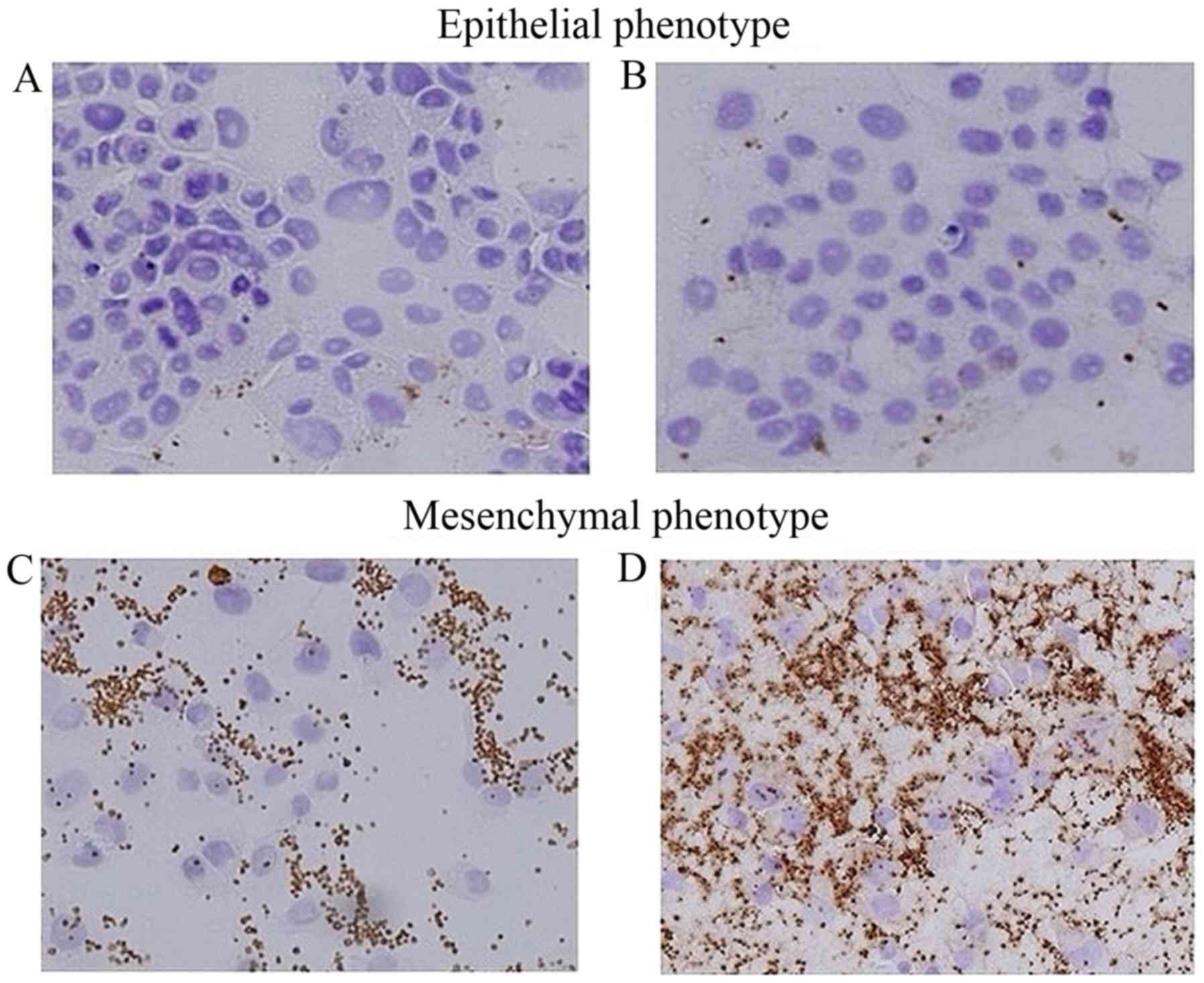
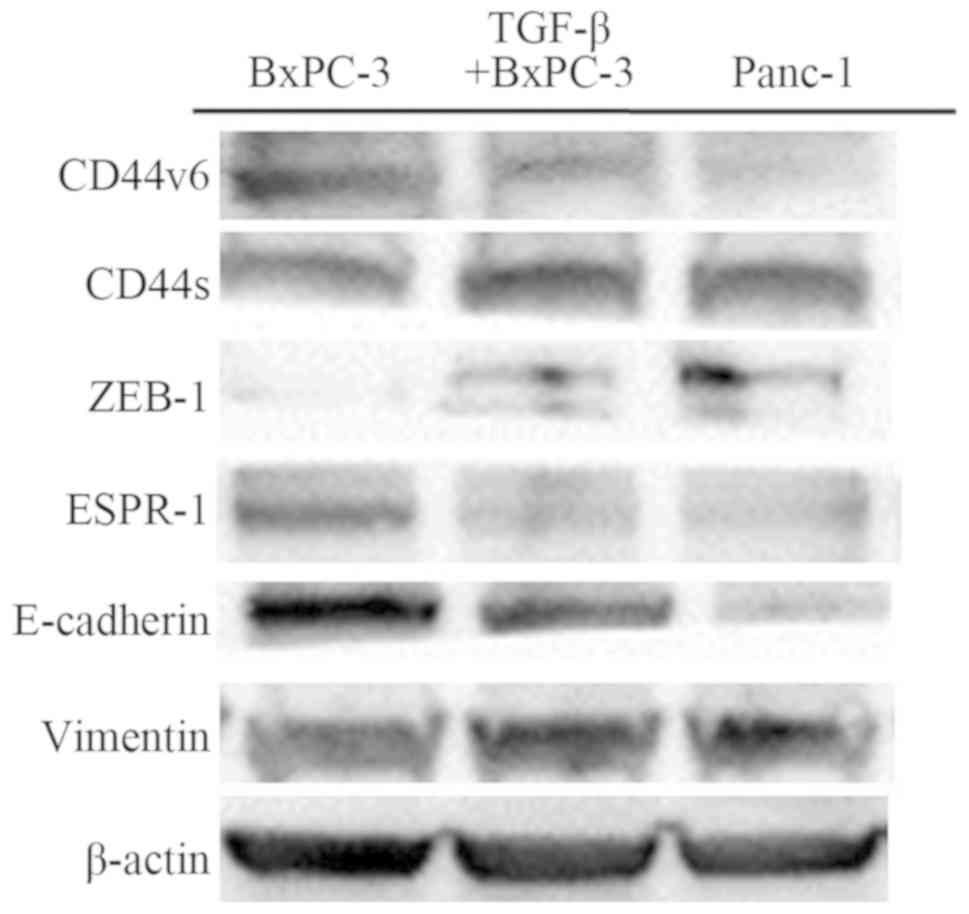
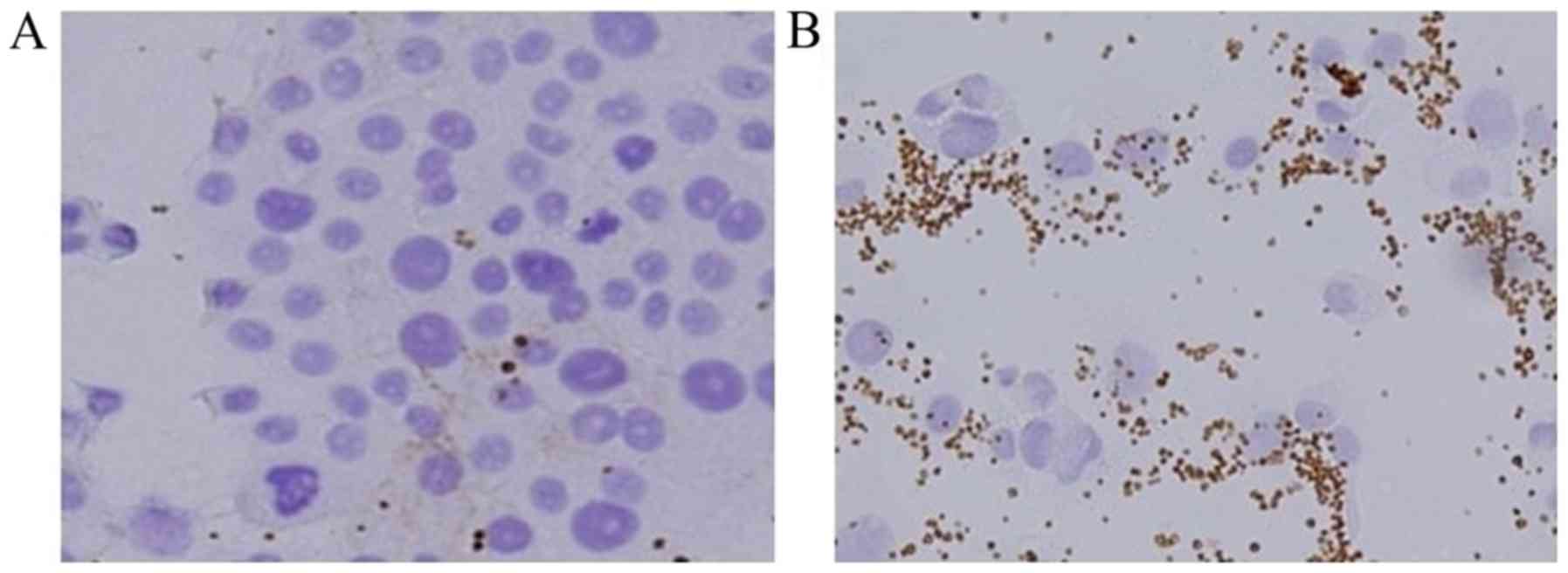
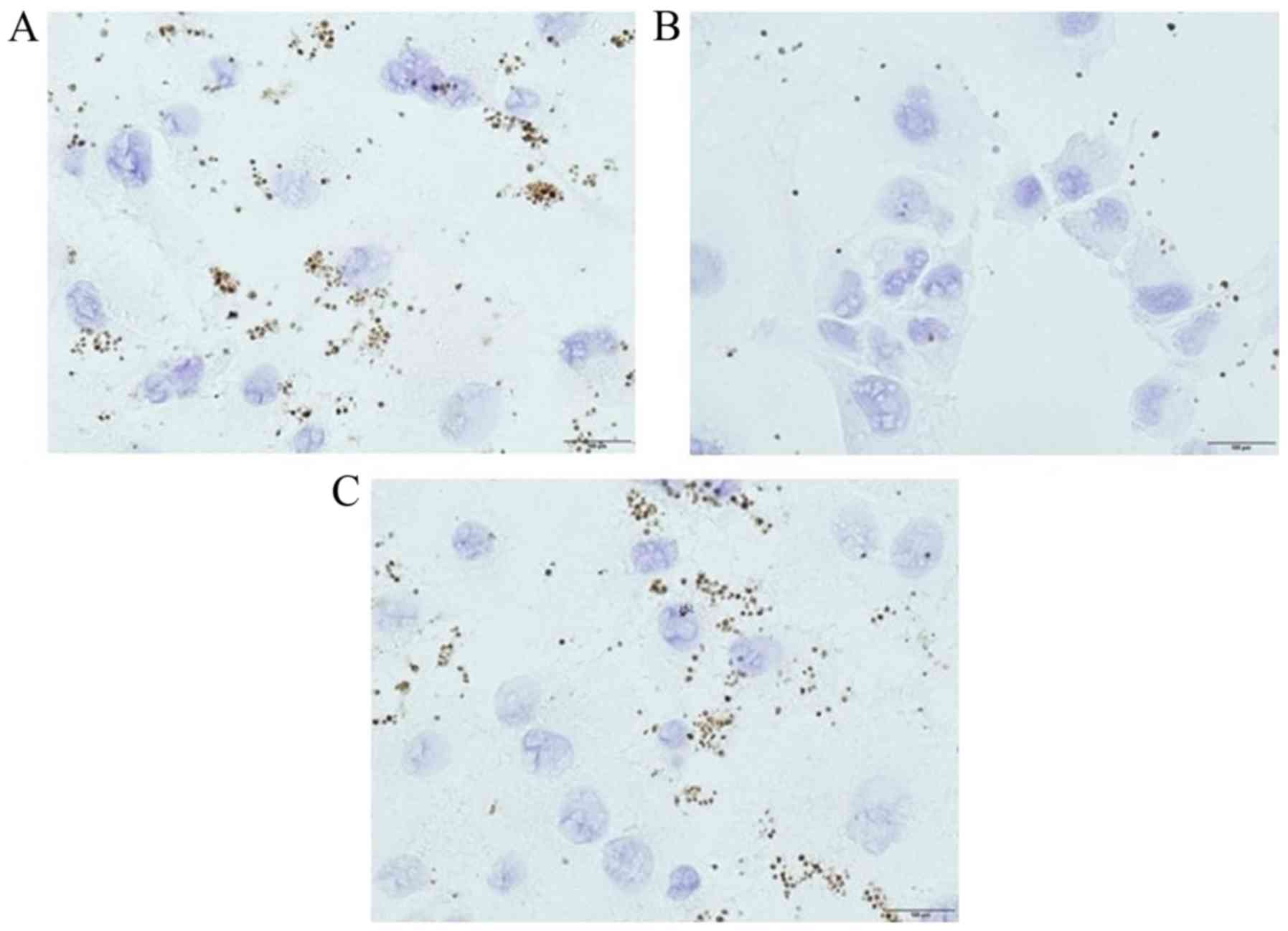
|
1
|
Pilleron S, Sarfati D, Janssen-Heijnen M,
Vignat J, Ferlay J, Bray F and Soerjomataram I: Global cancer
incidence in older adults, 2012 and 2035: A population-based study.
Int J Cancer. 144:49–58. 2019. View Article : Google Scholar
|
|
2
|
Carioli G, Malvezzi M, Bertuccio P, Hashim
D, Waxman S, Negri E, Boffetta P and La Vecchia C: Cancer mortality
in the elderly in 11 countries worldwide, 1970-2015. Ann Oncol.
30:1344–1355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farid SG, Aldouri A, Morris-Stiff G, Khan
AZ, Toogood GJ, Lodge JP and Prasad KR: Correlation between
postoperative infective complications and long-term outcomes after
hepatic resection for colorectal liver metastasis. Ann Surg.
251:91–100. 2010. View Article : Google Scholar
|
|
4
