Introduction
Renal cell carcinoma (RCC) is one of the 14 most
frequently diagnosed types of cancer worldwide and accounts for
approximately 3% of all adult malignancies (1). According to the World Health
Organization, the incidence and mortality rates of renal cell
carcinoma (RCC) are rapidly increasing worldwide (2). Patients with RCC exhibit a trend for
metastasis, and approximately 20% of them will develop metastasis
following treatment (3). Surgical
resection has been the major strategy in the treatment of RCC;
however, this method has several limitations regarding its efficacy
(4). Additionally, recent
pharmacological research has indicated that targeted therapy
results in the improved survival of patients with RCC (5). However, a number of patients present
with metastasis following treatment, which is a main reason for the
progression of RCC. Therefore, the development of novel therapeutic
strategies is crucial.
The primary bioactive component of feverfew, a
flowering plant in the daisy family, plant Tanacetum
parthenium, has traditionally been used as a medicinal herb.
Parthenolide has been revealed to exhibit anti-inflammatory
properties, and it has been used for the treatment of migraines
(6). Numerous studies have
indicated that parthenolide also exhibits antitumor activity
against a wide variety of solid tumors, including those of
osteosarcoma, pancreatic cancer and prostate cancer (7-9).
However, relevant studies have not been reported for the effects of
parthenolide on EMT in RCC and renal cancer stem cells.
Epithelial-mesenchymal transition (EMT) is characterized by
epithelial cells under the influence of certain factors, and by a
loss of cell polarity, cell connections and tight junctions, with
cells acquiring a mesenchymal cell morphology and characteristics.
EMT plays an important role in the regulation of tumorigenesis
(10). Furthermore, the main
hallmark of EMT is the loss of the expression of the adhesion
molecule, E-cadherin, and the acquisition of the expression of the
mesenchymal cell marker, N-cadherin (11), as well as the increase in the
expression of E-cadherin transcriptional suppressors, including
Snail, Twist, and Slug (11). It
has been demonstrated that the progression of metastatic RCC is
usually triggered by the activation of the embryonic development
program, EMT (12). Hence,
targeting an important molecule that leads to this process is key
to improving the treatment efficacy for RCC. It has been suggested
that renal cancer stem cells (CSCs) are primarily associated with
metastasis, recurrence and a poor prognosis. These cells have the
ability to resist tumor therapy (13).
Therefore, the aim of the present study was to
determine whether parthenolide alleviates RCC, and to examine
whether parthenolide may be an effective therapeutic drug for
RCC.
Materials and methods
Cell culture
786-O and ACHN (Dalian Medical University) cells and
were maintained in minimal essential medium (MEM) supplemented with
10% fetal bovine serum (FBS) and RPMI-1640 with 10% fetal bovine
serum (FBS) (all from HyClone™; Thermo Fisher Scientific, Inc.), at
37°C in a humidified atmosphere of 95% air and 5% CO2,
respectively.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) (Nanjing KeyGen
Biotech. Co., Ltd.) assay was used to examine cell viability. 786-O
and ACHN cells were plated at a density of 5×103
cells/well in 96-well plates and treated with various
concentrations (0, 1, 2, 4, 6, 8, 12, 16 and 20 µM) of
parthenolide (Sigma-Aldrich; Merck KGaA) for 24 and 48 h.
Parthenolide was first dissolved in DMSO and serum-free medium was
then used to dilute it to various concentrations (0, 1, 2, 4, 6, 8,
12, 16 and 20 µM). Thereafter, 10 µl CCK-8 solution
was added to each well, and the cells were further cultured at 37°C
for 2 h. The absorbance of each group was detected using a
microplate reader at a wavelength of 452 nm.
Colony-formation assay
The colony-formation assay was performed as follows:
Single-cell suspensions of 786-O and ACHN cells were seeded in
6-well culture plates (1,000 cells/well). They were subsequently
treated with parthenolide at various concentrations (0, 4 and 8
µM) for 24 h. Cells were cultured for a further 7 days for
colony formation. The cells were then washed with
phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde
for 30 min at room temperature. After washing, the cells were then
stained with 1% crystal violet for 20 min at room temperature, and
the number of colonies (minimum number of cells in a colony was
~50) was counted. The colonies were visualized via a light
microscope (magnification, ×400) and were photographed using a
camera.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
786-O and ACHN cells were incubated with
parthenolide for 24 h. After incubation, total RNA was extracted
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.), followed by isopropanol precipitation and chloroform
extraction. cDNA was synthesized using the Reverse Transcriptase
system (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. RT-PCR was performed using an iCycler™
Real Time system (Bio-Rad Laboratories, Inc.) and the SYBR Premix
EX Tag Master mixture kit (Takara Bio, Inc.) according to the
manufacturer's protocol. The PCR reactions were carried out under
the following conditions: 40 cycles of denaturation at 95°C for 10
sec, annealing at 60°C for 20 sec and extension at 72°C for 20
sec.
GAPDH mRNA was used as internal control. The primers
used in the present study were as follows: E-cadherin forward,
5′-GAA AAC AGC AAA AGG GCT TGG A-3′ and reverse, 5′-TTA GGG CTG TGT
ACG TGC TG-3′; Snail forward, 5′-CGA GTG GTT CTT CTG CGC TA-3′ and
reverse, 5′-AGG GCT GCT GGA AGG TAA AC-3′; GAPDH forward, 5′-CAC
CCA CTC CTC CAC CTT TG-3′ and reverse, 5′-CCA CCA CCC TGT TGC TGT
AG-3′. The samples were amplified in different wells and run in
triplicate. The relative expression of genes was calculated by
means of the 2-ΔΔCq relative quantification method
(14).
Mammosphere formation assay
Following treatment with various concentrations of
parthenolide for 24 h, 786-O and ACHN cell lines were inoculated
into ultra-low attachment 6-well plates (Corning, Inc.) at a
density of 0.1×106 cells/well, and grown in MEM and
RPMI-1640 supplemented with B27 (1:50, Invitrogen; Thermo Fisher
Scientific, Inc.), 20 ng/ml human recombinant EGF (Sigma-Aldrich;
Merck KGaA), 20 ng/ml bEGF (Sigma-Aldrich; Merck KGaA) and 5
µg/ml insulin (Sigma-Aldrich; Merck KGaA) for 14 days. Cell
colonies >60 µm in diameter were counted under an
inverted microscope (magnification, ×400) (Olympus
Corporation).
Transwell assay
Cell invasion and migration were evaluated by
Transwell assays (Corning, Inc.). An 8-µm Transwell was
pre-coated with diluted Matrigel (1:10 with serum-free medium; only
for the cell invasion assay) and dried for 2 h at room temperature
in an incubator. 786-O and ACHN cells were pretreated parthenolide
(0, 4 and 8 µM) for 24 h. Briefly, 1×105 cells
were seeded onto each upper chamber, while medium with 10% FBS was
added into the lower chamber for chemo-attraction and allowed to
invade for 24 h. The Transwell chamber was then removed, the
culture solution in the Transwell chamber was discarded and the
chamber was washed twice with calcium-free phosphate-buffered
saline (PBS). Then, the chamber was fixed in 3% methanol solution
for 30 min at room temperature and stained with 0.1% crystal violet
for 20 min at room temperature. The chamber was washed several
times with PBS, and the upper chamber liquid was aspirated. The
non-migrated/non-invasive cells in the upper layer were gently
wiped off using a cotton swab. The microporous membrane was removed
carefully with small tweezers and dried with the bottom side up.
Next, the membrane was transferred to a glass slide and sealed with
a neutral gum. Images were observed and collected by an inverted
optical microscope (magnification, ×200; Keyence Corporation).
