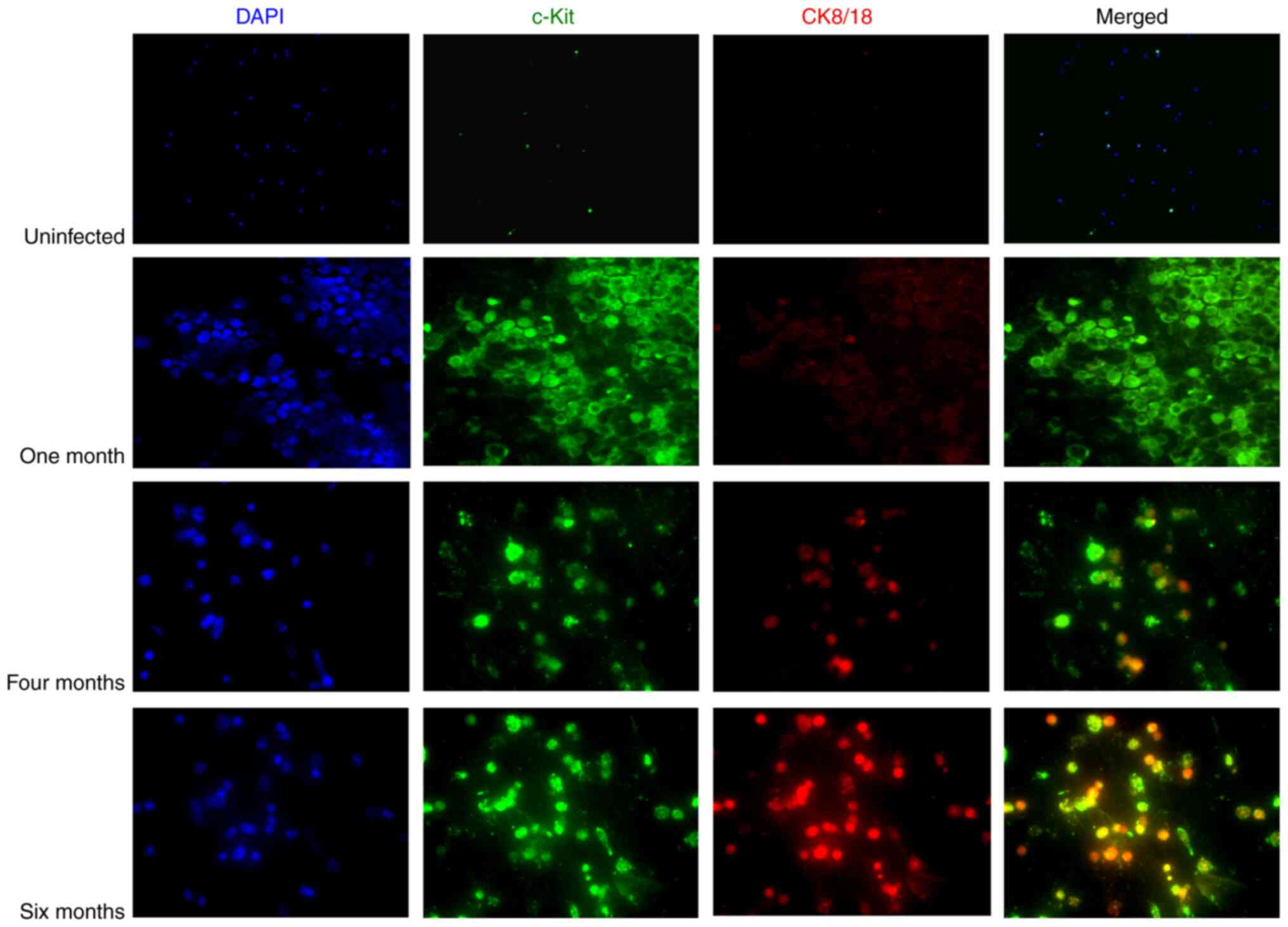
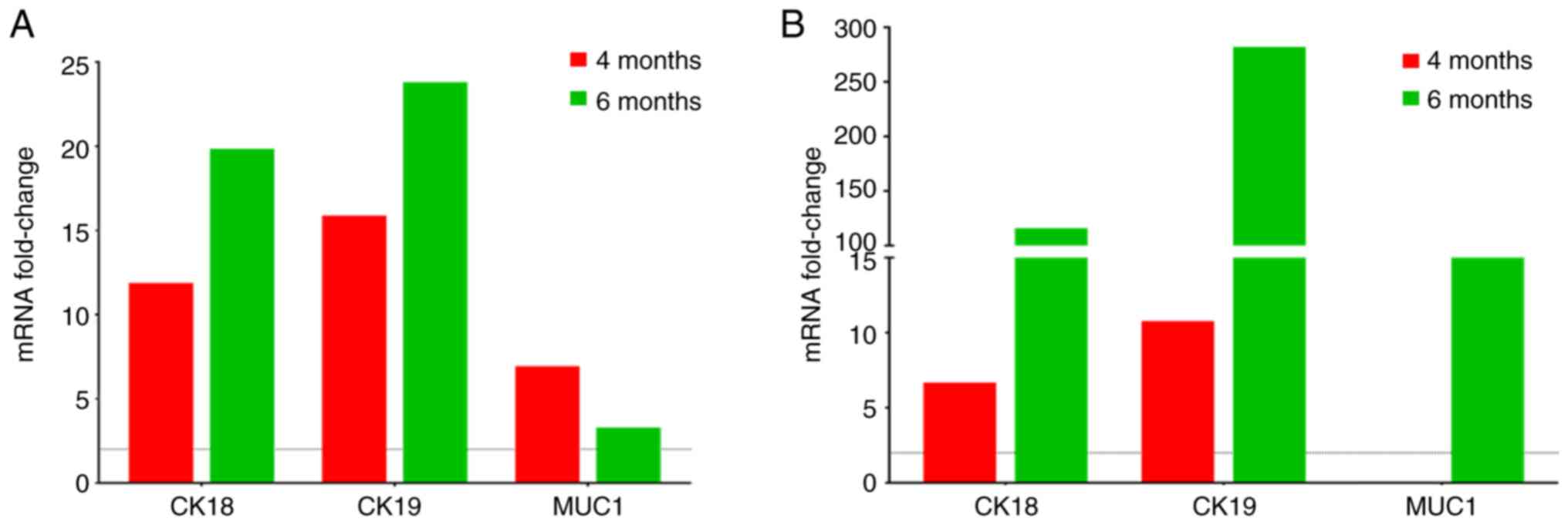
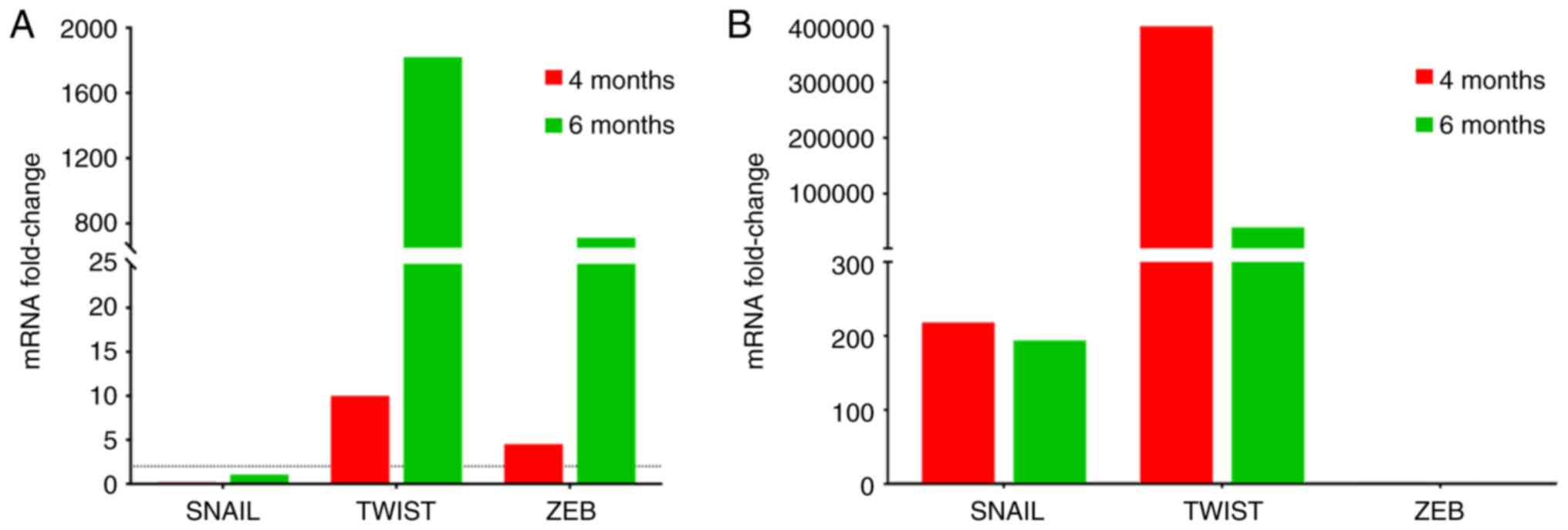
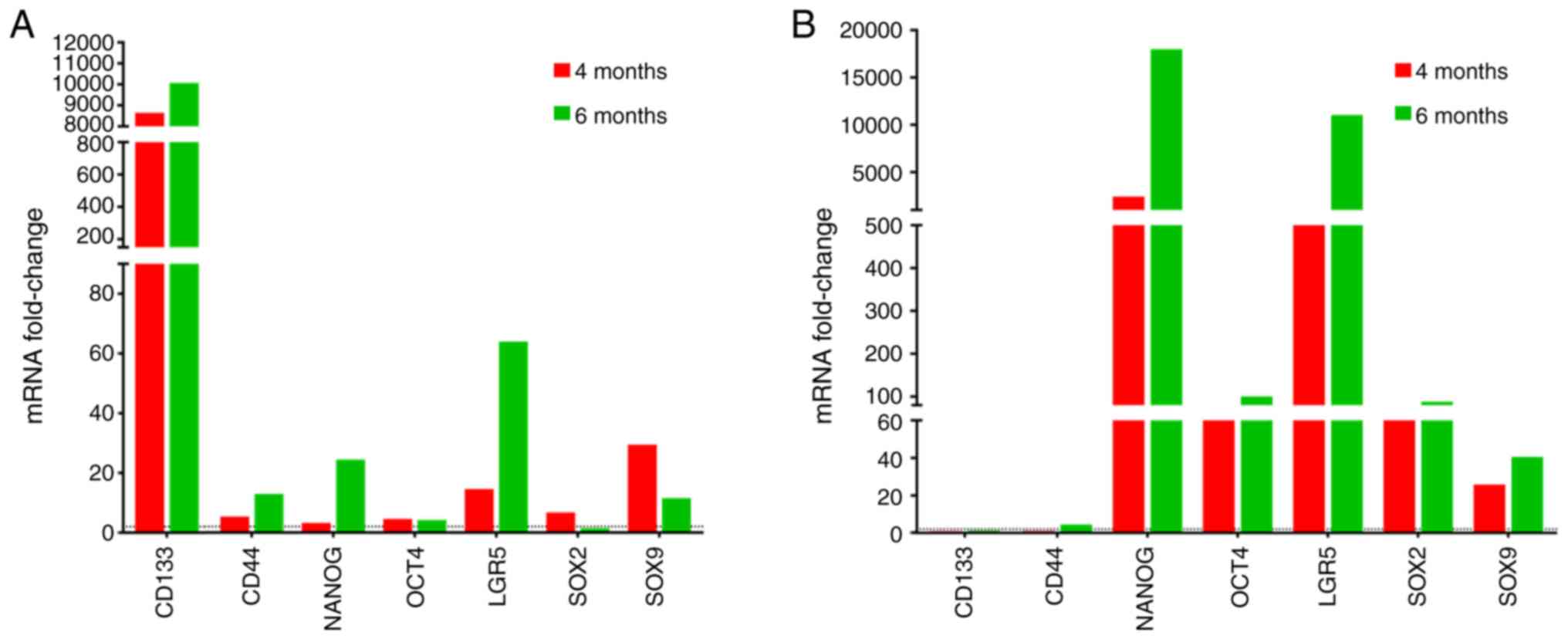
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar
|
|
2
|
Bessede E, Dubus P, Megraud F and Varon C:
Helicobacter pylori infection and stem cells at the origin of
gastric cancer. Oncogene. 34:2547–2555. 2015. View Article : Google Scholar
|
|
3
|
Bessède E, Staedel C, Acuña Amador LA,
Nguyen PH, Chambonnier L, Hatakeyama M, Belleannée G, Mégraud F and
Varon C: Helicobacter pylori generates cells with cancer stem cell
properties via epithelial-mesenchymal transition-like changes.
Oncogene. 33:4123–4131. 2014. View Article : Google Scholar
