Introduction
The phenomenon of 'living cell incorporation by
another cell (host cell)' was initially observed in 1925 (1). Humble et al (2) subsequently named this phenomenon
'emperipolesis', a Greek term meaning 'inside round about
wandering'. One of the histopathological characteristics of
emperipolesis is the formation of a 'clear halo' around the
engulfed living cells (3).
Emperipolesis is classified into three types: Megakaryocytic,
histiocytic and tumor cell emperipolesis (4). In detail, examples of emperipolesis
include lymphocyte incorporation by megakaryocytes (5), lymphocyte incorporation by
histiocytes in Rosai-Dolfman's disease (6,7) and
neutrophil incorporation (NI) by cancer cells (8). The most important criterion for
emperipolesis is that the engulfed cells are hematopoietic cells,
such as lymphocytes, plasma cells, erythrocytes or neutrophils
(3,4,8). A
similar halo formation around a living cell incorporated into host
cells is also observed in cell cannibalism; however, in this case
the halo-like structure around the incorporated cells is termed
'bird's eye' (9). In cell
cannibalism, the host cells use incorporated cells for their
nutritional support or incorporate immune cells to avoid immune
surveillance (9). Cell cannibalism
is frequently observed in several types of tumor, including lung
cancer, breast cancer and renal cell carcinoma (10,11).
In both emperipolesis and cell cannibalism, cell engulfment
involves active penetration into the host cells (12). By contrast, phagocytosis is a
process in which a cell surrounds and engulfs particles or dead
cells (13). Therefore, the
distinct halo formation in cell-in-cell phenomena (CiCP), including
emperipolesis and cell cannibalism, distinguishes them from
phagocytosis.
For cytological specimen evaluation, the focus is on
detection of atypical cells, and background cell characteristics,
such as components of inflammatory cells, are rarely considered
(14), for example in special
tumor cell types with lymphocyte infiltration such as Warthin tumor
of the salivary glands (15) and
seminoma of the testis/dysgerminoma of the ovary (16). Numerous studies describing
emperipolesis and cell cannibalism are either about experiments
that utilized cytological specimens or case reports (5,8,17-20).
However, in light of recent advancements in other techniques,
including immunocytochemistry, in-situ hybridization and reverse
transcription-quantitative PCR (21), cytological evaluations should
consider other characteristics, focusing on less frequently
observed findings such as CiCP, in addition to routinely observed
characteristics, including cellular atypia (22).
The present study aimed to analyze human cytological
specimens from the peritoneal cavity for CiCP and to evaluate its
significance in cytological evaluations.
Materials and methods
Ethics approval
The present study was approved by the Gunma
University Ethical Review Board (GUERB) for Medical Research
Involving Human Subjects of Gunma University School of Medicine,
and the written notification for the current study was presented
publicly on the webpage of Gunma University Hospital. Furthermore,
the possibility to decline participation in this study was provided
according to the Ethical Guidelines for Medical and Health Research
Involving Human Subjects of the Japanese government (Ministry of
Education, Culture, Sports, Science and Technology, and Ministry of
Health, Labour and Welfare) (23).
Informed consent was waived by the GUERB based on the
aforementioned guidelines due to the retrospective nature of the
study.
Cases and sample selection
The electronic health record system of Gunma
University Hospital (Maebashi, Japan) was reviewed, and 239
successive samples of peritoneal cavity fluid between January 2011
and December 2011 were selected for cytological specimen collection
of Papanicolaou staining (Pap smear), including ascites and
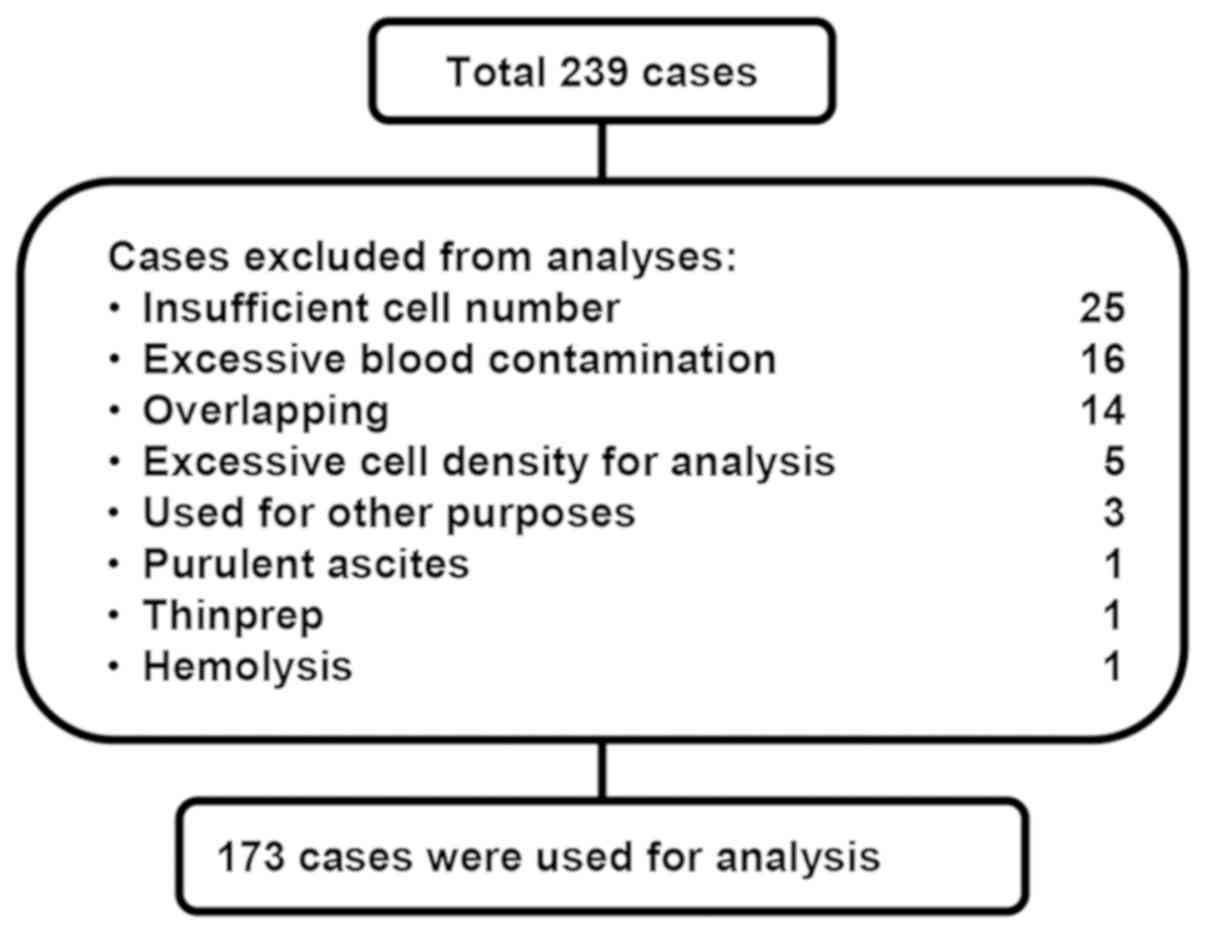
peritoneal washing. A total of 66 cases were excluded from the
present study for several reasons, including insufficient number of
cells or blood contamination (Fig.
