Tumor metastasis is the primary cause of cancer
morbidity and mortality. It is responsible for ~90% of
cancer-associated deaths (1).
Three theories have served a role in understanding the mechanism of
metastasis. In 1928, Stevens and Ewing (2) proposed the 'anatomical mechanism
theory' or 'metastatic fluid dynamics theory', which states that
the fluid mechanics and blood vessels are involved in tumor
metastasis. Fidler (3) noted that
tumor cells derived from a tumor site could metastasize to a
specific site, and the pre-metastatic microenvironment was formed
even before the tumor cells reached the site. In 1976, Bross and
Blumenson (4) proposed the
'metastatic waterfall theory', which states that tumor metastasis
is complex, dynamic and a continuous biological process. According
to the 'seed-soil' theory proposed by Paget (5) in 1989, tumor metastasis does not
occur randomly. Tumor metastasis occurs when a favorable
interaction occurs between metastatic tumor cells (the 'seed') and
target organ microenvironment (the 'soil') (5). These three theories have aided the
design of experiments and understanding of the basics of tumor
metastasis. However, these three theories do not explain how the
primary tumor drives target organ selection and metastasis.
Previous studies have demonstrated that the primary tumor induces
changes in the target organ microenvironment prior to metastasis
(6-8).
Exosomes contain various bioactive substances, such
as proteins and nucleic acids, that serve a role in cell-to-cell
communication (14). A previous
study revealed that exosomes act as signals and trigger the
formation of a pre-metastatic microenvironment (15). The majority of previous studies
have focused on the role of exosomal non-coding RNAs in metastasis.
To the best of our knowledge, the role of proteins in regulating
PMN formation is not yet fully understood. Therefore, the present
review focused on the role of exosomal proteins in PMN formation,
and evaluated the potential of using these proteins for cancer
detection and therapy.
The term 'exosome' was first used in 1981. It was
defined as a substance with 5'nuclease activity that is released
from tumor cell lines (16). In
1987, Johnstone et al (17) studied the differentiation of
reticulocytes and reported that exosomes were formed by the
invagination of the plasma membrane. The 'redundant' plasma
membrane released from cells was named 'exosome' (17,18). Exosomes are extracellular vesicles
with a diameter of 40-150 nm and can be secreted from a variety of
cells under physiological and pathological conditions (19). They are commonly found in body
fluids, such as amniotic fluid, ascites, saliva, serum, plasma,
breast milk, urine and cerebrospinal fluid (20). Therefore, they can serve as a
biomarker for tumor diagnosis and for monitoring treatment
(21,22). Extracellular vesicles can be
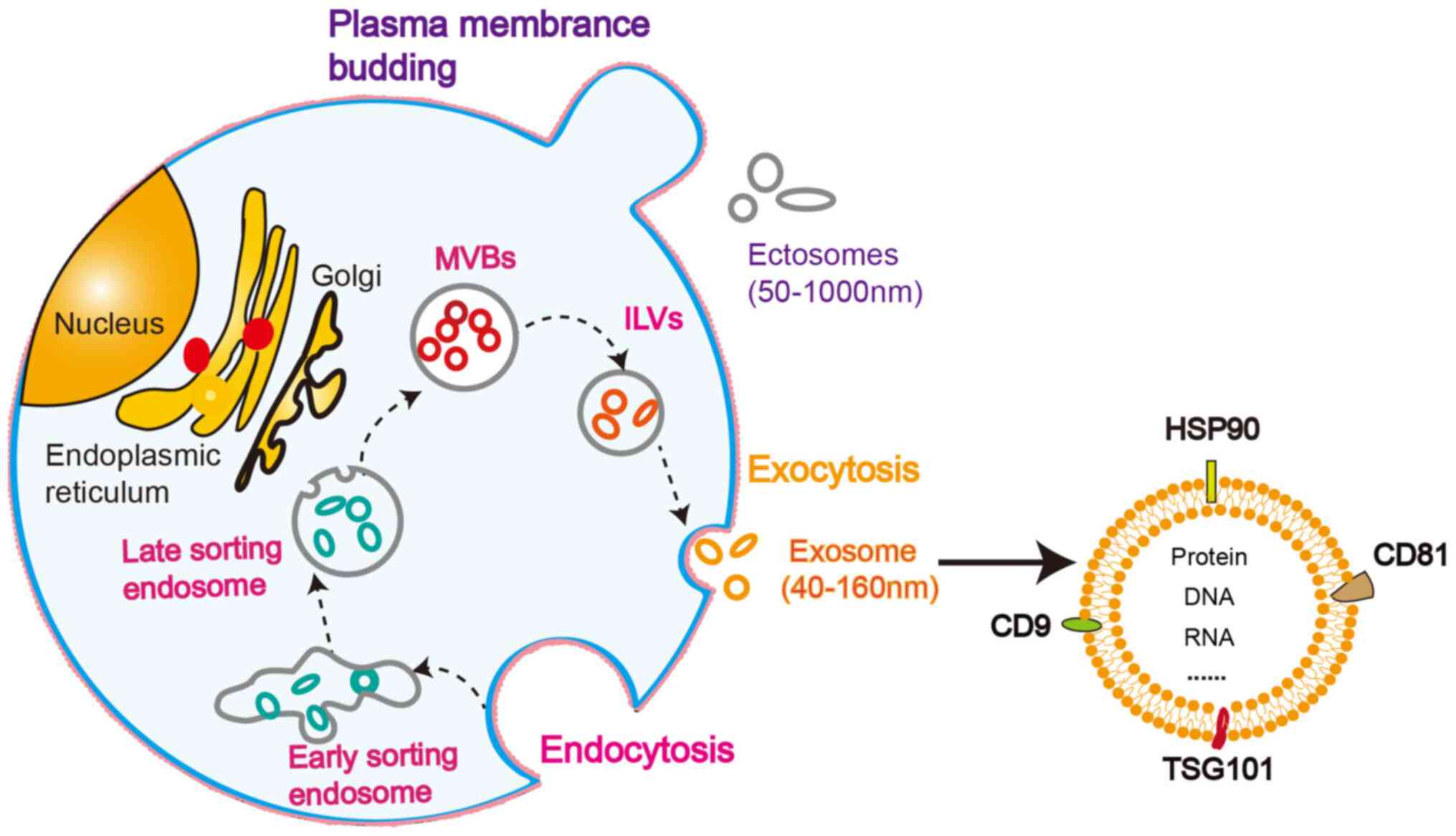
divided into two main categories: Ectosomes and exosomes. Ectosomes
are vesicles generated by the direct outward budding of the plasma
membrane and have a diameter of 50-1,000 nm (23,24). Exosomes are intraluminal vesicles
(ILVs) with a diameter of 40-160 nm formed through the fusion of
multivesicular bodies (MVBs) to the plasma membrane and exocytosis
(23). The formation of exosomes
is a complicated process (23).
The endogenous pathway can be divided into the following steps
(Fig. 1): The cell membrane sags
inward to form early sorting endosomes; the early sorting endosomes
mature into late sorting endosomes; inward invagination of the late
sorting endosomal limiting membrane leads to vesicles accumulation
in the cavity, thereby generating MVBs; the ILVs are released from
MVBs; the membranes of ILVs are fused with the cell membrane, and
the internal vesicles that are discharged are exosomes (25).
The composition of exosomes is relatively complex
and includes proteins, lipids, DNA, various types of RNAs,
metabolites and glycoconjugates (26,27). According to the statistics
released by Exocarta, 41,860 proteins, 1,116 lipid structures and
7,540 RNAs have been identified in exosomes (28). At present, increasing
high-throughput detection shows that tumor cells and
serum/plasma-derived exosomes of patients with cancer contain
different amounts of cargo compared with corresponding cells in
healthy individuals (29,30).
