Introduction
The small EF-hand calcium-binding protein S100A4, a
member of the S100 family, has been demonstrated to play important
roles in invasion and metastasis in a number of different types of
tumors (1,2). Furthermore, its high expression has
been shown to be associated with tumor aggressiveness and poor
outcomes in patients with a variety of cancers (3). Although the precise molecular
mechanisms underlying the S100A4-mediated enhancement of invasion
and metastasis remain to be completely elucidated, a number of
studies have revealed some of these mechanisms by focusing on the
discovery of effector proteins with which S100A4 interacts in a
Ca2+-dependent manner (3-6). To
date, dozens of such effector proteins have been reported. For
example, the authors previously identified methionine
aminopeptidase 2 (MetAP2) as a binding partner of S100A4 that plays
a key role in angiogenesis and acts as the target of the
antiangiogenic natural product fumagillin and its analogs, such as
TNP-470 (4,5). The authors have also recently
reported the association between S100A4 and the
metastasis-associated protein MTA1 in the cytoplasm of murine
endothelial cells, which resulted in the stabilization of the
S100A4 protein by the inhibition of its ubiquitin-proteasome
pathway-mediated degradation (6).
As such, MTA1 siRNA inhibited tumor angiogenesis and tumor growth
through the partial downregulation of S100A4 in a human pancreatic
cancer xenograft model, suggesting that the MTA1-S100A4 axis is a
target for cancer therapy. S100A4 also interacts with a variety of
effector proteins, including the heavy chain of non-muscle myosin
II-A (NMIIA), F-actin, tropomyosin, Liprin β1, the Rho-binding
domain of Rhotekin, the tumor suppressor p53 and Annexin A2,
through which the complex probably regulates cell motility and
apoptosis (6-14). Of note, previous studies have
demonstrated S100A4 as a regulator of epithelial-mesenchymal
transition and the properties of cancer stem cells (15-20).
Matrix metalloproteinases (MMPs) are zinc-dependent
endopeptidases involved in extracellular matrix (ECM) degradation
and remodeling (21,22). The roles of MMPs in cancer cell
invasion and metastasis have been extensively studied and have
become better understood. Among MMPs, MMP-14 (also known as
membrane-type 1 MMP, MT1-MMP) has been demonstrated to play
essential roles in the breakdown of ECM, cell proliferation and
cell motility (23,24). MMP-14 binds to tissue inhibitor of
metalloproteinases (TIMP)-2, which in turn binds pro-MMP-2 on the
cell surface, where a second unbound MMP-14 protein cleaves the
propeptide domain of pro-MMP-2, leading to its activation and
ultimately the degradation of type IV collagen within the basement
membrane (23-25). MMP-14 also activates MMP-13 and
degrades various ECM components, including type I, II and III
collagens, laminins and fibronectin (23,24).
Importantly, MMP-14 accumulates on the surface of invadopodia,
which are actin-rich membrane projections formed by invasive tumor
cells to degrade the basement membrane (26). In addition to its ECM-degradative
function, recent research has revealed novel non-ECM-degradative
functions of MMP-14. MMP-14 cleaves the N-terminal ligand-binding
portion of the Eph receptor tyrosine kinase EphA2, leading to the
loss of the suppressive effect of ligand-bound EphA2 on cell
growth, ultimately enhancing the growth, migration and metastasis
of cancer cells (27). The short
cytoplasmic tail of MMP-14 binds factor-inhibiting HIF-1 (FIH-1),
which activates the master regulator of the hypoxia response,
hypoxia-inducible factor-1 (HIF-1) (28).
The authors have previously demonstrated that a
synthetic peptide corresponding to the S100A4-binding domain of
MetAP2 (NBD peptide), but not a control synthetic peptide (CBD
peptide), efficiently blocked the S100A4-MetAP2 inter-action
(5). The NBD peptide is considered
to occupy the binding pocket of Ca2+-bound S100A4 and
hence block the binding of S100A4 to other effector proteins. The
NBD peptide was found to enhance the assembly of NMIIA filaments,
modulated the expression of angiogenesis-related genes, and inhibit
cell growth and capillary formation in murine endothelial cells
(5). Consequently, the NBD peptide
significantly inhibited tumor angiogenesis and hence retarded tumor
growth in a human prostate cancer xenograft model (4). As mentioned above, as metastatic
cancer cells express a large amount of S100A4, the NBD peptide may
also inhibit cancer cell invasion and metastasis. Given that the
NBD peptide amino acid sequences in mouse and human MetAP2 are
identical, the present study examined the unexplored possible
effects of the NBD peptide in highly metastatic human mammary
carcinoma cells.
Materials and methods
Cells
Highly metastatic human mammary carcinoma
MDA-MB-231-Luc-D3H2LN (RRID: CVCL_D257) and MDA-MB-468 cells
(ATCC® HTB-132™) were obtained from Caliper Life
Sciences (29) and the American
Type Culture Collection (ATCC), respectively. In addition, 293
cells (JCRB9068) were supplied by Health Science Research Resources
Bank. After confirming the absence of mycoplasma using the e-Myco
Mycoplasma PCR Detection kit (Cosmo Bio Co., Ltd.), the cells were
cultured in DMEM supplemented with 10% fetal bovine serum and 40
µg/ml gentamicin in a humidified atmosphere of 95% air/5%
CO2 at 37°C.
Peptide synthesis
The synthesis of the peptides (NBD corresponding to
MetAP2 (170-229) and CBD corresponding to MetAP2 (192-229)) was
carried out by the solid phase method with a Pioneer peptide
synthesis system (Applied Biosystems; Thermo Fisher Scientific,
Inc.), and the peptides were purified by reverse-phase
high-performance liquid chromatography on a C18 column as
previously described (5).
Peptide loading
The CBD and NBD peptides were introduced into the
cells using atelocollagen mixed with BioPORTER protein delivery
reagent (Genlantis, Inc.); throughout the experiment, the CBD
peptide was used as a control peptide for the NBD peptide as
previously described (5). For this
purpose, the peptides (12.5 µg) dissolved in 20 µl of
atelocollagen in Dulbecco's phosphate-buffered saline (DPBS) were
added to a dry film of BioPORTER reagent (equivalent to 10
µl of a BioPORTER solution) and mixed by pipetting.
Following incubation for 5 min at room temperature, the solution
was briefly vortexed and added to Opti-MEM (cat no. 31985070,
Thermo Fisher Scientific, Inc.) to yield a final volume of 1 ml,
which was then evenly distributed in a 60-mm dish. Following
incubation for 2 h in a CO2 incubator,
MDA-MB-231-Luc-D3H2LN or MDA-MB-468 cells (1.8×105
cells) were seeded in the dish and incubated at 37°C for 4 h. After
FBS was added to a final concentration of 10%, both the
MDA-MB-231-Luc-D3H2LN cells and MDA-MB-468 cells were further
cultured for 18 and 40 h, respectively.
Mithramycin A and fumagillin
treatment
MDA-MB-231-Luc-D3H2LN cells were treated with
solvent alone (DMSO), mithramycin A (cat no. M6891, Sigma-Aldrich;
Merck KGaA) or fumagillin (cat no. F6771, Calbiochem, Merck KGaA)
at the indicated concentrations and incubated at 37°C for 2
days.
RNA isolation and RT-qPCR
Total RNA was isolated using the RNAeasy Plus Mini
kit (Qiagen GmbH) according to the manufacture's protocol. The
quantity and purity of the RNA were measured using a NanoDrop 2000c
spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA was
synthesized using total RNA (1 µg) and the ReverTra Ace qPCR
RT kit (Toyobo Life Science,) in a 10 µl volume and then
diluted 10 times with distilled water. PCR reactions were performed
in 20 µl volumes containing 1 µl cDNA, 10 µl
PowerSYBR-Green PCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.), 0.3 µl of a mixture of forward and
reverse primers (10 pmoles each) and 8.7 µl RNase-free
water. qPCR was carried out for triplicate samples using the 7500
Real Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) in a PCR protocol consisting of an initial denaturation step
at 95°C for 1 min and 40 cycles of denaturation (95°C for 15 sec)
and extension (60°C for 1 min). The mRNA expression level of each
gene was normalized to that of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) using the 2−ΔΔCq method
(30). The sequences of the
specific primer sets are listed in Table SI.
Cell motility assay
A cell motility (wound healing) assay was performed
with the MDA-MB-231-Luc-D3H2LN cells treated with CBD and NBD.
