Introduction
Pancreatic cancer (PC) is one of the leading causes
of cancer-associated mortality and one of the most lethal
malignancies worldwide with a 5-year survival rate <10%
(1). Surgical resection is the
only available treatment for PC, followed by adjuvant chemotherapy
with gemcitabine plus albumin-bound paclitaxel (PTX) administration
following surgery (2). Because of
PC tumor biology, early metastasis, recurrence and resistance to
chemotherapy are common in patients with PC (2,3). It
is therefore crucial to develop effective therapies for patients
with PC following resection.
Cabazitaxel (CTX), PTX and docetaxel (DTX) are
taxane anticancer drugs that bind to tubulin and subsequently
suppress microtubule dynamics in cell division, leading therefore
to cancer cell death (4,5). CTX is mainly used to treat PC in
patients with resistance to PTX or DTX, due to its poor affinity
for P-glycoprotein (P-gp) compared with PTX and DTX (6). Previous studies demonstrated that CTX
is also suitable for the treatment of lung and breast cancers,
hepatocellular carcinoma and other types of cancer (7-10).
Two previous studies reported that CTX or modified CTX might be
effective in PC (11,12); however, whether CTX may be suitable
for the treatment of PC requires further investigation.
Nuclear factor-κB (NF-κB) is a transcription factor
involved in inflammation and immunity (13). However, numerous studies reported
that it could also regulates cell proliferation, apoptosis and cell
migration in various types of cancer cells. For example, activation
of the NF-κB pathway often acts as a cancer promoter by regulating
the anti-apoptotic B-cell lymphoma-2 (Bcl-2) gene (14,15).
Furthermore, the downregulation or inhibition of NF-κB activation
is an effective treatment option for certain types of cancer,
including colorectal cancer, glioblastoma, breast cancer and lung
cancer (16,17). It has been reported that NF-κB p65
directly bound to NME5 serves a central role in PC chemoresistance
by inhibiting gemcitabine-induced apoptosis and G1 phase arrest
(18). In particular, previous
studies reported that PTX and DTX can induce NF-κB activation
(19,20), suggesting that NF-κB might be a
crucial factor involved in PC chemoresistance. Combination of a
taxane anticancer drug with an NF-κB inhibitor may therefore be
considered as a more effective cancer treatment option. Caffeic
acid phenethyl ester (CAPE), which is a NF-κB inhibitor capable of
inhibiting the translocation of NF-κB p65 subunit to the nucleus,
was used in a previous study (21). The results from this study
demonstrated that the effect of CAPE on the inhibition of NF-κB
binding to DNA sequences is specific (21).
The present study aimed to investigate the
efficiency and potential mechanism of CTX in the treatment of
patients with PC. The results from this study may serve the
development of novel treatment plan for PC.
Materials and methods
Chemicals and antibodies
CTX and PTX were purchased from Dalian Meilun
Biology Technology Co., Ltd. CAPE was purchased from Selleck
Chemicals. Primary antibodies against GAPDH (1:2,000; cat. no.
ab181602), β-actin (1:2,000; cat. no. ab8227), Bcl-2 (1:2,000; cat.
no. ab196495), Histone H3 (1:2,000; cat. no. ab1791) and
proliferating cell nuclear antigen (PCNA) (1:5,000; cat. no.
ab18197) were obtained from Abcam. The NF-κB Pathway Sampler Kit
(cat. no. 9936) and Apoptosis Antibody Sampler Kit (containing
caspase-3, cleaved-caspase-3, poly(ADP-ribose) polymerase (PARP),
cleaved-PARP, caspase-9, cleaved-caspase-9, caspase-7,
cleaved-caspase-7, anti-rabbit IgG and anti-mouse IgG antibodies;
cat. no. 9915) were purchased from Cell Signaling Technology,
Inc.
Cell lines and cell culture
Two human PC cell lines, AsPC-1 and BxPC-3, were
used in the present study (China Center for Type Culture
Collection). Both cell lines were cultured in RPMI-1640 (Biological
Industries) supplemented with 10% fetal bovine serum (Biological
Industries) and placed at 37°C in a humidified incubator containing
5% CO2.
Cell proliferation assay
AsPC-1 and BxPC-3 cell proliferation was detected
using Cell Counting Kit-8 (MedChemExpress). A total of 5,000 cells
per well were seeded in 96-well plates and cultured at 37°C in an
incubator for 24 h. Cells were then treated with PTX (0, 1, 5, 10,
20, 40, 80, 100 or 120 nM) or CTX (0, 0.5, 1, 2, 4, 5, 7.5, 10, 20
or 40 nM) or CAPE (0, 1, 5, 10, 15, 20, 25, 30, 40 or 50 µM)
or CTX (0, 0.5, 1, 2, 4, 5, 7.5, 10, 20 or 40 nM) and 5 µM
CAPE or CTX (0, 0.8, 2, 4 or 8 nM) and CAPE (0, 1, 5, 10 or 15
µM) for 48 h (8,21). Cells were then incubated in 100 μl
medium containing 10% of Cell Counting Kit-8 (CCK-8) reagent for 1
h. Absorbance was read at 450 nM using a microplate reader (Bio-Rad
Laboratories, Inc.).
Colony formation assay
PC cells were seeded in 6-well plates at the density
of 2,000 cells per well and cultured for 24 h, followed by
treatment with or without drug for 48 h. Cells were maintained in
an incubator with 5% CO2 at 37°C for 7 days.
Subsequently, the medium was removed, and cells were washed twice
by PBS and fixed with methanol at room temperature for 10 min. Cell
clones were stained with Giemsa for 2 min, washed with deionized
water and photographed with a camera (Nikon D3500; Nikon
Corporation).
Cell transfection
The lentiviral vectors (CMV-MCS-EF1α-GFP-Puro)
containing human NF-κB p65 or a non-target control (Zorin) were
used to establish stable cell transfectants. PC cells were seeded
in 6-well plates at the density of 200,000 cells per well and were
infected by 2,000,000 UT lentivirus (MOI value of AsPC-1 and
BxPC-3=10) by using polybrene (Sigma-Aldrich; Merck KGaA; 5
µl/ml). Medium was changed after 8 h. Successfully
transfected PC cells were selected by using 5 µg/ml
puromycin (MedChemExpress) following 48 h transfection. The
lentivirus transfection efficiency was validated by western
blotting and with the expression of green fluorescent protein (488
nm).
Apoptosis analysis
AsPC-1 and BxPC-3 cells were harvested following
treatment with 5 and 10 nM CTX, or 5 µM CAPE, or 5 µM
CAPE and 5 nM CTX, or DMSO for 48 h. Cells were washed twice with
PBS and subsequently stained with 5 µl Annexin V-FITC and 5
µl propidium iodide (PI; cat. no. AD10; Dojindo Molecular
Technologies, Inc.) in the dark at room temperature for 15 min. PC
cell apoptosis was detected by using a FACSCalibur flow cytometer
and analyzed using FlowJo V10 software (BD Biosciences).
Cell cycle analysis
For cell cycle analysis, AsPC-1 and BxPC-3 cells
were harvested following treatment with 5 and 10 nM CTX, or 5
µM CAPE, or 5 µM CAPE and 5 nM CTX, or DMSO for 48 h.
Cells were washed twice with PBS and fixed with 75% ethanol at
−20°C overnight. Subsequently, cells were washed with PBS and were
stained with DNA Prep (Beckman Coulter, Inc.) for 30 min in the
dark. The percentage of cells in the G1, S and G2/M phases was
detected by FACSCalibur flow cytometry (BD Biosciences) and
analyzed using Modifit LT 5.0 software.
