Introduction
Neuroblastoma (NB), is an embryonal tumor derived
from precursors of the sympathetic peripheral nervous system with
heterogenous biology and genetics, as well as diverse clinical
presentation, ranging from spontaneous regression to aggressive
progressive metastatic disease (1). It is also the most common solid
extracranial tumor in children (1). While cure rates for low- and
intermediate risk NB are >90%, high-risk NB exhibits a 5-year
overall survival of only 40-50%, despite highly aggressive
multimodal therapy with multiagent chemotherapy, surgery,
radiotherapy, high-dose chemotherapy with autologous
bone-marrow-transplantation and immunotherapy (1,2).
Apart from treatment-related mortality, this is mainly due to the
fact that primary refractory, or relapsed NB responds poorly to
salvage chemotherapy and radiotherapy (1,3).
Therefore, there is an urgent medical need for novel treatment
strategies to: i) Reduce the incidence of refractory and recurring
NB; and ii) increase the therapeutic efficacy of salvage
treatments.
A subset of NB harbors somatic or germ-line
mutations of anaplastic lymphoma kinase (ALK), a gene recurrently
mutated or rearranged in particular in adenocarcinomas of the lung,
the colon and the breast, as well as in a number of other types of
cancer, including NB (4). The
prevalence of ALK alterations is up to 14% in high-risk NB, and the
association of ALK with a poor survival suggests that if functions
as an oncogenic driver in NB (5).
Even though the emergence of resistance is a concern, ALK
inhibitors have exhibited promising efficacy in individual patients
(6). Hence, the targeting of
specific oncogenic pathways appears to be a promising bona fide
strategy for NB.
Fibroblast growth factor receptors (FGFRs), a family
of tyrosine kinase receptors not extensively studied in child-hood
cancer, are recurrently mutated and deregulated in adult cancers,
and both unspecific and specific FGFR inhibitors targeting these
genes have been developed (7,8).
Likewise and at an even higher frequency, members of the
phosphoinositide 3 kinase (PI3K) family are dysregulated in a
number of types of cancer, including childhood cancer, and here as
well, several PI3K inhibitors with varying specificity with respect
to different subunits, have been developed (9-12);
several of these inhibitors have been evaluated in clinical trials
(13).
Recently, the authors examined 29 NB patient tumor
samples for possible mutations in
phosphatidylino-sitol-4,5-bisphosphate 3-kinase, catalytic subunit
alpha (PIK3CA), as well as in FGFR3 (14). It was found that these were not
commonly occurring; however, one FGFR mutation was identified in
one of the 29 patients with NB (14). Nonetheless, it was revealed that
the well-established NB cell lines, SK-N-AS, SK-N-BE(2)-C (MYCN
amplified and chemotherapy-resistant), SK-N-DZ (MYCN amplified and
a frameshift deletion of PIK3C2G), SK-N-FI and SK-N-SH (the only
cell line with wild-type TP53), exhibited dose dependent responses
to both PI3K (BKM120 and BEZ235) and FGFR (AZD4547) inhibitors
(14). Furthermore, combining the
2 types of inhibitors yielded more potent synergy. Given the
biological heterogeneity of NB cell lines tested with respect to
FGFR or PI3K mutations, MYCN amplifications, 11q deletions and
sensitivity to chemotherapy, the data suggest that NB is broadly
vulnerable to FGFR and PI3K inhibition (14-19).
While our recent studies were based on inhibitors at
a pre-clinical or early clinical trial stage, the FDA has recently
approved the PI3K inhibitor, alpelisib (BYL719), and the FGFR
inhibitor, erdafitinib (JNJ-42756493) (14,20-22).
The former is approved for certain types of breast cancer,
preferen-tially with PI3K mutations, while the other is used for
specific solid tumors, mainly with FGFR mutations, or chromosomal
rearrangements (21,22). Notably, however, erdafitinib is
currently tested in the pediatric MATCH phase II clinical trial,
including recurrent/relapsed neuroblastoma with FGFR mutations
(ClinicalTrials. gov, identifier: NCT03210714 and NCT03155620). As
approved drugs could directly be used for the treatment of NB in
for example, a compassionate use setting, the present study aimed
to investigate the anti-NB effects of alpelisib and erdafitinib
alone and in combination, as well as in combination with standard
NB cytotoxic drugs.
Materials and methods
Tumor cell lines, culture conditions and
cell seeding
The five 5 cell lines, SK-N-AS, SK-N-BE(2)-C,
SK-N-DZ, SK-N-FI and SK-N-SH, were used for the in vitro
experiments and were kindly provided by Professor Per Kogner,
Karolinska Institutet (15-19).
Short tandem repeat genetic profiling using the AmpFLSTR
Identifiler PCR Amplification kit (Applied Biosystems) in 2016 was
performed to verify the identities of the cell lines. None of the
cell lines used in the present study had any FGFR3 mutations
according to the Cancer Dependency Map (https://depmap.org/portal/), while only SK-N-DZ had a
frameshift deletion of PIK3C2G. The SK-N-DZ and SK-N-BE(2)-C cells
are MYCN-amplified and only the SK-N-SH cell line is TP53
wild-type. Some characteristics of the cell lines are summarized in
Table SI. The SK-N-BE(2)-C cell
line was derived from a previously treated relapsed patient and is
known to be chemoresistant, for example to doxorubicin (18).
Roswell Park Memorial Institute (RPMI; Gibco; Thermo
Fisher Scientific, Inc.), supplemented with 10% FBS (fetal bovine
serum; Gibco; Thermo Fisher Scientific, Inc.), 1% L-glutamine, 100
U/ml of penicillin and 100 µg/ml streptomycin was used for
the culture of all cell lines, and the cells were maintained at
37°C in a humidified incubator with 5% CO2.
In all assays, 5,000 cells were seeded in 90-200
µl medium/well (without penicillin and streptomycin to avoid
any interference with our drugs) in 96-well plates, and the edges
were filled with medium to avoid edge effects.
Inhibitor and cytostatic treatment
PI3K and FGFR inhibitors
The PI3K inhibitors, dactolisib (BEZ235, NVP-BEZ235)
and alpelisib (BYL719), and the FGFR inhibitors, AZD4547 and
JNJ-42756493 (erdafitinib), used in the present study were all
purchased from Selleckchem Chemicals. Dimethyl sulfoxide (DMSO;
Sigma-Aldrich; Merck KGaA) was used for the stock dilutions, which
were diluted further with PBS for the intended concentrations. The
cells were treated with the inhibitors 24 h after seeding and the
dose ranges used were as follows: AZD4547, 5.0-25 µM;
JNJ-42756493, 0.01-10 µM; BEZ235, 0.25-5.0 µM; and
BYL719, 0.25-10 µM.
Cytostatics
The cytostatics used for the current experiment were
as follows: Cisplatin (Accord Healthcare Ltd.), vincristine
(Oncovin, Pfizer) and doxorubicin (Accord Healthcare Ltd.). All the
cytostatic stock solutions were diluted in PBS and further diluted
in PBS prior to each experiment, and used at the following
concentrations: Cisplatin, 0.1-40 µM; vincristine, 0.001-1
µM; and doxorubicin, 0.1-5 µM.
WST-1 viability assay
A WST-1 assay (Roche Diagnostics) was used to
measure cell viability, which was followed for up to 72 h after
seeding according to a previously described protocol (20).
Proliferation, cell cytotoxicity and
apoptosis assays
Proliferation assays
Cells were seeded in 200 µl medium/well in a
96-well plate and were placed into the IncuCyte S3 Live-Cell
Analysis System (Essen Bioscience) for up to 72 h after seeding.
The machine was set to scan the plates and obtain images every 2 h.
PBS was used as a control and culture medium was used as the
background. Cell proliferation was observed by analyzing the
confluence of cell in the images (20).
Cell cytotoxicity and apoptosis
assays
IncuCyte Red Cytotoxicity reagent and IncuCyte
Caspase-3/7 Green Apoptosis assay (both from Essen Bioscience) were
used to measure cytotoxicity and apoptosis, respectively. At 24 h
after seeding, the medium was discarded and replaced with fresh
medium, which contained the cytotoxicity reagent (final
concentration of 250 nM per well) and the apoptosis reagent at a
ratio of 1:1,000. Subsequently, the indicated inhibitors or
chemotherapeutic agents were added either alone or combined and
simultaneously as indicated below. The plates were then incubated
at 37°C for up to 72 h following treatment in the IncuCyte S3
Live-Cell Analysis System (Essen Bioscience), where the machine
obtained images every 2 h [further details regarding this assay
have been previously described (20)].
Statistical analysis
The effects of treatments (single or combined) were
analyzed by a multiple t-test accompanied by a correction for
multiple comparison of the means confer-ring to the Holm-Sidak
method was performed. The 'Highest Single Agent' and
dose-effect-based approach 'median-effect method' (based on Loewe
Additivity) approach were used to analyze the combinational effects
of the drugs (23,24). This method describes whether the
achieved effect of a drug combination (EAB) is larger than the
effects obtained by any of the individual drugs (EA and EB). A
combination index (CI) was determined using the following formula:
CI=max(EA, EB)/EAB. A CI <1 was demarcated as a positive
combination effect and CI >1 as a negative combination effect.
Another method that we used to analyze the combinational effect was
the median-effect method of Chou (Chou-Talalay method) (24) by using ComboSyn software
(http://www.combosyn.com; ComboSyn, Inc.). The
dose-response curves were fitted to a linear model using the
median-effect equation, allowing for the calculation of a
median-effect value D (equivalent to IC50) and slope.
Goodness-of-fit was assessed with the linear correlation
coefficient, r; r>0.85 was required for the analysis to be
approved. The degree of drug interaction was rated using the CI for
mutually exclusive drugs: CI=d1/D1+d2/D2, where D1 and D2 represent
the concentration of drug 1 and 2 alone, respectively, that is
required to produce a certain effect, and d1 and d2 represent the
concentration of drugs 1 and 2 in combination that is required to
produce the same effect. CI <0.70 was defined as synergy and CI
>1.45 as antagonism, and values in between as additive effects,
according to the recommendations of the ComboSyn software. One-way
ANOVA with the Bonferroni post hoc test was utilized to analyze the
difference in means between the 2 single drugs and the
combinational treatment. A P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects following single-drug exposure of
SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH cells to PI3K
and FGFR inhibitors
All NB lines exhibited dose-dependent responses to
the PI3K inhibitor, BYL719 (0.25-10 µM), and the FGFR
inhibitor, JNJ-42756493 (0.01-10 µM), compared to treatment
with PBS, as shown by WST-1 assays, assessing cellular metabolic
capacity colorimetrically (viability/proliferation/cytotoxicity) by
absorbance. Data summarizing 3 experiments per NB cell line with
BYL719 and JNJ-42756493 at up to 72 h after treatment are presented
in Fig. 1. IC50 values from dose
response analysis for BYL719 and JNJ-42756493 for 24, 48 and 72 h
are presented in Table I. These
assays were subsequently complemented for BYL719 and JNJ-42756493,
with proliferation, cytotoxicity and apoptosis assays presented
below. Corresponding data were reported before for the PI3K
inhibitor, BEZ235, and the FGFR inhibitor, AZD4547 (14).