Immunofluorescence analysis
Cells were seeded in a 24-well plate
(1×104 cells/well) and allowed to attach overnight at
37°C in a humidified atmosphere of 95% air and 5% CO2.
Following treatment with parthenolide (0, 4 and 8 µM) for an
additional 24 h, the cells were permeabilized with 0.1% Triton
X-100 for 10 min and blocked with 5% BSA (Sigma-Aldrich; Merck
KGaA) for 1 h at room temperature and then incubated with primary
antibodies (E-cadherin, N-cadherin, vimentin, Snail) (Table I) overnight at 4°C. The following
day, the cells were washed and incubated with AlexaFluor 594
(1:500; product code ab150108) and AlexaFluor 488 (1:500; product
code ab150061; both from Abcam) secondary antibodies at room
temperature for 1 h. After washing, DNA was counter-stained with
DAPI (Sigma-Aldrich; Merck KGaA) at room temperature for 15 min,
and images of 3 high-expression fields were captured using a
fluorescence microscope (magnification, ×200).
 | Table IAntibodies. |
Table I
Antibodies.
| Primary
antibodies | Clonality | Catalogue
number | Company | Species | Dilution | Diluent |
|---|
| MMP2 | Monoclonal | 66366-1-Ig | ProteinTech Group,
Inc. | Mouse | 1:1,000 | Non-fat milk |
| MMP9 | Monoclonal | ab76003 | Abcam | Rabbit | 1:500 | Non-fat milk |
| E-cadherin | Monoclonal | 60335-1-Ig | ProteinTech Group,
Inc. | Mouse | 1:1,000 | Non-fat milk |
| N-cadherin | Monoclonal | 13116S | Cell Signaling
Technology, Inc. | Rabbit | 1:1,000 | Non-fat milk |
| Vimentin | Monoclonal | 60330-1-Ig | ProteinTech Group,
Inc. | Mouse | 1:1,000 | Non-fat milk |
| Snail | Monoclonal | ab216347 | Abcam | Rabbit | 1:500 | Non-fat milk |
| PI3K | Monoclonal | 60225-1-Ig | ProteinTech Group,
Inc. | Mouse | 1:1,000 | Non-fat milk |
| p-PI3K | Monoclonal | 17366S | Cell Signaling
Technology, Inc. | Rabbit | 1:500 | Non-fat milk |
| AKT | Monoclonal | 60203-2-Ig | ProteinTech Group,
Inc. | Mouse | 1:1,000 | Non-fat milk |
| p-AKT | Monoclonal | 66444-1-Ig | ProteinTech Group,
Inc. | Rabbit | 1:1,000 | Non-fat milk |
| ALD1H1 | Monoclonal | ab52492 | Abcam | Rabbit | 1:500 | Non-fat milk |
| ALD1H1 | Polyclonal | 22109-1-AP | ProteinTech Group,
Inc. | Rabbit | 1:500 | Non-fat milk |
| CD133 | Monoclonal | 66666-1-Ig | ProteinTech Group,
Inc. | Mouse | 1:500 | Non-fat milk |
| Oct-4 | Monoclonal | 60242-1-Ig | ProteinTech Group,
Inc. | Mouse | 1:1,000 | Non-fat milk |
| Sox2 | Monoclonal | 66411-1-Ig | ProteinTech Group,
Inc. | Mouse | 1:1,000 | Non-fat milk |
| GAPDH | Polyclonal | 10494-1-AP | ProteinTech Group,
Inc. | Rabbit | 1:500 | Non-fat milk |
| GAPDH | Monoclonal | 60004-1-Ig | ProteinTech Group,
Inc. | Mouse | 1:500 | Non-fat milk |
Western blot analysis
Antibodies raised against matrix metalloproteinase
(MMP)-2, MMP-9, E-cadherin, N-cadherin, vimentin, Snail, PI3K,
phosphorylated (p)-PI3K, AKT and p-AKT and CSC markers (ALDH1,
CD133, Oct4 and Sox2) were used in the present study. GAPDH was
selected as a reference protein. The information regarding the
antibodies is summarized in Table
I. The antibody of ALDH1 with cat. no. 22109-1-AP was used in
786-O cells, and the antibody of ALDH1 with product code ab52492
was used in ACHN cells. The antibody of GAPDH, cat. no. 10494-1-AP
was used in 786-O cells, and antibody cat. no. 60004-1-Ig was used
in ACHN cells. The 786-O and ACHN cells were cultured under normal
conditions, and were then treated with parthenolide at various
concentrations (0, 4 and 8 µM) for 24 h. The cells were
scraped on the ice, collected by centrifugation (12,000 × g, 10
min, 4°C) for protein extraction and incubated with freshly
prepared RIPA lysis (Merck KGaA) for 15 min, and then quantified
with a bicinchoninic acid (BCA) kit (Nanjing KeyGen Biotech. Co.,
Ltd.). The protein sample (20 µg) was mixed with loading
buffer and boiled for 8 min. Subsequently, the sample was separated
by a 10% SDS-PAGE gel and electrotransfered onto PVDF membranes
(Merck KGaA). The membranes were incubated for 1 h at room
temperature with 5% fat-free milk in Tris-buffered saline
containing 10% Tween-20, followed by incubation overnight at 4°C
with primary antibodies. The following day, the cells were washed
and incubated with secondary antibodies, AffiniPure goat
anti-rabbit IgG (cat. no. 111-035-003) or anti-mouse IgG (cat. no.
115-035-003; both 1:3,000; both from Jackson ImmunoResearch, Inc.),
at room temperature for 1 h. After washing, the protein bands were
detected using an enhanced chemiluminescence (ECL) kit (Advansta,
Inc.) and were analyzed by ImageJ software (v1.47; Rawak Software,
Inc.).
Statistical analysis
GraphPad Prism 5.0 software (GraphPad Software,
Inc.) was used for all statistical analyses. Results are presented
as the mean ± standard deviation (SD) or standard error of the
mean. Statistical significance was determined using ANOVA analysis
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Parthenolide suppresses the growth of
human renal cancer cells
Parthenolide (Fig.
1A) was revealed to exert an effect on the viability of human
786-O and ACHN cells, which was examined by CCK-8 assay. It was
revealed that the groups treated with various concentrations of
parthenolide exhibited reduced cell survival rates (Fig. 1B and C).
