Introduction
Multicellular tumor spheroids (MTSs) have been
established as an in vitro model for the systematic study of
tumor responses to therapy (1,2).
They are generally considered to be improved models when compared
with two-dimensional cell cultures for predicting in vivo
responses to drug treatment (3).
The MTS system has been widely used as a model to study
microenvironmental effects on basic biological mechanisms in cancer
research, such as proliferation, differentiation, cell death,
invasion and metastasis (4,5).
As the three-dimensional organization of cancer
cells in spheroids is different from the cellular organization of
cancer cells in monolayer cultures, a previous study has suggested
that the expression of cellular adhesion molecules (CAMs) known to
be responsible for cell-cell and cell-matrix interactions is
altered in MTSs compared with that in monolayer cultures (6). Given that alteration of CAM
expression is associated with invasion and metastasis (7), biological behaviors of cells in MTSs
may be different from those of cells grown in monolayer cultures,
particularly cellular migration and invasion.
In the early phase of tumor progression,
epithelial-mesenchymal transition (EMT) is induced by loss of
E-cadherin, a cell-cell adhesion molecule and epithelial marker,
with disaggregation of cancer cells from one another (8). However, previous studies have
revealed that loss of E-cadherin itself is not enough to induce EMT
or mediate EMT signaling functions that may induce cancer cells to
complete later steps of tumor progression more rapidly (9-12).
In addition, E-cadherin-mediated cell-cell adhesion is regulated by
downstream signaling of integrin-extracellular matrix (ECM)
adhesion (13). This crosstalk
between these two adhesions can modulate cellular motility
(14). Thus, regulation of EMT by
E-cadherin loss might be associated with the aberrated production
of integrin-mediated adhesion, which is closely related to the EMT
signaling molecules Snail or integrin-linked kinase (ILK) (15-17).
In the current study, it was hypothesized that
altered expression of adhesion molecules in MTSs might affect the
motility of spheroid-derived cells. To test this hypothesis, the
expression patterns of CAMs, E-cadherin and β1 integrin, and EMT
signaling markers were investigated in anaplastic thyroid carcinoma
(ATC) cells grown as monolayer cells or MTSs, and in E-cadherin
blocked cells. In addition, cell motility was monitored using
real-time phase-contrast imaging.
Materials and methods
Cell culture conditions
The ATC cell line, FRO, was generously gifted by Dr
Young Suk Jo (Yonsei University, Seoul, Korea) and showed a
negative mycoplasma result when tested using a Mycoplasma Detection
kit (Lonza Group, Ltd.). For monolayer culture, cells were grown in
RPMI-1640 medium (Welgene, Inc.) supplemented with L-glutamine, 100
U/ml penicillin-100 μg/ml streptomycin and 10%
heat-inactivated fetal bovine serum (Young In Frontier Co., Ltd.).
When cells reached 80-90% confluency, they were trypsinized and
subcul-tured to maintain the cell line. For experiments,
1x105 cells were cultured in six-well plates and
harvested on the designated day: Monolayer day (MLD) 2-5.
For spheroid cultures, 1×104 cells/well
in 200 μl RPMI-1640 medium were plated onto an
ultra-low-attachment-surface 96-well round-bottom plate (Corning
Inc.) and centrifuged at 440 × g for 3 min at room temperature
(RT). These spheroids were incubated at 37°C with 5% CO2
for up to 6 days: Spheroid day (SPD) 1-6. After plating, 100
μl medium was replaced with 100 μl fresh medium every
other day. Spheroids were examined every day with a Motic AE31
inverted microscope. They were harvested on SPD 2, 4 and 6.
In order to block E-cadherin, treatment with an
E-cadherin neutralizing antibody (DECMA, sc-59778, mouse monoclonal
anti-human; 5 μg/ml; Santa Cruz Biotechnology, Inc.) was
conducted for 24 h at 37°C in monolayer and spheroid cultures,
respectively.
Western blotting
For western blot analysis, monolayer cells or
spheroids were extracted with 5X Laemmli sample buffer and 5%
β-mercaptoethanol by boiling for 10 min. After determining
the protein concentration using bicinchoninic acid assay, the same
amount (20 μg/well) of the extracts were separated on 8 or
10% SDS-polyacrylamide gels (as specified for each antibody below)
and transferred to nitrocellulose membranes. Non-specific binding
sites on the membranes were blocked with skimmed milk for 90 min at
RT. Membranes were then blotted with primary antibody against
E-cadherin (rabbit polyclonal anti-human; sc-7870, 1:1,000, 8%;
Santa Cruz Biotechnology, Inc.), zonula accludens-1 (ZO-1; rabbit
polyclonal anti-human; cat. no. 61-7300, 1:1,000, 8%; Invitrogen;
Thermo Fisher Scientific, Inc.), occludin (rabbit polyclonal
anti-human; cat. no. 71-1500, 1:1,000, 10%; Invitrogen; Thermo
Fisher Scientific, Inc.), β1 integrin (rabbit polyclonal
anti-human; 4706S, 1:1,000, 8%; Cell Signaling Technology, Inc.),
ILK (rabbit polyclonal anti-human; PA5-27484; 1:1,000, 10%,
Invitrogen; Thermo Fisher Scientific, Inc.), Snail (rabbit
polyclonal anti-human; 3879S, 1:1,000, 10%; Cell Signaling
Technology, Inc.), paxillin (mouse monoclonal anti-human;
sc-365059, 1:1,000, 10%; Santa Cruz Biotechnology, Inc.) or focal
adhesion kinase (FAK; rabbit polyclonal anti-human; 3285S, 1:1,000,
8%; Cell Signaling Technology, Inc.) at 4°C overnight followed by
incubation with secondary antibody (peroxidase conjugated goat
anti-mouse, 115-036-003, 1:5,000; peroxidase conjugated goat
anti-rabbit, 711-036-152, 1:5,000; Jackson ImmunoResearch
Laboratories Inc.) for 6 h at 4°C. Blots were visualized using a
chemiluminescence kit (Santa Cruz Biotechnology, Inc.).
Chemiluminescence was captured with a ChemiDoc system (Bio-Rad
Laboratories, Inc.). Mouse monoclonal antibody to glyceraldehyde
3-phosphate dehydrogenase (GAPDH; mouse monoclonal anti-human;
sc-47724, 1:2,000, 10%; Santa Cruz Biotechnology, Inc.) was used to
detect GAPDH, which served as a loading control. The density of
each band was analyzed using ImageJ software (version 1.51i;
National Institutes of Health).
