BMPs serve important roles in tumourigenesis,
disease progression and the metastasis of various solid tumours
(7-10). BMP signalling has been found to be
both oncogenic and tumour suppressing, depending on context. For
example, studies have shown that BMPs are upregulated in certain
tumours, particularly those originating from soft tissues such as
osteosarcomas, chondrosarcoma, ameloblastoma and salivary tumours
(11-14). They are actively involved in cancer
development and metastasis (7-10).
BMP-6 overexpression in prostate cancer is associated with
osteoblastic bone metastasis (7).
BMP-4 may promote the invasion and motility of breast cancer cells
via upregulation of matrix metal-loproteinase (MMP)1 and C-X-C
chemokine receptor 4 (8). The
above studies indicate an oncogenic effect of BMPs in certain solid
tumours. In contrast, impairments in BMP signalling observed in
colorectal cancers and polyposis syndromes suggest a tumour
suppressor role in these situations (15). Our previous study reported that
BMP-10 inhibits prostate cancer cell growth by promoting apoptosis
via Smad-independent signalling, and that it can also reduce the
invasiveness and motility of cancer cells (9). BMP-4 can also reduce the capacity of
myeloid- derived suppressor cells to prevent metastasis of breast
cancer cells (10). It appears
that the same BMPs may have varied roles in different types of
tumour, potentially due to the involvement of distinct downstream
molecules.
As pleiotropic growth factors, BMPs are actively
involved in tumorigenesis, disease progression and metastasis, not
only directly due to their own signalling pathway, but also via
complex interactions with other growth factors and other signalling
pathways (16-24). More importantly, BMP-mediated
interactions between cancer cells and the local environment also
occur during the development of both the primary tumour and
metastasis, forming a large, intricate network that promotes the
epithelial to mesenchymal transition (EMT) of tumours, remodelling
of tumour-associated extracellular matrix (ECM), angiogenesis and
bone metastasis.
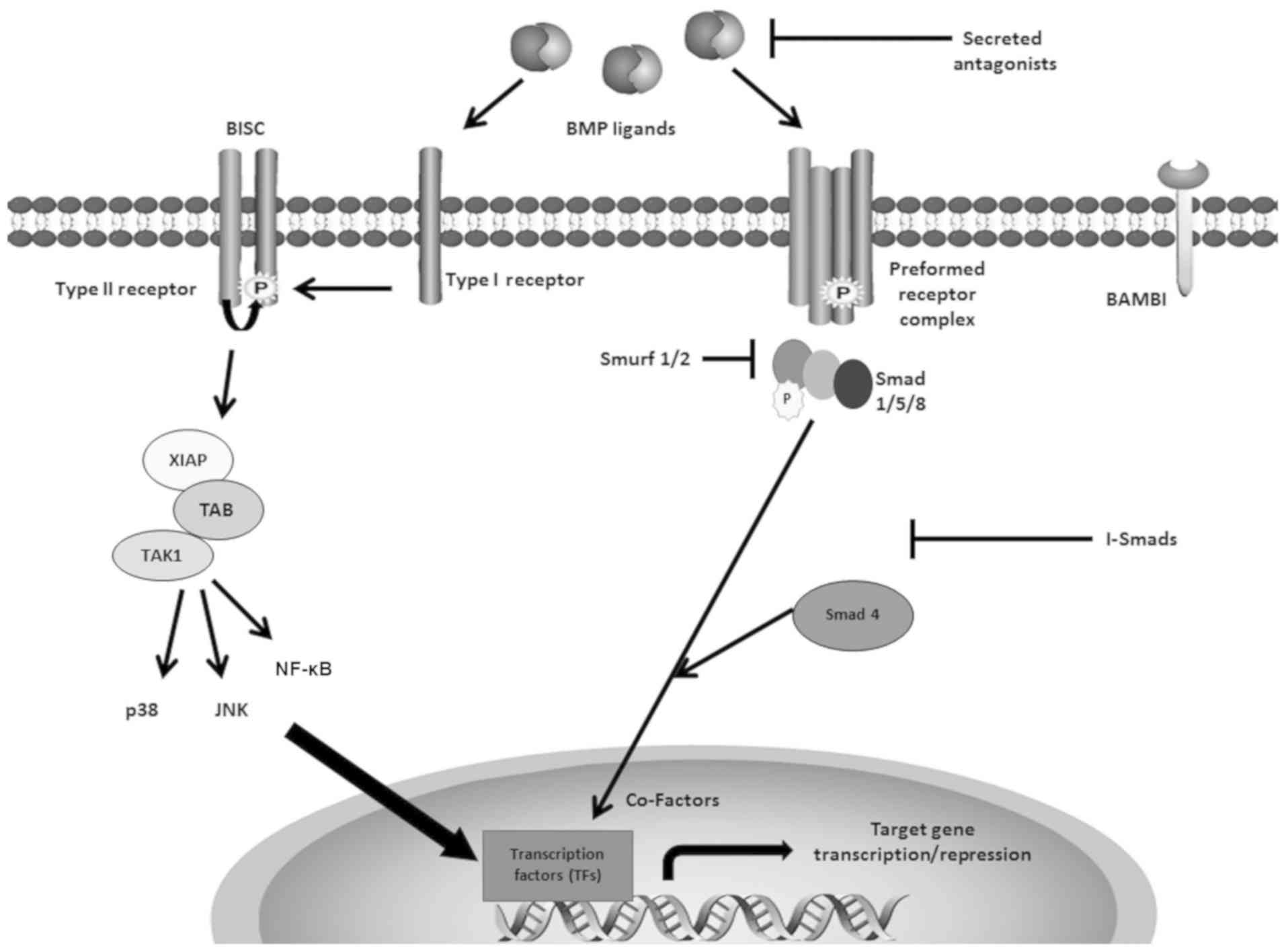
Both type I receptors [activin A receptor type I
(ACVR)-like 1, ACVR1, BMP receptor (BMPR)1A, ACVR1B, TGFβ receptor
(TGFβR)1, BMPR1B and ACVR1C] and type II receptors (TGFβR2, TGFβR3,
BMPR2, ACVR2A and ACVR2B) are indispensable for signal transduction
of TGFβ (25). The type I
receptors are also respectively known as activin receptor-like
kinase (ALK)1-7. Certain type I receptors (ALK1, ALK3 and ALK6)
exhibit a higher binding affinity to BMPs (25). Smad-dependent signalling will be
induced by the preformed hetero-oligomeric complexes (PFC) upon
binding with BMP ligands (26,27).
Alternatively, upon binding between BMP ligands and type I
receptors, type II receptors are then recruited, leading to the
formation of BMP-induced signalling complexes, which activate the
Smad-independent pathway (26,27).
As transcription factors, Smad proteins are vital
for intracellular transduction of BMP signalling (25,27,28).
There are three subgroups of Smad proteins: Smad 1, 2, 3, 5 and 8
are pathway-restricted Smads (R-Smads); Smad 4 is known as a common
mediator Smad; and Smad 6 and 7 are inhibitory Smads (I-Smads)
(27,28). After BMP homodimers or heterodimers
bind to the PFC, the glycine-serine region of type I receptors is
phosphorylated by the type II receptor, leading to the activation
and translocation of R-Smads (Smad 1, 5 and 8) into the nucleus,
and regulation of BMP-responsive genes such as Id1-3, Smad 6/7,
type I collagen, JunB and Mix.2 (25). Smad 4 translocates the signal
complex into the nucleus, and Smad 6 and 7 act as inhibitory
factors for the signal transduction through the Smad-dependent
pathway (Fig. 1) (25,29).
There is greater affinity between BMPs and type I
receptors compared with type II receptors (25). Thus, BMP ligands are also able to
bind to ALK3 or ALK6, and then recruit BMPR2 into a
hetero-oligomeric complex; this activates the Smad-independent
pathway (25-27). The X-linked inhibitor of apoptosis
protein acts as an adaptor protein to relay signalling from the
type I receptor to downstream TGFβ-activated binding protein,
leading to activation of TGFβ-activated tyrosine kinase 1 (30-32).
BMP-4 can induce apoptosis through this Smad-independent pathway,
in which p38, a mitogen-activated protein kinase (MAPK) (26,33,34),
Jun N-terminal kinases (JNKs), NF-κB and Nemo-like kinase (35-37)
are involved (Fig. 1).
Regulation of BMP pathway activity can be mediated
through several positive or negative modulators, which may be
extracellular when ligands bind to receptors, intracellular when
the signal is being relayed or intranuclear when modulating
R-Smad-mediated regulation of BMP-responsive genes (25).
Secreted extracellular BMP antagonists, including
Noggin, Gremlin, Chordin and twisted gastrulation-1, provide
important regulation (25). These
antagonists exert their regulatory role in two ways. BMP
antagonists can prevent BMPs from the binding to receptors by
binding directly to BMP ligands, thus preventing ligand-receptor
interaction (Fig. 1) (25). Antagonists are often target genes
of BMP signalling; thus, a negative regulatory feedback loop is
formed to ensure signalling homeostasis (38). For example, it has been shown that
BMP-2, 4 and 6 can induce Noggin expression in osteoblasts
(39). By upregulating their
antagonist expression, the BMPs are thus able to regulate their
activity (39).
Other factors also regulate BMP signalling
extracellularly, such as pseudoreceptors and co-receptors. For
example, BMP and activin membrane-bound inhibitor (BAMBI) acts as a
pseudoreceptor by competitively binding to the BMP ligands with its
extracellular domain, which shares high homology with type I
receptor; however, as it lacks intracellular domains, the signal is
not transduced (40). Similar to
the BMP antagonists, BAMBI can be induced by BMP-4 in mouse
embryonic fibroblasts, leading to negative feedback regulation of
BMP signalling (41).
In addition to these negative regulators, there are
positive regulators for the BMP pathway, such as co-receptors,
which enhance BMP signalling (25,42-44).
Previous studies showed that repulsive guidance molecules (RGMs;
including RGMA, RGMB and RGMC) are co-receptors for BMP-2 and
BMP-4. RGMB, also known as Dragon, can bind directly to BMP-2 and
BMP-4, enhancing signalling (42-44).
Among the intracellular regulatory factors, I-Smads
can prevent R-Smads from the binding to the activated type I
receptors, as well as blocking the recruitment of Smad 4 to the
activated R-Smads (Fig. 1). For
example, it has been reported that Smad 6 and 7 can weaken BMP
signalling by preventing Smad 1 and 5 activation by the type I
receptor, and that they can also prevent the interaction between
Smad 1/5 and Smad 4 (45). In
addition, BMP signalling can induce Smad 6/7 expression, enhancing
the negative regulation of further BMP signalling (46,47).
Secondly, as Smads exhibit low binding affinity to the Smad binding
elements (SBEs) of target genes, other transcription factors are
required for the regulation of BMP-responsive genes, such as Smad
interacting protein-1 (48),
activating transcription factor (ATF)2 (49), p53 (50), Runx (51) and Forkhead box HI (FOXHI); FOXHI
can specifically help recruit activated Smad 2/4 to the promoters
of target genes in TGFβ signalling (52). Additionally, the interactions
between certain transcriptional co-activators/repressors and the
MH2 binding domain of Smad have been shown to regulate BMP. For
example, P300 and CREB-binding protein interactions with Smads can
increase the transcription of target genes by making the
transcriptional machinery more accessible (53). However, transcriptional
co-repressors, including Ski and Ski related novel gene, ecotropic
viral integration site-1, TG interacting factor (TGIF)1 and TGIF2,
prevent Smad 3/4 from binding to the SBE of BMP-responsive genes
(54-57). Lastly, the BMP pathway can be
influenced by Smad ubiquitination regulatory factor (Smurf)1/2,
which induce degradation of Smads (Fig. 1) (58). The regulatory factors that
co-ordinate BMP signal transduction have been summarised previously
(59).
BMP and its signalling pathways are not isolated in
normal tissues and tumours, but are intricately linked to numerous
other growth factors, such as the epidermal growth factor (EGF)
receptor (EGFR) (16), receptor
tyrosine kinase (RTK)/MAPK (17-19),
PI3K/Akt (20-24,60),
Wnt (61-65) and hepatocyte growth factor
(HGF)/Met pathway (66,67); together, they form a vast network
that regulates various biological functions. There are multiple
levels where cross-talk can occur: By regulating ligands,
antagonists, receptors, or signalling components expression or
activities; by direct interactions with Smads or other signalling
components (68); and by
incorporating into transcription complexes that alter target gene
expression (69-71).
EGFR is regarded as an oncogenic factor belonging to
the ErbB RTK family, and is overexpressed in various types of
cancer, such as colorectal cancer, non-small cell lung cancer,
gastric cancer, esophagogastric cancer and pancreatic cancer
(72). Intracellular signalling of
EGFR is generally mediated through PI3K/Akt, Ras/MAPK and the
phospholipase C/protein kinase C (PKC) signalling cascades
(73), which are critical for cell
proliferation, differentiation, motility and survival (74).
Studies have shown that EGF can directly influence
the expression of BMPs. For example, BMP-6 in MCF-7 breast cancer
cells can be induced by EGF/EGFR signalling (16). The function of the BMP pathway can
also be indirectly regulated intracellularly by signalling
molecules downstream of the EGFR, including the RTK/MAPK pathway
and the PI3K/Akt pathway.
RTK/MAPK signalling can regulate BMP function.
Secretion of additional growth factors and cytokines which promote
EMT and cell invasion can often result from the interaction between
TGFβ and RTK/MAPK pathways (17-19,75,76).
ERK has been shown to upregulate Smad 3 in epithelial and smooth
muscle cells (77).
The linking region of Smad proteins plays a vital
role in interactions between BMP signalling and RTK/MAPK pathways.