|
|
4
|
Herrero R, Park JY and Forman D: The fight
against gastric cancer-the IARC Working Group report. Best Pract
Res Clin Gastroenterol. 28:1107–1114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Stoecklein NH, Lin PP and Gires O:
Circulating and disseminated tumor cells: Diagnostic tools and
therapeutic targets in motion. Oncotarget. 8:1884–1912. 2017.
View Article : Google Scholar :
|
|
6
|
Alix-Panabieres C, Riethdorf S and Pantel
K: Circulating tumor cells and bone marrow micrometastasis. Clin
Cancer Res. 14:5013–5021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joosse SA, Gorges TM and Pantel K:
Biology, detection, and clinical implications of circulating tumor
cells. EMBO Mol Med. 7:1–11. 2015. View Article : Google Scholar :
|
|
8
|
Lin H, Balic M, Zheng S, Datar R and Cote
RJ: Disseminated and circulating tumor cells: Role in effective
cancer management. Crit Rev Oncol Hematol. 77:1–11. 2011.
View Article : Google Scholar
|
|
9
|
Riethdorf S, Wikman H and Pantel K:
Review: Biological relevance of disseminated tumor cells in cancer
patients. Int J Cancer. 123:1991–2006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Messaritakis I, Politaki E, Kotsakis A,
Dermitzaki EK, Koinis F, Lagoudaki E, Koutsopoulos A, Kallergi G,
Souglakos J and Georgoulias V: Phenotypic characterization of
circulating tumor cells in the peripheral blood of patients with
small cell lung cancer. PLoS One. 12:e01812112017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tinhofer I, Saki M, Niehr F, Keilholz U
and Budach V: Cancer stem cell characteristics of circulating tumor
cells. Int J Radiat Biol. 90:622–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wülfing P, Borchard J, Buerger H, Heidl S,
Zänker KS, Kiesel L and Brandt B: HER2-positive circulating tumor
cells indicate poor clinical outcome in stage I to III breast
cancer patients. Clin Cancer Res. 12:1715–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Skelley AM, Merdek KD, Sprott KM,
Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP and Smirnov DA:
Microfluidics and circulating tumor cells. J Mol Diagn. 15:149–157.