Western blotting
AsPC-1 and BxPC-3 cells were treated with 5 and 10
nM CTX, or 5 µM CAPE, or 5 µM CAPE and 5 nM CTX, or
DMSO for 48 h, or stably transfected for NF-κB p65 overexpression
or infected by non-target control lentivirus. Cells were then lysed
using RIPA buffer (Thermo Fisher Scientific, Inc.) supplemented
with phosphatase inhibitors (1:100; Thermo Fisher Scientific, Inc.)
for 30 min on ice. Cell lysate was centrifuged at 14,000 × g for 15
min at 4°C to extract the proteins. The NE-PER Nuclear and
Cytoplasmic Extraction Reagent Kit (cat. no. 78835; Thermo Fisher
Scientific, Inc.) was used to separate the nuclear and cytoplasmic
proteins. The BCA Protein Assay Kit (Thermo Fisher Scientific,
Inc.) was used to quantify proteins in each fraction. Proteins (30
µg) were separated using SurePAGE™ gels 4-20% (GenScript)
and transferred onto polyvinylidene fluoride membranes (EMD
Millipore). Membranes were incubated with the blocking buffer (TBS
with Tween-20 containing 5% skim milk) at room temperature for 1 h.
Membranes were incubated with primary antibodies at 4°C overnight
and with horseradish peroxidase-linked secondary antibody at room
temperature for 1 h. Enhanced chemiluminescence reagent (Fdbio
Science) was used to detect the signal on the membrane.
Immunofluorescence
AsPC-1 and BxPC-3 cells were seeded in 24-well
plates at the density of 1.5×104 cells per well and
treated with 10 nM CTX for 0, 1, 3 and 6 h and washed with PBS.
Cells were subsequently fixed with 4% paraformaldehyde at room
temperature for 15 min and washed three times with PBS. Cells were
blocked with PBS containing 4% BSA (cat. no. abs9157; Absin) and
0.5% Triton at room temperature for 1 h. Cells were then incubated
with antibody against NF-κB p65 at 4°C overnight. Following
incubation with fluorescence-tagged secondary antibody (5
µg/ml; cat. no. A27034; Thermo Fisher Scientific, Inc.) for
1 h and staining with DAPI, cells were imaged using a fluorescent
microscope (magnification, ×200; Olympus Corporation).
Reverse transcription quantitative (RT-q)
PCR
Total RNA was extracted from AsPC-1 and BxPC-3 cells
using NucleoZOL (Macherey-Nagel GmbH) and reverse transcribed into
cDNA using Takara PrimeScript™ RT reagent Kit with gDNA Eraser
(Takara Bio Inc.) according to the manufacturer's instructions.
ChamQTM Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.)
was used and RT-qPCR was performed on an Applied Biosystems™ 7500
Fast Dx Real-Time PCR Instrument (Thermo Fisher Scientific, Inc.)
according to the following reactions: Step 1, 95°C for 30 sec; and
step 2, 40 cycles of 95°C for 10 sec and 60°C for 30 sec. The
sequences of the primers (Tsingke Biological Technology, Co., Ltd.)
were as follows: Bcl-2, forward, 5'-GCCCTGTGGATGACTGAGTA-3',
reverse, 5'-AGCCAGGAGAAATCAAACAGAG-3'; and GAPDH, forward,
5'-GAGCCAAAAGGGTCATCATCT-3', and reverse,
5'-TTCCACGATACCAAAGTTGTCA-3'. GAPDH was used as an internal control
for normalization. Each sample was analyzed in triplicate. The
relative expression levels were normalized to GAPDH and were
expressed as 2−ΔΔCq (22).
Antitumor activity in tumor-bearing
mice
A total of 25 male athymic nude (nu/nu) mice aged
4-5 weeks were provided by Shanghai SLAC Laboratory Animal Co.,
Ltd. Animal care and experiments were conducted according to the
Guidelines of the Zhejiang University Animal Care Committee (China)
and were approved by the Tab of Animal Experimental Ethical
Inspection of the First Affiliated Hospital, College of Medicine,
Zhejiang University. Briefly, BxPC-3 cells (4×106) in
100 µl PBS were injected into the right flank of the mice.
Two weeks after inoculation, mice were divided into five groups
(n=5 per group) according to the treatment they received as
follows: Control, no drug treatment; CAPE (10 mg/kg); PTX (10
mg/kg); CTX (10 mg/kg); and CTX (8 mg/kg) with CAPE (10 mg/kg).
Drugs were dissolved in a mixture of DMSO, Tween-80 and saline
(1:1:38 ratio), and the control group received only DMSO, Tween-80
and saline (1:1:38 ratio). The treatment was injected around the
tumors every three days and for three cycles. The width and length
of the tumors were measured every time before treatment. At the end
of the experiment, mice were sacrificed by cervical dislocation
following anesthesia by intraperitoneal injection of chloral
hydrate (400 mg/kg). Tumors were subsequently removed and preserved
in formalin. Tumor volume was calculated according to the following
formula: length × width2 × 0.5 (cm3).
Immunohistochemistry (IHC) and TUNEL
staining assay
To detect PC cell proliferation and apoptosis in
mice tumor, paraffin-embedded tissues were cut into 4-µm
slices, deparaffinized and rehydrated by dimethylbenzene and
ethanol. Antigen was retrieved by using citrate buffer (pH 6.0) for
15 min and slices were washed three times with PBS. Sections were
incubated with 3% H2O2 for 10 min at room
temperature and washed three times with PBS. One part of the slices
was used for IHC and the other parts were used for the TUNEL assay.
For IHC, slices were incubated with PCNA antibody (1:5,000)
overnight at 4°C, washed with PBS, and incubated with the secondary
antibody (cat. no. SAP9100; OriGene Technologies, Inc.) for 30 min
at 37°C. Sections were washed three times with PBS and stained with
the 3'-diaminobenzi-dine (DAB) for 1 min. Subsequently, DAB
staining was stopped by washing in running water and sections were
counterstained using hematoxylin. Sections were imaged using a
microscope (Olympus Corporation; magnification, ×200). For the
TUNEL assay, sections were stained according to the manufacturer's
instructions by using the One Step TUNEL Apoptosis Assay Kit
(Beyotime Institute of Biotechnology). Sections were imaged using a
fluorescent microscope (Olympus Corporation; magnification,
×200).
Statistical analysis
Data were presented as the means ± standard error of
the mean and were calculated using SPSS version 22.0 (IBM Corp.)