Reverse transcription-quantitative
polymerase chain reaction
Monolayer cells or spheroids were resuspended with
QIAzol lysis reagent (Qiagen, Inc.) to extract total RNAs by serial
treatments with chloroform (Sigma-Aldrich; Merck KGaA), isopropanol
(Duksan Pure Chemicals Co., Ltd.) and 75% ethanol in diethyl
pyrocarbonate water. mRNAs were then transcribed into cDNAs using a
cDNA Synthesis Master mix (amfiRivert cDNA synthesis Platinum
Master mix; GenDEPOT) with the following temperature protocol: 5
min at 25°C, 50 min at 42°C and 15 min at 70°C). The prepared cDNA
was amplified and quantified with a real time thermal cycler system
(TP800/TP860; Takara Bio, Inc.) using SYBR Green Master mix
(Qiagen, Inc.) with the following primers: E-cadherin, forward:
5′-TGC TCT TGC TGT TTC TTC GG-3′ and reverse: 5′-TGC CCC ATT CGT
TCA AGT AG-3′; β1 integrin, forward: 5′-TTC GAT GCC ATC ATG CAA GTT
G-3′ and reverse: 5′-CCA TCT CCA GCA AAG TGA AAC C-3′; and GAPDH,
forward: 5′-GAG TCA ACG GAT TTG GTC GT-3′ and reverse: 5′-TTG ATT
TTG GAG GGA TCT CG-3′. The thermal cycling conditions were 15 sec
at 95°C and 45 sec at 60°C. Data were analyzed using the
2-ΔΔCq method (18).
Immunocytochemistry
Spheroids were harvested from 96-well plates on days
SPD1, 3 and 6, and then fixed with 4% paraformaldehyde overnight at
RT. Fixed spheroids were then treated with ethanol and isopropanol
and embedded in paraffin blocks for tissue sectioning. After
deparaffinization and rehydration in descending grades of ethyl
alcohol, 5-μm sections were quenched with 0.3% hydrogen
peroxide in methanol for 30 min at RT. Non-specific sites were
blocked with 3% normal goat serum (Vector Laboratories, Inc.) for
30 min at RT. Slides were incubated with primary antibody to
E-cadherin (sc-7870, 1:50; Santa Cruz Biotechnology) overnight at
4°C. The next day, slides were treated with goat biotinylated
anti-rabbit IgG (H+L) secondary antibody (PK4001, 1:200; Vector
Laboratories Inc.) for 1 h at RT. Antigen-antibody complexes were
detected using an avidin-biotin complex detection system
(Vectastain ABC kit; Vector Laboratories, Inc.). Slides were
stained with DAB substrate kit (Vector Laboratories, Inc.), rinsed
in water, briefly counterstained with hematoxylin at RT for 10 sec,
and washed in water. After mounting, slides were examined under an
Olympus BX51 light microscope (magnification, x100). Pictures were
captured and controlled using Olympus DP72 and DP2-BSW software
(version 2.2).
Live cell time-lapse imaging and data
processing
To capture live cell time-lapse images to assess
cell motility, single cells dispersed from FRO spheroids on SPD2
and from monolayers of FRO cells on MLD3 were used. Monolayer cells
treated with DECMA (5 μg/ml) were also monitored. A culture
dish containing these cells was placed in a temperature (37°)- and
CO2 (5%)-regulated live-cell chamber mounted onto the
stage of an inverted microscope (IX71; Olympus) with an objective
lens (×20; numerical aperture 0.40). Time-lapse live cell images of
single cells were captured at 1-min intervals for 2 days using a
cooled CCD camera (ProgRes® MFcool; Jenoptic) with a
spatial resolution of ~0.5 mm/pixel. To trace the trajectory of a
crawling single cell, acquired images were analyzed using ImageJ
software (version 1.51i). The centroid of the cell body was
calculated for each frame. The sequence of centroid positions (x,
y) was converted into speed and displacement. Data from 10 cells
are expressed as mean ± SD.
Blocking of E-cadherin and spheroid
spreading assay
As mentioned above, DECMA (5 μg/ml) was used
to block E-cadherin for 24 h in spheroid culture. To determine the
effect of DECMA on the motility of MTSs, spheroids at day 2 were
transferred to an adhesive plate and the spreading ability was
monitored for 2 days. The area of the spheroid was measured using
ImageJ software (version 1.51i).
Statistical analysis
All statistical analyses were carried out using SPSS
for Windows (version 16.0.0; SPSS, Inc.). Data in graphs are
expressed as the mean ± SD of at least three independent
determinations. Differences between two samples were determined
using Mann-Whitney U-test. Differences with P<0.05 were
considered significant.
