Introduction
Osteosarcoma (OS), one of the most common primary
bone malignant tumors, usually occurs in children and adolescents
aged 10-20 years; it is associated with a high degree of morbidity,
and patients are prone to recurrence and metastasis, leading to a
poor prognosis (1-3). Osteosarcoma tends to occur in areas
where bone growth and bone turnover are more active, such as a
typical long-axis medullary bone tumor in growing adolescents.
Despite the innovative development of neoadjuvant chemotherapy and
surgery, which have the tremendous ability to shrink tumors and
eliminate small lesions to ensure complete surgical resection and
reduce tumor recurrence and metastasis, the 5-year survival rate of
patients is less than 70% (4,5).
This outcome may be related to various issues, such as complex
pathogenesis, tumor heterogeneity, lack of novel adjuvant drugs,
and an imperfect evaluation system. In addition, multidrug
resistance caused by cross-resistance of chemotherapeutic drugs is
a widely recognized problem (6).
Previous findings have shown that alterations in the combination of
chemotherapeutic drugs and the methods of administration do not
improve 5-year survival, even when the doses are increased
(7,8). The major limitations of current
clinical treatment regimens for osteosarcoma are recurrence and
primary or secondary chemical resistance (9). Therefore, since surgical strategies
have been developed, drug discovery is the key to improving
survival rates.
In a previous study, a cell-based and
phenotype-based high-throughput screening of approximately 2400
bioactive or clinically used compounds from the FDA-approved drug
library (Selleck Chem) was conducted and it was found that,
cetrimonium bromide (CTAB) has a tremendous inhibitory effect on
osteosarcoma. CTAB, a quaternary ammonium compound, is used as a
topical antiseptic and may play a variety of roles in cancer
treatment, exhibiting the ability to penetrate the hydrophobic
barriers of the plasma and mitochondrial membranes and accumulate
in mitochondria under a negative transmembrane potential, which
leads to mitochondrial toxicity (10,11). Furthermore, the cation part of
CTAB is the cause of bacterial cell wall damage, leading to the
leakage of essential cell components. Notably, no adverse effects
are observed in humans when CTAB is used as surgical lavage fluid,
which also lays the foundation for our exploration of its effect on
osteosarcoma (12,13).
In summary, due to the urgency of exploring new
drugs for osteosarcoma and the results of aforementioned studies,
in the present study the effects of CTAB on the proliferation,
apoptosis, invasion and metastasis of osteosarcoma and its
underlying mechanisms were examined for the first time.
Materials and methods
Cells and animals
The human osteosarcoma cells (HOS, MG63 and U2OS)
and human osteoblast line (hFOB1.19) used in this study were
obtained from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). All of the cell
lines were authenticated by Short Tandem Repeat (STR) profiling.
The simian virus 40-transfected hFOB1.19 cells were applied to
detect whether CTAB is toxic to normal human osteoblasts. The cells
were cultured in high-glucose Dulbecco's modified Eagle's medium
(DMEM; HyClone) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.), and 1%
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA), and incubated
at 37°C with 5% CO2. BALB/c nude mice (male, 4-5 weeks,
20-22 g) were obtained from the Shanghai SLAC Laboratory Animal Co
and housed in a standard animal environment (22-26 °C, 40-70%
humidity) with free access to water and food. All animal
experiments were approved by the Animal Research Committee of the
First Affiliated Hospital of Chinese Medical University.
Antibodies and reagents
CTAB was purchased from Sigma-Aldrich; Merck KGaA.
PI3K agonist 740 Y-P were purchased from MedChemExpress (MCE).
Antibodies against caspase-3 (1:1,000 dilution; cat. no. 9662),
cleaved caspase-3 (1:1,000 dilution; cat. no. 9661), caspase-8
(1:1,000 dilution; cat. no. 9746), cleaved caspase-8 (1:1,000
dilution; cat. no. 9496), caspase-9 (1:1,000 dilution; cat. no.
9502), cleaved caspase-9 (1:1,000 dilution; cat. no. 20750), PARP
(1:1,000 dilution; cat. no. 9532), cleaved PARP (1:1,000 dilution;
cat. no. 32563), AKT (1:1,000 dilution; cat. no. 9272) and p-AKT
(1:1,000 dilution; cat. no. 9271) were purchased from Cell
Signaling Technology. Antibodies against PI3K (1:1,000 dilution;
cat. no. 180967), p-PI3K (1:1,000 dilution; cat. no. 278545), Bcl-2
(1:1,000 dilution; cat. no. 32124), Bax (1:1,000 dilution; cat. no.
32503), cytochrome c (1:5,000 dilution; cat. no. 133504),
and β-actin (1:1,000 dilution; cat. no. 8226) and secondary
antibodies (1:5,000 dilution; cat. no. 96899 and 96879) were
obtained from Abcam. Phosphate-buffered saline (PBS) was obtained
from Gibco; Thermo Fisher Scientific, Inc. Radioimmunoprecipitation
assay (RIPA) lysis buffer was purchased from Santa Cruz
Biotechnology.