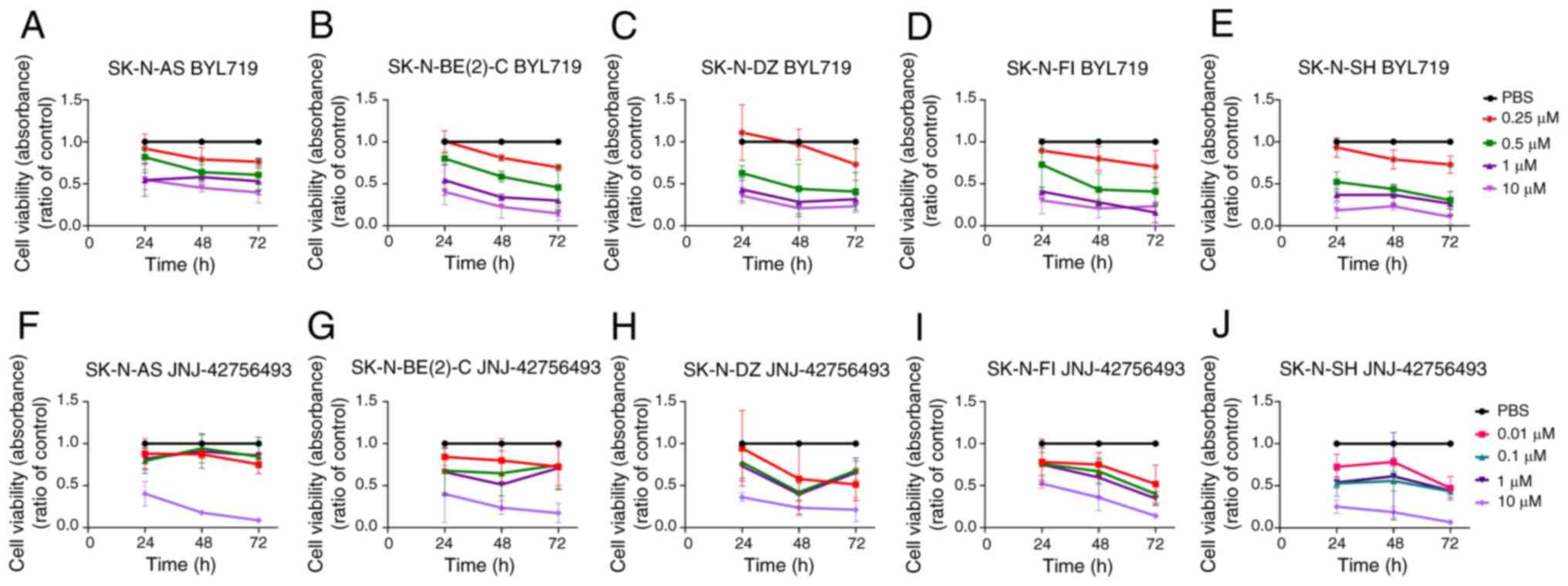 | Figure 1WST-1 viability assays on SK-N-AS,
SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH cell lines upon
treatment with the PI3K inhibitor, BYL719, and FGFR inhibitor,
JNJ-42756493. WST-1 viability assay measured the absorbance
following treatment for 24, 48 and 72 h of SK-N-AS, SK-N-BE(2)-C,
SK-N-DZ, SK-N-FI and SK-N-SH cells with (A-E) PI3K inhibitor,
BYL719, and (F-J) FGFR inhibitor, JNJ-42756493. The graphs
represent 3 experimental runs per cell line and results are
presented as the means ± standard deviation. |
 | Table IEstimation of IC50 values based on
WST-1 viability analysis following treatment with the FGFR
inhibitor, JNJ-42756493, PI3K inhibitor, BYL719, and the cytostatic
drugs cisplatin, vincristine and doxorubicin for 24, 48 and 72
h. |
Table I
Estimation of IC50 values based on
WST-1 viability analysis following treatment with the FGFR
inhibitor, JNJ-42756493, PI3K inhibitor, BYL719, and the cytostatic
drugs cisplatin, vincristine and doxorubicin for 24, 48 and 72
h.
| Drugs | Cell lines | IC50 (µM)
|
|---|
| 24 h | 48 h | 72 h |
|---|
| BYL | SK-N-AS | 4.78 | 1.56 | 1.14 |
| SK-N-BE(2)-C | 2.56 | 0.74 | 0.48 |
| SK-N-DZ | 1.42 | 0.66 | 0.49 |
| SK-N-FI | 1.19 | 0.52 | 0.38 |
| SK-N-SH | 0.79 | 0.60 | 0.38 |
| JNJ | SK-N-AS | 5.93 | 3.73 | 2.69 |
| SK-N-BE(2)-C | 3.38 | 0.84 | 1.99 |
| SK-N-DZ | 3.93 | 0.05 | 1.63 |
| SK-N-FI | 8.48 | 1.85 | 0.03 |
| SK-N-SH | 0.72 | 0.90 | 0.02 |
| CIS | SK-N-AS | 12.64 | 1.50 | 0.78 |
| SK-N-BE(2)-C | 12.08 | 8.10 | 3.07 |
| SK-N-DZ | 36.51 | 8.92 | 0.20 |
| SK-N-FI | 74.5a | 9.54 | 7.97 |
| SK-N-SH | 4.28 | 2.13 | 0.58 |
| VIN | SK-N-AS | 2.47a | 0.006 | 0.003 |
| SK-N-BE(2)-C | 0.33 | 0.07 | 0.07 |
| SK-N-DZ | 0.86 | 0.03 | 0.03 |
| SK-N-FI | 1.13 | <0.001b | 0.001 |
| SK-N-SH | 0.68 | 0.002 | <0.001b |
| DOXO | SK-N-AS | 7.69 | 0.77 | 0.15 |
| SK-N-BE(2)-C | 3.54 | 0.71 | 0.17 |
| SK-N-DZ | 4.12 | 0.36 | 0.09 |
| SK-N-FI | 3.18 | 0.71 | 0.35 |
| SK-N-SH | 0.38 | 0.09 | 0.04 |
BYL719
All NB lines presented decreased viability compared
to PBS early on following treatment with the majority of the BYL719
concentrations used (for all at least P<0.05), except at the
concentration of 0.25 µM BYL719 for all cell lines at 24 and
48 h, and apart from the SK-N-BE(2)-C cells at 48 h, and with 0.5
µM BYL719 at 24 h for the SK-N-FI and SK-N-AS cells
(Fig. 1A-E).
JNJ-42756493
The concentration of 10 µM JNJ-42756493
significantly decreased the viability of all NB cell lines compared
to PBS treatment early on following treatment at all recorded time
points (for all, at least P<0.05) (Fig. 1F-J). This was also observed at the
concentrations of 0.1-1 µM JNJ-42756493 for the SK-N-FI
cells (Fig. 1I), and with the
concentrations of 0.01-1 µM JNJ-42756493 for the SK-N-SH
cells (Fig. 1J) (for all, at least
P<0.05), while the SK-N-AS and SK-N-BE(2)-C cells tended to be
more resistant.
To conclude, all 4 NB lines exhibited dose-dependent
responses to all inhibitors, with IC50 values ranging from 0.38 to
4.78 µM for BYL719 and 0.02 to 8.48 µM for
JNJ-42756493, with the SK-N-SH cells generally being more
sensitive, and the SK-N-AS cells generally being more resistant to
both BYL719 and JNJ-42756493 (Table
I). Notably, NB cell lines with high-risk genetic alterations,
such as MYCN amplification, were in general not less sensitive to
the FGFR and PI3K inhibitors.
Effects following combined exposure of
SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH cells to PI3K
and FGFR inhibitors
All NB lines were treated with combinations of the
PI3K inhibitors, BYL719 (0.25-1 µM) and BEZ235 (0.25-1
µM), and the FGFR inhibitors, JNJ-42756493 (0.01-0.1
µM) and AZD4547 (5-10 µM), and assessed by WST-1
assays, since the combination of BEZ235 and AZD4547 has previously
shown synergy (14). In addition,
under these conditions, an enhanced efficacy was observed, despite
omitting the previously used highest concentration of the
inhibitors in the combination experiments. Data from 3 experiments
with the FDA-approved BYL719 and JNJ-42756493, as well as
additional combinations, including BEZ235 and AZD4547 with a read
out of 72 h following treatment are presented in Fig. 2.
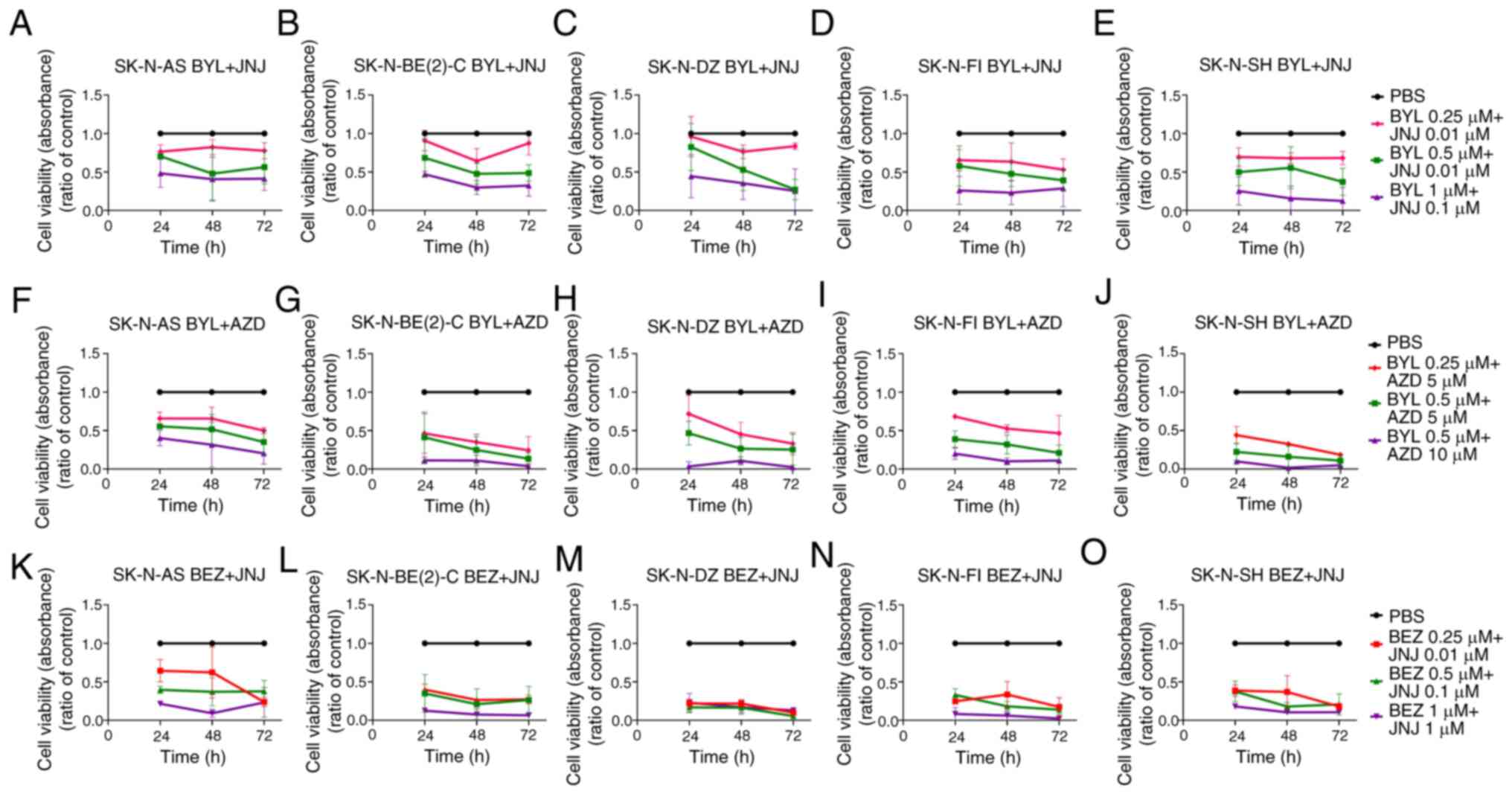 | Figure 2WST-1 viability assays on SK-N-AS,
SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH cell lines following
combined treatments with PI3K inhibitors (BYL719, BEZ235) and FGFR
inhibitors (JNJ-42756493, AZD4547). WST-1 viability assay measured
the absorbance following treatment for 24, 48 and 72 h of SK-N-AS,
SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH cells with (A-E) BYL719
and JNJ-42756493, (F-J) BYL719 and AZD4547, and (K-O) BEZ235 and
JNJ-42756493. The graphs represent 3 experimental runs per cell
line and results are presented as the means ± standard deviation.
