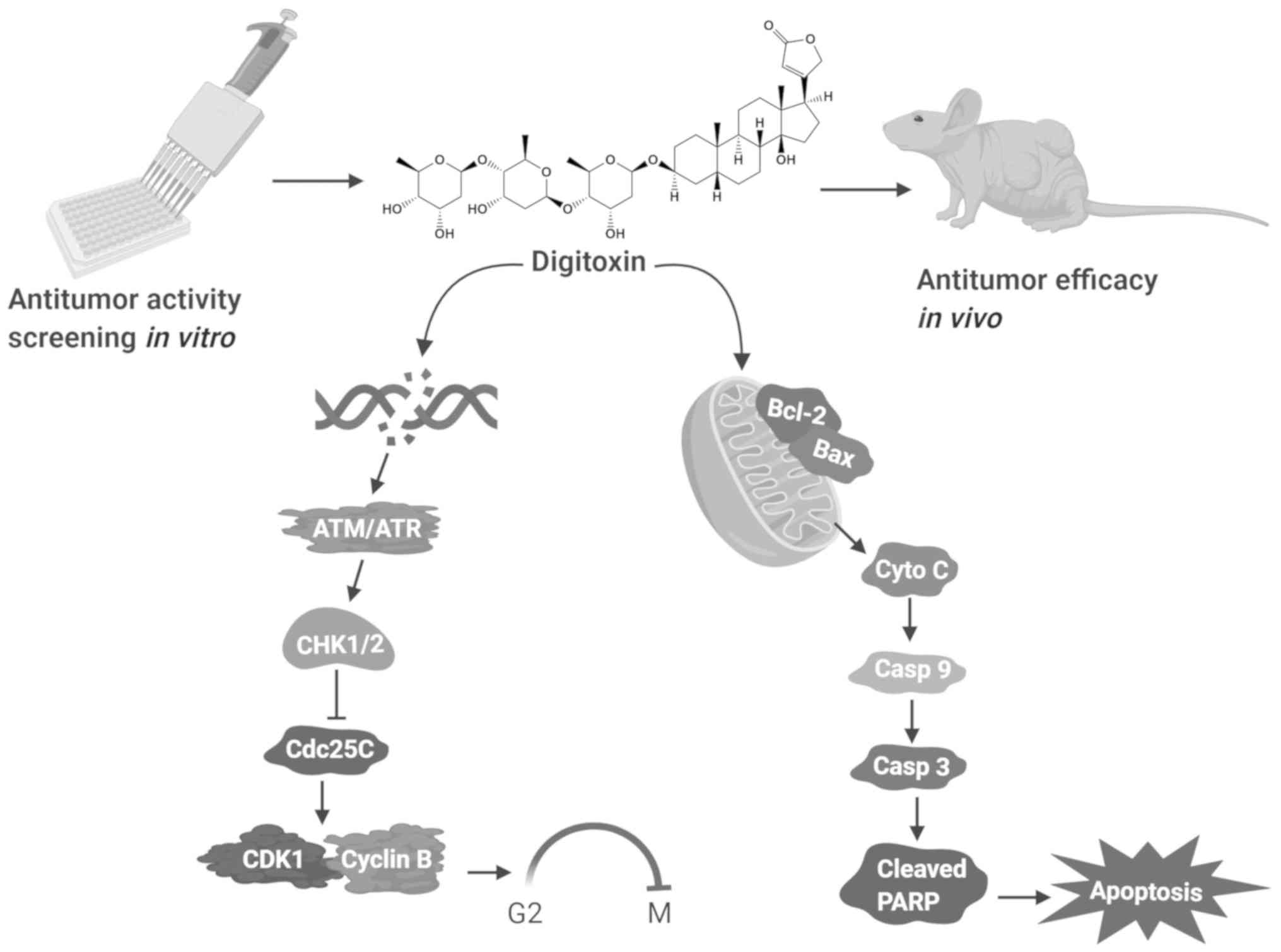
Introduction
Cervical cancer is the fourth most common
gynecological malignancy affecting the health of women worldwide,
with an estimated 570,000 new cases and 311,000 deaths in 2018.
Moreover, the incidence of cervical cancer among young women is
gradually increasing, and >85% of deaths related to cervical
cancer occur in the developing world, rendering this type of cancer
the second most common cause of cancer-related mortality in women
living in developing regions (1,2).
Although much progress has been made in the screening and
prevention of cervical cancer, such as vaccination, some patients
(approximately 6%) will inevitably be diagnosed with advanced,
recurrent or metastatic cervical cancer. For these patients,
chemotherapy, such as the combination of paclitaxel and cisplatin
or paclitaxel, cisplatin and bevacizumab, remains a cornerstone of
treatment (3-6). However, all these clinical
chemotherapies for cervical cancer exhibit only limited
effectiveness as tumor resistance eventually develops. Thus, the
development of effective chemotherapeutic drugs for the treatment
of cervical cancer has become an important issue in the medical
field.
Natural products remain an important and promising
source for the discovery of chemotherapeutic agents, such as the
antimalarial drug, artemisinin. Camptothecin (derived from
Camptotheca acuminata) and its clinical derivatives have
been mainly applied for the clinical treatment of colon, lung,
ovarian, breast, liver, pancreas and stomach cancers (7). Paclitaxel (isolated from Taxus
brevifolia) and its derivatives have been approved for the
management of metastatic breast cancer and metastatic breast cancer
(8). Furthermore, from 1981 to
2014, approximately 49% of FDA-approved anticancer drugs were
derived either directly from natural resources or from their
derivatives, including vinblastine and colchicine (9-14).
Digitoxin, a natural cardiac glycoside from
Digitalis, has been used in the treatment of cardiac
diseases for a number of years (15). Numerous experimental studies have
demonstrated that digitoxin exhibits significant antitumor
activities in vitro and in vivo, such as activities
against renal cancer, breast cancer, melanoma (16), lung cancer (17,18),
ovarian cancer (19,20), pancreatic cancer (21), glioma (22,23),
prostate cancer (24), liver
cancer (25) and colon cancer
(26). It has been reported that
the combination of digitoxin with anticancer agents leads to
synergistic effects (27-29). Mechanistic studies have revealed
that the promotion of apoptosis (21,22)
and autophagy (20,30), the inhibition of angiogenesis
(31), epithelial-mesenchymal
transition (EMT) (24) and
migration (19) and the
suppression of cancer cell stemness (22,23)
are involved in the anticancer effects of digitoxin. However, the
anticancer effects and molecular mechanisms of digitoxin against
HeLa cervical cancer cells have not yet been clearly defined.
In the present study, a library of natural compounds
composed of 78 single compounds was screened to identify potential
lead compounds with activity against cervical cancer. Several
compounds were found to be of interest, and digitoxin was further
evaluated in different malignant cell lines, including the cervical
cancer cell line, HeLa, the lung cancer cell line, A549, the
hepatoma cell line, MHCC97H, and the colon cancer cell line,
HCT116. Mechanistically, it was found that digitoxin inhibited the
proliferation of HeLa cells by blocking the cell cycle at the
G2/M phase via the ataxia telangiectasia mutated
serine/threonine kinase (ATM)/ATM and Rad3-related serine/threonine
kinase (ATR)-CHK1/checkpoint kinase 2 (CHK2)-Cdc25C pathway and
triggering the activation of the mitochondrial apoptotic pathway.
Furthermore, the in vivo anticancer effects of digitoxin
were confirmed in HeLa cell xenotransplantation models. The
findings of the present study provide support for the therapeutic
potential of digitoxin in the treatment of cervical cancer.
Materials and methods
Chemical agents and antibodies
The library of natural compounds in listed in Table
SI was obtained from Target Molecule Corp. The purities of these
compounds were >95%, as determined by HPLC/UV analysis (data not
shown). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was purchased from Guangzhou Xueyou Biotechnology
Co., Ltd. Propidium iodide (PI) and 4',6-dimidyl-2-phenyl-indole
(DAPI) were purchased from Roche Diagnostics. The Annexin V-FITC/PI
staining assay kit was obtained from Beyotime Institute of
Biotechnology. The antibodies listed in Table SII were mainly
purchased from Cell Signaling Technology and ProteinTech Group,
Inc. The BCA protein assay kit and the enhanced chemiluminescent
substrate were purchased from Jiangsu Keegan Biotechnology Co.,
Ltd.
