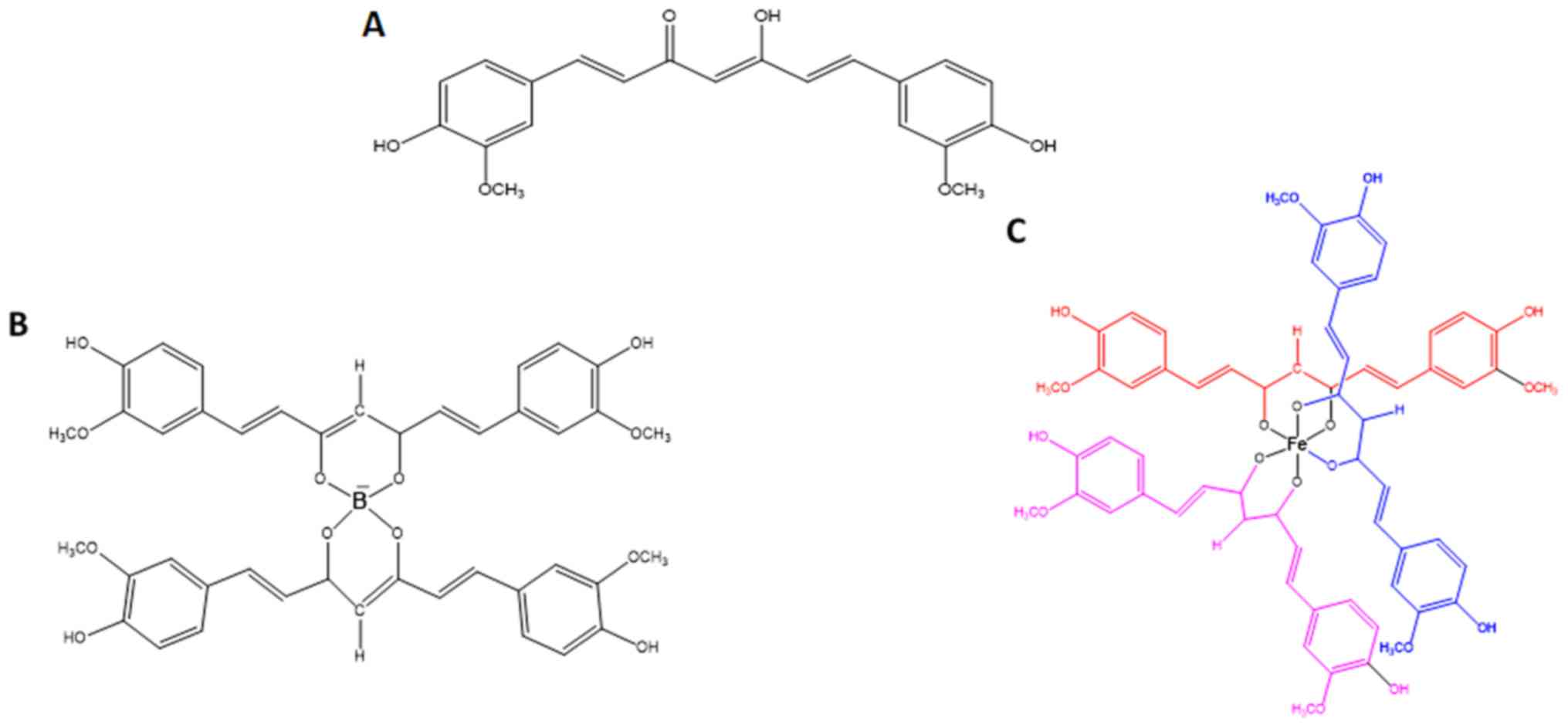
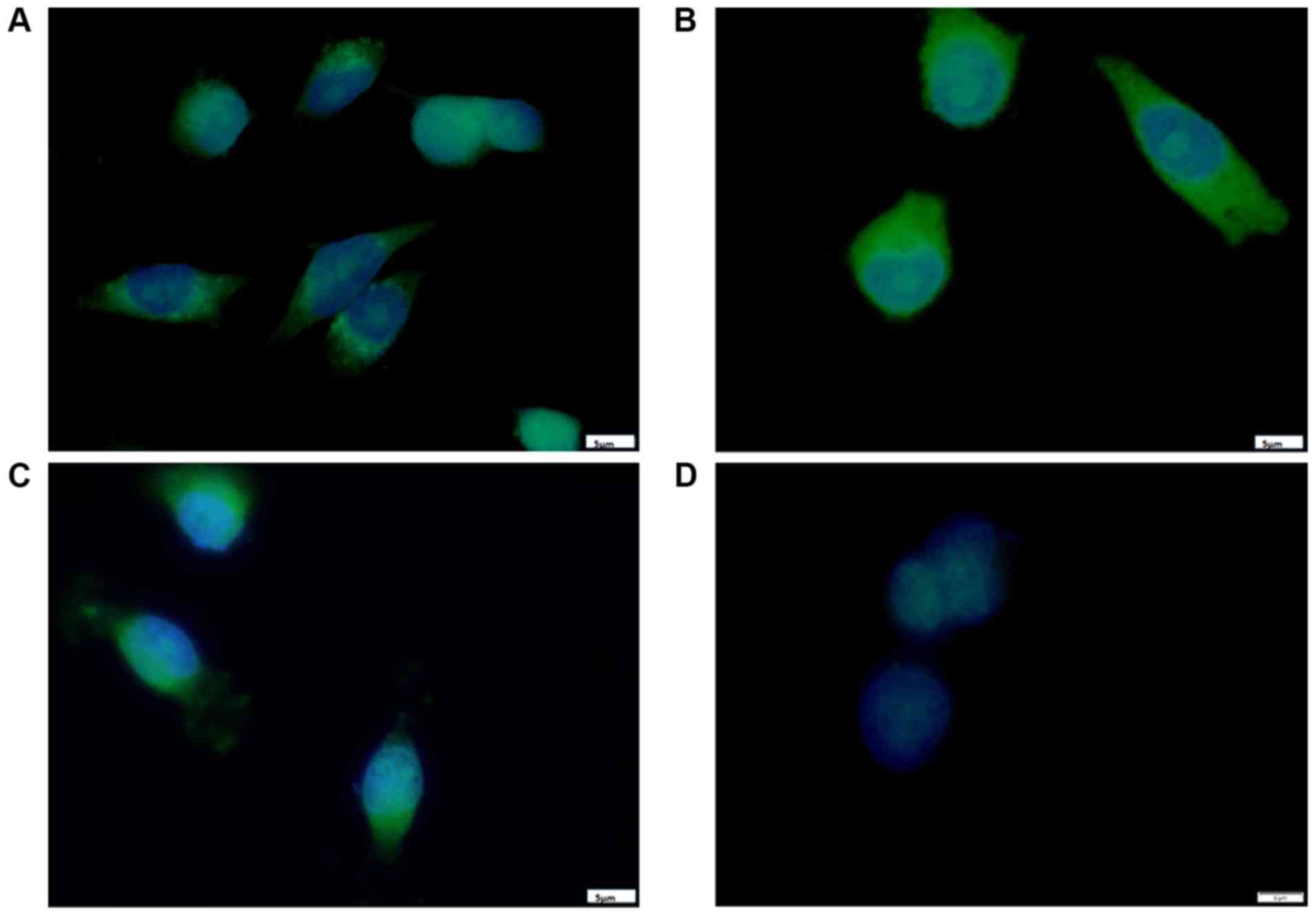
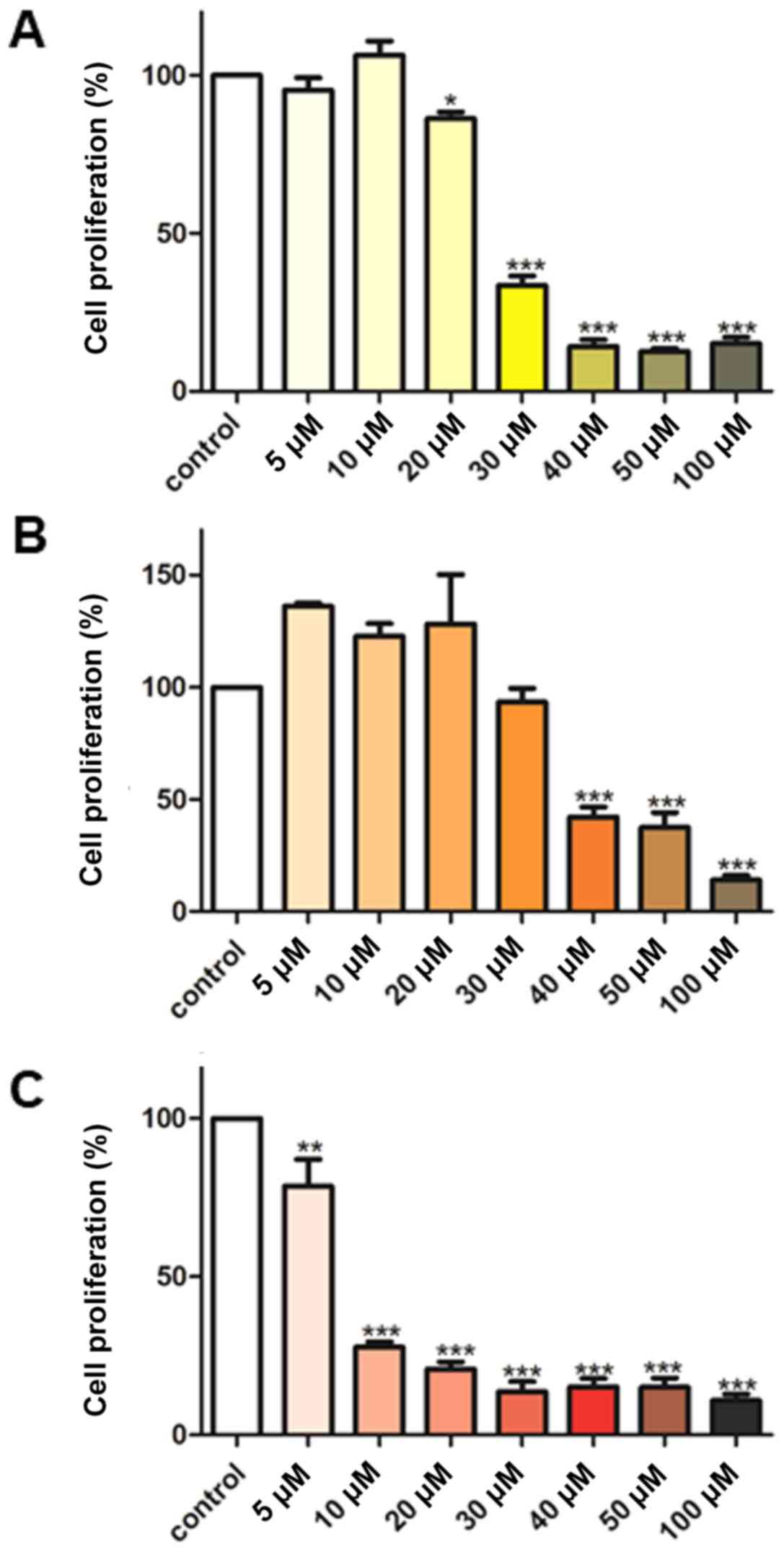
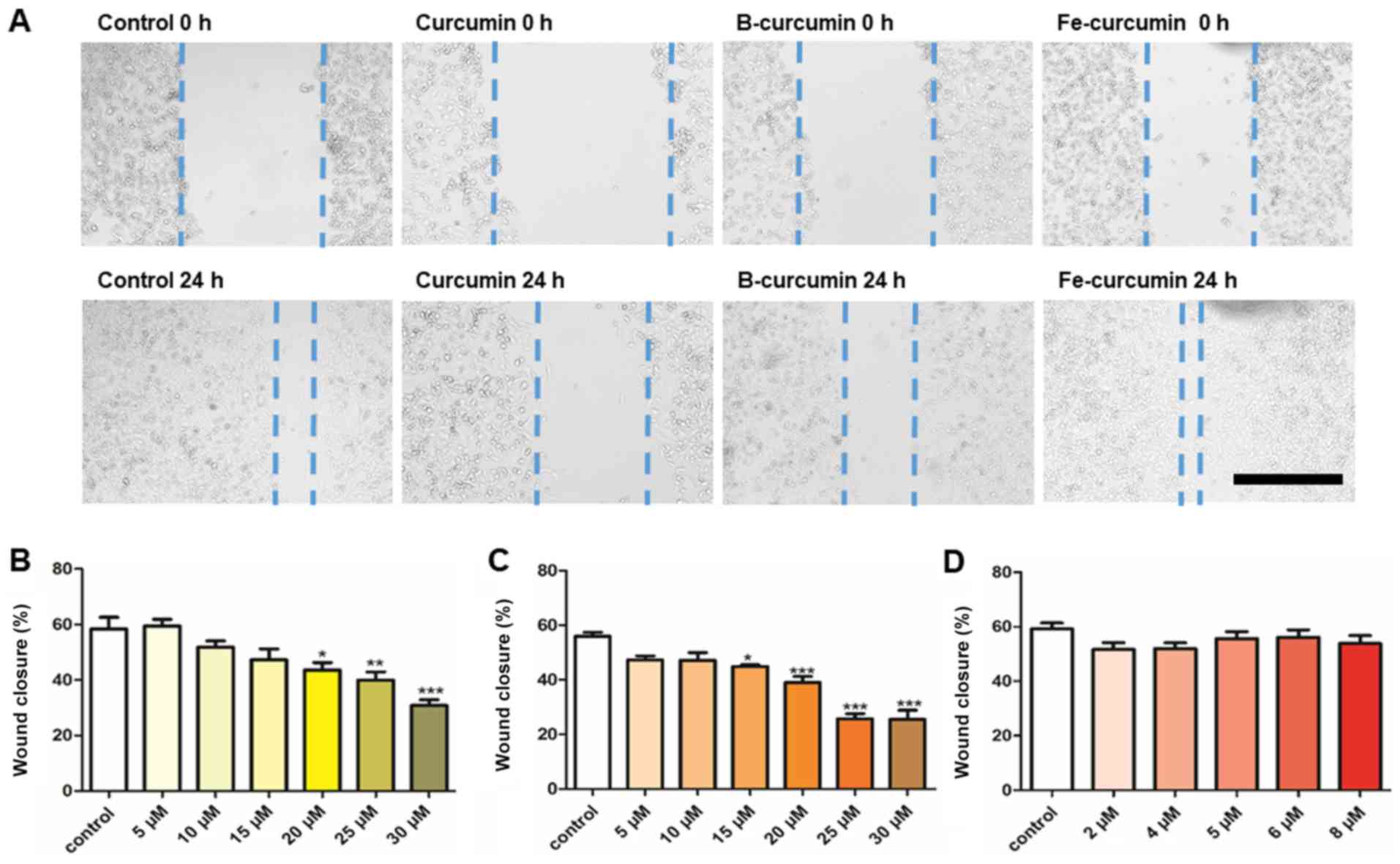
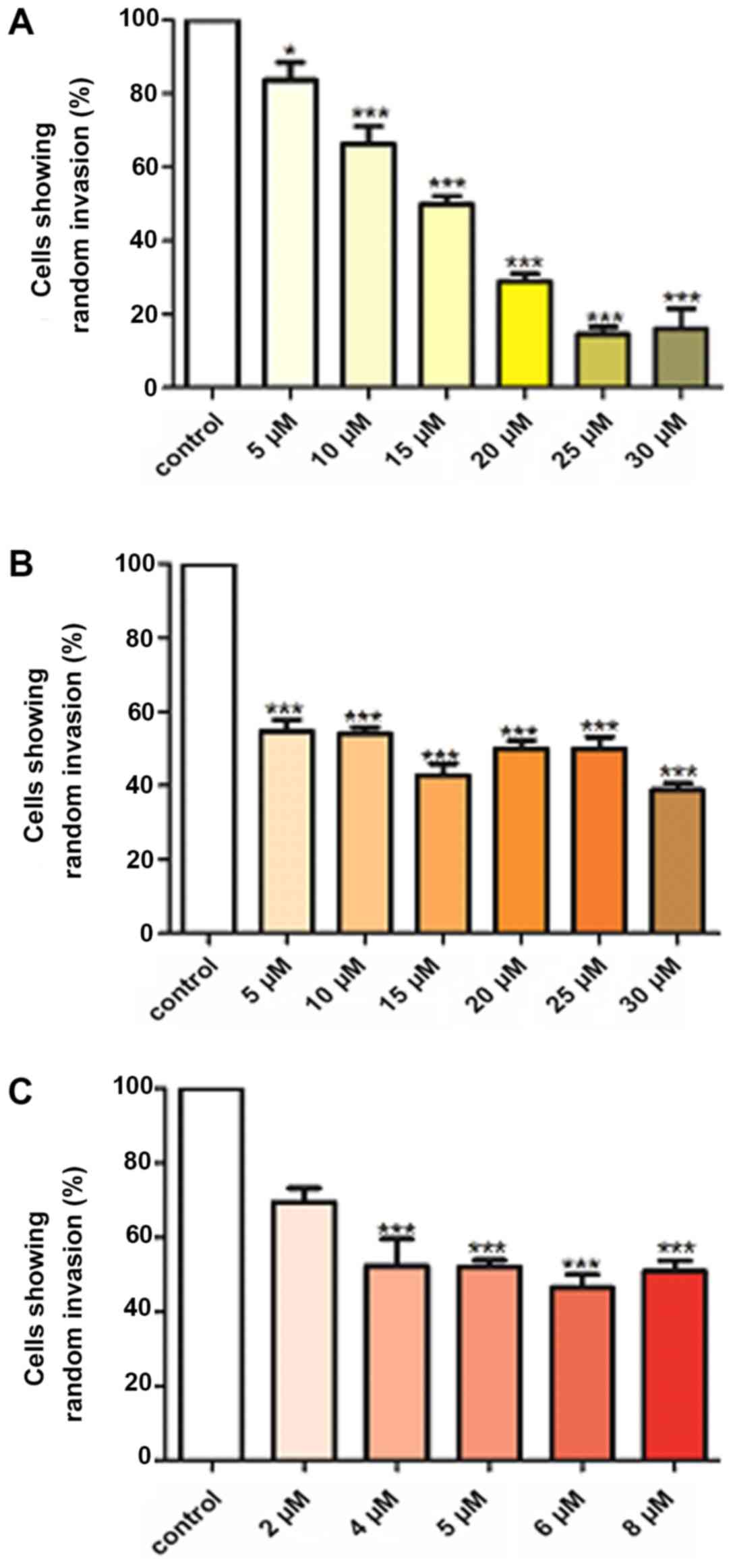
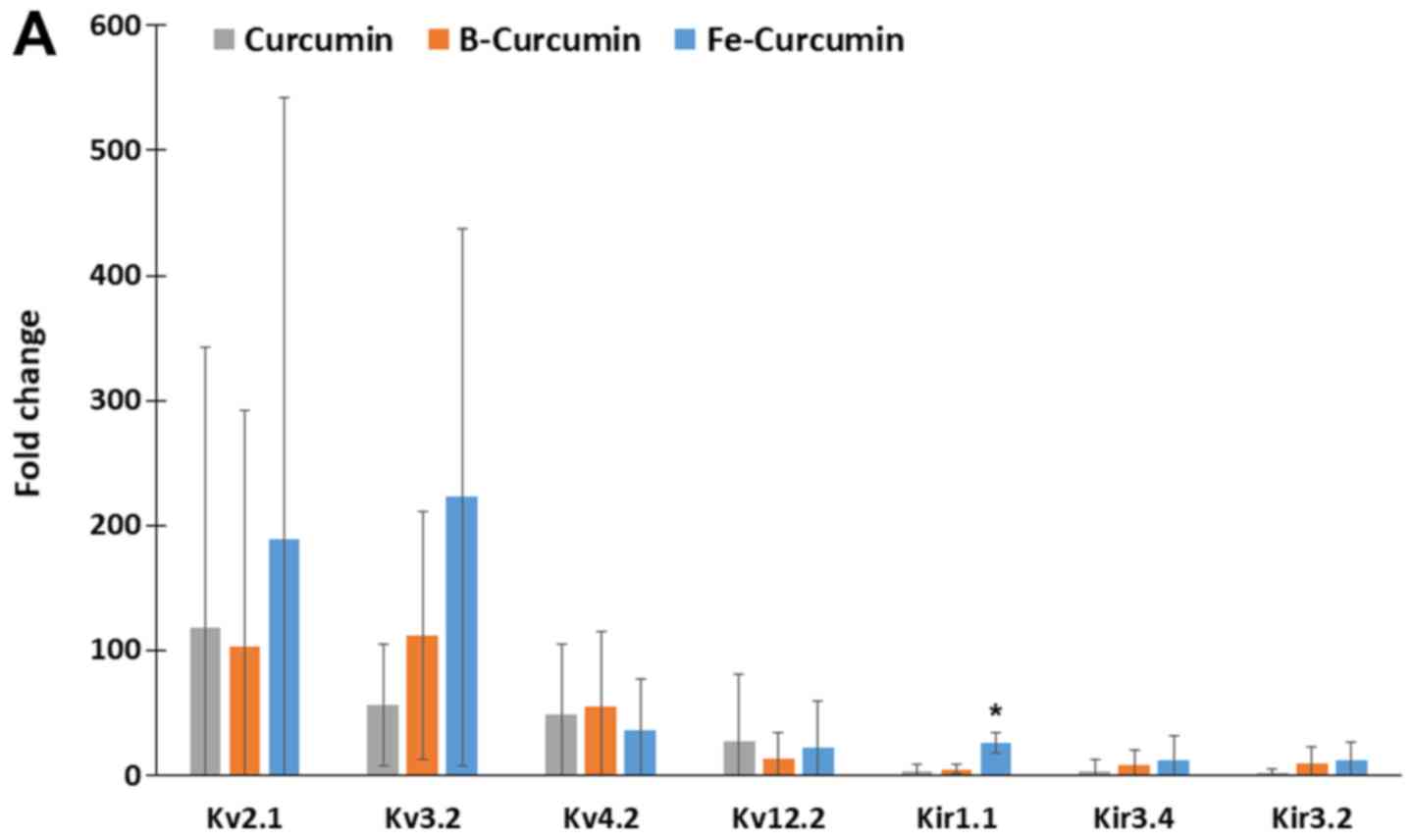
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blows FM, Driver KE, Schmidt MK, Broeks A,
van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO,
Blomqvist C, et al: Subtyping of breast cancer by
immunohistochemistry to investigate a relationship between subtype
and short and long term survival: A collaborative analysis of data
for 10,159 cases from 12 studies. PLoS Med. 7:pp. e10002792010,
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hugh J, Hanson J, Cheang MC, Nielsen TO,
Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, et
al: Breast cancer subtypes and response to docetaxel in
node-positive breast cancer: Use of an immunohistochemical
definition in the BCIRG 001 trial. J Clin Oncol. 27:1168–1176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nielsen TO, Hsu FD, Jensen K, Cheang M,
Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler
L, et al: Immunohistochemical and clinical characterization of the
basal-like subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Yang J, Peng L, Sahin AA, Huo L,
Ward KC, O'Regan R, Torres MA and Meisel JL: Triple-negative breast
cancer has worse overall survival and cause-specific survival than
non-triple-negative breast cancer. Breast Cancer Res Treat.