BYL, BYL719; JNJ, JNJ-42756493; BEZ, BEZ235; AZD, AZD4547. |
BYL719 and JNJ-42756493
All NB lines exhibited a significantly decreased
absorbance compared to PBS at all time points examined, with the
highest 1 µM BYL719 and 0.1 µM JNJ-42756493
combination (for all, at least P<0.05) (Fig. 2A-E). SK-N-SH was the most sensitive
cell line with a significantly lower absorption compared to PBS
with all combinations at all time points examined (at least,
P<0.05) (Fig. 2E). The other NB
lines also exhibited a decreased absorbance compared to PBS with
the lower concentrations of BYL719 and JNJ-42756493 used, but did
not reach statistical significance at all time points (Fig. 2A-D).
BYL719 and AZD4547, as well as, BEZ235
and JNJ-42756493
The FDA-approved inhibitors were also combined with
the previously tested inhibitors. A decreased absorbance compared
to PBS was observed for all NB lines at all time points with all
concentrations used, apart from the SK-N-DZ cells at 24 h following
treatment with 0.25 µM of BYL719 and 5 µM AZD4547,
and for the SK-N-AS cells at 48 h following treatment with 0.25
µM BEZ235 and 0.01 µM JNJ-42756493 (for all others,
at least P<0.05) (Fig.
2F-O).
Combinational effect analysis and the
dose-effect-based median-effect-principle were also calculated as
described below (23,24) and the findings are presented below.
Briefly, for the combinational effect analysis having a
combinatorial index (CI) CI <1 indicates a positive and a CI
>1 a negative effect. For the dose-effect-based median-effect
principle, a CI <0.75 indicates synergism, a CI >0.75, but
<1.45 indicates an additive effect, while a CI >1.45
indicates an antagonistic effect.
The CIs for the BYL719 and JNJ-42756493, BEZ235 and
AZD4547, BYL719 and AZD4547, as well as the BEZ235 and JNJ-42756493
combinations were calculated at 24 and 48 h following treatment,
and the CIs after 24 h are presented in Fig. 3. Data [for BEZ235 and AZD4547 data
were in line to what has been previously reported earlier (14)]. The overall combination effect was
positive and the majority of the drug combinations indicated a
positive effect (CI <1, i.e., an improved combinational effect
on viability than the best single drug) for the majority of the NB
cell lines (Fig. 3). The
SK-N-BE(2)-C and SK-N-DZ cells were less sensitive to some
concentration combinations of BYL719 and JNJ-42756493, and BEZ235
and JNJ-42756493, compared to the other NB cell lines (Fig. 3). Furthermore, while the SK-N-AS
cell line was sensitive to the BYL719 and JNJ-42756493 combination,
it tended over time to be less sensitive to the BYL719 and AZD4547,
and BEZ235 and JNJ-42756493 combinations (Fig. 3 and data not shown). To summarize,
the majority of the PI3K and FGFR inhibitor combinations exerted
additive or synergistic effects on all NB lines, with the former
being useful to avoid resistance, and the latter for dose
reduction.
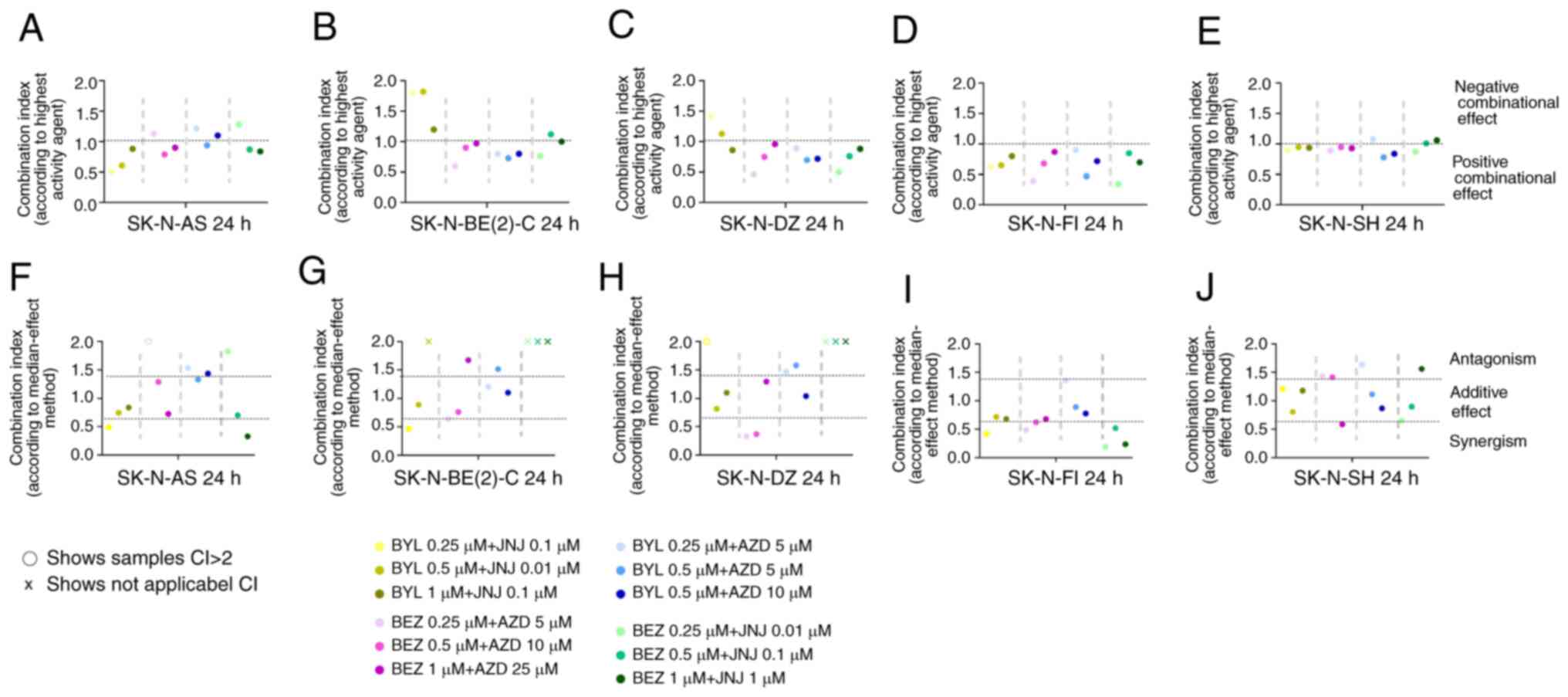 | Figure 3Combinational effects of PI3K
inhibitors, BYL719 and BEZ235, and FGFR inhibitors, JNJ-42756493
and AZD4547, on SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH
cell lines. CIs were obtained by (A-E) the highest single agent
approach, where CI >1 shows a negative combination effect, and
(F-J) the median effect method, where CI >1.45 indicates
antagonism, 0.7< CI >1.45 additive, and CI <0.7
synergistic combinational effects. CIs were calculated from the
mean of 3 experiments analyzed by WST 1, at 24 h following
treatment. x denotes r<0.85 in the median method; thus, the
analysis could not proceed; o denotes CI >2, which indicates a
negative combination effect. CI, combination index; NB,
neuroblastoma; BYL, BYL719; BEZ, BEZ235; AZD, AZD4547; JNJ,
JNJ-42756493. |
Effects of single cisplatin, vincristine
or doxorubicin treatment on SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI
and SK-N-SH cells
Single treatments of all NB lines with 0.1-40
µM cisplatin, 0.001-1 µM vincristine, and 0.1-5
µM doxorubicin was followed for 72 h by WST-1 assays, and
the data are presented in Fig. 4
and Table I. All NB lines
responded to treatment in a dose-dependent manner, although their
sensitivity varied, but was not associated with their sensitivity
to the PI3K and FGFR inhibitors, as demonstrated by IC50 values of
the different NB lines to the different drugs and inhibitors
(Table I). Nevertheless, the
SK-N-SH cell line seemed to be sensitive to both the inhibitors and
cytostatic drugs (Table I). The
effects of single cytostatic drug treatments were further analyzed
by proliferation, cytotoxicity and apoptosis assays (please see
below).
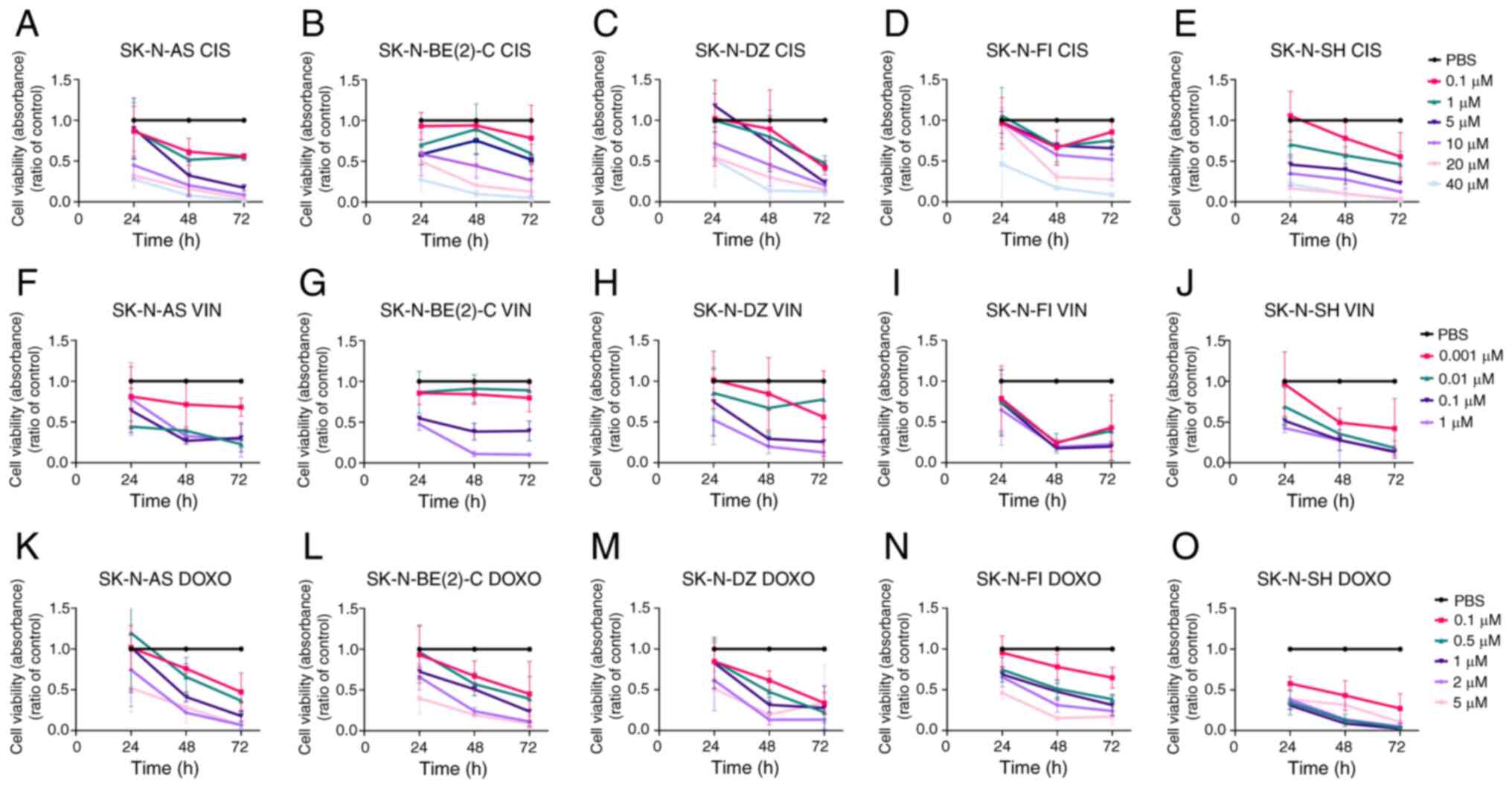 | Figure 4WST-1 viability assays on SK-N-AS,
SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH cell lines upon
treatment with cisplatin, vincristine and doxorubicin. WST-1
viability assay measured the absorbance following treatment for 24,
48 and 72 h of (A, F and K) SK-N-AS, (B, G and L) SK-N-BE(2)-C, (C,
H and M) SK-N-DZ, (D, I and N) SK-N-FI, and (E, J and O) SK-N-SH
cells treated with (A-E) cisplatin, (F-J) vincristine and (K-O)
doxorubicin. The graphs represent 3 experimental runs per cell line
and results are presented as the means ± standard deviation. CIS,
cisplatin; VIN, vincristine; DOXO, doxorubicin. |
Cisplatin
Concentration-dependent drug responses to cisplatin
were observed for all NB cell lines. At low concentrations, the
majority of the lines were resistant early on following treatment;
however, after 72 h, all exhibited a significantly decreased
absorbance compared to PBS for all cisplatin concentrations (0.1-40
µM), with the exception of the SK-N-BE(2)-C, SK-N-FI and
SK-N-SH cells treated with the 0.1 µM concentration (for all
remaining, at least P<0.05) Fig.
