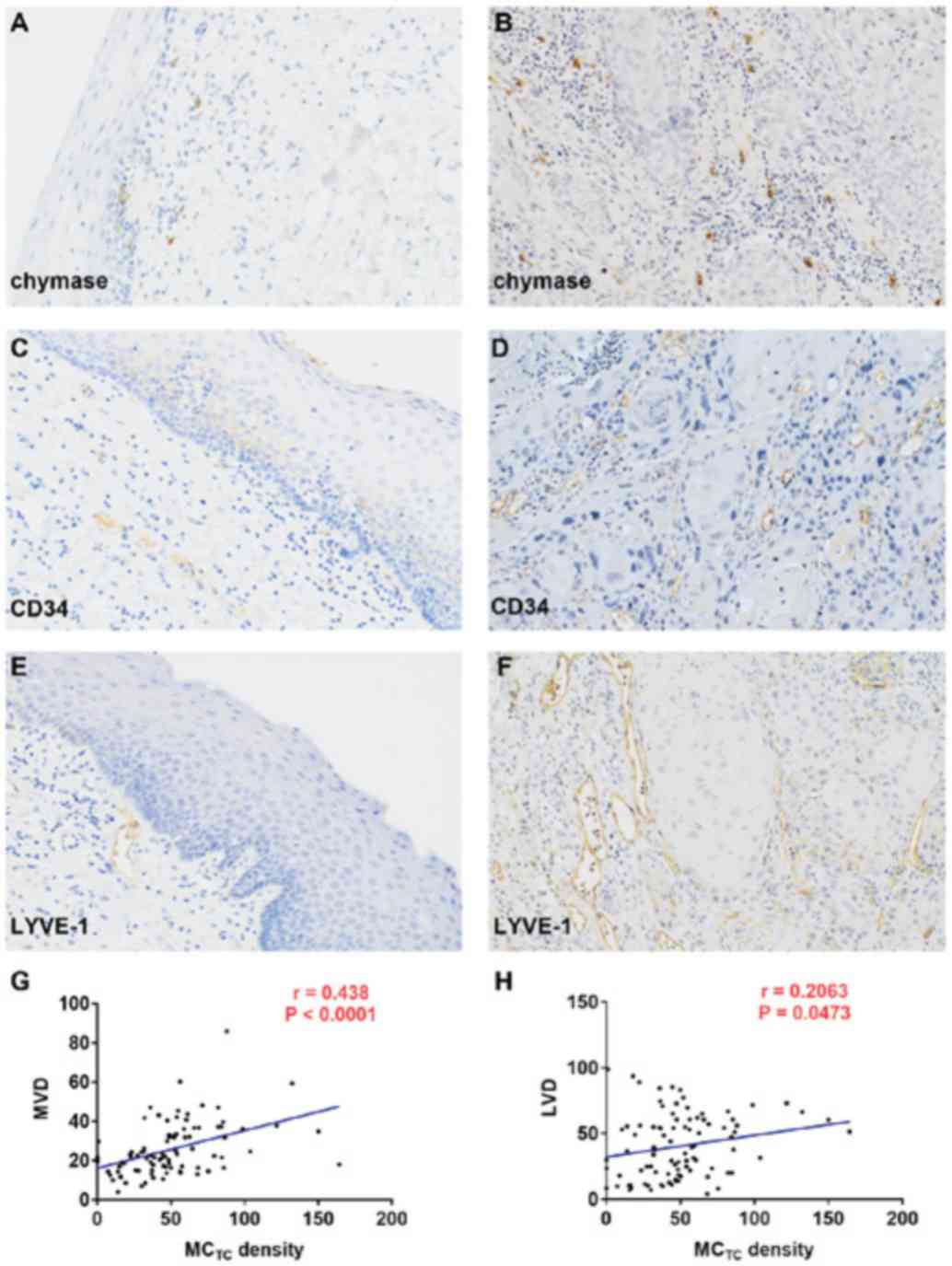 | Figure 1Expression of chymase, CD34 and LYVE1
in OSCC. (A and B) Chymase-positive mast cells, (C and D)
CD34-positive blood vessels and (E and F) LYVE-1-positive lymph
vessels in normal (A, C and E) mucosa and (B, D and F) OSCC.
Magnification, x400. MCTC density was significantly correlated with
(G) MVD and (H) LVD. OSCC, oral squamous cell carcinoma; LYVE1,
lymphatic vessel endothelial hyal-uronan receptor 1;
MCTC, mast cells expressing tryptase and chymase; MVD,
microvessel density; LVD, lymph vessel density. |
The clinicopathological relevance of MCTC
density in OSCC is presented in Table
I. Significantly higher density was observed in patients with
nodal metastasis compared with those without nodal metastasis
(P=0.0029). Significant relationships were also found between the
MCTC density and histological grade (P=0.0443), local
progression (T grade; P=0.0347), and clinical stage (P=0.0157). No
relationship was found between MCTC density and other
clinicopathological characteristics in OSCC. MCTC
density was also correlated with MVD (P<0.0001) and LVD
(P=0.0473) in OSCC (Fig. 1G and
H).
 | Table IRelationships between
chymase-positive MCTC density and clinicopathological
characteristics. |
Table I
Relationships between
chymase-positive MCTC density and clinicopathological
characteristics.
