Introduction
Breast cancer (BC) is the leading cause of
cancer-associated mortality among females worldwide (1,2). In
total, ~268,600 new cases of BC were diagnosed worldwide in 2019,
whereas BC is the second leading cause of cancer-associated
mortality (15%) after lung cancer; the incidence of BC is
increasing among Asian women, with an annual rate of 1.8% (3). Recently, the development of screening
tools and effective treatment approaches have greatly improved the
survival rate of patients with BC (4,5). The
treatment of early BC involves complex combinations among the three
main treatment modalities, namely surgery, systemic therapy and
radiation therapy (6). Notably,
the mortality rate of BC declined by 40% between 1989 and 2016;
despite advances in early detection and treatment of BC, 20-30% of
patients with early BC will develop recurrent disease and distant
metastases (3,7). Metastasis remains the leading cause
of death in patients with BC (3,7).
Molecular targeted therapeutic approaches have advanced current
knowledge concerning disease pathogenesis and individualized
treatment. For example, the monoclonal antibody trastuzumab,
targeted against human epidermal growth factor receptor, has been
shown to improve survival in patients with BC (8); however, the prognosis remains poor.
Therefore, more personalized therapies with less adverse events are
urgently required, further supporting the need for effective
therapeutic targets and prognostic biomarkers.
NUF2, a core component of the Ndc80 kinetochore
complex, also known as cell division cycle associated 1, was first
identified as a centromere protein (9). It has been reported that NUF2 may
interact with centromere-associated protein E (CENP-E) and
contribute to stable spindle kinetochore-micro-tubule attachment
(10,11). NUF2 downregulation promotes
kinetochore attachment defects and induces mitotic cell death
(10). Notably, NUF2 serves an
important role in regulating mitosis. NUF2 is upregulated in
several types of cancer, including colon, gastric, ovarian and
renal cell cancers (12-14). Furthermore, NUF2 overexpression is
associated with poor prognosis in patients with non-small cell lung
and colorectal cancers (14,15).
In colorectal and gastric cancer, it was reported that NUF2
knockdown attenuated tumor growth and significantly increased the
sub-G1 fraction of the cell cycle (12). These reports supported the possible
role of NUF2 in tumorigenesis.
The present study provided substantial evidence
regarding the important role of NUF2 in the malignant potential and
survival of BC, and revealed a promising prognostic biomarker and a
therapeutic target for BC.
Materials and methods
Cell culture
The human mammary carcinoma cell lines MCF-7,
MDA-MB-231, Hs578T and T47D, and the human breast epithelial cell
line MCF10A were purchased from the Cell Bank of Chinese Academy of
Sciences. MCF-7, MDA-MB-231, Hs578T and T47D cells were maintained
in DMEM (HyClone; Cytiva) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). MCF10A cells were
cultured in DMEM with Ham's F12 mixture (DMEM/F12) supplemented
with 6% equine serum (Gibco; Thermo Fisher Scientific, Inc.), 10
µg/ml insulin, 0.5 mg/ml hydrocortisone (both Sigma Aldrich;
Merck KGaA) and 1% penicillin-streptomycin (HyClone; Cytiva). All
cell lines were maintained at 37°C in a humidified atmosphere with
5% CO2.
Patients and tissue samples
BC tissues and corresponding adjacent normal tissues
samples were obtained from 49 patients with BC (mean age, 54.65±12
years; range, 33-86 years) under-going surgery at Shaoxing People's
Hospital (Shaoxing, China) between December 2015 and April 2016 for
immunohisto-chemistry (IHC). Clinical characteristics were
retrospectively reviewed, and prognostic information was recorded
on the basis of clinical follow-up visits or telephone (once every
6 months). The pathological stages of breast cancer patients were
determined based on the 7th edition of the American Joint Committee
on Cancer staging system (16).
Patients who had received neoadjuvant chemotherapy or radiotherapy,
or who had a history of other malignant tumors were excluded. The
Ethics Committee of Shaoxing People's Hospital approved the present
study, and written informed consent was obtained from all
participants.
IHC
IHC was conducted using 3-µm
paraffin-embedded tissue sections. The tissue samples were fixed in
10% neutral formalin at room temperature for 24 h. Briefly, the
tissue sections were heated at 65°C for deparaffination, and at
121°C for 120 sec in citrate buffer (pH 6.0) for antigen retrieval.
To block endogenous peroxidase activity, all sections were treated
with 3% (v/v) hydrogen peroxide at room temperature for 10 min.
Following blocking with 10% goat serum (Beyotime Institute of
Biotechnology) at 37°C for 30 min, tissue sections were then
incubated with an anti-NUF2 primary antibody (1:400; cat. no.
ab122962; Abcam) at 37°C for 1 h. Subsequently, the sections were
incubated at 37°C for 30 min with HRP-conjugated polymer as a
secondary antibody, stained with 3,3′-diaminobenzidine according to
the instructions of the GTVision™ III Detection System/Mo Rb kit
(GeneTech Biotechnology Co., Ltd.) and counterstained with
hematoxylin at room temperature for 2-3 min. The negative control
sections were incubated with phosphate buffered saline (PBS)
instead of the primary antibody. The immunostaining images were
acquired using a light microscope (magnification, ×100; Leica
DM3000; Leica Microsystems GmbH). All sections were evaluated
independently by two pathologists, who were blinded to the purpose
of the study.
The immunohistochemical staining score was
calculated by counting the percentage of positive cells in five
randomly selected fields from each slide. The staining positive
rate was defined as follows: 0, <25% positive cells; 1, 25-50%
positive cells; 2, 50-75% positive cells and 3, 75-100% positive
cells. In addition, staining intensity was scored as follows: 0, no
coloration; 1, light yellow; 2, yellow; and 3, brown. The two
scores were combined to obtain the overall score: 0, negative (−);
1-2, weakly positive (+); 3-4, moderate positive (++); and 5-6,
strongly positive (+++).
Bioinformatics analysis
The expression levels of NUF2 in patients with BCs
and normal controls were further compared using Gene Expression
Profiling Interactive Analysis (GEPIA2; http://gepia2.cancer-pku.cn/#index). GEPIA2 is an
online database for analyzing the gene expression profiles of 9,736
tumors and 8,587 normal samples from The Cancer Genome Atlas (TCGA;
https://cancergenome.nih.gov/) and the
genotype-tissue expression programs (https://www.gtexportal.org/) (17). The Kaplan-Meier Plotter database
(http://kmplot.com/analysis/) were used
to perform survival curve and log-rank test analyses to identify
the association between NUF2 expression and the prognosis of
patients with BC. Patients were divided into high and low
expression groups based on the median expression value.