For example, activation of oncogene Ras can restrict BMP-induced
Smad 2/3 signalling, including translocation into the nucleus and
binding to the target genes (78).
RTK-induced activation of ERK or JNK can phosphorylate endogenous
Smad 2/3 (75,76). Furthermore, Thr178, Ser203 and
Ser207 within the linker region of Smad 3 can be phosphorylated by
ERK, leading to suppression of nuclear translocation (79). However, in MCF10CA1h breast cancer
cells, p38 MAPK-induced phosphorylation of the Ser203 and Ser207
residues of Smad 3 facilitate, rather than inhibit, BMP-induced
growth inhibition (80). These
results suggest that varied phosphorylation of the Smad 2/3 linker
region can lead to different results depending on the specific
kinase, as well as the specificity of phosphorylation sites in
intracellular events downstream of those activated receptors
(81).
ERK1/2 can also prevent the nuclear translocation of
Smad 1/5 via similar phosphorylation of their linker region
(81). Furthermore, the oncogene
Ras can reduce the stability of Smad 4 via the ERK pathway
(82). Conversely, activation of
JNK and p38 can target a tumour-associated mutant Smad 4, leading
to degradation of the protein (83). There is suggested involvement of
ERK, JNK and p38 in the regulation of Smad 7 transcription
(84-86).
In addition to the above direct effects, MAPKs can
also indirectly affect the activity of the BMP pathway by
phosphorylating other nuclear transcription factors involved in the
pathway within the nucleus, including Jun and activator protein-1
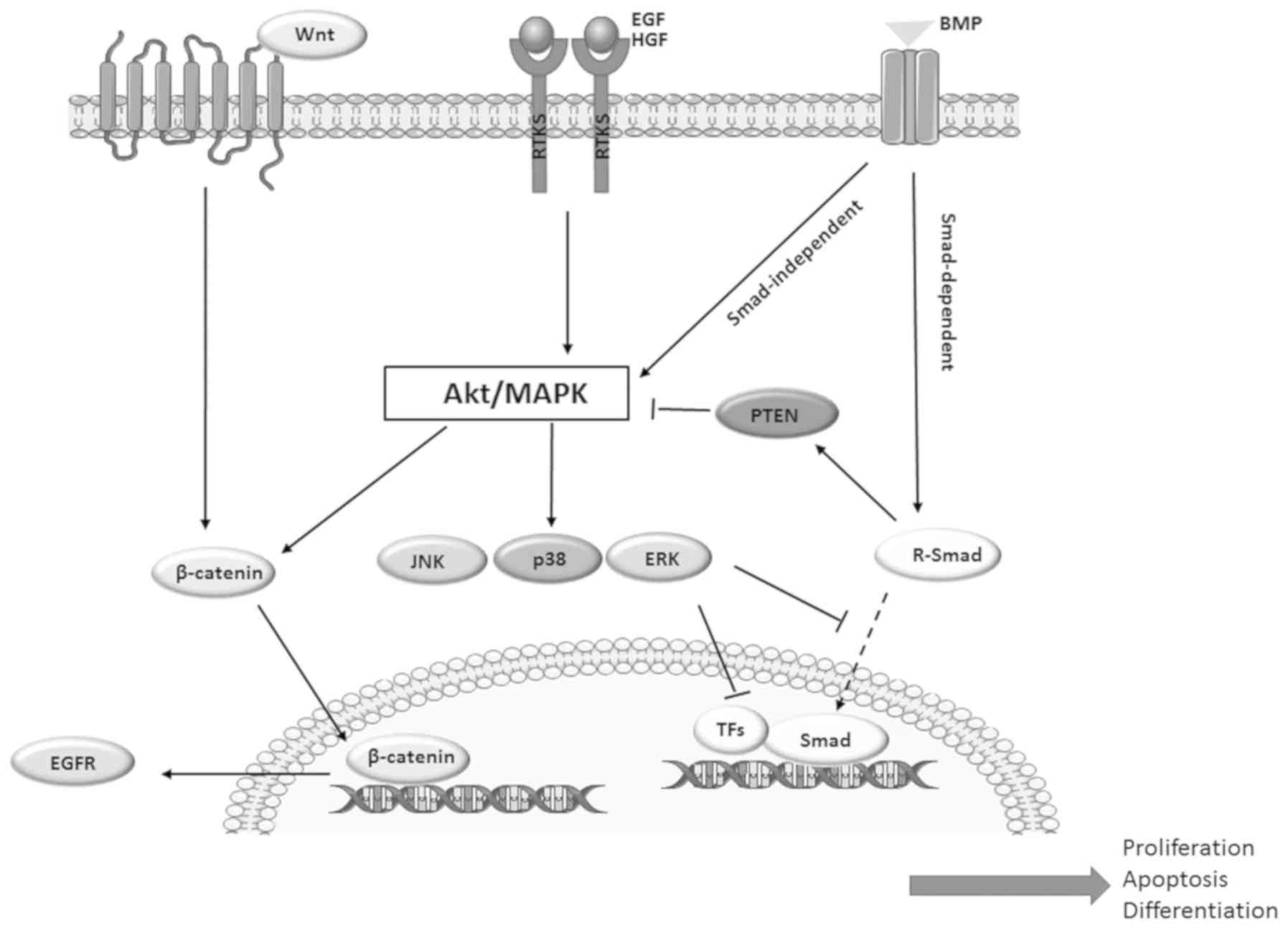
proteins such as Maf, Fos and ATFs (87). For example, p38 MAPK can activate
ATF1, ATF2 and ATF3, which bind Smads and participate in
BMP-regulated activities (Fig. 2)
(49,88-91).
Various studies have shown that BMP signalling can
regulate the PI3K/Akt pathway, affecting cell proliferation
(20), invasion (21), migration (22), EMT (92,93)
and differentiation (94). This
regulation can be achieved via activation of Smad-independent
pathways (23,24). Secondly, BMP signalling can
regulate the PI3K/Akt pathway by altering the transcriptional level
or activity of PTEN. For example, Beck and Carethers (60) showed that long-term exposure to
BMP-2 downregulated PTEN in Smad 4-null colon cancer cells through
the Ras/ERK pathway. Previous studies showed BMP signalling could
enhance PTEN activity (95,96).
Conversely, in hematopoietic cells, BMP/Smad signalling can also
suppress Akt activity via regulation of SH2 domain-containing 5′
inositol phosphatase, which is a lipid phosphatase targeting
phosphatidylinositol (3,4,5)-trisphosphate (Fig. 2) (97). Furthermore, PI3K/Akt activation
could promote the nuclear translocation of β-catenin (98,99),
increase transcription of EGFR and enhance EGFR signalling, forming
a vicious circle comprising Akt, β-catenin and EGFR (Fig. 2).
HGF is a regulator of cell motility, mitogenesis,
morphogenesis and angiogenesis (100). HGF and its receptor c-Met are
actively involved in tumour growth, invasion and metastasis
(101). Targeting HGF/c-Met can
inhibit the proliferation and invasion of cancer cells both in
vitro and in vivo (101-106).
There have been studies reporting an interaction
between the BMP and HGF signalling. For example, Ye et al
(29,100) reported that BMP-7, BMPRIB and
BMPR2 were upregulated in prostate cancer cells. Imai et al
(66) also showed that HGF was
able to regulate BMP receptors. A recent study showed that HGF
promoted bone regeneration and the formation of new blood
vasculature via upregulation of BMP-2 (67). However, the exact transcriptional
regulatory mechanism remains unclear. Further investigation is
required to determine how the interaction between BMP and HGF is
involved in bone metastasis.
The Wnt signalling pathway is essential for cell
proliferation, differentiation, migration, survival and other
processes (68). Dysregulated Wnt
signalling has been observed in colorectal cancer and leukaemia
(113). The Wnt signalling
pathway has been extensively studied and reviewed, and comprises
canonical and non-canonical pathways, the latter of which include
the planar cell polarity pathway and Wnt/calcium pathway (61).
In terms of the canonical pathway, upon binding with
Wnt ligand, Frizzled receptors and the transmembrane protein
low-density lipoprotein receptor-related protein 5/6 induce
intracellular signalling and regulation of responsive genes through
β-catenin (68). Outside of Wnt
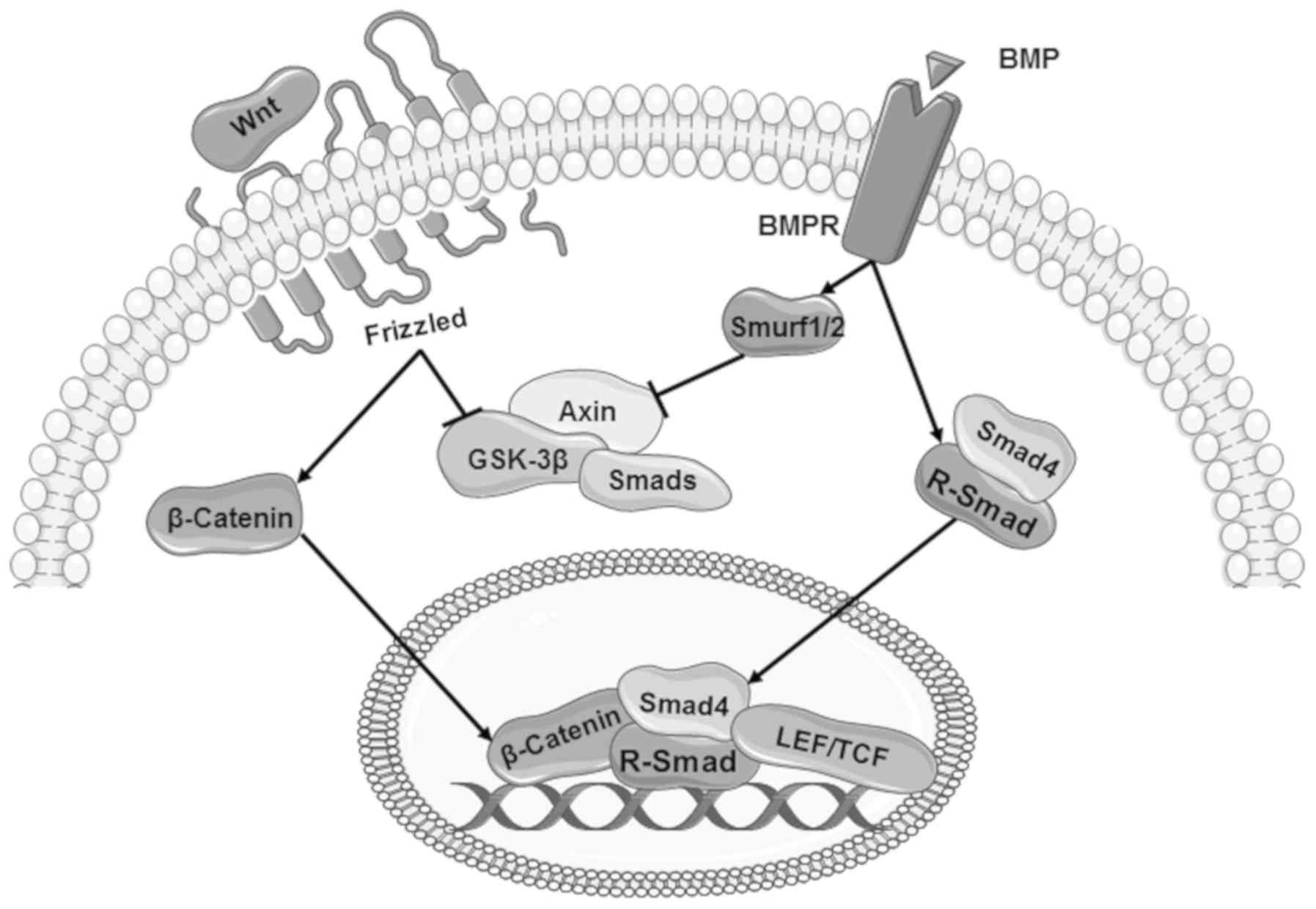
signalling, β-catenin is generally degraded by a protein complex
which comprises adenomatous polyposis coli, Axin, casein kinase 1α
and glycogen synthase kinase 3β (GSK-3β) (61). Degradation of β-catenin is
prevented when GSK-3β and Axin are recruited via the Wnt
signalling, leading to nuclear translocation and regulation of Wnt
target genes (62-65). Crosstalk between the BMP pathway
and the Wnt pathway can occur at multiple levels.
The Wnt signalling pathway can regulate the
expression of BMPs, BMP co-receptors or their antagonists during
embryonic development and in cancerous cells (81). Conversely, BMP-2 and BMP-4 are able
to regulate the expression of certain Wnt proteins, such as Wnt-7c
(89) and Wnt-8 (114).
GSK-3β can regulate the BMP pathway by
phosphorylating the linker region of Smad (68,115-117). In the absence of upstream
signalling, Smad 3 can be degraded by GSK-3β when it is recruited
into a protein complex comprising Axin and GSK-3β (68,116). GSK-3β can also target the
BMP-activated R-Smads, Smad 1 or Smad 3, leading to their
degradation and the inhibition of downstream signalling (68). However, the regulation of Smad by
GSK-3β can be prevented by Wnt signalling, leading to a
stabilisation of Smad proteins (Fig.
3) (68).
Certain molecules in the BMP pathway are also
involved in the regulation of Wnt signalling, such as Smurf1
(118) and Smurf2 (119). Smurf1 and Smurf2 are key
molecules in the degradation of Axin, which may consequently
disrupt the Wnt signalling. In addition, Smad 3 is also involved in
the nuclear translocation of β-catenin (Fig. 3) (120).
