Primary central nervous system (CNS) lymphoma
(PCNSL) is a rare subtype of extranodal non-Hodgkin lymphoma (NHL),
which is typically confined to the brain, spinal cord, eyes or
leptomeninges, without evidence of systemic spread. PCNSL was first
described by Bailey in 1929 as perivascular sarcoma (1). The symptoms of PCNSL often progress
rapidly and are non-specific and vary depending mainly on the
primary location of the lesions. Contrast-enhanced brain MRI is the
preferred method of diagnosis (2),
and the histological analysis of biopsy material, usually obtained
by imaging-guided stereo-tactic biopsy, is regarded as the gold
standard for the diagnosis of PCNSL (3). In total, ~95% of PCNSL cases
worldwide are classified histologically as diffuse large B-cell
lymphomas (DLBCL); however, PCNSL is unique and distinct from
systemic DLBCL (4,5). Considering the recent identification
of distinct genotypic and immunophenotypic features, with reference
to morphological and clinical characteristics, primary DLBCL of the
CNS (PCNS-DLBCL) has been classified as a specific lymphoma subtype
in the World Health Organization lymphoma classification system
since 2008 (6). Since the majority
of PCNSL cases are DLBCL and other pathological types are very
rare, PCNS-DLBCL and PCNSL are almost the same concept. The median
age at diagnosis of PCNSL is 65 years and the incidence is rising
rapidly in the elderly population (7,8).
Patients >60 years old account for >50% of all PCNSL cases
and up to 20% of all patients with PCNSL are aged ≥80 years
(2). Notably, age has been
identified as an independent poor prognostic factor for PCNSL
(9). For PCNSL, two major scoring
systems have been established and are widely used. Elderly patients
have an inferior prognosis compared with that of younger patients
and are more severely affected by iatrogenic toxicity; therefore,
they represent a unique and vulnerable treatment subgroup (10,11).
There is an unmet clinical need to define optimal treatment for
this population. In the present review, the available literature
has been reviewed to provide an improved understanding of the
epidemiology, clinical characteristics, diagnosis, prognosis and
management of PCNSL in the elderly; the present review focused on
the recent advances in prognosis and treatment.
PCNSL accounts for ~4% of all intracranial
malignancies, 5% of all extranodal lymphomas and <1% of all
non-Hodgkin lymphomas (12). The
incidence rate for PCNSL worldwide is 0.47 per 100,000 people per
year, occurring mostly in the 6th decade of life, with a
male:female ratio of 1.2:1.7 (7,13,14).
Moreover, a rising incidence has been observed in patients aged
>60 years in the last 10 years, with patients aged 70-79 years
old having the highest incidence (15). Notably, African-American
individuals tend to present with PCNSL at a younger age (<50
years), whereas Caucasian individuals usually present with the
disease when >50 years old (7).
PCNSL can affect immunocompetent and
immunocom-promised patients. Most cases occur sporadically;
however, a compromised immune system, both primary and acquired,
has been reported to be a predisposing factor for PCNSL (16). The risk of PCNSL has also been
reported to be 2-6% in patients with AIDS and 1-5% in those that
have received organ transplants (13). Primary immunodeficiency has been
reported to confer a 4% risk of developing PCNSL. In addition, the
incidence of PCNSL has been shown to be inversely correlated with
CD4 cell counts (17) and has
declined in HIV-infected individuals since the widespread use of
highly active antiretroviral therapy (7,18).
Currently, immunocompetent patients represent the vast majority of
patients with PCNSL (19); in
addition, these patients are often diagnosed between the ages of 50
and 70 years, whereas immunocompromised patients often present with
the disease earlier in life, in their 30s and 40s (20).
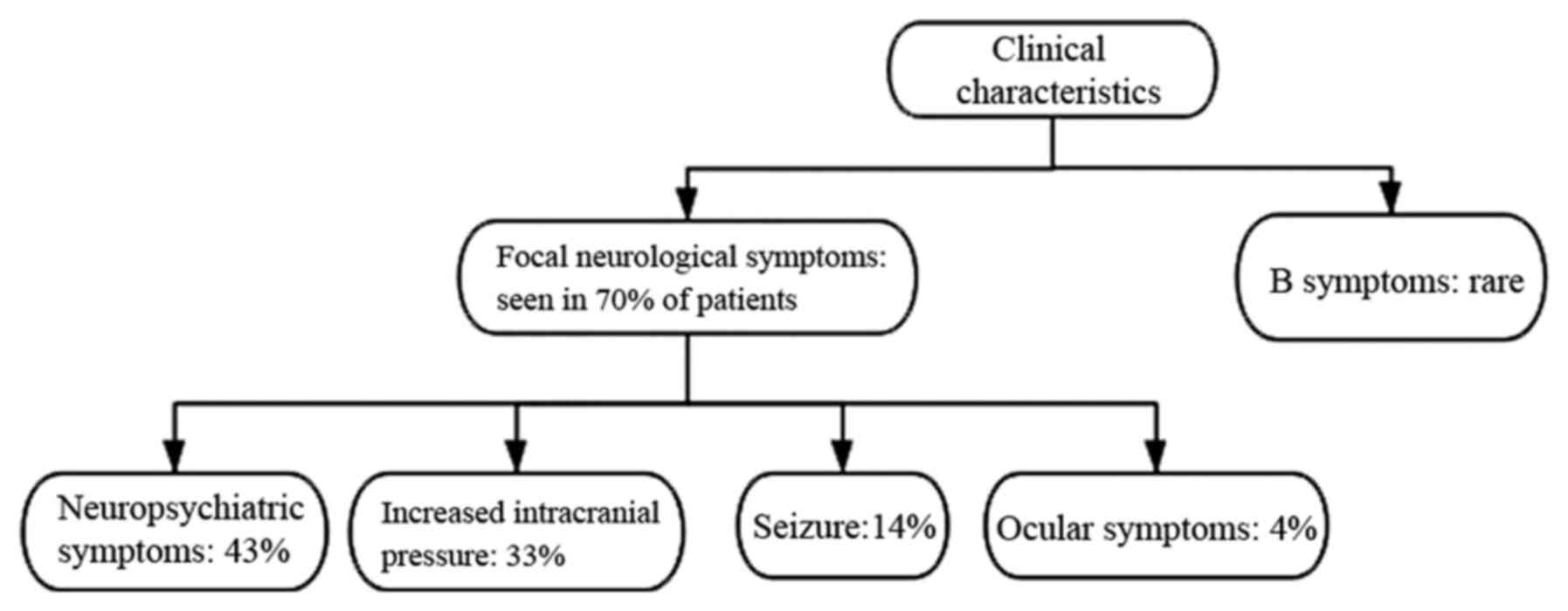
The clinical manifestation of PCNSL varies depending
mainly on the primary location of the lesions. The symptoms often
progress rapidly and are non-specific. The most common presentation
is focal neurological symptoms, which have been observed in 70% of
patients (21). In addition, a
total of 43% of patients presented with neuropsychiatric symptoms,
followed by signs of increased intracranial pressure, such as
headache and vomiting in 33%, seizures in 14% and ocular symptoms
in 4% of cases (21). In addition,
'B symptoms', such as weight loss, fever and night sweats have been
reported to be rare in PCNSL (21)
(Fig. 1).
PCNSL nearly always exhibits significant contrast
uniform enhancement, with or without necrosis, on computed
tomography (CT) and magnetic resonance imaging (MRI) scans
(21). Furthermore, linear
enhancement along perivascular spaces is highly characteristic of
PCNSL (22). On a CT scan, PCNSL
in immunocompetent individuals often presents as a single hyper- or
iso-attenuated lesion. On an MRI scan, PCNSL is typically
iso-hypointense on T1-weighted imaging and iso-hypointense to gray
matter on T2-weighted imaging; in a previous study, in 85% of
patients, a strong homogeneous pattern of enhancement was detected,
due to its hypercellularity (21).
However, PCNSL lesions may be non-contrast-enhancing, and can
contain atypical features of hemorrhage, calcification, cysts and
necrosis (23).
Lesions are often centrally located within cerebral
white matter, and are often found in the periventricular region. A
total of 87% of PCNSL lesions were previously reported to be
supratentorial, with 39% of them having frontoparietal involvement
in a retrospective analysis from French and Belgian medical centers
(12). A total of 9-25% of newly
diagnosed PCNSL were also demonstrated to have cerebellar
involvement (24,25). Malikova et al (26) demonstrated that parenchymal
involvement was much more common than previously hypothesized.
Leptomeningeal lesions of CNSL often involve the cranial nerves,
subependymal regions spinal cord or spinal nerve roots, whereas
dural involvement is rare in PCNSL or secondary CNSL (22).
While immunocompetent patients usually have solitary
lesions with homogenous enhancement, 20-40% of cases have been
reported to present with multiple lesions, and up to 13% with
ring-like enhancement (27). The
typical appearance of PCNSL in patients with immunodeficiency is
different from that in immunocompetent patients. A total of 30-80%
of immunodeficient patients have been shown to present with
multiple lesions that usually have necrosis, resulting in an
irregular ring-enhancing pattern and a higher propensity for
spontaneous hemorrhage (27).
Notably, ~95% of PCNSL cases are classified
histologically as DLBCL, and 5% as other histologies, including
T-cell, Burkitt, lymphoblastic and marginal zone lymphomas
(28). Gene expression profiling
has been used to establish three major DLBCL subtypes: Germinal
center B-cell-like (GCB), activated B-cell-like (ABC) and type 3.
The type 3 subgroup is not well defined, but the type 3 and ABC
subtypes appear to have a poor outcome and are often grouped
together as non-germinal center (non-GC) subtype (29). Further genomic sequencing revealed
that the pattern of somatic mutations in DLBCL was classified as
GCB tumors and non-GC tumors depending on the cell of origin. GCB
tumors were revealed to more likely have mutations in EZH2 and
GNA13, and translocations in bcl-2. Non-GC tumors were associated
with mutations in MyD88, CD79B, CARD11 and TNFAIP3, all of which
are involved in B-cell receptor (BCR) signaling activating NF-κB
(30). Tumors can be subdivided
into GCB and non-GC types based on the expression pattern of CD10,
bcl-6 and MUM-1/IRF4. Staining of PCNSL biopsies to distinguish
these DLBCL subgroups (CD10-, bcl-6+,
MUM-1/IRF4+) showed that the vast majority (>80%) of
PCNSL-DLBCLs were the non-GC immunophenotype (31). To the best of our knowledge,
age-related pathological characteristics in PCNSL tumor tissue have
not been identified.
PCNSL typically presents as an intracranial mass
lesion, alongside a combination of generalized symptoms, including
headaches, confusion and lethargy, and lateralizing symptoms, such
as hemiparesis. Focal neurological deficits have been reported to
affect 50% of patients, and PCNSL is usually misdiagnosed early on
as a cerebrovascular disorder (2).
