Introduction
Gastric cancer is the third leading cause of
cancer-related mortality worldwide (1). Helicobacter pylori (H.
pylori) infection is the most potent risk factor responsible
for the development of gastric cancer, leading to the recognition
of this bacterium by the World Health Organization (WHO) as class 1
carcinogen (2-4). H. pylori infections affect up
to 80% of the population in certain parts of the globe (4). Mortality due to gastric cancer,
similar to other common types of cancer, is the result of
metastasis (5). Moreover, there
are currently no available effective predictors for identifying
recurrence and metastasis in gastric cancer. Consequently, the
determination of factors that indicate the existence of metastasis
is critical for therapeutic interventions with the goal of
improving disease outcome. Cancer metastasis involves tumor cells
referred to as circulating tumor cells (CTCs), leaving the original
cancerous site by migrating to distant sites; these can be found in
peripheral blood and bone marrow (6-9). In
the bone marrow, these CTCs are referred to as disseminated cancer
cells (DTCs). CTCs have been used as biomarkers of metastasis in a
number of cancer types (8,9) and their presence is associated with a
poor prognosis (5,10-13).
In bone marrow, evidence of cancer cells at the time of surgical
intervention has been associated with metastasis (8,14).
While research into associating CTCs and DTCs with cancer
metastasis has been extensive for breast and lung cancer (5), research into this topic for gastric
cancer has been limited. Indeed, the CellSearch System (Veridex,
LLC) was approved by the US Food and Drug Administration for the
detection of CTCs in patients with breast, prostate and colorectal
cancer (9,15-17);
however, its use for the detection of CTCs in gastric cancer
continues to be controversial (18). This has led to a lack of enthusiasm
in studies detecting CTCs in gastric cancer patients, and
consequently, in their routine usage in gastric cancer management.
One of the most common methods for the detection of CTCs in solid
tumors is with the use of surface markers, such as cytokeratins and
mucin-1 (MUC1). Cytokeratins in general have been extensively
studied in epithelial cancers, such as breast cancer (19) and specifically, cytokeratins such
as CK8, CK18 and CK19 (20). These
markers are of particular interest due to their abundant expression
in epithelial cells and relatively low or no expression in
mesenchymal cells (10,21). Recently, other markers, such as
epithelial-to-mesenchymal transition (EMT)-related markers and
cancer stem cells (CSCs) have been shown to be major components of
CTCs due to their association with cancer progression (22-27).
EMT, which depicts changes in epithelial cells
towards a malignant phenotype (28) is considered a crucial step in
cancer progression (29). This
process disrupts crucial activities, such as cell-cell adhesion,
cell polarity (30) and extra
cellular matrix degradation (31).
There are a number of inducers of EMT, most notably factors, such
as cytokines, innate and adaptive immune responses, and growth
factors secreted by tumor microenvironment among others (32,33).
This EMT process is tightly regulated by transcription factors,
such as Snail, Twist and Zinc finger E-box-binding homeobox (ZEB).
Snail and Twist have previously been shown to be overexpressed in
H. pylori-infected patients (34). While patients with early stages of
cancer do not exhibit EMT phenotypes, gastric cancer cell motility
and metastasis are observed in the advanced stages of gastric
cancer, which is implicated in the EMT process (35). While the clinical significance of
EMT in other types of cancer has been confirmed (28,36,37),
in gastric cancer, although the expression of EMT-related proteins
has been studied, their significance remains questionable (38,39).
CSCs, which are also suggested to be components of CTCs, are
considered to contribute to a number of aggressive cancer
characteristics, such as metastasis, tumor invasion,
chemo-therapeutic resistance and relapse (40).
Knowledge of micrometastatic cells, including when
they arise and their detection, is critical since their
dissociation from the primary tumor microenvironment and
transportation to distant sites and finally, colonization is what
ultimately leads to death. These cells are therefore important for
the detection of metastasis or disease recurrence. The detection of
CTCs is therefore crucial in identifying patients that are likely
to relapse or develop metastases and can subsequently be targeted
for the suppression of metastasis. Previous studies by the authors
have demonstrated that the absence of myeloid differentiation
primary response gene 88 (MyD88−/−) leads to the
development of an aggressive form of Helicobacter-induced
gastric cancer, resulting in gastric cancer mouse models termed as
slow [wild-type (WT)] and fast (Myd88−/−)
'progressors' (41,42). Myd88−/− mice were
shown to exhibit a rapid progression to precancerous and cancerous
lesions in the stomach in response to infection with
Helicobacter felis (H. felis) when compared to WT
mice (41,42). In the present study, these gastric
cancer models were used to evaluate the kinetics of CTCs and DTCs
over a time span of 6 months. CTCs and DTCs were detected using
surface markers, cytokeretins and mucins; EMTs and CSC markers, in
the bone marrow and peripheral blood by employing
immunocytochemistry (ICC), and/or reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
data from the present study indicate that the early detection of
metastasis in aggressive gastric cancers may be useful for gastric
cancer management by providing information regarding proper
prognosis and treatment intervention guidelines.
Materials and methods
Animals
Mice at 6-10 weeks old (weighing 20±5 g) were used
in the present study. A total number of 40 male mice, WT (n=20) and
Myd88−/− mice (n=16) with a C57BL/6J background
were purchased from the Jackson Laboratory. In addition, some
Myd88−/− mice (n=4) were bred inhouse. All mice
were housed together prior to H. felis infection and for the
duration of the study in a biosafety level II (BSL-2) facility with
controlled temperature (23±2°C) and relative humidity (45-60%) and
had full access to food and water. All animal procedures were
approved by the Institutional Animal Care and Use Committee at the
University of California, San Diego, CA, USA. All procedures were
performed using accepted veterinary standards.
Bacterial strains and growth
conditions
H. felis strain CS1 (ATCC 49179) was used for
the mouse infections. This strain was originally purchased from the
American Type Culture Collection (ATCC). H. felis were
maintained in both solid and liquid medium. The solid medium was
composed of Columbia agar (BD Biosciences) supplemented with laked
blood (5%, Hardy Diagnostics) and Amphotericin B (1%; Mediatech,
Inc.). The liquid medium was composed of brain heart infusion (BHI;
BD Biosciences) supplemented with 10%, heat inactivated fetal
bovine serum (FBS). Bacteria were grown at 37°C under
microaerophilic conditions (5% O2, 10% CO2,
85% N2) as described in previous studies by the authors
(41,43). Bacteria maintained in solid media
were passaged every 2-3 days. Prior to the infection of the mice,
H. felis were grown in liquid medium for 48 h. Spiral
bacteria were counted using a Petroff-Hausser chamber.
Mouse infections
WT and Myd88−/− mice were infected
with H. felis grown in BHI. A total of 109
organisms in 300 µl were administered to each mouse (14 mice
from each mouse strain) by oral gavage every other day for a total
of 3 inoculations as described in previous studies by the authors
(41,43). Control mice (6 mice from each mouse
strain) received 300 µl of BHI. Myd88−/−
mice (2 or 3 mice) were euthanized each month for up to 6 months
post-infection. WT mice (2 or 3 mice) were euthanized at 5, 6 and 7
months post-infection. Bone marrow and peripheral blood was
aseptically removed and processed for experimental analysis.
