Introduction
Breast cancer is the most common malignant tumor
affecting women worldwide (1).
According to the expression of estrogen receptor (ER), progesterone
receptor (PR), human epidermal growth factor receptor 2 (HER-2) and
Ki-67 in breast cancer cells, breast cancer is divided into Luminal
A, Luminal B, HER-2-overexpressing and triple-negative breast
cancer (TNBC) subtypes (2). TNBC,
which is ER-, PR- and HER-2-negative, accounts for 15-20% of breast
cancer cases. TNBC is characterized by a low differentiation,
strong invasiveness, an increased likelihood of recurrence and
metastasis, and a poor prognosis (3,4). Due
to the lack of hormone receptor and HER‑2 expression, patients with
TNBC cannot benefit from endocrine therapy or other available
targeted agents. Therefore, the understanding of the underlying
molecular mechanisms of TNBC is crucial in order to be able to
identify novel therapeutic targets.
Platinum-based drugs are used extensively in the
treatment of malignant tumors. Carboplatin can reduce the
expression of FBP1 in ovarian cancer cells, and the silencing FBP1
can enhance the sensitivity of ovarian cancer cells to carboplatin
(5). Furthermore, a number of
clinical trials have demonstrated that platinum-based drugs can
significantly improve the pathological complete remission rate of
neoadjuvant chemotherapy in patients with TNBC (6-8),
particularly for patients with the BRCA1/2 mutation (9). Cisplatin is a commonly used
chemotherapeutic drug in patients with TNBC. Studies have reported
that cisplatin interacts with DNA to form intra-chain cross-linking
and inter-strand cross-linking, and exerts anti-tumor effects by
activating multiple DNA repair pathways and enhancing the DNA
damage repair processes (10,11).
However, the specific mechanisms underlying the effects of
cisplatin on TNBC and FBP1 expression in TNBC remain unknown.
The human far upstream element (FUSE) binding
protein 1 (FBP1) is a multifunctional DNA- and RNA-binding protein
involved in diverse cellular processes, which regulates
transcription, splicing and translation (12). FBP1 promotes cell proliferation,
enhances cell migration and inhibits apoptosis by modulating
complex networks (13). FBP1 is
overexpressed in a variety of malignant tumors, such as
hepatocellular carcinoma, ovarian cancer, nasopharyngeal carcinoma
and breast cancer (5,14-16).
The overexpression of FBP1 has been shown to be associated with a
lower overall survival rate in ovarian cancer and nasopharyngeal
carcinoma (5,16). Therefore, FBP1 is considered a
proto-oncogene. FBP1 was originally identified as a factor that
binds the FUSE motif in the promoter of the oncogene c-Myc
(13). Moreover, c-Myc, the
deubiquitinating enzyme ubiquitin specific peptidase 29 and the
cell cycle inhibitor p21, are regulated by FBP1 (17).
The present study hypothesized that FBP1 plays an
important role in promoting breast cancer development, and
therefore a lack of FBP1 may interfere with TNBC cells exiting the
cell cycle and migration. It was identified that the silencing of
FBP1 enhanced the sensitivity of TNBC cells to cisplatin.
Additionally, cisplatin treatment inhibited TNBC cell viability and
promoted cell apoptosis by inhibiting the expression of FBP1.
Therefore, FBP1 may be a potential novel biological target for the
treatment of TNBC.
Materials and methods
Clinical sample collection
Informed consents for the use of their samples in
scientific research were obtained from all patients. The present
study was conducted after the protocol was approved by the Medical
Ethics Committee of Guangzhou Red Cross Hospital of Jinan
University (approval no. 2015-045-01). For immunohistochemical
analysis, a total of 54 breast tissue samples, including 27 breast
cancer tissues and the corresponding 27 para-carcinoma normal
breast tissues, were collected from the Department of Breast,
Guangzhou Red Cross Hospital from January, 2015 to December, 2018
with a median age of 60 (from 47 to 85 years). None of the patients
had received any pre-operative therapies, such as chemotherapy,
radiotherapy, endocrine therapy, targeted therapy or immunotherapy
prior to the study.
Antibodies and reagents
GAPDH (#5174), c-Myc (#13987), cleaved caspase-3
(#9661), cyclin A2 (#91500), cyclin B1(#12231), cdc2 (#77055),
p-cdc2 (#4539) and matrix metalloproteinase (MMP)2 (#13132)
antibodies were purchased from Cell Signaling Technology, Inc. FBP1
(#ab213525) antibody was obtained from Abcam. All antibodies were
used at the concentrations recommended by the supplier. Fetal
bovine serum (FBS) and Dulbecco's modified Eagle's medium (DMEM)
were purchased from Gibco; Thermo Fisher Scientific, Inc.
Penicillin and streptomycin sulfate were obtained from Hyclone
(Logan, UT, USA). Cisplatin was purchased from Sigma-Aldrich; Merck
KGaA. The CellTiter 96® AQueous One Solution
proliferation assay kit
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt; MTS) was provided by Promega Corporation.
Immunohistochemical staining
Paraffin tissue sections (4-µm-thick) were dewaxed
by xylene, hydrated in a gradient alcohol and an aqueous solution,
and then subjected to antigen retrieval using a citric acid buffer
(pH 6.0). The peroxidase was blocked with 3%
H2O2 for 10 min and 10% BSA (Boster
Biological Technology Co. Ltd.) for 30 min at room temperature.
Rabbit anti-FBP1 antibody (diluted 1:500) was then incubated with
the sections overnight at 4°C. After washing with
phosphate-buffered saline (PBS: KH2PO4, 2 mM;
Na2HPO4, 8 mM; NaCl, 136 mM and KCl, 2.6 mM,
pH 7.2-7.4), the appropriate amount of secondary antibody (1:2,000;
IHC Detection Reagent (HRP, rabbit) #8114, Cell Signaling
Technology, Inc.) was incubated with the sections for 1 h at room
temperature. DAB (#ZLI-9018, Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) was used to display color reaction at room
temperature for 5 min, and hematoxylin (#ZLI-9620, Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.) was used for
counterstaining at room temperature for 10 min, and the slices were
then dehydrated by immersing them in 75, 80, 90, 95% and absolute
alcohol for 3 min each and sealed. Image processing and analysis
were performed using ImagePro Plus 6.0 software (Media Cybernetics,
Inc.). The intensity of the immunohistochemistry was expressed as
the integrated optical density (IOD) of the DAB brown reaction
product. Each sample was measured 5 times separately and the
results are expressed as the means ± standard deviation (SD).
Cell culture and generation of stable
cells in which FBP1 was knocked down
The TNBC cells, MDA-MB-231 and normal breast cells,
MCF-10A were purchased from the Chinese Academy of Sciences Cell
Bank. The cells were cultured in DMEM containing 10% FBS, 100 U/ml
penicillin, 100 µg/ml streptomycin in 37°C and 5% CO2
incubator. The lentivirus containing the FBP1 silenced sequence (sc
43760) and the control lentivirus (sc 108080) were purchased from
the Santa Cruz Biotechnology, Inc., and 10 µg each of these were
transfected into the MDA-MB-231 cells according to the protocol
provided by the manufacturer. After screening with 10.0 µg/ml of
puromycin for approximately 2 weeks, the cells were collected and
the silencing of FBP1 was determined by western blot analysis;
these cells were then termed as 'FBP1 knockdown' (FBP1-KD) and FBP1
normal control (FBP1-C) MDA-MB-231 cells, respectively.
