Introduction
Cancer is one of the most prevalent public health
issues and a lethal disease worldwide, posing a serious hazard to
the public (1,2). Based on statistics data available
from the Global Burden of Diseases, in 2018, ~18,100,000 new cancer
cases were diagnosed globally, and nearly a quarter of new cancer
cases in women worldwide are breast cancer (3). Furthermore, breast cancer has become
the most common malignancy in Chinese women, with an estimated
272,000 women diagnosed and ~70,000 cancer-associated deaths per
year (4,5). Alcohol abuse, obesity, a lack of
exercise, a later pregnancy or infertility and artificial feeding
have been reported to have a marked effect on breast cancer
incidence (5).
Currently, the available breast cancer therapeutic
strategies include surgery, chemotherapy, radiotherapy, and their
combinations (6). However, the
therapeutic effects are still limited, particularly with
triple-negative and advanced breast cancer. Therefore, novel
therapies or new and effective drugs are urgently required for
patients with metastatic breast cancer (7). Previous studies have reported that
natural medicines could be utilized in breast cancer therapy
(8). Compounds, such as
cordycepin, quercetin, berberine, polyphenols, and neem seed oil
have shown antitumor effects in human breast cancer cells (9-13).
Studies have shown that cordycepin could induce cancer cell death
by inhibiting RNA synthesis and DNA double-strand breaks (11). Neem seed oil inhibited breast
cancer cell growth by inducing apoptosis and cell cycle arrest at
G1 stage (13).
Ampelopsis megalophylla Diels et Gilg (A.
megalophylla) is a Chinese traditional herb, which is used as a
folk medicine. Its tender leaves and stems are commonly used as a
herbal tea and to prevent hypertension, particularly in the west of
Hubei province (14).
Pharmacological research has suggested that A. megalophylla
has hypertensive, antiinflammatory, antiviral, antitumor,
hyperglycemic, antimicrobial, hepatoprotective, neuroprotective,
and antioxidant activities (15-17).
Notably, its principal effective components are flavonoids and
total flavone extract (TFE), which contains compounds, such as
ampelopsin, myricetin and myricitrin. TFE was extracted and
isolated from A. megalophylla using the percolation method
(18). Statistically, ampelopsin
inhibited cell growth and induced apoptosis in the MCF-7,
MDA-MB-231, HepG2, PC-3, EJ, A549, MG-63, HCT-116 and HCT-8 cell
lines (19-25). Notably, out previous study found
that ampelopsin induced apoptosis in the HeLa cell line by the
mitochondrial signaling pathway (15). In addition, myricetin has been
widely investigated and demonstrated to have effective anticancer
activity (cell proliferation) in several types of cancer, including
papillary thyroid, anaplastic thyroid, ovarian, colon, prostate,
breast, liver, and lung cancers (26-33).
Furthermore, a high number of flavones have been reported to
exhibit antitumor effects in different types of cancer cells
(33-38). For example, the flavonoid
components of Radix Tetrastigma Hemsleyani and Hippophae
rhamnoides have demonstrated anti-proliferative activity in
lung cancer cells (34,35). The total flavones of
Choerospondias axillaris improved
ischemia/reperfusion-induced apoptosis via the MAPK signaling
pathway (38). Based on these
reports, it has been suggested that total flavones are a promising
candidate for treating malignancies (39). We hypothesized that TFE may inhibit
tumor cell proliferation and in the present study, its antitumor
activity and underlying molecular mechanisms in breast cancer cell
lines was investigated in vitro.
Materials and methods
Chemistry and reagents
DMEM (cat. no. AC10253739), RPMI-1640 (cat. no.
AC13431275), 100 IU/ml streptomycin and 100 IU/ml penicillin (cat.
no. J180014) and FBS (cat. no. 42G9072K), were purchased from
Thermo Fisher Scientific, Inc.. Newborn bovine serum (NBS; cat. no.
11011-7811) was obtained from Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd.. The Annexin V-FITC (cat. no.
KGA108) apoptosis detection kit was procured from the Nanjing
Jiancheng Bioengineering Institute. MTT (cat. no. M-2128), Hoechst
33258, PI (cat. no. P-4170), Rhodamine 123 (Rh 123; cat. no. C2007)
were purchased from Sigma-Aldrich (Merck KGaA). Cleaved-caspase-8
(43 kDa; cat. no. ab32351), Bcl-2-associated X protein (Bax; 20
kDa; cat. no. ab25901), B-cell lymphoma-2 (Bcl-2; 26 kDa; cat. no.
ab202068), apoptotic protease activating factor-1 (Apaf-1; 135 kDa;
cat. no. ab692), caspase-9 (49 kDa; cat. no. ab32539),
cleaved-caspase-9 (37 kDa; cat. no. ab133504), and
cleaved-caspase-3 (17 kDa; cat. no. ab2000) were all purchased from
Abcam. Caspase-3 (30 kDa; cat. no. 14220T) and caspase-8 (43 kDa;
cat. no. 4790T) were all purchased from Cell Signaling Technology
Inc.. GAPDH (36 kDa; cat. no. 60004-1-Ig) was purchased from
ProteinTech Group, Inc.
Cell lines and culture
The HeLa, A549, MCF-7, A2780, SW620 cell lines, and
the liver cancer cell line, HepG2 were purchased from the China
Center for Typical Culture Collection. The MDA-MB-231 cells were
purchased from Procell Life Science and Technology Co., Ltd.. The
HeLa cells were cultured in DMEM supplemented with 10% (v/v) NBS,
100 µg/ml streptomycin, and 100 IU/ml penicillin at 37°C in
a humidified incubator with 5% CO2. The A549 cells were
maintained in RPMI-1640, supplemented with 10% (v/v) FBS, while the
MCF-7, HepG2, A2780, SW620, MDA-MB-231 cells were maintained in
DMEM supplemented with 10% (v/v) FBS.
Plant materials
A. megalophylla was collected from Laifeng
(Hubei, China), which is a county in Hubei province, rich in
natural resources and open for the public, and was identified by
Professor Xiuqiao Zhang, Department of Pharmacognosy, School of
Pharmaceutical Sciences, Hubei University of Chinese Medicine
(Wuhan, China). The plant materials were air-dried and the samples
(100 g dry weight) were soaked in 70% ethanol for 24 h at 25°C. The
crude extract was prepared using the percolation method and
collected at 2 ml/min, and the alcohol was recovered using
decompression. The residual water was volatilized, followed by
vacuum drying at 60°C. Finally, TFE was obtained (672.38 mg/g in
the preliminary study), as previously described (18).
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay.
The HeLa, A549, MCF-7, HepG2, A2780 and SW620 cells were seeded in
96-well plates, at a density of 5×103 cells per well for
24 h. TFE, at final concentrations of 0, 5, 10, 20, 40, 80, and 100
µg/ml was added to the corresponding wells and incubated for
12, 24 and 48 h. Next, 20 µl MTT (5 mg/ml) was added to each
well, incubated for 4 h, then the medium was replaced with dimethly
sulfoxide (DMSO; cat. no. RNBF8134; 100 µl/well), to
dissolve the formazan crystals. The optical density (OD) was
measured at 490 nm using a microplate reader (Bio-Rad Laboratories,
Inc.). The experiment was repeated three times. The cell viability
was calculated as cell proliferation inhibition ratio
(%)=(1-ODtreated/ODcontrol) ×100%. Cytotoxicity was expressed as
the half-maximal inhibitory concentration (IC50), which
was calculated using Excel (v2010; Microsoft Corporation), defined
as the concentration of TFE inhibiting cell proliferation by
50%.