Bone marrow isolation
Following euthanasia, femurs and tibias were
aseptically removed from the mice taking care to remove any muscle
on or near the bones as described in previous studies by the
authors (44,45). Bone marrow cells were flushed using
a 22-gauge needle and phosphate-buffered saline (PBS) by cutting
the ends of the bones with sharp scissors. Cells were collected for
downstream applications.
ICC
Samples collected from bone marrow and peripheral
blood were deposited onto lysine coated slides using StatSpin
CytoFuge (Beckman Coulter, Inc.). Briefly, cell samples were loaded
onto cell concentrators with lysine coated slides. The
concentrators with sample were then placed into a cytofuge and spun
at 55 × g for 4 min at room temperature (RT). Once cell samples
were placed on slides, cells were fixed with 4% paraformaldehyde
for 10 min at RT. The cells were then incubated with 1% bovine
serum albumin (BSA) in 0.1% PBS supplemented with Tween-20 (PBST)
for 30 min at RT. Cells were immunostained with antibodies specific
for CK8/18 (ab215880), 1:2,000, Abcam and c-Kit, cluster of
differentiation (CD)117 (c-Kit, sc-168, 1:200, Santa Cruz
Biotechnology, Inc.). All primary antibodies were incubated in a
humidified chamber at 4°C overnight. After washing, the cells were
incubated with goat anti-rabbit secondary antibody (1:1,000) with
fluorochrome fluorochrome (Alexa Fluor 488 and 647; ab150077 and
ab150083, respectively, Abcam) for 1 h at RT in the dark and the
samples were then mounted with Fluoroshield mounting medium with
DAPI (4′,6-diamidino-2-phenylindole, Abcam). Slides were imaged
using the Keyence BZX-700 Fluorescent Microscope (UCSD Microscopy
Core).
Isolation of RNA and cDNA synthesis
RNA was isolated from the bone marrow and peripheral
blood samples using the Directzol RNA mini kit (Zymo Research Corp)
according to the manufacturer's instructions. Briefly, a total of
300 µl of TRI Reagent was added to bone marrow or blood
plasma in a volume of 3:1. The samples were vortexed vigorously
followed by RNA purification. The samples were passed through a
collection column and washed with the accompanying buffers. The
resulting RNA solution was passed through a filter cartridge and
RNA eluted using nuclease-free water. RNA quality was determined
using a Nanodrop system (Thermo Fisher Scientific, Inc.) by reading
the absorbance levels at 260 nm. A total of 2 µg of RNA per
sample was reverse transcribed into cDNA using the High Capacity
cDNA Reverse Transcription kit (cat. no. 4368814, Thermo Fisher
Scientific, Inc.).
qPCR
qPCR was performed as described in previous studies
by the authors (43,45,46).
The expression of select genes, including CD44, SRY-box
transcription factor (SOX)9, Prominin-1 (CD133), SOX2,
octamer-binding transcription factor 4 (OCT4), NANOG, leucinerich
repeat-containing G-protein coupled receptor 5 (LGR5), CK18, CK19,
MUC1, Snail, Twist and ZEB. Briefly, 2 µl of cDNA were used
per well in a 10 µl reaction mix for amplification using
Step One Real Time PCR (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The amplification conditions consisted of an
initial cycle of 95°C for 5 min followed by 40 cycles of
amplification with denaturation as follows: 95°C for 15 sec, 60°C
for 20 sec, 72°C for 40 sec. Gene expression levels were normalized
to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The data
collected was analyzed using comparative cycle threshold
calculations (ΔΔCT, Applied Biosystems; Thermo Fisher
Scientific, Inc.) and plotted using GraphPad Prism software. The
sequences of primers used are listed in Table SI.
Statistical analysis
Gene expression was analyzed using comparative cycle
threshold calculations (ΔΔCT, Applied Biosystems; Thermo
Fisher Scientific, Inc.) as described in previous studies by the
authors (41). A fold change of
>2 between infected and control mice was considered
significant.
Results
Detection of epithelial markers in bone
marrow and peripheral blood in response to Helicobacter
infection
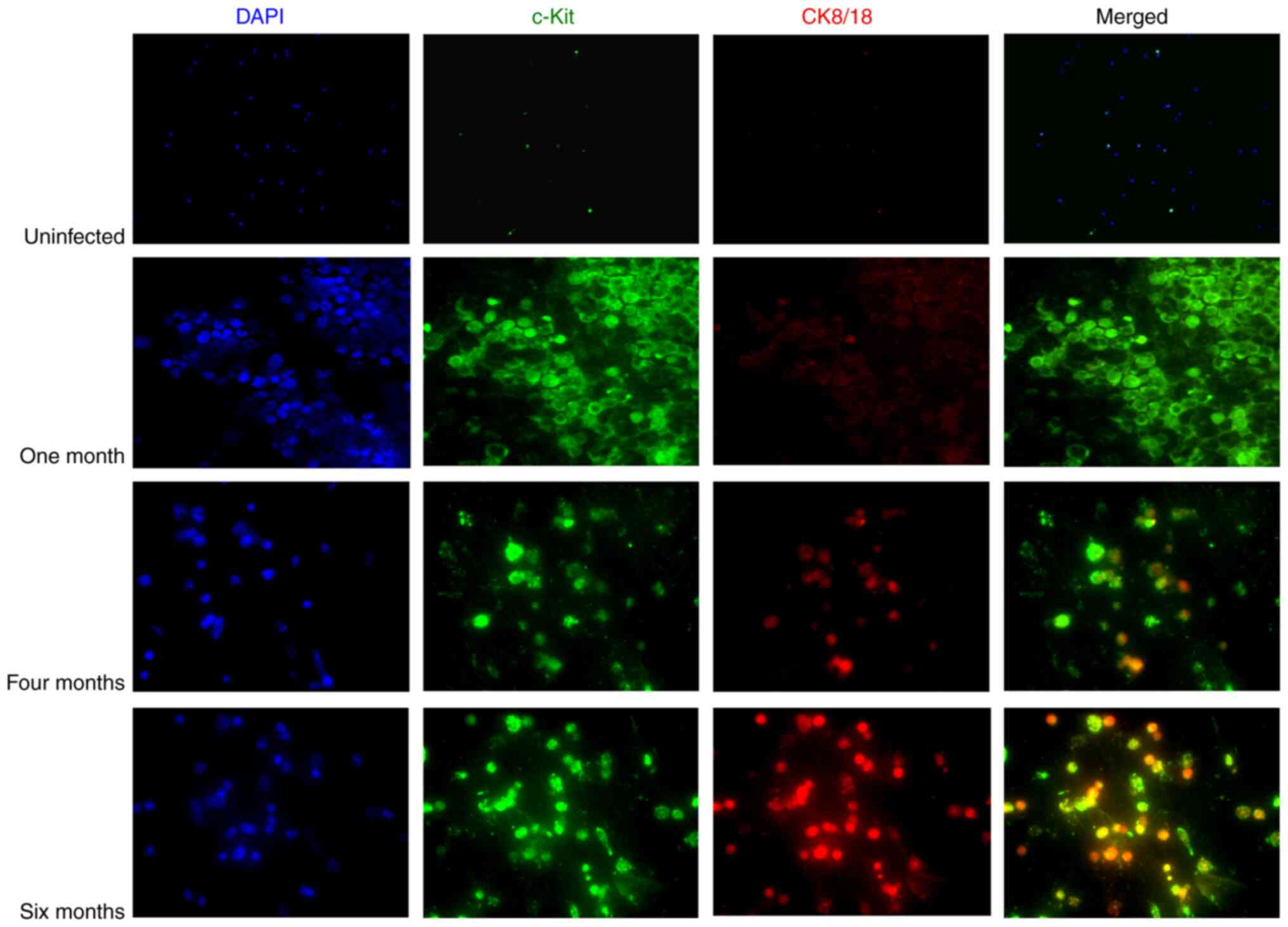
The epithelial markers, CK8/18, were used to detect
CTCs and DTCs in peripheral blood and bone marrow from mice in
response to infection with H. felis, respectively. c-Kit
(also known as CD117) was used as a standard surface marker
expressed in hematopoietic cells and progenitor cells in bone
marrow (47,48). In the present study, in the fast
'progressor' gastric cancer model, bone marrow was analyzed for
epithelial markers monthly for up to 6 months post-infection.
