Introduction
Choriocarcinoma (CC) is a pregnancy-associated tumor
characterized by massive hemorrhage and earlier hematogenous
metastasis compared with other female genital tumors (1). According to National Comprehensive
Cancer Network guidelines, with the development of chemotherapy,
the overall cure rate of patients with high-risk CC has reached
~90% worldwide (2). However,
multi-drug resistant CC still remains to be a challenge in the
clinical setting (3). Angiogenetic
ability is closely associated with the malignancy and
chemo-sensitivity of tumors (4).
Vasculogenic mimicry (VM) is the process by which aggressive cancer
cells can form highly patterned vascular channels without the
presence of endothelial cells (5),
which has been observed in various aggressive tumors, including
melanoma, hepatocellular carcinoma (HCC), breast and ovarian cancer
(5-8). Tumors that exhibit VM tend to
metastasize easily, develop advanced tumor stages and high tumor
grades, and present with a lower survival rate (9). Factors involved in regulating VM
formation and stability, such as ephrin-A2 (EphA2), vascular
endothelial cadherin (VE-cad) and matrix metalloproteinase (MMPs)
also participate in tumor metastasis (10). According to their morphology,
function and immunological factors, trophoblast cells could be
divided into three types during embryogenesis and differentiation:
cytotrophoblasts (CTBs), syncytiotrophoblasts (STBs) and
intermediate trophoblasts (ITBs). CTBs are undifferentiated and
proliferate to give rise to STBs and ITBs; thus, these cells are
termed trophoblast stem cells, which identifies their function as
tumor stem cells (11).
Trophoblast stem cells participating in the formation of the
placenta obtain endothelial cell phenotypes during the
differentiation process (12) and
are associated with a malignant tumor behavior (13). A previous morphological study of
clinical CC tissues has demonstrated that pseudovascular channels
are lined with STBs rather than CTBs or ITBs (14). These results suggested that
trophoblast cells utilized VM to support tumor development in CC
(14).
The cyclic adenosine monophosphate-protein kinase A
(cAMP-PKA) signaling pathway serves an important role in regulating
embryo implantation (15) combined
with the Notch pathway and differentiation of endothelial cells
(16). Forskolin is a typical
activator of the cAMP pathway by direct activation of adenylate
cyclase (17), and has been widely
used as an inducer of syncytiolization and expression of
invasion-associated molecules (18,19).
However, little is currently known about the role of this cAMP
activator and its mechanism in VM formation in CC.
The epithelial-to-mesenchymal transition (EMT) is a
process in which epithelial cells lose their epithelial features
and simultaneously acquire mesenchymal features (20). EMT serves an important role in
embryogenesis, wound healing, fibrosis and tumor metastasis
(21), and has recently been
demonstrated to be involved in VM formation in human HCC, breast
and colon cancer (7,22,23).
The Notch signaling pathway is highly conserved and has been
identified in arterial-venous specification (24). The Notch signaling pathway is
activated by the interaction of trans-membrane ligands of the
Jagged (Jagged1 and 2) and Delta (Delta-like 1, 3 and 4) families
with Notch receptors (Notch1-4). Blockage of Notch in the
endothelium permits unproductive angiogenesis and decelerates tumor
growth (25). A previous study has
demonstrated that Notch-1 signaling participates in the EMT process
initiated by hypoxic conditions in CC cells (26).
The present study aimed to test the hypothesis that
VM and metastatic ability would be affected during syncytiolization
of cytotrophoblast induced by forskolin, and also to explore
whether Notch-mediated EMT was involved in the regulation of these
biological phenomena in vitro and in vivo.
Materials and methods
Cell culture and reagents
Human CC cell lines JEG-3 (derived from a
gestational CC) and JAR (derived from a male fetus CC) were
purchased from the American Type Culture Collection, and the human
umbilical vein endothelial cell (HUVEC) line was kindly provided by
Dr Jer-Tsong Hsieh (University of Texas Southwestern Medical
Center, Dallas, TX, USA). These cells were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.). All cells were cultured at 37˚C in
a humidified atmosphere with 5% CO2. The cells used in
the present study were of the third generation after resuscitation
and passage. When cells reached 50% confluence, they were treated
with 100 µM forskolin (Abcam) or the vehicle (0.1% DMSO) for 48 h
at 37˚C. DMSO was purchased from Sigma-Aldrich; Merck KGaA. Notch-1
signaling was blocked with 10 µM DAPT (Abcam) for 24 h.
Wound healing assay
JAR and JEG-3 cells treated with forskolin or DMSO
were scratched in 6-well plates with a 200 µl pipette tip when the
cell confluence reached ~90%. Cells were incubated in serum-free
medium (SFM), and the wound was photographed at 0 and 24 h under a
phase-contrast microscope (×10 magnification; Olympus Corporation)
and the total wound area was measured.
Transwell migration and invasion
assays
The effects of forskolin on cell migratory and
invasive abilities were assessed using 8-µm-pore Transwell inserts
(EMD Millipore). For the migration assay, a 200 µl SFM suspension
with 5×104 JAR or JEG-3 cells pre-incubated with
forskolin or DMSO for 48 h were added to the upper chambers. The
lower chambers were filled with 800 µl medium containing 10% fetal
bovine serum. For cell invasion assay, the upper chambers were
pre-coated with 50 µl Matrigel (Sigma-Aldrich; Merck KGaA) for 4 h.
A 200 µl SFM suspension with 8×104 JAR or
5×104 JEG-3 cells pre-incubated with forskolin or DMSO
for 48 h were added to the upper chambers. For the HUVEC migration
assay, 200 µl HUVECs suspended in SFM at 2×105 cells/ml
were seeded into the upper chamber, and 1×104 CC cells
pretreated with forskolin or DMSO for 48 h were added to the lower
chamber. Following 24-h incubation at 37˚C, cells that had migrated
to the lower chamber were fixed with 4% paraformaldehyde for 25 min
and stained with crystal violet for 10 min at room temperature. The
upper chamber was wiped with a cotton swab, and cell numbers were
counted in five randomly selected fields per well under a
phase-contrast microscope (x200 magnification; Olympus
Corporation).
3D cultures and tubule formation
The assay was performed as previously described by
Zhan et al (6,27). A total of 200 µl Matrigel (BD
Biosciences) was added to 24-well plates and incubated at 37˚C for
1 h. CC cells (1×105 cells/well) suspended in SFM were
seeded into Matrigel-coated wells following treatment with
forskolin or DMSO for 48 h. After 6 and 24 h, tubule structure
formation was observed, and the number and completeness of the
tubule were assessed under a phase-contrast microscope (x200
magnification; Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was harvested using the TRIzol®
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol and quantified by spectrophotometry. The
samples were reverse-transcribed to cDNA using the PrimeScript™ RT
Reagent kit (Takara Bio, Inc.). The RNA sample was incubated with 2
µl 5X PrimeScript RT Master Mix at 37˚C for 15 min, followed by
85˚C for 5 sec and terminated at 4˚C. QPCR was performed using the
SYBR® Green PCR Master Mix (Takara Bio, Inc.) on a CFX96
real-time PCR system (Bio-Rad Laboratories, Inc.). The PCR protocol
was 94˚C for 10 min, followed by 40 cycles of 94˚C for 10 sec and
60˚C for 30 sec. The primer sequences used in the present study are
listed in Table I. Data were
normalized to GAPDH expression and all reactions were performed in
triplicate. Relative gene expression was calculated using the
2-ΔΔCq method (28).
 | Table IQuantitative PCR primers. |
Table I
Quantitative PCR primers.