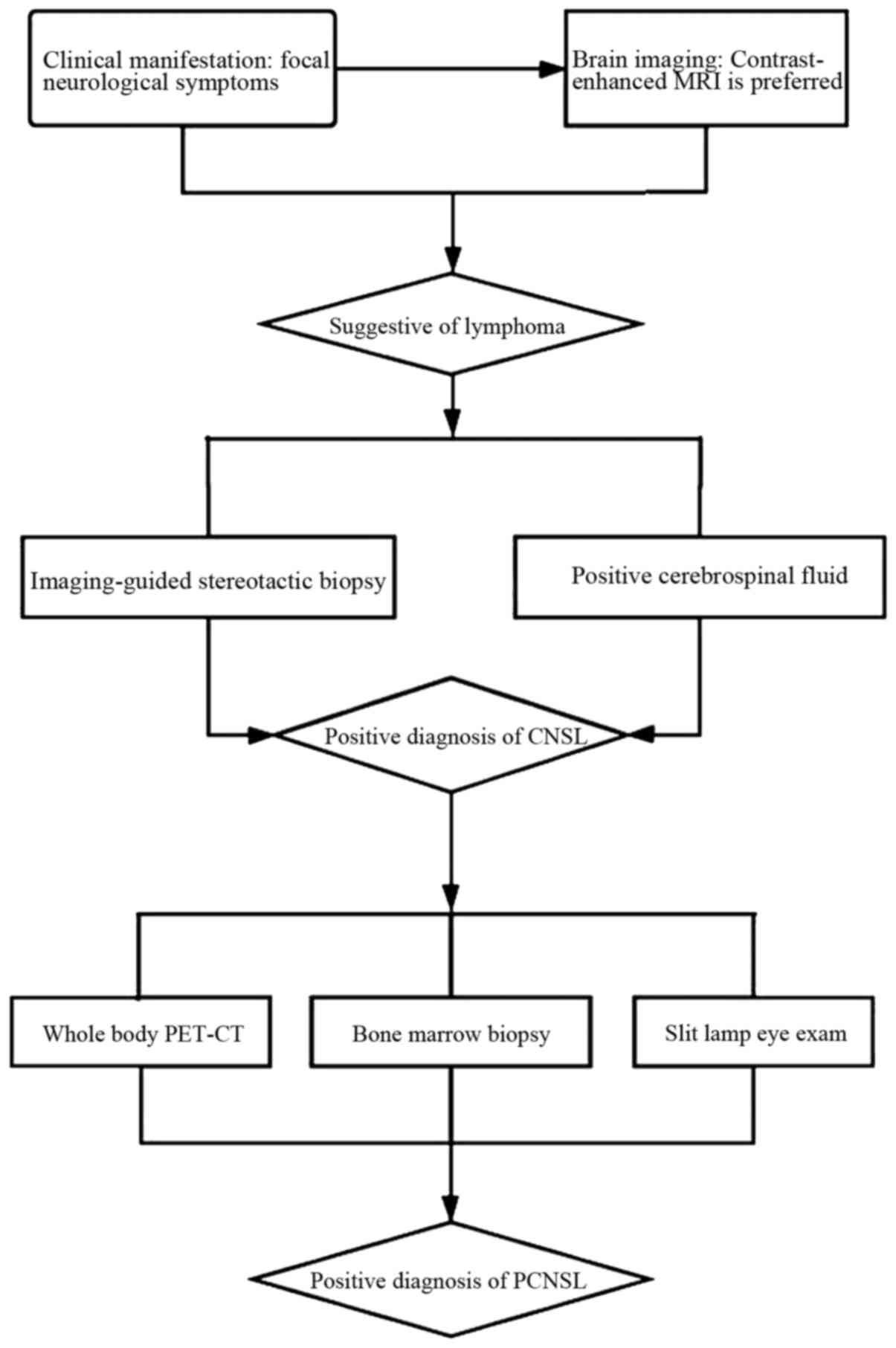
Contrast-enhanced brain MRI scan is the preferred method of
diagnosis (2). Definitive
diagnosis requires pathological confirmation. For the diagnosis of
CNSL, histological analysis of biopsy material, usually obtained by
imaging-guided stereotactic biopsy, is regarded as the gold
standard (3). An early brain
biopsy when CNSL is suspected is considered a valid option to
reduce diagnostic delay, with a high rate of definitive diagnosis
and a low complication rate (24).
A positive cerebrospinal fluid (CSF) test for lymphoma, referring
to the presence of lymphoma cells in CSF by flow cytometry and
cytology, can obviate the need for a surgical procedure (32). For the diagnosis of PCNSL, a body
positron emission tomography-computed tomography scan, bone marrow
biopsy and slit lamp eye exam should be performed to exclude
extraneural disease (Fig. 2).
Notably, PCNSL is sensitive to corticosteroids, and
pathological evaluation of corticosteroid-pretreated PCNSL has been
shown to lead to a final diagnosis in only 50-85% of cases
(33). Sensitivity may also be
significantly reduced when tissue is obtained through a
stereotactic biopsy after the patients were treated with
corticosteroids (24). Therefore,
corticosteroids should be avoided prior to biopsy as much as
possible, in order to decrease their impact on diagnosis.
When stereotactic biopsy is not possible or
conclusive, certain biomarkers have been shown to help establish
the diagnosis of CNSL (Table I).
In HIV-positive patients with CNSL, Epstein-Barr virus (EBV) DNA in
the CSF may be used as a diagnostic biomarker (34). In HIV-negative patients, several
diagnostic biomarkers, including inter-leukin (IL)-10, IL-6, CXC
chemokine ligand-13 (CXCL13), neopterin, β2-microglobulin (β2-MG),
osteopontin, soluble CD27, specific microRNAs and cell-free DNA,
have been described (35-45). Soluble transmembrane activator and
CAML interactor, and soluble B-cell maturation antigen in the CSF
have also been identified as promising novel biomarkers for the
diagnosis and treatment monitoring of PCNSL (46). In addition, a
proliferation-inducing ligand, alone or in combination with B-cell
activating factor, in the CSF may serve as a diagnostic biomarker
for patients with CNSL (3).
Furthermore, next-generation sequencing of circulating tumor DNA
isolated from CSF samples may provide a promising diagnostic
biomarker (47). Moreover, in
recent years the clinical significance of susceptibility-weighted
imaging has been established in the differential diagnosis of PCNSL
(48).
PCNS-DLBCL is an aggressive malignancy that has been
reported to be associated with an overall survival (OS) of 12-18
months (28,49,50).
Without treatment, OS has been shown to decrease to 1.5-3.3 months
(51). Since the introduction of
high-dose (HD)-methotrexate (MTX)-based chemotherapy regimens, OS
has increased substantially; it has been reported to be 16.3-66
months with a 2-year OS rate of 42-80.8% (52-56).
Outcomes for patients aged >60 years remain poor,
with 1-year progression-free survival (PFS) rates being reported at
~40% and a median OS in the range of 8-43 months in elderly
patients receiving multi-drug regimens, including HD-MTX (57-63).
Until now, only a few randomized controlled trials (10,59-65)
dedicated to treating PCNSL in the elderly population have been
performed (Table II). An
epidemiological analysis demonstrated that, while the median OS of
all patients with PCNSL doubled, from 12.5 months in the 1970s to
26 months in the 2010s, this progress was restricted to young
patients. Conversely, the median OS of patients with PCNSL aged ≥70
years has not improved in >40 years (6 months in the 1970s vs. 7
months in the 2010s) (66).
Furthermore, patients aged >70 years have been excluded from
several clinical trials; it has been reported that ~1/4 of patients
aged >70 years with PCNSL who survive for >3 months from
diagnosis do not receive chemotherapy at all in the US (53). Notably, in a previous study, the
fraction of patients who were not treated with chemotherapy
increased from 14 to 23, and to 44% in the of 61-70, 71-80 and
>80 years age groups, respectively (2). Therefore, the treatment of PCNSL,
particularly in elderly patients, remains challenging.
Although PCNSL is a significantly chemosensitive
neoplasm, often achieving complete response (CR) after initial
treatment, 30-50% of patients may not benefit from this intensive
treatment due to old age, delayed neurotoxicity, drug resistance or
relapse (67,68). Despite advances in induction and
consolidation regimens, relapse has been observed in 35-60% of
patients 2 years after the initial diagnosis, and in 4% of patients
5 years after the initial diagnosis (69). In a large population-based study,
overall prognosis was found to be poor following salvage therapy,
with the median PFS and OS following recurrence at 2.2 and 3.5
months, respectively, with elderly patients having the worse
outcome (67). In addition, ~1/3
of the patients with PCNSL had primary refractory disease, that is,
they failed to respond to the first-line treatment (32). In a French prospective cohort,
refractory patients were found to have a poor prognosis (median OS,
2.1 months) (68). Despite the
development of novel and intensified therapeutic regimens, PCNSL
has a very poor prognosis and its incidence in people aged ≥65
years is increasing in the US (8).
For PCNSL, two major scoring systems have been
established and are widely used. The Memorial Sloan-Kettering
Cancer Center prognostic model describes three groups, based on age
and Karnofsky performance score (KPS; Table III). The most relevant are poor
performance status and advanced age. Patients aged >50 years
with a KPS of <70 have been reported to have the worst
prognosis, with a median survival of 1.1 years (70). The International Extranodal
Lymphoma Study Group (IELSG) described five prognostic factors as
independent predictors of poor prognosis with a low OS (Table IV). Each factor was set at 1
point. According to the degree of integration, they were divided
into three groups, 0-1, 2-3 and 4-5. The 2-year survival of
patients with 0-1, 2-3 or 4-5 of these unfavorable factors was 80,
48 and 15%, respectively (71).
Although the aforementioned two prognostic scoring
systems already exist, risk assessment at the time of diagnosis
remains unsatisfactory. Several prognostic markers have been
reported in patients with PCNSL (Table
V).
Gross total resection following surgery is a
significant independent favorable prognostic marker for OS
(72). The completion of three
cycles of HD-MTX chemotherapy was also found to be a significant
independent prognostic factor for patient survival (73). In a previous study, patients with
CR following initial HD-MTX had a longer survival time (74). CR status following HD chemotherapy
with autologous stem cell transplantation (HDT/ASCT) and the use of
thiotepa in a HDT regimen have also been identified as independent
prognostic predictors for OS and PFS, respectively. Multivariate
analysis identified non-CR at HDT/ASCT as an independent prognostic
factor for poor OS (75). Notably,
patients treated with thiotepa-containing HDT had a significantly
superior 5-year PFS and OS compared with those receiving HDT
without thiotepa (75). Bcl-6
expression has also been determined to be a favorable prognostic
marker (76). Bcl-6 expression and
high KPS, as independent prognostic parameters, have been
associated with a favorable outcome (77). In addition, an Eastern Cooperative
Oncology Group (ECOG) score of ≤2, multiple brain lesions, a
maximum tumor diameter of <5 cm and CD10+ expression
were found to be significantly associated with a prolonged OS
(78). Furthermore, the texture
analysis of contrast-enhanced MRI may have the potential to predict
PCNSL prognosis. In a previous study, grey-level co-occurrence
matrix-homogeneity (<0.2864) was associated with a favorable
survival and could be considered an independent predictor (79). Alame et al (80) reported that the expression of
programmed death-1 protein (PD-1) on tumor-infiltrating lymphocytes
(TILs) and programmed death-1 ligand (PD-L1) on tumor-associated
macrophages was correlated with favorable survival. The serum level
of soluble PD-L1 (sPD-L1) has also been reported to act as a
reliable biomarker to predict the probability of relapse and
survival outcome in patients with PCNSL; sPD-L1 (<0.432 ng/ml)
has been associated with a longer OS and PFS (81). The expression of MHC II genes was
also found to predict a favorable outcome following chemotherapy
(82). The expression of bcl-6,
IMO2 and CD10 has also been associated with a favorable prognosis
(82).
Male sex, HIV infection and being of
African-American descent have been reported as independent
predictors of mortality in patients aged 0-49 years. In patients
aged >50 years, only advanced age was associated with decreased
survival (7). Furthermore, ECOG
>3 and multifocal lesions have been found to be significant
independent unfavorable prognostic markers for PFS (83). Infratentorial location and large
tumor volume (>11.4 cm3) were also revealed to be
associated with poor OS and PFS, respectively (84). CXCL13 and the presence of anemia
have also been found to be poor prognostic markers in PCNSL
(85,86). In addition, patients with
EBV-positive PCNSL had a shorter OS than those with EBV-negative
PCNSL (87). In a previous study,
the most important prognostic factors associated with a higher risk
of progression were ABCB1 rs1045642 CC genotype, ECOG performance
status >2 and older age (88).
Elevated CSF IL-10 and STAT3 phosphorylation have also been shown
to be associated with a worse prognosis (89). Furthermore, as demonstrated by
immunohistochemistry, concurrent expression of myc and bcl-2, also
known as double-expression lymphoma, was associated with inferior
OS and PFS (90). Bcl-2 gene
aberrations, bcl-2/c-myc gene double-hit and bcl-6 rearrangements
have also been associated with adverse outcomes (91,92).
In addition, the expression levels of MUM1, cyclin D2, p53, CD5,
FOXP1, ICAM1, HLA-DR and bcl-2 have been related to poor prognosis.
A strong FOXP1 positivity, myc and bcl-2 overexpression, bcl-6
translocations, and a high Ki-67 index have been associated with
unfavorable prognosis in a previous study (82). Takano et al (93) reported that MyD88 mutations
occurred more frequently in elderly patients and were associated
with poor prognosis. However, another previous study demonstrated
that MyD88 mutations were not associated with a change in PFS or
OS, whereas CD79b mutations were associated with improved PFS and
OS (94). The tumor expression
levels of activated STAT6, and elevated levels of CSF IL-4 and
IL-10 have been suggested as potential adverse prognostic
biomarkers for PCNSL (95). Serum
β2-MG (≥1.8 g/ml) may be associated with a shorter OS in PCNSL
(96). It has also been reported
that tumoral PD-L1 (tPD-L1) expression and the number of
PD-1+ TILs were independent prognostic factors for
PCNS-DLBCL. tPD-L1+ patients with a small number of
CD8+ or PD-1+ TILs exhibited the worst
prognosis, whereas tPD-L1- patients with a large number
of CD8+ or PD-1+ TILs exhibited the best
prognosis (97). Notably, in
HIV-related PCNSL, the CD4 count and HIV RNA viral load have been
found to be correlated with survival (98).