After the cells had reached sub-confluence (approximately 75%), the
cells were pre-treated with 5 µg/well of the CBD or NBD
peptide/atelocollagen complex for 30 min in serum-free Opti-MEM,
after which the cells were wounded by scraping the monolayer using
a pipette tip and grown in DMEM with 2% FBS for 24 h. The scratch
closure was monitored and imaged using a Keyence BZ-X710 microscope
(Keyence, Germany). The wound area was measured using NIH ImageJ
1.47 software. Wound closure percentage was calculated as follows:
Wound area (%)=AW/IW ×100, where IW represents the initial wound
area and AW the wound area at 24 h.
Gelatin invadopodia assay
The ECM-degrading activity of the peptide-loaded
cells was assessed using a QCM Gelatin Invadopodia Assay (Green)
kit (EMD Millipore) according to the manufacturer's protocol. The
MDA-MB-231-Luc-D3H2LN cells loaded with the NBD or CBD peptide or
mock-treated cells were seeded onto fluorescein-labeled gelatin and
cultured at 37°C for 24 h. Following fixation with 4% formaldehyde
in DPBS, the cells were stained with TRITC-phalloidin and DAPI at
room temperature for 1 h. Fluorescent images obtained using the TCS
SP8 confocal laser scanning micro-scope (Leica Microsystems, Inc.)
were analyzed using NIH ImageJ 1.52a software (31), and the degradation area (pixel
value) was normalized to the number of cells per field.
Invasion assay
Cell invasion assay for the MDA-MB-231-Luc-D3H2LN
cells was assessed using the CytoSelect 96-Well Cell Invasion Assay
(Cell Biolabs, Inc.). The cells were pre-treated with 5
µg/well of the CBD and NBD peptide/atelocollagen complex at
37°C for 30 min and then plated at 1×105 cells/well in
96-well plates. Cells that had invaded the Matrigel were stained
with hematoxylin (cat no. H9627, Sigma-Aldrich; Merck KGaA) at room
temperature for 5 min, and the number of pores with stained cells
was calculated. For the MDA-MB-468 cells, invasion assays were
performed with Corning BioCoat Matrigel Invasion Chambers (Corning,
Inc.), first by pre-treatment with either CBD or NBD
peptide/atelocollagen complex at 12.5 µg/well at 37°C for 18
h, followed by re-plating at 5×104 cells/well in 96-well
plates. Invasive cells were stained with 0.5% crystal violet (cat
no. V5265, Sigma-Aldrich; Merck KGaA) and quantified using a
microscope (CX23, Olympus Corporation) as per the manufacturer's
instructions.
Preparation of NBD peptide-encapsulated
glycoliposomes
NBD peptide-encapsulated glycoliposomes with sialyl
Lewis X on their surface (NBD-GlycoLipos) were prepared using an
improved cholate dialysis method and supplied by Katayama Chemical
Industries Co., Ltd. (32). The
lipid content of the obtained NBD-GlycoLipos was measured by
determining the total cholesterol content using a Cholesterol E
test Wako kit (FUJIFILM Wako Pure Chemical Corp.) after the
GlycoLipos were destroyed in the presence of 3% sodium dodecyl
sulfate. The total amount of fatty acids was then calculated from
the molar ratio of each lipid. The protein content in the presence
of 1% sodium dodecyl sulfate was determined using Pierce Micro BCA
Protein Assay Reagent (Thermo Fisher Scientific, Inc.). Particle
size and zeta potential at 25°C were measured using a Zetasizer
Nano-ZSP (Malvern Panalytical, Ltd.). The NBD-GlycoLipo solution
was diluted 50-fold with distilled water for measurements.
Spontaneous lung metastasis assay
Animal experiments were performed in compliance with
the guidelines of the Institute for Laboratory Animal Research,
National Cancer Center Research Institute (February to March,
2019). The protocol was approved by the National Cancer Center
Research Institute (Approval no. NCC-T17-038). On day 0, the
mammary glands of 6-week-old female CB-17 SCID mice (10 mice;
average body weight, 23.4 g; CLEA Japan) anesthetized by exposure
to 3% isoflurane were injected with 1.5×106
MDA-MB-231-Luc-D3H2LN cells expressing luciferase and randomly
divided into 3 groups. Mice were examined for their health by
monitoring activity, body weight, food/water intake and coat/skin
condition every 2 days during tumor progression following tumor
transplantation. In addition, the size of the tumor xenografts was
monitored so as not to exceed 10% of the animal's body weight at
the experimental endpoint (the maximum tumor weight expressed as a
percentage of the body weight in this study was 9.8% in a single
subject in the empty-GlycoLipos group). On days 7 and 14, the mice
were treated with only PBS only (n=2), empty glycoliposomes
(empty-GlycoLipos; lipid concentration, 3.6 mg/ml; n=4) or
glycoliposomes containing the NBD peptide (NBD-GlycoLipos; lipid
concentration, 3.6 mg/ml; peptide concentration, 0.32 mg/ml; n=4)
via the tail vein injection. On day 28, the mice were subjected to
imaging with an IVIS imaging system (Xenogen Corp.). For this, the
mice were intraperitoneally injected with D-luciferin (150 mg/kg;
Promega Corporation). After 10 min, photons from Firefly luciferase
were counted, and data were analyzed using LivingImage software
(version 2.50; Xenogen Corp.) according to the manufacturer's
instructions. During the process of live imaging, the mice were
anesthetized with 4% isoflurane by inhalation and maintained with
1.5% isoflurane. As for the animal euthanasia procedure, a
combination of anesthesia with isoflurane and cervical dislocation
by a trained person in compliance with an IACUC-approved protocol
was used. Animal death was confirmed by respiratory arrest and
verifying no heartbeat by palpation. The primary tumor and the lung
of each animal were resected at necropsy for tumor weight
measurement and immunohistological analysis, respectively.
Immunohistochemical analysis of CD31
Primary tumors were surgically removed and fixed in
a 10% phosphate-buffered formalin solution. The tissues were
embedded in paraffin and cut into 5-µm-thick sections.
Following heat-induced antigen retrieval by REAL Target Retrieval
Solution (DAKO) and the subsequent blocking of non-specific sites
with 0.1% normal goat serum/1% BSA at 4°C overnight, the tissue
sections were stained with rabbit monoclonal anti-CD31 (PECAM-1)
(D8V9E) XP antibody (diluted 1:100; cat no. #77699, Cell Signaling
Technology, Inc.) at room temperature for 1 h and visualized by
staining with Alexa Fluor 594-conjugated goat anti-rabbit IgG
(diluted 1:300; cat no. A32740, Thermo Fisher Scientific, Inc.) at
room temperature for 1 h and counterstaining with DAPI at room
temperature for 30 min. Image acquisition was performed on a Leica
TCS SC8 confocal laser scanning microscope (Leica Microsystems,
Inc.), and tumor vessel densities were calculated using pixel
values of CD31-positive regions in ImageJ software (National
Institutes of Health).
Evaluation of pulmonary metastases
The level of metastasis at the thoracic cavity was
evaluated based on immunohistochemistry. Lung tissues dissected
from the mice were fixed in a 4% paraformaldehyde phosphate buffer
solution (FUJIFILM Wako Pure Chemical Corporation) and embedded in
paraffin. Each section was deparaffinized and rehydrated, and
anti-gens were then activated by heating at 95°C for 45 min in
Immunosaver solution (Nissin EM). Human tumor cells were detected
by incubation with anti-human vimentin antibody (diluted 1:100; cat
no. VP-RM17, rabbit anti-human vimentin monoclonal antibody, Vector
Laboratories, Inc.) at 4°C over-night, followed by incubation with
ImmPRESS anti-rabbit IgG (diluted 1:200, cat. no. MP-7401, Vector
Laboratories) at room temperature for 1 h. DAB staining was carried
out using a Metal-Enhanced DAB Substrate kit (Thermo Fisher
Scientific, Inc.), followed by hematoxylin staining at room
temperature for 10 min and dehydration. The slides were subjected
to microscopic observation using a BZ-X710 microscope (Keyence
Corporation) with a 10X objective lens. The metastatic foci in the
lungs of each treatment group were quantified by counting using a
Digit Hand Tally Counter (AF-counter-2_hook+base, AFUNTA Technology
Development Co., Ltd.), and the numbers of metastatic foci per area
(cm2) were calculated.
