Introduction
Ovarian cancer is the third most common
gynecological malignancy (1).
Improvements in chemotherapeutics have only resulted in modest
increases in the survival rates. A large majority of patients is
diagnosed at an advanced stage of the disease (stage III or IV),
often with bowel obstruction and systemic involvement, which
explains the high lethality of this type of tumor among women
(2). At this stage, patients
present with widespread metastatic growth within the peritoneal
cavity, where the ascitic fluid enriched of growth factors and
inflammatory cytokines further contributes to the growth and
dissemination of cancer cells.
Cancer-associated fibroblasts (CAFs) in the tumor
microenvironment have been well-recognized for their potential to
induce cancer cell progression in ovarian cancer (3,4).
Ovarian CAFs secrete a variety of soluble factors that have been
shown to affect the metabolism and the phenotypes of ovarian cancer
cells, and in this respect interleukin (IL)-8 is one of the
cytokines playing a major role in this process (3).
The serum levels of IL-8 (and IL-10) closely
parallel the stage and prognosis of ovarian cancer (5). Additionally, the concentration of
IL-8 (and of IL-6) in the peritoneal fluid increases along with
ovarian cancer progression and represents a factor of poor
prognosis (6). At the biological
level, IL-8 has been shown to induce ovarian cancer cell migration,
invasion and epithelial-mesenchymal transition (EMT) (7-9),
and to promote the growth of ovarian cancer 3-dimensional spheroids
(10). Understanding the
mechanisms underlying ovarian cancer cell migration is challenging,
yet it is instrumental for designing effective therapeutic
strategies that could prevent the metastatic spreading of the tumor
and improve the prognosis of patients.
Autophagy, a lysosomal catabolic process with
homeostatic and pro-survival functions, influences the behavior of
ovarian cancer cells, affecting a variety of processes, such as
survival in metabolic harsh conditions, invasive growth, the
development of immune- and chemo-resistance, the maintenance of
stem-like properties and dormancy (3,11).
Autophagy is dysregulated in ovarian cancer (12,13), and the autophagy-related proteins
BECLIN-1 and LC3 are in fact prognostic factors for ovarian cancer
(14-17). The migration rate of ovarian
cancer cells increases upon autophagy gene knockdown (13). Several cytokines/chemokines from
ovarian CAFs have been proven to modulate autophagy (3). The authors have previously
demonstrated that IL-6 downregulates autophagy in ovarian cancer
cells, while promoting their motility (18). IL-8 has been shown to interrupt
the dormant state of ovarian cancer cells by attenuating
DIRAS3-mediated autophagy (19).
IL-8 has been shown to induce EMT in ovarian cancer cells and to
enhance ovarian cancer cell metastasis (9). Consistently, in vitro studies
have indicated that the overexpression and secretion of IL-8 in
ovarian cancer cells favor their anchorage-independent growth,
proliferation and invasion (20).
However, to date there are no data available showing a direct
effect of IL-8 secreted by ovarian cancer CAFs on the modulation of
autophagy and how this modulation affects ovarian cancer cell
migration. The present study aimed to provide knowledge on this
matter. To this end, primary cultured ovarian CAFs (OVCAFs) were
isolated from fresh surgical ovarian cancer tissues and their
secreted substances in the conditioned-media (OVCAF-CM) were
characterized. To the best of our knowledge, the present study
demonstrates for the first time that IL-8 is a major cytokine
driving ovarian cancer cell migration and that this effect is
mechanistically linked to the downregulation of autophagy in cancer
cells. The present findings indicate IL-8 as a therapeutic target
(e.g., with recombinant specific antibody) to hinder its activity
and restore autophagy in cancer cells, and by so doing prevent the
metastatic spreading of ovarian cancer.
Materials and methods
Human ovarian cancer cell lines and cell
culture
The human ovarian cancer cell lines, SKOV3 (ATCC,
Cell Systems & cGMP Biorepository) and Kuramochi (Japanese
Collection of Research Bioresources), were employed in the present
study. The SKOV3 cells and Kuramochi cells were grown in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) and RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.),
respectively. Culture media were supplemented with 10% (v/v)
fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). Cells were maintained in an incubator at 37°C with 5%
CO2. Cells at a passage of <10 and exhibiting >90%
viability (measured by trypan blue staining, data not shown) were
used in the experiments.
Primary culture of OVCAFs
Ovarian cancer tissues were obtained from patients
undergoing oophorectomy at Siriraj Hospital, Mahidol University.
Ethical approval was granted by the Siriraj Institution Board (COAs
no. si670/2013 and si246/2017). The patients released the informed
consent for the usage of their samples for research purposes in
written form. The samples were collected at Siriraj Hospital
(Bangkok) during the period of 2013-2019. Tissue sections of
approximately 10 mm3 were submerged in 2X
antibiotic-containing DMEM with 10% FBS overnight at 4°C to reduce
microbial contamination. The sample was rinsed with 1X
phosphate-buffered saline (PBS) and cut into small sections of 2
mm3. Cell debris was washed out with 10% FBS-containing
DMEM with 1X penicillin (100 U/ml)-streptomycin (100 µg/ml)
(Gibco; Thermo Fisher Scientific Inc.), amphotericin B (5 mg/ml)
(Amphotret™, Bharat Serums and Vaccines Limited Ltd.) and HEPES (20
mM) (Gibco; Thermo Fisher Scientific Inc.) (complete medium). The
tissue sections were then transferred and allowed to adhere to a
Petri dish at 37°C in a CO2 incubator. After the
fibroblast-like cells protruded from the underneath the tissues,
adherent cells were trypsinized with 0.25% trypsin/EDTA (Gibco;
Thermo Fisher Scientific Inc.) and re-plated. The complete medium
was replaced every 2-3 days. Upon reaching confluency, the cells
were trypsinized and re-plated. Cultures of fibroblasts at passages
4 to 17 were used in the experiments after confirming the CAF
biochemical and morphological features. These cells were named
OVCAFs. The normal-associated fibroblasts (OVNFs) were isolated
with the same method from adjacent non-tumoral ovarian tissues of
the same patient.
Characterization of primary culture of
OVCAFs
Immunocytochemistry of cytokeratin 19 (CK19),
α-smooth muscle actin (α-SMA), vimentin (VIM) and fibroblast
activation protein (FAP) was performed to verify the purity of
OVCAFs and OVNFs. Briefly, cells on sterile glass coverslips were
fixed in ice-cold methanol and then incubated overnight at 4°C in a
humid chamber with the indicated primary antibodies as follow:
1:200 mouse anti-human CK-19 antibody (sc-6278, Santa Cruz
Biotechnology Inc.), 1:500 anti-α-SMA antibody (A5228, Abcam),
1:500 anti-VIM antibody (sc-6260, Santa Cruz Biotechnology Inc.),
and 1:500 rabbit anti-human FAP (ab53066, Abcam). After washing out
the excess primary antibody, the coverslip was incubated for 3 h at
room temperature with the appropriate secondary fluorescent
antibody including goat anti-mouse IgG-Cy3 antibody (1:2,000,
#115-166-071, Jackson ImmunoResearch Laboratories Inc.) or the goat
anti-rabbit IgG-FITC antibody (1:2,000, ab6717, Abcam). Hoechst
33342 solution (Invitrogen; Thermo Fisher Scientific, Inc.) was
added to stain the nucleus. Fluorescence was captured using an
Inverted microscope model IX71 (Olympus Corporation).
Conditioned-media (CM) collection from
fibroblasts
OVCAFs and OVNFs were cultured in 75-cm2
flasks to reach 90-95% confluency in DMEM containing 10% FBS. Cells
were then washed twice with 1X PBS and twice with 1% (v/v) FBS-DMEM
and incubated for 24 h at room temperature. Subsequently, the CM
were collected and designated as 24 h CM. CMs were centrifuged at
2,000 × g at 4°C for 5 min to remove cell debris and the suspension
stored at −80°C until use. The Bradford assay kit (Bio-Rad
Laboratories, Inc.) was used to measure the amount of total protein
and adjusted to be equal when supplemented to the cells.
Wound healing migration assay
The SKOV3 and Kuramochi cells were cultured in a
6-well plate until they reached approximately 90% confluency. The
reference lines were drawn at the middle under the plate. Cells
were scratched using a sterile 1,000 µl pipette tip followed
by washing 3 times with serum-free medium to discard unattached
cells. The cells were treated with either OVCAF-CM or OVNF-CM or
with 0, 25, 250, 2,500 and 25,000 pg/ml recombinant human IL-8
(rhIL-8)/CXCL8 (11349086, ImmunoTools GmbH). Other treatments
included anti-IL-8/CXCL8 neutralizing antibody (MAB208, R&D
Systems, Inc.), 200 nM rapamycin (R8781, Sigma-Aldrich; Merck KGaA)
and 2 mM metformin hydrochloride (PHR1084, Sigma-Aldrich; Merck
KGaA), as indicated. Incubations were performed in medium
supplemented with 1 or 10% FBS, as indicated. The scratched areas
indicated by the reference line were recorded at the beginning and
24 h after treatment. The migration efficiency was determined as
the percentage of healing of the scratched area as calculated by
the following formula: % wound healing=wound space at 0 h-at the
end of the experiment ×100/wound space at 0 h.
Two dimensional (2D) transwell
Fluoroblok™ migration assay
The ovarian cancer cell lines were cultured in
25-cm2 flasks until they reached 80% confluency.
