Introduction
Cancer immunotherapy with anti-PD-1 or PDL-1
antibody has resulted in remarkable progress being made in the
treatment of a number of different types of advanced cancers
(1,2). By contrast, active specific
immunotherapy using either tumor-associated antigens or their
peptides capable of inducing cytotoxic T lymphocytes (CTLs) against
tumor cells has been failing to provide sufficient clinical
benefits in order to be approved for use, despite the large numbers
of clinical trials beginning in the 1990s, and the mechanisms
underlying this failure have not yet been clarified (3-5).
The authors have developed a novel approach to
immunotherapy termed personalized peptide vaccination (PPV), in
which peptides are selected for individual patients based on their
secondary immune responses (6-8).
Randomized phase II PPV trials achieved a longer overall survival
(OS) for patients with certain types of cancer (9,10).
However, no significant difference in OS was found between patients
receiving PPV and those receiving the placebo in either of two
randomized, double-blind, placebo-controlled phase III trials
(11,12). A biomarker study revealed that both
higher neutrophil and lower lymphocyte proportions prior to entry
were unfavorable predictive factors for OS of only patients
administered PPV with advanced prostate cancer (11). In another trial enrolling patients
with recurrent glioblastoma, either very low or very high MCP-1
levels prior to study entry were identified as another biomarker
discriminating between patients administered PPV exhibiting a
significantly shorter OS and those exhibiting a significantly
longer OS (12).
In the present study, these biomarker analyses of
the 2,588 patients enrolled in the phase II PPV studies for the
treatment of various types of cancer were extended, in order to
elucidate the mechanisms involved in the inhibition of clinical
benefits.
Patients and methods
Patients
From November, 2008 to March, 2017, phase II
clinical trials of PPV were conducted at the Kurume University
Cancer Vaccine Center, Kurume University Hospital, Sendai Kosei
Hospital or Naito Hospital in Japan. Patients receiving no
vaccination were excluded from the analysis, and thus a total of
2,588 patients were enrolled. Some of the results for certain
cancer types have been previously reported (9,10,13-16).
Eligibility criteria were the pathologically confirmed diagnosis of
cancer, positive pre-vaccination plasma IgG responses for at least
2 of the 31 warehouse peptides (Table
SI), positive status for HLA-A2, -A24, -A3supertypes (HLA-A3,
-A11, -A31, or -A33: HLA-A3 sup), or -A26, age >20 years,
Eastern Cooperative Oncology Group performance status (PS) of 0-2
and neurological 3 for only brain tumor patients, a life expectancy
of at least 12 weeks, and adequate bone marrow function, hepatic
function and renal function. Exclusion criteria were acute
infection, a history of severe allergic reactions, or other
systemic diseases. All patients provided written informed consent
for the study participation and data collection. All patient sample
collections, patient consent and recruitment followed protocols
approved by the institutional review board of Kurume University.
The study was conducted according to the principles of the
Declaration of Helsinki.
Peptides and clinical analysis
Patients were vaccinated with 2 to 4 peptides based
on their human leukocyte antigen (HLA) type and pre-existing
immunity by measuring peptide-specific IgG levels. Each of the
selected peptides was mixed with incomplete Freund's adjuvant
(Montanide ISA-51VG; Product Code 36508H; Seppic) and injected
subcutaneously into the inguinal, abdominal, or other sites. There
were 3 different protocols with regards to the vaccination
intervals. The details are presented as supplementary material
(Data S1). The cancer patients who became resistant to the standard
systemic therapies received 6 injections of PPV at 1-week intervals
(the first cycle) followed by 6 injections at 2-week intervals (the
second cycle); this protocol was termed PRT1 (for further details
please see Data S1). The cancer patients at any stages, including
those at the early stages of the disease, received 4 injections of
PPV at 1-week intervals and then 4 injections at 2-week intervals
(the first cycle), followed by 4 injections at 2-week intervals and
then 4 injections at 4-week intervals (the second cycle) (PRT2:
Data S1). Alternatively, the patients received 4 injections at
4-week intervals (the first cycle) followed by the same schedule
for the second cycle (PRT3: Data S1). Detailed protocols are
presented online only (https://upload.umin.ac.jp/cgi-open-bin/ctr/index.cgi).
The detailed information of the protocols is presented in Data S1.
All the trials were conducted in accordance with the Declaration of
Helsinki and Good Clinical Practice guidelines. This is a
retrospective study, i.e., all the patient data was already
available and ethical approval provided when the original
studies/trials were performed.
It was decided that only 2-4 peptides would be
injected among the 31 candidates, mainly due to the following 3
reasons: Each peptide was independently injected to avoid a
possible bias for CTL induction by different avidities of peptides
when they are mixed together, and a maximum of 4 peptides were then
independently injected every 7-14 days, which in turn was
associated with a substantial burden for the patients. Therefore,
>5 peptides did seem tolerable for certain patients. Secondly,
the OS of the patients who received only one peptide, as only 1 of
the 31 peptides was suitable for PPV due to lower baseline IgG
levels, was shorter than that of patients who received at least 2
peptides (17). Thirdly, these
antigens coding the 31 peptides were highly expressed on the
majority of the histologically different cancer cells (6-17), a
maximum of 4 peptides based on pre-existing immunity were expected
to be sufficient to induce potent anti-tumor immunity.
Immune responses and biomarker
analyses
T cell responses or IgG titers specific to the
antigen peptides in peripheral blood mononuclear cells (PBMCs) or
plasma were evaluated by interferon (IFN)y ELISPOT (Immunocyte IFNy
ELISPOT kit; code no. 8223; Medical & Biological Laboratories
Co., Ltd.) or bead-based multiplexed Luminex assay (Luminex; REF.
LHC6003M; Invitrogen; Thermo Fisher Scientific) as previously
described (8,9). For the measurement of the 35
different cytokines and proteins, bead-based multiplex assays or an
enzyme-linked-immunosorbent assay (ELISA) were used as described
previously (12). The vaccinated
peptides, immunological responses, OS during PPV treatment and
follow-up study were extracted from the database of the clinical
trials for 2,588 patients. For the biomarker studies, the factors
listed in the baseline characteristics and laboratory data at the
screening time (14 days prior to the first vaccination) was
provided.
Estimation of clinical efficacy of
individual peptides and statistical analysis
The Kaplan-Meier method, log-rank test, univariate
and multivariate proportional hazard regression models, Spearman's
rank correlation coefficient test, Student's t-test, Chi-square
test and Fisher's exact test were used for the statistical
analyses. OS was calculated as the time in months from the date of
study enrollment to death or to the date of last contact. The
clinical efficacy of individual peptides for prolonging OS was
evaluated by univariate and multivariate analyses with the Cox
proportional hazards regression model, and the HR and 95% CI values
were calculated. All reported P-values were two-sided, and P-values
<0.05 were considered to indicate statistically significant
differences. JMP version 12 or SAS version 9.4 software (SAS
Institute Inc.) was used to perform all analyses.