Western blot analysis
MDA-MB-231-Luc-D3H2LN or MDA-MB-468 cells cultured
in 6-well plates were mock-treated or treated with 5 µg/well
of the NBD or the CBD peptide/atelocollagen complex for 18 h and
then lysed in RIPA buffer (150 mM NaCl, 25 mM Tris-HCl, pH 7.6, 1%
NP-40, 1% sodium deoxycholate and 0.1% SDS) containing protease
inhibitor cocktail (Roche Applied Science). The lysates were
centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant
was used for western blot analysis. The protein concentration was
determined using the Bradford method. Proteins (40 µg
protein/lane) were denatured by boiling in SDS sample buffer and
separated by 10% SDS-PAGE under reducing conditions and transferred
to an Immobilon-P transfer membrane (EMD Millipore). The membrane
was blocked with BLOCK ACE (DS Pharma Biomedical Co., Ltd.) in
Tris-buffered saline and 0.1% Tween-20 (TBS-T) for 1 h at room
temperature, incubated with primary antibodies (diluted 1:1,000)
against MMP-14/MT1-MMP (cat. no. MAB9181-SP, mouse anti-human
MMP-14/MT1-MMP monoclonal antibody; R&D Systems, Inc.), Sp1
(cat. no. HPA001853, rabbit anti-human Sp1 polyclonal antibody;
Sigma-Aldrich; Merck KGaA), IκB-α (cat. no. 4812, rabbit anti-human
IκB-α monoclonal antibody; Cell Signaling Technology, Inc.),
phospho-IκB-α [Ser32; cat. no. 2859, rabbit anti-phospho-IκB-α
(Ser32) monoclonal antibody; Cell Signaling Technology, Inc.],
S100A4 (cat. no. ab27957, rabbit anti-human S100A4 polyclonal
antibody; Abcam) and β-actin (cat no. sc-47778, mouse anti-β-actin
monoclonal antibody, Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h or at 4°C overnight. The membrane was washed
extensively with TBS-T, and then incubated with HRP-conjugated
anti-rabbit IgG (diluted 1:3,000; cat no. #7074S, Cell Signaling
Technology, Inc.) or HRP-conjugated anti-mouse IgG (diluted
1:3,000; cat no. #7076S, Cell Signaling Technology, Inc.) for 1 h
at room temperature. Immunodetection was carried out using ECL Plus
Western Blotting Detection Reagent (Amersham Biosciences; Thermo
Fisher Scientific, Inc.). The density of the blots was analyzed
using NIH ImageJ 1.52a software.
Immunofluorescence staining
The MDA-MB-231-Luc-D3H2LN cells cultured in 6-well
plates were mock-treated or treated with the NBD or CBD
peptide/atelocollagen complex for 18 h and fixed for 10 min with 4%
formaldehyde in DPBS. Permeabilization of the cells was performed
by incubating the fixed cells with 0.5% Triton X-100 in DPBS for 4
min. To block non-specific binding, the cells were then incubated
with 3% BSA in DPBS containing 0.1% glycine for 1 h. After washing
with DPBS, the cells were incubated at room temperature for 1 h
with primary antibodies (diluted 1:200) against MMP-14/MT1-MMP
(cat. no. MAB9181-SP; R&D Systems, Inc.), Sp1 (cat. no.
HPA001853; Sigma-Aldrich; Merck KGaA), nuclear factor (NF)-κB p65
(cat. no. sc-372, rabbit anti-human NF-κB p65 polyclonal antibody;
Santa Cruz Biotechnology, Inc.), early growth response 1 (EGR1;
cat. no. 4153, rabbit anti-human EGR1 monoclonal antibody; Cell
Signaling Technology, Inc.) and ELK3 (cat. no. GTX114966, rabbit
anti-human ELK3 polyclonal antibody; GeneTex, Inc.). After rinsing
with DPBS, the cells were stained with an appropriate Alexa Fluor
488- or Alexa Fluor 594-conjugated secondary antibody (diluted
1:300; Thermo Fisher Scientific, Inc.) at room temperature for 1 h.
Nuclei were counterstained with 1 µg/ml DAPI at room
temperature for 15 min. The cells were observed under a confocal
laser scanning microscope (FluoView FV1000, Olympus
Corporation).
Statistical analysis
Data are expressed as the means ± standard
deviation. No statistical method was used to predetermine the
sample size. The researchers were not blinded to allocation during
experiments and outcome assessment. Statistical analyses were
conducted using one-way ANOVA and Tukey's test. A P-value of ≤0.05
was considered to indicate a statistically significant
difference.
Results
Inhibition of cell motility,
ECM-degrading activity and invasion by the NBD peptide in
MDA-MB-231 variants
The expression of S100A4 was first examined in
MDA-MB-231-Luc-D3H2LN cells by western blot analysis with 293 cells
(S100A4-negative per the Human Protein Atlas) as a control, and
found that there was a substantial amount of S100A4 expressed in
the cells; the NBD peptide and CBD peptide as a control were then
introduced into these cells (Fig.
1A). Of note, the NBD peptide seemed to decrease lamellipodia
formation compared to that following CBD peptide loading (Fig. 1B). In support of this finding,
staining of the cells with TRITC-phalloidin revealed that F-actin
was mainly localized at the periphery of the cells, probably at the
lamellipodia, in the mock-treated and CBD peptide-loaded cells,
while dense stress fibers were evident in the cell bodies of the
NBD peptide-loaded cells (Fig.
1C). The authors previously reported similar results in NBD
peptide-loaded endothelial cells, along with enhanced NMIIA
phosphorylation (5). As
lamellipodia extension is important for cell motility and invasion
(33), the motile activity and
ECM-degrading activity of the peptide-loaded cells was then
measured using a wound healing assay and a gelatin invadopodia
assay, respectively. The results revealed that the NBD peptide
markedly suppressed cell motility (Fig. 1D and E), and the ECM-degrading
activity of the NBD peptide-loaded cells was much weaker than that
of the CBD peptide-loaded cells and mock-treated cells (Fig. 1F and G). In line with these
results, the Matrigel invasion assay demonstrated a reduction in
the invasiveness of the NBD peptide-loaded cells compared with the
CBD peptide-loaded cells (Fig. 1H and
I).
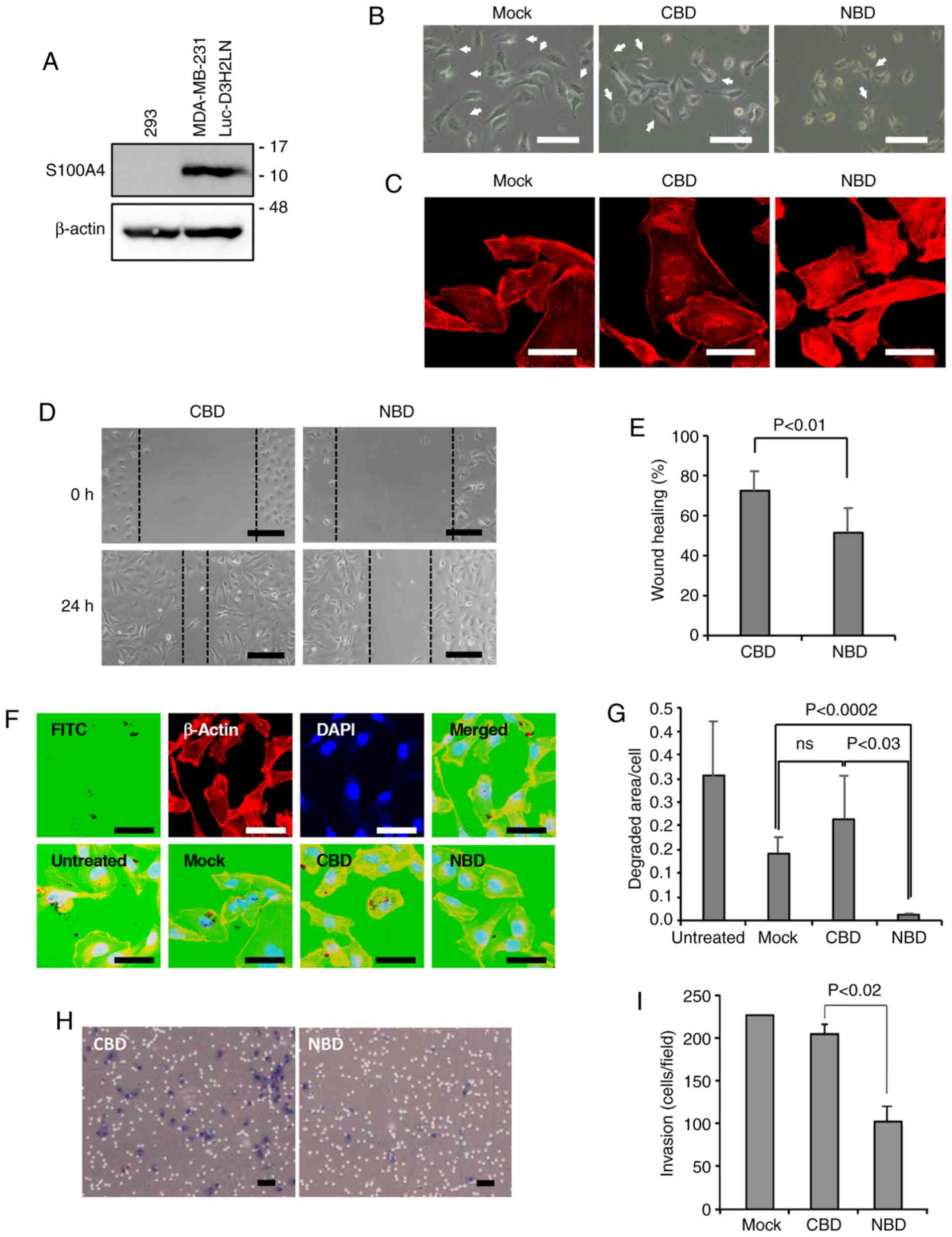 | Figure 1Inhibition of cell motility,
ECM-degrading activity and invasiveness of MDA-MB-231-Luc-D3H2LN
cells by the NBD peptide. (A) Western blot analyses of the
expression of S100A4. Full size images of the western blots are
shown in Fig. S6. (B) Morphology of mock-treated cells (only the
peptide loading reagent) or cells treated with the CBD or NBD
peptide. Bars: 100 µm. Arrows indicate examples of
lamellipodia. (C) Actin stress fiber formation. Visualization of
F-actin in mock-treated, CBD peptide-treated and NBD
peptide-treated cells by TRITC-phalloidin staining. Bars: 20
µm. (D) Cell motility. Images showing the cell motility of
the CBD peptide- and NBD peptide-treated cells examined by a wound
healing assay. The images were photographed at 0 h and after 24 h.