| Parameters | Number of
patients | MCTC density | P-value |
|---|
| Age (years) | | | 0.6436 |
| ≤60 | 26 (28.0%) | 52.1±36.9 | |
| >60 | 67 (72.0%) | 48.8±28.1 | |
| Sex | | | 0.1136 |
| Male | 43 (46.2%) | 55.1±36.7 | |
| Female | 50 (53.8%) | 45.0±23.7 | |
| Tumor site | | | 0.0869 |
| Tongue | 52 (55.9%) | 43.7±26.9 | |
| Gingiva | 27 (55.9%) | 54.8±32.3 | |
| Other | 14 (44.1%) | 61.9±34.4 | |
| Smoking habit | | | 0.7422 |
| Yes | 45 (48.4%) | 54.2±28.8 | |
| No | 48 (51.6%) | 56.2±29.6 | |
| Alcohol intake | | | 0.4700 |
| Habitual | 23 (24.7%) | 53.2±28.6 | |
| Social | 53 (57.0%) | 51.3±32.5 | |
| No drinking | 17 (18.3%) | 42.3±19.6 | |
| Histological
grade | | | 0.0443 |
| Well | 62 (66.7%) | 45.2±22.9 | |
| Moderate/Poor | 31 (33.3%) | 58.7±41.0 | |
| T grade | | | 0.0347 |
| T1 | 20 (21.5%) | 40.2±32.0 | |
| T2 | 51 (54.8%) | 47.8±27.6 | |
| T3-4 | 22 (23.7%) | 64.0±35.2 | |
| Clinical stage | | | 0.0157 |
| I | 20 (21.5%) | 40.2±32.0 | |
| II | 38 (40.9%) | 44.3±23.0 | |
| III-IV | 35 (37.6%) | 61.0±33.1 | |
| Nodal
metastasis | | | 0.0029 |
| Negative | 63 (67.7%) | 43.3±26.1 | |
| Positive | 30 (32.3%) | 63.2±35.2 | |
Relationship between MCTC
density and MIA gene family expression in OSCC specimens
It was previously reported that the MIA gene
family is associated with MVD and LVD in OSCC (20-24).
Thus, the expression levels of MIA, MIA2 and
TANGO were analyzed via RT-qPCR, and the correlations with
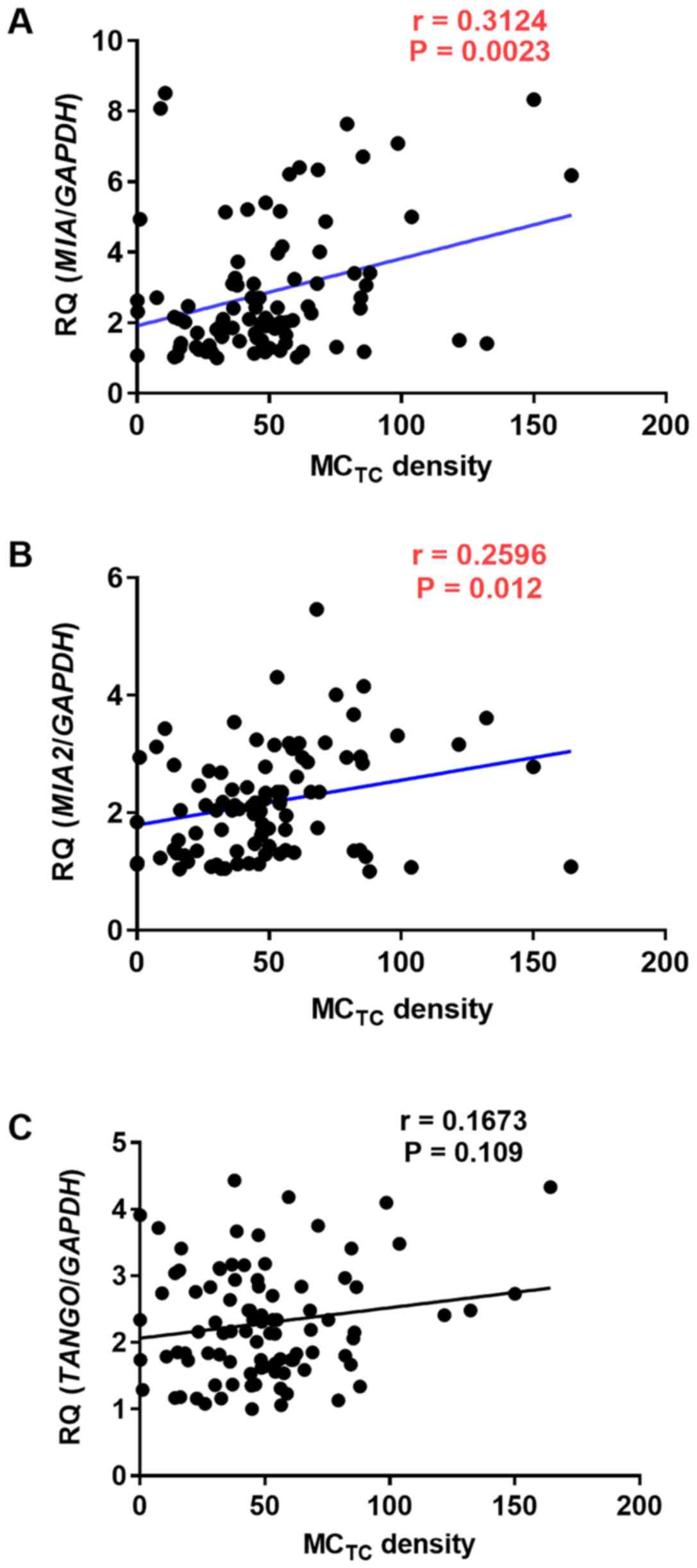
MCTC density in OSCC specimens were evaluated.
Expression levels of MIA (P=0.0023) and MIA2 (P=
0.012) were correlated with MCTC density, whereas those
of TANGO were not (Fig. 2A-C).