Results
Baseline characteristics
The baseline characteristics of the 2,588 cancer
patients and their median OS are presented in Table I. There were 399 patients with
lung, 354 with prostate, 344 with colon, 290 with pancreatic, 200
with gastric and 183 with breast cancers. The remaining 818 cancer
patients consisted of 139 patients with urothelial, 126 with
biliary tract cancer, 118 with ovarian cancer, 79 with uterine
cancer, 83 with hepatocellular carcinoma, 50 with head and neck
cancer, 49 with sarcoma, 39 with esophageal cancer, 35 with brain
cancer, 30 with renal and 70 with miscellaneous cancers (data not
shown). There were 1,520 male and 1,068 female subjects with a
median age of 63 years (Table I).
The median OS among male and female patients was 10.5 and 13.7
months, respectively, and the OS of the female patients with lung
or colon cancers was longer than that of male patients. Patients
with a better PS or with early stages exhibited a trend for a
longer OS in all types of cancer tested, respectively. No
significant differences in the median OS were found with regard to
different HLA-class I types. Table
I also presents the number of patients receiving systemic
therapies prior to the PPV vaccination and the number of patients
receiving systemic therapies combined with the PPV vaccination. The
median vaccination time was 11 months, ranging from 1 to 76 months,
while the median study period was 5.4 months in all 2,588 cases.
The median follow-up time was 11.3 months.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| All patients
(mOS) | Lung cancer
(mOS) | Prostate cancer
(mOS) | Colon cancer
(mOS) | Pancreatic cancer
(mOS) | Gastric cancer
(mOS) | Breast cancer
(mOS) | Other types of
cancer (mOS) |
|---|
| Number of
patients | 2588 | 399 | 354 | 344 | 290 | 200 | 183 | 818 |
| Age (years) | 63 | 64 | 69 | 61 | 64 | 64 | 56 | 62 |
| Sex | | | | | | | | |
| Male | 1,520 (10.5) | 252 (9.5) | 354 (18.7) | 188 (11.0) | 159 (5.6) | 121 (9.7) | 0 | 446 (9.8) |
| Female | 1,068 (13.7) | 147 (18.0)a | 0 | 156 (16.2)a | 131 (6.2) | 79 (8.6) | 183 (21.2) | 372 (14.8) |
| Performance
status | | | | | | | | |
| 0 | 2,072 (13.7) | 279 (17.7) | 291 (22.3) | 308 (14.8) | 230 (6.4) | 157 (10.3) | 150 (26.5) | 657 (13.1) |
| 1 | 491 (5.7) | 117 (5.9) | 61 (9.8) | 34 (5.2) | 58 (3.7) | 42 (4.1) | 33 (10.9) | 146 (5.2) |
| 2 | 22 (3.3) | 3 (2.4) | 2 (3.8) | 2 (2.0) | 2 (1.7) | 1 (3.3) | 0 | 12 (4.6) |
| 3 | 3 (8.6) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (8.6) |
| Numbers of
vaccinations Stage | 11 (1-76) | 10 (1-56) | 17 (1-76) | 11 (1-45) | 9 (1-60) | 9 (1-66) | 14 (1-49) | 11 (1-57) |
| I | 88 (58.9) | 30 (38.1) | 3 (41.7) | 3 (11.5) | 3 (-) | 6 (24.7) | 12 (-) | 31 (58.9) |
| II | 104 (29.5) | 19 (18.5) | 15 (71.3) | 6 (16.6) | 1 (-) | 8 (6.8) | 23 (32.3) | 32 (32) |
| III | 311 (15.4) | 95 (13.5) | 21 (26.1) | 53 (16.8) | 4 (53.5) | 41 (10.5) | 13 (21.9) | 84 (15.3) |
| IV | 1,753 (9.9) | 246 (10.0) | 315 (17.6) | 259 (12.3) | 246 (5.6) | 132 (7.7) | 78 (24.3) | 477 (8.3) |
| Recurrence | 332 (13.3) | 9 (22.9) | 0 | 23 (14.3) | 36 (6.1) | 13 (20.0) | 57 (12.2) | 194 (15.9) |
| Type of cancer | | | | | | | | |
| Lung cancer | - | 399 (11.9) | - | - | - | - | - | - |
| Prostate
cancer | - | - | 354 (18.7) | - | - | - | - | - |
| Colon cancer | - | - | | 344 (13.1) | - | - | - | - |