Results
Morphological examination and expression
of CAMs during spheroid formation by ATC cells
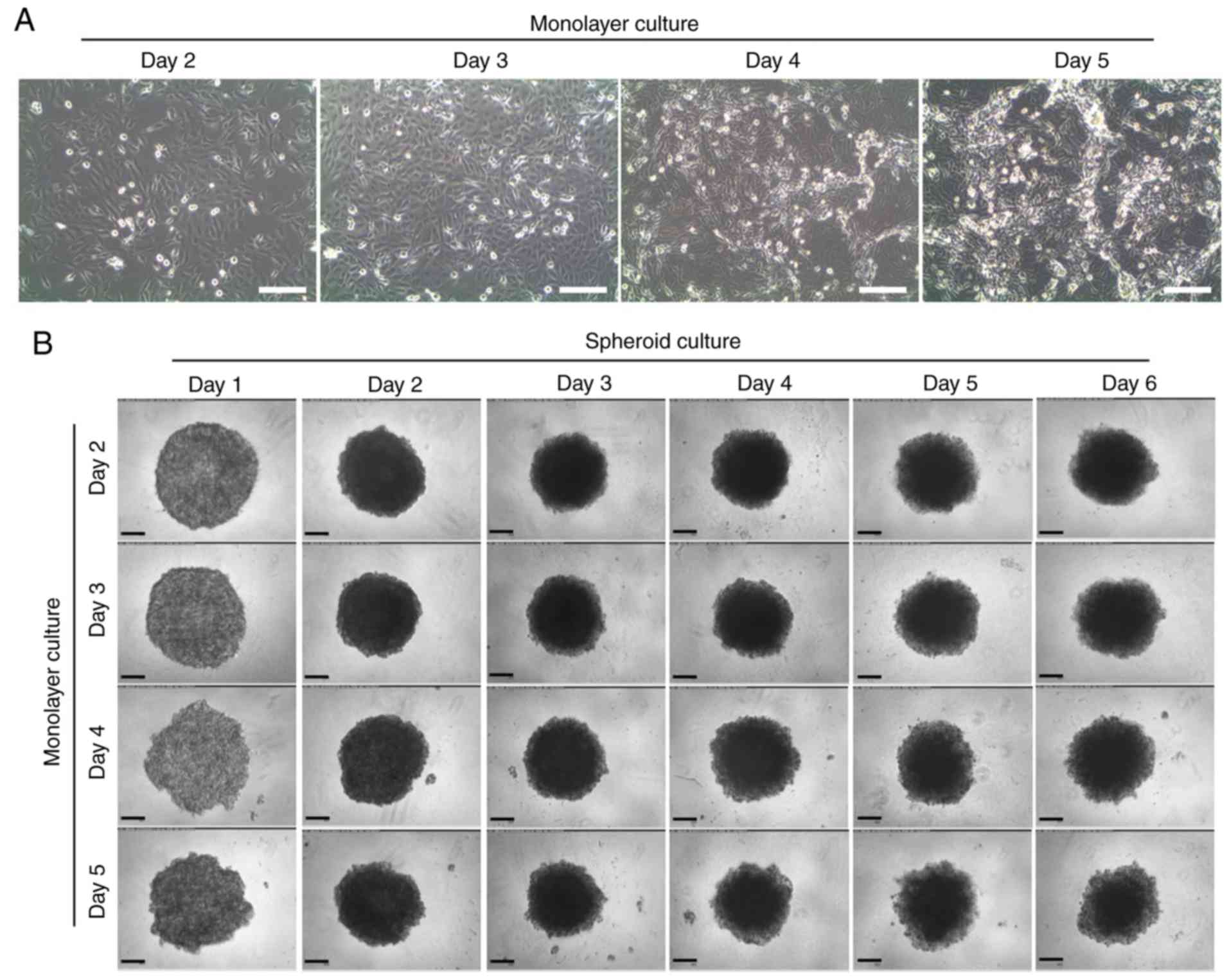
To understand the effect of the confluency of
monolayer cells on the formation of spheroids, MTSs were formed
from monolayer cells with different confluency. The MTSs were
packed tightly at SPD2 and sustained up to SPD6. When an MTS was
formed using MLD3 cells, which had been cultured for 3 days after
seeding single cells to culture plates, it maintained a relatively
uniform and round morphology. However, MTSs formed from monolayer
cells with higher confluency (MLD4 and MLD5) exhibited a rough
surface in the early stage of MTS formation, and the outer cells
were loosened easily (Fig. 1).
Expression levels of CAMs in monolayer cells
increased when cells were grown to confluency (Fig. 2A; MLD2-5). The expression of
E-cadherin was observed to increase in the early spheroid-forming
period (Fig. 2A and B; SPD2 and
SPD4) but decrease during late spheroid formation (Fig. 2A and B; SPD6) regardless of
monolayer cell confluency, indicating that a certain amount of
E-cadherin was required to form a spheroid. Notably, the
distribution of E-cadherin was dynamic as the spheroid formed. It
was distributed more centrally in the early period but was
distributed throughout the spheroid in the late period (Fig. 2C). However, the expression levels
of ZO-1 and occludin decreased sharply when spheroid formation
began (Fig. 2A).
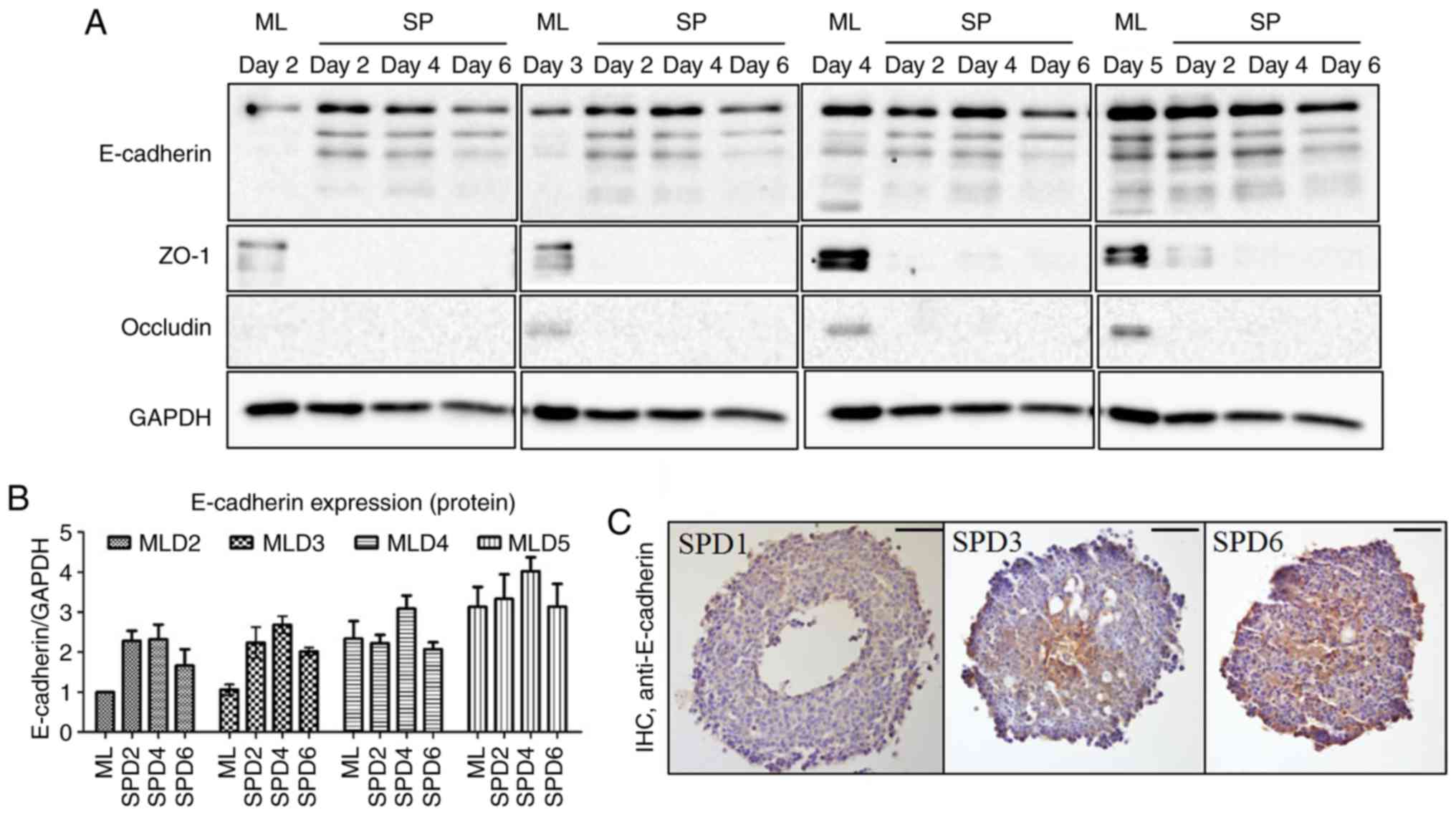 | Figure 2Expression of cell adhesion molecules
in MTS formation. (A and B) Expression levels of the cell-cell
adhesion molecules E-cadherin and ZO-1, and occludin were examined
by western blotting. (A) The spheroids were made from day 2 to day
5 monolayered cells. The expression of E-cadherin was sustained
