Introduction
Cervical cancer (CC) is one of the most common
malignant types of tumors in the female reproductive system, and
poses a major threat to the physical and mental health of women
(1). According to the World
Health Organization, there are ~530,000 new cases of CC each year,
with an annual number of CC fatalities of ~26,000, 90% of which are
from devel- oping countries (2,3).
The 5-year survival rate for early-stage CC is 91.5%, while for
patients with advanced-stage CC it is only 16.5% (4,5).
The main treatments for advanced CC include radiation therapy and
chemotherapy (6). Moreover,
progress has been achieved in understanding the molecular mechanism
in CC and novel therapeutic strategies are emerging. For instance,
targeting therapeutic molecules enhances the sensitivity of CC to
radiotherapy and chemotherapy (7).
The guanosine monophosphate synthase (GMPS) gene is
located on chromosome 3q24, GMPS is involved in the guanine
nucleotide synthesis process and converts xanthine 5′-monophosphate
to guanine monophosphate (8). In
addition to its classic functions in GMP synthesis, GMPS has also
been reported to be involved in chromatin and gene regulation in
fruit flies. It has been found that GMPS/ubiquitin-specific
protease 7 binds to ecdysone-regulated loci and mutants displays
severe misregulation of ecdysone target genes (9,10).
GMPS is upregulated in numerous malignant tumors, such as lung
squamous cell carcinoma, ovarian serous cystadenocarcinoma and head
and neck squamous cell carcinoma (11-13). A previous study revealed that GMPS
participates in radiotherapy resistance and plays a role in
inducing cell apoptosis of nasopharyngeal carcinoma (12). It has been reported that the
expression of GMPS strongly affects the invasive capacity of
melanoma cells and ultimately tumor growth, and angustmycin A, a
nucleoside-analog inhibitor of GMPS produced by Streptomyces
hygroscopicus efficiently suppresses melanoma cell invasion
in vitro and tumorigenicity in immunocompromised mice
(14).
To the best of our knowledge, there have been no
studies reporting the biological role of GMPS in CC. Therefore, the
present study aimed to investigate the expression of GMPS in CC and
to elucidate its effects on the proliferation, aging and apoptosis
of CC cells.
Materials and methods
Subjects
The enrolled subjects included patients with
cervical intraepithelial neoplasia (CIN) and CC. Patients were
diagnosed and treated at the Department of Gynecology of The First
Affiliated Hospital of Soochow University (Suzhou, China) between
January 2019 and June 2020. In this study, patients with other
malignant tumors or history of treatment for other malignant tumors
were excluded. The clinical and pathological data were collected.
Patients with other benign tumors who underwent total hysterectomy
were assigned as controls. The cervical pathological diagnosis was
normal in the controls. Written informed consent was obtained from
all patients, and the study was approved by the Ethics Committee of
The First Affiliated Hospital of Soochow University (approval no.
2020LS265).
Bioinformatics analysis
GMPS gene expression was analyzed using microarray
gene expression data from the Oncomine database (http://www.oncomine.org). Based on the Multi-cancer
Statistics by Pyeon et al (15), Cervix Statistics by Biewenga et
al (16), Cervix 2 Statistics
by Scotto et al (17) and
Cervix Statistics by Zhai et al (18), the four datasets were evaluated
via bioinformatics analysis. The differential expression of GMPS
was determined between CC, which was obtained from the database by
defining the type of cancer as CC, and normal cervical tissues. The
data type was 'mRNA', and the type of analysis was based on
filtering results of cancer vs. normal cervix. The Oncomine
algorithm was selected for comparative statistical analysis.
Immunohistochemistry (IHC)
The specimen was fixed with 10% formalin at room
temperature for 24 h, dehydrated using gradient ethanol and
embedded into paraffin blocks. Paraffin-embedded specimens were cut
on a microtome at a thickness of 4 μm and baked in an oven
at 60°C for 30 min. After the tissue sections were routinely
dewaxed and rehydrated, the sections were washed with PBS solution.
The sections were immersed in 0.01 M citrate buffer solution (pH
6.0). Following incubation for 3 min, sections were removed and
cooled. Endogenous peroxidase activity was blocked by adding one
drop of peroxidase blocking agent, and sections were washed again
with PBS solution. Subsequently, appropriately diluted GMPS
antibody (anti-GMP synthase antibody-C-terminal; cat. no. ab135538;
1:100; Abcam) was added at ~50 μl. The specimens were
incubated overnight in a wet box at 4°C in a refrigerator. Sections
were removed the next day, washed with PBS and incubated with 50
μl secondary antibody reagent (cat. no. GK600711; 1:100;
Shanghai South Gene Technology Co., Ltd.). Sections were washed
with PBS, stained with brown glucose oxidase-DAB and then incubated
with hematoxylin solution. Following dehydration with anhydrous
ethanol, the slide was sealed with neutral resin. All these
procedures were conducted at room temperature for 30 min. The
slides were observed under a light microscope. The IHC results were
interpreted by combining the staining intensity of the positive
reaction cells and the staining area.
Cellular staining intensity and cell staining area
were independently assessed by two pathologists, without prior
knowledge of patient information. The positive reaction cells were
defined as those with brown-yellow cytoplasmic staining. According
to the intensity, staining was defined as: i) Negative (0); ii)
mild positive (1); iii)
intermediate positive (2); and
iv) strong positive (3).
According to the percentage of positive cells, scoring was as
follows: i) <5% (0); ii) 5-25% (1); iii) 26-50% (2); iv) 51-75% (3); and v) >75% (4). Under low magnification, five fields
of view were randomly selected to determine the results. The
overall score was comprehensively evaluated using the staining
range and intensity. Counting cells required a clear structure,
good positioning and a light background. When the staining was
heterogeneous, sections were independently scored and the results
were comprehensively evaluated. For example, a specimen containing
75% of tumor cells with moderate intensity staining (total score:
3×2=6), with the remaining 25% of tumor cells exhibiting mild
intensity staining (total score: 1×1=1), the final score would be 7
(6+1=7). The staining index (0-12) was determined from the staining
intensity score and the stained area score. Scores in the range of
0-7 were defined as low expression, and 8-12 as high
expression.
Cell lines and small interfering
(si)RNA
Human CC cell lines (HeLa, SiHa, CaSki and C33A)
were purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. SiHa, C33A and CaSki cells were
cultured with RPMI-1640 medium (HyClone; Cytiva) containing 10% FBS
(Biosera) and 1% penicillin-streptomycin (Beyotime Institute of
Biotechnology). HeLa cells were cultured with 10% FBS and 1%
penicillin-streptomycin in DMEM (HyClone; Cytiva). HeLa, SiHa,
CaSki and C33A cells grown on a 6-well plate (30-50% confluence)
were transfected with siRNA (100 nM per well) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
Transfected cells were incubated at 37°C and 5% CO2 for
2-4 days for subsequent experiments. The chemically synthesized
GMPS specific double-stranded siRNA targeting sequences were as
follows: Control (Ctrl) siRNA sense, 5′-UGGUUUACAUGUCGACUAATT-3′
and antisense, 5′-UUAGUCGACAUGUAAACCATT-3′; siRNA#1 sense,
5′-GCAUGUGUCACAACGUUAACA-3′ and antisense,
5′-UUAACGUUGUGACACAUGCGA-3′; siRNA#2 sense,
5′-CGAAUUAUGUAUGACUUAACA-3′ and antisense,
5′-UUAAGUCAUACAUAAUUCGAG-3′; and siRNA#3 sense,
5′-UGGAGAUAGUGUAGACAAAGU-3′ and antisense,
5′-UUUGUCUACACUAUCUCCAUG-3′. The siRNAs were synthesized by
Shanghai Integrated Biotech Solutions Co., Ltd.
Western blotting
Total protein was extracted from the cells with RIPA
lysis buffer (Beyotime Institute of Biotechnology) after
transfection for 72 h and the concentration of the protein was
determined with a Pierce™ BCA Protein assay kit (Peirce; Thermo
Fisher Scientific, Inc.). Protein samples (15-30 μg/lane)
were separated via 10% SDS-PAGE, and then separated proteins were
transferred to PVDF membranes (EMD Millipore). The membranes were
blocked with 5% BSA (Guangzhou Saiguo Biotech Co., Ltd.) for 1 h at
room temperature. Subsequently, primary antibodies were added and
incubated overnight at 4°C. Following washes with TBS with 0.05%
Tween-20, the membranes were incubated with a secondary antibody
[anti-mouse (cat. no. A0216) and anti-rabbit (cat. no. A0208) IgG
(H+L) antibodies; 1:1,000; Beyotime Institute of Biotechnology] for
1 h at room temperature. Protein bands were visualized using an ECL
kit (Shanghai Yeasen Biotechnology Co., Ltd.). The following
primary antibodies were used: Anti-GMPS (1:1,000; cat. no. 14602;
Cell Signaling Technology, Inc.), anti-P53 (1:1,000; cat. no. 2527;
Cell Signaling Technology, Inc.), anti-phosphorylated (p)-P53
(1:1,000; cat. no. 2521; Cell Signaling Technology, Inc.),
anti-Stat3 (1:1,000; cat. no. 9139; Cell Signaling Technology,
Inc.), anti-p-Stat3 (1:1,000; cat. no. 9145; Cell Signaling
Technology, Inc.) and anti-GAPDH (1:2,000; cat. no. 97166; Cell
Signaling Technology, Inc.).
Reverse transcription-quantitative
(RT-q)PCR analysis
Tissue samples and total RNA from CC cell lines
transfected for 48 h were extracted with TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.) and reverse transcription was performed
using PrimeScript™ RT Reagent Kit with gDNA Eraser (cat. no.