Relationship between MCTC
density and OSCC prognosis
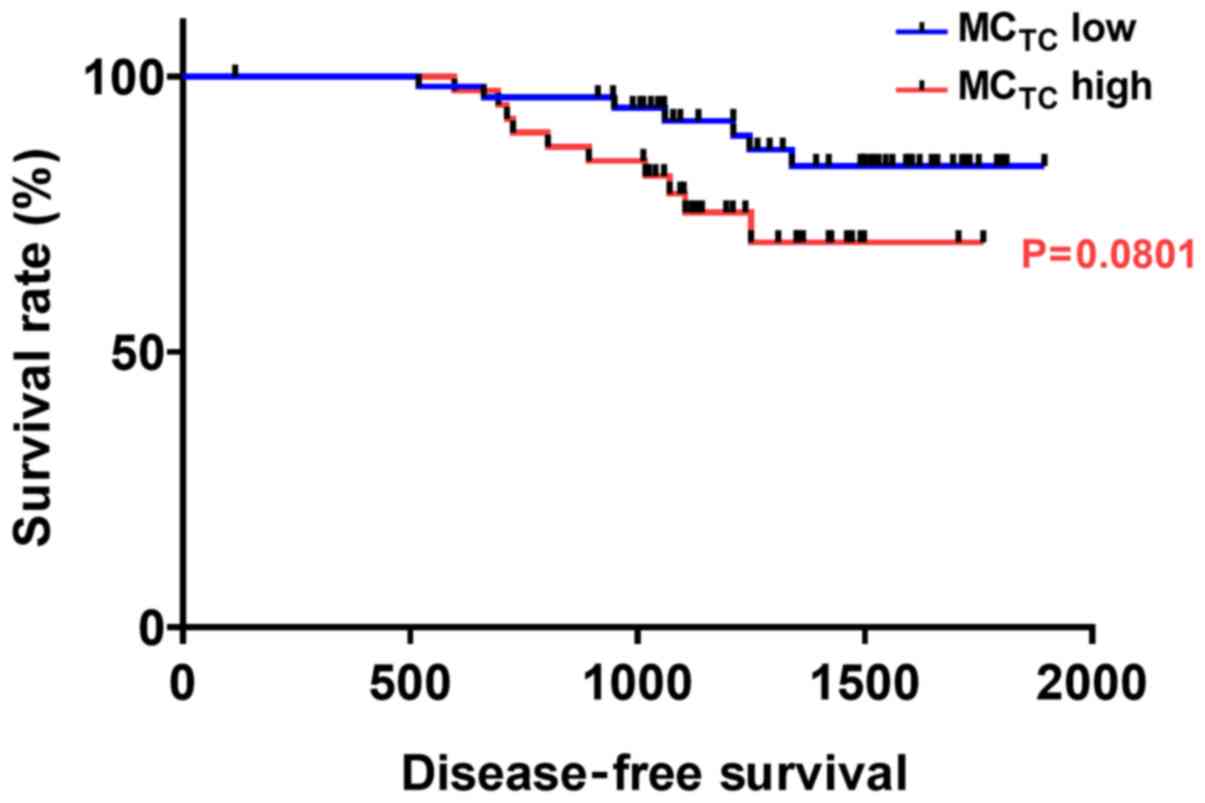
During the follow-up period, 17 of the 93 patients
presented with a local or metastatic recurrence of cancer. The
overall mean clinical follow-up time was 1,282 days, and ranged
from 115 to 1,895 days. The disease-free survival curves suggested
that cases with high MCTC density tended to have a
poorer prognosis than those with lower MCTC density;
however, there was no significant difference (P=0.0801; Fig. 3).
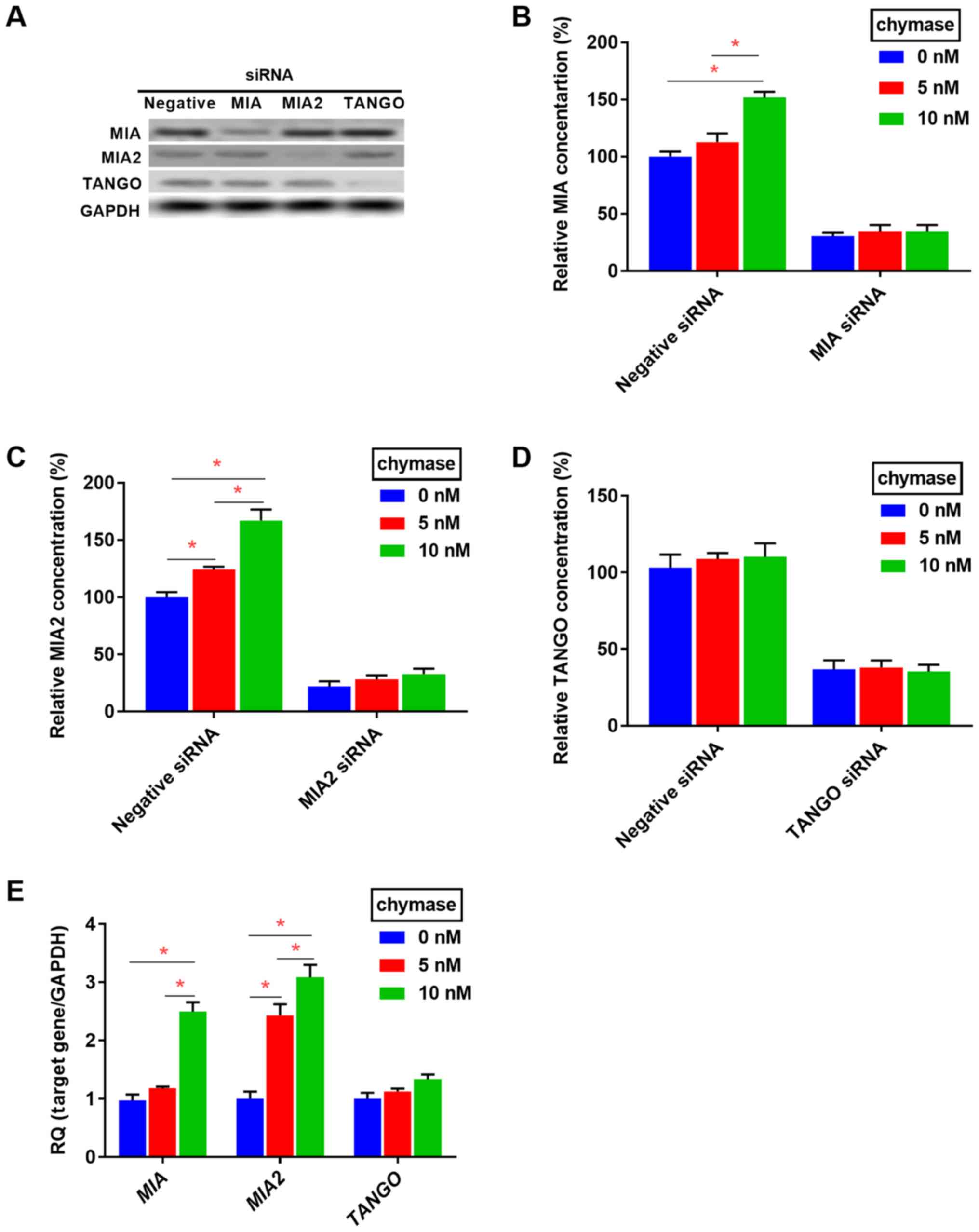
Effect of chymase on secretion of MIA
family proteins, and angiogenesis and lymphangiogenesis in OSCC
cells
HSC3 cells overexpress members of the MIA
family (20-23). As MIA, MIA2 and TANGO are secretory
proteins (4), the effect of