until day 6 in MTSs, unlike that of the other proteins tested. (B)
Quantification of the western blotting results. (C)
Immunocytochemistry revealed that E-cadherin was expressed at the
center of the MTS on day 3, and then evenly across the entire MTS
on day 6. ML, monolayer; SP, spheroid; D, day; MLD, monolayer day;
SPD, spheroid day; MTS, multicellular tumor spheroid; ZO-1, zonula
accludens-1. Scale bar, 100 μm. |
Motility of dispersed cells from ATC
spheroids is higher than that of dispersed cells from cell
monolayers
To evaluate changes in cellular motility after MTS
formation, cells dispersed from monolayer cells and spheroids on
SPD2 were cultured as single cells and monitored by time-lapse
image capture for 2 days. Microscopic morphology analysis showed
that dispersed cells from MTSs were longer and thinner than those
from monolayer cells (Fig. 3A;
Videos S1-S4). The speed and
displacement of the dispersed cells from MTSs increased
significantly during the first day (D1) compared to those of
monolayer cells. However, on the second day (D2), the speed and
displacement of the dispersed cells from MTSs were not markedly
different from those on D1; nor was there any difference between
dispersed cells from MTSs and monolayer single cells on D2
(Fig. 3B and C).
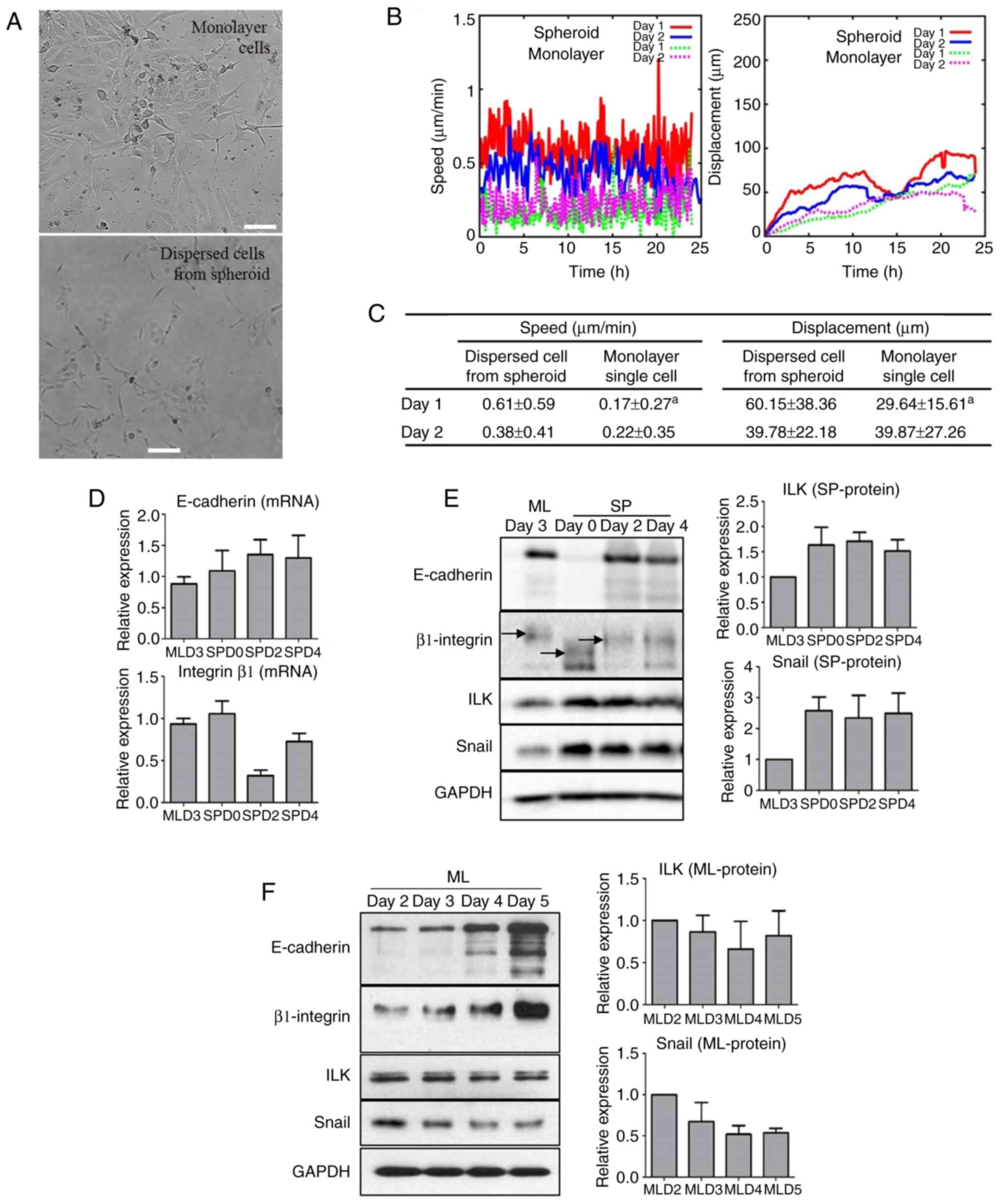 | Figure 3Increased motility of MTS cells with
increased E-cadherin and EMT signaling molecules. (A) Images of
single cells dispersed from cell monolayers and MTSs and cultured
for 48 h. (B) The speed and displacement of the dispersed cells
were monitored (D1, 0-24 h; D2, 24-48 h) and compared statistically
by analyzing 10 cells from each group. (C) The speed and
displacement of dispersed MTS cells were increased more than those
of dispersed monolayer cells after 24 h. aP<0.05 vs.
dispersed cell from spheroid on the same day (Mann-Whitney U test).