Animals and cell lines
A total of 15 female BALB/c (nu/nu) nude mice
(weighing 13-15 g, aged 4-5 weeks) were purchased from Vital River
Laboratory Animal Technology Co., Ltd. and were used for the tumor
xenograft experiments. Animals (5 animals/cage) were maintained at
22±2°C coupled with 55±10% humidity under a 12 h light/dark cycle
with free access to food and water. All animal experiments were
conducted in compliance with the ARRIVE guidelines and were
approved by the Experimental Animal Ethics Committee of Jinan
University (Guangzhou, China).
The human cervical cancer cell line, HeLa, the liver
cancer cell line, MHCC97H, the lung cancer cell line, A549 and the
colorectal cancer cell line, HCT116, were obtained from the Chinese
Academy of Sciences Cell Bank. All cells were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% (v/v) penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in an incubator with a
humidified atmosphere and 5% CO2. The HeLa cell line was
identified by short tandem repeat (STR) profiling.
Measurement of cytotoxic activity
The cytotoxicity of the 78 natural products in the
library against HeLa cells was screened by MTT assay. Cell
suspensions were seeded in 96-well plates containing DMEM with 10%
FBS and 1% (v/v) penicillin-streptomycin (PS) at a density of 5,000
cells per well. The plates were incubated at 37°C (5%
CO2) for 24 h, and the medium was then replaced with
fresh DMEM containing the test compounds (0.1 μM) for 72 h.
The cells were then incubated with 0.5 mg/ml MTT solution for 3 h.
The purple crystals were dissolved in dimethyl sulfoxide (DMSO),
and the absorbance of each well was measured at 570 nm using a
microplate reader (Epoch2, BioTek Instruments, Inc.).
Cell viability assay
HeLa, MHCC97H, A549 and HCT116 cells (5,000
cells/well) were plated in 96-well plates with various
concentrations of the digitoxin ranging from 4 to 1,000 nM for 24 h
and 48 h and then exposed to 0.5 mg/ml MTT for 3 h at 37°C. The
formazan crystals were dissolved in DMSO, and absorbance was
measured at 570 nm on a microplate reader (Epoch2, BioTek
Instruments, Inc.). The IC50 value was defined as the
concentration of digitoxin with which the percentage inhibition was
equal to 50 and was the mean from at least 3 independent
experiments.
Cell cycle analysis
The cell cycle distribution of the HeLa cells was
analyzed by PI staining assay. In brief, cells (200,000 cells/well)
in 6-well plates were treated overnight with various concentrations
of digitoxin (0, 4, 20, or 100 nM) for 24 h or with 20 nM digitoxin
for 12, 24, or 36 h and then fixed with pre‑cooled 75% ethanol at
4°C for 24 h. Cells were incubated with PI (0.2 mg/ml) for 15 min
at 37°C in the dark. The PI fluorescence of the cells was analyzed
using an EPICS‑X flow cytometry (Beckman Coulter, Inc.), and the
cell cycle distribution was analyzed using WINMDI v2.8 software
(The Scripps Research Institute).
Apoptosis assay
A total of 5×105 cells were seeded in
6-well plates and cultured overnight. The apoptotic rate of the
cells was determined after 48 h of digitoxin treatment using the
Annexin V-FITC/PI staining assay kit according to the
manufacturer's instructions. Briefly, the cells were harvested and
washed in PBS. The cells were then incubated with Annexin V for 15
min followed by PI for 5 min at 37°C in the dark, final analyzed in
an EPICS‑X flow cytometry (Beckman Coulter, Inc.), and cell
apoptosis was analyzed using WINMDI v2.8 software (The Scripps
Research Institute).
γH2AX staining
Immunofluorescence assays were performed as
previously described (32).
Briefly, cells (100,000 cells/dish) were incubated with digitoxin
at 37°C in a special culture dish used for confocal microscopy (20
mm) for 24 h and then fixed with 4% paraformaldehyde, permeabilized
and blocked with QuickBlock™ Blocking Buffer (Beyotime). The cells
were then incubated with γH2AX primary antibody (1:1,000) at 4°C
overnight. Subsequently, the cells were incubated for 2 h at room
temperature and mounted. Images were observed under a microscope
(Axio Vert. A1; Carl Zeiss AG).
Western blot analysis
Various concentrations of digitoxin (0, 4, or 20 nM)
were added to the HeLa cells (2,000,000 cells/dish) in culture
dishes (100 mm) for 24 h. Cellular proteins were then prepared, and
extracts were prepared using lysis buffer (KeyGen) according to the
manufacturer's instructions. The isolated cell lysate (40
μg) was separated by 10% SDS-PAGE and then transferred to
PVDF membranes. The membranes were blocked with QuickBlock™
Blocking Buffer (Beyotime) and further immunoblotted with primary
antibodies directed against ATM, ATR, CHK1, CHK2, Cdc25C, Bcl-2,
cytochrome c, cleaved PARP, CDK1, Cyclin B1, Bax, caspase-3,
caspase-9, cleaved caspase-3, cleaved caspase-9, and β-actin at a
dilution of 1:1,000 overnight at 4°C. A versatile imaging system
for use with enhanced chemiluminescent substrates was used to
visualize the protein bands. The membranes were then stripped with
stripping buffer (Beyotime) and reblotted with phosphorylation site
antibodies, including p-ATM (Ser1981), p-ATR (Ser428), p-Cdc25C
(Thr48), p-CDK1 (Thr14), p-CHK2 (Thr68), p-CHK1 (Ser286) at a
dilution of 1:1,000. The secondary antibody (1:5,000) consisting of
peroxidase-conjugated goat anti-rabbit or anti-mouse IgG for 1 hour
at room temperature. All the bands were visualized using enhanced
chemiluminescence reagents (Millipore). ImageJ and GraphPad Prism
v5.0 software were to measure the gray values and quantify the data
of the bands.
Xenograft assay in nude mice
A sample of 5,000,000 HeLa cells was subcutaneously
injected into the right flank of each mouse. When a tumor size of
approximately 300 mm3 was reached, the animals were
randomly divided into 3 treatment groups (n=5). The vehicle group
was intravenously administered 5% HS15 in saline. The
digitoxin-treated groups were intraperitoneally administered
digitoxin (dissolved in 5% HS15 in saline) at doses of 1 and 2
mg/kg. Tumor sizes were assessed every 2 days using calipers. Tumor
volume was calculated using the following formula: V = 0.5 × a ×
b2, where 'a' refers to the longer diameter and 'b'
refers to the shorter diameter of the tumor. The treatment was
terminated on day 19 as the size of one tumor was almost 2 cm in
diameter. Animals were anesthetized with 1.5% isoflurane and then
sacrificed by CO2 inhalation. The flow rate of
CO2 in the euthanasia system displaced 30% of the cage
volume/min. Tumors and hearts were collected, weighed and fixed in
4% paraformaldehyde. The tumor and heart tissues were further
examined by hematoxylin and eosin (H&E) staining and
immunohistochemistry (IHC).
Terminal deoxynucleotidyl
transferase‑mediated dUTP nick‑end labeling (TUNEL) assay
Sections were permeabilized with proteinase K
(Servicebio) working solution (20 μg/mL) for 25 min at 37°C.
After washing 3 times with PBS (pH 7.4) in a Rocker device
(Servicebio), terminal deoxynucleotidyl transferase (TdT) and dUTP
(Roche Diagnostics) were added to the sections followed by
incubation at 37°C for 2 h. The reaction was then stopped and
followed by colorization with DAPI (Servicebio) for 10 min, kept in
dark. Coverslips were subsequently mounted on glass slides with
anti-fade mounting medium (Servicebio). Finally, the sections were
analyzed under a light microscope (ECLIPSE C1, Nikon) and
photographs of the sections were obtained. The number of
TUNEL-positive cells were counted by an investigator who was
blinded to the experimental design using Image-Pro Plus v6.0
software.
H&E staining and IHC
Tumor xenografts were fixed and then embedded in
paraffin. Sections were cut at a thickness of 5 μm and
mounted on glass slides. For histological examination, the sections
were stained with H&E (Servicebio) 3 min at room temperature.
To analyze apoptotic cells, the sections were examined using an
in situ cell death detection kit (Roche Diagnostics) and
antibodies against cleaved caspase-3 (1:1,000) over 24 h at 4°C. To
determine the proliferative index, the sections were incubated with
a Ki-67 antibody (Cell Signaling Technology) (1:1,000) over 24 h at
4°C. The stained sections in the present study were examined by a
pathologist who was blinded to the treatment conditions. Images
were acquired using an Olympus DP2-SAL microscope. Apoptosis (%)
and the proliferative index (%) were analyzed using Image-Pro Plus
v6.0 software.