2013. View Article : Google Scholar
|
|
15
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller MC, Doyle GV and Terstappen LW:
Significance of circulating tumor cells detected by the cellsearch
system in patients with metastatic breast colorectal and prostate
cancer. J Oncol. 2010:6174212010. View Article : Google Scholar
|
|
17
|
Riethdorf S, Fritsche H, Müller V, Rau T,
Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, et al:
Significance of circulating tumor cells detected by the cellsearch
system in patients with metastatic breast colorectal and prostate
cancer. Detection of circulating tumor cells in peripheral blood of
patients with metastatic breast cancer: A validation study of the
CellSearch system. Clin Cancer Res. 13:920–928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang C, Zou K, Yuan Z, Guo T and Xiong B:
Prognostic value of circulating tumor cells detected with the
CellSearch system in patients with gastric cancer: Evidence from a
meta-analysis. Onco Targets Ther. 11:1013–1023. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soltani S, Mokarian F and Panjehpour M:
The expression of CK-19 gene in circulating tumor cells of blood
samples of metastatic breast cancer women. Res Pharm Sci.
10:485–496. 2015.
|
|
20
|
Andergassen U, Kolbl AC, Hutter S, Friese
K and Jeschke U: Detection of circulating tumour cells from blood
of breast cancer patients via RT-qPCR. Cancers (Basel).
5:1212–1220. 2013. View Article : Google Scholar
|
|
21
|
Zhao S, Yang H, Zhang M, Zhang D, Liu Y,
Liu Y, Song Y, Zhang X, Li H, Ma W and Zhang Q: Circulating tumor
cells (CTCs) detected by triple-marker EpCAM, CK19, and hMAM RT-PCR
and their relation to clinical outcome in metastatic breast cancer
patients. Cell Biochem Biophys. 65:263–273. 2013. View Article : Google Scholar
|
|
22
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grover PK, Cummins AG, Price TJ,
Roberts-Thomson IC and Hardingham JE: Circulating tumour cells: The
evolving concept and the inadequacy of their enrichment by
EpCAM-based meth-odology for basic and clinical cancer research.
Ann Oncol. 25:1506–1516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iinuma H, Watanabe T, Mimori K, Adachi M,
Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M
and Mori M: Clinical significance of circulating tumor cells,
including cancer stem-like cells, in peripheral blood for
recurrence and prognosis in patients with Dukes' stage B and C
colorectal cancer. J Clin Oncol. 29:1547–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kasimir-Bauer S, Hoffmann O, Wallwiener D,
Kimmig R and Fehm T: Expression of stem cell and
epithelial-mesenchymal transition markers in primary breast cancer
patients with circulating tumor cells. Breast Cancer Res.
14:R152012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu
SJ, Shi RY, Hu B, Zhou J and Fan J: Circulating stem cell-like
epithelial cell adhesion molecule-positive tumor cells indicate
poor prognosis of hepatocellular carcinoma after curative
resection. Hepatology. 57:1458–1468. 2013. View Article : Google Scholar
|
|
27
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moreno-Bueno G, Portillo F and Cano A:
Transcriptional regulation of cell polarity in EMT and cancer.
Oncogene. 27:6958–6969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singhal A, Deymier-Black AC, Almer JD and
Dunand DC: Effect of high-energy X-ray doses on bone elastic
properties and residual strains. J Mech Behav Biomed Mater.
4:1774–1786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eckert MA, Lwin TM, Chang AT, Kim J, Danis
E, Ohno-Machado L and Yang J: Twist1-induced invadopodia formation
promotes tumor metastasis. Cancer Cell. 19:372–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christie MJ, Bridge S, James LB and Beart
PM: Excitotoxin lesions suggest an aspartatergic projection from
rat medial prefrontal cortex to ventral tegmental area. Brain Res.