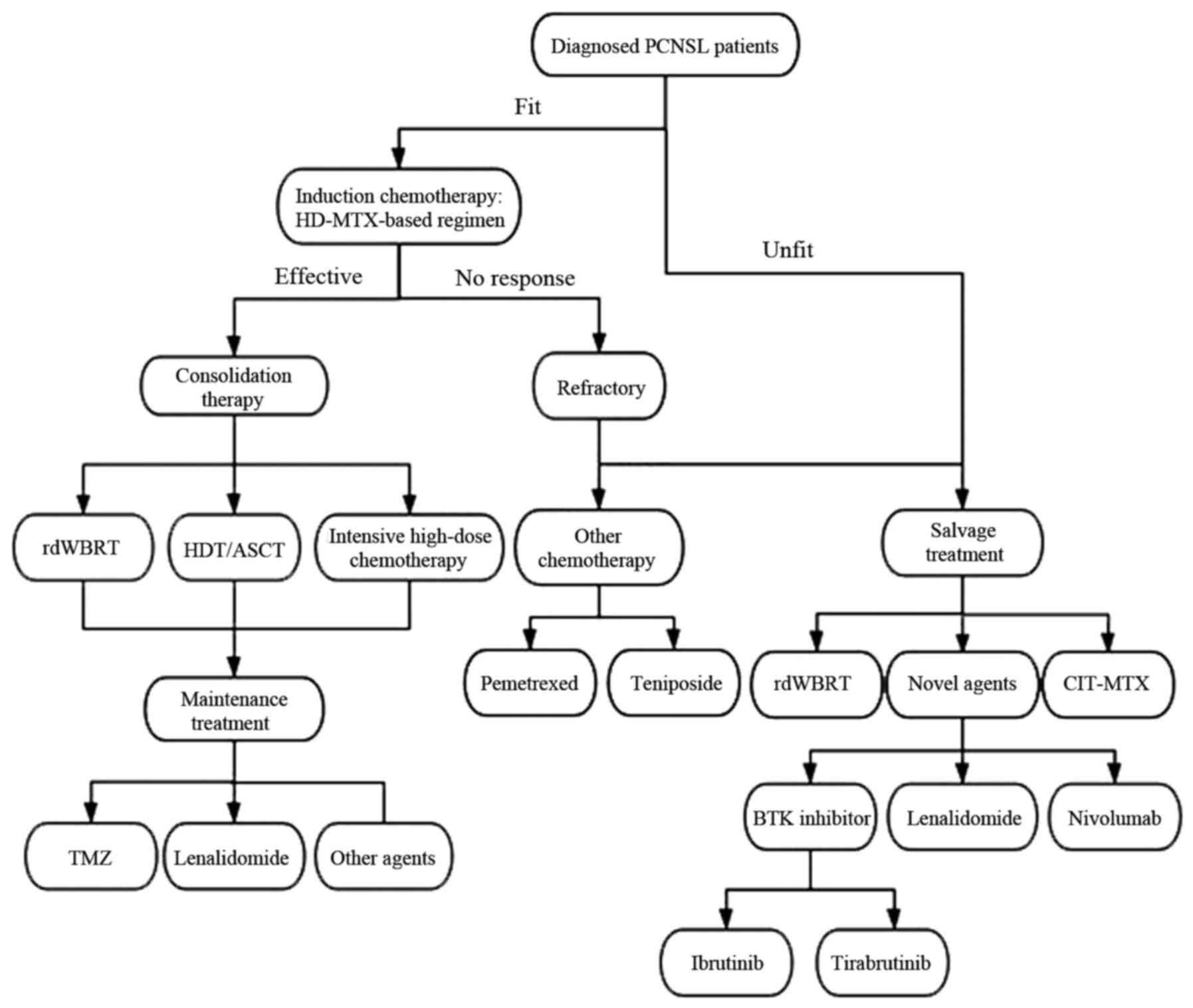
PCNSL often has a favorable response to chemotherapy
and radiotherapy (RT). Currently, the mainstay of treatment for
patients with PCNSL is induction chemotherapy, which aims for CR,
followed by consolidation therapy that aims to eradicate residual
disease and improve OS (99). The
treatment of PCNSL is based on age and performance status (100).
Older age is associated with an accumulation of
physiological deficits that alter the pharmacokinetics and
pharmacodynamics of therapies, and may increase the risk of
toxicity. Performance status should be considered an important
predictor of OS and drug toxicity. When selecting a treatment for
elderly patients, multiple parameters should be considered. The
American Society of Clinical Oncology Guidelines advocates for the
use of geriatric assessment tools in patients ≥65 years who have
received chemotherapy to identify vulnerabilities not routinely
captured in oncology assessments (101). These tools are useful in
predicting chemotherapeutic toxicity and mortality. The
chemotherapy risk assessment scale for high-age patients (CRASH)
score developed by Extermann et al (102) considers the specific chemotherapy
regimen to be used, laboratory values, and functional, mental and
nutritional status assessments. The CRASH score then stratifies
patients into four risk categories: Low, medium-low, medium-high
and high. The routine use of such a score may increase the
proportion of elderly patients that receive optimal treatment. For
example, a 'fit elderly' individual may tolerate standard doses and
schedules of chemotherapeutic drugs, and may thus reap the same
benefits as younger patients.
In the elderly population, an individualized
treatment that aims at prolonging survival while minimizing
toxicity is required (Fig. 3).
Despite the fact that the median OS of all patients has doubled
over the last 40 years, this survival benefit is restricted to
patients aged <70 years (66).
Therefore, identifying effective and tolerable treatment strategies
for elderly patients is one of the key future challenges.
PCNSL is sensitive to corticosteroids, with reported
response rates between 20 and 40% (103,104). Corticosteroids have been shown to
decrease tumor-associated edema and may result in a partial
radiographic regression of tumors. However, certain studies have
reported that the preoperative use of steroids can compromise the
efficacy of brain biopsy in patients with PCNSL (105,106). As such, the classic rationale
recommends withholding steroid treatment for ≥14 days prior to a
brain biopsy (24). An initial
response to corticosteroids has been reported to be associated with
a favorable outcome in PCNSL (107). As such, corticosteroids should be
avoided prior to biopsy as much as possible, in order to decrease
their impact on diagnosis. However, when the symptoms are serious
and life-threatening, corticosteroids should be used to relieve the
symptoms and prevent cerebral herniation. Treatment should be given
in the smallest possible dose and for the shortest possible time to
avoid long-term adverse reactions. In addition, almost all patients
relapse quickly after an initial response to corticosteroids
(108).
In newly diagnosed patients with PCNSL, the standard
management approach, according to the 2018 NCCN guidelines
(109), is HD-MTX-based
chemotherapy, followed by consolidation therapy with whole brain RT
(WBRT). MTX is an antifolate that suppresses DNA synthesis by
inhibiting dihydrofolate reductase activity in purine and thymidine
synthesis, which controls the expression of glucocorticoid
receptors in blood cells (110,111). HD-MTX, one of the few drugs able
to penetrate the blood-brain barrier (BBB), is commonly used as a
first-line treatment for PCNSL. When combined with other agents,
such as HD-cytarabine, temozolomide (TMZ) and rituximab, HD-MTX
combination therapy has been revealed to offer a better prognosis
compared with that of single therapeutics alone (55,112,113). Moreover, HD-MTX is considered a
relatively safe treatment for patients with PCNSL regardless of age
(114), and is even tolerable to
patients aged ≥80 years (115).
While HD-MTX-based treatment is widely accepted in clinical
settings, 50% of patients may have a risk of progression or
recurrence, and HD-MTX can cause renal dysfunction (116,117).
Pemetrexed is also an antifolate and is similar to
MTX in its ability to penetrate the CNS; however, it has an
advantage of targeting more than one site in folate metabolism.
Pemetrexed interrupts purine synthesis by inhibiting thymidylate
synthase and dihydrofolate reductase, and interrupts pyrimidine
synthesis via inhibition of glycinamide ribonucleotide
formyltransferase and aminoimidazole carboxamide for
methyltransferase (118). Raizer
et al (119) investigated
the antitumor activity and safety of pemetrexed in recurrent PCNSL,
and found that pemetrexed had single-agent activity in R/R PCNSL
and possible toxicities were due to the use of higher than standard
doses. Han et al (120)
studied 12 patients with newly diagnosed PCNSL that were >65
years old; four patients presented CR and six patients had partial
response (PR), with a median PFS of 9.0 months and a median OS of
19.5 months. This previous study demonstrated that the single agent
pemetrexed may be feasible, active and well tolerated in elderly
patients with PCNSL.
Rituximab is a large, monoclonal antibody against
CD20. Since ~98% of small molecule drugs and most macro-molecular
drugs are unable to pass through the BBB (121), the addition of rituximab to
HDMTX-based regimens remains controversial.
Several clinical studies have demonstrated that
rituximab can promote CR and prolong survival (113,122-125), an effect that occurs regardless
of age (126). Houillier et
al (58) reached the same
conclusion, and revealed that the addition of rituximab improved
the response rate of an MTX-based regimen (77 vs. 53% without
rituximab) with a good tolerance profile, except for an increased
rate of leucopenia. In addition, there is evidence that disruption
of the BBB by the presence of CNS lymphoma permits an enhanced
penetration of rituximab into the CNS compartment to reach
therapeutic concentrations (127). The international randomized trial
IELSG32 suggested that the addition of rituximab to HD-MTX and
HD-cytarabine improved response, PFS and OS (128). A meta-analysis suggested a
possible beneficial effect of rituximab on PFS and supported the
addition of rituximab when MTX-based chemotherapy was planned
(129).
Conversely, in the recently published HOVON trial,
rituximab was not found to have an effect on response or survival
in the treatment of PCNSL. There was no difference in survival or
response for patients treated with or without rituximab, after a
median follow up of 32.9 months (130).
Teniposide is a topoisomerase inhibitor, highly
lipopolysaccharide, which can cross the BBB and has been used in
the treatment of PCNSL (131,132). The penetration of teniposide has
been widely examined in normal brain tissue and in brain tumor
tissue (133). The phase II trial
by the European Organization for Research and Treatment of Cancer
Lymphoma Group indicated that the use of MTX, teniposide,
carmostine and methylprednisolone resulted in improved outcomes,
with a 3-year OS rate of 58% (132). A randomized phase II trial also
identified that a chemo-therapy regimen, which included
fotemustine, teniposide and dexamethasone, was an effective and
safe protocol for treating patients with newly diagnosed PCNSL
(134). In a previous
retrospective study, 56 patients were admitted, with a median age
of 54.5 years. The results indicated that the HD-MTX + teniposide
regimen, compared with HD-MTX alone, not only improved the CR rate
in the short-term, but also improved long-term efficacy in the
treatment of PCNSL (135).
A previous study demonstrated that the combination
of surgical excision and chemotherapy conferred a more favorable
outcome than chemotherapy alone, suggesting that multimodal
treatment might be more beneficial. With regard to the specific
extent of excision, a more extensive resection was revealed to be
associated with a more favorable survival (136). Kinslow et al (137) identified a significant survival
advantage for patients with PCNSL treated with adjuvant RT
following surgery.
It has been reported that a total of 20-30% of
patients with PCNSL relapse within 6 months, even after intensive
MTX treatment (113), indicating
consolidation therapy is necessary to eliminate minimal residual
disease in PCNSL. Until the beginning of this century, WBRT was the
only consolidation therapy available for PCNSL. Thereafter,
clinical trials investigating various types of consolidation have
fit into an emerging concept, which suggests that the treatment of
PCNSL is most efficient if HD-MTX-based treatment is followed by a
type of consolidation treatment (138).
WBRT is an important means of treating PCNSL with a
total response rate of 90%; however, the OS has been reported to be
only 12-16 months and it can be accompanied by marked neurotoxicity
(139). Radiation-induced
neurotoxicity includes progressive severe cognitive dysfunction,
ataxia and urinary incontinence. The elderly are also susceptible
to the detrimental cognitive side effects of WBRT. In a previous
study, neurotoxicity occurred in 19-83% of patients aged >60
years who received WBRT following MTX-based chemotherapy (2). Furthermore, neurotoxicity tends to
occur more severely and rapidly in patients with advanced age at
the time of treatment (2).