Following trypsinization, the cells were fluorescently labeled by
incubation with 5 µmol/l Green CMFDA Dye (Invitrogen; Thermo
Fisher Scientific, Inc.) for 30 min following the manufacturer's
protocol. The cells, 1×105 cells/well for SKOV3 and
2×105 cells/well for Kuramochi, were seeded onto the
coated 8 µm pore Fluoroblok™ membrane insert in the upper
chambers of 24-well companion plates (BD Biosciences). OVCAF-CM or
OVNF-CM was added to the cells in the upper chamber and into the
lower chamber. Fluoroblok™ plates were incubated in a humidified
atmosphere at 37°C in 5% CO2 in the Synergy H1
instrument (Biotek Instruments, Inc.). The measurement of the
relative fluorescence unit (RFU) at an excitation of 490
nm/emission 520 nm was measured for the migrated cells. A total of
3 independent experiments were performed.
Western blot analysis of LC3 and p62 in
ovarian cancer cells
The ovarian cancer cells were seeded in a 6-well
plate and cultured for 24 h in complete medium with 10% FBS as a
negative control for autophagy, or 1% FBS containing medium as a
positive control for autophagy, or in OVCAF-CM or OVNF-CM, as
indicated. Chloroquine (CLQ; 30 µM) (Sigma-Aldrich; Merck
KGaA) was added to CM 8 h prior to cell collection. The cells were
harvested and washed with 1X PBS, and lysed with in-house made
radioimmuno-precipitation assay (RIPA) buffer containing 150 mM
NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate,
0.1% SDS, 50 mM Tris, pH 8.0 and then heated for 5 min at 95°C to
denature proteins. Protein samples were separated by 15%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred
onto polyvinylidene fluoride (PVDF) membranes by TE 70 Semi-Dry
transfer units (GE Healthcare) for 1.5 h. The membranes were
blocked in a blocking solution containing 5% (w/v) skim milk in
0.1% (v/v) Tween 20 in TBS-T for 1 h at room temperature. The blots
were probed by overnight incubation at 4°C with rabbit anti-human
LC3B antibody (1:1,000, L7543, Sigma-Aldrich; Merck KGaA) or rabbit
anti-human p62 antibody (1:500, ab109012, Abcam). The blots were
then washed in TBS-T before being incubated with 1:2,000 goat
anti-rabbit IgG-HRP (ab6721, Abcam) for 1 h. For β-actin detection,
mouse anti-human β-actin monoclonal antibody (1:5,000) (sc-47778,
Santa Cruz Biotechnology Inc.) and 1:2,000 HRP-conjugated horse
anti-mouse IgG antibody (7076, Cell Signaling Technology Inc.) in
blocking solution were used as primary and secondary antibody,
respectively. The protein bands were developed by adding Clarity™
Western ECL substrate (170-5061, Bio-Rad Laboratories, Inc.) and
detected under Gel Document (Syngene®). The band
intensities were measured using ImageJ software version 1.52a. The
level of β-actin was used as an internal control to ensure equal
amounts of loading proteins.
Immunofluorescence detection of LC3 and
of E-cadherin in cells at the migration front
The ovarian cancer cells were allowed adhere on
sterile glass coverslips and cultured until they reached >90%
confluency. The cell monolayers were scratched using a 200
µl tip and then washed several times with 1X PBS to remove
the cell debris. OVCAF-CM and OVNF-CM were added to the cells for
24 h. Finally, the cells were fixed in ice-cold methanol and then
incubated overnight at 4°C in a humid chamber with anti-human LC3B
antibody (1:100) (L7543, Sigma-Aldrich; Merck KGaA) and anti-human
E-cadherin (2Q663, sc-71008, Santa Cruz Biotechnology, Inc.) After
washing out the excess of primary antibody, the coverslip was
incubated for 3 h at room temperature with the appropriate
fluorescence conjugated secondary antibody goat anti-rabbit
IgG-FITC antibody (1:2,000, ab6717, Abcam) and goat anti-mouse
IgG-CyTM3 antibody (115-166-071, Jackson ImmunoResearch
Laboratories, Inc.). Antibodies were diluted in 0.1% Triton X in 1X
PBS containing 10% FBS. Hoechst 33342 solution (Invitrogen; Thermo
Fisher Scientific, Inc.) was then added to stain the nuclei (30 min
at room temperature). Fluorescence signals were captured with a
Leica DMIRE2 confocal fluorescence microscope (Leica Microsystems
AG) equipped with Leica Confocal Software v. 2.61.
Protein array analysis of OVCAF-derived
secreted substances
The cytokines/chemokines secreted by the CAFs into
the CM were assayed using a ProHuman Cytokine Standard 27-Plex
assay, Group I (#M50-0KCAF0Y, Bio-Rad Laboratories, Inc.), which
monitors 27 human cytokines, including interleukin (IL)-1β (IL-1β),
IL-1 receptor α (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9,
IL-10, IL-12p70, IL-13, IL-15, IL-17A, eotaxin, basic fibroblast
growth factor (bFGF), granulocyte-colony stimulating factor
(G-CSF), granulocyte-monocyte-colony stimulating factor (GM-CSF),
interferon-γ (IFN-γ), interferon-γ-induced protein 10 (IP-10),
monocyte chemoattractant protein-1 (MCP-1), monocyte inhibiting
protein-1α (MIP-1α), monocyte inhibiting protein-1β (MIP-1β),
platelet-derived growth factor-BB (PDGF-BB), RANTES, tumor-necrosis
factor-α (TNF-α) and vascular endothelial growth factor (VEGF). The
detection process was performed following the instruction leaflet.
Briefly, 24 h CM from OVCAFs and OVNFs was centrifuged at 1,000 × g
for 10 min at 4°C and the concentration of cytokines/chemokines of
each sample was determined using the Bio-Plex 200 system (Bio-Rad
Laboratories, Inc.). Data were analyzed using Bio-Plex Manager™
Software Version 4.0 (Bio-Rad Laboratories, Inc.). The cytokine
concentration (pg/ml) represents the mean ± SD of 3 independent
experiments in triplicate.
Statistical analysis
All statistical analyses were performed using SPSS
version 23 (SPSS Inc.). The values are expressed as the means ± SD.
Statistical significance was determined by one-way ANOVA with the
Tukey's post hoc test when comparing multiple samples. The
Student's t-test was used when comparing each sample to the normal
control. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
OVCAF-derived CM induces ovarian cancer
cell migration and reduces autophagy
OVCAFs isolated from fresh ovarian cancer tissues
from 3 patients exhibited positivity for the specific markers,
including α-SMA, VIM and FAP, while they were negative for the
epithelial marker, CK19 (Fig. 1).
As a further confirmation, the OVCAFs, but not the normal
fibroblasts isolated from counterpart benign ovary tissues of the
same patients (OVNFs), expressed FAP (Fig. 1). The ability of the CM from
OVCAFs and from OVNFs isolated from the 3 patients to induce the
migration of fluorescently0labeled ovarian cancer SKOV3 and
Kuramochi cells through a porous membrane of the Transwell was
assayed (as described in the Materials and methods). As a baseline,
control medium supplemented with 1% FBS was employed to maintain
the level of serum factors to the minimum. Cell migration
stimulated by the OVCAF-CM was evidently greater than that
stimulated by the OVNF-CM counterpart, with statistical
significance at all incubation times tested (Fig. 2A and B).
