Introduction
Retinoblastoma (RB) is a primary malignant
intraocular tumor common among infants and children (1) that accounts for 4% of all pediatric
malignancies (2). RB can be
hereditary or non-hereditary, and the spontaneous inactivation of
the retinoblastoma gene (RB1) at 13q14 has been reported to be
responsible for the pathogenesis of the disease (3). Unilateral RB is observed in
approximately 2/3 of cases, while bilateral RB is observed in the
remaining 1/3 of cases, and the tumor size and tumor number per eye
can vary (4). The most common and
evident sign of RB is known as amaurotic cat's eye reflex or
leukocoria; this retinal pathology can be viewed through the pupil
and assists with accurate and early diagnosis (4). The present study aimed to investigate
and compare the toxicity and antitumor activity of the
third-generation platinum drugs lobaplatin with the
second-generation platinum drugs carbo-platin in vitro and in
vivo innovatively.
RB therapy is administered according to the
International Classification of Retinoblastoma (ICRB) guidelines
(5). Currently, available
therapies include eye enucleation, external beam radiotherapy,
thermotherapy, cryotherapy and systemic chemotherapy, among others.
Chemotherapy is the conventional and main therapeutic option for
shrinking RB tumors. One of the benefits of chemotherapy over
radiation therapy is that the development of secondary cancers and
various other complications associated with radiation can be
avoided (6). Chemotherapy can be
administered through several routes, such as the systemic,
intra-arterial, intravitreal and subconjunctival routes, and the
typical chemotherapeutic agents, vincristine, etoposide and
carboplatin (in the VEC protocol) (7) have contributed to improving the
survival rates to ≥95%. However, chemotherapeutic drugs influence
all cells in the body, not only cancer cells, inducing a series of
detrimental complications such as appetite and hair loss, nausea,
vomiting and sore mouth (8).
Despite recent advances in local delivery strategies for
chemotherapy, few agents have been incorporated into the
chemotherapeutic armamentarium for RB treatment (9). Thus, the exploration of novel
antitumor agents with reduced side-effects and enhanced therapeutic
effects in RB patients are crucial and are urgently required.
The representative 3rd-generation platinum drug,
lobaplatin (D-19466; 1,2-diamminomethylcyclobu-tane-platinum(II)
lactate), can cause DNA damage via GG and AG intrastrand
crosslinking to form DNA-drug adducts and to inhibit tumor
activity, which may influence the expression of certain genes in
tumor cells (10). Lobaplatin has
exhibited promising therapeutic effects in several clinical
studies; for example, it has been found to suppress proliferation
and peritoneal metastasis in a preclinical model of colorectal
cancer (11), to inhibit gastric
cancer cells by inducing apoptosis (12), and to arrest cells in S phase and
trigger apoptosis in the context of human non-small cell lung
cancer (13). Furthermore,
lobaplatin can be used to overcome the drug resistance associated
with cisplatin observed in several types of cancer as it exhibits
lower toxicity compared with cisplatin (14). Thus far, lobaplatin has been
approved in China for the treatment of small-cell lung and
metastatic breast cancer. Similarly, it has also been considered
effective for the treatment of chronic myelogenous leukemia
(15). Nonetheless, the effect of
lobaplatin on RB and its underlying mechanism are unknown.
Carboplatin is a second-generation platinum compound
that can directly inhibit DNA repair to attenuate tumor growth
(15). Carboplatin has been used
in the treatment of RB since the late 1980s. Currently, 6-10 cycles
of carboplatin, etoposide and vincristine are typically used for
systemic RB chemotherapy (16).
However, there are a number of side-effects associated with
systemic chemotherapy, such as autotoxicity, bone marrow
suppression, nephrotoxicity and alopecia. Similarly, acute myeloid
leukemia has also been found to occur in some cases, although the
affected patients were also administered high doses of etoposide
(17).
The present study prospectively compared the
therapeutic efficacies and toxicities of lobaplatin and carboplatin
in the context of RB treatment and examined the underlying
molecular mechanisms of these characteristics. Specifically, the
aim of the present study was to determine the effects of lobaplatin
and carboplatin on Y79 RB cell proliferation, apoptosis, cell cycle
progression, and tumor growth in vivo and in
vitro.
Materials and methods
Cell lines and culture
The human Y79 RB tumor cell line was obtained from
ATCC (The Global Bioresource Center). The tumor cells were then
cultured in RPMI-1640 medium. The culture medium was supplemented
with 10% fetal bovine serum (FBS) from Gibco; Thermo Fisher
Scientific, Inc., 100 µg/ml streptomycin and 100 U/ml
penicillin. The medium was maintained at 37°C in 5% CO2
and 95% humidified air to provide the appropriate conditions for
cell growth.
Cell viability assay
An MTT colorimetric assay, was used to assess cell
viability. Briefly, the Y79 cells were plated in 96-well plates at
a density of 1×105 cells per well in 100 µl of
complete culture medium. The cells were incubated for 24 h with 4
concentrations (20, 40, 60 and 80 µg/ml) of carboplatin and
4 concentrations (5, 10, 20 and 40 µg/ml) of lobaplatin.
Subsequently, 10 µl of MTT solution (5 mg/ml MTT in PBS)
were added, and the cells were incubated for 2 h at 37°C. A
microplate reader (BioTek, SynergyHT) was used to measure the
absorbance at 490 nm; the absorbance at 690 nm was measured to
correct for the background signal.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis assay
The Y79 cell line was subjected to apoptosis
analysis using a FITC Annexin V Apoptosis Detection kit. Cell cycle
analysis was performed according to the manufacturer's protocol
(KeyGEN Biotech Co., Ltd.). Specifically, Y79 cells
(1×106/ml) in 1,000 µl of complete RPMI-1640
medium were seeded in 6-well plates. The Y79 cells were incubated
in a controlled environment at 37°C for 2 days with 4 different
concentrations of carboplatin (20, 40, 60 and 80 µg/ml) and
lobaplatin (5, 10, 20 and 40 µg/ml); control cells were
incubated with no additives. Subsequently, as per the
manufacturer's instructions, flow cytometry was used to assess the
samples (1×104 cells). A Beckman Coulter FC 500 flow
cytometer was then used to measure the fluorescence intensities.
The percentage of early apoptotic plus late apoptotic cells was
considered as the apoptotic percentage. The cell cycle
distributions of the different groups were analyzed. This
experiment was repeated 3 times.
RNA sequencing (RNA-seq)
To analyze the transcriptomes of different Y79 RB
cells in vitro following treatment with carboplatin or
lobaplatin, RNA-seq was employed. Differential expression analysis
was also performed using the RNA-seq gene expression data, and a
negative binomial distribution-based model was used as a
statistical tool to address any potential issues associated with
the use of RNA-seq. The Benjamini-Hochberg method was then used to
adjust the resulting P-values to control the false discovery rate.
Differentially expressed genes were defined as those with P-values
<0.05. BioMart (http://www.biomart.org/) was then used to translate
the Ensemble gene IDs into official gene symbol IDs. Similarly, the
Clue GO program in Cystoscope software (https://cytoscape.org/) was employed to assess the
enrichment of the differentially expressed genes. The
Benjamini-Hochberg method was again used to correct the P-values,
and significant gene enrichment was indicated by a P-value
≤0.05.
Reverse transcription-quantitative
polymerase chain reaction (RT-PCR)
TRIzol reagent (Thermo Fisher Scientific, Inc.) was
used to isolate total RNA from tumors or cell pellets. A NanoDrop
2000C spectrophotometer (Thermo Fisher Scientific, Inc.) was
employed to quantify the isolated RNA. Subsequently, kits from
Fermentas (Thermo Fisher Scientific, Inc.) were used to reverse
transcribe the total RNA into cDNA with iScript Reverse
Transcription Supermix (Bio-Rad Laboratories, Inc.). The primer
sequences for human genes included the following: E2F1 forward,
5′GGGACTTTGCAG GCAGCGGC3′ and reverse, 3′GCCGCTGCCTGCAAAGT CCC5′
(reverse); Cdc25a forward, 5′ACTGAGCCGCTATTA CCGCG3′ and reverse,
3′CGCGGTAATAGCGGCTCAGT5′; and Cdk2 forward,
5′AACGCGGGAAGCAGGGGCGG3′ (forward) and reverse,
3′CCGCCCCTGCTTCCCGCGTT5′. The primer sequences for mouse genes
included the following: E2F1 forward, 5′CCGCCATGGGCCCGCGCCGC3′ and
reverse, 3′GCGGCGCGGGCCCATGGCGG5′; Cdc25a forward,
5′GGAGAAAAAAAGTGAGGCGA3′ and reverse, 3′TCGCCTCACTTTTTTTCTCC5′; and
Cdk2, forward, 5′TGGACAAATTGTCAAGGGCT3′ and reverse, 3′AGC
CCTTGACAATTTGTCCA5′. qPCR analysis was then performed using
SYBR-Green Master Mix and a CFX96 Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.) following the instructions of the
manufacturer. The comparative cycle threshold (Cq) (ΔΔCq) method
was used to analyze the data (18). The expression data for each sample
were normalized to GAPDH (forward, 5'AACTTTGGCATT GTGGAAGG3' and
reverse, 3' ACACATTGGGGGTAGGA ACA5').