Western blot analysis
After the TNBC MDA-MB-231 cells were treated without
or with cisplatin (20, 40 and 80 µM) for 48 h, they were collected
and lysed in a modified RIPA buffer [150 mM NaCl, 1% NP-40, 50 mM
Tris-Cl (pH 8.0) and 0.1% SDS] supplemented with PMSF (1 mM)
protease and phosphatase inhibitor. The homogenate was incubated in
ice for 30 min and centrifuged at 12,000 x g for 15 min at 4°C. The
protein concentrations were determined by double-acetyl acid
protein analysis (Pierce; Thermo Fisher Scientific, Inc.), and the
protein samples were then dissolved by a 12% SDS-PAGE gel and
transferred to a polyvinylidene difluoride filter membranes (PVDF)
(Merck KGaA). Following transfer, the membranes were blocked with
5% skim milk in TBS-Tween (0.05 M Tris, 0.15 M NaCl, pH 7.5, 0.2%
Tween-20) for 1 h and then incubated at 4°C overnight with the
primary antibodies (FBP1, 1:1,000; c-MYC, 1:500; Cyclin A2,
1:1,000; Cyclin B1, 1:1,000; p-cdc2, 1:1,000; cdc2, 1:1,000; MMP-2,
1:1,000; C-Caspase-3, 1:1,000; and GAPDH, 1:1,000). The membranes
were then washed 3 times with TBST and then incubated with
anti-rabbit/mouse HRP-labeled secondary antibodies (goat
anti-rabbit IgG antibody, 1:5,000, #ARG65351; goat anti-mouse IgG
antibody, 1:5,000, #ARG65350; Taiwan Arigo Biolaboratories Corp.)
for 1 h at room temperature, and detected using the ECL-Plus
detection system (Pierce; Thermo Fisher Scientific, Inc.). Relative
abundance was quantified by densitometry using Quantity One 4.6.7
software (Bio-Rad Laboratories, Inc.).
Reverse transcription‑quantitative
polymerase chain reaction (RT‑qPCR)
Total RNA was extracted from the FBP1-KD and FBP1-C
MDA-MB-231 cells using TRIzol reagent (Takara Biotechnology Co.,
Ltd.) and cDNA synthesis was carried out by reverse transcription
using PrimeScriptTM RT Master Mix (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. PCR amplification of
FBP1, c-Myc and GAPDH was carried out using TB GreenTM Premix Ex
TaqTM (Takara Biotechnology Co., Ltd.). The primers used were as
follows: FBP1 forward, 5'-GGAACTCCAATGGA CCAATACAAC-3' and reverse,
5'-AGTGAGGTAATAAG CAGCCAAG-3'; c-Myc forward, 5'-CGGTGCAGCCGTAT
TTCTACT-3' and reverse, 5'-TTCCAGATATCCTCGCT GGG-3'; and GAPDH
forward, 5'-GAGGTGAAGGTCGGA GTC-3' and reverse,
5'-GAGAGAGAGATGATGGGATTC-3'. The amplification conditions were as
follows: initial denaturation at 95°C for 5 min, followed by 40
cycles of denaturation 95°C for 5 sec, annealing at 60°C for 20
sec. All reactions were performed on an Applied Biosystems 7300 PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Relative quantification was performed using the 2-ΔΔCq
method (18). Each PCR
amplification was performed in triplicate to verify the
results.
Cell viability assay
For the cell viability assay, approximately
1x103 FBP1-C and FBP1-KD cells were added to a 96-well
plate and were incubated without or with cisplatin (20, 40 and 80
µM) for 24, 48 and 72 h. Cell viability was determined by MTS assay
in accordance with the CellTiter 96 Aqueous One Solution Viability
assay manual. The absorbance was read at 490 nm with an automated
plate reader (ELX800, BioTek Instruments Inc.). The experiment was
repeated at least 3 times.
Colony formation assay
The FBP1-C and FBP1-KD MDA-MB-231 cells
(5x103 cells/plate) were plated in 60 mm plates and
cultured for 14 days without or with 20, 40 and 80 µM of cisplatin.
Colonies were washed with PBS, fixed with 10% formalin for 10 min
at room temperature and stained with 1% (w/v) crystal violet
(#C8470, Beijing Solarbio Science & Technology Co., Ltd.) for
10 min at room temperature. The colony formation images were
captured using a scanner (x1 magnification, UMAX).
Cell cycle and cell apoptosis
analysis
Flow cytometry was used to analyze cell cycle
distribution and apoptosis. FBP1-C and FBP1-KD cells were treated
without or with cisplatin (20, 40 and 80 µM) for 48 h. The cells
were collected and washed twice with cold PBS, and the cells were
then resuspended into a single cell suspension with PBS,
supplemented with 200 µl binding buffer. This was followed by the
addition of 10 µl of Annexin V-FITC and 10 µl of 50 µg/ml propidium
iodide (PI; BD Biosciences) in PBS containing 1% Triton X-100 at
room temperature for 15 min. The data were acquired using a BD
FACSCAN flow cytometer (FACSAria II, BD Biosciences) and analyzed
using BD ModFit LT version 3.3 (BD Biosciences).
Wound healing and Transwell invasion
assays
The FBP1-C and FBP1-KD cells were treated with
mitomycin for 4 h, then cross-sectioned with 100 µl tips in a
6-well plate, and cultured for 48 h. After fixing with 4%
paraformaldehyde solution at room temperature for 15 min,
microscopic examination was performed and the cell migration
distance was determined. Matrigel was preliminarily spread in a
12-well Transwell, and the cultured FBP1-C and FBP1-KD cells were
prepared in a 1x105 cell/ml suspension with 1% FBS
culture solution. A total of 200 µl cell suspension was added to
the upper chamber, and 600 µl of 10% FBS cell culture medium was
added to the lower chamber. After the cells in the Transwell were
cultured at 37°C for 48 h, they were stained with 0.1% crystal
violet, and the number of invaded cells was then counted using a
microscope (BX63, Olympus Corporation). Images were acquired using
an Olympus inverted microscope (Olympus Corporation) at a
magnification of x200.