333:169–172. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Valcourt U, Carthy J, Okita Y, Alcaraz L,
Kato M, Thuault S, Bartholin L and Moustakas A: Analysis of
epithelial-mesenchymal transition induced by transforming growth
factor β. Methods Mol Biol. 1344:147–181. 2016. View Article : Google Scholar
|
|
34
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu AN, Zhu ZH, Chang SJ and Hang XS:
Twist expression associated with the epithelial-mesenchymal
transition in gastric cancer. Mol Cell Biochem. 367:195–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shioiri M, Shida T, Koda K, Oda K, Seike
K, Nishimura M, Takano S and Miyazaki M: Slug expression is an
independent prognostic parameter for poor survival in colorectal
carcinoma patients. Br J Cancer. 94:1816–1822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uchikado Y, Natsugoe S, Okumura H,
Setoyama T, Matsumoto M, Ishigami S and Aikou T: Slug Expression in
the E-cadherin preserved tumors is related to prognosis in patients
with esophageal squamous cell carcinoma. Clin Cancer Res.
11:1174–1180. 2005.PubMed/NCBI
|
|
38
|
Castro Alves C, Rosivatz E, Schott C,
Hollweck R, Becker I, Sarbia M, Carneiro F and Becker KF: Slug is
overexpressed in gastric carcinomas and may act synergistically
with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol.
211:507–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim MA, Lee HS, Lee HE, Kim JH, Yang HK
and Kim WH: Prognostic importance of epithelial-mesenchymal
transition-related protein expression in gastric carcinoma.
Histopathology. 54:442–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer stem cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Banerjee A, Thamphiwatana S, Carmona EM,
Rickman B, Doran KS and Obonyo M: Deficiency of the myeloid
differentiation primary response molecule MyD88 leads to an early
and rapid development of Helicobacter-induced gastric malignancy.
Infect Immun. 82:356–363. 2014. View Article : Google Scholar :
|
|
42
|
Lozano-Pope I, Sharma A, Matthias M, Doran
KS and Obonyo M: Effect of myeloid differentiation primary response
gene 88 on expression profiles of genes during the development and
progression of Helicobacter-induced gastric cancer. BMC Cancer.
17:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Obonyo M, Rickman B and Guiney DG: Effects
of myeloid differentiation primary response gene 88 (MyD88)
activation on Helicobacter infection in vivo and induction of a
Th17 response. Helicobacter. 16:398–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Obonyo M, Sabet M, Cole SP, Ebmeyer J,
Uematsu S, Akira S and Guiney DG: Deficiencies of myeloid
differentiation factor 88, Toll-like receptor 2 (TLR2), or TLR4
produce specific defects in macrophage cytokine secretion induced
by Helicobacter pylori. Infect Immun. 75:2408–2414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Obonyo M, Cole SP, Datta SK and Guiney DG:
Evidence for interleukin-1-independent stimulation of
interleukin-12 and down-regulation by interleukin-10 in
Helicobacter pylori-infected murine dendritic cells deficient in
the interleukin-1 receptor. FEMS Immunol Med Microbiol. 47:414–419.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thamphiwatana S, Gao W, Obonyo M and Zhang
L: In vivo treatment of Helicobacter pylori infection with
liposomal lino-lenic acid reduces colonization and ameliorates
inflammation. Proc Natl Acad Sci USA. 111:17600–17605. 2014.
View Article : Google Scholar
|
|
47
|
Edling CE and Hallberg B: c-Kit-a
hematopoietic cell essential receptor tyrosine kinase. Int J
Biochem Cell Biol. 39:1995–1998. 2007. View Article : Google Scholar
|
|
48
|
Escribano L, Ocqueteau M, Almeida J, Orfao
A and San Miguel JF: Expression of the c-kit (CD117) molecule in
normal and malignant hematopoiesis. Leuk Lymphoma. 30:459–466.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang B, Chen Q, Cao Y, Ma X, Yin C, Jia Y,
Zang A and Fan W: LGR5 is a gastric cancer stem cell marker
associated with stemness and the EMT signature genes NANOG,
NANOGP8, PRRX1, TWIST1, and BMI1. PLoS One. 11:e01689042016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoffmann W: Current status on stem cells
and cancers of the gastric epithelium. Int J Mol Sci.