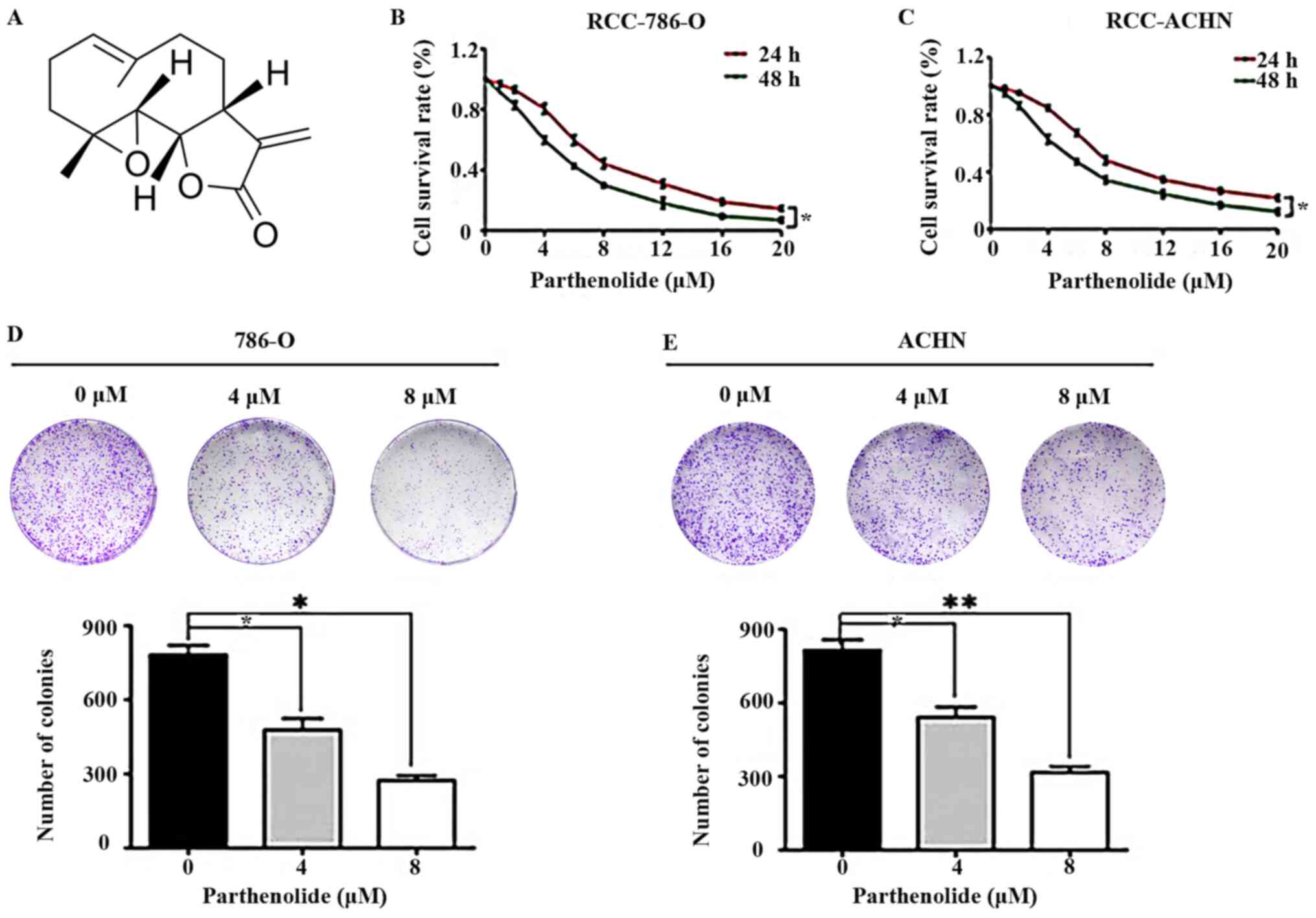 | Figure 1Effects of parthenolide on RCC. (A)
Chemical structure of parthenolide. (B and C) 786-O and ACHN cells
were treated with parthenolide (0, 1, 2, 4, 6, 8, 12, 16, 20
µM) for 24 and 48 h. Parthenolide suppressed the growth of
these two cell lines in a dose-dependent manner. (D and E)
Colony-formation assays demonstrated the inhibitory effects of
parthenolide on 786-O and ACHN. Cells were grown in 6-well plates
for 7 days following parthenolide (0, 4 and 8 µM) treatment.
Parthenolide inhibited the colony formation of the two RCC cell
lines in a dose-dependent manner. These experiments were performed
3 times. *P<0.05 and **P<0.01. RCC,
renal cell carcinoma. |
To further confirm the effects of parthenolide on
RCC, colony-formation assays were performed. Parthenolide
signifi-cantly inhibited the colony-formation abilities of the
786-O and ACHN cells in a dose-dependent manner (Fig. 1D and E). These results indicated
that parthenolide exerted an evident inhibitory effect on human
RCC.
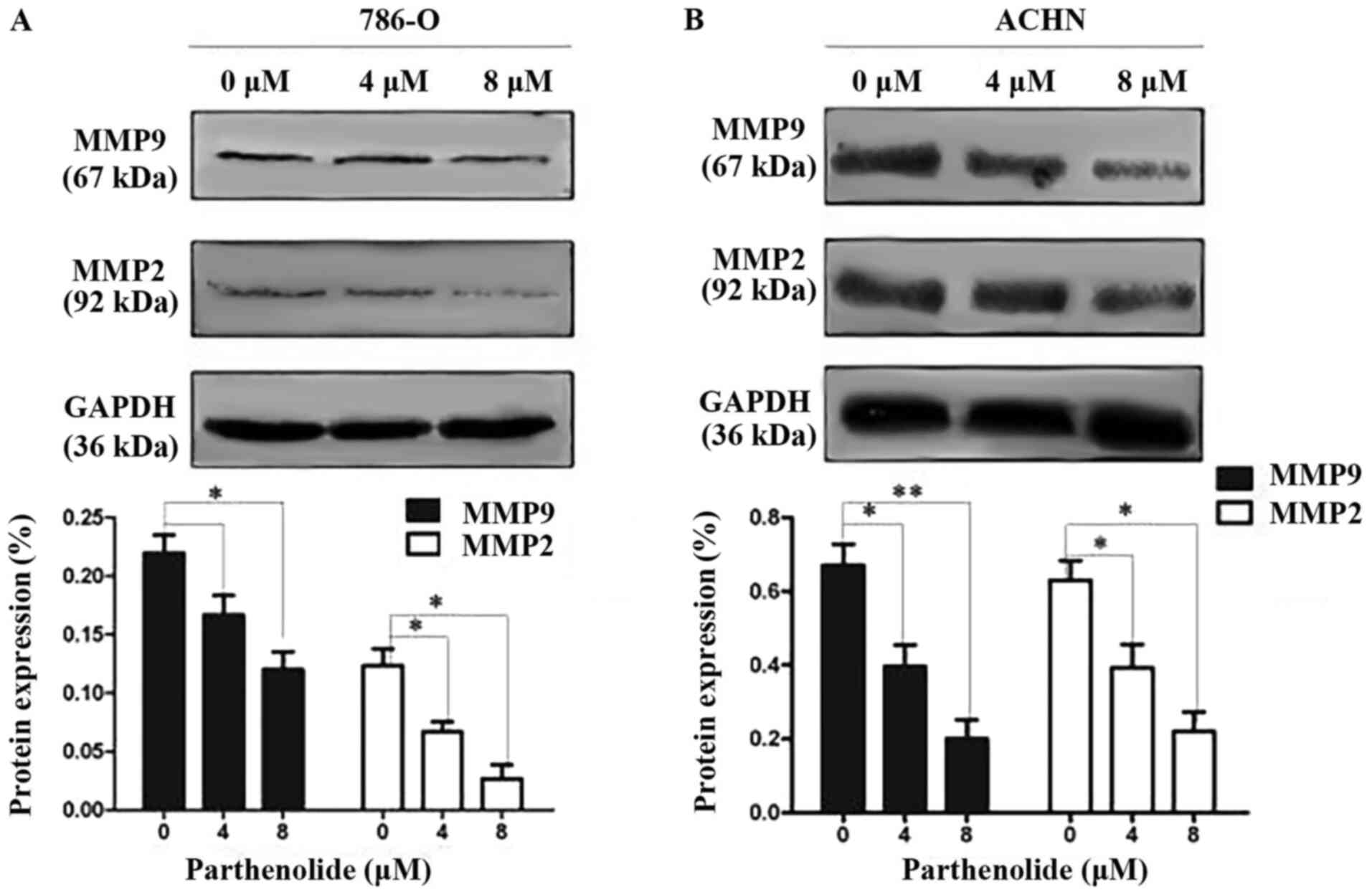
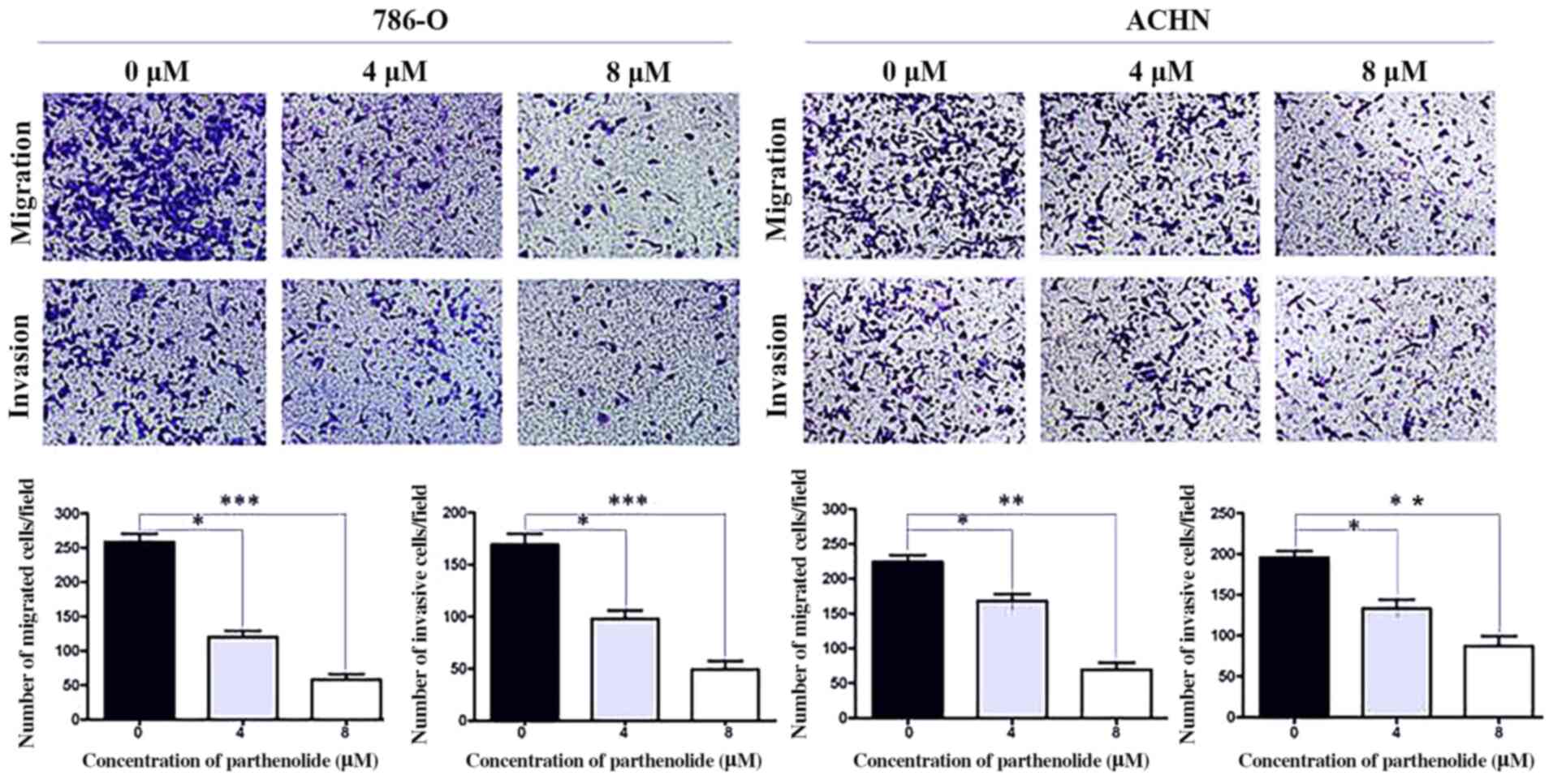
