Introduction
Isatin is an endogenous indole present in mammalian
brain tissues, body fluids, (including urine) and peripheral
tissues, as well as in extracts of Brassica oleracea and
Indigo naturalis (1). It is
the precursor of indirubin, a novel class I anticancer drug, or the
isomer of the melanin component involved in oxidative stress
(2). However, compared with
indirubin, isatin is a smaller molecule with a simpler structure
that can overcome steric hindrance and allow penetration into the
mucous epithelium (3). In
addition, isatin exerts an antioxidant effect via allosteric
inhibition of monoamine oxidase inhibitor, indicating its potential
as an anticancer agent (4).
Neuroblastoma is the most common extracranial solid
tumor diagnosed in infants and children up to 5-years old (5). It originates from the embryonic
neural crest cells that normally differentiates into the
sympathetic ganglia of the autonomic nervous system, or the
catecholamine-secreting cells of the adrenal glands (6). Therefore, neuroblastomas usually
arise in the adrenal medulla, neck, chest and spinal cord (6). The clinical manifestations and
outcomes of neuroblastoma vary, and patients with localized
neuroblastoma have a favorable prognosis and have an disease-free
survival rate of >90% 5 years after diagnosis (7). However, most cases are usually
diagnosed when the tumor cells have already metastasized, resulting
in a poor prognosis with a 5-year survival rate of ~50% (8-10).
At present, patients with high-risk neuroblastoma with early
metastasis are treated with high doses of combination chemo-therapy
(11), which has disadvantages
including severe side effects and recurrence.
Plant-derived compounds have gained considerable
attention in recent years to treat cancer owing to their minimal
toxicity and targeted antitumor effects (12). Isatin is a natural compound
(13) and the monomeric precursor
of indirubin (14). It is also a
constituent of the Daqingye and Qingqing formulations that have a
wide range of biological activities (15). Daqingye is widely used for the
treatment of influenza, viral pneumonia, mumps, pharyngitis, and
hepatitis and has anxio-genic, sedative, anticonvulsant,
antineoplastic, antimicrobial and antiviral properties (16).
Lysine-specific demethylase 1 (LSD1), is a
flavin-dependent demethylase. It is abnormally overexpressed in a
range of solid tumors, particularly in neuroblastoma (17). LSD1 is an established oncogene that
promotes metastasis in various cancer types, for example breast
cancer, prostate cancer and acute myeloid leukemia (18-20)
via epigenetic regulation of various pro-oncogenic and
pro-angiogenic pathways.
The present study analyzed the effect of isatin on
the malignant phenotype of neuroblastoma cells, and the findings
indicated that isatin is a promising therapeutic agent against
neuroblastoma through LSD1.
Materials and methods
Cell culture
SH-SY5Y neuroblastoma cells were purchased from
American Type Culture Collection, and STR profiling was conducted.
The cells cultured in high-glucose DMEM containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin/streptomycin under 5% CO2 at 37°C. The cells
were passaged once they were 80-90% confluent, and logarithmic
growth phase cultures were harvested for further analysis.
Cell transfection
The lentiviral construct with the Luc (GV260) tag
was synthesized by (Shanghai GenePharma Co., Ltd. After 48 h of
cell transfection, the 70-80% confluent cells were infected with
the virus at the MOI of 30. The stably transduced cells were
screened 48 h later using puromycin, and tested for Luc tag using
D-luciferin.
RNA extraction and quantitative
(q)PCR
RNA was isolated from the isatin-treated cells using
the TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions, and
analyzed using spectro-photometry (BioTek Instruments, Inc.).
Following reverse transcription (using a Prime-Script qPCR kit;
TransGen Biotech Co., Ltd.), the cDNA was amplified using
TransStart SYBR Probe qPCR SperMix (TransGen Biotech Co., Ltd.) on
a Bio-Rad One-Step Plus system (Bio-Rad Laboratories, Inc.). The
temperature protocol for reverse transcription was 42°C for 15min
and 85°C for 5 sec. The thermocycling conditions for qPCR were 94°C
for 30 sec, 94°C for 5 sec and 60°C for 30 sec, for 45 cycles.
Primer sequences for GAPDH, lysine-specific histone demethylase
(LSD)1 and p53 are provided in (Table
SI). Relative gene expression levels were calculated using the
2−∆∆Cq method (21).
Cell Counting Kit (CCK)-8 assay
Cells in the logarithmic growth phase were washed
twice with PBS, harvested using trypsin, and seeded in a 96-well
plate at the density of 3,000 cells/100 µl/well. After
treating with different concentrations (0, 25, 50, 100, 200, 300,
400, 500 and 800 µmol/l) of isatin for 3 days at 37°C, the
cells were washed and incubated at 37°C for 1 h with 10 µl
CCK8 according to the manufacturer's protocols (Beijing Solarbio
Science & Technology Co., Ltd.). Absorbance was measured at 450
nm using a microplate reader (Synergy H1; BioTek Instruments, Inc).
Each experiment was performed three times.
Cell cloning assay
The cells treated with isatin were seeded in a
six-well plate at a density of 100 cells per well and cultured for
2 weeks. The ensuing colonies were fixed with 4% paraformaldehyde,
stained with crystal violet (both at room temperature), air dried
and counted manually under a light microscope at ×200.
Apoptosis assay
Cells were seeded in six-well plates at the density
of 3×105 cells per well, and cultured until 80%
confluent. The cells were harvested and stained using the FITC/PI
Annexin V Apoptosis Detection kit I (BD Pharmingen; BD Biosciences)
to detect the level of apoptosis using a Accuri C6 flow cytometer
and analyzed with the corresponding software (BD Biosciences). The
percentage of early apoptotic cells was analyzed using flow
cytometry within 1 h of staining.
Wound scratch assay
The cells treated with isatin were seeded in a
6-well plate and cultured until a uniform monolayer had formed. The
layer was scratched with a sterile P200 pipette tip, and the wells
were rinsed with the aforementioned medium to remove all cellular
debris. Low-serum DMEM with mitomycin (2 µg/ml) was then
added to inhibit cell proliferation. Images (Nikon, TI-SM) of the
wound area were captured using a TI-SM light microscope
(magnification, ×100 or ×200) at 0, 24 and 48 h post scratching,
and the migration rate (%) was calculated as the percentage of area
covered by migrated cells divided by the total wound area.
Transwell assays
Precoating with Matrigel was conducted at 37°C for 4
h. The upper compartment of Transwell inserts were seeded with
cells in serum-free medium, and the lower chambers were filled with
600 µl complete medium with 30% FBS. After 12 to 24 h of
incubation, the inserts were removed, and the cells remaining on
the filter surface were swabbed. The cells that had
migrated/invaded to the other side the filter were fixed with
paraformaldehyde for 5 min at room temperature), stained with
crystal violet and images were captured using a light microscope
(magnification, ×100).
Western blotting
The cells treated with isatin were lysed in RIPA
buffer (Beijing Solarbio Science & Technology Co., Ltd.)
supplemented with a protease inhibitor cocktail (Sigma-Aldrich;
Merck KGaA) on ice for 30 min, and centrifuged for 20 min at 4°C
and 8,000 × g. The protein concentration in the cleared lysate was
measured using a BCA assay (Beyotime Institute of Biotechnology).
In total, 20 ng protein were loaded per lane onto a 10% gel,
resolved using SDS-PAGE and transferred to a PVDF membrane. After
blocking with 5% BSA in TBST for 2 h at room temperature, the
membranes were incubated overnight with the primary antibodies (all
1:1,000; Table I) at 4°C. The
blots were washed thrice with TBST, incubated with HRP-conjugated
secondary antibody (1:2,000; Wuhan Boster Biological Technology,
Ltd.), and washed again with TBST. The protein bands were detected
with an ECL detection system (Beijing Transgen Biotech Co., Ltd.).
The Fusion FX7 luminescence imaging system (Vilber) was used to
visualize and analyze protein bands.
 | Table IAntibody information. |
Table I
Antibody information.
| Antibody name | Supplier (catalog
number) |
|---|
| β-actin | Abcam (ab8226) |
| GAPDH | Abcam (ab8245) |
| LSD1 | Abcam
(ab129195) |
| P53 | Abcam (ab26) |
| H3K4Me1 | Abcam
(ab176877) |
| H3K4Me2 | Abcam
(ab32356) |
| Bax | Abcam
(ab32503) |
| Bcl-2 | Abcam
(ab59348) |
| MDM2 | Abcam
(ab259165) |
| TGFβ1 | Bioss
(bsm-33287M) |
| p-NF-κB | Abcam
(ab207297) |
| AffiniPure Rabbit
Anti-human IgG | Boster
(BA1041) |
| AffiniPure Mouse
Anti-Rabbit IgG | Boster
(BM2020) |
Co-immunoprecipitation
The cells treated with isatin were lysed (1 ml RIPA
buffer) at 4°C for 30 min, and the lysates were incubated overnight
with anti-LSD1 antibody (1:500; Abcam). After adding 40 µl
A/G sepharose (CST, 70024S), the lysates were incubated for 2 h at
4°C with constant agitation. Cells were centrifuged at 2,000 × g
for 5 min at 4°C and the supernatant was discarded. Cells were
washed six times with 500 µl wash buffer (50 Mm Tris-HCl,
150 mM NaCl, 1% Triton and 1% PMSF), then centrifuged at 4,000 × g
at 4°C for 5 min and the resulting supernatant was discarded. The
immunoprecipitates were separated using SDS-PAGE as aforementioned
after washing with the same buffer, and analyzed by immunoblotting
with the suitable antibodies.