RR047A; Takara Bio, Inc.) according to the manufacturer's protocol.
RT-qPCR was performed with SYBR-Green reagent (Takara Bio, Inc.).
The expression levels of GMPS in CC tissues, paired adjacent cancer
tissues and four CC cell lines were analyzed using the
2−ΔΔCq method (19).
β-actin was used as an internal reference. The reverse-transcribed
cDNA was used as a template. The primer sequences used were as
follows: GMPS forward, 5′-ATGGCTCTGTGCAACGGAG-3′ and reverse,
5′-CCTCACTCTTCGGTCTATGACT-3′; and β-actin forward,
5′-TGACGTGGACATCCGCAAAG-3′ and reverse, 5′-CTGGAAGGTGGACAGCGAGG-3′.
The thermocycling conditions were as follows: Initial denaturation
at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C
for 5 sec and annealing at 60°C for 30 sec. Analysis of the
dissolution stage curve was performed under the following
conditions: 95°C for 15 sec, 60°C for 1 min, 95°C for 30 sec and
60°C for 15 sec.
Cell Counting Kit-8 (CCK-8) assay
There were three groups in this CC cell line
experiment: i) Ctrl, siRNA negative control; ii) siRNA#1
transfection; and iii) siRNA#2 transfection. In total, three repeat
wells were used in each group. Cells were incubated for 1 h with
CCK-8 (New Cell & Molecular Biotech Co., Ltd.). The absorbance
at 450 nm was detected with a microplate reader at 24, 48, 72, 96
and 120 h after transfection. The cell growth curve was created
after zeroing the blank control group.
5-Ethynyl-2′-deoxyuridine (EdU) assay for
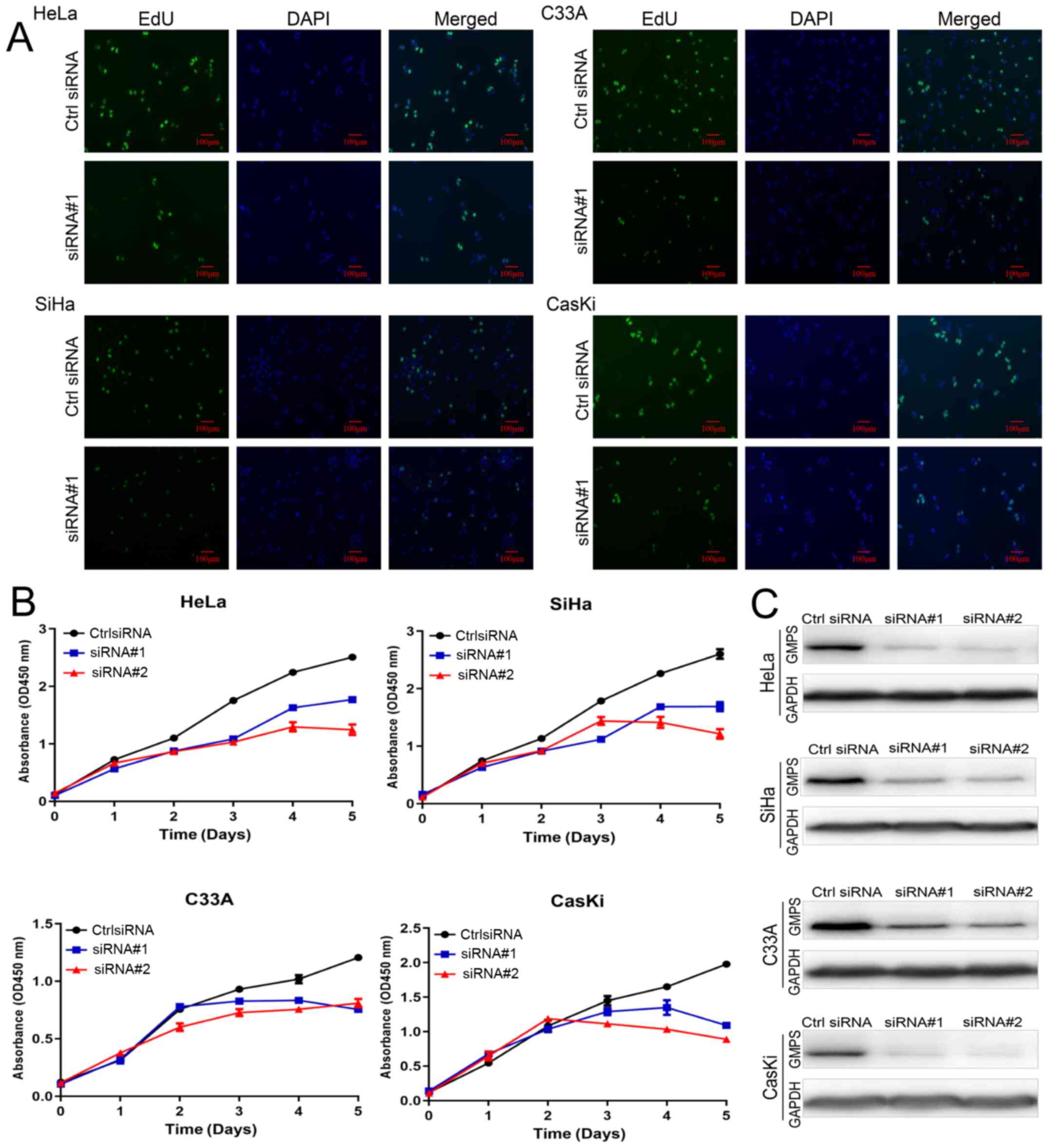
cell proliferation
There were two groups in this CC cell line
experiment: i) Ctrl, siRNA negative control; and ii) siRNA#1
transfection. In total, three repeat wells were used in each group.
At 72 h after cell transfection, 500 μl EdU medium (50
μM; Beyotime Institute of Biotechnology) was added to each
well and cells were incubated for 2 h. Then, the cells were fixed
with 4% paraformaldehyde for 15 min at room temperature and
permeated. A total of 200 μl Click working solution was
added to each well and incubated for 30 min. Cell nuclei were
stained with Hoechst for 10 min at room temperature, and imaged
with a fluorescence microscope (magnification, ×100).
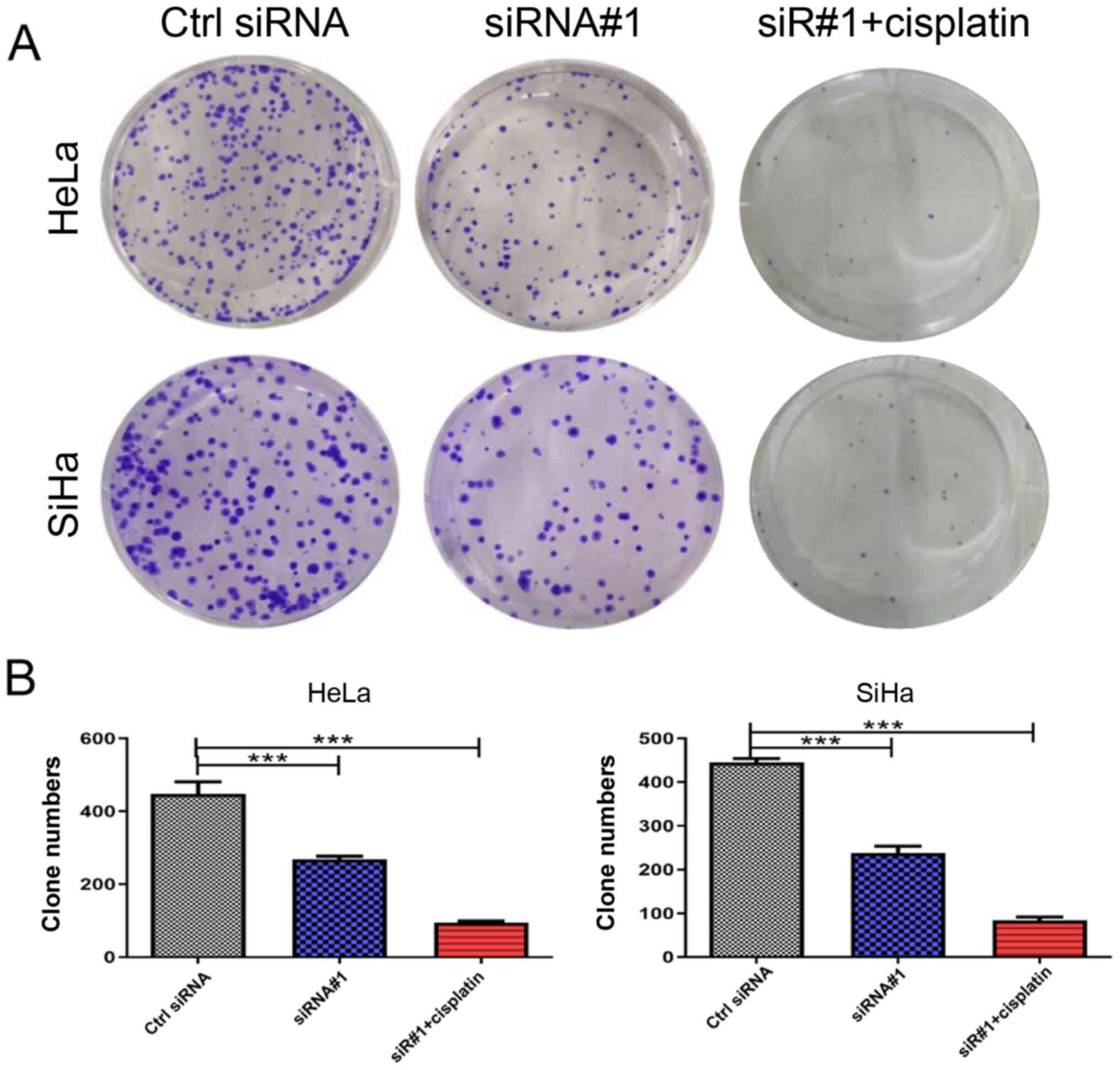
Clone formation assay to assess cell
proliferation
There were three experimental groups of CC cell
lines for this assay: i) Ctrl, siRNA negative control; ii) siRNA#1
transfection; and iii) siRNA#1 transfection combined with cisplatin
(3 μg/ml; Qilu Pharmaceutical Co., Ltd.). At 48 h after
transfection, 500 cells per well were added; three wells were used
in each group. The cells were stained with crystal violet solution
for 30 min and observed under an inverted microscope (CKX31SF;
Olympus Corporation) at ×100 magnification 7-10 days later. The
cell clones with >50 cells were counted as one monoclone, and
the mean number of clones on the plate was used to calculate the
cell clone-forming ability.