Statistical analysis
Each experiment was performed at least 3 times, and
the in vitro data are presented as the means ± SD and the
in vivo data are presented as the means ± SEM. Statistical
comparisons between groups were determined by one-way analysis of
variance followed by a Tukey's post hoc test to determine the
significant differences of means in multiple groups (n>2)
comparisons using GraphPad Prism v5.0. P<0.05 was considered to
indicate a statistically significant difference.
Results
Digitoxin is identified from the 78
natural products in the library screened against human cancer cells
in vitro
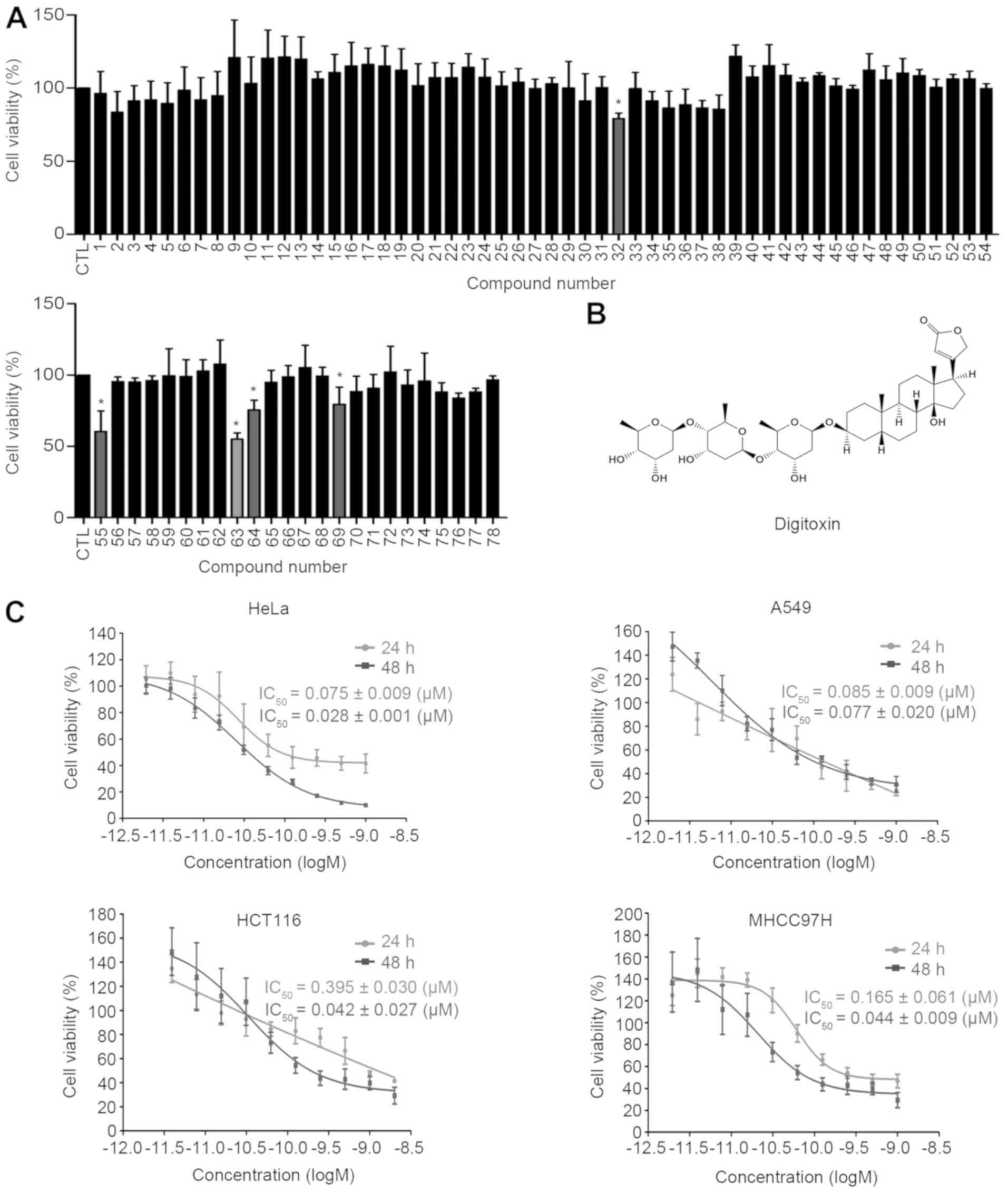
The inhibitory effects of 78 natural compounds were
tested in human cervical cancer HeLa cells by MTT assay. Based on
the extensive literature published by researchers in combination
with our own research experience, the concentration of 0.1
μM of the test compounds was selected (33-35).
The results are expressed as cell growth inhibition and, as shown
in Fig. 1A, the majority of the
compounds exhibited no cytotoxicity towards HeLa cells; however,
docetaxel trihydrate (no. 55), colchicine (no. 64), berberine
hydrochloride (no. 32) and doxorubicin hydrochloride (no. 69)
significantly inhibited the proliferation of HeLa cells, and the
cell growth inhibition rate was 60-80% at a concentration of 0.1
μM. Importantly, digitoxin (no. 63; chemical structure shown
in Fig. 1B) was identified as the
most cytotoxic compound in HeLa cells.
Subsequently, the growth inhibition curves of
digitoxin were further examined in several types of malignant
cells, including MHCC97H, A549, HCT116 and HeLa cells. As shown in
Fig. 1C, digitoxin potently
decreased the viability of these cancer cells in a dose- and
time-dependent manner, with the IC50 values ranging from
0.075 to 0.395 μM following digitoxin treatment for 24 h and
from 0.028 to 0.077 μM following digitoxin treatment for 48
h. When comparing the IC50 values, the HeLa and A549
cells exhibited a greater sensitivity to digitoxin than the other
cell lines, HCT116 and MHCC97H. These results indicate that
digitoxin has a broad spectrum of antitumor effects in
vitro. The HeLa cell line was selected for the investigation of
the anticancer mechanism of digitoxin, since it has the highest
sensitivity towards this natural compound.
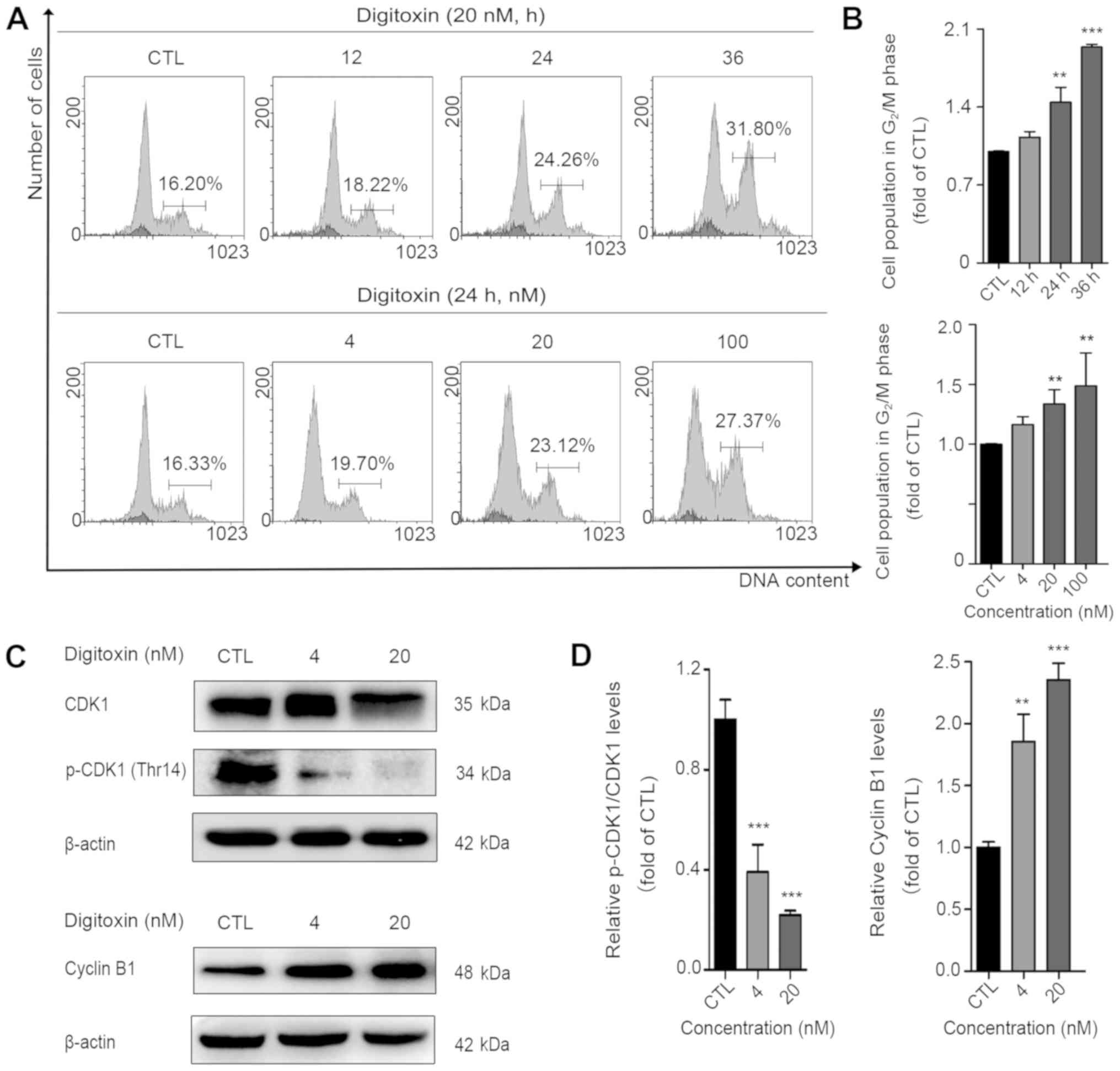
Digitoxin disrupts the cell cycle
To investigate whether digitoxin disrupts the cell
cycle, the DNA content of the digitoxin-treated cells was analyzed.