Cell chemiluminescence detection
After cells were transfected with luc-labelled
lentivirus for 48 h, cells in the logarithmic growth phase were
plated into a 96-well plate with 2,000 cells/well. After 24 h, the
original culture medium was removed and D-luciferin sodium working
solution was added (150 µg/ml) with 100 µl per well.
D-fluorescein sodium working solution was diluted to 1:200 using
D-fluorescein sodium stock solution (30 mg/ml) and pre-warmed
culture medium. Within 10-20 min, a microplate reader was used to
detect luminescence.
Establishment of in vivo tumor model and
treatment
In total, 20 4-week old female athymic nude mice
(14-15 g) were purchased from the Vital River Laboratory Animal
Technology Co. Ltd. The mice were anesthetized via inhalation of 2%
isoflurane, and inoculated with 1×106 tumor cells in 100
µl medium between the second and third ribs. The inoculated
mice were placed in a 37°C incubator until they became fully awake.
On day 3 post-injection, the mice were randomly divided into the
(A) control, (B) cyclophosphamide (CTX), (C) isatin (ISA) and (D)
combination (D) groups (n=5 each), and treated with 5 ml/kg 1.25%
Gummi Tragacanthae, 40 mg/kg CTX, 200 mg/kg ISA and 20 mg/kg CTX +
200 mg/kg ISA, respectively. The drugs were administered daily via
the intragastric route for 4 weeks. Tumor formation and metastasis
were observed using a live imager. All experimental procedures were
performed in compliance with the National Institutes of Health
Guide for Care and Use of Laboratory Animals (22), and were approved by The Animal
Ethics Committee of Qingdao University [approval no.
QDU20190506b0200610(031)].
Weights of the mice were checked every three days,
and fur gloss and behavior were also examined. After 3 days of
modeling, the drug was continuously administered for 4 weeks, and
mice were imaged in vivo. According to the results, it was
found that the tumor metastasis of the mice in the control group
had reached the standard, so the mice were sacrificed 4 weeks after
drug treatment, and the experiment ended. Animals were euthanized
with an overdose of 2% pentobarbital sodium (100 mg/kg) followed by
cervical dislocation.
Serum marker detection
The following ELISA kits were used to detect
specific markers in mouse serum samples: Mouse Matrix
metalloproteinase (MMP)2 (cat. no. ZN2705) mouse vascular
endothelial growth factor (cat. no. ZN2803), mouse MMP 9 (cat. no.
ZN2808), superoxide dismutase (SOD; cat. no. A001-1), glutathione
peroxidase (GSH-PX; cat. no. A005), malondialdehyde (MDA; cat. no.
A003-1), urea nitrogen (BUN; cat. no. C013-2), creatinine (CREA;
cat. no. C011-1), bilirubin (cat. no. C019-1-1), alkaline
phosphatase (AKP; cat. no. A059-1) and γ-GT (cat. no. C017) (all
Nanjing Jiancheng Bioengineering Institute). All operations are
performed according to the kit instructions.
Molecular docking
Molecular docking was performed using Glide
(Schrodinger Inc) with LSD1 crystal structure (PDB Entry: 4KUM) as
the receptor. Suitable structural modifications were made, and the
water molecules and the crystallized ligand in the protein
structure were removed. The refined structure was simulated in an
OPLS 2005 force field, and visualized using Maestro in Schrodinger
(Schrodinger Suite 2009). The active site was defined as a cubic
box containing residues around ligand FAD901 at a distance of 20 Å.
Other parameters were set at default levels.
Statistical analysis
The data were obtained from at least three
independent experiments and analyzed using SPSS 17.0 (SPSS, Inc)
for Windows. All data are expressed as mean ± SD or SEM. ANOVA
followed by Tukey's post hoc test was performed to comparing
differences between means in multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Isatin decreases the proliferative
activity of SH-SY5Y cells
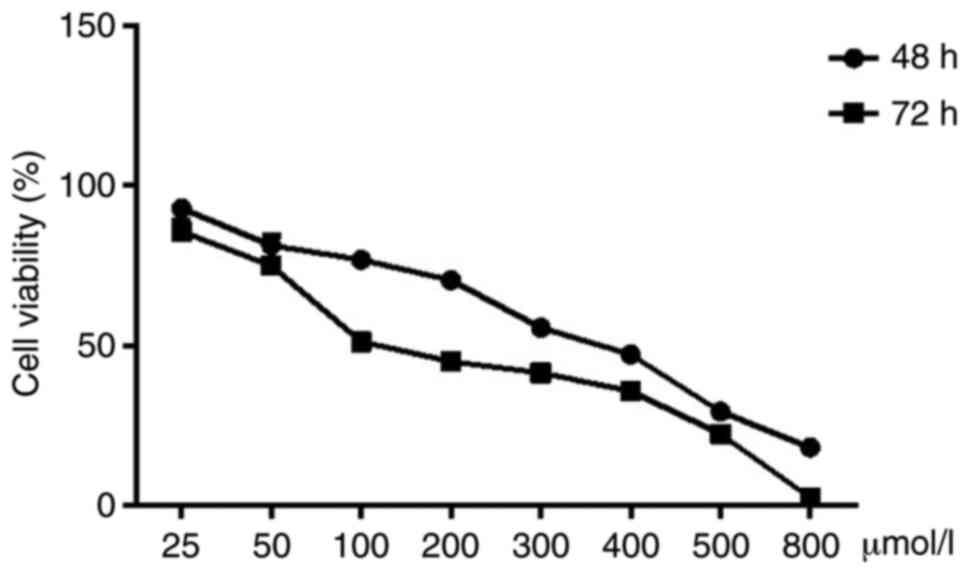
SH-SY5Y cells treated with 25, 50, 100, 200, 300,
400, 500 and 800 µM isatin for 48 and 72 h showed a marked
decrease in proliferation rates (Fig.
1 and Table SII).
Concentrations below 200 µM did not show any significant
effect on cellular morphology, whereas higher doses resulted in
aberrant morphological changes, obvious shrinkage and the number of
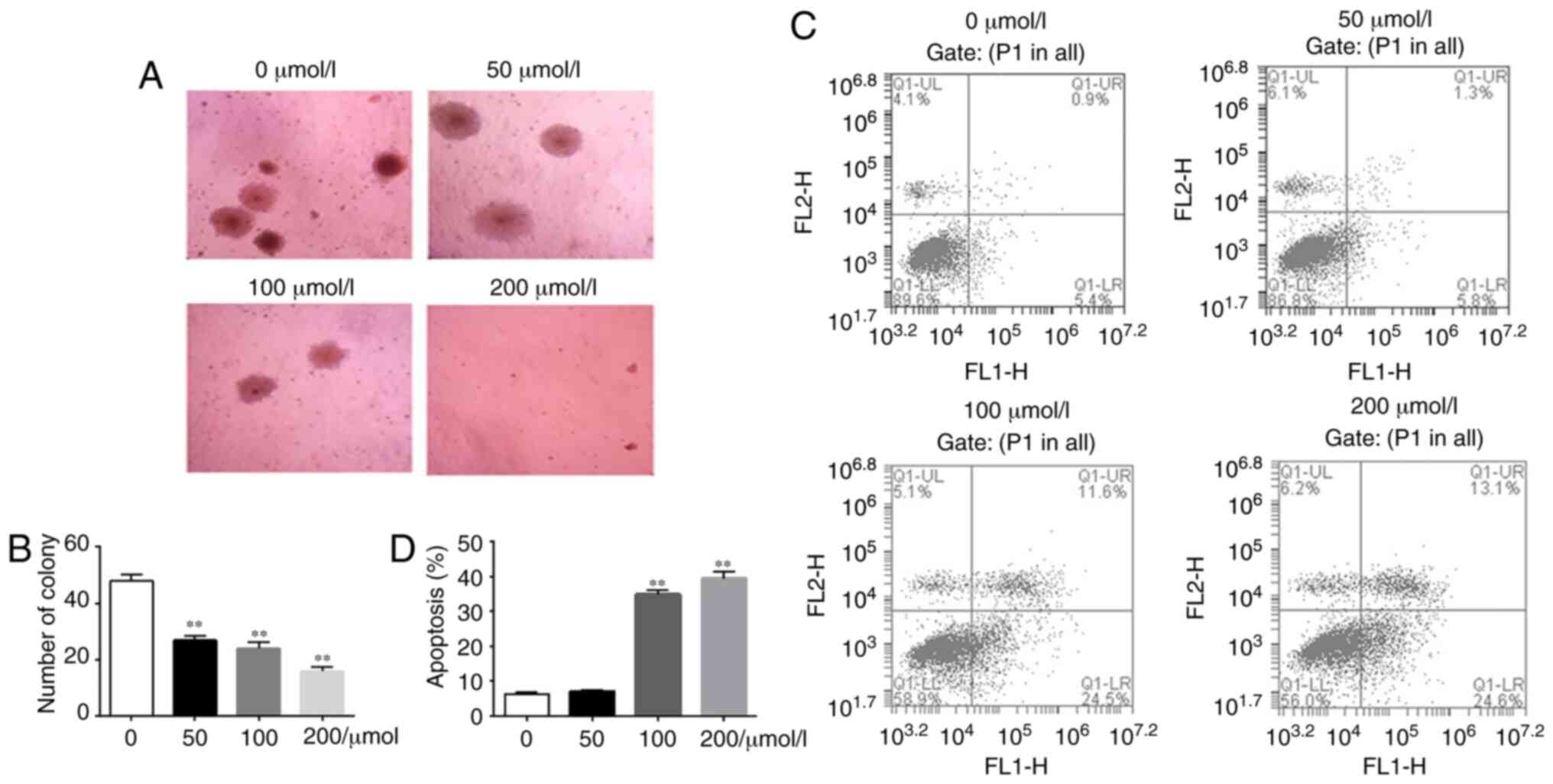
viable cells. Consistent with this, isatin also inhibited clonal
expansion of the SH-SY5Y cells in a concentration-dependent manner
(Fig. 2). Compared with the
untreated control, the number of colonies decreased significantly
following treatment with isatin (P<0.01) (Fig. 2). Similarly, apoptosis rates raised
obviously following treatment with 100 and 200 µM isatin
(P<0.01). Taken together, these data demonstrated that isatin
inhibited the proliferation of neuroblastoma cells.