1). The clinicopathological characteristics of each case are
summarized in Table I. The male to
female ratio of the utilized 173 samples was 38 to 135, and the
mean age was 57.22 years (age range, 13-89 years).
 | Table IClinicopathological summary of the
cases (n=173) used in the present study. |
Table I
Clinicopathological summary of the
cases (n=173) used in the present study.
| Organs | Histological
diagnosis | Cases, n |
|---|
| Stomach (n=43) |
Adenocarcinomaa | 41 |
| Gastrointestinal
stromal tumor | 2 |
| Uterine corpus
(n=30) | Endometrioid
carcinoma (n=24), serous carcinoma (n=2), mixed carcinoma
(n=1) | 27 |
| Carcinosarcoma
(n=2), endometrial stromal sarcoma (n=1) | 3 |
| Ovary (n=25) | Serous carcinoma
(n=7), endometrioid carcinomab
(n=5), clear cell carcinoma (n=4), Mucinous carcinoma (n=2),
malignant brenner tumor (n=1), carcinoma mixed subtypes (n=1) | 20 |
| Carcinosarcoma
(n=2), granulosa cell tumor (n=1), immature teratoma (n=1) | 4 |
| Malignant
(histology unknown) | 1 |
| Cervix (n=20) | Squamous cell
carcinoma (n=9) and carcinoma in situ (n=2) | 11 |
| Adenocarcinoma
(n=5) and adenocarcinoma in situ (n=2) | 7 |
| Adenosquamous
carcinoma | 2 |
| Others (malignant;
n=18) | Adenocarcinoma
(rectum n=3, bile duct n=1, pancreas n=1, total n=5), serous
carcinoma (fallopian tube, n=3), squamous cell carcinoma (esophagus
n=1, vagina n=1, total n=2), urothelial carcinoma (bladder, n=2),
mucinous neoplasm (appendix, n=1), Invasive ductal carcinoma
(breast, n=1), malignant lymphoma (lymph nodes, n=1) | 15 |
| Carcinoma of
unknown origin | 3 |
| Others
(borderline, | Borderline tumors
(ovary): mucinous (n=3), serous (n=2), endometrioid (n=1) | 6 |
| Precancerous;
n=8) | Cervical dysplasia:
squamous (n=1), glandular (n=1) | 2 |
| Others (benign;
n=22) | Benign (ovary)
teratoma (n=6), epithelial tumor (n=4), sex cord-stromal tumor
(n=2), cystic ovary (n=1) | 13 |
| Benign (uterine
corpus): leiomyoma (n=6), other lesions (n=3) | 9 |
| Unknown (n=7) | Unknown | 7 |
Evaluation of specimens, cytological
classification and grouping
Cytological classification was based on
Papanicolaou's classification (Table
II) (24). The specimens with
negative cytology, including 'normal' and 'reactive' cytology, were
defined as the 'negative group', while the specimens with
suspicious/malignant cytology, including 'suspicious', 'suspicious
for malignancy' and 'malignant' cytology, were defined as the
'positive group'.
 | Table IIGroups, classification and
definitions used in the present study for cytological evaluation
(n=173). |
Table II
Groups, classification and
definitions used in the present study for cytological evaluation
(n=173).
A, Negative group
|
|---|
| Cytological
classification | Definition | Cases, n |
|---|
| Normal | Absence of atypical
or abnormal cells | 9 |
| Reactive | Atypical cells
present but without abnormal features | 126 |
|
| B, Positive
group |
|
| Cytological
classification | Definition | Cases, n |
|
| Suspicious | Cells with abnormal
features suggestive but not conclusive for malignancy | 8 |
| Suspicious for
malignancy | Cells and cell
clusters fairly conclusive for malignancy | 3 |
| Malignant | Cells and cell
clusters conclusive for malignancy | 27 |
Screening and determination of CiCP
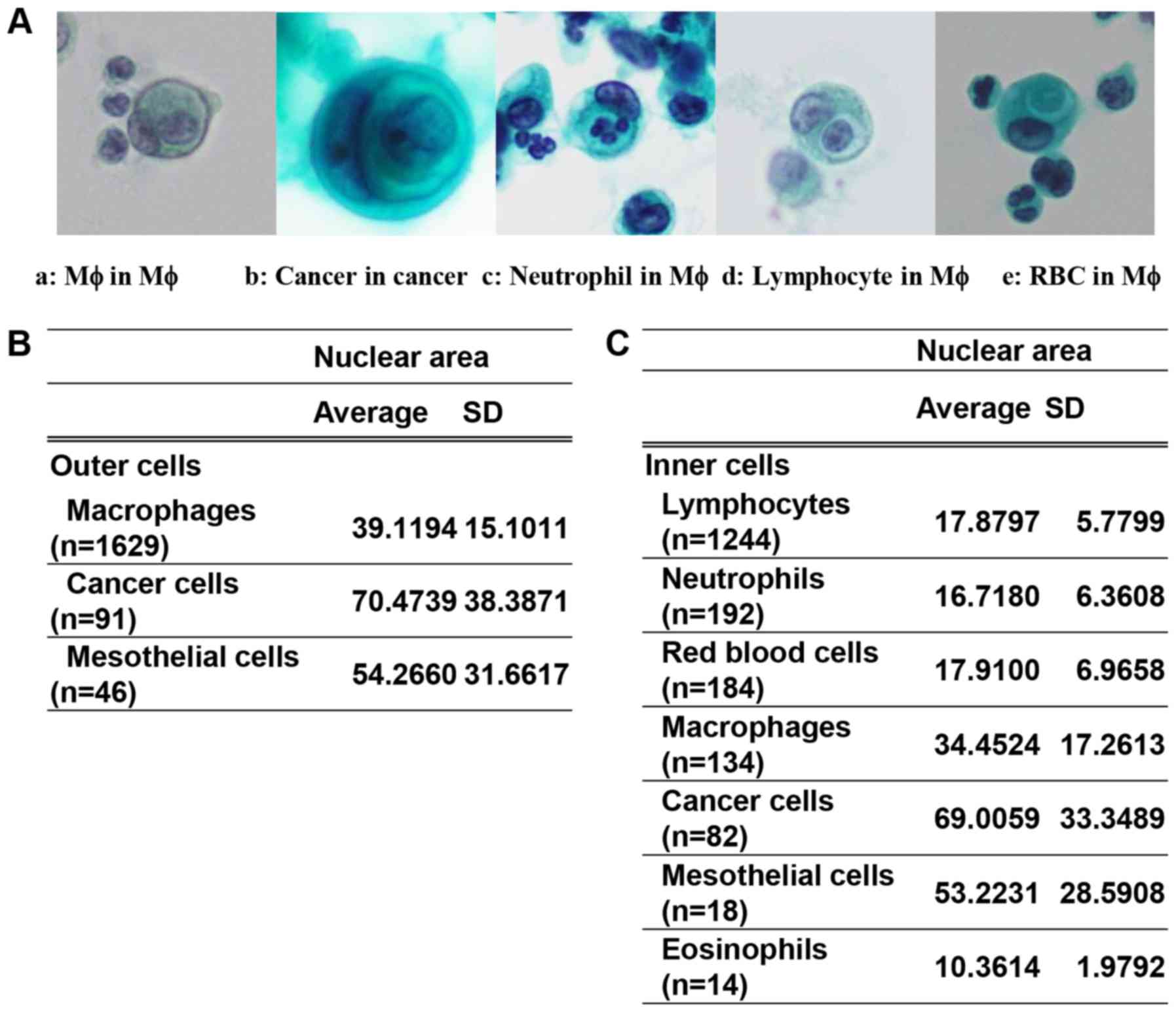
CiCP was defined as the presence of a clear
peri-cellular halo of the incorporated cells inside the host cells
(Fig. 2A). Smears from 173 cases,
prepared according to routine hospital protocols, were examined for
CiCP by manual screening of a ≥1-cm2 area of the smears.