4A-E.
Vincristine
Concentration-dependent drug responses to
vincristine were observed for all NB cell lines, and all presented
significantly decreased absorbance compared to PBS at 48 and 72 h
with the two highest concentrations (0.1 and 1.0 µM) (for
all, at least P<0.05) (Fig.
4F-J).
Doxorubicin
Concentration-dependent responses to doxorubicin
were also observed for all NB lines. The SK-N-SH cells exhibited a
decreased absorbance compared to PBS at all time points and
concentrations (at least P<0.01), and all the other NB lines
were also sensitive to doxorubicin (for most, at least P<0.05)
(Fig. 4K-O).
To conclude, all NB cell lines exhibited
concentration-dependent responses to the cytotoxic drugs and these
varied depending on the cell line and drug used; however, this was
not associated with their sensitivity to the FGFR and PI3K
inhibitors (Table I). SK-N-SH was
consistently the most chemo-sensitive cell line.
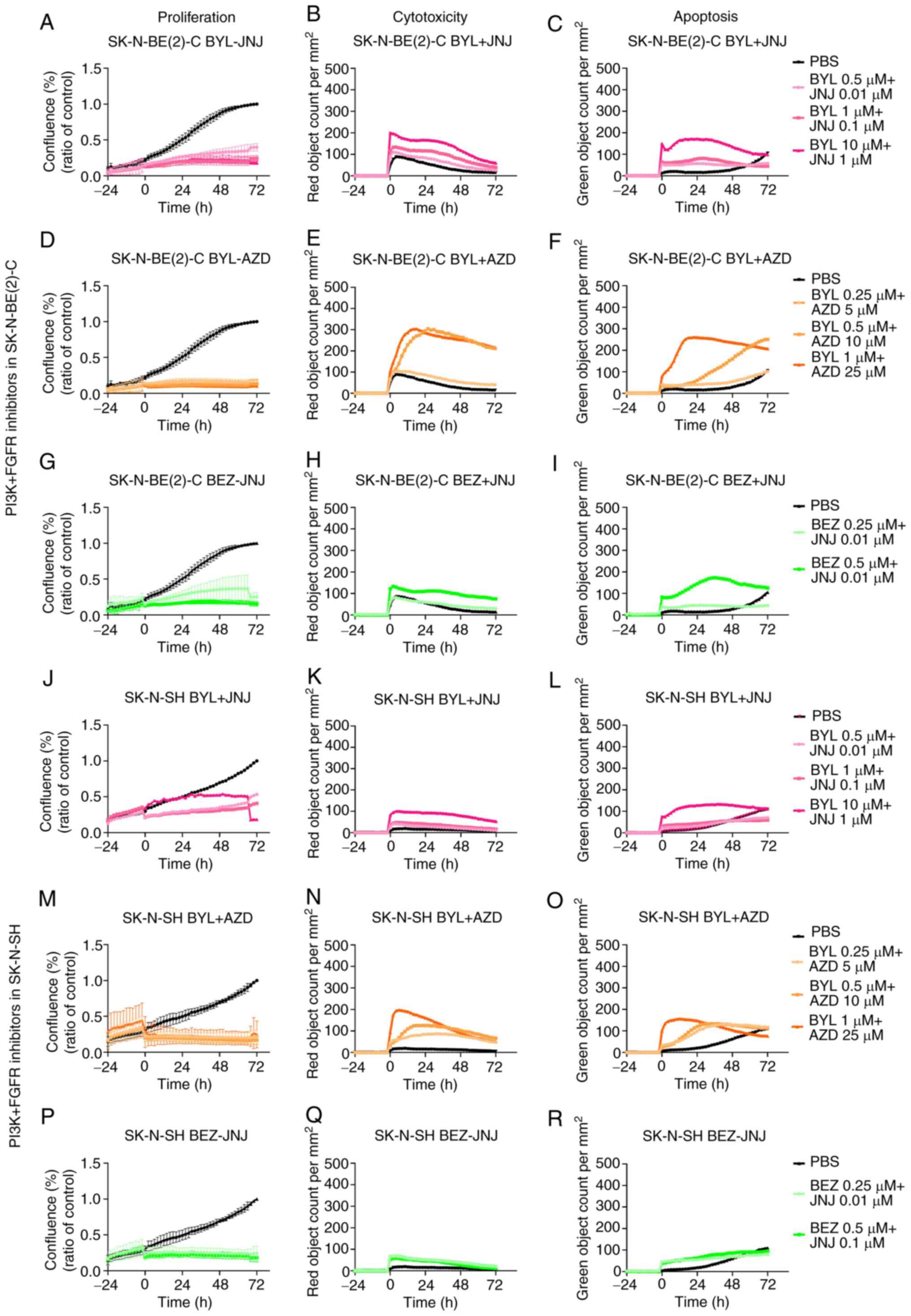
Effects of cisplatin, vincristine and
doxorubicin in combination with the PI3K and FGFR inhibitors,
BYL719 and JNJ-42756493, on the SK-N-BE(2)-C and SK-N-SH cells
Given the positive combined effects with PI3K and
FGFR inhibitors, the possible combined effects of the inhibitors
with cytostatic drugs used clinically (please see above) were then
examined. To this end, the NB lines, SK-N-BE(2)-C and SK-N-SH, were
selected, the former with a MYCN amplification, was established
from a relapsed, previously treated patient, and is regarded as
relatively chemo-resistant, while the latter with wild-type p53 was
sensitive to the majority of inhibitors and drugs tested. The
SK-N-BE(2)-C and SK-N-SH cells were exposed to either BYL719
(0.25-10 µM) or JNJ-42756493 (0.001-1.0 µM) combined
with cisplatin (1-10 µM), vincristine (0.001-0.1 µM),
or doxorubicin (0.1-1 µM) (Fig.
5).
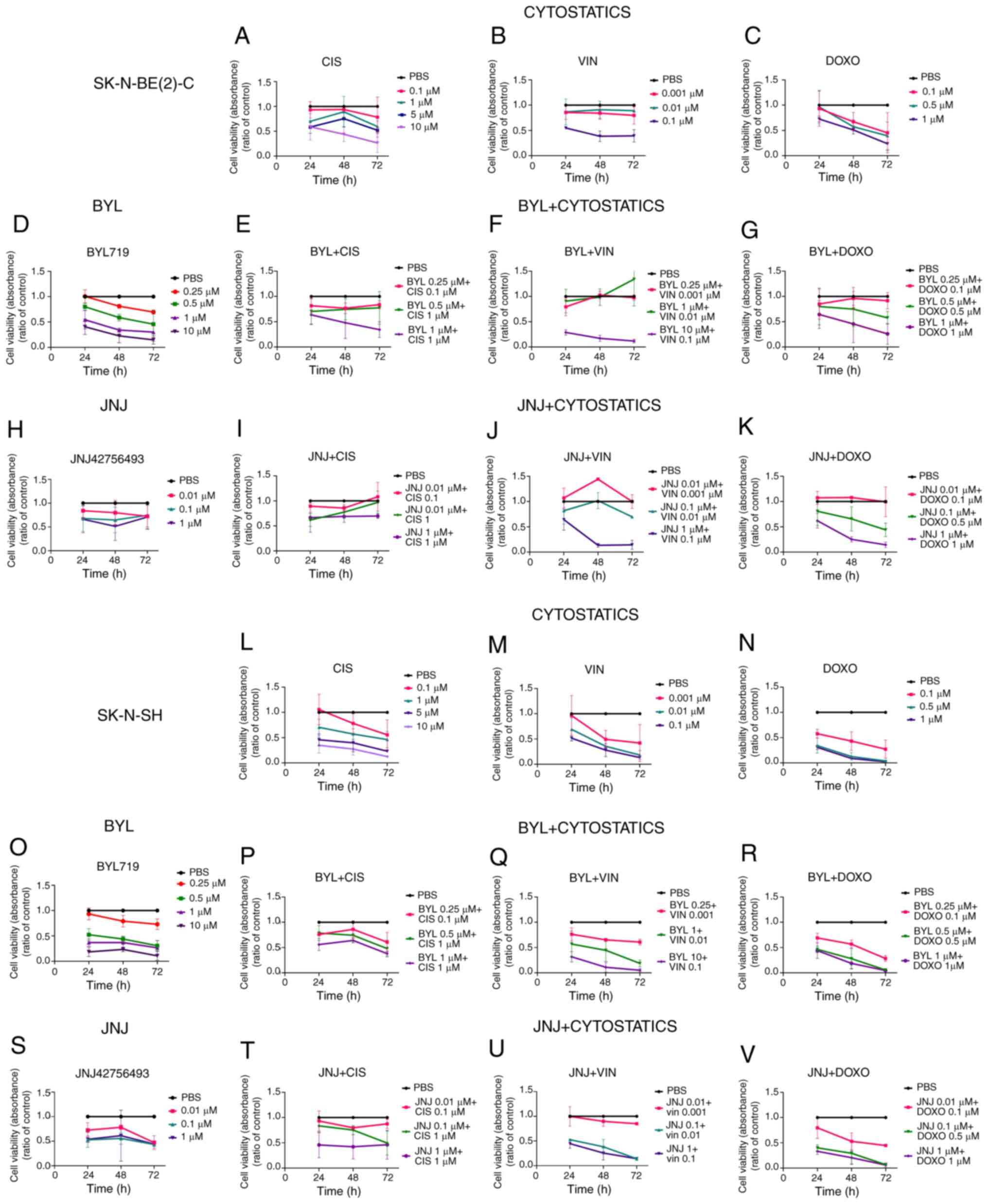 | Figure 5WST-1 viability assays on
SK-N-BE(2)-C and SK-N-SH cell lines upon treatment with BYL719 or
JNJ-42756493 with cisplatin, vincristine and doxorubicin. Viability
measured as absorbance, 24 48 and 72 h following treatment with
cisplatin, vincristine or doxorubicin alone of (A-C) SK-N-BE(2)-C
and (L-N) SK-N-SH cells, or (D) BYL719 treatment of SK-N-BE(2)-C
cells and (O) treatment of SK-N-SH cells with BYL719, or (H)
treatment of SK-N-BE(2)-C cells with JNJ-42756493, and (S)
treatment of SK-N-SH cells with JNJ-42756493. Combined effect on
the viability of (E) SK-NS-BE(2)-C and (P) SK-N-SH cells of BYL719
together with cisplatin. Combined effect on the viability of (F)
SK-NS-BE(2)-C and (Q) SK-N-SH cells of BYL719 with vincristine.