Immunofluorescence microscopy
Following incubation with carboplatin and
lobaplatin, 1X PBS was used to prepare the cells. Subsequently, the
cells were briefly fixed for 5 min using methanol and acetone (1:1,
v:v), and 3% BSA was used to block the cells for half a day at room
temperature. The cells were then incubated for half a day at 4°C
with anti-E2F1 (1:300; cat. no. HPA008003, Sigma-Aldrich; Merck
KGaA), anti-Cdc25a (1:300; cat. no. WH0000993M1, Sigma-Aldrich;
Merck KGaA) and anti-Cdk2 (1:100; cat. no. SAB5300328,
Sigma-Aldrich; Merck KGaA) antibodies. After washing, the cells
were incubated with an Invitrogen brand Alexa Fluor 488-conjugated
secondary antibody (1:500; cat. no. 913921 Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Subsequently, the
nuclei were stained for 5 min using the blue fluorescent stain DAPI
(Sigma-Aldrich; Merck KGaA). Finally, 1X PBS was used to wash the
samples, which were then observed using a Carl Zeiss AG
fluorescence microscope.
Animals and tumor xenograft growth
assay
All animal experiments complied with the guidelines
established by the Association for Research in Vision and
Ophthalmology for the use of animals in ophthalmic and vision
research and were approved by the Institutional Ethics Board of
Guangzhou Women and Children's Medical Center. BALB/c (nu/nu) nude
mice (4-5 weeks old) were procured from the Guangdong Medical
Laboratory Animal Center. There were 84 nude mice used and
euthanized in the present study to evaluate the tumor growth curve,
pathological changes, and the mRNA and protein levels of cytokines.
In the experiment, the mice were anesthetized intraperitoneally
with chloral hydrate (400 mg/kg) to minimize suffering. Mice were
housed under standard laboratory conditions (22±2°C, 60% relative
humidity, 12/12 h light/dark cycle, and provided with food and
water ad libitum), and animal health and behavior were daily
monitored.
Xenograft tumors were established bilaterally in
each mouse with a single subcutaneous injection in the right flank
consisting of 1×107/ml Y79 RB cells suspended in 0.3 ml
of a 1:1 mixture of ice-cold Matrigel basement membrane matrix (BD
Biosciences) and RPMI-1640 medium. Once the tumor masses became
visible in the 2nd week, the mice were randomly assigned to 4
groups and treated with different medications: Group 1 was the
control group, group 2 comprised mice injected with PBS (group 2a
received tail vein injections of PBS every 3 days for 1 week, and
group 2b received tail vein injections of PBS every 3 days for 2
weeks), group 3 consisted of mice injected with carboplatin (20
mg/kg; group 3a received tail vein injections of PBS every 3 days
for 1 week, and group 3b received tail vein injections of PBS every
3 days for 2 weeks) according to a previous study (19) and group 4 comprised mice injected
with lobaplatin (750 µg/kg; group 4a received tail vein
injections of PBS every 3 days for 1 week, and group 4b received
tail vein injections of PBS every 3 days for 2 weeks) according to
a previous study (20). There were
7 subgroups of 4 groups of mice, and the mice in each subgroup were
used in 4 different experiments, namely tumor growth curve
analysis, hematoxylin and eosin (H&E) staining, histological
analysis and immunohistochemistry (IHC) and RT-qPCR. Each
experiment contained 3 mice per subgroup, and independent
experiments were performed (7×3×4=84 mice). Two-dimensional
external measurements were obtained twice each week with calipers,
and the tumor volume was calculated with the equation volume
(mm3) = 4/3 × π × (L/2) × (W/2)2. The weights
of the mice were also recorded once each week. The mice were
euthanized by cervical dislocation following an intraperitoneal
injection of chloral hydrate (400 mg/kg). After sacrificing the
tumor-bearing mice, the tumors were collected. A combination of
methods was used to ensure the death of the mice, including a firm
toe pinch, a lack of visible respiration, a lack of digitally
palpable heartbeat or respiration, grey mucous membranes and the
loss of corneal reflex.
Histological analysis and IHC
Prior to paraffin-embedding, the eyes were fixed in
Bouin's solution or formalin for 1 day. The paraffin blocks were
sectioned at a thickness of 4 µm, and the sections were
stained with H&E; cat. no. C0105, Beyotime Institue of
Biotechnology). The specific steps included: i) Deparaffinization
of the sections: The slide was place on a burner and then in
xylene; ii) hydration: Tissue sections were hydrated by passing
through decreasing concentrations of alcohol baths and water (100,
90, 80 and 70%); iii) staining in hematoxylin for 3-5 min; iv)
washing under running tap water until the sections were 'blue' for
≤5 min; v) differentiation in 1% acid alcohol (1% HCl in 70%
alcohol) for 5 min; vi) washing udner running tap water until the
sections were again blue by dipping in an alkaline solution (e.g.,
ammonia water) followed by washing under tap water again; vii)
staining in 1% eosin Y for 10 min; viii) washing under tap water
for 1-5 min ix) dehydrationg in an increasing concentration of
alcohol and clearing in xylene; x) mounting in mounting media and
observation under a microscope. The following antibodies were used
in the immunohistochemical analysis: Anti-E2F1 (1:1,000; cat. no.
ABIN969516, Biocompare), anti-Cdc25a (1:100; cat. no. ab75743,
Abcam) and anti-Cdk2 (1:300; cat. no. ab6433, Abcam). The
paraffin-embedded sections were first treated with xylene, a graded
ethanol series and TBS Tween-20 (TBST); subsequently, antigen
retrieval was performed by microwaving the sections in sodium
citrate buffer (0.01 M, pH 6.0). H2O2 (3.5%)
was then employed to block endogenous peroxidase, and the sections
were incubated with primary antibodies overnight for
immunohistochemical analysis. Biotin-conjugated secondary
antibodies, goat anti-rabbit IgG H&L (HRP) (cat. no. ab205718,
Abcam), and goat anti-mouse IgG H&L (HRP) (cat. no. ab205719,
Abcam) were diluted 1:2,000 with a biotin peroxidase complex
(Vectastain ABC with DAB substrate; Vector Laboratories) for 1 h at
room temperature. The sections were then washed in PBS.
Subsequently, the sections were developed for 30 sec at room
temperature with a freshly prepared 3,3'-diaminoben-zidine solution
and counterstained using hematoxylin at room temperature for 45
sec. The sections were rehydrated through a graded series of
ethanol, washed with xylene and mounted at room temperature. A Carl
Zeiss light microscope (magnification, ×20) was used to estimate
the expression of E2F1, Cdc25a and Cdk2.