| Gene | Sequences
(5'→3') |
|---|
| GAPDH | F:
ATGGGGAAGGTGAAGGTCGG |
| R:
CACGGTGCCATGGAATTTGC |
| VE-cad | F:
GCGACTACCAGGACGCTTTCA |
| R:
CATGTATCGGAGGTCGATGGTG |
| EphA2 | F:
CTCTCACACCCCGTATGGCAAAG |
| R:
TCCTGGTCGCCAGACATCAC |
| MMP2 | F:
TACAGGATCATTGGCTACACACC |
| R:
GGTCACATCGCTCCAGACT |
| MMP9 | F:
TGTACCGCTATGGTTACACTCG |
| R:
GGCAGGGACAGTTGCTTCT |
| E-cadherin | F:
GGGCTCAAGTGACTCGTAACGA |
| R:
CAGCCGCTTTCAGATTTTCATC |
| CK19 | F:
AACGGCGAGCTAGAGGTGA |
| R:
GGATGGTCGTGTAGTAGTGGC |
| N-cadherin | F:
ATGAAAGACCCATCCACG |
| R:
CCTGCTCACCACCACTA |
| Vimentin | F:
CTTCCGCGCCTACGCCA |
| R:
GCCCAGGCGAGGTACTCC |
| ZEB1 | F:
GAAAGTGATCCAGCCAAATGGA |
| R:
TTTGGGCGGTGTAGAATCAGAG |
| HESI | F:
CTTCCGCGCCTACGCCA |
| R:
GCCCAGGCGAGGTACTCC |
| NICD | F:
GGAGGCATCCTACCCTTTTC |
| R:
TGTGTTGCTGGAGCTTCTTC |
Western blot analysis
Cells at 80-90% confluence were washed three times
with cold PBS and lysed in RIPA buffer (50 mM Tris pH 8.0, 150 mM
NaCl, 0.1% SDS, 1% NP-40, and 0.5% sodium deoxycholate) containing
protease inhibitors (1 mM PMSF; Sigma-Aldrich; Merck KGaA). Protein
samples were quantified by bicinchoninic acid assay, and 20 µg of
protein was loaded per lane, separated by SDS-PAGE (8-12% gel) and
electrophoretically transferred to nitrocellulose membranes (EMD
Millipore). The membranes were blocked with 5% skimmed milk at room
temperature for 1 h and incubated with primary antibodies against
GAPDH (cat. no. KG-5G4; Kangchen; 1:1,000), syncytin-1 (cat. no.
SC130888; Santa Cruz; 1:200), VEGFA (cat. no. ab185265; Abcam;
1:1,000), VE-cadherin (VE-cad; cat. no. ab205336; Abcam; 1:1,000),
MMP-2 (cat. no. ab37150; Abcam; 1:1,000), Hes family BHLH
transcription factor 1 (HES1; cat. no. ab108937; Abcam; 1:500),
cleaved Notch1 (NICD; cat. no. 4147; Cell Signaling Technology,
Inc.; 1:1,000), N-cadherin (N-cad; cat. no. 13116; Cell Signaling
Technology, Inc.; 1:500), zinc finger E-box-binding homeobox 1
(ZEB1; cat. no. 3396; Cell Signaling Technology, Inc.; 1:1,000),
E-cadherin (E-cad; cat. no. sc71009; Santa Cruz Biotechnology,
Inc.; 1:1,000), cyto-keratin (CK19; cat. no. sc376126; Santa Cruz
Biotechnology, Inc.; 1:1,000), vimentin (cat. no. sc80975; Santa
Cruz Biotechnology, Inc.; 1:400) and MMP-9 (cat. no. sc10737; Santa
Cruz Biotechnology, Inc.; 1:1,000) at 4˚C overnight, followed by
washing with TBS containing 0.1% Tween-20 and 1 h incubation with a
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H+L)
secondary antibody (cat. no. sc2301; Santa Cruz Biotechnology,
Inc.; 1:800) at room temperature. Protein bands were visualized
using the Odyssey detection system (LI-COR Biosciences) and
quantified using ImageLab software (version 4.1; Bio-Rad
Laboratories, Inc.).
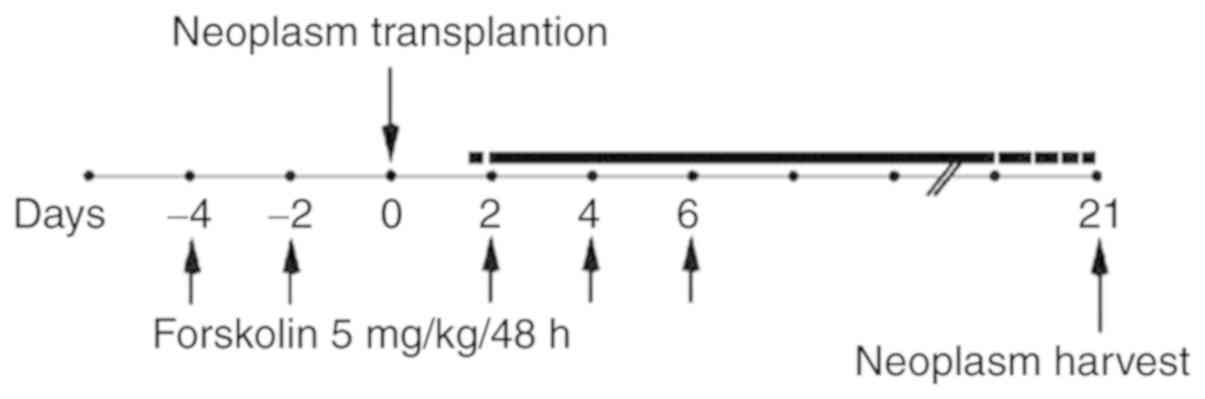
Xenograft tumor model
Animal care and protocols were performed according
to the guidelines of the Institutional Animal Care and Use
Committee of Xi'an Jiaotong University. A preliminary experiment
indicated that xenografts in nude mice were easier to establish
using JEG-3 cells compared with JAR cells (29); therefore, JEG-3 cells were used for
the in vivo study. A total of 12 5-week-old athymic nude
mice (Charles River Laboratories, Inc.) were randomly divided into
two groups; the mice in the forskolin group were injected
intra-peritoneally with 5 mg/kg forskolin diluted in a PBS/DMSO
solution (15:0.1) five times on days −4, −2, 2, 4 and 6 (relative
to JEG-3 cell injection), and the control groups were injected with
equal volumes of DMSO (30). A
total of 5×106 JEG-3 cells were injected subcutaneously
into nude mice to establish the xenograft model on day 0. Xenograft
tumors were harvested after 3 weeks and used for western blotting,
hematoxylin and eosin (H&E) staining and CD31/periodic
acid-Schiff (PAS) double staining (Fig. 1).
CD31/PAS double staining
Immunohistochemistry was performed on 4-µm-thick
sections. Sections were deparaf-finized using xylene and dehydrated
using ethanol (100, 95, 80, 70 and 50% for 3 min each). Antigen
retrieval was performed using citric acid for 5 min at 121˚C. After
blocking with 3% hydrogen peroxide for 30 min at room temperature,
the sections were incubated with an anti-CD31 antibody (cat. no.
ab28364; Abcam; 1:100) overnight at 4˚C and anti-rabbit ChemMate™
EnVision™/HRP secondary antibody for 1 h at room temperature,
followed by staining with DAB for 40 sec (cat. no. GK500705; Dako;
Agilent Technologies, Inc.). The sections were incubated with PAS
(0.5% periodic acid for 10 min and Schiff solution for 15 min) at
room temperature prior to H&E counterstaining (hematoxylin for
5 min and 0.5% eosin for 1-3 min). VM structures were defined as
CD31-negative PAS-positive structures.