Statistical analysis
Values are presented as the means ± SD. Data were
analyzed using the Student's t-test and two-way ANOVA (followed by
Tukey's post hoc test) using SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA). The correlation between FBP1 and c-Myc
expression was checked by Spearman's rank correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Overexpression of FBP1 and c‑Myc in
breast cancer tissues
To identify the association between FBP1 expression
and breast cancer development, the expression levels of FBP1 and
c-Myc, a target of FBP1, were measured in 4 pairs of breast cancer
tissues and their corresponding para-carcinoma normal tissues by
western blot analysis. The protein expression of FBP1 in breast
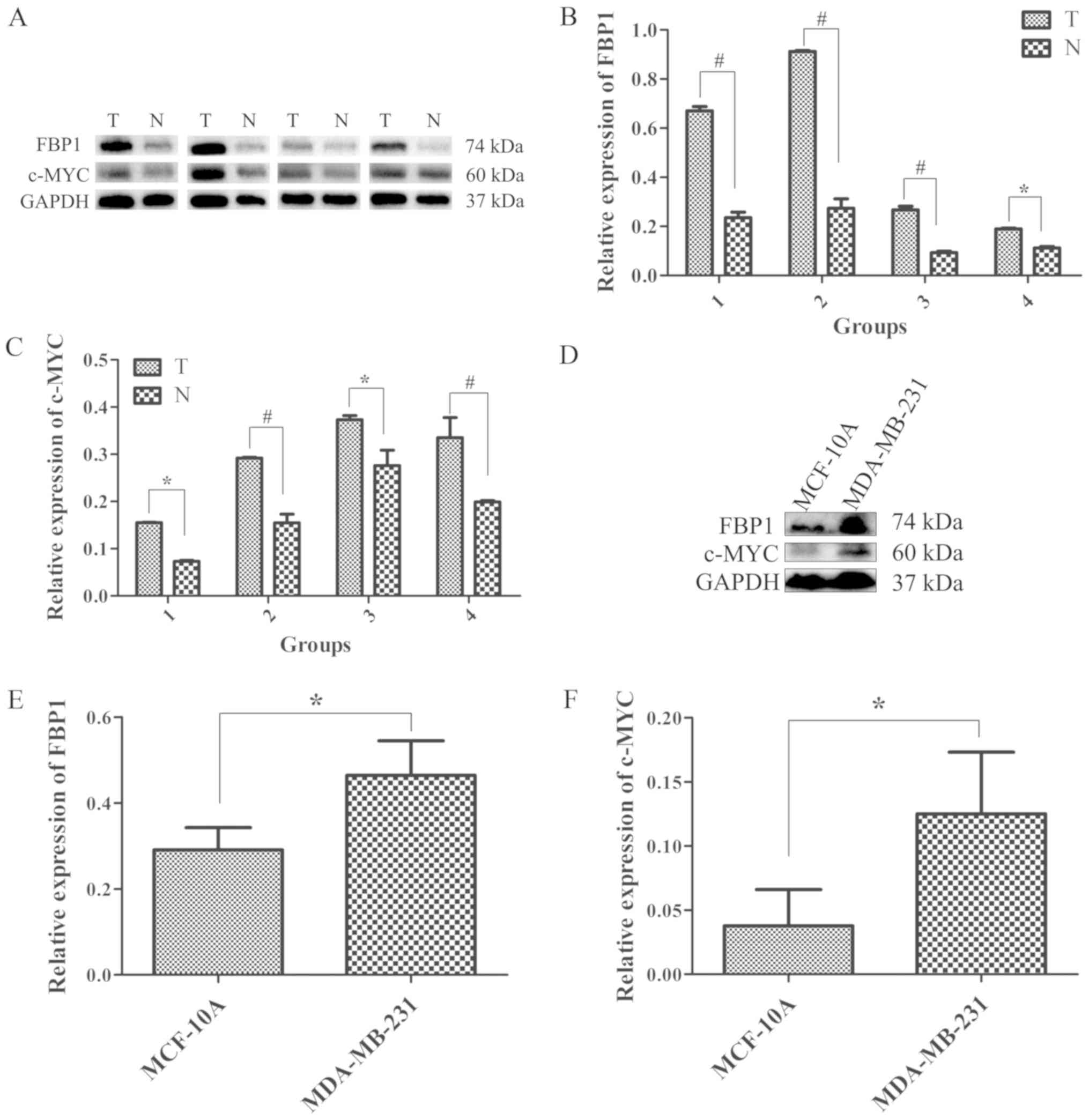
cancer tissues and normal breast tissues is shown in Fig. 1A. The relative protein expression
of FBP1 in cancer tissues was significantly higher compared with
that in normal tissues (Fig. 1B).
Since FBP1 binds to the FUSE DNA sequence upstream of the c-Myc
promoter and regulates its expression (19), the expression of c-Myc in these
breast cancer tissues was also examined. As was expected, the
protein expression of c-Myc was positively associated with FBP1
expression, and the expression of c-Myc in cancer tissues was
significantly higher compared with that in normal tissues (Fig. 1A-C).
It is critical to understand the underlying
molecular mechanisms of TNBC in order to be able to identify novel
therapeutic targets for its treatment. The present study compared
the expression of FBP1 and c-Myc in the normal breast cell line,
MCF-10A, and in the TNBC cancer cell line, MDA-MB-231. The protein
expression levels of FBP1 and c-Myc are illustrated in Fig. 1D. The protein expression of FBP1 in
the MDA-MB-231 cells was 1.66-fold higher than that in the MCF-10A
cells (Fig. 1D and E). The protein
expression of c-Myc in the MDA-MB-231 cells was 2.30-fold higher
than that of the MCF-10A cells (Fig.
1D and F).
To further investigate the expression of FBP1 and
c-Myc in a larger number of tissues, 54 samples from patients with
breast cancer were examined by immunohistochemical staining. As
shown in Fig. 1G, FBP1 was
predominantly localized in the nuclei of the breast cancer cells
(Fig. 1G). The IOD of FBP1 was
higher in breast cancer tissues compared with that in
para-carcinoma normal tissues (Fig.
1H). The results also demonstrated that c-Myc expression was
increased in accordance with the increased expression of FBP1. In
addition, the IOD of c-Myc was higher in breast cancer tissues
compared with breast para-carcinoma normal tissues (Fig. 1G and I). The correlation between
FBP1 and c-Myc expression was also investigated using Spearman's
rank correlation analysis, and c-Myc expression positively
correlated with FBP1 expression (Fig.
1J, r=0.6726, P=0.0001).
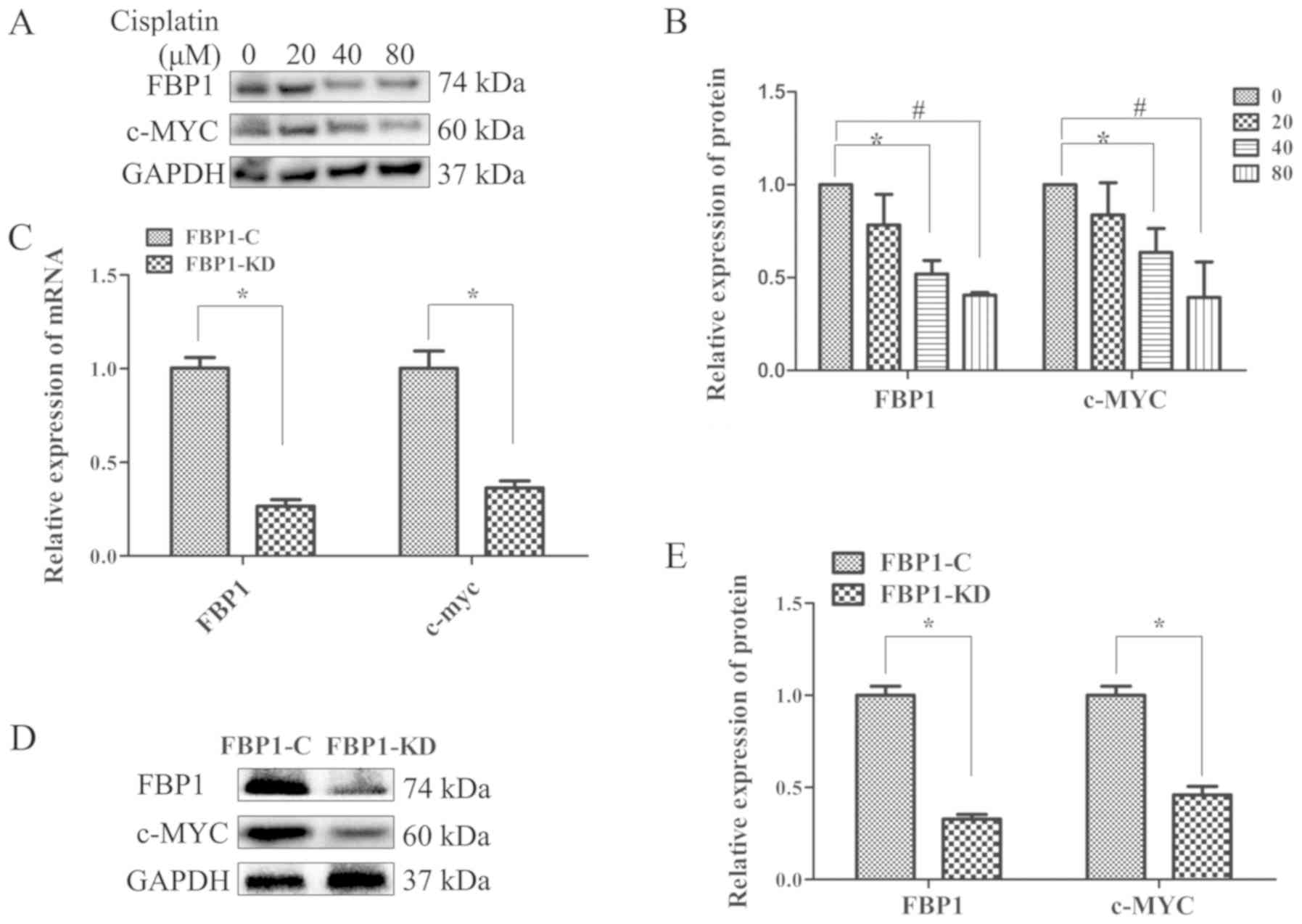
Cisplatin treatment decreases FBP1
expression in advanced TNBC cells
Cisplatin is frequently used in the treatment of
breast cancer, including TNBC. In the present study, to determine
the effects of cisplatin on FBP1 expression, western blot analysis
was performed to examine the expression of FBP1 in the MDA-MB-231
TNBC cells with or without cisplatin treatment. FBP1 expression was
downregulated by cisplatin in advanced TNBC cells (Fig. 2A). Cisplatin (40 and 80 µM)
significantly inhibited FBP1 expression (Fig. 2B). c-Myc expression was also
downregulated by cisplatin, and treatment with 40 and 80 µM of
cisplatin significantly inhibited c‑Myc expression. Both the
expression levels of FBP1 and c-Myc were decreased by cisplatin in
a dose-dependent manner (Fig. 2A and
B).