Statistical analysis
The statistical analysis was performed using SPSS
23.0 (SPSS Inc.). The data from the analysis of cell viability, the
apoptotic percentage and the number of cells in the S phase are
reported as the means ± standard error of the mean (SEM), and
one-way ANOVA test followed by Bonferroni's post hoc test was used
to determine the statistical significance of the differences among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Carboplatin and lobaplatin induce various
degrees of apoptosis and cell cycle changes in Y79 RB cells
To explore the toxicity of carboplatin and
lobaplatin toward Y79 RB cell viability, the cells were exposed to
various concentrations of carboplatin (0, 20, 40, 60 and 80
µg/ml) or lobaplatin (0, 5, 10, 20 and 40 µg/ml) for
2-3 days. Similarly, the inhibitory effects of carboplatin and
lobaplatin on Y79 cell proliferation in vitro were
determined using an MTT assay. Carboplatin and lobaplatin inhibited
Y79 cell viability in a dose-dependent manner (Fig. 1A and B, respectively). Cell
viability decreased gradually with the increasing concentrations of
carboplatin and lobaplatin; at 72 h, cell viabilities were
251.7±6.692, 207.0±6.658 (P<0.01), 185.0±6.658 (P<0.01),
104.0±5.859 (P<0.001) and 50.33±5.239 (P<0.001) in the 0, 20,
40, 60 and 80 µg/ml carboplatin groups, respectively
(Fig. 1A) and 554.0±8.718,
460.7±6.360 (P<0.001), 375.3±2.603 (P<0.001), 286.3±8.172
(P<0.001 and 258.7±10.91 (P<0.001) in the groups treated with
0, 5, 10, 20 and 40 µg/ml lobaplatin, respectively (Fig. 1B).
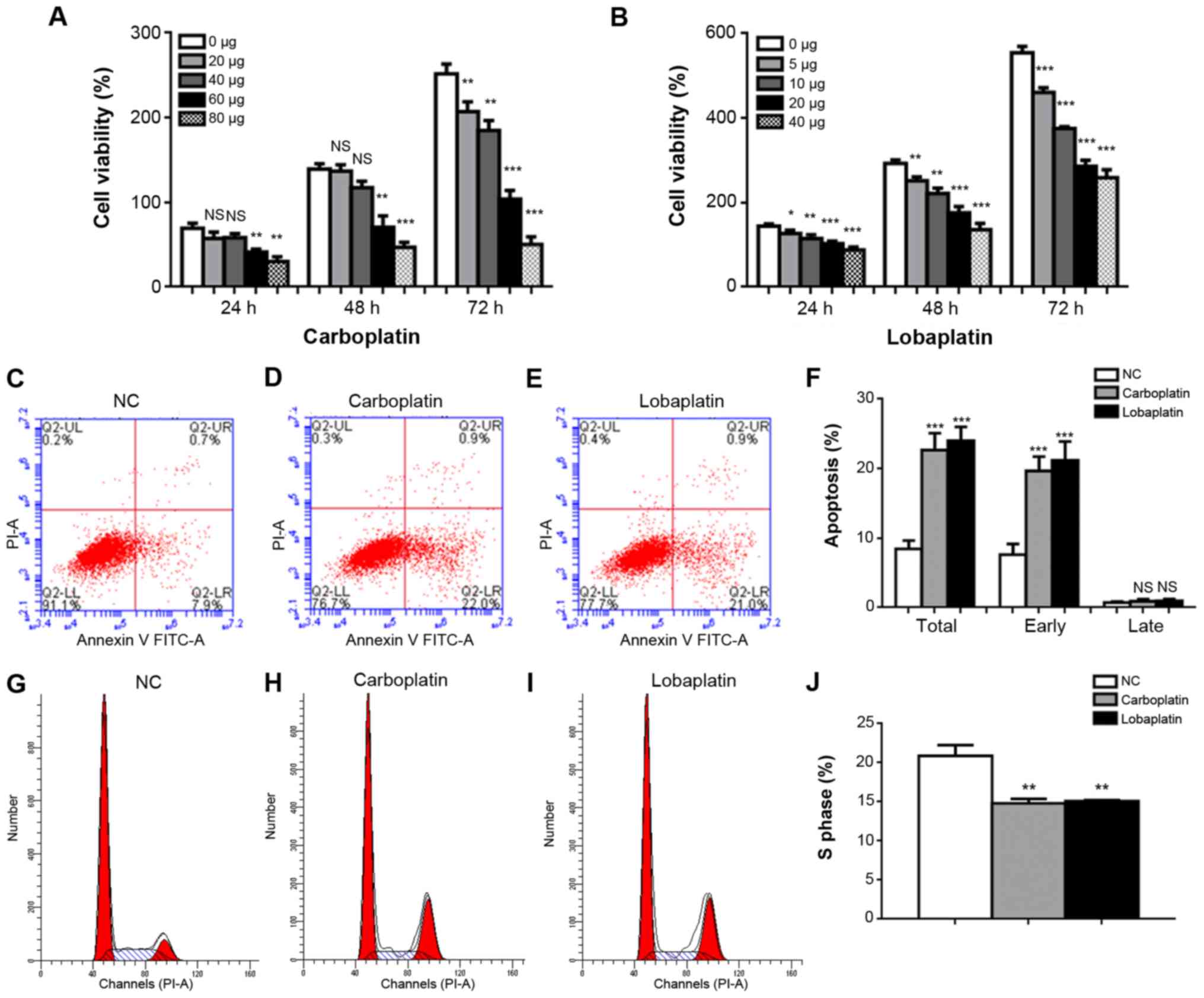 | Figure 1Carboplatin and lobaplatin induce
various degrees of apoptosis and cell cycle changes in Y79 cells.
Cell viability decreased gradually upon treatment with increasing
concentrations of (A) carboplatin (0, 20, 40, 60 and 80
µg/ml) or (B) lobaplatin (0, 5, 10, 20 and 40 µg/ml)
for 24, 48 or 72 h. The percentages of apoptotic and early
apoptotic cells in the carboplatin and lobaplatin groups differed
significantly from those in the NC group. (C-F) Representative
results of apoptosis in the NC, carboplatin, and lobaplatin groups.
(G-J) The proportions of cells in the S phase were lower in the
carboplatin and lobaplatin groups than in the NC group. NS, not
significant (P>0.05); *P<0.05,
**P<0.01 and ***P<0.001 vs. control (no
treatment) or NC group. |
Subsequently, flow cytometry was performed to assess
the effects of carboplatin and lobaplatin on Y79 cell early and
late apoptosis, and S phase cell cycle arrest. The total apoptotic
percentages were 8.467±0.696%, 22.67±1.419% (P<0.001 and
24.00±1.155% (P<0.001) in the control, carboplatin and
lobaplatin groups, respectively; the early apoptotic percentages
were 8.467±0.6960%, 21.63±1.450% (P<0.001) and 22.67±1.419%
(P<0.001), respectively; and the late apoptotic percentages were
0.7367±0.0857%, 0.9200±0.1620% (P= 0.814) and 0.9767±0.1690% (P=
0.769) in the control, carboplatin and lobaplatin groups,
respectively (Fig. 1C-F).
Additionally, the carboplatin and lobaplatin groups exhibited lower
proportions of Y79 cells arrested in the S phase than the negative
control (NC) group. The proportion of cells in S phase in the
control group was 22.49±1.51%, whereas the proportions in the
carboplatin and lobaplatin groups were 14.62±0.60% (P<0.01) and
14.99±1.20% (P<0.01), respectively. However, no significant
differences were observed between the carboplatin and lobaplatin
groups (P=0.8546) (Fig. 1G-J).