Statistical analysis
Data are presented as the mean ± standard error of
the mean of at least three independent experiments. GraphPad Prism
software (version 6.0; GraphPad Software, Inc.) was used to analyze
differences between two groups with Student's t-test, and among
multiple groups with one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
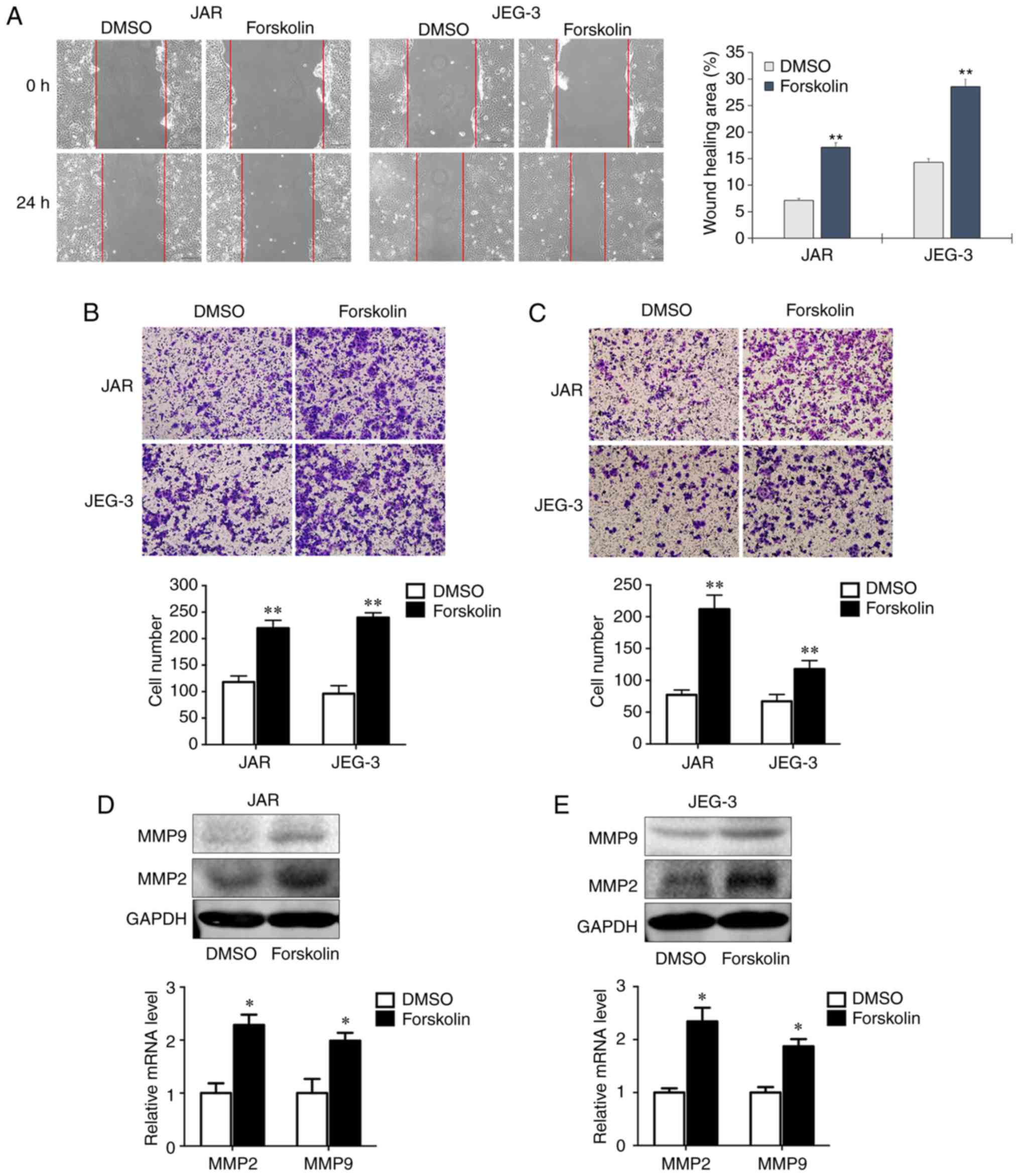
Forskolin promotes CC cell invasion and
migration in vitro
Cell motility is associated with tumor metastasis.
In order to assess whether forskolin could stimulate the invasion
and migration of JAR and JEG-3 cells, the present study used wound
healing and transwell assays. In the wound healing assay, the
migration rates of the forskolin groups at 24 h were significantly
higher compared with those of the DMSO groups (P<0.01; Fig. 2A). In the migration assay presented
in Fig. 2B, the numbers of migrant
cells were ~2-fold higher compared with the control groups when JAR
and JEG-3 cells were treated with forskolin (P<0.01). Similarly,
the invasive capacity was markedly enhanced when cells treated with
forskolin compared with the respective control groups (P<0.01;
Fig. 2C). Consistently, RT-qPCR
and western blot assays demonstrated significantly increased MMP2
and MMP9 expression following forskolin treatment in the two CC
cell lines compared with the corresponding controls (Fig. 2D and E).
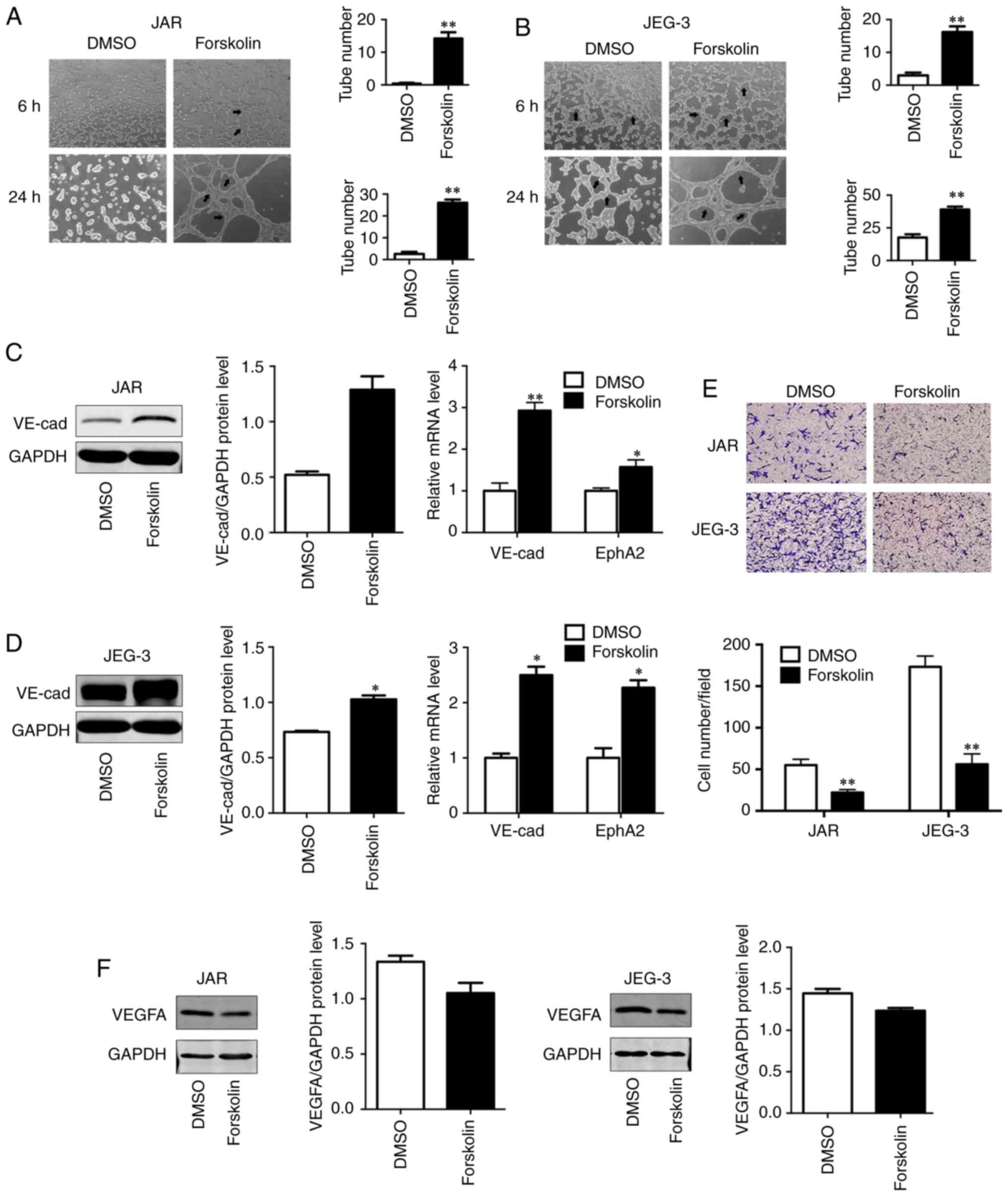
Forskolin promotes VM formation of CC
cells in vitro
VM has been demonstrated to be associated with cell
migration, invasion and capillary tube formation (6). In order to investigate the role of
forskolin in VM, the present study used a well-established in
vitro model of 3D culture. Compared with the control group, JAR
and JEG-3 cells treated with forskolin exhibited an enhanced