It has been revealed that the composition of
exosomes serves a role in cellular communication (23). The cargo is delivered between
cells in three ways: i) Through antigen presentation and
receptor-ligand interaction, thereby activating specific signaling
pathways and releasing protein and RNA contents into target cells;
ii) through a direct fusion of exosome membrane proteins with the
cell membrane of the target cell, so that the contents are released
into the cytoplasm of the target cell; and iii) through content
transport between cells via internalization mechanisms, such as
endocytosis by target cells, phagocytosis or receptor-mediated
endocytosis (31,32).
Exosomes contain a variety of biologically active
molecules (e.g., microRNA, long non-coding RNA, mRNA, protein and
lipids) (26,27). Proteins are the most abundant
component of exosomal cargo (33). The differential expression of
exosomal proteins between cancer and normal cells has been
intensively investigated (30).
Exosomes derived from malignant cancer cells have a distinctive
oncogenic protein profile and selectively enriched
metastasis-associated proteins (34). A few studies have directly
investigated whether metastasis-associated proteins are present in
exosomes derived from tumor cell lines (35,36). Metastasis-promoting lysyl
oxidase-like 4 has been identified in hepatocellular carcinoma
(HCC) cell exosomes (35). These
exosomes, when transferred to cancer cells and endothelial cells
(ECs), could promote cancer cell migration and angiogenesis
(35). It has been considered
that the ability of tumor exosomes to promote tumor metastasis is
associated with the metastatic ability of cancer cells (36). Previous studies screened the
protein profiles of exosomes derived from different cancer cell
phenotypes (37-39). A comparison of exosomal proteins
in mouse breast cancer cells with different metastatic abilities
revealed that highly metastatic cancer cell exosomes were enriched
in migration-, invasion- and angiogenesis-promoting proteins
(37). Additionally, membrane
proteins serving a crucial role in guiding target organ metastasis
were detected in exosomes derived from metastatic cancer cells
(37). Metastatic prostate cancer
cells deliver integrin (ITG) αvβ3 into the target cells via
exosomes and increase their migration capacity (38). Furthermore, screening the protein
profiles of exosomes helped analyze the differential
phosphorylation status of the proteins, such as Annexin A2 and
filamin-B (40). Weeraphan et
al (40) performed
phosphoproteomics and identified 43 differentially expressed
phosphoproteins between the highly invasive cholangiocarcinoma
cells and their corresponding control cells. Among these
phosphoproteins, heat shock protein 90 (HSP90) was finally
validated and associated with tumor metastasis (40).
A fluid biopsy is an important minimal invasive
approach for monitoring cancer and diagnosis. Body fluids,
including plasma, serum, urine and saliva, from patients with
cancer have been used to isolate exosomes, which were further
subjected to proteomics and compared with their corresponding
controls, to explore the potential role of exosomal protein cargo
for cancer detection (30).
Plasma exosomes from healthy donors were used to treat cancer cell
lines (41). Exosomes enhance
cancer cell migration and invasion in vitro and
dissemination in vivo (41). Metastasis-promoting proteins
involved in the activation of focal adhesion kinase signaling have
been identified on the surface of cancer cell exosomes (41). The proteome profiles of serum
exosomes from patients with papillary thyroid cancer (PTC) with or
without lymph node metastases (LNM) were compared, revealing that a
total of 697 proteins, including several well-known and tumor
metastasis-associated proteins, were selectively present in
patients with PTC and LNM (42).
Furthermore, 238 proteins were identified as the core urinary
exosome proteome by analyzing the intersection of proteins from
four urinary exosome proteome databases (43). Functional analysis of these
proteins revealed that they were involved in biological processes,
including regulation of cell movement and migration (43). A total of 86 proteins were
identified by exploring the proteome profile of saliva and serum
exosomes from patients with lung cancer. They were unique to lung
cancer and associated with cancer progression (44). Exosomes secreted by both invasive
breast cancer cells and glioma cells contain HSP90α, which
increases cancer cell motility by converting plasminogen into
plasmin (45).
It is well known that tumor stromal cells serve a
pivotal role in tumor progression (46). Fibroblasts are the most abundant
cell type in the tumor stroma. It has been demonstrated that the
proteins present in exosomes derived from cancer-associated
fibroblasts (CAFs) are also involved in tumor metastasis (47). Shimoda et al (48) generated tissue inhibitors of
metalloproteinases (Timp)-deficient fibroblasts, which acquired the
phenotype of CAFs. A disintegrin and metalloproteinase domain
(ADAM) 10, present in the exosomes derived from Timp-deficient
fibroblasts, is crucial for fibroblasts promoting breast cancer
motility (48). Chen et al
(49) reported that Wnt10b is
densely packed into exosomes derived from p85α-deficient breast
CAFs and mediates epithelial-to-mesenchymal transition of cancer
cells. Based on the available evidence, it is clear that, in
exosomes derived from body fluids of patients with cancer, cancer
cells and cancer-associated stromal cells, metastasis-associated
proteins are selectively enriched (Table I).
Studies have demonstrated that the integrins present
in exosomes derived from tumor cells are involved in the formation
of the PMN in target organs (39). Secretory integrins can be used as
a predictor of metastasis (39).
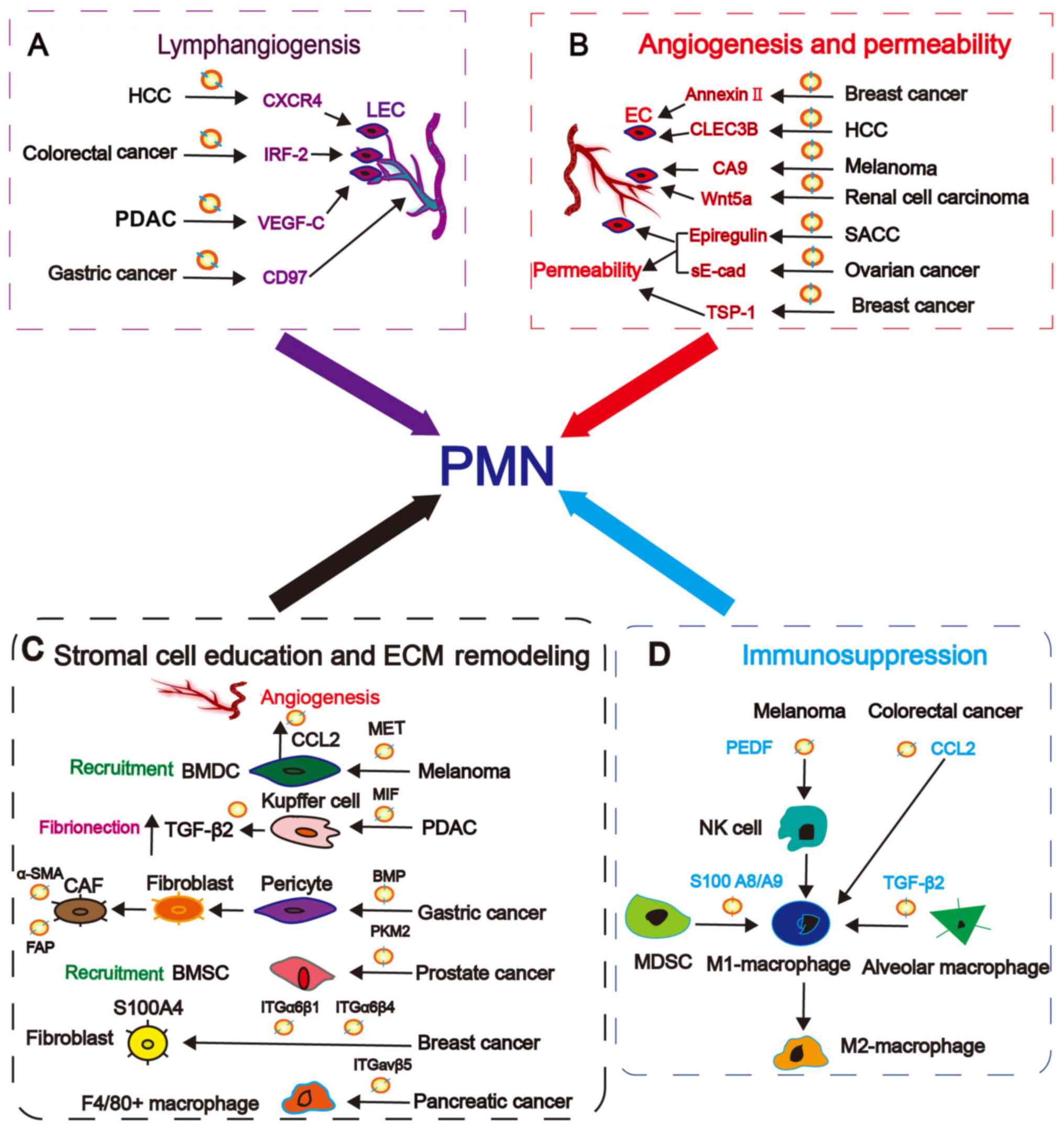
These findings emphasize the critical role of exosomal proteins in
the formation of PMN in target organs and metastasis. Exosomal
proteins are involved in the following important events, including
lymphangiogenesis, angiogenesis and permeability, stromal cell
education and extracellular matrix (ECM) remodeling and
immunosuppression, which can contribute to PMN formation.