In response to Wnt signalling and BMP signalling,
activated transcriptional factors such as Smads, T cell
factor/lymphoid enhancer-binding factor 1 and cofactors can
co-ordinate the regulation of target genes, including gastrin
Xtwin, Msh homeobox (Msx)2 and T-box transcription factor 6
(Fig. 3) (69-71).
In addition to the above, there are also
interactions between the BMP pathway and other pathways, including
the Hedgehog (Hh) pathway (121-124), Notch pathway (125-128), Janus kinase/STAT pathway
(129-133) and NF-κB pathway (134-136). For example, Smads can co-ordinate
Hh signalling through regulation of GLI (124). BMP and Notch orchestrate cell
differentiation and proliferation by targeting common genes
(125). BMP and NF-κB act against
each other in co-ordinating immune responses (133).
Overall, the BMP pathway is integrated into various
signalling networks through these interactions, thus orchestrating
cellular events in tumourigenesis and the progression of
malignancies.
Angiogenesis is essential for the tumour growth and
haematological dissemination of cancer cells (137,138). There are two stages in the
progression of neovascularisation, an activation phase and a late
phase (25). ALK1 and downstream
Smad signalling are involved in the activation phase, whilst ALK5
and Smad 2/3 promote maturation of the newly formed vascu-lature at
the late phase (139). It has
been shown that BMPs can affect angiogenesis via both direct and
indirect routes.
BMP-2, 4, 6 and 7, and growth differentiation factor
(GDF)-5 can directly regulate the proliferation and migration of
vascular endothelial cells (140-143). For instance, in a chorioallantoic
membrane assay, GDF-5 promotes angiogenesis (140). BMP-2 exhibits pro-angiogenic
effect in both in vivo tumour models (144) and in vitro functional
assays of vascular endothelial cells (145). In addition to direct effects on
vascular endothelial cells, BMP-2 can also promote the motility of
vascular smooth muscle cells (146). BMP-4 and BMP-7 can also promote
the migration of vascular smooth muscle cell (147,148). Of note, BMP-9/-10 elicit
concentration-dependent biphasic effects on angiogenesis,
specifically an inhibitory effect at high concentrations and a
promotive effect at lower concentrations (Fig. 4) (149).
BMP receptors are important mediators of the
pro-angiogenic BMP signal. For example, vascular endothelial cells
exhibited higher expression of BMPRIB and BMPR2 in an in
vitro tubule formation assay (150).
Studies have shown that distinct Smad pathways may
play opposing roles in angiogenesis, and that the same Smad may
also play different roles in angiogenesis for distinct types of
tissues. For example, Smad 3 mediates an upregulation of vascular
endothelial growth factor A (VEGFA), whereas Smad 2 is involved in
the regulation of thrombospondin-1 in rat proximal tubular cells
NRK52E (151). However, Smad
3-mediated repression of VEGF impaired angiogenesis induced by the
gastric cancer cell line SNU484 (152).
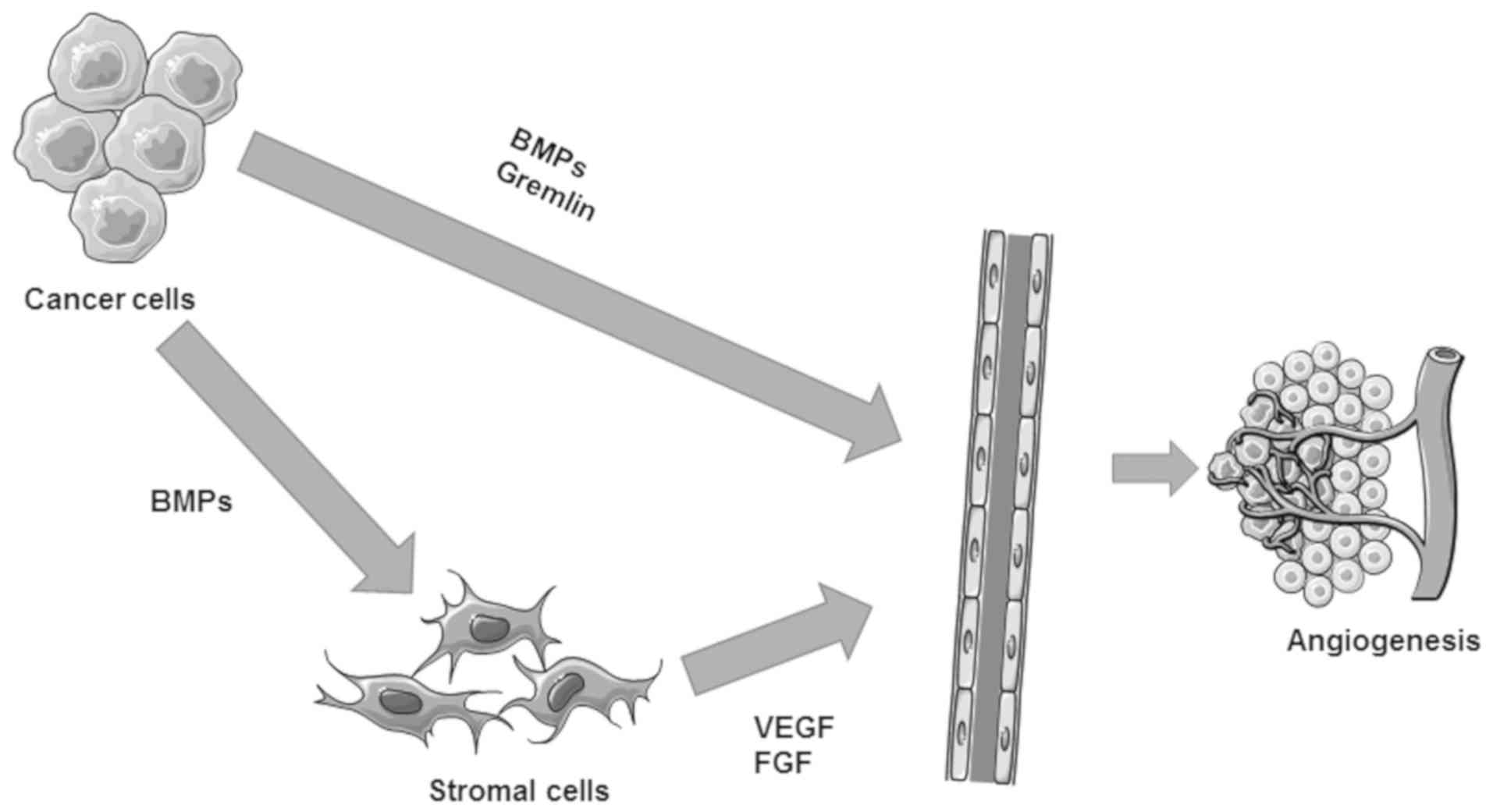
As antagonists of BMPs, Noggin and Gremlin are also
key regulators of tumour angiogenesis. Noggin can prevent
BMP-7-induced angiogenesis (153); conversely, Gremlin can promote
angiogenesis by directly targeting VEGF receptors (154).
In addition to these direct effects, BMPs can also
indirectly promote angiogenesis via upregulation of VEGF in other
cells, such as cancer cells and stromal cells (138). For example, BMP-7 is actively
involved in the bone metastasis of prostate cancer cells via
regulation of VEGF (153), in
addition to its direct regulation of VEGF receptor in vascular
endothelial cells (155). BMP-2
promotes tumour-associated angiogenesis via upregulation of VEGF
mediated by the p38 pathway in breast cancer (156). In contrast to most BMPs, BMP-9
elicits inhibition of the proliferation of vascular endothelial
cells through ALK-1 (157). In
addition, BMPs can indirectly induce VEGF (158), basic fibroblast growth factor and
TGFβ1 in stromal cells (Fig. 4)
(159).
EMT is pivotal for the carcinogenesis and aggressive
traits acquired by cancer cells during disease progression and
metastasis (160,161). BMP-regulated EMT has been
implicated in various studies regarding organ development (162,163) and cancer (164-167). In vitro, BMP-4 induces
EMT-like properties in mammary epithelial cells, transforming them
to express an invasive phenotype (165). BMP-2 can enhance the invasion and
migration of breast cancer cells (168,169), and the effect may be mediated by
the upregulation of ID-1 (170).
However, there are other BMPs that play opposing role, such as
BMP-7, which was not able to regulate the EMT in a murine mammary
epithelial cell line, NMuMG (166). BMP-7 can prevent EMT in breast
cancer cells by decreasing vimentin (171). BMP-6 can impair the metastatic
capacity of breast cancer cells by repressing miR-21 and zinc
finger E-box-binding homeobox 1 (ZEB1), which subsequently leads to
upregulation of E-cadherin (167,172,173). Both Smad-dependent (174-176) and Smad-independent pathways
(178,179) have been observed to be involved
in BMP-regulated EMT. For example, BMP signalling could directly
activate the transcription of Snail, Twist1 and Msx1/2 (174-176). Regarding the Smad-independent
pathway, BMP-2 could induce EMT via the PI3K/Akt pathway (177,178). Furthermore, BMPs could influence
tumour invasion by regulating MMPs, extracellular matrix
components, cytokines, and immune or inflammatory cells in the
tumour microenvironment (158).
BMP-4-regulated MMP3 and interleukin-6 are involved in the
fibroblast-stimulated invasion of breast cancer cells (179).
BMPs play an important role in co-ordinating the
interactions between cancer cells and the surrounding environment
in tumourigenesis and disease progression (158,180). For example, BMP released from
tumour-associated stromal cells can induce EMT in cancer cells via
the induction of ZEB1 (158).
Meanwhile, BMP-2 and BMP-4 secreted by breast cancer cells can
reciprocally act on stromal cells to synthesise more tenascin-W and
MMPs, which can further enhance their invasiveness (158,180). However, BMP-6, BMP-10 and BMP-15
are able to inhibit the invasion and motility of cancer cells,
while BMP-4 exhibits biphasic effects (158).
A number of cells located within tissues are
embedded in the ECM, which comprises collagens, proteoglycans and
adhesion proteins (181). The ECM
is very versatile and undergoes remodelling during tumour
development (181,182). Within the tumour stroma, both the
cancer cells and cancer-associated fibroblasts can remodel the ECM
(182). Growth factors and
cytokines will be released to the ECM, thus contributing to the
tumour-supporting microenvironment (182), which is actively involved in
disease progression and metastasis. Studies have shown that the
remodelling of ECM can be regulated by BMP (183,184). For example, secretion of collagen
type I and type III from hepatic stellate cells can be reduced by
recombinant human BMP-7 via inhibition of TGFβ1 and its signalling
(183). Another study showed that
Type I and type III collagen synthesis was significantly
up-regulated following BMP-2 treatment in human scleral fibroblasts
(184).
CCN proteins can directly interact with BMPs; for
example, binding of CCN2 to BMP-4 prevents its interaction with BMP
receptors, thus inhibiting BMP-induced cell proliferation (199). In addition, there have been
reported interactions between CCN3 and BMP-2 (200), CCN4 and BMP-2 (201), and CCN6 and BMP-4 (202). CCN proteins may act as both
antagonists and agonists for BMP signalling, depending on the
expression profile of related molecules (189,203,204). CCN2 promotes the proliferation of
chondrocytes via ERK and JNK signalling pathways, and induces
differentiation via p38 (189,203). BMP-2 can suppress the
phosphorylation of ERK1/2, which impairs CCN2-promoted
proliferation (204). Similarly,
CCN2 can abolish BMP-2-promoted cell proliferation by inhibiting
Smad-dependent and independent pathways (205).
In addition, studies have shown that certain
non-coding RNAs play roles in the interaction between the tumour
microenvironment and BMPs. For example, Xiao et al (206) reported that microRNA
(miRNA/miR)-885-3p inhibits the in vivo growth of HT-29
colon cells by disrupting angiogen-esis via targeting BMPR1A,
leading to a blockage of BMP signalling. Nishida et al
(207) found that miR-17-92a and
miR-106b-25 clusters were upregulated in colorectal cancer stromal
tissues compared with normal stroma; putative targets of these
miRNAs predicted by Target Scan were significantly downregulated in
cancer stromal tissues, including TGFβR2, Smad 2 and BMP family
genes.
BMPs enriched in bone matrix are the most potent
factors to induce the formation of new bone (58). Numerous studies have reported that
BMPs are expressed to varying degrees in a range of benign and
malignant bone tumours, such as osteoid osteoma (208), fibrous dysplasia (209), giant-cell tumours (210) and osteosarcoma (211). BMP expression was detected in
both human osteosarcoma cell lines (212,213) and human osteosarcoma specimens
(214,215). Furthermore, differential
expression of BMPs was evident in different histopathological
subtypes (215). For example,
Yoshikawa et al (215)
found that high-grade osteosarcoma with a malignant fibre
histio-sarcoma-type pattern exhibited the strongest expression of
BMP-2/4. Additionally Sulzbacher et al (216) reported that BMPs are expressed in
osteosarcoma specimens, and their expression is related with
osteosarcoma histopathological subtype; high expression of BMP-6
was detected in osteosar-comas with chondroblastic differentiation.
Aside from this aberrant expression, little is known regarding the
biological function of BMPs in bone tumour cells. Li et al
(217) showed that BMP-9
inhibited tumour growth and migration by blocking the PI3K/AKT
signalling pathway in an osteosarcoma cell line.
