Introduction
Non-small cell lung cancer (NSCLC) is the most
frequent type of lung cancer, comprising at least 80% of all lung
cancer diagnoses (1). NSCLC is
primarily divided into two subtypes, namely squamous cell carcinoma
and adenocarcinoma, which are derived from the epithelial cells
lining the larger and peripheral small airways, respectively
(2). Despite the advances in NSCLC
treatment, the prognosis of NSCLC patients remains poor, mainly due
to tumor metastasis (3,4). Thus, exploring the mechanisms
underlying the development of NSCLC is crucial.
Long non-coding RNAs (lncRNAs) are a class of RNA
molecules>200 nucleotides in length, which have no
protein-coding capacity (5).
Recently, lncRNAs have been demonstrated to play key roles in
various biological processes, including cell proliferation,
migration and invasion (6-8). A large number of lncRNAs have been
reported to be involved in the development of NSCLC, such as CRNDE,
AWPPH and BANCR (9-11). Cancer susceptibility candidate 7
(CASC7) is a ~9.3-kb lncRNA, the function of which is largely
unknown (12). Certain studies
have demonstrated the involvement of CASC7 in severe asthma and
spinal cord ischemia-reperfusion injury (13,14).
In addition, CASC7 was reported to act as a tumor suppressor in
colorectal cancer (CRC) and glioma tissues (15,16),
but the precise mechanism of action of this cancer-related lncRNA
remains unclear. Moreover, there are currently no reports on the
role or mechanism of action of CASC7 in hepatocellular carcinoma
(HCC).
LncRNAs have been shown to act as competing
endogenous RNAs (ceRNAs) by sponging specific miRNAs, thereby
preventing targeted transcripts of these miRNAs from being degraded
(17). An example of this type of
regulation is the lncRNA MNX1-AS1, which was shown to interact with
miR-218-5p and regulate the expression of COMMD8 in HCC cells
(18). Similarly, lncRNA NEAT1
acts as a ceRNA to regulate the expression of oncogene SOX2 through
sponging miR-132 in glioma cells (19). In NSCLC, lncRNA 1308 has been
identified as an oncogenic lncRNA that acts through regulating
miR-124 (20). Notably, lncRNA
CASC7 has been reported to inhibit growth and invasion of CRC cells
through upregulating ING3 expression via sequestration of miR-21
(16). In addition, CASC7 was
previously reported to target miR-21 in airway smooth muscle cells
(13). Therefore, it was
hypothesized that CASC7 may affect the development of NSCLC via its
ceRNA role, which has not been previously investigated.
In the present study, the expression level and
clinical significance of CASC7 in NSCLC were investigated.
Furthermore, the regulatory role and underlying mechanism of action
of CASC7 in the proliferation and invasion of NSCLC cells were
examined, in order to determine whether CASC7 may serve as a new
potential therapeutic target in NSCLC.
Materials and methods
Patients and samples
NSCLC tissues (n=80) and matched normal adjacent
tissues (n=80) were obtained from patients who had undergone
surgical resection at the Department of Thoracic Surgery, Changhai
Hospital, between January 2016 and December 2017. The samples were
snap-frozen and stored at -80°C prior to RNA extraction. The
clinicopathological characteristics are summarized in Table I. Written informed consent was
obtained from each patient. The protocol of the present study was
approved by the Ethics Committee of Changhai Hospital (approval no.
2016-00113).
 | Table ICorrelation between lncRNA CASC7 and
the clinicopathological characteristics of patients with non-small
cell lung cancer. |
Table I
Correlation between lncRNA CASC7 and
the clinicopathological characteristics of patients with non-small
cell lung cancer.
|
Characteristics | All cases
(n=80) | CASC7 expression
| P-value |
|---|
| High (n=31) | Low (n=49) |
|---|
| Sex | | | | 0.7787 |
| Male | 48 | 18 | 30 | |
| Female | 32 | 13 | 19 | |
| Age (years) | | | | 0.9097 |
| ≥60 | 51 | 20 | 31 | |
| <60 | 29 | 11 | 18 | |
| Tumor size
(cm) | | | | 0.0758 |
| ≥5 | 46 | 14 | 32 | |
| <5 | 34 | 17 | 17 | |
| Smoking | | | | 0.7336 |
| No | 25 | 9 | 16 | |
| Yes | 55 | 22 | 33 | |
| Differentiation
degree | | | | 0.1096 |
| Moderate | 30 | 15 | 15 | |
| Poor | 50 | 16 | 34 | |
| Clinical stage | | | | 0.0188 |
| I-II | 31 | 17 | 14 | |
| III-IV | 49 | 14 | 35 | |
| Distant
metastasis | | | | 0.0002b |
| No | 36 | 22 | 14 | |
| Yes | 44 | 9 | 35 | |
| Lymph node
involvement | | | | 0.0439a |
| No | 21 | 12 | 9 | |
| Yes | 59 | 19 | 40 | |
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated from NSCLC tissues and cell
lines using TRIzol reagent (TaKaRa Bio, Inc.). The RT of miR-92a
was performed using the miScript II RT kit (Invitrogen; Thermo
Fisher Scientific, Inc.), and the RT of lncRNA CASC7 and
phosphatase and tensin homolog (PTEN) was performed using the
SuperScript III First-Strand Synthesis System kit (Invitrogen;
Thermo Fisher Scientific, Inc.). RT-qPCR assays were carried out
using SYBR Premix Ex Taq II reagent (TaKaRa Bio, Inc.) on the 7900
HT Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction mixtures were denatured at 95°C for
3 min, followed by 40 two-step cycles of 95°C for 10 sec and at
60°C for 30 sec. The primer sequences were as follows: CASC7
forward, 5′-GCT GCC AGG AGA AGG CAA GGA TC-3′ and reverse, 5′-AGG
GTT AGA GCA GCC TTC GGA CT-3′; PTEN forward, 5′-TGG AAA GGG ACG AAC
TGG TG-3′ and reverse, 5′-CAT AGC GCC TCT GAC TGG GA-3′; GAPDH
forward, 5′-GAA GAT GGT GAT GGG ATT TC-3′ and reverse, 5′-AAC GCT
TCA CGA ATT TGC GT-3′; miR-92a forward, 5′-CAC CTA TAT TGC ACT TGT
CC-3′ and reverse, 5′-TGC GTG TCG TGG AGT C-3′; and U6 forward,
5′-CTC GCT TCG GCA GCA CA-3′ and reverse, 5′-GTC ATA CTC CTG CTT
GCT GAT-3′. GAPDH was used as the internal reference for CASC7 and
PTEN and U6 was used as reference for miR-92a. The relative gene
expression was calculated using the 2−ΔΔCq method
(21).
In situ hybridization (ISH)
ISH detection for CASC7 was performed on NSCLC and
normal tissues using a commercial ISH Detection kit (cat. no.
AR0149; Boster Biological Technology, Ltd.). ISH staining was
evaluated by two pathologists in a blinded manner.
Cell lines and cell culture
The A549, H358 and H2170 NSCLC cell lines and 293T
cells, were obtained from ATCC. Normal human bronchial epithelial
cells (16HBE) were also obtained from ATCC. All cells were
maintained in DMEM supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) in a 5% CO2 incubator at 37°C.
Cell transfection
The miR-92a mimics (5′-UAU UGC ACU UGU CCC GGC CUG
U-3′), mimics negative control (NC; 5′-UUC UCC GAA CGU GUC ACG
UTT-3′), miR-92a inhibitor (5′-ACA GGC CGG GAC AAG UGC AAU A-3′)
and inhibitor NC (5′-CAG UAC UUU UGU GUA GUA CAA-3′) were obtained
from RiBoBio. The CASC7-overexpressing vector pcDNA-CASC7 and pcDNA
vector were constructed by Qiagen, Inc. In addition, CASC7 siRNA
(si-CASC7) and corresponding negative control siRNA (si-Scramble)
were purchased from RiboBio. The sequences were as follows:
si-CASC7, 5′-TGG AAC ACA TGG TCC AGC ACT TTA A-3′; and si-Scramble,
5′-TGG ACA CTG GTG ACC TCA CTA ATA A-3′.