chymase on the secretion and expression of the MIA gene
family was evaluated in HSC3 cells. Secretion and expression of MIA
and MIA2 was increased by treatment with recombinant human chymase,
whereas MIA or MIA2 knockdown abolished the
chymase-induced increase in MIA or MIA2 secretion (Fig. 4A-E). The secretion levels of TANGO
were not affected by treatment with different concentrations of
chymase in HSC-3 cells (Fig. 4D and
E). These results suggested that chymase increases the
expression and secretion of MIA and MIA2 in a paracrine manner in
OSCC cells.
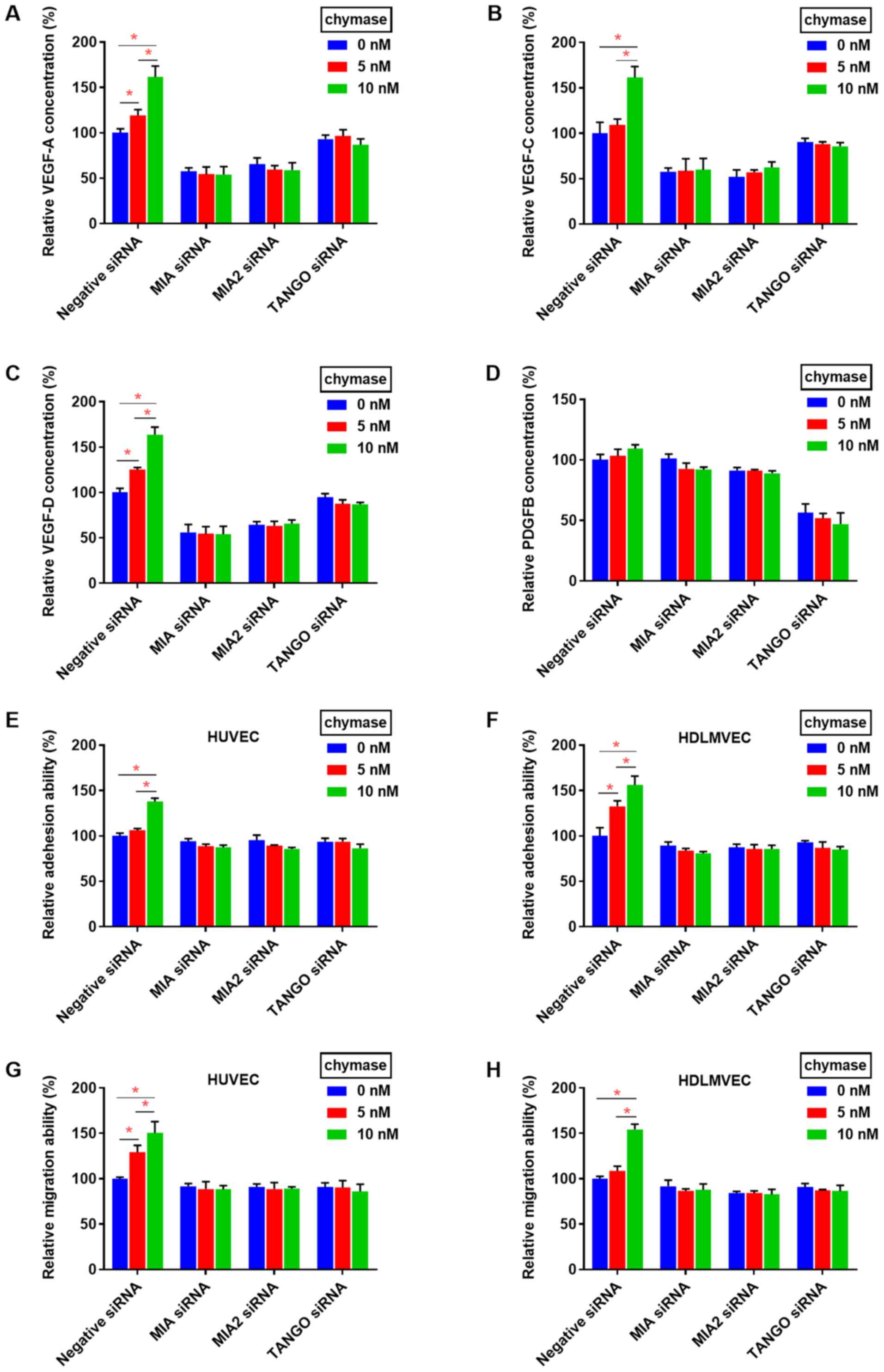
MIA and MIA2 are angiogenic and lymphangiogenic
factors, inducing VEGF family activation, whereas TANGO promotes
angiogenesis and lymphangiogenesis via the activation of PDGFB in
HSC3 cells (4,20-23).