Cell proliferation assay
OS cells and osteoblasts were cultured
(1×104 cells/well) in 96-well plates (Thermo Fisher
Scientific, Inc.) and treated with CTAB at different concentrations
(0, 1, 2, 3, 4, 5, 6, 7, and 8 µM) for 24, 48 and 72 h in
vitro. After the specified incubation time, 10 µl of
CCK-8 (Dojindo Molecular Technologies, Inc.) was added to the
plate, the cells were incubated at 37°C for 1-4 h, and the
absorbance was measured at 450 nm using an ELISA microplate reader
(Bio-Rad). Cells without CTAB treatment were used as negative
controls.
Cell cycle analysis
After incubation with a concentration gradient of
CTAB at 37°C for 48 h, the cells were collected, washed twice with
PBS, and then fixed overnight with 70% cold ethanol at 4°C. After
the cells had been washed with PBS again, they were resuspended in
0.5 ml stain buffer containing 100 µg/ml RNase A and 50
µg/ml PI (Beyotime Biotech) in the dark at room temperature
for 30 min and analyzed by a flow cytometer (Becton-Dickinson).
Moreover, CytExpert (version 2.3) was used for subsequent data
analysis.
Cell metastasis assay
OS cell migration and invasion were detected by the
wound-healing and Transwell assays, respectively. The cells were
seeded in 6-well plates at a density of 5×105
cells/well. After the cell monolayer was formed, a micropipette tip
was used to create a wound. Then, the cells were washed with PBS,
and the medium was replaced with serum-free high-glucose DMEM
containing different concentrations (0, 1, 2 and 4 µM) of
CTAB. Cells migrating to the wound area were photographed with an
inverted microscope at 0, 6, 18 and 24 h (the average wound size
represented the relative migration of cells). A 24-well Transwell
chamber (Corning Costar) coated with matrix gel (Sigma-Aldrich;
Merck KGaA) was used for cell invasion analysis. HOS and MG63 cells
(5×104 cells/well) were inoculated with medium
containing different concentrations (0 and 4 uM) of CTAB (without
FBS) in the upper part of the Transwell chamber, and the lower
chamber was filled with complete medium containing 10% FBS. After
24 h of treatment, the cells in the upper chamber were removed, and
the remaining cells that had invaded through the Matrigel matrix
were fixed with 4% paraformaldehyde, stained with 0.1% crystal
violet, and counted under an inverted fluorescence microscope
(Nikon Eclipse Ti-S).
Apoptosis analysis by flow cytometry
The effect of CTAB on the apoptosis of OS cells was
analyzed by using a membrane protein V-FITC apoptosis detection kit
(BD Biosciences). HOS and MG63 cells were inoculated in 6-well
plates for 24 h and then treated with CTAB at a concentration of 0,
2, 4 or 6 µM for 48 h. The cells were washed twice with
precooled PBS, resuspended in 100 µl 1X buffer and then
incubated with FITC-labeled Annexin V as well as PI for 15 min at
room temperature in the dark. Finally, 400 µl 1X binding
buffer was added to the reaction system, and cell apoptosis was
assessed by flow cytometry (Becton-Dickinson) and CytExpert
(version 2.3).
Western blot analysis
After treatment of HOS and MG63 cells with different
doses of CTAB (0, 2, 4 and 6 µM) for 48 h, the cells were
washed twice with PBS and lysed with RIPA buffer comprising
protease/phosphatase inhibitors to extract total protein.
Mitochondrial and cytosolic proteins were isolated according to the
protocol provided by the mitochondrial extract kit (Thermo Fisher
Scientific Inc.). A BCA protein assay kit (Beyotime) was used to
determine the protein concentration. Then, 10 and 12% SDS-PAGE gels
were used to separate the proteins (30 µg per lane), which
were then transferred onto polyvinylidene fluoride (PVDF)
membranes. The membranes were blocked with 5% bovine serum albumin
solution at room temperature for 2 h and incubated with all the
primary antibodies overnight at 4°C. Then, the membranes were
incubated with secondary antibody for 2 h at room temperature.
Subsequently, the results were visualized with an enhanced
chemiluminescence (ECL) system (UVP Inc.) and a Protein Blotting
Detection and Imaging Scanner (Bio-Rad). ImageJ (NIH) software was
used for quantitative analysis.
Mitochondrial membrane potential (MMP)
assay
The change in the mitochondrial membrane potential
(MMP) of osteosarcoma cells after intervention with CTAB was
assessed by the JC-1 Analysis Kit (Beyotime). HOS and MG63 cells
were seeded in 6-well plates (5×105/well) overnight and
then treated with different doses of CTAB for 24 h. The next day,
the supernatant was removed, and the cells were washed with PBS and
1X JC-1 buffer. Then, the cells were resuspended in 500 µl
JC-1 staining solution in an incubator for 20 min and analyzed by
flow cytometry (Beckham).