LNM has been demonstrated to be an independent
prognostic indicator of various types of cancer, including oral
squamous cell carcinoma and cancer (50,51). Lymphatic vessels act as the
initial route for tumor cell dissemination to the lymphatic system
(52-54). Studies have demonstrated that LNM
is a critical step for the systemic spread of tumor cells (51) by providing abundant blood vessels
for tumor cells entering the blood circulation in lymph nodes
(55). Furthermore,
lymphangiogenesis in distant organs enhances the dissemination of
tumor cells to these organs (51). Therefore, lymphangiogenesis is an
important event involved in PMN; however, to the best of our
knowledge, its underlying mechanism is not yet fully
understood.
Recent studies have demonstrated that exosomal
proteins serve a role in lymphangiogenesis (56,57). Lymphatic endothelial cells (LECs)
are essential for lymphangiogenesis (58). Interferon regulatory factor 2,
which is present in colorectal cancer cell exosomes, induces the
secretion of vascular endothelial growth factor (VEGF) C by
macrophages, thereby promoting LEC proliferation and lymphatic
network formation required for sentinel LNM (56). Exosomal C-X-C chemokine receptor
type 4 derived from mouse hepatocarcinoma Hca-F cells with high
metastatic ability enhances LEC proliferation and lymphatic tube
formation (57). Pancreatic
ductal adenocarcinoma (PDAC) exosomes enhance lymphangiogenesis and
tumor cell dissemination via the delivery of VEGFC into LECs
(59). These findings suggest
that LECs are important for exosomal protein-mediated
lymphangiogenesis. Additionally, exosomal CD97 derived from
SGC-7901 gastric cancer cells with high lymphatic metastatic
potential increases the LNM capability of cancer cells with weak
metastasis potential (60). This
is accompanied by the induction of PMN-associated molecule
expression in lymph nodes (60).
Although there is no direct experimental evidence for
lymphangiogenesis in this study (60), the levels of proteins, such as
transforming growth factor-β induced protein, which is an important
lymphangiogenesis-promoting factor (61), are increased. Representative
exosomal proteins modulating lymphangiogenesis are presented in
Fig. 2A.
The generation of new blood vessels and increased
vascular permeability are key steps in disseminating tumor cells.
They provide tumor cells with the necessary nutrients and oxygen,
and also increase vascular permeability, facilitating tumor cell
intravasation and extravasation (62). Therefore, angiogenesis and
increased vascular leaking have been defined as one of the
characteristics of PMN (11).
Studies have demonstrated the involvement of exosomal proteins in
these two pathological processes (63,64).
Annexin II loaded in exosomes derived from malignant
breast cancer cells promotes angiogenesis in vitro as shown
by EC migration, invasion and tube formation assays, and in
vivo as observed in a Matrigel plug assay (63). Additionally, exosomes collected
from metastatic breast cancer cell lines with knockdown of annexin
II have a reduced capacity in guiding targeted metastasis (63). Thrombospondin-1-enriched exosomes
derived from metastatic breast cancer cells suppress the expression
of tight junction proteins, including vascular endothelial cadherin
(VE-cadherin) and zona occluden-1, leading to the increased leakage
of vessels and enhanced transendothelial migration (64). The aforementioned two studies
(63,64) explored the unilateral role of
exosomal proteins in angiogenesis and permeability.
Studies have demonstrated that a few exosomal
proteins serve a regulatory role in both angiogenesis and
permeability (64-66). Exosomes from highly metastatic
ovarian cancer cells contain soluble E-cadherin (sE-cad) (67). Exosomal sE-cad interacts with
VE-cadherin to activate β-catenin and NF-κB signaling in human
umbilical vein ECs (HUVECs), thereby promoting HUVEC migration,
tube formation and permeability in vitro (67). Furthermore, sE-cad promotes
angiogenesis in vivo and is positively associated with
ascites formation and dissemination of tumor cells (67). Exosomal epiregulin derived from
salivary adenoid cystic carcinoma cells increases the migration of
human pulmonary microvascular ECs, tube formation and permeability
in vitro and in vivo, leading to lung metastasis
(65). In addition to the
proteins enriched in exosomes, reduced levels of C-type lectin
domain family 3 member B in exosomes derived from HCC cells
promotes EC migration and invasion and increases VEGF release from
HCC cells and ECs by activating adenosine
5'-monophosphate-activated protein kinase signaling to
synergistically induce angiogenesis (66).
Cell migration-inducing and hyaluronan-binding
protein (CEMIP) is specifically present in brain metastatic cancer
cell exosomes (68). The delivery
of CEMIP+ exosomes into brain endothelial and microglial
cells promotes EC branching and induces the expression of several
pro-inflammatory cytokines that facilitate brain vascular
remodeling and metastasis (68).
Hypoxia is one of the main driving forces of tumor angiogenesis. It
can stimulate the release of exosomes containing high levels of
pro-angiogenesis proteins by tumor cells (69). Additionally, treatment of renal
carcinoma cells with hypoxia-mimicking agent cobalt chloride
results in elevated exosomal carbonic anhydrase 9 levels in HUVECs,
and promotes HUVEC migration, tube formation and MMP2 expression
(70). In addition to hypoxia
serving as an inducer of pro-angiogenesis proteins in exosomes,
exosomal Wnt5a from melanoma cells could increase the exosomal
content of IL6, VEGF and MMP2, which confers increased
pro-angiogenic function to malignant melanoma cells as observed by
blood vessel formation assays (71). Representative exosomal proteins
involved in angiogenesis and permeability are presented in Fig. 2B.
When cancer cells metastasize to distant target
organs, they have to adapt to the new environment. The main
components of these distant organ microenvironments are stromal
cells and the ECM. Stromal cell education and ECM remodeling in
target organs determine whether tumor cells can successfully
colonize and metastasize (72,73).