Scale bars, 100 µm. (E) Quantification of cell motility. The
motility of CBD peptide-treated and NBD peptide-treated cells was
evaluated using the images obtained by a wound healing assay (n=6
for each). (F) Gelatin invadopodia assay. Upper panels represent
images showing the degradation of FITC-labeled gelatin (leftmost),
TRITC-phalloidin staining (second from left), and DAPI staining
(third from left) and a merged image (rightmost). Lower panels
represent merged images of FITC-gelatin staining, TRITC-phalloidin
staining and DAPI staining of untreated (leftmost), mock-treated
(second from left), CBD peptide-treated (third from left) and NBD
peptide-treated (rightmost) cells. Scale bars, 20 µm. (G)
Quantification of the degraded area/cell in the invadopodia assay.
The degradation area (pixel value) was normalized to the number of
cells per field (Untreated, n=10 fields; Mock, n=10 fields; CBD,
n=12 fields; NBD, n=13 fields); ns, not significant. (H) Matrigel
invasion assay. Images showing the invasion of the CBD peptide- and
NBD peptide-treated cells. Scale bars, 100 µm. (I)
Quantification of the number of invaded cells (n=4 membranes for
each). |
Decrease in the expression of MMP-14 in
the NBD peptide-loaded cells
To obtain insights into the mechanisms underlying
the reduction in cell motility and invasiveness in NBD
peptide-loaded MDA-MB-231-Luc-D3H2LN cells, the expression levels
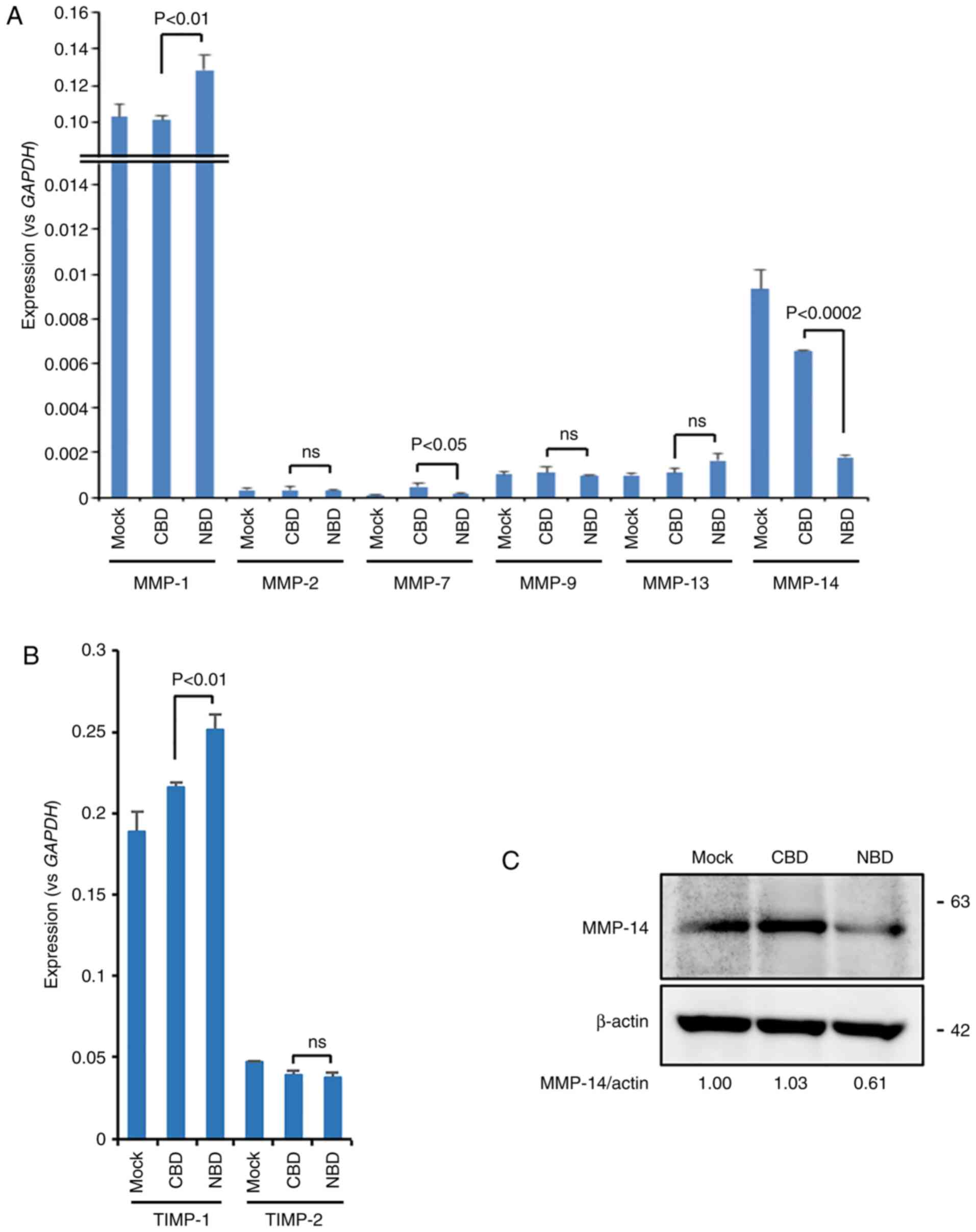
of MMPs and their inhibitors, TIMPs, were examined. Notably, the
results of RT-qPCR revealed that MMP-14 expression was
significantly downregulated, while MMP-7 expression was slightly,
yet significantly downregulated in the NBD peptide-loaded cells
compared to the CBD peptide-loaded cells (Fig. 2A). MMP-1 and TIMP-1 expression was
significantly increased. The expression levels of other MMPs
(MMP-2, MMP-9 and MMP-13) and TIMP-2 were only slightly altered by
the NBD peptide (Fig. 2A and B).
The reduction in MMP-14 expression was also confirmed at the
protein level (Fig. 2C).