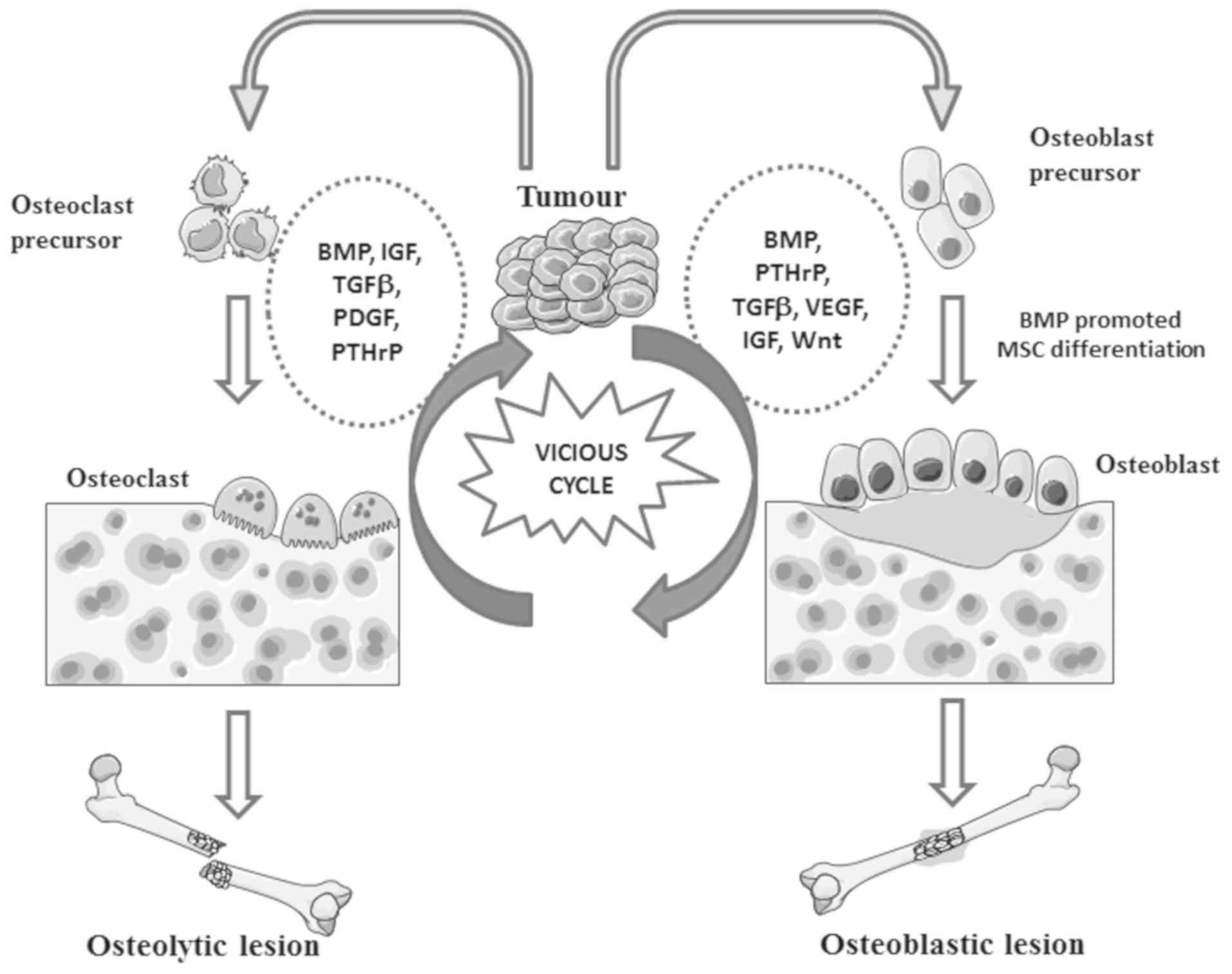
In bone metastatic tumours, BMPs can be synthesised
by both cancer cells and osteoblasts (218). There is increasing evidence
showing that BMPs are implicated in bone metastases of prostate and
breast cancer (156,219,220). BMPs are expressed in both primary
prostate tumours and bone metastases with different phenotypic
patterns. For example, BMP-7 and GDF-15 are reduced in or absent
from primary prostate tumours, but overexpression of both molecules
is evident in the bone metastases (219,220). In contrast, BMP-6 is consistently
expressed at high levels in both primary tumours and bone
metastases of prostate cancer (138). The expression profiles of BMP in
primary tumours and bone metastases reflects an adaptive phenotype
acquired by the cancer cells during disease progression based upon
requirements at different metastatic locations. Elevated expression
of BMP in cancer cells is more likely to result in osteoblastic
bone lesion by enhancing bone formation (138). In addition to BMP ligands, the
BMP antagonist Noggin has been associated with the osteolytic bone
lesions of prostate cancer in a murine model (221). Moreover, loss of Noggin can also
enhance osteoblastic activity in bone metastasis (222).
BMPs released from cancer cells can regulate
osteoblastic or osteoclastic activities in bone lesions, leading to
bone formation or resorption. BMPs secreted by
osteoblasts/osteoclasts or released from disrupted bone can
reciprocally induce EMT in cancer cells, promoting the development
of bone lesions (218). These
interactions form a vicious cycle during the development of bone
metastasis (Fig. 5). However, the
exact machinery underlying the regulation of BMP signalling
utilised by the cancer cells requires more intensive
investigation.
In addition to direct stimulation, BMPs can also
enhance the vicious cycle during bone metastasis via regulation of
other factors. For example, osteoprotegerin can be upregu-lated by
BMP-2 in PC-3 cells, acting as a pseudo-receptor for receptor
activator of NF-κB (RANK) ligand (RANKL) to prevent
RANKL/RANK-induced osteoclastogenesis (223). BMP-7 can enhance osteoblastic
activity via upregulation of VEGF in cancer cells (59). As angiogenic factors, BMPs can also
facilitate the formation of bone metastasis by promoting
tumour-associated angiogenesis.
The role played by BMP signalling in cancer
progression, metastasis and angiogenesis has raised interest in
developing targeted therapies. ALK1 appears to be the most
attractive target for preventing tumour-associated new vasculature.
PF-03446962, a monoclonal antibody against ALK1 from Pfizer, has
exhibited dose-dependent anti-angiogenic effects (224). ALK1-Fc, known as Dalantercept or
ACE-041, which exhibits high binding affinity to BMP-9 and BMP-10,
has demonstrated an inhibitory effect on angiogenesis and thus
tumour growth (225). These
anti-angiogenic therapies are currently being evaluated for their
therapeutic potential in the treatment of advanced cancers and
metastases in different clinical trials (Table I). In addition to ALK1, CD105, a
co-receptor for BMP-9, has been targeted with a monoclonal
antibody, TRC105, to prevent angiogenesis (226). In a recent analysis of BMP and
BMP receptors in gastric cancer in our lab (227), it was shown that elevated
expression levels of BMP receptors in GC were highly associated
with tumour-associated angiogenesis and lymphangiogenesis, which
facilitate the tumour growth, expansion and spread. However, BMP
signalling is only part of the orchestrated signalling required for
the formation of new vasculature in tumours, with interactions with
other pro-angiogenic factors and pathways, such as HGF, VEGF and
fibroblast growth factor, also involved (138). More targeted and specific
therapeutic approaches to meet the requirements of each individual
patient are expected when improved understanding of the exact
underlying mechanisms has been obtained. Therefore, the side
effects, adverse effects, and imbalances between BMPs and BMP
antagonists should be comprehensively considered when they are
evaluated as targets to prevent bone metastasis. Additionally,
antibodies or small inhibitors targeting the BMP pathway may affect
human bone formation during development and tissue repair. Relevant
side effects should be considered in future clinical studies.
In contrast to the development of anti-angiogenic
therapies, BMPs have been evaluated for their direct anti-cancer
potential with caution. This is mainly as a result of their
biphasic effects in both primary tumours and secondary tumours.
Most BMPs elicit inhibition of proliferation while also acting as
potent inducers of EMT through Smad signalling (2). In bone metastases, imbalanced BMP
signalling may facilitate either osteoblastic or osteolytic
lesions. None of these will likely result in a favourable outcome
in patients with solid tumours (158). More intensive research is
required to elucidate the precise role played by BMP signalling in
more specific windows of malignancy.
BMPs play a role in tumorigenesis and disease
progression, not only from the activation of BMP signalling
pathways (25-28), but also from BMP-mediated crosstalk
between tumour cells and local environments comprising vascular
endothelial cells (140-143), fibroblasts, ECM (183,184), osteoclasts and osteoblasts
(137,217,219). BMPs can directly induce
angiogenesis by acting on vascular endothelial cells (140-141), and also indirectly promote the
synthesis and secretion of pro-angiogenic factors in both cancer
cells and stromal cells (138,153). BMP-2 and BMP-4 secreted by breast
cancer cells can facilitate their invasiveness via upregulation of
tenascin-W and MMPs in adjacent fibroblasts (158,180). BMPs can also alter the ECM by
promoting the secretion of ECM components, generating a
tumour-supporting tumour microenvironment (183,184). BMPs also play an important part
in the vicious cycle of forming metastatic bone lesions (59,218,223,228). Emerging evidence shows that the
BMP signalling is also involved in the regulation of immunity. For
example, BMP signalling can regulate the activation and
differentiation of T cells (229,230). BMP-2 could robustly activate
macrophages through Smad 1/5/8 signalling pathway. However,
potential roles of BMPs in immunotherapies targeted against
malignancies remain to be fully investigated.
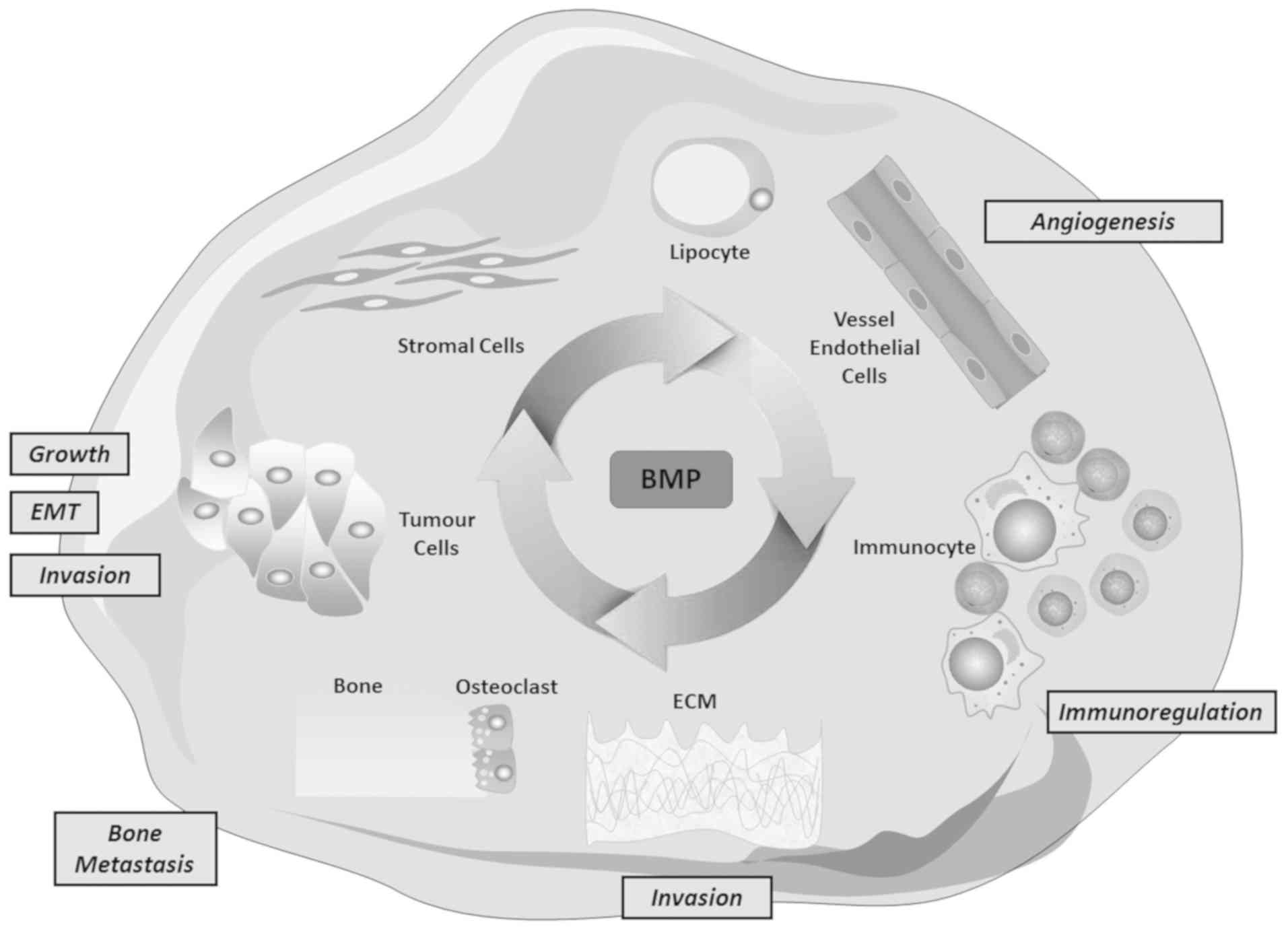
Collectively, BMP, tumour cells and the tumour
microenvironment constitute a large, intricate network that
regulates tumour proliferation, EMT, invasion, angiogenesis,
development of metastasis and immune regulation (Fig. 6).
Not applicable.
This work was supported by a Chinese Scholarship
from Cardiff University, and sponsorship by Peking University
Cancer Hospital and Institute.
Not applicable.
ZS, SC, CZ, CL and LY prepared the figures and
drafted the manuscript. ZS, CZ and LY revised the manuscript. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Urist MR: Bone: Formation by
autoinduction. Science. 150:893–899. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ye L, Bokobza SM and Jiang WG: Bone
morphogenetic proteins in development and progression of breast
cancer and therapeutic potential (review). Int J Mol Med.
24:591–597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L, Meng F, Ma D, Xie W and Fang M:
Bridging Decapentaplegic and Wingless signaling in Drosophila wings
through repression of naked cuticle by Brinker. Development.