After A540 and H358 cells in 6-well plates had grown
to ~80% confluence, miR-92a mimics (20 nmol/l), miR-92a inhibitor
(20 nmol/l), si-CASC7 (30 nM) or 2 µg pcDNA-CASC7 were
transfected into cells at 37°C for 24 h using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.).
Cell proliferation
The antiproliferative effect of CASC7 on NSCLC cells
was evaluated by using MTT assay. At the end of transfection, 20
µl MTT solution (Sigma-Aldrich; Merck KGaA) was added to
each well, and A540 and H358 cells (1×105/well) were
cultured for 2 h. Subsequently, the absor-bance of the samples at
450 nm was detected by an iMark microplate reader (Bio-Rad
Laboratories, Inc.).
Immunofluorescence
Following transfection, the cells were fixed in
absolute ethyl alcohol for 30 min at room temperature. After
washing twice with PBS, the fixed cells were stained with primary
antibody against cleaved caspase-3 (1:100; cat. no. ab49822; Abcam)
for 1 h at room temperature. Subsequently, secondary antibody
conjugated with FITC (1:200, cat. no. ab116639; Abcam) was added
for 2 h in the dark, and fluorescence images were captured and
analyzed using an inverted fluorescence microscope (EVOS FL;
AMF-4306, Thermo Fisher Scientific, Inc.; magnification, 100×).
Cell apoptosis
The apoptosis of A549 and H358 cells was examined
using flow cytometry. Following transfection for 48 h, cells were
collected and the apoptotic cells were identified using an Annexin
V-FITC Apoptosis Detection kit (Abcam) according to the
manufacturer's protocol. The fluorescence signals were collected by
a FACScan flow cytometer (Beckman Coulter, Inc.) and then analyzed
by FlowJo 8.7.1 software (FlowJo LLC).
Cell invasion
Transwell chambers (24-well, 8-µm pore
polyethylene terephthalate membrane; BD Biosciences) coated with
Matrigel (BD Biosciences) were used for the invasion assay.
Briefly, A549 and H358 cell suspension containing 8×104
cells were added in the top chamber with DMEM, while the lower
chamber contained DMEM supplemented with 20% FBS. After 24 h of
incubation, the cells were stained with 0.1% crystal violet
solution for 10 min at room temperature and photographed with a
CKX41 inverted microscope (Olympus Corporation) at a magnification
of ×200.
Wound healing assay
A540 and H358 cells (2×106/well) were
seeded in 6-well plates coated with 50 µg/ml poly-D-lysine
(Sigma-Aldrich; Merck KGaA) overnight at 37°C to allow cells to
attach. When the cell reached ~90%, the cells were starved in
serum-free medium for 24 h. Then, the cell monolayers were
scratched using a 200-µl pipette tip and images from each
well were captured at 0 and 48 h with a CKX41 inverted microscope
(Olympus Corporation) at a magnification of ×200.
Dual-luciferase reporter assay
A total of 100 ng pGL3-CASC7 wild-type (wt) or
pGL3-CASC7 mutant (mut) plasmid (Shanghai Jima Industrial Co.,
Ltd.) were co-transfected in cells with miR-92a mimics, together
with 20 ng Renilla luciferase vector (Promega Corporation) as an
internal normalization control in 24-well plates
(2×105/well) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). At 24 h post-transfection, the
luciferase activities were analyzed using the Dual-Luciferase
Reporter Assay system (Promega Corporation) according to the
manufacturer's protocol. Transfections were performed in duplicate
and repeated three times.
Western blot analysis
Western blotting was performed to detect the
expression of proteins, as described previously (22). Briefly, protein was extracted from
cells using RIPA lysis buffer (Beyotime Institute of Biotechnology)
with proteinase inhibitor. The concentrations of total cellular
protein were determined using a BCA assay kit (Beyotime Institute
of Biotechnology). Proteins (40 µg) were separated by 10%
SDS-PAGE and transferred onto a PVDF membrane (EMD Millipore). The
membrane was then blocked with 5% skimmed milk at 4°C overnight and
probed with antibodies against PTEN (cat. no. 32199; 1:1,000),
proliferating cell nuclear antigen (PCNA; cat. no. 92552; 1:1,000),
E-cadherin (cat. no. 194982; 1:1,000), N-cadherin (cat. no. 202030;
1:1,000), fibronectin (cat. no. 32419; 1:1,000), vimentin (cat. no.
92547; 1:1,000) and β-actin (cat. no. 179467; 1:2,000) overnight at
4°C, followed by horseradish peroxidase-conjugated goat anti-rabbit
IgG (cat. no. 205718; 1:10,000) for 1 h at room temperature. All
antibodies were purchased from Abcam. The protein bands were
developed using an ECL kit (GE Healthcare) and blot bands were
quantified with ImageJ software (version 1.46; Rawak Software,
Inc.).
Statistical analysis
Statistical analysis was performed by SPSS 18.0
(SPSS, Inc.). All data are presented as mean ± standard deviation.
The overall survival was analyzed using the Kaplan-Meier method and
log-rank test. Correlations between clinical characteristics and
CASC7 expression were evaluated using the χ2 test. The
correlations between CASC7 and miR-92a levels, and between CASC7
and PTEN levels, were evaluated using Pearson's coefficient.
Comparisons among data were performed by one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate
statistically significant differences.
Results
LncRNA CASC7 is downregulated in NSCLC
tissues and cell lines
To investigate the roles of CASC7 in NSCLC, the
expression levels of CASC7 were quantified in 80 pairs of NSCLC and
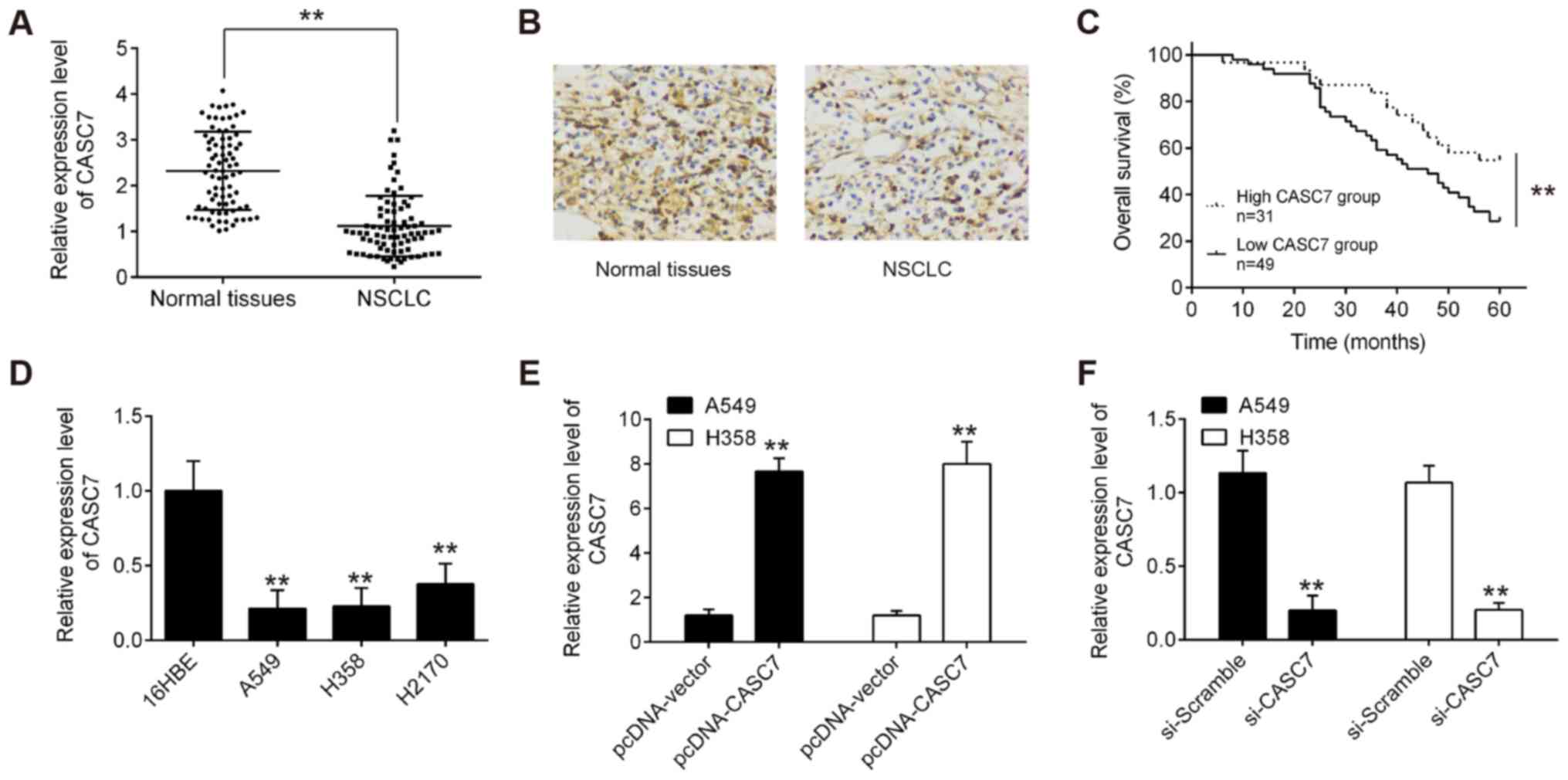
matched normal adjacent tissues using RT-qPCR. As shown in Fig. 1A, CASC7 expression was
significantly lower in tumor tissues compared with that in normal
adjacent tissues. Furthermore, downregulation of CASC7 in NSCLC
tissues was also observed by ISH assay (Fig. 1B). The correlations between the
clinicopathological characteristics and CASC7 expression in 80
NSCLC patients are summarized in Table
I. CASC7 expression was found to be negatively associated with
distant metastasis and lymph node involvement. Next, Kaplan-Meier
analysis demonstrated that patients with lower levels of CASC7
expression had poorer overall survival (OS) rates compared with
those with higher levels of CASC7 expression (Fig. 1C). These data indicated that low
CASC7 expression may play an important role in NSCLC
progression.