capability of forming typical capillary-like structures on 3D
Matrigel medium (P<0.01; Fig. 3A
and B), which appeared more typical in JEG-3. As VE-cad and
EphA2 act in a coordinated manner as key regulatory elements during
the process of VM (31), these two
VM-associated markers were detected via western blotting or
RT-qPCR; both VE-cad protein levels and EphA2 mRNA levels were
upregulated in the forskolin-treated group compared with the
control group (P<0.01; Fig. 3C;
P<0.05; Fig. 3D), suggesting
that forskolin was involved in the formation of vasculogenic-like
networks in CC cells. In addition, to reveal the potential roles of
forskolin in angiogenesis, an endothelial recruitment assay was
performed, which revealed a decreased ability of forskolin-treated
CC cells to recruit HUVECs compared with that of DMSO-treated cells
(P<0.01; Fig. 3E). In line with
these results, the expression of vascular endothelial growth factor
α (VEGFA), which serves a key role in tumor angiogenesis (32), was also effectively inhibited by
forskolin treatment (Fig. 3F).
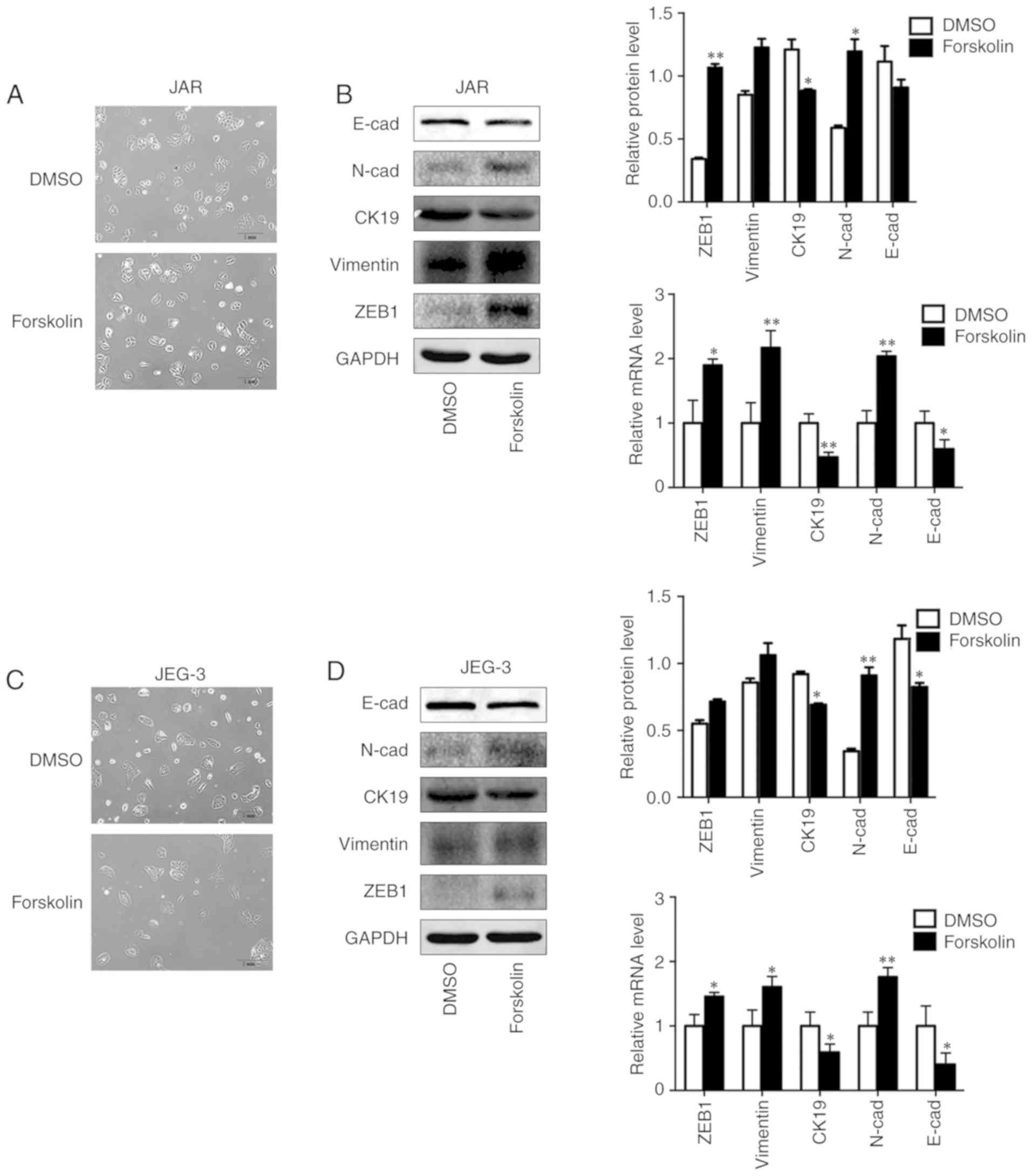
Forskolin induces EMT of CC cells in
vitro
In order to investigate the biological variation of
trophoblasts during syncytiolization induced by forskolin in CC
cells, the morphological changes and EMT markers were observed and
detected in the present study. The results demonstrated that the
cells were converted to a spindle-like shape and lost cellular
cohesiveness following administration of forskolin for 48 h
(Fig. 4A and C), and the
expression levels of epithelial markers E-cad and CK19 were
significantly downregulated, whereas those of mesenchymal markers,
including N-cad, Vimentin and ZEB1, were significantly upregulated
(Fig. 4B and D).