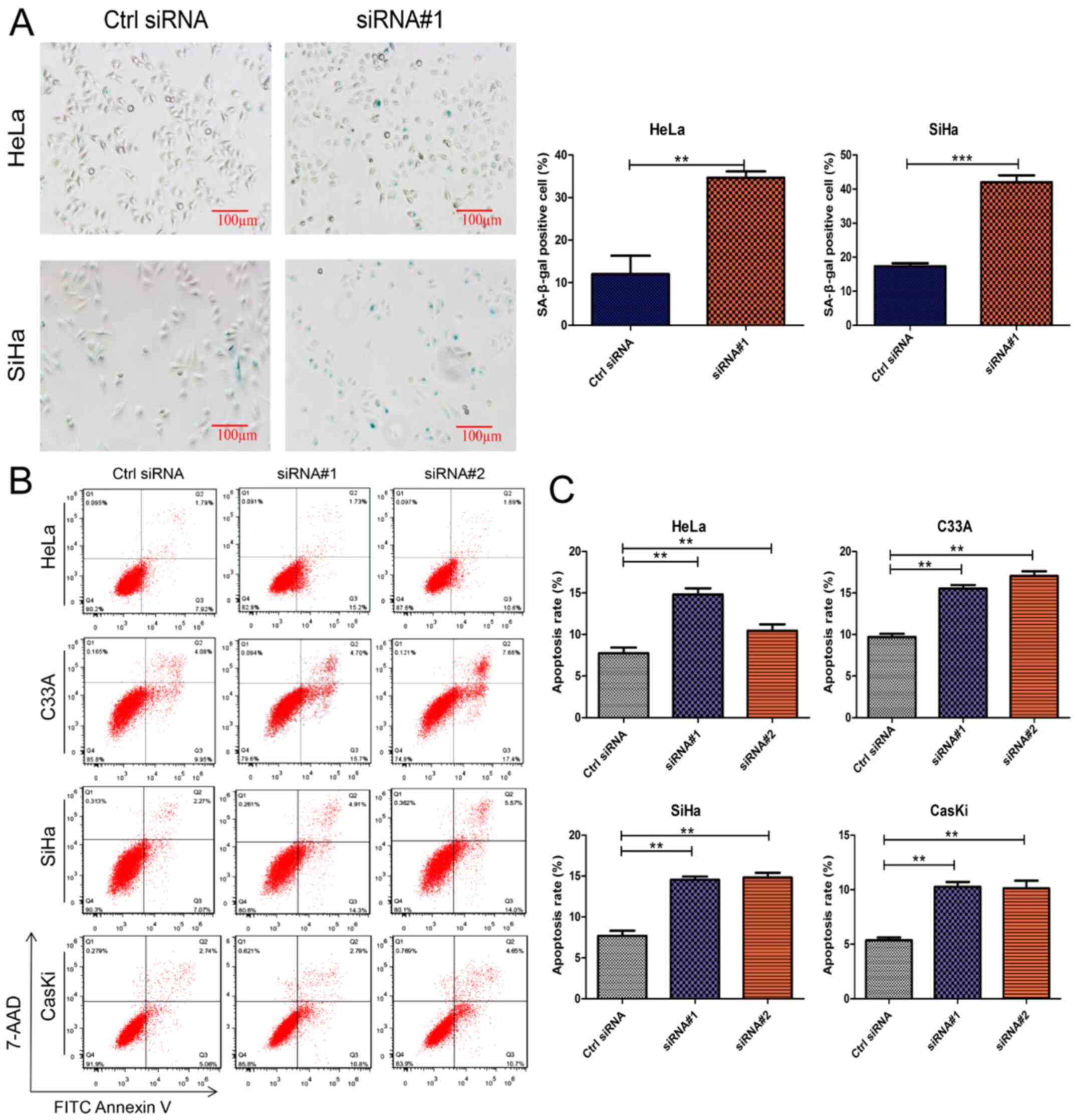
Flow cytometry for analysis of
apoptosis
There were three experimental groups of CC cell
lines for this analysis: i) Ctrl, siRNA negative control; ii)
siRNA#1 transfection; and iii) siRNA#2 transfection. HeLa, C33A,
SiHa and CaSki cells in the logarithmic growth phase were collected
and added to 6-well plates, and then transfected with siRNA at
30-50% confluence. Cells were digested and the transient
transfection was terminated after 72 h to create a single-cell
suspension. Cells were centrifuged at 500 × g for 5 min, and washed
twice with PBS. Then, 100 μl Annexin V-FITC binding solution
(BD Biosciences) was added to re-suspend the cells. After adding 5
μl PE-Annexin V and 5 μl 7-AAD staining solution,
cells were incubated at room temperature for 15 min in the dark.
Then, 400 μl Annexin V-FITC was added and the cells were
detected with a FC 500 flow cytometer (Beckman Coulter, Inc.). The
results were analyzed using FlowJo v10 software (FlowJo LLC) to
create a scatter plot. The living cells were on the lower left
quadrant (FITC-/7-AAD-), early apoptotic
cells on the lower right quadrant and late apoptotic and dead cells
on the upper right quadrant.
β-galactosidase staining for cell
senescence
HeLa and SiHa cells were selected for
β-galactosidase staining. Cells were divided into two groups: i)
Ctrl, siRNA negative control; and ii) siRNA#1 transfection. A cell
senescence β-galactosidase staining kit (Jiangsu Biyuntian
Biotechnology Research Institute) was used for staining and fixing.
Cells were cultured in a 6-well plate and transfected at 30-50%
confluence. Each group of cells was collected, washed once with
PBS, incubated with β-galactosidase staining fixative and fixed at
room temperature for 15 min. After aspirating the fixative, washing
with PBS three times and adding 1 ml staining solution to each
well, the cells were incubated at 37°C overnight. The results were
observed and images were captured under an inverted microscope
(magnification, ×100).
Immunofluorescence staining
There were two experimental groups of HeLa and SiHa
cells in this part of the study: i) Ctrl, siRNA negative control;
and ii) siRNA#1 transfection. CC cells were seeded at a density of
5×104/well in a 24-well cell plate with small glass slides. Cells
were transfected with siRNA for 48 h, according to their
experimental groups. Cells were washed three times with pre-chilled
PBS and fixed with paraformaldehyde (4%) for 15 min at room
temperature. Cells were washed twice with ice-cold PBS, incubated
with 0.5% Triton for 10 min, washed twice with PBS and blocked with
5% BSA (Guangzhou Saiguo Biotech Co., Ltd.) in PBS at room
temperature for 1 h. Cells were removed from the small round glass
and inverted onto the parafilm, which was coated with the primary
antibodies (1:100; cat. nos. 2521 and 9145; Cell Signaling
Technology, Inc.). After incubation overnight at 4°C, the slide was
removed and placed in a 12-well plate. After being washed with PBS,
the small round glass was removed and the plate was incubated with
a secondary antibody (cat. no. A0423; 1:500; Beyotime Institute of
Biotechnology) at room temperature for 1 h. The washing steps were
the same as aforementioned. The nuclei were stained with DAPI
solution in the dark for 30 min. Under the same condition in the
dark, the small round glass slide was inverted, placed on a glass
slide and an anti-fluorescent quencher was added dropwise. The
slides were observed and imaged using a fluorescence microscope
(magnification, ×200).