Two observers independently screened each CiCP candidate on the
glass slides and marked it using a thin-tipped indelible marker.
CiCP candidate cells were imaged under a light microscope using a
×40 magnification (BX51; Olympus Corporation) and a digital camera
(DP-22; Olympus Corporation), and captured with auto-white balance
as a TIFF file (1,920×1,140 pixels) using an image capture software
(cellSens Standard v2.1; Olympus Corporation) and manual focusing.
Dead cells or unclear samples were excluded, while samples
exhibiting a clear 'peri-cellular halo of incorporated cells inside
host cells' were selected as CiCP. Furthermore, a cytopathologist
and a cytotechnologist characterized the types of host cells and
engulfed cells on the basis of their morphological
characteristics.
Evaluation of nuclear area (NA) for host
cell and incorporated cell in CiCP
The NA of each host and incorporated cell was
manually traced on the TIFF files and calculated using Image Pro
v10 image analysis software (Nippon Roper K.K.).
Whole-slide imaging and detection of a
singly detectable nucleus (SDN)
Whole-slide imaging of specimens was performed using
a Nanozoomer SQ Virtual Slide Scanner (VS; Hamamatsu Photonics
K.K.) with a ×40 magnification. The specifications of the
Nanozoomer SQ were as follows: Objective lens, ×20 with 0.75
numerical aperture; scan mode, ×40 mode; maximum capture size,
26×76 mm; pixel size, 0.23 µm/pixel; light source,
light-emitting diode; image-saving format, JPEG with compression;
and focus mode, autofocus. The VS image files were converted to
MRXS files using Slide converter (v1.14; 3DHISTECK Ltd.) and were
then analyzed using Pannoramic Viewer v1.15.4 and the Quant Center
HistoQuant module (both 3DHISTECK Ltd.). For identification of a
SDN using the aforementioned softwares, two general protocols were
used for small and large cells (10-85 mm2 and 86-150
mm2, respectively) in 100 specimens due to the
processing capacity of the computer used for analysis.
Subsequently, the separately analyzed data were combined. However,
for 26 specimens, different protocols were used for SDN detection
based on the condition of the specimens, for example the color of
the cells, the background color or debris. All image analysis
protocols are summarized in Table
SI. A total of 47 specimens were excluded due to impediments
arising from excessive background mucus, red blood cells,
hemosiderin-incorporated macrophages, pale cells, cell aggregates
or overlapping cells. The CiCP emergence rate (CER) per SDN was
defined as follows: CER/SDN (%)=total CiCP number/total SDN
×00.
Evaluation of white blood cell count
Data on white blood cell counts of the peripheral
blood samples collected at the closest date before ascites or
peritoneal washing sample collection were extracted from the
electronic health record system of Gunma University Hospital.
Statistical analysis
Statistical analyses were performed using JMP Pro
v12.2.0 software (SAS Institute, Inc.). The associations between
two categorical variables were calculated using the χ2
test, and the differences between the mean of two groups were
calculated using Welch's unpaired t-test. For non- parametric
comparisons between two groups, Wilcoxon rank-sum test was used.
For multiple pair-wise comparison, Steel-Dwass test was used. In
order to determine the cut-off value, receiver operating
characteristic (ROC) analysis was performed, and the value that
gave the highest sensitivity and 1-specificity was regarded as the
cut-off value. The data were presented as box plots (median and
interquartile range) or as the mean and SD. P<0.05 was
considered to indicate a statistically significant difference.
Results
Results of manual evaluation and
identification of incorporated cells and host cells in CiCP are
associated with those of the image analysis for NA evaluation
Among the 173 cases examined in the present study,
CiCP were detected in 105 cases. In these cases, as shown in
Fig. 2B and C, the host cells
included macrophages, mesothelial cells and cancer cells, while the
incorporated cells included lymphocytes, neutrophils, eosinophils,
mesothelial cells, macrophages, erythrocytes and cancer cells. A
total of 13 cases exhibited cell cannibalism: Adenocarcinoma of the
stomach (3 cases); serous carcinoma of the uterine corpus (2
cases); serous carcinoma of the ovary (2 cases); endometrioid
carcinoma of the ovary (1 case); carcinosarcoma of the ovary (1
case); granulosa cell tumor (1 case); malignant ovarian tumor
(histology unknown; 1 case); high-grade urothelial carcinoma (1
case); and carcinoma of unknown origin (1 case) (Table SII). Comparison of the NAs of the
host and incorporated cells among the groups revealed significant
differences, except for the pairwise comparison of incorporated
cells between erythrocytes and neutrophils, mesothelial cells and
cancer cells, and erythrocytes and lymphocytes (Table SIII). The present results
suggested mutual agreement between the results of manual evaluation
and image analysis.