As WBRT is associated with a substantial risk for
cognitive impairment, alternative consolidation methods have been
used, including reduced-dose WBRT (rdWBRT). A study that used
rdWBRT as consolidation therapy reported good PFS and OS rates
following treatment (140).
Furthermore, a recent study demonstrated that rdWBRT (≤23.4 Gy)
combined with HD-MTX exhibited no statistical difference in terms
of OS and PFS, as compared with higher-dose WBRT, and suggested
that patients aged <60 years might benefit from rdWBRT (141). The value of consolidation WBRT
and the optimal dose of RT remain controversial, particularly in
older patients.
Based on the available data, HDT/ASCT as
consolidation therapy for patients with PCNSL is considered an
effective and feasible strategy, with less overall neurotoxicity
compared with WBRT; however a higher risk of treatment-related
mortality has been reported with HDT/ASCT compared with WBRT
(142). Conditioning regimens
that contain CNS-penetrant agents, including carmustine, thiotepa
and busulfan, have exhibited the most encouraging results. In a
retrospective study, patients treated with thiotepa-containing
HDT/ASCT were found to have a significantly better 2-year PFS (62%)
and OS (70.8%); however, the associated toxicity limited their use
in elderly patients. Therefore, for selected 'biologically young'
patients, MTX-based chemotherapy with HDT/ASCT as consolidation
therapy is a viable option (143).
Intensive HD chemotherapy as consolidation therapy
may also be a reasonable option for those patients who respond to
first-line chemotherapy, but the advanced age and poor general
condition of most patients are major obstacles to this approach.
While intensive consolidation may improve outcomes in patients aged
<70 years, HD chemotherapy is not an option for the majority of
elderly patients with PCNSL (144). A previous study showed no
additional benefit of a prolonged consolidation treatment with
cytarabine following HD-MTX-based chemotherapy in the elderly
(58).
In elderly patients who cannot tolerate
consolidation therapy, maintenance treatment may serve as a
feasible alternative approach to prolonging remission, delaying
relapses and maintaining tumor dormancy. Several available and
promising chemotherapy and targeted agents, such as TMZ,
procarbazine, lenalidomide and ibrutinib may serve a role in
maintenance therapy for PCNSL (145). To the best of our knowledge,
there has been no randomized clinical trial conducted proving that
maintenance treatment is beneficial in PCNSL.
TMZ, an oral alkylating agent, is known to
effectively penetrate the BBB and have a tolerable toxicity
profile. It is generally an easily administered drug, given orally
for 5 days for a 4-week cycle. TMZ has been incorporated into
induction treatment for elderly patients with PCNSL (10,64).
It has been reported that TMZ may be effective as single-agent
maintenance therapy against PCNSL in elderly patients to maintain
quality of life and functional independence with the best
neurocognitive preservation results (65). Notably, a previous study introduced
TMZ as a maintenance treatment in elderly patients with promising
results (65). It is a potential
option for elderly patients with PCNSL, who have achieved CR or PR
and wish to avoid WBRT neurotoxicity, particularly those considered
unsuitable for ASCT (117).
Despite advances in induction and consolidation
regimens, 10-15% of patients with PCNSL have been reported to
become refractory to initial treatment and 35-60% relapse within
1-2 years (9,146). The ideal approach to treat
patients with R/R PCNSL is unclear.
CIT-MTX has been reported to be a promising therapy
not only for patients treated with conventional common therapy, but
also those at high risk from HD-MTX. A better prognosis in patients
treated with CIT-MTX may be derived from the stable concentration
of MTX in the CSF (147).
HDT/ASCT has been used to treat patients with R/R
PCNSL with a 3-year event-free survival of 53% and an OS of 64%
(148). In relapsed patients,
HDT/ASCT appears to have an equivalent or improved efficacy
compared with other salvage therapies; however, the risk of
treatment-related mortality is a consideration (85). A previous study suggested that
HDT/ASCT and rdWBRT may be associated with white matter
abnormalities in patients with PCNSL who have achieved long-term
remission, and that patients treated with rdWBRT may be at greater
risk (149). Moreover, HDT/ASCT
cannot be used to treat frail elderly patients.
Allo-HCT has been studied in R/R PCNSL; however, to
the best of our knowledge, the only available data is from two case
reports by Atilla et al (150) and Varadi et al (151). The results of these studies were
encouraging, suggesting Allo-HCT might be an alternative option for
R/R PCNSL. Evaluating the safety and efficacy of allo-HCT in PCNSL
requires further clinical trials, particularly phase III randomized
trials.
Cumulative data have revealed that immunotherapy
with CART-cells provides hope for a high response rate in patients
with R/R DLBCL (152). However,
clinical trials on CART-cell therapy usually rule out PCNSL because
of the occurrence of lethal cerebral edema after CART-cell therapy.
A case report from Tu et al (152) reported that combination treatment
with CD19- and CD70-specific CART-cells may effectively target
PCNSL and maintain disease-free survival without inducing cytokine
release syndrome or CART-related encephalopathy syndrome, thus
indicating that CART-cell therapy may be a potentially promising
treatment for patients with R/R PCNS-DLBCL.
Based on new insights into the pathogenesis of
PCNSL, novel agents have been introduced to treat PCNSL. These
agents, such as ibrutinib (153),
tirabrutinib (154), lenalidomide
(137), pomalidomide (155) and nivolumab (156), are currently being evaluated both
as single agents and in combination with other agents in clinical
trials. The incor-poration of novel agents into the treatment of
PCNSL may enable the application of precision medicine in the
treatment of patients with R/R PCNSL.
BTK, a nonreceptor protein kinase, is important for
the amplification of B-cell signaling. BTK integrates BCR and
Toll-like receptor signaling, linking them to downstream NF-κB
signaling; therefore, it may be considered an attractive candidate
for targeted inhibition (4,157).
Mutations leading to activation of the NF-κB signaling pathway,
such as mutations in MyD88, CARD11 and CD79, and TNFAIP3 and
TBL1XR1 deletions, have been observed in almost all PCNSL cases,
thus indicating that NF-κB activation may serve a role in the
pathogenesis of PCNS-DLBCL and could represent a potential
therapeutic target (4,157).
Ibrutinib, a small molecule BTK inhibitor, has been
identified as a potential novel therapeutic agent for the treatment
of PCNSL. A small retrospective study in R/R PCNSL demonstrated the
feasibility of ibrutinib treatment, with promising responses
reported in several patients (158). Even patients without genomic
alterations in the BCR pathway have been shown to respond to
ibrutinib (159); however, as a
single drug, ibrutinib is prone to drug resistance, limiting PFS. A
phase II study on ibrutinib revealed treatment resistance in the
absence of a CARD11 mutation and presence of a mutation in the BCR
pathway (160). A phase Ib trial
investigating ibru-tinib monotherapy for 2 weeks, followed by its
combination with conventional chemotherapies, including adjusted
TMZ, etoposide, doxorubicin, dexamethasone, cytarabine and
rituximab, demonstrated a PR rate of 83% prior to the initiation of
the combination regimen, with a subsequent CR rate of 86% following
combination chemotherapy in the patients with R/R PCNSL (153). Resistance to ibrutinib
monotherapy could be overcome through combination regimens with
chemotherapy and/or targeted biological agents.
Tirabrutinib is a second-generation, potent, highly
selective, irreversible oral BTK inhibitor. A phase I/II study
evaluated the safety, tolerability, efficacy and pharmacokinetics
of tirabrutinib in Japanese patients with R/R PCNSL, and displayed
an objective response rate (ORR) of 64% and PFS of 2.9 months.
Furthermore, median OS was not reached, indicating a favorable
efficacy of tirabrutinib in patients with R/R PCNSL (154).
IMIDs, such as lenalidomide and pomalidomide, are
thalidomide-derived agents with antiproliferative and
immunomodulatory properties. These drugs have been tested in
recurrent PCNSL. A previous study indicated that lenalidomide may
exert cytotoxic effects relevant to PCNSL, including the inhibition
of IRF4 and myc pro-survival signals (161). Lenalidomide has been used as a
single agent in the treatment of patients with relapsing PCNSL,
with two patients achieving CR in a small retrospective cohort
(162). The activity was
confirmed in a phase I trial, with 9/14 patients with R/R PCNSL
treated with lenalidomide monotherapy exhibiting objective response
(163). A phase II study
evaluated lenalidomide in combination with rituximab in 50 patients
with R/R PCNSL or primary vitreoretinal lymphoma, and found an ORR
of 35% (164). Maintenance
therapy with lenalidomide alone was recommended to responding
patients, and the median PFS and OS were 7.8 and 17.7 months,
respectively (164). In addition,
a previous study revealed that reduced-dose MTX-based treatment
followed by low-dose lenalidomide maintenance was well-tolerated in
patients aged ≥70 years, and associated with excellent PFS and OS
(136). Pomalidomide is a novel
immunomodulatory drug with anti-lymphoma activity. The combination
of pomalidomide with dexamethasone resulted in an ORR of 48% and a
median PFS of 5.3 months (155).
Mutations and copy number variations of PIK3CA and
PTEN have been detected in next-generation sequencing analyses of
PCNSL (165,166). These genes are associated with
the PI3K/AKT/mTOR signaling pathway, indicating the potential role
of this pathway in the pathogenesis of PCNSL (167). PI3K/AKT/mTOR inhibitors have also
been used to treat R/R PCNSL. In a formal phase II trial, the first
targeted agent investigated, the mTOR inhibitor temsirolimus, was
used to treat 37 patients with R/R PCNSL, with an acceptable ORR of
54%, a median OS of 3.7 months, and a poor median PFS of 2.1 months
(168). Moreover, in a small
retrospective series, the response rate to the PI3K inhibitor
buparlisib was even lower (1/4 of patients responded) (169). These findings indicate that
PI3K/AKT/mTOR inhibitors may not be appropriate as a single agent
for R/R PCNSL treatment.
Notably, it is common for the active PI3K/AKT/mTOR
pathway in PCNSL to carry a CD79B mutation (159). In addition, in an ABC-DLBCL
model, ibrutinib was reported to achieve a synergistic effect when
combined with PI3K/AKT/mTOR inhibitors through concerted inhibition
of multiple pathways (170,171).
PD-1 is an inhibitory receptor expressed on
activated T cells, and its interaction with PD-L1 produces an
immunosuppressive activity, protecting tumor cells from antitumor
immunity and possibly releasing sPD-L1 from PD-L1-expressing tumor
cells. Since immune checkpoint inhibitors that block PD-1 have been
reported to be effective in patients with R/R lymphoma, the role of
PD-1 inhibitors as a salvage treatment has been emerging in
patients with R/R PCNSL. sPD-L1 in the serum may serve as a
feasible biomarker for determining a risk-adapted treatment
strategy for patients with PCNSL (91).
Nivolumab, an antibody targeting PD-1, and
dendritic cell vaccinations have been reported to lead to CR in one
case (172). In addition, in a
small case series, a response was recorded in four patients with
PCNSL treated with nivolumab (156). Low-dose nivolumab has been
reported to induce durable CR in relapsed PCNS-DLBCL (173). In summary, PD-1 blockade with
nivolumab could be considered an option in the treatment of R/R
PCNSL.
The biallelic deletion of CDKN2A has been observed
in 44% of PCNSL cases, suggesting that CDKN2A loss may be an early
clonal event in PCNSL evolution, raising the possibility of
investigating the efficacy of CDK4/6 inhibitors in patients with
PCNSL (94).
The incidence of PCNSL in immunocompetent elderly
patients, particularly men, is increasing. Notably, the symptoms of
PCNSL and brain imaging are often non-specific. For the diagnosis
of CNSL, imaging-guided stereotactic biopsy is regarded as the gold
standard; if stereotactic biopsy is not possible or conclusive,
certain biomarkers can help establish a diagnosis. PCNSL has a very
poor prognosis in the elderly, even though several prognostic
scoring systems have been generated and numerous prognostic markers
for PCNSL have been reported. Therefore, the treatment of elderly
patients with PCNSL remains challenging. An individualized
treatment that aims at prolonging survival while minimizing
toxicity is required. It is unlikely that a novel agent could have
curative effects as a monotherapy; however, its rational
combination with chemotherapeutic agents or its combination with
other new drugs may have therapeutic potential. Consequently,
patients should be urged to enter clinical trials, whenever
possible. Further clinical studies should offer more evidence of
optimal doses or combinations of polychemotherapeutic regimens and
new drugs in the elderly.
No funding was received.
Not applicable.