16:19153–19169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Braun S and Naume B: Circulating and
disseminated tumor cells. J Clin Oncol. 23:1623–1626. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dardaei L, Shahsavani R, Ghavamzadeh A,
Behmanesh M, Aslankoohi E, Alimoghaddam K and Ghaffari SH: The
detection of disseminated tumor cells in bone marrow and peripheral
blood of gastric cancer patients by multimarker (CEA, CK20, TFF1
and MUC2) quantitative real-time PCR. Clin Biochem. 44:325–330.
2011. View Article : Google Scholar
|
|
54
|
Moll R, Divo M and Langbein L: The human
keratins: Biology and pathology. Histochem Cell Biol. 129:705–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tuffaha MSA, Guski H and Kristiansen G:
Immunohistochemistry in Tumor Diagnostics. Springer; Cham, New
York, NY: pp. 49–58. 2018, View Article : Google Scholar
|
|
56
|
Guang W, Czinn SJ, Blanchard TG, Kim KC
and Lillehoj EP: Genetic regulation of MUC1 expression by
Helicobacter pylori in gastric cancer cells. Biochem Biophys Res
Commun. 445:145–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ng GZ, Menheniott TR, Every AL, Stent A,
Judd LM, Chionh YT, Dhar P, Komen JC, Giraud AS, Wang TC, et al:
The MUC1 mucin protects against Helicobacter pylori pathogenesis in
mice by regulation of the NLRP3 inflammasome. Gut. 65:1087–1099.
2016. View Article : Google Scholar
|
|
58
|
Linden SK, Sheng YH, Every AL, Miles KM,
Skoog EC, Florin TH, Sutton P and McGuckin MA: MUC1 limits
Helicobacter pylori infection both by steric hindrance and by
acting as a releasable decoy. PLoS Pathog. 5:e10006172009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
McGuckin MA, Every AL, Skene CD, Linden
SK, Chionh YT, Swierczak A, McAuley J, Harbour S, Kaparakis M,
Ferrero R and Sutton P: Muc1 mucin limits both Helicobacter pylori
colonization of the murine gastric mucosa and associated gastritis.
Gastroenterology. 133:1210–1218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kharbanda A, Rajabi H, Jin C, Tchaicha J,
Kikuchi E, Wong KK and Kufe D: Targeting the oncogenic MUC1-C
protein inhibits mutant EGFR-mediated signaling and survival in
non-small cell lung cancer cells. Clin Cancer Res. 20:5423–5434.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ross JS and Slodkowska EA: Circulating and
disseminated tumor cells in the management of breast cancer. Am J
Clin Pathol. 132:237–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kodack DP, Farago AF, Dastur A, Held MA,
Dardaei L, Friboulet L, von Flotow F, Damon LJ, Lee D, Parks M, et
al: Primary patient-derived cancer cells and their potential for
personalized cancer patient care. Cell Rep. 21:3298–3309. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee HS, Lee HK, Kim HS, Yang HK, Kim YI
and Kim WH: MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric
carcinomas: Their roles as prognostic indicators. Cancer.
92:1427–1434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang HK, Zhang QM, Zhao TH, Li YY and Yi
YF: Expression of mucins and E-cadherin in gastric carcinoma and
their clinical significance. World J Gastroenterol. 10:3044–3047.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
von Mensdorff-Pouilly S, Snijdewint FG,
Verstraeten AA, Verheijen RH and Kenemans P: Human MUC1 mucin: A
multi-faceted glycoprotein. Int J Biol Markers. 15:343–356. 2000.
View Article : Google Scholar
|
|
66
|
Deng M, Qin Y, Chen X, Wang Q and Wang J:
MiR-206 inhibits proliferation, migration, and invasion of gastric
cancer cells by targeting the MUC1 gene. Onco Targets Ther.