Gene expression changes in the
carboplatin- and lobaplatin-treated groups
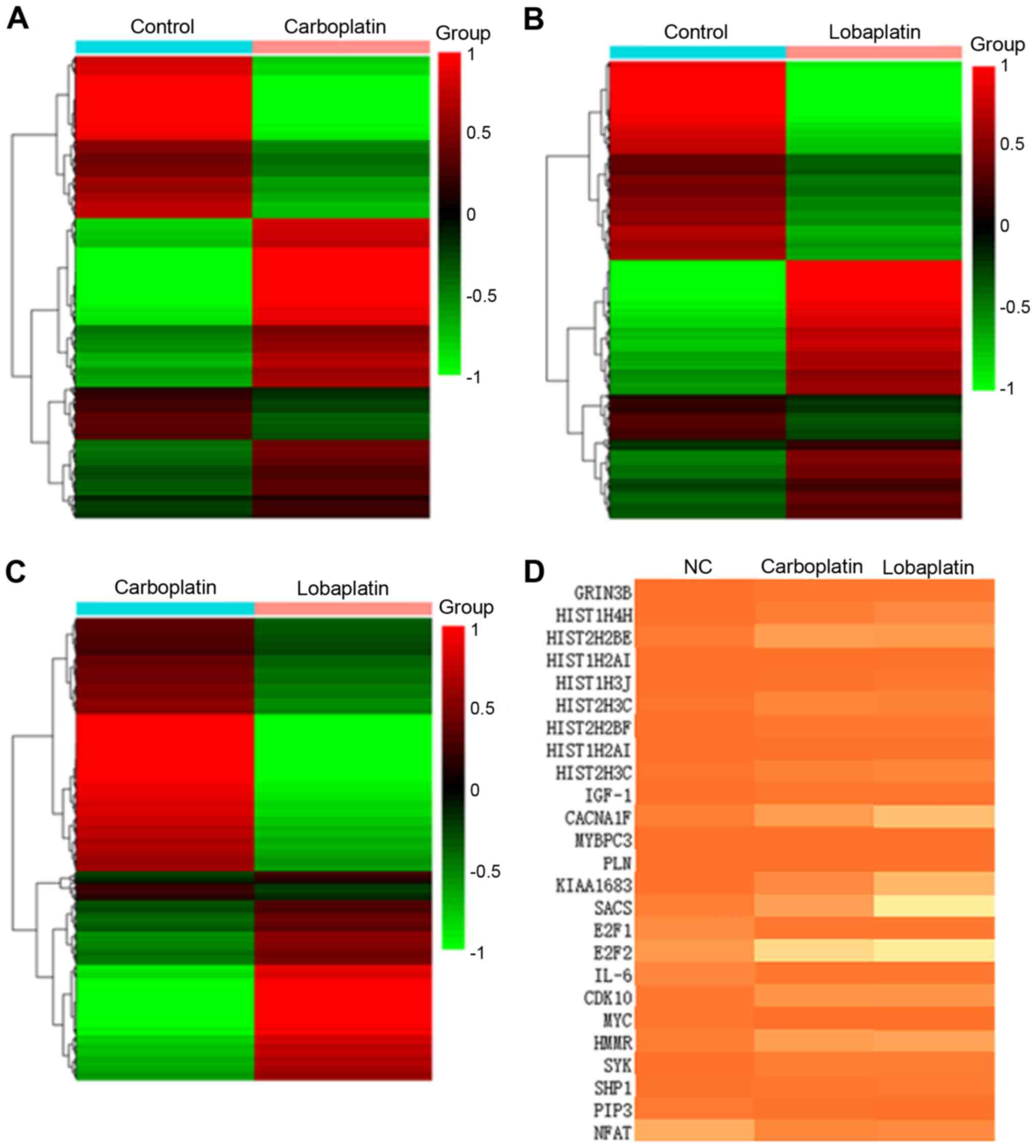
RNA-seq was performed to identify mRNA expression
patterns in the different groups (Fig.
2A-C) to assess the effects of lobaplatin and carboplatin on
Y79 cells. Cluster analysis revealed differences among the 3
groups, confirming that the RNA-seq data of the present study were
suitable for differential expression analysis (Fig. 2D). In total, 2,525 genes were
differentially expressed in the carboplatin group compared to the
control group (Fig. S1A-a),
including 1,353 upregulated genes and 1,172 downregulated genes. In
addition, 5,553 genes were differentially expressed in the
lobaplatin group compared to the control group (Fig. S1A-b), including 2,582 upregulated
genes and 2,971 downregulated genes, while 3,724 genes were
differentially expressed in the carboplatin group compared to the
lobaplatin group, including 1,534 upregulated genes and 2,190
downregulated genes (Fig. S1A-c).
The important biological functions were assessed in these 3 groups
through Gene Ontology (GO) enrichment analysis (Fig. S1B-a-c), and the results revealed
enrichment for 'E2F/IGF1/CACNA1F/TNNC1/TGFB3/CACNG6/
MYBPC3/CACNA2D3/ADCY1/PLN', 'E2F/IGF1/NUPR1/
HIST1H3J/HIST2H3C/HIST2H3A/HIST1H3B/HIST1H3E/ HIST1H3H/HIST1H3D'
and 'E2F/MYC/CDKN2B/HIPK4/ CALML6' in the groups (Fig. 2D). Notably, E2F1 was a significant
gene in all groups (Fig. 2D).
Expression of the transcription factors,
E2F1, Cdc25a and Cdk2, is decreased in carboplatin-treated and
lobaplatin-treated Y79 RB cells
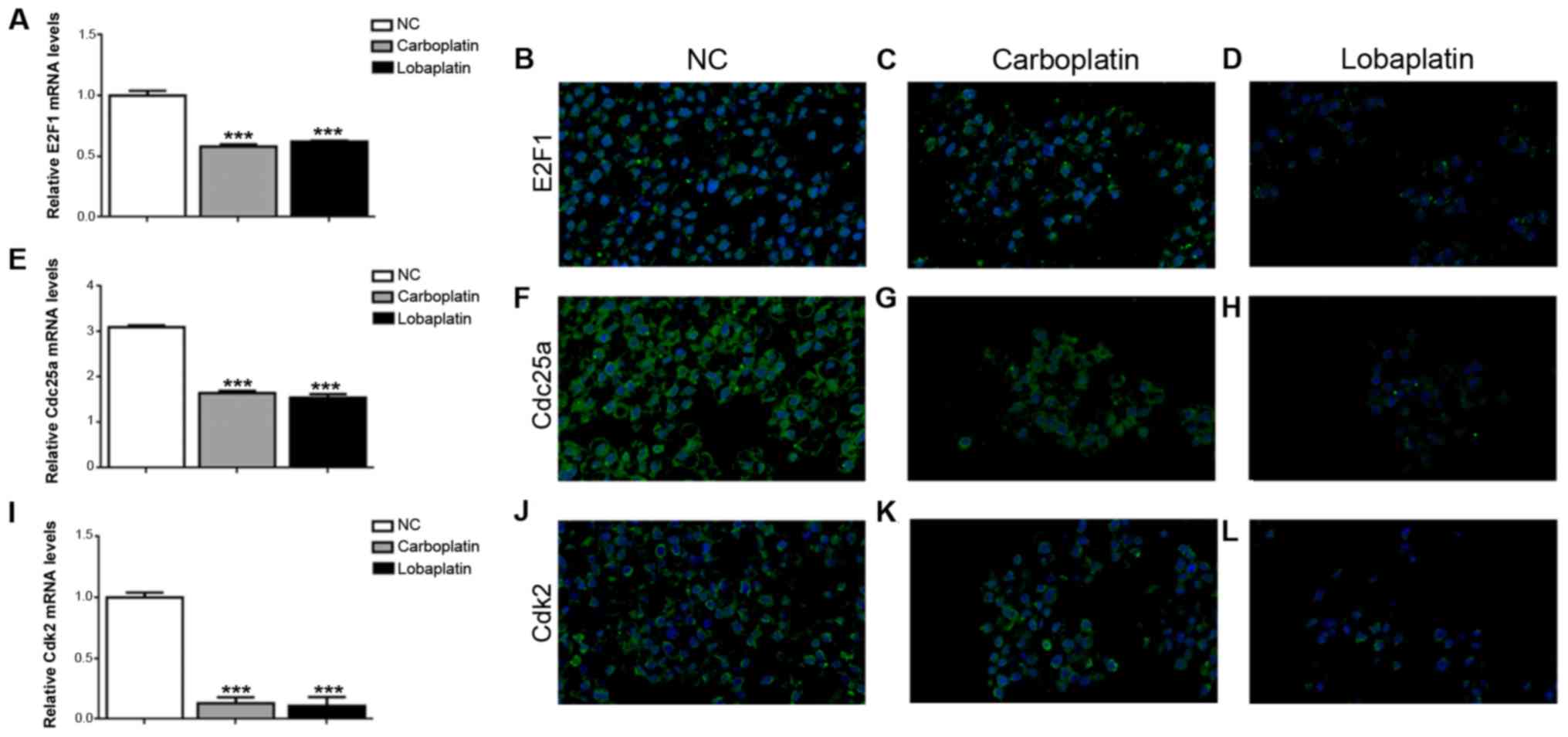
E2F1 is a transcriptional regulator of genes
essential for RB, and the present study detected the decreased
release of nuclear E2F1 in both the carboplatin-treated and
lobaplatin-treaded Y79 cells, further verifying the results of the
RNA-seq analysis. The protein and mRNA levels of E2F1 were
downregulated in the Y79 cells treated with carboplatin or
lobaplatin compared to the normal control Y79 cells, with mRNA
expression levels of 0.53±0.02 in the carboplatin group, lower than
those of 0.56±0.01 in the lobaplatin group (P<0.001) and
1.00±0.04 in the NC group (P<0.001) (Fig. 3A). The results of
immunofluorescence staining revealed similar tendencies in the E2F1
protein levels in these 3 groups (Fig.