Tumor xenografts and histopathology
HOS cells (2×106) suspended in 100
µl PBS were subcutaneously inoculated into the dorsal area
of 4-week-old male nude mice. Three days after inoculation, the
mice were randomly divided into 3 groups (n=4). Then, CTAB (10 or
20 mg/kg/d) was injected intraperitoneally every 3 days into each
mouse for a total of 20 days. The control group was treated with
the same volume of physiological saline. During the administration
period, the body weights and tumor sizes of the mice were monitored
every 3 days. The mice were sacrificed by cervical dislocation
after 20 days of CTAB treatment, and the tumors and major organs,
including the liver, lung, kidney, spleen and heart, were collected
from each group of mice and immersed in 4% formalin for
immunohistochemical staining and hematoxylin and eosin (H&E)
staining. The following formula was used to calculate the tumor
volume: Volume=1/2 (length x width2). The tissue samples
were fixed with 10% formalin (for 24 h at room temperature),
embedded in paraffin, and cut into 4 µm sections. The
sections were deparaffinized with xylene and then dehydrated with
gradient ethanol solutions. Then, the slides were stained with
hematoxylin for 10 min and eosin for 5 min. Tissue damage was
observed under a microscope (Nikon) after the slides were sealed
with neutral resin.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). GraphPad Prism 7.0 software was used for statistical
analysis, and Student's t-test or one-way analysis of variance
(ANOVA) followed with Tukey post-hoc test was used to analyze
differences between groups. P<0.05 was considered statistically
significant. All the cell experiments were performed in
triplicate.
Results
CTAB inhibited the proliferation of and
induced cell cycle arrest in osteosarcoma cells
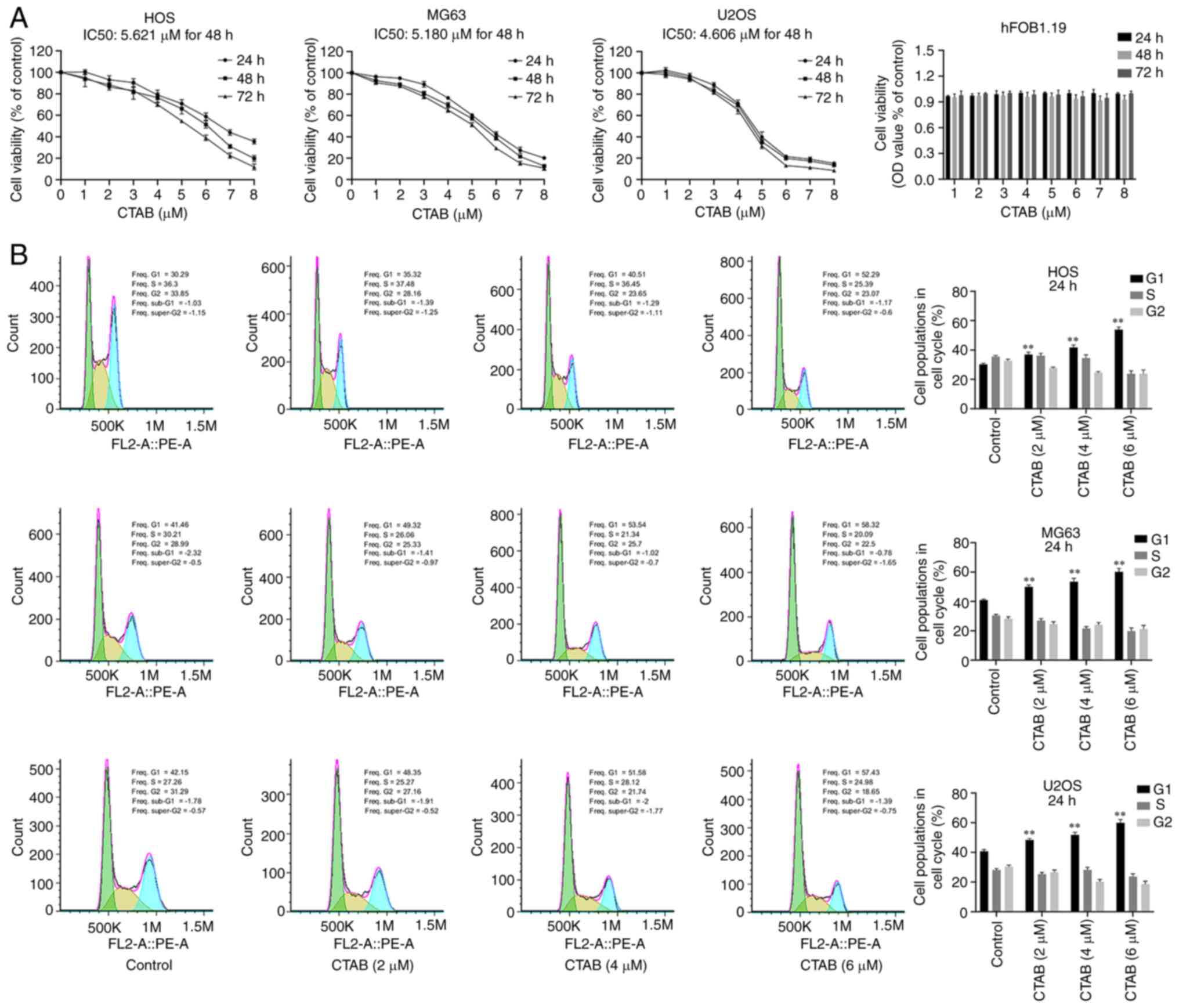
To investigate the effect of CTAB on the
proliferation of OS cells, we treated HOS, MG63 and U2OS cells with
different concentrations of CTAB in vitro for 24, 48 or 72
h. The results showed that CTAB significantly inhibited the
activity of osteosarcoma cells in a time- and
concentration-dependent manner (Fig.