| Pancreatic
cancer | - | - | - | - | 290 (5.7) | - | - | - |
| Gastric
cancer | - | - | - | - | - | 200 (9.1) | - | - |
| Breast cancer | - | - | - | - | - | - | 183 (21.2) | - |
| Other | - | - | - | - | - | - | - | 818 (11.3) |
| HLA status | | | | | | | | |
| A24 | 1,550 (12.0) | 233 (12.1) | 225 (20.2) | 209 (13.6) | 167 (5.7) | 119 (8.6) | 119 (23.5) | 478 (11.3) |
| A2 | 1,050 (11.5) | 164 (11.7) | 142 (18.3) | 133 (14.4) | 123 (5.6) | 89 (8.9) | 61 (18.8) | 338 (11.7) |
| A3 | 1,214 (11.1) | 164 (12.1) | 165 (18.2) | 168 (11.5) | 153 (5.8) | 90 (9.1) | 94 (26.5) | 380 (10.8) |
| A26 | 552 (12.4) | 107 (12.6) | 72 (22.4) | 71 (13.8) | 56 (4.7) | 36 (7.2) | 32 (23.7) | 178 (12.3) |
| Prior systemic
therapy | | | | | | | | |
| Operation | 1,574 | 155 | 86 | 317 | 126 | 127 | 160 | 603 |
| Chemotherapy | 2,185 | 362 | 303 | 308 | 241 | 167 | 180 | 624 |
| Radiation | 771 | 185 | 119 | 45 | 49 | 9 | 102 | 262 |
| Combination
therapy | | | | | | | | |
| Operation | 74 | 3 | 3 | 17 | 11 | 1 | 12 | 27 |
| Chemotherapy | 1,787 | 256 | 321 | 228 | 249 | 149 | 164 | 420 |
| Radiation | 272 | 76 | 29 | 25 | 20 | 4 | 33 | 85 |
| Local therapy | 99 | 1 | 5 | 8 | 4 | 2 | 7 | 72 |
| Study periods | 5.4 | 5.8 | 8.9 | 5.7 | 3.8 | 4.8 | 8.3 | 4.9 |
| mOS (95% CI) | 11.6
(11.0-12.3) | 11.9
(10.0-13.5) | 18.7
(16.1-22.3) | 13.1
(11.3-15.5) | 5.7 (5.1-6.7) | 9.1 (7.6-10.4) | 21.2
(15.6-28.4) | 11.3
(9.9-12.5) |
Circulating blood cells and OS
The association between the number or proportion of
pre-vaccination circulating blood cells and OS was examined
(Table II). The median OS of the
patients administered PPV with greater than the median numbers of
neutrophils, monocytes, or platelets was significantly shorter than
that of the patients administered PPV with lower numbers, whereas
the opposite was true for lymphocytes or red blood cells. Similar
trends were obtained in the cellular proportions with more
sensitive HRs as compared to those of the cellular numbers. The
most potent unfavorable and favorable factors for OS were the
median percentage of neutrophils >64.8% (HR, 1.70, Table II) or percentage lymphocytes
>25.1% (HR, 0.53, Table II)
with correlation coefficients (R2) of 0.98 and
0.92 (Fig. S1A and B),
respectively. The median OS for 1,522 of the 2,588 cancer patients
who met either or both the cut-offs of neutrophils >64.8% and
lymphocytes <25.1% was significantly shorter than that of the
remaining 1,066 patients who exhibited both <64.8% neutrophils
and >25.1% lymphocytes together (8.1 months; vs. 17.8 months;
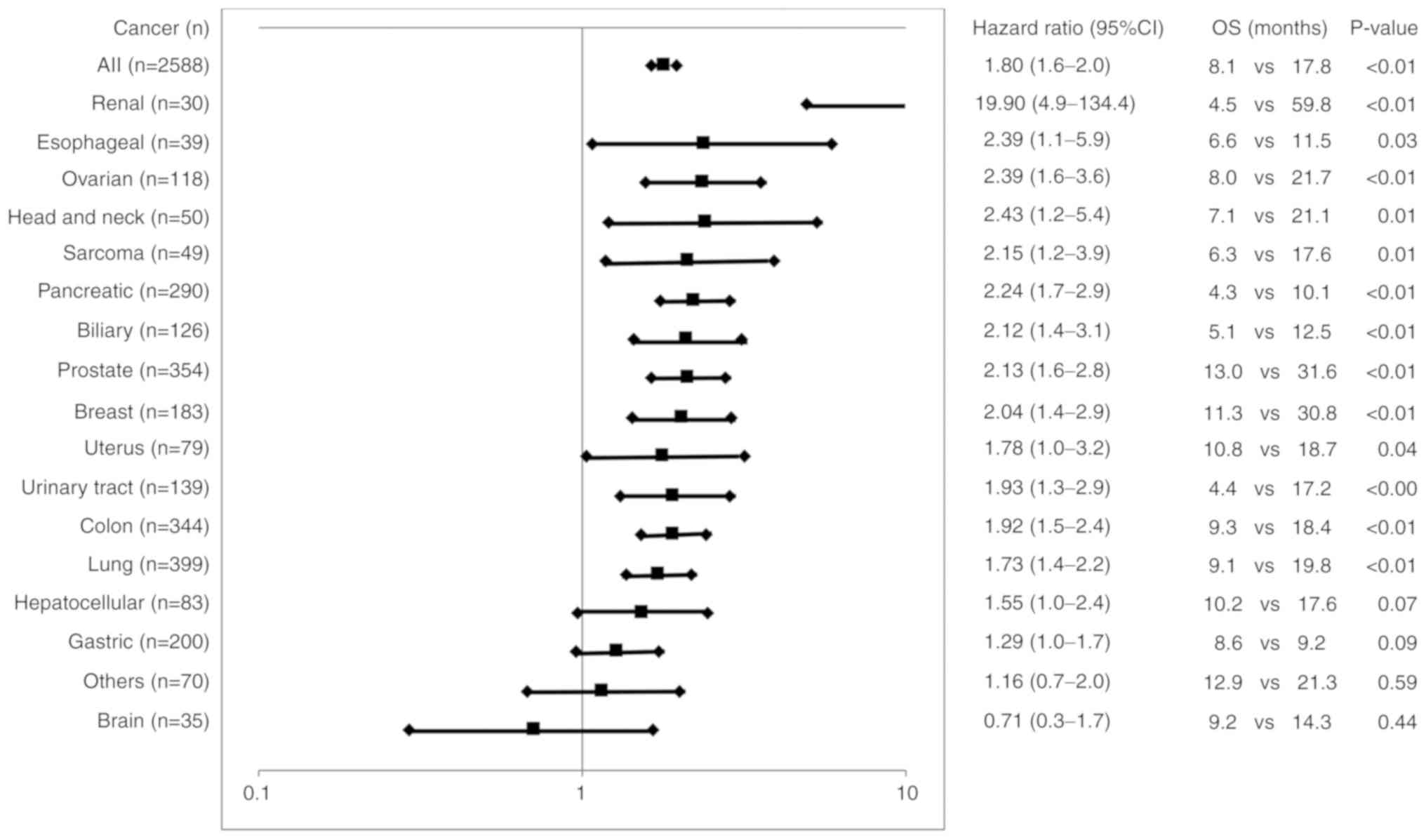
HR, 1.8, 95% CI, 1.6-2.0; P<0.01) (Fig. 1). Significantly shorter OS times
were also obtained in patients with all different cancer types
tested, apart from gastric cancer, brain cancer, or other
miscellaneous cancer types (Fig.
1). The median OS of the patients administered PPV with a
greater than or equal to median neutrophil-lymphocyte-ratio (NLR)
was also significantly shorter than that of the patients
administered PPV with a less than median NLR (7.6 vs. 17.2 months;
HR, 1.85; P<0.01) (Table II)
with an (R2) value of 0.57 (Fig. S1C).
 | Table IICirculating blood cells and immune
responses. |
Table II
Circulating blood cells and immune
responses.
| Factors (no. of
patients) | Median value | OS: Median < vs.