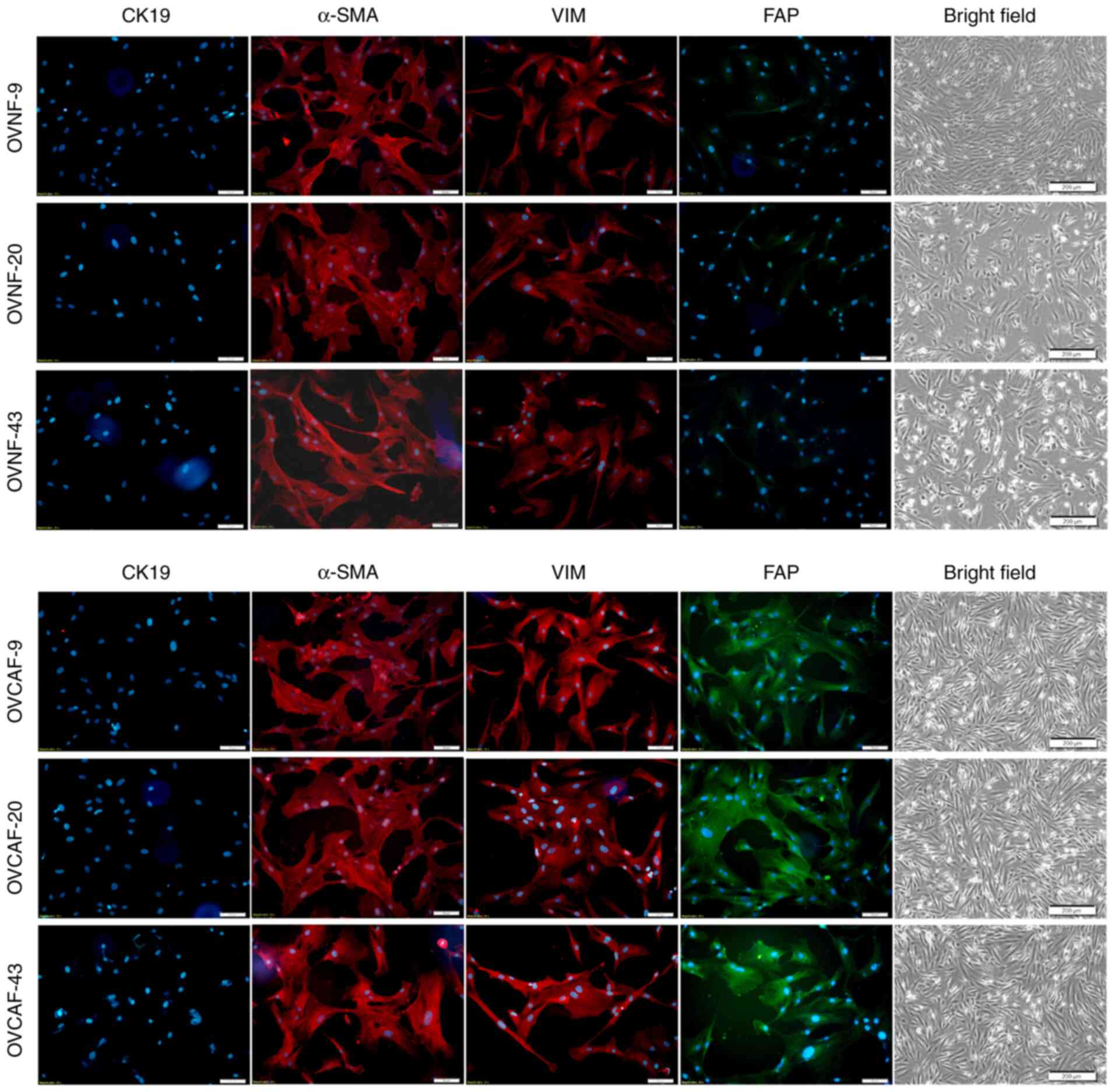 | Figure 1Quality control of the purity of
OVNFs and OVCAFs. Fibroblasts were isolated from ovarian cancer
tissues (OVCAF) and from adjacent healthy ovarian tissues (paired
OVNFs) and assayed for purity by immunofluorescence using
antibodies directed to specific markers for myofibroblasts (α-SMA,
red fluorescence), epithelial cells (CK19, red fluorescence),
fibroblast intermediate filament (VIM, red fluorescence) and
fibroblast-associated protein (FAP, green fluorescence). The nuclei
were counterstained with Hoechst. The unstained cells of each cell
type are shown. All images shown in the figure are representative
of 3 independent experiments. Scale bar, 50 µM. OVNFs,
ovarian non-tumor-associated fibroblasts; OVCAFs, ovarian
cancer-associated fibroblasts; α-SMA, α smooth muscle actin; CK19,
cytokeratin 19; FAP, fibroblast activation protein. |
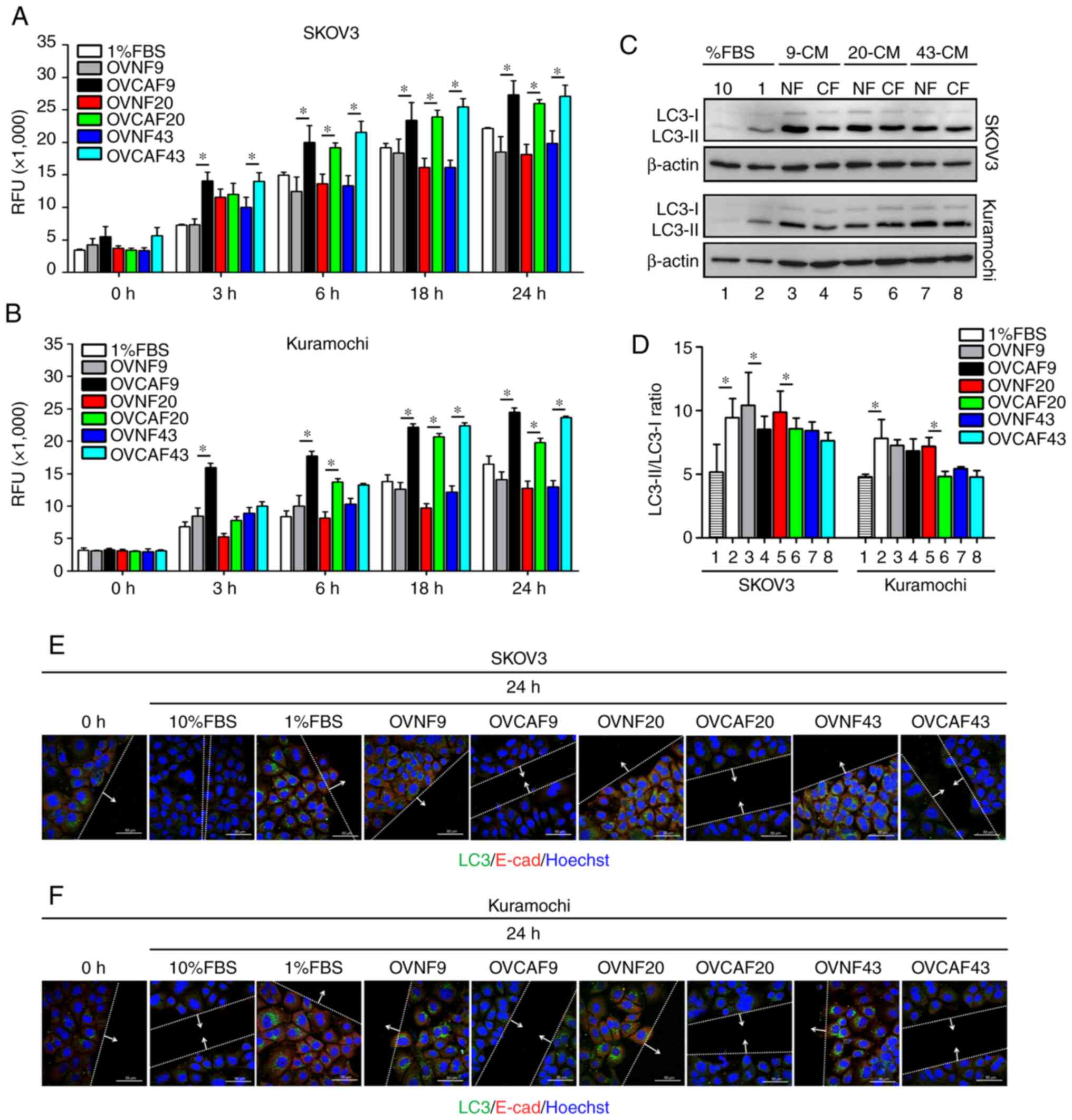 | Figure 2OVCAF-derived CM induced ovarian
cancer cell migration and reduced autophagy. (A and B)
Representative RFUs of SKOV3 and Kuramochi migrated cells. The
cells were seeded in 24-Fluoroblok™ membrane Transwell plates and
treated with OVNFs-CM or OVCAFs-CM. The graphs report the rate of
migration cells for each time point (0, 3, 6, 18 and 24 h). (C)
Western blot analysis showing the level of LC3 expressed by SKOV3
and Kuramochi cells exposed to OVNFs-CM or OVCAFs-CM, compared with
10% FBS and 1% FBS control media and in the presence of 30
µM CLQ (added 8 h before cell lysis). (D) Densitometry of
the bands is reported as the ratio of LC3-II/LC3-I. Bar graphs
represent mean ± SD of 3 independent experiments. Student's t-test
showing the significant differences of OVCAF compared to normal
fibroblasts, *P<0.05. (E and F) Double immunostaining
for LC3 (green fluorescence) and E-cad (red fluorescence). Images
show the co-localization of LC3 with E-cad in cells at the
migration front under OVNFs-CM or OVCAFs-CM-treatment compared with
10% FBS and 1% FBS media control and in the presence of 30
µM CLQ. The cells at the migration front in the wound area
were photographed under the confocal fluorescence microscope. Scale
bar, 50 µM; magnification, ×63. RFU, relative fluorescence
unit; LC3, light chain 3; E-cad, E-cadherin; OVNFs, ovarian
non-tumor-associated fibroblasts; OVCAFs, ovarian cancer-associated
fibroblasts. |
Given the role of autophagy in ovarian cancer cell
migration (18,21), the present study assessed the
level of cellular autophagy in the treated cancer cells. The cells
were incubated with medium as indicated in the presence of CLQ to
prevent autophagosome degradation (22), and the expression of the autophagy
marker, LC3, was analyzed in the homogenates by western blot
analysis. Upon the induction of autophagy, the cytosolic LC3-I
isoform is converted into the autophagosomal LC3-II isoform
(22). The LC3-II/LC3-I ratio,
considered a marker of the induction of autophagy (22), was decreased in both ovarian
cancer cell lines exposed to OVCAF-CM (from 3 patients) compared to
the OVNF-CM of the respective patients (Fig. 2C and D). The downregulation of
autophagy in the SKOV3 cells was significant with the CM of OVCAF-9
and of OVCAF-20, whereas in the Kuramochi cells, the CM from
OVCAF-20 was more effective. Notably, the cancer cells cultured in
control culture medium containing 10% FBS exhibited a lower level
of autophagy as compared to the cells cultured with 1% FBS
(Fig. 2C and D). Taken together,
these results indicate a link between low autophagy and a high
migration rate, and suggest that OVCAFs-CM induce ovarian cancer
cell migration by reducing autophagy in the cancer cells. To
confirm this link, the expression of vacuolar LC3 (corresponding to
the autophagosomal LC3-II isoform) and of the epithelial marker
E-cadherin (E-cad) was assessed by immunofluorescence staining in
the ovarian cancer cells at the migration front. It is expected
that E-cad, which mediates cell-cell adhesion, would be
downregulated in cells that begin to migrate.