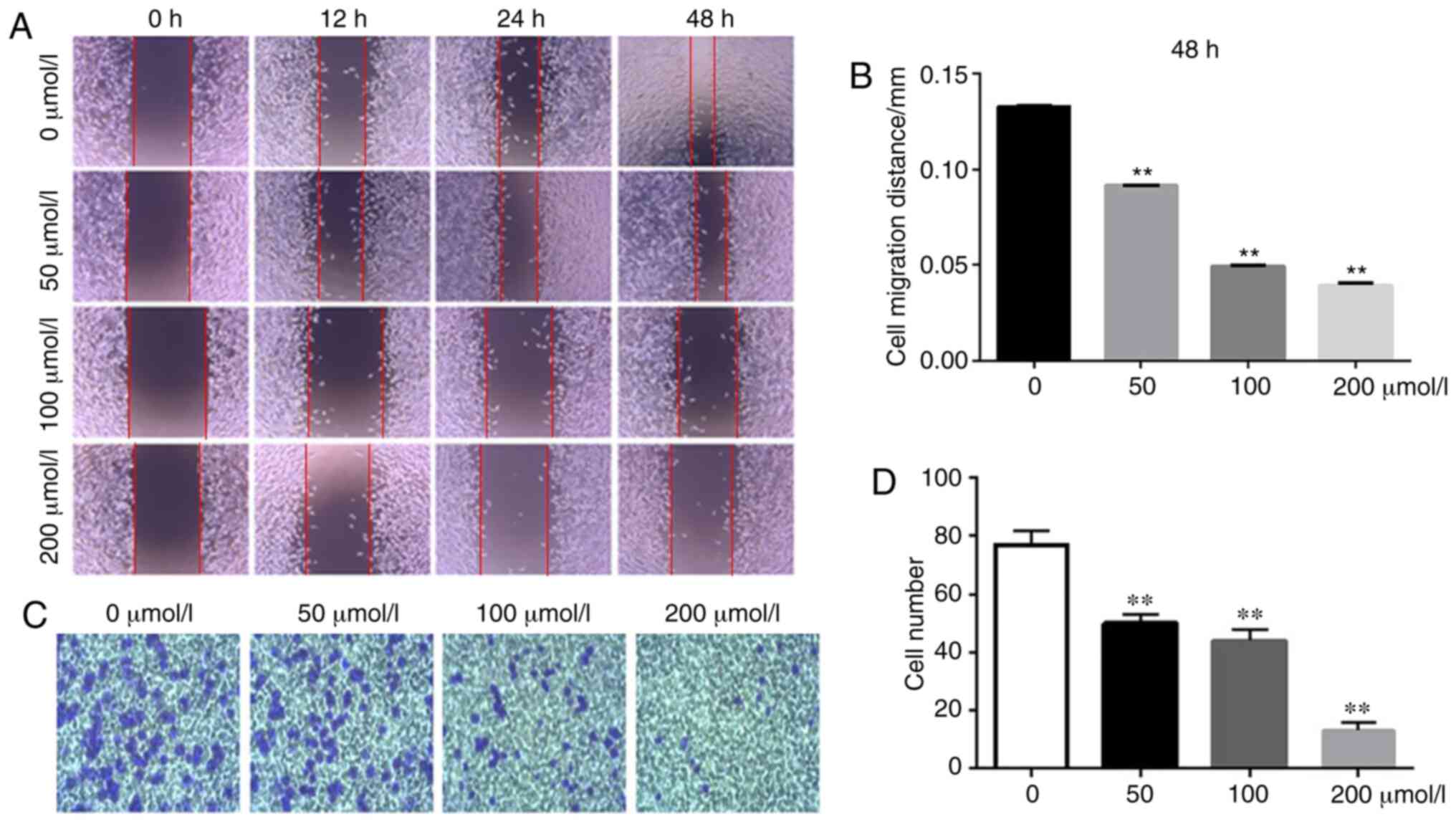
Isatin inhibits the migration and
invasion of neuroblastoma cells
The effect of isatin on tumor cell migration and
invasion was assessed using wound healing and Transwell assays. The
rate of wound healing was significantly slower in the SH-SY5Y cells
treated with isatin compared with the untreated cells. At 48 h,
wound coverage was almost complete in the control group but a
noticeable gap remained in the all isatin-treated groups
(P<0.01; Fig. 3A and B).
Furthermore, isatin also significantly decreased the invasion
capacity of the SH-SY5Y cells through the Matrigel-coated Transwell
insert (Fig. 3C and D) by
35.22±4.21, 42.14±1.58 and 83.42±3.67% at 200 µM
(P<0.01), the same level of significance was also observed at 50
and 100 µM (P<0.01).
Molecular mechanisms underlying the
antitumor effects of isatin
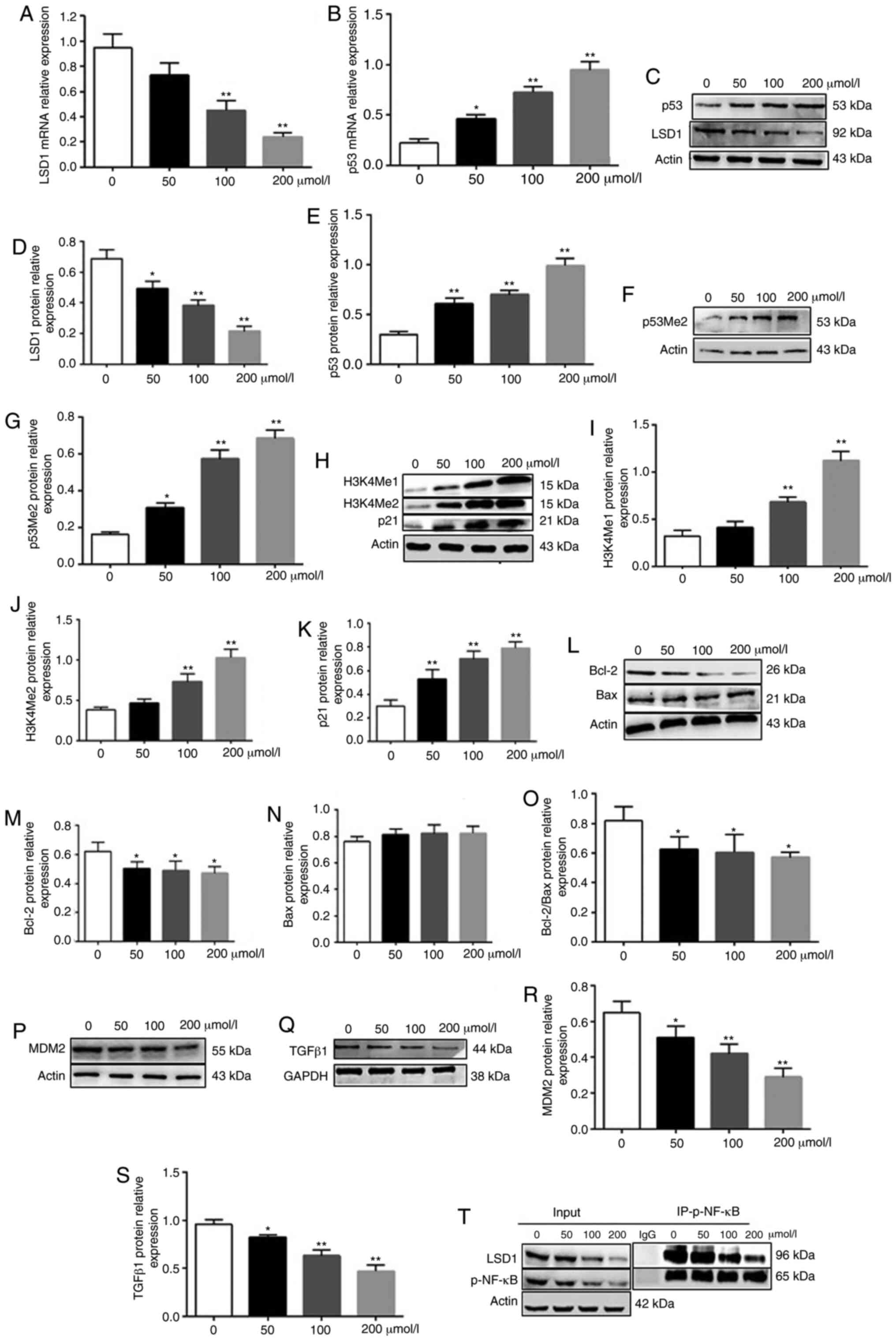
LSD1 is an oncogene that inhibits the tumor
suppressor p53 by demethylating the lysine 370 residue. Consistent
with this, LSD1 is overexpressed in neuroblastoma tissues and cell
lines, and is associated with the grade of tumor malignancy
(17). Isatin significantly
decreased the expression of LSD1 mRNA (P<0.01, 100 and 200
µmol/l vs. control) and protein (P<0.05, 50 µmol/l
vs. control; P<0.01, 100 and 200 µmol/l vs. control), and
increased that of p53 mRNA (P<0.05, 50 µmol/l vs.