median > value | HR (95% CI) | P-value |
|---|
| Pre-vaccination
cell counts | | | | |
| White blood cells
(2,588) | 5,600 | 15.3/8.9 | 1.47
(1.35-1.60) | <0.01 |
| Red blood cells
(2,588) | 384 | 8.7/16.4 | 0.61
(0.56-0.66) | <0.01 |
| Platelets
(2,588) | 21.4 | 13.7/10.1 | 1.29
(1.19-1.40) | <0.01 |
| Neutrophils
(2,587) | 3,519 | 16.1/8.3 | 1.62
(1.49-1.76) | <0.01 |
| % Neutrophils
(2,588) | 64.8 | 16.5/8.2 | 1.70
(1.56-1.85) | <0.01 |
| Lymphocytes
(2,588) | 1,346 | 10.2/13.6 | 0.78
(0.72-0.85) | <0.01 |
| % Lymphocyte
(2,588) | 25.1 | 7.6/17.5 | 0.53
(0.48-0.57) | <0.01 |
| Basophiles
(2,555) | 20.6 | 11.8/11.7 | 0.97
(0.89-1.05) | 0.43 |
| % Basophil
(2,555) | 0.4 | 10.3/12.8 | 0.82
(0.75-0.89) | <0.01 |
| Eosinophils
(2,562) | 101 | 11.6/11.7 | 0.98
(0.90-1.07) | 0.71 |
| % Eosinophils
(2,562) | 1.9 | 10.8/12.4 | 0.90
(0.83-0.98) | 0.01 |
| Monocytes
(2,575) | 352 | 16.5/8.5 | 1.67
(1.54-1.82) | <0.01 |
| % Monocyte
(2,575) | 6.2 | 13.7/10.1 | 1.28
(1.18-1.40) | <0.01 |
| %
Neutrophil-lymphocyte ratio (2,588) | 2.6 | 17.2/7.6 | 1.85
(1.70-2.02) | <0.01 |
| Pre-vaccination IgG
(FIU) | | | | |
| To 31 peptides
(2,588) | 2,251 | 12.0/11.1 | 1.07
(0.98-1.16) | 0.12 |
| To vaccinated
peptides (2,588) | 561 | 12.1/11.1 | 1.09
(1.01-1.19) | 0.04 |
| Post-vaccination
IgG (FIU) | | | | |
| To 31 peptides
(2,116) | 27,266 | 9.9/22.3 | 0.48
(0.44-0.53) | <0.01 |
| To vaccinated
peptides (2,116) | 22,716 | 9.8/22.3 | 0.48
(0.43-0.52) | <0.01 |
| Increased IgG
levels (FIU) | | | | |
| To 31 peptides
(2,116) | 20,949 | 9.6/22.5 | 0.46
(0.42-0.51) | <0.01 |
| To vaccinated
peptides (2,116) | 19,309 | 9.5/22.6 | 0.45
(0.41-0.50) | <0.01 |
| Pre-vaccination CTL
to vaccinated peptides (IFNγ spots) (525) | 22 | 16.0/16.8 | 0.98
(0.82-1.19) | 0.86 |
| Post-vaccination
CTL to vaccinated peptides (IFNγ spots) (525) | 68 | 11.4/19.5 | 0.68
(0.56-0.82) | <0.01 |
| Increased CTL
levels to vaccinated peptides (IFNγ spots) (525) | 33 | 11.3/19.5 | 0.72
(0.60-0.87) | <0.01 |
Soluble factors and OS
A total of 35 cytokine or protein levels were
provided for the analyses of the association between the
pre-vaccination levels and the OS of patients administered PPV from
whom samples were available for the biomarker analysis. The total
numbers tested were 1,432 for C-reactive protein (CRP) and 250 to
504 for the others. A cancer type having <20 tested samples was
excluded from the analysis to avoid any biases. Higher levels of
CRP (n=1,432), a typical protein involved in inflammation (18), were inversely associated with the
OS of patients for all the cancer types tested (Fig. 2 and Table SII). Similar results were obtained
for interleukin (IL)-6, B-cell activating factor (BAFF),
haptoglobin (Hp), hepatocyte growth factor (HGF), vascular
endothelial growth factor (VEGF), IL2-R and MIG levels, soluble
factors involved in inflammatory responses (19), although the levels did not differ
significantly in the majority of cancer types. Higher levels of
IL-8, macrophage inflammatory protein (MIP)-1ß,
interferon-inducible protein 10 (IP-10), or granulocyte-colony
stimulating factor (G-CSF) were inversely associated with the OS of
patients with only urinary tract, lung, or breast cancers,
respectively (Table SII).
Discrepant results were obtained for several
cytokines. Higher levels of IL-2, a cytokine for T cell activation
(18), were positively associated
with the OS of patients with certain types of cancer (urinary
tract, breast and colon) (indicated by HR values <0.63), but
were inversely associated with the OS of patients with prostate
cancer (indicated by HR value of 2.15) (Table SII). Similar results were obtained
for IL-1β or GM-CSF (cytokines involved in immune activation) or
IL-10 (an anti-inflammatory cytokine) (18) (indicated by HR values of <0.63
for a positive association or HR values of >2.25 for an inverse
association). Namely higher levels of IL-1β were positively
associated with the OS of patients with urinary tract and breast
cancers, but were inversely associated with the OS of patients with
prostate cancer. Similarly, higher levels of GM-CSF or IL-10 were
positively associated with the OS of patients with urinary tract or
gastric cancer respectively, but were inversely associated with the
OS of patients with lung or biliary cancer respectively. Higher
levels of IFNa IFNy, IL-17a, or eotaxin were positively associated
with the OS of patients with only certain types of cancer
(indicated by HR values of <0.60). Pre-vaccination levels of the
remaining 15 soluble factors tested [transforming growth factor
(TGF), IL-21, fibroblast growth factor (FGF)-basic, IL-13, IL-12,
IL-1RA, tumor necrosis factor (TNF)a, IL-7, IL-4, RANTES, MIP-1a,
MCP-1, IL-15, epidermal growth factor (EGF), IL-5] had no
significant effect on the OS of patients (Table SII).
Immune responses and OS
The median peptide-specific IgG levels in
pre-vaccination plasma for all 31 of the peptides or for only the
peptides selected for vaccination were 2,251 or 561 fluorescence
intensity units (FIU) with no significant association between IgG
levels to all the peptides and OS (Table II). The median IgG levels in
post-vaccination plasma for all 31 of the peptides and for only the
peptides selected for vaccination were 27,266 and 22,716 FIU, and
lower IgG levels were inversely associated with OS in both the
cases. Similar results were obtained with regard to the increase in
IgG levels following vaccination. The median peptide-specific CTL
activity in pre-vaccination PBMCs in response to the vaccinated
peptides was 22 IFNy-spots with no association between higher
levels and OS (Table II). The
median CTL activity in post-vaccination PBMCs in response to the
vaccinated peptides was 68 IFNy-spots with a positive association
between the higher levels and OS. Similar results were obtained
with regard to the increase in CTL levels following
vaccination.
Immune responses to the 31 peptides
The pre-vaccination positive rates of the patients
exhibiting >10 FIU (n=2,588) were largely different among the 31
peptides (12 to 87%) (Table
III). The magnitude of IgG titers of the 31 peptides among the
patients exhibiting detectable levels also differed between the
peptides. Post-vaccination lgG titers were measured at the end of
both the first cycle and second cycle, and the numbers of patients
exhibiting a >2-fold increase in the IgG titer against a peptide
at either the end of the first or second cycle as compared to the
pre-vaccination IgG titer are listed in Table III as the number of positive
patients. A portion of the patients (472 of 2,588 patients, 18.3%)
were dropped from the clinical trials prior to the end of the first
cycle due to a rapid disease progressive. The rates of patients
exhibiting positive IgG responses among the 2,116 patients who
completed at least the first cycle were largely different among the
31 peptides (Table III). The
magnitudes of IgG titers of the 31 peptides among the patients
exhibiting positive responses were also largely different from each
other. Despite these large diversities, it was commonly observed
that the patients exhibiting immune boosting to each of the
vaccinated peptides had a longer survival than those without such
immune boosting.
 | Table IIIImmune responses and association
between 31 warehouse peptides and the OS of 2,588 patients. |
Table III
Immune responses and association
between 31 warehouse peptides and the OS of 2,588 patients.