In both the SKOV3 and Kuramochi cells, incubation
OVCAF-CM led to a reduced positivity for vacuolar LC3 and for
membrane E-cad compared to the cells incubated with the counterpart
OVNF-CM (Fig. 2E and F). This
pattern is characteristic of migratory cells (18,21). Serum factors are known to
stimulate cell proliferation and cell migration (22) and to downregulate basal autophagy
(23). Therefore, the present
study assayed the expression of these proteins in cultures
incubated in standard culture medium supplemented with low (1%) or
high (10%) FBS as controls. A high LC3 and high E-cad expression
pattern was observed in the cells cultured with 1% FBS medium and
exhibiting a low invasive capability (Fig. 2A-D), whereas the opposite pattern
(low LC3 and low E-cad expression) was observed in the cells
cultured in 10% FBS medium exhibiting migratory features (Fig. 2E, F). These data consistently
demonstrate that cancer cell migration induced by OVCAF-CM and by
high serum (10% FBS) is associated with the downregulation of
autophagy.
OVCAF-derived secretion contains higher
amounts of IL-8 compared to the OVNF counterpart
Secreted substances in OVCAF-CM and in OVNF-CM from
6 patients (case nos. 9, 20, 26, 34, 43 and 48) were assayed using
a cytokine array for 27 cytokines/chemokines including IL-1β,
IL-1rα, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70,
IL-13, IL-15, IL-17A, eotaxin, bFGF, G-CSF, GM-CSF, IFN-γ, IP-10,
MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α and VEGF. The raw
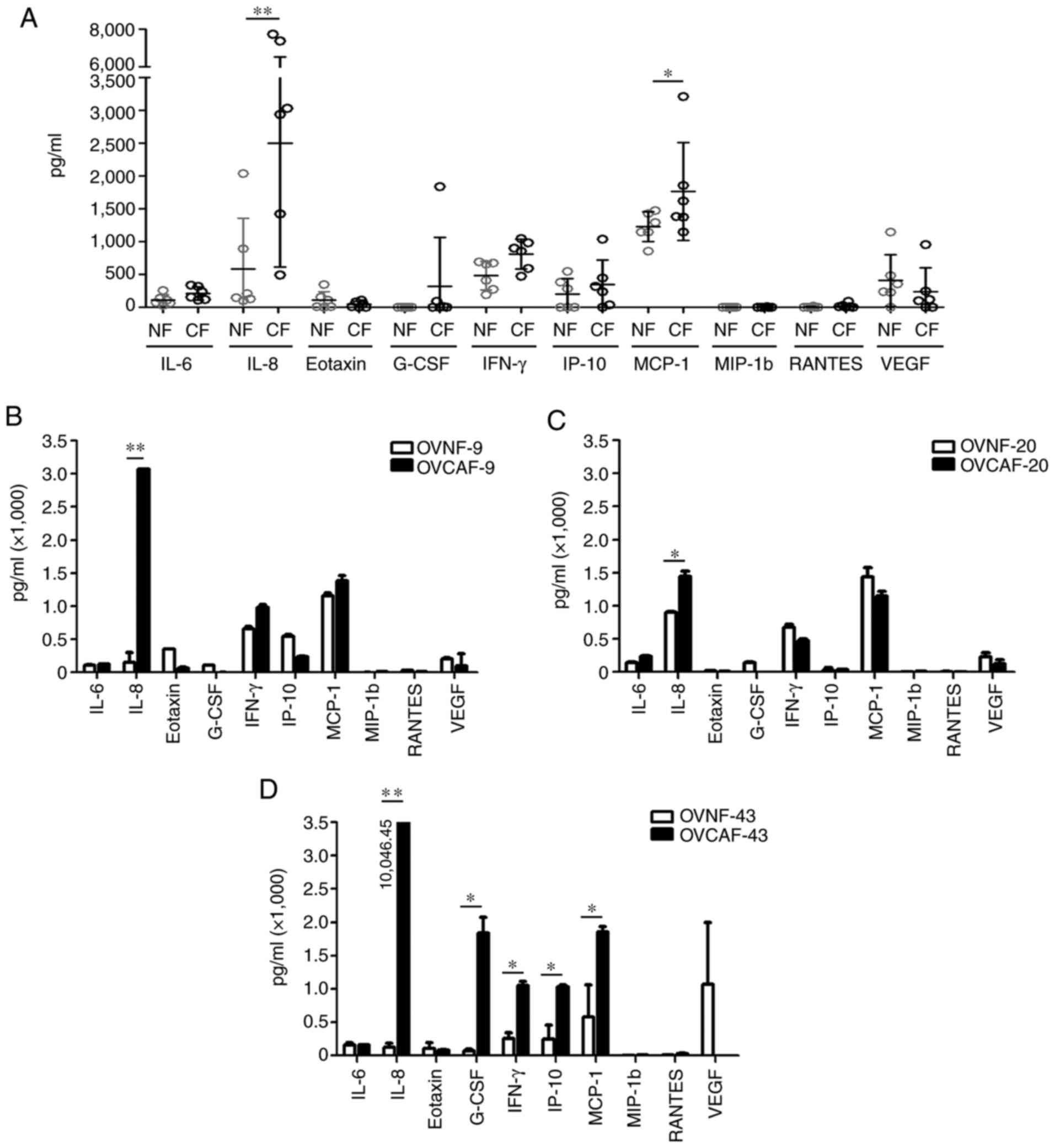
data of the cytokine levels are presented in Table I. In total, 10 cytokines, namely
IL-6, IL-8, eotaxin, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1b, RANTES
and VEGF, were in the detectable range. Of the 6 OVCAF-CM, only 7
from the 10 total detectable cytokines, including IL-6, IL-8,
G-CSF, IFN-γ, IP-10, MCP-1 and VEGF, exhibited a significantly
increased level (P-value <0.05 and <0.01) (Fig. S1). All 6 OVCAF-CM exhibited
statistically significant higher levels of IL-8 (P-value <0.01)
and MCP-1 (P-value <0.05) compared with the 6 pooled OVNF-CM
(Fig. 3A). Since a different
genetic background can influence the gene expression and the
secretion of cytokines, the matched OVNF-CM and OVCAF-CM from the
same patients were selected and compared. The following were
included in the assay: OVCAF-9 and OVNF-9, OVCAF-20 and OVNF-20,
and OVCAF-43 and OVNF-43. The results revealed a significantly
higher level of IL-8 in all 3 pairings of OVCAF-CM compared to
OVNF-CM. In addition, the GM-CSF, IFN-γ, IP-10 and MCP-1 levels
were increased in OVCAF-43-CM compared to those in the normal
counterpart with statistical significance (Fig. 3B-D).
 | Table ICytokine levels in 6 OVNF-CM and 6
OVCAF-CM. |
Table I
Cytokine levels in 6 OVNF-CM and 6
OVCAF-CM.