140:413–422. 2013. View Article : Google Scholar
|
|
4
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar
|
|
5
|
Willet SG and Mills JC: Stomach organ and
cell lineage differentiation: From embryogenesis to adult
homeostasis. Cell Mol Gastroenterol Hepatol. 2:546–559. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Todisco A: Regulation of gastric
metaplasia, dysplasia, and neoplasia by bone morphogenetic protein
signaling. Cell Mol Gastroenterol Hepatol. 3:339–347. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tamada H, Kitazawa R, Gohji K and Kitazawa
S: Epigenetic regulation of human bone morphogenetic protein 6 gene
expression in prostate cancer. J Bone Miner Res. 16:487–496. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo D, Huang J and Gong J: Bone
morphogenetic protein 4 (BMP4) is required for migration and
invasion of breast cancer. Mol Cell Biochem. 363:179–190. 2012.
View Article : Google Scholar
|
|
9
|
Ye L, Kynaston H and Jiang WG: Bone
morphogenetic protein-10 suppresses the growth and aggressiveness
of prostate cancer cells through a Smad independent pathway. J
Urol. 181:2749–2759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Y, Slaney CY, Bidwell BN, Parker BS,
Johnstone CN, Rautela J, Eckhardt BL and Anderson RL: BMP4 inhibits
breast cancer metastasis by blocking myeloid-derived suppressor
cell activity. Cancer Res. 74:5091–5102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raval P, Hsu HH, Schneider DJ, Sarras MP
Jr, Masuhara K, Bonewald LF and Anderson HC: Expression of bone
morphogenetic proteins by osteoinductive and non-osteoinductive
human osteosarcoma cells. J Dent Res. 75:1518–1523. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo W, Gorlick R, Ladanyi M, Meyers PA,
Huvos AG, Bertino JR and Healey JH: Expression of bone
morphogenetic proteins and receptors in sarcomas. Clin Orthop Relat
Res. 175–183. 1999. View Article : Google Scholar
|
|
13
|
Gao YH and Yang LY: In situ hybridization
and immunohistochemical detection of bone morphogenetic protein
genes in ameloblastomas. Zhonghua Yi Xue Za Zhi. 74:621–623.
6471994.In Chinese.
|
|
14
|
Kusafuka K, Luyten FP, De Bondt R, Hiraki
Y, Shukunami C, Kayano T and Takemura T: Immunohistochemical
evaluation of cartilage-derived morphogenic protein-1 and -2 in
normal human salivary glands and pleomorphic adenomas. Virchows
Arch. 442:482–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hardwick JC, Kodach LL, Offerhaus GJ and
van den Brink GR: Bone morphogenetic protein signalling in
colorectal cancer. Nat Rev Cancer. 8:806–812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clement JH, Sanger J and Hoffken K:
Expression of bone morphogenetic protein 6 in normal mammary tissue
and breast cancer cell lines and its regulation by epidermal growth
factor. Int J Cancer. 80:250–256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lehmann K, Janda E, Pierreux CE, Rytömaa
M, Schulze A, McMahon M, Hill CS, Beug H and Downward J: Raf
induces TGFbeta production while blocking its apoptotic but not
invasive responses: A mechanism leading to increased malignancy in
epithelial cells. Genes Dev. 14:2610–2622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oft M, Peli J, Rudaz C, Schwarz H, Beug H
and Reichmann E: TGF-beta1 and Ha-Ras collaborate in modulating the
phenotypic plasticity and invasiveness of epithelial tumor cells.
Genes Dev. 10:2462–2477. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yue J and Mulder KM: Requirement of
Ras/MAPK pathway activation by transforming growth factor beta for
transforming growth factor beta 1 production in a Smad-dependent
pathway. J Biol Chem. 275:30765–30773. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wilkes MC, Mitchell H, Penheiter SG, Doré
JJ, Suzuki K, Edens M, Sharma DK, Pagano RE and Leof EB:
Transforming growth factor-beta activation of phosphatidylinositol
3-kinase is independent of Smad2 and Smad3 and regulates fibroblast
responses via p21-activated kinase-2. Cancer Res. 65:10431–10440.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Liao J, Lu Y, Duan X and Sun W:
Activation of the PI3K/Akt pathway mediates bone morphogenetic
protein 2-induced invasion of pancreatic cancer cells Panc-1.
Pathol Oncol Res. 17:257–261. 2011. View Article : Google Scholar
|
|
22
|
Wang SE, Shin I, Wu FY, Friedman DB and
Arteaga CL: HER2/Neu (ErbB2) signaling to Rac1-Pak1 is temporally
and spatially modulated by transforming growth factor beta. Cancer
Res. 66:9591–9600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL,
Kim JS and Yoo YA: Metastatic function of BMP-2 in gastric cancer
cells: The role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9
expression. Exp Cell Res. 317:1746–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Ye Y, Long X, Xiao P, Ren X and
Yu J: BMP signaling and its paradoxical effects in tumorigenesis
and dissemination. Oncotarget. 7:78206–78218. 2016.PubMed/NCBI
|
|
25
|
Ye L, Mason MD and Jiang WG: Bone
morphogenetic protein and bone metastasis, implication and
therapeutic potential. Front Biosci (Landmark Ed). 16:865–897.
2011. View Article : Google Scholar
|
|
26
|
Nohe A, Hassel S, Ehrlich M, Neubauer F,
Sebald W, Henis YI and Knaus P: The mode of bone morphogenetic
protein (BMP) receptor oligomerization determines different BMP-2
signaling pathways. J Biol Chem. 277:5330–5338. 2002. View Article : Google Scholar
|
|
27
|
Nohe A, Keating E, Knaus P and Petersen
NO: Signal transduction of bone morphogenetic protein receptors.
Cellular Signal. 16:291–299. 2004. View Article : Google Scholar
|
|
28
|
Shi Y and Massague J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye L, Lewis-Russell JM, Davies G, Sanders
AJ, Kynaston H and Jiang WG: Hepatocyte growth factor up-regulates
the expression of the bone morphogenetic protein (BMP) receptors,
BMPR-IB and BMPR-II, in human prostate cancer cells. Int J Oncol.
30:521–529. 2007.PubMed/NCBI
|
|
30
|
Shibuya H, Yamaguchi K, Shirakabe K,
Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E and Matsumoto K:
TAB1: An activator of the TAK1 MAPKKK in TGF-beta signal
transduction. Science. 272:1179–1182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamaguchi K, Nagai S, Ninomiya-Tsuji J,
Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H and
Matsumoto K: XIAP, a cellular member of the inhibitor of apoptosis
protein family, links the receptors to TAB1-TAK1 in the BMP
signaling pathway. EMBO J. 18:179–187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamaguchi K, Shirakabe K, Shibuya H, Irie
K, Oishi I, Ueno N, Taniguchi T, Nishida E and Matsumoto K:
Identification of a member of the MAPKKK family as a potential
mediator of TGF-beta signal transduction. Science. 270:2008–2011.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kimura N, Matsuo R, Shibuya H, Nakashima K
and Taga T: BMP2-induced apoptosis is mediated by activation of the
TAK1-p38 kinase pathway that is negatively regulated by Smad6. J
Biol Chem. 275:17647–17652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moriguchi T, Kuroyanagi N, Yamaguchi K,
Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto
K, et al: A novel kinase cascade mediated by mitogen-activated
protein kinase kinase 6 and MKK3. J Biol Chem. 271:13675–13679.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishitani T, Ninomiya-Tsuji J, Nagai S,
Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers
H, Shibuya H and Matsumoto K: The TAK1-NLK-MAPK-related pathway
antagonizes signalling between beta-catenin and transcription
factor TCF. Nature. 399:798–802. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee SW, Han SI, Kim HH and Lee ZH:
TAK1-dependent activation of AP-1 and c-Jun N-terminal kinase by
receptor activator of NF-kappaB. J Biochem Mol Biol. 35:371–376.
2002.PubMed/NCBI
|
|
37
|
Shirakabe K, Yamaguchi K, Shibuya H, Irie
K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K and Nishida E: TAK1
mediates the ceramide signaling to stress-activated protein
kinase/c-Jun N-terminal kinase. J Biol Chem. 272:8141–8144. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alarmo EL and Kallioniemi A: Bone
morphogenetic proteins in breast cancer: Dual role in
tumourigenesis? Endocr Relat Cancer. 17:R123–R139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gazzerro E, Gangji V and Canalis E: Bone
morphogenetic proteins induce the expression of noggin, which
limits their activity in cultured rat osteoblasts. J Clin Invest.
102:2106–2114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Onichtchouk D, Chen YG, Dosch R, Gawantka
V, Delius H, Massagué J and Niehrs C: Silencing of TGF-beta
signalling by the pseudoreceptor BAMBI. Nature. 401:480–485. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grotewold L, Plum M, Dildrop R, Peters T
and Ruther U: Bambi is coexpressed with Bmp-4 during mouse
embryogenesis. Mech Dev. 100:327–330. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Samad TA, Rebbapragada A, Bell E, Zhang Y,
Sidis Y, Jeong SJ, Campagna JA, Perusini S, Fabrizio DA, Schneyer
AL, et al: DRAGON, a bone morphogenetic protein co-receptor. J Biol
Chem. 280:14122–14129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Babitt JL, Zhang Y, Samad TA, Xia Y, Tang
J, Campagna JA, Schneyer AL, Woolf CJ and Lin HY: Repulsive
guidance molecule (RGMa), a DRAGON homologue, is a bone
morphogenetic protein co-receptor. J Biol Chem. 280:29820–29827.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Babitt JL, Huang FW, Wrighting DM, Xia Y,
Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, et
al: Bone morphogenetic protein signaling by hemojuvelin regulates
hepcidin expression. Nat Genet. 38:531–539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hayashi H, Abdollah S, Qiu Y, Cai J, Xu
YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL
and Falb D: The MAD-related protein Smad7 associates with the
TGFbeta receptor and functions as an antagonist of TGFbeta
signaling. Cell. 89:1165–1173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takase M, Imamura T, Sampath TK, Takeda K,
Ichijo H, Miyazono K and Kawabata M: Induction of Smad6 mRNA by
bone morphogenetic proteins. Biochem Biophys Res Commun. 244:26–29.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishisaki A, Yamato K, Hashimoto S, Nakao
A, Tamaki K, Nonaka K, ten Dijke P, Sugino H and Nishihara T:
Differential inhibition of Smad6 and Smad7 on bone morphogenetic
protein- and activin-mediated growth arrest and apoptosis in B
cells. J Biol Chem. 274:13637–13642. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feng XH, Lin X and Derynck R: Smad2, Smad3
and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in
response to TGF-beta. EMBO J. 19:5178–5193. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sano Y, Harada J, Tashiro S,
Gotoh-Mandeville R, Maekawa T and Ishii S: ATF-2 is a common
nuclear target of Smad and TAK1 pathways in transforming growth
factor-beta signaling. J Biol Chem. 274:8949–8957. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cordenonsi M, Montagner M, Adorno M,
Zacchigna L, Martello G, Mamidi A, Soligo S, Dupont S and Piccolo
S: Integration of TGF-beta and Ras/MAPK signaling through p53
phosphorylation. Science. 315:840–843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miyazono K, Maeda S and Imamura T:
Coordinate regulation of cell growth and differentiation by
TGF-beta superfamily and Runx proteins. Oncogene. 23:4232–4237.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Germain S, Howell M, Esslemont GM and Hill
CS: Homeodomain and winged-helix transcription factors recruit
activated Smads to distinct promoter elements via a common Smad
interaction motif. Genes Dev. 14:435–451. 2000.PubMed/NCBI
|
|
53
|
Miyazono K, ten Dijke P and Heldin CH:
TGF-beta signaling by Smad proteins. Adv Immunol. 75:115–157. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Durand SH, Romeas A, Couble ML, Langlois
D, Li JY, Magloire H, Bleicher F, Staquet MJ and Farges JC:
Expression of the TGF-beta/BMP inhibitor EVI1 in human dental pulp
cells. Arch Oral Biol. 52:712–719. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luo K, Stroschein SL, Wang W, Chen D,
Martens E, Zhou S and Zhou Q: The Ski oncoprotein interacts with
the Smad proteins to repress TGFbeta signaling. Genes Dev.
13:2196–2206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Spagnoli FM and Brivanlou AH: The Gata5
target, TGIF2, defines the pancreatic region by modulating BMP
signals within the endoderm. Development. 135:451–461. 2008.
View Article : Google Scholar
|
|
57
|
Wotton D and Massague J: Smad
transcriptional corepressors in TGF beta family signaling. Curr Top
Microbiol Immunol. 254:145–164. 2001.PubMed/NCBI
|
|
58
|
Zhu H, Kavsak P, Abdollah S, Wrana JL and
Thomsen GH: A SMAD ubiquitin ligase targets the BMP pathway and
affects embryonic pattern formation. Nature. 400:687–693. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ye L, Lewis-Russell JM, Kyanaston HG and
Jiang WG: Bone morphogenetic proteins and their receptor signaling
in prostate cancer. Histol Histopathol. 22:1129–1147.
2007.PubMed/NCBI
|
|
60
|
Beck SE and Carethers JM: BMP suppresses
PTEN expression via RAS/ERK signaling. Cancer Biol Ther.