Parthenolide inhibits the migration and
invasion abilities of RCC cells
To elucidate the underlying mechanisms of the
effects of parthenolide on cell migration and invasion, the
expression of related proteins was examined in the 786-O and ACHN
cells by western blot analysis. The results revealed that
parthenolide decreased the expression of MMP-2 and MMP-9 in the
786-O and ACHN cells (Fig. 2).
Transwell assays demonstrated that the
parthenolide-treated 786-O and ACHN cells exhibited suppressed
migratory and invasive abilities compared with the control group
(Fig. 3).
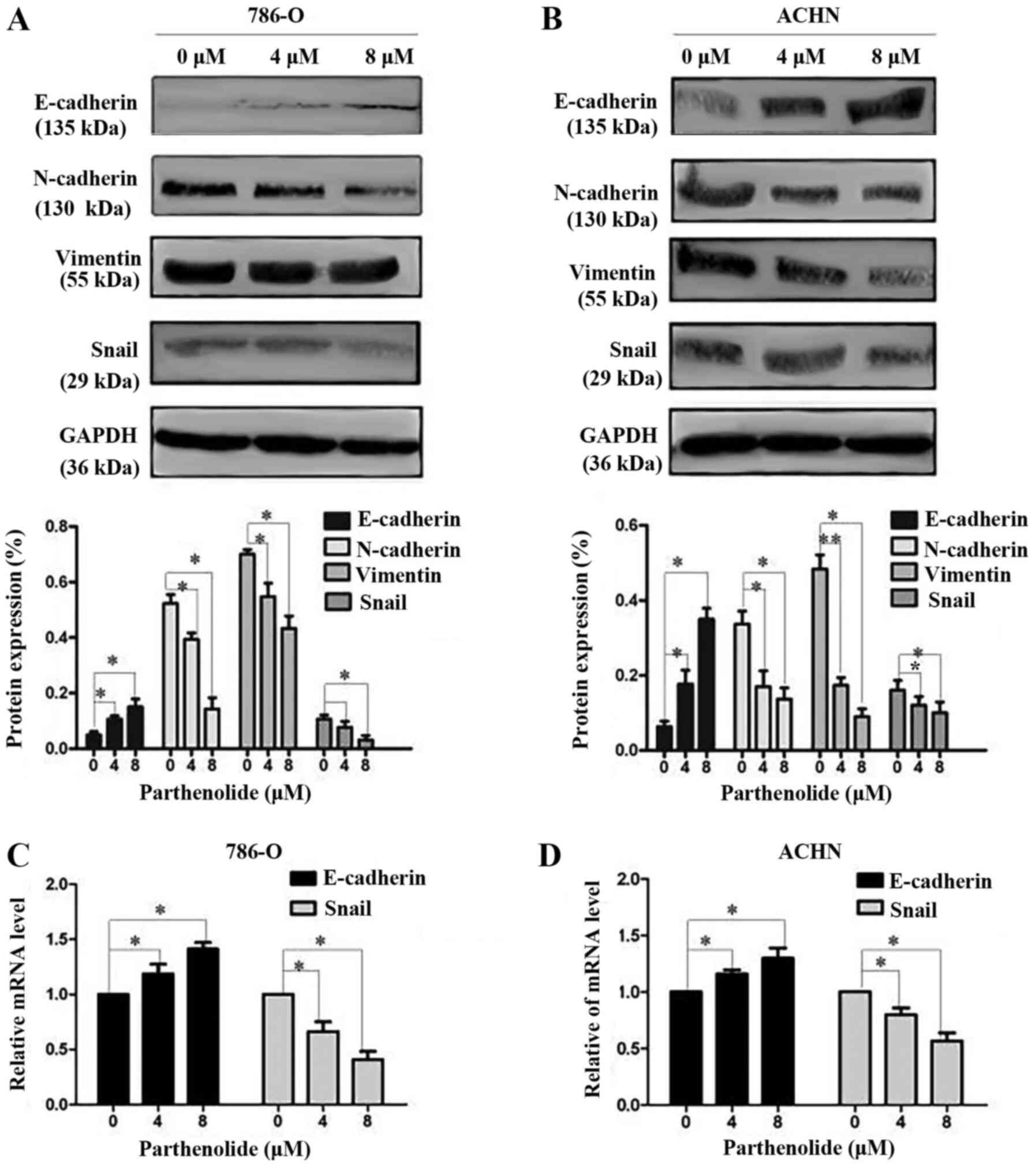
Effect of parthenolide on EMT of RCC
cells
E-cadherin, N-cadherin and vimentin are important
biomarkers of EMT. Western blot analysis was thus performed to
examine the occurrence of EMT. The results revealed a high
expression level of E-cadherin, and a low expression level of
N-cadherin and vimentin in the 786-O and ACHN cells treated with
parthenolide in a dose-dependent manner (Fig. 4A and B). The expression of
E-cadherin and Snail was also assessed by RT-qPCR. The results