Exosomes derived from different site-specific tropic
cancer cells could be internalized by distinct resident stromal
cells (39). Lung-tropic exosomes
are mainly taken up by S100 calcium-binding protein A (S100A)
4-fibroblasts and surfactant protein C-epithelial cells in the
lungs, while liver-tropic exosomes are mostly internalized by
Kupffer cells in the liver (39).
Brain-tropic exosomes are preferentially taken up by CD31-ECs in
the brain (39). Specific
integrins packaged into these exosomes induce distinct stromal
cells to express S100 genes, which facilitate tumor metastasis
(39). Prostate cancer exosomes
selectively deliver pyruvate kinase M2 (PKM2) to bone marrow
stromal cells (BMSCs) and educated BMSCs by inducing the secretion
of C-X-C motif chemokine ligand 12 (CXCL12), which promotes cancer
cell seeding and growth in the bone marrow to form bone metastasis
(74). The exosomal transfer of
MET proto-oncogene, receptor tyrosine kinase from melanoma cells
with high metastatic ability to bone marrow-derived progenitor
cells induces the acquisition of a pro-vasculogenic phenotype,
which may further elicit cancer cell metastasis (75).
In addition to directly affecting tumor cells, the
educated stromal cells are responsible for ECM remodeling,
triggering a cascade effect and promoting PMN formation (74-77). Macrophage migration inhibitory
factor (MIF) expressing PDAC-derived exosomes induces the secretion
of TGF-β by Kupffer cells, which subsequently upregulates
fibronectin generation in hepatic stellate cells (76). Such a fibrotic microenvironment
induces the infiltration of bone marrow-derived macrophages that
promote liver metastasis (76).
Exosomal bone morphogenetic protein (BMP) derived from gastric
cancer cells induces pericyte transition into CAFs by increasing
the secretion of CAF markers (α-smooth muscle actin and fibroblast
activation protein) in pericytes (77). The two markers are important for
cancer cell motility (78). CAFs
promote malignant tumor progression by regulating multiple cellular
events, such as angiogenesis, ECM remodeling and metabolism
(79). Exosomal BMP is an
important signaling molecule that confers pericytes with CAF-like
functions during cancer progression (77). Additionally, tumor-associated
leukocytes induce breast cancer cells to express fibronectin and
generate fibronectin-positive exosomes (80). The uptake of these exosomes by
cancer cells produces pro-inflammatory cytokines and MMP9, which
further promotes cancer cell invasion (80). Furthermore, exosomal proteins
could directly remodel the ECM by altering the related protein and
protease levels in target sites. Under hypoxia, prostate cancer
cell exosomes selectively increase MMP activity and expression, and
promote fibronectin and collagen IV expression at the PMN (81). Additionally, the differential
expression of proteins in exosomes has been screened and analyzed
before and after inducing hypoxia, and the results suggested that
exosomal protein-mediated ECM remodeling served an important role
in the formation of the PMN (81). ITGα6β4 and ITGα6β1 enriched in
lung-tropic exosomes interact with S100A4-fibroblasts in the
laminin-rich lung tissues, whereas liver-tropic exosomes packaged
with ITGαvβ5 interact with F4/80+ macrophages in
fibronectin-rich liver tissues (39). Despite no direct evidence
indicating how ECM remodeling is regulated, the altered composition
of the ECM is likely caused by exosomal integrins, further
suggesting that the interaction between integrin and the ECM
determines the target site for metastasis (Fig. 2C) (33).
Exosomes released by tumors and stromal cells serve
an important role in modulating the host immune response and
creating an immunosuppressive microenvironment (82). In 2003, researchers revealed that
the nonclassical human leucocyte antigen-G class I molecule, an
immunosuppressive molecule, could be loaded into exosomes to help
cancer cell escape from immunosurveillance (83). At present, immunosuppression is
deemed as another important characteristic of the PMN (11). The immunosuppressed
microenvironment shaped by exosomal proteins in primary lesions has
been investigated (84). However,
few studies have focused on the roles of exosomal protein-mediated
immunosuppression in secondary metastasis sites. By contrast,
exosomal proteins modulate the immunosuppressive microenvironment
at the PMN mainly by recruiting immunosuppressive cells and
polarizing the tumor-promoting phenotypes of immune cells (11,85-88).
Recruiting immunosuppressive cells is an important
aspect contributing to the permissive microenvironment at the PMN
(11). Myeloid-derived suppressor
cells (MDSCs) are classic immunosuppressive cells recruited to the
PMN (85). MDSC recruitment
triggered by exosomal proteins at the PMN has been reported in two
studies (86,87). S100A8/A9 enriched in MDSC-derived
exosomes at the PMN could enhance chemotactic activity and further
recruitment of MDSCs (87).
Macrophages have the M1 phenotype with antitumor activity and M2
phenotype with tumor-promoting activity (89). Their phenotypic shift has been
extensively studied in the formation of the immunosuppressed PMN
(88,90). Colorectal cancer cell exosomal C-C
motif chemokine ligand 2 (CCL2) recruits macrophages and confers
the M2 phenotype to them in liver PMN (90). Exosomes derived from highly
metastatic osteosarcoma cells exhibit an enhanced ability to induce
alveolar macrophage secretion of TGF-β2 and transition into
tumor-promoting M2 phenotype, thus suppressing their ability to
kill tumor cells (88). However,
this study (88) did not reveal
which component in the exosomes mediates the immunosuppression
regulation and this should be investigated in the future.
The role of exosomal protein-mediated
immunosuppression in the PMN has been studied extensively. However,
Plebanek et al (91)
revealed that pigmented epithelium-derived factor, an outer surface
protein of exosomes derived from less metastatic melanoma cells,
could expand Ly6Clow patrolling monocytes (PMo) in the
bone marrow, recruit natural killer cells, induce the
differentiation of PMo into macrophages and M1 polarization, thus
enhancing the capacity of PMo to kill tumor cells in lung tissues
and suppressing lung metastasis (91). The contradictory aspect of
exosomal proteins in immune response regulation increases the
complexity of the PMN, indicating that the diversity of exosomal
proteins in immune regulation should be further clarified (Fig. 2D).
Exosomal proteins have been demonstrated to serve an
essential role in PMN formation and target organ metastasis.
Therefore, it is important to know whether they have potential
clinical applications. A review of the current literature revealed
that most studies also paid attention to the potential prognostic
and therapeutic implications of exosomal proteins. A study by
Hoshino et al (39) was
the first to uncover the role and molecular mechanism of exosomal
proteins in organ-specific metastasis by inducing PMN. It was
determined that different integrins were associated with
site-specific metastasis, and integrins were specifically knocked
down or integrin binding was blocked using peptides, and it was
observed that integrins could efficiently suppress the arrival of
exosomes to target organs and internalization of exosomes by
stromal cells, and reduce metastasis. Notably, Hoshino et al
(39) identified the upregulation
of specific lung metastasis-associated ITGβ4 expression in exosomes
derived from plasma of patients with lung metastasis. Patients
whose plasma exosome contained a higher amount of ITGβ4 developed
lung metastasis (39). The
detection of binding partner ITGαV and liver metastasis-associated
ITGβ5 in exosomes derived from plasma of patients with liver
metastasis revealed similar results (39). MIF was critical for PDAC-derived
exosomes creating fibrotic PMN in the liver (76). The knockdown of MIF blocked a
series of events primed by PDAC exosomes involved in liver PMN
formation and reduced liver metastasis (76). The plasma exosomal MIF level was
increased in patients with PDAC with disease progression compared
with those with no evidence of disease, indicating that exosomal
MIF could be used as a prognostic marker in patients with PDAC
(76). CEMIP is specifically
enriched in exosomes of cancer cells with brain-metastatic ability
and remodels brain vascular metastasis (68). Knockdown of CEMIP markedly reduces
brain metastasis (68). Its
content in exosomes derived from primary tumor cells could be used
to predict the risk of brain metastasis and survival for patients
with cancer (68). Prostate
cancer cell exosomes deliver PKM2 into BMSCs and induce the
secretion of CXCL12 for bone-specific metastasis (74). Targeted inhibition of BMSC
education eliminates exosome-mediated bone metastasis (74). Higher levels of PKM2 in serum
exosomes are associated with metastases (74). In addition to being a potential
circulating biomarker for cancer detection, exosomal S100A8/A9
mediates MDSCs contributing to the immune-suppressive niche for
metastasis and has been explored as an imaging marker for PMN
formation (87). These findings
suggested that proteins in exosomes involved in regulating PMN
formation could be used as therapeutic targets and predictors of
target organ metastasis.