Transfection
The small interfering RNA (siRNA) clone targeting
human NUF2 gene (NM_031423; siNUF2) was purchased from Guangzhou
RiboBio Co., Ltd. [siRNA-1, cat. no. siB1533140940; siRNA-2, cat.
no. siB1533141009; siRNA-3, cat. no. siG151013041657; negative
control siRNA (siNC), cat. no. siN05815122147]. siRNA-1 was
selected as the siNUF2 for subsequent experiments after evaluating
transfection efficiency. MCF-7 and MDA-MB-231 cells were seeded
into 6-well plates at a density of 2×105 cells/well
prior to transfection, and when they reached the log growth phase
and 60-70% confluence, cells were transfected with siNUF2 or siNC
(50 nM) using Lipofection 6000 (Beyotime Institute of
Biotechnology), according to the manufacturer's instructions.
Following transfection for 24-48 h, the cells were washed with PBS
and collected for reverse transcription-quantitative (RT-q)PCR and
western blot analysis.
RT-qPCR analysis
Total RNA was extracted from MCF-7 and MDA-MB-231
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and RT-qPCR was conducted with a One Step TB
Green™ PrimeScript™ RT-PCR kit II (Takara Bio, Inc.) according to
the manufacturer's protocol: 42°C for 5 min and 95°C for 10 sec;
followed by 40 cycles of 95°C for 5 sec and 60°C for 20 sec; and
finally 65°C for 15 sec. The mRNA expression levels were determined
on a Roche LightCycler® 480 instrument (Roche
Diagnostics) using the following pairs of primers: NUF2 forward,
5′-TAC CAT TCA GCA ATT TAG TTA CT-3′ and reverse, 5′-TAG AAT ATC
AGC AGT CTC AAA G-3′; and β-actin (internal control) forward,
5′-CAT GTA CGT TGC TAT CCA GGC-3′ and reverse, 5′-CTC CTT AAT GTC
ACG CAC GAT-3′. All experiments were performed in triplicate, and
the mRNA expression levels of NUF2 were calculated using the
2−ΔΔCq method (18).
Western blot analysis
Total proteins isolated from MCF-7, MDA-MB-231,
Hs578T, T47D and MCF10A cells were used for western blot analysis.
Briefly, cultured cells were lysed with RIPA buffer (Beyotime
Institute of Biotechnology) supplemented with 1 mM PMSF and 0.25
U/µl RNase, and the protein concentration was then
determined using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Subsequently, equal quantities of proteins (25-30
µg/lane) were separated via 10% SDS-PAGE, and were then
transferred onto PVDF membranes (cat. no. IPVH00010; EMD
Millipore). The membranes were blocked with 5% skimmed milk at room
temperature for 2 h, and probed with anti-NUF2 (1:1,000; cat. no.
ab180945; Abcam), β-actin (1:5,000; cat. no. BS6007M; Bioworld
Technology, Inc.), anti-Bax (1:1,000; cat. no. 2772; Cell Signaling
Technology, Inc.), anti-Bcl-2 (1:1,000; cat. no. 4223; Cell
Signaling Technology, Inc.) and anticyclin B1 (1:1,000; cat. no.
ab7957; Abcam) antibodies at 4°C overnight. Membranes were then
washed with TBS-0.1% Tween 20 and incubated with the corresponding
HRP-conjugated secondary goat anti-rabbit IgG antibody (1:5,000;
cat. no. BS13278; Bioworld Technology, Inc.) at room temperature
for 1 h. Protein bands were detected with BeyoECL Plus Enhanced
Chemiluminescence reagent (Beyotime Institute of Biotechnology) and
analyzed on a LAS-4000 Science Imaging System (Fujifilm Holdings
Corporation). Densitometry of protein bands was measured and
analyzed with ImageJ software (v1.52a; National Institutes of
Health).
Colony formation assay
For the colony formation assay, following siRNA
transfection for 24 h, 103 cells/well were seeded into a
new 6-well plate, and cultured in 2 ml DMEM supplemented with 10%
FBS for 10 days or until colonies were visible to the naked eye.
Colonies were then washed with PBS, fixed in 95% ethanol for 15 min
at room temperature, and stained with 0.1% crystal violet for 15
min at room temperature. After being washed twice with PBS,
colonies (>50 cells/colony) were counted using ImageJ v1.52a
software.
Cell proliferation assay
To evaluate the effect of NUF2 knockdown on BC cell
proliferation, cell proliferation assays were conducted in both
MCF-7 and MDA-MB-231 cell lines using a Cell Counting Kit-8 (CCK-8;
Jiangsu Kaiji Bio-Technology Co., Ltd.) according to the
manufacturer's instructions. Briefly, cells were seeded into
96-well plates at a density of 1×103 cells/well in
quintuple and cultured at 37°C for 1, 2, 3 or 4 days. Subsequently,
10 µl CCK-8 reagent was added into each well, and cells were
then incubated for an additional 1 h. Cell proliferation was
determined by measuring the absorbance at 450 nm using a microplate
reader (Bio-Rad Laboratories, Inc.).
Cell cycle analysis
The cell cycle was evaluated in MCF-7 and MDA-MB-231
cells via flow cytometry following cell staining with propidium
iodide (PI). Briefly, 24 h after siRNA transfection, MCF-7 and
MDA-MB-231 cells (2×105/well) were seeded into 6-well
plates and incubated at 37°C. Subsequently, cells were harvested
and fixed in 75% (v/v) ethanol overnight at 4°C. Fixed cells were
washed twice with ice cold PBS, resuspended in RNase A solution and
incubated at 37°C for 30 min. The cell suspension was then
supplemented with PI and incubated at room temperature for 30 min.
The cell cycle was performed on a flow cytometer (FACS Calibur; BD
Biosciences). Data were analyzed using ModFit LT 4.0 (Verity
Software House).