1A), and the IC50 values of HOS, MG63, and U2OS cells were
4.949, 3.500, and 4.212 µM, respectively (Fig. 1A). Notably, CTAB treatment did not
affect the viability of hFOB1.19 cells (Fig. 1A), indicating that CTAB at a
concentration of 1-8 µM had no significant toxicity compared
to normal human osteoblasts. Based on the above results, 2, 4 and 6
µM were selected as the effective drug concentrations for
subsequent analysis. To determine whether CTAB inhibits cell
proliferation by inducing cell cycle arrest, we evaluated the cell
cycle distribution of osteosarcoma cells using flow cytometry. The
proportion of G1 phase cells increased from 30.29 to 53.34%, 40.23
to 61.55% and 39.95 to 60.58% in HOS, MG63 and U2OS cells treated
with CTAB (2, 4 or 6 µM), respectively (Fig. 1B). These data suggest that CTAB
has an inhibitory effect on OS cells and induces G1 phase
arrest.
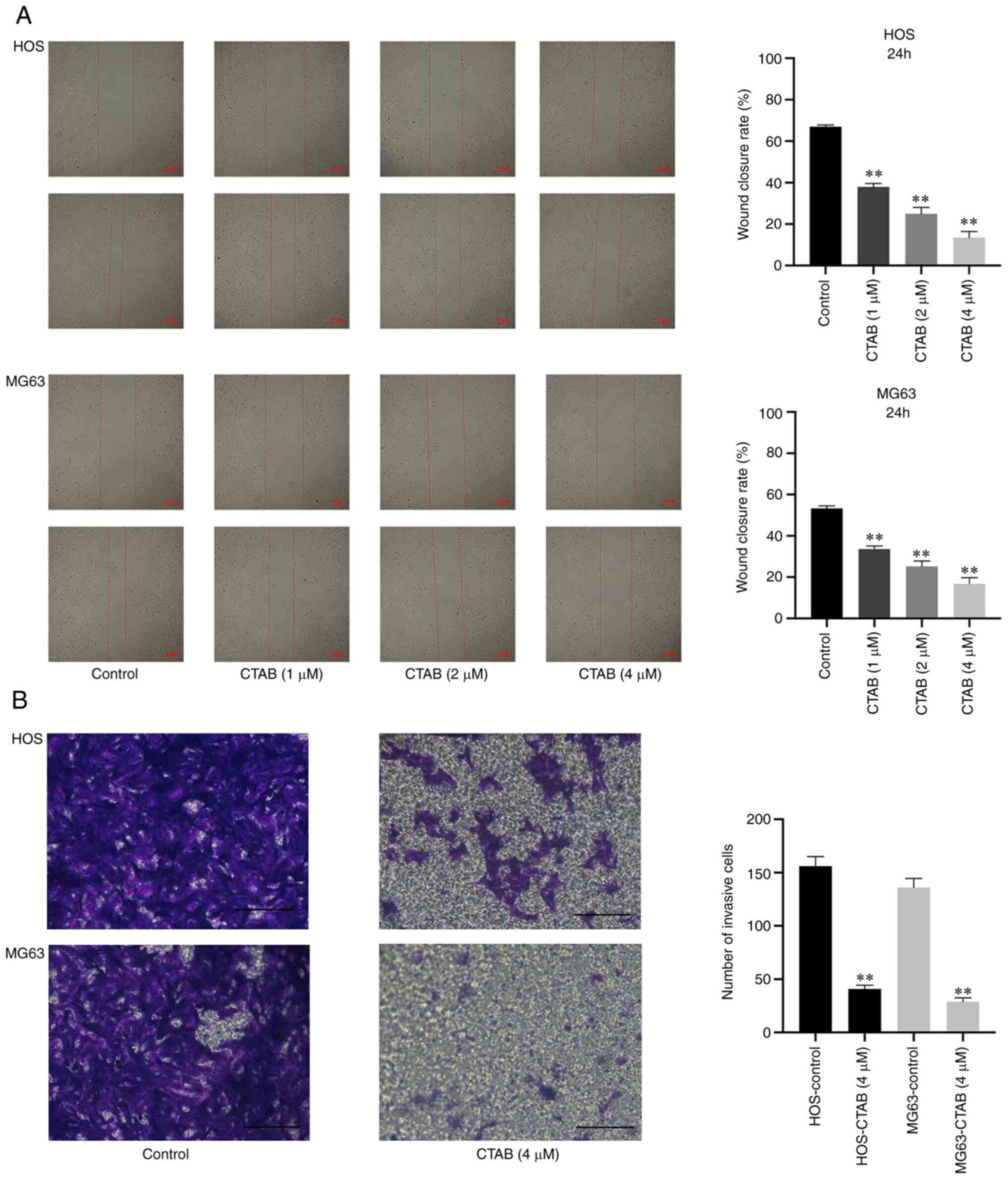
CTAB inhibits the migration and invasion
of osteosarcoma cells
Tumor metastasis is the most common factor affecting
the survival of patients. Therefore, inhibiting tumor migration and
invasion may be an effective strategy to prevent tumor metastasis.