Decreased Sp1 expression is involved in
the downregulation of MMP-14 expression in the NBD peptide-loaded
cells
The transcription of the MMP-14 gene is regulated by
several transcription factors, including Sp1, NF-κB, EGR1 and ELK3
(34-38). The present study then examined the
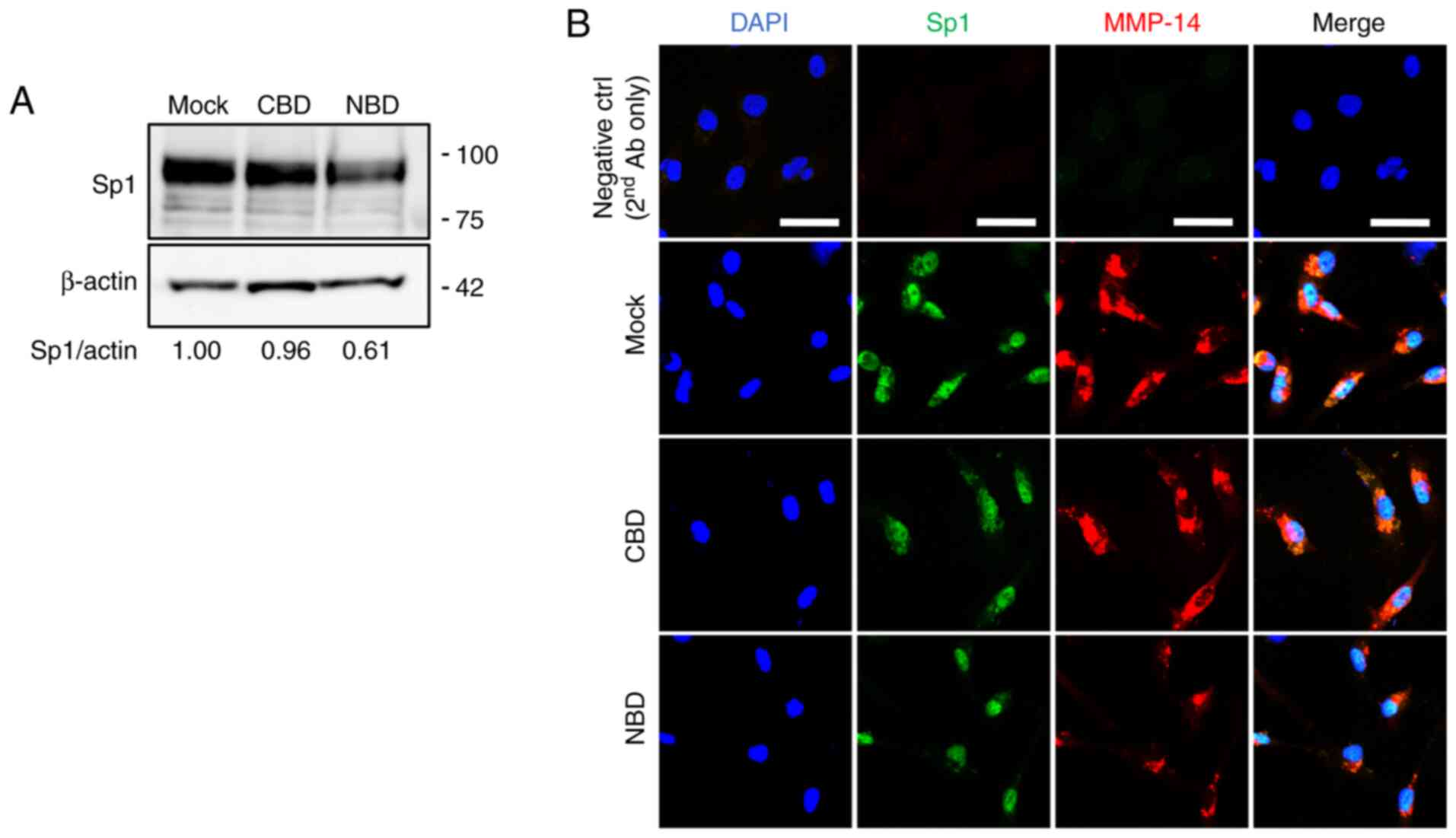
expression of these transcription factors and it was found that Sp1
was downregulated in the NBD peptide-loaded MDA-MB-231-Luc-D3H2LN
cells (Fig. 3A). This was
corrob-orated by double immunostaining experiments in which the
expression levels of Sp1 and MMP-14 were decreased in the NBD
peptide-loaded cells (Fig. 3B). No
marked changes were detected in either the expression or
intracellular localization of NF-κB or the phosphorylation of IκB-α
(Fig. S1). Likewise, no marked
alterations were observed in the expression level or localization
of EGR1 or ELK3, as assessed by immunofluorescence staining
(Fig. S2), although western blot
analysis might allow for the more detailed characterization of the
two proteins. The involvement of Sp1 in the regulation of MMP-14 in
MDA-MB-231-Luc-D3H2LN cells was also demonstrated by the inhibition
of MMP-14 expression in the cells treated with the Sp1 inhibitor,
mithramycin A (Fig. S3).
Decrease in MMP-14 and Sp1 expression
levels in the NBD peptide-loaded MDA-MB-468 cells
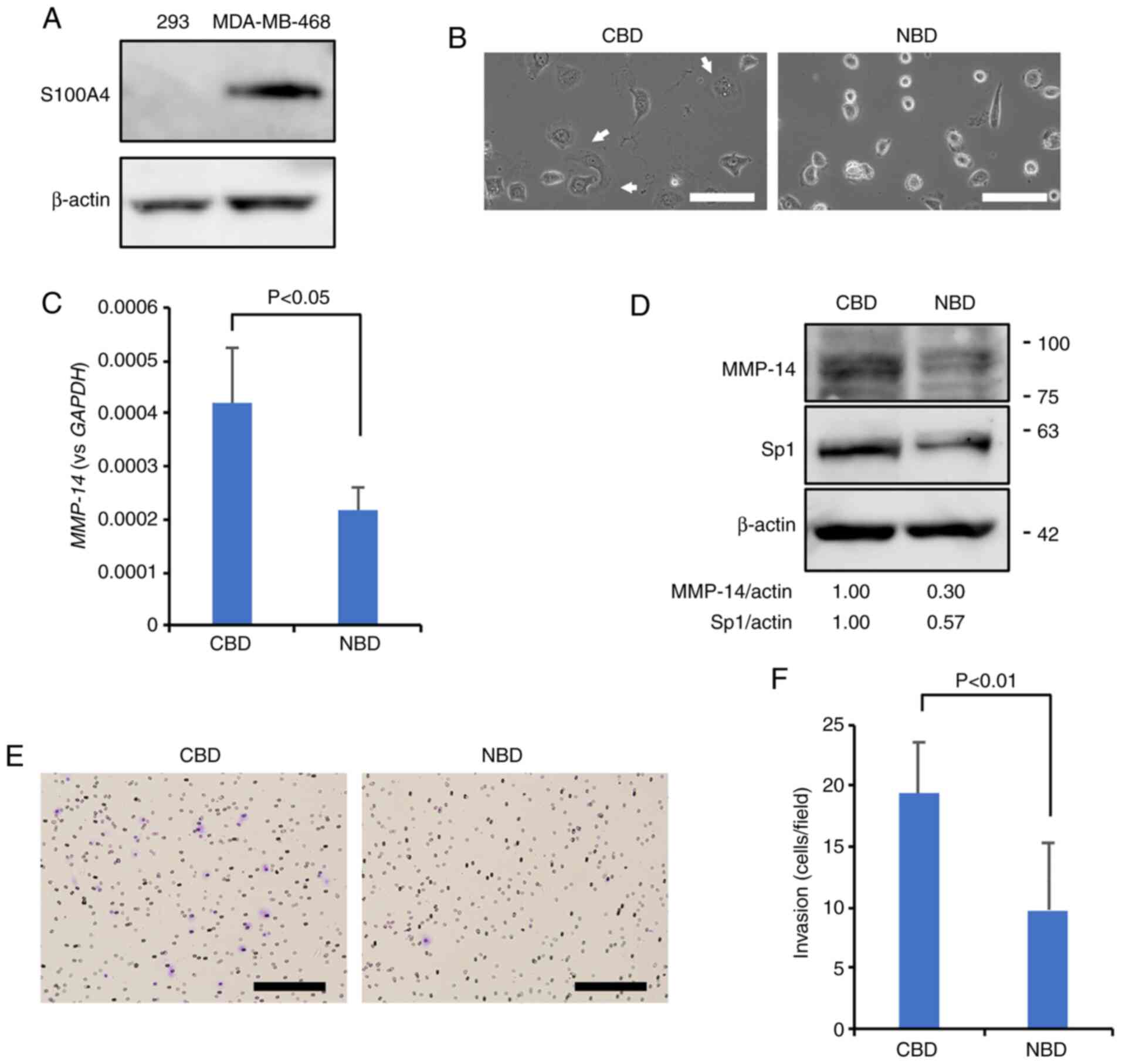
To address whether the NBD peptide also suppresses
MMP-14 and Sp1 expression levels in other mammary carcinoma cell
lines, both NBD peptide and CBD (control) peptides were loaded into
MDA-MB-468 cells that were confirmed to express S100A4 (Fig. 4A), and the suppression of
lamellipodia formation (Fig. 4B)
was observed in the samples that received NBD; additionally, it was
also noted that the NBD peptide decreased the expression of MMP-14
and Sp1 compared to the CBD control (Fig. 4C and D). The Matrigel invasion
assay also demonstrated a significant reduction in the invasiveness
of the NBD peptide-loaded cells compared to the CBD peptide-loaded
control cells (Fig. 4E and F).