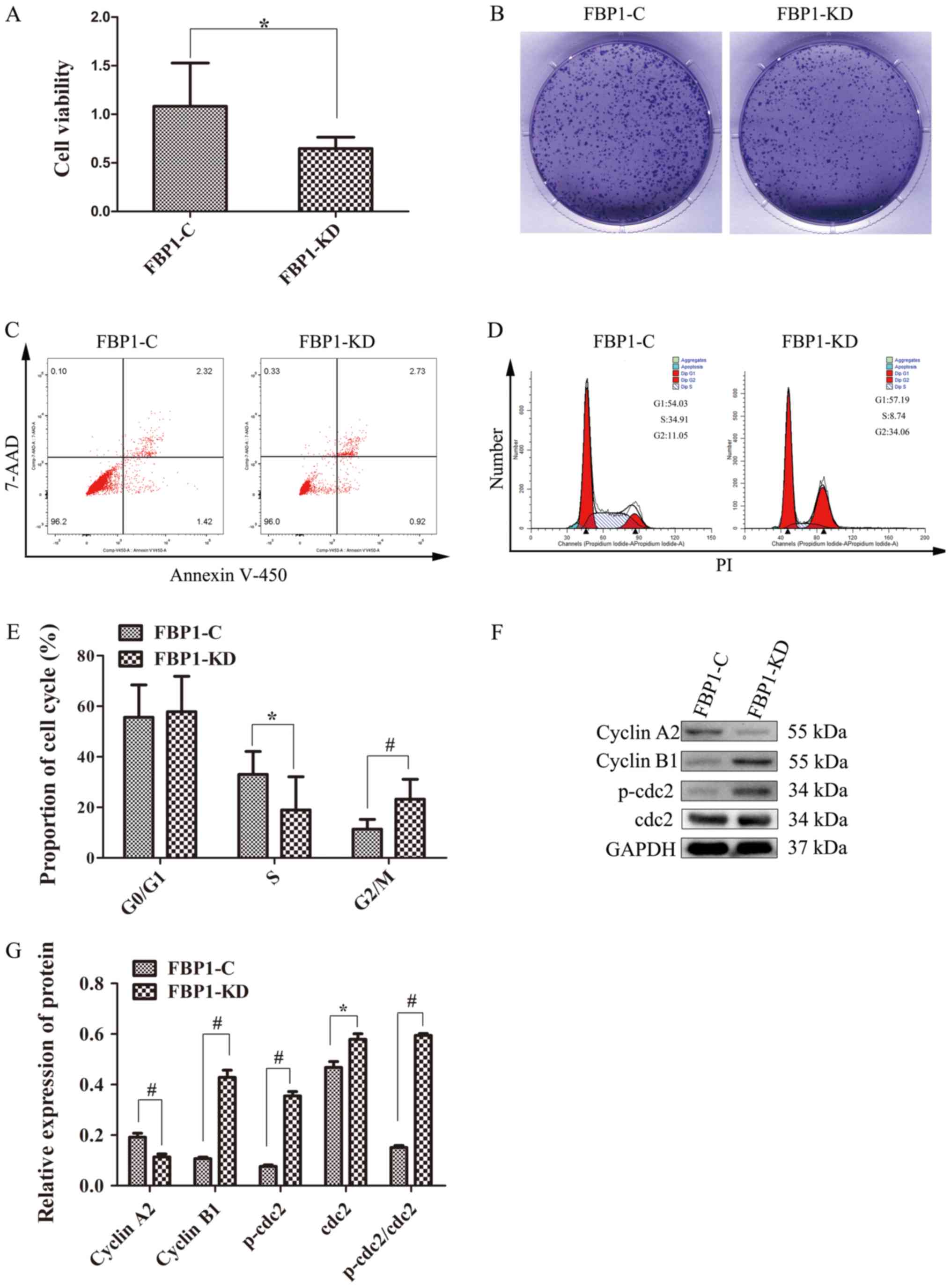
FBP1 promotes cell proliferation and G2/M phase
transition in TNBC cells. Since FBP1 was positively associated with
breast cancer development, it was hypothesized that FBP1 may play a
role in cell proliferation, cell cycle progression and the
apoptosis of breast cancer cells. To investigate the possible roles
of FBP1 in cell proliferation, the MDA-MB-231 cells were
transfected with shRNA to create a cell line in which FBP1 was
knockdown, which was termed FBP1-KD cells. FBP1 normal cells served
as the control group and were termed FBP1-C cells. The expression
of FBP1 and c-Myc in the FBP1‑KD MDA‑MB‑231 cells was significantly
decreased compared with its expression in the FBP1-C MDA-MB-231
cells, as determined by RT-qPCR and western blot analysis (Fig. 2C-E). These results demonstrated
that a stable FBP1-KD cell line was constructed.
As illustrated in Fig.
3A and B, the proliferation of the FBP1‑KD cells was
significantly decreased compared with that of the FBP1-C cells,
according to MTS assay and colony formation assay. The colonies of
FBP1‑KD cells were significantly smaller and fewer than those of
the FBP1-C cells (Fig. 3B).
However, Annexin-V450/7‑ADD flow cytometry revealed that the
percentage of apoptotic cells was ~5.0% in both the FBP1-C and
FBP1-KD cells (Fig. 3C). These
data indicated that FBP1 knockdown affected the proliferation of
the TNBC MDA-MB-231 cells, but did not affect cell apoptosis.
To certify the function of FBP1 in cell cycle
transition, the cell cycle phase distribution of FBP1-C and FBP1-KD
MDA-MB-231 cells was analyzed by flow cytometry. As shown in
Fig. 3D and E, the percentage of
cells in the G2 phase was 11.05 and 34.06% in the FBP1-C and
FBP1-KD cells, respectively. By contrast, the percentages of cells
in the S and G1 phases were 34.91 and 54.03% in the FBP1-C cells,
and 8.74 and 57.19% in the FBP1-KD cells, respectively. These data
indicated that the transition from the G2/M/G1 to the S phase was
significantly inhibited by FBP1 knockdown. Additionally, the
expression levels of G2 phase markers, such as cyclin A2, cyclin
B1, p-cdc2 and cdc2 were measured. The protein expression of cyclin
A2 was lower in the FBP1-KD cells compared with the FBP1-C cells.