Apoptosis analysis
Apoptosis was analyzed by flow cytometry. Briefly,
MDA-MB-231 and MCF-7 cells were seeded into 6-well plates at a
density of 2×105 cells/well. Following transfection with
siRNA clones for 24 h, cells were harvested, washed with ice-cold
PBS and resuspended in 1X binding buffer. The apoptosis rate was
quantitatively determined by detecting phosphatidylserine on
apoptotic cells using an Annexin V/PI double-staining apoptosis
assay kit (cat. no. KGA511; Nanjing KeyGen Biotech Co., Ltd.)
according to the manufacturer's instructions. Cells were incubated
with Annexin V and PI for 30 min, and analyzed with a flow
cytometer (FACS Calibur; BD Biosciences) and ModFit software. The
apoptotic rate was calculated as the sum of the percentage of early
and late apoptotic cells. All experiments were performed in
triplicate.
Statistical analysis
Statistical analyses were performed with SPSS 23.0
(IBM Corp.) and GraphPad Prism 7 software (GraphPad Software,
Inc.). All experiments were performed in triplicate. The IHC
results with respect to patient characteristics were analyzed using
Fisher's exact test, while comparisons of IHC scores between two
paired groups were performed by Wilcoxon signed-rank test.
Bioinformatics comparison of expression in patients and controls
was performed using Student's t-test. Comparisons between three or
more groups were performed by one-way ANOVA followed by Dunnett's
post hoc test. Univariate and multivariate analyses of clinical
indicators of cancer-associated mortality were conducted using the
Cox proportional hazard model. P<0.05 was considered to indicate
a statistically significant difference.
Results
NUF2 is associated with poor prognosis in
BC
The expression of NUF2 was detected in 49 BC tissue
specimens via IHC. Furthermore, its expression pattern was
classified in tissues based on staining from negative, to weakly,
moderately and strongly positive (Fig.
1). Positive staining was observed in 43/49 BC cases (87.8%),
including 29 cases (59.2%) with strongly positive staining and 14
cases (28.6%) with weakly or moderately positive staining, in
addition to 6 cases (12.2%) negative for NUF2 staining (Table I). Subsequently, the association
between NUF2 expression and clinical characteristics was assessed.
The number of distant metastasis in patients with BC was too small
to adequately perform association analysis. For those variables
that could be analyzed, it was determined that NUF2 was
significantly associated with estrogen receptor (ER) status only
(P=0.042; Table I). Furthermore,
univariate analysis was performed to evaluate the association
between prognosis and other factors, including age (≥53 vs. <53
years), lymph node stage (N1+N2 vs. N0), tumor stage (T3+T4 vs.
T1+T2) and NUF2 expression status (strong vs. weak/absent). Among
these parameters, strong NUF2 expression (P=0.0334) and advanced
age (P=0.0456) were significantly associated with a less favorable
prognosis. In multivariate analysis, strong NUF2 expression
(P=0.0183) and advanced age (P=0.0263) were identified as
independent prognostic factors (Table
II).
 | Table IAssociation between NUF2 expression
and clinical characteristics of patients with breast cancer
(n=49). |
Table I
Association between NUF2 expression
and clinical characteristics of patients with breast cancer
(n=49).
| Characteristic | Number | NUF2 expression
| P-value |
|---|
| − | +/++ | +++ |
|---|
| Age, years | | | | | |
| <53 | 24 | 3 | 7 | 14 | 0.993 |
| ≥53 | 25 | 3 | 7 | 15 | |
| Tumor size, cm | | | | | |
| <2 | 10 | 1 | 2 | 7 | 0.732 |
| ≥2 | 39 | 5 | 12 | 22 | |
| TNM stage | | | | | |
| I | 13 | 0 | 3 | 10 | 0.095 |
| II | 24 | 4 | 10 | 10 | |
| III | 12 | 2 | 1 | 9 | |
| ER status | | | | | |
| Positive | 37 | 4 | 14 | 19 | 0.042 |
| Negative | 12 | 2 | 0 | 10 | |
| PR status | | | | | |
| Positive | 30 | 3 | 11 | 16 | 0.281 |
| Negative | 19 | 3 | 3 | 13 | |
| HER2 status | | | | | |
| Positive | 47 | 6 | 13 | 28 | 0.733 |
| Negative | 2 | 0 | 1 | 1 | |
| Lymph node
metastasis | | | | | |
| Yes | 25 | 4 | 6 | 15 | 0.617 |
| No | 24 | 2 | 8 | 14 | |
| Tumor stage | | | | | |
| T1 | 23 | 3 | 4 | 16 | 0.483 |
| T2 | 19 | 3 | 8 | 8 | |
| T3 | 2 | 0 | 1 | 1 | |
| T4 | 5 | 0 | 1 | 4 | |
| Lymph node
stage | | | | | |
| N0 | 25 | 2 | 7 | 16 | 0.536 |
| N1 | 13 | 2 | 5 | 6 | |
| N2 | 5 | 0 | 1 | 4 | |
| N3 | 6 | 2 | 1 | 3 | |
 | Table IICox proportional hazards model
analysis of prognostic factors in patients with breast cancer. |
Table II
Cox proportional hazards model
analysis of prognostic factors in patients with breast cancer.