In this study, the wound-healing assay showed that CTAB treatment
significantly reduced the wound closure rate of HOS and MG63 cells
in a dose-dependent manner (Fig.
2A). The invasion of tumor cells was analyzed by the Transwell
assay, in which Matrigel matrix effectively simulates the invasion
microenvironment of tumor cells. The digestion and penetration of
Matrigel matrix by tumor cells is a good reflection of invasion
ability. Compared with the control, CTAB significantly reduced the
number of invasive HOS and MG63 cells (Fig. 2B).
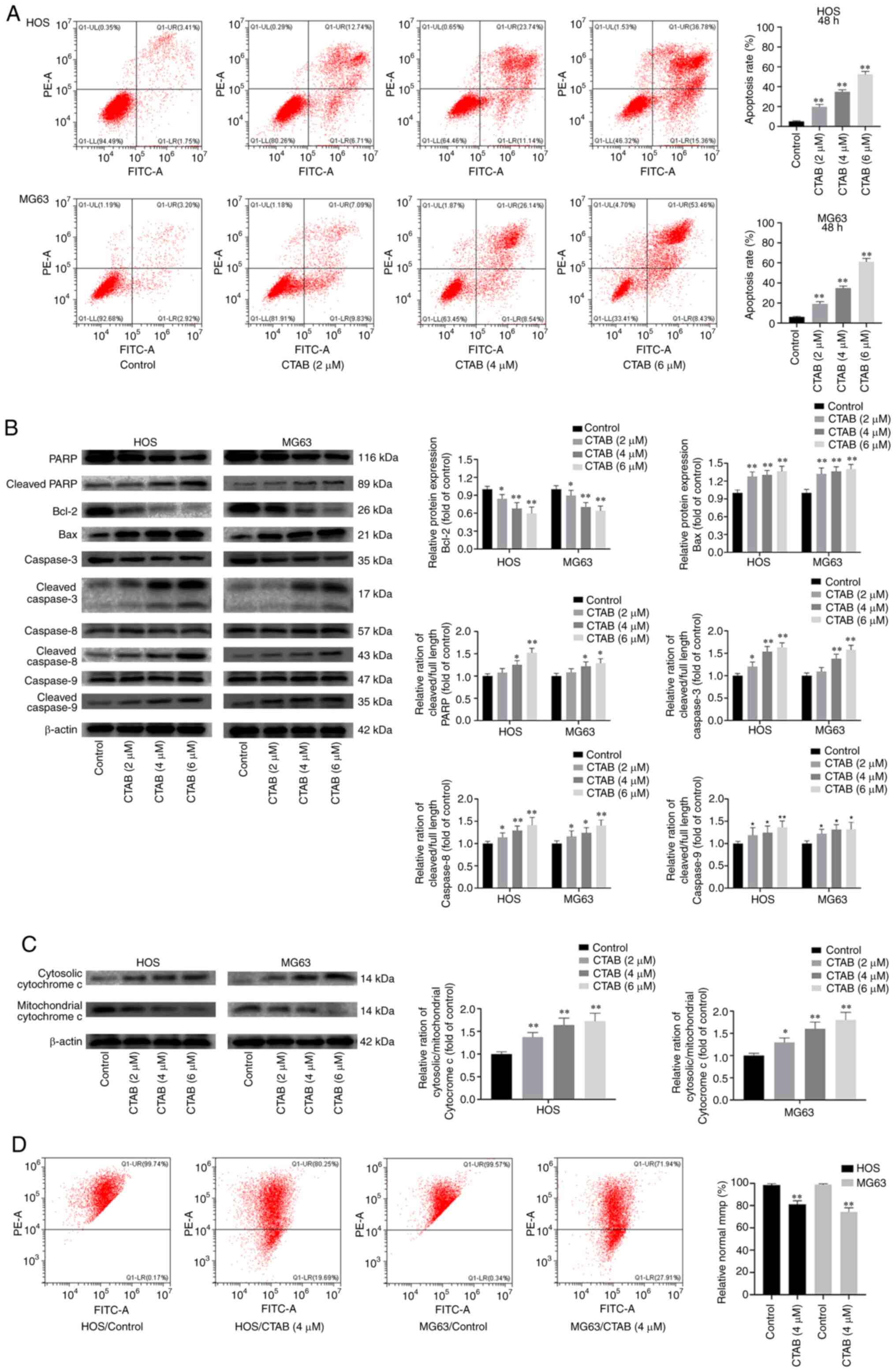
CTAB induced apoptosis of osteosarcoma
cells
The inhibitory effect of CTAB on the growth of
osteosarcoma cells resulting from apoptosis was verified by flow
cytometry. The percentage of apoptotic OS cells increased
significantly after treatment with different concentrations of CTAB
compared with control treatment for 48 h (Fig. 3A). The expression level of
downstream apoptotic proteins was further measured by western blot
analysis, and as shown in Fig. 3B and
C, CTAB-treated OS cells exhibited downregulated expression of
the antiapoptotic protein PARP and Bcl-2 but upregulated expression
of cytosolic cytochrome c (cyto-c) and the proapoptotic
protein Bax. In addition, CTAB significantly promoted the cleavage
of PARP, caspase-3, caspase-8 and caspase-9. These results suggest
that CTAB triggers apoptosis in OS cell lines. Disturbance of the
mitochondrial membrane potential (MMP, ΔΨm) is an early sign of
apoptosis that affects the permeability of mitochondrial membranes
(14). An increased mitochondrial
membrane permeability leads to the release of mitochondrial
apoptotic factors, such as cyto-c, which is transferred from the
mitochondria to the cytoplasm and activates a series of apoptotic