and Prism GraphPad 6.02 (GraphPad Software, Inc.). Differences
between two groups were calculated using Student's t-test. ANOVA
followed by Tukey's test were used to calculate the differences
among multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
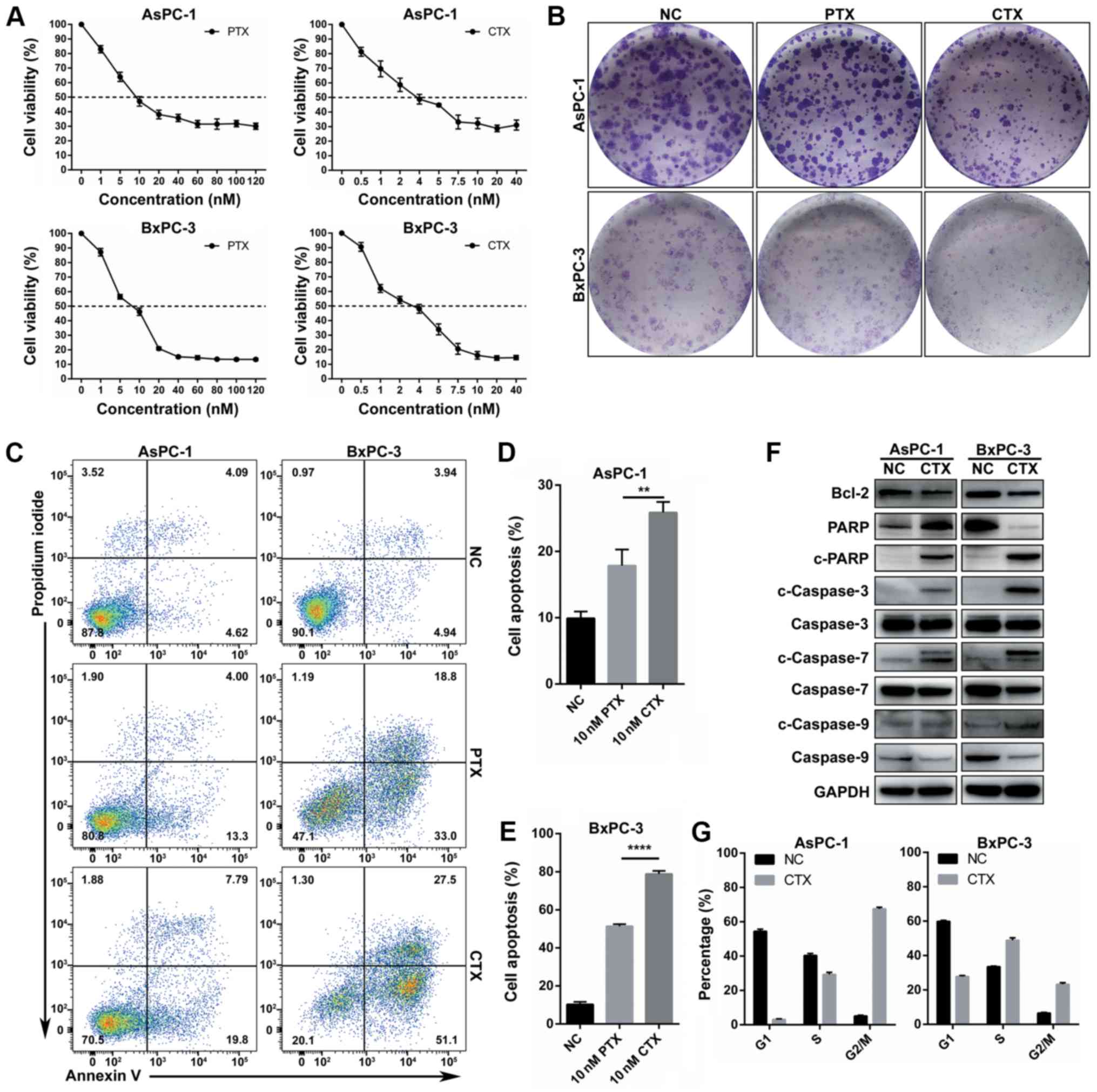
CTX is more effective than PTX in PC
cells
The effect of CTX and PTX on the proliferation of
the PC cell lines AsPC-1 and BxPC-3 was evaluated by using the
CCK-8 assay. As presented in Fig.
1A and Table I, after
assessing the inhibition curve of cell proliferation in a drug
concentration gradient after 48 h of drug treatment, the results
demonstrated that CTX had a lower IC50 value compared
with PTX in the two PC cell lines. Furthermore, the results from
the colony formation assay demonstrated that the colony number and
size in the CTX group was lower and smaller, respectively, compared
with the PTX group (10 nM; Fig.
1B). Following PC cell treatment with 10 nm PTX or CTX for 48
h, Annexin V-FITC and PI staining was used to assess apoptosis. The
results demonstrated that apoptosis was significantly higher in the
CTX group compared with the PTX group in the two PC cell lines
(Fig. 1C-E). Expression of
apoptosis-related proteins was also evaluated. The results from
western blotting demonstrated that CTX led to a decline expression
of the anti-apoptotic protein Bcl-2 and an increased cleavage of
caspase-3, caspase-7, caspase-9 and PARP (Fig. 1F). Furthermore, the effect of
treatment with 10 nM CTX on cell cycle arrest was examined in the
AsPC-1 and BxPC-3 cell lines. The results demonstrated that cell
cycle arrest in the AsPC-1 and BxPC-3 cell lines, and the
proportion of cells in the G2/M phase was increased (Fig. 1G and S1). Taken together, these findings
suggested that CTX may inhibit PC cell proliferation mostly by
promoting cell apoptosis and cell cycle arrest; however, no
association between cell apoptosis and cell cycle arrest was
determined.
 | Table IDrug sensitivity of AsPC-1 and BxPC-3
cell lines to cabazitaxel. |
Table I
Drug sensitivity of AsPC-1 and BxPC-3
cell lines to cabazitaxel.
| Drug (48 h
treatment) | AsPC-1
IC50 nM | BxPC-3
IC50 nM |
|---|
| Paclitaxel | 13.010±1.780 | 7.056±0.636 |
| Cabazitaxel | 3.772±0.465 | 2.507±0.262 |
| Cabazitaxel + 5
µM caffeic acid phenethyl ester | 1.440±0.273 | 0.917±0.187 |
CTX promotes the translocation of NF-κB
p65 to the nucleus and activates the NF-κB pathway
Previous studies reported that taxanes can induce
the translocation of NF-κB to the nucleus, which is crucial to the
activation of the NF-κB pathway (19,20).
In the present study, cytoplasmic and nuclear proteins were
extracted from PC cells following treatment with 10 nM CTX for 0,
1, 3 and 6 h, and NF-κB p65 expression was detected in both
fractions by using western blotting. The results demonstrated that
treatment with CTX induced an increase in NF-κB p65 protein
expression in the nucleus compared within the cytoplasm in the two
PC cell lines (Fig. 2A). In
addition, the results from IHC demonstrated that the translocation
of NF-κB to the nucleus increased with CTX treatment (Fig. 2C). In addition, numerous proteins
involved in the NF-κB pathway were also examined by western
blotting, including phospho-inbibitor of κB kinase (IKK)α/β,
phospho-inhibitor of κB (IκB)α and phospho-NF-κB p65. The results
demonstrated that the expression of all these proteins was
increased following treatment with CTX (Fig. 2B). These findings suggested that
NF-κB pathway may influence CTX treatment efficiency.