| No. | Level (mean ± SD;
pg/ml)
|
|---|
| Cytokine | OVNF-9 | OVNF-20 | OVNF-22 | OVNF-28 | OVNF-41 | OVNF-43 | OVCAF-9 | OVCAF-20 | OVCAF-26 | OVCAF-34 | OVCAF-43 | OVCAF-48 |
|---|
| 1 | IL-1β | 0.62±0.18 | 0.48±0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | IL-1rα | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 3 | IL-2 | 2.08±1.18 | 1.28±0.47 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | IL-4 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.28±0.23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5 | IL-5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 6 | IL-6 | 109.35± 5.71 | 138.18± 8.00 | 254.73± 6.730 | 75.01± 1.60 | 67.63± 18.43 | 29.73± 18.43 | 121.24± 5.00 | 237.22± 11.70 | 340.87± 14.35 | 109.69± 23.40 | 159.50± 3.00 | 324.25± 26.67 |
| 7 | IL-7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8 | IL-8 | 148.23± 149.71 | 898.42± 10.86 | 2,040.38±
140.36 | 209.82± 7.82 | 127.89± 1.72 | 105.32± 21.82 | 3,069.50± 5.00 | 1,441.13±
86.16 | 26,800.33±
720.15 | 508.46± 279.82 | 9,451.45±
595.00 | 2,857.73±
74.42 |
| 9 | IL-9 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | IL-10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 11 | IL-12p70 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 12 | IL-13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 13 | IL-15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 14 | IL-17A | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 15 | Eotaxin | 352.91± 1.27 | 14.77± 6.00 | 11.32± 0.30 | 6.64± 0.21 | 111.72± 90.22 | 158.17± 0.21 | 62.88± 16.00 | 6.49± 1.20 | 6.85± 0.70 | 110.81± 88.34 | 83.89± 1.00 | 9.99± 1.81 |
| 16 | bFGF | 3.25±1.30 | 3.01±0.37 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 17 | G-CSF | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15±0.00 | 0.15±0.00 | 98.94±29.5 | 15.33±16.07 | 0.00 | 0.00 |
| 18 | GM-CSF | 109.37± 5.77 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1,835.54±
236.00 | 0.00 |
| 19 | IFN-γ | 658.45± 37.54 | 638.17± 8.00 | 697.30± 61.44 | 412.05± 37.37 | 197.87± 55.84 | 280.47± 37.37 | 984.54± 26.31 | 476.06± 26.31 | 592.67± 45.75 | 855.37± 124.16 | 1,049.90±
67.00 | 905.41± 92.53 |
| 20 | IP-10 | 548.21± 21.41 | 31.99± 27.00 | 0.00 | 0.00 | 286.01± 4.50 | 385.27± 36.84 | 544.95± 72.74 | 38.98± 0.00 | 450.60± 32.35 | 319.03± 3.35 | 1,038.29±
21.00 | 0.00 |
| 21 | MCP-1 | 1,154.40±
78.33 | 1,439.28±
135.32 | 1,480.06±
95.80 | 1,153.75±
70.03 | 857.89± 4.72 | 581.36± 378.97 | 1,386.52±
43.00 | 1,144.95±
72.74 | 3,210.20±
384.20 | 1,624.66±
601.16 | 1,859.67±
73.40 | 1,378.97±
119.62 |
| 22 | MIP-1α | 2.47±1.10 | 0.22±0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 23 | MIP-1β | 4.15±1.78 | 4.04±1.00 | 7.35±1.41 | 0.00 | 0.00 | 0.00 | 6.50±3.00 | 7.96±1.70 | 8.86±0.83 | 3.44±0.95 | 8.67±0.00 | 14.53±1.60 |
| 24 | PDGF-BB | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 25 | RANTES | 23.58±1.15 | 3.32±0.31 | 0.00 | 0.00 | 5.21±0.00 | 6.70±2.64 | 12.36±1.74 | 3.54±1.74 | 91.92±9.00 | 11.71±2.63 | 31.16±1.00 | 8.93±1.71 |
| 26 | TNF-α | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 27 | VEGF | 238.05± 14.01 | 233.37± 63.28 | 488.33± 15.81 | 358.61± 84.87 | 1,150.41±
21.35 | 1,150.41±
1,121.35 | 101.88± 180.00 | 124.91± 59.04 | 0.00 | 251.76± 60.47 | 0.00 | 961.57± 49.52 |
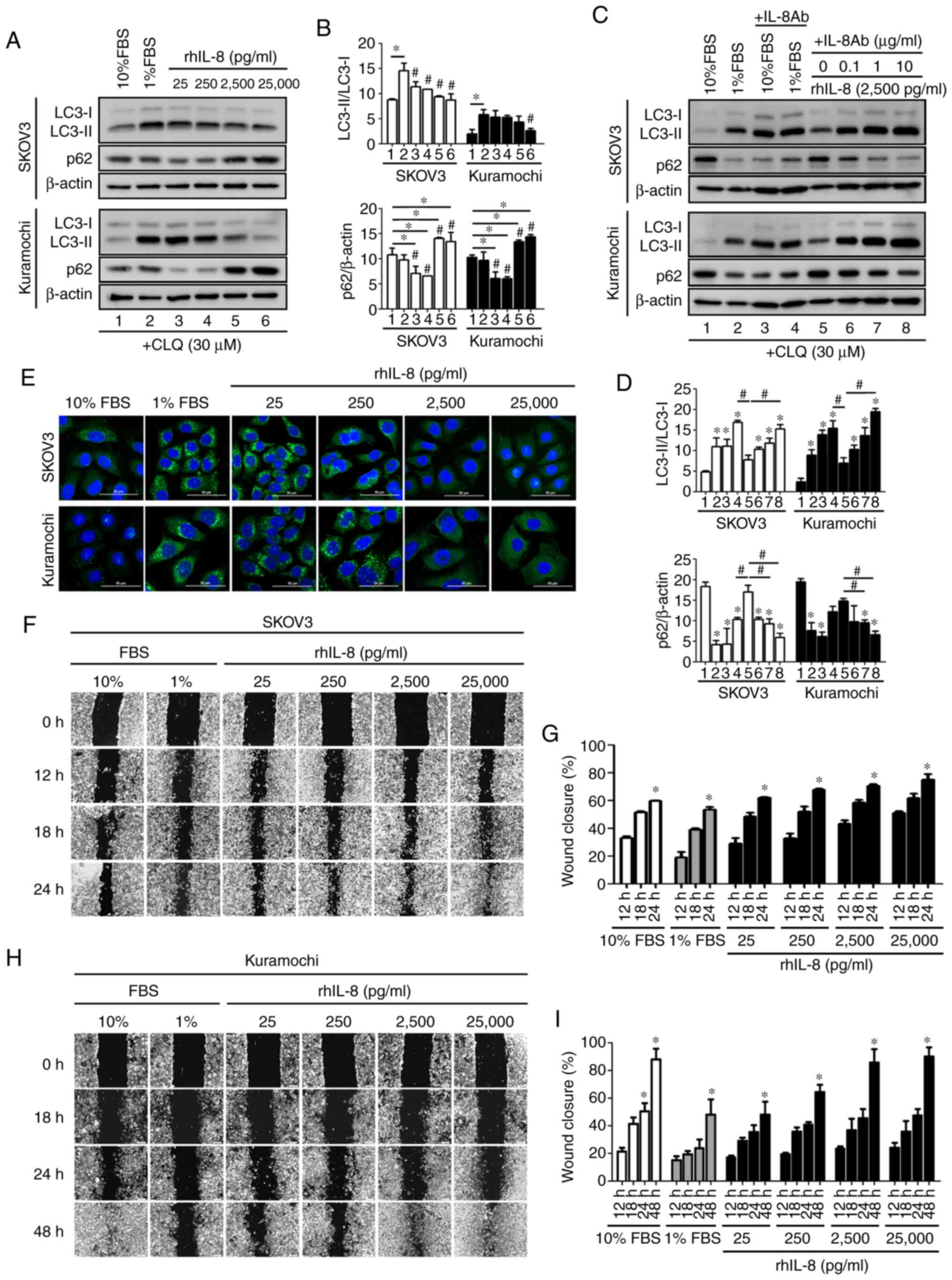
IL-8 promotes ovarian cancer cell
migration and reduces autophagy
According to the consistent finding of high IL-8 in
all OVCF-CM compared to their normal counterpart CM, it was
hypothesized that IL-8 may be the most relevant secreted substance
in OVCAF-CM driving cell migration in association with the
suppression of autophagy. To examine this hypothesis, the effect of
rhIL-8 was assayed. The positive control of high basal autophagy
was detected in cells cultured with 1% FBS containing medium
compared to that in the cells grown in 10% FBS. CLQ was added to
discriminate LC3-II accumulation arising from increased
autophagosome formation from that due to impaired autophagosome
degradation (24). As shown by
the results of western blot analysis of the LC3-II/LC3-I ratio, it
was evident that rhIL-8 significantly inhibited basal autophagy
both in SKOV3 cells (at 25 pg/ml to 25,000 pg/ml) and in Kuramochi
cells (at 25,000 pg/ml) (LC3, Fig. 4A
and B). The p62/SQSTM1 protein, a receptor for cargo destined
to be degraded by autophagy, accumulates in cells when the
autophagic flux is hampered (24). In the present study, p62 protein
significantly accumulated in the cells treated with high
concentrations of rhIL-8 (2,500 and 25,000 pg/ml) (p62, Fig. 4A and B). Taken together, the
results with both autophagy markers (LC3 and p62) support the
conclusion that rhIL-8 decreases the level of basal autophagy in
ovarian cancer cells.
To further support this contention, neutralizing
anti-IL8 antibody was applied to the culture medium supplemented
with FBS or rhIL-8. The addition of anti-IL8 to the culture medium
restored the autophagic flux, as evidenced by the high LC3-II/LC3-I
ratio and the reduced accumulation of p62, both in the SKOV3 and
Kuramochi cells cultured in the presence of rhIL-8 or 10% FBS
(Fig. 4C and D).
Immunofluorescence staining of LC3 further confirmed that IL-8
effectively inhibited autophagy in ovarian cancer cells (Fig. 4E). Of note, the fact that
anti-IL-8 restored autophagy in the cells cultured with 10% FBS to
the level of that in control cells cultured with 1% FBS suggests
that FBS contains some IL-8 (Fig. 4C
and D, lane 3 vs. lane 2). In a wound-healing assay, the
addition of rhIL-8 markedly promoted wound closure, much alike in
the culture with 10% FBS, supporting the hypothesis that FBS
contains IL-8 (Fig. 4F and H;
with respective quantification data shown in Fig. 4G and I). The data also
demonstrated that the SKOV3 cells were more responsive than the
Kuramochi cells to IL-8. Indeed, the Kuramochi cells exhibited less
motility and required a longer time (up to 48 h) for wound healing
in the presence of a high FBS or IL-8 concentration compared to the
SKOV3 cells.