Next, the relationship between chymase and MIA gene family-induced
angiogenesis and lymphangiogenesis was investigated. The secretion
of VEGF-A, VEGF-C, and VEGF-D was increased following treatment
with chymase in a dose-dependent manner in HSC3 cells, but not in
MIA or MIA2 knockdown HSC3 cells (Fig. 5A-C). TANGO siRNA similarly
attenuated chymase-induced increases in VEGF-A, VEGF-C and VEGF-D.
Moreover, secretion levels of PDGFB were not affected by chymase
treatment (Fig. 5D). Finally,
although the adhesion and transmigration abilities of HSC3 cells to
endothelial cells were increased by chymase treatment, these
abilities were inhibited by MIA, MIA2 or TANGO siRNA (Fig. 5E-H).
Collectively, the present resulted suggested that
chymase promotes OSCC progression by inducing MIA- and
MIA2-dependent angiogenic and lymphangiogenic mechanisms.
Discussion
The TME is proposed to substantially contribute to
cancer progression and metastasis (4). During this process, angio-genesis and
lymphangiogenesis are pivotal events (21). Our previous study showed that high
MVD and LVD, resulting from activation of the VEGF family, are
strongly associated with T grade, clinical stage, nodal metastasis,
local recurrence and poor prognosis in OSCC (21). MCs are present in the TME, and
induce positive or negative effects on cancer (5,10-17).
In human OSCC, an increase in MCT density is strongly
associated with MVD (28).
Mohtasham et al (29) also
reported a gradual increase in MCT from oral dysplasia
to OSCC. In addition, a separate study observed a significant
increase in the numbers of MCTC in OSCC compared with
healthy oral mucosa (30).
Conversely, another study showed that the numbers of MCT
decrease in OSCC and leukoplakia compared with normal oral mucosa
(31). Hence, there remains
substantial uncertainty regarding the role of MCs in OSCC,
particularly the relevance of MCTC to
lymphangiogenesis.
Chymase is a component of the renin-angiotensin
system and plays a key role in blood pressure regulation (32,33).
In malignancies, chymase can cleave pro-MMP9 to produce MMP9,
generate Ang II from Ang I, and induce angiogenesis via the
activation of VEGF-A (5). Other
studies have suggested that MCT promote
lymphangiogenesis by releasing VEGF-C and VEGF-D in mild and
moderate periodontitis (7,8,34).
In the present study, it was revealed that MCTC density
is associated with tumor progression and nodal metastasis in OSCC.
Moreover, a significant correlation was observed between
MCTC density, and MVD, LVD, and expression levels of
MIA and MIA2 in OSCC specimens. In OSCC cells,
chymase promoted angiogenesis and lymphangiogenesis through the
secretion of VEGF-A, VEGF-C, and VEGF-D via MIA and MIA2
activation.
MIA/MIA2-integrin α4/α5 signaling is regulated by
the phosphorylation of mitogen-activated protein kinase p38 and
c-Jun N-terminal kinase, and promotes OSCC progression,
angiogenesis and lymphangiogenesis via upregulation of VEGF family
activity (4,20,21,24).
As TANGO activates PDGFB-dependent neovasculogenesis in OSCC
(23), it is proposed that chymase
does not promote angiogenesis and lymphangiogenesis via TANGO. The
interaction of VEGF family members secreted by OSCC cells and
MCTC may augment the potential for angiogenesis and
lymphangiogenesis. A recent study suggested that chymase promotes
cell detachment by decreasing E-cadherin, and facilitates the
destruction of the extracellular matrix (ECM) through the
activation of MMP-9 in human lung adenocarcinoma and squamous cell
carcinoma cells (35). In cancer
cells, MIA can bind to fibronectin, a major component of the ECM,
and both MMP and MIA gene family receptors are cell surface
integrins (4,6,19,36).
Chymase in the TME may enhance ECM destruction, angiogenesis and
lymphangiogenesis by interacting with MIA secreted from cancer
cells. Therefore, further studies are warranted to further
understand the mechanisms underlying this process.
In general, the release of histamine, interleukin-10
and tumor necrosis factor-α from MCT leads to the
suppression of tumor immunity in several malignancies (37). Cancer cells also suppress tumor
immunity and create an environment in which they can easily grow
(20,37,38).
Previous results have indicated that tumor-derived MIA may
contribute to immune escape mechanisms frequently seen in patients
with melanoma by suppressing the activation of immune cells and
their associated antitumor cytotoxicity (38). Moreover, our previous study
reported that MIA2 expression in OSCC is promoted by a disturbance
in tumor immunity via the suppression of cytotoxic T lymphocytes
and a relative increase in regulatory T lymphocytes (20). MCTC may also disrupt
tumor immunity via interactions with MIA and MIA2 in OSCC cells.
Additional studies are required to elucidate the immunosuppression
observed following MCTC activation.
The functions and roles of chymase in tumor tissue
remain controversial. Two polymorphisms in the chymase gene
(CMA), CMA/A and CMA/B, are localized on
chromosome 14 and associated with chymase expression (39). Sugimoto et al (32,33)
reported that the CMA/B polymorphism in leukocytes is
strongly associated with an increased risk of gastric cancer
development and progression in Japanese populations. Conversely,
Shimomoto et al (40)
observed that the expression of MC chymase is upregulated in colon
cancer-derived HT29 and CT26 cells, and the cytoplasm of colon
cancer specimens. To our knowledge, there is no other report that
chymase is expressed in epithelial cells. Therefore, there are
questions regarding the findings of the aforementioned study, and
further studies are required to determine the accurate expression
of chymase in cancer cells.