revealed the increased expression of E-cadherin and the decreased
expression of Snail (Fig. 4C and
D) in the cells treated with parthenolide. Furthermore, the
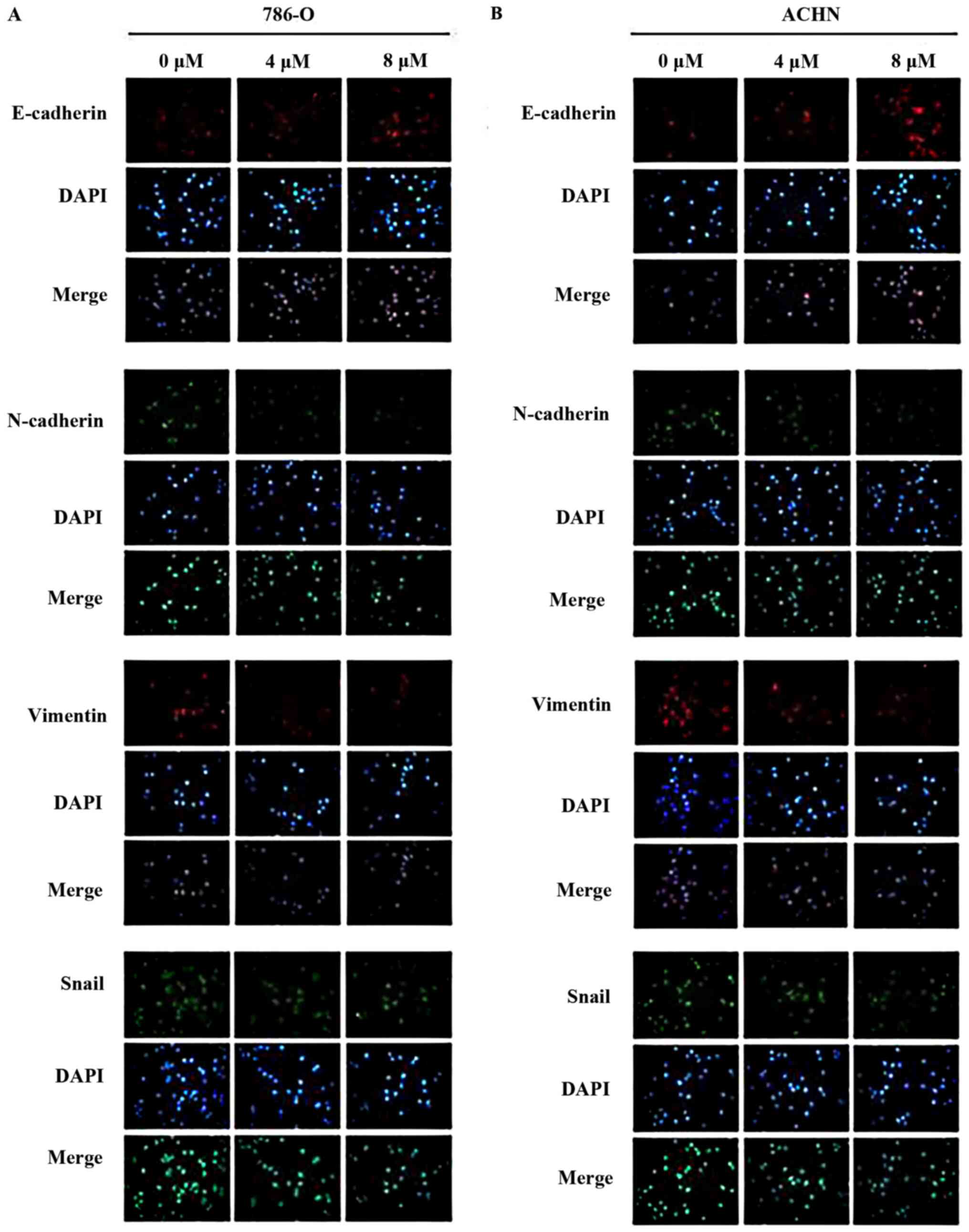
present study confirmed that parthenolide treatment induced high
expression levels of E-cadherin, and decreased expression levels of
N-cadherin and vimentin, as revealed by immunofluorescence
(Fig. 5).
Additionally, the expression of the EMT
transcriptional factor, Snail, was assessed by western blot
analysis, which revealed that parthenolide treatment of the 786-O
and ACHN cells inhibited the expression of Snail (Fig. 4A and B). The inhibited expression
of Snail was also observed in the 786-O and ACHN cells treated with
parthenolide by immunofluorescence (Fig. 5).
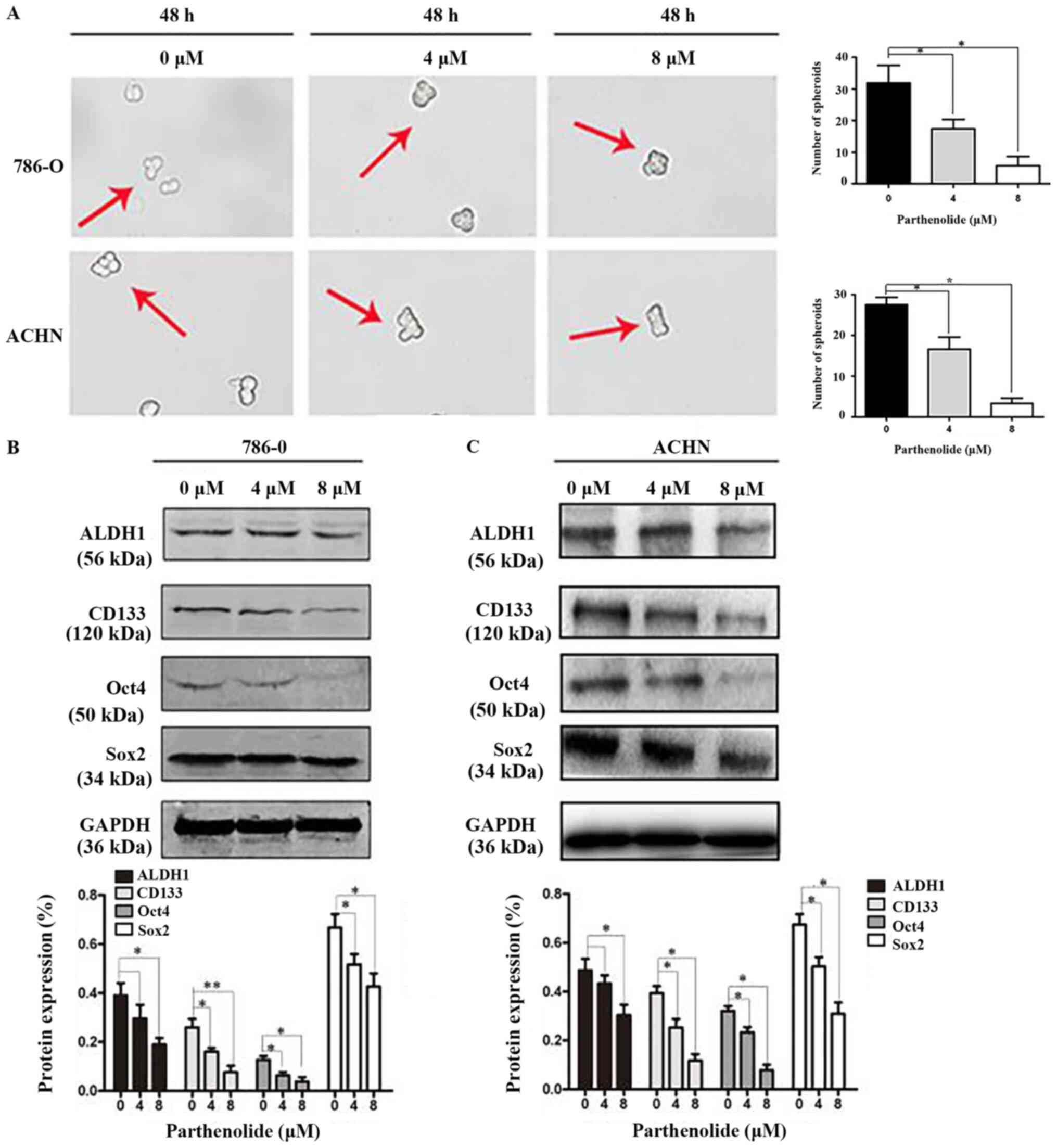
Parthenolide suppresses RCC cells
stemness
Mammosphere formation is a typical cancer stem-cell
property, which can reflect the self-renewal potential ability
(15). Mammosphere formation
assays were performed and they revealed that parthenolide
significantly inhibited the number of spheres that derived from
786-O and ACHN cells (Fig. 6A).