control; P<0.01, 100 and 200 µmol/l vs. control) and
protein (P<0.01 vs. control) (Fig.
4A-E). In addition, isatin also upregulated the levels of
p53Me2 (P<0.05, 50 µmol/l vs. control; P<0.01, 100 and
200 µmol/l vs. control) (Fig.
4F and G). Given that LSD1 specifically demethylates histone
H3K4 and transcriptionally inhibits the target genes (23), the levels of H3K4Me1 and H3K4Me2
were also analyzed, demonstrating that isatin treatment
significantly upregulated H3k4Me1 (P<0.01, 100 and 200
µmol/l vs. control) and H3K4Me2 (P<0.01, 100 and 200
µmol/l vs. control) (Fig.
4H-J). Furthermore, the down-stream pro-apoptotic protein p21
was significantly increased by isatin (P<0.01 vs. control)
(Fig. 4H and K) while the p53
destabilizing MDM2 (P<0.05, 50 µmol/l vs. control;
P<0.01, 100 and 200 µmol/l vs. control) and antiapoptotic
Bcl-2 were downregulated (P<0.05 vs control) (Fig. 4L, M, O, P and R). TGFβ1 may
activate LSD1 via the ERK/NF-κB pathway (23). Consistent with the aforementioned
results, isatin not only decreased the levels of TGFβ1 (P<0.05,
50 µmol/l vs. control; P<0.01, 100 and 200 µmol/l
vs. control) protein in neuroblastoma cells (Fig. 4Q and S) but also inhibited the
co-precipitation of LSD1 and phosphorylated (p-)NF-κB and decreased
the expression level of p-NF-κB (Fig.
4T). Taken together, these data demonstrated that isatin
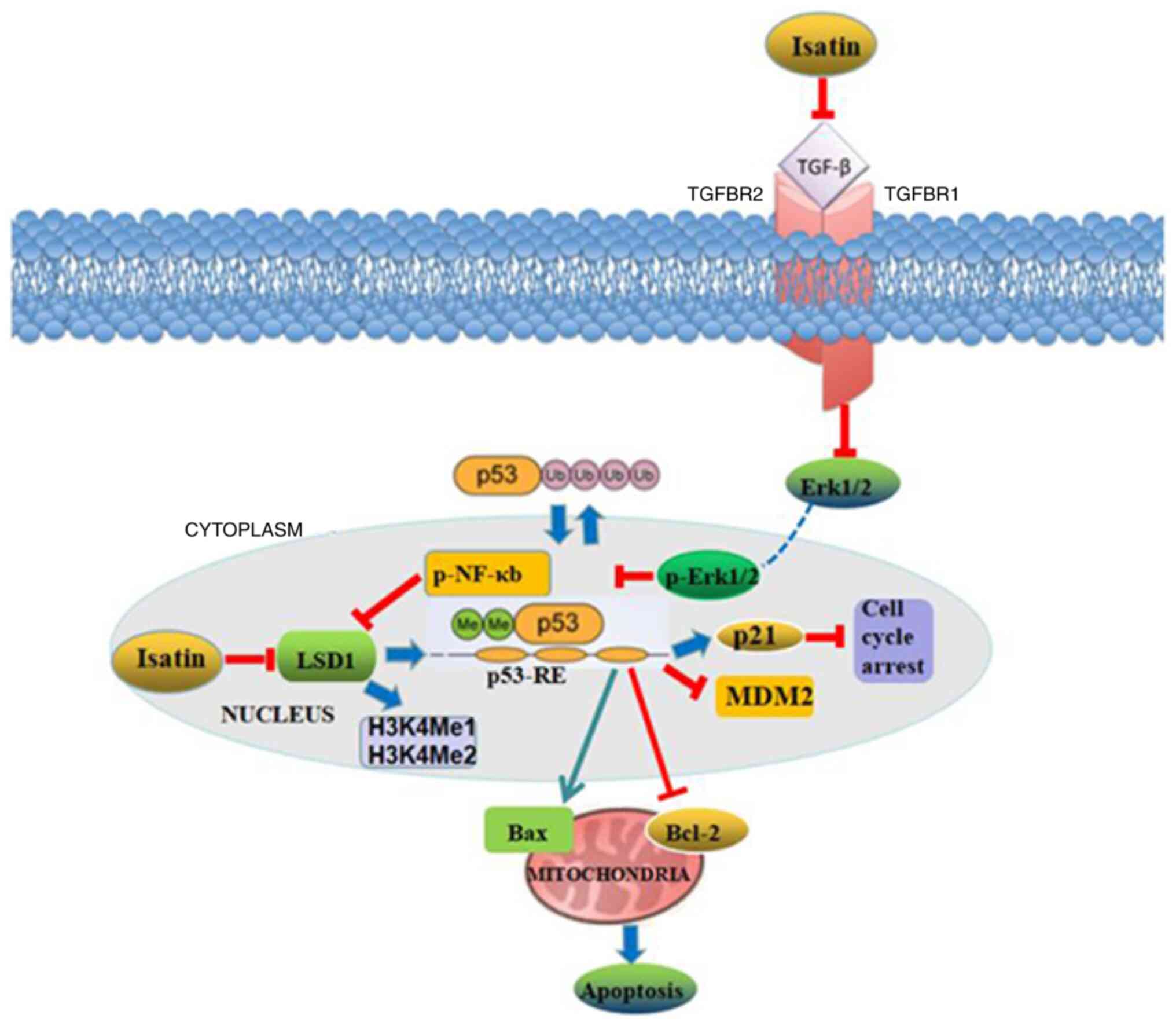
inhibits the expression of TGFβ1/NF-κB/LSD1.
Isatin inhibits the metastasis of
neuroblastoma SH-SY5Y cells in nude mice
The Luc-SH-SY5Y cells were injected in nude mice to
establish an in vivo neuroblastoma model, and metastasis was
tracked using real-time fluorescence imaging. Luc labeling has no
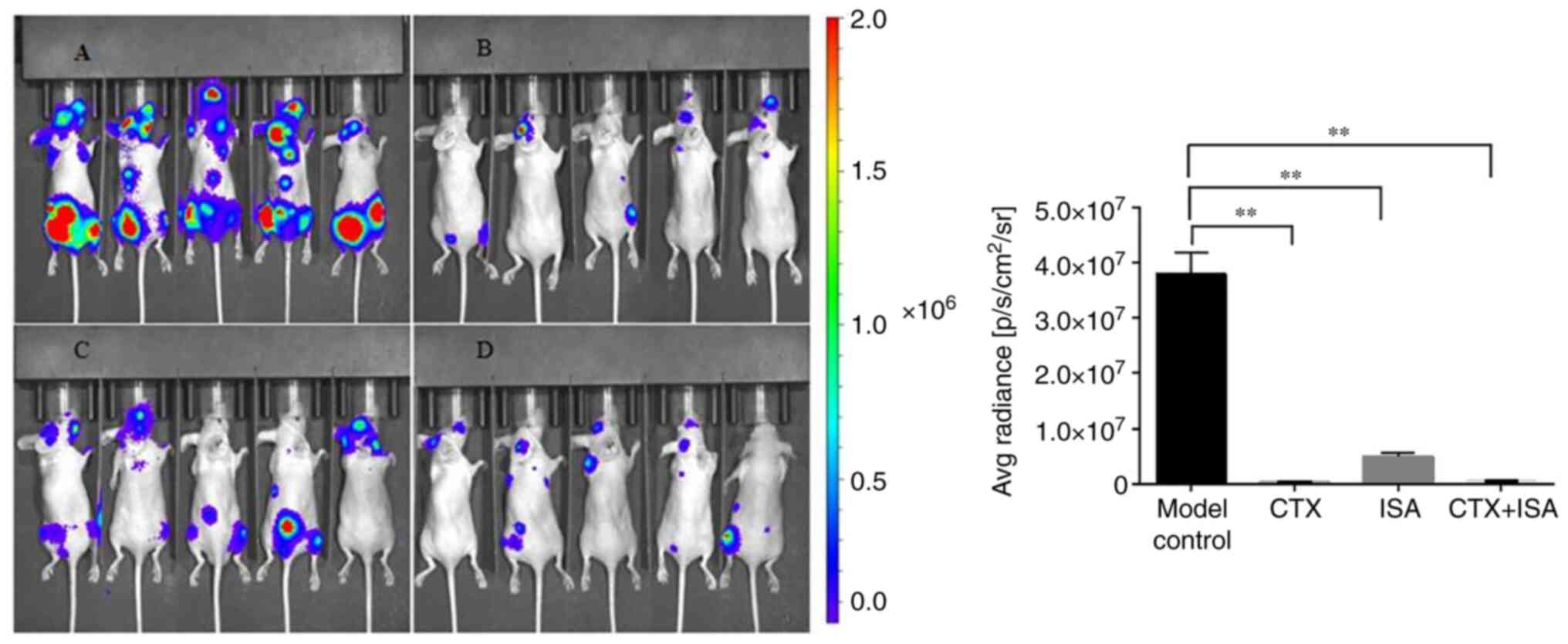
direct effect on cell proliferation (Fig. S1). As shown in Fig. 5, the untreated tumor-bearing mice
showed strong fluorescence signals in the cervical vertebrae,
spine, scapula, pelvic bone and extremities of long bones,
indicating that distant metastasis of the tumor cells had occurred.