Cell apoptosis assay
Apoptotic cells were determined using the Annexin
V-FITC apoptosis detection kit. Briefly, the MCF-7 cells were
treated with TFE (0, 5, 10, 20 and 30 µg/ml) and
5-fluorouracil (5-Fu; cat. no. E1712174) for 10 h. The cells were
collected, washed twice with cold PBS (cat. no. 8118334), then
resuspended in 500 µl binding buffer. Finally, 5 µl
Annexin V-FITC and 5 µl PI was added to the cells, then left
in the dark for 10 min at 37°C. The stained cells were analyzed
using a flow cytometer (Accuri C6) and the data was analyzed using
the FlowJo software (v10) (both from BD Biosciences).
Morphological changes in the apoptotic cells were
examined under a microscope after staining the nuclei with Hoechst
33258. After treatment with TFE (0, 5, 10, 20 and 30 µg/ml)
and 5-Fu for 10 h, the cells were washed twice with cold PBS and
fixed in methanol/acetic acid (3:1 v/v) for 13 min at room
temperature. Next, 500 µl Hoechst 33258 (5 µg/ml)
solution was added to each well and incubated in the dark for 30
min for staining at room temperature. Finally, changes in the cell
nuclei were observed and images were obtained using a
charge-coupled device camera (DP70; Olympus Corporation) attached
to a fluorescent microscope (IX51; Olympus Corporation).
Detection of the cell cycle
The MCF-7 cells were treated as aforementioned. To
analyze the cell cycle, the cells were washed twice with PBS and
fixed with cold 75% ethanol over-night at −20°C. The cells were
then labeled with PI (50 µg/ml) in the presence of 0.1%
RNAse A for 30 min at room temperature. The stained cells were
detected using a flow cytometer (Accuri C6) and the DNA content was
analyzed using FlowJo software (v10) (both from BD
Biosciences).
Detection of mitochondrial transmembrane
potential (ΔΨm) variation using flow cytometry
The ΔΨm was determined using Rh-123. The MCF-7 cells
were treated with TFE (0, 5, 10, 20 and 30 µg/ml) and 5-Fu
for 10 h, harvested, then Rh-123 solution (1.0 µg/ml) was
subsequently added to the cells and were incubated at 37°C for 30
min. The results were detected using a flow cytometer (Accuri C6)
and analyzed using the FlowJo software (v10) (both from BD
Biosciences).
Western blot analysis
For western blot analysis, the TFE-treated MCF-7
cells (0, 5, 10, 20 and 30 µg/ml for 12 h, or with 20
µg/ml for 6, 12, 24 h) were washed twice with PBS and lysed
in RIPA lysis buffer (PAB180006; Bioswamp Wuhan Beinle
Biotechnology Co., Ltd.). The cell debris was removed using
centrifugation at 13,523 × g for 10 min at 4°C. The supernatant was
harvested and the concentration was measured using a BCA protein
assay kit (PAB180007; Shanghai, China). Protein samples (10
µg) were separated using 15% SDS-PAGE, performed at 80 V for
40 min, followed by 120 V for 50 min. After separation, the
proteins were transferred onto PVDF membranes (IPVH00010; EMD
Millipore). The membranes were blocked in TBS-Tween-20 (TBST)
buffer containing 5% (w/v) skimmed milk for 1.5 h at room
temperature and incubated overnight at 4°C with the primary
antibodies (caspase-8, 1:1,000; cleaved-caspase-8, 1:1,000;
caspase-9, 1:2,000; cleaved-caspase-9, 1:1,000; caspase-3, 1:5,000;
cleaved-caspase-3, 1:1,000; Bax, 1:1,000; Bcl-2, 1:500; Apaf-1,
1:1,000; GAPDH, 1:5,000) diluted in TBST, followed by incubation
with HRP-conjugated secondary antibodies (goat anti-rabbit IgG,
PAB160011; 1:10,000; goat anti-mouse IgG, PAB160009; 1:10,000)
(both from Bioswamp Wuhan Beinle Biotechnology Co., Ltd.) for 1 h
at room temperature. Finally, the signal was developed using an
enhanced chemi-luminescence plus kit (EMD Millipore) and detected
using the ChemiDoc Touch imaging system (Tanon-5200; Tanon Science
and Technology Co., Ltd.). The ImageJ software 1.48v (National
Institutes of Health) was used to calculate the relative density of
the proteins.
Statistical analysis
All the data are presented as the mean ± SD and
analyzed using the SPSS v17.0 (SPSS Inc.,). The experiments were
performed in triplicate. One-way ANOVA followed by Tukey's post hoc
test was used to compare the differences between continuous data
(>3 groups), while differences between 2 groups were compared
using a Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
TFE inhibits the proliferation of the
MCF-7 cells
First, the effect of TFE on the proliferation of the
HeLa, A549, MCF-7, HepG2, A2780, and SW620 cells was
investigated using a MTT assay. It was found that the six types of
cell lines treated with TFE (0, 5, 10, 20, 40, 60, 80, and 100
µg/ml) at 3 time intervals (12, 24 and 48 h) demonstrated a
notable decrease in cell proliferation (Fig. 1A). In particular, the
IC50 values were 31.01±1.93, 31.77±3.06, 27.32±0.24,
53.05±3.15, 16.98±1.56 and 21.39±1.47 µg/ml for HeLa, A549,
MCF-7, HepG2, A2780 and SW620 cells, respectively at 48
h (Table I).