Rate of CiCP-positive cases (RCPCs) and
CiCP emergence rate (CER) per SDN are significantly lower in
peritoneal washing than in ascites samples
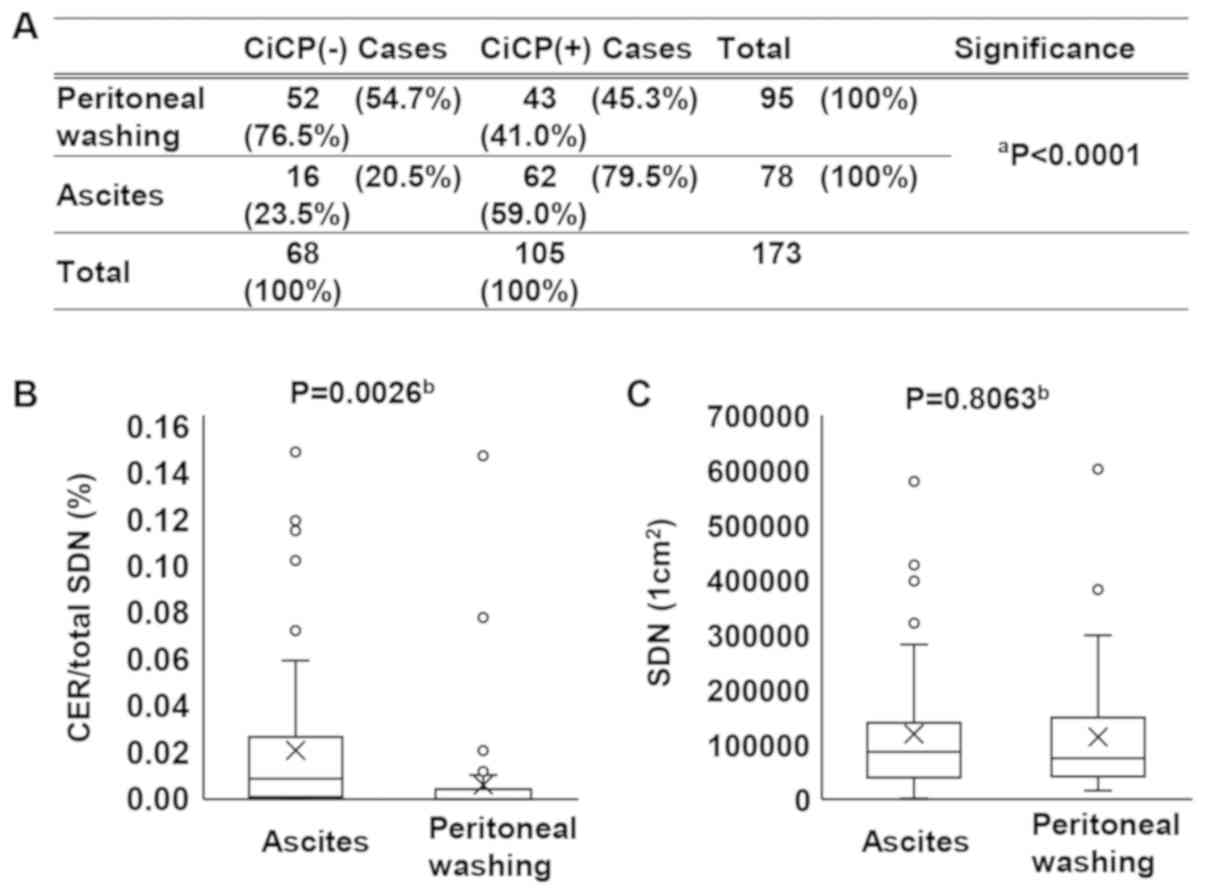
The RCPCs and CER per SDN were analyzed for both
peritoneal washing and ascites samples. The RCPCs and CER/SDN ratio
were significantly lower in the peritoneal washing samples than in
ascites samples. The percentage of the RCPCs was 45.3% in the
peritoneal washing samples and 79.5% in the ascites samples
(P<0.0001; Fig. 3A). On the
other hand, the CER/SDN was 0.006% in the peritoneal washing
samples and 0.020% in the ascites samples, as shown in Fig. 3B (P=0.0026; Fig. 3B). The average SDN number was
103,906 cells/cm2 in the peritoneal washing samples and
119,508 cells/cm2 in the ascites samples, but the
difference was not statistically significant (P=0.8063; Fig. 3C). The present results indicated
that in ascites, both the RCPCs and CER/SDN ratio were increased
compared with in peritoneal washing samples, and CiCP was present
in visually undetectable ascites (59% of CiCP-positive cases were
derived from ascites and 41% from peritoneal washing samples) in
the cytological specimens.
RCPCs and CER per SDN are significantly
higher in the cytologically positive group than in the negative
group
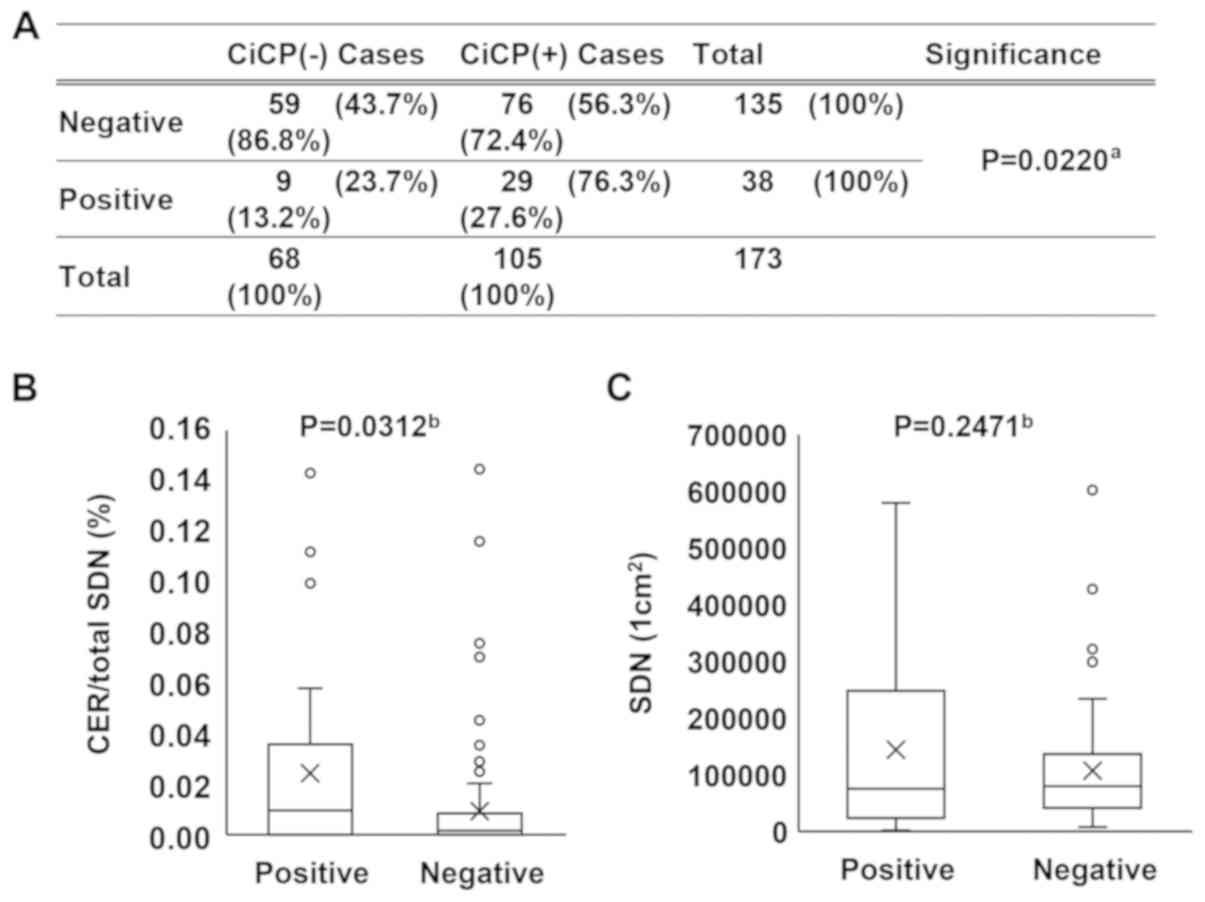
The RCPCs and CER/SDN ratio were analyzed for the
cytologically positive and negative groups. Fig. 4A indicated that there was a
significant association between CiCP status and cytological status
(P=0.0220). On the other hand, the CER/SDN was 0.009% in the
negative group and 0.024% in the positive group, as shown in
Fig. 4B (P=0.0312; Fig. 4B). The average number of SDN, as
shown in Fig. 4C, was 107,242
cells/cm2 in the negative group and 144,508
cells/cm2 in the positive group, but the difference was
not statistically significant (P=0.2471; Fig. 4C). The present results indicated
that both the RCPCs and CER/SDN ratio were increased in malignant
cytological samples.