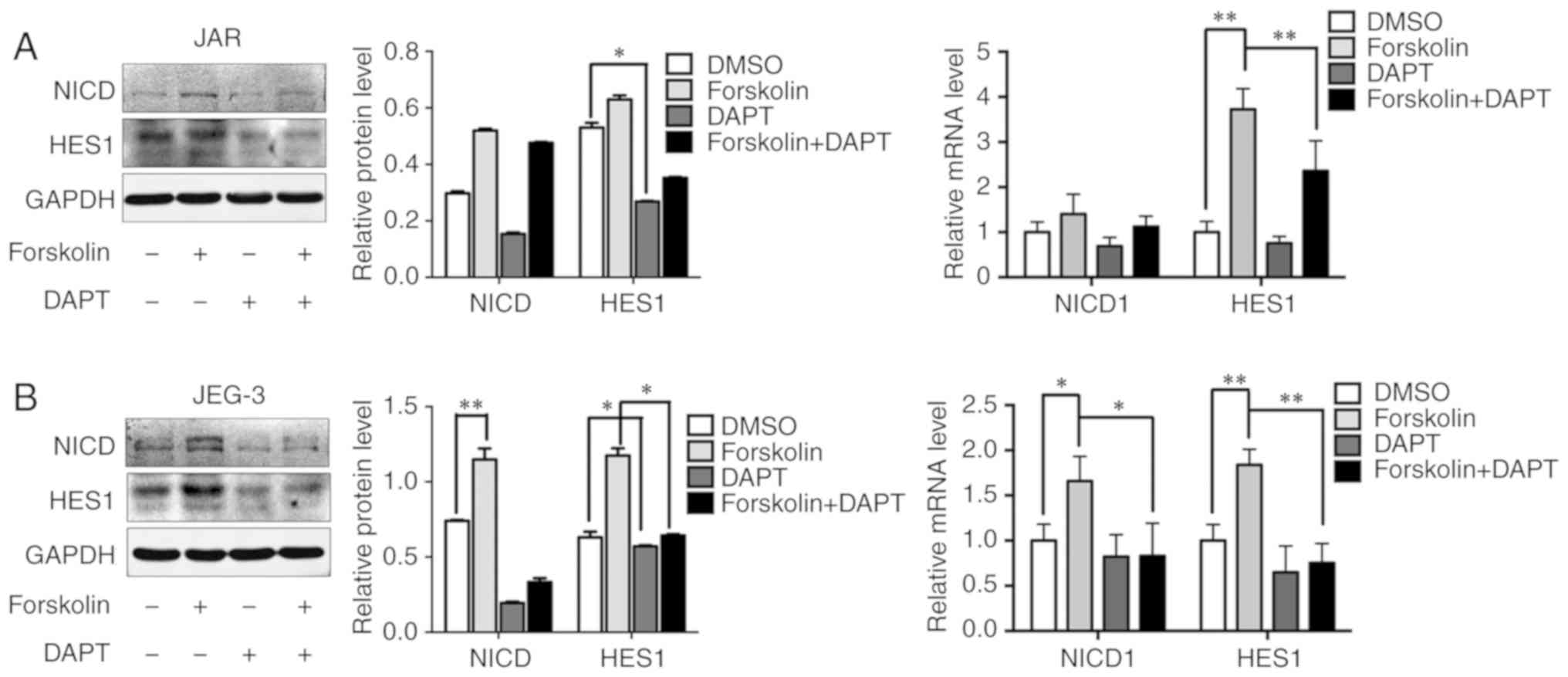
Notch-mediated EMT is activated in
forskolin-induced syncytiolization in vitro
A previous study has demonstrated that Notch-1
signaling is involved in gestational trophoblastic neoplasia
(26). Based on the result that
the expression levels of NICD and HES1 were upregulated after
forskolin treatment, 10 µM DAPT was added into the culture medium
to evaluate whether the Notch-1 signaling pathway was involved in
regulating metastatic and VM capabilities in CC cells (Fig. 5A and B). In addition, DAPT could
reverse the upregulating of NICD and HES. Consistently, inhibition
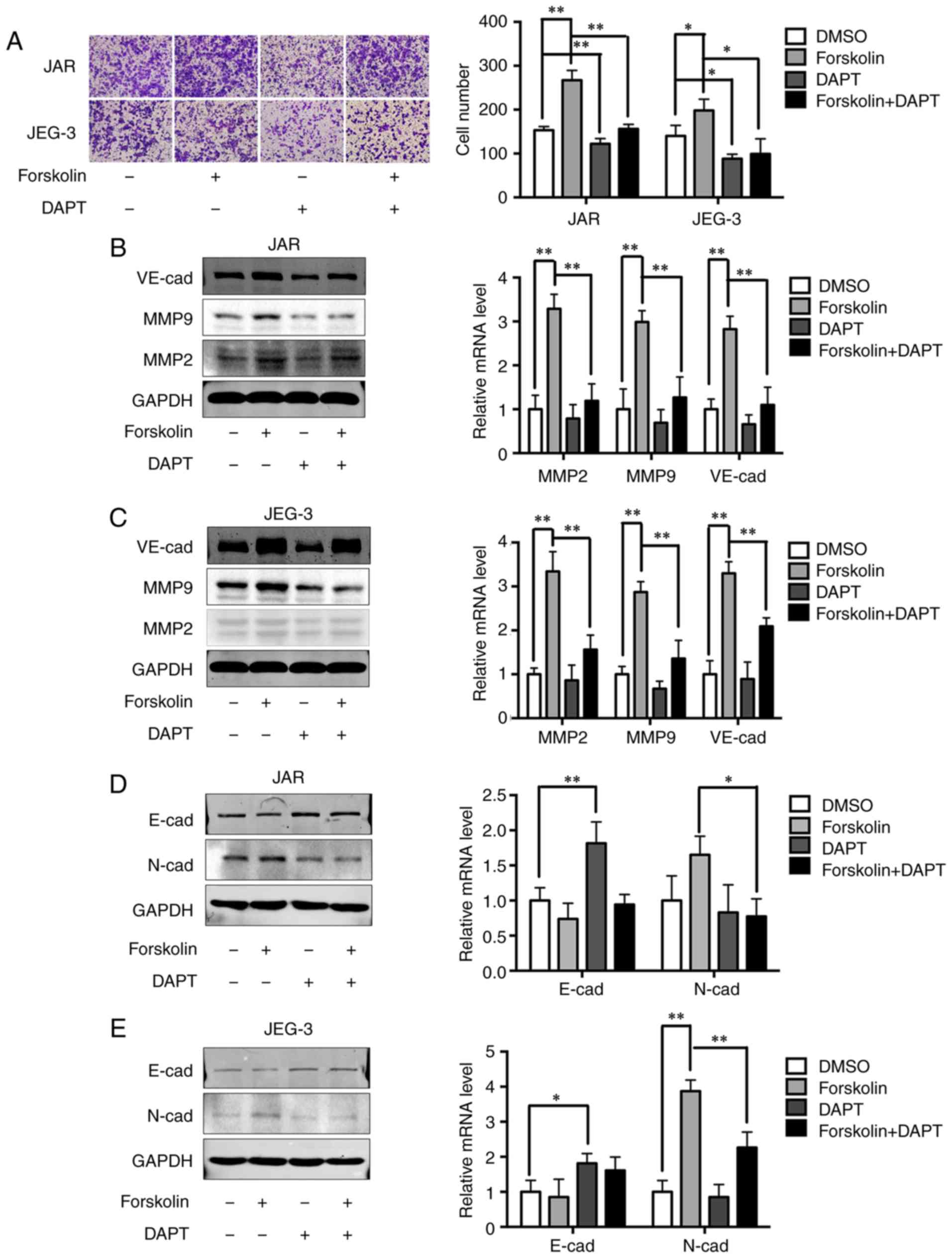
of the Notch-1 signaling pathway also significantly attenuated the
invasive ability of cells in the forskolin and DMSO groups compared
with cells without DAPT treatment (Fig. 6A). Upregulation of MMP2, MMP9 and
VE-cad expression levels by forskolin was also reversed by DAPT
(P<0.01; Fig. 6B and C).