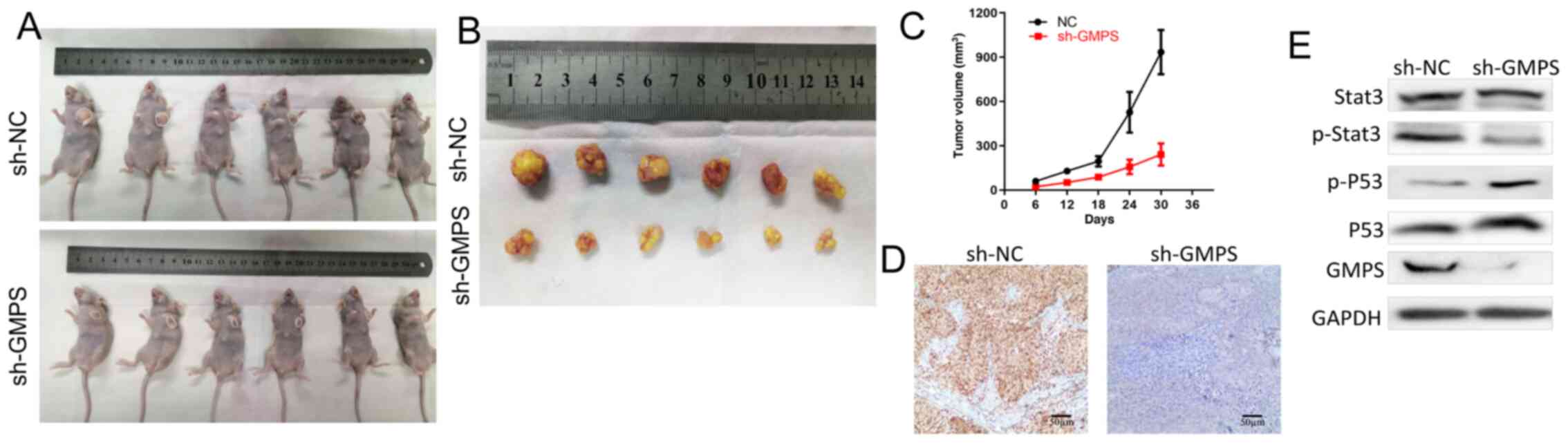
Animal experiments
A total of 12 female athymic BALB/c nude mice (age,
4-5 weeks; weight, 16-18 g) were obtained from the Model Animal
Research Center of Soochow University (Suzhou, China). The mice
were housed in specific pathogen-free barrier facilities in a
temperature-controlled room (24°C) with a 12 h light/dark cycle and
35-40% relative humidity, and were permitted free access to food
and drinking water. The body weight of each mouse was measured
every 2 days. The mice were humanely sacrificed via cervical
dislocation at the study endpoint. All experiments were performed
in accordance with the Guide for the Care and Use of Laboratory
Animals (NIH publication 80-23, revised 1996) (20), with the approval of the Ethics
Committee of Soochow University (approval no. SUDA20201027A01).
To obtain stable cell lines, lentivirus supernatant
of sh-GMPS and sh-control (GeneChem, Inc.) were added to HeLa cells
at MOI 10, according to the manufacturer's instructions, and
incubated at 37°C and 5% CO2 for 48 h, followed by
screening with 4 μg/ml puromycin for 2 weeks. Control HeLa
cells and GMPS-knockdown HeLa cells (2×106 cells) were
suspended in 100 μl PBS media and were subcutaneously
inoculated into the flanks of the mice. There were six mice in each
group (control and sh-GMPS). Mice were monitored every 2 days for
tumor growth, and tumor size was measured using a caliper. The
tumor volume was calculated using the modified ellipsoid formula:
Volume=1/2 × (length × width2). After 5 weeks, the mice
were sacrificed and tumor weight was measured. The maximum volume
of the tumors was 1.17 cm3, and the maximum diameter of
the tumors was 1.21 cm. A section of each tumor tissue was fixed in
10% formalin at room temperature for 24 h, and used for IHC. The
remaining tumor tissues were used for protein and mRNA expression
analyses, and the experiment methods were the same as above.
Statistical analysis
The data are presented as the mean ± standard
deviation. The clinical data are presented as percentages and
statistical analysis was performed using Pearson Chi-square and
Fisher's exact probability method. Each experiment was repeated ≥3
times independently. The χ2 segmentation method or
Fisher's exact probability method was used to further compare the
two sample frequencies. The differences between two groups were
examined using an unpaired Student's t-test and a one-way analysis
of variance for normally distributed data, and using the
Mann-Whitney U test for non-normally distributed data. SNK and
Dunnett's post hoc tests were performed following ANOVA. P<0.05
(two-sided) was considered to indicate a statistically significant
difference. All data analysis was performed using SPSS 22.0
software (IBM Corp.).
Results
Clinical characteristics of the
patients
The median age of the patients was 51 years (age
range, 25-76 years). There were 20 cases of normal cervical
tissues, 11 cases with Grade CIN I, 16 cases with Grade CIN II, 22
cases with Grade CIN III and 129 cases with CC. A total of 198
patient specimens were collected in this study (among which, paired
cancer and adjacent tissues were obtained from 32 cases). The
histopathological types of CC included squamous cell carcinoma
(n=88), adenocarcinoma (n=31), adenosquamous carcinoma (n=6) and
other special types (n=4). Clinical staging of CC was based on
International Federation of Gynecology and Obstetrics (FIGO 2014)
staging criteria (21). There
were 101 cases of early-stage CC (stage Ia n=8, stage Ib1 n=37,
stage Ib2 n=29 and stage IIa n=27). In total, 28 cases were at an
advanced stage (5 cases of stage IIb, 15 cases of stage III and 8
cases of stage IV) (Tables I and
II).
 | Table IGMPS expression as determined via
immunohistochemistry increased with the cervical histological
grading. |
Table I
GMPS expression as determined via
immunohistochemistry increased with the cervical histological
grading.
| Histopathological
diagnosis | n | GMPS expression, %
(n)
| P-value |
|---|
| Low | High |
|---|
| Normal | 20 | 20 (100.00) | 0 (0.00) | P<0.001 |
| CINI | 11 | 10 (90.91) | 1 (9.09) | |
| CINII | 16 | 13 (81.25) | 3 (18.75) | |
| CINIII | 22 | 14 (63.64) | 8 (36.36) | |
| CC | 129 | 55 (42.64) | 74 (57.36) | |
 | Table IIAssociation between GMPS expression,
assessed by immunohistochemistry, and clinicopathological
parameters in patients with cervical cancer. |
Table II
Association between GMPS expression,
assessed by immunohistochemistry, and clinicopathological
parameters in patients with cervical cancer.
| Clinical
parameters | n | GMPS expression, %
(n)
| P-value |
|---|
| Low | High |
|---|
| Age, years | | | | 1.0000 |
| ≤50 | 70 | 30 (42.86) | 40 (57.14) | |
| >50 | 59 | 25 (42.37) | 34 (57.63) | |
| Serum SCCA | | | | 0.1711 |
| ≤5 | 72 | 25 (34.72) | 47 (65.28) | |
| >5 | 44 | 25 (56.82) | 19 (43.18) | |
| Unknown | 13 | 5 (38.46) | 8 (61.54) | |
| FIGO staging | | | | 0.0661 |
| Ia | 8 | 6 (75.00) | 2 (25.00) | |
| Ib1 | 37 | 22 (59.46) | 15 (40.54) | |
| Ib2 | 29 | 7 (24.14) | 22 (75.86) | |
| IIa | 27 | 9 (33.33) | 18 (66.67) | |
| IIb | 5 | 2 (40.00) | 3 (60.00) | |
| III | 15 | 6 (40.00) | 9 (60.00) | |
| IV | 8 | 3 (37.50) | 5 (62.50) | |
| Pathological
type | | | | 0.7029 |
| Squamous cell
carcinoma | 88 | 38 (43.18) | 50 (56.82) | |
|
Adenocarcinoma | 31 | 12 (38.71) | 19 (61.29) | |
| Adenosquamous
carcinoma | 6 | 2 (33.33) | 4 (66.67) | |
| Others | 4 | 3 (75.00) | 1 (25.00) | |
| Early tumor
metastasis | | | | |
| Stage Ib Yes | 31 | 15 (48.39) | 16 (51.61) | 0.6202 |
| Stage Ib No | 35 | 14 (40.00) | 21 (60.00) | |
| Stage IIa Yes | 11 | 5 (45.45) | 6 (54.55) | 0.4105 |
| Stage IIa No | 16 | 4 (25.00) | 12 (75.00) | |
| Tumor
differentiation | | | | <0.0001a |
| Low-to-medium | 86 | 25 (29.07) | 61 (70.93) | |
| High | 43 | 30 (69.77) | 13 (30.23) | |
| Early infiltration
depth | | | | |
| Stage Ib deep
muscular layer | 28 | 7 (25.00) | 21 (75.00) | 0.0118a |
| Stage Ib
superficial layer | 38 | 22 (57.89) | 16 (42.11) | |
| Stage IIa deep
muscular layer | 19 | 5 (26.32) | 14 (73.68) | 0.1972 |
| Stage IIa
superficial layer | 8 | 4 (50.00) | 4 (50.00) | |
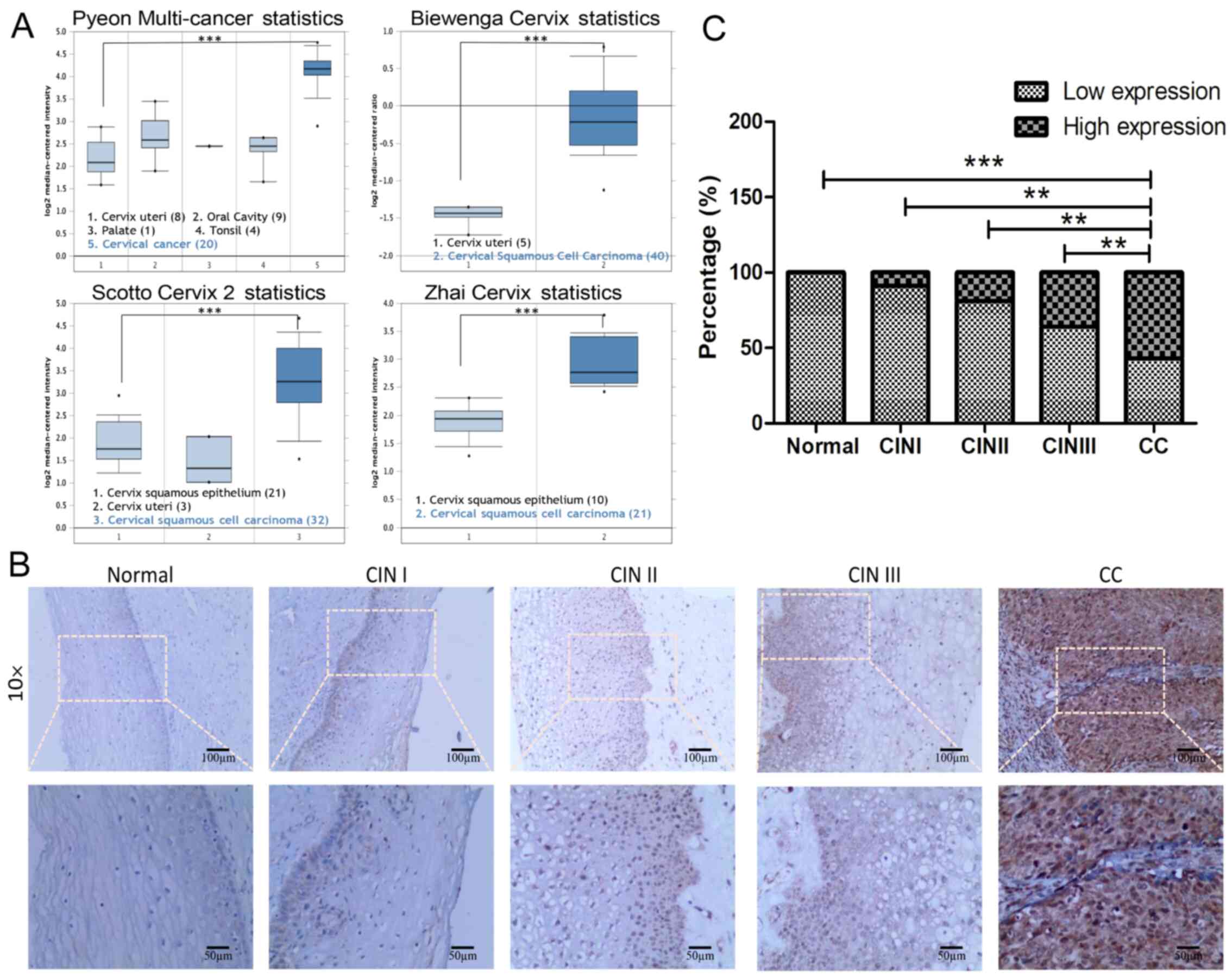
Bioinformatics analysis of the abnormal
expression of GMPS in CC
In order to elucidate the association between GMPS
and CC, the differential expression of GMPS mRNA in CC tissue and
normal cervical tissue was determined by analyzing the Oncomine
microarray gene expression dataset. The results demonstrated that
the expression of GMPS in CC tissues was significantly higher
compared with that in the corresponding normal cervical tissues
(Fig. 1A).