|
Ito H, Are C, Gonen M, D'Angelica M,
Dematteo RP, Kemeny NE, Fong Y, Blumgart LH and Jarnagin WR: Effect
of postoperative morbidity on long-term survival after hepatic
resection for meta-static colorectal cancer. Ann Surg.
247:994–1002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kataoka K, Takeuchi H, Mizusawa J, Igaki
H, Ozawa S, Abe T, Nakamura K, Kato K, Ando N and Kitagawa Y:
Prognostic impact of postoperative morbidity after esophagectomy
for esophageal cancer: Exploratory analysis of JCOG9907. Ann Surg.
265:1152–1157. 2017. View Article : Google Scholar
|
|
6
|
Lerut T, Moons J, Coosemans W, Van
Raemdonck D, De Leyn P, Decaluwé H, Decker G and Nafteux P:
Postoperative complications after transthoracic esophagectomy for
cancer of the esophagus and gastroesophageal junction are
correlated with early cancer recurrence: Role of systematic grading
of complications using the modified Clavien classification. Ann
Surg. 250:798–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braunwarth E, Primavesi F, Göbel G,
Cardini B, Oberhuber R, Margreiter C, Maglione M, Schneeberger S,
Öfner D and Stättner S: Is bile leakage after hepatic resection
associated with impaired long-term survival? Eur J Surg Oncol.
45:1077–1083. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:11–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogura M, Takeuchi H, Kawakubo H, Nishi T,
Fukuda K, Nakamura R, Takahashi T, Wada N, Saikawa Y, Omori T, et
al: Clinical significance of CXCL-8/CXCR-2 network in esophageal
squamous cell carcinoma. Surgery. 154:512–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamura A, Takeuchi H, Matsuda S, Ogura M,
Miyasho T, Nakamura R, Takahashi T, Wada N, Kawakubo H, Saikawa Y,
et al: Factors affecting cytokine change after esopha-gectomy for
esophageal cancer. Ann Surg Oncol. 22:3130–3135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pachot A, Cazalis MA, Venet F, Turrel F,
Faudot C, Voirin N, Diasparra J, Bourgoin N, Poitevin F, Mougin B,
et al: Decreased expression of the fractalkine receptor CX3CR1 on
circulating monocytes as new feature of sepsis-induced
immunosuppression. J Immunol. 180:6421–6429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Panis Y, Ribeiro J, Chrétien Y and
Nordlinger B: Dormant liver metastases: An experimental study. Br J
Surg. 79:221–223. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hackl C, Neumann P, Gerken M, Loss M,
Klinkhammer-Schalke M and Schlitt HJ: Treatment of colorectal liver
metastases in Germany: A ten-year population-based analysis of 5772
cases of primary colorectal adenocarcinoma. BMC Cancer. 14:8102014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J and Chen L: Current status and
progress in gastric cancer with liver metastasis. Chin Med J
(Engl). 124:445–456. 2011.