161:279–287. 2017. View Article : Google Scholar
|
|
8
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Massat NJ, Dibden A, Parmar D, Cuzick J,
Sasieni PD and Duffy SW: Impact of screening on breast cancer
mortality: The UK program 20 years on. Cancer Epidemiol Biomarkers
Prev. 25:455–462. 2016. View Article : Google Scholar
|
|
10
|
Kalager M, Zelen M, Langmark F and Adami
HO: Effect of screening mammography on breast-cancer mortality in
Norway. N Engl J Med. 363:1203–1210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choudhuri T, Pal S, Das T and Sa G:
Curcumin selectively induces apoptosis in deregulated cyclin
D1-expressed cells at G2 phase of cell cycle in a p53-dependent
manner. J Biol Chem. 280:20059–20068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pal S, Bhattacharyya S, Choudhuri T, Datta
GK, Das T and Sa G: Amelioration of immune cell number depletion
and potentiation of depressed detoxification system of
tumor-bearing mice by curcumin. Cancer Detect Prev. 29:470–478.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shim JS, Kim JH, Cho HY, Yum YN, Kim SH,
Park HJ, Shim BS, Choi SH and Kwon HJ: Irreversible inhibition of
CD13/aminopeptidase N by the antiangiogenic agent curcumin. Chem
Biol. 10:695–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, Thomas DP, Zhang X, Culver BW,
Alexander BM, Murdoch WJ, Rao MN, Tulis DA, Ren J and Sreejayan N:
Curcumin inhibits platelet-derived growth factor-stimulated
vascular smooth muscle cell function and injury-induced neointima
formation. Arterioscler Thromb Vasc Biol. 26:85–90. 2006.
View Article : Google Scholar
|
|
16
|
Zeitlin P: Can curcumin cure cystic
fibrosis? N Engl J Med. 351:606–608. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carroll RE, Benya RV, Turgeon DK, Vareed
S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C,
Meyskens FL Jr and Brenner DE: Phase IIa clinical trial of curcumin
for the prevention of colorectal neoplasia. Cancer Prev Res
(Phila). 4:354–364. 2011. View Article : Google Scholar
|
|
18
|
He ZY, Shi CB, Wen H, Li FL, Wang BL and
Wang J: Upregulation of p53 expression in patients with colorectal
cancer by administration of curcumin. Cancer Invest. 29:208–213.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hejazi J, Rastmanesh R, Taleban FA, Molana
SH, Hejazi E, Ehtejab G and Hara N: Effect of curcumin
supplementation during radiotherapy on oxidative status of patients
with prostate cancer: A double blinded, randomized,
placebo-controlled study. Nutr Cancer. 68:77–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Epstein J, Sanderson IR and Macdonald TT:
Curcumin as a therapeutic agent: The evidence from in vitro, animal
and human studies. Br J Nutr. 103:1545–1557. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Durgaprasad S, Pai CG, Vasanthkumar,
Alvres JF and Namitha S: A pilot study of the antioxidant effect of
curcumin in tropical pancreatitis. Indian J Med Res. 122:315–318.
2005.
|
|
22
|
Sahin Kavaklı H, Koca C and Alıcı O:
Antioxidant effects of curcumin in spinal cord injury in rats. Ulus
Travma Acil Cerrahi Derg. 17:14–18. 2011. View Article : Google Scholar
|
|
23
|
Meng B, Li J and Cao H: Antioxidant and
antiinflammatory activities of curcumin on diabetes mellitus and
its complications. Curr Pharm Des. 19:2101–2113. 2013.
|
|
24
|
Lüer S, Troller R and Aebi C:
Antibacterial and antiinflammatory kinetics of curcumin as a
potential antimucositis agent in cancer patients. Nutr Cancer.
64:975–981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beevers CS and Huang S: Pharmacological
and clinical properties of curcumin. Botanics Targets Ther. 1:5–18.
2011.
|
|
26
|
Shishodia S, Sethi G and Aggarwal BB:
Curcumin: Getting back to the roots. Ann N Y Acad Sci.
1056:206–217. 2005. View Article : Google Scholar
|
|
27
|
Aggarwal BB, Kumar A and Bharti AC:
Anticancer potential of curcumin: Preclinical and clinical studies.
Anticancer Res. 23:363–398. 2003.PubMed/NCBI
|
|
28
|
Tønnesen HH, Karlsen J and van Henegouwen
GB: Studies on curcumin and curcuminoids. VIII. Photochemical
stability of curcumin. Z Lebensm Unters Forsch. 183:116–122. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS,
Hsieh CY and Lin JK: Stability of curcumin in buffer solutions and
characterization of its degradation products. J Pharm Biomed Anal.
15:1867–1876. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cartiera MS, Ferreira EC, Caputo C, Egan
ME, Caplan MJ and Saltzman WM: Partial correction of cystic
fibrosis defects with PLGA nanoparticles encapsulating curcumin.
Mol Pharm. 7:86–93. 2010. View Article : Google Scholar :
|
|
31
|
Liang G, Shao L, Wang Y, Zhao C, Chu Y,
Xiao J, Zhao Y, Li X and Yang S: Exploration and synthesis of
curcumin analogues with improved structural stability both in vitro
and in vivo as cytotoxic agents. Bioorg Med Chem. 17:2623–2631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wanninger S, Lorenz V, Subhan A and
Edelmann FT: Metal complexes of curcumin-synthetic strategies,
structures and medicinal applications. Chem Soc Rev. 44:4986–5002.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Greish K, Pittalà V, Taurin S, Taha S,
Bahman F, Mathur A, Jasim A, Mohammed F, El-Deeb IM, Fredericks S
and Rashid-Doubell F: Curcumin-copper complex nanoparticles for the
management of triple-negative breast cancer. Nanomaterials (Basel).
8. pp. E8842018, View Article : Google Scholar
|
|
34
|
Mohammed F, Rashid-Doubell F, Cassidy S
and Henari F: A comparative study of the spectral, fluorometric
properties and photostability of natural curcumin, iron- and
boron-complexed curcumin. Spectrochim Acta A Mol Biomol Spectrosc.