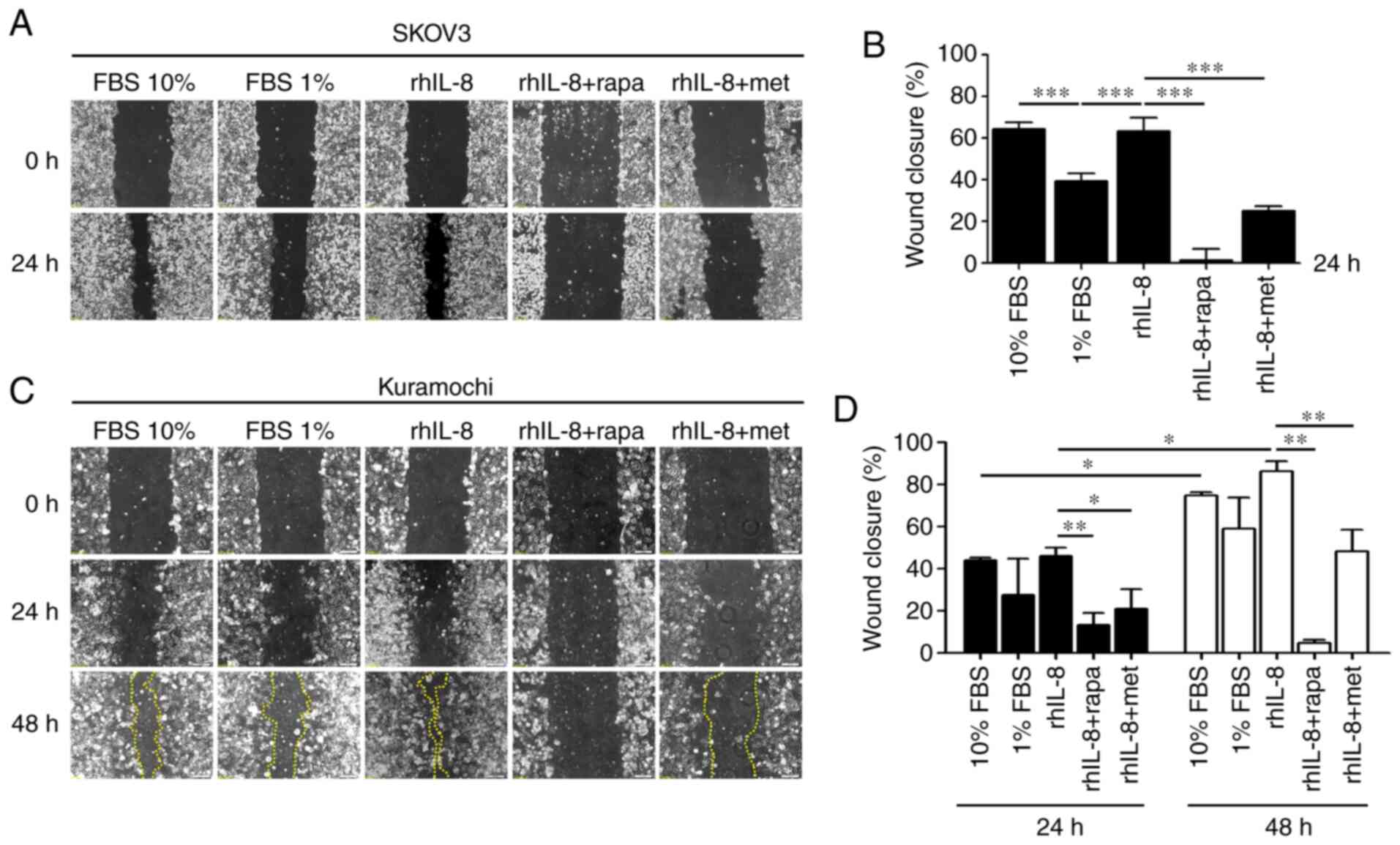
Finally, a wound-healing assay was performed using
cells incubated with rhIL-8 in the presence of rapamycin or
metformin, two drugs known to induce autophagy via mTOR inhibition
and AMPK activation, respectively. Both autophagy inducers
neutralized the migratory capability of the SKOV3 (Fig. 5A and B) and Kuramochi (Fig. 5C and D) ovarian cancer cells
exposed to IL-8. These findings confirm that the pro-migratory
effect of IL-8 is mechanistically linked to the downregulation of
autophagy.
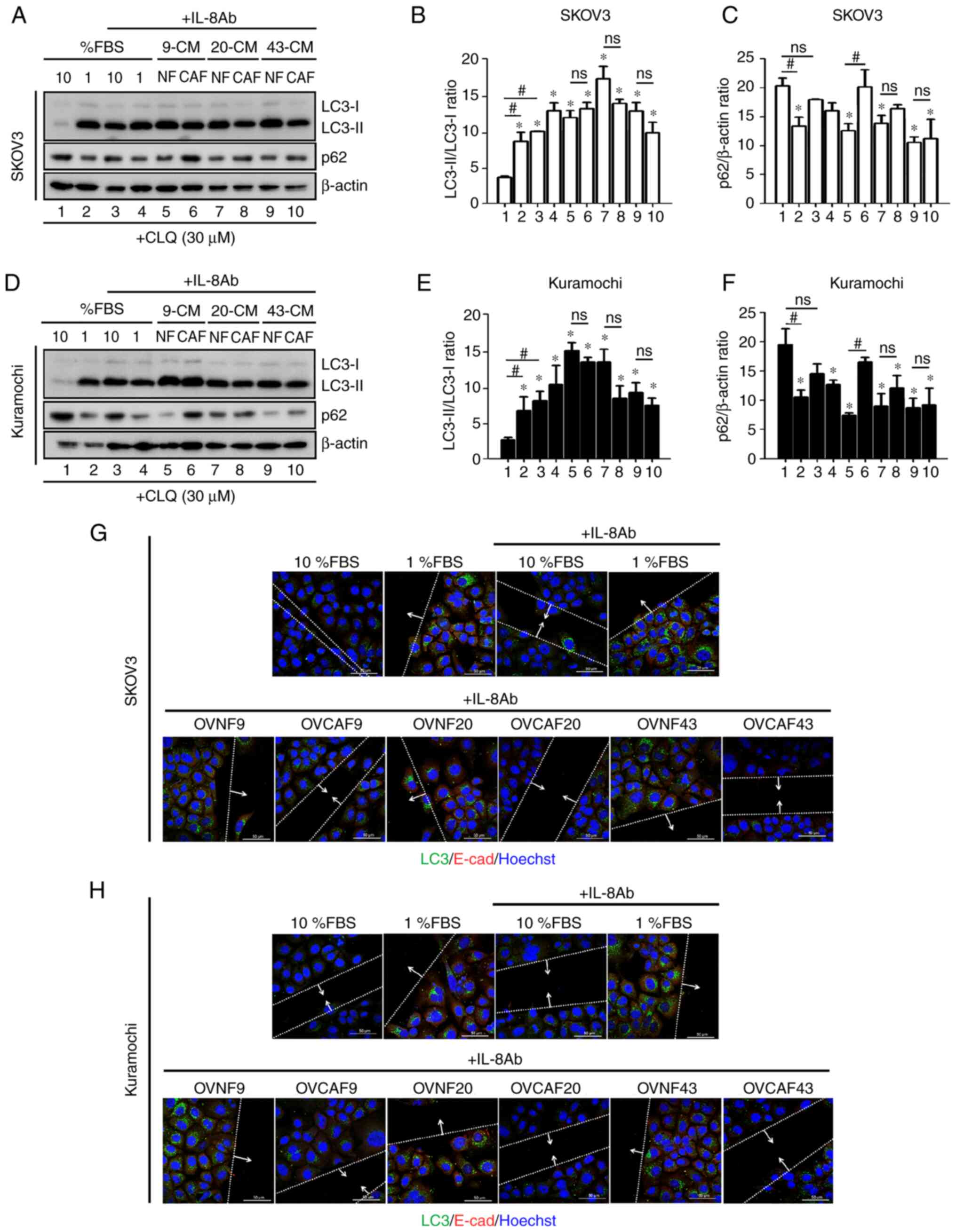
OVCAF-CM reduces autophagy and mediates
cancer cell migration through IL-8
The possible involvement of IL-8 in the modulation
of autophagy by FBS and the CM from normal and CAFs was
investigated further. To this end, the cellular levels of LC3-II
and p62 in the SKOV-3 cells (Fig.
6A-C) and in Kuramochi cells (Fig. 6D-F) cultured in 10 or 1% FBS or in
CM from OVNFs or OVCAFs in the presence of the neutralizing
anti-IL-8 antibody was assayed. CLQ was added to prevent
autophagosome degradation. Consistent with the data in Fig. 4C and D, in both ovarian cancer
cell lines the anti-IL-8 antibody restored autophagy in the cancer
cells cultured in 10% FBS medium to the level found in the control
(no anti-IL-8) cells cultured in 1% FBS medium (Fig. 6A and B, and D and E; lane 3 vs.
lane 2), and this effect was statistically significant. Moreover,
anti-IL-8 abrogated the negative effect of CAF-CM on autophagy
modulation (reduced autophagy), maintaining the LC3-II/LC3-I levels
at values comparable to those observed in cells incubated with
OVNF-CM or in control 1% FBS medium (Fig. 6A and B, and D and E; lanes 5-10
compared to lane 2). A decreased accumulation of p62 was observed
in the OVCAF-CM (cases no 20 and 43)-treated cells upon the
addition of anti-IL-8 antibody compared to that in the control
cells incubated in 10% FBS medium (Fig. 6A-C, and D-F; lanes 5-10 compared
to lane 2), indicating that anti-IL-8 antibody restored the
autophagic flux, reverting the block induced by OVCAF-CM.
Finally, to link this observation with the
functional effect on cell migration, the expression levels of LC3
and E-cad in the cells at the migration front in the cancer cells
cultured as described above were assayed. Neutralizing anti-IL-8
antibody attenuated the migration of both ovarian cancer cells
cultured in 10% FBS medium (Fig. 6G
and H). Anti-IL8 antibody increased the expression of LC3 and
of E-cad in the cells incubated in 1% FBS, while this effect was
less prominent in the cells cultured in 10% FBS. Similarly,
anti-IL8 antibody blocked the cell migration induced by OVCAFs-CM
concomitantly, with the upregulation of LC3 expression (indicative
of autophagy) and the downregulation of E-cad expression
(indicative of migratory cells) in the cells at the migration front
(Fig. 6G and H). From these data,
it can be concluded that OVCAF-CM reduces autophagy and mediates
cancer cell migration through IL-8.