The fluorescence intensities decreased significantly in the ISA and
CTX-treated groups, with a more substantial inhibition in the
latter (P<0.01), and were weakest in the CTX+ISA group and
limited the brain, spine and pelvis. The CTX dose in the
combination regimen was half of that in the monotherapy group,
which indicated a synergistic effect of combining CTX and ISA. The
nude mice were weighed once every 2 days. No significant changes
were found in the body weight of the nude mice in each group
(Fig. S2).
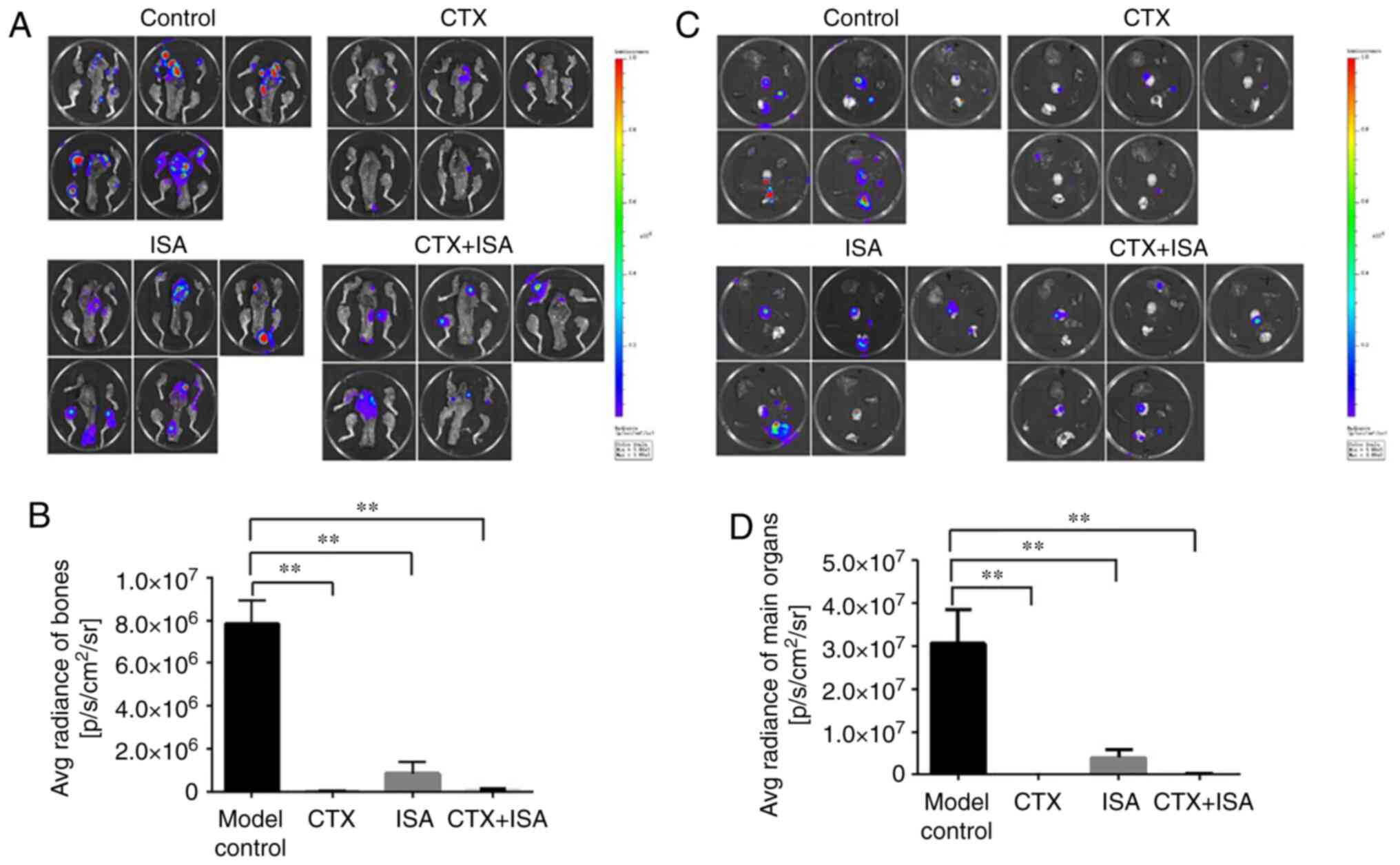
A statistical analysis on the fluorescence signal
intensity of bone metastasis in each group was made, as shown in
Fig. 6A. The results showed that
compared with the model group, the fluorescence intensity of bone
metastasis in the other three groups was significantly decreased
(P<0.01 vs. control; Fig. 6B).
Compared with the ISA group, the fluorescence intensity of bone
metastases in the CTX group and the combination group was reduced,
but there was no statistical difference. There was no significant
difference between CTX group and combination group.
The intensity of the transfer fluorescence signal in
the main organs of each group of nude mice was analyzed, as shown
in Fig. 6C. The results showed
that compared with the model group, the fluorescence intensity of
the main organs (heart, liver, lung, kidney and spleen) in the
other three groups were significantly decreased (P<0.01;
Fig. 6D). Compared with the ISA
group, the fluorescence intensity of the main organs in the CTX
group and the combination group were significantly decreased
(P<0.01; Fig. 6D). There was no
significant difference between CTX group and combination group.
However, no visible solid tumor tissues were found in any of the
mice.
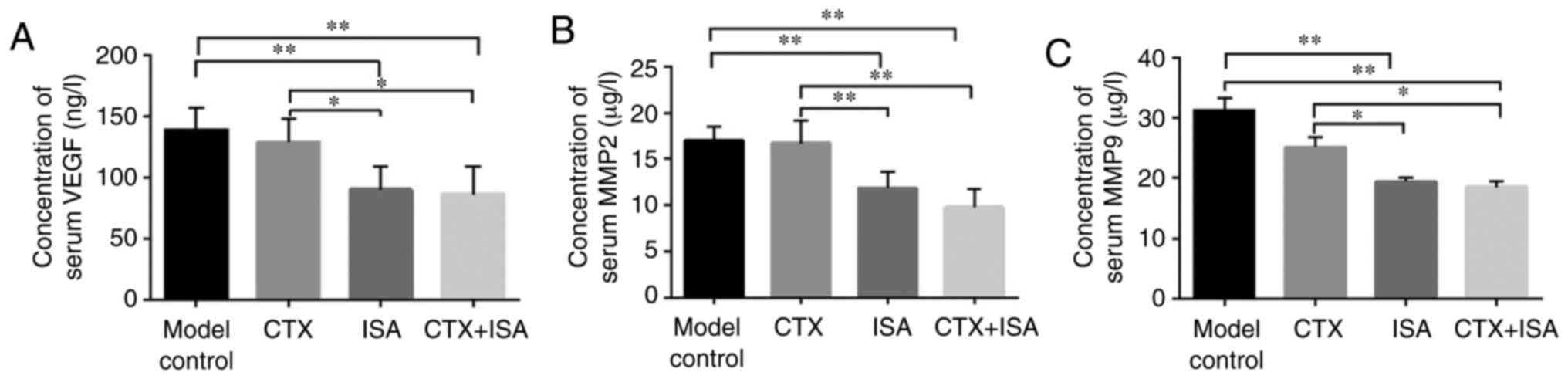
The levels of the angiogenic VEGF, and
pro-metastatic MMP2 and MMP9 in the sera of the
differentially-treated tumor-bearing mice were measured. The
expression of VEGF is positively correlated with tumor microvessel
density. It can accelerate tumor invasion and early metastasis by
promoting tumor angiogenesis to meet the nutrient and oxygen supply
needed by rapid growth tumor (24). VEGF levels were significantly
decreased in the ISA and ISA+CTX groups compared with the model
(P<0.01) and CTX groups (P<0.05) (Fig. 7A). MMPs aid tumor cell invasion and
metastasis by degrading the extracellular matrix (25), MMP2 decreased significantly in ISA
and CTX+ISA group (P<0.01, vs. control or CTX), simultaneously,
MMP9 has obviously decreased in ISA and CTX+ISA group (P<0.01,
vs. control, P<0.05, vs. CTX). Taken together, these data
suggested that isatin might inhibit tumor invasion, metastasis and
angiogenesis by targeting the MMPs and VEGF.