Epithelial cell markers were detected in the bone marrow as early
as 3 months and their expression levels increased as the disease
progressed, with maximum expression observed at 6 months
post-infection (Fig. 1). These
markers were not detected at 1 (Fig.
1) or 2 months (Fig. S1).
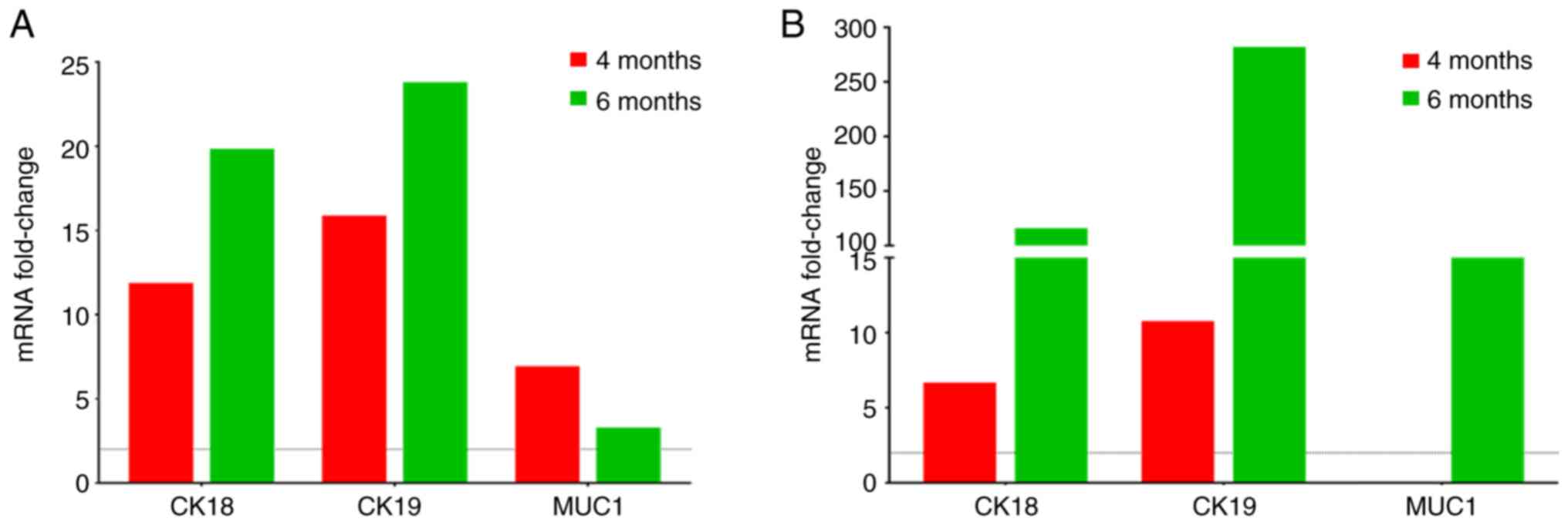
CK8/18 were not detected in peripheral blood. However, the
increased expression of CK18 and CK19 in both peripheral blood and
bone marrow (Fig. 2) was observed
as the disease progressed using RT-qPCR. Moreover, MUC1 expression
was observed in peripheral blood at both at 4 and 6 months, whereas
its expression in bone marrow was only observed at 6 months
(Fig. 2B). On the other hand, no
epithelial markers were detected in the slow 'progressor' gastric
cancer model (H. felis-infected WT mice) at 5 and 6 months
post-infection (Fig. S2).
Therefore, all subsequent experiments were only performed in the
fast 'progressor' gastric cancer model (H. felis-infected
Myd88−/− mice).
Evidence of epithelial transition to a
mesenchymal phenotype
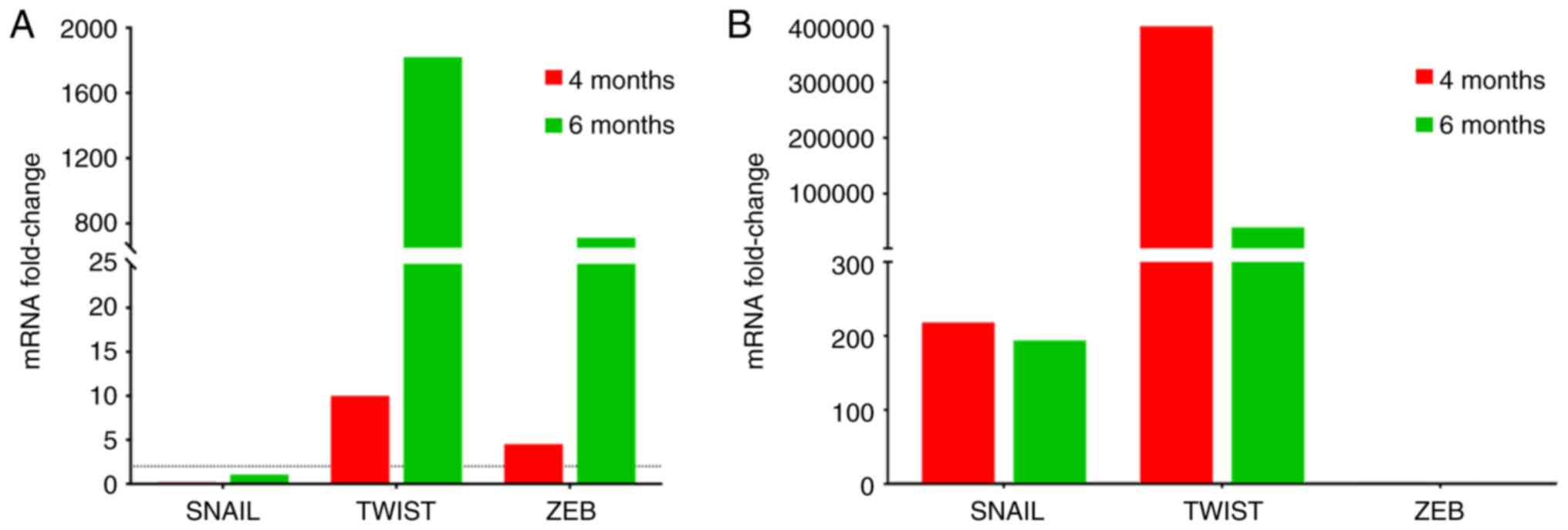
The EMT transcription factors, Snail, Twist and ZEB,
were analyzed to determine their expression during H.