These observations suggest that the NBD peptide affects the
Sp1/MMP-14 axis in different mammary carcinoma cell lines.
MetAP2 enzyme activity is dispensable for
the reduction in MMP-14
The NBD peptide may dissociate the S100A4-MetAP2
complex, releasing MetAP2 in the cytoplasm. To address whether the
enzyme activity of MetAP2 is necessary for the reduction in MMP-14,
we treated MDA-MB-231-Luc-Luc-D3H2LN cells with fumagillin. The
results revealed that MMP-14 expression was not affected by this
treatment (Fig. S4), indicating
that the enzyme activity is dispensable.
Inhibition of lung metastasis of
MDA-MB-231-Luc-D3H2LN cells by NDB peptide-loaded particles
To examine whether the NBD peptide suppresses the
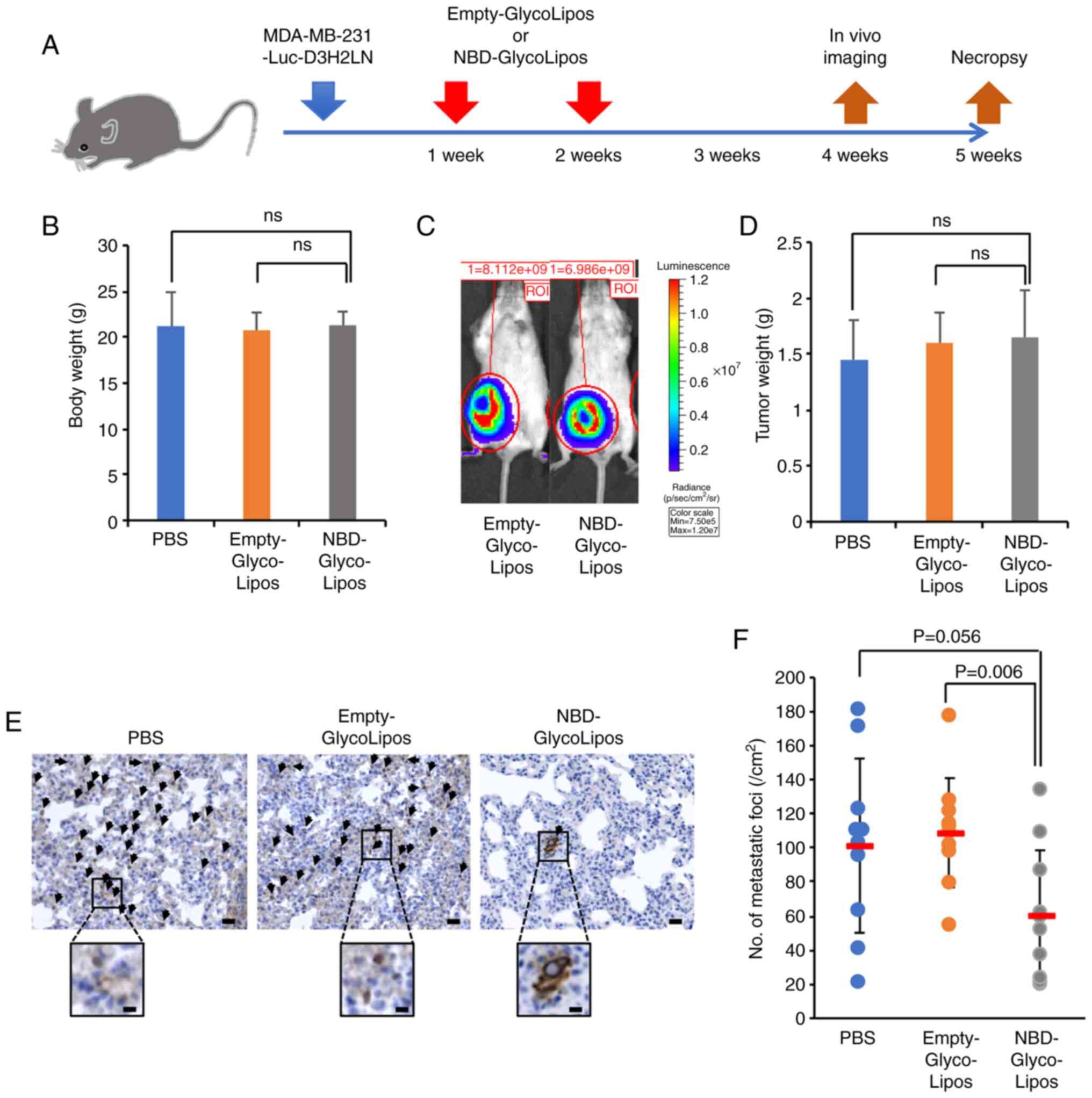
spontaneous metastasis of MDA-MB-231-Luc-D3H2LN cells, the cells
were implanted into the mammary glands of SCID mice on day 0. The
mice then received intravenous injections of PBS, Empty-GlycoLipos
or NBD-GlycoLipos on days 7 and 14 following implantation (Fig. 5A). The body weight of mice was
comparable among the treatment groups (Fig. 5B). Compared to the Empty-GlycoLipos
group, NBD-GlycoLipos was not found to inhibit primary tumor
growth, either in vivo imaging on day 28 (Fig. 5C) or by tumor weight assessment on
day 35 (Fig. 5D). The NBD peptide
also did not suppress angiogenesis, which was assessed by CD31
immunohisto-chemical staining (Fig.
S5). The present study was unable to observe evidence of
metastases in the lungs in whole-body bioluminescent images due to
the oversaturation of strong luminescence intensity emitted from
parts of the primary tumor. Subsequently, to observe disseminated
tumor cells in the lungs, immunohistochemistry was performed with
sections of the lungs using anti-human vimentin antibody (Fig. 5E). As a result, a significant
reduction in the number of metastatic foci was observed in the NBD
peptide-loaded particle-treated mice compared with the control
particle-treated mice (Fig.
5F).
Discussion
The present study demonstrated that the NBD peptide
significantly inhibited the invasiveness of highly metastatic
mammary carcinoma MDA-MB-231-Luc-D3H2LN cells through the
suppression of cell motility and invasiveness. Simultaneously, the
expression of MMP-14, which plays a central role in cancer cell
invasion and metastasis, was suppressed by the peptide. Most
importantly, the intravenous injection of liposomes containing the
NBD peptide appeared to significantly inhibit the spontaneous
metastasis of these cells. Although the authors previously reported
that the intratumoral injection of the NBD peptide complexed with
atelocollagen could potentially inhibit tumor angiogenesis
(5), the present study was unable
to find any measurable difference in vessel density in the primary
tumors of mice injected with the NBD peptide containing-liposomes
compared to the control group, most likely due to the differences
in the formulation, the injection route as well as the local
concentration of the peptide. It was therefore hypothesized that a
likely reason for the suppressive effect by the liposomes was due
to the inhibition of the extravasation of the tumor cells or tumor
angiogenesis in the lungs, rather than the inhibition of tumor
angiogenesis in the primary tumors. This hypothesis was
corroborated by the fact that no significant difference was
observed in the primary tumor growth rate between the control and
the NBD peptide-treated groups. Taken together, these results
suggest that the NBD peptide can suppress tumor cell invasion and
metastasis when administered in vivo.
In the present study, the CBD peptide was used as a
control peptide as previously described (5), and a mild suppression of MMP-14
expression was noted in the CBD-peptide-loaded cells compared to
the mock-treated cells (Fig. 2A);
therefore, the presence of non-specific effects from CBD peptide
introduction should not be discounted. Nevertheless, it was noted
that the suppressive effect of the NBD peptide was reproducibly
more potent compared to that of CBD in both the MDA-MB-231 and
MDA-MB-468 cells, a result that highlighted the specific action of
the NBD peptide.
S100A4 interacts with the C-terminus of NMIIA, and
this interaction controls myosin filament assembly through the
regulation of NMIIA phosphorylation at S1943 (8,39).
In a previous study by the authors, the NBD peptide enhanced NMIIA
phosphorylation at S1943 in endothelial cells and stimulated myosin
filament assembly and actin stress fiber formation (5). Therefore, NMIIA filaments were also
considered to be assembled in MDA-MB-231-Luc-D3H2LN cells treated
with the NBD peptide. On the other hand, actin assembly with
non-muscle tropomyosin assembly has been reported to recruit NMIIA
(40). This may indicate the
formation of thicker and more stable actin stress fibers, as
observed in stationary cells compared to highly motile cells
(41). Indeed, prominent actin
stress fiber formation was observed in the cell bodies of the NBD
peptide-loaded cells. This change may also suppress the formation
of invadopodia, where the branched F-actin network, cortactin and
MMP-14 are concentrated, resulting in a reduction in focal
degradation of the ECM (26).