The cell population in the G2/M phase increased from
16.27 to 18.36, 23.46 and 31.51% in the presence of digitoxin (20
nM) for 12, 24 and 36 h, respectively (data presented in the text
are the average of 3 experiments). Moreover, when the cells were
exposed to digitoxin at concentrations of 4, 20 and 100 nM for 24
h, the cell population in the G2/M phase markedly
increased from 16.27 to 28.07% (data presented in the text are the
average of 3 experiments; Fig. 2A and
B). In animal cells, the G2/M transition is
regulated by the activity of CDK1, which is regulated by
phosphorylation, and the concentration of cyclin B (36). In the present study, to further
reveal the molecular mechanisms responsible for digitoxin-induced
G2/M arrest, the levels of these two key regulators,
CDK1 and cyclin B1, were examined. As shown in Fig. 2C and D, digitoxin significantly
decreased the protein expression levels of total CDK1 and
phosphorylated CDK1 (p-CDK1 Thr14). Digitoxin treatment led to a
marked accumulation of the cyclin B1 protein, further suggesting
that HeLa cells treated with digitoxin are mainly blocked at the
G2/M phase.
Digitoxin induces G2/M phase
arrest via the activation of the ATM pathway
It is well known that DNA damage may be responsible
for G2/M cell cycle arrest (37). In the present study, to investigate
whether the digitoxin-induced cell cycle arrest at the
G2/M phase was related to DNA lesions, an
immunofluorescence staining assay was performed to measure the
expression level of p-γH2AX, a marker of DNA double-stranded breaks
(DSBs) (38). The results revealed
the accumulation of γH2AX (Fig. 3A and
B). ATM and ATR are activated by phosphorylation following DNA
damage, and phosphorylated ATM and ATR block the cell cycle partly
through the activation of the checkpoint kinases CHK1 and CHK2.
Active CHK1 and CHK2 then decrease Cdc25C activity, which prevents
the dephosphorylation of CDK1 (Tyr15 and Thr14) to maintain the
CDK1-Cyclin B1 complex in an inactive state (37,39-41).
In the present study, as shown in Fig.
3C and D, the levels of p-ATM (Ser1981), p-ATR (Ser428), p-CHK1
(Ser286) and p-CHK2 (Thr68) were significantly upregulated in the
digitoxin‑treated cells. The level of phosphorylated Cdc25C was
decreased in the digitoxin-treated HeLa cells. Collectively, these
results demonstrated that digitoxin caused DNA damage to block the
cell cycle at the G2/M phase by triggering the
activation of the ATM/ATR-CHK1/CHK2-Cdc25C signaling pathway.
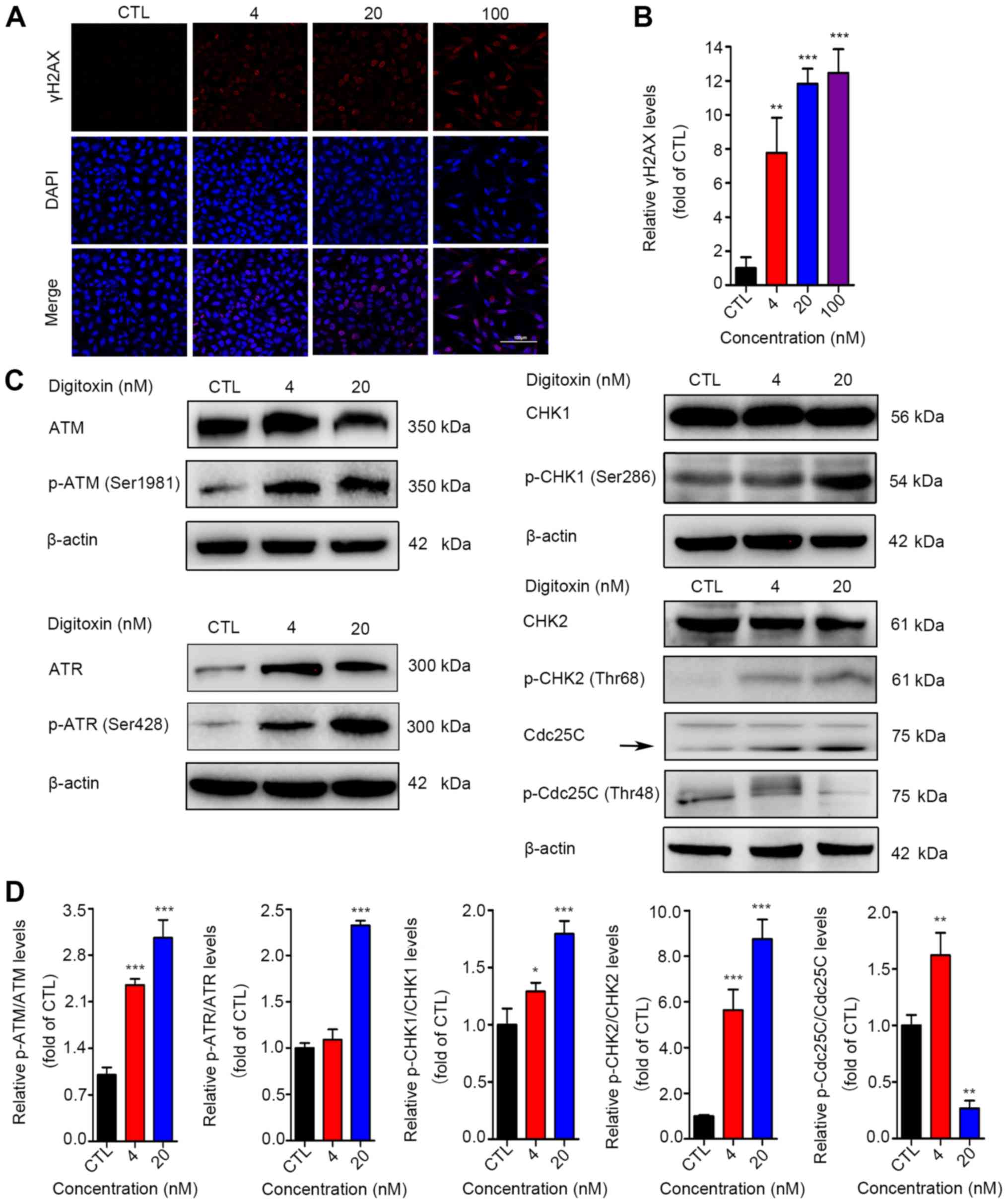 | Figure 3Digitoxin-induced G2/M
phase arrest via the ATM pathway. (A) Immunofluorescence of γH2AX.