| Variable | Hazard ratio | 95% CI |
Unfavorable/favorable | P-value |
|---|
| Univariate
analysis | | | | |
| NUF2
expression | 5.144 | 1.137-23.271 | strong/weak,
absent | 0.0334a |
| Age, years | 8.738 | 1.043-73.203 | ≥53/<53 | 0.0456a |
| Tumor stage | 0.239 | 0.053-1.085 | T3+T4/T1+T2 | 0.0637 |
| Lymph node
stage | 0.400 | 0.089-1.793 | N1+N2/N0 | 0.2313 |
| Multivariate
analysis | | | | |
| Age, years | 0.074 | 0.008-0.736 | ≥53/<53 | 0.0263a |
| NUF2
expression | 8.412 | 1.434-49.357 | strong/weak,
absent | 0.0183a |
NUF2 is highly expressed in BC tissues
and cell lines
The NUF2 expression data from 1,085 patients with BC
were analyzed using the GEPIA2 database. The results indicated that
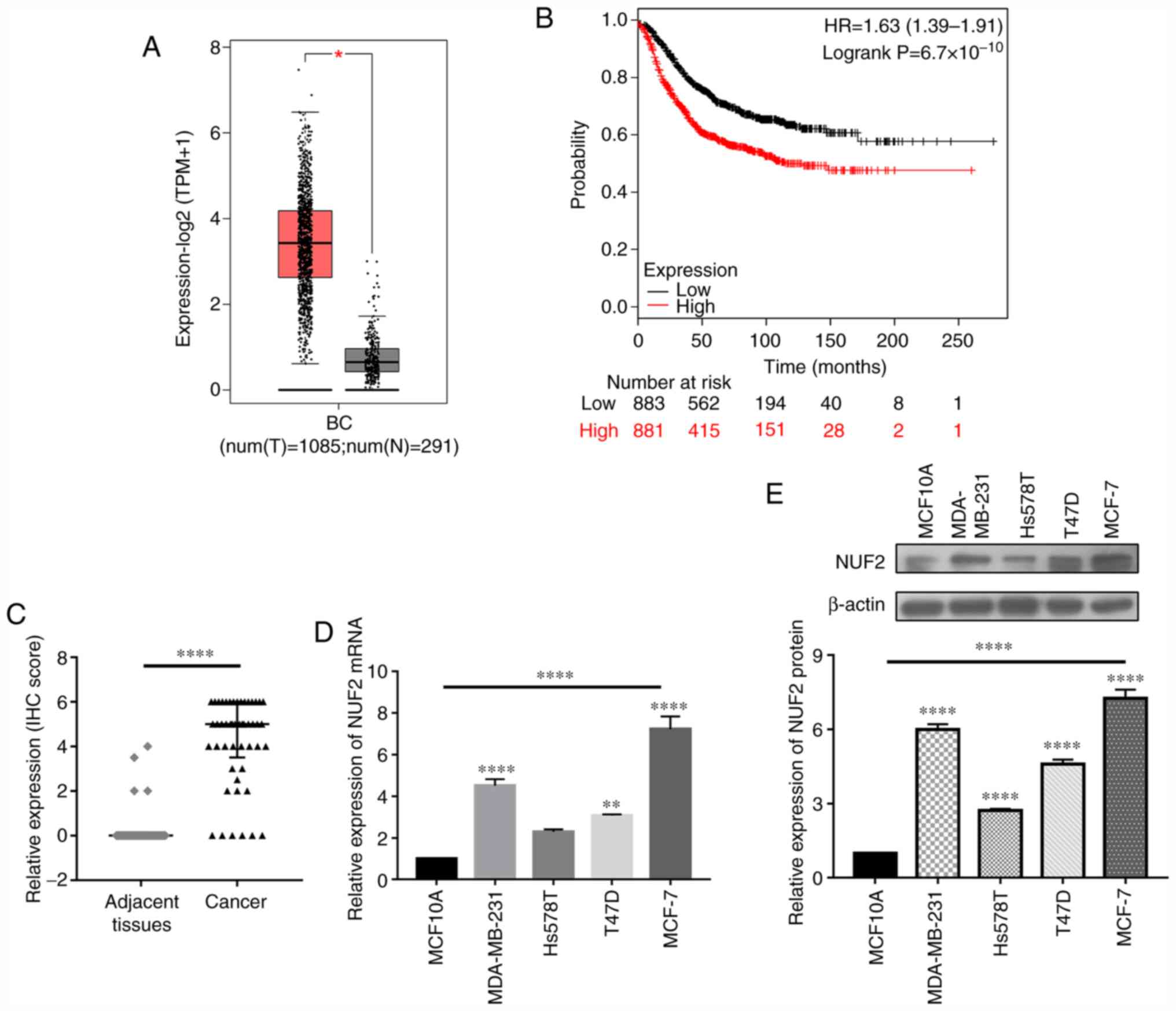
NUF2 was upregulated in patients with BC (Fig. 2A). Kaplan-Meier plotter database
analysis revealed that increased expression of NUF2 in patients
with BC was closely associated with a poor prognosis (Fig. 2B). Additionally, IHC results
revealed that NUF2 was upregulated in tumor tissues compared with
adjacent normal tissues (Fig. 2C).
Consistently, the mRNA and protein expression levels of NUF2 were
increased in BC cell lines compared with non-tumorigenic epithelial
MCF-10A cells, as demonstrated by RT-qPCR and western blot
analysis, respectively (Fig. 2D and
E). These findings indicated that NUF2 was significantly
upregulated in BC tissues and cell lines. Among different BC cell
lines, MCF-7 and MDA-MB-231 cells exhibited relatively higher NUF2
expression; therefore, these cell lines were considered suitable
for subsequent loss-of-function experiments.
NUF2 knockdown in BC cells using
siRNA
To evaluate the effects of NUF2 in BC cells, MCF-7
and MDA-MB-231 cells were transfected with siNUF2 to silence its
expression. As shown in Fig. 3,
compared with the siNC group, the protein and mRNA expression
levels of NUF2 were significantly decreased following siNUF2
transfection in both MDA-MB-231 and MCF-7 cells. Based on the
transfection efficiencies of different siNUF2 sequences, siRNA-1
was selected for subsequent experiments.