YL is responsible for writing the review. QY
revised the review. FZ contributed to finalize the draft. QY and FZ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Bailey P: Intracranial sarcomatous tumors
of leptomeingeal origin. Arch Surg. 18:1359–1402. 1929. View Article : Google Scholar
|
|
2
|
Siegal T and Bairey O: Primary CNS
lymphoma in the elderly: The challenge. Acta Haematol. 141:138–145.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mulazzani M, Huber M, Borchard S, Langer
S, Angele B, Schuh E, Meinl E, Dreyling M, Birnbaum T, Straube A,
et al: APRIL and BAFF: Novel biomarkers for central nervous system
lymphoma. J Hematol Oncol. 12:1022019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Braggio E, Van Wier S, Ojha J, McPhail E,
Asmann YW, Egan J, da Silva JA, Schiff D, Lopes MB, Decker PA, et
al: Genome-wide analysis uncovers novel recurrent alterations in
primary central nervous system lymphomas. Clin Cancer Res.
21:3986–3994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapuy B, Roemer MG, Stewart C, Tan Y, Abo
RP, Zhang L, Dunford AJ, Meredith DM, Thorner AR, Jordanova ES, et
al: Targetable genetic features of primary testicular and primary
central nervous system lymphomas. Blood. 27:869–881. 2016.
View Article : Google Scholar
|
|
6
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Villano JL, Koshy M, Shaikh H, Dolecek TA
and McCarthy BJ: Age, gender, and racial differences in incidence
and survival in primary CNS lymphoma. Br J Cancer. 105:1414–1418.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Neill BP, Decker PA, Tieu C and Cerhan
JR: The changing incidence of primary central nervous system
lymphoma is driven primarily by the changing incidence in young and
middle-aged men and differs from time trends in systemic diffuse
large B-cell non-Hodgkin's lymphoma. Am J Hematol. 88:997–1000.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deangelis LM and Iwamoto FM: An update on
therapy of primary central nervous system lymphoma. Hematol Am Soc
Hematol Educ Program. 2006:311–316. 2006. View Article : Google Scholar
|
|
10
|
Omuro AM, Taillandier L, Chinot O, Carnin
C, Barrie M and Hoang-Xuan K: Temozolomide and methotrexate for
primary central nervous system lymphoma in the elderly. J Neuro
Oncol. 85:207–211. 2007. View Article : Google Scholar
|
|
11
|
Roth P and Hoang-Xuan K: Challenges in the
treatment of elderly patients with primary central nervous system
lymphoma. Curr Opin Neurol. 27:697–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grommes C and DeAngelis LM: Primary CNS
lymphoma. J Clin Oncol. 35:2410–2418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schabet M: Epidemiology of primary CNS
lymphoma. J Neurooncol. 43:199–201. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Enblad G, Martinsson G, Baecklund E,
Hesselager G, Sundström C, Amini RM and Hagberg H: Population-based
experience on primary central nervous system lymphoma 2000-2012:
The incidence is increasing. Acta Onco. 156:599–607. 2017.
View Article : Google Scholar
|
|
15
|
Reni M, Ferreri AJ, Guha-Thakurta N, Blay
JY, Dell'Oro S, Biron P and Hochberg FH: Clinical relevance of
consolidation radiotherapy and other main therapeutic issues in
primary central nervous system lymphomas treated with upfront
high-dose methotrexate. Int J Radiat Oncol Biol Phys. 51:419–425.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan SM, Hutnik CM, Heathcote JG, Orton RB
and Banerjee D: Iris lymphoma in a pediatric cardiac transplant
recipient: Clinicopathologic findings. Ophthalmology.
107:1479–1482. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matinella A, Lanzafame M, Bonometti MA,
Gajofatto A, Concia E, Vento S, Monaco S and Ferrari S:
Neurological complications of HIV infection in pre-HAART and HAART
era: A retrospective study. J Neurol. 262:1317–1327. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engels EA, Biggar RJ, Hall HI, Cross H,
Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS
and Goedert JJ: Cancer risk in people infected with human
immunodeficiency virus in the United States. Int J Cancer.
123:187–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiels MS, Pfeiffer RM, Besson C, Clarke
CA, Morton LM, Nogueira L, Pawlish K, Yanik EL, Suneja G and Engels
EA: Trends in primary central nervous system lymphoma incidence and
survival in the U.S. Br J Haematol. 174:417–424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bathla G and Hegde A: Lymphomatous
involvement of the central nervous system. Clin Radiol. 71:602–609.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morell AA, Shah AH, Cavallo C, Eichberg
DG, Sarkiss CA, Benveniste R, Ivan ME and Komotar RJ: Diagnosis of
primary central nervous system lymphoma: A systematic review of the
utility of CSF screening and the role of early brain biopsy.
Neurooncol Pract. 6:415–423. 2019.PubMed/NCBI
|
|
22
|
Miller B, Sirotkin I and Martinez C:
Review of radiologic considerations in an immunocompetent patient
with primary central nervous system lymphoma. Fed Pract. 36(Suppl
5): S51–S53. 2019.PubMed/NCBI
|
|
23
|
Lin X, Khan IRA, Seet YHC, Lee HY and Yu
WY: Atypical radiological findings of primary central nervous
system lymphoma. Neuroradiology. 62:669–676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scott BJ, Douglas VC, Tihan T, Rubenstein
JL and Josephson SA: A systematic approach to the diagnosis of
suspected central nervous system lymphoma. JAMA Neurol. 70:311–319.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferreri AJM: Therapy of primary CNS
lymphoma: Role of intensity, radiation, and novel agents.
Hematology Am Soc Hematol Educ Program. 2017:565–577. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malikova H, Burghardtova M, Koubska E,
Mandys V, Kozak T and Weichet J: Secondary central nervous system
lymphoma: Spectrum of morphological MRI appearances. Neuropsychiatr
Dis Treat. 14:733–740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Green K and Hogg JP: Central Nervous
System Lymphoma. StatPearls. StatPearls Publishing; Treasure
Island, FL: 2020
|
|
28
|
Blay JY, Ongolo-Zogo P, Sebban C, Carrie
C, Thiesse P and Biron P: Primary cerebral lymphomas: Unsolved
issues regarding first-line treatment, follow-up, late neurological
toxicity and treatment of relapses. The FNCLCC. French Fédération
Nationale des Centres de Lutte contre le Cancer. Ann Oncol.
11(Suppl 1): S39–S44. 2000. View Article : Google Scholar
|
|
29
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identifified by
gene expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pasqualucci L and Dalla-Favera R: The
genetic landscape of diffuse large B-cell lymphoma. Semin Hematol.
52:67–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar
|
|
32
|
Hoang-Xuan K, Bessell E, Bromberg J,
Hottinger AF, Preusser M, Rudà R, Schlegel U, Siegal T, Soussain C,
Abacioglu U, et al: Diagnosis and treatment of primary CNS lymphoma
in immunocompetent patients: Guidelines from the European
Association for Neuro-Oncology. Lancet Oncol. 16:e322–e332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
von Baumgarten L, Illerhaus G, Korfel A,
Schlegel U, Deckert M and Dreyling M: The diagnosis and treatment
of primary CNS LYmphoma. Dtsch Arztebl Int. 115:419–426.
2018.PubMed/NCBI
|
|
34
|
Antinori A, De Rossi G, Ammassari A,
Cingolani A, Murri R, Di Giuda D, De Luca A, Pierconti F,
Tartaglione T, Scerrati M, et al: Value of combined approach with
thallium-201 single-photon emission computed tomography and
Epstein-Barr virus DNA polymerase chain reaction in CSF for the
diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol.
17:554–560. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sasagawa Y, Akai T, Tachibana O and Iizuka
H: Diagnostic value of interleukin-10 in cerebrospinal fluid for
diffuse large B-cell lymphoma of the central nervous system. J
Neurooncol. 121:177–183. 2015. View Article : Google Scholar
|
|
36
|
Song Y, Zhang W, Zhang L, Wu W, Zhang Y,
Han X, Yang C, Zhang L and Zhou D: Cerebrospinal fluid IL-10 and
IL-10/IL-6 as accurate diagnostic biomarkers for primary central
nervous system large B-cell lymphoma. Sci Rep. 6:386712016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rubenstein JL, Wong VS, Kadoch C, Gao HX,
Barajas R, Chen L, Josephson SA, Scott B, Douglas V, Maiti M, et
al: CXCL13 plus interleukin 10 is highly specific for the diagnosis
of CNS lymphoma. Blood. 121:4740–4748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Viaccoz A, Ducray F, Tholance Y, Barcelos
GK, Thomas-Maisonneuve L, Ghesquières H, Meyronet D, Quadrio I,
Cartalat-Carel S, Louis-Tisserand G, et al: CSF neopterin level as
a diagnostic marker in primary central nervous system lymphoma.
Neuro Oncol. 17:1497–1503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Caudie C, Bancel J, Dupont M, Matanza D,
Poitevin F and Honnorat J: CSF levels and diagnostic utility of
cerebrospinal fluid beta2-microglobulin. Ann Biol Clin (Paris).
63:631–637. 2005.
|
|
40
|
Strehlow F, Bauer S, Martus P, Weller M,
Roth P, Schlegel U, Seidel S, Scheibenbogen C, Korfel A and Kreher
S: Osteopontin in cerebrospinal fluid as diagnostic biomarker for
central nervous system lymphoma. J Neurooncol. 129:165–171. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kersten MJ, Evers LM, Dellemijn PL, van
den Berg H, Portegies P, Hintzen RQ, van Lier RA, von dem Borne AE
and van Oers RH: Elevation of cerebrospinal fluid soluble CD27
levels in patients with meningeal localization of lymphoid
malignancies. Blood. 87:1985–1989. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baraniskin A, Kuhnhenn J, Schlegel U, Chan
A, Deckert M, Gold R, Maghnouj A, Zöllner H, Reinacher-Schick A,
Schmiegel W, et al: Identification of microRNAs in the
cerebrospinal fluid as marker for primary diffuse large B-cell
lymphoma of the central system. Blood. 117:3140–3146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng J, Xu J, Ma S, Sun X, Geng M and
Wang L: Clinicopathological study of gene rearrangement and
microRNA expression of primary central nervous system diffuse large
B-cell lymphomas. Int J Clin Exp Pathol. 6:2048–2055.
2013.PubMed/NCBI
|
|
44
|
Yu X, Li Z, Shen J, Chan MT and Wu WK:
Role of microRNAs in primary central nervous system lymphomas. Cell
Prolif. 49:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rimelen V, Ahle G, Pencreach E, Zinniger
N, Debliquis A, Zalmaï L, Harzallah I, Hurstel R, Alamome I, Lamy
F, et al: Tumor cell-free DNA detection in CSF for primary CNS
lymphoma diagnosis. Acta Neuropathol Commun. 7:432019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thaler FS, Laurent SA, Huber M, Mulazzani
M, Dreyling M, Ködel U, Kümpfel T, Straube A, Meinl E and von
Baumgarten L: Soluble TACI and soluble BCMA as biomarkers in
primary central nervous system lymphoma. Neuro Oncol. 19:1618–1627.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ho KG and Grommes C: Molecular profiling
of primary central nervous system lymphomas-predictive and
prognostic value? Curr Opin Neurol. 32:886–894. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deguchi S, Nakashima K, Muramatsu K,
Mitsuya K, Oishi T, Shirata K, Hayashi N, Sugino T, Endo M and
Nakasu Y: Pretreatment intratumoral susceptibility signals
correlate with response to high-dose methotrexate and
progression-free survival in primary central nervous system
lymphoma. J Clin Neurosci. 69:43–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Phillips EH, Fox CP and Cwynarski K:
Primary CNS lymphoma. Curr Hematol Malig Rep. 9:243–253. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bataille B, Delwail V, Menet E,
Vandermarcq P, Ingrand P, Wager M, Guy G and Lapierre F: Primary
intracerebral malignant lymphoma: Report of 248 cases. J Neurosurg.
92:261–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lim T, Kim SJ, Kim K, Lee JI, Lim DH, Lee
DJ, Baek KK, Lee HY, Han B, Uhm JE, et al: Primary CNS lymphoma
other than DLBCL: A descriptive analysis of clinical features and
treatment outcomes. Ann Hematol. 90:1391–1398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Van Der Meulen M, Dinmohamed AG, Visser O,
Doorduijn JK and Bromberg JEC: Improved survival in primary central
nervous system lymphoma up to age 70 only: A population-based study
on incidence, primary treatment and survival in the Netherlands,
1989-2015. Leukemia. 31:1822–1825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fallah J, Qunaj L and Olszewski AJ:
Therapy and outcomes of primary central nervous system lymphoma in
the United States: Analysis of the national cancer database. Blood
Adv. 1:112–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Graham MS and DeAngelis LM: Improving
outcomes in primary CNS lymphoma. Best Pract Res Clin Haematol.