However, the expression levels of cyclin B1, p-cdc2 and cdc2 were
higher in the FBP1-KD cells compared with the FBP1-C cells
(Fig. 3F and G). The relative
ratio of p-cdc2 (p-cdc2/GAPDH) against cdc2 (cdc2/GAPDH) in the
FBP1-KD cells was higher than that in the FBP1-C cells (Fig. 3F and G). These data suggested that
FBP1 knockdown affected the cell cycle transition by arresting
cells in the G2/G1 phase.
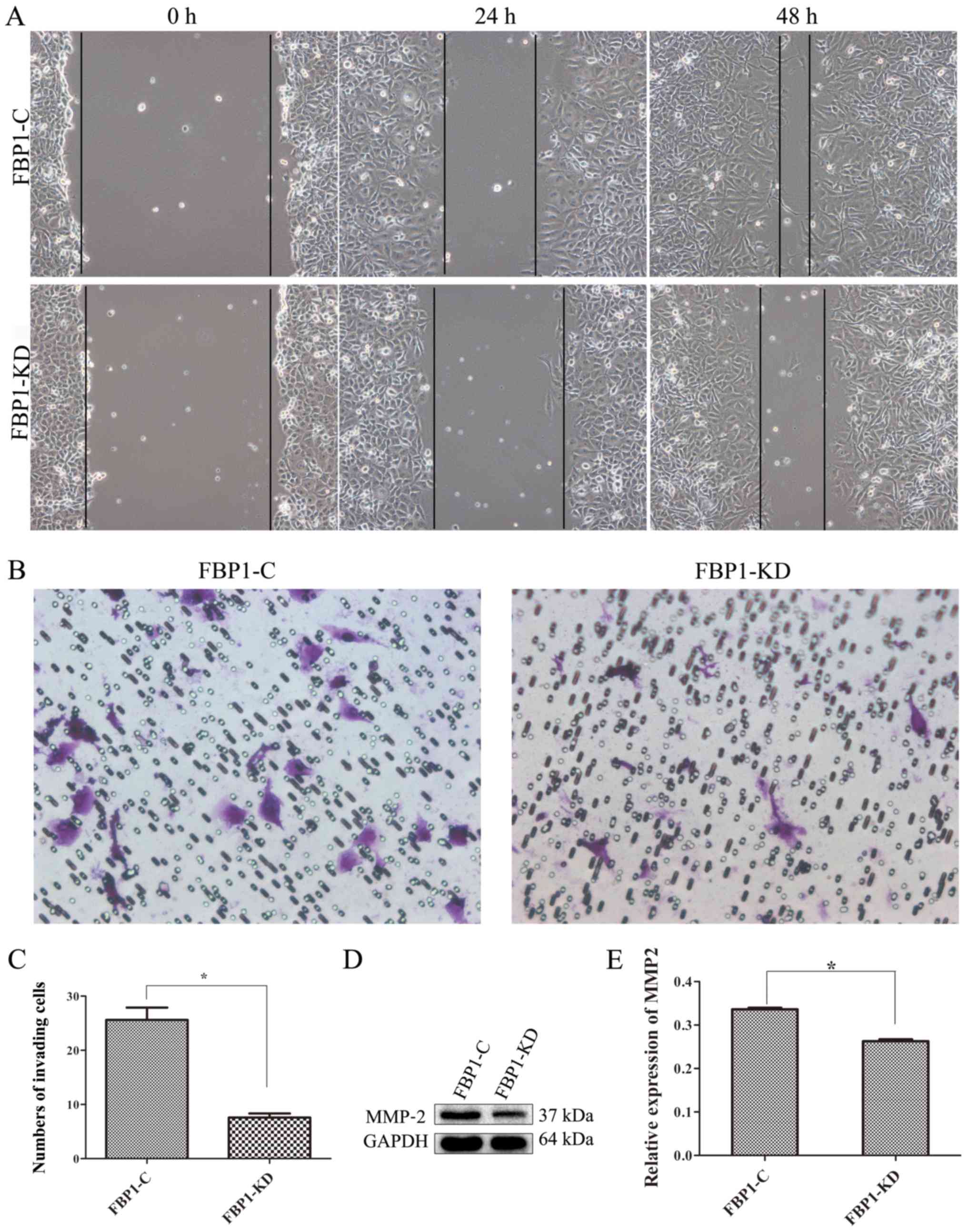
FBP1 knockdown inhibits cell migration
and invasion
Cell migration and invasion are critical for cancer
progression. A previous study by the authors demonstrated that FBP1
contributed to tumor cell migration and invasion in endothelial
cancer (24). A wound healing
assay in the present study revealed that FBP1 knockdown decreased
the migration of MDA-MB-231 cells. As shown in Fig. 4A, the migration rate of the FBP1-KD
MDA-MB-231 cells was slower compared with that of the FBP1-C cells
at 24 and 48 h. Additionally, the results from Transwell assay
revealed that FBP1 knockdown significantly inhibited cell invasion
to the bottom chambers (Fig. 4B and
C).
A previous study demonstrated that MMPs are key
regulators of cell migration (20). The present study thus investigated
the expression of MMP-2 in the FBP1 C and FBP1-KD MDA-MB-231 cells.
FBP1 knockdown inhibited the expression of MMP-2 (Fig. 4D and E). These data suggest that
FBP1 promotes cancer cell migration and facilitates cell
metastasis.
FBP1 knockdown enhances the sensitivity
of TNBC cells to cisplatin
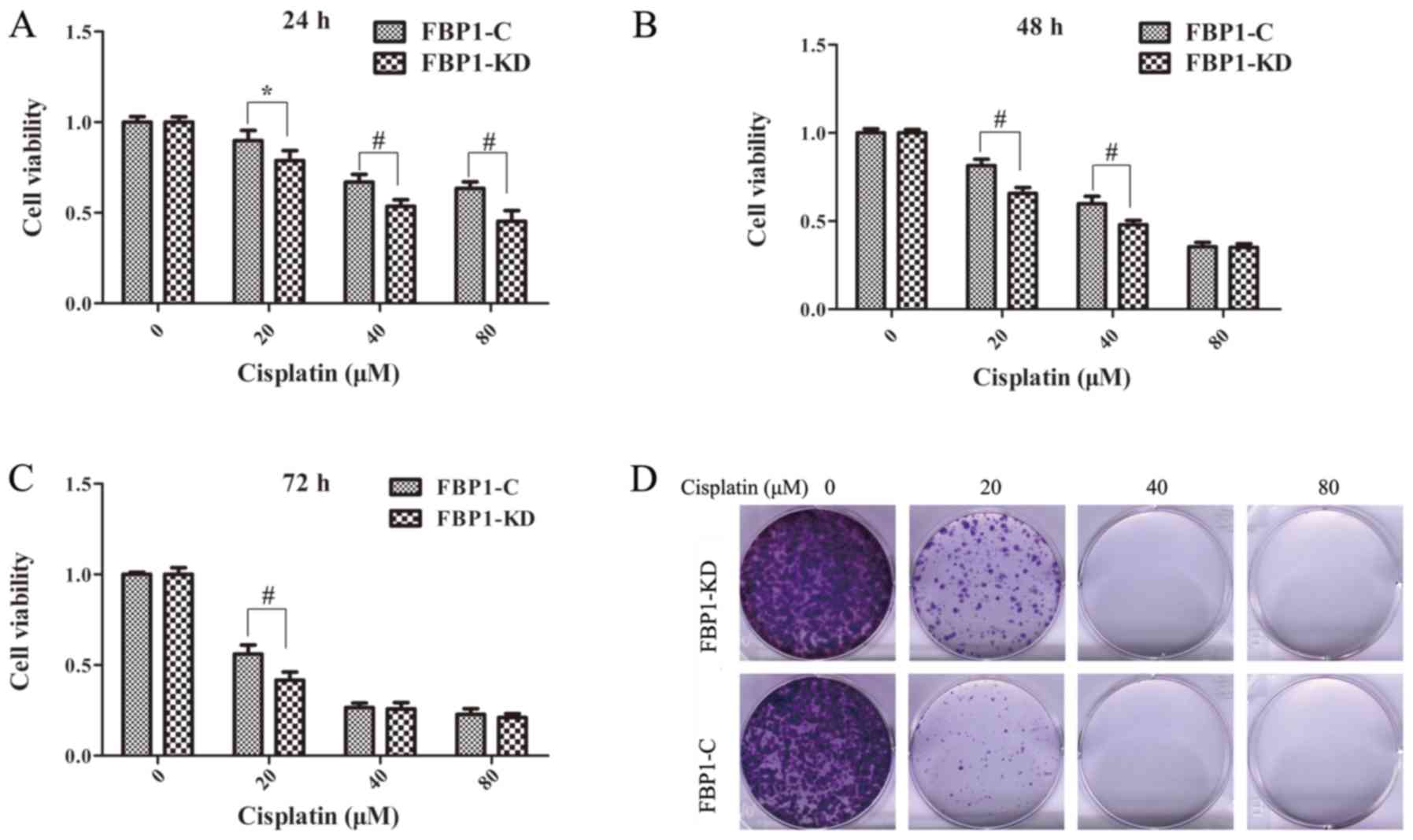
The sensitivity of TNBC cells to drugs is low and
this is postulated as one of the reasons for the poor prognosis of
patients with TNBC. In the present study, to investigate the role
of FBP1 in the drug sensitivity of TNBC cells, the effects of FBP1
on the cisplatin-induced toxicity of MDA-MB-231 cells were
examined. The FBP1-C and FBP1-KD MDA-MB-231 cells were incubated
with gradient concentrations (20, 40 and 80 µM) of cisplatin for 24
to 72 h. Cell viability, which is used to assess drug sensitivity,