12:849–859. 2019. View Article : Google Scholar :
|
|
67
|
Gnemmi V, Bouillez A, Gaudelot K, Hémon B,
Ringot B, Pottier N, Glowacki F, Villers A, Vindrieux D, Cauffiez
C, et al: MUC1 drives epithelial-mesenchymal transition in renal
carcinoma through Wnt/β-catenin pathway and interaction with SNAIL
promoter. Cancer Lett. 346:225–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jung H, Kim B, Moon BI and Oh ES:
Cytokeratin 18 is necessary for initiation of TGF-β1-induced
epithelial-mesenchymal transition in breast epithelial cells. Mol
Cell Biochem. 423:21–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Okabe H, Ishimoto T, Mima K, Nakagawa S,
Hayashi H, Kuroki H, Imai K, Nitta H, Saito S, Hashimoto D, et al:
CD44s signals the acquisition of the mesenchymal phenotype required
for anchorage-independent cell survival in hepatocellular
carcinoma. Br J Cancer. 110:958–966. 2014. View Article : Google Scholar :
|
|
72
|
Satelli A, Brownlee Z, Mitra A, Meng QH
and Li S: Circulating tumor cell enumeration with a combination of
epithelial cell adhesion molecule- and cell-surface vimentin-based
methods for monitoring breast cancer therapeutic response. Clin
Chem. 61:259–266. 2015. View Article : Google Scholar
|
|
73
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kletukhina S, Neustroeva O, James V,
Rizvanov A and Gomzikova M: Role of mesenchymal stem cell-derived
extracellular vesicles in epithelial-mesenchymal transition. Int J
Mol Sci. 20:48132019. View Article : Google Scholar
|
|
75
|
Rattigan Y, Hsu JM, Mishra PJ, Glod J and
Banerjee D: Interleukin 6 mediated recruitment of mesenchymal stem
cells to the hypoxic tumor milieu. Exp Cell Res. 316:3417–3424.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Soen B, Vandamme N, Berx G, Schwaller J
and Vlierberghe PV: ZEB proteins in leukemia: Friends, Foes, or
Friendly Foes? HemeSphere. 2:e432018. View Article : Google Scholar
|
|
81
|
Pantel K and Alix-Panabieres C:
Circulating tumour cells in cancer patients: Challenges and
perspectives. Trends Mol Med. 16:398–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang ZY and Ge HY: Micrometastasis in
gastric cancer. Cancer Lett. 336:34–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bock C, Rack B, Huober J, Andergassen U,
Jeschke U and Doisneau-Sixou S: Distinct expression of cytokeratin,
N-cadherin and CD133 in circulating tumor cells of metastatic
breast cancer patients. Future Oncol. 10:1751–1765. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen Q, Cao HZ and Zheng PS: LGR5 promotes
the proliferation and tumor formation of cervical cancer cells
through the Wnt/β-catenin signaling pathway. Oncotarget.
5:9092–9105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ishimoto T, Oshima H, Oshima M, Kai K,
Torii R, Masuko T, Baba H, Saya H and Nagano O: CD44+
slow-cycling tumor cell expansion is triggered by cooperative
actions of Wnt and pros-taglandin E2 in gastric tumorigenesis.
Cancer Sci. 101:673–678. 2010. View Article : Google Scholar
|
|
86
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: Migrating cancer stem cells-an integrated
concept of malignant tumour progression. Nat Rev Cancer. 5:744–749.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hollier BG, Evans K and Mani SA: The
epithelial-to-mesenchymal transition and cancer stem cells: A
coalition against cancer therapies. J Mammary Gland Biol Neoplasia.
14:29–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jeter CR, Yang T, Wang J, Chao HP and Tang
DG: Concise review: NANOG in cancer stem cells and tumor
development: An update and outstanding questions. Stem Cells.
33:2381–2390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Torres-Padilla ME and Chambers I:
Transcription factor heterogeneity in pluripotent stem cells: A
stochastic advantage. Development. 141:2173–2181. 2014. View Article : Google Scholar : PubMed/NCBI
|