GMPS expression gradually increases from
normal cervical to CIN to CC tissues
To verify the abnormal expression of the GMPS gene
during the progression of CC, IHC experiments were conducted in
normal cervical, CIN I, CIN II, CIN III and CC tissues. During the
progression through different stages of cancer, the expression rate
of GMPS exhibited a significant increasing trend (Table I; Fig. 1B and C). Among them, the high
expression rate was 0.00% (0/10), 9.09% (1/11), 18.75% (3/16),
36.36% (8/22) and 57.36% (74/129) in normal cervical, CIN I, CIN
II, CIN III and CC tissues, respectively.
In CC, there were 88 cases of squamous cell
carcinoma, 31 cases of adenocarcinoma, 6 cases of adenosquamous
carcinoma and 4 cases of other special types. The IHC results
demonstrated that the high GMPS expression rate was 57.36% (74/129)
in the CC cases (Table II;
Fig. 2A and B).
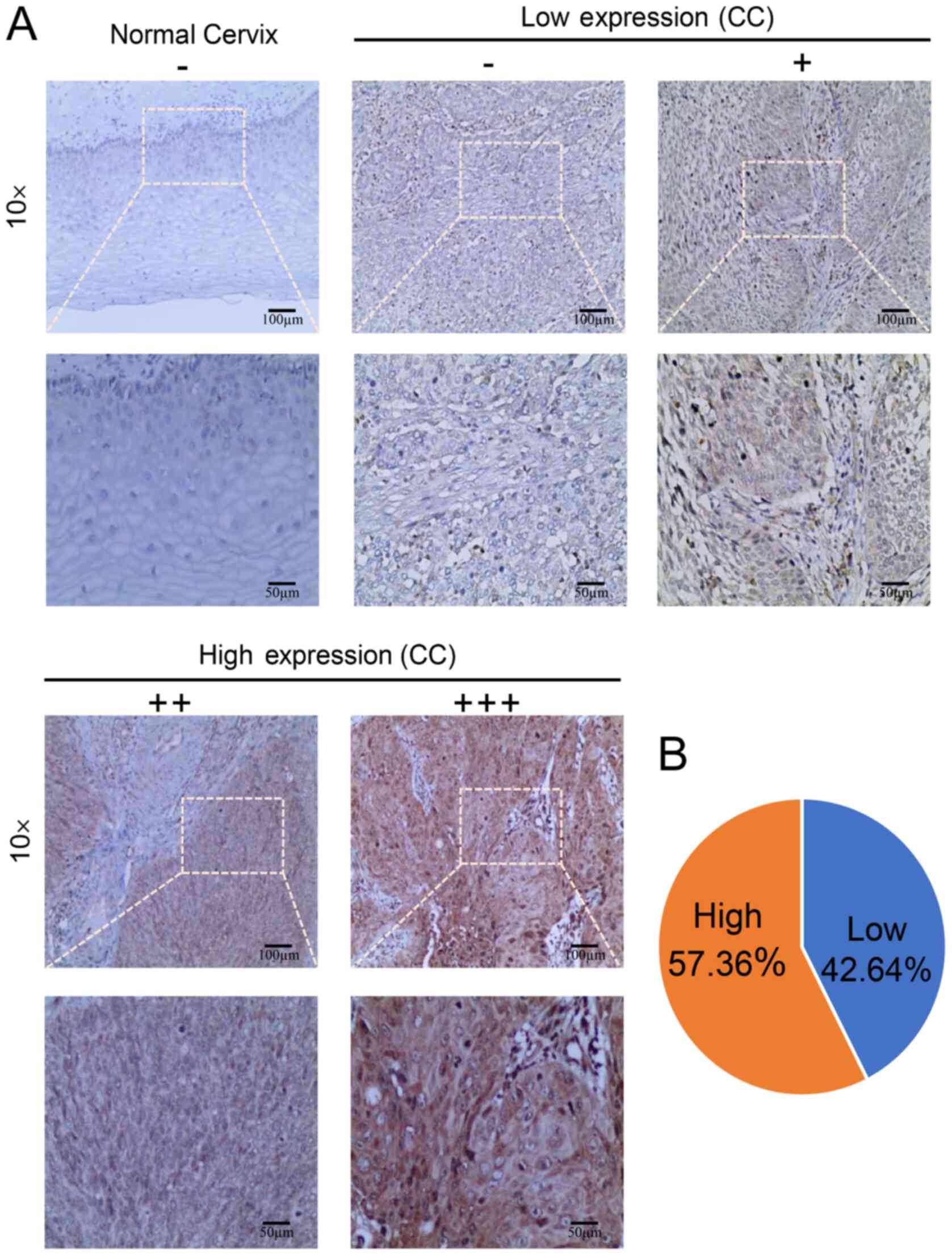
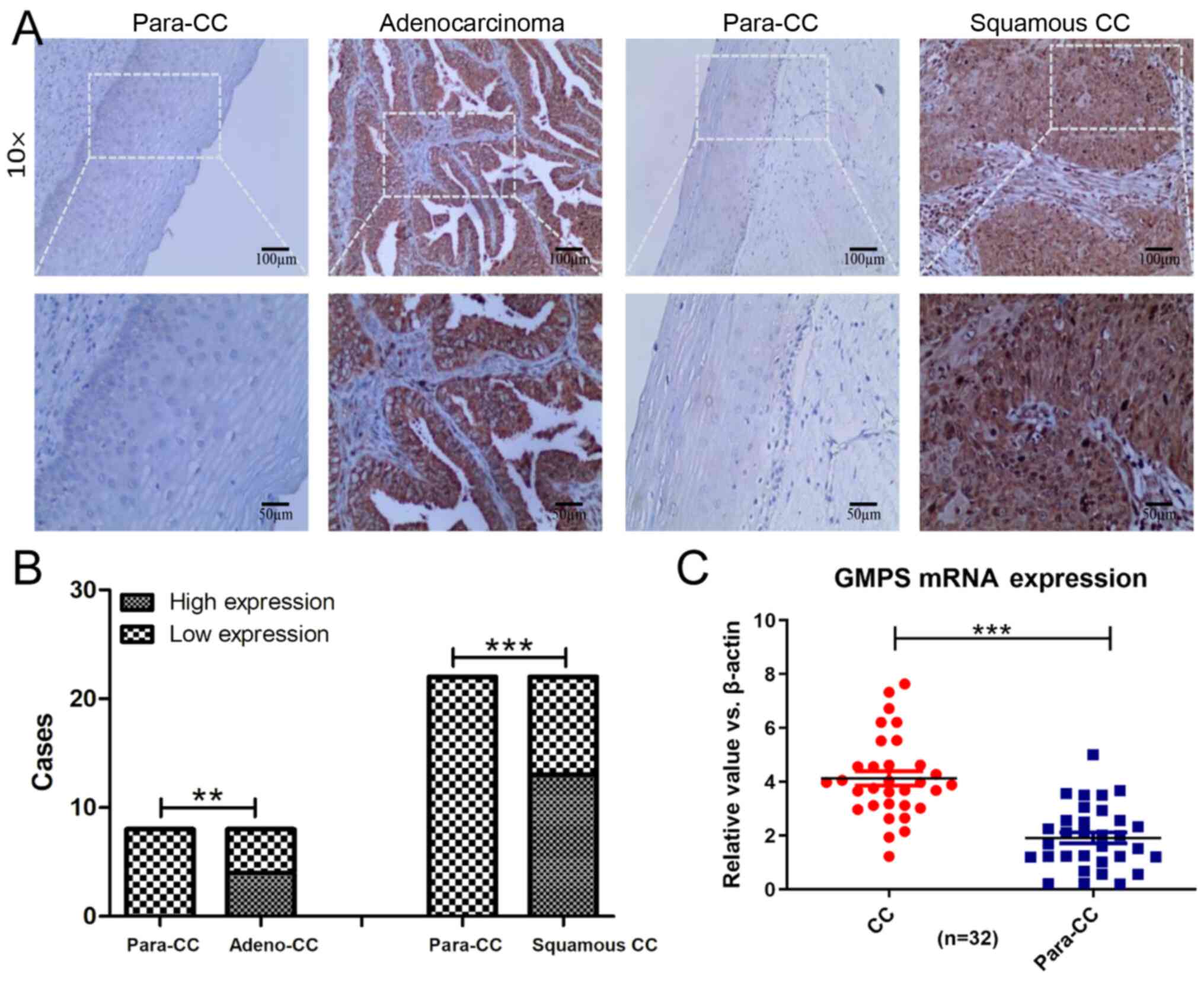
GMPS expression in CC tissues is
significantly higher compared with that in adjacent tissues
IHC results revealed that, in 32 cases of paired
cancer and adjacent tissues, the expression rate of GMPS in cancer
tissues was significantly higher compared with that in adjacent
tissues. In 10 cases of cervical adenocarcinoma tissues, the high
expression rate of GMPS was 50.00% (5/10) in adenocarcinoma tissue.