Overexpression of lncRNA CASC7 suppresses
NSCLC cell proliferation in vitro
To further examine the biological role of CASC7 in
NSCLC, the expression of CASC7 was first assessed in NSCLC cell
lines. It was observed that the level of CASC7 was markedly
decreased in NSCLC cell lines compared with that in normal human
bronchial epithelial cells (16HBE) (Fig. 1D). As the expression of CASC7 was
relatively lower in A549 (a widely used lung adenocarcinoma cell
line) and H358 (a commonly used lung squamous cell carcinoma cell
line) cells, CASC7 was overexpressed using pcDNA CASC7 or knocked
down using si-CASC7 in these two cell lines. The pcDNA-CASC7
plasmids were added to both A549 and H358 cells, and it was
observed that CASC7 was effectively upregulated compared with the
pcDNA vector group (Fig. 1E). In
addition, CASC7 expression was successfully reduced by si-CASC7 in
A549 and H358 cells (Fig. 1F). The
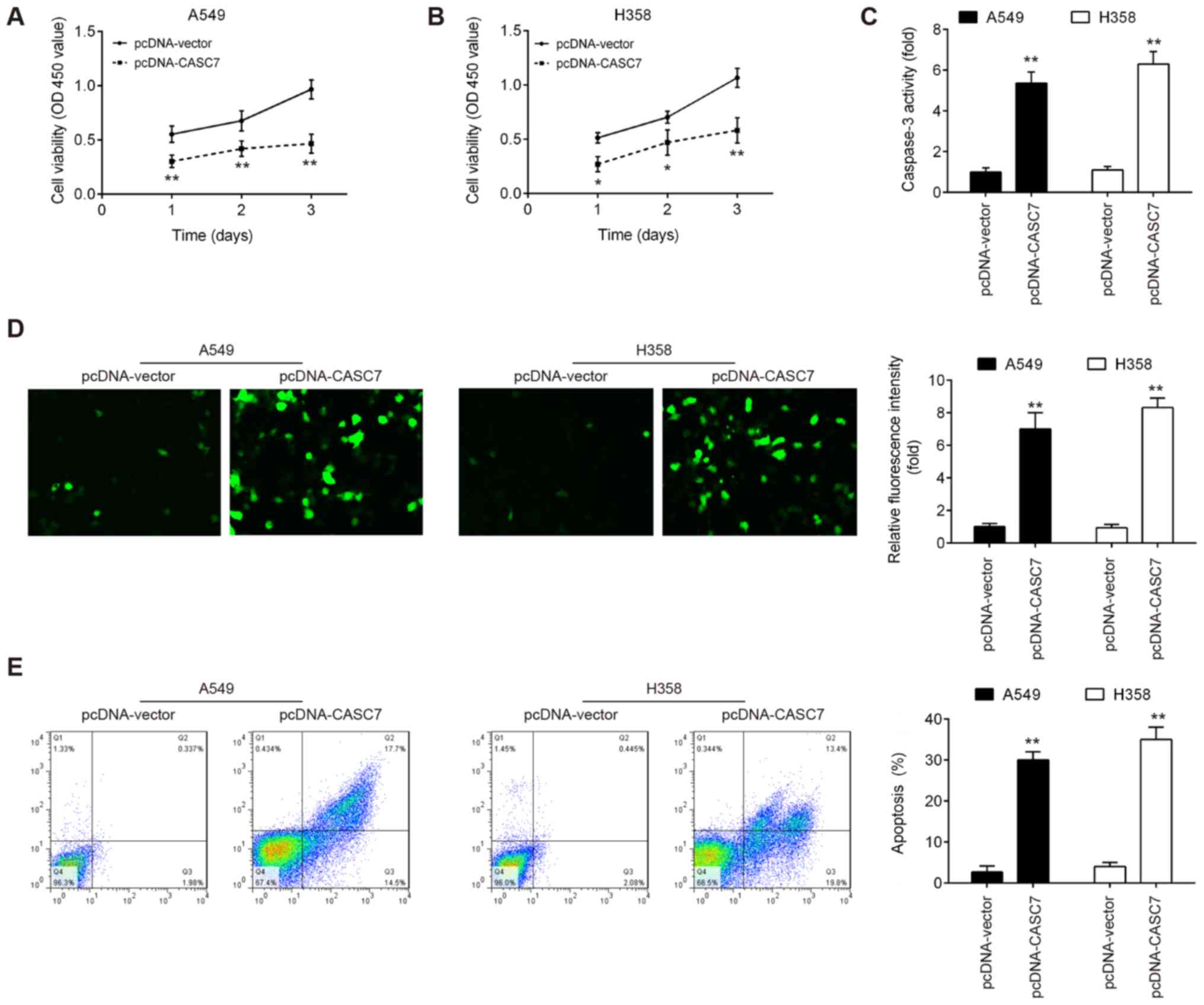
MTT assay revealed that overexpression of CASC7 signifi-cantly
suppressed the proliferation of both A549 and H358 cells (Fig. 2A and B). Moreover, the activity and
expression of caspase-3 in both NSCLC cell lines were markedly
enhanced by lncRNA CASC7 overexpression (Fig. 2C and D). The effect of lncRNA CASC7
on cell apoptosis was also examined, and the results demonstrated
that CASC7 overexpression promoted the apoptosis of A549 and H358
cells compared with the control group (Fig. 2E). Taken together, these results
suggest that upregulation of CASC7 may exert suppressive effects on
cell proliferation and promote apoptosis in NSCLC cells.