felis-induced disease progression. Increased expression levels
of Snail were observed in bone marrow as compared to negligible or
below threshold levels at both 4 months and 6 months in peripheral
blood (Fig. 3). On the other hand,
although Twist was expressed above threshold levels at both 4 and 6
months post-infection, the peak levels differed, peaking at 6
months in peripheral blood (Fig.
3A) and at 4 months in bone marrow (Fig. 3B). ZEB was expressed in peripheral
blood; however, its expression was undetectable in bone marrow
(Fig. 3).
Expression of cancer stem cells markers
in bone marrow and peripheral blood
LGR5 is the most well-known gastric cancer stem cell
marker and has been extensively studied to validate its importance
in gastric cancer (49). In the
present study, the expression of LGR5 increased gradually from 4 to
6 months in peripheral blood and bone marrow, with higher
expression levels observed in bone marrow (Fig. 4). The expression of CD44, which was
the first gastric cancer stem cell marker identified (50,51)
peaked at 6 months in the bone marrow. On the other hand, CD133 was
expressed at high levels in peripheral blood (Fig. 4A) but was undetectable in the bone
marrow (Fig. 4B). Other cancer
markers evaluated included OCT4, NANOG, SOX2 and SOX9; their
expression levels were detected in both bone marrow and peripheral
blood (Fig. 4).
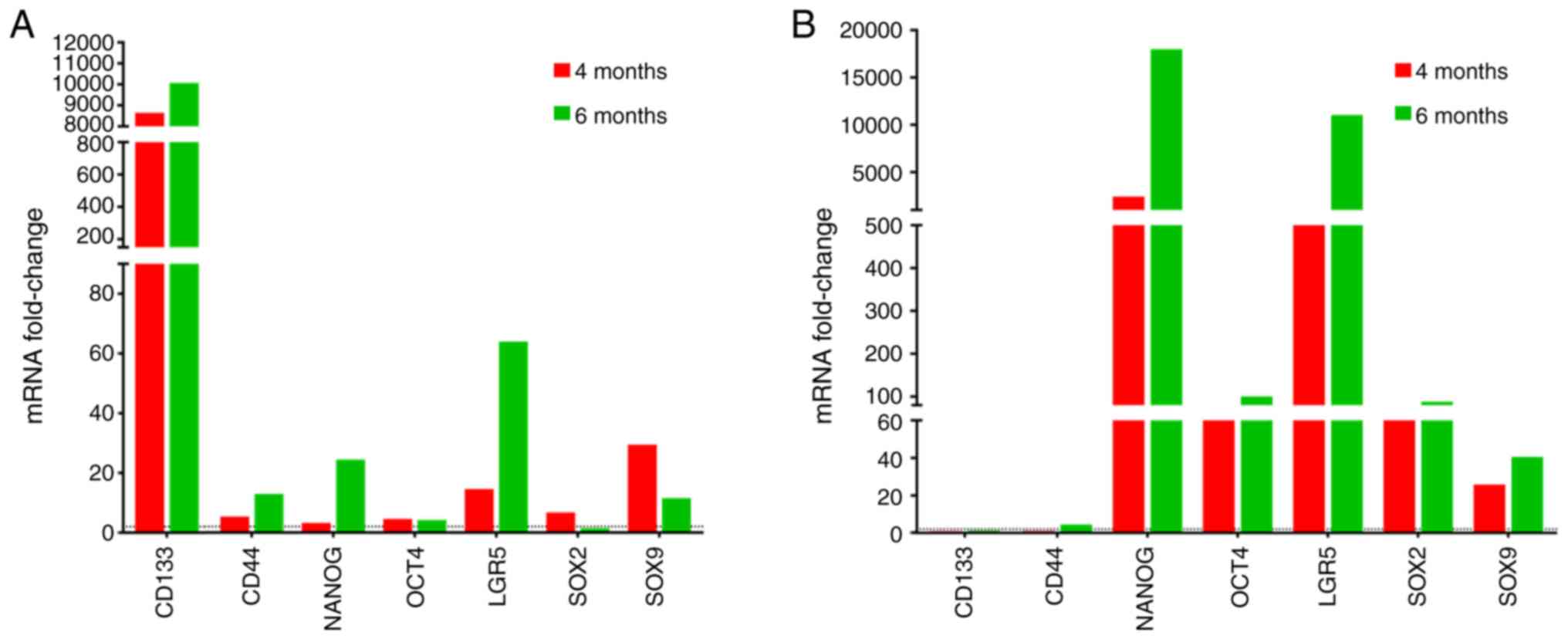 | Figure 4Quantification of cancer stem cell
marker levels. CD133, CD44, NANOG, OCT4, LGR5, SOX2 and SOX9 levels
were assessed in (A) peripheral blood and (B) bone marrow from
Helicobacter felis-infected Myd88−/− mice
at 4- and 6-months using RT-qPCR. Data are reported as the fold
induction of uninfected Myd88−/− mice.
Myd88−/−, myeloid differentiation primary
response 88- deficient; CD133, cluster of differentiation 133
(Prominin 1); OCT4, octamer-binding transcription factor 4; LGR5,
leucine-rich repeat-containing G-protein coupled receptor 5, SOX,
SRY-box transcription factor. |
Discussion
Examination and diagnostic tools for confirming the
presence of gastric cancer are often invasive with endoscopy being
the main test used to detect stomach cancer. At times, signs and
symptoms are not very distinguishable for many patients and with no
protocol in place in countries where incidence of gastric cancer is
low, the chance of early stage detection is very minimal. For the
majority of patients, gastric cancer is diagnosed in the
locally-advanced or late stages, as either screening is not
performed or the disease is detected only following the development
of symptoms. Early detection will help increase patient survival by
decreasing the chance for metastatic progression. Indeed, detection
of CTCs in peripheral blood and bone marrow in gastric cancer
patients has been suggested to be indicative of metastasis
(52,53). However, the clinical significance
of CTCs and DTCs as indicators of metastasis has not been
appropriately utilized in gastric cancer compared to breast and
lung cancer (5). In the present
study, three subsets of biomarkers were utilized, namely
cytokeretins, EMTs and CSCs, to detect CTCs and DTCs indicative of
metastasis using a previously established fast 'progressor' gastric
cancer model at an early stage (41). CTCs were detectable as early as 3
months compared to our slow 'progressor' model (WT type) where they
were undetectable even at 6 months. This suggests that in an
aggressive form of cancer the transformed cells, CTCs begin moving
to secondary locations even before the cancer is well established
at the primary site. The presence of epithelial gastric surface
markers within bone marrow and peripheral blood indicate that not
only have tumor-like cells left the microenvironment of the gastric
mucosa, but have successfully begun infiltrating these areas
leading to micro metastatic tumors throughout the body (19). CK8, CK18 and CK19 have previously
been identified as markers whose expression is found in almost all
epithelial-based carcinomas (48,54).