| Peptides | Pre vaccination IgG
| Post vaccination
IgG
| Association between
immune responses and OS
| Association between
vaccination and OS
|
|---|
|
Positive/negative | Median of positive
patients (FIU) | Number of
vaccinated patients (No. of positive patients) | Median (FIU) |
|---|
| Positive
(mOS)/Negative (mOS) | P-value | Vaccinate cases
(mOS) | Non vaccinate cases
(mOS) | HR | HR (95% CI) | HR (P-value) |
|---|
| SART2-93 | 2,248/340 | 66 | 1,137 (362) | 3,435 | 362 (21.7)/568
(12.3) |
<0.01 | 1,137 (12.3) | 413 (11.5) | 0.9 | 0.8 1.1 | 0.31 |
| Lck-486 | 2,184/404 | 42 | 879 (573) | 15,147 | 573 (19.4)/186
(7.2) |
<0.01 | 879 (12.3) | 671 (11.3) | 0.9 | 0.8 1.0 | 0.01 |
| Lck-488 | 2,147/441 | 60 | 1,103 (635) | 11,594 | 635 (20.6)/277
(7.8) |
<0.01 | 1,103 (12.0) | 447 (12.0) | 0.9 | 0.8 1.0 | 0.17 |
| Lck-90 | 2,087/501 | 44 | 502 (187) | 3,350 | 187 (20.9)/222
(10.3) |
<0.01 | 502 (11.3) | 712 (11.0) | 1.0 | 0.9 1.1 | 0.92 |
| SART3-734 | 2,041/547 | 111 | 625 (225) | 4,816 | 225 (22.3)/280
(11.0) |
<0.01 | 625 (10.9) | 589 (11.3) | 1.0 | 0.9 1.2 | 0.52 |
| PSA-248 | 2,035/553 | 48 | 283 (200) | 13,165 | 200 (20.2)/40
(7.0) |
<0.01 | 283 (13.8) | 1,267 (11.6) | 0.9 | 0.8 1.1 | 0.24 |
| SART3-511 | 1,967/621 | 41 | 439 (145) | 921 | 145 (22.6)/213
(10.8) |
<0.01 | 439 (11.0) | 775 (11.2) | 1.0 | 0.9 1.1 | 0.93 |
| SART3-309 | 1,921/667 | 35 | 328 (158) | 3,070 | 158 (21.4)/124
(10.5) |
<0.01 | 328 (12.1) | 722 (11.1) | 0.9 | 0.8 1.1 | 0.25 |
| WHSC2-141 | 1,908/680 | 39 | 325 (173) | 13,078 | 173 (19.0)/94
(7.7) |
<0.01 | 325 (12.1) | 725 (11.1) | 1.0 | 0.9 1.2 | 0.85 |
| CypB-129 | 1,888/700 | 31 | 483 (222) | 2,483 | 222 (23.8)/192
(9.0) |
<0.01 | 483 (12.3) | 1,458 (11.2) | 0.9 | 0.8 1.1 | 0.24 |
| Lck-246 | 1,815/773 | 49 | 366 (169) | 10,806 | 169 (19.3)/128
(8.3) |
<0.01 | 366 (11.4) | 684 (11.6) | 1.0 | 0.9 1.2 | 0.68 |
| WHSC2-103 | 1,673/915 | 43 | 729 (222) | 827 | 222 (19.6)/374
(12.1) |
<0.01 | 729 (11.5) | 1,465 (11.6) | 1.0 | 0.9 1.1 | 0.52 |
| SART3-302 | 1,640/948 | 84 | 408 (271) | 14,958 | 271 (18.2)/78
(9.1) |
<0.01 | 408 (12.7) | 642 (10.5) | 0.9 | 0.8 1.0 | 0.10 |
| MRP3-1293 | 1,558/1,030 | 27 | 324 (157) | 6,003 | 157 (20.6)/113
(8.6) |
<0.01 | 324 (11.9) | 1,226 (12.1) | 1.1 | 0.9 1.2 | 0.31 |
| EGF-R-800 | 1,542/1,046 | 30 | 390 (156) | 715 | 156 (24.3)/188
(12.4) |
<0.01 | 390 (14.3) | 1,160 (11.1) | 0.8 | 0.7 1.0 | 0.01 |
| PAP-213 | 1,422/1,166 | 33 | 279 (196) | 14,912 | 196 (21.7)/52
(7.0) |
<0.01 | 279 (16.6) | 1,271 (11.4) | 0.9 | 0.8 1.0 | 0.06 |
| Lck-449 | 1,406/1,182 | 23 | 127 (67) | 18,919 | 67 (19.5)/44
(6.2) |
<0.01 | 127 (13.7) | 1,087 (10.8) | 0.9 | 0.8 1.1 | 0.55 |
| ppMAPkkk-432 | 1,308/1,280 | 51 | 375 (97) | 757 | 97 (23.8)/203
(12.0) |
<0.01 | 375 (11.1) | 1,087 (11.9) | 1.0 | 0.9 1.2 | 0.51 |
| HNRPL-140 | 1,295/1,293 | 39 | 200 (111) | 8,576 | 111 (23.9)/62
(9.8) |
<0.01 | 200 (12.9) | 850 (11.1) | 0.8 | 0.7 0.9 | 0.01 |
| PAP-248 | 1,286/1,302 | 37 | 147 (62) | 2,136 | 62 (21.1)/63
(9.9) | 0.01 | 147 (11.4) | 1,067 (11.1) | 1.0 | 0.8 1.2 | 0.76 |
| UBE2V-43 | 1,238/1,350 | 34 | 230 (159) | 25,121 | 159 (18.5)/40
(7.0) |
<0.01 | 230 (11.7) | 820 (11.5) | 0.9 | 0.8 1.0 | 0.15 |
| SART3-109 | 1,206/1,382 | 31 | 474 (223) | 7,875 | 223 (19.0)/191
(10.3) |
<0.01 | 474 (12.3) | 1,948 (11.5) | 1.0 | 0.9 1.1 | 0.88 |
| HNRPL-501 | 1,189/1,399 | 45 | 355 (223) | 10,405 | 223 (19.3)/74
(6.6) |
<0.01 | 355 (12.1) | 1,107 (11.6) | 1.0 | 0.9 1.1 | 0.75 |
| SART2-161 | 947/1,641 | 29 | 195 (61) | 312 | 61 (20.6)/109
(12.6) | 0.05 | 195 (12.3) | 13,552 (11.9) | 1.0 | 0.8 1.1 | 0.68 |
| PSMA-624 | 950/1,638 | 24 | 101 (50) | 7,772 | 50 (21.7)/37
(5.5) |
<0.01 | 101 (11.7) | 1,449 (12.0) | 1.0 | 0.8 1.3 | 0.74 |
| PTHrP-102 | 886/1,702 | 27 | 204 (99) | 1,423 | 98 (20.0)/81
(11.4) | 0.01 | 204 (13.3) | 1,346 (11.9) | 0.9 | 0.8 1.1 | 0.22 |
| Lck-208 | 560/2,028 | 30 | 134 (64) | 4,597 | 64 (30.5)/62
(16.0) | 0.01 | 134 (19.7) | 1,416 (11.4) | 0.9 | 0.8 1.0 | 0.06 |
| EZH2-735 | 558/2,030 | 23 | 69 (51) | 17,691 | 51 (24.7)/11
(5.3) |
<0.01 | 69 (13.3) | 1,481 (11.9) | 0.9 | 0.7 1.1 | 0.24 |
| MRP3-503 | 467/2,121 | 37 | 133 (85) | 6,401 | 85 (20.6)/36
(9.3) | 0.08 | 133 (14.1) | 1,417 (11.9) | 0.9 | 0.8 1.1 | 0.55 |
| UBE2V-85 | 362/2,226 | 28 | 51 (26) | 3,941 | 26 (16.8)/18
(8.4) | 0.09 | 51 (9.6) | 999 (11.6) | 1.2 | 0.9 1.5 | 0.32 |
| Lck-422 | 316/2,272 | 33 | 92 (20) | 436 | 20 (18.5)/60
(11.7) | 0.10 | 92 (12.0) | 1,849 (11.4) | 1.2 | 0.9 1.4 | 0.22 |
The association between each of the 31 peptides and
OS was evaluated by the Cox proportional hazards regression model.