31:262–269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Glass J, Won M, Schultz CJ, Brat D,
Bartlett NL, Suh JH, Werner-Wasik M, Fisher BJ, Liepman MK,
Augspurger M, et al: Phase I and II study of induction chemotherapy
with methotrexate, rituximab, and temozolomide, followed by
whole-brain radiotherapy and postirradiation temozolomide for
primary CNS lymphoma: NRG oncology RTOG 0227. J Clin Oncol.
34:1620–1625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Royer-Perron L and Hoang-Xuan K:
Management of primary central nervous system lymphoma. Presse Med.
47:e213–e244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kasenda B, Ferreri AJ, Marturano E, Forst
D, Bromberg J, Ghesquieres H, Ferlay C, Blay JY, Hoang-Xuan K,
Pulczynski EJ, et al: First-line treatment and outcome of elderly
patients with primary central nervous system lymphoma (PCNSL)-a
systematic review and individual patient data meta-analysis. Ann
Oncol. 26:1305–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Houillier C, Ghesquières H, Chabrot C,
Soussain C, Ahle G, Choquet S, Nicolas-Virelizier E, Bay JO,
Vargaftig J, Gaultier C, et al: Rituximab, methotrexate,
procarbazine, vincristine and intensifified cytarabine
consolidation for primary central nervous system lymphoma (PCNSL)
in the elderly: A LOC network study. J Neurooncol. 133:315–320.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fritsch K, Kasenda B, Schorb E, Hau P,
Bloehdorn J, Möhle R, Löw S, Binder M, Atta J, Keller U, et al:
High-dose methotrexate-based immune-chemotherapy for elderly
primary CNS lymphoma patients (PRIMAIN study). Leukemia.
31:846–852. 2017. View Article : Google Scholar
|
|
60
|
Hoang-Xuan K, Taillandier L, Chinot O,
Soubeyran P, Bogdhan U, Hildebrand J, Frenay M, De Beule N,
Delattre JY and Baron B; European Organization for Research and
Treatment of Cancer Brain Tumor Group: Chemotherapy alone as
initial treatment for primary CNS lymphoma in patients older than
60 years: A multicenter phase II study (26952) of the European
organization for research and treatment of cancer brain tumor
group. J Clin Oncol. 21:2726–2731. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Illerhaus G, Marks R, Müller F, Ihorst G,
Feuerhake F, Deckert M, Ostertag C and Finke J: High-dose
methotrexate combined with procarbazine and CCNU for primary CNS
lymphoma in the elderly: Results of a prospective pilot and phase
II study. Ann Oncol. 20:319–325. 2009. View Article : Google Scholar
|
|
62
|
Roth P, Martus P, Kiewe P, Möhle R, Klasen
H, Rauch M, Röth A, Kaun S, Thiel E, Korfel A and Weller M: Outcome
of elderly patients with primary CNS lymphoma in the G-PCNSL-SG-1
trial. Neurology. 79:890–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Olivier G, Clavert A, Lacotte-Thierry L,
Gardembas M, Escoffre-Barbe M, Brion A, Cumin I, Legouffe E,
Solal-Celigny P, Chabin M, et al: A phase 1 dose escalation study
of idarubicin combined with methotrexate, vindesine, and
prednisolone for untreated elderly patients with primary central
nervous system lymphoma. The GOELAMS LCP 99 trial. Am J Hematol.
89:1024–1029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Omuro A, Chinot O, Taillandier L,
Ghesquieres H, Soussain C, Delwail V, Lamy T, Gressin R, Choquet S,
Soubeyran P, et al: Methotrexate and temozolomide vs. methotrexate,
procarbazine, vincristine, and cytarabine for primary CNS lymphoma
in an elderly population: An intergroup ANOCEF-GOELAMS randomized
phase 2 trial. Lancet Haematol. 2:e251–e259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pulczynski EJ, Kuittinen O, Erlanso M,
Hagberg H, Fosså A, Eriksson M, Nordstrøm M, Østenstad B, Fluge Ø,
Leppä S, et al: Successful change of treatment strategy in elderly
patients with primary central nervous system lymphoma by
de-escalating induction and introducing temozolomide maintenance:
Results from a phase II study by the Nordic Lymphoma Group.
Haematologica. 100:534–540. 2015. View Article : Google Scholar :
|
|
66
|
Mendez JS, Ostrom QT, Gittleman H, Kruchko
C, DeAngelis LM, Barnholtz-Sloan JS and Grommes C: The elderly left
behind-changes in survival trends of primary central nervous system
lymphoma over the past 4 decades. Neuro Oncol. 20:687–694. 2018.
View Article : Google Scholar
|
|
67
|
Langner-Lemercier S, Houillier C, Soussain
C, Ghesquières H, Chinot O, Taillandier L, Soubeyran P, Lamy T,
Morschhauser F, Benouaich-Amiel A, et al: Primary CNS lymphoma at
first relapse/progression: Characteristics, management, and outcome
of 256 patients from The French LOC network. Neuro Oncol.
18:1297–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jahnke K, Thiel E, Martus P, Herrlinger U,
Weller M, Fischer L and Korfel A; German Primary Central Nervous
System Lymphoma Study Group: Relapse of primary central nervous
system lymphoma: Clinical features, outcome and prognostic factors.
J Neurooncol. 80:159–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nayak L, Hedvat C, Rosenblum MK, Abrey LE
and DeAngelis LM: Late relapse in primary central nervous system
lymphoma: Clonal persistence. Neuro Oncol. 13:525–529. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Abrey LE, Ben-Porat L, Panageas KS,
Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta
M and DeAngelis LM: Primary central nervous system lymphoma: The
memorial Sloankettering cancer Center prognostic model. J Clin
Oncol. 24:5711–5715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ferreri AJ, Blay JY, Reni M, Pasini F,
Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi
A, et al: Prognostic scoring system for primary CNS lymphomas: The
International extranodal lymphoma study group experience. J Clin
Oncol. 21:266–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Weller M, Martus P, Roth P, Thiel E and
Korfel A; German PCNSL Study Group: Surgery for primary CNS
lymphoma? Challenging a paradigm. Neuro Oncol. 14:1481–1484. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Makino K, Nakamura H, Hide T, Kuroda J,
Yano S and Kuratsu J: Prognostic impact of completion of initial
high-dose methotrexate therapy on primary central nervous system
lymphoma: A single institution experience. Int J Clin Oncol.
20:29–34. 2015. View Article : Google Scholar
|
|
74
|
Nakasu Y, Mitsuya K, Hayashi N, Okamura I,
Mori K, Enami T, Tatara R, Nakasu S and Ikeda T: Response-adapted
treatment with upfront high-dose chemotherapy followed by
autologous stem-cell transplantation rescue or consolidation phase
high-dose methotrexate for primary central nervous system lymphoma:
A long-term mono-center study. Springerplus. 5:3072016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kondo E, Ikeda T, Izutsu K, Chihara D,
Shimizu-Koresawa R, Fujii N, Sakai T, Kondo T, Kubo K, Kato Y, et
al: High dose chemotherapy with autologous stem cell
transplantation in primary central nervous system lymphoma: Data
from the Japan Society for Hematopoietic Cell Transplantation
(JSHCT) registry. Biol Blood Marrow Transplant. 25:899–905. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Levy O, DeAngelis LM, Filippa DA, Panageas
KS and Abrey LE: Bcl-6 predicts improved prognosis in primary
central nervous system lymphoma. Cancer. 112:151–156. 2008.
View Article : Google Scholar
|
|
77
|
Preusser M, Woehrer A, Koperek O,
Rottenfusser A, Dieckmann K, Gatterbauer B, Roessler K, Slavc I,
Jaeger U, Streubel B, et al: Primary central nervous system
lymphoma: A clinicopathological study of 75 cases. Pathology.
42:547–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Niparuck P, Boonsakan P, Sutthippingkiat
T, Pukiat S, Chantrathammachart P, Phusanti S, Boonyawat K,
Puavilai T, Angchaisuksiri P, Ungkanont A, et al: Treatment outcome
and prognostic factors in PCNSL. Diagn Pathol. 14:562019.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen C, Zhuo H, Wei X and Ma X:
Contrast-enhanced MRI texture parameters as potential prognostic
factors for primary central nervous system lymphoma patients
receiving high-dose methotrexate-based chemotherapy. Contrast Media
Mol Imaging. 2019:54814912019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Alame M, Pirel M, Costes-Martineau V,
Bauchet L, Fabbro M, Tourneret A, De Oliveira L, Durand L, Roger P,
Gonzalez S, et al: Characterisation of tumour microenvironment and
immune checkpoints in primary central nervous system diffuse large
B cell lymphomas. Virchows Arch. 476:891–902. 2020. View Article : Google Scholar
|
|
81
|
Cho I, Lee H, Yoon SE, Ryu KJ, Ko YH, Kim
WS and Kim SJ: Serum levels of soluble programmed death-ligand 1
(sPD-L1) in patients with primary central nervous system diffuse
large B-cell lymphoma. BMC Cancer. 20:1202020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cambruzzi E: Primary intra-axial diffuse
large b-cell lymphoma in immunocompetent patients: Clinical impact
of molecular analysis and histogenetic evaluation. World Neurosurg.
134:215–220. 2020. View Article : Google Scholar
|
|
83
|
Yuan XG, Huang YR, Yu T, Xu Y, Liang Y,
Zhang XH, Sun CR and Zhao XY: Primary central nervous system
lymphoma in China: A single-center retrospective analysis of 167
cases. Ann Hematol. 99:93–104. 2020. View Article : Google Scholar
|
|
84
|
Tabouret E, Houillier C, Martin-Duverneuil
N, Blonski M, Soussain C, Ghesquières H, Houot R, Larrieu D,
Soubeyran P, Gressin R, et al: Patterns of response and relapse in
primary CNS lymphomas after first-line chemotherapy: Imaging
analysis of the ANOCEF-GOELAMS prospective randomized trial. Neuro
Oncol. 19:422–429. 2017.
|
|
85
|
Chunsong H, Yuling H, Li W, Jie X, Gang Z,
Qiuping Z, Qingping G, Kejian Z, Li Q, Chang AE, et al: CXC
chemokine ligand 13 and CC chemokine ligand 19 cooperatively render
resistance to apoptosis in B cell lineage acute and chronic
lymphocytic leukemia CD23+CD5+ B cells. J Immunol. 177:6713–6722.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Le M, Garcilazo Y, Ibáñez-Juliá MJ, Younan
N, Royer-Perron L, Benazra M, Mokhtari K, Houillier C, Hoang-Xuan K
and Alentorn A: Pretreatment hemoglobin as an independent
prognostic factor in primary central nervous system lymphomas.
Hematologic malignancies. Oncologist. 24:e898–e904. 2019.
View Article : Google Scholar
|
|
87
|
Oyama T, Yamamoto K, Asano N, Oshiro A,
Suzuki R, Kagami Y, Morishima Y, Takeuchi K, Izumo T, Mori S, et
al: Age-related EBV associated B-cell lymphoproliferative disorders
constitute a distinct clinicopathologic group: A study of 96
patients. Clin Cancer Res. 13:5124–5132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wu T, Kang H, Zhuang D, Ma Y, Lin Z,
Suolitiken D, Chen B and Xu X: The role of ABCB1 polymorphism as a
prognostic marker for primary central nervous system lymphoma. Ann
Hematol. 98:923–930. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yang X, Wang Y, Sun X, Bai X, Cui Q, Zhu
H, Qian J, Chen Y, Sun S, Ji N, et al: STAT3 activation is
associated with IL-10 expression and survival in primary central
nervous system lymphoma. World Neurosurg. 134:e1077–e1084. 2020.
View Article : Google Scholar
|
|
90
|
Hatzl S, Posch F, Deutsch A, Beham-Schmid
C, Stöger H, Greinix H, Pichler M, Neumeister P and Prochazka KT:
Immunohistochemistry for c-myc and bcl-2 overexpression improves
risk stratification in primary central nervous system lymphoma
(PCNSL). Hematol Oncol. 38:277–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yin W, Xia X, Wu M, Yang H, Zhu X, Sun W
and Ge M: The impact of BCL-2/MYC protein expression and gene
abnor-mality on primary central nervous system diffuse large B-cell
lymphoma. Int J Clin Exp Pathol. 12:2215–2223. 2019.
|
|
92
|
Villa D, Tan KL, Steidl C, Ben-Neriah S,
Al Moosawi M, Shenkier TN, Connors JM, Sehn LH, Savage KJ, Scott
DW, et al: Molecular features of a large cohort of primary central
nervous system lymphoma using tissue microarray. Blood Adv.