Rate of NI in the total CiCP cells is
significantly higher in the cytologically positive group than in
the negative group
The components of CiCP for host cells and
incorporated cells were evaluated in each case based on the
classification of cytologically positive and negative groups, and
summarized in Table III.
Briefly, the rate of incorporated lymphocytes among the total
incorporated cells was significantly lower in the positive group
than in the negative group (P<0.0001; Table III), while the rate of
incorporated neutrophils among the total incorporated cells was
significantly higher in the positive group than in the negative
group (P=0.0288; Table III). By
contrast, the rate of incorporating host macrophages in the total
host cells was significantly lower in the positive group than in
the negative group (P=0.0083; Table
III). Except for cancer cells, rates of the other incorporated
cells among the total incorporated cells and the other
incorporating host cells (such as mesothelial cells) among the
total incorporating cells were not significantly different
(Table III). Neutrophils were
mostly incorporated by macrophages (data not shown). However, NI by
cells that were not macrophages was seldom observed (three
mesothelial cells and one cancer cell as host cells). Therefore,
the present data suggested that NI by macrophages, or neutrophil
emperipolesis, may be increased in malignant samples.
 | Table IIIComponents of cell-in-cell phenomena
in cytologically negative (n=76) and positive (n=29) groups. |
Table III
Components of cell-in-cell phenomena
in cytologically negative (n=76) and positive (n=29) groups.
A, Host cells
(outer cells)
|
|---|
| Cells | Mean component
ratio, % (SD)
| P-valuea |
|---|
| Cytologically
negative group | Cytologically
positive groups |
|---|
| Macrophages | 95.39 (16.88) | 76.00 (40.15) | 0.0083 |
| Cancer cells | 0 | 23.72 (39.79) | <0.0001 |
| Mesothelial
cells | 4.61 (16.88) | 0.29 (1.27) | 0.1964 |
|
| B, Incorporated
cells (inner cells) |
|
| Cells | Mean component
ratio, % (SD)
| P-valuea |
| Cytologically
negative group | Cytologically
positive group |
|
| Lymphocytes | 68.64 (34.56) | 35.82 (35.62) | <0.0001 |
| Neutrophils | 10.15 (22.80) | 17.56 (26.09) | 0.0288 |
| Macrophages | 8.92 (17.83) | 17.09 (29.06) | 0.1938 |
| Cancer cells | 0 | 18.44 (35.49) | <0.0001 |
| Red blood
cells | 8.11 (19.55) | 10.85 (23.85) | 0.2881 |
| Mesothelial
cells | 3.87 (16.47) | 0.26 (1.12) | 0.2569 |
| Eosinophils | 0.31 (2.68) | 0 | 0.5368 |
High NI rate in CiCP cells implicates
cytological malignancy and high peripheral blood leukocyte (PBL)
count
As the NI rate per total CiCP cells was increased in
malignant samples as shown in Table
III, a cut-off value of NI rate was determined per total CiCP
cells to distinguish cytologically positive samples from negative
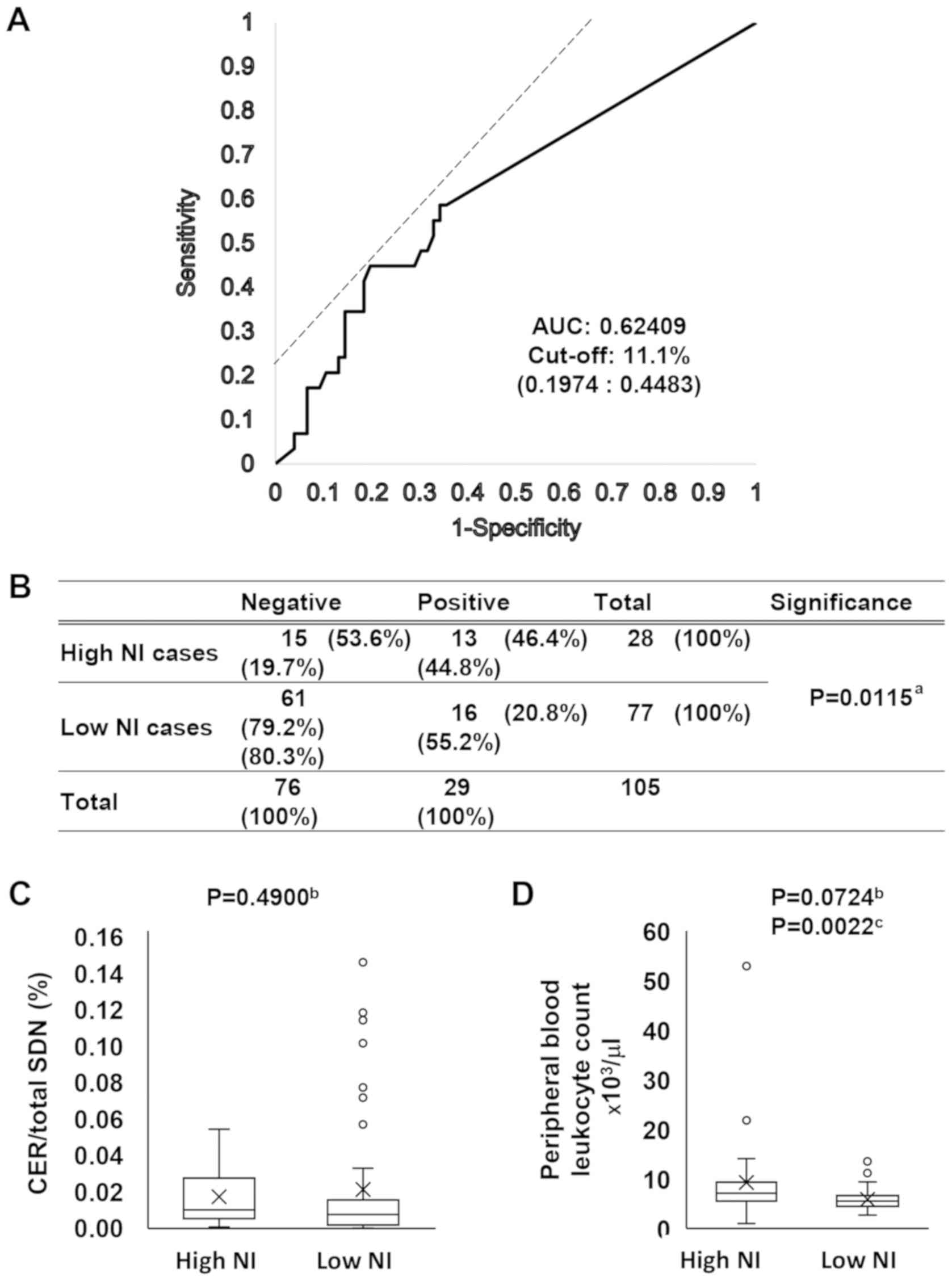
samples. Firstly, the ROC curve between the NI rate per total CiCP
(NI/CiCP) and cytological classification (negative or positive) was
evaluated. As shown in Fig. 5A and
Table SIV, the cut-off value for
NI/CiCP to distinguish the positive group from the negative group
was 11.1%, and the area under the ROC curve was 0.62409. Samples
were classified according to this cut-off value and the percentages
of a positive cytology rate were compared. As shown in Fig. 5B, the positive cytology rate was
46.4% in high NI/CiCP specimens and 20.8% in low NI/CiCP specimens
(P=0.0115). The CER per total SDN was 0.017% in the high NI/CiCP
and 0.021% in the low NI/CiCP specimens, but the difference was not
statistically significant (P=0.4900; Fig. 5C). Fig. 5D presents the numbers of PBLs at
the nearest date before the cytological sample was collected. The
PBL count was 9.30×103 cells/µl in the high
NI/CiCP specimens and 5.84×103 cells/µl in the
low NI/CiCP specimens, but the difference was not statistically
significant (P=0.0724), whereas a statistically significant
difference was observed using the Wilcoxon rank-sum test
(P=0.0022). The present findings suggested that a change in the
immune condition of a patient may result in a high NI.