Western blot assays were also carried out to confirm whether
parthenolide has an effect on cancer stem cell markers. The results
revealed that parthenolide inhibited the expression of ALDH1,
CD133, Oct4 and Sox2 (Fig.
6B).
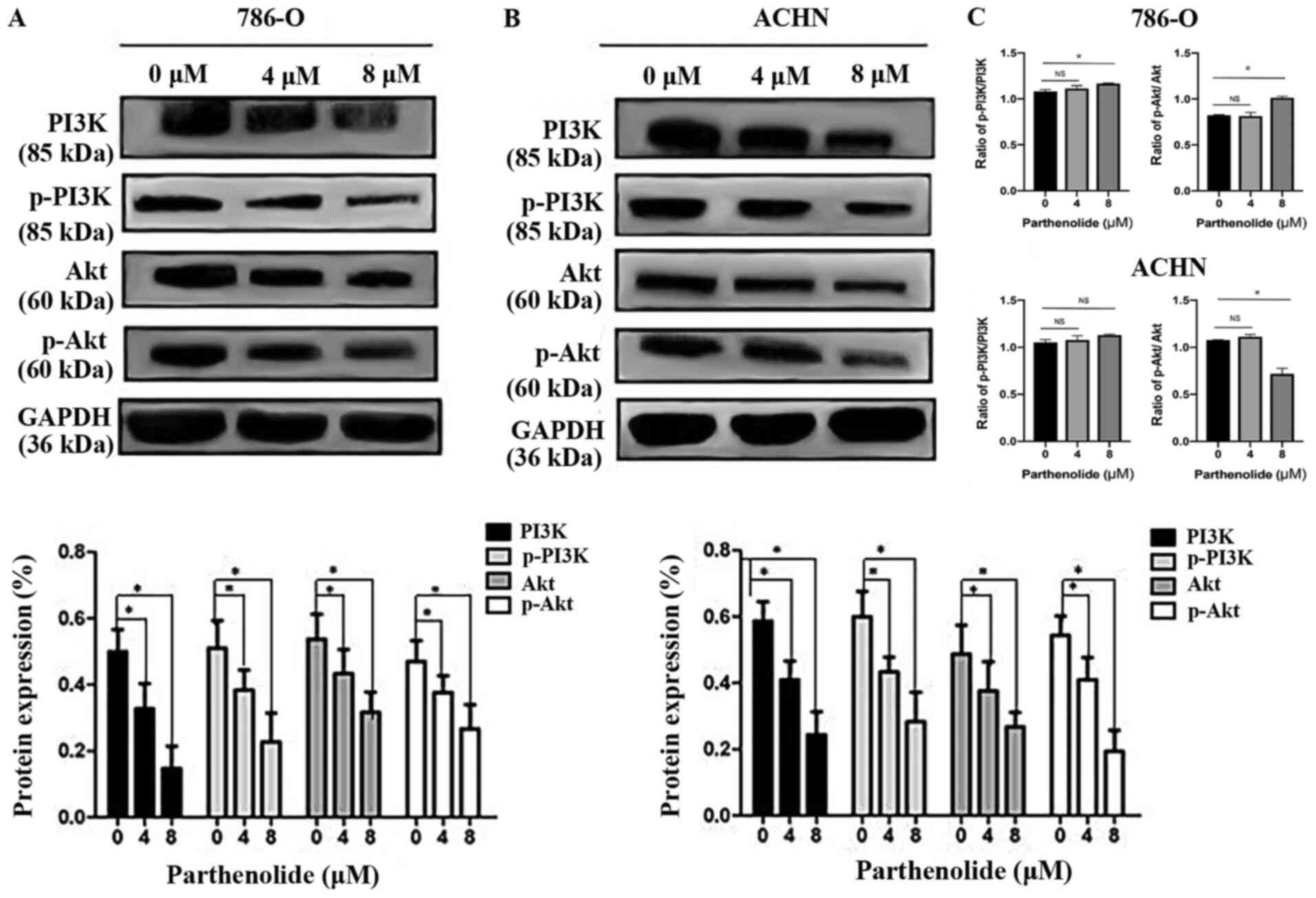
Parthenolide suppresses the PI3K/AKT
pathway
The PI3K/AKT pathway is one of the most frequently
mutated or altered pathways in RCC and plays an important role in
tumorigenesis, proliferation, and cancer progression. PI3K and AKT,
which are the major components of this signaling pathway, are both
increased in RCC (16).
Parthenolide decreased the levels of components of the PI3K/AKT
pathway including the expression of p-PI3K, and p-AKT (Fig. 7A and B). The total protein levels
of PI3K, and AKT were also inhibited by parthenolide, as determined
by western blot analysis in 786-O and ACHN cells (Fig. 7A and B). The ratio of p-PI3K/PI3K
and p-AKT/AKT in 786-O and ACHN cells was also analyzed (Fig. 7C). There was no significant
difference in the ratio of p-PI3K/PI3K between the control group
and the group treated with 4 µM parthenolide in 786-O cells.
The ratio of p-PI3K/PI3K was significant between the control group
and the group treated with 8 µM parthenolide in 786-O cells.
Similarly, no significant difference was observed in the ratio of
p-AKT/AKT between the control group and the group treated with 4
µM parthenolide in 786-O cells. A significant difference was
detected in the ratio of p-AKT/AKT between the control group and
the group treated with 8 µM parthenolide in 786-O cells. In
addition, there was no significant difference observed in the ratio
of p-PI3K/PI3K between the control group and the groups treated
with 4 and 8 µM with parthenolide in ACHN cells. However, a
significant difference was observed in the ratio of p-AKT/AKT
between the control group and the group treated with 8 µM
parthenolide in ACHN cells.
Discussion
Renal cell carcinoma (RCC) comprises approximately
4.2% of all new cancer diagnoses, and RCC accounts for
approximately 2-3% of adult malignant tumors and 80-90% of adult
kidney malignancies (17).
Therefore, it is of utmost importance to identify novel treatment
strategies for RCC.