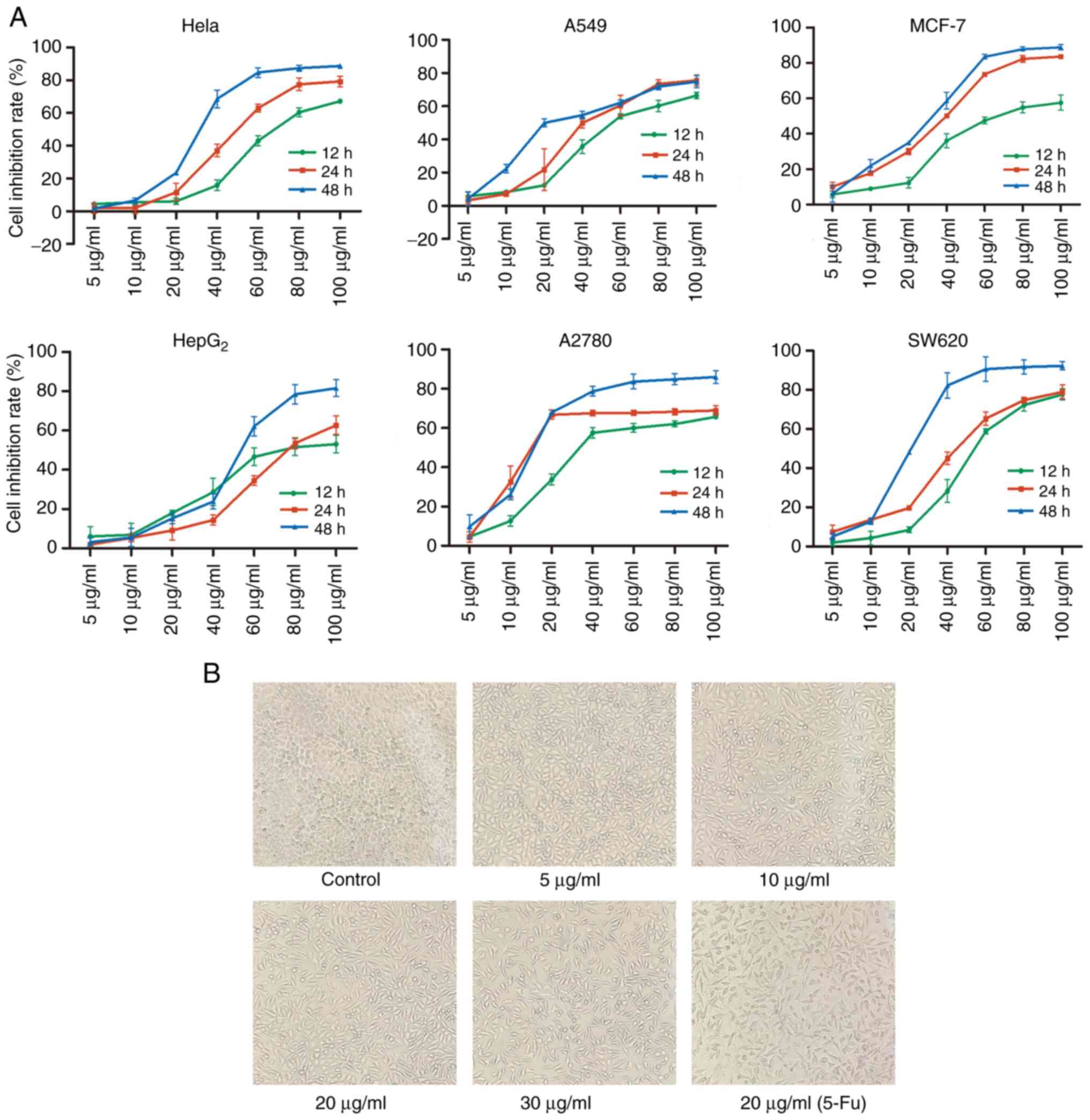 | Figure 1Inhibition of cell growth by TFE in
six cell lines and morphological changes in the MCF-7 cell line
treated with TFE. (A) MTT assay analysis of the inhibition ratio in
six cell lines following 12, 24, 48 h treatment with TFE at
different concentrations (0, 5, 10, 20 40, 60, 80 and 100
µg/ml). (B) The MCF-7 cells were treated with different
concentrations of TFE (0, 5, 10, 20 and 30 µg/ml) and 20
µg/ml 5-Fu for 10 h, then observed using a light microscope
(magnification, ×100). TFE, total flavone extract; 5-Fu,
5-fluorouracil. |
 | Table IThe IC50 values in six
cell lines following TFE treatment for 12, 24 and 48 h. |
Table I
The IC50 values in six
cell lines following TFE treatment for 12, 24 and 48 h.
| Cell lines | IC50
values, µg/ml
|
|---|
| 12 h | 24 h | 48 h |
|---|
| HeLa | 70.27±2.85 | 49.11±1.4a | 31.01±1.93a |
| A549 | 59.68±3.04 | 43.66±2.24a | 31.77±3.06a |
| MCF-7 | 70.98±7.04 | 33.45±1.09a | 27.32±0.24a |
| HepG2 | 80.5±10.38 | 79.14± 3.98a | 53.05±3.15a |
| A2780 | 43.41±3.29 | 22.74±2.3a | 16.98±1.56a |
| SW620 | 55.14±2.48 | 42.73±2.05a | 21.39±1.47a |
The A2780 and SW620 cells showed sensitivity to TFE
treatment compared with that in the MCF-7 cell line. Notably, the
mechanism of apoptosis for ampelopsin/myricetin (principal
effective components of TFE) have been reported in ovarian and
colon cancer (22,32). Therefore, the breast cancer cell
line was selected as a priority, as it had not been investigated in
this cell line before. To investigate the efficacy of TFE in breast
cancer, the MDA-MB-231 cell line was assessed using the MTT assay.
The IC50 values were 84.74±0.747% at 48 h.
Furthermore, separately and simultaneously, the
cellular morphological changes were observed using light
microscopy. Increasing concentrations of TFE, caused cell
shrinkage, cell size reduction, sloughing and increased cell death;
however, the control cells were normal. Morphological changes (cell
shrinkage) were also observed in the positive control
(Fu-5-treated) cells (Fig. 1B).
Therefore, further experiments were performed using the MCF-7 cell
line and TFE concentration between 5 and 30 µg/ml.
TFE induces apoptosis of MCF-7 cells
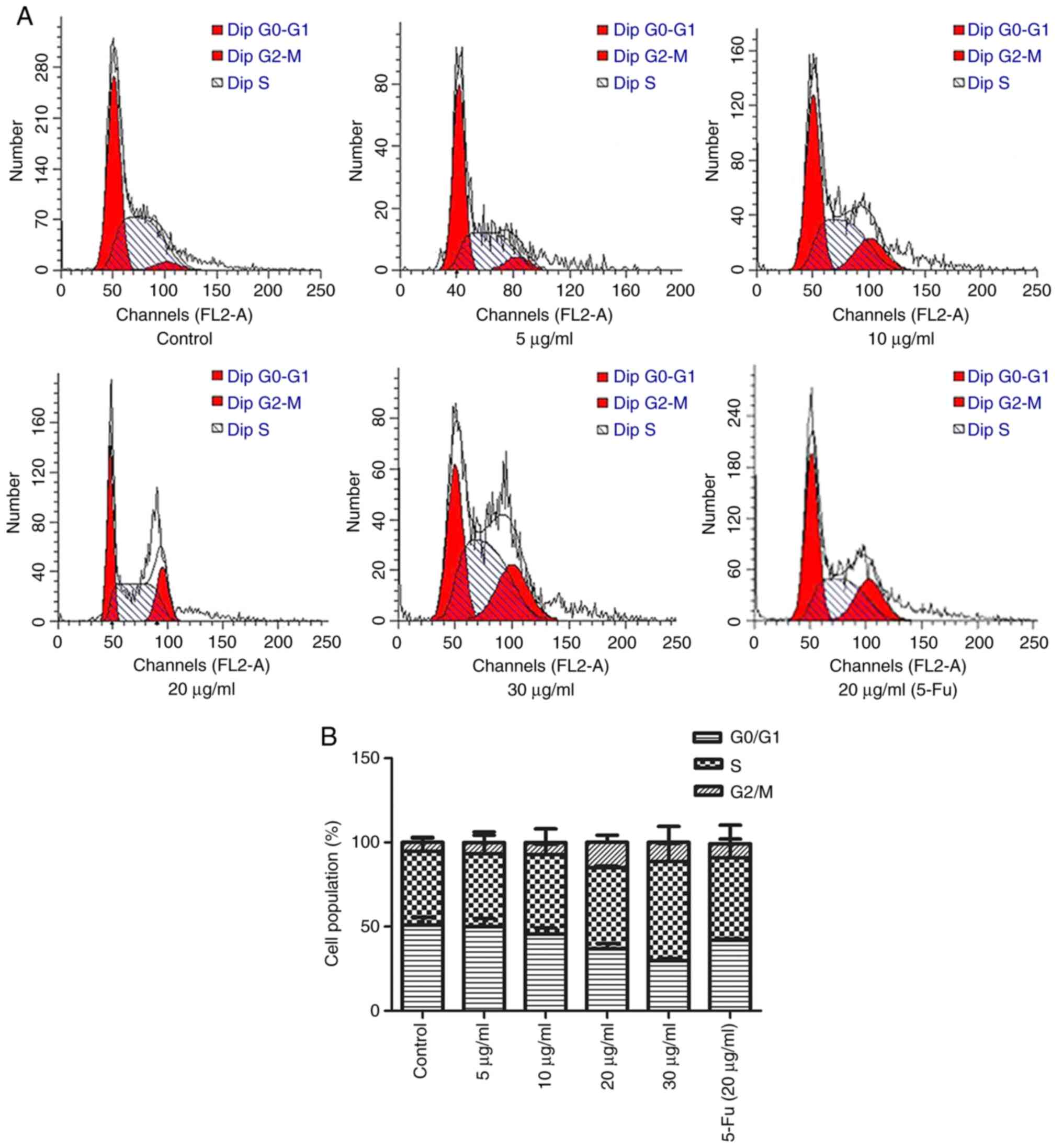
The cell cycle plays a significant role in
apoptosis, particularly in the intrinsic mitochondrial-related
pathway. Therefore, the effect of TFE treatment on cell cycle
arrest in the MCF-7 cell line was investigated using PI staining.