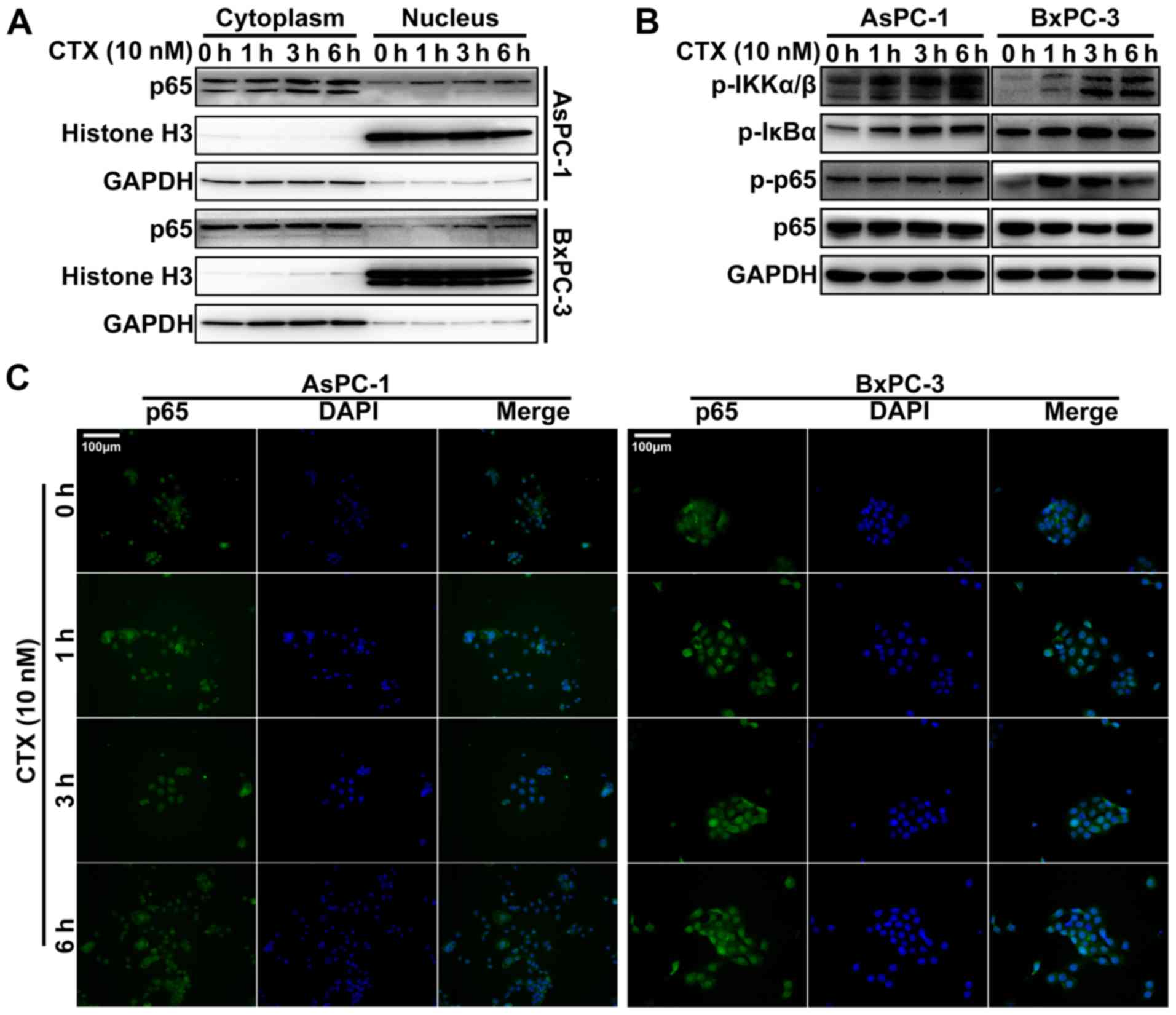 | Figure 2NF-κB was activated in PC cells
following treatment with CTX. (A) NF-κB p65 protein expression in
the nucleus was detected by western blotting in PC cells following
treatment with 10 nM CTX for 0, 1, 3 and 6 h. (B) Protein
expression of the NF-κB pathway proteins phospho-IKKα/β,
phospho-IκBα, phospho-NF-κB p65 and NF-κB p65 assessed by western
blotting. (C) NF-κB p65 location was observed by
immunofluorescence. Magnification, x200. Blue staining, nucleus by
DAPI; green staining, NF-κB p65. Bcl-2, B-cell lymphoma-2; CTX,
cabazitaxel; p-p65, phospho-NF-κB p65; NF-κB, nuclear factor-κB;
PC, prostate cancer; p-IKKα/β, phospho-IKKα/β; p-IκBα,
phospho-IκBα. |
NF-κB p65 overexpression attenuates the
effect of CTX
To evaluate the role of NF-κB p65 in CTX treatment,
AsPC-1 and BxPC-3 cell lines overexpressing NF-κB p65 were
constructed. Transfection efficiency was evaluated by western
blotting. The results demonstrated that phospho-NF-κB p65 was
increased in NF-κB p65-overexpressing cells (Fig. 3A); however, no change in NF-κB p65
expression was observed in the nucleus (Fig. 3B). Subsequently, control and
transfected were treated with the same concentration gradient of
CTX for 48 h in 96-well plates and cell proliferation was assessed
by using CCK-8 assay. The results demonstrated a decrease in
sensitivity to CTX treatment in the NF-κB p65-overexpressing cells
compared with control cells (Fig.
3C). These findings suggested that NF-κB p65 may affect CTX
resistance.
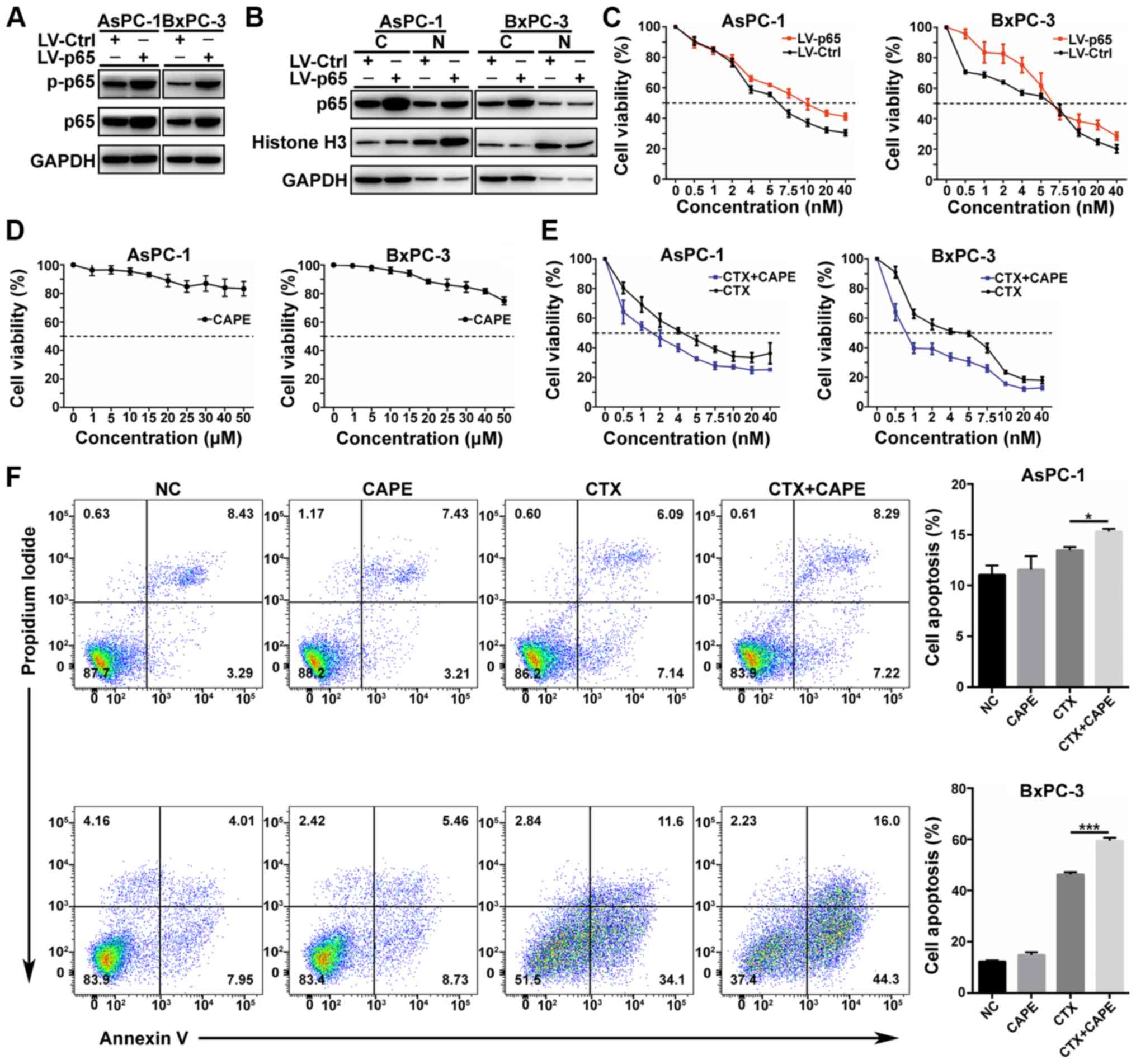 | Figure 3NF-κB affected CTX toxicity in PC
cells. (A) Efficacy of NF-κB p65 (RELA) overexpression in the
AsPC-1 and BxPC-3 cell lines and phospho-NF-κB p65 expression were
examined by western blotting. (B) NF-κB p65 protein expression in
the nucleus was detected by western blotting following NF-κB p65
overexpression. (C) CCK-8 assay was used to detect the sensitivity
of the two cell lines to CTX in the control and NF-κB p65
overexpression groups. (D) CAPE cytotoxicity determined with the
CCK-8 assay. (E) Effects of CTX combined to 5 µM CAPE on PC
cell proliferation. (F) Apoptosis analysis of PC cells treated with
CAPE 5 µM CAPE, 5 nM CTX or 5 nM CTX and 5 µM CAPE
(left panel), and quantification of cell apoptosis in AsPC-1 and
BxPC-3 cell lines (right panel). Data were presented as the means ±
standard error of the mean of three independent experiments. In
AsPC-1 cell line, the apoptosis rate of CTX group was compared with
CTX+CAPE group *P<0.05. In BxPC-3 cell line, the
apoptosis rate of CTX group was compared with CTX+CAPE group
**P<0.01. C, cytoplasm; CAPE, caffeic acid phenethyl
ester; CCK-8, Cell Counting Kit-8; CTX, cabazitaxel; PC, pancreatic
cancer; NF-κB, nuclear factor-κB; N, nucleus; p-p65, phospho-NF-κB
p65; NC, negative control. |
Inhibiting NF-κB p65 translocation to the
nucleus enhances CTX efficiency by downregulating Bcl-2
To further evaluate the role of NF-κB p65 in CTX
treatment efficiency, the NF-κB inhibitor CAPE was used in
combination with CTX in AsPC-1 and BxPC-3 cells. The toxicity of