(D) Transcriptional expression of E-cadherin was increased in MTS
cells but that of β1 integrin was variable. (E) During MTS
formation, the molecular weight of β1 integrin changed and
expression levels of EMT signaling molecules increased. (F) Protein
levels of cell adhesion molecules increased in monolayered cells at
day 2 to day 5 while those of EMT signaling molecules decreased.
MTS, multicellular tumor spheroid; EMT, epithelial-mesenchymal
transition; ML, monolayer; SP, spheroid; D, day; MLD, monolayer
day; SPD, spheroid day. Scale bar, 50 μm. |
Expression of EMT molecules in MTS
formation is different from that in monolayer cells
When the expression of EMT molecules was evaluated
during spheroid formation, the transcriptional expression of
E-cadherin increased while the protein level of E-cadherin markedly
decreased at SPD0 but then increased to a value comparable with
that in the monolayer at SPD2. Regarding the expression of β1
integrin, a complicated aspect was observed during spheroid
formation. Although the expression of β1 integrin increased when
spheroid formation began, its molecular weight (MW) critically
decreased. As spheroid formation proceeded, the expression of β1
integrin decreased and the reduction in the MW of β1 integrin was
sustained. Interestingly, the expression levels of ILK and Snail
increased with spheroid formation, which was not associated with
the expression level of β1 integrin but did appear to be associated
with the MW of β1 integrin (Fig. 3D
and E). By contrast, in monolayer FRO cell culture, the
expression levels of the CAMs E-cadherin and β1 integrin increased,
whereas those of the EMT molecules ILK and Snail decreased with
increasing confluency (Fig.
3F).
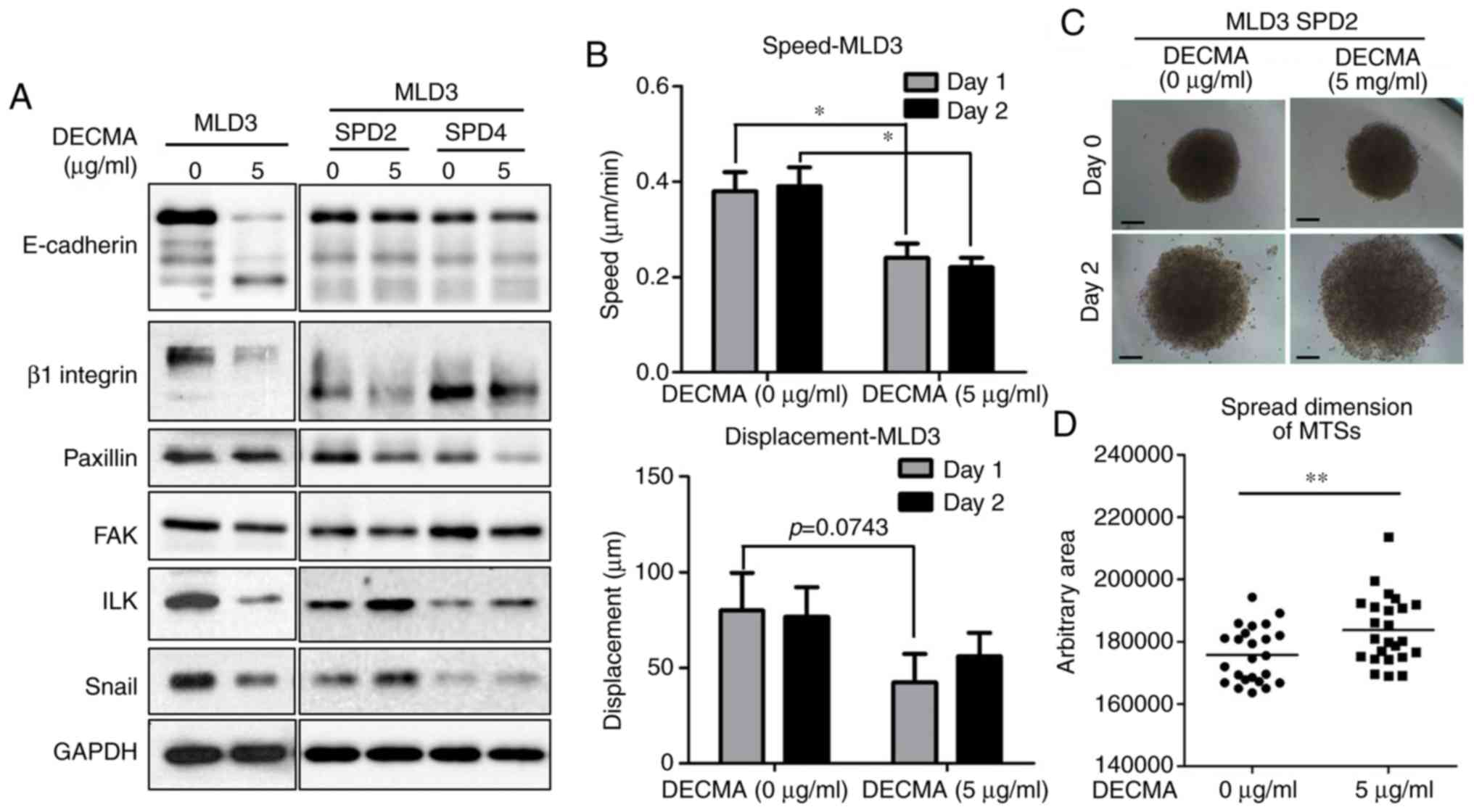
Altered expression of CAMs and EMT
molecules by blocking E-cadherin in MTSs enhances cell
motility
When the E-cadherin neutralizing antibody DECMA was
used to treat monolayers or MTSs derived from FRO cells, the
expression levels of E-cadherin and β1 integrin decreased in both
culture conditions. In addition, the EMT markers paxillin and FAK
were expressed at lower levels in DECMA-treated cells. However, the
expression levels of EMT signaling molecules ILK and Snail
decreased in monolayer cells but increased in MTSs (Fig. 4A). To validate the different
reactions to E-cadherin neutralizing antibody between monolayer
cells and MTSs, motility assays were performed. E-cadherin-blocked
monolayer cells moved more slowly than non-treated mono-layer
cells, whereas DECMA-treated MTS cells migrated further than
non-treated MTS cells (Fig.