183:439–450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pröhl M, Schubert US, Weigand W and
Gottschaldt M: Metal complexes of curcumin and curcumin derivatives
for molecular imaging and anticancer therapy. Coord Chem Rev.
307:32–41. 2016. View Article : Google Scholar
|
|
36
|
Cailleau R, Olivé M and Cruciger QV:
Long-term human breast carcinoma cell lines of metastatic origin:
Preliminary characterization. In Vitro. 14:911–915. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chavez KJ, Garimella SV and Lipkowitz S:
Triple negative breast cancer cell lines: One tool in the search
for better treatment of triple negative breast cancer. Breast Dis.
32:35–48. 2010. View Article : Google Scholar
|
|
38
|
Osborne CK: Tamoxifen in the treatment of
breast cancer. N Engl J Med. 339:1609–1618. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chiu TL and Su CC: Curcumin inhibits
proliferation and migration by increasing the Bax to Bcl-2 ratio
and decreasing NF-kappaBp65 expression in breast cancer MDA-MB-231
cells. Int J Mol Med. 23:469–475. 2009.PubMed/NCBI
|
|
41
|
Lv ZD, Liu XP, Zhao WJ, Dong Q, Li FN,
Wang HB and Kong B: Curcumin induces apoptosis in breast cancer
cells and inhibits tumor growth in vitro and in vivo. Int J Clin
Exp Pathol. 7:2818–2824. 2014.PubMed/NCBI
|
|
42
|
Kim HI, Huang H, Cheepala S, Huang S and
Chung J: Curcumin inhibition of integrin (alpha6beta4)-dependent
breast cancer cell motility and invasion. Cancer Prev Res (Phila).
1:pp. 385–391. 2008, View Article : Google Scholar
|
|
43
|
Yee NS: Roles of TRPM8 Ion channels in
cancer: Proliferation, survival, and invasion. Cancers (Basel).
7:2134–2146. 2015. View Article : Google Scholar
|
|
44
|
Pardo LA and Stühmer W: The roles of K(+)
channels in cancer. Nat Rev Cancer. 14:39–48. 2014. View Article : Google Scholar
|
|
45
|
Urrego D, Tomczak AP, Zahed F, Stühmer W
and Pardo LA: Potassium channels in cell cycle and cell
proliferation. Philos Trans R Soc Lond B Biol Sci.
369:201300942014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Litan A and Langhans SA: Cancer as a
channelopathy: Ion channels and pumps in tumor development and
progression. Front Cell Neurosci. 9:862015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Djamgoz MB and Onkal R: Persistent current
blockers of voltage-gated sodium channels: A clinical opportunity
for controlling metastatic disease. Recent Pat Anticancer Drug
Discov. 8:66–84. 2013. View Article : Google Scholar
|
|
48
|
Wang Y, Yang Z, Meng Z, Cao H, Zhu G, Liu
T and Wang X: Knockdown of TRPM8 suppresses cancer malignancy and
enhances epirubicin-induced apoptosis in human osteosarcoma cells.
Int J Biol Sci. 10:90–102. 2013. View Article : Google Scholar
|
|
49
|
Hoffmann EK and Lambert IH: Ion channels
and transporters in the development of drug resistance in cancer
cells. Philos Trans R Soc Lond B Biol Sci. 369:201301092014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang M and Brackenbury WJ: Membrane
potential and cancer progression. Front Physiol. 4:1852013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bortner CD and Cidlowski JA: Ion channels
and apoptosis in cancer. Philos Trans R Soc Lond B Biol Sci.
369:201301042014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Felipe A, Bielanska J, Comes N, Vallejo A
and Roig S: Targeting the voltage-dependent K(+) channels Kv1.3 and
Kv1.5 as tumor biomarkers for cancer detection and prevention. Curr
Med Chem. 19:661–674. 2012. View Article : Google Scholar
|
|
53
|
Szabò I, Zoratti M and Gulbins E:
Contribution of voltage-gated potassium channels to the regulation
of apoptosis. FEBS Lett. 584:2049–2056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Szabó I, Bock J, Grassmé H, Soddemann M,
Wilker B, Lang F, Zoratti M and Gulbins E: Mitochondrial potassium
channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc
Natl Acad Sci USA. 105:14861–14866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Banderali U, Belke D, Singh A, Jayanthan
A, Giles WR and Narendran A: Curcumin blocks Kv11.1 (erg) potassium
current and slows proliferation in the infant acute monocytic
leukemia cell line THP-1. Cell Physiol Biochem. 28:1169–1180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lian YT, Yang XF, Wang ZH, Yang Y, Yang Y,
Shu YW, Cheng LX and Liu K: Curcumin serves as a human kv1.3
blocker to inhibit effector memory T lymphocyte activities.
Phytother Res. 27:1321–1327. 2013. View Article : Google Scholar
|
|
57
|
Aréchiga-Figueroa IA, Delgado-Ramirez M,
Morán-Zendejas R and Rodriguez-Menchaca AA: Modulation of Kv2.1
channels inactivation by curcumin. Pharmacol Rep. 67:1273–1279.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Khalil MI, AL-Zahem AM and Qunaibit MM:
Synthesis, characterization, and antitumor activity of binuclear
curcumin-metal(II) hydroxo complexes. Med Chem Res. 23:1683–1689.
2014. View Article : Google Scholar
|
|
59
|
Skehan P, Storeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Heit B and Kubes P: Measuring chemotaxis
and chemokinesis: The under-agarose cell migration assay. Sci STKE.
2003:PL52003.PubMed/NCBI
|
|
61
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
62
|
Hope-Roberts M and Horobin RW: A review of
curcumin as a biological stain and as a self-visualizing
pharmaceutical agent. Biotech Histochem. 92:315–323. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kunwar A, Barik A, Mishra B, Rathinasamy
K, Pandey R and Priyadarsini KI: Quantitative cellular uptake,
localization and cytotoxicity of curcumin in normal and tumor
cells. Biochim Biophys Acta. 1780:673–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sarkar T, Butcher RJ, Banerjee S,
Mukherjee S and Hussain A: Visible light-induced cytotoxicity of a
dinuclear iron(III) complex of curcumin with low-micromolar IC50
value in cancer cells. Inorganica Chimica Acta. 439:8–17. 2016.