HeLa cells were treated with digitoxin (4, 20, or 100 nM) or not
for 24 h. γH2AX‑positive cells were identified by
immunofluorescence staining. Images were obtained at x200
magnification (scale bar, 100 μm). (B) Quantitative data of
the γH2AX fluorescence intensities. Data are expressed as the means
± SD (n=3). **P<0.01, ***P<0.001 vs.
the control group. (C) Digitoxin activated the ATM signaling
pathway. The expression levels of ATM, p-ATM (Ser1981), ATR, p-ATR
(Ser428), CHK1, p-CHK1 (Ser286), CHK2, p-CHK2 (Thr68), Cdc25C and
p-Cdc25C (Thr48) were examined by western blot analysis. β-actin
served as the reference protein. (D) Quantitative data of the
relative protein expression are illustrated as the means ± SD
(n=3). *P<0.05, **P<0.01,
***P<0.001 vs. the control group. |
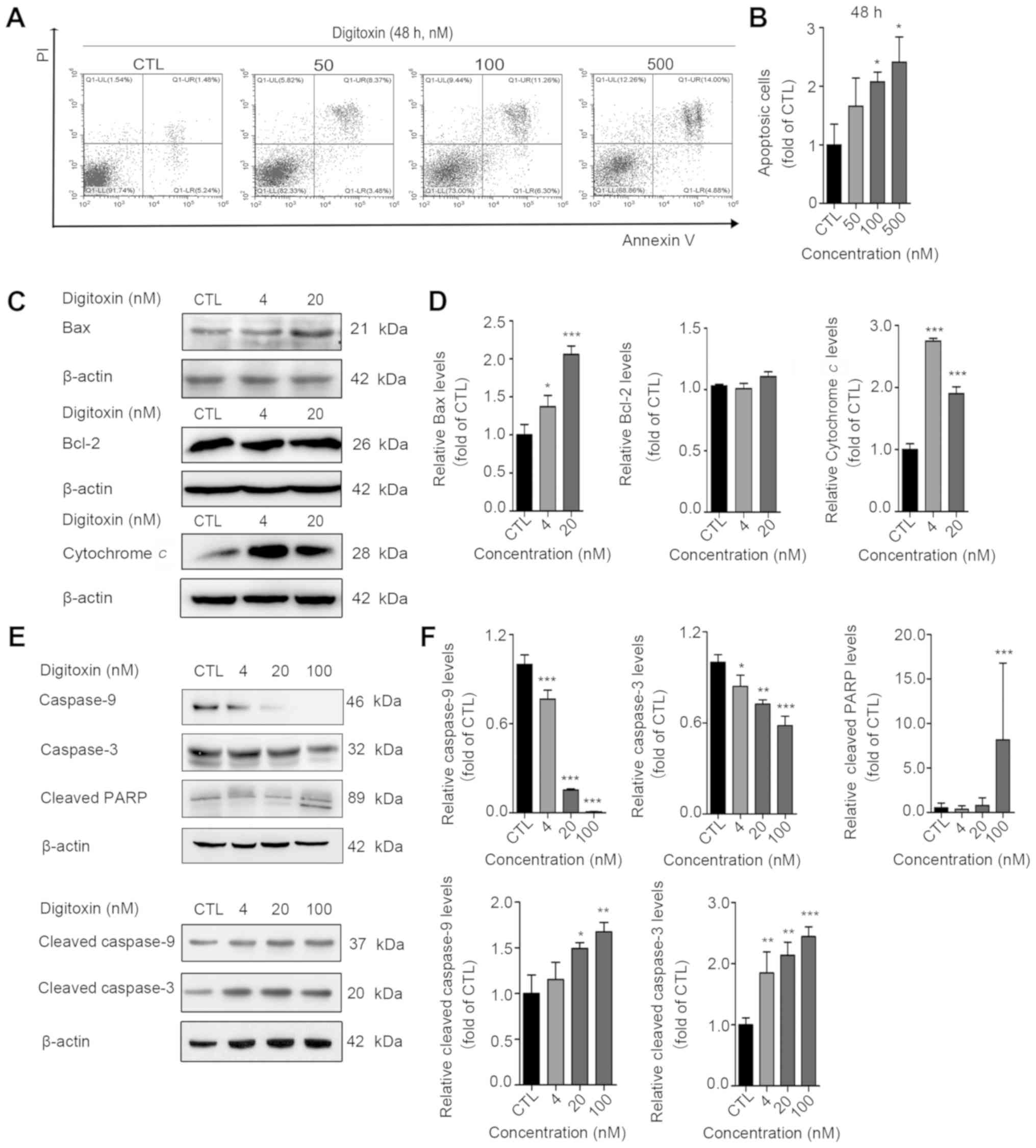
Digitoxin activates mitochondrial
apoptosis
To determine whether digitoxin induces cell
apoptosis, an Annexin V-FITC/PI double staining assay was
performed. HeLa cells were treated with increasing concentrations
of digitoxin (20, 100 and 500 nM) for 48 h. The apoptotic ratio of
HeLa cells increased by approximately 2-fold from 8.95 to 23.77% at
48 h (data in the text are the average of 3 experiments; Fig. 4A and B). To investigate whether
digitoxin-induced apoptosis is mediated by the mitochondrial
pathway, the changes in the levels of Bax/Bcl-2 were analyzed. As
was expected, Bax expression was upregulated and Bcl-2 expression
was almost unaltered in the digitoxin-treated cells (Fig. 4C and D). Moreover, it was found
that the expression of cytochrome c was significantly
increased in the digitoxin‑treated cells (Fig. 4C and D). In addition, the caspase
signaling pathway was activated, which was characterized by the
downregulation of caspase-9 and caspase-3, and the upregulation of
cleaved caspase-9, cleaved Caspase-3 and cleaved poly(ADP-ribose)
polymerase (PARP) (Fig. 4E and F).
Taken together, these results indicated that digitoxin triggered
the activation of the mitochondrial apoptotic pathway in HeLa
cells.
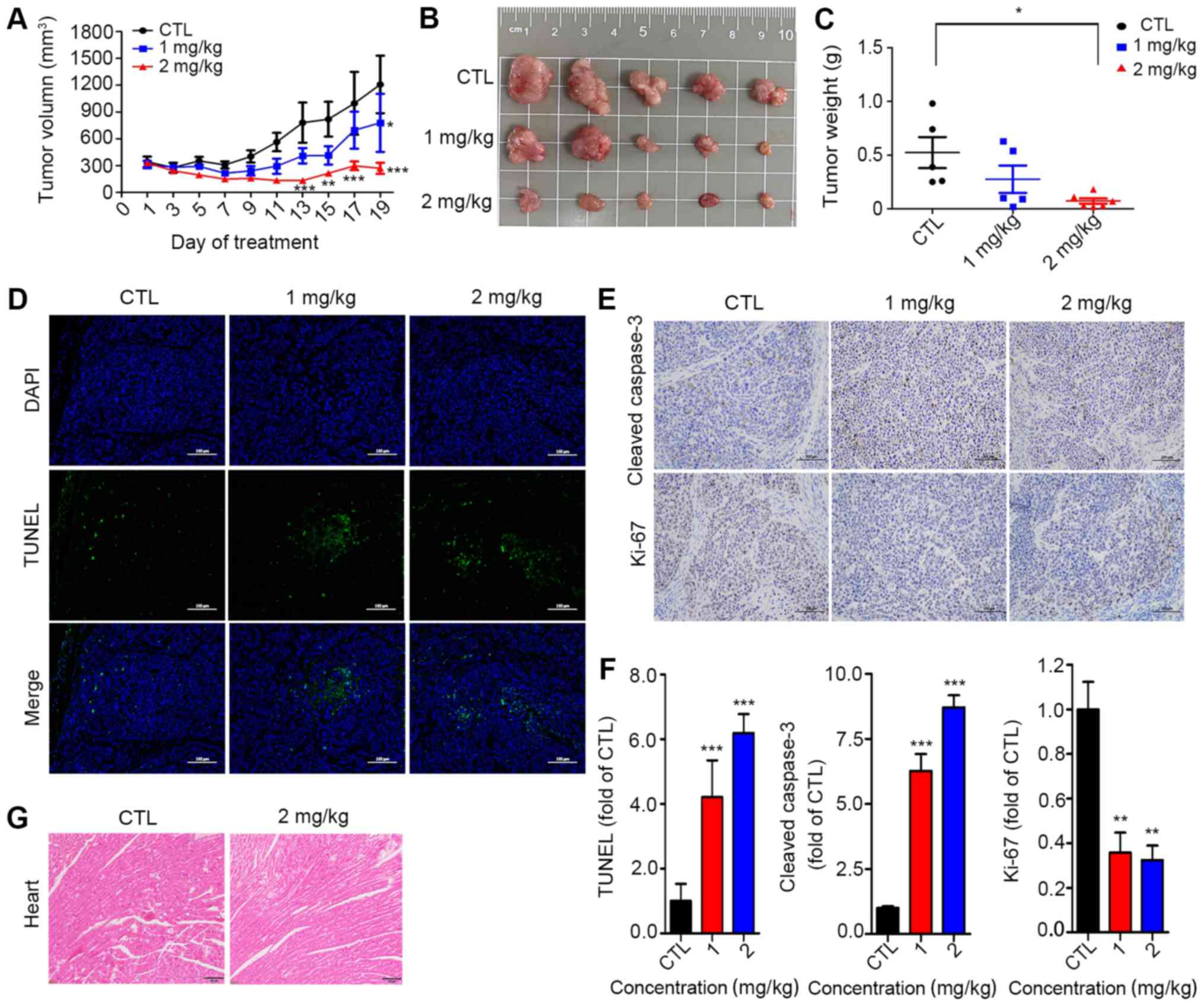
Anticancer effects of digitoxin in
vivo
The antitumor effects of digitoxin were examined in
nude mice harboring HeLa tumor xenografts. As shown in Fig. 5A and B, the tumor volume in the
vehicle control group increased from 344.78±39.25 to 1054.18±414.04
mm3, while the tumor volume in the digitoxin-treated
group (2 mg/kg) increased from 330.71±45.61 to 214.56.93±73.25
mm3, demonstrating that digitoxin treatment exerted a
tumor-suppressive effect. The tumor weight of the digitoxin-treated
group was much lower than that of the control group, with an
inhibitory rate of approximately 80% (Fig. 5C). Compared with those in the
vehicle group, TUNEL-positive cells were observed approximately
3-fold more clearly in the tumors in the digitoxin-treated groups
(Fig. 5D). To further confirm
whether digitoxin exerts its anticancer effects in vivo by
activating the caspase pathway, the protein levels of cleaved
caspase-3 were detected in tumor tissues. As was expected, cleaved
caspase-3 was strongly increased (Fig.