4B-D).
Discussion
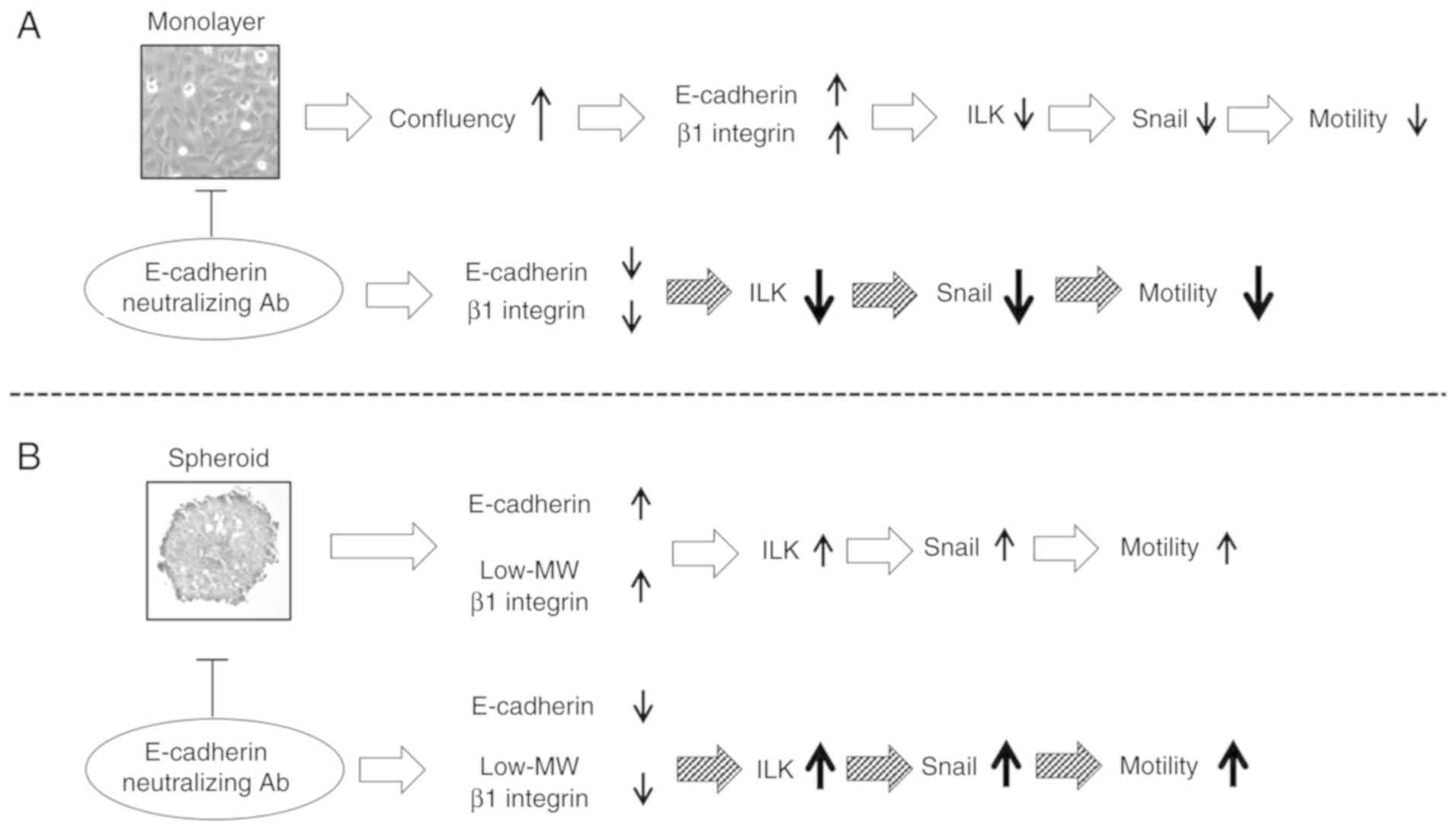
In the present study, MTS cells expressed low-MW β1
integrin and upregulated EMT signaling molecules, including ILK and
Snail, which increasing the motility of the cells. By contrast,
highly confluent cell monolayers exhibited low expression levels of
ILK and Snail but high expression levels of CAMs; these changes
decreased cellular motility. In monolayer and MTS culture
conditions, when E-cadherin was blocked with neutralizing antibody,
the expression levels of E-cadherin and β1 integrin decreased;
however, those of ILK and Snail differed according to the culture
conditions: Upregulation of ILK and Snail and increased motility
were observed in MTSs; downregulation of ILK and Snail and
decreased motility were observed in monolayer cells (Fig. 5). These results demonstrate that
when a two-dimensional cell condition was changed to a
three-dimensional cell condition, in addition to simple structural
changes, EMT signals were also upregulated by low-MW β1
integrin.
ATC is one of the most lethal human neoplasms.
Previous studies have reported a median survival time ranging from
3 to 10 months (19). Although ATC
comprises <2% of all thyroid carcinomas, it is responsible for
14-50% of annual deaths associated with thyroid cancer (20). The majority of patients with ATC
present with advanced disease with regional and systemic
metastases. ATC is the most undifferentiated and aggressive thyroid
cancer. It is characterized by high metastatic spread (21). Among diverse differentiation levels
of thyroid carcinomas, poorly differentiated ATC shows
significantly lower expression of E-cadherin than other well- or
moderately differentiated carcinomas (22,23).
Thus, an undifferentiated ATC cell line, FRO, expected to show high
cell motility due to reduced levels of E-cadherin was used in the
present study.
The expression of E-cadherin increased in proportion
to the confluency of the monolayered cells and increased further
when MTSs were formed. In addition to the expression level of
E-cadherin, the cytological examination of MTSs suggested the
importance of the differential distribution of E-cadherin in MTS
formation. However, the motilities of cells dispersed from cell
monolayers and MTSs were significantly different.