3B-D). The Cdc25a mRNA expression levels were 3.10±0.04 in the
NC group, markedly higher than the levels of 1.65±0.05 (P<0.001)
and 1.55±0.07 (P<0.001) in the carboplatin and lobaplatin
groups, respectively (Fig. 3E),
and it displayed the least negative staining in the lobaplatin
group compared with the carboplatin and control groups (Fig. 3F-H). In addition, the Cdk2 mRNA
expression level was 1.00±0.04 in the NC group, markedly higher
than the levels of 0.12±0.003 (P<0.001) and 0.08±0.005
(P<0.001) in the carboplatin and lobaplatin groups, respectively
(Fig. 3I), in which the protein
levels exhibited similar same expression trends (Fig. 3J-L).
Carboplatin and lobaplatin at
pharmacological doses successfully inhibit the growth of human RB
xenografts in vivo
The mean tumor sizes determined at necropsy on days
7, 10, 14, 17, 21, 24 and 28 were 0.65±0.001, 5.63±0.002,
14.98±0.003, 26.47±0.001, 50±0.001, 115±0.002 and 220±0003,
respectively, in the control group; 0.73±0.001, 5.79±0.002,
15.12±0.003, 28.47±0.001, 48.61±0.001, 110±0.002 and 223±0003,
respectively, in the NC 1-week (W) group; 0.70±0.001, 5.74±0.002,
15.19±0.003, 28.17±0.001, 48.42±0.001, 118.44±0.002 and
213.52±0003, respectively, in the NC 2 W group; 0.72±0.001,
5.54±0.002, 15.31±0.003, 14.23±0.001, 6.21±0.001, 2.91±0.002 and
0.51±0003, respectively, in the carboplatin 1 W group; 0.76±0.001,
5.58±0.002, 14.85±0.003, 13.33±0.001, 5.98±0.001, 0±0.002 and
0±0003, respectively, in the carboplatin 2 W group; 0.76±0.001,
5.57±0.002, 15.48±0.003, 12.08±0.001, 5.01±0.001, 1.69±0.002 and
0.20±0003, respectively, in the lobaplatin 1 W group; and
0.70±0.001, 5.69±0.002, 15.21±0.003, 10.41±0.001, 5.21±0.001,
0±0.002 and 0±0003, respectively, in the lobaplatin 2 W group
(Fig. 4B and C).
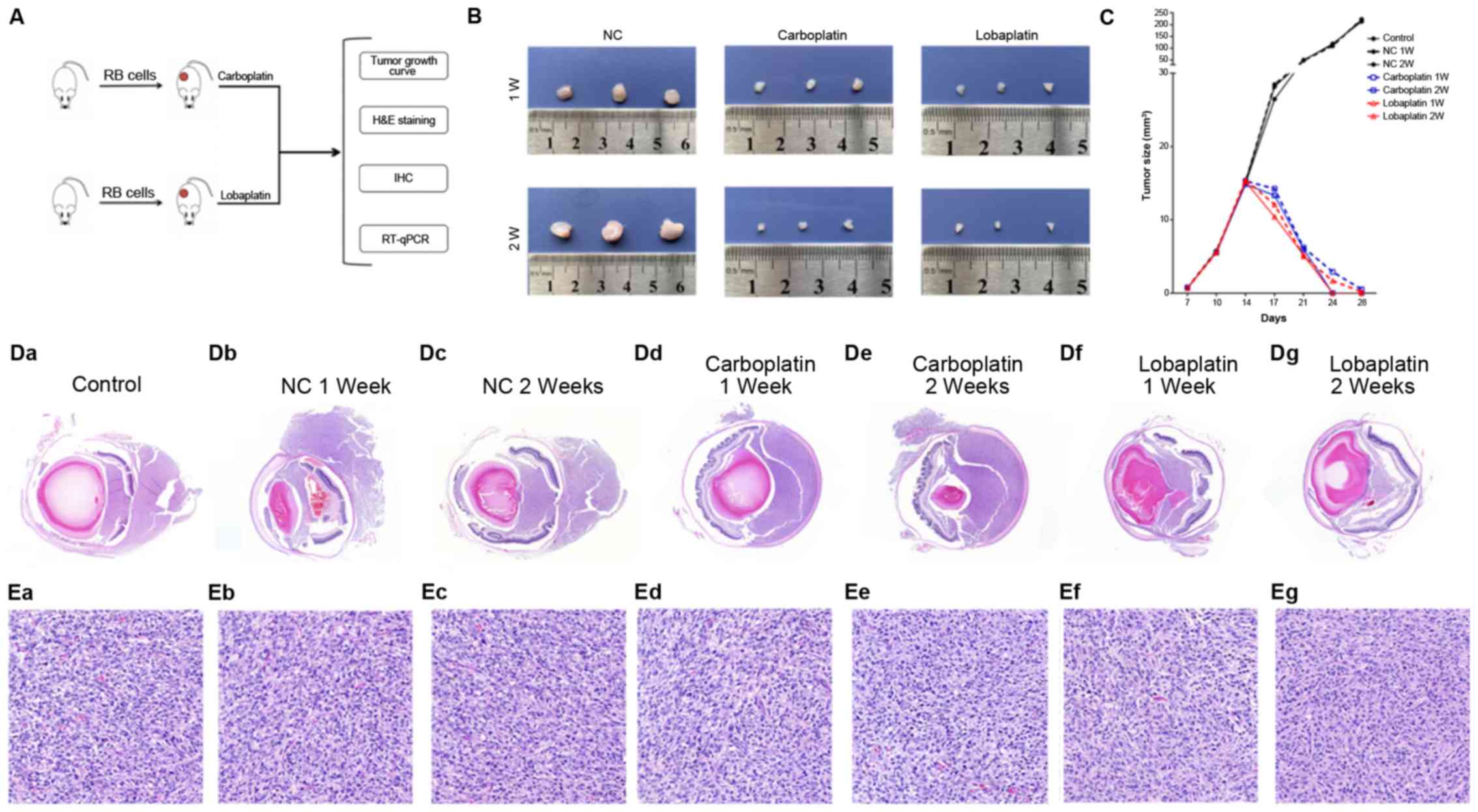 | Figure 4Carboplatin and lobaplatin at
pharmacologic doses successfully inhibit the growth of human RB
xenografts in vivo. (A) Animal experiments were divided into
4 analyses, including tumor growth curve generation, H&E
staining, immunohistochemistry, western blot analysis and RT-qPCR.
(B and C) Tumor size in nude mice was monitored after the injection
of carboplatin or lobaplatin from day 7 to day 28. H&E staining
of the (D-a-g) eyeball and (E-a-g) tumor tissue samples revealed
that tumor growth was significantly inhibited after the drug
injection, and the degree of inhibition mediated by lobaplatin was
greater than that mediated by carboplatin (E-a-g). In (D and E)
panels a-g represent the NC, control at 1 week, control at 2 weeks,
carboplatin at 1 week, carboplatin at 2 weeks, lobaplatin at 1 week
and lobaplatin at 2 weeks, respectively. Magnification, ×1 for
tumor size; ×20 for subcutaneous tissue H&E staining and ×2 for
eyeball H&E staining. H&E, hematoxylin and eosin. |
H&E staining revealed that cell proliferation
was significantly inhibited in both ocular and subcutaneous tumors.
In the control group and NC group, the tumor cells broke through
the basement membrane, and extradermal growth occurred. However,
the injection of carboplatin or lobaplatin significantly inhibited
tumor growth (Fig. 4D) and
significantly decreased cell division in subcutaneous tumors
(Fig. 4E).
E2F1, Cdc25a and Cdk2 expression is
decreased following carboplatin or lobaplatin treatment
The mRNA and protein levels of E2F1, Cdc25a and Cdk2
were evaluated following carboplatin or lobaplatin treatment. In
the 2nd week, the E2F1 mRNA expression level was 1.00±0.04 in the
NC group, evidently higher than the levels of 0.62±0.02
(P<0.001) and 0.54± 0.01 (P<0.001) in the carboplatin and
lobaplatin groups, respectively (Fig.