6:1313–1317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Veeman MT, Axelrod JD and Moon RT: A
second canon. Functions and mechanisms of beta-catenin-independent
Wnt signaling. Dev Cell. 5:367–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mosimann C, Hausmann G and Basler K:
Beta-catenin hits chromatin: Regulation of Wnt target gene
activation. Nat Rev Mol Cell Biol. 10:276–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Moon RT: Wnt/beta-catenin pathway. Sci
STKE. 2005:cm12005.PubMed/NCBI
|
|
65
|
Teo JL and Kahn M: The Wnt signaling
pathway in cellular proliferation and differentiation: A tale of
two coactivators. Adv Drug Deliv Rev. 62:1149–1155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Imai Y, Terai H, Nomura-Furuwatari C,
Mizuno S, Matsumoto K, Nakamura T and Takaoka K: Hepatocyte growth
factor contributes to fracture repair by upregulating the
expression of BMP receptors. J Bone Miner Res. 20:1723–1730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhen R, Yang J, Wang Y, Li Y, Chen B, Song
Y, Ma G and Yang B: Hepatocyte growth factor improves bone
regeneration via the bone morphogenetic protein2mediated NFκB
signaling pathway. Mol Med Rep. 17:6045–6053. 2018.PubMed/NCBI
|
|
68
|
Luo K: Signaling cross talk between
TGF-β/smad and other signaling pathways. Cold Spring Harb Perspect
Biol. 9:pii: a022137. 2017. View Article : Google Scholar
|
|
69
|
Labbe E, Letamendia A and Attisano L:
Association of Smads with lymphoid enhancer binding factor 1/T
cell-specific factor mediates cooperative signaling by the
transforming growth factor-beta and wnt pathways. Proc Natl Acad
Sci USA. 97:8358–8363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nishita M, Hashimoto MK, Ogata S, Laurent
MN, Ueno N, Shibuya H and Cho KW: Interaction between Wnt and
TGF-beta signalling pathways during formation of Spemann's
organizer. Nature. 403:781–785. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hussein SM, Duff EK and Sirard C: Smad4
and beta-catenin co-activators functionally interact with
lymphoid-enhancing factor to regulate graded expression of Msx2. J
Biol Chem. 278:48805–48814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Weng X, Zhang H, Ye J, Kan M, Liu F, Wang
T, Deng J, Tan Y, He L and Liu Y: Hypermethylated Epidermal growth
factor receptor (EGFR) promoter is associated with gastric cancer.
Sci Rep. 5:101542015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar :
|
|
75
|
de Caestecker MP, Parks WT, Frank CJ,
Castagnino P, Bottaro DP, Roberts AB and Lechleider RJ: Smad2
transduces common signals from receptor serine-threonine and
tyrosine kinases. Genes Dev. 12:1587–1592. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Brown JD, DiChiara MR, Anderson KR,
Gimbrone MA Jr and Topper JN: MEKK-1, a component of the stress
(stress-activated protein kinase/c-Jun N-terminal kinase) pathway,
can selectively activate Smad2-mediated transcriptional activation
in endothelial cells. J Biol Chem. 274:8797–8805. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ross KR, Corey DA, Dunn JM and Kelley TJ:
SMAD3 expression is regulated by mitogen-activated protein kinase
kinase-1 in epithelial and smooth muscle cells. Cell Signal.
19:923–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kretzschmar M, Doody J, Timokhina I and
Massague J: A mechanism of repression of TGFbeta/ Smad signaling by
oncogenic Ras. Genes Dev. 13:804–816. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Matsuura I, Wang G, He D and Liu F:
Identification and characterization of ERK MAP kinase
phosphorylation sites in Smad3. Biochemistry. 44:12546–12553. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kamaraju AK and Roberts AB: Role of
Rho/ROCK and p38 MAP kinase pathways in transforming growth
factor-beta-mediated Smad-dependent growth inhibition of human
breast carcinoma cells in vivo. J Biol Chem. 280:1024–1036. 2005.
View Article : Google Scholar
|
|
81
|
Guo X and Wang XF: Signaling cross-talk
between TGF-beta/BMP and other pathways. Cell Res. 19:71–88. 2009.
View Article : Google Scholar
|
|
82
|
Saha D, Datta PK and Beauchamp RD:
Oncogenic ras represses transforming growth factor-beta /Smad
signaling by degrading tumor suppressor Smad4. J Biol Chem.
276:29531–29537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liang M, Liang YY, Wrighton K,
Ungermannova D, Wang XP, Brunicardi FC, Liu X, Feng XH and Lin X:
Ubiquitination and proteolysis of cancer-derived Smad4 mutants by
SCFSkp2. Mol Cell Biol. 24:7524–7537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Brodin G, Ahgren A, ten Dijke P, Heldin CH
and Heuchel R: Efficient TGF-beta induction of the Smad7 gene
requires cooperation between AP-1, Sp1, and Smad proteins on the
mouse Smad7 promoter. J Biol Chem. 275:29023–29030. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dowdy SC, Mariani A and Janknecht R:
HER2/Neu- and TAK1-mediated up-regulation of the transforming
growth factor beta inhibitor Smad7 via the ETS protein ER81. J Biol
Chem. 278:44377–44384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Uchida K, Suzuki H, Ohashi T, Nitta K,
Yumura W and Nihei H: Involvement of MAP kinase cascades in Smad7
transcriptional regulation. Biochem Biophys Res Commun.
289:376–381. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shaulian E and Karin M: AP-1 as a
regulator of cell life and death. Nat Cell Biol. 4:E131–E136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hanafusa H, Ninomiya-Tsuji J, Masuyama N,
Nishita M, Fujisawa J, Shibuya H, Matsumoto K and Nishida E:
Involvement of the p38 mitogen-activated protein kinase pathway in
transforming growth factor-beta-induced gene expression. J Biol
Chem. 274:27161–27167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jin EJ, Lee SY, Choi YA, Jung JC, Bang OS
and Kang SS: BMP-2-enhanced chondrogenesis involves p38
MAPK-mediated down-regulation of Wnt-7a pathway. Mol Cells.
22:353–359. 2006.
|
|
90
|
Thomas DA and Massague J: TGF-beta
directly targets cytotoxic T cell functions during tumor evasion of
immune surveillance. Cancer Cell. 8:369–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Monzen K, Hiroi Y, Kudoh S, Akazawa H, Oka
T, Takimoto E, Hayashi D, Hosoda T, Kawabata M, Miyazono K, et al:
Smads, TAK1, and their common target ATF-2 play a critical role in
cardiomyocyte differentiation. J Cell Biol. 153:687–698. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lamouille S and Derynck R: Cell size and
invasion in TGF-beta-induced epithelial to mesenchymal transition
is regulated by activation of the mTOR pathway. J Cell Biol.
178:437–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ghosh-Choudhury N, Abboud SL, Nishimura R,
Celeste A, Mahimainathan L and Choudhury GG: Requirement of
BMP-2-induced phosphatidylinositol 3-kinase and Akt
serine/threonine kinase in osteoblast differentiation and
Smad-dependent BMP-2 gene transcription. J Biol Chem.
277:33361–33368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
He XC, Zhang J, Tong WG, Tawfik O, Ross J,
Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al: BMP
signaling inhibits intestinal stem cell self-renewal through
suppression of Wnt-beta-catenin signaling. Nat Genet. 36:1117–1121.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tian Q, He XC, Hood L and Li L: Bridging
the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3zeta. Cell
Cycle. 4:215–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Valderrama-Carvajal H, Cocolakis E,
Lacerte A, Lee EH, Krystal G, Ali S and Lebrun JJ: Activin/TGF-beta
induce apoptosis through Smad-dependent expression of the lipid
phosphatase SHIP. Nat Cell Biol. 4:963–969. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lu Z, Ghosh S, Wang Z and Hunter T:
Downregulation of caveolin-1 function by EGF leads to the loss of
E-cadherin, increased transcriptional activity of beta-catenin, and
enhanced tumor cell invasion. Cancer Cell. 4:499–515. 2003.
View Article : Google Scholar
|
|
99
|
Ji H, Wang J, Nika H, Hawke D, Keezer S,
Ge Q, Fang B, Fang X, Fang D, Litchfield DW, et al: EGF-induced ERK
activation promotes CK2-mediated disassociation of alpha-Catenin
from beta-Catenin and transactivation of beta-Catenin. Mol Cell.
36:547–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ye L, Lewis-Russell JM, Sanders AJ,
Kynaston H and Jiang WG: HGF/SF up-regulates the expression of bone
morphogenetic protein 7 in prostate cancer cells. Urol Oncol.
26:190–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jiang WG, Martin TA, Parr C, Davies G,
Matsumoto K and Nakamura T: Hepatocyte growth factor, its receptor,
and their potential value in cancer therapies. Crit Rev Oncol
Hematol. 53:35–69. 2005. View Article : Google Scholar
|
|
102
|
Davies G, Mason MD, Martin TA, Parr C,
Watkins G, Lane J, Matsumoto K, Nakamura T and Jiang WG: The HGF/SF
antagonist NK4 reverses fibroblast- and HGF-induced prostate tumor
growth and angiogenesis in vivo. Int J Cancer. 106:348–354. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Martin TA, Parr C, Davies G, Watkins G,
Lane J, Matsumoto K, Nakamura T, Mansel RE and Jiang WG: Growth and
angio-genesis of human breast cancer in a nude mouse tumour model
is reduced by NK4, a HGF/SF antagonist. Carcinogenesis.
24:1317–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tomioka D, Maehara N, Kuba K, Mizumoto K,
Tanaka M, Matsumoto K and Nakamura T: Inhibition of growth,
invasion, and metastasis of human pancreatic carcinoma cells by NK4
in an orthotopic mouse model. Cancer Res. 61:7518–7524.
2001.PubMed/NCBI
|
|
105
|
Abounader R, Ranganathan S, Lal B,
Fielding K, Book A, Dietz H, Burger P and Laterra J: Reversion of
human glioblastoma malignancy by U1 small nuclear RNA/ribozyme
targeting of scatter factor/hepatocyte growth factor and c-met
expression. J Natl Cancer Inst. 91:1548–1556. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jiang WG, Grimshaw D, Martin TA, Davies G,
Parr C, Watkins G, Lane J, Abounader R, Laterra J and Mansel RE:
Reduction of stromal fibroblast-induced mammary tumor growth, by
retroviral ribozyme transgenes to hepatocyte growth factor/scatter
factor and its receptor, c-MET. Clin Cancer Res. 9:4274–4281.
2003.PubMed/NCBI
|
|
107
|
Grenier A, Chollet-Martin S, Crestani B,
Delarche C, El Benna J, Boutten A, Andrieu V, Durand G,
Gougerot-Pocidalo MA, Aubier M and Dehoux M: Presence of a
mobilizable intracellular pool of hepatocyte growth factor in human
polymorphonuclear neutrophils. Blood. 99:2997–3004. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Taieb J, Delarche C, Paradis V, Mathurin
P, Grenier A, Crestani B, Dehoux M, Thabut D, Gougerot-Pocidalo MA,
Poynard T and Chollet-Martin S: Polymorphonuclear neutrophils are a
source of hepatocyte growth factor in patients with severe
alcoholic hepatitis. J Hepatol. 36:342–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jaffre S, Dehoux M, Paugam C, Grenier A,
Chollet-Martin S, Stern JB, Mantz J, Aubier M and Crestani B:
Hepatocyte growth factor is produced by blood and alveolar
neutrophils in acute respiratory failure. Am J Physiol Lung Cell
Mol Physiol. 282:L310–L315. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nakamura T, Nawa K and Ichihara A: Partial
purification and characterization of hepatocyte growth factor from
serum of hepatectomized rats. Biochem Biophys Res Commun.
122:1450–1459. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nakashiro K, Hayashi Y and Oyasu R:
Immunohistochemical expression of hepatocyte growth factor and
c-Met/HGF receptor in benign and malignant human prostate tissue.
Onco Rep. 10:1149–1153. 2003.
|
|
112
|
Nakashiro K, Hara S, Shinohara Y, Oyasu M,
Kawamata H, Shintani S, Hamakawa H and Oyasu R: Phenotypic switch
from paracrine to autocrine role of hepatocyte growth factor in an
androgen-independent human prostatic carcinoma cell line, CWR22R.
Am J Pathol. 165:533–540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Janovska P and Bryja V: Wnt signalling
pathways in chronic lymphocytic leukaemia and B-cell lymphomas. Br
J Pharmacol. 174:4701–4715. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hoppler S and Moon RT: BMP-2/-4 and Wnt-8
cooperatively pattern the Xenopus mesoderm. Mech Dev. 71:119–129.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fuentealba LC, Eivers E, Ikeda A, Hurtado
C, Kuroda H, Pera EM and De Robertis EM: Integrating patterning
signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal.
Cell. 131:980–993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Millet C, Yamashita M, Heller M, Yu LR,
Veenstra TD and Zhang YE: A negative feedback control of
transforming growth factor-beta signaling by glycogen synthase
kinase 3-mediated Smad3 linker phosphorylation at Ser-204. J Biol
Chem. 284:19808–19816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Aragon E, Goerner N, Zaromytidou AI, Xi Q,
Escobedo A, Massagué J and Macias MJ: A Smad action turnover switch
operated by WW domain readers of a phosphoserine code. Genes Dev.
25:1275–1288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Fei C, Li Z, Li C, Chen Y, Chen Z, He X,
Mao L, Wang X, Zeng R and Li L: Smurf1-mediated Lys29-linked
nonproteolytic polyubiquitination of axin negatively regulates
Wnt/β-catenin signaling. Mol Cell Biol. 33:4095–4105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kim S and Jho EH: The protein stability of
Axin, a negative regulator of Wnt signaling, is regulated by Smad
ubiquitination regulatory factor 2 (Smurf2). J Biol Chem.