Overexpression of lncRNA CASC7 suppresses
NSCLC cell invasion and migration in vitro
The effect of CASC7 on NSCLC cell invasion and
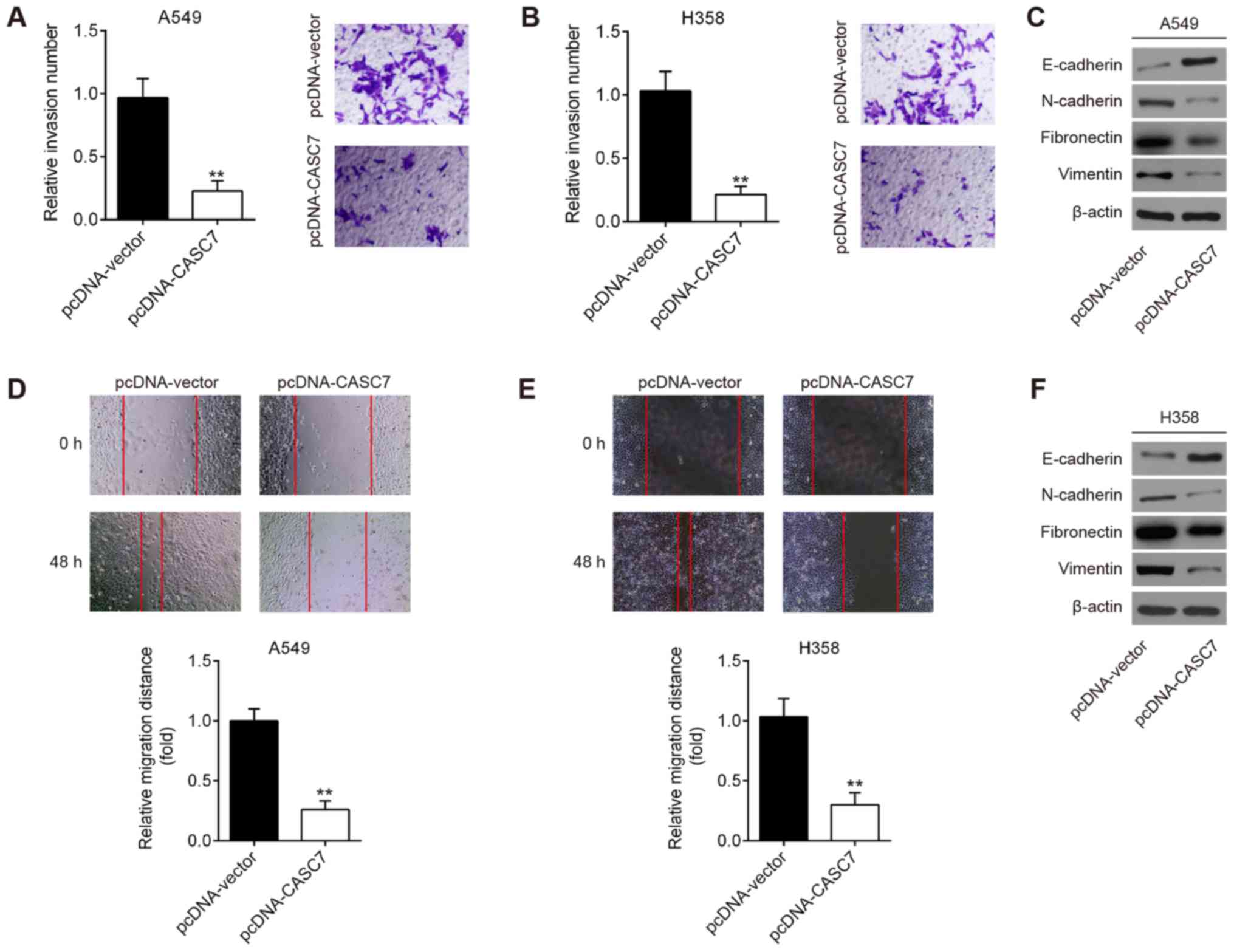
migration was next assessed. Transwell and wound healing assays
demonstrated that CASC7 overexpression suppressed the invasive and
migratory capacities of A549 cells (Fig. 3A and D). Since
epithelial-to-mesenchymal transition (EMT) is known to be a key
pro-metastatic event, the expression of EMT markers was detected by
western blotting. As shown in Fig.
3C, overexpression of CASC7 increased the expression of
E-cadherin, whereas it decreased the expression of N-cadherin,
fibronectin and vimentin, suggesting that CASC7 overexpression
inhibits EMT in NSCLC cells. Similar results were observed in H358
cells (Fig. 3B, E and F). These
data demonstrated that CASC7 overexpression exerted a significant
suppressive effect on the invasion and migration of NSCLC cells
in vitro.
LncRNA CASC7 acts as a ceRNA for miR-92a
in NSCLC cells
It is well-known that lncRNAs are likely to function
as ceRNAs for special miRNAs, thus reversing the effects of miRNAs
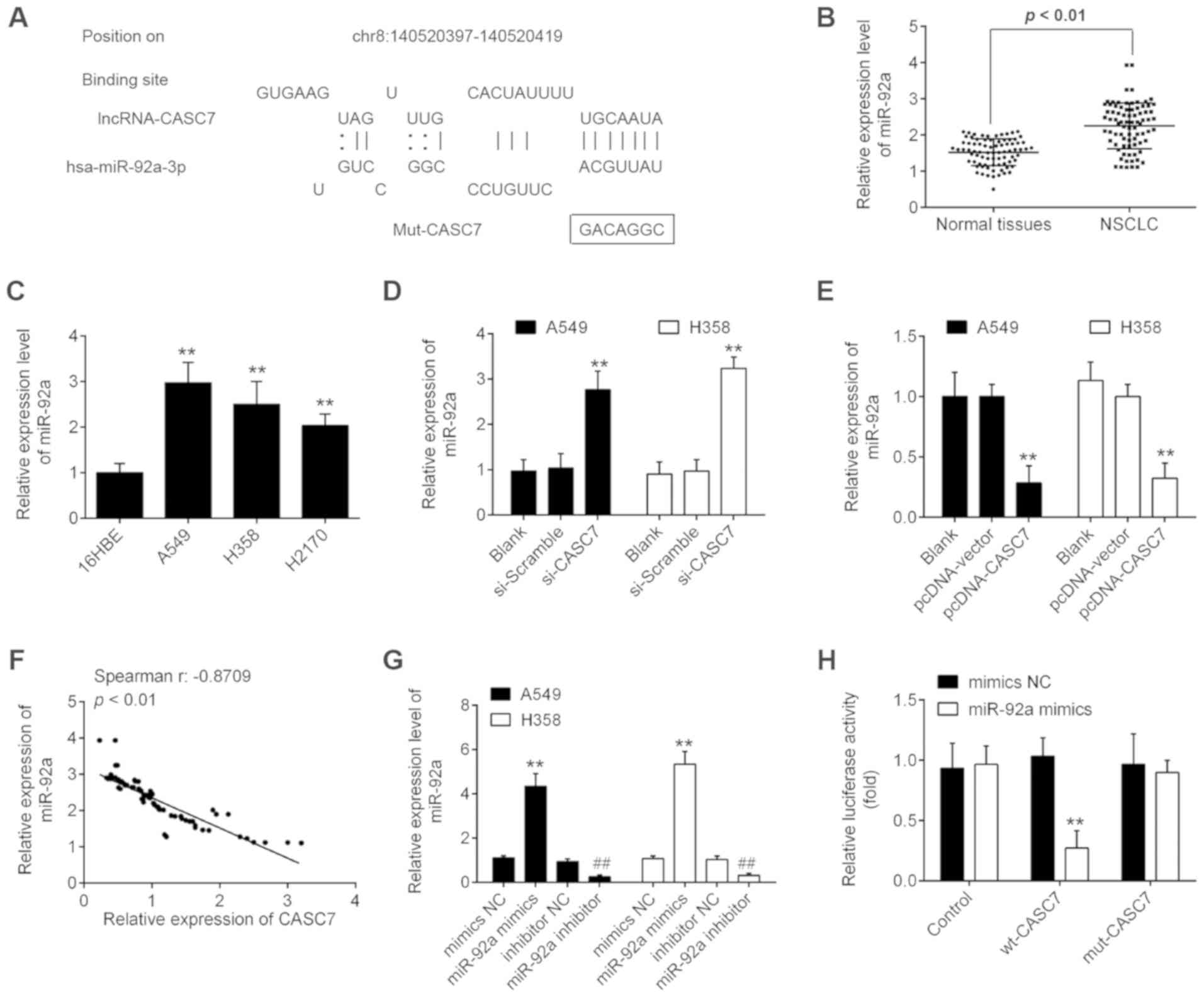
on the target genes (23,24). In the present study, starbase v2.0
(http://starbase.sysu.edu.cn/) was used
to predict the potential targets of CASC7. As shown in Fig. 4A, miR-92a had a putative binding
site with CASC7. miR-92a has been previously reported to be among
the cancer-associated miRNAs (25-27).