Effect of drugs on liver and kidney
function in animals
The potential adverse effects of ISA were assessed
in terms of oxidative stress, renal function and liver function.
The results of SOD activity showed that the CTX group was
significantly lower compared with the other three groups (P<0.05
CTX+ISA vs. CTX, P<0.01 ISA vs. CTX). There was no significant
difference in SOD activity between the model, CTX and CTX+ISA
groups (Fig. 8A). The activity of
GSH-PX enzyme in CTX+ISA group was significantly higher compared
with control (P<0.01), compared with CTX group, GSH-PX activity
was higher in ISA and CTX+ISA group (P<0.01) (Fig. 8B). The serum MDA content of the
nude mice in CTX group was significantly higher compared with other
groups (P<0.01; Fig. 8C).
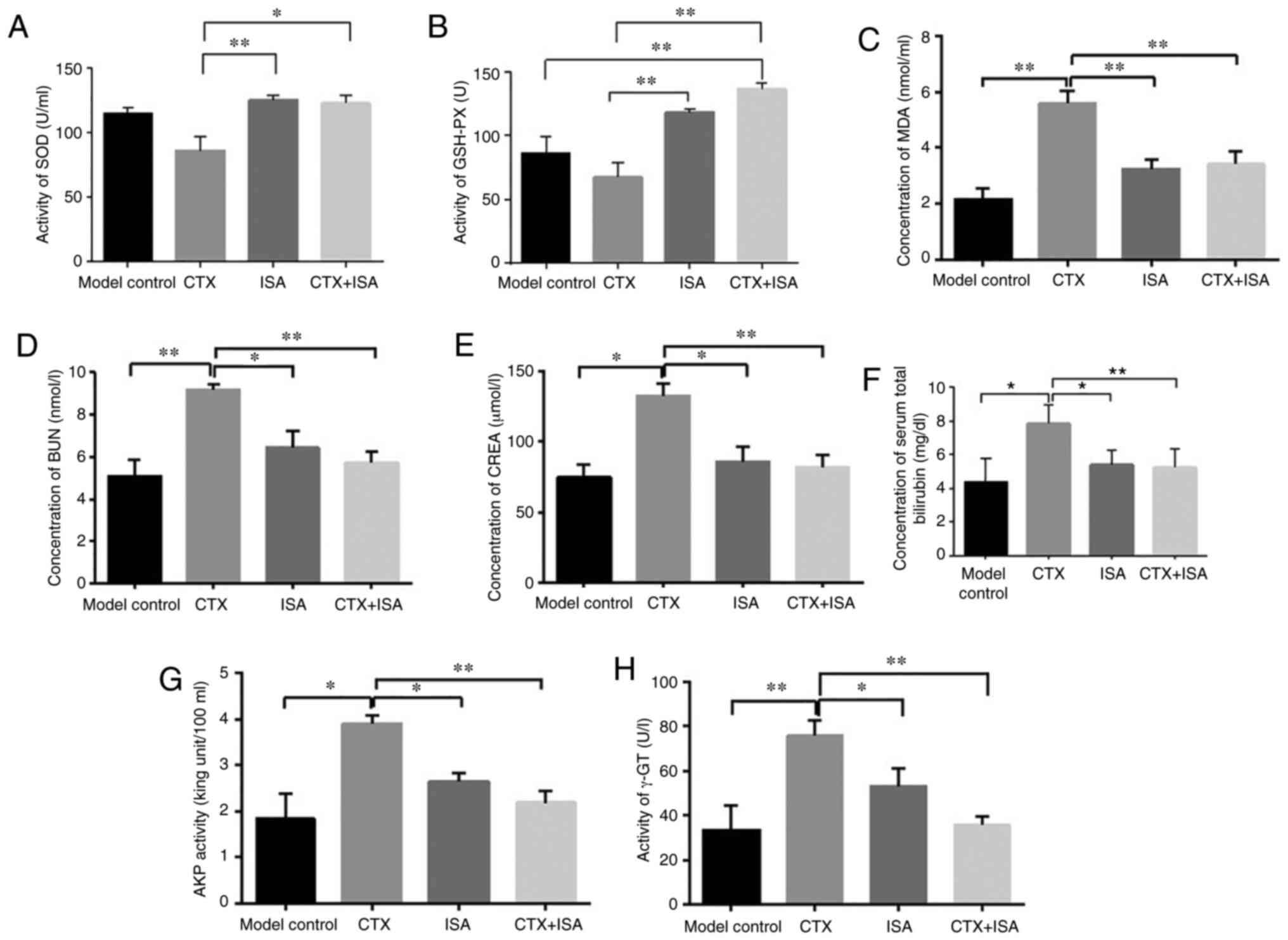 | Figure 8Effect of ISA on liver and kidney
function in tumor-bearing mice. Activity of (A) SOD and (B) GSH-PX.
Levels of (C) MDA and (D) BUN. (E) CREA activity, (F) bilirubin
levels, (G) AKP activity and (H) γ-GT activity in the sera of the
differentially treated mice. *P<0.05 vs. model
control, ISA, CTX or CTX+ISA. **P<0.01 vs. model
control, ISA, CTX or CTX+ISA. CTX, cyclophosphamide; ISA, isatin;
SOD, superoxide dismutase; GSH-PX, glutathione peroxidase; MDA,
malondialdehyde; BUN, urea nitrogen; CREA, creatinine; AKP,
alkaline phosphatase; γ-GT. |
The BUN content of the CTX group was significantly
higher compared with the other three groups (P<0.01 vs. control
and CTX+ISA groups, P<0.05 vs. ISA group; Fig. 8D). There was no significant
difference in BUN content between model and CTX+ISA group (Fig. 8D). The results showed that the
concentration of creatinine (CREA) in the CTX group was
dignificantly higher compared with other group (P<0.05, vs.
control or ISA, P<0.01 vs. CTX+ISA; Fig. 8E). In addition, the concentration
of serum bilirubin in the CTX group was significantly higher
compared with that in the model group (P<0.05), and total
bilirubin in the ISA and CTX+ISA groups was significantly lower
compared with that in the CTX group (P<0.05 and P<0.01,
respectively; Fig. 8F). Serum AKP
results showed that the AKP activity in the control, ISA and CT+ISA
groups was significantly lower compared with that in the CTX group
(P<0.05 vs. control or ISA, P<0.01, vs.CTX+ISA; Fig. 8G). In addition, the γ-GT activity
of the CTX group was also significantly higher compared with other
groups (P<0.05 vs. ISA, P<0.01 vs. control or CTX+ISA;
Fig. 8H).
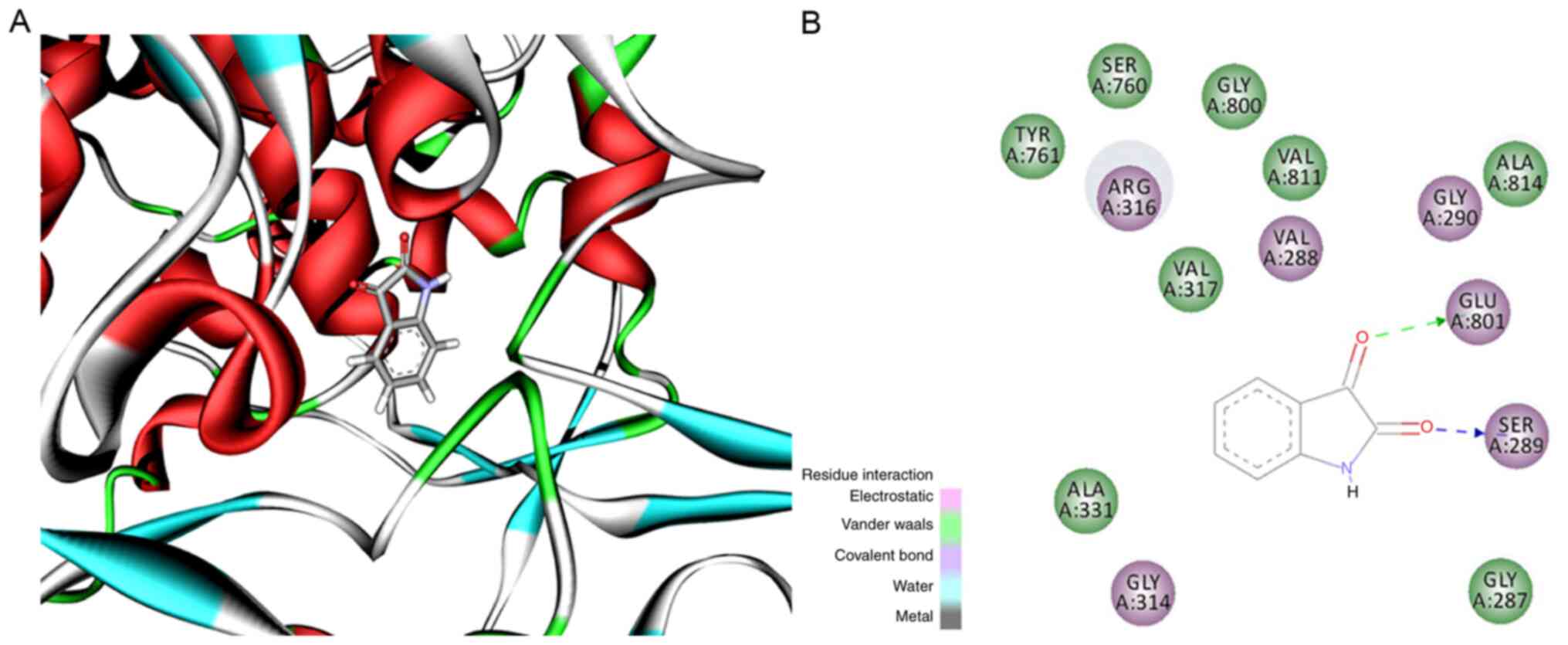
Docking results
Since isatin is a monoamine oxidase inhibitor and
LSD1 is a monoamine oxidase [Triazole-dithiocarbamate based
selective lysine specific demethylase 1 (LSD1) inactivators inhibit
gastric cancer cell growth, invasion, and migration,
10.1021/jm401002r], the binding ability was predicted using
molecular docking of isatin to the LSD1 crystal structure. As shown
in Fig. 9, isatin bound to the
surrounding amino acids (colored green) in LSD1 protein via
hydrophobic interactions. In addition, electrostatic interactions
were also seen at the ligand-receptor binding site (purple amino
acids of LSD1). Finally, hydrogen bonds were formed between two
carbonyl groups of isatin and the NH of Glu801 and OH of Ser289. It
was hypothesized that isatin can bind to LSD1 with high affinity
and inhibit its function.