This analysis revealed that the patients administered PPV that
included each of the 3 peptides (Lch-486, EGFR-800 or HNRPL-140
peptide) exhibited a significantly longer survival than the
patients whose PPV did not include each of these peptides with HR
values of 0.9, 0.8 or 0.8, respectively (Table III). The HR values of the 25
peptides were between 0.8 to 1.0, and those of the remaining 3
peptides were 1.1 to 1.2; however, none of these were statistically
significant.
Discussion
The results of the present study demonstrated that
both higher neutrophil and lower lymphocyte proportions prior to
entry were unfavorable biomarkers for the OS of patients
administered PPV. When the most relevant cut-off levels in terms of
inhibiting the clinical benefits of PPV were set as neutrophils
>64% and lymphocytes <26%, the median OS of more than half of
the cancer patients who met either or both the cut-offs of
neutrophils >64% and lymphocytes <26% was significantly
shorter than that of the remaining patients who exhibited both
<64% neutrophils and >26% lymphocyte together. These results
are consistent with those of the randomized, placebo-controlled,
phase III trial (11). It has been
previously reported that both circulating myeloid-derived
suppressor cells (MDSCs) and CD4+CD45RA- activated T
cells prior to study entry suppress or promote the OS of patients
administered PPV in the other phase III study of PPV (12). The abnormal circulating
granulocytes at the gene expression level, as well as the increase
in granulocytic MDSCs following PPV treatment have been shown to
negatively contribute to the OS of certain patients receiving PPV
(20,21). These findings suggest that certain
types of circulating neutrophils or lymphocytes suppress or promote
the PPV-induced clinical benefits, respectively. However, the
underlying immunological mechanisms are unclear. Cellular subset
analyses of the PBMCs, regional lymph nodes and tumor sites of
cancer patients are required to obtain a better understanding of
the mechanisms.
The neutrophil-to-lymphocyte ratio (NLR) has been
reported to be a risk factor for the OS of advanced cancers
(22), which was consistent with
the results of the present study. However, the co-efficiency
(R2) between HR of the OS and NLR (0.57) was much lower
than that between HR and the neutrophil (0.97) or lymphocyte
proportion (0.92). NLR was less sensitive as compared to the
proportion of neutrophils or lymphocytes as a biomarker to predict
the efficacy of PPV when the OS was compared between the PPV and
placebo patients in the randomized phase III trial (11). The results suggest that the
pre-vaccination neutrophil or lymphocyte proportion is more
suitable than pre-vaccination NLR as a biomarker for the clinical
efficacy of PPV.
The present study indicated that higher
pre-vaccination levels of CRP along with IL-6, BAFF, Hp, HGF, VEGF,
IL2-R and MIG were inversely associated with the OS of cancer
patients receiving PPV regardless of the cancer types tested.
However, the higher levels of IL-2, IL-1β, GM-CSF and IL-10 were
well associated with the OS of patients with certain cancer types,
but were inversely associated with those with other cancer types.
The mechanisms involved in this discrepancy are also presently
unknown, and will need to be confirmed in a relatively large number
of cancer patients in the future. Furthermore, all these results
with the exception of CRP need to be confirmed with a larger number
of samples.
Discrepant results with regard to the cytokine
biomarkers are one of the unsolved hot points in cancer
immunotherapy, and the more in depth underlying mechanisms reason
cannot be explained at present. The hypothesis is that the
discrepancy is due in part to the ambiguity of cytokines with
regard to the promotion and inhibition of immune responses. For
example, IL-2 and IFN-γ are key cytokines for both cytotoxic and
helper T cell activation, which play a role in tumor elimination,
but they also activate other types of immune cells (suppressor
macrophages and T regulatory cells) that have roles in immune
suppression in certain types of cancers that depend on the doses of
IL-2 or IFN-γ. Similarly, IL-10 can suppress T regulatory cells,
but can also inhibit the TNFa production that is needed for the
infiltration of T cells into cancers IL-10 dose-dependently. This
hypothesis is based in part on a recent randomized double-blind,
phase III trial of personalized peptide vaccination for recurrent
glioblastoma (12). It was found
that the median OS of the patients with very low or high CCL2
levels (CCL2low/high) or IL-6 (IL-6low/high) was shorter than that
of the patients with an intermediate level of CCL2 (CCL2im) or IL-6
(IL-6im). By contrast, the median level of CCL2 or IL-6 failed to
discriminate one from the other. Instead, CCL4 was an indicator to
discriminate one from the other, with higher levels favorable to
longer OS. Further studies need to be conducted to clarify this
issue.
Higher CCL2 levels were unfavorable for the OS of
patients (n=338; HR, 1.5, 95% CI, 0.7-3.5; P=0.06), but not for the
OS of each cancer type tested, including brain tumor (n=35) (data
not shown). These results were also consistent with the results of
the randomized PIII study of recurrent glioblastoma (12).
The results shown in Table I were also evaluated with the
multivariate proportional hazard regression model. The 10 factors
consisting of 5 higher HRs and 5 lower HRs in the univariate
analysis (Table I) were provided
for the multivariate analysis, and the results are presented in
Table SIII. The HR values of red
blood cell numbers (0.56; 95% CI, 0.46-0.68; P<0.01) or monocyte
numbers (1.40; 95% CI, 1.13-1.74; P<0.01) were statistically
significant, respectively (Table
SIIIA). The red blood cell number was a prognostic risk factor
for the OS of both the PPV and placebo groups in the double-blind
placebo control phase III study for advanced prostate cancer,
whereas the monocyte number was a risk factor for the OS of only
the placebo group (11). By
contrast, the lymphocyte ratio or neutrophil ratios were predictive
a favorable or unfavorable factor for the OS of only the PPV group
(11). This analysis also revealed
that the lymphocyte (P=0.09) or neutrophil ratio (P=0.16) was a
favorable or unfavorable factor for the OS of the PPV group,
although not statistically significant. Both the post-vaccination
increment of peptide-specific IgG levels (P<0.01) and CTL
activities (P=0.04) were favorable factors for OS, as expected from
previous studies (6,7). The results of the soluble factors
shown in Table SII were then
evaluated with the multivariate proportional hazard regression
models. The 10 factors consisting of 5 higher HRs and 5 lower HRs
in the univariate analysis using >300 test cases (Table SII) were provided for the
multivariate analysis, and the results are presented in Table SIIIB of the revised manuscript.