CAPE on PC cells was determined. The results demonstrated an
absence of toxicity of CAPE, and the concentration of 5 µM
was chosen for further experiments (Figs. 3D and S2A). Subsequently, the effect of CTX
combined with 5 µM CAPE on PC cell proliferation was
determined. The results demonstrated that CAPE enhanced cell
sensitivity to CTX (Fig. 3E;
Table I). Furthermore, cell
apoptosis was increased following treatment with 5 nmol CTX and 5
µmol CAPE for 48 h, compared with treatment with 5 nmol CTX
alone (Fig. 3F). No significant
difference in the cell cycle arrest was observed between treatment
with CTX alone and combined treatment with CTX and CAPE (Fig. S2B). Furthermore, the results from
drug combination assay (combination of CTX and CAPE at different
concentration to treat AsPC-1 and BxPC-3) indicated that CAPE
synergized with CTX (Fig. 4A and
B). To evaluate the underlying mechanism driving apoptosis in
PC cells following treatment with combination of CTX and CAPE, the
protein expression of apoptosis-related proteins was detected. The
results demonstrated a decline in Bcl-2 expression and an increase
in cleaved-PARP expression (Fig.
4C). Subsequently, the role of NF-κB p65 in the regulation of
Bcl-2 protein expression was evaluated by using RT-qPCR. The
results demonstrated that Bcl-2 mRNA level was significantly
decreased following treatment with CTX and CAPE compared with CTX
treatment alone (Fig. 4D). In
addition, Bcl-2 protein expression was upregulated following NF-κB
p65 overexpression (Fig. S2C).
These findings suggested that CAPE may downregulate Bcl-2, mostly
by inhibiting NF-κB p65 translocation into the nucleus, leading to
the enhancement of CTX pro-apoptotic effect.
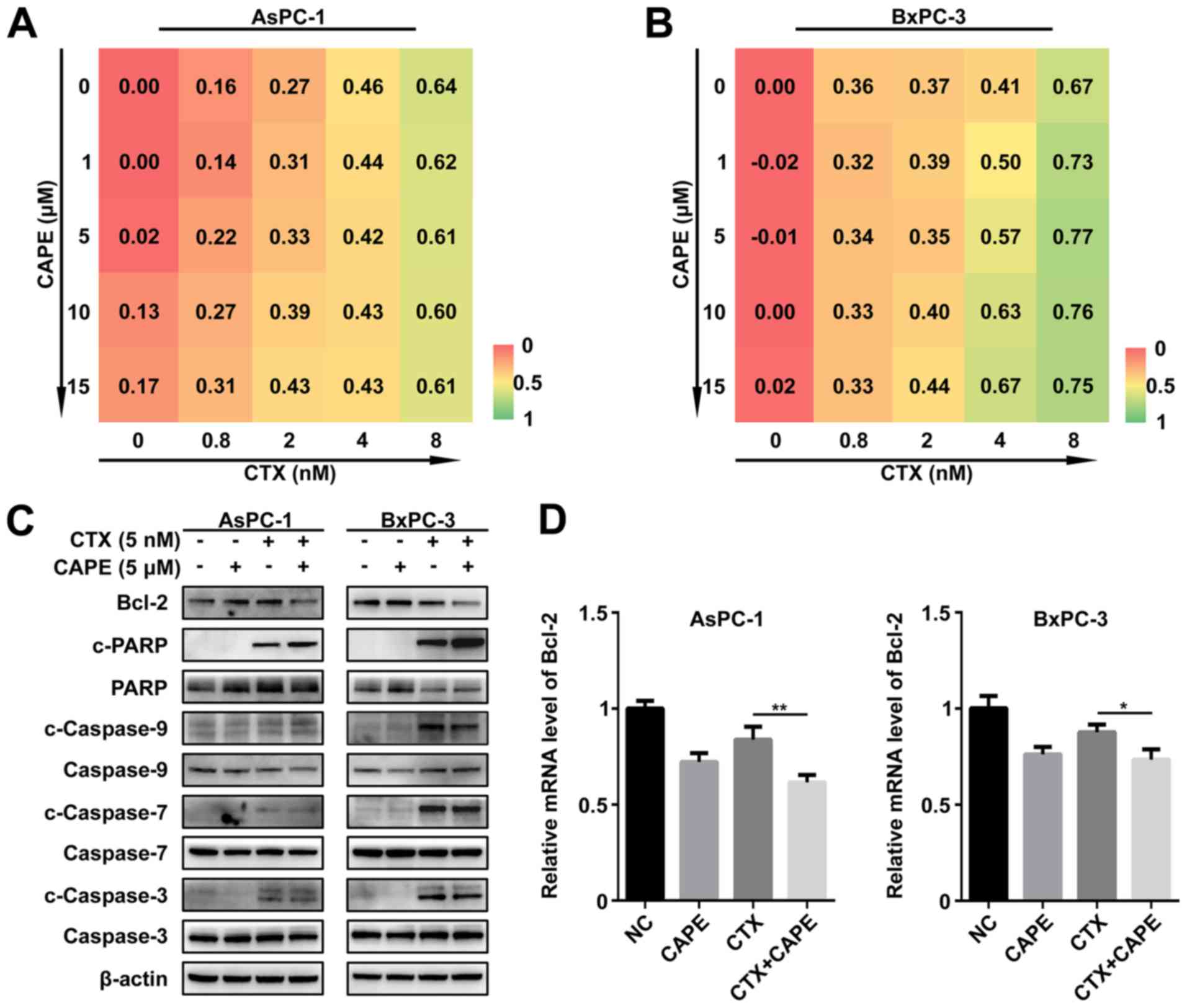 | Figure 4CAPE increased PC cell apoptosis by
downregulating Bcl-2. (A and B) Drug combination assay revealed the
synergy between CAPE and CTX. (C) Protein expression of
apoptosis-related proteins between the negative control, 5
µM CAPE, 5 nM CTX, and 5 nM CTX plus 5 µM CAPE groups
assessed by western blotting. (D) Relative mRNA level of Bcl-2 in
determined by reverse transcription quantitative PCR. Data were
presented as the mean ± standard error of the mean of three
independent experiments. In AsPC-1 cell line, the Bcl-2 mRNA level
of CTX group was compared with CTX+CAPE group
**P<0.01. In BxPC-3 cell line, the Bcl-2 mRNA level
of CTX group was compared with CTX+CAPE group
*P<0.05. Bcl-2, B-cell lymphoma-2; c, cleaved; CAPE,
caffeic acid phenethyl ester; CTX, cabazitaxel; NC, negative
control; PARP, poly (ADP-ribose) polymerase; PC, pancreatic
cancer. |
Combination of CTX and CAPE is more
effective in mouse xenograft models
Following mice treatment with drugs, the results
demonstrated that combination of CTX and CAPE was more effective in
inhibiting tumor growth according to the ratio of tumor weight to
body weight compared with CTX treatment alone; however, there was
no significant difference in the tumor volume between CTX and
combination groups. In addition, CTX was significantly more
effective at inhibiting tumor growth in mouse xenograft models
compared with PTX (Fig. 5A-C).