The majority of studies have focused on exosomal
protein cargo-mediated PMN formation. Lin et al (92) investigated the upstream regulators
of pro-metastatic exosome generation and determined that aspartate
β-hydroxylase (ASPH) specifically guides exosomal protein content
(MMPs and ADAMs) assembly. Using an ASPH inhibitor could
efficiently block its pro-metastatic effect in vitro and
in vivo (92).
Lobos-Gonzalez et al (93)
revealed that knockdown of antisense non-coding mitochondrial RNA
altered the protein cargo in breast cancer cell exosomes, which
abrogated the regulatory role of exosomes in PMN formation and
suppressed cancer metastasis. Chairoungdua et al (94) demonstrated that CD82 and CD9, as
suppressors of tumor metastasis, inhibit tumor intracellular
Wnt/β-catenin signaling by increasing the export of β-catenin into
exosomes. Based on the universality of the intercellular transfer
of exosomal proteins, the therapeutic effect of the upregulation of
CD82 and CD9 may be limited.
Gene knockout can effectively inhibit the
pro-metastatic effect of proteins in exosomes (90,95). Chen et al (90) explored the therapeutic effect of
traditional Chinese medicine in targeting a pro-metastatic protein
in exosomes. It was revealed that Dahuang Zhechong Pill suppressed
CCL2 expression in colon cancer cell exosomes and subsequently
reduced macrophage infiltration and transition to the M1 phenotype
and ECM remodeling in distant organs (liver), thus impairing PMN
formation for liver metastasis (90). The aforementioned studies
(39,68,74,76,87,90,92-94) suggested that the factors mediating
metastasis-promoting protein cargo assembly and the cargo proteins
can be potential therapeutic targets to inhibit metastasis.
Representative exosomal proteins that can serve as potential
biomarkers for metastasis detection, prediction and therapy are
listed in Table II.
Tumor metastasis, particularly distant metastasis,
has been widely acknowledged as the main cause of cancer-associated
mortality and the bottleneck of tumor cure. In the last decades,
scientists have paid attention to this field and have performed
numerous studies, aiming to elucidate the molecular mechanism of
tumor metastasis. Based on their findings, several classical
metastasis-related hypotheses have been developed, emphasizing the
interaction between 'seeds' and 'soil' and establishing the concept
of the PMN (9). Tumor cell
exosome-loaded integrins have been revealed to serve a decisive
role in directing tumor cells to form secondary metastasis by
reshaping permissive PMN in target organs (39). These findings suggest the
following: i) Distant metastasis is not random but directional; ii)
PMN formation in secondary organs is crucial for organ-specific
metastasis of tumor cells; and iii) tumor cell-derived exosomes are
important mediators that trigger and establish the PMN in target
metastatic organs. Subsequently, studies analyzed the role and
mechanism of action of tumor cell exosomes in PMN formation in
different tumors with organ-specific metastases. They further
demonstrated that exosomes serve a critical role in dictating PMN
formation by affecting a number of aspects, and different exosomal
cargos are involved in this process.
Due to the complexity of exosomal cargo, different
studies have focused on different cargos. At present, non-coding
RNAs and proteins are the two most extensively studied components
encapsulated by exosomes mediating PMN formation; however, the
former have received more attention. Non-coding RNAs are of
numerous types, among which microRNAs and long non-coding RNAs in
exosomes have been under intensive investigation and have been
demonstrated to serve vital roles in PMN formation (96,97). Non-coding RNAs perform their
biological functions through complex and diverse regulatory
mechanisms, and their downstream proteins are the real effector
molecules. By contrast, the function of proteins is much clearer
than that of non-coding RNAs. Furthermore, high-throughput
proteomics and protein function annotation have demonstrated that
metastatic tumor cell exosomes contain metastasis-promoting PMN
formation-related proteins, thus providing a basis for studying the
key role of exosomal proteins in PMN formation. Therefore,
understanding exosomal protein-mediated PMN formation in directed
metastasis is an important direction in the field of tumor
metastasis research. The synergistic effects of exosomal proteins
and other molecules within exosomes should be evaluated, since this
can facilitate an improved understanding of the underlying
mechanisms of tumor metastasis. It could provide information on
potential effective molecular targets for the prediction and
treatment of secondary organ-specific metastasis. Furthermore, it
is worth noting that exosomal proteins from stromal cells have been
demonstrated to be involved in PMN formation. Investigations should
not be restricted to tumor cell-derived exosomes. In other words,
the role of exosomal proteins from other cells in PMN formation
should not be ignored in the future.
In summary, metastasis-associated proteins have been
demonstrated to be enriched in exosomes. However, few studies have
been conducted to explore the role of exosomal proteins in the PMN.
The findings demonstrated that exosomal proteins promote PMN
formation and mediate site-specific metastasis of tumor cells by
inducing lymphangiogenesis, angiogenesis and permeability,
educating stromal cells, remodeling the ECM, and suppressing the
antitumor immune response. Exosomal proteins mediating PMN
formation have great potential in predicting organ-directed
metastasis and prognosis, as well as in cancer therapy.
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81772641 and
81902510).
Not applicable.
MW and XZ collected the related papers, drafted the
manuscript, initiated the study, revised and finalized the
manuscript. FH, LW, JH, ZG and WY participated in the design of the
review. MW and XZ were responsible for confirming the authenticity
of the raw data. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81772641 and 81902510).
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stevens AR and Ewing J: Adenocarcinoma of
the Testis in the Adult. Ann Surg. 88:1074–1078. 1928. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fidler IJ: Selection of successive tumour
lines for metastasis. Nat New Biol. 242:148–149. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bross ID and Blumenson LE: Screening
random asymptomatic women under 50 by annual mammographies: Does it
make sense? J Surg Oncol. 8:437–445. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
6
|
Gupta GP and Massague J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Henrich SE, McMahon KM, Plebanek MP,
Calvert AE, Feliciano TJ, Parrish S, Tavora F, Mega A, De Souza A,
Carneiro BA and Thaxton CS: Prostate cancer extracellular vesicles
mediate intercellular communication with bone marrow cells and
promote metastasis in a cholesterol-dependent manner. J Extracell
Vesicles. 10:e120422020. View Article : Google Scholar
|
|
8
|
Mazumdar A, Urdinez J, Boro A, Arlt MJE,
Egli FE, Niederöst B, Jaeger PK, Moschini G, Muff R, Fuchs B, et
al: Exploring the role of osteosarcoma-derived extracellular
vesicles in Pre-metastatic niche formation and metastasis in the
143-B Xenograft mouse osteosarcoma model. Cancers (Basel).
12:34572020. View Article : Google Scholar
|
|
9
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao J, Yang G, Zhou W, Qiu J, Chen G, Luo
W, Zhao F, You L, Zheng L, Zhang T and Zhao Y: Targeting hypoxic
tumor microenvironment in pancreatic cancer. J Hematol Oncol.