Cytokeratins, such as 8 and 18 are found in >90% of gastric
cancer tumors (55) rendering them
reasonable targets to evaluate as positive markers of gastric
metastasis. In addition, the present study detected MUC1 in both
the peripheral blood and bone marrow. MUC1 is an oncoprotein found
in a number of adenocarcinomas (59), which under normal conditions is
known to protect the gastric epithelium (56-59).
However, in the presence of H. pylori, MUC1 expression has
been shown to be considerably decreased (57). MUC1 is one of the markers used for
detecting CTCs and DTCs in epithelial solid cancers (5) and has been linked to cancers such as
non-small cell lung cancer (60),
as well as DTCs within the bone marrow of breast cancer patients
(61). The role of MUC1 in
carcinogenesis has not been well elucidated and in particular, its
role in gastric cancer is contradictory (62-64);
however, its overexpression has been associated with cancer
metastases (65). Recent studies
have suggested regulation of MUC1 by mir-206 inhibits proliferation
and migration of gastric cancer cells (66). Thus, reinforcing the role of MUC1
as a gastric cancer metastases biomarker. Moreover, MUC1 promotes
cell proliferation by Wnt signaling pathway and EMT activation
through Snail in renal carcinoma (67).
EMT is described as the transition of cells from an
epithelial to a mesenchymal state that is associated with the
suppression of E-cadherin resulting in an invasive cell phenotype
(27-29,68,69).
This change in expression is induced by EMT-transcription factors
(EMT-TFs), which include Snail, Twist and ZEB. The increased
expression of these EMT markers is associated with the transition
of the epithelium into a malignant phenotype (28). As gastric cancer progresses,
epithelial cells begin to lose these phenotypic markers and begin
to acquire a mesenchymal phenotype (70), which is associated with the loss of
cell-cell adhesion of epithelial cells, as well as changes in cell
polarity, which eventually allows for the easy migration of cells
(69). The concomitant expression
of these EMT markers with epithelial markers in our gastric cancer
model indicates that these EMT markers may be used as indicators of
metastasis in gastric cancer. Recent findings suggest that
acquisition of mesenchymal markers in tumors is a poor prognostic
cancer factor (27,71,72).
Hypoxic conditions in tumors are suggested to trigger mesenchymal
stem cell (MSC) migration (73-76).
The presence of these MSCs in the tumor stroma is associated with
EMT stimulation. Once stimulated it is indicated that these MSCs
may promote the invasion and spread of tumor cells in systemic
circulation (77). As an example,
studies carried out by Yang et al, 2004 (78), in breast cancer have suggested that
high levels of the continued expression of Twist are essential for
metastasis. The findings of the present study also demonstrated the
continued expression of Twist in both peripheral blood and bone
marrow, thus, suggesting a role of Twist in metastasis in an
aggressive form of gastric cancer. Snail is a potent suppressor of
E-cadherin and is closely associated with cancer metastasis and
tumor progression via the Wnt pathway (79). Previous research on breast cancer
has demonstrated that Snail is required for lymph node metastasis
(28). High expression levels of
Snail in the bone marrow may indicate DTCs in the bone marrow. ZEB,
in addition to its function as an EMT inducer, also plays a role in
hematopoiesis. ZEB has been associated with acquisition of cancer
stem cell (CSC) properties (80).
Thus, the expression of ZEB, also shown in the present study,
indicates early metastasis in fast-progressing gastric cancer.
The present study also detected CSCs, including
LGR5, CD44, CD133, OCT4, SOX2, SOX9 and NANOG in peripheral blood
and bone marrow during disease progression. Cancer stem cells play
a vital role in cancer metastasis (12,40).
For all tumor-associated stem cell markers detected in the current
fast-progressing gastric cancer model, the expression levels were
always greater in the bone marrow than in the peripheral blood.
This is in line with research on challenges associated with the
detection of CTCs in blood due to very low numbers of tumor cells
in blood (81). Indeed, the levels
of CTCs are generally lower in peripheral blood compared to bone
marrow (82). Notably, CD133 was
highly expressed in peripheral blood and undetectable in bone
marrow. The reason for this differential expression remains to be
investigated. CD133 is a known cancer stem cell marker in cancers,
such as colorectal cancer and liver cancer and for is its role in
metastasis in these types of cancer (83). Of all these CSC markers, LGR5 and
CD44 are well-known targets of the Wnt signaling pathway, and have
been implicated in cancer invasion and metastasis through their
involvement in tumor formation and proliferation (84-86).
LGR5 induces the Wnt/β-catenin pathway, enhancing tumor formation
and cancer cell proliferation (84). The gradual increase in the
expression of LGR5 observed in the present study was similar to
that observed for cervical cancer (84). LGR5 expression increased as the
disease progressed. The expression of CD44 also gradually increased
as the disease progressed although the level of expression was
lower compared to LGR5. The findings of the present study
demonstrating a close association in expression between EMT
transcriptional factors and stem cells markers are in agreement
with those of studies indicating a link between EMT and acquisition
of stem cell properties (87,88).
In addition, studies have indicated that EMT facilitates the
generation of CSC traits for metastasis, but also for self-renewal
properties required for initiating secondary tumors attributed to
NANOG, OCT 4, SOX2 to name a few (89-91).
These provide credence to the current observation that these
markers can be used to detect gastric cancer metastasis and predict
aggressive and fast-progressing gastric cancers. Future studies are
required to confirm that these cells are indeed cancer cells by use
of a xenograft mouse model. In considering the future translation
of the current study to humans, the data suggest that the detection
of tumor cell biomarkers could be performed when H. pylori
infection is diagnosed in a patient, given that currently, gastric
cancer is diagnosed when it has already progressed to the late
stages of the disease. Granted that bone marrow biopsy is an
invasive procedure, performing peripheral blood tumor cell
biomarker detection screens alone would be a better prophylactic
approach at first and depending on the results, further
confirmation for gastric cancer could be performed by bone marrow
aspiration procedures. Therefore, including peripheral blood tumor
cell biomarker detection in regular health screens upon diagnosis
of H. pylori infection is potentially a good preventive
measure.