|
|
15
|
Murakami Y, Satoi S, Sho M, Motoi F,
Matsumoto I, Kawai M, Honda G, Uemura K, Yanagimoto H, Shinzeki M,
et al: National comprehensive cancer network resectability status
for pancreatic carcinoma predicts overall survival. World J Surg.
39:2306–2314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krenkel O and Tacke F: Liver macrophages
in tissue homeostasis and disease. Nat Rev Immunol. 17:306–321.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guilliams M, Dutertre CA, Scott CL,
McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger
M, De Prijck S, et al: Unsupervised high-dimensional analysis
aligns dendritic cells across tissues and species. Immunity.
45:669–684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nolan JP: The role of intestinal endotoxin
in liver injury: A long and evolving history. Hepatology.
52:1829–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crispe IN: The liver as a lymphoid organ.
Annu Rev Immunol. 27:147–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jenne CN and Kubes P: Immune surveillance
by the liver. Nat Immunol. 14:996–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies LC, Jenkins SJ, Allen JE and Taylor
PR: Tissue-resident macrophages. Nat Immunol. 14:986–995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y and Zychlinsky A:
Neutrophil extracellular traps kill bacteria. Science.
303:1532–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brinkmann V and Zychlinsky A: Neutrophil
extracellular traps: Is immunity the second function of chromatin?
J Cell Biol. 198:773–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Erpenbeck L and Schön MP: Neutrophil
extracellular traps: Protagonists of cancer progression? Oncogene.
36:2483–2490. 2017. View Article : Google Scholar
|
|
25
|
Chen R, Kang R, Fan XG and Tang D: Release
and activity of histone in diseases. Cell Death Dis. 5:e13702014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakurai K, Miyashita T, Okazaki M,
Yamaguchi T, Ohbatake Y, Nakanuma S, Okamoto K, Sakai S, Kinoshita
J, Makino I, et al: Role for neutrophil extracellular traps (NETs)
and platelet aggregation in early sepsis-induced hepatic
dysfunction. In Vivo. 31:1051–1058. 2017.PubMed/NCBI
|
|
27
|
Miyashita T, Ahmed AK, Nakanuma S, Okamoto
K, Sakai S, Kinoshita J, Makino I, Nakamura K, Hayashi H, Oyama K,
et al: A three-phase approach for the early identification of acute
lung injury induced by severe sepsis. In Vivo. 30:341–349.
2016.PubMed/NCBI
|
|
28
|
Placke T, Örgel M, Schaller M, Jung G,
Rammensee HG, Kopp HG and Salih HR: Platelet-derived MHC class I
confers a pseudonormal phenotype to cancer cells that subverts the
anti-tumor reactivity of natural killer immune cells. Cancer Res.