14:142021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y and Cao X: Characteristics and
Significance of the Pre-metastatic Niche. Cancer Cell. 30:668–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seubert B, Grunwald B, Kobuch J, Cui H,
Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N,
et al: Tissue inhibitor of metalloproteinases (TIMP)-1 creates a
premetastatic niche in the liver through SDF-1/CXCR4-dependent
neutrophil recruitment in mice. Hepatology. 61:238–248. 2015.
View Article : Google Scholar
|
|
13
|
Domaschenz R, Kurscheid S, Nekrasov M, Han
S and Tremethick DJ: The histone variant H2A.Z is a master
regulator of the epithelial-mesenchymal transition. Cell Rep.
21:943–952. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan W and Jiang S: Immune cell-derived
exosomes in the cancer-immunity cycle. Trends Cancer. 6:506–517.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lobb RJ, Lima LG and Moller A: Exosomes:
Key mediators of metastasis and pre-metastatic niche formation.
Semin Cell Dev Biol. 67:3–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trams EG, Lauter CJ, Salem N Jr and Heine
U: Exfoliation of membrane ecto-enzymes in the form of
micro-vesicles. Biochim Biophys Acta. 645:63–70. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Z, Fan J, Hsu YS, Lyon CJ, Ning B and
Hu TY: Extracellular vesicles as cancer liquid biopsies: From
discovery, validation, to clinical application. Lab Chip.
19:1114–1140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bebelman MP, Bun P, Huveneers S, van Niel
G, Pegtel DM and Verweij FJ: Real-time imaging of multivesicular
body-plasma membrane fusion to quantify exosome release from single
cells. Nat Protoc. 15:102–121. 2020. View Article : Google Scholar
|
|
20
|
Simpson RJ, Jensen SS and Lim JW:
Proteomic profiling of exosomes: Current perspectives. Proteomics.
8:4083–4099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Niel G, D'Angelo G and Raposo G:
Shedding light on the cell biology of extracellular vesicles. Nat
Rev Mol Cell Biol. 19:213–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lane RE, Korbie D, Hill MM and Trau M:
Extracellular vesicles as circulating cancer biomarkers:
Opportunities and challenges. Clin Transl Med. 7:142018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar :
|
|
24
|
Gao Y and Raj JU: Extracellular vesicles
as unique signaling messengers: Role in lung diseases. Compr
Physiol. 11:1351–1369. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mashouri L, Yousefi H, Aref AR, Ahadi AM,
Molaei F and Alahari SK: Exosomes: Composition, biogenesis, and
mechanisms in cancer metastasis and drug resistance. Mol Cancer.
18:752019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mathieu M, Martin-Jaular L, Lavieu G and
Thery C: Specificities of secretion and uptake of exosomes and
other extracellular vesicles for cell-to-cell communication. Nat
Cell Biol. 21:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keerthikumar S, Chisanga D, Ariyaratne D,
Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M,
Chilamkurti N, et al: ExoCarta: A Web-based compendium of exosomal
cargo. J Mol Biol. 428:688–692. 2016. View Article : Google Scholar :
|
|
29
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: A new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W, Li C, Zhou T, Liu X, Liu X, Li X and
Chen D: Role of exosomal proteins in cancer diagnosis. Mol Cancer.
16:1452017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bebelman MP, Smit MJ, Pegtel DM and Baglio
SR: Biogenesis and function of extracellular vesicles in cancer.
Pharmacol Ther. 188:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lindenbergh MFS and Stoorvogel W: Antigen
presentation by extracellular vesicles from professional
antigen-presenting cells. Annu Rev Immunol. 36:435–459. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi D, Rak J and Gho YS: Isolation of
extracellular vesicles for proteomic profiling. Methods Mol Biol.
2261:193–206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Emmanouilidi A, Paladin D, Greening DW and
Falasca M: Oncogenic and Non-malignant pancreatic exosome cargo
reveal distinct expression of oncogenic and prognostic factors
involved in tumor invasion and metastasis. Proteomics.
19:e18001582019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li R, Wang Y, Zhang X, Feng M, Ma J, Li J,
Yang X, Fang F, Xia Q, Zhang Z, et al: Exosome-mediated secretion
of LOXL4 promotes hepatocellular carcinoma cell invasion and
metastasis. Mol Cancer. 18:182019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alharbi M, Lai A, Guanzon D, Palma C,
Zuñiga F, Perrin L, He Y, Hooper JD and Salomon C: Ovarian
cancer-derived exosomes promote tumour metastasis in vivo: An
effect modulated by the invasiveness capacity of their originating
cells. Clin Sci (Lond). 133:1401–1419. 2019. View Article : Google Scholar
|
|
37
|
Gangoda L, Liem M, Ang CS, Keerthikumar S,
Adda CG, Parker BS and Mathivanan S: Proteomic profiling of
exosomes secreted by breast cancer cells with varying metastatic
potential. Proteomics. 17:2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh A, Fedele C, Lu H, Nevalainen MT,
Keen JH and Languino LR: Exosome-mediated Transfer of αvβ3 integrin
from tumorigenic to nontumorigenic cells promotes a migratory
phenotype. Mol Cancer Res. 14:1136–1146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weeraphan C, Phongdara A, Chaiyawat P,
Diskul-Na-Ayudthaya P, Chokchaichamnankit D, Verathamjamras C,
Netsirisawan P, Yingchutrakul Y, Roytrakul S, Champattanachai V, et
al: Phosphoproteome profiling of isogenic cancer cell-derived
exosome reveals HSP90 as a potential marker for human
cholangiocarcinoma. Proteomics. 19:e18001592019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shtam T, Naryzhny S, Samsonov R, Karasik
D, Mizgirev I, Kopylov A, Petrenko E, Zabrodskaya Y, Kamyshinsky R,
Nikitin D, et al: Plasma exosomes stimulate breast cancer
metastasis through surface interactions and activation of FAK
signaling. Breast Cancer Res Treat. 174:129–141. 2019. View Article : Google Scholar
|
|
42
|
Luo D, Zhan S, Xia W, Huang L, Ge W and
Wang T: Proteomics study of serum exosomes from papillary thyroid
cancer patients. Endocr Relat Cancer. 25:879–891. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Erozenci LA, Bottger F, Bijnsdorp IV and
Jimenez CR: Urinary exosomal proteins as (pan-)cancer biomarkers:
Insights from the proteome. FEBS Lett. 593:1580–1597. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun Y, Liu S, Qiao Z, Shang Z, Xia Z, Niu
X, Qian L, Zhang Y, Fan L, Cao CX and Xiao H: Systematic comparison
of exosomal proteomes from human saliva and serum for the detection
of lung cancer. Anal Chim Acta. 982:84–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McCready J, Sims JD, Chan D and Jay DG:
Secretion of extracellular hsp90alpha via exosomes increases cancer
cell motility: A role for plasminogen activation. BMC Cancer.
10:2942010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mazurkiewicz J, Simiczyjew A, Dratkiewicz
E, Zietek M, Matkowski R and Nowak D: Stromal cells present in the
melanoma niche affect tumor invasiveness and its resistance to
therapy. Int J Mol Sci. 22:5292021. View Article : Google Scholar :
|
|
47
|
Zhou L, Li J, Tang Y and Yang M: Exosomal
LncRNA LINC00659 transferred from cancer-associated fibroblasts
promotes colorectal cancer cell progression via miR-342-3p/ANXA2
axis. J Transl Med. 19:82021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shimoda M, Principe S, Jackson HW, Luga V,
Fang H, Molyneux SD, Shao YW, Aiken A, Waterhouse PD, Karamboulas
C, et al: Loss of the Timp gene family is sufficient for the
acquisition of the CAF-like cell state. Nat Cell Biol. 16:889–901.