Additionally, our previous study demonstrated that miR-92a acts as
an oncogene in the progression of NSCLC (28). Therefore, miR-92a was selected for
further investigation. The expression levels of miR-92a were
significantly upregulated in tumor tissues and NSCLC cell lines
compared with those in adjacent normal tissues and 16HBE cells
(Fig. 4B and C). Moreover,
knockdown of CASC7 by si-CASC7 significantly increased miR-92a
expression, while NSCLC cells transfected with pcDNA-CASC7
exhibited a marked inhibition of miR-92a expression (Fig. 4D and E). In addition, further
correlation analysis revealed that the expression of CASC7 was
inversely correlated with the expression of miR-92a in NSCLC
tissues (Fig. 4F). In addition,
the expression of miR-92a was detected by RT-qPCR 48 h after
transfection of miR-92a mimics, miR-92a inhibitor, and their
respective NCs. As shown in Fig.
4G, the expression of miR-92a was signifi-cantly increased
following transfection of miR-92a mimics, whereas it was markedly
decreased following transfection of miR-92a inhibitor, compared
with their respective NCs.
Next, luciferase reporter assay was employed to
validate the binding of miR-92a to lncRNA CASC7. As shown in
Fig. 4H, overexpression of miR-92a
reduced the luciferase activity of wt-CASC7, but did not affect the
luciferase activity of mut-CASC7 in 293T cells. These data
indicated that CASC7 interacts with miR-92a in NSCLC cells.
LncRNA CASC7 increases the expression of
PTEN by acting as a ceRNA of miR-92a
PTEN is a well-known tumor suppressor, and plays an
important role in cancer initiation and progression (29). Notably, several studies have
demonstrated that miR-92a targets PTEN and suppresses its
translation in different types of cancer cells (30-32).
Given the association between miR-92a and CASC7, it was further
investigated whether CASC7 acts a tumor suppressor through
restoring the expression of PTEN via sequestration of miR-92a.
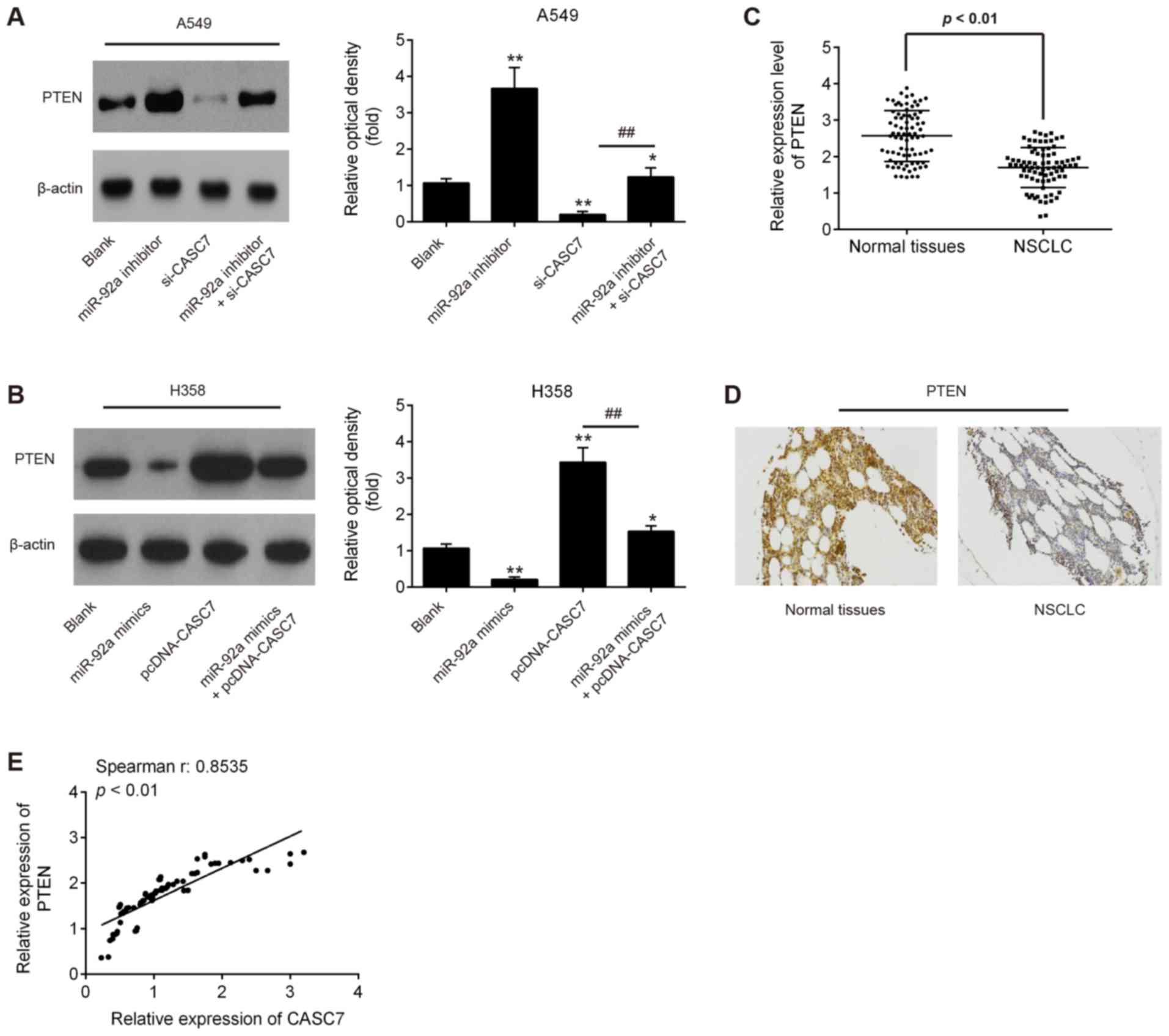
Western blot assay demonstrated that the CASC7 knockdown by
si-CASC7 led to a significant decrease of PTEN expression in A549
cells, whereas miR-92a knockdown reversed the inhibitory effect of
CASC7 on the expression of PTEN (Fig.
5A). It was also observed that CASC7 overexpression induced a
marked increase of PTEN expression in H358 cells, while miR-92a
overexpression attenuated the promoting effect of CASC7 on PTEN
protein levels (Fig. 5B).
Furthermore, the levels of PTEN in NSCLC tissues were detected by
RT-qPCR. As shown in Fig. 5C, the
expression of PTEN was significantly decreased in NSCLC compared
with that in adjacent tissues. In addition, using
immunohistochemistry, a significantly lower expression of PTEN was
observed in NSCLC tissues compared with that in adjacent tissues
(Fig. 5D). Moreover, a positive
correlation was detected between CASC7 and PTEN expression in NSCLC
tissues (Fig. 5E). These data
indicate that CASC7 may regulate the expression of PTEN by acting
as a ceRNA of miR-92a.