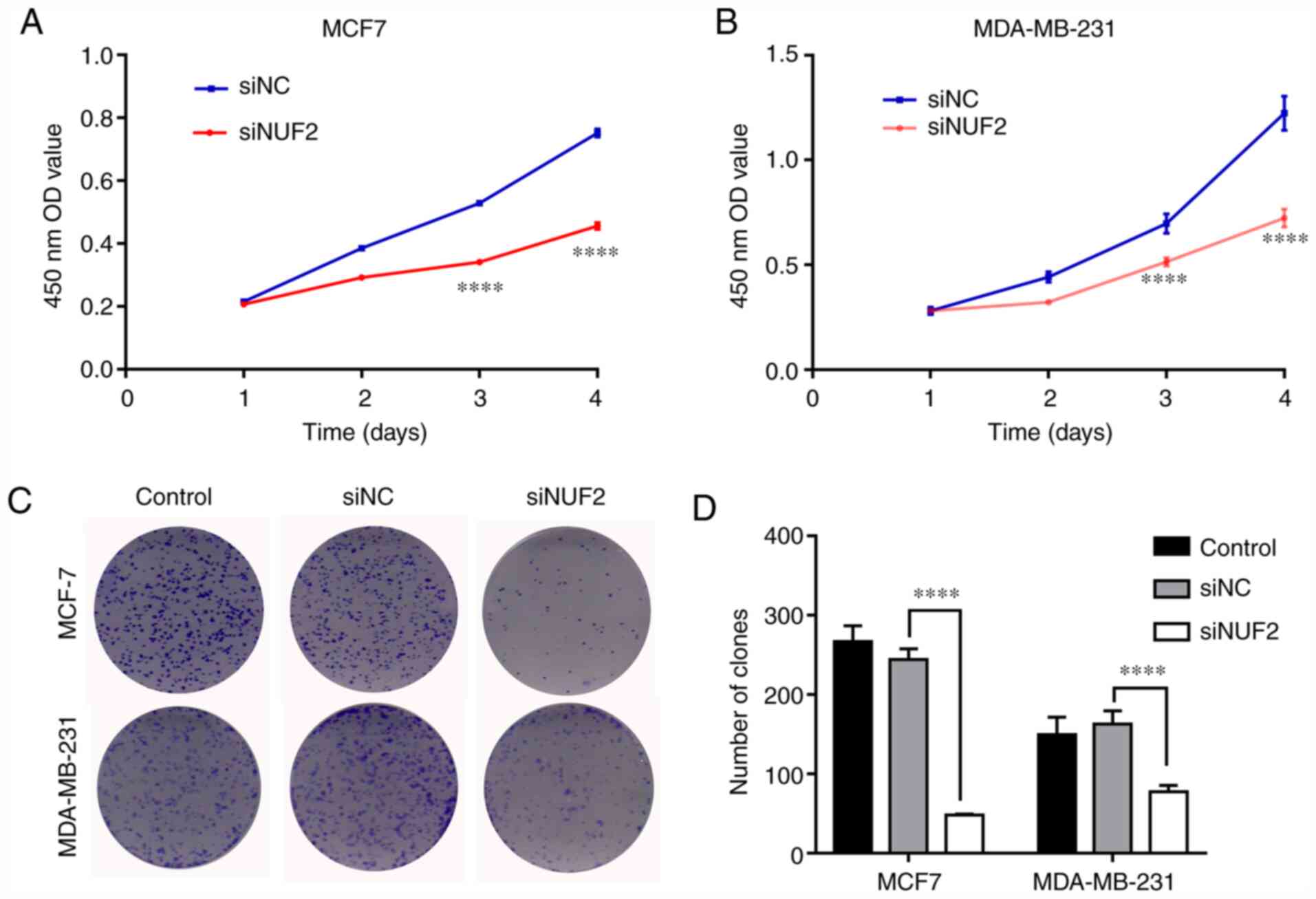
Knockdown of NUF2 inhibits BC cell
proliferation and colony formation
To investigate the biological effect of NUF2 in BC
cell growth, a CCK-8 assay over a 4-day period was conducted in
MDA-MB-231 and MCF-7 cells. NUF2 knockdown significantly attenuated
MDA-MB-231 cell proliferation compared with siNC-transfected cells
on days 3 and 4 (Fig. 4A).
Similarly, NUF2 knockdown inhibited the proliferation of MCF-7
cells on days 3 and 4 (Fig. 4B).
These results suggested that knockdown of NUF2 could potently
suppress BC cell proliferation. Furthermore, the effect of NUF2
expression on long-term cell proliferation capacity was evaluated
using a colony formation assay. The results revealed that colony
formation ability was affected in MDA-MB-231 and MCF-7 cells
following siRNA-mediated NUF2 knockdown. As presented in Fig. 4C and D, the number of colonies in
the siNUF2 group was significantly reduced compared with the siNC
group. These findings indicated that silencing of NUF2 expression
served an important role in inhibiting BC cell growth.
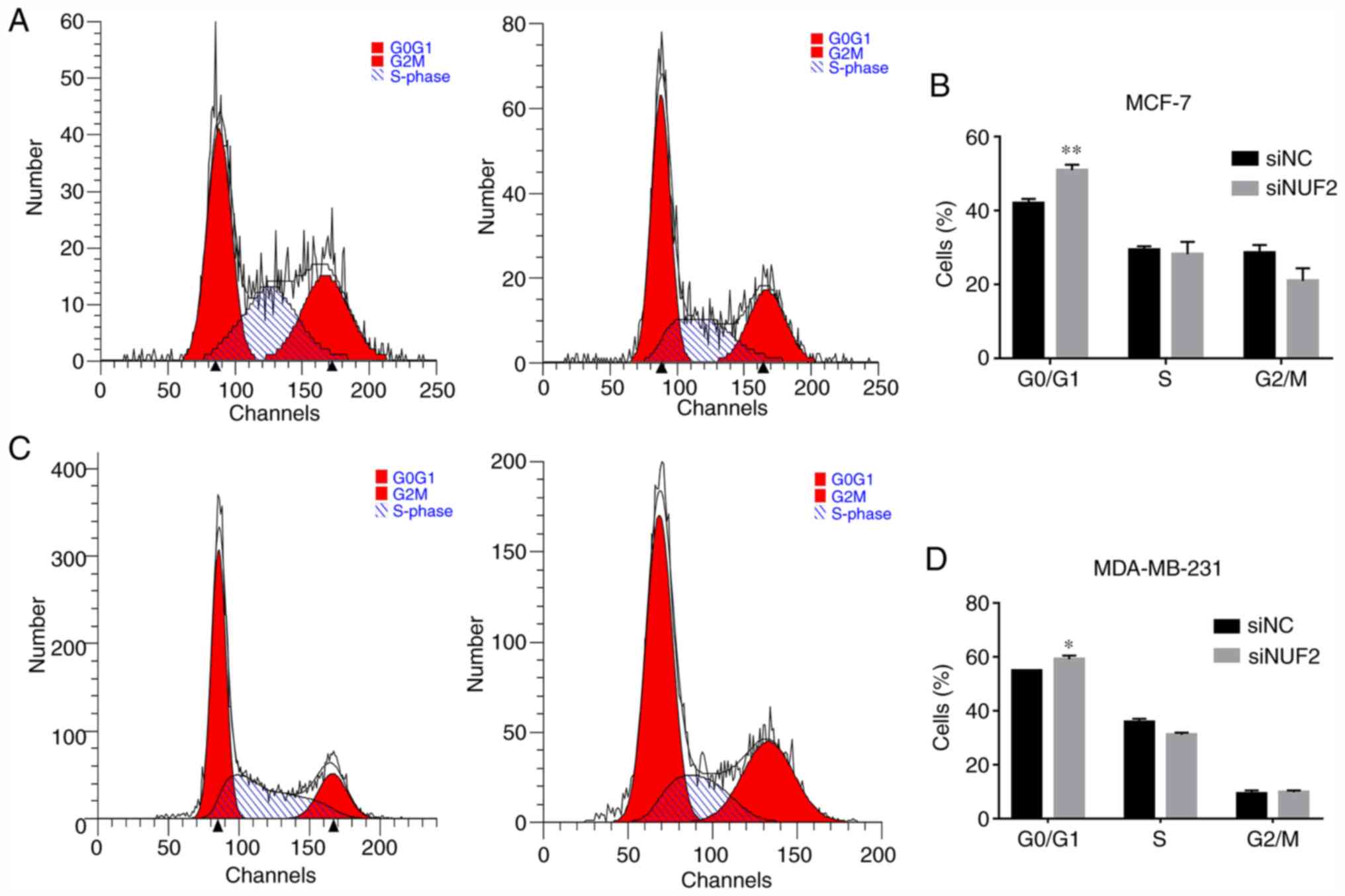
Suppression of NUF2 induces cell cycle
arrest and downregulates the expression of cell cycle
regulators
NUF2 is a key mediator of kinetochore-microtubule
attachment (9); therefore, NUF2
knockdown-mediated inhibition of BC cell proliferation may occur
due to impediment of cell cycle progression or enhanced apoptosis.