Discussion
CAFs in the tumor microenvironment have been
well-recognized for their potential in inducing cancer cell
progression in ovarian cancer (3,4).
Several cytokines/chemokines from ovarian CAFs have been proven to
modulate autophagy (3). The
migration rate of ovarian cancer cells increases upon autophagy
gene knockdown (13). However, to
date, to the best of our knowledge, there are no data demonstrating
a direct effect of cytokines/chemokines secreted by ovarian cancer
CAFs on the modulation of autophagy and how this modulation can
affect ovarian cancer cell migration. In the present study,
cytokines and chemokines, including IL-6, IL-8, eotaxin, GM-CSF,
IFN-γ, IP-10, MCP-1, MIP-1b, RANTES and VEGF were detected in the
conditioned-medium of 3 primary CAFs from ovarian cancer,
indicating their possible release in the microenvironment and their
participation in ovarian cancer progression. In fact, IFN-γ from
tumor infiltrating lymphocytes has been shown to regulate the
expression of programed death ligand-1 (PD-L1) in ovarian cancer
cells (25). Moreover, IFN-γ,
TNF-α, IL-10 and IL-6 released from tumor-associated macrophages
have been shown to stimulate the expression of PD-L1 at the surface
of the cancer cells (26). As
shown in a previous study, MCP-1 promoted the invasion and adhesion
of ovarian cancer cells, and a CCR2 antagonist attenuated the
effects of MCP-1 in vitro (27). High concentrations of IP-10 and
MCP-1 have been detected in both ascites and tumor cells of ovarian
cancer patients (28). This
evidence supports the tumorigenic promoting effect of the
substances released from CAFs in ovarian cancer.
In the present study, OVCAFs were characterized by
the presence of (29). The lack
of positivity for the epithelial marker CK19 in CAF culture ensures
no contamination by cancer cells. In a previous study, CK19 was
found to be highly expressed at the same level of CK7 in three
ovarian cancer cells (Caov-3, OVCAR-3 and SKOV3), including the one
used in the present study (30).
By contrast, CK7 was not expressed in other ovarian cancer cell
lines (PA-1 and A2780ADR) that however expressed CK19 (30). Additionally, the upregulation of
CK19 has been shown to be associated with the proliferation,
migration and invasion of ovarian cancer cells, and is in fact
considered a potential therapeutic target (31,32). These data confirm that CK19 is a
reliable marker for identifying ovarian cancer cells and support
its use for examining epithelial contamination in OVCAF primary
culture.
CAFs are activated fibroblasts in the tumor
microenvironment and their metastasis-promoting functions has been
well-established (33). CAFs can
promote the tumor growth by modulating the actual level of
autophagy in ovarian cancer cells through the secretion of
pro-inflammatory cytokines and the release of autophagy-derived
metabolites and substrates (3).
Several cytokines, including IFN-γ, IL-4, IL-6 and IL-13, have been
shown to be associated with autophagy regulation, yet the majority
of studies has focused on IL-6, as this is one of the main
cytokines released in the tumor microenvironment. Apparently, this
cytokine exerts pleiotropic effects on autophagy regulation that
likely are cell- and extracellular environment-context dependent.
In a previous study, human IL-6 recombinant protein induced
autophagy in HepG2 liver cancer cells through the induction of
NF-κB mediated signaling pathway (34). Yet, other studies have
demonstrated an inhibitory effect of IL-6 on autophagy regulation
in cancer cells. For instance, IL-6 has been shown to reduce the
expression of LC3-II and BECLIN-1, thus inhibiting autophagy in
starved U937 cells through the STAT3 signaling pathway (35). IL-6 has also been shown to promote
cancer cell migration via the inhibition of autophagy. The
conditioned-medium from human primary cholangiocarcinoma CAFs,
which has been shown to contain high levels of IL-6 and IL-8, cab
promote the migration of human cholangiocarcinoma cells through the
inhibition of autophagy in cancer cells, and this effect is
abolished when the secretion of IL-6 in the CAFs conditioned medium
is prevented (21). With
reference to ovarian cancer, it has been shown that IL-6 can
promote cell migration through the inhibition of BECLIN-1-dependent
autophagy in the cells at the migration front (18). While the role of IL-6 in
modulating autophagy and cell migration has been well addressed in
ovarian cancer cells and in other types of cancer, little is known
about the role played by IL-8.
E-cadherin plays a pivotal role in cell-cell
adhesion, and it is not expressed on the membrane and it is
epigenetically silenced in the cells that disaggregate to begin
moving. The switch from E- to N-cadherin expression on cell surface
is a marker of EMT. This is likely to be more evident in the cells
that lose the cell-cell contact and start to migrate, as also
demonstrated in a previous study by the authors (18), beside others (7,9).
On the other hand, it is not expected that all cells would exhibit
a downregulation of E-cadherin to the same extent, as indicated by
the lack of a typical scatter phenomenon. In fact, cytokines
downregulate autophagy and E-cadherin expression preferentially in
the cells at the migration front (18). Therefore, western blot analysis of
the whole homogenate would provide an average level of reduced
expression. For these reasons, it is more informative to examine
the expression of E-cadherin on the membrane of the cells at the
migration front as a confirmation of their acquired ability to
move.
IL-8 has been shown to attenuate the DIRAS3-mediated
autophagy (19), induce EMT and
enhance ovarian cancer cell metastasis (7,9).
Consistently, in vitro analyses have indicated that the
overexpression and secretion of IL-8 in ovarian cancer cells favor
their anchorage-independent growth, proliferation and invasion
(20). In the present study, IL-8
was found highly expressed in and secreted by OVCAFs compared to
normal fibroblasts. The CM from CAFs isolated from 3 primary
ovarian cancers were shown to stimulate cell migration in 2 ovarian
cancer cell lines, and this effect was parallel to the
downregulation of autophagy in the cancer cells. These effects were
abolished when the CAFs-CM was supplemented with anti-IL8
neutralizing antibody. Of note, FBS at 10% elicited a similar
effect in promoting cell migration along with the suppression of
autophagy. Again, the addition of anti-IL8 neutralizing antibody to
the medium with 10% FBS restored autophagy and blocked cell
migration to the level observed in the control cells cultured in 1%
FBS medium, thus suggesting the presence of IL-8 in FBS. Although
the involvement of IL-8 in the migration and invasion of ovarian
cancer SKOV3 cells has previously been reported (8), to the best of our knowledge, this is
the first study to demonstrate that CAF-derived IL-8 negatively
regulates autophagy and concomitantly induces the migration of 2
ovarian cancer cell lines. The mechanistic link between the
downregulation of autophagy and increased cell migration was
definitively proven, showing that the over-stimulation of autophagy
with rapamycin and metformin counteracted the promigratory activity
of rhIL-8. These results are consistent with previous evidence
indicating that the knockdown of autophagy-related genes favors
ovarian cancer cell migration (13). IL-8 can negatively regulate
autophagy via the activation of the PI3K/AKT and MAPK/ERK signaling
pathways (19).
In conclusion, the present study demonstrates that
CAF-derived IL-8 negatively regulates autophagy and concomitantly
induces ovarian cancer cell migration. The findings presented
herein indicate that antibody targeting IL-8 released by CAFs and
other stromal cells may prove to be an additional therapeutic tool
with which to limit ovarian cancer progression.
Supplementary Data
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
CT, PT and CI designed the experiments. SThongchot,
PJ, STherasakvichya, MW and AF performed the experiments,
elaborated the data, performed the quantification and statistical
analysis, and prepared the figures. PT, CT and CI interpreted the
results. SThongchot, PT and CT drafted the manuscript. CT and CI
revised and finalized the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Miss Vijakhana
Pilaisangsuree, Mahidol University for providing technical support.
The authors would also like to thank Miss Marisa Royrod, Mahidol
University for providing technical assistance with autophagy
detection.
Funding
The present study was supported by the Siriraj Cancer
Foundation, Siriraj Hospital, Mahidol University and Research
Grants from Siriraj Research Division (R016033015) to CT. AF is
recipient of a post-doctoral fellowship 'Paolina Troiano' (id.
24094) granted by Associazione Italiana per la Ricerca sul Cancro
(AIRC, Milan, Italy).