The results indicated that the cells were arrested at the
G2/M phase (Fig. 2A).
There was a notable increase in the proportion of cells in the
G2/M phase (from 7.64±0.98 to 22.24±1.43%) and a
decrease in the number of cells in the G0/G1
phase (from 51.79±2.03 to 31.13±1.03%; Fig. 2B) compared with that in the control
group. These results indicated that TFE induced cell cycle arrest
in the MCF-7 cells.
Next, it was investigated whether TFE induced
apoptosis in the MCF-7 cells. Annexin V-FITC/PI staining was used
to evaluate the apoptotic rate of MCF-7 cells following TFE
treatment for 10 h. The results showed an increased in the
apoptotic rate, and the percentage of apoptotic cells significantly
increased from 12.49±3.72 (5 µg/ml) to 15.45±2.07,
29.28±2.45, and 30.11±3.87% after treatment with 10, 20 and 30
µg/ml TFE, respectively. The apoptotic rate of the positive
control was 26.40±6.17%, whereas in the control cells it was
7.47±1.24% (P<0.05) (Fig. 3A and
B).
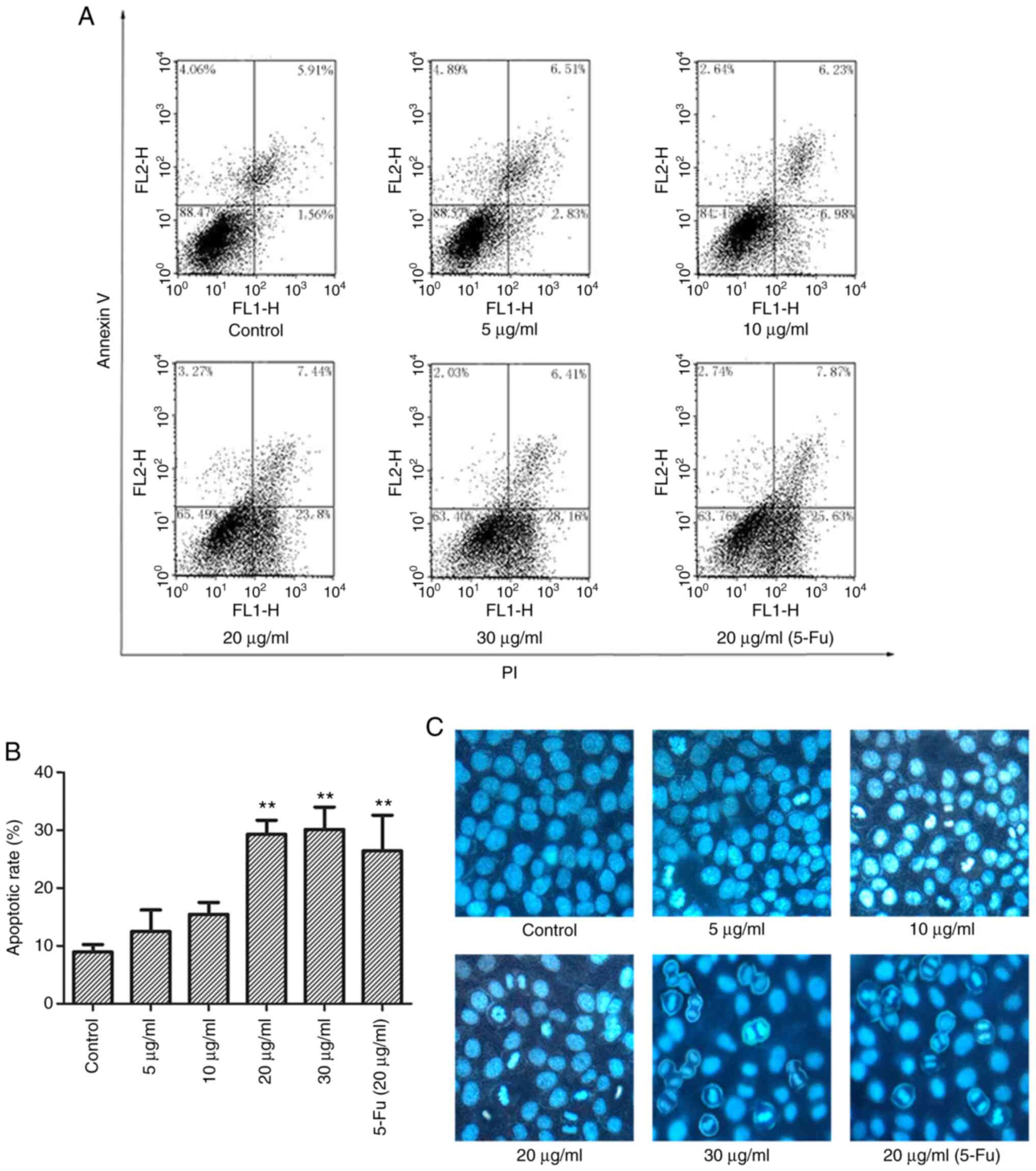 | Figure 3TFE induces MCF-7 cell apoptosis. (A)
Representative flow cytometry plots of apoptosis in the TFE-treated
(0, 5, 10, 20 and 30 µg/ml) MCF-7 cells and with 20
µg/ml 5-Fu. (B) The apoptotic rate in TFE-treated MCF-7
cells was statistically analyzed. (C) Nuclei staining with Hoechst
33258 in TFE-treated (0, 5, 10, 20 and 30 µg/ml) MCF-7
cells, and with 20 µg/ml 5-Fu. The data are presented as the
mean ± SD. **P<0.01. vs. Con. TFE, total flavone
extract; 5-Fu, 5-fluorouracil; Con, control. |
To visually observe apoptosis, the MCF-7 cells were
treated with TFE and Fu-5 (20 µg/ml), then stained with
Hoechst 33258. As shown in Fig.