3:3953–3961. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Takano S, Hattori K, Ishikawa E, Narita Y,
Iwadate Y, Yamaguchi F, Nagane M, Akimoto J, Oka H, Tanaka S, et
al: MyD88 mutation in elderly predicts poor prognosis in primary
central nervous system lymphoma: Multi-institutional analysis.
World Neurosurg. 112:e69–e73. 2018. View Article : Google Scholar
|
|
94
|
Nayyar N, White MD, Gill CM, Lastrapes M,
Bertalan M, Kaplan A, D'Andrea MR, Bihun I, Kaneb A, Dietrich J, et
al: MYD88 L265P mutation and CDKN2A loss are early mutational
events in primary central nervous system diffuse large B-cell
lymphomas. Blood Adv. 3:375–383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mondello P, Cuzzocrea S, Arrigo C, Pitini
V, Mian M and Bertoni F: STAT6 activation correlates with
cerebrospinal fluid IL-4 and IL-10 and poor prognosis in Primary
Central Nervous System Lymphoma. Hematol Oncol. 38:106–110. 2020.
View Article : Google Scholar
|
|
96
|
Hyung J, Hong JY, Kim S, Ryu JS, Huh J and
Suh C: Beta-2 microglobulin as a prognostic factor of primary
central nervous system lymphoma. Blood Res. 54:285–288. 2019.
View Article : Google Scholar
|
|
97
|
Kim S, Nam SJ, Park C, Kwon D, Yim J, Song
SG, Ock CY, Kim YA, Park SH, Kim TM, et al: High tumoral PD-L1
expression and low PD-1 + or CD8 + tumor-infiltrating lymphocytes
are predictive of a poor prognosis in primary diffuse large B-cell
lymphoma of the central nervous system. Oncoimmunology.
8:e16266532019. View Article : Google Scholar
|
|
98
|
Gopal S, Martin KE, Richards KL and Eron
JJ: Clinical presentation, treatment, and outcomes among 65
patients with HIV-associated lymphoma treated at the University of
North Carolina 2000-2010. AIDS Res Hum Retroviruses. 28:798–805.
2012. View Article : Google Scholar :
|
|
99
|
Han CH and Batchelor TT: Diagnosis and
management of primary central nervous system lymphoma. Cancer.
123:4314–4324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Corry J, Smith JG, Wirth A, Quong G and
Liew KH: Primary central nervous system lymphoma: Age and
performance status are more important than treatment modality. Int
J Radiat Oncol Biol Phys. 41:615–620. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mohile SG, Dale W, Somerfield MR,
Schonberg MA, Boyd CM, Burhenn PS, Canin B, Cohen HJ, Holmes HM,
Hopkins JO, et al: Practical Assessment and Management of
Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO
Guideline for Geriatric Oncology. J Clin Oncol. 36:2326–2347. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Extermann M, Boler I, Reich RR, Lyman GH,
Brown RH, DeFelice J, Levine RM, Lubiner ET, Reyes P, Schreiber FJ
III and Balducci L: Predicting the risk of chemotherapy toxicity in
older patients: The chemotherapy risk assessment scale for high-age
patients (CRASH) score. Cancer. 118:3377–3386. 2012. View Article : Google Scholar
|
|
103
|
Hiraga S, Arita N, Ohnishi T, Kohmura E,
Yamamoto K, Oku Y, Taki T, Sato M, Aozasa K and Yoshimine T: Rapid
infusion of high-dose methotrexate resulting in enhanced
penetration into cerebrospinal fluid and intensified tumor response
in primary central nervous system lymphomas. J Neurosurg.
91:221–230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Glass J, Shustik C, Hochberg FH, Cher L
and Gruber ML: Therapy of primary central nervous system lymphoma
with pre-irradiation methotrexate, cyclophosphamide, doxorubicin,
vincristine, and dexamethasone (MCHOD). J Neurooncol. 30:257–265.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Binnahil M, Au K, Lu JQ, Wheatley BM and
Sankar T: The influence of Cortico-steroids on diagnostic accuracy
of biopsy for primary central nervous system lymphoma. Can J Neurol
Sci. 43:721–725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Porter AB, Giannini C, Kaufmann T,
Lucchinetti CF, Wu W, Decker PA, Atkinson JL and O'Neill BP:
Primary central nervous system lymphoma can be histologically
diagnosed after previous corticosteroid use: A pilot study to
determine whether corticosteroids prevent the diagnosis of primary
central nervous system lymphoma. Ann Neurol. 63:662–667. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mathew BS, Carson KA and Grossman SA:
Initial response to glucocorticoids: A potentially important
prognostic factor in patients with primary CNS lymphoma. Cancer.
106:383–387. 2006. View Article : Google Scholar
|
|
108
|
Batchelor TT: Primary central nervous
system lymphoma: A curable disease. Hematol Oncol. 37(Suppl 1):
S15–S18. 2019. View Article : Google Scholar
|
|
109
|
Nabors LB, Portnow J, Baehring J, Brem H,
Butowski N, Forsyth P, Hattangadi-Gluth J, Holdhoff M, Horbinski C,
Howard S, et al: NCCN Clinical Practice Guidelines in Oncology.
Central Nervous System Cancers. 1:32–35. 2018.
|
|
110
|
Rushworth D, Mathews A, Alpert A and
Cooper LJ: Dihydrofolate reductase and thymidylate synthase
transgenes resistant to methotrexate interact to permit novel
transgene regulation. J Biol Chem. 290:22970–22976. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Goecke IA, Alvarez C, Henríquez J, Salas
K, Molina ML, Ferreira A and Gatica H: Methotrexate regulates the
expres-sion of glucocorticoid receptor alpha and beta isoforms in
normal human peripheral mononuclear cells and human lymphocyte cell
lines in vitro. Mol Immunol. 44:2115–2123. 2007. View Article : Google Scholar
|
|
112
|
Madle M, Krämer I, Lehners N, Schwarzbich
M, Wuchter P, Herfarth K, Egerer G, Ho AD and Witzens-Harig M: The
influence of rituximab, high-dose therapy followed by autologous
stem cell transplantation, and age in patients with primary CNS
lymphoma. Ann Hematol. 94:1853–1857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rubenstein JL, Hsi ED, Johnson JL, Jung
SH, Nakashima MO, Grant B, Cheson BD and Kaplan LD: Intensive
chemotherapy and immunotherapy in patients with newly diagnosed
primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol.
31:3061–3068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jahnke K, Korfel A, Martus P, Weller M,
Herrlinger U, Schmittel A, Fischer L and Thiel E; German Primary
Central Nervous System Lymphoma Study Group (G-PCNSL-SG): High-dose
methotrexate toxicity in elderly patients with primary central
nervous system lymphoma. Ann Oncol. 16:445–449. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Welch MR, Omuro A and Deangelis LM:
Outcomes of the oldest patients with primary CNS lymphoma treated
at Memorial Sloan-Kettering Cancer Center. Neuro Oncol.
14:1304–1311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Illerhaus G, Schorb E and Kasenda B: Novel
agents for primary central nervous system lymphoma: Evidence and
perspectives. Blood. 132:681–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Faivre G, Butler MJ, Le I and Brenner A:
Temozolomide as a single agent maintenance therapy in elderly
patients with primary CNS lymphoma. Clin Lymphoma Myeloma Leuk.
19:665–669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Shih C, Chen VJ, Gossett LS, Gates SB,
MacKellar WC, Habeck LL, Shackelford KA, Mendelsohn LG, Soose DJ,
Patel VF, et al: LY231514, a pyrrolo[2,3-d] pyrimidine-based
antifolate that inhibits multiple folate-requiring enzymes. Cancer
Res. 57:1116–1123. 1997.PubMed/NCBI
|
|
119
|
Raizer JJ, Rademaker A, Evens AM, Rice L,
Schwartz M, Chandler JP, Getch CC, Tellez C and Grimm SA:
Pemetrexed in the treatment of relapsed/refractory primary central
nervous system lymphoma. Cancer. 118:3743–3748. 2012. View Article : Google Scholar
|
|
120
|
Han S, Wang M, Liu B and Yu J: Pemetrexed
for primary central nervous system lymphoma in the elderly. Clin
Transl Oncol. 18:138–143. 2016. View Article : Google Scholar
|
|
121
|
Pardridge WM: BBB-genomics: Creating new
openings for brain-drug targeting. Drug Discovery Today. 6:381–383.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Thiel E, Korfel A, Martus P, Kanz L,
Griesinger F, Rauch M, Röth A, Hertenstein B, von Toll T,
Hundsberger T, et al: High-dose methotrexate with or without whole
brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase
3, randomised, non-inferiority trial. Lancet Oncol. 11:1036–1047.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Birnbaum T, Stadler EA, von Baumgarten L
and Straube A: Rituximab significantly improves complete response
rate in patients with primary CNS lymphoma. J Neurooncol.
109:285–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Holdhoff M, Ambady P, Abdelaziz A, Sarai
G, Bonekamp D, Blakeley J, Grossman SA and Ye X: High-dose
methotrexate with or without rituximab in newly diagnosed primary
CNS lymphoma. Neurology. 83:235–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Miyakita Y, Ohno M, Takahashi M, Muragaki
Y, Katai H and Narita Y: Immunochemotherapy using rituximab (RTX)
and high-dose methotrexate (HD-MTX): An evaluation of the addition
of RTX to HD-MTX in recurrent primary central nervous system
lymphoma (PCNSL). Jpn J Clin Oncol. 47:919–924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Fritsch K, Kasenda B, Hader C, Nikkhah G,
Prinz M, Haug V, Haug S, Ihorst G, Finke J and Illerhaus G:
Immunochemotherapy with rituximab, methotrexate, procarbazine, and
lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann
Oncol. 22:2080–2085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Iwamoto FM, Schwartz J, Pandit-Taskar N,
Peak S, Divgi CR, Zelenetz AD, Humm J and Abrey LE: Study of
radiolabeled indium-111 and yttrium-90 ibritumomab tiuxetan in
primary central nervous system lymphoma. Cancer. 110:2528–2534.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ferreri AJ, Cwynarski K, Pulczynski E,
Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosée PL, Schorb
E, et al: Chemoimmunotherapy with methotrexate, cytarabine,
thiotepa, and rituximab (MATRix regimen) in patients with primary
CNS lymphoma: Results of the first randomisation of the
International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2
trial. Lancet Haematol. 3:e217–e227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Schmitt AM, Herbrand AK, Fox CP, Bakunina
K, Bromberg JEC, Cwynarski K, Doorduijn JK, Ferreri AJM, Illerhaus
G, Issa S, et al: Rituximab in primary central nervous system
lymphoma-a systematic review and Meta-analysis. Hematol Oncol.
37:548–557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Bromberg JEC, Issa S, Bakunina K, Minnema
MC, Seute T, Durian M, Cull G, Schouten HC, Stevens WBC, Zijlstra
JM, et al: Rituximab in patients with primary CNS lymphoma (HOVON
105/ALLG NHL 24): A randomised, open-label, phase 3 inter-group
Study. Lancet Oncol. 20:216–228. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Han X, Ji Y, Ouyang M, Zhu T and Zhou D:
Efficacy and safety of HD-MTX based systemic chemotherapy regimens:
Retrospective study of induction therapy for primary central
nervous system lymphoma in Chinese. Sci Rep. 7:170532017.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Poortmans PM, Kluin-Nelemans HC,
Haaxma-Reiche H, Van't Veer M, Hansen M, Soubeyran P, Taphoorn M,
Thomas J, Van den Bent M, Fickers M, et al: High-dose
methotrexate-based chemotherapy followed by consolidating
radiotherapy in non-AIDS-related primary central nervous system
lymphoma: European organization for research and treatment of
cancer lymphoma group phase II trial 20962. J Clin Oncol.