Cells from MTSs exhibited increased motility despite
the high level of E-cadherin. In particular, MTS cells expressed
low-MW β1 integrin. Generally, when glycosylation of some proteins
is changed, the function of these proteins in cancer cells may be
affected, thus influencing several metastatic processes such as
EMT, migration and invasion (24).
A previous study has suggested that the glycosylation of integrins
serves an important role in the metastatic process of cancer cells
(25). In addition to
glycosylation, several posttranslational modifications of integrin,
including phosphorylation and palmitoylation, have been reported to
regulate integrin activity in cancer cells (26,27).
Despite the diversity of the posttranslational modifications of
integrin, glycosylation is important for numerous biological
functions of β1 integrin, including cell adhesion and migration
(28,29). Therefore, it is hypothesized that
the enhancement of cancer cell motility with low-MW β1 integrin
observed in the present study may be associated with the aberrant
glycosylation of β1 integrin. However, this was not demonstrated
empirically due to the complexity of the glycosylation mechanism
and the diversity of the enzymes involved. Furthermore,
glycosylation of integrins is regulated in thyroid cancer cells by
several enzymes, including α1-6 fucosyltransferase and
N-acetylglucosaminyltransferase, that are less expressed in
ATCs (30). Therefore, reduced MW
β1 integrin in FRO MTSs may represent a clinical state of
undifferentiated carcinoma with increased metastatic ability.
Suppressive roles of E-cadherin in cancer
progression have been well reviewed. The loss of E-cadherin is
thought to enhance tumor invasiveness and increase the metastatic
ability of many human carcinomas. In addition, as tumor cells
metastasize to distant sites, cancer cells can change their ability
to interact with the surrounding ECM and adjacent tumor cells
(11,31). The crosstalk between the cell-cell
adhesion molecule E-cadherin and cell-ECM adhesion molecules of the
integrin family contributes to the effective migration of tumor
cells (13). In the present study,
to mimic the early phase of metastasis, a commercial neutralizing
monoclonal antibody, DECMA, that can recognize specific epitopes
within the extracellular domain of E-cadherin was applied to
disag-gregate FRO cells from one another. The neutralizing strategy
is considered to be better than a gene-silencing method because the
preservation of E-cadherin synthesis is important for
mesenchymal-epithelial transition, the reverse process of EMT, and
the completion step of metastasis.
Although the disruption of cell-cell adhesion by
blocking E-cadherin induced the downregulation of β1 integrin in
monolayer and spheroid cultures, this reduction caused differential
effects on the expression of EMT signaling molecules and cell
motility in the two culture conditions. These contradictory results
might be elicited by the reduced MW of β1 integrin during MTS
formation because the expression levels of E-cadherin and β1
integrin in MTSs were not markedly different from those in
monolayer cells.
As a cell adhesion receptor, integrin is linked to
diverse metastatic functions through intracellular signaling to
other adhesion molecules or EMT molecules (32). Generally, β1 inte-grin is linked to
ILK, which stimulates the expression of Snail, an EMT signaling
molecule. A recent study has reported that aberrant glycosylation
of β1 integrin decreases the cell-surface transport of β1 integrin
and changes the expression of ILK and Snail in hypoxic conditions
(17). In fact, MTS has a
spherical geometry similar to that of avascular tumor nodules in
which proliferating cells are arranged at the periphery but
non-proliferating cells are arranged in deeper regions under
hypoxic conditions (5). Similar to
these previous studies, the present study demonstrated that the
expression level of ILK and Snail was enhanced by low-MW β1
integrin, which may be regarded as aberrantly glycosylated β1
integrin. Additionally, DECMA elicited the upregulation of ILK and
Snail and accelerated the spread of MTSs.
Although the present study demonstrates that a
three-dimensional culture is a more relevant model than a
two-dimensional culture for studying tumor aggressiveness and
metastatic potential, detailed molecular mechanisms underlying the
differences in the expression of CAMs and EMT signaling molecules
between these two types of cell culture environments were not
elucidated. Thus, further studies are required to identify key
molecular mechanisms that regulate these expression patterns during
spheroid formation and confirm the in vitro results by
comparing the tumorigenesis and metastasis of MTS cells with
monolayer cells in vivo. Furthermore, co-culture of cancer
cells with endothelial cells, fibroblasts or immune cells during
spheroid formation might yield more complex models to advance
metastasis research.
In conclusion, the expression levels of CAMs and the
MW of β1 integrin in MTS cells differed from those in monolayer
cells, causing enhanced motility of MTS cells. The EMT signaling
molecules Snail and ILK were expressed at higher levels in MTSs
compared with cell monolayers. Furthermore, reduced E-cadherin
binding ability increased the expression levels of EMT signaling
molecules and the motility of cells. Consequently, changing
monolayers to three-dimensional MTS not only leads to morphological
changes, but also elicits marked differences in the expression of
adhesion- and EMT-associated molecules.
Supplementary Data
Abbreviations:
|
ATC
|
anaplastic thyroid carcinoma
|
|
CAM
|
cell adhesion molecule
|
|
ECM
|
extracellular matrix
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FAK
|
focal adhesion kinase
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
ILK
|
integrin-linked kinase
|
|
MTS
|
multicellular tumor spheroid
|
|
ZO-1
|
zonula accludens-1
|
Acknowledgments
Not applicable.
Funding
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant nos. NRF2
016R1D1A1A02937362 and 2018R1D1A1A09083263), Science and Technology
and the Ministry of Science, ICT & Future Planning
(2017R1A2B2003575); the Korea Health Technology R&D Project
(grant nos. HI14C0748 and HI17C0387) through the Korea Health
Industry Development Institute (KHIDI) by the Ministry of Health
& Welfare; the Clinical Trial Center of Korea University Anam
Hospital (grant no. I1502411). This research was also supported by
a grant from Korea University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The authors made substantial contributions to the
study as follows: BK, THK and SB contributed to study conception
and design; BK, NI, TDY and JK acquired data; BK, THK and SB
analyzed the data; BK, TDY, KJ, THK, SB interpreted the data. BK,
THK and SB drafted the article and all other authors contributed to
revising the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Inch WR, McCredie JA and Sutherland RM:
Growth of nodular carcinomas in rodents compared with multi-cell
spheroids in tissue culture. Growth. 34:271–282. 1970.PubMed/NCBI
|
|
2
|
Sutherland RM, McCredie JA and Inch WR:
Growth of multicell spheroids in tissue culture as a model of
nodular carcinomas. J Natl Cancer Inst. 46:113–120. 1971.PubMed/NCBI
|
|
3
|
Breslin S and O'Driscoll L:
Three-dimensional cell culture: The missing link in drug discovery.