2014. View Article : Google Scholar
|
|
49
|
Chen Y, Zeng C, Zhan Y, Wang H, Jiang X
and Li W: Aberrant low expression of p85α in stromal fibroblasts
promotes breast cancer cell metastasis through exosome-mediated
paracrine Wnt10b. Oncogene. 36:4692–4705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang S, Zhu Y, Cai H, Zhang Y and Hou J:
Impact of lymphovascular invasion in oral squamous cell carcinoma:
A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. Nov
5–2020.Epub ahead of print. PubMed/NCBI
|
|
51
|
Ma Q, Dieterich LC, Ikenberg K, Bachmann
SB, Mangana J, Proulx ST, Amann VC, Levesque MP, Dummer R, Baluk P,
et al: Unexpected contribution of lymphatic vessels to promotion of
distant metastatic tumor spread. Sci Adv. 4:eaat47582018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sleeman JP: The lymph node pre-metastatic
niche. J Mol Med (Berl). 93:1173–1184. 2015. View Article : Google Scholar
|
|
53
|
Qian CN, Berghuis B, Tsarfaty G, Bruch M,
Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, et
al: Preparing the 'soil': The primary tumor induces vasculature
reorganization in the sentinel lymph node before the arrival of
metastatic cancer cells. Cancer Res. 66:10365–10376. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ogawa F, Amano H, Eshima K, Ito Y, Matsui
Y, Hosono K, Kitasato H, Iyoda A, Iwabuchi K, Kumagai Y, et al:
Prostanoid induces premetastatic niche in regional lymph nodes. J
Clin Invest. 124:4882–4894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brown M, Assen FP, Leithner A, Abe J,
Schachner H, Asfour G, Bago-Horvath Z, Stein JV, Uhrin P, Sixt M
and Kerjaschki D: Lymph node blood vessels provide exit routes for
metastatic tumor cell dissemination in mice. Science.
359:1408–1411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sun B, Zhou Y, Fang Y, Li Z, Gu X and
Xiang J: Colorectal cancer exosomes induce lymphatic network
remodeling in lymph nodes. Int J Cancer. 145:1648–1659. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y,
Wang L, Li L, Wang B, Shen J, et al: Horizontal transfer of
exosomal CXCR4 promotes murine hepatocarcinoma cell migration,
invasion and lymphangiogenesis. Gene. 676:101–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rondon-Galeano M, Skoczylas R, Bower NI,
Simons C, Gordon E, Francois M, Koltowska K and Hogan BM: MAFB
modulates the maturation of lymphatic vascular networks in mice.
Dev Dyn. 249:1201–1216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang CA, Chang IH, Hou PC, Tai YJ, Li WN,
Hsu PL, Wu SR, Chiu WT, Li CF, Shan YS and Tsai SJ: DUSP2 regulates
extracellular vesicle-VEGF-C secretion and pancreatic cancer early
dissemination. J Extracell Vesicles. 9:17465292020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu D, Li C, Trojanowicz B, Li X, Shi D,
Zhan C, Wang Z and Chen L: CD97 promotion of gastric carcinoma
lymphatic metastasis is exosome dependent. Gastric Cancer.
19:754–766. 2016. View Article : Google Scholar :
|
|
61
|
Lin T, Zhang X, Lu Y and Gong L: TGFBIp
mediates lymphatic sprouting in corneal lymphangiogenesis. J Cell
Mol Med. 23:7602–7616. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Carraway RE and Cochrane DE: Enhanced
vascular permeability is hypothesized to promote
inflammation-induced carcinogenesis and tumor development via
extravasation of large molecular proteins into the tissue. Med
Hypotheses. 78:738–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Maji S, Chaudhary P, Akopova I, Nguyen PM,
Hare RJ, Gryczynski I and Vishwanatha JK: Exosomal Annexin II
promotes angiogenesis and breast cancer metastasis. Mol Cancer Res.
15:93–105. 2017. View Article : Google Scholar
|
|
64
|
Cen J, Feng L, Ke H, Bao L, Li LZ, Tanaka
Y, Weng J and Su L: Exosomal Thrombospondin-1 disrupts the
integrity of endothelial intercellular junctions to facilitate
breast cancer cell metastasis. Cancers (Basel). 11:19462019.
View Article : Google Scholar
|
|
65
|
Yang WW, Yang LQ, Zhao F, Chen CW, Xu LH,
Fu J, Li SL and Ge XY: Epiregulin promotes lung metastasis of
salivary adenoid cystic carcinoma. Theranostics. 7:3700–3714. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dai W, Wang Y, Yang T, Wang J, Wu W and Gu
J: Downregulation of exosomal CLEC3B in hepatocellular carcinoma
promotes metastasis and angiogenesis via AMPK and VEGF signals.
Cell Commun Signal. 17:1132019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tang MKS, Yue PYK, Ip PP, Huang RL, Lai
HC, Cheung ANY, Tse KY, Ngan HYS and Wong AST: Soluble E-cadherin
promotes tumor angiogenesis and localizes to exosome surface. Nat
Commun. 9:22702018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rodrigues G, Hoshino A, Kenific CM, Matei
IR, Steiner L, Freitas D, Kim HS, Oxley PR, Scandariato I,
Casanova-Salas I, et al: Tumour exosomal CEMIP protein promotes
cancer cell colonization in brain metastasis. Nat Cell Biol.
21:1403–1412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Park JE, Tan HS, Datta A, Lai RC, Zhang H,
Meng W, Lim SK and Sze SK: Hypoxic tumor cell modulates its
microenvironment to enhance angiogenic and metastatic potential by
secretion of proteins and exosomes. Mol Cell Proteomics.
9:1085–1099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Horie K, Kawakami K, Fujita Y, Sugaya M,
Kameyama K, Mizutani K, Deguchi T and Ito M: Exosomes expressing
carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res
Commun. 492:356–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ekstrom EJ, Bergenfelz C, von Bulow V,
Serifler F, Carlemalm E, Jönsson G, Andersson T and Leandersson K:
WNT5A induces release of exosomes containing pro-angiogenic and
immunosuppressive factors from malignant melanoma cells. Mol
Cancer. 13:882014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C,
Wang W, Wang G, Wang H, Yuan W, et al: Effects of exosomes on
pre-metastatic niche formation in tumors. Mol Cancer. 18:392019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hoye AM and Erler JT: Structural ECM
components in the premetastatic and metastatic niche. Am J Physiol
Cell Physiol. 310:C955–C967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dai J, Escara-Wilke J, Keller JM, Jung Y,
Taichman RS, Pienta KJ and Keller ET: Primary prostate cancer
educates bone stroma through exosomal pyruvate kinase M2 to promote
bone metastasis. J Exp Med. 216:2883–2899. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ning X, Zhang H, Wang C and Song X:
Exosomes released by gastric cancer cells induce transition of
pericytes into cancer-associated fibroblasts. Med Sci Monit.
24:2350–2359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ren Y, Zhou X, Liu X, Jia HH, Zhao XH,
Wang QX, Han L, Song X, Zhu ZY, Sun T, et al: Reprogramming
carcinoma associated fibroblasts by AC1MMYR2 impedes tumor
metastasis and improves chemotherapy efficacy. Cancer Lett.
374:96–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Deng Z, Cheng Z, Xiang X, Yan J, Zhuang X,
Liu C, Jiang H, Ju S, Zhang L, Grizzle W, et al: Tumor cell cross
talk with tumor-associated leukocytes leads to induction of tumor
exosomal fibronectin and promotes tumor progression. Am J Pathol.