5A). Similarly, in the 2nd week, the Cdc25a mRNA expression
level was 3.10±0.04 in the NC group, evidently higher than the
levels of 1.66±0.05 (P<0.001) and 1.54±0.07 (P<0.001) in the
carboplatin and lobaplatin groups, respectively (Fig. 5B). In the 2nd week, the levels of
Cdk2 mRNA expression were 1.00±0.03 in the NC group, evidently
higher than the levels of 0.82±0.05 (P<0.01) and 0.77±0.07
(P<0.01) in the carboplatin and lobaplatin groups, respectively
(Fig. 5C). IHC further revealed
significant positive staining for E2F1 (Fig. 5D), Cdc25a (Fig. 5E) and Cdk2 (Fig. 5F) (depicted as brown dots) in
ocular and subcutaneous tumors. However, substantially less
positive staining was observed in both the carboplatin and
lobaplatin groups compared with the control and NC groups in the
1st and 2nd weeks after treatment.
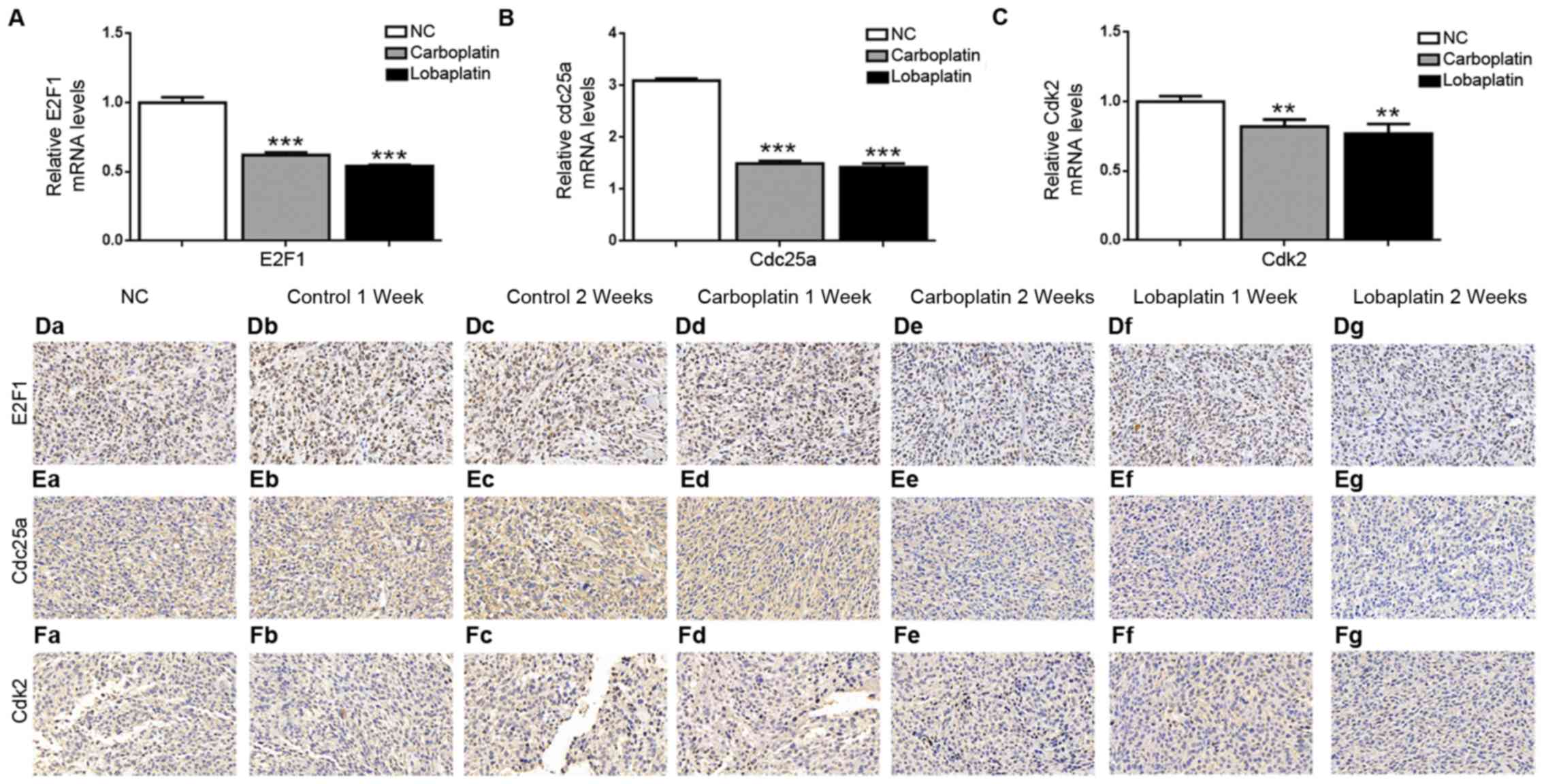 | Figure 5E2F1, Cdc25a and Cdk2 expression is
decreased following carboplatin or lobaplatin treatment in
vivo. RT-qPCR was employed to determine the mRNA levels of (A)
E2F1, (B) Cdc25a and (C) Cdk2 in the NC, carboplatin and lobaplatin
groups two weeks after the mice were treated. Immunohistochemical
staining of tumor tissue samples revealed marked decreases in
(D-a-g) E2F1-, (E-a-g) Cdc25a- and (F-a-g) Cdk2-positive cell
frequencies; panels a-g represent the NC, control at 1 week,
control at 2 weeks, carboplatin at 1 week, carboplatin at 2 weeks,
lobaplatin at 1 week and lobaplatin at 2 weeks, respectively.
Magnification, ×20. NS, not significant (P>0.05);
**P<0.01 and ***P<0.001 vs. NC
group. |
Discussion
RB, a malignant intraocular tumor known to be most
common among children (21), is
often associated with distant metastasis and can be fatal if left
untreated (22). Chemotherapy with
drugs, such as carboplatin, etoposide and vincristine along with
local consolidation treatment is the primary treatment strategy for
RB. This strategy has improved patient survival rates to ≥95%
higher (23). Recently, novel
drugs have been developed for the treatment of RB. Platinum drugs
are commonly used to treat malignancies; however, toxicity and
resistance have limited their application in the clinical settings
(24). Carboplatin is a
conventional chemotherapeutic drug that has been used in the past
few years for the treatment of RB; it has achieved good results in
past research and clinical applications, but is accompanied by
substantial side-effects (25,26).
The third-generation platinum derivative lobaplatin is a novel
chemotherapeutic drug that has shown strong antitumor activity in
different malignancies with fewer side-effects than carboplatin
(10). However, to the best of our
knowledge, no study to date has demonstrated the therapeutic effect
of lobaplatin on RB. In the present study, it was confirmed that
carboplatin and lobaplatin both exert antitumor effects on RB by
inhibiting the E2F1 signaling pathway and that lobaplatin has lower
cytotoxicity and a higher efficacy than carboplatin. The antitumor
activity of lobaplatin results from the formation of DNA-drug
adducts, mainly as GG and AG intra-strand cross-links. Lobaplatin
influences the expression of the c-myc gene, which is involved in
oncogenesis, apoptosis and cell proliferation. Carboplatin activity
is very similar to lobaplatin, as it binds with DNA and affects
replication; however, it can cause severe complications. In the
course of clinical treatment, it is always accompanied by certain
side-effects, such as autotoxicity, bone marrow suppression,
nephrotoxicity and alopecia. To further explore more effective
therapy for RB, the present study investigated the third-generation
platinum derivative lobaplatin, a novel chemotherapeutic drug, that
has exhibited potent antitumor activity with fewer side-effects
than carboplatin in non-small-cell lung and metastatic breast
cancer (27,28). In addition, the present study
compared the therapeutic effects with a two-sided inequality test
and concluded that lobaplatin exhibited lower cytotoxicity and
exerted more prominent therapeutic effects than carboplatin on RB
cells in vitro and in mice in vivo.
Several preclinical studies have established the
antitumor activity of lobaplatin and have investigated the
underlying mechanisms in multiple malignancies (11,29-31);
for example, lobaplatin has been found to induce caspase-dependent
apoptosis and to increase the Bax/Bcl-2 ratio in esophageal
squamous cell carcinoma (29).