Drug Discov Today. 18:240–249. 2013. View Article : Google Scholar
|
|
4
|
Kimlin LC, Casagrande G and Virador VM: In
vitro three-dimensional (3D) models in cancer research: An update.
Mol Carcinog. 52:167–182. 2013. View
Article : Google Scholar
|
|
5
|
Mueller-Klieser W: Tumor biology and
experimental therapeutics. Crit Rev Oncol Hematol. 36:123–139.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rainaldi G, Calcabrini A, Arancia G and
Santini MT: Differential expression of adhesion molecules (CD44,
ICAM-1 and LFA-3) in cancer cells grown in monolayer or as
multicellular spheroids. Anticancer Res. 19:1769–1778.
1999.PubMed/NCBI
|
|
7
|
Erler JT and Weaver VM: Three-dimensional
context regulation of metastasis. Clin Exp Metastasis. 26:35–49.
2009. View Article : Google Scholar :
|
|
8
|
Frixen UH, Behrens J, Sachs M, Eberle G,
Voss B, Warda A, Löchner D and Birchmeier W: E-cadherin-mediated
cell-cell adhesion prevents invasiveness of human carcinoma cells.
J Cell Biol. 113:173–185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen A, Beetham H, Black MA, Priya R,
Telford BJ, Guest J, Wiggins GA, Godwin TD, Yap AS and Guilford PJ:
E-cadherin loss alters cytoskeletal organization and adhesion in
non-malignant breast cells but is insufficient to induce an
epithelial-mesenchymal transition. BMC Cancer. 14:5522014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hollestelle A, Peeters JK, Smid M,
Timmermans M, Verhoog LC, Westenend PJ, Heine AA, Chan A, Sieuwerts
AM, Wiemer EA, et al: Loss of E-cadherin is not a necessity for
epithelial to mesenchymal transition in human breast cancer. Breast
Cancer Res Treat. 138:47–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeanes A, Gottardi CJ and Yap AS:
Cadherins and cancer: How does cadherin dysfunction promote tumor
progression? Oncogene. 27:6920–6929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borghi N, Lowndes M, Maruthamuthu V,
Gardel ML and Nelson WJ: Regulation of cell motile behavior by
crosstalk between cadherin- and integrin-mediated adhesions. Proc
Natl Acad Sci USA. 107:13324–13329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao D, Tang XF, Yang K, Liu JY and Ma XR:
Over-expression of integrin-linked kinase correlates with aberrant
expression of Snail, E-cadherin and N-cadherin in oral squamous
cell carcinoma: Implications in tumor progression and metastasis.
Clin Exp Metastasis. 29:957–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takei N, Yoneda A, Sakai-Sawada K, Kosaka
M, Minomi K and Tamura Y: Hypoxia-inducible ERO1α promotes cancer
progression through modulation of integrin-β1 modification and
signalling in HCT116 colorectal cancer cells. Sci Rep. 7:93892017.
View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
De Crevoisier R, Baudin E, Bachelot A,
Leboulleux S, Travagli JP, Caillou B and Schlumberger M: Combined
treatment of anaplastic thyroid carcinoma with surgery,
chemotherapy, and hyperfractionated accelerated external
radiotherapy. Int J Radiat Oncol Biol Phys. 60:1137–1143. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors Cancer. 103:1330–1335. 2005.
|
|
21
|
Kondo T, Ezzat S and Asa SL: Pathogenetic
mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer.
6:292–306. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ivanova K, Ananiev J, Aleksandrova E,
Ignatova MM and Gulubova M: Expression of E-cadherin/beta-catenin
in epithelial carcinomas of the thyroid gland. Open Access Maced J
Med Sci. 5:155–159. 2017.PubMed/NCBI
|
|
23
|
Brabant G, Hoang-Vu C, Cetin Y, Dralle H,
Scheumann G, Mölne J, Hansson G, Jansson S, Ericson LE and Nilsson
M: E-cadherin: A differentiation marker in thyroid malignancies.
Cancer Res. 53:4987–4993. 1993.PubMed/NCBI
|
|
24
|
Oliveira-Ferrer L, Legler K and
Milde-Langosch K: Role of protein glycosylation in cancer
metastasis. Semin Cancer Biol. 44:141–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo HB, Lee I, Kamar M, Akiyama SK and
Pierce M: Aberrant N-glycosylation of beta1 integrin causes reduced
alpha5beta1 integrin clustering and stimulates cell migration.
Cancer Res. 62:6837–6845. 2002.PubMed/NCBI
|
|
26
|
Gahmberg CG, Gronholm M and Uotila LM:
Regulation of integrin activity by phosphorylation. Adv Exp Med
Biol. 819:85–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coleman DT, Soung YH, Surh YJ, Cardelli JA
and Chung J: Curcumin prevents palmitoylation of integrin β4 in
breast cancer cells. PLoS One. 10:e01253992015. View Article : Google Scholar
|
|
28
|
Isaji T, Sato Y, Fukuda T and Gu J:
N-glycosylation of the I-like domain of beta1 integrin is essential
for beta1 integrin expression and biological function:
Identification of the minimal N-glycosylation requirement for
alpha5beta1. J Biol Chem. 284:12207–12216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marsico G, Russo L, Quondamatteo F and
Pandit A: Glycosylation and integrin regulation in cancer. Trends
Cancer. 4:537–552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyoshi E, Ito Y and Miyoshi Y:
Involvement of aberrant glycosylation in thyroid cancer. J Oncol.
2010:8165952010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong SHM, Fang CM, Chuah LH, Leong CO and
Ngai SC: E-cadherin: Its dysregulation in carcinogenesis and
clinical implications. Crit Rev Oncol Hematol. 121:11–22. 2018.
View Article : Google Scholar
|
|
32
|
Ganguly KK, Pal S, Moulik S and Chatterjee
A: Integrins and metastasis. Cell Adh Migr. 7:251–261. 2013.
View Article : Google Scholar : PubMed/NCBI
|