There were 22 cases of cervical squamous cell carcinoma. The high
expression rate of GMPS was 59.09% (13/22) in squamous cell
carcinoma tissues, while GMPS expression was not detected in
adjacent tissues (Fig. 3A and B).
RT-qPCR analysis was used to detect mRNA in 32 cases. The results
indicated that the mean expression level of GMPS mRNA in CC tissues
was significantly higher compared with that in matched
para-cancerous tissues (Fig.
3C).
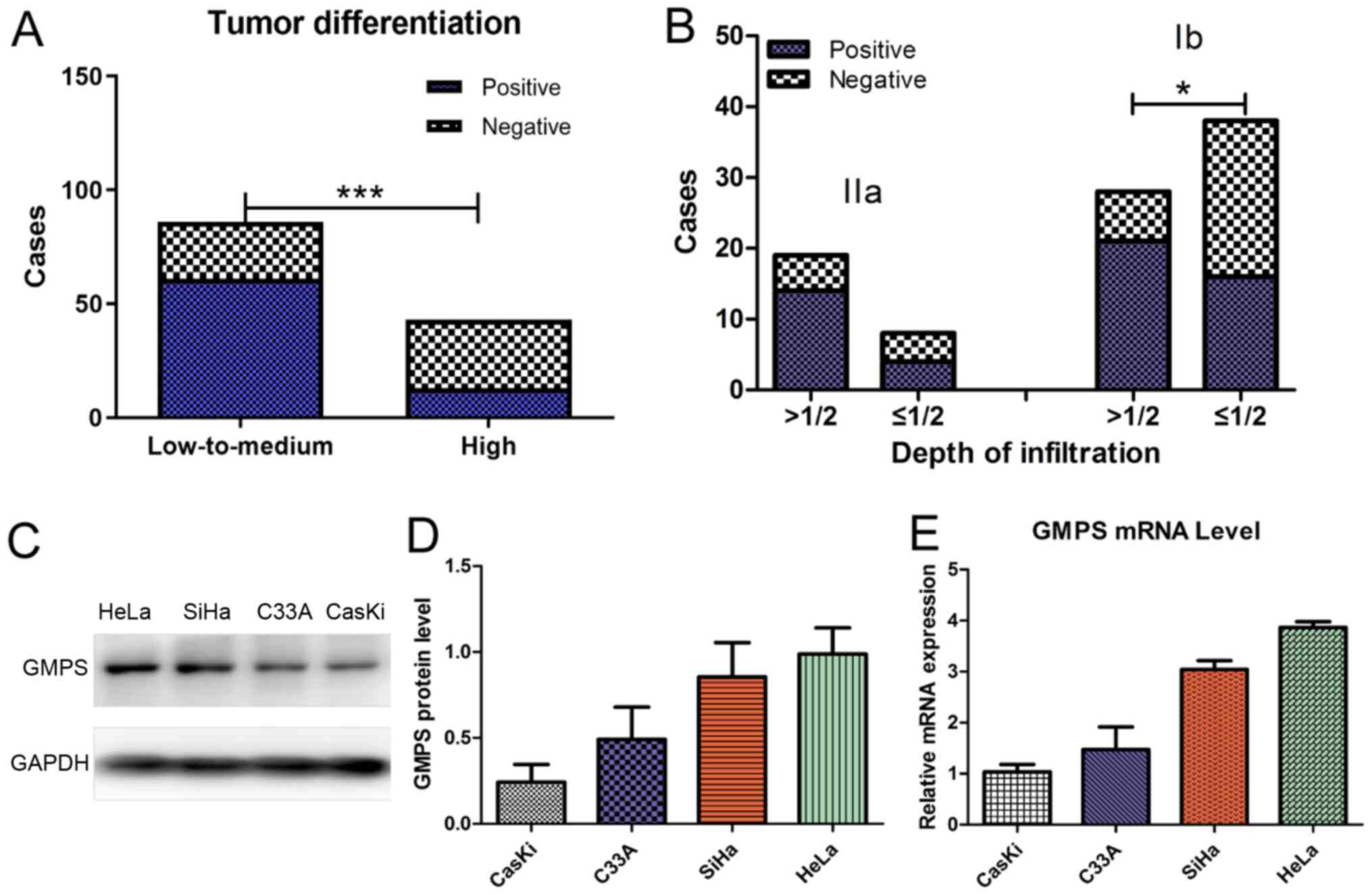
Association between abnormal expression
of GMPS and clinicopathological characteristics
A total of 129 CC tissue specimens were collected.
Among them, the high expression rate of GMPS was 70.93% (61/86) in
patients with low-to-medium differentiation of cancer cells, and
30.23% (13/43) in patients with high differentiation of cancer
cells. The results demonstrated that, compared with the high
differentiation of cancer cells, the expression rate of GMPS was
increased in the group with low-to- medium differentiation of
cancer cells (Table II; Fig. 4A).
In total, 66 patients had CC stage Ib. According to
postoperative pathology, 28 patients had deep myometrial invasion
(tumor invasion of >1/2 the thickness of the myometrium), and 38
patients had superficial myometrial invasion (myometrium invasion
≤1/2 of the total thickness). The findings indicated that, for
stage Ib disease, the high expression rate of GMPS was increased in
patients with tumors invading deep into the myometrium compared
with those with superficial myometrial invasion (Table II; Fig. 4B).
GMPS expression in CC cell lines
Western blotting and RT-qPCR analysis were performed
to evaluate the expression of GMPS in the four CC cell lines (SiHa,
HeLa, C33A and CaSki; Fig. 4C).
GMPS was variably expressed in the four CC cell lines. GMPS protein
and mRNA expression levels were markedly higher in SiHa and HeLa
cells in comparison with C33A and CaSki cells (Fig. 4D and E).
Effect of GMPS knockdown on the
proliferation and colony formation of CC cells
HeLa, SiHa, C33A and CaSki cells were transfected
with Ctrl siRNA, siRNA#1 and siRNA#2. The results suggested that,
compared with the Ctrl group, the cells in the GMPS siRNA knockdown
group grew slowly at 0-5 days (Fig.
5A-C). The CC cells HeLa and SiHa were transfected with Ctrl
siRNA, siRNA#1, and siRNA#1 combined with cisplatin. The results of
the clone formation experiments indicated that GMPS knockdown could
significantly decrease the colony-forming ability of the two CC
cell lines, the group of siRNA#1 combined with cisplatin was the
most obviously decreased compared with the Ctrl siRNA group
(Fig. 6A and B).
Effect of GMPS knockdown on the
senescence and apoptosis of CC cells
HeLa and SiHa cells were transfected with Ctrl siRNA
and siRNA#1. The senescence of the cells in each group was
observed, and it was found that GMPS knockdown could induce the
senescence of HeLa and SiHa cells (Fig. 7A).
HeLa, SiHa, C33A and CaSki cells were transfected
with Ctrl siRNA, siRNA#1 and siRNA#2. After 72 h of transfection,
cells were detected via flow cytometry to analyze the effects of
GMPS knockdown on CC cell apoptosis. Transfection with GMPS siRNA
specifically knocked down the expression of GMPS in CC cells. Flow
cytometry experiments demonstrated that knockdown of GMPS could
increase the proportion of apoptotic cells (Fig. 7B and C). These findings indicated
that GMPS knockdown facilitated apoptosis, and that GMPS may
enhance the malignant phenotype of tumors via the apoptotic
pathway.
Knockdown of GMPS plays a
tumor-suppressive role in CC
In the animal experiments, female nude mice were
subcutaneously inoculated with HeLa (low GMPS expressing cell
strain) cells transfected with short hairpin RNA (sh)-GMPS and HeLa
sh-NC cells. The experimental results demonstrated that the tumor
growth rate of the sh-GMPS group was significantly slower compared
with that of the HeLa sh-NC group (Fig. 8A-C). GMPS was highly expressed in
tumors of the sh-NC group, but its expression was low in tumors of
the sh-GMPS group (Fig. 8D).