To date, at least to the best of our knowledge,
there are no published data available showing the stage at which
gastric cancer metastasizes. Using the present mouse models of
gastric cancer (41), the
expression of EMT, stem cell markers and cytokeratins was detected
in the fast 'progressors' gastric cancer model by 4 months, but not
in the slow 'progressors', suggesting that these factors are
involved in the early events of tumorigenesis; thus, these factors
may represent early indicators of disease dissemination and
therefore, metastasis. The present study using mice suggests that
dysplastic gastric epithelial cells begin seeding themselves in
other tissues, including the bone marrow early during the disease
progression to gastric cancer and before the emergence of gastric
cancer in situ. In addition, the present study revealed an
association between cytokeratins, EMTs and CSCs with an aggressive
form of gastric cancer. Studies on tumor cell biomarker detection
in human gastric cancer patients are required to confirm these
findings. Nonetheless, the present study sets up a proof of concept
that longitudinal monitoring of CTCs as an indicator of metastasis
in gastric cancer is an achievable goal similar to the current
management of breast cancer (19,20,27).
Therefore, the findings of the present study may lead to the
development of early detection strategies for CTCs in patients with
an aggressive form of gastric cancer, so that appropriate treatment
can be provided in a timely manner.
Supplementary Data
Funding
The present study was supported by the National
Cancer Institute of the National Institute of Health under award
R21CA210227.
Availability of data and materials
The datasets generated and analyzed during this
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MO designed the study concept. ILP and CP acquired
the data. PB, ILP and MO analyzed and interpreted the data. PB and
IL drafted the manuscript. PB and MO edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors wish to thank the UCSD School of
Medicine Microscopy Core for access to the Keyence
Immunofluorescence Microscope, which is supported by NINDS P30
Grant (NS047101). This manuscript has been released as a pre-print
at bioRxiv (https://biorxiv.org/cgi/content/short/2020.01.29.925727v1).
Abbreviations:
|
H. pylori
|
Helicobacter pylori
|
|
WHO
|
World Health Organization
|
|
BMDCs
|
bone marrow-derived cells
|
|
Myd88−/−
|
myeloid differentiation primary
response 88-deficient
|
|
H. felis
|
Helicobacter felis
|
|
CTCs
|
circulating tumor cells
|
|
DTCs
|
disseminating tumor cells
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
CK8/18/19
|
cytokeratin 8/18/19
|
|
WT
|
wild-type
|
|
c-Kit
|
cluster of differentiation 117
(CD117)
|
|
MUC1
|
mucin 1
|
|
ICC
|
immunocytochemistry
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
BHI
|
brain heart infusion
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
CD133
|
cluster of differentiation 133
(prominin 1)
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
MSCs
|
mesenchymal stem cells
|
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar
|
|
2
|
Bessede E, Dubus P, Megraud F and Varon C:
Helicobacter pylori infection and stem cells at the origin of
gastric cancer. Oncogene. 34:2547–2555. 2015. View Article : Google Scholar
|
|
3
|
Bessède E, Staedel C, Acuña Amador LA,
Nguyen PH, Chambonnier L, Hatakeyama M, Belleannée G, Mégraud F and
Varon C: Helicobacter pylori generates cells with cancer stem cell
properties via epithelial-mesenchymal transition-like changes.
Oncogene. 33:4123–4131. 2014. View Article : Google Scholar
|
|
4
|
Herrero R, Park JY and Forman D: The fight
against gastric cancer-the IARC Working Group report. Best Pract
Res Clin Gastroenterol. 28:1107–1114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Stoecklein NH, Lin PP and Gires O:
Circulating and disseminated tumor cells: Diagnostic tools and
therapeutic targets in motion. Oncotarget. 8:1884–1912. 2017.
View Article : Google Scholar :
|
|
6
|
Alix-Panabieres C, Riethdorf S and Pantel
K: Circulating tumor cells and bone marrow micrometastasis. Clin
Cancer Res. 14:5013–5021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joosse SA, Gorges TM and Pantel K:
Biology, detection, and clinical implications of circulating tumor
cells. EMBO Mol Med. 7:1–11. 2015. View Article : Google Scholar :
|
|
8
|
Lin H, Balic M, Zheng S, Datar R and Cote
RJ: Disseminated and circulating tumor cells: Role in effective
cancer management. Crit Rev Oncol Hematol. 77:1–11. 2011.
View Article : Google Scholar
|
|
9
|
Riethdorf S, Wikman H and Pantel K:
Review: Biological relevance of disseminated tumor cells in cancer
patients. Int J Cancer. 123:1991–2006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Messaritakis I, Politaki E, Kotsakis A,
Dermitzaki EK, Koinis F, Lagoudaki E, Koutsopoulos A, Kallergi G,
Souglakos J and Georgoulias V: Phenotypic characterization of
circulating tumor cells in the peripheral blood of patients with
small cell lung cancer. PLoS One. 12:e01812112017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tinhofer I, Saki M, Niehr F, Keilholz U
and Budach V: Cancer stem cell characteristics of circulating tumor
cells. Int J Radiat Biol. 90:622–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wülfing P, Borchard J, Buerger H, Heidl S,
Zänker KS, Kiesel L and Brandt B: HER2-positive circulating tumor
cells indicate poor clinical outcome in stage I to III breast
cancer patients. Clin Cancer Res. 12:1715–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Skelley AM, Merdek KD, Sprott KM,
Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP and Smirnov DA:
Microfluidics and circulating tumor cells. J Mol Diagn. 15:149–157.
2013. View Article : Google Scholar
|
|
15
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller MC, Doyle GV and Terstappen LW:
Significance of circulating tumor cells detected by the cellsearch
system in patients with metastatic breast colorectal and prostate
cancer. J Oncol. 2010:6174212010. View Article : Google Scholar
|
|
17
|
Riethdorf S, Fritsche H, Müller V, Rau T,
Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, et al:
Significance of circulating tumor cells detected by the cellsearch
system in patients with metastatic breast colorectal and prostate
cancer. Detection of circulating tumor cells in peripheral blood of
patients with metastatic breast cancer: A validation study of the
CellSearch system. Clin Cancer Res. 13:920–928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang C, Zou K, Yuan Z, Guo T and Xiong B:
Prognostic value of circulating tumor cells detected with the
CellSearch system in patients with gastric cancer: Evidence from a
meta-analysis. Onco Targets Ther. 11:1013–1023. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soltani S, Mokarian F and Panjehpour M:
The expression of CK-19 gene in circulating tumor cells of blood
samples of metastatic breast cancer women. Res Pharm Sci.
10:485–496. 2015.
|
|
20
|
Andergassen U, Kolbl AC, Hutter S, Friese
K and Jeschke U: Detection of circulating tumour cells from blood
of breast cancer patients via RT-qPCR. Cancers (Basel).