Discussion
In the present study, the term CiCP was used for
emperipolesis and cell cannibalism since both phenomena have
similar mechanisms in terms of the formation of a distinct halo
structure around the incorporated cells in the host cells (4). Emperipolesis involves hematopoietic
cells, whereas cell cannibalism involves tumor cells (11). Therefore, if the host tumor cells
incorporate hematopoietic cells, the phenomenon can be termed
either emperipolesis or cell cannibalism. In the present study,
cell cannibalism was observed in 13 cases (Table SII). Among them, the host tumor
cells incorporated hematopoietic cells in six specimens (data not
shown). In addition, few CiCP were detected that involved
mesothelial cells, either as ingested or host cells, although
neither emperipolesis nor cell cannibalism generally involves
mesothelial cells. Therefore, in the present study, CiCP is the
correct term to explain the internalization of viable cells by host
cells.
It is important to recognize the usefulness of image
analysis. The present study focused on NA image analysis to
evaluate manual classification of CiCP by consensus agreement among
the evaluators since reproducibility of pathological diagnosis is
often reported in the literature (25,26).
Image analysis revealed that host cells were composed of three cell
types: Macrophages, mesothelial cells and tumor cells. The NA of
these three cell types was markedly different from each other.
Notably, the NAs of the incorporated cells were also markedly
different among most of the cell types. In pathological practice, a
number of cellular features, including NA, nuclear shape, nuclear
density and nuclear/cytoplasmic ratio, are used to distinguish one
cell type from another (14). This
kind of comprehensive evaluation enables differentiation of cells
via manual observation. However, the present image analysis data
suggested that NA has an important central role in distinguishing
cell types. Manual observation and software-based image analysis
can detect most cells at the level of 103 cells
(27). However, in the present
study, a virtual slide-format-based image was used to count cells,
which allowed to count >105 cells in one sample and
to calculate the emergence rate of a low emergence rate phenomenon,
such as CiCP. Therefore, virtual slide-format-based image analysis
may provide an improved method for specimen evaluation.
Finally, the significance of CiCP in patients with
cancer should be discussed. Three notable results were found in
patients with cancer: CER was increased in malignant cytological
specimens of the peritoneal cavity fluid, NI was increased in
malignant cytological specimens and high NI specimens exhibited a
high PBL count. The present results indicated that CiCP may alter
the immune response of a patient. Macrophages were the main host
cell type in CiCP, and increased CiCP was observed in the tumor
specimens. Historically, macrophages have been classified into
several subtypes according to their activation status (28), frequently as 'Th1 cytokine-based
activation phenotype' M1 and 'Th2 cytokine-based activation
phenotype' M2 (29). In general,
M1 macrophages are considered as antitumor and immune promoting,
while M2 macrophages are considered as pro-tumor (30). Tumor-associated macrophages (TAMs)
are predominantly the M2 phenotype and help promote tumor
angiogenesis, tumor survival and tumor metastasis (31). Additionally, the infiltration of
high numbers of TAMs into a tumor site has been associated with a
poor prognosis in numerous types of tumor (32), including breast (33), prostate (34), endometrial (35) and urinary bladder (36) cancer, malignant melanoma (37) and malignant lymphoma (38). Additionally, peritoneal macrophages
can acquire an M2-tumor-promoting function (39). The present findings suggested that
peritoneal macrophages may have an M2 pheno-type and may
incorporate more immune cells and help tumor cells avoid immune
surveillance. The present study could not definitely determine
whether CiCP was one of the characteristics of M2-type macrophages.