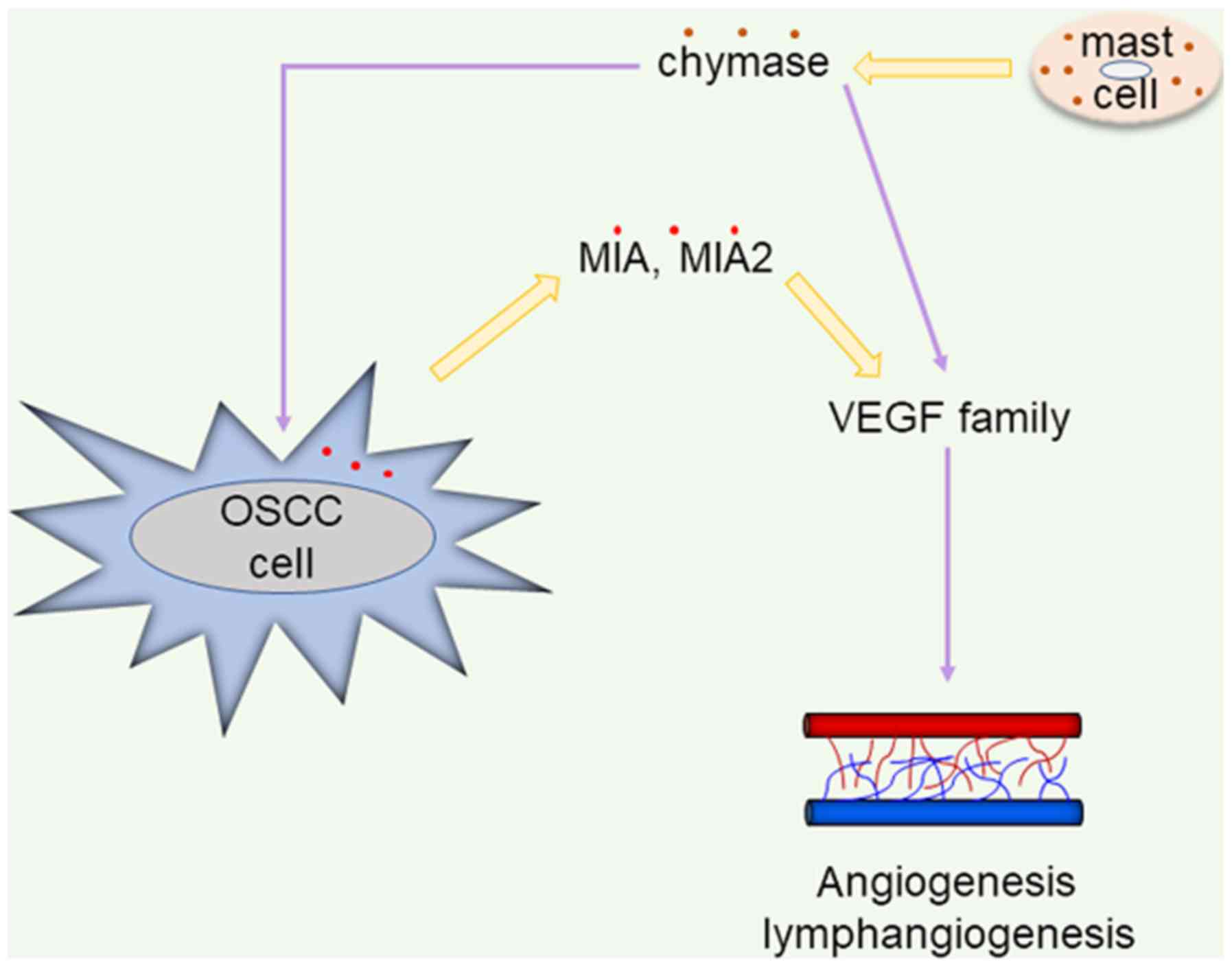
Fig. 6 presents a
proposed schematic of the mechanisms of MCTC in OSCC
based on the findings of the present study. MCTC may
accelerate angiogenesis and lymphangiogen-esis via the activation
of the VEGF family. Moreover, it is suggested that OSCC cells
stimulated by chymase induce VEGF family secretion through MIA and
MIA2, and promote angiogenesis and lymphangiogenesis in combination
with MCTC. Consequently, it is proposed that
MCTC act as tumor promoting factors by upregulating MIA
and MIA2 activity in OSCC. A previous study suggested that
cimeti-dine inhibits the activation of MCs in patients with breast
cancer (41). Additionally, c-kit
signaling is critical for MCs and the antitumor effects of
imatinib, a tyrosine kinase inhibitor with activity against c-kit,
which are mediated via mast cell inhibition (42,43).
The antiangiogenic agents, sunitinib, sorafenib and nilotinib, have
similar effects to imatinib, and may also be effective in
suppressing cancer cells by regulating MC functions (37). Angiogenesis and lymphangiogenesis
are pivotal events in tumor progression, and the resulting
irregular neoplastic blood and lymphatic vessels interfere with the
delivery of anticancer drugs (4).
Normalization of the tumor vessels by using antiangiogenic and
antilymphan-giogenic treatments targeting the chymase-MIA
gene family network may be useful methods for malignancies.
However, the function of MCTC may vary depending on the
type of cancer and clinical stage. More detailed studies are
required to elucidate the varied roles of MCs; however, the present
findings indicated the relevance of MCTC as a diagnostic
and therapeutic target in OSCC.
Funding
This work was supported by JSPS KAKENHI (grant nos.
JP 17K11621 and 18K09796).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS, AKB and TK conceived and designed the study.