Changes in EMT-associated proteins were also evaluated following
DAPT interference. Inhibition of the Notch-1 signaling pathway
partially reversed the downregulation of E-cad and significantly
reversed the upregulation of N-cad in forskolin-treated cells
(Fig. 6D and E), which indicated
that the Notch-1 signaling pathway may mediate EMT during the
syncytiolization process induced by forskolin.
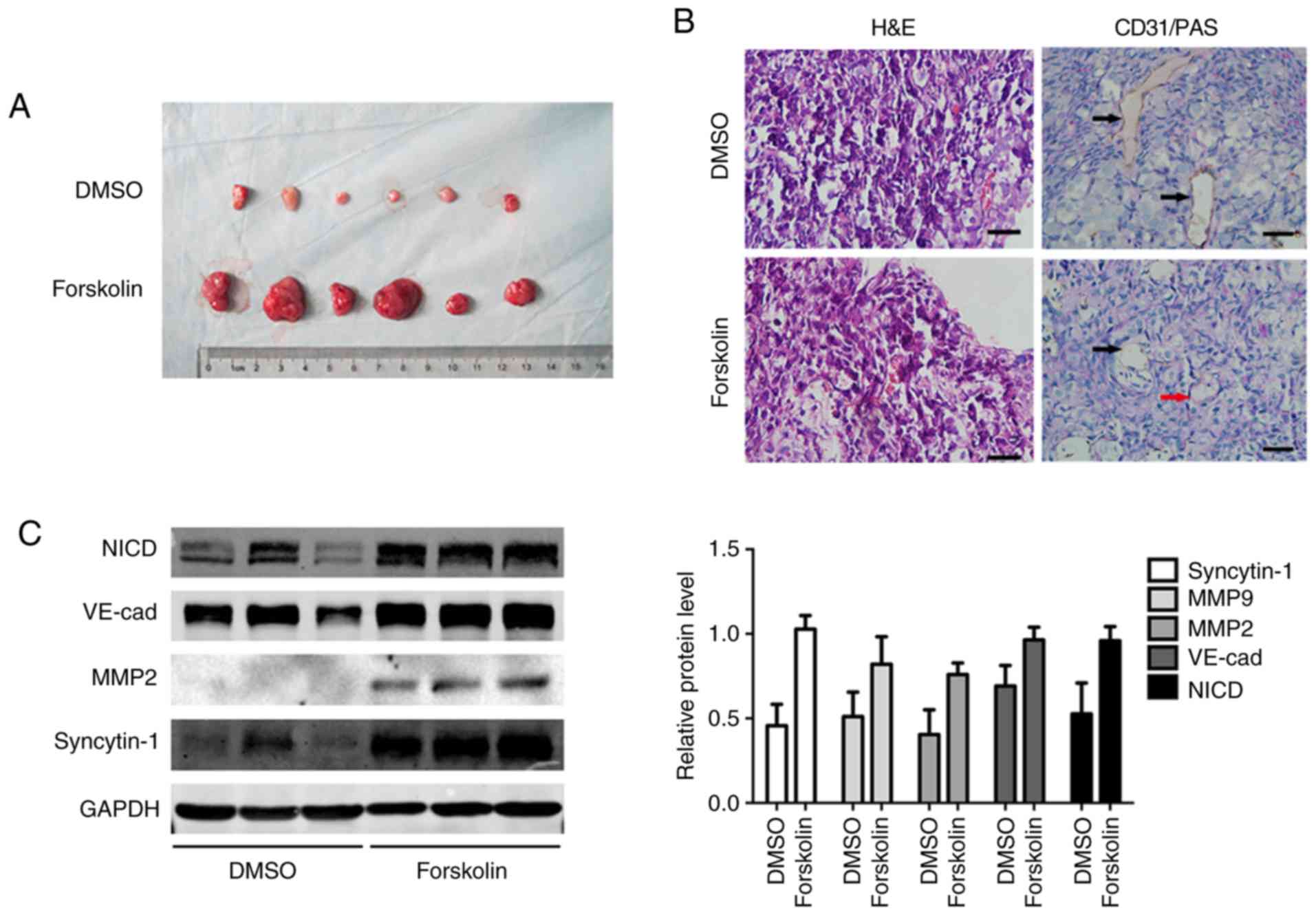
Forskolin promotes cell proliferation and
VM formation in vivo
In order to validate the function of forskolin in
vivo, JEG-3 cells were subcutaneously implanted into the flanks
of nude mice. After 3 weeks, the subcutaneous tumor diameter in the
forskolin group was larger compared with that in the DMSO group
(Fig. 7A). Tumor sections were
stained with CD31 to observe tumor angiogenesis by counting the
number of vessels; VM structures formed by tumor cells exhibited a
PAS-positive basement membrane (25). Endothelium-dependent vessels and VM
channels were observed in the excised tumors; PAS-positive tubular
structures lined with CD31-negative cells were indicated, and
intratumoral blood vessels were detected in all specimens (Fig. 7B). Consistent with the observation
in vitro, forskolin treatment resulted in high expression of
MMP2, MMP-9 and VE-cad, as well as the activation of the Notch-1
signaling pathway (Fig. 7C).
Discussion
CC is an aggressive solid tumor, which is
characterized by abundant blood supply and earlier blood metastasis
compared with other female genital tumors, and can develop a
vascular system containing fragile blood vessels (16). VM is a pattern of tumor
micro-circulation important for the growth and progression of solid
tumors (5-8). VM was initially reported in melanomas
and occasionally in other solid tumors, including breast, ovarian
and prostate cancer (5-8,32).
Channels in VM are similar to the embryonic microcirculatory
networks (33). The capacity of
STBs to perform endothelium-like functions, such as controlling
substrate exchange across the villous surface, has been well
established (14,34). A previous study of VM revealed that
tumor cells established their own pseudovascular networks by STBs
in clinical tissues from patients with CC (14). Our previous study demonstrated that
compared with normal villus, the level of syncytiolization in CC
tissues was higher and associated with the malignancy of
gestational trophoblastic disease (Supplementary Material).
However, little is currently known about the role of
syncyti-olization of cytotrophoblast cells in VM formation.
Forskolin, an activator of the cAMP pathway, is widely used as an
inducer of syncytiolization of trophoblasts; it elevates cAMP
levels and causes accumulation of VE-cad inside the cell-cell
junctions on HUVECs (35). VE-cad
and EphA2 act in a coordinated manner as a key element in the
process of VM (31). To assess
whether VM occurred in the process of syncytiolization and the
underlying mechanisms, CC cell lines JEG-3 and JAR were treated
with 100 µM forskolin for 48 h in the present study, and a positive
association between forskolin and VM was observed. Compared with
the control group cells treated with DMSO, forskolin not only
enhanced the migratory and invasive abilities of CC cells, but also
promoted VM formation and decreased tube formation detected by a 3D
system accompanied by increased VE-cad and EphA2 expression. The
behavior of trophoblasts is similar to aggressive tumors during
differentiation and implantation (36), and excessive fusion of
cytotrophoblasts to syncytiotrophoblasts leads to gestational
trophoblastic neoplasia, including CC (37,38).