5:1212–1220. 2013. View Article : Google Scholar
|
|
21
|
Zhao S, Yang H, Zhang M, Zhang D, Liu Y,
Liu Y, Song Y, Zhang X, Li H, Ma W and Zhang Q: Circulating tumor
cells (CTCs) detected by triple-marker EpCAM, CK19, and hMAM RT-PCR
and their relation to clinical outcome in metastatic breast cancer
patients. Cell Biochem Biophys. 65:263–273. 2013. View Article : Google Scholar
|
|
22
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grover PK, Cummins AG, Price TJ,
Roberts-Thomson IC and Hardingham JE: Circulating tumour cells: The
evolving concept and the inadequacy of their enrichment by
EpCAM-based meth-odology for basic and clinical cancer research.
Ann Oncol. 25:1506–1516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iinuma H, Watanabe T, Mimori K, Adachi M,
Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M
and Mori M: Clinical significance of circulating tumor cells,
including cancer stem-like cells, in peripheral blood for
recurrence and prognosis in patients with Dukes' stage B and C
colorectal cancer. J Clin Oncol. 29:1547–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kasimir-Bauer S, Hoffmann O, Wallwiener D,
Kimmig R and Fehm T: Expression of stem cell and
epithelial-mesenchymal transition markers in primary breast cancer
patients with circulating tumor cells. Breast Cancer Res.
14:R152012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu
SJ, Shi RY, Hu B, Zhou J and Fan J: Circulating stem cell-like
epithelial cell adhesion molecule-positive tumor cells indicate
poor prognosis of hepatocellular carcinoma after curative
resection. Hepatology. 57:1458–1468. 2013. View Article : Google Scholar
|
|
27
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moreno-Bueno G, Portillo F and Cano A:
Transcriptional regulation of cell polarity in EMT and cancer.
Oncogene. 27:6958–6969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singhal A, Deymier-Black AC, Almer JD and
Dunand DC: Effect of high-energy X-ray doses on bone elastic
properties and residual strains. J Mech Behav Biomed Mater.
4:1774–1786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eckert MA, Lwin TM, Chang AT, Kim J, Danis
E, Ohno-Machado L and Yang J: Twist1-induced invadopodia formation
promotes tumor metastasis. Cancer Cell. 19:372–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christie MJ, Bridge S, James LB and Beart
PM: Excitotoxin lesions suggest an aspartatergic projection from
rat medial prefrontal cortex to ventral tegmental area. Brain Res.
333:169–172. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Valcourt U, Carthy J, Okita Y, Alcaraz L,
Kato M, Thuault S, Bartholin L and Moustakas A: Analysis of
epithelial-mesenchymal transition induced by transforming growth
factor β. Methods Mol Biol. 1344:147–181. 2016. View Article : Google Scholar
|
|
34
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu AN, Zhu ZH, Chang SJ and Hang XS:
Twist expression associated with the epithelial-mesenchymal
transition in gastric cancer. Mol Cell Biochem. 367:195–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shioiri M, Shida T, Koda K, Oda K, Seike
K, Nishimura M, Takano S and Miyazaki M: Slug expression is an
independent prognostic parameter for poor survival in colorectal
carcinoma patients. Br J Cancer. 94:1816–1822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uchikado Y, Natsugoe S, Okumura H,
Setoyama T, Matsumoto M, Ishigami S and Aikou T: Slug Expression in
the E-cadherin preserved tumors is related to prognosis in patients
with esophageal squamous cell carcinoma. Clin Cancer Res.
11:1174–1180. 2005.PubMed/NCBI
|
|
38
|
Castro Alves C, Rosivatz E, Schott C,
Hollweck R, Becker I, Sarbia M, Carneiro F and Becker KF: Slug is
overexpressed in gastric carcinomas and may act synergistically
with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol.
211:507–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim MA, Lee HS, Lee HE, Kim JH, Yang HK
and Kim WH: Prognostic importance of epithelial-mesenchymal
transition-related protein expression in gastric carcinoma.
Histopathology. 54:442–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer stem cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Banerjee A, Thamphiwatana S, Carmona EM,
Rickman B, Doran KS and Obonyo M: Deficiency of the myeloid
differentiation primary response molecule MyD88 leads to an early
and rapid development of Helicobacter-induced gastric malignancy.
Infect Immun. 82:356–363. 2014. View Article : Google Scholar :
|
|
42
|
Lozano-Pope I, Sharma A, Matthias M, Doran
KS and Obonyo M: Effect of myeloid differentiation primary response
gene 88 on expression profiles of genes during the development and
progression of Helicobacter-induced gastric cancer. BMC Cancer.
17:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Obonyo M, Rickman B and Guiney DG: Effects
of myeloid differentiation primary response gene 88 (MyD88)
activation on Helicobacter infection in vivo and induction of a
Th17 response. Helicobacter. 16:398–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Obonyo M, Sabet M, Cole SP, Ebmeyer J,
Uematsu S, Akira S and Guiney DG: Deficiencies of myeloid
differentiation factor 88, Toll-like receptor 2 (TLR2), or TLR4
produce specific defects in macrophage cytokine secretion induced
by Helicobacter pylori. Infect Immun. 75:2408–2414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Obonyo M, Cole SP, Datta SK and Guiney DG:
Evidence for interleukin-1-independent stimulation of
interleukin-12 and down-regulation by interleukin-10 in
Helicobacter pylori-infected murine dendritic cells deficient in
the interleukin-1 receptor. FEMS Immunol Med Microbiol. 47:414–419.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thamphiwatana S, Gao W, Obonyo M and Zhang
L: In vivo treatment of Helicobacter pylori infection with
liposomal lino-lenic acid reduces colonization and ameliorates
inflammation. Proc Natl Acad Sci USA. 111:17600–17605. 2014.
View Article : Google Scholar
|
|
47
|
Edling CE and Hallberg B: c-Kit-a
hematopoietic cell essential receptor tyrosine kinase. Int J
Biochem Cell Biol. 39:1995–1998. 2007. View Article : Google Scholar
|
|
48
|
Escribano L, Ocqueteau M, Almeida J, Orfao
A and San Miguel JF: Expression of the c-kit (CD117) molecule in
normal and malignant hematopoiesis. Leuk Lymphoma. 30:459–466.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang B, Chen Q, Cao Y, Ma X, Yin C, Jia Y,
Zang A and Fan W: LGR5 is a gastric cancer stem cell marker
associated with stemness and the EMT signature genes NANOG,
NANOGP8, PRRX1, TWIST1, and BMI1. PLoS One. 11:e01689042016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoffmann W: Current status on stem cells
and cancers of the gastric epithelium. Int J Mol Sci.
16:19153–19169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Braun S and Naume B: Circulating and
disseminated tumor cells. J Clin Oncol. 23:1623–1626. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dardaei L, Shahsavani R, Ghavamzadeh A,
Behmanesh M, Aslankoohi E, Alimoghaddam K and Ghaffari SH: The
detection of disseminated tumor cells in bone marrow and peripheral
blood of gastric cancer patients by multimarker (CEA, CK20, TFF1
and MUC2) quantitative real-time PCR. Clin Biochem. 44:325–330.