View Article : Google Scholar
|
|
65
|
Ganguly KK, Sen T, Pal S, Biswas J and
Chatterjee A: Studies on focal adhesion kinase in human breast
cancer cell MDA-MB-231. Adv Biol Chem. 2:29–42. 2012. View Article : Google Scholar
|
|
66
|
Garrido C, Galluzzi L, Brunet M, Puig PE,
Didelot C and Kroemer G: Mechanisms of cytochrome c release from
mitochondria. Cell Death Differ. 13:1423–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xiao Y, Xia J, Wu S, Lv Z, Huang S, Huang
H, Su X, Cheng J and Ke Y: Curcumin inhibits acute vascular
inflammation through the activation of heme oxygenase-1. Oxid Med
Cell Longev. 2018:32958072018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu SY, Lee YR, Huang CC, Li YZ, Chang YS,
Yang CY, Wu JD and Liu YW: Curcumin-induced heme oxygenase-1
expression plays a negative role for its anti-cancer effect in
bladder cancers. Food Chem Toxicol. 50:3530–3536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee WY, Chen YC, Shih CM, Lin CM, Cheng
CH, Chen KC and Lin CW: The induction of heme oxygenase-1
suppresses heat shock protein 90 and the proliferation of human
breast cancer cells through its byproduct carbon monoxide. Toxicol
Appl Pharmacol. 274:55–62. 2014. View Article : Google Scholar
|
|
70
|
Park J and Conteas CN: Anti-carcinogenic
properties of curcumin on colorectal cancer. World J Gastrointest
Oncol. 2:169–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Balogun E, Hoque M, Gong P, Killeen E,
Green CJ, Foresti R, Alam J and Motterlini R: Curcumin activates
the haem oxygenase-1 gene via regulation of Nrf2 and the
antioxidant-responsive element. Biochem J. 371:887–895. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
McNally SJ, Harrison EM, Ross JA, Garden
OJ and Wigmore SJ: Curcumin induces heme oxygenase 1 through
generation of reactive oxygen species, p38 activation and
phosphatase inhibition. Int J Mol Med. 19:165–172. 2007.
|
|
73
|
Mimche PN, Taramelli D and Vivas L: The
plant-based immunomodulator curcumin as a potential candidate for
the development of an adjunctive therapy for cerebral malaria.
Malar J. 10(Suppl 1): pp. S102011, View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nitti M, Piras S, Marinari UM, Moretta L,
Pronzato MA and Furfaro AL: HO-1 induction in cancer progression: A
matter of cell adaptation. Antioxidants(Basel). 6. pp. E292017,
View Article : Google Scholar
|
|
75
|
Jeon WK, Hong HY, Seo WC, Lim KH, Lee HY,
Kim WJ, Song SY and Kim BC: Smad7 sensitizes A549 lung cancer cells
to cisplatin-induced apoptosis through heme oxygenase-1 inhibition.
Biochem Biophys Res Commun. 420:288–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kongpetch S, Kukongviriyapan V, Prawan A,
Senggunprai L, Kukongviriyapan U and Buranrat B: Crucial role of
heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to
chemotherapeutic agents. PLoS One. 7:pp. e349942012, View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lv X, Song DM, Niu YH and Wang BS:
Inhibition of heme oxygenase-1 enhances the chemosensitivity of
laryngeal squamous cell cancer Hep-2 cells to cisplatin. Apoptosis.
21:489–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tracey N, Creedon H, Kemp AJ, Culley J,
Muir M, Klinowska T and Brunton VG: HO-1 drives autophagy as a
mechanism of resistance against HER2-targeted therapies. Breast
Cancer Res Treat. 179:543–555. 2020. View Article : Google Scholar :
|
|
79
|
Zhao Z, Xu Y, Lu J, Xue J and Liu P: High
expression of HO-1 predicts poor prognosis of ovarian cancer
patients and promotes proliferation and aggressiveness of ovarian
cancer cells. Clin Transl Oncol. 20:491–499. 2018. View Article : Google Scholar
|
|
80
|
Becker JC, Fukui H, Imai Y, Sekikawa A,
Kimura T, Yamagishi H, Yoshitake N, Pohle T, Domschke W and
Fujimori T: Colonic expression of heme oxygenase-1 is associated
with a better long-term survival in patients with colorectal
cancer. Scand J Gastroenterol. 42:852–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Loboda A, Jozkowicz A and Dulak J: HO-1/CO
system in tumor growth, angiogenesis and metabolism-targeting HO-1
as an anti-tumor therapy. Vascul Pharmacol. 74:11–22. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Maines MD and Abrahamsson PA: Expression
of heme oxygenase-1 (HSP32) in human prostate: Normal,
hyperplastic, and tumor tissue distribution. Urology. 47:727–733.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Busserolles J, Megías J, Terencio MC and
Alcaraz MJ: Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via
activation of Akt pathway. Int J Biochem Cell Biol. 38:1510–1517.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tanaka S, Akaike T, Fang J, Beppu T, Ogawa
M, Tamura F, Miyamoto Y and Maeda H: Antiapoptotic effect of haem
oxygenase-1 induced by nitric oxide in experimental solid tumour.
Br J Cancer. 88:902–909. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cherrington JM, Strawn LM and Shawver LK:
New paradigms for the treatment of cancer: The role of
anti-angiogenesis agents. Adv Cancer Res. 79:1–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Price JT and Thompson EW: Mechanisms of
tumour invasion and metastasis: Emerging targets for therapy.
Expert Opin Ther Targets. 6:217–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lin Q, Weis S, Yang G, Weng YH, Helston R,
Rish K, Smith A, Bordner J, Polte T, Gaunitz F and Dennery PA: Heme
oxygenase-1 protein localizes to the nucleus and activates
transcription factors important in oxidative stress. J Biol Chem.