Certainly, the gelatin invadopodia assay showed a marked reduction
in ECM degradation.
The mechanism of the transcriptional regulation of
the MMP-14 gene has yet not been fully resolved; however, a number
of transcription factors involved in regulating MMP-14, such as
NF-κB, Sp1, EGR1 and ELK3, have been reported (34-38).
Among these, Sp1 was specifically downregulated in the NBD
peptide-loaded cells. Although the involvement of Sp1 in the
regulation of MMP-14 in MDA-MB-231-Luc-D3H2LN cells was confirmed
by the mithramycin A1 experiment, Sp1 has also been reported to
regulate transcription of the MMP-2, MMP-9,
TIMP-1 and TIMP-2 genes (42-44).
Although the reasons for the fact that MMP-14 was mainly
downregulated in the NBD peptide-loaded cells cannot be explained
at present, the transcription of the MMP-14 gene may be more
dependent on Sp1 than that of other MMP and TIMP
genes. This issue warrants further investigation in the future.
It should be noted that the NBD peptide also
suppressed lamellipodia formation and the expression levels of
MMP-14 and Sp1 in the MDA-MB-468 cells, suggesting the likelihood
of the Sp1-MMP-14 axis being inhibited in S100A4-expressing cancer
cells; this in turn led to the suppression of the invasion and
metastasis of mammary carcinoma cells. Future studies into the
involvement of Sp1-MMP-14 axis in invasion and metastasis by Sp1
and MMP-14 knockdown would be able to further elucidate the
underlying mechanisms.
The NBD peptide may dissociate the S100A4-MetAP2
complex, releasing MetAP2 in the cytoplasm. The authors previously
reported that the binding of S100A4 to MetAP2 did not affect
methionine aminopeptidase activity, but altered the intracellular
localization of MetAP2 (4).
Therefore, it is possible that the enzyme activity of MetAP2 in the
right place is necessary for the reduction in MMP-14. To address
this issue, in the present study, cells were treated with
fumagillin. However, MMP-14 expression was not affected by this
treatment These results suggest that MetAP2 activity is dispensable
and that NBD peptide-mediated blockade of the binding of other
effector proteins with a lower affinity to the binding pocket in
S100A4 is important for the decrease in Sp1 and MMP-14 expression.
In this context, it is interesting to note that S100A4 binds MTA1
in the cytoplasm and that MTA1 induces expression of the tumor
suppressor alternative reading frame (ARF) through corecruitment of
c-Jun onto the ARF promoter (5,45).
ARF interacts with Sp1 and promotes its proteasomal degradation by
enhancing its interaction with the proteasome subunit regulatory
particle ATPase 6 (45).
Subsequently, if the NBD peptide blocks the interaction between
S100A4 and MTA1, free MTA1 may translocate into the nucleus and
induce ARF, in turn promoting Sp1 degradation. An examination of
this possibility may be worthwhile in the future.
Therapeutic peptides have attracted attention as
promising cancer therapeutics (46). The results of the present study in
conjunction with those of previous observations highlight the NBD
peptide as a peptide drug that can simultaneously inhibit tumor
angiogenesis and cancer cell invasiveness. The NBD peptide is 60
amino acids in length. To improve the solubility, cell permeability
and efficacy of the NBD peptide, identification of an essential
core amino acid sequence capable of inhibiting the S100A4-MetAP2
interaction is undoubtedly required. Accordingly, the authors
recently reported a peptide smaller than NBD, NBD-ΔN10, a core
peptide that can inhibit the S100A4-MetAP2 interaction (47). Further studies, including
investigations of the effects of NBD-ΔN10 on tumor angiogenesis,
invasion and metastasis, are warranted to develop an S100A4-based
peptide drug that can efficiently inhibit tumor angiogenesis and
tumor growth.
Supplementary Data
Funding
The present study was supported in part by the Japan
Arteriosclerosis Research Foundation. The funding bodies had no
role in the design of the study, or data collection, analysis, or
interpretation, or in the preparation of the manuscript.
Availability of data and materials
All data generated or analyzed are included in this
published article (and in the included supplementary files).
Authors' contributions
HE conceived the experiments. KT and TO designed and
performed the experiments and analyzed the data. KT wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were performed in compliance with
the guidelines of the Institute for Laboratory Animal Research,
National Cancer Center Research Institute (February to March,
2019). The protocol was approved by the National Cancer Center
Research Institute (Approval no. NCC-T17-038).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor J. Inoue
of the Institute of Medical Science, the University of Tokyo, for
providing helpful discussions.
Abbreviations:
|
DPBS
|
Dulbecco's phosphate-buffered
saline
|
|
MMP
|
matrix metalloproteinase
|
|
MTA1
|
metastasis-associated protein 1
|
|
MetAP2
|
methionine aminopeptidase 2
|
|
NMIIA
|
non-muscle myosin II-A
|
|
Sp1
|
specificity protein 1
|
|
TIMP
|
tissue inhibitor of
metalloproteinases
|
References
|
1
|
Bjornland K, Winberg JO, Odegaard OT,
Hovig E, Loennechen T, Aasen AO, Fodstad O and Maelandsmo GM:
S100A4 involvement in metastasis: Deregulation of matrix
metalloproteinases and tissue inhibitors of matrix
metalloproteinases in osteosarcoma cells transfected with an
anti-S100A4 ribozyme. Cancer Res. 59:4702–4708. 1999.PubMed/NCBI
|
|
2
|
Buetti-Dinh A, Pivkin IV and Friedman R:
S100A4 and its role in metastasis-simulations of knockout and
amplification of epithelial growth factor receptor and matrix
metalloproteinases. Mol Biosyst. 11:2247–2254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boye K and Maelandsmo GM: S100A4 and
metastasis: A small actor playing many roles. Am J Pathol.
176:528–535. 2010. View Article : Google Scholar :
|
|
4
|
Endo H, Takenaga K, Kanno T, Satoh H and
Mori S: Methionine aminopeptidase 2 is a new target for the
metastasis-associated protein, S100A4. J Biol Chem.
277:26396–26402. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ochiya T, Takenaga K, Asagiri M, Nakano K,
Satoh H, Watanabe T, Imajoh-Ohmi S and Endo H: Efficient inhibition
of tumor angiogenesis and growth by a synthetic peptide blocking
S100A4-methionine aminopeptidase 2 interaction. Mol Ther Methods
Clin Dev. 2:150082015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishikawa M, Osaki M, Yamagishi M, Onuma K,
Ito H, Okada F and Endo H: Correlation of two distinct
metastasis-associated proteins, MTA1 and S100A4, in angiogenesis
for promoting tumor growth. Oncogene. 38:4715–4728. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kriajevska MV, Cardenas MN, Grigorian MS,
Ambartsumian NS, Georgiev GP and Lukanidin EM: Non-muscle myosin
heavy chain as a possible target for protein encoded by
metastasis-related mts-1 gene. J Biol Chem. 269:19679–19682. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kriajevska M, Bronstein IB, Scott DJ,
Tarabykina S, Fischer-Larsen M, Issinger O and Lukanidin E:
Metastasis-associated protein Mts1 (S100A4) inhibits CK2-mediated
phosphorylation and self-assembly of the heavy chain of nonmuscle
myosin. Biochim Biophys Acta. 1498:252–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe Y, Usada N, Minami H, Morita T,
Tsugane S, Ishikawa R, Kohama K, Tomida Y and Hidaka H:
Calvasculin, as a factor affecting the microfilament assemblies in
rat fibroblasts transfected by src gene. FEBS Lett. 324:51–55.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takenaga K, Nakamura Y, Sakiyama S,
Hasegawa Y, Sato K and Endo H: Binding of pEL98 protein, an
S100-related calcium-binding protein, to nonmuscle tropomyosin. J
Cell Biol. 124:757–768. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kriajevska M, Fischer-Larsen M, Moertz E,
Vorm O, Tulchinsky E, Grigorian M, Ambartsumian N and Lukanidin E:
Liprin beta 1, a member of the family of LAR transmembrane tyrosine
phosphatase-interacting proteins, is a new target for the
metastasis-associated protein S100A4 (Mts1). J Biol Chem.
277:5229–5235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen M, Bresnick AR and O'Connor KL:
Coupling S100A4 to rhotekin alters Rho signaling output in breast
cancer cells. Oncogene. 32:3754–3764. 2013. View Article : Google Scholar
|
|
13
|
Grigorian M, Andresen S, Tulchinsky E,
Kriajevska M, Carlberg C, Kruse C, Cohn M, Ambartsumian N,
Christensen A, Selivanova G and Lukanidin E: Tumor suppressor p53
protein is a new target for the metastasis-associated Mts1/S100A4
protein: Functional consequences of their interaction. J Biol Chem.