LncRNA CASC7 regulates miR-92a to
suppress NSCLC cell growth and invasion
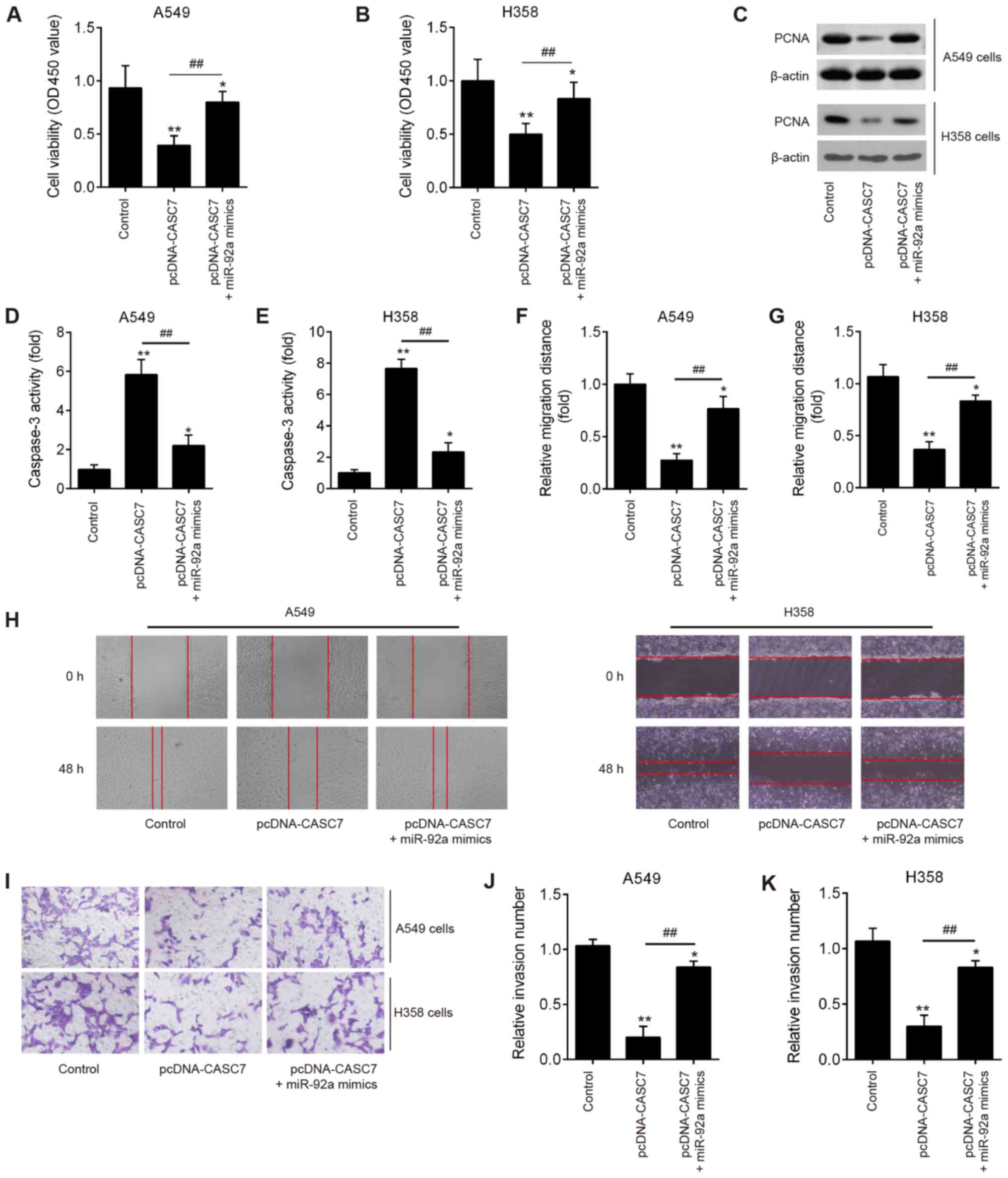
To further investigate whether miR-92a is involved
in lncRNA CASC7-mediated inhibition of proliferation and invasion
in NSCLC cells, pcDNA-CASC7 and miR-92a mimics were co-transfected
into A549 and H358 cells. Compared with the control group, CASC7
overexpression significantly suppressed cell proliferation, whereas
miR-92a overexpression partially reversed this inhibitory effect of
CASC7 (Fig. 6A and B). In
addition, the expression of a well-known marker of cell
proliferation, PCNA, was significantly decreased in the pcDNA-CASC7
group compared with the control group in A549 and H358 cells, while
this inhibitory effect was also reversed by overexpression of
miR-92a (Fig. 6C). The activity of
caspase-3 was also measured and found to be obviously increased by
CASC7 overexpression compared with the control group, whereas
upregulation of miR-92a attenuated this promoting effect (Fig. 6D and E). Wound healing and cell
invasion experiments revealed that the decreased migration distance
and reduced number of invading cells induced by CASC7
overexpression was partially abrogated by overexpression of miR-92a
(Fig. 6F-K). These data indicated
that CASC7 suppresses NSCLC cell growth and invasion by regulating
miR-92a expression.
Discussion
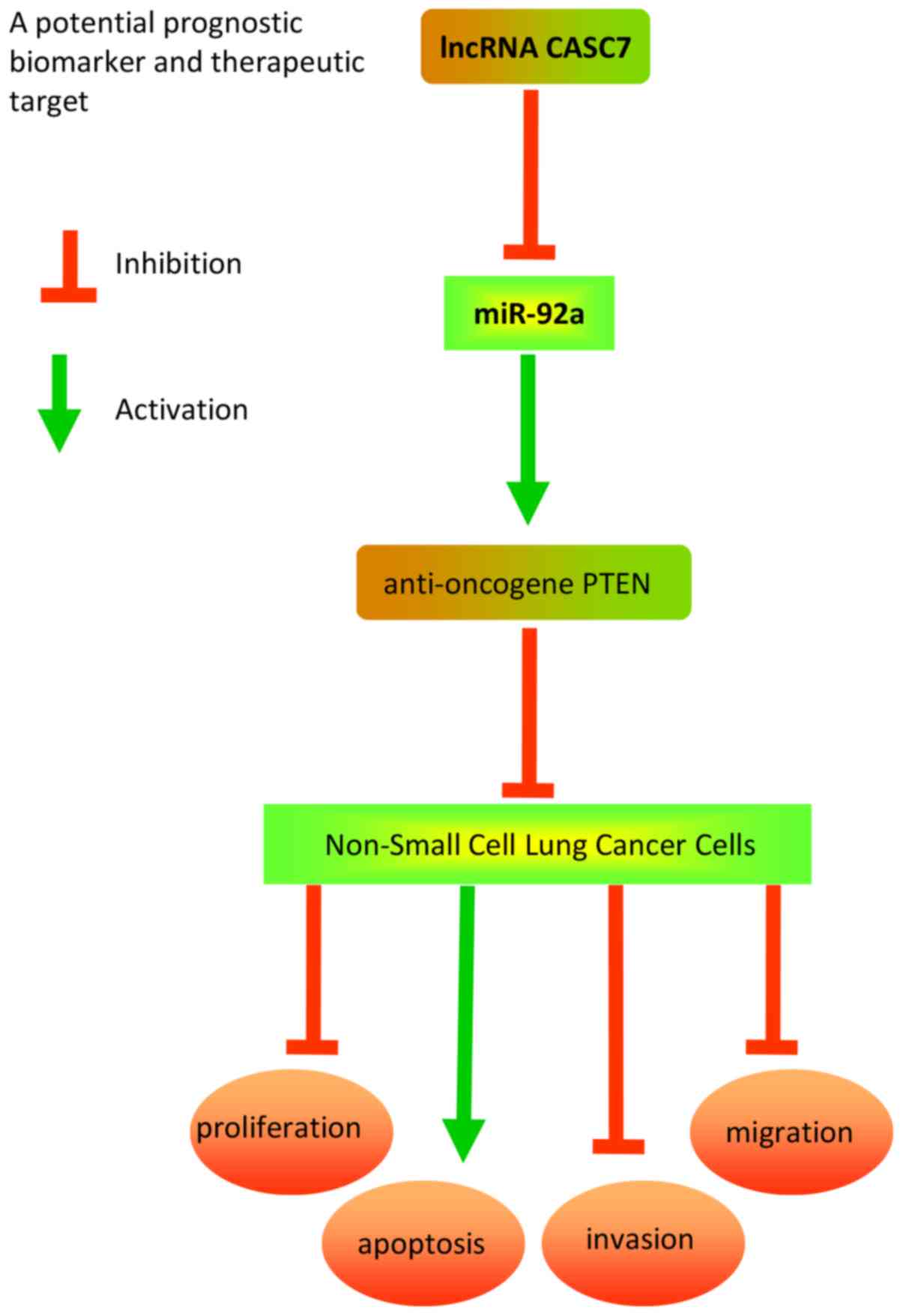
The present study demonstrated that CASC7 was
down-regulated in NSCLC tissues and cell lines, and its low
expression was closely correlated with poor prognosis and shorter
survival in NSCLC patients. Furthermore, CASC7 overexpression
sponged miR-92a to suppress cancer cell proliferation, invasion and
migration via upregulating the expression of the anti-oncogene
PTEN. Collectively, the results of the present study may provide
new insight into the role of the CASC7/miR-92a/PTEN axis in NSCLC,
which may prove to be a promising molecular target for NSCLC
treatment. A schematic presentation summarizing the mechanism of
action of CASC7 in NSCLC is presented in Fig. 7.