180:390–398. 2012. View Article : Google Scholar
|
|
81
|
Deep G, Jain A, Kumar A, Agarwal C, Kim S,
Leevy WM and Agarwal R: Exosomes secreted by prostate cancer cells
under hypoxia promote matrix metalloproteinases activity at
pre-metastatic niches. Mol Carcinog. 59:323–332. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Herrera M, Galindo-Pumarino C,
Garcia-Barberan V and Pena C: A snapshot of the tumor
microenvironment in colorectal cancer: The liquid biopsy. Int J Mol
Sci. 20:60162019. View Article : Google Scholar :
|
|
83
|
Riteau B, Faure F, Menier C, Viel S,
Carosella ED, Amigorena S and Rouas-Freiss N: Exosomes bearing
HLA-G are released by melanoma cells. Hum Immunol. 64:1064–1072.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Whiteside TL: Exosomes and tumor-mediated
immune suppression. J Clin Invest. 126:1216–1223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang Y, Ding Y, Guo N and Wang S: MDSCs:
Key criminals of tumor Pre-metastatic Niche Formation. Front
Immunol. 10:1722019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Burke M, Choksawangkarn W, Edwards N,
Ostrand-Rosenberg S and Fenselau C: Exosomes from myeloid-derived
suppressor cells carry biologically active proteins. J Proteome
Res. 13:836–843. 2014. View Article : Google Scholar
|
|
87
|
Eisenblaetter M, Flores-Borja F, Lee JJ,
Wefers C, Smith H, Hueting R, Cooper MS, Blower PJ, Patel D,
Rodriguez-Justo M, et al: Visualization of tumor-immune
interaction-target-specific imaging of S100A8/A9 reveals
pre-metastatic niche establishment. Theranostics. 7:2392–2401.
2017. View Article : Google Scholar :
|
|
88
|
Wolf-Dennen K, Gordon N and Kleinerman ES:
Exosomal communication by metastatic osteosarcoma cells modulates
alveolar macrophages to an M2 tumor-promoting phenotype and
inhibits tumoricidal functions. Oncoimmunology. 9:17476772020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chen C, Yao X, Xu Y, Zhang Q, Wang H, Zhao
L, Wen G, Liu Y, Jing L and Sun X: Dahuang Zhechong Pill suppresses
colorectal cancer liver metastasis via ameliorating exosomal CCL2
primed pre-metastatic niche. J Ethnopharmacol. 238:1118782019.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Plebanek MP, Angeloni NL, Vinokour E, Li
J, Henkin A, Martinez-Marin D, Filleur S, Bhowmick R, Henkin J,
Miller SD, et al: Pre-metastatic cancer exosomes induce immune
surveillance by patrolling monocytes at the metastatic niche. Nat
Commun. 8:13192017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lin Q, Chen X, Meng F, Ogawa K, Li M, Song
R, Zhang S, Zhang Z, Kong X, Xu Q, et al: ASPH-notch Axis guided
Exosomal delivery of Prometastatic Secretome renders breast Cancer
multi-organ metastasis. Mol Cancer. 18:1562019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lobos-Gonzalez L, Bustos R, Campos A,
Silva V, Silva V, Jeldes E, Salomon C, Varas-Godoy M,
Cáceres-Verschae A, Duran E, et al: Exosomes released upon
mitochondrial ASncmtRNA knockdown reduce tumorigenic properties of
malignant breast cancer cells. Sci Rep. 10:3432020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chairoungdua A, Smith DL, Pochard P, Hull
M and Caplan MJ: Exosome release of β-catenin: A novel mechanism
that antagonizes Wnt signaling. J Cell Biol. 190:1079–1091. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhao L, Gu C, Gan Y, Shao L, Chen H and
Zhu H: Exosome-mediated siRNA delivery to suppress postoperative
breast cancer metastasis. J Control Release. 318:1–15. 2020.
View Article : Google Scholar
|
|
96
|
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J,
Zhou K, Liu X, Ren X, Wang F, et al: Cancer-derived exosomal
miR-25-3p promotes pre-metastatic niche formation by inducing
vascular permeability and angiogenesis. Nat Commun. 9:53952018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z,
Zhang X, Huang B, Xu X, Zheng J and Cao X: Tumor exosomal RNAs
promote lung Pre-metastatic niche formation by activating alveolar
epithelial TLR3 to recruit neutrophils. Cancer Cell. 30:243–256.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nakamura K, Sawada K, Kinose Y, Yoshimura
A, Toda A, Nakatsuka E, Hashimoto K, Mabuchi S, Morishige KI,
Kurachi H, et al: Exosomes promote ovarian cancer cell invasion
through transfer of CD44 to peritoneal mesothelial cells. Mol
Cancer Res. 15:78–92. 2017. View Article : Google Scholar
|
|
99
|
Zhang W, Ou X and Wu X: Proteomics
profiling of plasma exosomes in epithelial ovarian cancer: A
potential role in the coagulation cascade, diagnosis and prognosis.
Int J Oncol. 54:1719–1733. 2019.PubMed/NCBI
|
|
100
|
Ji H, Greening DW, Barnes TW, Lim JW,
Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W, et al:
Proteome profiling of exosomes derived from human primary and
metastatic colorectal cancer cells reveal differential expression
of key metastatic factors and signal transduction components.
Proteomics. 13:1672–1686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liang B, Peng P, Chen S, Li L, Zhang M,
Cao D, Yang J, Li H, Gui T, Li X and Shen K: Characterization and
proteomic analysis of ovarian cancer-derived exosomes. J
Proteomics. 80:171–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
DeRita RM, Zerlanko B, Singh A, Lu H,
Iozzo RV, Benovic JL and Languino LR: c-Src, Insulin-like growth
Factor I receptor, G-protein-coupled receptor kinases and focal
adhesion kinase are enriched into prostate cancer cell exosomes. J
Cell Biochem. 118:66–73. 2017. View Article : Google Scholar :
|
|
103
|
Li Y, Zhang Y, Qiu F and Qiu Z: Proteomic
identification of exosomal LRG1: A potential urinary biomarker for
detecting NSCLC. Electrophoresis. 32:1976–1983. 2011. View Article : Google Scholar
|
|
104
|
Lu J, Li J, Liu S, Wang T, Ianni A, Bober
E, Braun T, Xiang R and Yue S: Exosomal tetraspanins mediate cancer
metastasis by altering host microenvironment. Oncotarget.
8:62803–62815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chen Y, Xie Y, Xu L, Zhan S, Xiao Y, Gao
Y, Wu B and Ge W: Protein content and functional characteristics of
serum-purified exosomes from patients with colorectal cancer
revealed by quantitative proteomics. Int J Cancer. 140:900–913.
2017. View Article : Google Scholar
|
|
106
|
Greening DW, Ji H, Chen M, Robinson BW,
Dick IM, Creaney J and Simpson RJ: Secreted primary human malignant
mesothelioma exosome signature reflects oncogenic cargo. Sci Rep.
6:326432016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang Z, Sun H, Provaznik J, Hackert T and
Zoller M: Pancreatic cancer-initiating cell exosome message
transfer into noncancer-initiating cells: The importance of CD44v6
in reprogramming. J Exp Clin Cancer Res. 38:1322019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jung T, Castellana D, Klingbeil P, Cuesta
Hernández I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P and
Zöller M: CD44v6 dependence of premetastatic niche preparation by
exosomes. Neoplasia. 11:1093–1105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
You Y, Shan Y, Chen J, Yue H, You B, Shi
S, Li X and Cao X: Matrix metalloproteinase 13-containing exosomes
promote nasopharyngeal carcinoma metastasis. Cancer Sci.
106:1669–1677. 2015. View Article : Google Scholar
|