The levels of only CRP, and not those of the other cytokines (IL-6,
VEGF, MIG, IL-2, IL1β, IP-10, GM-CSF, IFNa and IFNy) were
significantly (P<0.05) associated with OS. It has previously
been reported that CRP is an unfavorable factor for the OS of
patients administered PPV (6,7).
Collectively, the results with the multivariate analyses revealed
that the well-known pre-vaccination unfavorable factors (red blood
cell numbers, monocyte numbers and CRP levels) and well-known
post-vaccination favorable markers as the statistically significant
biomarkers for the OS of the patients administered PPV. Further
studies are required to identify novel biomarkers influencing the
OS of cancer patients administered PPV in order to better
understand the mechanisms involved the long-lasting failure of the
peptide-based cancer vaccine with regard to the clinical
efficacy.
Inflammatory responses associated with tumors are
considered to be one of the major events involved in cancer
development (22-26), which in turn may be responsible for
the hindering of the clinical benefits of PPV in patients when
pre-vaccination levels of inflammatory soluble factors are higher
than the median levels, as shown in the present study. However, the
immunological mechanisms responsible for this soluble
factor-mediated inhibition of clinical benefits are presently
unknown. One might think that higher levels of inflammatory soluble
factors cause the higher neutrophil or lymphocyte proportion, which
in turn may be responsible for the inhibition of clinical efficacy.
However, the results of the present study suggested that this was
not the case, since the R2 values by Spearman's
rank correlation coefficient test between the CRP level and the
proportion of neutrophils and lymphocytes in the 1,432 patients
tested were as low as 0.007 and 0.005, respectively (data not
shown). Cellular subset analyses of the PBMCs, regional lymph
nodes, and tumor sites of cancer patients and the correlation of
each of these parameters and the soluble factor levels are required
to better understand the immunological mechanisms.
Both the antibody positivity rate and the magnitude
of IgG titers in pre-vaccination samples were largely different
among the 31 peptides. These divergence among the peptides was also
observed in the post-vaccination samples (the positive IgG
responses and magnitude of IgG titers). However, it was commonly
observed that the patients showing immune boosting to each of the
vaccinated peptides had a longer survival than those without it,
suggesting that all 31 of the peptides used in this study
maintained their ability to prolong clinical benefits through
immune boosting. Furthermore, the patients administered PPV that
included certain peptides exhibited a significantly longer survival
than the patients administered PPV without these peptides.
Therefore, the divergence among the peptides used for PPV may not
be a risk factor hampering the clinical benefits of PPV.
The results revealed that, among the vaccinated
peptides, only three peptides (Lck-486, EGFR-800 and HNRPL-140)
were significantly associated with OS. Although the underlying
mechanisms remain unclear, it was reported that a monoclonal
antibody reacting to the Lck-486 peptide exhibited an antitumor
activity in a murine model with suppression of T regulatory cells
at tumor sites (27). Lck (a
member of the Src family of non-receptor protein tyrosine kinases
expressed in both activated T lymphocytes and metastatic cancers
cells with oncogenic properties in human cancers), is pivotal for T
regulatory cells (Tregs) and program death-1-positive T-cell
activities (26). The anti-Lck-486
antibody augmented by the vaccination may have promoted the
antitumor activity in the present study on PPV. Monoclonal
antibodies to the other peptides, including EGFR-800 and HNRPL-140
have not yet been created.
The results revealed that the baseline neutrophil
and lymphocyte ratios affected the clinical benefits. Although the
reasons why the baseline neutrophil and lymphocyte ratios most
strongly affected the clinical benefits cannot be explained, the
following hypothesis could be considered. A tumor mass causes
inflammation to escape from immune attack (28) and promotes the production of
inflammatory cytokines (IL-6, VEGF, CRP and haptoglobin, etc.),
which in turn results in the circulation of a few lymphocytes along
with many neutrophils. Subsequently, fewer peptide-specific T cells
may enter the vaccinated lymph nodes for binding to HLA-peptide
complex on dendritic cells, which in turn results in the induction
of tolerogenic dendritic cells, suppressive macrophages and T
regulatory cells (29). The normal
range of the baseline lymphocyte ratio in cancer patients may thus
be one of the keys for successful immune induction (28-30).
The present study demonstrated that pre-vaccination
inflammatory signatures, but not those of post-vaccination immune
induction, were associated with lower clinical benefits of PPV. The
major limitation of the present study is the exploratory nature of
the retrospective analyses on 2,588 patients under PPV treatment,
which means that our results are not clinically applicable. Thus,
the present results are purely informative, but may facilitate the
better understanding of one of the mechanisms involved in the
long-lasting failure of peptide-based cancer vaccines to provide
clinical benefits enough to be approved in clinical trials since
the 1990s.
Supplementary Data
Abbreviations:
|
OS
|
overall survival
|
|
PPV
|
personalized peptide vaccination
|
|
HLA
|
human leukocyte antigen
|
|
CTLs
|
cytotoxic T lymphocytes
|
|
PS
|
performance status
|
|
MHC
|
major histocompatibility complex
|
|
FIU
|
fluorescence intensity units
|
|
NLR
|
neutrophil to lymphocyte ratio
|
|
MDSCs
|
myeloid derived suppressor cells
|
|
(R2)
|
correlation coefficients
|
Acknowledgements
Not applicable.
Funding
The present study did not receive specific funding,
but was performed as part of the Kurume University, Kurume Fukuoka,
Japan, and in part by Sendai Kousei Hospital, Sendai, Japan.
Availability of data and materials
The data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
SSuekane, SShichijo, SY, MNo and KI were involved in
the conception and design of the study. SSuekane, SY, SShichijo,
AY, SM, KI, NK and MNo were involved in the collection and assembly
of the data. SSuekane, SShichijo, MNo and KI were involved in data
analysis and interpretation, and were involved in the writing of
the manuscript. SSuekane, SY, TS, ST, UT, KK, KY, SSakamoto,
SSugawara, TY, MNa, MT, TM, KI and MNo were involved in the
clinical studies by treating cancer patients under personalized
peptide vaccination. All these 19 authors were involved in the
provision of study materials or patients. All have read and
approved the final manuscript.
Ethics approval and consent to
participate
All patient sample collections, patient consent and
recruitment followed protocols approved by the institutional review
board of Kurume University. The study was conducted according to
the principles of the Declaration of Helsinki. All patients
provided written informed consent for the study participation and
data collection.
Patient consent for publication
Not applicable.
Competing interests
MN has served as an advisory board consultant for
BrightPath Biotherapeutics Co. Ltd. TS received a grant from
BrightPath Biotherapeutics Co. AY is a part-time executive of
BrightPath Biotherapeutics Co. KI received research funding from
Taiho Pharmaceutical Company. KI and SShichijo gained income by
selling stock of BrightPath Biotherapeutics Co., Ltd. The other
authors have no competing interests to declare.
References
|
1
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Eng J Med.