72:440–448. 2012. View Article : Google Scholar
|
|
29
|
Miyashita T, Tajima H, Makino I,
Nakagawara H, Kitagawa H, Fushida S, Harmon JW and Ohta T:
Metastasis-promoting role of extravasated platelet activation in
tumor. J Surg Res. 193:289–94. 2015. View Article : Google Scholar
|
|
30
|
Ishikawa S, Miyashita T, Inokuchi M,
Hayashi H, Oyama K, Tajima H, Takamura H, Ninomiya I, Ahmed AK,
Harman JW, et al: Platelets surrounding primary tumor cells are
related to chemoresistance. Oncol Rep. 36:787–774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
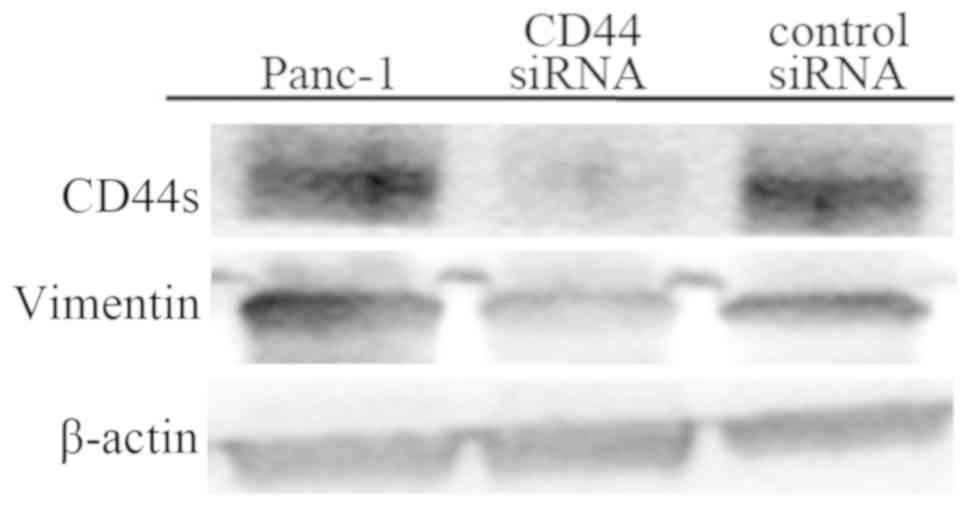
Zhao S, Chen C, Chang K, Karnad A,
Jagirdar J, Kumar AP and Freeman JW: CD44 expression level and
isoform contributes to pancreatic cancer cell plasticity,
invasiveness, and response to therapy. Clin Cancer Res.
15:5592–5604. 2016. View Article : Google Scholar
|
|
32
|
Ohta T, Nakagawara H, Arakawa H, Futagami
F, Tsukioka Y, Kitagawa H, Kayahara M, Nagakawa T and Miyazaki I: A
new strategy for the therapy of pancreatic cancer invasion and
metastasis by protease inhibitor and protein pump inhibitor agents.
Jpn J Gastroenterol Surg. 29:888–892. 1996. View Article : Google Scholar
|
|
33
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. 123:3446–3458. 2013.
View Article : Google Scholar
|
|
34
|
Qiao Y, Li J, Shi C, Wang W, Qu X, Xiong
M, Sun Y, Li D, Zhao X and Zhang D: Prognostic value of circulating
tumor cells in the peripheral blood of patients with esophageal
squamous cell carcinoma. Onco Targets Ther. 10:1363–1373. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Groot Koerkamp B, Rahbari NN, Büchler MW,
Koch M and Weitz J: Circulating tumor cells and prognosis of
patients with resectable colorectal liver metastases or widespread
meta-static colorectal cancer: A meta-analysis. Ann Surg Oncol.
20:2156–2165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rahbari NN, Aigner M, Thorlund K, Mollberg
N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M and Weitz
J: Meta-analysis shows that detection of circulating tumor cells
indicates poor prognosis in patients with colorectal cancer.
Gastroenterology. 138:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Negin BP and Cohen SJ: Circulating tumor
cells in colorectal cancer: Past, present, and future challenges.
Curr Treat Options Oncol. 11:1–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dotan E, Alpaugh RK, Ruth K, Negin BP,
Denlinger CS, Hall MJ, Astsaturov I, McAleer C, Fittipaldi P,
Thrash-Bingham C, et al: Prognostic significance of MUC-1 in
circulating tumor cells in patients with metastatic pancreatic
adenocarcinoma. Pancreas. 45:1131–1135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kurihara T, Itoi T, Sofuni A, Itokawa F,
Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tsuchida A, Kasuya K, et
al: Detection of circulating tumor cells in patients with
pancreatic cancer: A preliminary result. J Hepatobiliary Pancreat
Surg. 15:189–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tien YW, Kuo HC, Ho BI, Chang MC, Chang
YT, Cheng MF, Chen HL, Liang TY, Wang CF, Huang CY, et al: A high
circulating tumor cell count in portal vein predicts liver
metastasis from periampullary or pancreatic cancer: A high portal
venous CTC count predicts liver metastases. Medicine (Baltimore).