Therefore, to evaluate the mechanisms underlying the effect of NUF2
knockdown on cell cycle progression, the cell cycle distribution of
both MCF-7 and MDA-MB-231 cells was evaluated via flow cytometry,
and the percentage of cells in each phase of the cell cycle was
determined. Compared with the siNC group, cell cycle progression
was inhibited in the siNUF2 group, as determined based on the
significant increase in the percentage of MCF-7 and MDA-MB-231
cells in G0/G1 phase (Fig. 5).
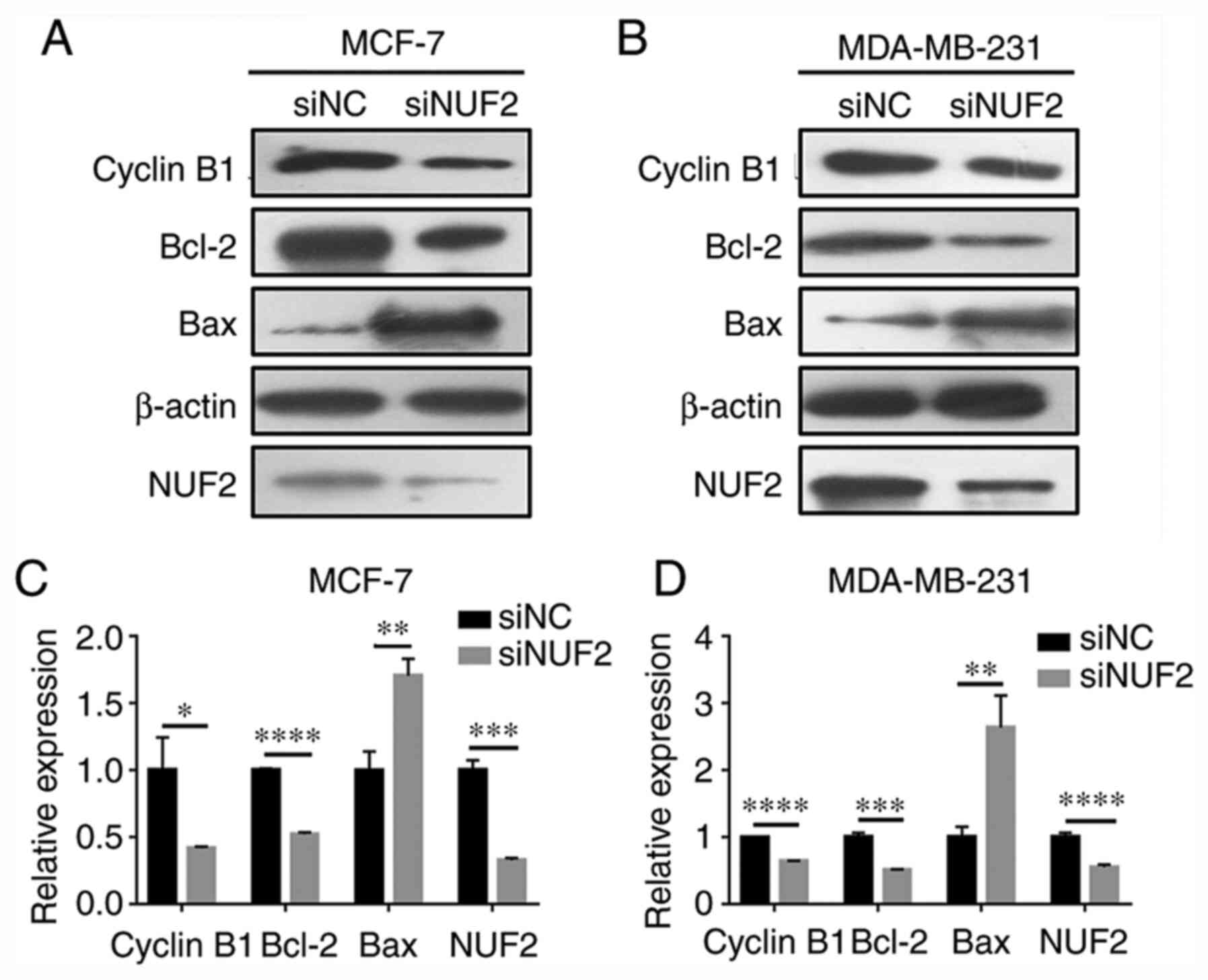
Furthermore, the protein expression levels of the cell
cycle-associated protein cyclin B1 were decreased following NUF2
knockdown (Fig. 6). These results
suggested that NUF2 knockdown mediated cell cycle arrest at the
G0/G1 phase via cyclin B1 downregulation.