These results were consistent with in vitro results from the
present study (Figs. 1 and
3). Furthermore, BxPC-3 xenograft
tumors were tested by IHC for PCNA. As presented in Fig. 5D, the presence of PCNA-positive
cells was decreased in the combination group compared with the
other groups, suggesting a decline in PC cell proliferation
(Fig. 5D). Furthermore, the
results from TUNEL assay demonstrated that the combination group
was more efficient at inducing cell apoptosis (Fig. 5E).
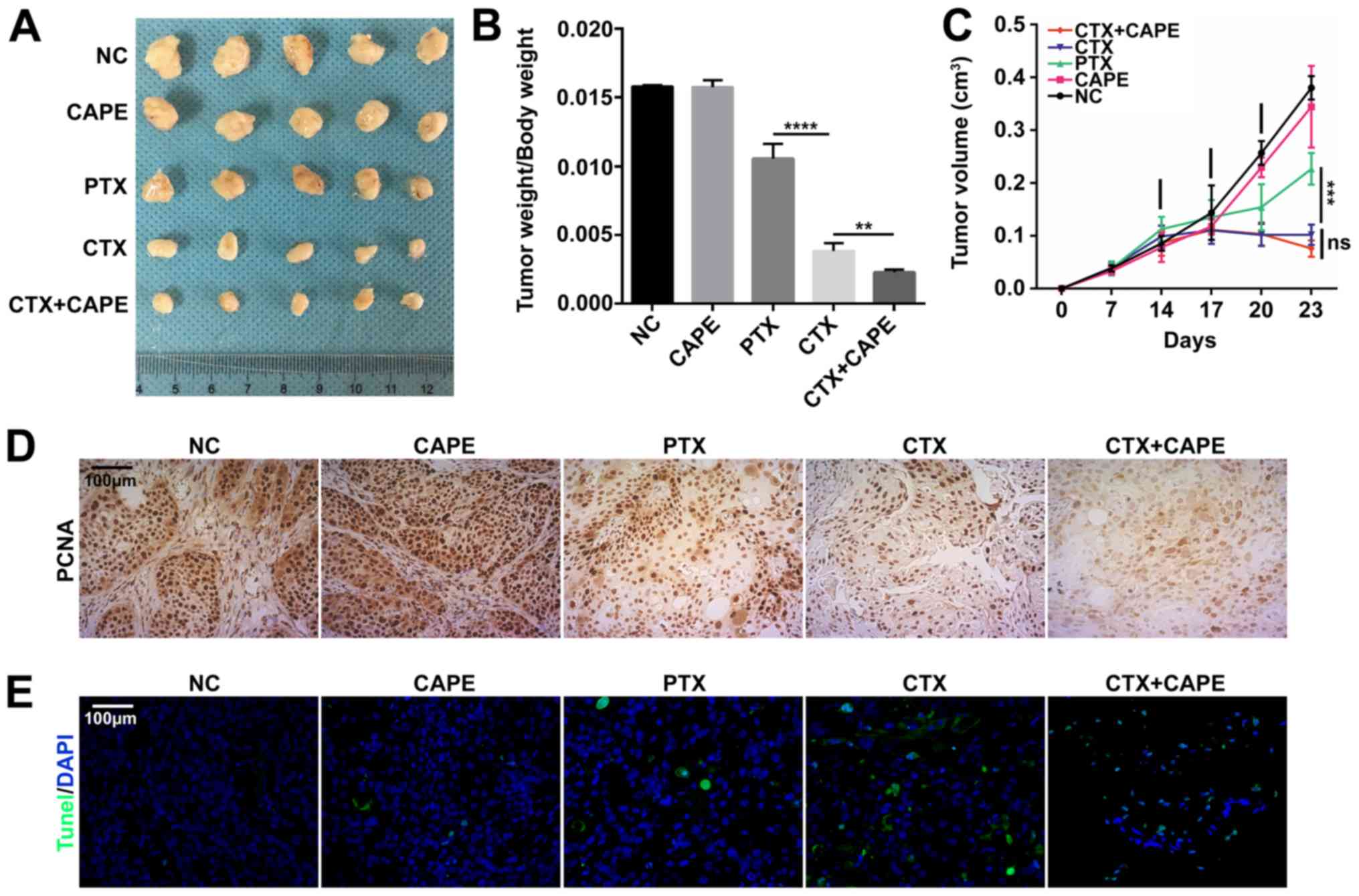 | Figure 5Effect of CAPE, CTX and PTX on PC
tumor growth in vivo. (A) Tumor size collected in the five
treatment groups (saline, CAPE at 10 mg/kg, PTX at 10 mg/kg, CTX at
10 mg/kg and CTX at 8 mg/kg plus CAPE 10 mg/kg) at the end of the
experiment. (B) Ratio of tumor weight to body weight and (C) tumor
volume were compared between the five groups. Data were presented
as the mean ± standard error of the mean of five independent
experiments. Tumor weight/body weight rate of CTX group was
compared with CTX+CAPE group **P<0.01. Tumor
weight/body weight rate of PTX group was compared with CTX group.
****P<0.0001. (D) Immunohistochemistry of tumor
tissues stained for PCNA. Magnification, x200. (E) TUNEL staining
of tumor tissues. The nucleus was stained blue by DAPI; green
staining represents the TUNEL staining. Magnification, x200. CAPE,
caffeic acid phenethyl ester; CTX, cabazitaxel; PCNA, proliferating
cell nuclear antigen; PC, prostate cancer; PTX, paclitaxel. |
Discussion
At present, the early diagnosis of PC remains
difficult, and patients with PC present with a high recurrence rate
following surgical resection. Administration of an effective
adjuvant chemotherapy following resection is therefore crucial.
Gemcitabine combined with albumin-bound PTX remians the main
therapeutic option for patients with PC following surgical
resection, or for patients with a good performance status and who
are physically unable to undergo the procedure (2,23).
However, the high resistance rate of gemcitabine and PTX in PC
remains a challenge. The main cause for drug resistance in PC is
the upregulation of multidrug resistance-associated P-gp, which
acts as a drug efflux pump (24-26).
In addition, gemcitabine and PTX resistance is associated with P-gp
high expression (2,27). CTX is a semi-synthetic taxane that
was developed to overcome PTX and DTX resistance (6,28-30).
Compared with the other two types of taxanes, CTX has a poor
affinity for P-gp, suggesting that patients treated with CTX might
be less likely to develop resistance (4,31).
It is therefore crucial to develop a treatment alternative to PTX
for patients with PC. In the present study, CTX sensitivity was
compared with PTX in PC cell lines. The results from CCK-8 and
colony formation assays demonstrated that CTX was more effective
than PTX in inhibiting AsPC-1 and BxPC-3 cell proliferation.
Furthermore, in PC cell lines, CTX was efficient at inducing cell
cycle arrest in the G2/M phase and promoting apoptosis at low
concentration. In vivo, CTX also significantly inhibited
tumor growth. However, treatment with same dose of PTX did not have
the same effects. These findings suggested that CTX may be used in
replacement of PTX in the treatment of PC.