Lobaplatin may prevent cell cycle progression; similarly, it may
play significant roles in stimulating apoptosis, altering the
proteome and impeding invasion and migration. In addition,
lobaplatin can reduce E2F1, cyclin D1, matrix metalloproteinase
(MMP)-2, MMP-9, CDK4, CDK6 and Bcl-2 expression and/or upregulate
p53, Bax, poly(ADP-ribose) polymerase (PARP), caspase-3, caspase-8
and caspase-9 expression. The present study revealed that in the
Y79 RB cells, both carboplatin and lobaplatin function as potent
antitumor agents. The cytotoxic effects of carboplatin and
lobaplatin against Y79 RB cells were determined using cell
viability assays. The results revealed that carboplatin and
lobaplatin diminished Y79 cell proliferation in a dose- and
time-dependent manner and caused the cells to swell significantly.
The colony formation of the Y79 cells was also significantly
reduced by carboplatin and lobaplatin. Over time, low-dose
lobaplatin (≤10 µg/ml) inhibited tumor cell growth to a
greater degree than low-dose carboplatin, although high-dose
carboplatin (≥60 μg/ml) inhibited tumor cell growth to a greater
degree than high-dose lobaplatin.
Tumorigenesis is associated with malfunctions in
apoptosis, the induction of which is a vital mechanism of antitumor
agents (32). Apoptosis induction
and apoptosis levels are commonly determined to assess antitumor
drugs. It has been found that lobaplatin causes substantial
apoptosis in a number of cancer cell lines at various
concentrations (32). In the
present study, the apoptotic percentage of the Y79 RB cells was
found to increase several-fold following treatment with carboplatin
or lobaplatin in a dose-dependent manner. The total cell apoptotic
percentage for carboplatin was higher than that for lobaplatin,
although lobaplatin induced significantly higher rates of early
apoptosis than carboplatin, with no significant difference in the
late apoptotic percentage. The analysis of the effects of
carboplatin and lobaplatin on the cell cycle in cultured cells
revealed that the cells were arrested in either the G1 phase or S
phase, and/or that the increases in the proportions of cells in the
sub-G0/G1 populations were cell type-dependent. Similarly, cell
cycle analysis revealed that carboplatin and lobaplatin treatment
substantially increased the proportions of Y79 cells in the G0/G1
phase, while it reduced the proportions in the S phase. The results
of the present study regarding cell cycle distribution are fairly
consistent with those of another study which used lobaplatin for
the treatment of non-small cell lung cancer (33).
A number of signaling pathways that play roles in
tumor progression have been described, and a number of different
cytokines can promote or inhibit tumorigenesis (34-36).
Through gene screening and signaling pathway analyses, it was found
that carboplatin and lobaplatin both suppressed tumor cell
proliferation by inhibiting the E2F1/Cdc25a/Cdk2 pathway,
particularly E2F1, which plays a significant and unique role in
promoting the proliferation of tumor cells. Cell cycle progression
is mediated by 2 regulators: E2F1 and pRB. These regulators
determine the progression of cells through the G1/S and G2/M
checkpoints, which indicate whether a cell can proceed with DNA
replication and cell division, respectively. The phosphorylation of
pRB at specific amino acid residues by cyclin-dependent kinases
(CDKs) inhibits heterodimerization with E2F1, while allowing E2F1
to be transcriptionally active. On the other hand, the
dephosphorylation of pRB encourages heterodimerization with E2F1,
while hindering E2F1 activity (37). E2F1 is an important transcription
factor for a number of key proteins that can move cells through the
G1/S transition and the S phase; thus, the progression of the cell
cycle may be stalled if the activity of E2F1 activity is hindered.
Out-of-control cell growth may be triggered by the overexpression
of E2F1, which leads to tumorigenesis and cancer (38). By contrast, tumor growth, DNA
repair issues and other anomalies may result from the blockade of
E2F1 expression via the inhibition of apoptosis (39,40).
Neoplastic cell growth may also result from the E2F1-mediated
activation of a transactivator of DNA synthesis genes. In the
present study, the expression levels of E2F1 were significantly
lower in both the carboplatin and lobaplatin groups than in the
control group. The levels of Cdc25a and Cdk2, which can promote
tumor proliferation, were also significantly decreased following
treatment with carboplatin or lobaplatin; thus, it was deduced that
the E2F1/Cdc25a/Cdk2 pathway may be relevant to the therapeutic
effects of carboplatin and lobaplatin on RB.
In the present study, in in vivo experiments,
both carbo-platin and lobaplatin were found to exert beneficial
effects against tumor formation; the nude mice in the control group
exhibited very rapid tumor growth, while those in the carbo-platin
and lobaplatin groups were sensitive to treatment. At the same
time, it was found that the inhibitory effects of lobaplatin on
tumors were more potent than those of carboplatin at both 1 and 2
weeks following treatment. The degree of tumor regression following
lobaplatin treatment was significantly higher than that following
carboplatin treatment. This finding indicates that both carboplatin
and lobaplatin inhibit tumor growth and that the therapeutic
effects of lobaplatin were more prominent than those of
carboplatin. In previous studies, lobaplatin has been shown to be
effective in inhibiting esophageal squamous cell carcinoma and
colorectal cancer tumors (11,31).
Thus, it can be concluded that lobaplatin exerts similar inhibitory
effects on RB.
H&E staining of the eyeball and tumor tissue
samples revealed that tumor growth was significantly inhibited
following treatment and that lobaplatin caused a greater degree of
inhibition than carboplatin. These results suggest that these drugs
inhibit tumor growth. According to the immunohistochemical analysis
of the tumor tissue, the expression levels of E2F1, Cdc25a and Cdk2
in the tumors were significantly reduced following the injection of
carboplatin or lobaplatin, and the reductions were in direct
proportion to the degrees of tumor inhibition. In a previous study,
arsenic was found to inhibit the phosphorylation of pRB at
particular sites due to inhibition of the actions of E2F1 and
CyclinE2/CDK2, contributing to decreases in the transcription of
CCNE2 and the phosphatase activity of CDC25a and thus preventing
dephosphorylation of CDK2 (35).
Therefore, it can be speculated that these drugs negatively
regulate tumors through the E2F1/Cdc25a/Cdk2 signaling pathway.
In conclusion, the present study demonstrates that
lobaplatin significantly inhibited the growth of RB with lower
cytotoxicity and a higher efficiency than carboplatin, suppressing
Y79 tumor cell proliferation by inhibiting the E2F1/Cdc25a/Cdk2
signaling pathway. However, a limitation of the present study has
to be stated in that only 1 stem cell line was used in the in
vitro experiments and thus further studies using other cell
lines are required to verify the therapeutic effects of lobaplatin
is meaningful clinically. On the whole, it can be concluded that
lobaplatin may prove to be an efficient and high-performing drug
for the treatment of RB and that further clinical evaluations
should be conducted.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study supported by the Natural Science
Foundation of Guangdong Province (grant no. 2015A03033878).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available at https://pan.baidu.com/s/1VDY1qaM_niaYpCEE7up4eA with
the password 'oyah'.
Authors' contributions
ZZ and JZ conceived and designed the experiments. ZZ
and JX performed the experiments. ZZ, HJ and JX analyzed the data.
ZZ and HJ wrote the manuscript. ZZ, HJ, JX and JZ modified the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments complied with the guidelines
established by the Association for Research in Vision and
Ophthalmology for the use of animals in ophthalmic and vision
research and were approved by the Institutional Ethics Board of
Guangzhou Women and Children's Medical Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hayden B, Jockovich ME, Murray TG,
Kralinger MT, Voigt M, Hernandez E, Feuer W and Parel JM:
Iontophoretic delivery of carboplatin in a murine model of
retinoblastoma. Invest Ophthalmol Vis Sci. 47:3717–3721. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Castillo BV Jr and Kaufman L: Pediatric
tumors of the eye and orbit. Pediatr Clin North Am. 50:149–172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiman KG: The retinoblastoma gene: Role in
cell cycle control and cell differentiation. FASEB J. 7:841–845.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
MacCarthy A, Birch JM, Draper GJ,
Hungerford JL, Kingston JE, Kroll ME, Onadim Z, Stiller CA, Vincent
TJ and Murphy MF: Retinoblastoma in Great Britain 1963-2002. Br J
Ophthalmol. 93:33–37. 2009. View Article : Google Scholar
|
|
5
|
Shields CL, Mashayekhi A, Au AK, Czyz C,
Leahey A, Meadows AT and Shields JA: The International
Classification of Retinoblastoma predicts chemoreduction success.