Discussion
Neuroblastoma is one of the most commonly diagnosed
pediatric solid tumors (26), and
originates from the neuroectodermal tissue that normally develops
into the central and peripheral nervous systems (27). In total, >90% of
cancer-associated deaths in patients with solid tumors are caused
by metastases rather than the primary tumor (28). The mortality rates associated with
neuroblastoma can also be attributed to its high degree of
malignancy and early metastasis, therefore it is important to
target the metastatic cells to improve patient prognosis (29). In spite of aggressive chemotherapy
and targeted therapy, the prognosis for patients with advanced
neuroblastoma remains poor (30).
Therefore, the focus of research has shifted to natural compounds
that target tumor cells with minimal toxicity to the normal
tissues.
The natural compound isatin is a potent antioxidant
with neuroprotective and antitumor effects (31), and has the advantages of low
molecular weight, oral administration, targeted inhibition of tumor
cells and low toxicity (32).
Previous studies have shown that isatin increases the apoptosis of
neuroblastoma cells in vivo and in vitro (33,34),
and inhibits SH-SY5Y cell proliferation and invasion by
downregulating MMP-2/MMP-9 and p-STAT3 in a concentration-dependent
manner (35). Furthermore, a
previous microarray assay showed that isatin regulates the
mTOR-mediated autophagy of SH-SY5Y cells to promote invasion
(36). Consistent with this, it
was found that isatin inhibits the proliferation, invasion and
migration abilities of SH-SY5Y cells in a dose-dependent manner,
and promotes apoptosis and G1 phase arrest (35). In the present study, isatin
significantly reduced the distant metastasis of neuroblastoma cells
in tumor-bearing mice, and synergized with CTX resulting in greater
antimetastatic effects but minimal systemic toxicity.
Mechanistically, isatin significantly decreased the circulating
levels of MMP2, MMP9 and VEGF in the tumor-bearing mice, and
increased the activity of the antioxidant enzymes SOD and GSH. The
present study compared ISA (200 mg/kg) with the positive control
drug CTX (40 mg/kg) and found they have similar antitumor
metastasis effects, but it must be emphasized that the natural
small molecule compound isatin not only has antimetastatic effects,
but also protects normal cells from free radicals. Although the
dosage of isatin was higher compared with the chemotherapeutic drug
CTX, isatin has lower toxicity, fewer side effects and can be taken
orally while the toxicity and side effects of CTX are more severe.
Therefore, these data showed that the protective effect of isatin
should be the focus of future research.
LSD1 is a histone demethylase that removes methyl
groups from H3K4 via the flavin adenine dinucleotide-dependent
oxidative reaction (37). LSD1 is
an established oncogene that promotes metastasis in various cancer
types, for example breast cancer, prostate cancer and acute myeloid
leukemia (18-20) via epigenetic regulation of various
pro-oncogenic and pro-angiogenic pathways. The tumor suppressor p53
is regulated by numerous post-translational modifications,
including lysine methylation. LSD1-mediated demethylation of p53
protein represses the expression of p53 downstream targets and
inhibits apoptosis (38). LSD1 can
remove one (K370me1) or both (K370me2) methyl groups of p53
(19), which is reversed by isatin
via LSD1 inhibition. Furthermore, isatin also upregulates p53 and
its downstream protein p21, which blocks cell cycle progression in
the G1 phase by inhibiting cyclin-dependent kinases
(CDK), including CDK2 and CDK4 (39). The pro-apoptotic Bax and
antiapoptotic Bcl-2 and MDM2 targets of p53 are also affected by
isatin. In the present cellular experiments, it was demonstrated
that isatin decreased the expression of Bcl-2 protein, ratio of
Bcl-2 to Bax, protein expression of MDM2 and increased expression
of p53, which indicated that isatin promoted apoptosis. In
addition, isatin also downregulates TGFβ1, and its downstream
components of the ERK/NF-kB cascade (40), which in turn inhibits LSD1 and the
expression of its target genes. TGFβ signaling controls numerous
cellular processes, such as proliferation, differentiation,
apoptosis and migration (41), and
is the central inducer of epithelial mesenchymal transition of
tumor cells (42) and subsequent
metastatic spread, especially that of breast cancer and prostate
cancer cells to bone and lung (43). Therefore, the antimetastatic effect
of isatin observed in vivo likely involves TGFβ1 inhibition.
Since the molecular docking experiments of the present study
suggested putative binding sites between isatin and LSD1, it was
inferred that isatin can not only inhibit LSD1 via direct binding
but also indirectly through the TGFβ1/ERK/NF-kB pathway (Fig. 10).
Overall, isatin significantly inhibits the malignant
phenotype of neuroblastoma cells and is a promising therapeutic
agent against metastatic neuroblastoma either as a candidate drug
or as an adjuvant of other chemotherapeutic drugs. However, the
exact target of isatin was not confirmed, and there may even be
multiple targets. Deeper and more overall research is still needed
in this respect. In addition, as a natural small molecule lead
compound, a new generation of isatin derivatives need to be
developed to improve their antitumor activity.
Supplementary Data
Funding
This study was supported by The National Natural
Science Foundation of China (grant no. 81472542), The Focus on
Research and Development Plan in Shandong Province (grant no.
2019GSF107025), The Clinical Medicine+X Project of the Medical
Department of Qingdao University and Innovation Team of Qingdao,
The University Medical School Youth Teacher Training Project (grant
no. 600201304) and Qingdao Startup and Innovation Leader Talent
Plan (grant no. 13-CX-3,201409-201709).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LH conceived and designed the study. LZ and YH
collected and analyzed the data and analysis and wrote the final
paper. YH interpretated the data. NZ, JZ, ZZ and NL collected the
data and performed the experiments. JW, WZ, XL and FW analyzed the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mr. Shaun Judge from
Cure Edit for help with the writing and editing of this
manuscript.
References
|
1
|
Vine KL, Matesic L, Locke JM, Ranson M and
Skropeta D: Cytotoxic and anticancer activities of isatin and its
derivatives: A comprehensive review from 2000-2008. Anticancer
Agents Med Chem. 9:397–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blažević T, Heiss EH, Atanasov AG, Breuss
JM, Dirsch VM and Uhrin P: Indirubin and indirubin derivatives for
counteracting proliferative diseases. Evid Based Complement
Alternat Med. 2015:6540982015. View Article : Google Scholar
|
|
3
|
Medvedev A, Buneeva O and Glover V:
Biological targets for isatin and its analogues: Implications for
therapy. Biologics. 1:151–162. 2007.PubMed/NCBI
|
|
4
|
Premanathan M, Radhakrishnan S,
Kulangiappar K, Singaravelu G, Thirumalaiarasu V, Sivakumar T and
Kathiresan K: Antioxidant & anticancer activities of isatin
(1H-indole-2,3-dione), isolated from the flowers of Couroupita
guianensis Aubl. Indian J Med Res. 136:822–826. 2012.
|
|
5
|
Stafman LL and Beierle EA: Cell
proliferation in neuroblastoma. Cancers (Basel). 8:132016.