3C, apoptotic morphological changes were observed, including
nuclear chromatin condensation and fragmentation, with increase in
the light blue fluorescence observed compared with that in the
control, and decrease in normal cell number.
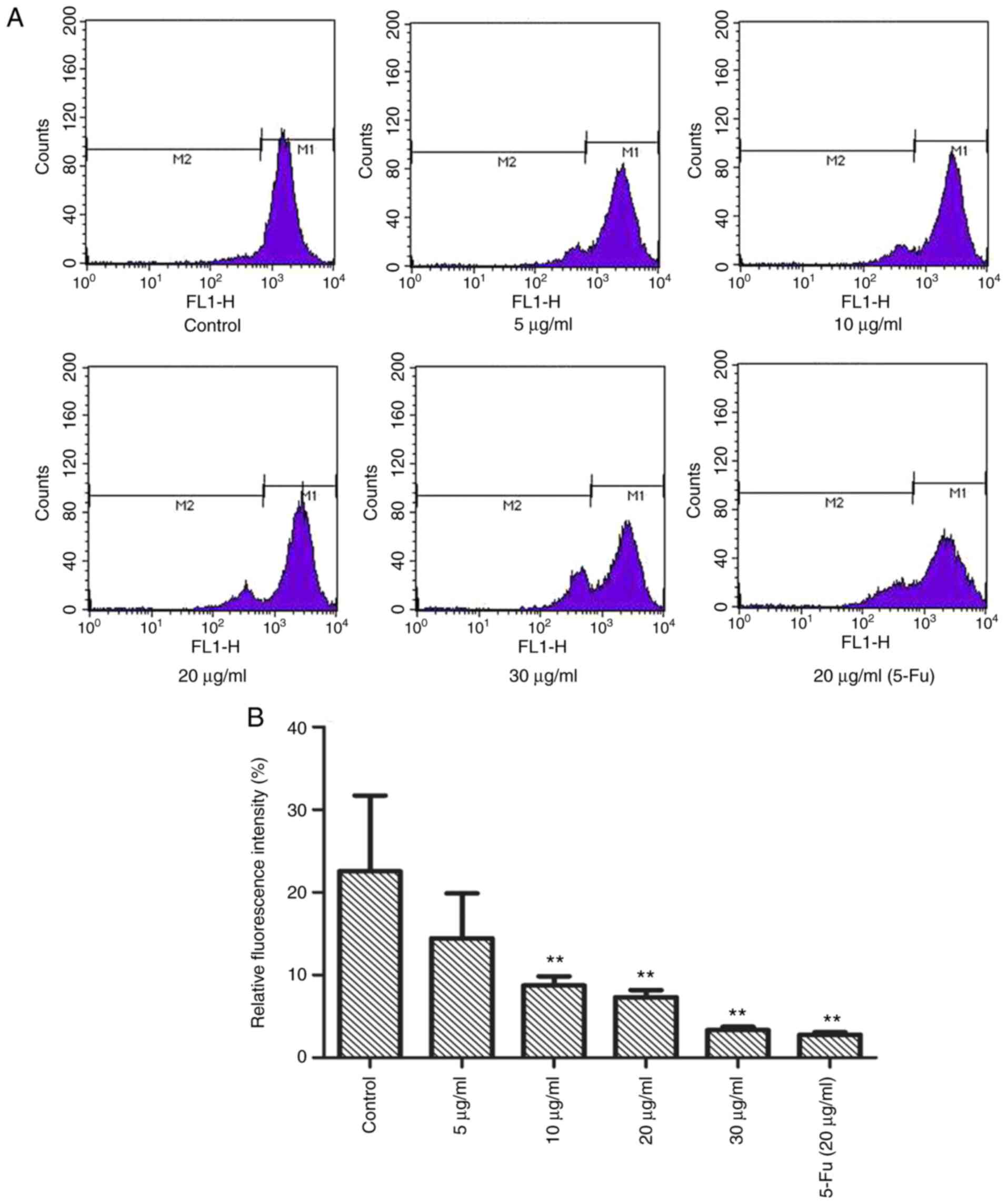
TFE ΔΨm of the MCF-7 cells
Subsequently, it was investigated whether TFE
altered the ΔΨm using Rh-123 staining. As shown in Fig. 4A and B, compared with that in the
control cells, with a relative fluorescence intensity of
22.54±2.16%, the MCF-7 cells, treated with different TFE
concentrations (5, 10, 20, 30 µg/ml), demonstrated decreased
relative fluorescence intensity, from 14.44±5.45 to 3.34±0.41%,
indicating a notable decrease in ΔΨm.
TFE regulates relative protein expression
level in apoptosis
To investigate the possible mechanism of TFE-induced
apoptosis in the MCF-7 cells, the expression level of related
proteins, including anti-apoptotic Bcl-2, pro-apoptotic Bax and
caspase-3. In addition, several other proteins involved in the
related pathways were also investigated, such as caspase-8, Apaf-1
and caspase-9. The results, shown in Fig. 5A and C, demonstrated that treatment
with TFE decreased the protein expression level of Bcl-2 and
increased the expression level of Bax, caspase-3, -8 and -9 and
Apaf-1. TFE demonstrated regulatory effects on these
apoptosis-related proteins in a time-dependent manner (Fig. 5B and D).