95:e34072016. View Article : Google Scholar
|
|
42
|
Richardson JJR, Hendrickse C, Gao-Smith F
and Thickett DR: Neutrophil extracellular trap production in
patients with colorectal cancer in vitro. Int J Inflam.
2017:49150622017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Engelmann B and Massberg S: Thrombosis as
an intravascular effector of innate immunity. Nat Rev Immunol.
13:34–45. 2013. View Article : Google Scholar
|
|
44
|
Nakanuma S, Miyashita T, Hayashi H, Tajima
H, Takamura H, Tsukada T, Okamoto K, Sakai S, Makino I, Kinoshita
J, et al: Extravasated platelet aggregation in liver zone 3 may
correlate with the progression of sinusoidal obstruction syndrome
following living donor liver transplantation: A case report. Exp
Ther Med. 9:1119–1124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miyashita T, Nakanuma S, Ahmed AK, Makino
I, Hayashi H, Oyama K, Nakagawara H, Tajima H, Takamura H, Ninomiya
I, et al: Ischemia reperfusion-facilitated sinusoidal endothelial
cell injury in liver transplantation and the resulting impact of
extravasated platelet aggregation. Eur Surg. 48:92–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Keysar SB and Jimeno A: More than markers:
Biological significance of cancer stem cell-defining molecules. Mol
Cancer Ther. 9:2450–2457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zoller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brabletz T: EMT and MET in metastasis:
Where are the cancer stem cells? Cancer Cell. 22:699–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alix-Panabières C, Mader S and Pantel K:
Epithelial-mesenchymal plasticity in circulating tumor cells. J Mol
Med (Berl). 95:133–142. 2017. View Article : Google Scholar
|
|
50
|
Bourcy M, Suarez-Carmona M, Lambert J,
Francart ME, Schroeder H, Delierneux C, Skrypek N, Thompson EW,
Jérusalem G, Berx G, et al: Tissue factor induced by
epithelial-mesenchymal transition triggers a procoagulant state
that drives metastasis of circulating tumor cells. Cancer Res.
76:4270–4282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cortés-Hernández LE, Eslami-S Z and
Alix-Panabières C: Circulating tumor cells as the functional aspect
of liquid biopsy to understand the metastatic cascade in solid
cancer. Mol Aspects Med. 72:1008162020. View Article : Google Scholar
|
|
52
|
Aceto N, Toner M, Maheswaran S and Haber
DA: En route to metastasis: Circulating tumor cell clusters and
epithelial-to-mesenchymal transition. Trends Cancer. 1:44–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu H, Ni YN, Liang ZA, Liang BM and Wang
Y: The effect of aspirin in preventing the acute respiratory
distress syndrome/acute lung injury: A meta-analysis. Am J Emerg
Med. 36:1486–1491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Boyle AJ, Di Gangi S, Hamid UI, Mottram
LJ, McNamee L, White G, Cross LJ, McNamee JJ, O'Kane CM and McAuley
DF: Aspirin therapy in patients with acute respiratory distress
syndrome (ARDS) is associated with reduced intensive care unit
mortality: A prospective analysis. Crit Care. 19:1092015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liao X, Lochhead P, Nishihara R, Morikawa
T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, et
al: Aspirin use, tumor PIK3CA mutation, and colorectal-cancer
survival. N Engl J Med. 367:1596–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sahasrabuddhe VV, Gunja MZ, Graubard BI,
Trabert B, Schwartz LM, Park Y, Hollenbeck AR, Freedman ND and
McGlynn KA: Nonsteroidal anti-inflammatory drug use, chronic liver
disease, and hepatocellular carcinoma. J Natl Cancer Inst.
104:1808–1814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Takada S, Miyashita T, Yamamoto Y, Kanou
S, Munesue S, Ohbatake Y, Nakanuma S, Okamoto K, Sakai S, Kinoshita
J, et al: Soluble thrombomodulin attenuates endothelial cell damage
in hepatic sinusoidal obstruction syndrome. In Vivo. 32:1409–1417.
2018. View Article : Google Scholar : PubMed/NCBI
|