As a newly identified lncRNA, there is very little
research on the role of CASC7 in cancer. In glioma, CASC7 was found
to be downregulated and exerted its tumor-suppressive effects via
inactivation of the Wnt/β-catenin pathway (15). In CRC, CASC7 overexpression
inhibited proliferation, migration and invasion, and promoted
apoptosis in CRC cells, suggesting that CASC7 may be considered as
a novel diagnostic marker of CRC (16). However, to the best of our
knowledge, no data on the expression and role of CASC7 in NSCLC
have been reported to date. In the present study, CASC7 was found
to be significantly decreased in NSCLC tissues and cell lines, and
its low expression was correlated with the clinicopathological
characteristics of NSCLC patients, particularly distant metastasis
and lymph node involvement. Furthermore, the high expression of
CASC7 in the NSCLC cell lines A549 and H358 was found to suppress
cancer cell proliferation, invasion and migration, and promote
apoptosis. Collectively, these data indicate that CASC7 plays a key
role in suppressing NSCLC development and progression; however, the
underlying mechanism remains elusive.
Recently, ceRNA regulation has been implicated in
lung carcinogenesis. For example, Dong et al demonstrated
that lncRNA GAS5 acted as a tumor suppressor via the miR-205/PTEN
axis in NSCLC (33). Jin et
al reported that the lncRNA SNHG20 suppressed NSCLC growth in
in vivo through suppressing miR-154 and enhancing ZEB2 and
RUNX2 expression (34). Zhang
et al demonstrated that the lncRNA FENDRR was downregulated
in NSCLC tissues, whereas FENDRR overexpression inhibited the
malignant phenotypes of NSCLC cell by competitively binding to
miR-761 (35). Of note, it has
been reported that CASC7 may act as a ceRNA to sponge miR-21 and
regulate the expression of ING3 in CRC (16). Therefore, it was inferred that
lncRNA CASC7 may act as a ceRNA, participating in NSCLC
development. The present study, using bioinformatics analysis,
identified miR-92a as a potential CASC7-binding miRNA, which had
been previously demonstrated to be an oncogene in several cancers,
such as HCC (36), CRC (37) and gastric cancer (38). Interestingly, our previous study
had demonstrated that the expression of miR-92a was markedly
upregulated in NSCLC, and overexpression of miR-92a promoted tumor
progression through activating the PTEN/PI3K/AKT signaling pathway
(28). As expected, the results of
the present study demonstrated that CASC7 directly targeted miR-92a
and negatively regulated the expression of miR-92a in vitro.
Furthermore, the expression of CASC7 was found to be inversely
correlated with the expression of miR-92a in NSCLC tissues.
Importantly, CASC7 inhibited cell proliferation, invasion and
migration through suppressing miR-92a expression in NSCLC cells.
Collectively, these data suggest that the CASC7/miR-92a axis plays
a key role in NSCLC growth and development. Of note, the
overexpression of miR-92a could only partially reverse the
promoting effects of CASC7 on PTEN expression, as well as its
inhibitory effects on the proliferation, invasion and migration of
NSCLC cells. All these results suggest that the tumor-suppressive
effects of CASC7 may also be mediated by other targets, and future
research should focus on the identification of other targets of
CASC7.
It is well-known that the PTEN gene plays a
tumor-suppressive role in several human cancers, including NSCLC
(39,40). For example, Perumal et al
demonstrated that PTEN inactivation induced migration and invasion
by regulation of β-catenin and Snail/Slug in NSCLC cells (41). Liu et al reported that PTEN
promoted G0/G1 arrest and apoptosis in NSCLC cells by regulating
the expression levels of Skp2 (42). It has also been reported that
miR-92a exerts its oncogenic effects by directly targeting PTEN in
several types of cancer, including NSCLC (28,30,43,44).
Given that both lncRNA CASC7 and PTEN interact with miR-92a, lncRNA
CASC7 may regulate PTEN expression by competitively binding to
miR-92a. In the present study, it was observed that CASC7
upregulated the expression of PTEN in A549 and H358 cells by
inhibiting the expression of miR-92. Furthermore, PTEN expression
was found to be markedly downregulated, and was positively
correlated with CASC7 expression levels in NSCLC tissues. Taken
together, these data strongly suggest that there is a ceRNA
functional association among CASC7, PTEN and miR-92a in NSCLC.
To the best of our knowledge, the present study is
the first to demonstrate that the expression of CASC7 is low in
NSCLC tissues and cell lines, and that this low expression is
closely associated with distant metastasis and lymph node
involvement. There is currently no treatment regulating the
expression of CASC7 in NSCLC. Thus, in the future, more clinical
samples must be collected in order to determine whether
chemotherapeutic drugs affect the expression levels of CASC7 in
NSCLC.
In conclusion, we herein demonstrated that CASC7
acts as a tumor suppressor by upregulating PTEN by acting as a
ceRNA of miR-92a in NSCLC cells. These findings suggest that CASC7
may be a promising target for the treatment of NSCLC.
Funding
The present study was supported by the National
Natural Science Foundation of China Grants (grant no.
81802288).
Availability of data and materials
All the datasets generated and/or analyzed during
the present study are included in this published article.
Authors' contributions
LC, XL, YZ and JZ performed the experiments,
contributed to data analysis and wrote the manuscript. LC, XL, YZ
and JZ analyzed the data. CL and LY conceptualized the study
design, contributed to data analysis and experimental materials.
All authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
All individuals provided informed consent regarding
the use of their specimens for the purposes of clinical research.
The present study was approved by the Ethics Committee of Changhai
Hospital (approval no. 2016-00113).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Chen G and Yi XH: Pathology and genetics
of disease and tumours of the lung, pleura in China. Zhonghua Bing
Li Xue Za Zhi. 34:490–493. 2005.In Chinese. PubMed/NCBI
|
|
3
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mostafa AA and Morris DG: Immunotherapy
for lung cancer: Has it finally arrived? Front Oncol. 4:2882014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang R, Ma Z, Feng L, Yang Y, Tan C, Shi
Q, Lian M, He S, Ma H and Fang J: LncRNA MIR31HG targets HIF1A and
P21 to facilitate head and neck cancer cell proliferation and
tumori-genesis by promoting cell-cycle progression. Mol Cancer.
17:1622018. View Article : Google Scholar
|
|
7
|
Zeng C, Liu S, Lu S, Yu X, Lai J, Wu Y,
Chen S, Wang L, Yu Z, Luo G and Li Y: The c-Myc-regulated lncRNA
NEAT1 and paraspeckles modulate imatinib-induced apoptosis in CML
cells. Mol Cancer. 17:1302018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang G, Sun J, Zhao H and Li H: Long
non-coding RNA (lncRNA) growth arrest specific 5 (GAS5) suppresses
esophageal squamous cell carcinoma cell proliferation and migration
by inactivating phosphatidylinositol 3-kinase (PI3K)/AKT/Mammalian
target of rapamycin (mTOR) signaling pathway. Med Sci Monit.
24:7689–7696. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan YF, Yu ZP and Cui XY: lncRNA
colorectal neoplasia differentially expressed (CRNDE) promotes
proliferation and inhibits apoptosis in non-small cell lung cancer
cells by regulating the miR-641/CDK6 axis. Med Sci Monit.
25:2745–2755. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song Z, Du J, Zhou L and Sun B: lncRNA
AWPPH promotes proliferation and inhibits apoptosis of non-small
cell lung cancer cells by activating the Wnt/β-catenin signaling
pathway. Mol Med Rep. 19:4425–4432. 2019.PubMed/NCBI
|
|
11
|
Zhang Y, Li Y, Han L, Zhang P and Sun S:
SUMO1P3 is associated clinical progression and facilitates cell
migration and invasion through regulating miR-136 in non-small cell
lung cancer. Biomed Pharmacother. 113:1086862019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Heesch S, van Iterson M, Jacobi J,
Boymans S, Essers PB, de Bruijn E, Hao W, MacInnes AW, Cuppen E and
Simonis M: Extensive localization of long noncoding RNAs to the
cytosol and mono- and polyribosomal complexes. Genome Biol.
15:R62014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu JH, Li C, Zhang CH and Zhang ZH:
LncRNA-CASC7 enhances corticosteroid sensitivity via inhibiting the
PI3K/AKT signaling pathway by targeting miR-21 in severe asthma.