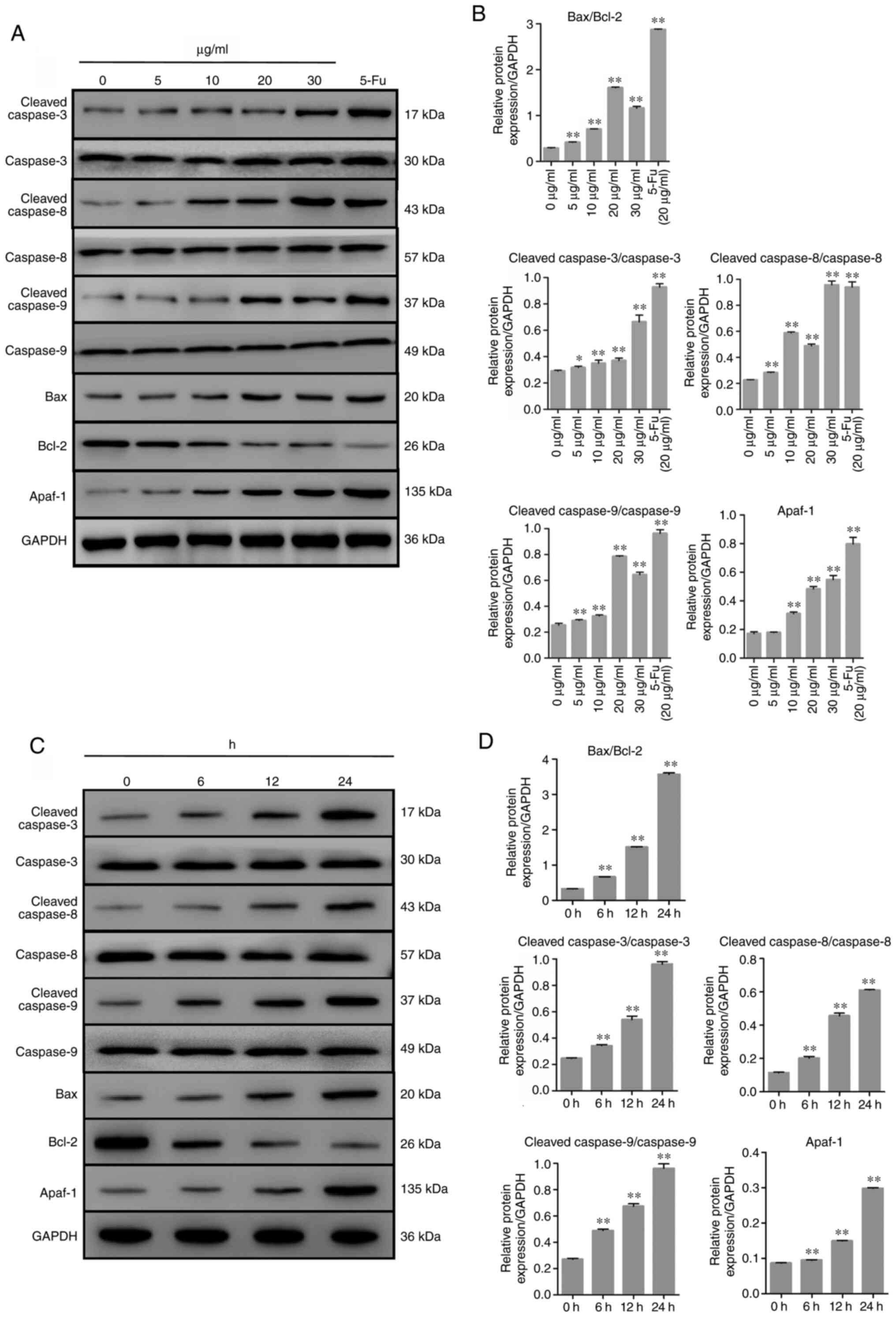 | Figure 5Expression level of the
apoptosis-related proteins in TFE-treated MCF-7 cells. (A and C)
The relative expression levels of the apoptotic proteins in
TFE-treated (0, 5, 10, 20 and 30 µg/ml for 12 h, or with 20
µg/ml for 6, 12, 24 h) MCF-7 cells. (B and D) Quantification
of the relative protein expression level in the TFE-treated MCF-7
cells. The data are presented as the mean ± SD.
*P<0.05, **P<0.01. vs. Con. TFE, total
flavone extract; 5-Fu, 5-fluorouracil; Con, control. |
Discussion
Breast cancer, which has been reported to be the
most malignant tumor in women, has no specific drug treatment.
Furthermore, triple-negative breast cancer is the worst type and
has poor therapeutic response due to the lack of specific receptors
(25,30). Presently, targeted drugs, such as
inhibitors and antagonists are used in clinical practice. However,
these types of drugs are susceptible to resistance (7). Currently, an increasing number of
natural products are under investigation in cancer research. A.
megalophylla, is a Chinese traditional herb used as a folk
medicine and it has a long history of use in Hubei province. Total
flavonoids are the major components extracted from A.
megalophylla (18).
In the present study, the antitumor activity of TFE
in six cancer cell lines (HeLa, A549, MCF-7, HepG2, A2780 and
SW620) was investigated using the MTT assay and followed by
microscopic observations. Furthermore, the flow cytometric analysis
suggested that TFE notably inhibited tumor cell proliferation and
induced apoptosis in the MCF-7 cells.
In addition, these findings were supported by
western blot analysis and Rh-123 staining, which revealed the
expression of key apoptotic proteins and the change in ΔΨm induced
by TFE. The Bcl-2 family members are critical regulators of
apoptosis, including the anti-apoptotic proteins Bcl-2 and
pro-apoptotic Bax proteins (33).
The increased of Bcl-2 has been associated with the induction of
apoptosis by the alteration in ΔΨm (36). As shown in Fig. 5 there was an upregulation in the
Bax/Bcl-2 ratio in TFE-treated MCF-7 cells, indicating apoptosis.
Notably, the relative fluorescence intensity was decreased
following TFE treatment (Fig. 4).
These results indicated that TFE treatment of the MCF-7 cells
induced apoptosis, possibly through the mitochondrial pathway.
Apoptosis is a complex process, that involves
several signaling pathways (10).
Based on the results from the present study, the mitochondrial
pathway was the main target of TFE; however, further research is
important to elucidate the pathway mediating the effects of TFE in
the MCF-7 cells. Subsequently, the caspase family members and
mitochondria-related proteins were also investigated to verify that
apoptosis occurred and to determine the possible role of this
pathway. Caspase-3 activates apoptosis by cleaving a number of
cellular proteins and is characteristically proteolyzed during the
apoptotic process (37). TFE
induced a dose-dependent decrease of ΔΨm and the expression level
of Bcl-2 (Fig. 5). Bcl-2 is
expressed in the membranes of the nucleus and endoplasmic reticulum
(5). It has been reported that
cytochrome c sequentially binds to Apaf-1 to stimulate the
apoptotic protease cascade, forming an activation complex with
caspase-9 and activating caspase-3 (30,33).
The activation of pro-caspase-8 cleaves the pro-apoptotic Bcl-2
family member (40). In the
current study, TFE treatment significantly upregulated cleaved
caspase-8 and Bax, and downregulated Bcl-2 expression level, and
promoted Apaf-1 expression level (Fig.