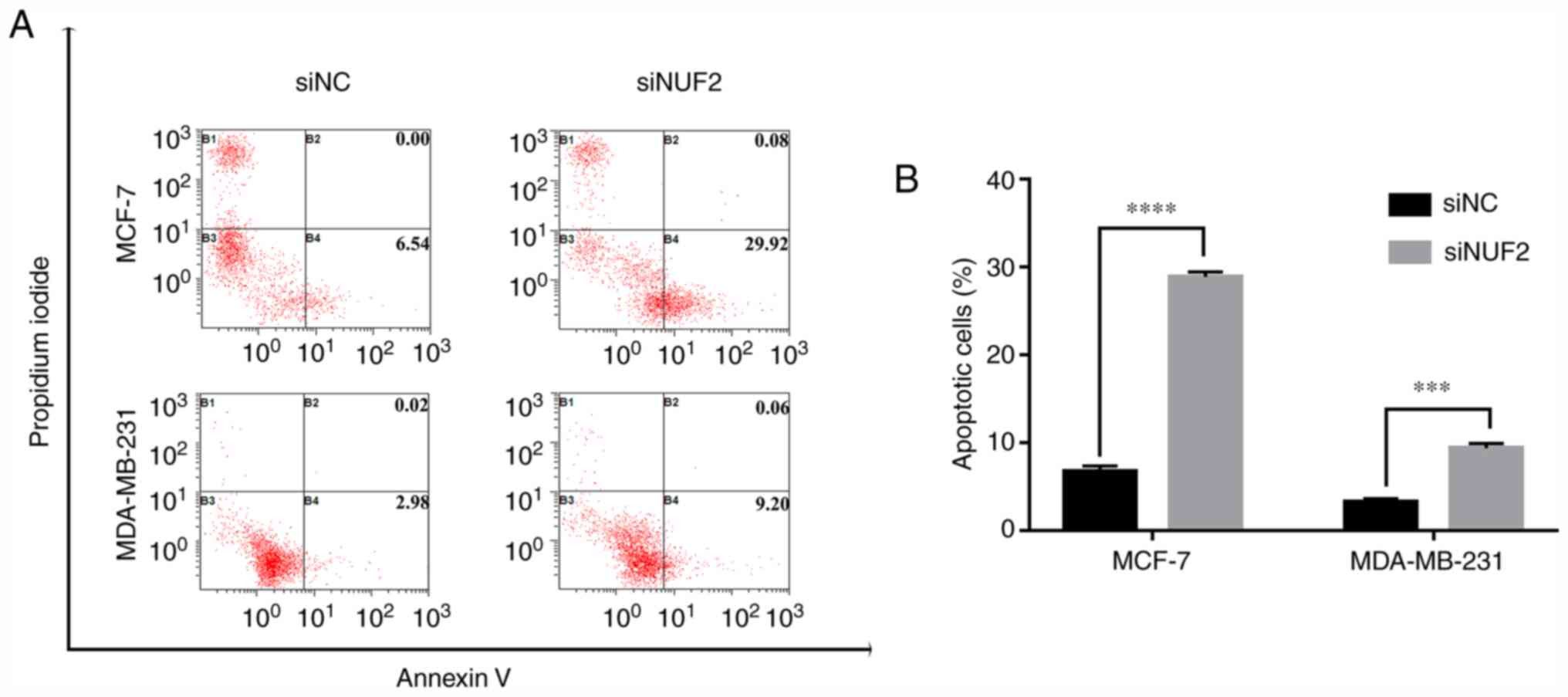
NUF2 knockdown attenuates BC cell
apoptosis
Cell apoptosis was examined by flow cytometry using
Annexin V/PI double staining. Following NUF2 silencing, the results
revealed a significant increase in the apoptotic rate of MCF-7
cells (Fig. 7). Similarly, the
apoptotic rate was significantly increased in MDA-MB-231 cells in
the siNUF2 group compared with the siNC group (Fig. 7). In addition, the expression
levels of the apoptosis-associated proteins Bax and Bcl-2 were
significantly upregulated and downregulated, respectively, in BC
cells transfected with siNUF2 compared with siNC (Fig. 6). These findings indicated that
NUF2 knockdown exerted a pro-apoptotic effect on BC cells.
Discussion
In the Ndc80-NUF2 complex, NUF2 is required to
maintain the integrity of the centromere and the stability of the
micro-tubule-binding site in the outer plate of the centromere
(19). It has been reported that
the Ndc80-NUF2 complex is involved in the development of several
types of human cancer, including hepatocellular, lung, prostate and
oral carcinomas (20-23). Although marked progress has been
achieved in molecular targeted therapy for BC in the previous few
years, current anti-cancer agents are characterized by limited
efficacy and drug resistance (8).
Therefore, the development of novel therapeutic targets to improve
the clinical outcome of patients with BC is urgently required.
In the present study, IHC analysis revealed that
NUF2 was upregulated in BC tissues compared with normal adjacent
tissues (43/49 BC cases). This finding was consistent with results
obtained from the GEPIA2 database analysis. Furthermore,
Kaplan-Meier and Cox proportional hazard model analysis
demonstrated that NUF2 overexpression was associated with a poor
prognosis in patients with BC. NUF2 was also upregulated in BC cell
lines. These data supported the role of NUF2 as a prognostic
biomarker for BC. To further investigate the biological effect of
NUF2 in BC cells, MDA-MB-231 and MCF-7 cells were transfected with
siNUF2 to silence NUF2 expression. NUF2 downregulation
significantly attenuated BC cell growth by inhibiting cell
proliferation and colony formation ability, and induced apoptosis
in BC cells. However, in the present study, the upregulation of
NUF2 was not associated with tumor size. It is proposed that the
number of patients enrolled in the study may have been too small to
observe such an association, or there may have been inaccuracies in
tumor size measurement, amongst other possible factors.
In addition, NUF2 silencing affected the expression
levels of apoptosis- and cell cycle-associated proteins, including
Bax, Bcl-2 and cyclin B1, in BC cells. Cyclin B1 serves an
important role in the initiation of mitosis, and inhibition of
cyclin B1 may result in cell cycle blockage and eventually
apoptosis (24). In the present
study, cell cycle analysis showed that the percentage of cells in
G0/G1 phase was significantly increased following NUF2 knockdown,
which was accompanied with decreased cyclin B1 expression levels.
These results indicated that cell cycle blockage was associated
with decreased cell proliferation and increased apoptosis.
NUF2 is a highly conversed component of the mitotic
cycle complex, which serves an important role in maintaining
spindle microtubule-kinetochore attachment (25). Decreased NUF2 expression inhibits
the attachment of the kinetochore to the spindle microtubules,
resulting in abnormal chromosome segregation, which in turn results
in mitotic arrest and cell death (10,11,26).
In the early stages of mitosis, kinetic particles are attached to
spindle microtubules to form a stable complex; however, impaired
binding of kinetic particles to microtubules leads to complex
instability and shortened complex half-life, eventually resulting
in abnormal cell division and cell death (10,11).