enzymes (15). The results of
flow cytometry (Fig. 3D) showed
that after 24 h of treatment with 4 µM CTAB, the percentage
of HOS and MG63 cells with a normal MMP was significantly reduced.
These results indicate that CTAB may promote OS apoptosis by
interfering with the MMP and disrupting mitochondrial membrane
permeability.
CTAB promoted osteosarcoma apoptosis by
inhibiting the PI3K-AKT pathway
The PI3K/AKT signaling pathway is the main
intracellular signaling pathway that regulates the proliferation,
apoptosis and migration of tumor cells (16). Previous findings have reported
that excessive activation of the PI3K/AKT pathway is closely
related to the negative regulation of tumor cell apoptosis
(17). Therefore, we further
analyzed whether CTAB-induced OS cell apoptosis depends on the
PI3K/AKT signaling pathway. As shown in Fig. 4A, the expression levels of p-PI3k
and p-AKT in HOS and MG63 cells treated with different
concentrations of CTAB decreased significantly. We further
investigated whether the antitumor effect of CTAB is mediated
through inhibition of the PI3K/AKT signaling pathway, and we found
that 740Y-P (a PI3K agonist) partially reversed the inhibitory
effect of CTAB on OS cells (Fig.
4B). Specifically, 740Y-P reversed CTAB-induced alterations in
the abundance of p-PI3K, p-Akt, Bax and cleaved caspase-9 in HOS
and MG63 cells (Fig. 4B). In
addition, the flow cytometry showed that 740Y-P decreased the
apoptosis rate of OS cells (Fig.
4C). After treatment with CTAB (4 µM), the apoptosis
rates of HOS and MG63 cells were 41.9 and 50.2%, respectively, but
the combination of CTAB and 740Y-P reduced the apoptosis rate to
18.7 and 21.5%, respectively. These results confirmed that CTAB
induces apoptosis by inhibiting the PI3K/AKT signaling pathway.