Trophoblasts are highly invasive due to the secretion of
extracellular proteases, such as MMPs. MMP2 and MMP9 are key
enzymes associated with trophoblast invasion (39,40)
and malignancy (41,42). In the present study, increases in
MMP-2 and MMP-9 expression were observed in forskolin-treated cells
compared with the control cells, which was consistent with high
cell invasiveness.
Previously research has revealed that VM could be
observed in surgical specimens from patients with CC (14). In the present study, animal
xenograft tumor models also exhibited enhanced function of
proliferation and VM in vivo, and intratumoral blood vessels
were easily detected. This may have resulted in part from the
instability of forskolin concentration in local tumors in
vivo, and these results may explain why blood metastasis is
more easily observed in patients with CC. Forskolin can promote the
differentiation of CTB to STB and the formation of VM structure,
and it is reported that red blood cells are surrounded by STB in CC
cells without endothelial cells (14). In addition, the blood supply by VM
may not be as effective as intratumoral blood vessels, which may
explain why CC tissue is characterized by massive tumor necrosis
and hemorrhage.
EMT is a crucial process in cancer progression and
is closely associated with the remodeling of vascular endothe-lial
cells (43). Epithelial tumor
cells with the capacity of VM exhibit certain endothelial
phenotypes of mesenchymal cells, which are similar to the EMT
process (44). To comprehensively
understand the formation of VM mechanism during forskolin-induced
differentiation of trophoblasts, the present study evaluated the
switch of EMT. The results revealed that forskolin significantly
decreased the expression of epithelial markers and enhanced the
expression of mesenchymal markers, which was in agreement with the
results of a previous study (44).
These findings suggested that forskolin enhanced the formation of
VM channels via the induction of EMT.
Notch signaling influences trophoblastic
differentiation (45,46) and vascular development in several
types of human cancer, including CC (25). Notch-1 knockouts are lethal for the
embryo due to vascular and somatic defects (47), and activation of Notch can enhance
cell differentiation by upregu-lating anti-apoptotic genes
(48). The present study aimed to
investigate the signaling pathway involved in EMT during
forskolin-induced syncytiolization. Increased expression levels of
Notch-1 signaling markers were observed in CC cells treated with
forskolin, suggesting that this process was under the control of
the Notch signaling pathway. Since Notch-1 activation is dependent
on γ-secretase, the present study utilized DAPT to block the
activation of Notch-1 and assessed the NICD- and Notch-1-targeting
genes. HES1 was significantly decreased, along with the attenuation
in invasion and VM markers. The invasive ability and VM formation
was weakened and the process of EMT was reversed by DAPT. In
addition, our previous study also demonstrated that overexpression
of hypoxia-inducible factor 1α induced migration and invasion
through Notch signaling (26).
Therefore, the inhibitor of Notch signaling pathway maybe used to
block the syncytiolization of trophoblast cells indirectly,
although the side effects and exact functions need to be determined
in further research.
In conclusion, the present study revealed that
forskolin may act as a tumor promoter in CC through enhancing cell
metastasis and VM formation via the activation of Notch-mediated
EMT during syncytiolization. As forskolin induced CC
syncytiolization and increased human chorionic gonadotro-phin
secretion, a cell model stably expressing hCG may be established to
further study the crosstalk between Notch-1 signaling and hCG. The
results of the present study provided an additional rationale to
explain why massive hemorrhage and hematogenous metastasis commonly
occur in patients with CC, which suggested that inhibition of
syncytiolization ability of trophoblast cells may be an effective
way to inhibit tumor metastasis.
Supplementary Data
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81671491), the Shaanxi
Natural Science Basic Research Program-General Projects (grant no.
2017JM8055) and the Science Foundation of The First Affiliated
Hospital of Xi'an Jiaotong University (grant no. 2015YK8).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YX, RS and RA conceived and designed the study,
developed the methodology, wrote and reviewed the manuscript. YX,
RS, LY and WZ acquired, analyzed and interpreted the data. YX and
RA provided administrative, technical and material support and
supervised the study. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All animal experiments and the use of human tissue
samples were approved by Xi'an Jiaotong University Ethics
Committee. Animal experiments were in accordance with the
principles of Laboratory Animal Care and the Practice Guidelines
for Laboratory Animals of China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
The authors would like to thank Mr. XY Wang, Ms. S
Xu and Mr. K Wang (Oncology Research Lab, Key Laboratory of
Environment and Genes Related to Disease of the First Affiliated
Hospital of Xi'an Jiaotong University, Xi'an, China) for providing
technical support.
References
|
1
|
Stevens FT, Katzorke N, Tempfer C, Kreimer
U, Bizjak GI, Fleisch MC and Fehm TN: Gestational trophoblastic
disorders: An update in 2015. Geburtshilfe Frauenheilkd.
75:1043–1050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abu-Rustum NR, Yashar CM, Bean S, Bradley
K, Campos SM, Chon HS, Chu C, Cohn D, Crispens MA, Damast S, et al:
Gestational trophoblastic neoplasia, version 2.2019, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
17:1374–1391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alazzam M, Tidy J, Osborne R, Coleman R,
Hancock BW and Lawrie TA: Chemotherapy for resistant or recurrent
gestational trophoblastic neoplasia. Cochrane Database Syst Rev.
13:CD0088912012.
|
|
4
|
Viallard C and Larrivee B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao N, Sun H, Sun B, Zhu D, Zhao X, Wang
Y, Gu Q, Dong X, Liu F, Zhang Y and Li X: miR-27a-3p suppresses
tumor metastasis and VM by down-regulating VE-cadherin expression
and inhibiting EMT: An essential role for Twist-1 in HCC. Sci Rep.
6:230912016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang D, Sun B, Zhao X, Ma Y, Ji R, Gu Q,
Dong X, Li J, Liu F, Jia X, et al: Twist1 expression induced by
sunitinib accelerates tumor cell vasculogenic mimicry by increasing
the population of CD133+ cells in triple-negative breast cancer.
Mol Cancer. 13:2072014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su M, Fan C, Gao S, Shen A, Wang X and
Zhang Y: An HCG-rich microenvironment contributes to ovarian cancer
cell differentiation into endothelioid cells in a three-dimensional
culture system. Oncol Rep. 34:2395–2402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Qiao L, Liang N, Xie J, Luo H,
Deng G and Zhang J: Vasculogenic mimicry and tumor metastasis. J
BUON. 21:533–541. 2016.PubMed/NCBI
|
|
10
|
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu
D, Yu X and Tian Y: Advanced research on vasculogenic mimicry in
cancer. J Cell Mol Med. 19:315–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okae H, Toh H, Sato T, Hiura H, Takahashi
S, Shirane K, Kabayama Y, Suyama M, Sasaki H and Arima T:
Derivation of human trophoblast stem cells. Cell Stem Cell.
22:50–63. 2018. View Article : Google Scholar
|
|
12
|
Hendrix MJ, Seftor EA, Hess AR and Seftor
RE: Vasculogenic mimicry and tumour-cell plasticity: Lessons from
melanoma. Nat Rev Cancer. 3:411–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Red-Horse K, Zhou Y, Genhacev O,
Prakobphol A, Foulk R, McMaster M and Fisher SJ: Trophoblast
differentiation during embryo implantation and formation of the
maternal-fetal interface. J Clin Invest. 114:744–754. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih IeM: Trophoblastic vasculogenic
mimicry in gestational choriocarcinoma. Mod Pathol. 24:646–652.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keryer G, Alsat E, Tasken K and
Evain-Brion D: Cyclic AMP-dependent protein kinases and human
trophoblast cell differentiation in vitro. J Cell Sci.