was evaluated by MTS assay. The viability of the FBP1-C and FBP1-KD
cells decreased following cisplatin treatment and the decrease was
dose-dependent (Fig. 5A-C). The
viability of the FBP1-KD cells decreased more prominently that that
of the FBP1-C cells following treatment with 20, 40 and 80 µM
cisplatin for 24 h (P<0.05 and P<0.01), 20 and 40 µM of
cisplatin for 48 h (P<0.01), and 20 µM of cisplatin for 72 h
(P<0.01). In addition, a colony formation assay was used to
further confirm that FBP1 knockdown enhanced the sensitivity of
TNBC cells to cisplatin. The FBP1-KD MDA-MB-231 cells formed fewer
colonies than the FBP1-C MDA-MB-231 cells following treatment with
20 µM cisplatin (Fig. 5D). These
results demonstrated that FBP1 knockdown enhanced the sensitivity
of TNBC cells to cisplatin.
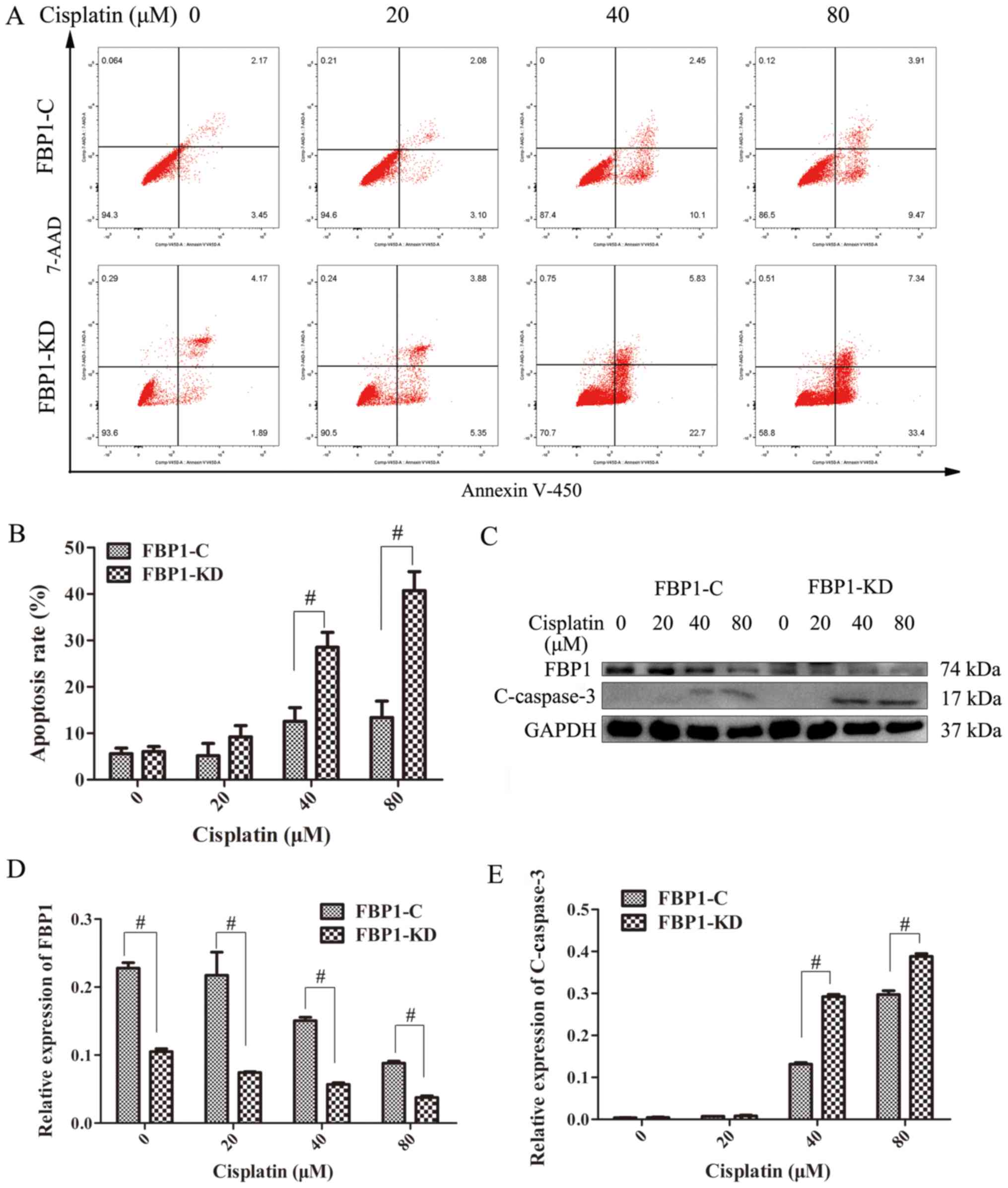
FBP1 suppresses the cisplatin‑induced
apoptosis of TNBC cells
To verify the possible mechanisms underlying the
high sensitivity of TNBC cells in which FBP1 was knocked down to
cisplatin, the effects of cisplatin on the apoptosis of the FBP1-C
and FBP1-KD MDA-MB-231 cells were examined by flow cytometry. As
shown in Fig. 6A and B, the
percentage of apoptotic cells was significantly higher in the
FBP1-KD cells compared with that in the FBP1-C cells when the cells
were treated with 40 and 80 µM cisplatin for 48 h. The expression
of cleaved caspase-3 (C-Caspase-3), a typical characteristic of
cell apoptosis, was examined by western blot analysis in the FBP1-C
and FBP1-KD MDA-MB-231 cells. As shown in Fig. 6C and E, C-Caspase-3 expression was
increased in both the FBP1-C and in FBP1-KD cells following
treatment with 40 and 80 µM cisplatin for 48 h. Compared with the
expression of C-Caspase-3 in the FBP1-C cells, the expression of
C-Caspase-3 in the FBP1-KD cells was significantly higher. The
expression of FBP1 in the FBP1-C and FBP1-KD cells decreased as the
concentration of cisplatin increased (Fig. 6D). Based on these data, it was
considered that FBP1 knockdown promoted cisplatin-induced
apoptosis.