282:20621–20633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Biswas C, Shah N, Muthu M, La P, Fernando
AP, Sengupta S, Yang G and Dennery PA: Nuclear heme oxygenase-1
(HO-1) modulates subcellular distribution and activation of Nrf2,
impacting metabolic and anti-oxidant defenses. J Biol Chem.
289:26882–26894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Was H, Dulak J and Jozkowicz A: Heme
oxygenase-1 in tumor biology and therapy. Curr Drug Targets.
11:1551–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Duckers HJ, Boehm M, True AL, Yet SF, San
H, Park JL, Clinton Webb R, Lee ME, Nabel GJ and Nabel EG: Heme
oxygenase-1 protects against vascular constriction and
proliferation. Nat Med. 7:693–698. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Taillé C, Almolki A, Benhamed M, Zedda C,
Mégret J, Berger P, Lesèche G, Fadel E, Yamaguchi T, Marthan R, et
al: Heme oxygenase inhibits human airway smooth muscle
proliferation via a bilirubin-dependent modulation of ERK1/2
phosphorylation. J Biol Chem. 278:27160–27168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ozawa N, Goda N, Makino N, Yamaguchi T,
Yoshimura Y and Suematsu M: Leydig cell-derived heme oxygenase-1
regulates apoptosis of premeiotic germ cells in response to stress.
J Clin Invest. 109:457–467. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu XM, Chapman GB, Wang H and Durante W:
Adenovirus-mediated heme oxygenase-1 gene expression stimulates
apoptosis in vascular smooth muscle cells. Circulation. 105:79–84.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hill M, Pereira V, Chauveau C, Zagani R,
Remy S, Tesson L, Mazal D, Ubillos L, Brion R, Asghar K, et al:
Heme oxygenase-1 inhibits rat and human breast cancer cell
proliferation: Mutual cross inhibition with indoleamine
2,3-dioxygenase. FASEB J. 19:1957–1968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lin CW, Shen SC, Hou WC, Yang LY and Chen
YC: Heme oxygenase-1 inhibits breast cancer invasion via
suppressing the expression of matrix metalloproteinase-9. Mol
Cancer Ther. 7:1195–1206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Andreadi CK, Howells LM, Atherfold PA and
Manson MM: Involvement of Nrf2, p38, B-Raf, and nuclear
factor-kappaB, but not phosphatidylinositol 3-kinase, in induction
of hemeoxy-genase-1 by dietary polyphenols. Mol Pharmacol.
69:1033–1040. 2006. View Article : Google Scholar
|
|
97
|
Pal SK, Takimoto K, Aizenman E and Levitan
ES: Apoptotic surface delivery of K+ channels. Cell Death Differ.
13:661–667. 2006. View Article : Google Scholar :
|
|
98
|
Comes N, Bielanska J, Vallejo-Gracia A,
Serrano-Albarrás A, Marruecos L, Gómez D, Soler C, Condom E, Ramón
Y, Cajal S, et al: The voltage-dependent K(+) channels Kv1.3 and
Kv1.5 in human cancer. Front Physiol. 4:2832013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lowinus T, Heidel FH, Bose T, Nimmagadda
SC, Schnöder T, Cammann C, Schmitz I, Seifert U, Fischer T,
Schraven B and Bommhardt U: Memantine potentiates
cytarabine-induced cell death of acute leukemia correlating with
inhibition of Kv1.3 potassium channels, AKT and ERK1/2 signalin.
Cell Commun Signal. 17:52019. View Article : Google Scholar
|
|
100
|
Teisseyre A, Palko-Labuz A, Sroda-Pomianek
K and Michalak K: Voltage-gated potassium channel Kv1.3 as a target
in therapy of cancer. Front Oncol. 9:9332019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wu J, Chen Z, Liu Q, Zeng W, Wu X and Lin
B: Silencing of Kv1.5 gene inhibits proliferation and induces
apoptosis of osteo-sarcoma cells. Int J Mol Sci. 16:26914–26926.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pal S, Hartnett KA, Nerbonne JM, Levitan
ES and Aizenman E: Mediation of neuronal apoptosis by Kv2.1-encoded
potassium channels. J Neurosci. 23:4798–4802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Al-Owais MM, Dallas ML, Boyle JP, Scragg
JL and Peers C: Heme oxygenase-1 influences apoptosis via
CO-mediated inhibition of K+ channels. Adv Exp Med Biol.
860:343–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Al-Owais MM, Scragg JL, Dallas ML, Boycott
HE, Warburton P, Chakrabarty A, Boyle JP and Peers C: Carbon
monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in
medulloblastoma DAOY cells via K+ channel inhibition. J Biol Chem.
287:24754–24764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lan M, Shi Y, Han Z, Hao Z, Pan Y, Liu N,
Guo C, Hong L, Wang J, Qiao T and Fan D: Expression of delayed
rectifier potassium channels and their possible roles in
proliferation of human gastric cancer cells. Cancer Biol Ther.
4:1342–1347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Song MS, Park SM, Park JS, Byun JH, Jin
HJ, Seo SH, Ryu PD and Lee SY: Kv3.1 and Kv3.4, are involved in
cancer cell migration and invasion. Int J Mol Sci. 19:pp.
E10612018, View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Schwab A, Fabian A, Hanley PJ and Stock C:
Role of ion channels and transporters in cell migration. Physiol
Rev. 92:1865–1913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Duvvuri U, Shiwarski DJ, Xiao D, Bertrand
C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K,
et al: TMEM16A induces MAPK and contributes directly to
tumorigenesis and cancer progression. Cancer Res. 72:3270–3281.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Spitzner M, Martins JR, Soria RB,
Ousingsawat J, Scheidt K, Schreiber R and Kunzelmann K: Eag1 and
Bestrophin 1 are up-regulated in fast-growing colonic cancer cells.
J Biol Chem. 283:7421–7428. 2008. View Article : Google Scholar : PubMed/NCBI
|