MKS, TS and HS acquired data. MKS, TS, HS and AKB analyzed and
interpreted data. TS, MKS, AKB and TK drafted, revised and/or
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical
Ethical Committee of the Nara Medical University (approval no.
719). The study protocol using human samples was performed
according to the ethical standards laid out in the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shield KD, Ferlay J, Jemal A,
Sankaranarayanan R, Chaturvedi AK, Bray F and Soerjomataram I: The
global incidence of lip, oral cavity, and pharyngeal cancers by
subsite in 2012. CA Cancer J Clin. 67:51–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasahira T and Kirita T: Hallmarks of
cancer-related newly prognostic factors of oral squamous cell
carcinoma. Int J Mol Sci. 19:192018. View Article : Google Scholar
|
|
5
|
de Souza DA Jr, Santana AC, da Silva EZ,
Oliver C and Jamur MC: The role of mast cell specific chymases and
tryptases in tumor angiogenesis. BioMed Res Int.
2015:1423592015.
|
|
6
|
Bauer R, Humphries M, Fässler R,
Winklmeier A, Craig SE and Bosserhoff AK: Regulation of integrin
activity by MIA. J Biol Chem. 281:11669–11677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Detoraki A, Staiano RI, Granata F,
Giannattasio G, Prevete N, de Paulis A, Ribatti D, Genovese A,
Triggiani M and Marone G: Vascular endothelial growth factors
synthesized by human lung mast cells exert angiogenic effects. J
Allergy Clin Immunol. 123:1142–1149. 11491e1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sammarco G, Varricchi G, Ferraro V,
Ammendola M, De Fazio M, Altomare DF, Luposella M, Maltese L, Currò
G, Marone G, et al: Mast cells, angiogenesis and lymphangiogenesis
in human gastric cancer. Int J Mol Sci. 20:202019. View Article : Google Scholar
|
|
9
|
Jachetti E, Cancila V, Rigoni A,
Bongiovanni L, Cappetti B, Belmonte B, Enriquez C, Casalini P,
Ostano P, Frossi B, et al: Cross-talk between myeloid-derived
suppressor cells and mast cells mediates tumor-specific
immunosuppression in prostate cancer. Cancer Immunol Res.
6:552–565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagata M, Shijubo N, Walls AF, Ichimiya S,
Abe S and Sato N: Chymase-positive mast cells in small sized
adenocarcinoma of the lung. Virchows Arch. 443:565–573. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ibaraki T, Muramatsu M, Takai S, Jin D,
Maruyama H, Orino T, Katsumata T and Miyazaki M: The relationship
of tryptase- and chymase-positive mast cells to angiogenesis in
stage I non-small cell lung cancer. Eur J Cardiothorac Surg.
28:617–621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carlini MJ, Dalurzo MC, Lastiri JM, Smith
DE, Vasallo BC, Puricelli LI and Lauría de Cidre LS: Mast cell
phenotypes and microvessels in non-small cell lung cancer and its
prognostic significance. Hum Pathol. 41:697–705. 2010. View Article : Google Scholar
|
|
13
|
Shikotra A, Ohri CM, Green RH, Waller DA
and Bradding P: Mast cell phenotype, TNFα expression and
degranulation status in non-small cell lung cancer. Sci Rep.
6:383522016. View Article : Google Scholar
|
|
14
|
Kondo K, Muramatsu M, Okamoto Y, Jin D,
Takai S, Tanigawa N and Miyazaki M: Expression of chymase-positive
cells in gastric cancer and its correlation with the angiogenesis.
J Surg Oncol. 93:36–42; discussion 42-43. 2006. View Article : Google Scholar
|
|
15
|
Siiskonen H, Poukka M, Bykachev A,
Tyynelä-Korhonen K, Sironen R, Pasonen-Seppänen S and Harvima IT:
Low numbers of tryptase+ and chymase+ mast cells associated with
reduced survival and advanced tumor stage in melanoma. Melanoma
Res. 25:479–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehdawi L, Osman J, Topi G and Sjölander
A: High tumor mast cell density is associated with longer survival
of colon cancer patients. Acta Oncol. 55:1434–1442. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Glajcar A, Szpor J, Pacek A, Tyrak KE,
Chan F, Streb J, Hodorowicz-Zaniewska D and Okoń K: The
relationship between breast cancer molecular subtypes and mast cell
populations in tumor microenvironment. Virchows Arch. 470:505–515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stieglitz D, Lamm S, Braig S, Feuerer L,
Kuphal S, Dietrich P, Arndt S, Echtenacher B, Hellerbrand C, Karrer
S, et al: BMP6-induced modulation of the tumor micro-milieu.
Oncogene. 38:609–621. 2019. View Article : Google Scholar
|
|
19
|
Bosserhoff AK: Melanoma inhibitory
activity (MIA): An important molecule in melanoma development and
progression. Pigment Cell Res. 18:411–416. 2005.PubMed/NCBI
|
|
20
|
Kurihara M, Kirita T, Sasahira T, Ohmori
H, Matsushima S, Yamamoto K, Bosserhoff AK and Kuniyasu H:
Protumoral roles of melanoma inhibitory activity 2 in oral squamous
cell carcinoma. Br J Cancer. 108:1460–1469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasahira T, Kirita T, Kurihara M, Yamamoto
K, Bhawal UK, Bosserhoff AK and Kuniyasu H: MIA-dependent
angiogenesis and lymphangiogenesis are closely associated with
progression, nodal metastasis and poor prognosis in tongue squamous
cell carcinoma. Eur J Cancer. 46:2285–2294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasahira T, Kirita T, Oue N, Bhawal UK,
Yamamoto K, Fujii K, Ohmori H, Luo Y, Yasui W, Bosserhoff AK, et
al: High mobility group box-1-inducible melanoma inhibitory
activity is associated with nodal metastasis and lymphangiogenesis
in oral squamous cell carcinoma. Cancer Sci. 99:1806–1812.