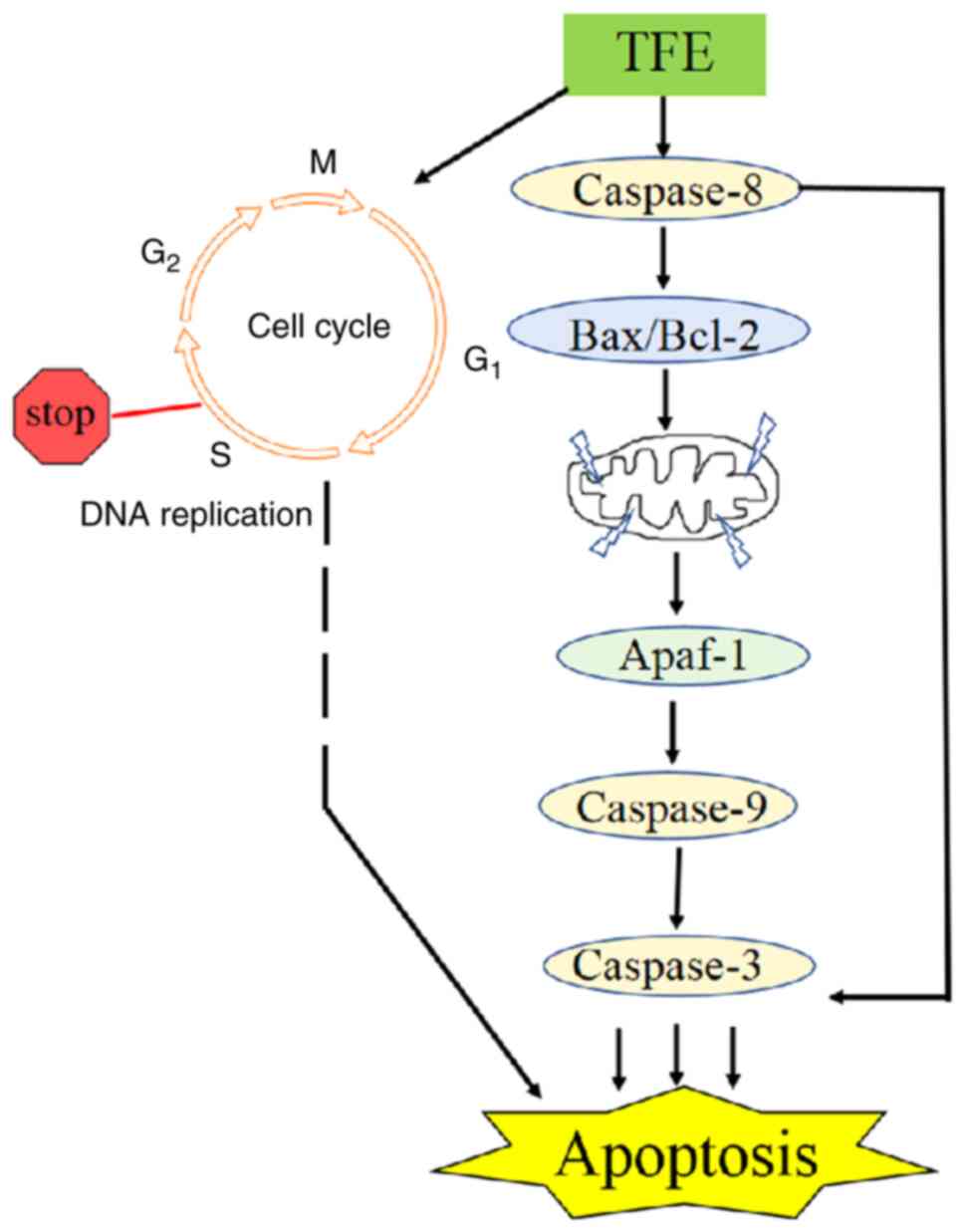
5). Collectively, the results from the present study indicated
that treatment with TFE induced caspase-8, which may have directly
activated Bax/Bcl-2, caused ΔΨm loss, and the binding of Apaf-1, to
then activate the caspase-9/3 cascade. These effects ultimately
induced apoptosis of the MCF-7 cells (Fig. 6).
In the present study, on the one hand the effect of
cell cycle stage was only examined; therefore, further
investigation is required into the mechanism involved. For example,
the expression levels of cell cycle and apoptosis-associated
proteins could be detected with immunohistochemistry. On the other
hand, to further elucidate the signal pathway in-depth, detailed
studies into the mitochondrial apoptosis pathway, the expression
level of the cytochrome c protein in mitochondria/cytoplasm
fractions, and into the activity and mechanism of TFE in
vivo are also required in the future.
In summary, it was found that TFE inhibited seven
cancer cell lines (HeLa, A549, MCF-7, HepG2, A2780, SW620 and
MDA-MB-231). These findings indicated that the
mitochondria-mediated apoptotic pathway was involved in TFE-induced
apoptosis of the MCF-7 cells. Furthermore, the results provided an
experimental basis for the use of TFE, as an agent for the
treatment of breast cancer. To further elucidate this signal
pathway in-depth, in vivo studies are in progress to
investigate the activity of TFE.
Funding
This research was supported by the National Natural
Science Foundation of China (grant no. 31170335), the Foundation of
the Scientific and Technological Bureau of Wuhan (grant no.
2018060401011308) and the Foundation of 'Beanstalk Program' of
Hubei University of Chinese Medicine.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
XZ and BH conceived and designed the study. CG, CZ,
JH and LG performed all of the experiments. CG and CZ analyzed the
data. CZ, XX and JX performed the statistical analysis. CG wrote
the manuscript. CG, BH and XZ confirm the authenticity of all the
raw data. All authors have reviewed the manuscript and read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Czarnomysy R, Surażyński A, Muszynska A,
Gornowicz A, Bielawska A and Bielawski K: A novel series of
pyrazole-platinum(II) complexes as potential anti-cancer agents
that induce cell cycle arrest and apoptosis in breast cancer cells.
J Enzyme Inhib Med Chem. 33:1006–1023. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Global Burden of Disease Cancer
Collaboration; Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the Global Burden of Disease Study. JAMA Oncol.
3:524–548. 2017. View Article : Google Scholar
|
|
4
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi T, Min M, Sun C, Zhang Y, Liang M and
Sun Y: Periodontal disease and susceptibility to breast cancer: A
meta-analysis of observational studies. J Clin Periodontol.
45:1025–1033. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao W, Li JQ, Hao QY, Jaydutt VV and Wu Y:
AMP-activated protein kinase: A potential therapeutic target for
triple-negative breast cancer. Breast Cancer Res. 21:292019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tejashree M and Manu L: From natural
products to designer drugs: Development and molecular mechanisms
action of novel anti-microtubule breast cancer therapeutics. Curr
Top Med Chem. 17:2559–2568. 2017.
|
|
8
|
Tsao AS, Kim ES and Hong WK:
Chemoprevention of cancer. CA Cancer J Clin. 54:150–180. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alimohammadi M, Lahiani MH, McGehee D and
Khodakovskaya M: Polyphenolic extract of InsP 5-ptase expressing
tomato plants reduce the proliferation of MCF-7 breast cancer
cells. PLoS One. 12:e1757782017. View Article : Google Scholar
|
|
10
|
Khorsandi L, Orazizadeh M, Niazvand F,
Abbaspour MR, Mansouri E and Khodadadi A: Quercetin induces
apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl Lek
Listy. 118:123–128. 2017.PubMed/NCBI
|
|
11
|
Lee HJ, Burger P, Vogel M, Friese K and
Bruning A: The nucleo-side antagonist cordycepin causes DNA double
strand breaks in breast cancer cells. Invest New Drugs.
30:1917–1925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan Y, Zhang F, Zhao Y, Shao D, Zheng X,
Chen Y, He K, Li J and Chen L: Berberine enhances chemosensitivity
and induces apoptosis through dose-orchestrated AMPK signaling in
breast cancer. J Cancer. 8:1679–1689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma R, Kaushik S, Shyam H, Agarwal S
and Balapure AK: Neem seed oil induces apoptosis in MCF-7 and MDA
MB-231 human breast cancer cells. Asian Pac J Cancer Prev.
18:2135–2140. 2017.PubMed/NCBI
|
|
14
|
Xie X, Wang J and Zhang H:
Characterization and antitumor activities of a water-soluble
polysaccharide from Ampelopsis megalophylla. Carbohydr Polym.
129:55–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng P, Gui C, Huang J, Xia Y, Fang Y, Da
GZ and Zhang XQ: Molecular mechanisms of ampelopsin from Ampelopsis
megalophylla induces apoptosis in HeLa cells. Oncol Lett.