285:36420–36426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jian H, Shen X, Liu I, Semenov M, He X and
Wang XF: Smad3-dependent nuclear translocation of beta-catenin is
required for TGF-beta1-induced proliferation of bone marrow-derived
adult human mesenchymal stem cells. Genes Dev. 20:666–674. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Aza-Blanc P and Kornberg TB: Ci: A complex
transducer of the hedgehog signal. Trends Genet. 15:458–462. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hepker J, Blackman RK and Holmgren R:
Cubitus inter-ruptus is necessary but not sufficient for direct
activation of a wing-specific decapentaplegic enhancer.
Development. 126:3669–3677. 1999.PubMed/NCBI
|
|
123
|
Muller B and Basler K: The repressor and
activator forms of Cubitus interruptus control Hedgehog target
genes through common generic gli-binding sites. Development.
127:2999–3007. 2000.PubMed/NCBI
|
|
124
|
Dennler S, Andre J, Alexaki I, Li A,
Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F and Mauviel A:
Induction of sonic hedgehog mediators by transforming growth
factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression
in vitro and in vivo. Cancer Res. 67:6981–6986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Blokzijl A, Dahlqvist C, Reissmann E, Falk
A, Moliner A, Lendahl U and Ibáñez CF: Cross-talk between the Notch
and TGF-beta signaling pathways mediated by interaction of the
Notch intracellular domain with Smad3. J Cell Biol. 163:723–728.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Asano N, Watanabe T, Kitani A, Fuss IJ and
Strober W: Notch1 signaling and regulatory T cell function. J
Immunol. 180:2796–2804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Samon JB, Champhekar A, Minter LM, Telfer
JC, Miele L, Fauq A, Das P, Golde TE and Osborne BA: Notch1 and
TGFbeta1 cooperatively regulate Foxp3 expression and the
maintenance of peripheral regulatory T cells. Blood. 112:1813–1821.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ostroukhova M, Qi Z, Oriss TB,
Dixon-McCarthy B, Ray P and Ray A: Treg-mediated immunosuppression
involves activation of the Notch-HES1 axis by membrane-bound
TGF-beta. J Clin Invest. 116:996–1004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ulloa L, Doody J and Massague J:
Inhibition of transforming growth factor-beta/SMAD signalling by
the interferon-gamma/STAT pathway. Nature. 397:710–713. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ishida Y, Kondo T, Takayasu T, Iwakura Y
and Mukaida N: The essential involvement of cross-talk between
IFN-gamma and TGF-beta in the skin wound-healing process. J
Immunol. 172:1848–1855. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Jenkins BJ, Grail D, Nheu T, Najdovska M,
Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, et
al: Hyperactivation of Stat3 in gp130 mutant mice promotes gastric
hyperproliferation and desensitizes TGF-beta signaling. Nat Med.
11:845–852. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
132
|
Huang M, Sharma S, Zhu LX, Keane MP, Luo
J, Zhang L, Burdick MD, Lin YQ, Dohadwala M, Gardner B, et al: IL-7
inhibits fibroblast TGF-beta production and signaling in pulmonary
fibrosis. J Clin Invest. 109:931–937. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Letterio JJ and Roberts AB: Regulation of
immune responses by TGF-beta. Annu Rev Immunol. 16:137–161. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordón-Cardo C, Guise TA and Massagué J: A multigenic
program mediating breast cancer metastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kang Y, He W, Tulley S, Gupta GP,
Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL and
Massagué J: Breast cancer bone metastasis mediated by the Smad
tumor suppressor pathway. Proc Natl Acad Sci USA. 102:13909–13914.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Fong YC, Maa MC, Tsai FJ, Chen WC, Lin JG,
Jeng LB, Yang RS, Fu WM and Tang CH: Osteoblast-derived TGF-beta1
stimulates IL-8 release through AP-1 and NF-kappaB in human cancer
cells. J Bone Miner Res. 23:961–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tseng JC, Chen HF and Wu KJ: A twist tale
of cancer metastasis and tumor angiogenesis. Histol Histopathol.
30:1283–1294. 2015.PubMed/NCBI
|
|
138
|
Ye L and Jiang WG: Bone morphogenetic
proteins in tumour associated angiogenesis and implication in
cancer therapies. Cancer Lett. 380:586–597. 2016. View Article : Google Scholar
|
|
139
|
Goumans MJ, Valdimarsdottir G, Itoh S,
Rosendahl A, Sideras P and ten Dijke P: Balancing the activation
state of the endothelium via two distinct TGF-beta type I
receptors. EMBO J. 21:1743–1753. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Yamashita H, Shimizu A, Kato M, Nishitoh
H, Ichijo H, Hanyu A, Morita I, Kimura M, Makishima F and Miyazono
K: Growth/differentiation factor-5 induces angiogenesis in vivo.
Exp Cell Res. 235:218–226. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Mori S, Yoshikawa H, Hashimoto J, Ueda T,
Funai H, Kato M and Takaoka K: Antiangiogenic agent (TNP-470)
inhibition of ectopic bone formation induced by bone morphogenetic
protein-2. Bone. 22:99–105. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yeh LC and Lee JC: Osteogenic protein-1
increases gene expression of vascular endothelial growth factor in
primary cultures of fetal rat calvaria cells. Mol Cell Endocrinol.
153:113–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Glienke J, Schmitt AO, Pilarsky C,
Hinzmann B, Weiss B, Rosenthal A and Thierauch KH: Differential
gene expression by endothelial cells in distinct angiogenic states.
Eur J Biochem. 267:2820–2830. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Langenfeld EM and Langenfeld J: Bone
morphogenetic protein-2 stimulates angiogenesis in developing
tumors. Molc Cancer Res. 2:141–149. 2004.
|
|
145
|
Finkenzeller G, Hager S and Stark GB:
Effects of bone morpho-genetic protein 2 on human umbilical vein
endothelial cells. Microvasc Res. 84:81–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Willette RN, Gu JL, Lysko PG, Anderson KM,
Minehart H and Yue T: BMP-2 gene expression and effects on human
vascular smooth muscle cells. J Vasc Re. 36:120–125. 1999.
View Article : Google Scholar
|
|
147
|
Dorai H, Vukicevic S and Sampath TK: Bone
morphogenetic protein-7 (osteogenic protein-1) inhibits smooth
muscle cell proliferation and stimulates the expression of markers
that are characteristic of SMC phenotype in vitro. J Cell Physiol.
184:37–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Morrell NW, Yang X, Upton PD, Jourdan KB,
Morgan N, Sheares KK and Trembath RC: Altered growth responses of
pulmonary artery smooth muscle cells from patients with primary
pulmonary hypertension to transforming growth factor-beta(1) and
bone morphogenetic proteins. Circulation. 104:790–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
David L, Mallet C, Mazerbourg S, Feige JJ
and Bailly S: Identification of BMP9 and BMP10 as functional
activators of the orphan activin receptor-like kinase 1 (ALK1) in
endothelial cells. Blood. 109:1953–1961. 2007. View Article : Google Scholar
|
|
150
|
Regazzoni C, Winterhalter KH and Rohrer L:
Type I collagen induces expression of bone morphogenetic protein
receptor type II. Biochem Biophys Res Commun. 283:316–322. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Nakagawa T, Li JH, Garcia G, Mu W, Piek E,
Böttinger EP, Chen Y, Zhu HJ, Kang DH, Schreiner GF, et al:
TGF-beta induces proangiogenic and antiangiogenic factors via
parallel but distinct Smad pathways. Kidney Int. 66:605–613. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Han SU, Kim HT, Seong DH, Kim YS, Park YS,
Bang YJ, Yang HK and Kim SJ: Loss of the Smad3 expression increases
susceptibility to tumorigenicity in human gastric cancer. Oncogene.
23:1333–1341. 2004. View Article : Google Scholar
|
|
153
|
Dai J, Kitagawa Y, Zhang J, Yao Z,
Mizokami A, Cheng S, Nör J, McCauley LK, Taichman RS and Keller ET:
Vascular endothe-lial growth factor contributes to the prostate
cancer-induced osteoblast differentiation mediated by bone
morphogenetic protein. Cancer Res. 64:994–999. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Stabile H, Mitola S, Moroni E, Belleri M,
Nicoli S, Coltrini D, Peri F, Pessi A, Orsatti L, Talamo F, et al:
Bone morphogenic protein antagonist Drm/gremlin is a novel
proangiogenic factor. Blood. 109:1834–1840. 2007. View Article : Google Scholar
|
|
155
|
Akiyama I, Yoshino O, Osuga Y, Shi J,
Harada M, Koga K, Hirota Y, Hirata T, Fujii T, Saito S and Kozuma
S: Bone morphogenetic protein 7 increased vascular endothelial
growth factor (VEGF)-a expression in human granulosa cells and VEGF
receptor expression in endothelial cells. Reprod Sci. 21:477–482.
2014. View Article : Google Scholar :
|
|
156
|
Raida M, Clement JH, Leek RD, Ameri K,
Bicknell R, Niederwieser D and Harris AL: Bone morphogenetic
protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res
Clin Oncol. 131:741–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Scharpfenecker M, van Dinther M, Liu Z,
van Bezooijen RL, Zhao Q, Pukac L, Löwik CW and ten Dijke P: BMP-9
signals via ALK1 and inhibits bFGF-induced endothelial cell
proliferation and VEGF-stimulated angiogenesis. J Cell Sci.
120:964–972. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Zabkiewicz C, Resaul J, Hargest R, Jiang
WG and Ye L: Bone morphogenetic proteins, breast cancer, and bone
metastases: Striking the right balance. Endocr Relat Cancer.
24:R349–R366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ramoshebi LN and Ripamonti U: Osteogenic
protein-1, a bone morphogenetic protein, induces angiogenesis in
the chick chorioallantoic membrane and synergizes with basic
fibroblast growth factor and transforming growth factor-beta1. Anat
Rec. 259:97–107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3' kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Nakajima Y, Yamagishi T, Hokari S and
Nakamura H: Mechanisms involved in valvuloseptal endocardial
cushion formation in early cardiogenesis: Roles of transforming
growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat
Rec. 258:119–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Romano LA and Runyan RB: Slug is an
essential target of TGFbeta2 signaling in the developing chicken
heart. Dev Biol. 223:91–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Yang S, Zhong C, Frenkel B, Reddi AH and
Roy-Burman P: Diverse biological effect and Smad signaling of bone
morpho-genetic protein 7 in prostate tumor cells. Cancer Res.
65:5769–5777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Montesano R: Bone morphogenetic protein-4
abrogates lumen formation by mammary epithelial cells and promotes
invasive growth. Biochem Biophys Res Commun. 353:817–822. 2007.
View Article : Google Scholar
|
|
166
|
Piek E, Moustakas A, Kurisaki A, Heldin CH
and ten Dijke P: TGF-(beta) type I receptor/ALK-5 and Smad proteins
mediate epithelial to mesenchymal transdifferentiation in NMuMG
breast epithelial cells. J Cell Sci. 112:4557–4568. 1999.PubMed/NCBI
|
|
167
|
Yang S, Du J, Wang Z, Yuan W, Qiao Y,
Zhang M, Zhang J, Gao S, Yin J, Sun B and Zhu T: BMP-6 promotes
E-cadherin expression through repressing deltaEF1 in breast cancer
cells. BMC Cancer. 7:2112007. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Clement JH, Raida M, Sanger J, Bicknell R,
Liu J, Naumann A, Geyer A, Waldau A, Hortschansky P, Schmidt A, et
al: Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion
and in vivo hormone independent growth of breast carcinoma cells.
Int J Oncol. 27:401–407. 2005.PubMed/NCBI
|
|
169
|
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y,
Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K and Imamura T:
Bone morpho-genetic protein signaling enhances invasion and bone
metastasis of breast cancer cells through Smad pathway. Oncogene.
27:6322–6333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Gautschi O, Tepper CG, Purnell PR, Izumiya
Y, Evans CP, Green TP, Desprez PY, Lara PN, Gandara DR, Mack PC and
Kung HJ: Regulation of Id1 expression by SRC: Implications for
targeting of the bone morphogenetic protein pathway in cancer.
Cancer Res. 68:2250–2258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Buijs JT, Henriquez NV, van Overveld PG,
van der Horst G, Que I, Schwaninger R, Rentsch C, Ten Dijke P,
Cleton-Jansen AM, Driouch K, et al: Bone morphogenetic protein 7 in
the development and treatment of bone metastases from breast
cancer. Cancer Res. 67:8742–8751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Du J, Yang S, An D, Hu F, Yuan W, Zhai C
and Zhu T: BMP-6 inhibits microRNA-21 expression in breast cancer
through repressing deltaEF1 and AP-1. Cell Res. 19:487–496. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
de Boeck M, Cui C, Mulder AA, Jost CR,
Ikeno S and Ten Dijke P: Smad6 determines BMP-regulated invasive
behaviour of breast cancer cells in a zebrafish xenograft model.
Sci Rep. 6:249682016. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Luna-Zurita L, Prados B, Grego-Bessa J,
Luxán G, del Monte G, Benguría A, Adams RH, Pérez-Pomares JM and de
la Pompa JL: Integration of a Notch-dependent mesenchymal gene
program and Bmp2-driven cell invasiveness regulates murine cardiac
valve formation. J Clin Invest. 120:3493–3507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Ma L, Lu MF, Schwartz RJ and Martin JF:
Bmp2 is essential for cardiac cushion epithelial-mesenchymal
transition and myocardial patterning. Development. 132:5601–5611.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Dyer L, Lockyer P, Wu Y, Saha A, Cyr C,
Moser M, Pi X and Patterson C: BMPER promotes
epithelial-mesenchymal transition in the developing cardiac
cushions. PLoS One. 10:e01392092015. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Kang MH, Kang HN, Kim JL, Kim JS, Oh SC
and Yoo YA: Inhibition of PI3 kinase/Akt pathway is required for
BMP2-induced EMT and invasion. Oncol Rep. 22:525–534.