2008.PubMed/NCBI
|
|
23
|
Sasahira T, Kirita T, Yamamoto K, Ueda N,
Kurihara M, Matsushima S, Bhawal UK, Bosserhoff AK and Kuniyasu H:
Transport and Golgi organisation protein 1 is a novel tumour
progressive factor in oral squamous cell carcinoma. Eur J Cancer.
50:2142–2151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasahira T, Nishiguchi Y, Fujiwara R,
Kurihara M, Kirita T, Bosserhoff AK and Kuniyasu H: Storkhead box 2
and melanoma inhibitory activity promote oral squamous cell
carcinoma progression. Oncotarget. 7:26751–26764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Sullivan B: Head and neck tumours. UICC
TNM classification of malignant tumours. Brierley JD, Gospodarowicz
MK and Wittekind C: 8th edition. Wiley; Chichester: pp. 17–54.
2017
|
|
26
|
Sloan P, Gale N, Hunter K, Lingen M,
Nylander K, Reibel J, Salo T and Zain RB: Malignant surface
epithelial tumors. WHO classification of head and neck tumors.
El-Naggar AK, Chan JKC, Grandis JR, et al: 4th edition. IARC Press;
Lyon: pp. 17–54. 2017
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Iamaroon A, Pongsiriwet S, Jittidecharaks
S, Pattanaporn K, Prapayasatok S and Wanachantararak S: Increase of
mast cells and tumor angiogenesis in oral squamous cell carcinoma.
J Oral Pathol Med. 32:195–199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mohtasham N, Babakoohi S, Salehinejad J,
Montaser-Kouhsari L, Shakeri MT, Shojaee S, Sistani NS and Firooz
A: Mast cell density and angiogenesis in oral dysplastic epithelium
and low- and high-grade oral squamous cell carcinoma. Acta Odontol
Scand. 68:300–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yadav A, Desai RS, Bhuta BA, Singh JS,
Mehta R and Nehete AP: Altered immunohistochemical expression of
mast cell tryptase and chymase in the pathogenesis of oral
submucous fibrosis and malignant transformation of the overlying
epithelium. PLoS One. 9:e987192014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oliveira-Neto HH, Leite AF, Costa NL,
Alencar RC, Lara VS, Silva TA, Leles CR, Mendonça FE and Batista
AC: Decrease in mast cells in oral squamous cell carcinoma:
Possible failure in the migration of these cells. Oral Oncol.
43:484–490. 2007. View Article : Google Scholar
|
|
32
|
Sugimoto M, Furuta T, Kodaira C, Nishino
M, Yamade M, Ikuma M, Sugimura H and Hishida A: Polymorphisms of
matrix metalloproteinase-7 and chymase are associated with
susceptibility to and progression of gastric cancer in Japan. J
Gastroenterol. 43:751–761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sugimoto M, Furuta T, Shirai N, Ikuma M,
Sugimura H and Hishida A: Influences of chymase and angiotensin
I-converting enzyme gene polymorphisms on gastric cancer risks in
Japan. Cancer Epidemiol Biomarkers Prev. 15:1929–1934. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raica M, Cimpean AM, Popovici RA, Balica
AR, Vladau M and Gaje PN: Mast cells stimulate lymphangiogenesis in
the gingiva of patients with periodontal disease. In Vivo.
29:29–34. 2015.PubMed/NCBI
|
|
35
|
Jiang Y, Wu Y, Hardie WJ and Zhou X: Mast
cell chymase affects the proliferation and metastasis of lung
carcinoma cells in vitro. Oncol Lett. 14:3193–3198. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rolli M, Fransvea E, Pilch J, Saven A and
Felding-Habermann B: Activated integrin alphavbeta3 cooperates with
metallopro-teinase MMP-9 in regulating migration of metastatic
breast cancer cells. Proc Natl Acad Sci USA. 100:9482–9487. 2003.
View Article : Google Scholar
|
|
37
|
Dyduch G, Kaczmarczyk K and Okoń K: Mast
cells and cancer: Enemies or allies? Pol J Pathol. 63:1–7.
2012.PubMed/NCBI
|
|
38
|
Jachimczak P, Apfel R, Bosserhoff AK,
Fabel K, Hau P, Tschertner I, Wise P, Schlingensiepen KH,
Schuler-Thurner B and Bogdahn U: Inhibition of immunosuppressive
effects of melanoma-inhibiting activity (MIA) by antisense
techniques. Int J Cancer. 113:88–92. 2005. View Article : Google Scholar
|
|
39
|
Pfeufer A, Osterziel KJ, Urata H, Borck G,
Schuster H, Wienker T, Dietz R and Luft FC: Angiotensin-converting
enzyme and heart chymase gene polymorphisms in hypertrophic
cardiomyopathy. Am J Cardiol. 78:362–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimomoto T, Ohmori H, Luo Y, Chihara Y,
Denda A, Sasahira T, Tatsumoto N, Fujii K and Kuniyasu H:
Diabetes-associated angiotensin activation enhances liver
metastasis of colon cancer. Clin Exp Metastasis. 29:915–925. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bowrey PF, King J, Magarey C, Schwartz P,
Marr P, Bolton E and Morris DL: Histamine, mast cells and tumour
cell proliferation in breast cancer: Does preoperative cimetidine
administration have an effect? Br J Cancer. 82:167–170. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J,
Li X, Yang X, Knowles S, Horn W, Li Y, et al: Nf1-dependent tumors
require a microenvironment containing Nf1+/−- and
c-kit-dependent bone marrow. Cell. 135:437–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pittoni P, Piconese S, Tripodo C and
Colombo MP: Tumor-intrinsic and -extrinsic roles of c-Kit: Mast
cells as the primary off-target of tyrosine kinase inhibitors.
Oncogene. 30:757–769. 2011. View Article : Google Scholar
|