5E and F). In addition, Ki-67 staining was used to examine the
effects of digitoxin on tumor proliferation, and the results
demonstrated that the number of Ki-67-positive cells were reduced
by >50% in the digitoxin-treated groups compared with the
control group (Fig. 5F). Of note,
compared with the control group, digitoxin treatment did not lead
to a reduction in body weight at the end of treatment (Fig. S1), and mice in the digitoxin group
did not exhibit any abnormalities in food intake or behavior,
suggesting that digitoxin may exhibit low or no toxicity at the
doses used in the present study. Furthermore, pathological analysis
of the heart demonstrated that there were no apparent changes in
the digitoxin-treated tumor-bearing mice (Fig. 5G).
In summary, a library of 78 natural products was
screened, and digitoxin exhibited the highest cytotoxicity against
cervical cancer HeLa cells. Mechanistically, digitoxin caused DNA
DSBs and then blocked the cell cycle at the G2/M phase
via the ATM/ATR-CHK1/CHK2-Cdc25C pathway. In addition, the
accumulation of cytochrome c caused by the digitoxin-induced
activation of caspase-9 and caspase-3, ultimately triggered the
mitochondrial apoptosis of HeLa cells (Fig. 6). Moreover, the in vivo
anticancer effects of digitoxin were confirmed in HeLa cell
xenotransplantation models. These data indicate that digi-toxin is
an effective therapeutic agent for cervical cancer.
Discussion
Traditionally, natural products, such as colchicine,
doxorubicin hydrochloride, vinblastine (VBL) and paclitaxel
(Taxol®), have been one of the primary sources of drug
discovery in the field of cancer research and are among the most
effective cancer chemotherapeutics that are currently available
(42-44). In previously published data, the
IC50 values of colchicine and doxorubicin hydrochloride
against HeLa cells were shown to be 1.08 and 0.374 μM
(45,46), respectively. In the present study,
digitoxin displayed potent antiproliferative activity with an
IC50 value of 0.028 μM. According to these data,
digitoxin appears to have at least as much antitumor activity as
colchicine and doxorubicin hydrochloride against HeLa cells in
vitro. Of note, Hosseini et al reported that digitoxin
exerted cytotoxic effects against HeLa cells with an
IC50 value of 5.62 μg/ml (47), which is different from the value
observed in the present study. There may be several reasons for
this difference, including the cell culture conditions, the
experimental details of the cell viability assay, and the storage
concentration of digitoxin.
In detail, it was found that digitoxin induced DSBs
and then triggered the DNA damage response ATM/ATR-
CHK1/CHK2-Cdc25C pathway in human cervical cancer HeLa cells. It
has been reported that a number of small molecules can arrest the
cell cycle at the G1/S or S phase to prevent incorrect
DNA replication or at the G2/M phase to prevent entry
into mitosis with damaged DNA (48). The present study found that
digitoxin impeded cell cycle progression at the G2/M
phase, suggesting that digitoxin may not block DNA replication, but
instead induce DNA damage. The DNA damage factors, phosphorylated
ATM, ATR and γH2AX, accumulate upon the activation of DNA damage
checkpoints (49,50), as observed in the present study.
CHK2 is phosphorylated by ATM (39), and CHK1 is activated by the
ATR-dependent phosphorylation (40). Activated CHK2 and CHK1 inactive
Cdc25C to maintain the CDK1-Cyclin B1 complex in an inactivate
state in the G2 phase, thereby inhibiting the
G2/M transition (41).
In the present study, the CHK1 and CHK2 kinases were activated, and
the levels of the Cdc25C phosphatase were downregulated. Overall,
these data demonstrated that digitoxin induced DNA damage and
ultimately led to G2/M cell cycle arrest via the
ATM/ATR-CHK1/CHK2-Cdc25C pathway in HeLa cells.
It has been reported that digitoxin blocks the cell
cycle at the G2/M phase by decreasing the expression of
both cyclin B1 and CDK1 in NCI-H460 and H1975 lung cancer cells
(17,51). Accordingly, the present study also
found that digitoxin significantly decreased the protein expression
levels of total CDK1 and phosphorylated (p-CDK1 Thr14). However,
digitoxin treatment led to a marked accumulation of cyclin B1
protein in HeLa cells. This evidence indicates that the molecular
mechanisms through which digitoxin modulates the cell cycle may be
context-dependent. In eukaryotic cells, the expression of cyclin B1
is very low in the G1 phase, is synthesized and
significantly increased in the S phase, and peaks at the late
G2 phase and early mitosis. When cells enter late
mitosis, the expression of cyclin B1 is significantly decreased
(52–56). In the present study, the cell
population in the G2/M phase was increased in the
presence of digitoxin, suggesting that digitoxin consistently
triggered G2/M phase arrest, and the accumulation of
cyclin B1 protein in digitoxin-treated HeLa cells further suggested
that digitoxin led to G2/M arrest. Nevertheless, the
reasons for this differential effect of digitoxin are complex and
warrant further investigation in the future.
Digitoxin, as a Na+/K+-ATPase
inhibitor, is widely applied in the clinical management of heart
diseases, such as congestive heart failure and cardiac arrhythmias
(57). It should be noted that
digitoxin may cause cardiac side-effects. It has been demonstrated
that digitoxin leads to poisoning at serum concentrations of
108-205 ng/ml (approximately 140-270 nM) (15,58).
Other studies have reported that digitoxin exerts anticancer
effects at concentrations ranging from 20 to 33 nM, and no notable
toxicity has been observed in cardiac patients (16,59).
As previously demonstrated, the growth of tumors was attenuated
effectively in mice bearing M214 melanomas after 27 days digitoxin
treatment and no cardiac side-effects were observed (60). In the present study, it was found
that digitoxin exhibited cytotoxicity in HeLa cells with an
IC50 value of approximately 28 nM and the mechanism
involved was digitoxin-induced G2/M phase cell cycle
arrest at concentrations of 4 and 20 nM. In vivo, compared
with the control treatment, digitoxin treatment did not lead to a
reduction in body weight at the end of treatment, and mice in the
digitoxin group did not exhibit any abnormalities in food intake or
behavior, and heart pathological analysis demonstrated that there
were no apparent changes in digitoxin-treated tumor-bearing mice.