Combined effect on the viability of (G) SK-NS-BE(2)-C and (R)
SK-N-SH cells of BYL719 and doxorubicin. Combined effect on the
viability of (I) SK-N-BE(2)-C and (T) SK-N-SH cells of JNJ-42756493
together with cisplatin. Combined effect on the viability of (J)
SK-NS-BE(2)-C and (U) SK-N-SH cells of JNJ-42756493 and
vincristine. Combined effect on the viability of (K) SK-N-BE(2)-C
and (V) SK-N-SH cells of JNJ-42756493 and doxorubicin. BYL, BYL719;
JNJ, JNJ-42756493; CIS, cisplatin; VIN, vincristine; DOXO,
doxorubicin. |
The calculations of the combinational effect
analysis and according to the dose-effect-based
median-effect-principle after 24 h are presented in Fig. 6. In contrast to the predominantly
positive combinational effects combining PI3K and FGFR inhibitors
(Fig. 3), the combination of the
inhibitors with the cytostatic drugs, resulted in more diverse
effects, including neutral, positive and adverse effects (Figs. 5 and 6).
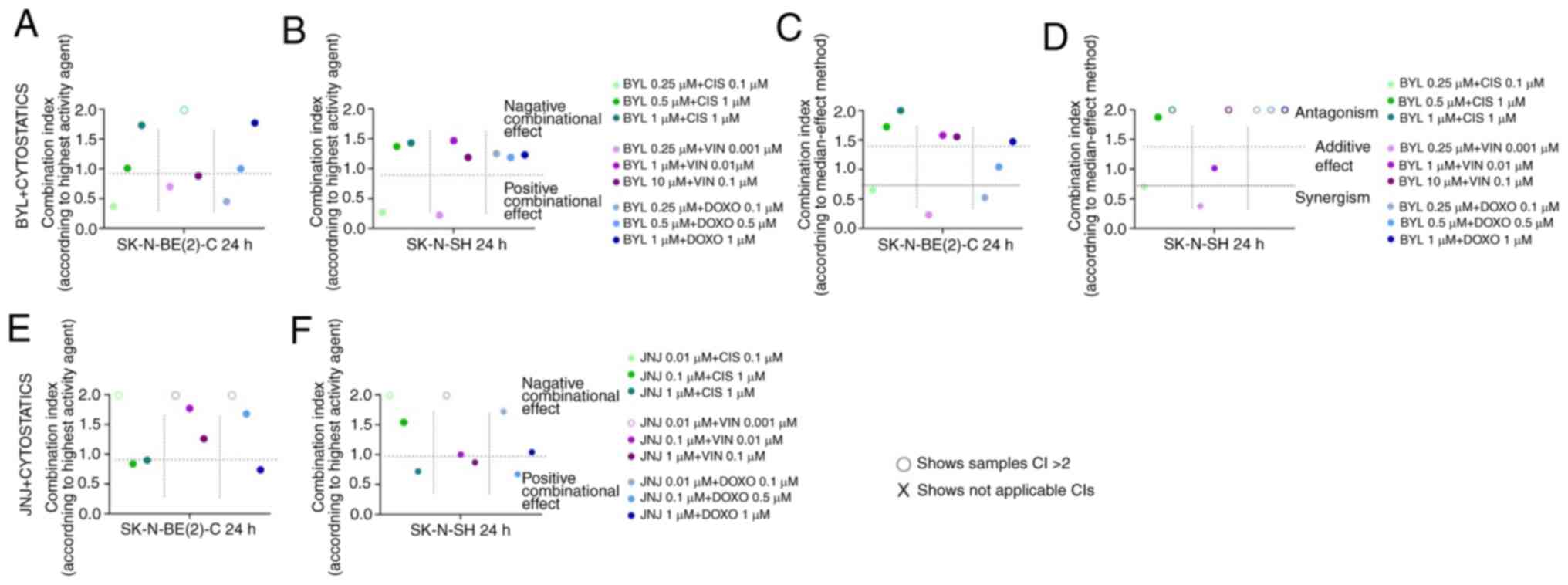 | Figure 6Combinational effects of the PI3K
inhibitor, BYL719, and FGFR inhibitor, JNJ-42756493, with
cisplatin, vincristine and doxorubicin on SK-N-BE(2)-C and SK-N-SH
cell lines. CIs were obtained by the highest single agent approach
following treatment of (A and E) SK-N-BE(2)-C and (B and F) SK-N-SH
cells with (A and B) BYL719 or (E and F) JNJ-42756493 and
cytostatic drugs. CIs were calculated also only for BYL719 and
cytostatic drugs by using the median effect method for (C)
SK-N-BE(2)-C and (D) SK-N-SH cells, since for JNJ-42756493
r<0.85 in median method, so the analysis could not proceed. CIs
were calculated from the mean of 3 experiments, analyzed by WST 1.
o denotes CI >2, which shows a negative combination effect. CI,
combination index; BYL, BYL719; JNJ, JNJ-42756493; CIS, cisplatin;
VIN, vincristine; DOXO, doxorubicin. |
PI3K inhibitor, BYL719, in combination
with cisplatin, vincristine or doxorubicin
SK-N-BE(2)-C cells
Single effects for comparison to the combinational
effects of cisplatin, vincristine, or doxorubicin with BYL719
evaluated by WST-1 assays of the SK-N-BE(2)-C cells are shown in
Fig. 5A-G (for all at least
P<0.05). The combination of BYL719 with cisplatin, vincristine
or doxorubicin yielded dose-dependent responses, with diverse
changes in absorbance compared to PBS, exhibiting additive, neutral
or adversary effects of the drug combinations compared to the
single-drug exposures (Fig. 5E-G).
As shown in Fig. 6A and C, some
positive combinational effects (CI <1) e.g., synergistic effects
for the SK-N-BE(2)-C cells, with 0.25 µM BYL719 and 0.1
µM cisplatin, 0.25 µM BYL719 and 0.001 µM
vincristine, and 0.25 µM BYL719 and 0.1 µM
doxorubicin were observed at 24 h following treatment, while the
remaining data indicated neutral or adverse outcomes.
SK-N-SH cells
Single effects for comparison to the combinational
effects of cisplatin, vincristine, doxorubicin and BYL719 on the
viability of SK-N-SH cells are shown in Fig. 5L-R. The majority of the
combinations led to significant decreases in absorbance at all time
points compared to PBS (for all at least P<0.05) (Fig. 5P-R). Positive combinatory effects
were however, not common; in fact, neutral or adverse outcomes were
more frequent or equally present. This was confirmed by the
calculation of the combinational effects and dose-effect-based
median-effect-principle 24 h following treatment, as shown in
Fig. 6B and D. Herein, 0.25
µM BYL719 and 0.1 µM cisplatin, and 0.25 µM
BYL719 and 0.001 µM vincristine exhibited synergy, while the
remaining combinations led to either neutral or adverse
consequences. For BYL719 and doxorubicin, the dose-effect-based
median-effect-principle could not be calculated.
To conclude, the combinations of BYL719 with
cisplatin, vincristine and doxorubicin used on the SK-N-BE(2)-C and
SK-N-SH cells resulted in variable effects with both positive,
neutral and adverse combinatory effects (Figs. 5 and 6). It was not possible to consistently
state that any combination was the optimal for any of the cell
lines. Notably, however, the lowest BYL719-cisplatin and lowest
BYL719-vincristine combinations exerted a synergistic effect for
both SK-N-BE(2)-C and SK-N-SH cells.
FGFR inhibitor, JNJ-42756493, in
combination with cisplatin, vincristine and doxorubicin
SK-N-BE(2)-C cells
The single and combined effects of cisplatin,
vincristine, doxorubicin with JNJ-42756493 on the viability of
SK-N-BE(2)-C cells are shown in Fig.
5A-C, H and I-K, respectively. No clear-cut enhanced
sensitivity was observed upon the combination of JNJ-42756493 with
the cytostatic drugs. This was confirmed by the calculation of the
combinational effects after 24 h, where only some rare positive
effects were observed (Fig. 6E),
while the dose-effect-based median-effect-principle could not be
calculated. Positive effects were observed after 24 h with the 0.01
and 0.1 µM, and 0.1 and 1 µM JNJ-42756493 and
cisplatin combinations, and the 1 µM JNJ-42756493 and 1
µM doxorubicin combination, while remaining outcomes tended
to be neutral or adverse (Fig.
6E).
SK-N-SH cells
The single and combinational effects of cisplatin,
vincristine, doxorubicin and JNJ-42756493 on the viability of
SK-N-SH cells are shown in Fig. 5L-N,
S and T-V, respectively. All combinations significantly
decreased viability compared to PBS at 72 h following treatment
except for the 0.01 JNJ-43756493 and 0.1 µM cisplatin
combination (for all, P<0.05 at least), although positive
effects were not dominant (Figs.
5T-V, and 6F). Positive
combinational effects were observed after 24 h for the 1 µM
JNJ-42756493 and µM cisplatin combination, the 0.1 µM
JNJ-42756493 and 1 µM vincristine combination, and the 0.1
µM JNJ-42756493 and 0.5 µM doxorubicin combination,
while the remaining outcomes were neutral or adverse (Fig. 6F). Dose-effect-based
median-effect-principle could not be calculated
To sum up, the combined effects of JNJ-42756493 with
cisplatin, vincristine and doxorubicin on the SK-N-BE(2)-C and
SK-N-SH cells yielded variable outcomes with positive, neutral and
adverse effects (Figs. 5 and
6). It was not possible to state a
specific combination as the most effective for inhibiting viability
in any of the cell lines (Fig.
6).
Proliferation, apoptosis and cytotoxicity
following single and combined treatment of SK-N-BE(2)-C and SK-N-SH
cells with PI3K and FGFR inhibitors
After conducting WST-1 assays (as described above),
single and combined inhibitor treatments were performed and
proliferation, apoptosis and cytotoxicity were examined using the
IncuCyte S3 Live-Cell Analysis System on the BYL719 and
JNJ-42756493, and SK-N-BE(2)-C and SK-N-SH cells. This analysis
system could more specifically reflect upon the joint data
reflected in the WST-1 assay, by distinguishing proliferation,
cytotoxicity in a more specific manner.