276:22699–22708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Semov A, Moreno MJ, Onichtchenko A,
Abulrob A, Ball M, Ekiel I, Pietrzynski G, Stanimirovic D and
Alakhov V: Metastasis-associated protein S100A4 induces
angiogenesis through interaction with Annexin II and accelerated
plasmin formation. J Biol Chem. 280:20833–20841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okada H, Danoff TM, Kalluri R and Neilson
EG: Early role of Fsp1 in epithelial-mesenchymal transformation. Am
J Physiol. 273:F563–F754. 1997.PubMed/NCBI
|
|
16
|
Ning Q, Li F, Wang L, Li H, Yao Y, Hu T
and Sun Z: S100A4 amplifies TGF-β-induced epithelial-mesenchymal
transition in a pleural mesothelial cell line. J Investig Med.
66:334–339. 2018. View Article : Google Scholar
|
|
17
|
Chow KH, Park HJ, George J, Yamamoto K,
Gallup AD, Graber JH, Chen Y, Jiang W, Steindler DA, Neilson EG, et
al: S100A4 is a biomarker and regulator of glioma stem cells that
is critical for mesenchymal transition in glioblastoma. Cancer Res.
77:5360–5373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lo JF, Yu CC, Chiou SH, Huang CY, Jan CI,
Lin SC, Liu CJ, Hu EY and Yu YH: The epithelial-mesenchymal
transition mediator S100A4 maintains cancer-initiating cells in
head and neck cancers. Cancer Res. 71:1912–1923. 2011. View Article : Google Scholar
|
|
19
|
Guo J, Bian Y, Wang Y, Chen L, Yu A and
Sun X: S100A4 influences cancer stem cell-like properties of MGC803
gastric cancer cells by regulating GDF15 expression. Int J Oncol.
49:559–568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Y, Zhou Y, Zhou X, Guo Y, Huang D,
Zhang J, Wang C and Cai L: S100A4 suppresses cancer stem cell
proliferation via interaction with the IKK/NF-κB signaling pathway.
BMC Cancer. 18:7632018. View Article : Google Scholar
|
|
21
|
Conlon GA and Murray GI: Recent advances
in understanding the roles of matrix metalloproteinases in tumour
invasion and metastasis. J Pathol. 247:629–640. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castro-Castro A, Marchesin V, Monteiro P,
Lodillinsky C, Rosse C and Chavrier P: Cellular and molecular
mechanisms of MT1-MMP-dependent cancer cell invasion. Annu Rev Cell
Dev Biol. 32:555–576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zarrabi K, Dufour A, Li J, Kuscu C,
Pulkoski-Gross A, Zhi J, Hu Y, Sampson NS, Zucker S and Cao J:
Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer
cell migration. J Biol Chem. 286:33167–33177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kayano K, Shimada T, Shinomiya T, Nakai S,
Hisa Y, Aoki T, Seiki M and Okada Y: Activation of pro-MMP-2
mediated by MT1-MMP in human salivary gland carcinomas: Possible
regulation of pro-MMP-2 activation by TIMP-2. J Pathol.
202:403–411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meirson T and Gil-Henn H: Targeting
invadopodia for blocking breast cancer metastasis. Drug Resist
Updat. 39:1–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koshikawa N, Hoshino D, Taniguchi H,
Minegishi T, Tomari T, Nam SO, Aoki M, Sueta T, Nakagawa T,
Miyamoto S, et al: Proteolysis of EphA2 converts it from a tumor
suppressor to an oncoprotein. Cancer Res. 75:3327–3339. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakamoto T and Seiki M: Integrated
functions of membrane-type 1 matrix metalloproteinase in regulating
cancer malignancy: Beyond a proteinase. Cancer Sci. 108:1095–1100.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishida-Aoki N, Tominaga N, Kosaka N and
Ochiya T: Altered biodistribution of deglycosylated extracellular
vesicles through enhanced cellular uptake. J Extracell Vesicles.
9:17135272020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to ImageJ: 25 years of image analysis. Nature Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirai M, Minematsu H, Kondo N, Oie K,
Igarashi K and Yamazaki N: Accumulation of liposome with Sialyl
Lewis X to inflammation and tumor region: Application to in vivo
bio-imaging. Biochem Biophys Res Commun. 353:553–558. 2007.
View Article : Google Scholar
|
|
33
|
Condeelis JS, Wyckoff JB, Bailly M,
Pestell R, Lawrence D, Backer J and Segall JE: Lamellipodia in
invasion. Semin Cancer Biol. 11:119–128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sroka IC, Nagle RB and Bowden GT:
Membrane-type 1 matrix metalloproteinase is regulated by sp1
through the differential activation of AKT, JNK, and ERK pathways
in human prostate tumor cells. Neoplasia. 9:406–417. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong IK, Byun HJ, Lee J, Jin YJ, Wang SJ,
Jeoung DI, Kim YM and Lee H: The tetraspanin CD81 protein increases
melanoma cell motility by up-regulating metalloproteinase MT1-MMP
expression through the pro-oncogenic Akt-dependent Sp1 activation
signaling pathways. J Biol Chem. 289:15691–15704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takino T, Nakada M, Li Z, Yoshimoto T,
Domoto T and Sato H: Tip60 regulates MT1-MMP transcription and
invasion of glioblastoma cells through NF-κB pathway. Clin Exp
Metastasis. 33:45–52. 2016. View Article : Google Scholar
|
|
37
|
Haas TL, Stitelman D, Davis SJ, Apte SS
and Madri JA: Egr-1 mediates extracellular matrix-driven
transcription of membrane type 1 matrix metalloproteinase in
endothelium. J Biol Chem. 274:22679–22685. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heo SH, Lee JY, Yang KM and Park KS: ELK3
Expression correlates with cell migration, invasion, and membrane
type 1-matrix metalloproteinase expression in MDA-MB-231 breast
cancer cells. Gene Expr. 16:197–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dulyaninova NG, Malashkevich VN, Almo SC
and Bresnick AR: Regulation of myosin-IIA assembly and Mts1 binding
by heavy chain phosphorylation. Biochemistry. 44:6867–6876. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masedunskas A, Appaduray MA, Lucas CA,
Cagigas ML, Heydecker M, Holliday M, Meiring JCM, Hook J, Kee A,
White M, et al: Parallel assembly of actin and tropomyosin, but not
myosin II, during de novo actin filament formation in live mice. J
Cell Sci. 131:jcs2126542018. View Article : Google Scholar
|
|
41
|
Lehtimaki J, Hakala M and Lappalainen P:
Actin filament structures in migrating cells. Handb Exp Pharmacol.
235:123–152. 2017. View Article : Google Scholar
|
|
42
|
Tatematsu N, Waguri-Nagaya Y, Kawaguchi Y,
Oguri Y, Ikuta K, Kobayashi M, Nozaki M, Asai K, Aoyama M and
Otsuka T: Mithramycin has inhibitory effects on gliostatin and
matrix metalloproteinase expression induced by gliostatin in
rheumatoid fibroblast-like synoviocytes. Mod Rheumatol. 28:495–505.
2018. View Article : Google Scholar
|
|
43
|
Lee M, Song SU, Ryu JK and Suh JK:
Sp1-dependent regulation of the tissue inhibitor of
metalloproteinases-1 promoter. J Cell Biochem. 91:1260–1268. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Clerck YA, Darville MI, Eeckhout Y and
Rousseau GG: Characterization of the promoter of the gene encoding
human tissue inhibitor of metalloproteinases-2 (TIMP-2). Gene.
139:185–191. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li DQ, Pakala SB, Reddy SDN, Ohshiro K,
Zhang JX, Wang L, Zhang Y, de Alborán IM, Pillai MR, Eswaran J and
Kumar R: Bidirectional autoregulatory mechanism of metastasis-
associated protein 1-alternative reading frame pathway in
oncogenesis. Proc Natl Acad Sci USA. 108:8791–8796. 2011.
View Article : Google Scholar
|
|
46
|
Lau JL and Dunn MK: Therapeutic peptides:
Historical perspectives, current development trends, and future
directions. Bioorg Med Chem. 26:2700–2707. 2018. View Article : Google Scholar
|
|
47
|
Katagiri N, Nagatoishi S, Tsumoto K and
Endo H: Structural features of methionine aminopeptidase2-active
core peptide essential for binding with S100A4. Biochem Biophys Res
Commun. 516:1123–1129. 2019. View Article : Google Scholar : PubMed/NCBI
|