111:995–1004. 1998.PubMed/NCBI
|
|
16
|
Yurugi-Kobayashi T, Itoh H, Schroeder T,
Nakano A, Narazaki G, Kita F, Yanagi K, Hiraoka-Kanie M, Inoue E,
Ara T, et al: Adrenomedullin/cyclic AMP pathway induces Notch
activation and differentiation of arterial endothelial cells from
vascular progenitors. Arterioscler Thromb Vasc Biol. 26:1977–1984.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto T, Matsumoto K, Kurachi H,
Okamoto Y, Nishio Y, Sakata M, Tasaka K and Murata Y: Progesterone
inhibits transcriptional activation of human chorionic
gonadotropin-alpha gene through protein kinase A pathway in
trophoblast cells. Mol Cell Endocrinol. 182:215–224. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belkacemi L, Beall MH, Magee TR,
Pourtemour M and Ross MG: AQP1 gene expression is upregulated by
arginine vasopressin and cyclic AMP agonists in trophoblast cells.
Life Sci. 82:1272–1280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Staun-Ram E, Goldman S and Shalev E: Ets-2
and p53 mediate cAMP-induced MMP-2 expression, activity and
trophoblast invasion. Reprod Biol Endocrinol. 7:1352009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesen-chymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW,
Chen N, Wang XH, Du J, Liu YX and Sun BC: Expression and functional
significance of Twist1 in hepatocellular carcinoma: Its role in
vasculogenic mimicry. Hepatology. 51:545–556. 2010. View Article : Google Scholar
|
|
23
|
Liu Z, Sun B, Qi L, Li H, Gao J and Leng
X: Zinc finger E-box binding homeobox 1 promotes vasculogenic
mimicry in colorectal cancer through induction of
epithelial-to-mesen-chymal transition. Cancer Sci. 103:813–820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shawber CJ and Kitajewski J: Notch
function in the vasculature: Insights from zebrafish, mouse and
man. Bioessays. 26:225–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vartanian A, Gatsina G, Grigorieva I,
Solomko E, Dombrovsky V, Baryshnikov A and Stepanova E: The
involvement of Notch signaling in melanoma vasculogenic mimicry.
Clin Exp Med. 13:201–209. 2013. View Article : Google Scholar
|
|
26
|
Tian Q, Xue Y, Zheng W, Sun R, Ji W, Wang
X and An R: Overexpression of hypoxia-inducible factor 1α induces
migration and invasion through Notch signaling. Int J Oncol.
47:728–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang J, Wang J, Fan L, Li X, Liu N, Luo W,
Wang J and Wang Y and Wang Y: cRGD inhibits vasculogenic mimicry
formation by down-regulating uPA expression and reducing EMT in
ovarian cancer. Oncotarget. 7:24050–24062. 2016.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Grummer R, Donner A and Winterhager E:
Characteristic growth of human choriocarcinoma xenografts in nude
mice. Placenta. 20:547–553. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Follin-Arbelet V, Hofgaard PO, Hauglin H,
Naderi S, Sundan A, Blomhoff R, Bogen B and Blomhoff HK: Cyclic AMP
induces apoptosis in multiple myeloma cells and inhibits tumor
development in a mouse myeloma model. BMC Cancer. 11:3012011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hess AR, Seftor EA, Gruman LM, Kinch MS,
Seftor RE and Hendrix MJ: VE-cadherin regulates EphA2 in aggressive
melanoma cells through a novel signaling pathway: Implications for
vasculogenic mimicry. Cancer Biol Ther. 5:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paulis YW, Soetekouw PM, Verheul HM,
Tjan-Heijnen VC and Griffioen AW: Signalling pathways in
vasculogenic mimicry. Biochim Biophys Acta. 1806:18–28.
2010.PubMed/NCBI
|
|
33
|
Cross JC, Hemberger M, Lu Y, Nozaki T,
Whiteley K, Masutani M and Adamson SL: Trophoblast functions,
angiogenesis and remodeling of the maternal vasculature in the
placenta. Mol Cell Endocrinol. 187:207–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kojima T, Katsumi A, Yamazaki T, Muramatsu
T, Nagasaka T, Ohsumi K and Saito H: Human ryudocan from
endothelium-like cells binds basic fibroblast growth factor,
midkine, and tissue factor pathway inhibitor. J Biol Chem.
271:5914–5920. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beese M, Wyss K, Haubitz M and Kirsch T:
Effect of cAMP deri-vates on assembly and maintenance of tight
junctions in human umbilical vein endothelial cells. BMC Cell Biol.
11:682010. View Article : Google Scholar
|
|
36
|
Staun-Ram E and Shalev E: Human
trophoblast function during the implantation process. Reprod Biol
Endocrinol. 3:562005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Langbein M, Strick R, Strissel PL, Vogt N,
Parsch H, Beckmann MW and Schild RL: Impaired cytotrophoblast
cell-cell fusion is associated with reduced Syncytin and increased
apoptosis in patients with placental dysfunction. Mol Reprod Dev.
75:175–183. 2008. View Article : Google Scholar
|
|
38
|
Boize PA, Patrier S, Cheynet V, Oriol G,
Massardier J, Hajri T, Guillotte M, Bossus M, Sanlaville D, Golfier
F and Mallet F: Expression patterns of ERVWE1/Syncytin-1 and other
placentally expressed human endogenous retroviruses along the
malignant transformation process of hydatidiform moles. Placenta.
39:116–124. 2016. View Article : Google Scholar
|
|
39
|
Staun-Ram E, Goldman S, Gabarin D and
Shalev E: Expression and importance of matrix metalloproteinase 2
and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol
Endocrinol. 2:592004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cohen M, Ribaux P, Epiney M and Irion O:
Expression of metal-loproteinases 1, 2, 7, 9, and 12 in human
cytotrophoblastic cells from normal and preeclamptic placentas.
Neuro Endocrinol Lett. 33:406–411. 2012.
|
|
41
|
Bischof P, Martelli M, Campana A, Itoh Y,
Ogata Y and Nagase H: Importance of matrix metalloproteinases in
human trophoblast invasion. Early Pregnancy. 1:263–269.
1995.PubMed/NCBI
|
|
42
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L and Li W: Epithelial-mesenchymal
transition in human cancer: Comprehensive reprogramming of
metabolism, epigenetics, and differentiation. Pharmacol Ther.
150:33–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fan YL, Zheng M, Tang YL and Liang XH: A
new perspective of vasculogenic mimicry: EMT and cancer stem cells
(Review). Oncol Lett. 6:1174–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cuman C, Menkhorst E, Winship A, Van
Sinderen M, Osianlis T, Rombauts LJ and Dimitriadis E:
Fetal-maternal communication: The role of Notch signalling in
embryo implantation. Reproduction. 147:R75–R86. 2014. View Article : Google Scholar
|
|
46
|
Haider S, Pollheimer J and Knofler M:
Notch signaling in placental development and gestational disease.
Placenta. 56:65–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huppert SS, Le A, Schroeter EH, Mumm JS,
Saxena MT, Milner LA and Kopan R: Embryonic lethality in mice
homozygous for a processing-deficient allele of Notch1. Nature.
405:966–970. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jasinska A, Strakova Z, Szmidt M and
Fazleabas AT: Human chorionic gonadotropin and decidualization in
vitro inhibits cytochalasin-D-induced apoptosis in cultured
endometrial stromal fibroblasts. Endocrinology. 147:4112–4121.
2006. View Article : Google Scholar : PubMed/NCBI
|