The sesquiterpene parthenolide has traditionally
been used primarily for the treatment of fever, migraine and
arthritis (6,18) and no significant side-effects have
been reported in humans (19). In
recent years, it has been revealed that parthenolide exerts
anticancer effects against various types of tumors, such as breast
cancer, cholangiocarcinoma, pancreatic, bladder and prostate
cancer, as well as leukemia, and melanoma (20), which may be related to feverfew
lactone; its structural formula contains a-methylene-γ-lactone ring
and epoxide structure (21). As
these structures can interact with enzymes and some functional
proteins containing sulfhydryl groups, they can subsequently affect
biological processes, such as cell signaling pathways, cell
proliferation and apoptosis (22).
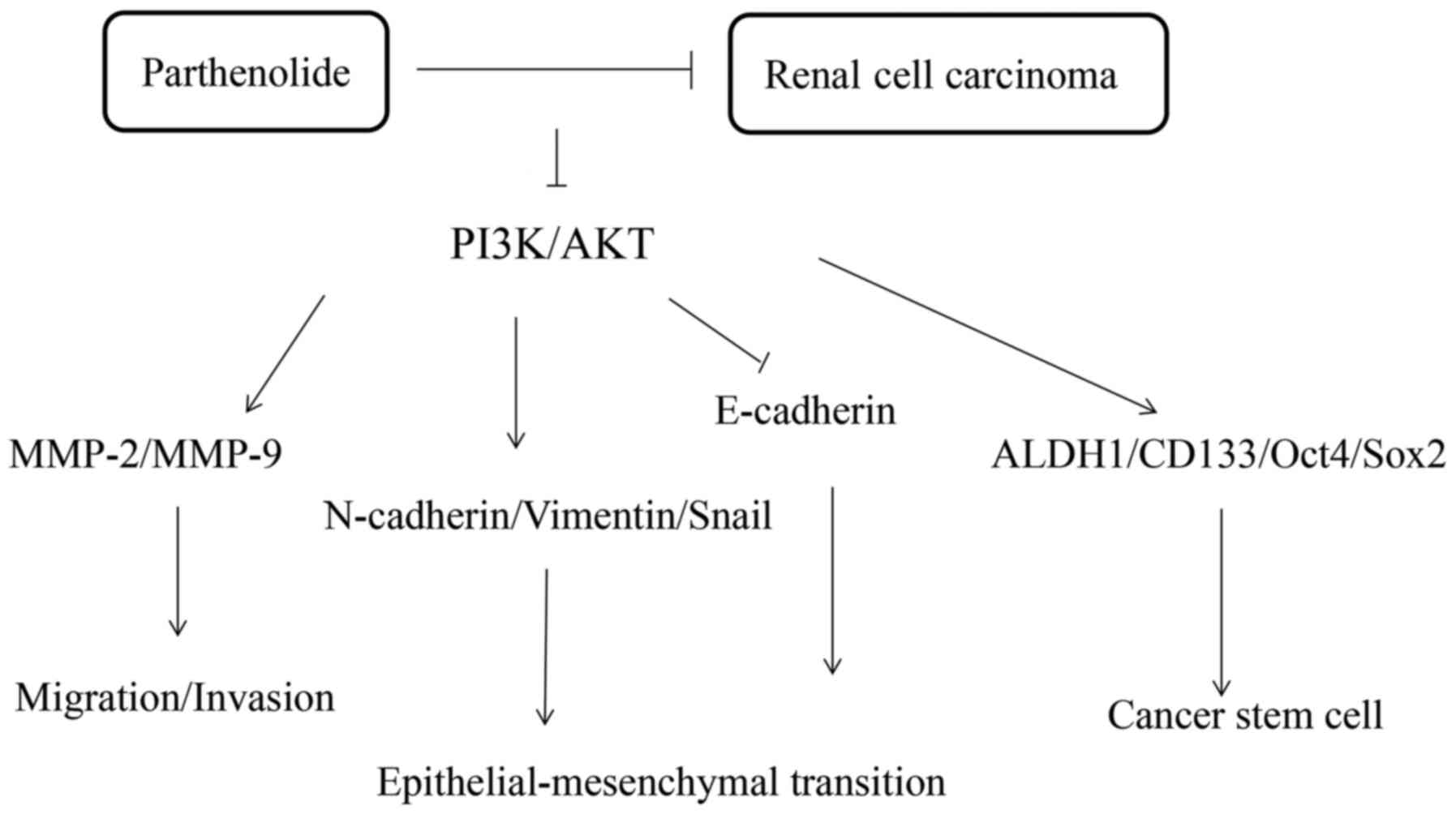
The present study revealed that parthenilide
markedly inhibited the tumorous characteristics of 786-O and ACHN
cells (Fig. 8). The results of
CCK-8 assays indicated a marked decrease in the viability of cancer
cells following treatment with parthenolide, which occurred in a
dose-dependent manner. Additionally, a colony-formation assay
revealed that parthenolide suppressed the growth of the cells.
Transwell assays revealed that parthenolide suppressed the
migratory and invasive abilities of the 786-O and ACHN cells.
Collectively, these data indicated that treatment with parthenolide
may be a novel therapeutic strategy for RCC. In the present study,
it was demonstrated that parthenolide suppressed the formation of
mammospheres, which indicated parthenolide can inhibit renal cancer
stem cell-like properties. In addition, the results of western blot
analysis revealed that parthenolide exerted a significant effect on
biomarkers of metastasis and EMT.
Invasion and metastasis are important biological
features of malignant tumors. These biological processes can cause
cancer cells to invade and spread to other tissues and organs, and
represent a major obstacle to treatment (23). MMPs are zinc-dependent
endopeptidases that play an important role in tumorigenesis and
cancer cell development (24).
They can degrade the main components of the basal membrane and
extracellular matrix, such as collagen IV and fibronectin (25), causing a partial defect to the
basement membrane, which promotes the passage of cancer cells,
migration into blood vessels and lymphatic vessels, or other parts
of the body cavity, followed by extended growth. It has been
demonstrated that the overexpression of MMP-2 and MMP-9 is
associated with a poor prognosis of RCC (26).
EMT refers to the loss of polarity of cells
exhibiting an epithelial-like phenotype, demonstrating an enhanced
mobility, the ability to move freely within the cell matrix and the
transformation process of a fibroid phenotype (27). It has been demonstrated that EMT is
closely related to the primary and secondary metastases of multiple
tumor cells (28). The most
significant change associated with EMT is the decrease in
E-cadherin expression as an epithelial marker and the increase in
N-cadherin expression as a mesenchymal marker (29). It is usually accompanied by the
increased expression of stromal cell-derived proteins, such as
vimentin, α-smooth muscle actin and fibronectin (30). EMT activation is regulated by
transcription factors, such as Snail. It has been revealed that
Snail is a binding protein containing a zinc finger structure
(31). It can recognize and
combine with the F-box sequence of the E-cadherin gene promoter to
inhibit the expression of E-cadherin and lead to the occurrence of
EMT (32). The highly conserved
Twist can promote the expression of Snail and further promote the
tumor cells to undergo EMT (33).
The PI3K/AKT pathway is closely associated with
tumorigenesis, growth, apoptosis, invasion, metastasis, EMT and the
stem-like phenotype of cancer cells (34). The present results revealed that
parthenolide could inhibit the PI3K/AKT pathway, which may be a
potential mechanism of suppressing cancer cells. We only carried
out the study in vitro, it is important and necessary to
observe the function of parthenolide in vivo. In additional,
the mechanism of parthenolide should be further studied in RCC.