Therefore, it was suggested that knockdown of GMPS exerted a
tumor-suppressive effect on CC. In summary, knockdown of GMPS could
suppress cancer growth rate in vitro and in vivo.
GMPS mediates the aging and apoptosis of
CC cells via the Stat3/P53 pathway
HeLa and C33A cells were transfected with Ctrl
siRNA, siRNA#1, siRNA#2 and siRNA#3. Western blotting results
indicated that, compared with the control group, Stat3 and p-Stat3
protein expression levels were markedly decreased in the GMPS siRNA
knockdown group. Moreover, the expression levels of the apoptotic
protein P53 and p-P53 were increased (Fig. 9B). These findings were also
confirmed in immunofluorescence experiments (Fig. 9A).
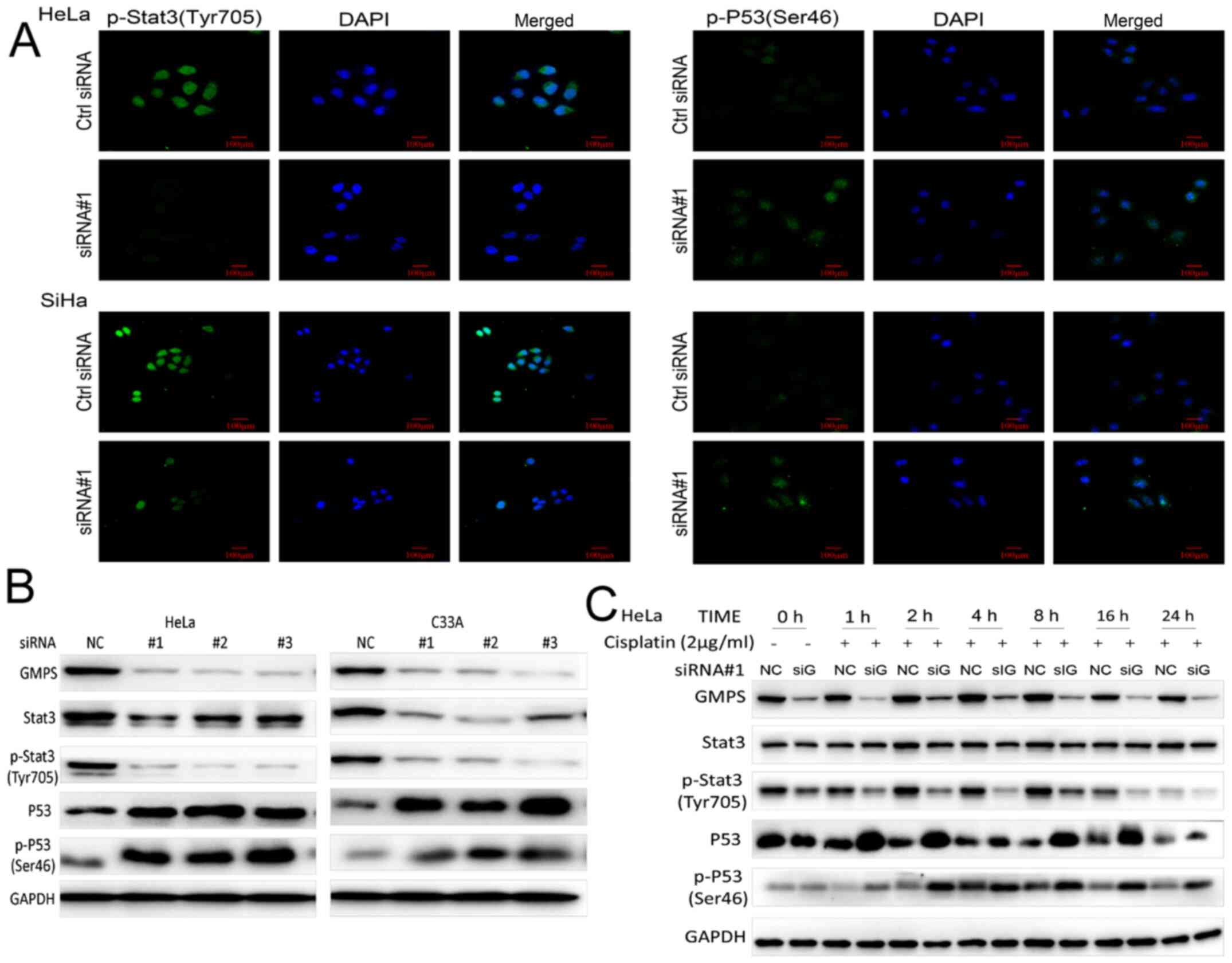 | Figure 9GMPS mediates the aging and apoptosis
of cervical cancer cells via the Stat3/P53 pathway. (A) HeLa and
SiHa cells were transfected with Ctrl siRNA and siRNA#1.
Immunofluorescence identified that p-Stat3 expression level was
decreased in the GMPS siRNA knockdown group, compared with the
control group. However, p-P53 expression level was increased. (B)
HeLa and C33A cells were transfected with Ctrl siRNA, siRNA#1,
siRNA#2 and siRNA#3. Western blotting results demonstrated that
Stat3 and p-Stat3 protein expression levels were decreased, and
those of P53 and p-P53 were increased. (C) HeLa cells were
transfected with Ctrl siRNA and siRNA#1, and then treated with
cisplatin. Western blotting was conducted to detect Stat3 and P53
protein expression levels at different time points. The results
suggested that the expression levels of Stat3 and p-Stat3 in GMPS
siRNA and cisplatin intervention groups were notably lower compared
with those in single cisplatin group and the control group. P53 and
p-P53 expression levels were notably higher in GMPS siRNA and
cisplatin intervention groups. GMPS, guanosine monophosphate
synthase; NC, negative control; p-, phosphorylated; Ctrl, control;
siRNA, small interfering RNA. |
HeLa cells were transfected with Ctrl siRNA and
siRNA#1, and the cells were treated with or without cisplatin.
Western blotting was used to detect Stat3 and P53 protein
expression levels. The protein expression levels of Stat3 and
p-Stat3 in the siRNA#1 + cisplatin treatment group were notably
lower compared with those in the other groups. Furthermore, the
protein expression levels of P53 and p-P53 were notably higher in
the transfected and cisplatin-treated cells compared with those in
the other groups (Fig. 9C).
In the animal experiments, the western blotting
results demonstrated that, compared with the control group, the
expression levels of P53 and p-P53 were upregulated in the sh-GMPS
group, while the expression levels of Stat3 and p-Stat3 were
downregulated (Fig. 8E).
The results indicated that GMPS may participate in
the regulation of CC cell senescence and apoptosis via the
Stat3/P53 pathway.
Discussion
GMPS is a classic biosynthetic enzyme that promotes
cell proliferation and DNA replication, most of the existing
research on GMPS has been performed in bacteria and insects, it has
been reported that GMPS is involved in chromatin and gene
regulation (8,12,22). A previous study revealed that GMPS
metabolism serves an important role in controlling the invasion and
tumorigenicity of human melanoma cells. Furthermore, GMPS is
associated with the degree of malignancy of tumors and is expected
to become a novel target for anticancer therapy (14). The present study demonstrated that
GMPS expression was upregulated at the mRNA and protein levels in
four human CC cell lines. Moreover, GMPS was highly expressed in
CC, with an expression rate of 57.36% in clinical specimens.
However, in normal cervical and para-cancerous tissue, its
expression was lower. The expression rate of GMPS was consecutively
increased from normal cervical, to CIN I, CIN II, CIN III and CC
tissues. Therefore, the present results suggested that GMPS may be
involved in the progression of CC.
GMPS may participate in DNA replication, cell
proliferation and abnormal cell division (12,23). Thus, this multi-function enzyme
may facilitate the development of novel therapeutic strategies in
cancer and other diseases. Examination of clinical specimens of
metastatic melanoma revealed that GMPS and guanosine monophosphate
reductase were not associated the disease outcome, but were
associated with cancer aggressiveness (8,23).
The present study indicated that, among the 129 patients with CC,
the expression rate of GMPS was higher in patients with
low-to-medium differentiation of cancer cells compared with those
with high cancer cell differentiation. Among the 66 patients with
CC stage Ib, the expression rate of GMPS was higher in patients
with tumor invasion of the deep muscularis layer of the myometrium.
These results suggested that GMPS may be associated with the
biological behavior of CC.
Apoptosis refers to the process of programmed cell
death. Dysregulation of the apoptotic mechanism is a sign of cancer
development. Apoptosis is associated with not only the occurrence
and development of cancer, but also the treatment resistance of
cancer (24,25). The mechanism of apoptosis is
complex and involves multiple signaling pathways. In general,
regulating the balance between pro-apoptotic and anti-apoptotic
proteins is a key factor in determining whether cells are apoptotic
(26). Targeting molecules
involved in apoptosis resistance is an effective treatment strategy
for cancer (27-29). After knocking down GMPS using
siRNA, the present study examined CC cell proliferation,
colony-forming ability, senescence and apoptosis. The results
demonstrated that knockdown of GMPS could significantly inhibit the
proliferation and colony-forming abilities of CC cells, as well as
promote cell aging and apoptosis. The animal experimental results
showed that the tumor growth rate of the sh-GMPS group was
significantly slower than that of the HeLa sh-NC group. These
findings indicated that GMPS may inhibit the senescence and
apoptosis of CC cells; however, the exact underlying mechanisms
remain unknown.