Pulmonology. 26:18–26. 2020. View Article : Google Scholar
|
|
14
|
Liu Y, Pan L, Jiang A and Yin M: Hydrogen
sulfide upregulated lncRNA CasC7 to reduce neuronal cell apoptosis
in spinal cord ischemia-reperfusion injury rat. Biomed
Pharmacother. 98:856–862. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong X, Liao X and Huang M: LncRNA CASC7
inhibits the progression of glioma via regulating Wnt/β-catenin
signaling pathway. Pathol Res Pract. 215:564–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Fu C, Xu Q and Wei X: Long
non-coding RNA CASC7 inhibits the proliferation and migration of
colon cancer cells via inhibiting microRNA-21. Biomed Pharmacother.
95:1644–1653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji D, Wang Y, Sun B, Yang J and Luo X:
Long non-coding RNAMNX1-AS1 promotes hepatocellular carcinoma
proliferation and invasion through targeting miR-218-5p/COMMD8
axis. Biochem Biophys Res Commun. 513:669–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou K, Zhang C, Yao H, Zhang X, Zhou Y,
Che Y and Huang Y: Knockdown of long non-coding RNA NEAT1 inhibits
glioma cell migration and invasion via modulation of SOX2 targeted
by miR-132. Mol Cancer. 17:1052018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Guo X, Li Q, Ran P, Xiang X, Yuan Y,
Dong T, Zhu B, Wang L, Li F, et al: Long non-coding RNA1308
promotes cell invasion by regulating the miR-124/ADAM 15 axis in
non-small-cell lung cancer cells. Cancer Manag Res. 10:6599–6609.
2018. View Article : Google Scholar :
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
E C, Li C, Li H and Yang J: Silencing of a
novel lncRNA LOC105369748 suppresses the progression of
hepatocel-lular carcinoma by sponging miR-5095 from MBD2. J Cell
Physiol. 234:18504–18512. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang F, Qi W, Wang Y, Wang W and Fan L:
lncRNA PEG10 promotes cell survival, invasion and migration by
sponging miR-134 in human bladder cancer. Biomed Pharmacother.
114:1088142019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi H, Wen B, Wu Q, Cheng W, Lou J, Wei J,
Huang J, Yao X and Weng G: Long noncoding RNA SNHG7 accelerates
prostate cancer proliferation and cycle progression through cyclin
D1 by sponging miR-503. Biomed Pharmacother. 102:326–332. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu H, Song H, Liu L, Hu S, Liao Y, Li G,
Xiao X, Chen X and He S: MiR-92a modulates proliferation,
apoptosis, migration and invasion of osteosarcoma cell lines by
targeting Dickkopf-related protein 3. Biosci Rep.
39:BSR201904102019. View Article : Google Scholar
|
|
26
|
Sun L, Jin X, Xie L, Xu G, Cui Y and Chen
Z: Swainsonine represses glioma cell proliferation, migration and
invasion by reduction of miR-92a expression. BMC Cancer.
19:2472019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun B, Zhang J, Liu M and Guan L: Alkannin
inhibits proliferation, migration and invasion of hepatocellular
carcinoma cells via regulation of miR-92a. Biomed Pharmacother.
114:1087822019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu C, Shan Z, Hong J and Yang L:
MicroRNA-92a promotes epithelial-mesenchymal transition through
activation of PTEN/PI3K/AKT signaling pathway in non-small cell
lung cancer metastasis. Int J Oncol. 51:235–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang L, Wang X, Sun Q, Papakonstantinou E,
S'ng C, Tamm M, Stolz D and Roth M: IgE Downregulates PTEN through
MicroRNA-21-5p and stimulates airway smooth muscle cell remodeling.
Int J Mol Sci. 20:E8752019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Cao H, Xu D and Zhu K:
MicroRNA-92a promotes metastasis of nasopharyngeal carcinoma by
targeting the PTEN/AKT pathway. Onco Targets Ther. 9:3579–3588.
2016.PubMed/NCBI
|
|
31
|
Ke TW, Wei PL, Yeh KT, Chen WT and Cheng
YW: MiR-92a promotes cell metastasis of colorectal cancer through
PTEN-Mediated PI3K/AKT pathway. Ann Surg Oncol. 22:2649–2655. 2015.
View Article : Google Scholar
|
|
32
|
Zhang G, Zhou H, Xiao H, Liu Z, Tian H and
Zhou T: MicroRNA-92a functions as an oncogene in colorectal cancer
by targeting PTEN. Dig Dis Sci. 59:98–107. 2014. View Article : Google Scholar
|
|
33
|
Dong L, Li G, Li Y and Zhu Z: Upregulation
of long noncoding RNA GAS5 inhibits lung cancer cell proliferation
and metastasis via miR-205/PTEN axis. Med Sci Monit. 25:2311–2319.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin L, Jiang X, He G, Shi J, Su F and Zhu
H: SNHG20 knockdown suppresses proliferation, migration and
invasion, and promotes apoptosis in non-small cell lung cancer
through acting as a miR-154 sponge. Biomed Pharmacother.
112:1086482019. View Article : Google Scholar
|
|
35
|
Zhang MY, Zhang ZL, Cui HX, Wang RK and Fu
L: Long non-coding RNA FENDRR inhibits NSCLC cell growth and
aggressiveness by sponging miR-761. Eur Rev Med Pharmacol Sci.
22:8324–8332. 2018.PubMed/NCBI
|
|
36
|
Shigoka M, Tsuchida A, Matsudo T, Nagakawa
Y, Saito H, Suzuki Y, Aoki T, Murakami Y, Toyoda H, Kumada T, et
al: Deregulation of miR-92a expression is implicated in
hepatocellular carcinoma development. Pathol Int. 60:351–357. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu GH, Zhou ZG, Chen R, Wang MJ, Zhou B,
Li Y and Sun XF: Serum miR-21 and miR-92a as biomarkers in the
diagnosis and prognosis of colorectal cancer. Tumour Biol.
34:2175–2181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu Q, Yang Z, Wang F, Hu S, Yang L, Shi Y
and Fan D: MiR-19b/20a/92a regulates the self-renewal and
proliferation of gastric cancer stem cells. J Cell Sci.
126:P4220–P4229. 2013. View Article : Google Scholar
|
|
39
|
Zhao YB, Zhao J, Zhang LJ, Shan RG, Sun
ZZ, Wang K, Chen JQ and Mu JX: MicroRNA-370 protects against
myocardial isch-emia/reperfusion injury in mice following
sevoflurane anesthetic preconditioning through PLIN5-dependent PPAR
signaling pathway. Biomed Pharmacother. 113:1086972019. View Article : Google Scholar
|
|
40
|
Ding K, Wu Z, Wang N, Wang X, Wang Y, Qian
P, Meng G and Tan S: MiR-26a performs converse roles in
proliferation and metastasis of different gastric cancer cells via
regulating of PTEN expression. Pathol Res Pract. 213:467–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perumal E, So Youn K, Sun S, Seung-Hyun J,
Suji M, Jieying L and Yeun-Jun C: PTEN inactivation induces
epithelial-mesenchymal transition and metastasis by intranuclear
translocation of β-catenin and snail/slug in non-small cell lung
carcinoma cells. Lung Cancer. 130:25–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu L, Huang L, He J, Cai S, Weng Y, Huang
S and Ma S: PTEN inhibits non-small cell lung cancer cell growth by
promoting G0/G1 arrest and cell apoptosis.
Oncol Lett. 17:1333–1340. 2019.PubMed/NCBI
|
|
43
|
Qin LB, Li ZY, Li H, Fan XQ, Liu HG, Dong
XM and Jia WY: Inhibitive effects of microRNA-34a on protecting
against ischemia-reperfusion injury of vital organs in hemorrhagic
shock pregnant mice. Eur Rev Med Pharmacol Sci. 22:1812–1818.
2018.PubMed/NCBI
|
|
44
|
Xiao J, Yu W, Hu K, Li M, Chen J and Li Z:
MiR-92a promotes tumor growth of osteosarcoma by targeting PTEN/AKT
signaling pathway. Oncol Rep. 37:2513–2521. 2017. View Article : Google Scholar : PubMed/NCBI
|