In conclusion, the antitumor effects of parthenolide
on RCC were demonstrated. The biological function of parthenoliode
on renal cancer cells may be related to the regulation of the
PI3K/AKT pathway. These findings provide a promising treatment
approach for RCC.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DL and YH contributed to the design and writing of
the study. LeL and XR acquired and analyzed the data. DL, YH, HZ
performed the experiments. SF and TQ made substantial contributions
to the interpretation and analysis of the data. YH and LiL
contributed to drafting the manuscript, revising it critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We are grateful to the members of the Department of
Pathology of Dalian Medical University (Dalian, China) for their
discussion and suggestions during the course of this study.
References
|
1
|
Erickson LA: Clear cell renal cell
carcinoma. Mayo Clin Proc. 93:813–814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu L, Wang Q, Mao J, Qin T, Sun Y, Yang
J, Han Y, Li L and Li Q: Salinomycin suppresses cancer cell
stemness and attenuates TGF-β-induced epithelial-mesenchymal
transition of renal cell carcinoma cells. Chem Biol Interact.
296:145–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: Bevacizumab plus interferon alfa-2a for
treatment of metastatic renal cell carcinoma: A randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singer EA, Gupta GN and Srinivasan R:
Update on targeted therapies for clear cell renal cell carcinoma.
Curr Opin Oncol. 23:283–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bork PM, Schmitz ML, Kuhnt M, Escher C and
Heinrich M: Sesquiterpene lactone containing Mexican Indian
medicinal plants and pure sesquiterpene lactones as potent
inhibitors of transcription factor NF-kappaB. FEBS Lett. 402:85–90.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang C, Yang QO, Kong QJ, Yuan W and Ou
Yang YP: Parthenolide induces reactive oxygen species-mediated
autophagic cell death in human osteosarcoma cells. Cell Physiol
Biochem. 40:146–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Wang X, Sun J, Yang Y, Li W and
Song J: Parthenolide suppresses pancreatic cell growth by
autophagy-mediated apoptosis. Onco Targets Ther. 10:453–461. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marino S, Bishop RT, Carrasco G, Logan JG,
Li B and Idris AI: Pharmacological inhibition of NFκB reduces
prostate cancer related osteoclastogenesis in vitro and osteolysis
ex vivo. Calcif Tissue Int. 105:193–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe? Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bai Y, Sha J and Kanno T: The role of
carcinogenesis-related biomarkers in the Wnt pathway and their
effects on epithelial-mesenchymal transition (EMT) in oral squamous
cell carcinoma. Cancers (Basel). 12:5552020. View Article : Google Scholar
|
|
12
|
Han X, Piao L, Yuan X, Wang L, Liu Z and
He X: Knockdown of NSD2 suppresses renal cell carcinoma metastasis
by inhibiting epithelial-mesenchymal transition. Int J Med Sci.
16:1404–1411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corrò C, Healy ME, Engler S, Bodenmiller
B, Li Z, Schraml P, Weber A, Frew IJ, Rechsteiner M and Moch H:
IL-8 and CXCR1 expression is associated with cancer stem cell-like
properties of clear cell renal cancer. J Pathol. 248:377–389. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Xu C, Sun X, Qin S, Wang H, Zheng Z, Xu S,
Luo G, Liu P, Liu J, Du N, et al: Let-7a regulates mammosphere
formation capacity through Ras/NF-κB and Ras/MAPK/ERK pathway in
breast cancer stem cells. Cell Cycle. 14:1686–1697. 2015.
View Article : Google Scholar :
|
|
16
|
Gargalionis AN, Sarlani E, Stofas A,
Malakou LS, Adamopoulos C, Bamias A, Boutati E, Constantinides CA,
Stravodimos KG, Piperi C, et al: Polycystin-1 induces activation of
the PI3K/AKT/mTOR pathway and promotes angiogenesis in renal cell
carcinoma. Cancer Lett. 489:135–143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murphy JJ, Heptinstall S and Mitchell JR:
Randomised double-blind placebo-controlled trial of feverfew in
migraine prevention. Lancet. 2:189–192. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghorbani-Abdi-Saedabad A, Hanafi-Bojd MY,
Parsamanesh N, Tayarani-Najaran Z, Mollaei H and Hoshyar R:
Anticancer and apoptotic activities of parthenolide in combination
with epirubicin in mda-mb-468 breast cancer cells. Mol Biol Rep.
47:5807–5815. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berdan CA, Ho R, Lehtola HS, To M, Hu X,
Huffman TR, Petri Y, Altobelli CR, Demeulenaere SG, Olzmann JA, et
al: Parthenolide covalently targets and inhibits focal adhesion
kinase in breast cancer cells. Cell Chem Biol. 26:1027–1035.e22.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dandawate PR, Subramaniam D, Jensen RA and
Anant S: Targeting cancer stem cells and signaling pathways by
phytochemicals: Novel approach for breast cancer therapy. Semin
Cancer Biol. 40-41:192–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Freund RRA, Gobrecht P, Moser P, Fischer D
and Arndt HD: Synthesis and biological profiling of parthenolide
ether analogs. Org Biomol Chem. 17:9703–9707. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao L, Zhang L, Yang M, Wang X, Huang W,
Wu X, Pan H, Yuan L, Huang W, Wu Y and Guan J: Expression profile
of SYNE3 and bioinformatic analysis of its prognostic value and
functions in tumors. J Transl Med. 18:3552020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Kong L, Yang Q, Duan A, Ju X, Cai B,
Chen L, An T and Li Y: Parthenolide inhibits ubiquitin-specific
peptidase 7 (USP7), Wnt signaling, and colorectal cancer cell
growth. J Biol Chem. 295:3576–3589. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gonzalez-Avila G, Sommer B,
García-Hernández AA and Ramos C: Matrix metalloproteinases' role in
tumor microenvironment. Adv Exp Med Biol. 1245:97–131. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bates AL, Pickup MW, Hallett MA, Dozier
EA, Thomas S and Fingleton B: Stromal matrix metalloproteinase 2
regulates collagen expression and promotes the outgrowth of
experimental metastases. J Pathol. 235:773–783. 2015. View Article : Google Scholar :
|
|
27
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo F, Zhao Y and Liu J: Cell adhesion
molecule 4 suppresses cell growth and metastasis by inhibiting the
Akt signaling pathway in non-small cell lung cancer. Int J Biochem
Cell Biol. 123:1057502020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakamoto K, Imanishi Y, Tomita T, Shimoda
M, Kameyama K, Shibata K, Sakai N, Ozawa H, Shigetomi S, Fujii R,
et al: Overexpression of SIP1 and downregulation of E-cadherin
predict delayed neck metastasis in stage I/II oral tongue squamous
cell carcinoma after partial glossectomy. Ann Surg Oncol.
19:612–619. 2012. View Article : Google Scholar
|
|
30
|
Xi W, Sonam S, Beng Saw T, Ladoux B and
Teck Lim C: Emergent patterns of collective cell migration under
tubular confinement. Nat Commun. 8:15172017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dattoli AA, Hink MA, DuBuc TQ, Teunisse
BJ, Goedhart J, Röttinger E and Postma M: Domain analysis of the
Nematostella vectensis SNAIL ortholog reveals unique nucleolar
localization that depends on the zinc-finger domains. Sci Rep.
5:121472015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Georgakopoulos-Soares I, Chartoumpekis DV,
Kyriazopoulou V and Zaravinos A: EMT factors and metabolic pathways
in cancer. Front Oncol. 10:4992020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Škovierová H, Okajčeková T, Strnádel J,
Vidomanová E and Halašová E: Molecular regulation of
epithelial-to-mesenchymal transition in tumorigenesis (Review). Int
J Mol Med. 41:1187–1200. 2018.
|
|
34
|
Jiang N, Dai Q, Su X, Fu J, Feng X and
Peng J: Role of PI3K/AKT pathway in cancer: The framework of
malignant behavior. Mol Biol Rep. 47:4587–4629. 2020. View Article : Google Scholar : PubMed/NCBI
|