Combining the results from others laboratories with the current
experimental data, it is suggested that digitoxin does not cause
cardiac side-effects at the concentration used in the present
study. Furthermore, digitoxin has been demonstrated to exert a
notable killing effect on HeLa cells (IC50 = 5.62
μg/ml), but to cause almost no damage to normal human
lymphocyte cells (IC50 = 412.94 μg/ml) (47). Previous studies have proven that
the cardiac glycosides are generally more toxic to cancer cells
than normal peripheral blood mononuclear cells (34,61,62).
Thus, digitoxin has few adverse side-effects on normal human cells
at the concentration used in the present study. However,
prospective clinical trials need to be performed to determine
whether digitoxin is useful as an anticancer agent.
Several trials related to the use of digitoxin in
combination with other anticancer agents are currently reported.
For example, the combination of digitoxin with standard
chemotherapeutic agents in clinical practice, such as
5‑fluorouracil, cisplatin and oxaliplatin, has additive effects
against colon cancer HT-29 and HCT116 cells (63). Digitoxin and its synthetic analog,
MonoD, exert potent anti-proliferative effects at clinically
relevant concentrations in serum-starved conditions, while
paclitaxel, hydroxyurea and colchicine were only active in lung
cancer cells growing in routine culture conditions. Furthermore,
digitoxin and its analog have been shown to potentiate the effects
of hydroxyurea or paclitaxel (64). These data indicate that digitoxin
has potential clinical applications in translational oncology
particularly in combination with other drugs.
In conclusion, the present study screened a library
of natural compounds composed of 78 single compounds to identify
potential lead compounds with activity against cervical cancer, and
digitoxin exhibited the highest cytotoxicity in the different
malignant cell lines. Mechanistically, digitoxin causes DNA DSBs,
blocks the cell cycle at the G2/M phase via the
ATM/ATR-CHK1/CHK2-Cdc25C pathway, and ultimately triggers
mitochondrial apoptosis. Furthermore, the in vivo anticancer
effects of digitoxin were confirmed in HeLa cell
xenotransplantation models. These results shed new light on the
mechanisms of digitoxin-induced cell cycle arrest, which is
valuable for the further study of the application of digitoxin to
anticancer chemotherapy in clinical practice.
Supplementary Data
Funding
The present study was supported by the National
Science Foundation of China (grant nos. 81803790, 81630104 and
81973748), the National Natural Science Foundation of Guangdong
(grant no. 2020A1515011090) and the Huang Zhendong Research Fund
for Traditional Chinese Medicine of Jinan University.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author upon reasonable request.
Authors' contributions
LD and JC designed the study and revised the
manuscript. HG, MQ and CC performed the experiments and drafted the
manuscript. PL, YL and GY assisted with the in vitro
research experiments. AL and FX contributed to the flow cytometry
experiments. WY and DZ assisted with the design of the study and
revised the manuscript. DL assisted with the revision of the
manuscript and performed experiments to update the data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in compliance
with the ARRIVE guidelines and were approved by the Experimental
Animal Ethics Committee of Jinan University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
DSB
|
DNA double-stranded break
|
Acknowledgements
Not applicable.
References
|
1
|
Bonde JH, Sandri MT, Gary DS and Andrews
JC: Clinical utility of human papillomavirus genotyping in cervical
cancer screening: A systematic review. J Low Genit Tract Dis.
24:1–13. 2020. View Article : Google Scholar :
|
|
2
|
Wang R, Pan W, Jin L, Huang W, Li Y, Wu D,
Gao C, Ma D and Liao S: Human papillomavirus vaccine against
cervical cancer: Opportunity and challenge. Cancer Lett.
471:88–102. 2020. View Article : Google Scholar
|
|
3
|
Eskander RN and Tewari KS: Chemotherapy in
the treatment of metastatic, persistent, and recurrent cervical
cancer. Curr Opin Obstet Gynecol. 26:314–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monk BJ and Tewari KS: Evidence-based
therapy for recurrent cervical cancer. J Clin Oncol. 32:2687–2690.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liontos M, Kyriazoglou A, Dimitriadis I,
Dimopoulos MA and Bamias A: Systemic therapy in cervical cancer: 30
years in review. Crit Rev Oncol Hematol. 137:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27:e432016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gokduman K: Strategies targeting DNA
topoisomerase I in cancer chemotherapy: Camptothecins, nanocarriers
for camp-tothecins, organic non-camptothecin compounds and metal
complexes. Curr Drug Targets. 17:1928–1939. 2016. View Article : Google Scholar
|
|
8
|
Khanna C, Rosenberg M and Vail DM: A
review of paclitaxel and novel formulations including those
suitable for use in dogs. J Vet Intern Med. 29:1006–1012. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carter GT: Natural products and Pharma
2011: Strategic changes spur new opportunities. Nat Prod Rep.
28:1783–1789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cragg GM and Newman DJ: Natural products:
A continuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harvey AL, Edrada-Ebel R and Quinn RJ: The
re-emergence of natural products for drug discovery in the genomics
era. Nat Rev Drug Discov. 14:111–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mishra BB and Tiwari VK: Natural products:
An evolving role in future drug discovery. Eur J Med Chem.
46:4769–4807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patel S: Plant-derived cardiac glycosides:
Role in heart ailments and cancer management. Biomed Pharmacother.
84:1036–1041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
López-Lázaro M, Pastor N, Azrak SS, Ayuso
MJ, Austin CA and Cortés F: Digitoxin inhibits the growth of cancer
cell lines at concentrations commonly found in cardiac patients. J
Nat Prod. 68:1642–1645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YZ, Chen X, Fan XX, He JX, Huang J,
Xiao DK, Zhou YL, Zheng SY, Xu JH, Yao XJ, et al: Compound library
screening identified cardiac glycoside digitoxin as an effective
growth inhibitor of gefitinib‑resistant non‑small cell lung cancer
via downregulation of α-tubulin and inhibition of microtubule
formation. Molecules. 21:3742016. View Article : Google Scholar
|
|
18
|
Iyer AKV, Zhou M, Azad N, Elbaz H, Wang L,
Rogalsky DK, Rojanasakul Y, O'Doherty GA and Langenhan JM: A direct
comparison of the anticancer activities of digitoxin
MeON-Neoglycosides and O-Glycosides: Oligosaccharide chain
length-dependent induction of caspase-9-mediated apoptosis. ACS Med
Chem Lett. 1:326–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trenti A, Boscaro C, Tedesco S, Cignarella
A, Trevisi L and Bolego C: Effects of digitoxin on cell migration
in ovarian cancer inflammatory microenvironment. Biochem Pharmacol.
154:414–423. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu IL, Chou CY, Wu YY, Wu JE, Liang CH,
Tsai YT, Ke JY, Chen YL, Hsu KF and Hong TM: Targeting FXYD2 by
cardiac glycosides potently blocks tumor growth in ovarian clear
cell carcinoma. Oncotarget. 7:62925–62938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prassas I, Karagiannis GS, Batruch I,
Dimitromanolakis A, Datti A and Diamandis EP: Digitoxin-induced
cytotoxicity in cancer cells is mediated through distinct kinase
and interferon signaling networks. Mol Cancer Ther. 10:2083–2093.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shang Z and Zhang L: Digitoxin increases
sensitivity of glioma stem cells to TRAIL-mediated apoptosis.
Neurosci Lett. 653:19–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee DH, Cheul Oh S, Giles AJ, Jung J,
Gilbert MR and Park DM: Cardiac glycosides suppress the maintenance
of stemness and malignancy via inhibiting HIF-1α in human glioma
stem cells. Oncotarget. 8:40233–40245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pollard BS, Suckow MA, Wolter WR, Starr
JM, Eidelman O, Dalgard CL, Kumar P, Battacharyya S, Srivastava M,
Biswas R, et al: Digitoxin Inhibits
epithelial-to-mesenchymal-transition in hereditary castration
resistant prostate cancer. Front Oncol. 9:6302019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu M, Feng LX, Sun P, Liu W, Mi T, Lei M,
Wu W, Jiang B, Yang M, Hu L, et al: Knockdown of apolipoprotein E
enhanced sensitivity of Hep3B cells to cardiac steroids via
regulating Na+/K+-ATPase signalosome. Mol Cancer Ther.
15:2955–2965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Felth J, Rickardson L, Rosén J, Wickström
M, Fryknäs M, Lindskog M, Bohlin L and Gullbo J: Cytotoxic effects
of cardiac glycosides in colon cancer cells, alone and in
combination with standard chemotherapeutic drugs. J Nat Prod.