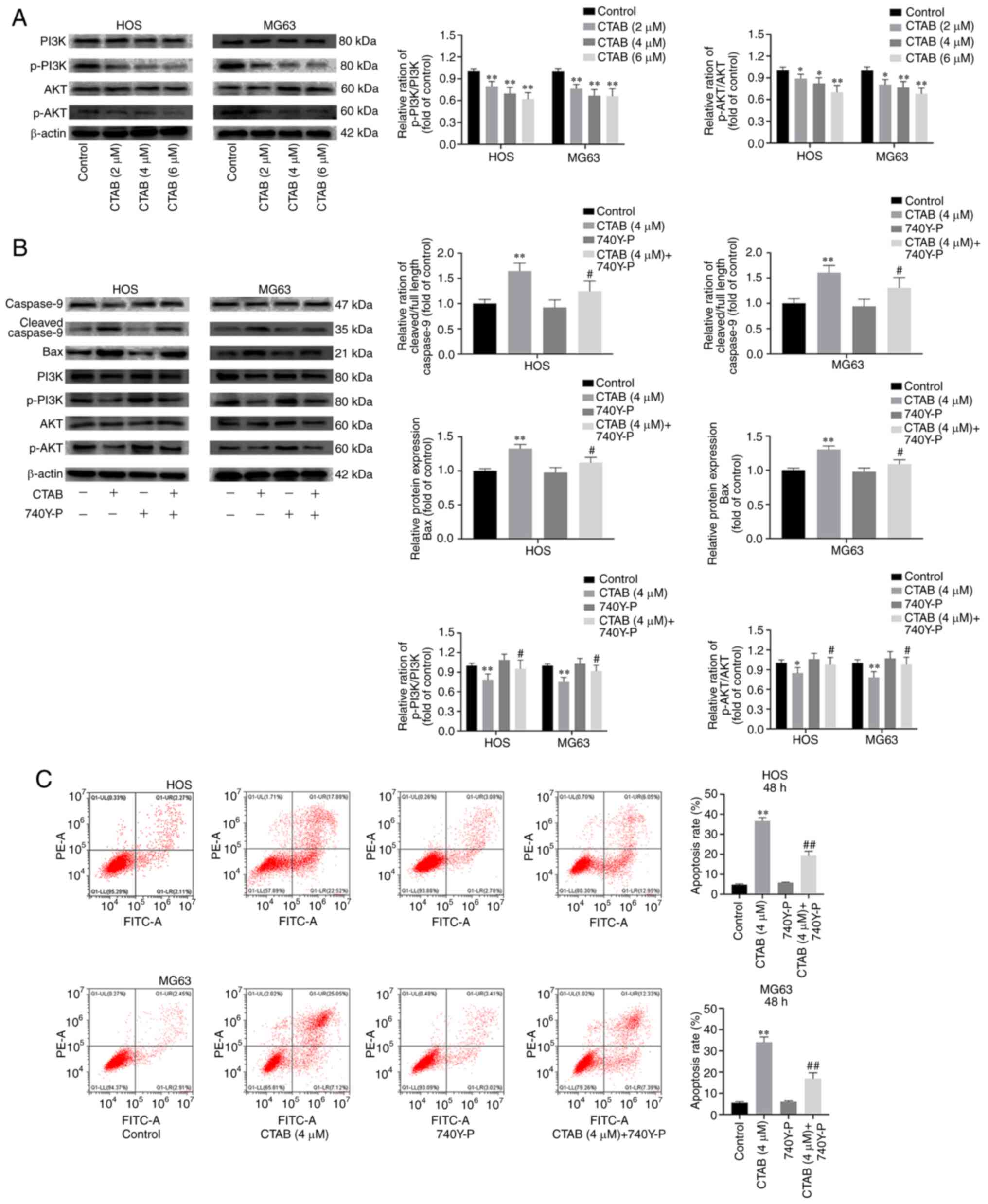 | Figure 4CTAB promotes osteosarcoma cell
apoptosis by inhibiting the PI3K-AKT signaling pathway. (A) The
expression of PI3K, p-PI3K, AKT and p-AKT in HOS and MG63 cells
treated with different concentrations of CTAB for 48 h was analyzed
by western blot analysis. (B) The expression of caspase-9, cleaved
caspase-9, Bax, PI3K, p-PI3K, AKT and p-AKT in HOS and MG63 cells
treated with CTAB (4 µM) or CTAB (4 µM) + 740Y-P (20
µM) for 48 h was measured by western blot analysis. (C)
Apoptosis of HOS and MG63 cells was evaluated by flow cytometry
(n=3, *P<0.05 and **P<0.01, CTAB
compared with the control; #P<0.05 and ##P<0.01,
CTAB compared with CTAB + 740Y-P). |
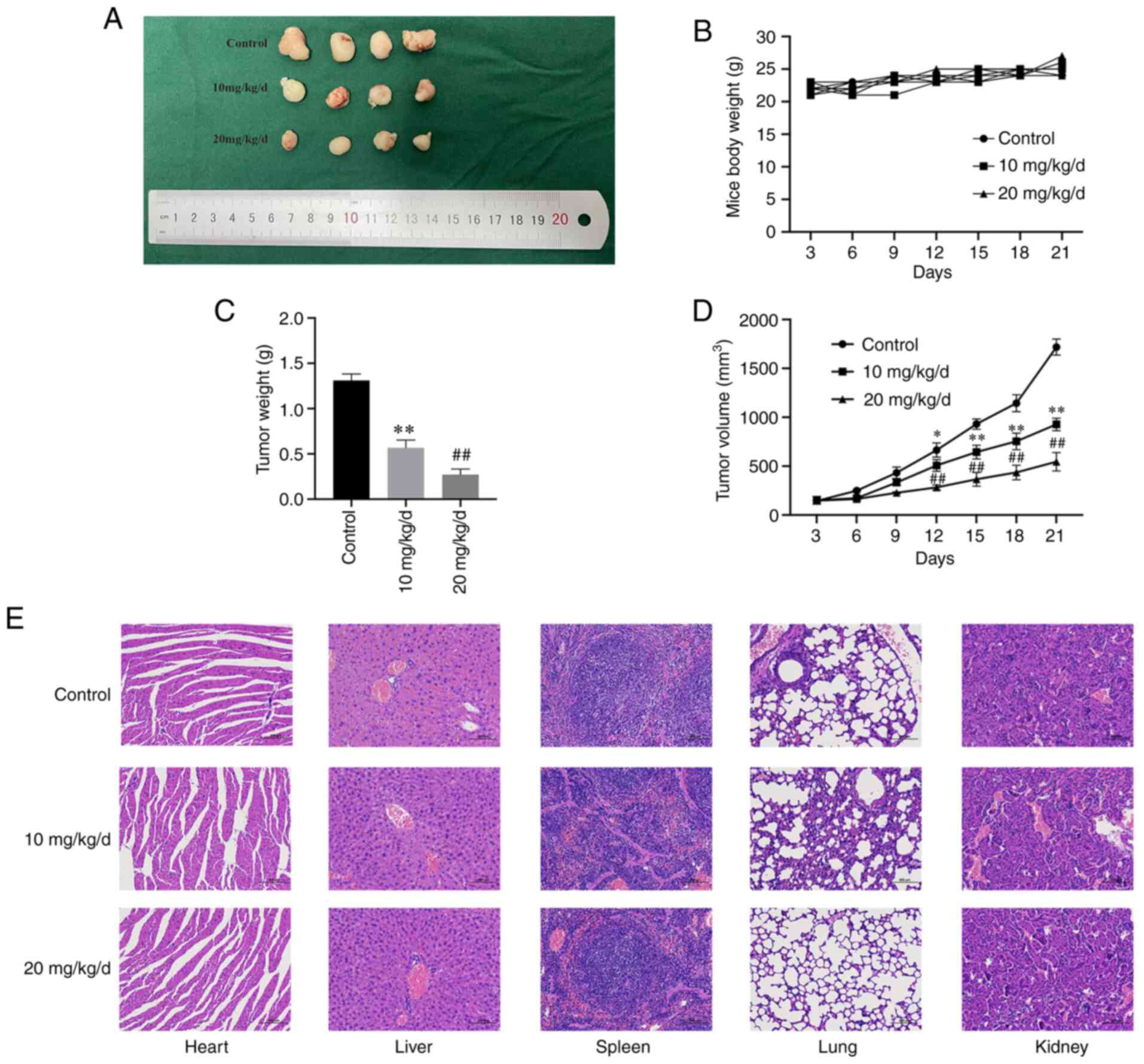
CTAB prevented the growth of OS cells in
vivo
Since CTAB can inhibit OS cell proliferation and
metastasis and induce apoptosis in vitro, we examined
whether CTAB can inhibit the growth of OS (HOS) in xenograft
tumors. As shown in Fig. 5A-D,
CTAB treatment resulted in a significant reduction in tumor volume
and weight in a dose-dependent manner. The average tumor volume of
the control group was 1718±97 mm3, while that of the 10
mg/kg/d CTAB-treated group was 987±107 mm3 and that of
the 20 mg/kg/d CTAB-treated group was 545±105 mm3. In
addition, the mean tumor weight of the control group was 1380±87
mg, while that of the 10 mg/kg/d CTAB-treated group was 566±101 mg
and that of the 20 mg/kg/d-treated group was 270±75 mg. In
addition, there was no significant organ toxicity in the CTAB
group, indicating that it safely exerts antitumor effects in
vivo (Fig. 5E).
Discussion
With the rapid development of medical science and
technology, the rate of limb salvage in osteosarcoma is greater
than 80% in the clinic, and limb salvage has gradually replaced
amputation in the majority of cases (18). Currently, the widely accepted