NF-κB serves a crucial role in inflammation and
immunity, and a previous study reported that NF-κB pathway
activation is also important for tumor development (14). Reticuloendotheliosis (REL) protein
family provided the main evidence linking NF-κB to cancer. The main
type of REL includes RELA, also known as p65. The first step of the
classical NF-κB activation pathway is the activation of the IKK
complex inhibitor. Subsequently, IKK phosphorylates the NF-κB-bound
IκB, which contributes to the ubiquitin-dependent degradation of
IκB, resulting in the nuclear translocation of the p65-p50 dimer
(14). This process induces cancer
cell proliferation and inhibit their apoptosis. Certain stimuli,
including tumor necrosis factor-α, CD40L, interleukin-1 or
lipopolysaccha-rides, can cause NF-κB pathway activation (13,32).
However, numerous studies reported that PTX can NF-κB pathway
activation in immune or tumor cells (19,20).
Both CTX and PTX are taxanes, and CTX must be identical to PTX in
some ways. In the present study, NF-κB activation was determined in
PC cell lines treated by CTX by western blotting and
immuno-fluorescence. The results demonstrated and increased nuclear
translocation of NF-κB p65. In addition, phospho-IKKα/β,
phospho-IκBα and phospho-NF-κB p65 were also upregulated in PC
cells treated with CTX. Subsequently, it was important to determine
whether NF-κB pathway activation may affect CTX cytotoxicity. To do
so, AsPC-1 and BxPC-3 cell lines overexpressing NF-κB p65 were
designed, and cell proliferation following treatment with different
concentrations of CTX was evaluated. The results demonstrated that
NF-κB p65 overexpression attenuated CTX cytotoxicity, suggesting
that NF-κB may serve a crucial role in PC cell sensitivity to CTX.
Over the last decades, preclinical and clinical studies reported
that NF-κB activation serves a central role in drug resistance
(33,34). Subsequently, it is hypothesized
that NF-κB may influence CTX resistance. CTX exerts its effects
mainly by binding to tubulin and therefore suppressing microtubule
dynamics during cell division (31); however, the activation of NF-κB
offset part of the effects that CTX made on the expression of cell
apoptosis-related proteins and G2/M phase arrest-related proteins
(14,35). As a nuclear factor, NF-κB might
regulate the transcription of apoptosis-related proteins and G2/M
phase arrest-related proteins, leading to CTX resistance. The
results from the present study demonstrated that Bcl-2 may be
regulated by NF-κB; however, further investigation is required to
confirm this hypothesis. At present, there are only a few reports
on CTX resistance, and no cell or animal models of CTX resistance
have been studied.
In the present study, an inhibitor of NF-κB was
selected to explore the pharmacological effects of CTX. CAPE is a
specific inhibitor of NF-κB activation that doesn't function by
blocking the degradation of IκBα, but by directly suppressing NF-κB
protein interaction with DNA (21,36,37).
The present study demonstrated that combination of CTX with CAPE
had better effects on the inhibition of PC cell proliferation and
the stimulation of PC cell apoptosis compared with CTX alone.
Furthermore, results from in vivo experiments demonstrated
that tumor growth inhibition was significantly increased in the
combination group compared with the group treated with CTX alone.
Previous clinical studies reported that CTX can greatly improve the
survival of patients with prostate cancer (6,11);
however, some adverse effects are observed when patients received
intravenous injection of 25 mg/m2 CTX over 1 h every 3
week (38), which is also the case
with other taxanes. The present study demonstrated therefore that
the combination of CTX and CAPE may allow the diminution of CTX
dose, which may alleviate the potential onset of side effects.
Previous studies reported that Bcl-2 expression is regulated by
NF-κB signaling (39,40). The present study demonstrated that
Bcl-2 was downregulated following treatment with the NF-κB
inhibitor CAPE, and the increased cleaved-PARP expression could
explain the increased apoptosis in the combination group. The
findings from the present study highlighted the potential synergy
between CTX and CAPE, and suggested that CAPE may enhance CTX
pharmacological effects in patients with PC.
CTX is a drug without any modification used in our
study, however, albumin-bound PTX is a clinical drug that uses
albumin as a carrier for PTX. In order to eliminate the effect of
albumin on the experiment, PTX was chosen in the present study.
Previous studies have reported nanoparticle-CTX delivery (41-43).
The present study provided evidence for the use of modified CTX to
replace albumin-bound PTX in PC treatment, due to its low
resistance rate and its strong effect on tumor growth inhibition.
The present study also highlighted the crucial role of NF-κB
activation in PC cell sensitivity to CTX. NF-κB inhibition enhanced
CTX-induced toxicity in PC cells, suggesting that activation of
NF-κB may influence CTX resistance. However, further investigation
is required to validate this hypothesis. In addition, combining CTX
with a NF-κB inhibitor may be considered as an effective way to
reduce CTX dosage, which may therefore decrease CTX-mediated
adverse effects. Clinical trial including patients with PC is
therefore required to improve the response prediction of CTX and
optimize therapeutic options for patients. The results from the
present study indicated that CTX may be used in the clinical
treatment of patients with PC.
Supplementary Data
Funding
This study was supported by the Innovative Research
Groups of National Natural Science Foundation of China (grant no.
81721091), the Major program of National Natural Science Foundation
of China (grant no. 91542205), the National Natural Science
Foundation of China (grant nos. 81570575 and 81870434) and the
National S&T Major Project (grant no. 2017ZX10203205).
Availability of data and materials
All data analyzed during this study are included in
this published article.
Authors' contributions
ZL and ZX designed the study. ZL wrote the
manuscript. ZL, JC and SZ performed cell experiments, western
blotting, RT-qPCR, apoptosis and cell cycle analyses. WS, CJ and MZ
performed animal experiments. ZL and WS contributed to statistical
analysis and designed the table and figures. PS and SZ were
involved in project management and supervised the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Tab of Animal
Experimental Ethical Inspection of the First Affiliated Hospital,
College of Medicine, Zhejiang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
CTX
|
cabazitaxel
|
|
CAPE
|
caffeic acid phenethyl ester
|
|
DTX
|
docetaxel
|
|
PC
|
pancreatic cancer
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
PTX
|
paclitaxel
|
References
|
1
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartwig W, Werner J, Jäger D, Debus J and
Büchler MW: Improvement of surgical results for pancreatic cancer.
Lancet Oncol. 14:e476–e485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galsky MD, Dritselis A, Kirkpatrick P and
Oh WK: Cabazitaxel. Nat Rev Drug Discov. 9:677–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seruga B and Tannock IF:
Chemotherapy-based treatment for castration-resistant prostate
cancer. J Clin Oncol. 29:3686–3694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dorff TB and Quinn DI: Cabazitaxel in
prostate cancer: Stretching a string. Lancet. 376:1119–1120. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotsakis A, Matikas A, Koinis F,
Kentepozidis N, Varthalitis II, Karavassilis V, Samantas E,
Katsaounis P, Dermitzaki EK, Hatzidaki D, et al: A multicentre
phase II trial of cabazitaxel in patients with advanced
non-small-cell lung cancer progressing after docetaxel-based
chemotherapy. Br J Cancer. 115:784–788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen R, Cheng Q, Owusu-Ansah KG, Chen J,
Song G, Xie H, Zhou L, Xu X, Jiang D and Zheng S: Cabazitaxel, a
novel chemotherapeutic alternative for drug-resistant
hepatocellular carcinoma. Am J Cancer Res. 8:1297–1306.
2018.PubMed/NCBI
|
|
9
|
Zhong T, He B, Cao HQ, Tan T, Hu HY, Li YP
and Zhang ZW: Treating breast cancer metastasis with
cabazitaxel-loaded polymeric micelles. Acta Pharmacol Sin.