72:1969–1974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao Y, Yan W, Guo L, Meng C, Li B, Neves
H, Chen PC, Li L, Huang Y, Kwok HF, et al: Digitoxin synergizes
with sorafenib to inhibit hepatocelluar carcinoma cell growth
without inhibiting cell migration. Mol Med Rep. 15:941–947. 2017.
View Article : Google Scholar
|
|
28
|
Einbond LS, Shimizu M, Ma H, Wu HA,
Goldsberry S, Sicular S, Panjikaran M, Genovese G and Cruz E:
Actein inhibits the Na+-K+-ATPase and enhances the growth
inhibitory effect of digitoxin on human breast cancer cells.
Biochem Biophys Res Commun. 375:608–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Einbond LS, Wu HA, Sandu C, Ford M, Mighty
J, Antonetti V, Redenti S and Ma H: Digitoxin enhances the growth
inhibitory effects of thapsigargin and simvastatin on ER negative
human breast cancer cells. Fitoterapia. 109:146–154. 2016.
View Article : Google Scholar
|
|
30
|
Kulkarni YM, Kaushik V, Azad N, Wright C,
Rojanasakul Y, O'Doherty G and Iyer AK: Autophagy-induced apoptosis
in lung cancer cells by a novel digitoxin analog. J Cell Physiol.
231:817–828. 2016. View Article : Google Scholar :
|
|
31
|
Trenti A, Zulato E, Pasqualini L,
Indraccolo S, Bolego C and Trevisi L: Therapeutic concentrations of
digitoxin inhibit endothelial focal adhesion kinase and
angiogenesis induced by different growth factors. Br J Pharmacol.
174:3094–3106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng LJ, Peng QL, Wang LH, Xu J, Liu JS,
Li YJ, Zhuo ZJ, Bai LL, Hu LP, Chen WM, et al: Arenobufagin
intercalates with DNA leading to G2 cell cycle arrest via ATM/ATR
pathway. Oncotarget. 6:34258–34275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Silva IT, Munkert J, Nolte E, Zanchett
Schneider NF, Carvalho Rocha S, Pacheco Ramos AC, Kreis W, Castro
Braga F, de Pádua RM, et al: Cytotoxicity of AMANTADIG - a
semi-synthetic digitoxigenin derivative - alone and in combination
with docetaxel in human hormone-refractory prostate cancer cells
and its effect on Na+/K+-ATPase inhibition.
Biomed Pharmacother. 107:464–474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johansson S, Lindholm P, Gullbo J, Larsson
R, Bohlin L and Claeson P: Cytotoxicity of digitoxin and related
cardiac glycosides in human tumor cells. Anticancer Drugs.
12:475–483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marzo-Mas A, Barbier P, Breuzard G,
Allegro D, Falomir E, Murga J, Carda M, Peyrot V and Marco JA:
Interactions of long-chain homologues of colchicine with tubulin.
Eur J Med Chem. 126:526–535. 2017. View Article : Google Scholar
|
|
36
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Löbrich M, Shibata A, Beucher A, Fisher A,
Ensminger M, Goodarzi AA, Barton O and Jeggo PA: gammaH2AX foci
analysis for monitoring DNA double-strand break repair: Strengths,
limitations and optimization. Cell Cycle. 9:662–669. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuoka S, Rotman G, Ogawa A, Shiloh Y,
Tamai K and Elledge SJ: Ataxia telangiectasia-mutated
phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA.
97:10389–10394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao H and Piwnica-Worms H: ATR-mediated
checkpoint pathways regulate phosphorylation and activation of
human Chk1. Mol Cell Biol. 21:4129–4139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perdiguero E and Nebreda AR: Regulation of
Cdc25C activity during the meiotic G2/M transition. Cell Cycle.
3:733–737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gupta S and Bhattacharyya B:
Antimicrotubular drugs binding to vinca domain of tubulin. Mol Cell
Biochem. 253:41–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jordan MA, Thrower D and Wilson L, Jordan
MA, Thrower D and Wilson L: Mechanism of inhibition of cell
proliferation by Vinca alkaloids. Cancer Res. 51:2212–2222.
1991.PubMed/NCBI
|
|
44
|
Johnson IS, Armstrong JG, Gorman M and
Burnett JP Jr: The vinca alkaloids, a new class of oncolytic
agents. Cancer Res. 23:1390–1427. 1963.PubMed/NCBI
|
|
45
|
Sadeghi-Aliabadi H, Minaiyan M and
Dabestan A: Cytotoxic evaluation of doxorubicin in combination with
simvastatin against human cancer cells. Res Pharm Sci. 5:127–133.
2010.PubMed/NCBI
|
|
46
|
Yin Y, Lian BP, Xia YZ, Shao YY and Kong
LY: Design, synthesis and biological evaluation of
resveratrol-cinnamoyl derivates as tubulin polymerization
inhibitors targeting the colchicine binding site. Bioorg Chem.
93:1033192019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hosseini M, Taherkhani M and Ghorbani
Nohooji M: Introduction of Adonis aestivalis as a new source of
effective cytotoxic cardiac glycoside. Nat Prod Res. 33:915–920.
2019. View Article : Google Scholar
|
|
48
|
Laiho M and Latonen L: Cell cycle control,
DNA damage checkpoints and cancer. Ann Med. 35:391–397. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Harper JW and Elledge SJ: The DNA damage
response: Ten years after. Mol Cell. 28:739–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Durocher D and Jackson SP: DNA-PK, ATM and
ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell
Biol. 13:225–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Elbaz HA, Stueckle TA, Wang HYL, O'Doherty
GA, Lowry DT, Sargent LM, Wang L, Dinu CZ and Rojanasakul Y:
Digitoxin and a synthetic monosaccharide analog inhibit cell
viability in lung cancer cells. Toxicol Appl Pharmacol. 258:51–60.
2012. View Article : Google Scholar :
|
|
52
|
Nurse P: A long twentieth century of the
cell cycle and beyond. Cell. 100:71–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bloom J and Cross FR: Multiple levels of
cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol.
8:149–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fisher DL and Nurse P: A single fission
yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and
mitosis in the absence of G1 cyclins. EMBO J. 15:850–860. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hayles J, Fisher D, Woollard A and Nurse
P: Temporal order of S phase and mitosis in fission yeast is
determined by the state of the p34cdc2-mitotic B cyclin complex.
Cell. 78:813–822. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gould KL and Nurse P: Tyrosine
phosphorylation of the fission yeast cdc2+ protein kinase regulates
entry into mitosis. Nature. 342:39–45. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rahimtoola SH: Digitalis therapy for
patients in clinical heart failure. Circulation. 109:2942–2946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lapostolle F, Borron SW, Verdier C,
Taboulet P, Guerrier G, Adnet F, Clemessy JL, Bismuth C and Baud
FJ: Digoxin‑specific Fab fragments as single first‑line therapy in
digitalis poisoning. Crit Care Med. 36:3014–3018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Haux J, Klepp O, Spigset O and Tretli S:
Digitoxin medication and cancer; case control and internal
dose-response studies. BMC Cancer. 1:112001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Eskiocak U, Ramesh V, Gill JG, Zhao Z,
Yuan SW, Wang M, Vandergriff T, Shackleton M, Quintana E, Johnson
TM, et al: Synergistic effects of ion transporter and MAP kinase
pathway inhibitors in melanoma. Nat Commun. 7:123362016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Daniel D, Süsal C, Kopp B, Opelz G and
Terness P: Apoptosis-mediated selective killing of malignant cells
by cardiac steroids: maintenance of cytotoxicity and loss of
cardiac activity of chemically modified derivatives. Int
Immunopharmacol. 3:1791–1801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
López-Lázaro M: Digitoxin as an anticancer
agent with selectivity for cancer cells: Possible mechanisms
involved. Expert Opin Ther Targets. 11:1043–1053. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao B, Kim J, Ye X, Lai ZC and Guan KL:
Both TEAD-binding and WW domains are required for the growth
stimulation and oncogenic transformation activity of yes-associated
protein. Cancer Res. 69:1089–1098. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yakisich JS, Azad N, Venkatadri R,
Kulkarni Y, Wright C, Kaushik V, O'Doherty GA and Iyer AK:
Digitoxin and its synthetic analog MonoD have potent
antiproliferative effects on lung cancer cells and potentiate the
effects of hydroxyurea and paclitaxel. Oncol Rep. 35:878–886. 2016.
View Article : Google Scholar :
|