14:2691–2698. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kou X, Shen K, An Y, Qi S, Dai WX and Yin
Z: Ampelopsin inhibits H2O2-induced apoptosis
by ERK and Akt signaling pathways and up-regulation of heme
oxygenase-1. Phytother Res. 26:988–994. 2012. View Article : Google Scholar
|
|
17
|
Xie XF, Wang JW, Zhang HP, Li QX and Chen
BY: Chemical composition, antimicrobial and antioxidant activities
of essential oil from Ampelopsis megalophylla. Nat Prod Res.
28:853–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He XY, Yang RJ, Zhu ZK, Jiang C, Zhang XQ
and Liu YW: Determination of total flavonoids in different extracts
of Ampelopsis megalophylla. Herald Med. 31:211–212. 2012.
|
|
19
|
Chen XM, Xie XB, Zhao Q, Wang F, Bai Y,
Yin JQ, Jiang H, Xie XL, Jia Q and Huang G: Ampelopsin induces
apoptosis by regulating multiple c-Myc/S-phase kinase-associated
protein 2/F-box and WD repeat-containing protein 7/histone
deacety-lase 2 pathways in human lung adenocarcinoma cells. Mol Med
Rep. 11:105–112. 2015. View Article : Google Scholar
|
|
20
|
Liu B, Tan X, Liang J, Wu S, Liu J, Zhang
Q and Zhu R: ERRATUM: A reduction in reactive oxygen species
contributes to dihydromyricetin-induced apoptosis in human
hepatocellular carcinoma cells. Sci Rep. 5:79402015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu M, Huang W, Bao N, Zhou G and Zhao J:
The flavonoid ampelopsin inhibited cell growth and induced
apoptosis and G0/G1 arrest in human osteosarcoma MG-63 cells in
vitro. Pharmazie. 70:388–393. 2015.PubMed/NCBI
|
|
22
|
Ni F, Gong Y, Li L, Abdolmaleky HM and
Zhou JR: Flavonoid ampelopsin inhibits the growth and metastasis of
prostate cancer in vitro and in mice. PLoS One. 7:e388022012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park GB, Jeong JY and Kim D:
Ampelopsin-induced reactive oxygen species enhance the apoptosis of
colon cancer cells by activating endoplasmic reticulum
stress-mediated AMPK/MAPK/XAF1 signaling. Oncol Lett. 14:7947–7956.
2017.PubMed/NCBI
|
|
24
|
Zhang B, Dong S, Cen X, Wang X, Liu X,
Zhang H, Zhao X and Wu Y: Ampelopsin sodium exhibits antitumor
effects against bladder carcinoma in orthotopic xenograft models.
Anticancer Drugs. 23:590–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Y, Liang X, Chang H, Shu F, Wu Y,
Zhang T, Fu Y, Zhang Q, Zhu JD and Mi M: Ampelopsin-induced
autophagy protects breast cancer cells from apoptosis through
Akt-mTOR pathway via endoplasmic reticulum stress. Cancer Sci.
105:1279–1287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao J, Chen H, Lu W, Wu Y, Wu X, Xia D and
Zhu J: Myricetin induces protective autophagy by inhibiting the
phosphorylation of mTOR in HepG2 cells. Anat Rec (Hoboken).
301:786–795. 2018. View
Article : Google Scholar
|
|
27
|
Ha TK, Jung I, Kim ME, Bae SK and Lee JS:
Anti-cancer activity of myricetin against human papillary thyroid
cancer cells involves mitochondrial dysfunction-mediated apoptosis.
Biomed Pharmacother. 91:378–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jo S, Ha TK, Han SH, Kim ME, Jung I, Lee
HW, Bae SK and Lee JS: Myricetin induces apoptosis of human
anaplastic thyroid cancer cells via mitochondria dysfunction.
Anticancer Res. 37:1705–1710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jose J, Dhanya AT, Haridas KR, Sumesh
Kumar TM, Jayaraman S, Variyar EJ and Sudhakaran S: Structural
characterization of a novel derivative of myricetin from Mimosa
pudica as an anti-proliferative agent for the treatment of cancer.
Myricetin induces apoptosis of human anaplastic thyroid cancer
cells via mitochondria dysfunction. Biomed Pharmacother.
84:1067–1077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knickle A, Fernando W, Greenshields AL,
Rupasinghe H and Hoskin DW: Myricetin-induced apoptosis of
triple-negative breast cancer cells is mediated by the
iron-dependent generation of reactive oxygen species from hydrogen
peroxide. Food Chem Toxicol. 118:154–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma L, Cao X, Wang H, Lu K, Wang Y, Tu C,
Dai Y, Meng Y, Li Y, Yu P, et al: Discovery of myricetin as a
potent inhibitor of human flap endonuclease 1, which potentially
can be used as sensitizing agent against HT-29 human colon cancer
cells. J Agric Food Chem. 67:1656–1665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye C, Zhang C, Huang H, Yang B, Xiao G,
Kong D, Tian Q, Song Q, Song Y, Tan H, et al: The natural compound
myricetin effectively represses the malignant progression of
prostate cancer by inhibiting PIM1 and disrupting the PIM1/CXCR4
Interaction. Cell Physiol Biochem. 48:1230–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng AW, Chen YQ, Zhao LQ and Feng JG:
Myricetin induces apoptosis and enhances chemosensitivity in
ovarian cancer cells. Oncol Lett. 13:4974–4978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang ZR, Wang L, Yin HH, Yang FJ, Gao YQ
and Zhang ZJ: Effect of total flavonoids of Hippophae rhamnoides on
contractile mechanics and calcium transfer in stretched myocyte.
Space Med Eng (Beijing). 13:6–9. 2000.
|
|
35
|
Zhong LR, Chen X and Wei KM: Radix
tetrastigma hemsleyani flavone induces apoptosis in human lung
carcinoma A549 cells by modulating the MAPK pathway. Asian Pac J
Cancer Prev. 14:5983–5987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gong WY, Wu JF, Liu BJ, Zhang HY, Cao YX,
Sun J, Lv YB, Wu X and Dong JC: Flavonoid componentsin Scutellaria
baicalensis inhibit nicotine-induced proliferation metastasis and
lung cancer-associated inflammation in vitro. Int J Oncol.
44:1561–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li-Weber M: New therapeutic aspects of
flavones: The anti- cancer properties of Scutellaria and its main
active constituents wogonin, baicalein and baicalin. Cancer Treta
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
38
|
Li CM, He J, Gao YL, Xing YL, Hou J and
Tian JW: Preventive effect of total flavones of choerospondias
axillaries on ischemia/reperfusion-induced myocardial
infarction-related MAPK signaling pathway. Cardiovasc Toxicol.
14:145–152. 2014. View Article : Google Scholar
|
|
39
|
Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M,
Dong Q, Liu Y and Xu H: The traditional chinese medicine baicalein
potently inhibits gastric cancer cells. J Cancer. 7:453–461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Q, Ren FQ, Yang CL, Zhou LM, Liu YY,
Xiao J, Zhu L and Wang ZG: Anti-proliferation effects of
isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac J
Cancer Prev. 16:3035–3042. 2015. View Article : Google Scholar : PubMed/NCBI
|