38:924–930. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunos CA, Stefan T and Jacobberger JW:
Cabazitaxel-induced stabilization of microtubules enhances
radiosensitivity in ovarian cancer cells. Front Oncol. 3:2262013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abidi A: Cabazitaxel: A novel taxane for
metastatic castration-resistant prostate cancer-current
implications and future prospects. J Pharmacol Pharmacother.
4:230–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Bteich J and Li SD: Current update
of a carboxymeth-ylcellulose-PEG conjugate platform for delivery of
insoluble cytotoxic agents to tumors. AAPS J. 19:386–396. 2017.
View Article : Google Scholar
|
|
13
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Awasthee N, Rai V, Chava S, Nallasamy P,
Kunnumakkara AB, Bishayee A, Chauhan SC, Challagundla KB and Gupta
SC: Targeting IκappaB kinases for cancer therapy. Semin Cancer
Biol. 56:12–24. 2019. View Article : Google Scholar
|
|
16
|
Rajagopal C, Lankadasari MB, Aranjani JM
and Harikumar KB: Targeting oncogenic transcription factors by
polyphenols: A novel approach for cancer therapy. Pharmacol Res.
130:273–291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zaidi AH, Raviprakash N, Mokhamatam RB,
Gupta P and Manna SK: Profilin potentiates chemotherapeutic agents
mediated cell death via suppression of NF-κB and upregulation of
p53. Apoptosis. 21:502–513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Yang G, Feng M, Zheng S, Cao Z, Qiu
J, You L, Zheng L, Hu Y, Zhang T, et al: NF-κB in pancreatic
cancer: Its key role in chemoresistance. Cancer Lett. 421:127–134.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Das KC and White CW: Activation of
NF-kappaB by anti-neoplastic agents. Role of protein kinase C. J
Biol Chem. 272:14914–14920. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perera PY, Qureshi N and Vogel SN:
Paclitaxel (Taxol)-induced NF-kappaB translocation in murine
macrophages. Infect Immun. 64:878–884. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Natarajan K, Singh S, Burke TR Jr,
Grunberger D and Aggarwal BB: Caffeic acid phenethyl ester is a
potent and specific inhibitor of activation of nuclear
transcription factor NF-kappa B. Proc Natl Acad Sci USA.
93:9090–9095. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Mohammed S, Van Buren G II and Fisher WE:
Pancreatic cancer: Advances in treatment. World J Gastroenterol.
20:9354–9360. 2014.PubMed/NCBI
|
|
24
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hagmann W, Faissner R, Schnölzer M, Löhr M
and Jesnowski R: Membrane drug transporters and chemoresistance in
human pancreatic carcinoma. Cancers (Basel). 3:106–125. 2010.
View Article : Google Scholar
|
|
26
|
Lu Z, Kleeff J, Shrikhande S, Zimmermann
T, Korc M, Friess H and Büchler MW: Expression of the
multidrug-resistance 1 (MDR1) gene and prognosis in human
pancreatic cancer. Pancreas. 21:240–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ezrahi S, Aserin A and Garti N: Basic
principles of drug delivery systems - the case of paclitaxel. Adv
Colloid Interface Sci. 263:95–130. 2019. View Article : Google Scholar
|
|
28
|
Di Lorenzo G, Buonerba C, Autorino R, De
Placido S and Sternberg CN: Castration-resistant prostate cancer:
Current and emerging treatment strategies. Drugs. 70:983–1000.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng YY, Zeng YJ, Zhang NN, Li CX, Xie T
and Zeng ZW: The preparation, determination of a flexible complex
liposome co-loaded with cabazitaxel and β-elemene, and animal
pharmacodynamics on paclitaxel-resistant lung adenocarcinoma.
Molecules. 24:242019. View Article : Google Scholar
|
|
30
|
Vrignaud P, Sémiond D, Lejeune P, Bouchard
H, Calvet L, Combeau C, Riou JF, Commerçon A, Lavelle F and Bissery
MC: Preclinical antitumor activity of cabazitaxel, a semisynthetic
taxane active in taxane-resistant tumors. Clin Cancer Res.
19:2973–2983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villanueva C, Bazan F, Kim S, Demarchi M,
Chaigneau L, Thiery-Vuillemin A, Nguyen T, Cals L, Dobi E and Pivot
X: Cabazitaxel: A novel microtubule inhibitor. Drugs. 71:1251–1258.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang CY, Mayo MW and Baldwin AS Jr: TNF-
and cancer therapy-induced apoptosis: Potentiation by inhibition of
NF-kappaB. Science. 274:784–787. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ju HQ, Li H, Tian T, Lu YX, Bai L, Chen
LZ, Sheng H, Mo HY, Zeng JB, Deng W, et al: Melatonin overcomes
gemcitabine resistance in pancreatic ductal adenocarcinoma by
abrogating nuclear factor-κB activation. J Pineal Res. 60:27–38.
2016. View Article : Google Scholar
|
|
34
|
Qiao Q, Sun C, Han C, Han N, Zhang M and
Li G: Endoplasmic reticulum stress pathway PERK-eIF2α confers
radioresistance in oropharyngeal carcinoma by activating NF-κB.
Cancer Sci. 108:1421–1431. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng J, Hamanishi J, Matsumura N, Abiko K,
Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, et
al: Chemotherapy induces programmed cell death-ligand 1
overexpression via the nuclear factor-κB to foster an
immuno-suppressive tumor microenvironment in ovarian cancer. Cancer
Res. 75:5034–5045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu J, Omene C, Karkoszka J, Bosland M,
Eckard J, Klein CB and Frenkel K: Caffeic acid phenethyl ester
(CAPE), derived from a honeybee product propolis, exhibits a
diversity of anti-tumor effects in pre-clinical models of human
breast cancer. Cancer Lett. 308:43–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park MH, Kang DW, Jung Y, Choi KY and Min
S: Caffeic acid phenethyl ester downregulates phospholipase D1 via
direct binding and inhibition of NFκB transactivation. Biochem
Biophys Res Commun. 442:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: TROPIC Investigators: Prednisone plus cabazitaxel or
mito-xantrone for metastatic castration-resistant prostate cancer
progressing after docetaxel treatment: A randomised open-label
trial. Lancet. 376:1147–1154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kong R, Sun B, Jiang H, Pan S, Chen H,
Wang S, Krissansen GW and Sun X: Downregulation of nuclear
factor-kappaB p65 subunit by small interfering RNA synergizes with
gemcitabine to inhibit the growth of pancreatic cancer. Cancer
Lett. 291:90–98. 2010. View Article : Google Scholar
|
|
40
|
Yuan Z, Jiang H, Zhu X, Liu X and Li J:
Ginsenoside Rg3 promotes cytotoxicity of Paclitaxel through
inhibiting NF-κB signaling and regulating Bax/Bcl-2 expression on
triple-negative breast cancer. Biomed Pharmacother. 89:227–232.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie B, Wan J, Chen X, Han W and Wang H:
Preclinical evaluation of a cabazitaxel prodrug using nanoparticle
delivery for the treatment of taxane-resistant malignancies. Mol
Cancer Ther. 19:822–834. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng F, Sun Y, Lee RJ, Wang G, Zheng X,
Zhang H, Fu Y, Yan G, Wang Y, Deng W, et al: Folate
receptor-targeted albumin nanoparticles based on microfluidic
technology to deliver cabazitaxel. Cancers (Basel). 11:112019.
View Article : Google Scholar
|
|
43
|
Ren T, Wang Q, Xu Y, Cong L, Gou J, Tao X,
Zhang Y, He H, Yin T, Zhang H, et al: Enhanced oral absorption and
anticancer efficacy of cabazitaxel by overcoming intestinal mucus
and epithelium barriers using surface polyethylene oxide (PEO)
decorated positively charged polymer-lipid hybrid nanoparticles. J
Control Release. 269:423–438. 2018. View Article : Google Scholar
|