Single treatments of SK-N-BE(2)-C and
SK-N-SH cells with PI3K and FGFR inhibitors
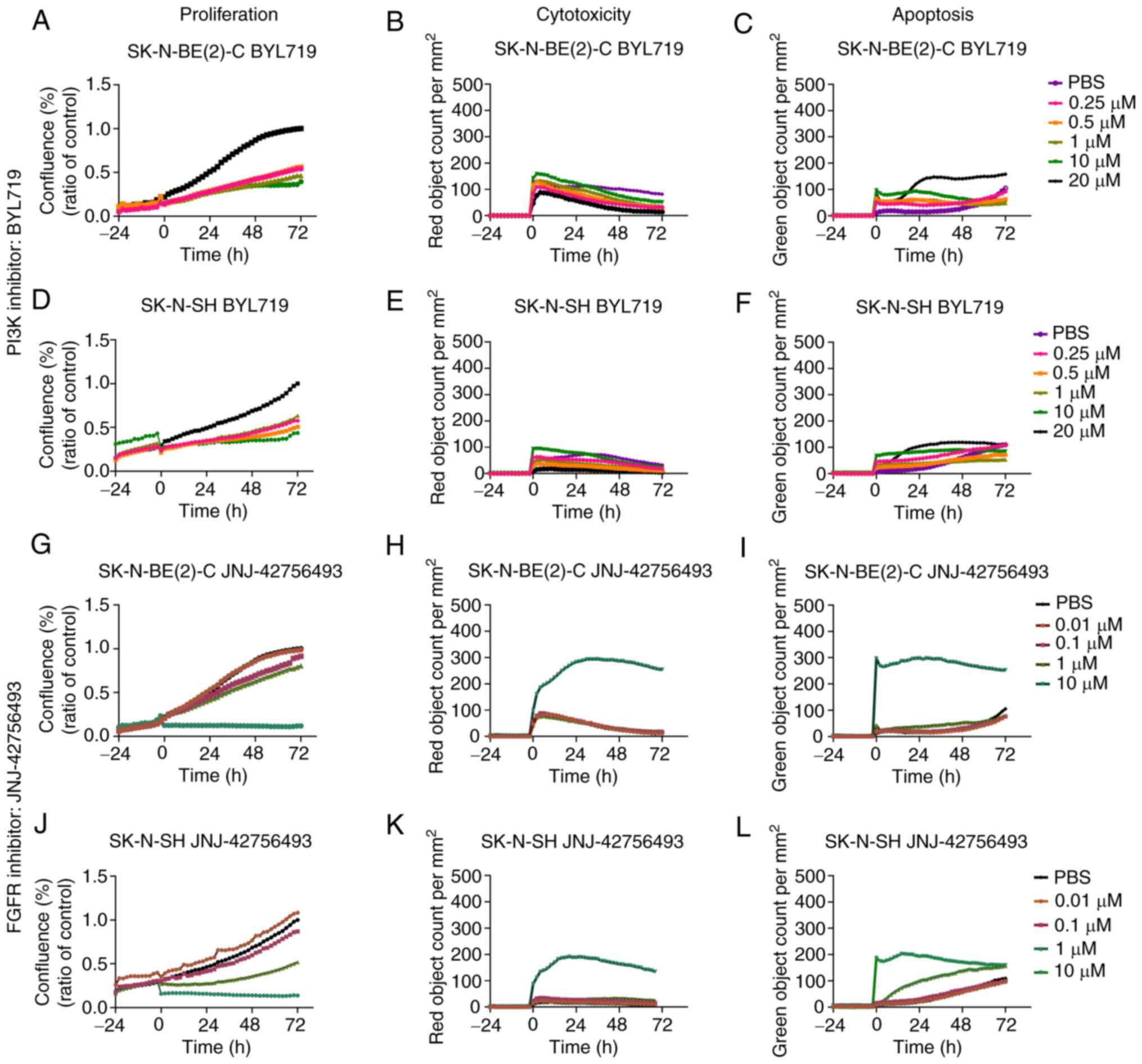
BYL719 and JNJ-42756493
The proliferation, apoptosis and cytotoxicity of the
SK-N-BE(2)-C and SK-N-SH cells were observed 72 h following
treatment with BYL719 (0.25-20 µM) and JNJ-42756493 (0.01-10
µM). Both cell lines exhibited dose-dependent decreases in
proliferation to both drugs, and with the drug concentrations used,
the highest JNJ-42756493 concentration induced very marked
cytotoxicity and apoptosis, with the SK-N-BE(2)-C cells tending to
present higher cytotoxicity and apoptosis than the SK-N-SH cells
(Fig. 7). Images of the effects on
proliferation are depicted in Fig.
S1.
BEZ235 and AZD4547
Both BEZ235 and AZD4547 have previously been
reported to induce dose-dependent proliferation inhibition, and, in
line with the present study, the FGFR inhibitor, AZD4547, induced
more pronounced effects than the PI3K inhibitor on cytotoxicity and
the apoptosis of SK-N-BE(2)-C and SK-N-SH cells (14).
Combined treatments of SK-N-BE(2)-C and
SK-N-SH cells with PI3K and FGFR inhibitor
Effects on proliferation, cytotoxicity and apoptosis
upon combined treatment with PI3K and FGFR inhibitors are presented
for the SK-N-BE(2)-C and SK-N-SH cells.
BYL719 and JNJ-42756493
The proliferation, apoptosis and cytotoxicity of the
SK-N-BE(2)-C and SK-N-SH cells were observed for 72 h following
treatment with combinations of BYL719 (0.5-10 µM) and
JNJ-42756493 (0.01-1 µM). Both cell lines exhibited a marked
decrease in proliferation, while the effects on cytotoxicity and
apoptosis were consistently more pronounced for the SK-N-BE(2)-C
than for the SK-N-SH cells (Fig. 8A-C
and J-L, respectively), with images on proliferation presented
in Fig. S1.
BYL719 and AZD4547, as well as BEZ235 and
JNJ-42756493
The FDA-approved inhibitors were also combined with
the previously tested inhibitors at the drug concentrations
indicated above. For both the SK-N-BE(2)-C and SK-N-SH cells, an
enhanced decrease in proliferation was noted at all time points
with all concentrations (Fig. 8D, G
and M, and P, respectively). For all combinations, cytotoxicity
and apoptosis were consistently observed more readily for the
SK-N-BE(2)-C cells when compared to the SK-N-SH cells (Fig. 8).
To conclude, in both the SK-N-BE(2)-C and SK-N-SH
cells, the combined use of PI3K and FGFR inhibitors resulted in an
enhanced inhibition of proliferation, in parallel with data
obtained from the WST-1 assays. Effects on cytotoxicity and
apoptosis were consistently observed more readily in the
SK-N-BE(2)-C cells than in the SK-N-SH cells.
Proliferation, apoptosis and cytotoxicity
following treatment of SK-N-BE(2)-C and SK-N-SH cells with single
cytostatic drugs, or cytostatic drugs combined with PI3K and FGFR
inhibitors
After conducting WST-1 assays, with the single
cytostatic drugs, cisplatin, vincristine and doxorubicin, or in
combination with BYL719 and JNJ-42756493, their effects on
proliferation, apoptosis and cytotoxicity in the SK-N-BE(2)-C and
SK-N-SH cells were observed (Fig.
9). Images of proliferation are presented in Fig. S2.
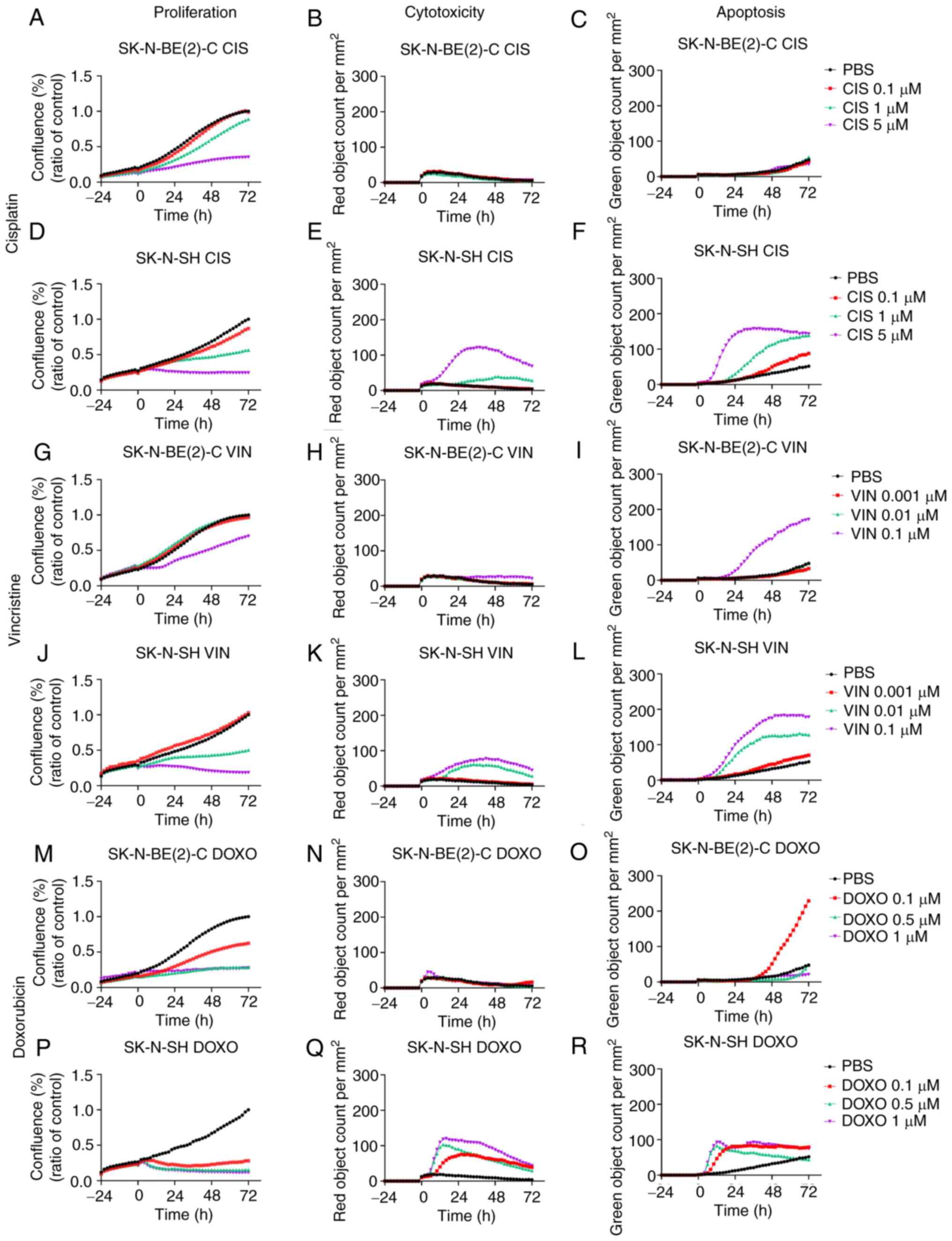 | Figure 9Proliferation, cytotoxicity and
apoptosis on SK-N-BE(2)-C and SK-N-SH cell lines upon treatment
with cisplatin, vincristine or doxorubicin. Effects on
proliferation, cytotoxicity and apoptosis of cisplatin, vincristine
and doxorubicin, respectively on SK-N-BE(2)-C cells (A-C, G-I and
M-O, respectively), and on SK-N-SH cells (D-F, J-L and P-R,
respectively). The graphs represent 3 experimental runs per cell
line. CIS, cisplatin; VIN, vincristine; DOXO, doxorubicin. |
Single treatments of SK-N-BE(2)-C and
SK-N-SH cells with cytostatic drugs
Cisplatin
The effects on proliferation, cytotoxicity and
apoptosis following treatment with 0.1-5 µM cisplatin were
observed for 72 h for the SK-N-BE(2)-C and SK-N-SH cells and the
results are depicted in Fig. 9A-C and
D-F, respectively. Dose-dependent effects on proliferation were
observed for both cell lines, while the effects on cytotoxicity and
apoptosis were minimal for the SK-N-BE(2)-C cells as compared to
those for the SK-N-SH cells.
Vincristine
The effects on proliferation, cytotoxicity and
apoptosis following treatment with 0.001-0.1 µM vincristine
were observed for 72 h for the SK-N-BE(2)-C and SK-N-SH cells and
are depicted in Fig. 9G-I and J-L,
respectively. Dose-dependent effects on proliferation were observed
for both lines, with the SK-N-SH cells being more sensitive to the
lower drug concentrations than the SK-N-BE(2)-C cells, in
concordance with the higher cytotoxicity and apoptotic levels of
the SK-N-SH cells compared to the SK-N-BE(2)-C cells.