Discussion
According to the report of the American Cancer
Society (21), breast cancer
accounts for 29% of all new cancer diagnoses and was the leading
cause of cancer-associated mortality in women aged 20-59 years in
2016. Although the treatments for breast cancer have improved over
the past decades, the treatment efficacy is still limited due to
drug toxicity and resistance, as well as the lack of dependable
predictive and prognostic biomarkers (22). TNBC is a particular type of breast
cancer that is ER‑, PR‑ and HER‑2‑negative and cannot benefit from
endocrine therapy. Thus, the investigation and identification of
novel biomarkers and the relevant mechanisms of TNBC is of utmost
importance.
FBP1 is an anti-apoptotic and anti-proliferative
oncoprotein that acts by modulation of complex networks (12). Studies have indicated that FBP1 is
overexpressed in a variety of malignant tumors, such as liver
cancer, gastric cancer, esophageal squamous cell carcinoma, ovarian
cancer and nasopharyngeal carcinoma, and FBP1 is associated with
both a lower disease-free and overall survival in patients
(5,14-17,23,24).
In a previous study by the authors, it was demonstrated that FBP1
expression was higher in epithelial ovarian cancer (EOC) tissues
compared with in para-tissues, and that a higher expression of FBP1
was associated with EOC progression (25). The present study identified that
the progression of breast cancer was positively associated with
FBP1 expression, even though the exact numbers of clinical samples
from Luminal A, Luminal B, HER-2 overexpression and TNBC patients
were not know. In the future, the authors aim to collect more 4
types of clinical breast cancer samples, in order to analyze the
effect of FBP1 on the development of breast cancer.
Cancer progression may be associated with cell
viability, and a decreased cell viability may be induced by the
suppression of cell cycle transition and the activation of cell
death (25). The present study
demonstrated that FBP1 knockdown significantly inhibited colony
formation and increased the percentage of cells in the G2 phase.
Cyclin B1 and p-CDC2, which are considered to be G2/M phase
transition inhibiting proteins, were induced by FBP1 knockdown.
Conversely, cyclinA1, which is considered to be a G2/M phase
transition promoting protein, was suppressed by FBP1 knockdown.
FBP1 knockdown did not affect cell apoptosis. These results suggest
that FBP1 knockdown significantly suppresses the G2/M phase
transition. However, FBP1 did not affect the death of TNBC
cells.
Metastasis is a complex, multistep process that
requires cancer cells to detach from the primary tumor, travel,
survive and proliferate in distant organs (26,27).
MMPs have traditionally been considered to regulate a number of
processes, including cell migration, angiogenesis, tumor expansion
and metastasis (28,29). In general, the expression of MMPs
is low and can be upregulated during inflammation, tissue
remodeling, wound healing and cancer progression (30). The present study demonstrated that
FBP1 knockdown inhibited the migration and metastasis of TNBC
cells, as well as the expression of MMP-2, which is an important
member of the MMP family. These results suggested that FBP1
knockdown decreased the migration and metastasis via the inhibition
of MMP-2 expression. It has been reported that the major mechanism
regulating MMP expression is transcription. The majority of members
of the MMP family share common cis-acting elements in their
promoters and the promoters contain multiple elements that
cooperate to either induce or repress gene expression including
E2F1 (31,32). As regards the mechanisms underlying
the regulatory effects of FBP1 on MMP-2, further investigations are
warranted to determine this in the future.
Cisplatin is a commonly used chemotherapeutic drug
in patients with TNBC. A recent study on patients with refractory
breast cancer demonstrated that tumor profiling based therapy
resulted in a survival benefit (33). Therefore, enhancing our
understanding of the mechanisms implicated in cisplatin treatment
may improve the management of TNBC. The present study demonstrated
that cisplatin reduced FBP1 expression in cisplatin-treated
advanced TNBC cells, and FBP1 knockdown enhanced the sensitivity of
TNBC cells to cisplatin. Cisplatin treatment upregulated the
expression of C-Caspase-3 in the FBP1-KD cells to a greater extent
than in the FBP1-C cells. This indicated that cisplatin treatment
induced more prominent apoptosis in the FBP1-KD cells compared with
the FBP1-C cells. The inhibition on FBP1 expression may be one of
the reasons that cisplatin abrogates breast cancer development.
However, the high expression of FBP1 in cancer cells/tissues
inhibits cancer cell apoptosis resulting from cisplatin treatment.
There may be a negative feedback loop between FBP1 expression and
the therapeutic efficacy of cisplatin in tumors. The mechanisms
through which cisplatin regulates FBP1 warrant further
clarification in the future.
The clinical treatment of TNBC remains challenging
due to the lack of available targets. Chemotherapy has
significantly improved from previously administered treatments. In
the present study, the progression of breast cancer was
demonstrated to be positively associated with FBP1 expression. FBP1
knockdown inhibited cell viability, cell cycle transition and cell
migration, and increased the sensitivity of TNBC cells to
cisplatin. As a highly expressed protein in breast cancer tissues,
FBP1 promotes cell proliferation and cell migration, and
neutralizes the sensitivity of TNBC cells to cisplatin, but has no
effect on apoptosis. Based on the above-mentioned facts, FBP1 may
be a potential adjuvant drug target rather than a potential
chemotherapeutic target for breast cancer treatment. In the future,
the authors aim to perform further studies on the synergistic
effects between FBP1 and radiation, chemicals, etc.
In conclusion, FBP1 is a potential adjuvant drug
target for breast cancer treatment and the downregulation of FBP1
may be a potential strategy for the development of novel TNBC
treatments.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272222 to ZL and
81902802 to XX), the Guangdong Provincial Natural Science
Foundation of China (grant no. 2016A030313425 to ZZ), the Medical
Science and Technology Research Foundation of Guangdong (grant nos.
B2016018 to XX and A2018063 to XX), the research grants of
Guangdong Bureau of Traditional Chinese Medicine (nos. 20181206 to
ZL and 20191260 to XX), the Medical and Health Science and
Technology Project of Guangzhou (grant no. 20191A011016 to WL), the
research grants of Guangzhou Municipal Health and Family Planning
Commission (nos. 20161A010019 to XX and 20181A010017 to XX).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
WL, XX, ZL, XL and ZZ were involved in the initial
experimental design. WL, WC and XH prepared the tissue samples. XX
and WL performed the experiments. WL and XX wrote the manuscript.
ZL and ZZ analyzed the data and wrote the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consents for the use of their samples in
scientific research were obtained from all patients. The present
study was conducted after the protocol was approved by the Medical
Ethics Committee of Guangzhou Red Cross Hospital of Jinan
University (approval no. 2015-045-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
Acknowledgements
The authors would like to thank Dr Siyu Liu from
Rutgers, the State University of New Jersey, USA for her assisting
in the writing of the manuscript. Flow cytometric analysis was
performed with the assistance of Guangdong Provincial Key
Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun
Yat-Sen Memorial Hospital, Sun Yat-Sen University.