To the best of our knowledge, no previous pathology- or
cytology-associated study has reported cytological findings of
CiCP, which may be due to the very low emergence rate of CiCP in
specimens. In addition, since most host cells of CiCP were
macrophages in the present study (Table III), from a cytological
viewpoint, an important finding of the current study was that CiCP
in macrophages may be a novel characteristic of TAMs in ascites of
patients with cancer. Recent studies on tumor immunity have
revealed that tumor-bearing conditions can alter myeloid
differentiation and lead to the induction of TAMs, as well as
dendritic cells, myeloid-derived suppressor cells and neutrophils,
to sustain the immunosuppressive environment of tumor tissues
(40-42). Tumor-associated neutrophils (TANs)
promote tumor angiogenesis (43)
and epithelial to mesenchymal transition (44) in gastric cancer. In addition,
previous clinical studies revealed that a high
neutrophil-to-lymphocyte ratio of PBLs predicted an unfavorable
disease-specific survival (45,46).
In addition, Araki et al (47) reported that a low absolute
lymphocyte count in PBLs revealed a poor prognosis in patients with
advanced breast cancer. The aforementioned reports suggest that the
pro-tumor function of TANs, as well as an increased neutrophil
ratio in PBLs, can lead to a poor prognosis. In the current study,
the increased number of PBLs was one of the reasons for increased
NI in tumor ascites. Notably, there are no reports on CiCP
involving NI by macrophages, although NI by tumor cells has been
demonstrated in numerous types of tumor, including anaplastic
carcinoma of the gall bladder, adenocarcinoma of the small
intestine and pancreas, infiltrating duct carcinoma of the breast,
squamous cell carcinoma of the larynx, small cell carcinoma of the
lung and malignant lymphoma (8).
In conclusion, the present findings shed light on
tumor-immunity-associated phenomena, especially since increased
emperipolesis by macrophages in a tumor microenvironment may allow
to detect novel characteristics of TAMs in the peritoneal cavity
fluid. Therefore, during cytological specimen screening for
atypical cells, it is very important to observe other background
findings such as emperipolesis and cannibalism, in addition to
atypical cells. In the present study, macrophage characteristics,
such as M1/M2 polarization, were not further investigated.
Therefore, future studies should investigate macrophage
characteristics using freshly collected ascites.
Supplementary Data
Funding
The present study was supported by Gunma
University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MF conducted clinical data collection and specimen
screening. MS developed the experimental design. MF and MS
participated in discussions in meetings to reach a consensus,
performed nuclear tracing and nuclear area analysis, digital
imaging of the specimens by virtual slide scanner, image analysis
for SDN, statistical analysis and figure and manuscript
preparation. HT and SF performed specimen screening. SK
participated in discussions in meetings to reach a consensus,
digital imaging of the specimens by virtual slide scanner,
determination of condition for SDN in image analysis and figure
preparation. YN assisted in clinical data collection, determination
of condition for SDN in image analysis and manuscript preparation.
JH, TO and TF assisted in cytological review of the cases and
manuscript reviewing. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present research was approved by the Gunma
University Ethical Review Board (GUERB) for Medical Research
Involving Human Subjects of Gunma University School of Medicine
(Maebashi, Japan), and the possibility of publishing the study
results was stated on the public webpage of the hospital as part of
an information disclosure document. Additionally, the possibility
to decline participation in the study was provided according to the
Ethical Guidelines for Medical and Health Research Involving Human
Subjects of the Japanese government (Ministry of Education,
Culture, Sports, Science and Technology, and the Ministry of
Health, Labor and Welfare). Informed consent was waived by the
GUERB based on the aforementioned guidelines due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lewis W: The engulfment of living blood
cells by others of the same type. Anatomical Record. 31:43–49.
1925. View Article : Google Scholar
|
|
2
|
Humble JG, Jayne WH and Pulvertaft RJ:
Biological interaction between lymphocytes and other cells. Br J
Haematol. 2:283–294. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta N, Jadhav K and Shah V:
Emperipolesis, entosis and cell cannibalism: Demystifying the
cloud. J Oral Maxillofac Pathol. 21:92–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rastogi V, Sharma R, Misra SR, Yadav L and
Sharma V: Emperipolesis-a review. J Clin Diagn Res. 8:ZM01–ZM02.
2014.PubMed/NCBI
|
|
5
|
Samii K and Pasteur E: Images in
hematology. Emperipolesis Am J Hematol. 59:641998. View Article : Google Scholar
|
|
6
|
Rosai J and Dorfman RF: Sinus
histiocytosis with massive lymphadenopathy. A newly recognized
benign clinicopathological entity. Arch Pathol. 87:63–70.
1969.PubMed/NCBI
|
|
7
|
Foucar E, Rosai J and Dorfman R: Sinus
histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease):
Review of the entity. Semin Diagn Pathol. 7:19–73. 1990.PubMed/NCBI
|
|
8
|
Singhal N, Handa U, Bansal C and Mohan H:
Neutrophil phagocytosis by tumor cells-a cytological study. Diagn
Cytopathol. 39:553–555. 2011. View
Article : Google Scholar
|
|
9
|
Kale A: Cellular Cannibalism. J Oral
Maxillofac Pathol. 19:7–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bansal C, Tiwari V, Singh U, Srivastava A
and Misra J: Cell cannibalism: A cytological study in effusion
samples. J Cytol. 28:57–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma N and Dey P: Cell cannibalism and
cancer. Diagn Cytopathol. 39:229–233. 2011.PubMed/NCBI
|
|
12
|
Fais S: Cannibalism: A way to feed on
metastatic tumors. Cancer Lett. 258:155–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Richards DM and Endres RG: How cells
engulf: A review of theoretical approaches to phagocytosis. Rep
Prog Phys. 80:1266012017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koss L and Melamed M: Fundamental concepts
of neoplasia: Benign tumor and cancer. Koss's diagnostic cytology
and its histopathologic bases. Koss L and Melamed M: Lippincott
Williams & Wilkins; Philadelphia: pp. 143–179. 2006
|
|
15
|
Al-Abbadi M: Warthin's tumor. Salivary
gland Cytology. Al-Abbadi M: Wiley-Blackwell; Hobokwnm NJ: pp.
69–75. 2011, View Article : Google Scholar
|
|
16
|
Kini S: Gonads (Ovaries ans Testis). Color
atlas of diagnosis in exfoliative and aspiration cytopathology.
Wolters Kluwer/Lipponcott Williams & Wilkins; Philadelphia, PA:
pp. 852–869. 2011
|
|
17
|
Saxena S, Beena KR, Bansal A and Bhatnagar
A: Emperipolesis in a common breast malignancy. A case report Acta
Cytol. 46:883–886. 2002. View Article : Google Scholar
|
|
18
|
Rane SR, Parkhi M, Vishwasrao S and Nakate
L: Non-Hodgkin's lymphoma with extensive emperipolesis mimicking
Rosai-Dorfman disease: A rare case report. Indian J Pathol
Microbiol. 62:319–322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meykler S, Baloch ZW and Barroeta JE: A
case of marginal zone lymphoma with extensive emperipolesis
diagnosed on pleural effusion cytology with immunocytochemistry and
flow cytometry. Cytopathology. 27:70–72. 2016. View Article : Google Scholar
|
|
20
|
Lopes LF, Bacchi MM, Coelho KI, Filho AA
and Bacchi CE: Emperipolesis in a case of B-cell lymphoma: A rare
phenomenon outside of Rosai-dorfman disease. Ann Diagn Pathol.