Both Stat3 and P53 are important regulators of cell
proliferation and survival. These serve an important role in the
stability and differentiation of cells, as well as participate in
the carcinogenesis of numerous types of cells (30-32). Stat3 is activated in multiple
types of human solid tumors and hematological malignancies, and it
has been suggested that Stat3 may provide novel molecular targets
for tumor treatments (33,34).
As a cytoplasmic transcription factor, nuclear transport of Stat3
requires phosphorylation, and Stat3 is activated primarily by
phosphorylation of the conserved tyrosine Tyr705 residue (32,35). Since the early seminal work in the
preclinical and clinical development of cisplatin, numerous
analogues have been synthesized and tested for properties that may
enhance their therapeutic index (36). The present study used siRNA to
knock down GMPS, and CC cells were simultaneously treated with
cisplatin. Compared with the control group, the expression levels
of Stat3 and p-Stat3 in the GMPS siRNA knockdown group were
decreased. P53 is a human tumor suppressor protein that serves a
vital role in cell cycle control and apoptosis (37,38). Previous studies have shown that
Stat3 inhibition could activate p53 expression in human cancer
cells and induce p53-mediated tumor cell apoptosis (39). The present study demonstrated
that, after knocking down GMPS, both Stat3 and p-Stat3 expression
levels were decreased, which was accompanied by increased P53 and
p-P53 expression levels, resulting in the induction of senescence
and apoptosis in CC cells. These same changes were observed in the
animal experiments. Based on these findings, it was suggested that
overexpression of GMPS may inhibit the senescence and apoptosis of
CC cells via the Stat3/P53 molecular pathway.
In conclusion, the present study demonstrated that
GMPS was highly expressed in CC, and was associated with tumor
differentiation and depth of invasion. It was also suggested that
GMPS may be implicated in the processes of CC cell proliferation,
colony formation, aging and apoptosis. Furthermore, GMPS may
inhibit the senescence and apoptosis of CC cells via the Stat3/P53
molecular pathway. Thus, a novel therapeutic target may be
developed based on GMPS for the treatment of CC.
Funding
The present study was funded by the National Natural
Science Foundation of China, (grant nos. 81672560, 81772773 and
81302275), the Project of Suzhou Minsheng Science and Technology
(grant no. SYSD2019204) and the Project of Suzhou Science and
Technology Development Plan (grant no. SLJ202006).
Availability of data and materials
The data generated and analyzed during the current
study are available from the corresponding author on reasonable
request. Public data and data repositories are referenced within
the manuscript.
Authors' contributions
JW, YWu, YL, FS and YWa performed the research. YWu,
YL and YWa analyzed the data. JW, YWu and YL wrote the paper. JZ
and YC conceived and designed the study, and they have seen and can
confirm the authenticity of the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all patients, and
the study was approved by the Ethics Committee of The First
Affiliated Hospital of Soochow University (approval no. 2019LS083;
Suzhou, China). All animal experiments were performed with the
approval of the Ethics Committee of Soochow University (approval
no. SUDA20201027A01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China, (grant nos. 81672560, 81772773 and 81302275),
the Project of Suzhou Minsheng Science and Technology (grant no.
SYSD2019204) and the Project of Suzhou Science and Technology
Development Plan (grant no. SLJ202006).
References
|
1
|
Melan K, Janky E, Macni J, Ulric-Gervaise
S, Dorival MJ, Veronique-Baudin J and Joachim C: Epidemiology and
survival of cervical cancer in the French West-Indies: Data from
the Martinique Cancer Registry (2002-2011). Glob Health Action.
10:13373412017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vu M, Yu J, Awolude OA and Chuang L:
Cervical cancer worldwide. Curr Probl Cancer. 42:457–465. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar :
|
|
5
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe Estimates
for 40 countries in 2012. Eur J Cancer. 49:1374–1403. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27:e432016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reddy BA, van der Knaap JA, Bot AG,
Mohd-Sarip A, Dekkers DH, Timmermans MA, Martens JW, Demmers JA and
Verrijzer CP: Nucleotide biosynthetic enzyme GMP synthase is a
TRIM21-controlled relay of p53 stabilization. Mol Cell. 53:458–470.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van der Knaap JA, Kozhevnikova E,
Langenberg K, Moshkin YM and Verrijzer CP: Biosynthetic enzyme GMP
synthetase cooperates with ubiquitin-specific protease 7 in
transcriptional regulation of ecdysteroid target genes. Mol Cell
Biol. 30:736–744. 2010. View Article : Google Scholar :
|
|
10
|
van der Knaap JA, Kumar BR, Moshkin YM,
Langenberg K, Krijgsveld J, Heck AJ, Karch F and Verrijzer CP: GMP
synthetase stimulates histone H2B deubiquitylation by the
epigenetic silencer USP7. Mol Cell. 17:695–707. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang P, Zhang Z, Ma Y, Lu J, Zhao H, Wang
S, Tan J and Li B: Prognostic values of GMPS, PR, CD40, and p21 in
ovarian cancer. PeerJ. 7:e63012019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang P, Li X, He Q, Zhang L, Song K, Yang
X, He Q, Wang Y, Hong X, Ma J, et al: TRIM21–SERPINB5. aids GMPS
repression to protect nasopharyngeal carcinoma cells from
radiation-induced apoptosis. J Biomed Sci. 27:302020. View Article : Google Scholar
|
|
13
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multi-dimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bianchi-Smiraglia A, Wawrzyniak JA, Bagati
A, Marvin EK, Ackroyd J, Moparthy S, Bshara W, Fink EE, Foley CE,
Morozevich GE, et al: Pharmacological targeting of guanosine
monophosphate synthase suppresses melanoma cell invasion and
tumorigenicity. Cell Death Differ. 22:1858–1864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pyeon D, Newton MA, Lambert PF, den Boon
JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH,
Smith EM, et al: Fundamental differences in cell cycle deregulation
in human papillomavirus-positive and human papillomavirus-negative
head/neck and cervical cancers. Cancer Res. 67:4605–4619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Biewenga P, Buist MR, Moerland PD, Ver
Loren van Themaat E, van Kampen AH, ten Kate FJ and Baas F: Gene
expression in early stage cervical cancer. Gynecol Oncol.
108:520–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scotto L, Narayan G, Nandula SV,
Arias-Pulido H, Subramaniyam S, Schneider A, Kaufmann AM, Wright
JD, Pothuri B, Mansukhani M, et al: Identification of copy number
gain and overexpressed genes on chromosome arm 20q by an
integrative genomic approach in cervical cancer: Potential role in
progression. Genes Chromosomes Cancer. 47:755–765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhai Y, Kuick R, Nan B, Ota I, Weiss SJ,
Trimble CL, Fearon ER and Cho KR: Gene expression analysis of
preinvasive and invasive cervical squamous cell carcinomas
identifies HOXC10 as a key mediator of invasion. Cancer Res.
67:10163–10172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
National Research Council: Institute for
Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press; Washington, DC: pp.
80–83. 1996
|
|
21
|
FIGO staging for carcinoma of the vulva,
cervix, and corpus uteri(the official organ of the International
Federation of Gynaecology and Obstetrics). Int J Gynaecol Obstet.
125:97–98. 2014. View Article : Google Scholar
|
|
22
|
Kozhevnikova EN, van der Knaap JA,
Pindyurin AV, Ozgur Z, van Ijcken WF, Moshkin YM and Verrijzer CP:
Metabolic enzyme IMPDH is also a transcription factor regulated by
cellular state. Mol Cell. 47:133–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wawrzyniak JA, Bianchi-Smiraglia A, Bshara
W, Mannava S, Ackroyd J, Bagati A, Omilian AR, Im M, Fedtsova N,
Miecznikowski JC, et al: A purine nucleotide biosynthesis enzyme
guanosine monophosphate reductase is a suppressor of melanoma
invasion. Cell Rep. 5:493–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar
|
|
26
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baig S, Seevasant I, Mohamad J, Mukheem A,
Huri HZ and Kamarul T: Potential of apoptotic pathway-targeted
cancer therapeutic research: Where do we stand? Cell Death Dis.
7:e20582016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fulda S: Targeting apoptosis for
anticancer therapy. Semin Cancer Biol. 31:84–88. 2015. View Article : Google Scholar
|
|
29
|
Giménez-Bonafé P, Tortosa A and
Pérez-Tomás R: Overcoming drug resistance by enhancing apoptosis of
tumor cells. Curr Cancer Drug Targets. 9:320–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Avalle L, Camporeale A, Camperi A and Poli
V: STAT3 in cancer: A double edged sword. Cytokine. 98:42–50. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar :
|
|
32
|
Galoczova M, Coates P and Vojtesek B:
STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett.
23:122018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buettner R, Mora LB and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
34
|
Yu H and Jove R: The STATs of cancer–new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Decker T and Kovarik P: Transcription
factor activity of STAT proteins: Structural requirements and
regulation by phosphorylation and interacting proteins. Cell Mol
Life Sci. 55:1535–1546. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chatterjee K, Das P, Chattopadhyay NR, Mal
S and Choudhuri T: The interplay between Epstein-Bar virus (EBV)
with the p53 and its homologs during EBV associated malignancies.
Heliyon. 5:e026242019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sullivan KD, Galbraith MD, Andrysik Z and
Espinosa JM: Mechanisms of transcriptional regulation by p53. Cell
Death Differ. 25:133–143. 2018. View Article : Google Scholar
|
|
39
|
Niu G, Wright KL, Ma Y, Wright GM, Huang
M, Irby R, Briggs J, Karras J, Cress WD, Pardoll D, et al: Role of
Stat3 in regulating p53 expression and function. Mol Cell Biol.
25:7432–7440. 2005. View Article : Google Scholar : PubMed/NCBI
|