References
|
1
|
Tobin NP, Harrell JC, Lövrot J, Egyhazi
Brage S, Frostvik Stolt M, Carlsson L, Einbeigi Z, Linderholm B,
Loman N, Malmberg M, et al: TEX Trialists Group: Molecular subtype
and tumor characteristics of breast cancer metastases as assessed
by gene expression significantly influence patient post‑relapse
survival. Ann Oncol. 26:81–88. 2015. View Article : Google Scholar
|
|
2
|
Omarini C, Guaitoli G, Pipitone S,
Moscetti L, Cortesi L, Cascinu S and Piacentini F: Neoadjuvant
treatments in triple-negative breast cancer patients: Where we are
now and where we are going. Cancer Manag Res. 10:91–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurvitz S and Mead M: Triple-negative
breast cancer: Advancements in characterization and treatment
approach. Curr Opin Obstet Gynecol. 28:59–69. 2016.
|
|
4
|
Chalakur-Ramireddy NKR and Pakala SB:
Combined drug therapeutic strategies for the effective treatment of
Triple Negative Breast Cancer. Biosci Rep. 38:382018. View Article : Google Scholar
|
|
5
|
Pénzváltó Z, Lánczky A, Lénárt J,
Meggyesházi N, Krenács T, Szoboszlai N, Denkert C, Pete I and
Győrffy B: MEK1 is associated with carboplatin resistance and is a
prognostic biomarker in epithelial ovarian cancer. BMC Cancer.
14:8372014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loibl S, O'Shaughnessy J, Untch M, Sikov
WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag
D, et al: Addition of the PARP inhibitor veliparib plus carboplatin
or carboplatin alone to standard neoadjuvant chemotherapy in
triple-negative breast cancer (BrighTNess): A randomised, phase 3
trial. Lancet Oncol. 19:497–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
von Minckwitz G, Schneeweiss A, Loibl S,
Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S,
Gerber B, et al: Neoadjuvant carboplatin in patients with
triple-negative and HER2-positive early breast cancer (GeparSixto;
GBG 66): A randomised phase 2 trial. Lancet Oncol. 15:pp. 747–756.
2014, View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poggio F, Bruzzone M, Ceppi M, Pondé NF,
La Valle G, Del Mastro L, de Azambuja E and Lambertini M:
Platinum-based neoadjuvant chemotherapy in triple-negative breast
cancer: A systematic review and meta-analysis. Ann Oncol.
29:1497–1508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tutt A, Tovey H, Cheang MCU, Kernaghan S,
Kilburn L, Gazinska P, Owen J, Abraham J, Barrett S, Barrett-Lee P,
et al: Carboplatin in BRCA1/2-mutated and triple-negative breast
cancer BRCAness subgroups: The TNT Trial. Nat Med. 24:628–637.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmad S: Platinum-DNA interactions and
subsequent cellular processes controlling sensitivity to anticancer
platinum complexes. Chem Biodivers. 7:543–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim H and D'Andrea AD: Regulation of DNA
cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev.
26:pp. 1393–1408. 2012, View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Debaize L and Troadec MB: The master
regulator FUBP1: Its emerging role in normal cell function and
malignant development. Cell Mol Life Sci. 76:259–281. 2019.
View Article : Google Scholar
|
|
13
|
Steiner M, Schneider L, Yillah J, Gerlach
K, Kuvardina ON, Meyer A, Maring A, Bonig H, Seifried E, Zörnig M,
et al: FUSE binding protein 1 (FUBP1) expression is upregulated by
T-cell acute lymphocytic leukemia protein 1 (TAL1) and required for
efficient erythroid differentiation. PLoS One. 14:pp. e02105152019,
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malz M, Bovet M, Samarin J, Rabenhorst U,
Sticht C, Bissinger M, Roessler S, Bermejo JL, Renner M, Calvisi
DF, et al: Overexpression of far upstream element (FUSE) binding
protein (FBP)-interacting repressor (FIR) supports growth of
hepatocellular carcinoma. Hepatology. 60:1241–1250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venturutti L, Cordo Russo RI, Rivas MA,
Mercogliano MF, Izzo F, Oakley RH, Pereyra MG, De Martino M,
Proietti CJ, Yankilevich P, et al: MiR-16 mediates trastuzumab and
lapatinib response in ErbB-2-positive breast and gastric cancer via
its novel targets CCNJ and FUBP1. Oncogene. 35:6189–6202. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu ZH, Hu JL, Liang JZ, Zhou AJ, Li MZ,
Yan SM, Zhang X, Gao S, Chen L, Zhong Q, et al: Far upstream
element-binding protein 1 is a prognostic biomarker and promotes
nasopharyngeal carcinoma progression. Cell Death Dis. 6:pp.
e19202015, View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J and Chen QM: Far upstream element
binding protein 1: A commander of transcription, translation and
beyond. Oncogene. 32:2907–2916. 2013. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Duncan R, Bazar L, Michelotti G, Tomonaga
T, Krutzsch H, Avigan M and Levens D: A sequence-specific,
single-strand binding protein activates the far upstream element of
c-myc and defines a new DNA‑binding motif. Genes Dev. 8:pp.
465–480. 1994, View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rodríguez D, Morrison CJ and Overall CM:
Matrix metalloproteinases: What do they not do? New substrates and
biological roles identified by murine models and proteomics.
Biochim Biophys Acta. 1803:39–54. 2010. View Article : Google Scholar
|
|
21
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo
X, Wei Q, Wang J, Xiong H, Chen C, Xu B, et al: Exosome: Emerging
biomarker in breast cancer. Oncotarget. 8:41717–41733. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Zhu JY, Zhang JG, Bao BJ, Guan CQ,
Yang XJ, Liu YH, Huang YJ, Ni RZ and Ji LL: Far upstream
element-binding protein 1 (FUBP1) is a potential c-Myc regulator in
esophageal squamous cell carcinoma (ESCC) and its expression
promotes ESCC progression. Tumour Biol. 37:4115–4126. 2016.
View Article : Google Scholar
|
|
24
|
Ding Z, Liu X, Liu Y, Zhang J, Huang X,
Yang X, Yao L, Cui G and Wang D: Expression of far upstream element
(FUSE) binding protein 1 in human glioma is correlated with c-Myc
and cell proliferation. Mol Carcinog. 54:405–415. 2015. View Article : Google Scholar
|
|
25
|
Xiong X, Zhang J, Hua X, Cao W, Qin S, Dai
L, Liu W, Zhang Z, Li X and Liu Z: FBP1 promotes ovarian cancer
development through the acceleration of cell cycle transition and
metastasis. Oncol Lett. 16:1682–1688. 2018.PubMed/NCBI
|
|
26
|
Talmadge JE and Fidler IJ: AACR centennial
series: the biology of cancer metastasis: historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Massagué J and Obenauf AC: Metastatic
colonization by circulating tumour cells. Nature. 529:298–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Butler GS and Overall CM: Updated
biological roles for matrix metalloproteinases and new
'intracellular' substrates revealed by degradomics. Biochemistry.
48:10830–10845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zitka O, Kukacka J, Krizkova S, Huska D,
Adam V, Masarik M, Prusa R and Kizek R: Matrix metalloproteinases.
Curr Med Chem. 17:3751–3768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vincenti MP and Brinckerhoff CE: Signal
transduction and cell‑type specific regulation of matrix
metalloproteinase gene expression: Can MMPs be good for you? J Cell
Physiol. 213:355–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Guo Y, Jiang H, Zhang T, Jin C,
Young CY and Yuan H: Differential regulation of MMPs by E2F1, Sp1
and NF-kappa B controls the small cell lung cancer invasive
phenotype. BMC Cancer. 14:2762014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jameson GS, Petricoin EF, Sachdev J,
Liotta LA, Loesch DM, Anthony SP, Chadha MK, Wulfkuhle JD,
Gallagher RI, Reeder KA, et al: A pilot study utilizing multi-omic
molecular profiling to find potential targets and select
individualized treatments for patients with previously treated
metastatic breast cancer. Breast Cancer Res Treat. 147:579–588.
2014. View Article : Google Scholar : PubMed/NCBI
|