7:310–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pinto D and Schmitt F: Current
applications of molecular testing on body cavity fluids. Diagn
Cytopathol. Mar 30–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Renshaw AA: Quality improvement in
cytology: Where do we go from here? Arch Pathol Lab Med.
135:1387–1390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ministry of Education Culture, Sports,
Science and Technology, and Ministry of Health, Labor and Welfare:
Ethical guidelines for medical and health research involving human
subjects. https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukousei-kagakuka/0000080278.pdfuri.
Accessed March 31, 2015.
|
|
24
|
Smith P and Gray W: Cervical
intraepithelial neoplasia and squamous cell carcinoma of the
cervix. Diagnostic Cytopathology. Gray W and Kocjan G: Churchill
Livingstone Elsevier; London: pp. 609–644. 2010, View Article : Google Scholar
|
|
25
|
Nakazato Y, Maeshima AM, Ishikawa Y,
Yatabe Y, Fukuoka J, Yokose T, Tomita Y, Minami Y, Asamura H,
Tachibana K, et al: Interobserver agreement in the nuclear grading
of primary pulmonary adenocarcinoma. J Thorac Oncol. 8:736–743.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grilley-Olson JE, Hayes DN, Moore DT,
Leslie KO, Wilkerson MD, Qaqish BF, Hayward MC, Cabanski CR, Yin X,
Socinski MA, et al: Validation of interobserver agreement in lung
cancer assessment: Hematoxylin-eosin diagnostic reproducibility for
non-small cell lung cancer: The 2004 World Health Organization
classification and therapeutically relevant subsets. Arch Pathol
Lab Med. 137:32–40. 2013. View Article : Google Scholar
|
|
27
|
Lindauer K, Bartels T, Scherer P and
Kabiri M: Development and validation of an image analysis system
for the measurement of cell proliferation in mammary glands of
rats. Toxicol Pathol. 47:634–644. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yunna C, Mengru H, Lei W and Weidong C:
Macrophage M1/M2 polarization. Eur J Pharmacol. 877:1730902020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sica A, Larghi P, Mancino A, Rubino L,
Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P and Mantovani
A: Macrophage polarization in tumour progression. Semin Cancer
Biol. 18:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leek RD, Landers RJ, Harris AL and Lewis
CE: Necrosis correlates with high vascular density and focal
macrophage infiltration in invasive carcinoma of the breast. Br J
Cancer. 79:991–995. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lissbrant IF, Stattin P, Wikstrom P,
Damber JE, Egevad L and Bergh A: Tumor associated macrophages in
human prostate cancer: Relation to clinicopathological variables
and survival. Int J Oncol. 17:445–451. 2000.PubMed/NCBI
|
|
35
|
Ohno S, Ohno Y, Suzuki N, Kamei T, Koike
K, Inagawa H, Kohchi C, Soma G and Inoue M: Correlation of
histological localization of tumor-associated macrophages with
clinico-pathological features in endometrial cancer. Anticancer
Res. 24:3335–3342. 2004.PubMed/NCBI
|
|
36
|
Hanada T, Nakagawa M, Emoto A, Nomura T,
Nasu N and Nomura Y: Prognostic value of tumor-associated
macrophage count in human bladder cancer. Int J Urol. 7:263–269.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mäkitie T, Summanen P, Tarkkanen A and
Kivelä T: Tumor-infiltrating macrophages (CD68(+) cells) and
prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci.
42:1414–1421. 2001.PubMed/NCBI
|
|
38
|
Farinha P, Masoudi H, Skinnider BF,
Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM and
Gascoyne RD: Analysis of multiple biomarkers shows that
lymphoma-associated macro-phage (LAM) content is an independent
predictor of survival in follicular lymphoma (FL). Blood.
106:2169–2174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ko SY, Ladanyi A, Lengyel E and Naora H:
Expression of the homeobox gene HOXA9 in ovarian cancer induces
peritoneal macrophages to acquire an M2 tumor-promoting phenotype.
Am J Pathol. 184:271–281. 2014. View Article : Google Scholar :
|
|
40
|
Law AMK, Valdes-Mora F and Gallego-Ortega
D: Myeloid-derived suppressor cells as a therapeutic target for
cancer. Cells. 9:5612020. View Article : Google Scholar :
|
|
41
|
Mukaida N, Sasaki SI and Baba T: Two-faced
roles of tumor-associated neutrophils in cancer development and
progression. Int J Mol Sci. 21:34572020. View Article : Google Scholar :
|
|
42
|
Schupp J, Krebs FK, Zimmer N, Trzeciak E,
Schuppan D and Tuettenberg A: Targeting myeloid cells in the tumor
sustaining microenvironment. Cell Immunol. 343:1037132019.
View Article : Google Scholar
|
|
43
|
Li TJ, Jiang YM, Hu YF, Huang L, Yu J,
Zhao LY, Deng HJ, Mou TY, Liu H, Yang Y, et al:
Interleukin-17-producing neutrophils link inflammatory stimuli to
disease progression by promoting angiogenesis in gastric cancer.
Clin Cancer Res. 23:1575–1585. 2017. View Article : Google Scholar
|
|
44
|
Li S, Cong X, Gao H, Lan X, Li Z, Wang W,
Song S, Wang Y, Li C, Zhang H, et al: Tumor-associated neutrophils
induce EMT by IL-17a to promote migration and invasion in gastric
cancer cells. J Exp Clin Cancer Res. 38:62019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang SC, Chou JF, Strong VE, Brennan MF,
Capanu M and Coit DG: Pretreatment neutrophil to lymphocyte ratio
independently predicts disease-specific survival in resectable
gastroesophageal junction and gastric adenocarcinoma. Ann Surg.
263:292–297. 2016. View Article : Google Scholar :
|
|
46
|
Ock CY, Nam AR, Lee J, Bang JH, Lee KH,
Han SW, Kim TY, Im SA, Kim TY, Bang YJ, et al: Prognostic
implication of anti-tumor immunity measured by the
neutrophil-lymphocyte ratio and serum cytokines and angiogenic
factors in gastric cancer. Gastric Cancer. 20:254–262. 2017.
View Article : Google Scholar
|
|
47
|
Araki K, Ito Y, Fukada I, Kobayashi K,
Miyagawa Y, Imamura M, Kira A, Takatsuka Y, Egawa C, Suwa H, et al:
Predictive impact of absolute lymphocyte counts for
progression-free survival in human epidermal growth factor receptor
2-positive advanced breast cancer treated with pertuzumab and
trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer.
18:9822018. View Article : Google Scholar : PubMed/NCBI
|