Doxorubicin
The effects on proliferation, cytotoxicity and
apoptosis following treatment with 0.1-1 µM doxorubicin were
observed for 72 h for the SK-N-BE(2)-C and SK-N-SH cells and the
results are depicted in Fig. 9M-O and
P-R, respectively. Dose-dependent effects on proliferation were
observed for both cell lines, with the SK-N-SH cells being more
sensitive to the lower drug concentrations than the SK-N-BE(2)-C
cells, in concordance also with the higher cytotoxicity and
apoptotic levels of the SK-N-SH cells as compared to the
SK-N-BE(2)-C cells.
To conclude, single cisplatin, vincristine and
doxorubicin treatments, although not including the highest
concentrations present in the WST-1 assays, exerted dose-dependent
effects on both the SK-N-BE(2)-C and SK-N-SH cells. The SK-N-SH
cells, being the more chemo-sensitive line as compared to the
SK-N-BE(2)-C cells, was consistently more sensitive to the lower
drug concentrations than the SK-N-BE(2)-C cells, with regard to the
inhibition of proliferation and effects on cytotoxicity and
apoptosis.
Combined treatment of SK-N-BE(2)-C and
SK-N-SH cells with PI3K or FGFR inhibitor with cytostatic
drugs
The effects on proliferation, cytotoxicity and
apoptosis with cisplatin (0.1-1 µM), vincristine (0.001-0.1
µM), or doxorubicin (0.1-1 µM), combined with BYL719,
(0.25-10 µM) or JNJ-42756493 (0.01-10 µM), i.e.,
excluding higher single concentrations, on the SK-N-BE(2)-C and
SK-N-SH cells were observed for 72 h (data not shown). In the
proliferation analysis, dose-dependent effects were obtained with
all combinations, but with no clear-cut positive nor negative
effects of the combinations, while the effects on cytotoxicity and
apoptosis were moderate with the drug concentrations used.
To summarize, dose-dependent effects on
proliferation, were observed upon combining BYL719 or JNJ-42756493
with either cisplatin, vincristine or doxorubicin; however, no
clear-cut positive effects were acquired and modifications of the
effects on cytotoxicity and apoptosis were moderate or minimal with
the inhibitor and drug doses used.
Discussion
In the present study, the recently FDA-approved
drugs, alpelisib (PI3K inhibitor) and erdafitinib (FGFR inhibitor),
were shown to exert dose-dependent effects with decreased viability
and proliferation on the 5 NB cell lines, SK-N-AS, SK-N-BE(2)-C,
SK-N-DZ, SK-N-FI and SK-N-SH. Importantly, this was also the case
for NB cell lines with specific high-risk mutations or MYCN
amplification. Moreover, upon combination with the inhibitors,
additive/synergistic effects were observed with a similar decrease
in viability and proliferation using lower concentrations of the
inhibitors.
The 5 NB cell lines were also shown to exhibit
dose-dependent effects with a decreased viability and proliferation
upon exposure to cisplatin, vincristine and doxorubicin, commonly
used clinically, although e.g., the SK-N-BE(2)-C and SK-N-DZ cells
with MYCN amplifications were relatively more resistant.
Subsequently, the SK-N-BE(2)-C cells, with MYCN amplification, and
presumed drug-resistant, and the SK-N-SH cells, with wild-type p53
and presumed drug-sensitive, were examined for their sensitivity to
inhibitor drug combinations; however, when combining drugs and
inhibitors, more complex effects were noted.
The results of the inhibitors, alpelisib and
erdafitinib, were consistent with the effects on viability and
proliferation, and the cytotoxicity of the previously tested
inhibitors, BEZ235 (PI3K inhibitor) and AZD4547 (FGFR inhibitor),
on NB and medulloblastoma (MB) cell lines (14,20),
suggesting on-target effects of alpelisib and erdafitinib. Hence,
the FDA-approved PI3K and FGFR inhibitors may be of interest for
future clinical evaluation in children with refractory or recurrent
NB or MB.
The fact that all NB lines exhibited drug- dependent
dose responses and decreases in viability and proliferation, to
FDA-approved alpesilib (BYL719) and erdafitinib (JNJ-42756493), and
that the effects were enhanced upon combining the two drugs was not
unexpected, since they had responded similarly to analogous
inhibitors, BEZ235 and AZD4547 (14). Upon combined treatments with BYL719
and JNJ-42756493, the SK-N-SH and SK-N-FI cells tended to be the
most sensitive lines; however, with the majority inhibitor
combinations, all NB lines seemed susceptible, with possibly the
SK-N-AS cells being marginally more resistant. It is possible that
that the sensitivity of the SK-N-SH cells is due to the fact that
these cells have a normal p53 expression, while the relative
resistance of the SK-N-BE(2)-C and SK-N-DZ cells may be due to the
fact that these cells have MYCN amplifications (14). Thus far however, there is no
specific explanation for the relative sensitivity of the SK-N-FI
cells, and the relatively greater resistance of the SK-N-AS cells
to the above-mentioned inhibitors, since both do not have MYCN
amplification and have mutated p53. Additional, studies would be
required to elucidate the influence of the different inhibitors on
specific mechanisms of action in the signaling pathways of the
different NB cell lines.
Notably, the data described above emphasize the fact
that NB cell lines, despite their heterogeneity and without having
FGFR or PI3K mutations, can be sensitive to PI3K and FGFR
inhibitors. This has also been supported by previous studies by
others and us, where different tumors and tumor lines have been
reported to be sensitive to PI3K and FGFR inhibitors, despite not
having PI3K and or FGFR mutations or chromosomal rearrangements
(14,20,25-33).
More specifically, in some reports, it was shown that having
mutations conferred enhanced drug vulnerability, while this was not
at all the case in other studies (32,33).
Combinatorial studies showing an enhanced efficacy on the
inhibition of viability and proliferation, when combining BYL719
and JNJ-42756493 also emphasized consistency with previous data,
indicating that PI3K and FGFR inhibitors can be combined and show
synergistic activity (14,20,25).
Moreover, apart from increasing the antitumor
efficacy, synergistic combinations might also allow the use of
lower concentrations of the single drugs, thereby possibly reducing
side-effects. Eventually, targeting NB with 2 different mechanisms
might reduce the risk of the development of resistance. That
JNJ-42756493, at the concentrations used, exerted more prominent
effects on cytotoxicity and apoptosis compared to BYL719, was not
unexpected either, and was in line with reports on AZD4547 (FGFR
inhibitor) being superior to the included PI3K inhibitors with
regard to inducing cytotoxicity and apoptosis (11,14,20,25).
Thus, collectively, our data of the present study showing synergy
of the 2 FDA-approved inhibitors, BYL719 and JNJ-42756493, allowing
for the use of lower concentrations of the drugs and avoiding
resistance, suggest that they indeed could be of clinical interest
for the treatment of refractory or recurrent NB.
The 5 NB cell lines were also tested for their
sensitivity to single therapies with cisplatin, vincristine and
doxorubicin. Notably, herein, the SK-N-AS and SK-N-SH were the most
sensitive cell lines to cisplatin and vincristine, including the
SK-N-FI cells for the latter, while the SK-N-BE(2)-C cells were
generally more resistant. These findings were not entirely
unexpected, since several reports have investigated the sensitivity
of NB cell lines to cytostatic drugs and repeatedly found
SK-N-BE(2)-C being relatively more chemo-resistant and SK-N-SH
being more chemo-sensitive (34-36).
This was also reflected by the generally more prominent effects on
cytotoxicity and apoptosis the cytotoxic drugs had on the latter,
as compared to the former (Fig.
9), while the opposite was observed for the inhibitors
(Fig. 7). Nevertheless, of note,
all NB cell lines tended to be relatively more sensitive to
doxorubicin at the concentrations used in the present study; in
addition, herein, the SK-N-SH cell line was the most sensitive, a
finding which is consistent with a previous report (37).
Therefore, when investigating possible additive
effects using canonical cytostatic drugs combined with BYL719 or
JNJ-42756493, the 2 cell lines, SK-N-BE(2)-C, which had an MYCN
amplification and were fairly chemo-resistant, and SK-N-SH, which
was generally chemo-sensitive to most drugs, were selected for
comparison. Herein, a more complex image was obtained, with
synergistic, additive, neutral and adverse effects. Of note
however, were the potentially synergistic combinations of 0.25
µM BYL719 and 0.1 µM cisplatin, 0.25 µM BYL719
and 0.001 µM vincristine for both cell lines, as well as
0.25 µM BYL719 and 0.1 µM doxorubicin for the
SK-N-BE(2)-C cells (Fig. 6C and
D). These data suggest that experimentally, synergistic effects
with low concentrations of inhibitors and drugs, could be easier to
disclose, particularly on cell lines that are more resistant to
both inhibitors and drugs, than using higher concentrations and
more sensitive cell lines.
To the best of our knowledge, in an experimental
setting, potential synergism between alpesilib and erdafitinib and
cisplatin, vincristine and doxorubicin has not been tested
previously in NB, and there are only limited reports on other cell
lines with some of the present combinations. One report on
nasopharyngeal cancer cell lines, demonstrated neutral or very mild
additive effects upon combining similar doses of BYL719 and
cisplatin to those used herein (38). The fact that the present study
obtained not only positive, but also neutral and adverse effects,
could at least partially be explained by the fact that BYL719 tends
to induce G0/G1 arrest, and thereby could have inhibited some of
the cytotoxic effects that e.g., cisplatin has (38,39).
Other studies have explored combinations of alpesilib with olaparib
and erdafitinib with check point inhibitors (29,40).
While there is an apparent plethora of possible combinations with
other anti-neoplastic agents, the present study focused on
combinations with established NB drugs as this might more directly
lead to clinical translation.
There are some limitations to the present study.
Although 5 NB cell lines were examined, additional cell lines could
have been included. Nevertheless, these NB lines are representative
of the ones commonly used by the scientific community (15-18,35).
Furthermore, the present study mainly focused on the effects the
inhibitors and the cytotoxic drugs on viability, cytotoxicity and
apoptosis, using well-established methods, rather than a more
detailed analysis of signaling pathways, which the authors also
plan to pursue in the future. Nonetheless, importantly, the data
indicate that drug-drug interactions of PI3K and FGFR inhibitors
with cytotoxic drugs are of a complex nature, and can paradoxically
result in either synergistic or antagonistic interaction. While
broader concentration ranges, modified incubation times, and
sequential drug exposure might shed further light on the
determinants of the quality of drug-drug interactions with respect
to their anti-tumor efficacy, the drug and inhibitor concentrations
used herein adhere to commonly used standard conditions and
therefore allow more direct comparisons (19,21,32,38,39).
As mentioned above, further studies are required to
elucidate the mechanistic details of how the tested drug
combinations exert synergistic or antagonistic effects to provide a
pre- clinical rationale of how to test these combinations
clinically.
To conclude, the present study provides evidence
that the combined use of the FDA-approved drugs, alpelisib and
erdafitinib, enhances their individual efficacy on viability and
proliferation of well-established NB-cell lines, indicating their
use could be helpful for the treatment of refractory or recurrent
NB. In addition, the present study indicates that the incorporation
of alpelisib and erdafitinib into clinical chemotherapy regimens,
will require careful consideration in order to obtain the best
efficacy.
Supplementary Data
Funding
The present study was supported by the Swedish
Childhoods Cancer Fond (PR2017-0042, PR2017-0052), the Swedish
Cancer Society (180440, 2017/658), the Stockholm Cancer Society
(181053), the Swedish Cancer and Allergy Foundation (190), the
Royal Swedish Academy of Sciences (2017-2018), the Stockholm City
Council (20180037), Stiftelsen AnnaBrita o Bo Casters Minne
(Lindhés Advokatbyrå) (LA2019-0080, LA2020-0012), Svenska
Läkaresällskapets (SLS-934161), and Karolinska Institutet, Sweden
(2018:0007).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
ONK and SH, performed the majority of the
experiments, interpreted the data, calculated the statistics and
contributed to the writing of the manuscript. ML collaborated with
ONK and SH and performed some experiments, and ML contributed
together with ONK and SH in preparing the graphs of the manuscript.