Ophthalmology. 113:2276–2280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abramson DH, Lawrence SD, Beaverson KL,
Lee TC, Rollins IS and Dunkel IJ: Systemic carboplatin for
retinoblastoma: Change in tumour size over time. Br J Ophthalmol.
89:1616–1619. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yanık Ö, Gündüz K, Yavuz K, Taçyıldız N
and Ünal E: Chemotherapy in retinoblastoma: Current approaches.
Turk J Ophthalmol. 45:259–267. 2015. View Article : Google Scholar
|
|
8
|
Smith SJ, Smith BD and Mohney BG: Ocular
side effects following intravitreal injection therapy for
retinoblastoma: A systematic review. Br J Ophthalmol. 98:292–297.
2014. View Article : Google Scholar
|
|
9
|
Winter U, Buitrago E, Mena HA, Del Sole
MJ, Laurent V, Negrotto S, Francis J, Arana E, Sgroi M and Croxatto
JO: et a l: Pharmacokinetics, safety, and efficacy of intravitreal
digoxin in preclinical models for retinoblastoma. Invest Ophthalmol
Vis Sci. 56:4382–4393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McKeage MJ: Lobaplatin: A new antitumour
platinum drug. Expert Opin Investig Drugs. 10:119–128. 2001.
View Article : Google Scholar
|
|
11
|
Shan L, Bai B, Lv Y, Xie B, Huang X and
Zhu H: Lobaplatin suppresses proliferation and peritoneal
metastasis of colorectal cancer in a preclinical model. Biomed
Pharmacother. 108:486–491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin CY, Lin XL, Tian L, Ye M, Yang XY and
Xiao XY: Lobaplatin inhibits growth of gastric cancer cells by
inducing apoptosis. World J Gastroenterol. 20:17426–17433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie CY, Xu YP, Jin W and Lou LG: Antitumor
activity of lobaplatin alone or in combination with antitubulin
agents in non-small-cell lung cancer. Anticancer Drugs. 23:698–705.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Cao H, Yu C and Feng Y: Lobaplatin
inhibits prostate cancer progression in part by impairing AR and
ERG signal. Fundam Clin Pharmacol. 32:548–557. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wheate NJ, Walker S, Craig GE and Oun R:
The status of platinum anticancer drugs in the clinic and in
clinical trials. Dalton Trans. 39:8113–8127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Q, Cheng Y, Huang L, Bai Y, Liang J
and Li X: Inhibitory effect of carboplatin in combination with
bevacizumab on human retinoblastoma in an in vitro and in vivo
model. Oncol Lett. 14:5326–5332. 2017.PubMed/NCBI
|
|
17
|
Gombos DS, Hungerford J, Abramson DH,
Kingston J, Chantada G, Dunkel IJ, Antoneli CB, Greenwald M, Haik
BG, Leal CA, et al: Secondary acute myelogenous leukemia in
patients with retinoblastoma: Is chemotherapy a factor?
Ophthalmology. 114:1378–1383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Moisan F, Francisco EB, Brozovic A, Duran
GE, Wang YC, Chaturvedi S, Seetharam S, Snyder LA, Doshi P and
Sikic BI: Enhancement of paclitaxel and carboplatin therapies by
CCL2 blockade in ovarian cancers. Mol Oncol. 8:1231–1239. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang H, Liu HZ, Wang HB, Zhong JY, Yang
CX and Zhang B: Dexmedetomidine protects against cisplatin induced
acute kidney injury in mice through regulating apoptosis and
inflammation. Inflamm Res. 66:399–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tamboli A, Podgor MJ and Horm JW: The
incidence of retinoblastoma in the United States 1974 through 1985.
Arch Ophthalmol. 108:128–132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim DY, Choi JA, Koh JY and Yoon YH:
Efficacy and safety of aflibercept in in vitro and in vivo models
of retinoblastoma. J Exp Clin Cancer Res. 35:1712016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balmer A, Zografos L and Munier F:
Diagnosis and current management of retinoblastoma. Oncogene.
25:5341–5349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dilruba S and Kalayda GV: Platinum-based
drugs: Past, present and future. Cancer Chemother Pharmacol.
77:1103–1124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buckingham R, Fitt J and Sitzia J:
Patients' experiences of chemotherapy: Side-effects of carboplatin
in the treatment of carcinoma of the ovary. Eur J Cancer Care
(Engl). 6:59–71. 1997. View Article : Google Scholar
|
|
26
|
Kooijmans EC, Bökenkamp A, Tjahjadi NS,
Tettero JM, van Dulmen-den Broeder E, van der Pal HJ and Veening
MA: Early and late adverse renal effects after potentially
nephrotoxic treatment for childhood cancer. Cochrane Database Syst
Rev. 3:CD0089442019.PubMed/NCBI
|
|
27
|
Zhang H, Chen R, Wang X, Zhang H, Zhu X
and Chen J: Lobaplatin-induced apoptosis requires p53-mediated
p38MAPK activation through ROS generation in non-small-cell lung
cancer. Front Oncol. 9:5382019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Xu XY, Yan F, Sun WL, Zhang Y, Liu
DL and Shen B: Retrospective study of the efficacy and toxicity of
lobaplatin in combined chemotherapy for metastatic breast cancer.
OncoTargets Ther. 12:4849–4857. 2019. View Article : Google Scholar
|
|
29
|
Du L, Fei Z, Song S and Wei N: Antitumor
activity of lobaplatin against esophageal squamous cell carcinoma
through caspase dependent apoptosis and increasing the Bax/Bcl 2
ratio. Biomed Pharmacother. 95:447–452. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hua S, Kong X, Chen B, Zhuang W, Sun Q,
Yang W, Liu W and Zhang Y: Anticancer mechanism of lobaplatin as
monotherapy and in combination with paclitaxel in human gastric
cancer. Curr Mol Pharmacol. 11:316–325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan S, Sun Y, Sui D, Yang T, Fu S, Wang J,
Hui B, Xi R, He C and Zhang X: Lobaplatin promotes
radiosensitivity, induces apoptosis, attenuates cancer stemness and
inhibits proliferation through PI3K/AKT pathway in esophageal
squamous cell carcinoma. Biomed Pharmacother. 102:567–574. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun X, Lou LG, Sui DH and Wu XH:
Preclinical activity of loba-platin as a single agent and in
combination with taxanes for ovarian carcinoma cells. Asian Pac J
Cancer Prev. 15:9939–9943. 2014. View Article : Google Scholar
|
|
33
|
Zhang CY, Bao W and Wang LH:
Downregulation of p16(ink4a) inhibits cell proliferation and
induces G1 cell cycle arrest in cervical cancer cells. Int J Mol
Med. 33:1577–1585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korah J, Canaff L and Lebrun JJ: The
retinoblastoma tumor suppressor protein (pRb)/E2 promoter binding
factor 1 (E2F1) pathway as a novel mediator of TGFbeta induced
autophagy. J Biol Chem. 291:2043–2054. 2016. View Article : Google Scholar
|
|
35
|
Sheldon LA: Inhibition of E2F1 activity
and cell cycle progression by arsenic via retinoblastoma protein.
Cell Cycle. 16:2058–2072. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Y and Peng XW: The silencing of long
non-coding RNA ANRIL suppresses invasion, and promotes apoptosis of
retino-blastoma cells through the ATM-E2F1 signaling pathway.
Biosci Rep. 38:BSR201805582018. View Article : Google Scholar
|
|
37
|
Dyson N: The regulation of E2F by
pRB-family proteins. Genes Dev. 12:2245–2262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kent LN, Bae S, Tsai SY, Tang X,
Srivastava A, Koivisto C, Martin CK, Ridolfi E, Miller GC, Zorko
SM, et al: Dosage-dependent copy number gains in E2f1 and E2f3
drive hepatocellular carcinoma. J Clin Invest. 127:830–842. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Müller H, Bracken AP, Vernell R, Moroni
MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD and
Helin K: E2Fs regulate the expression of genes involved in
differentiation, development, proliferation, and apoptosis. Genes
Dev. 15:267–285. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Poppy Roworth A, Ghari F and La Thangue
NB: To live or let die - complexity within the E2F1 pathway. Mol
Cell Oncol. 2:e9704802015. View Article : Google Scholar : PubMed/NCBI
|