Abbreviations:
|
α-SMA
|
α-smooth muscle actin
|
|
CAFs
|
cancer-associated fibroblasts
|
|
OVCAF-CM
|
conditioned-medium of
cancer-associated fibroblasts
|
|
CK19
|
cytokeratin 19
|
|
FAP
|
fibroblast activation protein
|
|
IL
|
interleukin
|
|
bFGF
|
basic fibroblast growth factor
|
|
G-CSF
|
granulocyte-colony stimulating
factor
|
|
GM-SCF
|
granulocyte-monocyte-colony
stimulating factor
|
|
IFN-γ
|
interferon-γ
|
|
IP-10
|
interferon-γ-induced protein 10
|
|
MCP-1
|
monocyte chemoattractant protein-α
|
|
MIP-1β
|
monocyte inhibiting protein-1a
|
|
MIP-1β
|
monocyte inhibiting protein-1β
|
|
NFs
|
normal fibroblasts
|
|
PDGF-BB
|
platelet-derived growth factor-BB
|
|
RANTES
|
regulated on activation, normal T cell
expressed and secreted
|
|
TNF-α
|
tumor-necrosis factor-α
|
|
VEGF
|
vascular endothelial growth factor
|
|
VIM
|
vimentin
|
References
|
1
|
Ahmed N, Kadife E, Raza A, Short M,
Jubinsky PT and Kannourakis G: Ovarian cancer, cancer stem cells
and current treatment strategies: A potential role of magmas in the
current treatment methods. Cells. 9:7192020. View Article : Google Scholar :
|
|
2
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thuwajit C, Ferraresi A, Titone R,
Thuwajit P and Isidoro C: The metabolic cross-talk between
epithelial cancer cells and stromal fibroblasts in ovarian cancer
progression: Autophagy plays a role. Med Res Rev. 38:1235–1254.
2018. View Article : Google Scholar
|
|
4
|
Jiang Y, Wang C and Zhou S: Targeting
tumor microenvironment in ovarian cancer: Premise and promise.
Biochim Biophys Acta Rev Cancer. 1873:1883612020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Liu W, Wang X, Wang X and Sun H:
Prognostic value of serum IL-8 and IL-10 in patients with ovarian
cancer under-going chemotherapy. Oncol Lett. 17:2365–2369.
2019.PubMed/NCBI
|
|
6
|
Rodrigues ISS, Martins-Filho A, Micheli
DC, Lima CA, Tavares-Murta BM, Murta EFC and Nomelini RS: IL-6 and
IL-8 as prognostic factors in peritoneal fluid of ovarian cancer.
Immunol Invest. 49:510–521. 2020. View Article : Google Scholar
|
|
7
|
Wen J, Zhao Z, Huang L, Wang L, Miao Y and
Wu J: IL-8 promotes cell migration through regulating EMT by
activating the Wnt/β-catenin pathway in ovarian cancer. J Cell Mol
Med. 24:1588–1598. 2020. View Article : Google Scholar
|
|
8
|
Li Y, Liu L, Yin Z, Xu H, Li S, Tao W,
Cheng H, Du L, Zhou X and Zhang B: Effect of targeted silencing of
IL-8 on in vitro migration and invasion of SKOV3 ovarian cancer
cells. Oncol Lett. 13:567–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin J, Zeng F, Wu N, Kang K, Yang Z and
Yang H: Interleukin-8 promotes human ovarian cancer cell migration
by epithelial-mesenchymal transition induction in vitro. Clin
Transl Oncol. 17:365–370. 2015. View Article : Google Scholar
|
|
10
|
Uddin MM, Gaire B and Vancurova I:
Interleukin-8 induces proliferation of ovarian cancer cells in 3D
spheroids. Methods Mol Biol. 2108:117–124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferraresi A, Girone C, Esposito A, Vidoni
C, Vallino L, Secomandi E, Dhanasekaran DN and Isidoro C: How
autophagy shapes the tumor microenvironment in ovarian cancer.
Front Oncol. 10:5999152020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peracchio C, Alabiso O, Valente G and
Isidoro C: Involvement of autophagy in ovarian cancer: A working
hypothesis. J Ovarian Res. 5:222012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delaney JR, Patel CB, Bapat J, Jones CM,
Ramos-Zapatero M, Ortell KK, Tanios R, Haghighiabyaneh M, Axelrod
J, DeStefano JW, et al: Autophagy gene haploinsufficiency drives
chromosome instability, increases migration, and promotes early
ovarian tumors. PLoS Genet. 16:e10085582020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai M, Hu Z, Liu J, Gao J, Liu C, Liu D,
Tan M, Zhang D and Lin B: Beclin 1 expression in ovarian tissues
and its effects on ovarian cancer prognosis. Int J Mol Sci.
15:5292–5303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Chen S, Gou WF, Xiao LJ, Takano Y
and Zheng HC: Aberrant Beclin 1 expression is closely linked to
carcinogenesis, differentiation, progression, and prognosis of
ovarian epithelial carcinoma. Tumour Biol. 35:1955–1964. 2014.
View Article : Google Scholar
|
|
16
|
Valente G, Morani F, Nicotra G, Fusco N,
Peracchio C, Titone R, Alabiso O, Arisio R, Katsaros D, Benedetto C
and Isidoro C: Expression and clinical significance of the
autophagy proteins BECLIN 1 and LC3 in ovarian cancer. Biomed Res
Int. 2014:4626582014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spowart JE, Townsend KN, Huwait H, Eshragh
S, West NR, Ries JN, Kalloger S, Anglesio M, Gorski SM, Watson PH,
et al: The autophagy protein LC3A correlates with hypoxia and is a
prognostic marker of patient survival in clear cell ovarian cancer.
J Pathol. 228:437–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferraresi A, Phadngam S, Morani F, Galetto
A, Alabiso O, Chiorino G and Isidoro C: Resveratrol inhibits
IL-6-induced ovarian cancer cell migration through epigenetic
up-regulation of autophagy. Mol Carcinog. 56:1164–1181. 2017.
View Article : Google Scholar
|
|
19
|
Mao W, Peters HL, Sutton MN, Orozco AF,
Pang L, Yang H, Lu Z and Bast RC Jr: The role of vascular
endothelial growth factor, interleukin 8, and insulinlike growth
factor in sustaining autophagic DIRAS3-induced dormant ovarian
cancer xenografts. Cancer. 125:1267–1280. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Xu RC, Zhang XL, Niu XL, Qu Y, Li
LZ and Meng XY: Interleukin-8 secretion by ovarian cancer cells
increases anchorage-independent growth, proliferation, angiogenic
potential, adhesion and invasion. Cytokine. 59:145–155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thongchot S, Ferraresi A, Vidoni C,
Loilome W, Yongvanit P, Namwat N and Isidoro C: Resveratrol
interrupts the pro-invasive communication between cancer associated
fibroblasts and cholangiocarcinoma cells. Cancer Lett. 430:160–171.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heger JI, Froehlich K, Pastuschek J and
Schmidt A, Baer C, Mrowka R, Backsch C, Schleußner E, Markert UR
and Schmidt A: Human serum alters cell culture behavior and
improves spheroid formation in comparison to fetal bovine serum.
Exp Cell Res. 365:57–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Sun C, Sun J, Yang MH, Zuo R, Liu C,
Lan WR, Liu MH, Huang B and Zhou Y: Autophagy mediates serum
starvation- induced quiescence in nucleus pulposus stem cells by
the regulation of P27. Stem Cell Res Ther. 10:1182019. View Article : Google Scholar
|
|
24
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abiko K, Matsumura N, Hamanishi J,
Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I
and Mandai M: IFN-γ from lymphocytes induces PD-L1 expression and
promotes progression of ovarian cancer. Br J Cancer. 112:1501–1509.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu QX, Xie F, Huang Q and Zhang XG:
Membranous and cytoplasmic expression of PD-L1 in ovarian cancer
cells. Cell Physiol Biochem. 43:1893–1906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furukawa S, Soeda S, Kiko Y, Suzuki O,
Hashimoto Y, Watanabe T, Nishiyama H, Tasaki K, Hojo H, Abe M and
Fujimori K: MCP-1 promotes invasion and adhesion of human ovarian
cancer cells. Anticancer Res. 33:4785–4790. 2013.PubMed/NCBI
|
|
28
|
Rådestad E, Klynning C, Stikvoort A,
Mogensen O, Nava S, Magalhaes I and Uhlin M: Immune profiling and
identification of prognostic immune-related risk factors in human
ovarian cancer. Oncoimmunology. 8:e15357302019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lai D, Ma L and Wang F: Fibroblast
activation protein regulates tumor-associated fibroblasts and
epithelial ovarian cancer cells. Int J Oncol. 41:541–550. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stimpfl M, Schmid BC, Schiebel I, Tong D,
Leodolter S, Obermair A and Zeillinger R: Expression of mucins and
cytokeratins in ovarian cancer cell lines. Cancer Lett.
145:133–141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu HH, Wang PH, Yeh JY, Chen YJ, Yen MS,
Huang RL, Tsai YJ and Yuan CC: Serum cytokeratin-19 fragment (Cyfra
21-1) is a prognostic indicator for epithelial ovarian cancer.
Taiwan J Obstet Gynecol. 53:30–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu Q, Qu H, Lou T, Liu C and Zhang Z: CK19
promotes ovarian cancer development by impacting on Wnt/β-catenin
pathway. Onco Targets Ther. 13:2421–2431. 2020. View Article : Google Scholar :
|
|
33
|
Kwa MQ, Herum KM and Brakebusch C:
Cancer-associated fibroblasts: How do they contribute to
metastasis? Clin Exp Metastasis. 36:71–86. 2019.PubMed/NCBI
|
|
34
|
Lu H, Han M, Yuan X, Tursun K, Zhang Y, Li
Y, Li Z, Feng S, Zhou L, Pan Z, et al: Role of IL-6-mediated
expression of NS5ATP9 in autophagy of liver cancer cells. J Cell
Physiol. 233:9312–9319. 2018. View Article : Google Scholar
|
|
35
|
Qin B, Zhou Z, He J, Yan C and Ding S:
IL-6 inhibits starvation-induced autophagy via the STAT3/Bcl-2
signaling pathway. Sci Rep. 5:157012015. View Article : Google Scholar : PubMed/NCBI
|