21:4483–4488. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
van Tellingen O, Boogerd W, Nooijen WJ and
Beijnen JH: The vascular compartment hampers accurate determination
of teniposide penetration into braintumor tissue. Cancer Chemother
Pharmacol. 40:330–334. 1997. View Article : Google Scholar
|
|
134
|
Wu J, Duan L, Zhang L, Sun Z, Fu X, Li X,
Li L, Wang X, Zhang X, Li Z, et al: Fotemustine, teniposide and
dexametha-sone versus high-dose methotrexate plus cytarabine in
newly diagnosed primary CNS lymphoma: A randomised phase 2 trial. J
Neurooncol. 140:427–434. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wang YX, Huang Y, Xu XP, Chen BB, Lin ZG,
Ma Y, Ding TL and Wang Q: Curative effect of methotrexate combined
with teniposide in the treatment of primary central nervous system
lymphoma. Oncol Lett. 19:2097–2106. 2020.PubMed/NCBI
|
|
136
|
Deng X, Xu X, Lin D, Zhang X, Yu L, Sheng
H, Yin B, Zhang N and Lin J: Real-world impact of surgical excision
on overall survival in primary central nervous system lymphoma.
Front Oncol. 10:1312020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kinslow CJ, Rae AI, Neugut AI, Adams CM,
Cheng SK, Sheth SA, McKhann GM, Sisti MB, Bruce JN, Iwamoto FM, et
al: Surgery plus adjuvant radiotherapy for primary central nervous
system lymphoma. Br J Neurosurg. 34:690–696. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Korfel A and Schlegel U: Diagnosis and
treatment of primary CNS lymphoma. Nat Rev Neurol. 9:317–327. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Schlegel U and Korfel A: Is whole-brain
radiotherapy still a standard treatment for primary central nervous
system lymphomas? Curr Opin Neurol. 31:733–739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Shah GD, Yahalom J, Correa DD, Lai RK,
Raizer JJ, Schiff D, LaRocca R, Grant B, DeAngelis LM and Abrey LE:
Combined immunochemotherapy with reduced whole-brain radiotherapy
for newly diagnosed primary CNS lymphoma. J Clin Oncol.
25:4730–4735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lee TH, Lee JH, Chang JH, Ye SJ, Kim TM,
Park CK, Kim IH, Kim BH and Wee CW: Reduced-dose whole-brain
radiotherapy with tumor bed boost after upfront high-dose
methotrexate for primary central nervous system lymphoma. Radiat
Oncol J. 38:35–43. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Gaut D and Schiller GJ: Hematopoietic stem
cell transplantation in primary central nervous system lymphoma: A
review of the literature. Int J Hematol. 109:260–277. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Schorb E, Fox CP, Fritsch K, Isbell L,
Neubauer A, Tzalavras A, Witherall R, Choquet S, Kuittinen O,
De-Silva D, et al: High-dose thiotepa-based chemotherapy with
autologous stem cell support in elderly patients with primary
central nervous system lymphoma: A European retrospective study.
Bone Marrow Transplant. 52:1113–1119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Vu K, Mannis G, Hwang J, Geng H and
Rubenstein JL: Low-dose lenalidomide maintenance after induction
therapy in older patients with primary central nervous system
lymphoma. Br J Haematol. 186:180–183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Bairey O and Siegal T: The possible role
of maintenance treatment for primary central nervous system
lymphoma. Blood Rev. 32:378–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Reni M and Ferreri AJ: Therapeutic
management of refractory or relapsed primary central nervous system
lymphomas. Ann Hematol. 80(Suppl 3): B113–B117. 2001.
|
|
147
|
Otani R, Yamada R, Kushihara Y, Inazuka M
and Shinoura N: Continuous intrathecal injection therapy of
methotrexate is a therapeutic option in primary CNS lymphoma. J
Clin Neurosci. 69:26–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Soussain C, Suzan F, Hoang-Xuan K, Cassoux
N, Levy V, Azar N, Belanger C, Achour E, Ribrag V, Gerber S, et al:
Results of intensive chemotherapy followed by hematopoietic
stem-cell rescue in 22 patients with refractory or recurrent
primary CNS lymphoma or intraocular lymphoma. J Clin Oncol.
19:742–749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Correa DD, Braun E, Kryza-Lacombe M, Ho
KW, Reiner AS, Panageas KS, Yahalom J, Sauter CS, Abrey LE,
DeAngelis LM, et al: Longitudinal cognitive assessment in patients
with primary CNS lymphoma treated with induction chemotherapy
followed by reduced-dose whole-brain radiotherapy or autologous
stem cell transplantation. J Neurooncol. 144:553–562. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Atilla E, Sahin U, Atilla PA, Merter M,
Ozyurek E, Ceyhan K and Bozdag SC: Allogeneic stem cell
transplantation for relapsed primary central nervous system
lymphoma: Is it feasible? Hematol Oncol Stem Cell Ther. 12:220–225.
2019. View Article : Google Scholar
|
|
151
|
Varadi G, Or R, Kapelushnik J, Naparstek
E, Nagler A, Brautbar C, Amar A, Kirschbaum M, Samuel S, Slavin S
and Siegal T: Graft-versus-lymphoma effect after allogeneic
peripheral blood stem cell transplantation for primary central
nervous system lymphoma. Leuk Lymphoma. 34:185–190. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Tu S, Zhou X, Guo Z, Huang R, Yue C, He Y,
Li M, Chen Y, Liu Y, Chang LJ and Li Y: CD19 and CD70 Dual-target
chimeric antigen receptor T-cell therapy for the treatment of
relapsed and refractory primary central nervous system diffuse
large B-cell lymphoma. Front Oncol. 9:13502019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lionakis MS, Dunleavy K, Roschewski M,
Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C,
Higham CS, et al: Inhibition of B cell receptor signaling by
Ibrutinib in primary CNS lymphoma. Cancer Cell. 31:833–843.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Narita Y, Nagane M, Mishima K, Terui Y,
Arakawa Y, Yonezawa H, Asai K, Fukuhara N, Sugiyama K, Shinojima N,
et al: Phase 1/2 study of tirabrutinib, a second-generation
Bruton's tyro-sine kinase inhibitor, in relapsed/refractory primary
central nervous system lymphoma. Neuro Oncol. noaa1452020.
View Article : Google Scholar : Epub ahead of
print.
|
|
155
|
Tun HW, Johnston PB, DeAngelis LM,
Atherton PJ, Pederson LD, Koenig PA, Reeder CB, Omuro AMP, Schiff
D, O'Neill B, et al: Phase I study of pomalidomide and
dexamethasone for relapsed/refractory primary CNS or vitreoretinal
lymphoma. Blood. 132:2240–2248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Nayak L, Iwamoto FM, LaCasce A, Mukundan
S, Roemer MGM, Chapuy B, Armand P, Rodig SJ and Shipp MA: PD-1
blockade with nivolumab in relapsed/refractory primary central
nervous system and testicular lymphoma. Blood. 129:3071–3073. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ferry JA: The diversity of diffuse large
B-cell lymphoma in extranodal sites: Overview and update. J
Hematopathol. 7:57–70. 2014. View Article : Google Scholar
|
|
158
|
Chamoun K, Choquet S, Boyle E, Houillier
C, Larrieu-Ciron D, Al Jijakli A, Delrieu V, Delwail V,
Morschhauser F, Hoang-Xuan K and Soussain C: Ibrutinib monotherapy
in relapsed/refractory CNS lymphoma: A retrospective case series.
Neurology. 88:101–102. 2017. View Article : Google Scholar
|
|
159
|
Grommes C, Pastore A, Palaskas N, Tang SS,
Campos C, Schartz D, Codega P, Nichol D, Clark O, Hsieh WY, et al:
IbrutInib unmasks critical role of Bruton tyrosine kinase in
primary CNS lymphoma. Cancer Discov. 7:1018–1029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Soussain C, Choquet S, Blonski M, Leclercq
D, Houillier C, Rezai K, Bijou F, Houot R, Boyle E, Gressin R, et
al: Ibrutinib monotherapy for relapse or refractory primary CNS
lymphoma and primary vitreoretinal lymphoma: Final analysis of the
phase II 'proof-of-concept' iLOC study by the Lymphoma study
association (LYSA) and the French oculo-cerebral lymphoma (LOC)
network. Eur J Cancer. 117:121–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Yang Y, Shaffer AL III, Emre NC, Ceribelli
M, Zhang M, Wright G, Xiao W, Powell J, Platig J, Kohlhammer H, et
al: Exploiting synthetic lethality for the therapy of ABC diffuse
large B cell lymphoma. Cancer Cell. 21:723–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Houillier C, Choquet S, Touitou V,
Martin-Duverneuil N, Navarro S, Mokhtari K, Soussain C and
Hoang-Xuan K: Lenalidomide monotherapy as salvage treatment for
recurrent primary CNS lymphoma. Neurology. 84:325–326. 2015.
View Article : Google Scholar
|
|
163
|
Rubenstein JL, Geng H, Fraser EJ, Formaker
P, Chen L, Sharma J, Killea P, Choi K, Ventura J, Kurhanewicz J, et
al: Phase 1 investigation of lenalidomide/rituximab plus outcomes
of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv.
2:1595–1607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ghesquieres H, Chevrier M, Laadhari M,
Chinot O, Choquet S, Moluçon-Chabrot C, Beauchesne P, Gressin R,
Morschhauser F, Schmitt A, et al: Lenalidomide in combination with
intrave-nous rituximab (REVRI) in relapsed/refractory primary CNS
lymphoma or primary intraocular lymphoma: A multicenter prospective
'proof of concept' phase II study of the French Oculo-Cerebral
lymphoma (LOC) Network and the Lymphoma Study Association (LYSA).
Ann Oncol. 30:621–628. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Takashima Y, Sasaki Y, Hayano A, Homma J,
Fukai J, Iwadate Y, Kajiwara K, Ishizawa S, Hondoh H, Tokino T and
Yamanaka R: Target amplicon exomesequencing identifies promising
diagnosis and prognostic markers involved in RTK-RAS and PI3K-AKT
signaling as central oncopathways in primary central nervous system
lymphoma. Oncotarget. 9:27471–27486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Todorovic Balint M, Jelicic J, Mihaljevic
B, Kostic J, Stanic B, Balint B, Pejanovic N, Lucic B, Tosic N,
Marjanovic I, et al: Gene mutation profiles in primary diffuse
large B cell lymphoma of central nervous system: Next generation
sequencing analyses. Int J Mol Sci. 17:6832016. View Article : Google Scholar :
|
|
167
|
Zhang X and Liu Y: Targeting the the
PI3K/AKT/mTOR signaling pathway in primary central nervous system
lymphoma: Current status and future prospects. CNS Neurol Disord
Drug Targets. 19:165–173. 2020. View Article : Google Scholar
|
|
168
|
Korfel A, Schlegel U, Herrlinger U,
Dreyling M, Schmidt C, von Baumgarten L, Pezzutto A, Grobosch T,
Kebir S, Thiel E, et al: Phase II trial of temsirolimus for
Relapsed/Refractory primary CNS lymphoma. J Clin Oncol.
34:1757–1763. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Grommes C, Pentsova E, Nolan C, Wolfe J,
Mellinghoff IK and Deangelis L: Phase II study of single agent
buparlisib in recurrent/refractory primary (PCNSL) and secondary
CNS lymphoma (SCNSL). Ann Oncol. 27(Suppl 6): vi103–vi113.
2016.
|
|
170
|
Ezell SA, Mayo M, Bihani T, Tepsuporn S,
Wang S, Passino M, Grosskurth SE, Collins M, Parmentier J, Reimer
C, et al: Synergistic induction of apoptosis by combination of BTK
and dual mTORC1/2 inhibitors in diffuse large B cell lymphoma.
Oncotarget. 5:4990–5001. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Tarantelli C, Gaudio E, Arribas AJ, Kwee
I, Hillmann P, Rinaldi A, Cascione L, Spriano F, Bernasconi E,
Guidetti F, et al: PQR309 is a novel dual PI3K/mTOR inhibitor with
preclinical antitumor activity in lymphomas as a single agent and
in combination therapy. Clin Cancer Res. 24:120–129. 2018.
View Article : Google Scholar
|
|
172
|
Furuse M, Nonoguchi N, Omura N, Shirahata
M, Iwasaki K, Inui T, Kuroiwa T, Kuwabara H and Miyatake SI:
Immunotherapy of Nivolumab with dendritic cell vaccination is
effective against intractable recurrent primary central nervous
system lymphoma: A case report. Neurol Med Chir (Tokyo).
57:191–197. 2017. View Article : Google Scholar
|
|
173
|
Chan TSY, Khong PL, Au-Yeung R, Kwong YL
and Tse E: Low-dose nivolumab induced durable complete response in
relapsed primary central nervous system diffuse large B cell
lymphoma. Ann Hematol. 98:2227–2230. 2019. View Article : Google Scholar : PubMed/NCBI
|