View Article : Google Scholar
|
|
6
|
Buhagiar A and Ayers D: Chemoresistance,
cancer stem cells, and miRNA influences: The case for
neuroblastoma. Anal Cell Pathol (Amst). 2015:1506342015.
|
|
7
|
Strother DR, London WB, Schmidt ML,
Brodeur GM, Shimada H, Thorner P, Collins MH, Tagge E, Adkins S,
Reynolds CP, et al: Outcome after surgery alone or with restricted
use of chemotherapy for patients with low-risk neuroblastoma:
Results of Children's Oncology Group study P9641. J Clin Oncol.
30:1842–1848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kreissman SG, Seeger RC, Matthay KK,
London WB, Sposto R, Grupp SA, Haas-Kogan DA, Laquaglia MP, Yu AL,
Diller L, et al: Purged versus non-purged peripheral blood
stem-cell transplantation for high-risk neuroblastoma (COG A3973):
A randomised phase 3 trial. Lancet Oncol. 14:999–1008. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu AL, Gilman AL, Ozkaynak MF, London WB,
Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay
KK, et al: Anti-GD2 antibody with GM-CSF, inter-leukin-2, and
isotretinoin for neuroblastoma. N Engl J Med. 363:1324–1334. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pinto NR, Applebaum MA, Volchenboum SL,
Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F,
Schleiermacher G, Park JR, et al: Advances in risk classification
and treatment strategies for neuroblastoma. J Clin Oncol.
33:3008–3017. 2016. View Article : Google Scholar
|
|
11
|
Hara J: Development of treatment
strategies for advanced neuro-blastoma. Int J Clin Oncol.
17:196–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mazalovska M and Kouokam JC: Plant-derived
lectins as potential cancer therapeutics and diagnostic tools.
Biomed Res Int. 2020:16313942020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han K, Zhou Y, Liu F, Guo Q, Wang P, Yang
Y, Song B, Liu W, Yao Q, Teng Y and Yu P: Design, synthesis and in
vitro cytotoxicity evaluation of 5-(2-carboxyethenyl)isatin
derivatives as anticancer agents. Bioorg Med Chem Lett. 24:591–594.
2014. View Article : Google Scholar
|
|
14
|
Pakravan P, Kashanian S, Khodaei MM and
Harding FJ: Biochemical and pharmacological characterization of
isatin and its derivatives: From structure to activity. Pharmacol
Rep. 65:313–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watkins P, Clow A, Glover V, Halket J,
Przyborowska A and Sandler M: Isatin, regional distribution in rat
brain and tissues. Neurochem Int. 17:321–323. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou P and Koh HL: Determination of
indican, isatin, indirubin and indigotin in Isatis indigotica by
liquid chromatography/electrospray ionization tandem mass
spectrometry. Rapid Commun Mass Spectrom. 21:1239–1246. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schulte JH, Lim S, Schramm A, Friedrichs
N, Koster J, Versteeg R, Ora I, Pajtler K, Klein-Hitpass L,
Kuhfittig-Kulle S, et al: Lysine-specific demethylase 1 is strongly
expressed in poorly differentiated neuroblastoma: Implications for
therapy. Cancer Res. 69:2065–2071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakane C, Okitsu T, Wada A, Sagami H and
Shidoji Y: Inhibition of lysine-specific demethylase 1 by the
acyclic diterpenoid geranylgeranoic acid and its derivatives.
Biochem Biophys Res Commun. 444:24–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rotili D and Mai A: Targeting histone
demethylases A new avenue for the fight against cancer. Genes
Cancer. 2:663–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu
W, Liang J, Sun L, Yang X, Shi L, et al: LSD1 is a subunit of the
NuRD complex and targets the metastasis programs in breast cancer.
Cell. 138:660–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue M, Liang H, Ji X, Liu Y and Sun T:
Fucoidan prevent murine autoimmune diabetes via suppression
TLR4-signaling pathways, regulation DC/Treg induced immune
tolerance and improving gut microecology. Nutr Metab (Lond).
16:872019. View Article : Google Scholar
|
|
23
|
Soleimani A, Khazaei M, Ferns GA, Ryzhikov
M, Avan A and Hassanian SM: Role of TGF-β signaling regulatory
microRNAs in the pathogenesis of colorectal cancer. J Cell Physiol.
Jan 26–2019.Epub ahead of print. View Article : Google Scholar
|
|
24
|
Rössler J, Breit S, Havers W and
Schweigerer L: Vascular endothelial growth factor expression in
human neuroblastoma: Up-regulation by hypoxia. Int J Cancer.
81:113–117. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamamura R, Nagatsuka H, Siar CH, Katase
N, Naito I, Sado Y and Nagai N: Comparative analysis of basal
lamina type IV collagen α chains, matrix metalloproteinases-2 and
-9 expressions in oral dysplasia and invasive carcinoma. Acta
Histochem. 115:113–119. 2013. View Article : Google Scholar
|
|
26
|
Lanza C, Galeazzi V, Carboni N, De
Berardinis A, De Marino L, Barile A and Giovagnoni A: Neuroblastoma
image-defined risk factors in adrenal neuroblastoma: Role of
radiologist. Gland Surg. 8(Suppl 3): S168–S177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orr KE and McHugh K: The new international
neuroblastoma response criteria. Pediatr Radiol. 49:1433–1440.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belczacka I, Latosinska A, Siwy J, Metzger
J, Merseburger AS, Mischak H, Vlahou A, Frantzi M and Jankowski V:
Urinary CE-MS peptide marker pattern for detection of solid tumors.
Sci Rep. 8:52272018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zage PE: Novel therapies for relapsed and
refractory neuroblastoma. Children (Basel). 5:1482018.
|
|
30
|
Morandi F, Frassoni F, Ponzoni M and
Brignole C: Novel immunotherapeutic approaches for neuroblastoma
and malignant melanoma. J Immunol Res. 2018:80973982018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gencer N, Sonmez F, Demir D, Arslan O and
Kucukislamoglu M: Synthesis, structure-activity relationships and
biological activity of new isatin derivatives as tyrosinase
inhibitors. Curr Top Med Chem. 14:1450–1462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma Z, Hou L, Jiang Y, Chen Y and Song J:
The endogenous oxindole isatin induces apoptosis of MCF7 breast
cancer cells through a mitochondrial pathway. Oncol Rep.
32:2111–2117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin H, Ju C, Zhang J, Song J, Ge Y and
Wang Y: Antitumor effects of Isatin on human neuroblastoma cell
line (SH-SY5Y) and the related mechanism. Eur J Pharmacol.
589:27–31. 2008. View Article : Google Scholar
|
|
34
|
Song J, Hou L, Ju C, Zhang J, Ge Y and Yue
W: Isatin inhibits proliferation and induces apoptosis of SH-SY5Y
neuroblastoma cells in vitro and in vivo. Eur J Pharmacol.
702:235–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu P, Hou L, Ju C, Zhang Z, Sun W, Zhang
L, Song J, Lv Y, Liu L, Chen Z and Wang Y: Isatin inhibits the
proliferation and invasion of SH-SY5Y neuroblastoma cells. Mol Med
Rep. 13:2757–2762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Sun W, Cao Y, Hou L and Wang X:
Isatin inhibits the invasion of human neuroblastoma SH-SY5Y cells,
based on microarray analysis. Mol Med Rep. 20:1700–1706.
2019.PubMed/NCBI
|
|
37
|
Schildhaus HU, Riegel R, Hartmann W,
Steiner S, Wardelmann E, Merkelbach-Bruse S, Tanaka S, Sonobe H,
Schüle R, Buettner R and Kirfel J: Lysine-specific demethylase 1 is
highly expressed in solitary fibrous tumors, synovial sarcomas,
rhabdomyosarcomas, desmoplastic small round cell tumors, and
malignant peripheral nerve sheath tumors. Hum Pathol. 42:1667–1675.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khandanpour C and Möröy T: Growth factor
independence 1 (Gfi1) as a regulator of p53 activity and a new
therapeutical target for ALL. Oncotarget. 4:374–375. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bandopadhyay M, Sarkar N, Datta S, Das D,
Pal A, Panigrahi R, Banerjee A, Panda CK, Das C, Chakrabarti S and
Chakravarty R: Hepatitis B virus X protein mediated suppression of
miRNA-122 expression enhances hepatoblastoma cell proliferation
through cyclin G1-p53 axis. Infect Agent Cancer. 11:402016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun W, Zhang L, Hou L, Ju C, Zhao S and
Wei Y: Isatin inhibits SH-SY5Y neuroblastoma cell invasion and
metastasis through MAO/HIF-1α/CXCR4 signaling. Anti Cancer Drugs.
28:645–653. 2017. View Article : Google Scholar
|
|
41
|
Itatani Y, Kawada K and Sakai Y:
Transforming growth factor-β signaling pathway in colorectal cancer
and its tumor microenvi-ronment. Int J Mol Sci. 20:58222019.
View Article : Google Scholar
|
|
42
|
Lee HJ: The role of tripartite motif
family proteins in TGF-β signaling pathway and cancer. J Cancer
Prev. 23:162–169. 2018. View Article : Google Scholar
|
|
43
|
Xie F, Ling L, van Dam H, Zhou F and Zhang
L: TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin
(Shanghai). 50:121–132. 2018. View Article : Google Scholar
|