CV and CP initiated the experiments and the interpretation of the
initial experiments and contributed to the writing of the material
and methods section, all under the supervision of ONK. MW assisted
in the combinational analyses and in the final interpretation of
the data. NH assisted in the analysis of the data and in their
clinical interpretation. TD and ONK made substantial contributions
to the conception and design of the study, the acquisition of data,
analysis and interpretation of data, and were involved in drafting
the manuscript and revising it critically for important
intellectual content. TD also provided the sources for the
performance of the experiments. TD and ONK provided the financial
support for conducting the research. All authors critically read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ward E, DeSantis C, Robbins A, Kohler B
and Jemal A: Childhood and adolescent cancer statistics, 2014. CA
Cancer J Clin. 64:83–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park JR, Bagatell R, London WB, Maris JM,
Cohn SL, Mattay KK and Hogarty M: COG Neuroblastoma Committee.
Children's Oncology Group's 2013 blueprint for research:
Neuroblastoma. Pediatr Blood Cancer. 60:985–993. 2013. View Article : Google Scholar
|
|
3
|
London WB, Bagatell R, Weigel BJ, Fox E,
Guo D, Van Ryn C, Naranjo A and Park JR: Historical time to disease
progression and progression-free survival in patients with
recurrent/refractory neuroblastoma treated in the modern era on
Children's Oncology Group early-phase trials. Cancer.
123:4914–4923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
AACR Project GENIE: Powering precision
medicine through an international consortium. Cancer Discov.
7:818–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trigg RM and Turner SD: ALK in
neuroblastoma: Biological and therapeutic implications. Cancers
(Basel). 10:1132018. View Article : Google Scholar
|
|
6
|
Guan J, Fransson S, Siaw JT, Treis D, Van
den Eynden J, Chand D, Umapathy G, Ruuth K, Svenberg P, Wessman S,
et al: Clinical response of the novel activating ALK-I1171T
mutation in neuroblastoma to the ALK inhibitor ceritinib. Cold
Spring Harb Mol Case Stud. 4:a0025502018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wesche J, Haglund K and Haugsten EM:
Fibroblast growth factors and their receptors in cancer. Biochem J.
437:199–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parker BC, Engels M, Annala M and Zhang W:
Emergence of FGFR family gene fusions as therapeutic targets in a
wide spectrum of solid tumours. J Pathol. 232:4–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaughan L, Clarke PA, Barker K, Chanthery
Y, Gustafson CW, Tucker E, Renshaw J, Raynaud F, Li X, Burke R, et
al: Inhibition of mTOR-kinase destabilizes MYCN and is a potential
therapy for MYCN-dependent tumors. Oncotarget. 7:57525–57544. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chesler L, Schlieve C, Goldenberg DD,
Kenney A, Kim G, McMillan A, Matthay KK, Rowitch D and Weiss WA:
Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn
protein and blocks malignant progression in neuroblastoma. Cancer
Res. 66:8139–8146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Segerström L, Baryawno N, Sveinbjörnsson
B, Wickström M, Elfman L, Kogner P and Johnsen JI: Effects of small
molecule inhibitors of PI3K/Akt/mTOR signaling on neuroblastoma
growth in vitro and in vivo. Int J Cancer. 129:2958–2965. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klempner SJ, Myers AP and Cantley LC: What
a tangled web we weave: Emerging resistance mechanisms to
inhibition of the phosphoinositide 3-kinase pathway. Cancer Discov.
3:1345–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Nie J, Ma X, Wei Y, Peng Y and Wei
X: Targeting PI3K in cancer: Mechanisms and advances in clinical
trials. Mol Cancer. 18:262019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kostopoulou ON, Holzhauser S, Lange BKA,
Ohmayer A, Andonova T, Bersani C, Wickström M and Dalianis T:
Studies of FGFR3 and PIK3CA mutations in neuroblastomas and the
effects of the corresponding inhibitors in neuroblastoma cell
lines. Int J Oncol. 55:1372–1384. 2019.PubMed/NCBI
|
|
15
|
Biedler JL, Helson L and Spengler BA:
Morphology and growth, tumorigenicity, and cytogenetics of human
neuroblastoma cells in continuous culture. Cancer Res.
33:2643–2652. 1973.PubMed/NCBI
|
|
16
|
Biedler JL and Spengler BA: A novel
chromosome abnormality in human neuroblastoma and
antifolate-resistant Chines hamster cell lives in culture. J Natl
Cancer Inst. 57:683–695. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugimoto T, Tatsumi E, Kemshead JT, Helson
L, Green AA and Minowada J: Determination of cell surface membrane
antigens common to both human neuroblastoma and leukemia-lymphoma
cell lines by a panel of 38 monoclonal antibodies. J Natl Cancer
Inst. 73:51–57. 1984.PubMed/NCBI
|
|
18
|
Helson L and Helson C: Human neuroblastoma
cells and 13-cis-retinoic acid. Neurooncol. 3:39–41. 1985.
|
|
19
|
LaQuaglia MP, Kopp EB, Spengler BA, Meyers
MB and Biedler JL: Multidrug resistance in human neuroblastoma
cells. J Pediatr Surg. 26:1107–1112. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holzhauser S, Lukoseviciute M, Andonova T,
Ursu RG, Dalianis T, Wickström M and Kostopoulou ON: Targeting
fibroblast growth factor receptor (FGFR) and phosphoinositide
3-kinase (PI3K) signaling pathways in medulloblastoma cell lines.
Anticancer Res. 40:53–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
André F, Ciruelos E, Rubovszky G, Campone
M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, et al:
Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced
breast cancer. N Engl J Med. 380:1929–1940. 2019. View Article : Google Scholar
|
|
22
|
Bahleda R, Italiano A, Hierro C, Mita A,
Cervantes A, Chan N, Awad M, Calvo E, Moreno V, Govindan R, et al:
Multicenter phase I study of erdafitinib (JNJ-42756493), Oral
Pan-fibroblast growth factor receptor inhibitor, in patients with
advanced or refractory solid tumors. Clin Cancer Res. 25:4888–4897.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Foucquier J and Guedj M: Analysis of drug
combinations: Current methodological landscape. Pharmacol Res
Perspect. 3:e001492015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holzhauser S, Kostopoulou ON, Ohmayer A,
Lange BKA, Ramqvist T, Andonova T, Bersani C, Wickström M and
Dalianis T: In vitro antitumor effects of FGFR and PI3K inhibi-tors
on human papillomavirus positive and negative tonsillar and base of
tongue cancer cell lines. Oncol Lett. 18:6249–6260. 2019.PubMed/NCBI
|
|
26
|
Wang L, Šuštić T, Leite de Oliveira R,
Lieftink C, Halonen P, van de Ven M, Beijersbergen RL, van den
Heuvel MM, Bernards R and van der Heijden MS: A functional genetic
screen identifies the phosphinositide 3-kinase pathway as a
determinant of resistance to fibroblast grown factor receptor
inhibitors in FGFR mutant urothelial cell carcinoma. Eur Urol.
71:858–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Mikse O, Liao RG, Li Y, Tan L,
Janne PA, Gray NS, Wong KK and Hammerman PS: Ligand-associated
ERBB2/3 activation confers acquired resistance to FGFR inhibition
in FGFR3-dependent cancer cells. Oncogene. 34:2167–2177. 2015.
View Article : Google Scholar
|
|
28
|
Herrera-Abreu MT, Pearson A, Campbell J,
Shnyder SD, Knowles MA, Ashworth A and Turner NC: Parallel RNA
interference screens identify EGFR activation as an escape
mechanism in FGFR3-mutant cancer. Cancer Discov. 3:1058–1071. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brands RC, Knierim LM, De Donno F,
Steinacker V, Hartmann S, Seher A, Kübler AC and Müller-Richter
UDA: Targeting VEGFR and FGFR in head and neck squamous cell
carcinoma in vitro. Oncol Rep. 38:1877–1885. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singleton KR, Hinz TK, Kleczko EK, Marek
LA, Kwak J, Harp T, Kim J, Tan AC and Heasley LE: Kinome RNAi
screens reveal synergistic targeting of MTOR and FGFR1 pathways for
treatment of lung cancer and HNSCC. Cancer Res. 75:4398–4406. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai W, Song B and Ai H: Combined
inhibition of FGFR and mTOR pathways is effective in suppressing
ovarian cancer. Am J Transl Res. 11:1616–1625. 2019.PubMed/NCBI
|
|
32
|
Munster P, Aggarwal R, Hong D, Schellens
JH, van der Noll R, Specht J, Witteveen PO, Werner TL, Dees EC,
Bergsland E, et al: First-in-Human phase I study of GSK2126458, an
Oral Pan-class I Phosphatidylinositol-3-Kinase inhibitor, in
patients with advanced solid tumor malignancies. Clin Cancer Res.
22:1932–1939. 2016. View Article : Google Scholar
|
|
33
|
Tamura R, Yoshihara K, Saito T, Ish imura
R, Martínez-Ledesma JE, Xin H, Ishiguro T, Mori Y, Yamawaki K, Suda
K, et al: Novel therapeutic strategy for cervical cancer harboring
FGFR3-TACC3 fusions. Oncogenesis. 7:42018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulda S, Honer M, Menke-Moellers I and
Berthold F: Antiproliferative potential of cytostatic drugs on
neuroblastoma cells in vitro. Eur J Cancer. 31A:616–621. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng X, Naiditch J, Czurylo M, Jie C,
Lautz T, Clark S, Jafari N, Qiu Y, Chu F and Madonna MB:
Differential effect of long-term drug selection with doxorubicin
and vorinostat on neuroblastoma cells with cancer stem cell
characteristics. Cell Death Dis. 4:e7402013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eschenburg G, Luckert C, Reinshagen K and
Bergholz R: Taurolidine cooperates with antineoplastic drugs in
neuroblastoma cells. Genes Cancer. 5:460–469. 2014. View Article : Google Scholar
|
|
37
|
Wickström M, Johnsen JI, Ponthan F,
Segerström L, Sveinbjörnsson B, Lindskog M, Lövborg H, Viktorsson
K, Lewensohn R, Kogner P, et al: The novel melphalan prodrug J1
inhibits neuroblastoma growth in vitro and in vivo. Mol Cancer
Ther. 6:2409–2417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wong CH, Ma BB, Cheong HT, Hui CW, Hui EP
and Chan AT: Preclinical evaluation of PI3K inhibitor BYL719 as a
single agent and its synergism in combination with cisplatin or MEK
inhibitor in nasopharyngeal carcinoma (NPC). Am J Cancer Res.
5:1496–1506. 2015.PubMed/NCBI
|
|
39
|
Keam B, Kim S, Ahn YO, Kim TM, Lee SH, Kim
DW and Heo DS: In vitro anticancer activity of PI3K alpha selective
inhibitor BYL719 in head and neck cancer. Anticancer Res.
35:175–182. 2015.PubMed/NCBI
|
|
40
|
Konstantinopoulos PA, Barry WT, Birrer M,
Westin SN, Cadoo KA, Shapiro GI, Mayer EL, O'Cearbhaill RE, Coleman
RL, Kochupurakkal B, et al: Olaparib and α-specific PI3K inhibitor
alpelisib for patients with epithelial ovarian cancer: A
dose-escalation and dose-expansion phase 1b trial. Lancet Oncol.
20:570–580. 2019. View Article : Google Scholar : PubMed/NCBI
|