Consistent with the these observations, NUF2 knockdown in BC cells
resulted in reduced cell proliferation and increased apoptosis.
The present study had certain limitations, such as
the absence of NUF2 overexpression experiments. Another potential
limitation is that the specific underlying molecular mechanisms of
NUF2-promoted cell proliferation were not revealed. Furthermore,
the association between NUF2 expression in patients with BC and
clinicopathological characteristics such as distant metastasis
requires a greater number of patients. Further follow-up studies
are required to more rigorously address these issues.
In conclusion, the present study demonstrated that
NUF2 may serve an important role in the development and progression
of BC, thus providing a potential target for molecular targeted
therapy against BC.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Public
Technology Research Project of Zhejiang Province (grant no.
LGF18H200006) and the Medicines Health Technology Plan Project of
Zhejiang Province (grant no. 2018PY073).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL, WX and XD were involved in the study design. SL
and WX contributed to data collection and analysis. SL, JZ and YZ
performed the RT-qPCR experiments and analyzed the data. All
authors were involved in drafting the manuscript. XD critically
reviewed the manuscript and provided final approval of the
published version. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved and supervised by the
Ethics Committee of Shaoxing People's Hospital. Written informed
consent was obtained from all participants.
Patient consent for publication
Consent for publication was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and Trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar
|
|
2
|
Siegel RK, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arnaout A, Lee J, Gelmon K, Poirier B, Lu
FI, Akra M, Boileau JF, Tonkin K, Li H, Illman C, et al:
Neoadjuvant therapy for breast cancer: Updates and proceedings from
the seventh annual meeting of the canadian consortium for locally
advanced breast cancer. Curr Oncol. 25:e490–e498. 2018. View Article : Google Scholar :
|
|
7
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sandoo A, Kitas GD and Carmichael AR:
Breast cancer therapy and cardiovascular risk: Focus on
trastuzumab. Vasc Health Risk Manag. 11:223–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nabetani A, Koujin T, Tsutsumi C,
Haraguchi T and Hiraoka Y: A conserved protein, Nuf2, is implicated
in connecting the centromere to the spindle during chromosome
segregation: A link between the kinetochore function and the
spindle checkpoint. Chromosoma. 110:322–334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeLuca JG, Moree B, Hickey JM, Kilmartin
JV and Salmon ED: hNuf2 inhibition blocks stable
kinetochore-microtubule attachment and induces mitotic cell death
in HeLa cells. J Cell Biol. 159:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu D, Ding X, Du J, Cai X, Huang Y, Ward
T, Shaw A, Yang Y, Hu R, Jin C and Yao X: Human NUF2 interacts with
centromere-associated protein E and is essential for a stable
spindle microtubule-kinetochore attachment. J Biol Chem.
282:21415–21424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneko N, Miura K, Gu Z, Karasawa H,
Ohnuma S, Sasaki H, Tsukamoto N, Yokoyama S, Yamamura A, Nagase H,
et al: siRNA-mediated knockdown against CDCA1 and KNTC2, both
frequently overexpressed in colorectal and gastric cancers,
suppresses cell proliferation and induces apoptosis. Biochem
Biophys Res Commun. 390:1235–1240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sethi G, Pathak HB, Zhang H, Zhou Y,
Einarson MB, Vathipadiekal V, Gunewardena S, Birrer MJ and Godwin
AK: An RNA interference lethality screen of the human druggable
genome to identify molecular vulnerabilities in epithelial ovarian
cancer. PLoS One. 7:e470862012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayama S, Daigo Y, Kato T, Ishikawa N,
Yamabuki T, Miyamoto M, Ito T, Tsuchiya E, Kondo S and Nakamura Y:
Activation of CDCA1-KNTC2, members of centromere protein complex,
involved in pulmonary carcinogenesis. Cancer Res. 66:10339–10348.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi Y, Takano A, Miyagi Y, Tsuchiya
E, Sonoda H, Shimizu T, Okabe H, Tani T, Fujiyama Y and Daigo Y:
Cell division cycle-associated protein 1 overexpression is
essential for the malignant potential of colorectal cancers. Int J
Oncol. 44:69–77. 2014. View Article : Google Scholar
|
|
16
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A III: AJCC cancer staging manual. 7th
edition. Springer; pp. 2372009
|
|
17
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nuclc Acids Res. 45:W98–W102.
2017. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Wigge PA and Kilmartin JV: The Ndc80p
complex from Saccharomyces cerevisiae contains conserved centromere
components and has a function in chromosome segregation. J Cell
Biol. 152:349–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Tan PY, Handoko YA, Sekar K, Shi
M, Xie C, Jiang XD, Dong QZ, Goh BKP, Ooi LL, et al: NUF2 is a
valuable prognostic biomarker to predict early recurrence of
hepatocellular carcinoma after surgical resection. Int J Cancer.
145:662–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan W, Xie S, Wang M, Pan S, Huang X,
Xiong M, Xiao RJ, Xiong J, Zhang QP and Shao L: Bioinformatic
analysis of prognostic value of ZW10 interacting protein in lung
cancer. Onco Targets Ther. 11:1683–1695. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Obara W, Sato F, Takeda K, Kato R, Kato Y,
Kanehira M, Takata R, Mimata H, Sugai T, Nakamura Y and Fujioka T:
Phase I clinical trial of cell division associated 1 (CDCA1)
peptide vaccination for castration resistant prostate cancer.
Cancer Sci. 108:1452–1457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thang PM, Takano A, Yoshitake Y, Shinohara
M, Murakami Y and Daigo Y: Cell division cycle associated 1 as a
novel prognostic biomarker and therapeutic target for oral cancer.
Int J Oncol. 49:1385–1393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Menon VR, Peterson EJ, Valerie K, Farrell
NP and Povirk LF: Ligand modulation of a dinuclear platinum
compound leads to mechanistic differences in cell cycle progression
and arrest. Biochem Pharmacol. 86:1708–1720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sundin LJ, Guimaraes GJ and Deluca JG: The
NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to
kinetochore-microtubule attachment in mitosis. Mol Biol Cell.
22:759–768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
DeLuca JG, Howell BJ, Canman JC, Hickey
JM, Fang G and Salmon ED: Nuf2 and Hec1 are required for retention
of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr
Biol. 13:2103–2109. 2003. View Article : Google Scholar : PubMed/NCBI
|