2011. View Article : Google Scholar
|
|
54
|
Moll R, Divo M and Langbein L: The human
keratins: Biology and pathology. Histochem Cell Biol. 129:705–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tuffaha MSA, Guski H and Kristiansen G:
Immunohistochemistry in Tumor Diagnostics. Springer; Cham, New
York, NY: pp. 49–58. 2018, View Article : Google Scholar
|
|
56
|
Guang W, Czinn SJ, Blanchard TG, Kim KC
and Lillehoj EP: Genetic regulation of MUC1 expression by
Helicobacter pylori in gastric cancer cells. Biochem Biophys Res
Commun. 445:145–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ng GZ, Menheniott TR, Every AL, Stent A,
Judd LM, Chionh YT, Dhar P, Komen JC, Giraud AS, Wang TC, et al:
The MUC1 mucin protects against Helicobacter pylori pathogenesis in
mice by regulation of the NLRP3 inflammasome. Gut. 65:1087–1099.
2016. View Article : Google Scholar
|
|
58
|
Linden SK, Sheng YH, Every AL, Miles KM,
Skoog EC, Florin TH, Sutton P and McGuckin MA: MUC1 limits
Helicobacter pylori infection both by steric hindrance and by
acting as a releasable decoy. PLoS Pathog. 5:e10006172009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
McGuckin MA, Every AL, Skene CD, Linden
SK, Chionh YT, Swierczak A, McAuley J, Harbour S, Kaparakis M,
Ferrero R and Sutton P: Muc1 mucin limits both Helicobacter pylori
colonization of the murine gastric mucosa and associated gastritis.
Gastroenterology. 133:1210–1218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kharbanda A, Rajabi H, Jin C, Tchaicha J,
Kikuchi E, Wong KK and Kufe D: Targeting the oncogenic MUC1-C
protein inhibits mutant EGFR-mediated signaling and survival in
non-small cell lung cancer cells. Clin Cancer Res. 20:5423–5434.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ross JS and Slodkowska EA: Circulating and
disseminated tumor cells in the management of breast cancer. Am J
Clin Pathol. 132:237–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kodack DP, Farago AF, Dastur A, Held MA,
Dardaei L, Friboulet L, von Flotow F, Damon LJ, Lee D, Parks M, et
al: Primary patient-derived cancer cells and their potential for
personalized cancer patient care. Cell Rep. 21:3298–3309. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee HS, Lee HK, Kim HS, Yang HK, Kim YI
and Kim WH: MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric
carcinomas: Their roles as prognostic indicators. Cancer.
92:1427–1434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang HK, Zhang QM, Zhao TH, Li YY and Yi
YF: Expression of mucins and E-cadherin in gastric carcinoma and
their clinical significance. World J Gastroenterol. 10:3044–3047.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
von Mensdorff-Pouilly S, Snijdewint FG,
Verstraeten AA, Verheijen RH and Kenemans P: Human MUC1 mucin: A
multi-faceted glycoprotein. Int J Biol Markers. 15:343–356. 2000.
View Article : Google Scholar
|
|
66
|
Deng M, Qin Y, Chen X, Wang Q and Wang J:
MiR-206 inhibits proliferation, migration, and invasion of gastric
cancer cells by targeting the MUC1 gene. Onco Targets Ther.
12:849–859. 2019. View Article : Google Scholar :
|
|
67
|
Gnemmi V, Bouillez A, Gaudelot K, Hémon B,
Ringot B, Pottier N, Glowacki F, Villers A, Vindrieux D, Cauffiez
C, et al: MUC1 drives epithelial-mesenchymal transition in renal
carcinoma through Wnt/β-catenin pathway and interaction with SNAIL
promoter. Cancer Lett. 346:225–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jung H, Kim B, Moon BI and Oh ES:
Cytokeratin 18 is necessary for initiation of TGF-β1-induced
epithelial-mesenchymal transition in breast epithelial cells. Mol
Cell Biochem. 423:21–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Okabe H, Ishimoto T, Mima K, Nakagawa S,
Hayashi H, Kuroki H, Imai K, Nitta H, Saito S, Hashimoto D, et al:
CD44s signals the acquisition of the mesenchymal phenotype required
for anchorage-independent cell survival in hepatocellular
carcinoma. Br J Cancer. 110:958–966. 2014. View Article : Google Scholar :
|
|
72
|
Satelli A, Brownlee Z, Mitra A, Meng QH
and Li S: Circulating tumor cell enumeration with a combination of
epithelial cell adhesion molecule- and cell-surface vimentin-based
methods for monitoring breast cancer therapeutic response. Clin
Chem. 61:259–266. 2015. View Article : Google Scholar
|
|
73
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kletukhina S, Neustroeva O, James V,
Rizvanov A and Gomzikova M: Role of mesenchymal stem cell-derived
extracellular vesicles in epithelial-mesenchymal transition. Int J
Mol Sci. 20:48132019. View Article : Google Scholar
|
|
75
|
Rattigan Y, Hsu JM, Mishra PJ, Glod J and
Banerjee D: Interleukin 6 mediated recruitment of mesenchymal stem
cells to the hypoxic tumor milieu. Exp Cell Res. 316:3417–3424.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Soen B, Vandamme N, Berx G, Schwaller J
and Vlierberghe PV: ZEB proteins in leukemia: Friends, Foes, or
Friendly Foes? HemeSphere. 2:e432018. View Article : Google Scholar
|
|
81
|
Pantel K and Alix-Panabieres C:
Circulating tumour cells in cancer patients: Challenges and
perspectives. Trends Mol Med. 16:398–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang ZY and Ge HY: Micrometastasis in
gastric cancer. Cancer Lett. 336:34–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bock C, Rack B, Huober J, Andergassen U,
Jeschke U and Doisneau-Sixou S: Distinct expression of cytokeratin,
N-cadherin and CD133 in circulating tumor cells of metastatic
breast cancer patients. Future Oncol. 10:1751–1765. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen Q, Cao HZ and Zheng PS: LGR5 promotes
the proliferation and tumor formation of cervical cancer cells
through the Wnt/β-catenin signaling pathway. Oncotarget.
5:9092–9105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ishimoto T, Oshima H, Oshima M, Kai K,
Torii R, Masuko T, Baba H, Saya H and Nagano O: CD44+
slow-cycling tumor cell expansion is triggered by cooperative
actions of Wnt and pros-taglandin E2 in gastric tumorigenesis.
Cancer Sci. 101:673–678. 2010. View Article : Google Scholar
|
|
86
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: Migrating cancer stem cells-an integrated
concept of malignant tumour progression. Nat Rev Cancer. 5:744–749.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hollier BG, Evans K and Mani SA: The
epithelial-to-mesenchymal transition and cancer stem cells: A
coalition against cancer therapies. J Mammary Gland Biol Neoplasia.
14:29–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jeter CR, Yang T, Wang J, Chao HP and Tang
DG: Concise review: NANOG in cancer stem cells and tumor
development: An update and outstanding questions. Stem Cells.
33:2381–2390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Torres-Padilla ME and Chambers I:
Transcription factor heterogeneity in pluripotent stem cells: A
stochastic advantage. Development. 141:2173–2181. 2014. View Article : Google Scholar : PubMed/NCBI
|