2009.PubMed/NCBI
|
|
178
|
Kang MH, Kim JS, Seo JE, Oh SC and Yoo YA:
BMP2 accelerates the motility and invasiveness of gastric cancer
cells via activation of the phosphatidylinositol 3-kinase
(PI3K)/Akt pathway. Exp Cell Res. 316:24–37. 2010. View Article : Google Scholar
|
|
179
|
Owens P, Polikowsky H, Pickup MW, Gorska
AE, Jovanovic B, Shaw AK, Novitskiy SV, Hong CC and Moses HL: Bone
Morphogenetic Proteins stimulate mammary fibroblasts to promote
mammary carcinoma cell invasion. PLoS One. 8:e675332013. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Scherberich A, Tucker RP, Degen M,
Brown-Luedi M, Andres AC and Chiquet-Ehrismann R: Tenascin-W is
found in malignant mammary tumors, promotes alpha8
integrin-dependent motility and requires p38MAPK activity for BMP-2
and TNF-alpha induced expression in vitro. Oncogene. 24:1525–1532.
2005. View Article : Google Scholar
|
|
181
|
Giussani M, Triulzi T, Sozzi G and
Tagliabue E: Tumor extracellular matrix remodeling: New
perspectives as a circulating tool in the diagnosis and prognosis
of solid tumors. Cells. 8:pii: E81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Eble JA and Niland S: The extracellular
matrix in tumor progression and metastasis. Clin Exp Metastasis.
36:171–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Zhong L, Wang X, Wang S, Yang L, Gao H and
Yang C: The anti-fibrotic effect of bone morphogenic
protein-7(BMP-7) on liver fibrosis. Int J Med Sci. 10:441–450.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Li H, Cui D, Zhao F, Huo L, Hu J and Zeng
J: BMP-2 is involved in scleral remodeling in myopia development.
PLoS One. 10:e01252192015. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Chen CC and Lau LF: Functions and
mechanisms of action of CCN matricellular proteins. Int J Biochem
Cell Biol. 41:771–783. 2009. View Article : Google Scholar :
|
|
186
|
Leask A and Abraham DJ: All in the CCN
family: Essential matricellular signaling modulators emerge from
the bunker. J Cell Sci. 119:4803–4810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Holbourn KP, Acharya KR and Perbal B: The
CCN family of proteins: Structure-function relationships. Trends
Biochem Sci. 33:461–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Kireeva ML, Mo FE, Yang GP and Lau LF:
Cyr61, a product of a growth factor-inducible immediate-early gene,
promotes cell proliferation, migration, and adhesion. Mol Cell
Biol. 16:1326–1334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Yosimichi G, Nakanishi T, Nishida T,
Hattori T, Takano-Yamamoto T and Takigawa M: CTGF/Hcs24 induces
chondrocyte differentiation through a p38 mitogen-activated protein
kinase (p38MAPK), and proliferation through a p44/42
MAPK/extracellular-signal regulated kinase (ERK). Eur J Biochem.
268:6058–6065. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Baguma-Nibasheka M and Kablar B: Pulmonary
hypoplasia in the connective tissue growth factor (Ctgf) null
mouse. Dev Dyn. 237:485–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Chen CC, Chen N and Lau LF: The angiogenic
factors Cyr61 and connective tissue growth factor induce adhesive
signaling in primary human skin fibroblasts. J Biol Chem.
276:10443–10452. 2001. View Article : Google Scholar
|
|
192
|
Liu H, Dong W, Lin Z, Lu J, Wan H, Zhou Z
and Liu Z: CCN4 regulates vascular smooth muscle cell migration and
proliferation. Mol Cells. 36:112–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Schutze N, Schenk R, Fiedler J, Mattes T,
Jakob F and Brenner RE: CYR61/CCN1 and WISP3/CCN6 are
chemoattractive ligands for human multipotent mesenchymal stroma
cells. BMC Cell Biol. 8:452007. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Leu SJ, Lam SC and Lau LF: Pro-angiogenic
activities of CYR61 (CCN1) mediated through integrins alphavbeta3
and alpha6beta1 in human umbilical vein endothelial cells. J Biol
Chem. 277:46248–46255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Todorovic V, Chen CC, Hay N and Lau LF:
The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J
Cell Biol. 171:559–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Kubota S and Takigawa M: CCN family
proteins and angiogen-esis: from embryo to adulthood. Angiogenesis.
10:1–11. 2007. View Article : Google Scholar
|
|
197
|
Kular L, Pakradouni J, Kitabgi P, Laurent
M and Martinerie C: The CCN family: A new class of inflammation
modulators? Biochimie. 93:377–388. 2011. View Article : Google Scholar
|
|
198
|
Bai T, Chen CC and Lau LF: Matricellular
protein CCN1 activates a proinflammatory genetic program in murine
macrophages. J Immunol. 184:3223–3232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Abreu JG, Ketpura NI, Reversade B and De
Robertis EM: Connective-tissue growth factor (CTGF) modulates cell
signalling by BMP and TGF-beta. Nat Cell Biol. 4:599–604. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Minamizato T, Sakamoto K, Liu T, Kokubo H,
Katsube K, Perbal B, Nakamura S and Yamaguchi A: CCN3/NOV inhibits
BMP-2-induced osteoblast differentiation by interacting with BMP
and Notch signaling pathways. Biochem Biophys Res Commun.
354:567–573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Ono M, Inkson CA, Kilts TM and Young MF:
WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J
Bone Miner Res. 26:193–208. 2011. View Article : Google Scholar
|
|
202
|
Nakamura Y, Weidinger G, Liang JO,
Aquilina-Beck A, Tamai K, Moon RT and Warman ML: The CCN family
member Wisp3, mutant in progressive pseudorheumatoid dysplasia,
modulates BMP and Wnt signaling. J Clin Invest. 117:3075–3086.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Kubota S, Kawaki H, Kondo S, Yosimichi G,
Minato M, Nishida T, Hanagata H, Miyauchi A and Takigawa M:
Multiple activation of mitogen-activated protein kinases by
purified independent CCN2 modules in vascular endothelial cells and
chondrocytes in culture. Biochimie. 88:1973–1981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Maeda A, Nishida T, Aoyama E, Kubota S,
Lyons KM, Kuboki T and Takigawa M: CCN family 2/connective tissue
growth factor modulates BMP signalling as a signal conductor, which
action regulates the proliferation and differentiation of
chondrocytes. J Biochem. 145:207–216. 2009. View Article : Google Scholar :
|
|
205
|
Maeda S: An impact of CCN2-BMP-2 complex
upon chondrocyte biology: Evoking a signalling pathway bypasses ERK
and Smads? J Biochem. 150:219–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Xiao F, Qiu H, Cui H, Ni X, Li J, Liao W,
Lu L and Ding K: MicroRNA-885-3p inhibits the growth of HT-29 colon
cancer cell xenografts by disrupting angiogenesis via targeting
BMPR1A and blocking BMP/Smad/Id1 signaling. Oncogene. 34:1968–1978.
2015. View Article : Google Scholar
|
|
207
|
Nishida N, Nagahara M, Sato T, Mimori K,
Sudo T, Tanaka F, Shibata K, Ishii H, Sugihara K, Doki Y and Mori
M: Microarray analysis of colorectal cancer stromal tissue reveals
upregulation of two oncogenic miRNA clusters. Clin Cancer Res.
18:3054–3070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Okuda S, Myoui A, Nakase T, Wada E,
Yonenobu K and Yoshikawa H: Ossification of the ligamentum flavum
associated with osteoblastoma: A report of three cases. Skeletal
Radiol. 30:402–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Khurana JS, Ogino S, Shen T, Parekh H,
Scherbel U, DeLong W, Feldman MD, Zhang PJ, Wolfe HJ and Alman BA:
Bone morphogenetic proteins are expressed by both bone-forming and
non-bone-forming lesions. Arch Pathol Lab Med. 128:1267–1269.
2004.PubMed/NCBI
|
|
210
|
Kudo N, Ogose A, Ariizumi T, Kawashima H,
Hotta T, Hatano H, Morita T, Nagata M, Siki Y, Kawai A, et al:
Expression of bone morphogenetic proteins in giant cell tumor of
bone. Anticancer Res. 29:2219–2225. 2009.PubMed/NCBI
|
|
211
|
Urist MR, Grant TT, Lindholm TS, Mirra JM,
Hirano H and Finerman GA: Induction of new-bone formation in the
host bed by human bone-tumor transplants in athymic nude mice. J
Bone Joint Surg Am. 61:1207–1216. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Anderson HC, Hsu HH, Raval P, Hunt TR,
Schwappach JR, Morris DC and Schneider DJ: The mechanism of bone
induction and bone healing by human osteosarcoma cell extracts.
Clin Orthop Relat Res. 129–134. 1995.PubMed/NCBI
|
|
213
|
Hara A, Ikeda T, Nomura S, Yagita H,
Okumura K and Yamauchi Y: In vivo implantation of human
osteosarcoma cells in nude mice induces bones with human-derived
osteoblasts and mouse-derived osteocytes. Lab Invest. 75:707–717.
1996.PubMed/NCBI
|
|
214
|
Ishiyama M, Relyea-Chew A, Longstreth WT
and Lewis DH: Impact of decompressive craniectomy on brain
perfusion scin-tigraphy as an ancillary test for brain death
diagnosis. Ann Nucl Med. 33:842–847. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Yoshikawa H, Rettig WJ, Takaoka K,
Alderman E, Rup B, Rosen V, Wozney JM, Lane JM, Huvos AG and
Garin-Chesa P: Expression of bone morphogenetic proteins in human
osteo-sarcoma. Immunohistochemical detection with monoclonal
antibody. Cancer. 73:85–91. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Sulzbacher I, Birner P, Trieb K,
Pichlbauer E and Lang S: The expression of bone morphogenetic
proteins in osteosarcoma and its relevance as a prognostic
parameter. J Clin Pathol. 5:381–385. 2002. View Article : Google Scholar
|
|
217
|
Li B, Yang Y, Jiang S, Ni B, Chen K and
Jiang L: Adenovirus-mediated overexpression of BMP-9 inhibits human
osteosarcoma cell growth and migration through downregulation of
the PI3K/AKT pathway. Int J Oncol. 41:1809–1819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Ye L, Kynaston HG and Jiang WG: Bone
metastasis in prostate cancer: Molecular and cellular mechanisms
(Review). Int J Mol Med. 20:103–111. 2007.PubMed/NCBI
|
|
219
|
Masuda H, Fukabori Y, Nakano K, Takezawa
Y, C Suzuki T and Yamanaka H: Increased expression of bone
morphoge-netic protein-7 in bone metastatic prostate cancer.
Prostate. 54:268–274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Thomas R, True LD, Lange PH and Vessella
RL: Placental bone morphogenetic protein (PLAB) gene expression in
normal, pre-malignant and malignant human prostate: Relation to
tumor development and progression. Int J Cancer. 93:47–52. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Secondini C, Wetterwald A, Schwaninger R,
Thalmann GN and Cecchini MG: The role of the BMP signaling
antagonist noggin in the development of prostate cancer osteolytic
bone metastasis. PLoS One. 6:e160782011. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Schwaninger R, Rentsch CA, Wetterwald A,
van der Horst G, van Bezooijen RL, van der Pluijm G, Löwik CW,
Ackermann K, Pyerin W, Hamdy FC, et al: Lack of noggin expression
by cancer cells is a determinant of the osteoblast response in bone
metastases. Am J Pathol. 170:160–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Brubaker KD, Corey E, Brown LG and
Vessella RL: Bone morphogenetic protein signaling in prostate
cancer cell lines. J Cell Biochem. 91:151–160. 2004. View Article : Google Scholar
|
|
224
|
Necchi A, Giannatempo P, Mariani L, Farè
E, Raggi D, Pennati M, Zaffaroni N, Crippa F, Marchianò A, Nicolai
N, et al: PF-03446962, a fully-human monoclonal antibody against
transforming growth-factor β (TGFβ) receptor ALK1, in pre-treated
patients with urothelial cancer: An open label, single-group, phase
2 trial. Invest New Drugs. 32:555–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Mitchell D, Pobre EG, Mulivor AW, Grinberg
AV, Castonguay R, Monnell TE, Solban N, Ucran JA, Pearsall RS,
Underwood KW, et al: ALK1-Fc inhibits multiple mediators of
angiogenesis and suppresses tumor growth. Mol Cancer Ther.
9:379–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Liu Y, Tian H, Blobe GC, Theuer CP,
Hurwitz HI and Nixon AB: Effects of the combination of TRC105 and
bevacizumab on endothelial cell biology. Invest New Drugs.
32:851–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Sun Z, Liu C, Jiang WG and Ye L:
Deregulated bone morpho-genetic proteins and their receptors are
associated with disease progression of gastric cancer. Comput
Struct Biotechnol J. 18:177–188. 2020. View Article : Google Scholar :
|
|
228
|
Hullinger TG, Taichman RS, Linseman DA and
Somerman MJ: Secretory products from PC-3 and MCF-7 tumor cell
lines upregulate osteopontin in MC3T3-E1 cells. J Cell Biochem.
78:607–616. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Yoshioka Y, Ono M, Osaki M, Konishi I and
Sakaguchi S: Differential effects of inhibition of bone morphogenic
protein (BMP) signalling on T-cell activation and differentiation.
Eur J Immunol. 42:749–759. 2012. View Article : Google Scholar
|
|
230
|
Martinez VG, Sacedon R, Hidalgo L,
Valencia J, Fernández-Sevilla LM, Hernández-López C, Vicente A and
Varas A: The BMP pathway participates in human naive CD4+ T cell
activation and homeostasis. PLoS One. 10:e01314532015. View Article : Google Scholar : PubMed/NCBI
|