366:2443–2454. 2012. View Article : Google Scholar
|
|
2
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Eng J Med. 366:2455–2465. 2012. View Article : Google Scholar
|
|
3
|
Rosenberg SA, Yang JC, Schwartzentruber
DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME,
Schwarz SL, Spiess PJ, et al: Immunologic and therapeutic
evaluation of a synthetic peptide vaccine for the treatment of
patients with metastatic melanoma. Nat Med. 4:321–327. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwartzentruber DJ, Lawson DH, Richards
JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K,
Pockaj B, et al: gp100 peptide vaccine and interleukin-2 in
patients with advanced melanoma. N Engl J Med. 364:2119–2127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bezu L, Kepp O, Cerrato G, Pol J, Fucikova
J, Spisek R, Zitvogel L, Kroemer G and Galluzzi L: Trial watch:
Peptide-based vaccines in anticancer therapy. OncoImmunol.
7:e15115062018. View Article : Google Scholar
|
|
6
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: A new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013. View Article : Google Scholar
|
|
7
|
Sasada T, Yamada A, Noguchi M and Itoh K:
Personalized peptide vaccine for treatment of advanced cancer. Curr
Med Chem. 21:2332–2345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kibe S, Yutani S, Motoyama S, Nomura T,
Tanaka N, Kawahara A, Yamaguchi T, Matsueda S, Komatsu N, Miura M,
et al: Phase II study of personalized peptide vaccination for
previously treated advanced colorectal cancer. Cancer Immunol Res.
2:1154–1162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noguchi M, Matsumoto K, Uemura H, Arai G,
Eto M, Naito S, Ohyama C, Nasu Y, Tanaka M, Moriya F, et al: An
open-label, randomized phase II trial of personalized peptide
vaccination in patients with bladder cancer that progressed after
platinum-based chemotherapy. Clin Cancer Res. 22:54–60. 2016.
View Article : Google Scholar
|
|
10
|
Yoshimura K, Minami T, Nozawa M, Kimura T,
Egawa S, Fujimoto H, Yamada A, Itoh K and Uemura H: A phase 2
randomized controlled trial of personalized peptide vaccine
immunotherapy with low-dose dexamethasone versus dexamethasone
alone in chemotherapy-naive castration-resistant prostate cancer.
Eur Urol. 70:35–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noguchi M, Fujimoto K, Arai G, Uemura H,
Hashine K, Matsumoto H, Fukasawa S, Nakatsu H, Takenaka A, Fujisawa
M, et al: Personalized peptide vaccination for castration-resistant
prostate cancer progressing after docetaxel: A randomized,
double-blind, placebo-controlled, phase III trial. J Clin Oncol.
37(Suppl 15): S50332019. View Article : Google Scholar
|
|
12
|
Narita Y, Arakawa Y, Yamasaki F, Nishikawa
R, Aoki T, Kanamori M, Nagane M, Kumabe T, Hirose Y, Ichikawa T, et
al: A randomized, double-blind, phase III trial of personalized
peptide vaccination for recurrent glioblastoma. Neuro Oncol.
21:348–359. 2019. View Article : Google Scholar :
|
|
13
|
Shirahama T, Muroya D, Matsueda S, Yamada
A, Shichijo S, Naito M, Yamashita T, Sakamoto S, Okuda K, Itoh K,
et al: A randomized phase II trial of personalized peptide vaccine
with low dose cyclophosphamide in biliary tract cancer. Cancer Sci.
108:838–845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suekane S, Ueda K, Nishihara K, Sasada T,
Yamashita T, Koga N, Yutani S, Shichijo S, Itoh K, Igawa T and
Noguchi M: Personalized peptide vaccination as second-line
treatment for metastatic upper tract urothelial carcinoma. Cancer
Sci. 108:2430–2437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takayama K, Sugawara S, Saijo Y, Maemondo
M, Sato A, Takamori S, Harada T, Sasada T, Kakuma T, Kishimoto J,
et al: Randomized phase II study of docetaxel plus personalized
peptide vaccination versus docetaxel plus placebo for patients with
previously treated advanced wild type EGFR non-small-cell lung
cancer. J Immunol Res. 2016:17451082016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noguchi M, Koga N, Moriya F, Suekane S,
Yutani S, Yamada A, Shichijo S, Kakuma T and Itoh K: Survival
analysis of multiple peptide vaccination for the selection of
correlated peptides in urological cancers. Cancer Sci.
109:2660–2669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mine T, Sato Y, Noguchi M, Sasatomi T,
Gohara R, Tsuda N, Tanaka S, Shomura H, Katagiri K, Rikimaru T, et
al: Humoral responses to peptides correlate with overall survival
in advanced cancer patients vaccinated with peptides based on
pre-existing, peptide-specific cellular responses. Clin Cancer Res.
10:929–937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sproston NR and Ashworth JJ: Role of
C-reactive protein at sites of inflammation and infection. Front
Immunol. 9:7542018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murphy K and Weaver C: Immunobiology
Zayets E (eds) Chapter 9, Garland New York and London. Science
Taylor and Francis Group; pp. 345–398. 2017
|
|
20
|
Komatsu N, Matsueda S, Tashiro K, Ioji T,
Shichijo S, Noguchi M, Yamada A, Doi A, Suekane S, Moriya F, et al:
Gene expression profiles in peripheral blood as a biomarker in
cancer patients receiving peptide vaccination. Cancer.
118:3208–3221. 2012. View Article : Google Scholar
|
|
21
|
Noguchi M, Moriya F, Koga N, Matsueda S,
Sasada T, Yamada A, Kakuma T and Itoh K: A randomized phase II
clinical trial of personalized peptide vaccination with metronomic
low-dose cyclophosphamide in patients with metastatic
castration-resistant prostate cancer. Cancer Immunol Immunother.
65:151–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dolan RD, Laird BJA, Horgan PG and
McMillan DC: The prognostic value of the systemic inflammatory
response in randomised clinical trials in cancer: A systematic
review. Crit Rev Oncol Hematol. 132:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shaul ME and Fridlender ZG: Cancer-related
circulating and tumor-associated neutrophils-subtypes, sources and
function. FEBS J. 285:4316–4342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ritter B and Greten FR: Modulating
inflammation for cancer therapy. J Exp Med. 216:1234–1243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsueda S, Itoh K and Shichijo S:
Antitumor activity of antibody against cytotoxic T lymphocyte
epitope peptide of lymphocyte-specific protein tyrosine kinase.
Cancer Sci. 109:611–617. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dvorak HF: Tumors: Wounds that do not
heal-redux. Cancer Immunol Res. 3:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steinman RM, Howiger D and Nussenzweig MC:
Tolerogenic dendritic cells. Annu Rev Immunol. 21:685–711. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Basile D, Garattini SK, Bonotto M, Ongaro
E, Casagrande M, Cattaneo M, Fanotto V, De Carlo E, Loupakis F,
Urbano F, et al: Immunotherapy for colorectal cancer: Where are we
heading? Expert Opin Biol Ther. 17:709–721. 2017. View Article : Google Scholar : PubMed/NCBI
|