strategy for osteosarcoma treatment is surgery combined with
neoadjuvant chemotherapy (19,20). Different chemotherapy regimens
include the use of two to seven drugs, of which the four classic
drugs that show consistent effects are cisplatin, doxorubicin, and
high-dose methotrexate with leucovorin and ifosfamide with or
without etoposide (21). With the
development and application of neoadjuvant chemotherapies, the
5-year survival rate of patients with osteosarcoma has increased
from 20% to more than 60%. Moreover, another great value of
neoadjuvant chemotherapy is that it provides time for patients to
undergo artificial replacement without amputation. Therefore,
preoperative chemotherapy-surgery-postoperative chemotherapy is the
current standard treatment for osteosarcoma. However, attempts to
target specific cell receptors and intracellular signaling
molecules have not further improved survival rates due to the
extreme genetic polymorphism of osteosarcoma cells. It is not
difficult to determine that the key to improving the survival rate
is drug therapy, which is also a challenging and controversial
issue for orthopedic and oncology experts globally. We identified
CTAB through high-throughput drug screening. CTAB is a known
component of ctrimetide, which has been clinically used at
clinically well-tolerated concentrations as a bactericidal adjuvant
or tumor suppressor for hydatid cysts and during colorectal surgery
(12,13). However, no studies have explored
the role of CTAB in osteosarcoma and its biochemical mechanisms.
Our data suggest that CTAB significantly inhibits the proliferation
of osteosarcoma cells, leading to G1 arrest, suppresses metastasis
and induces apoptosis.
One of the clinical features of osteosarcoma is the
tendency to form metastatic lesions, and the degradation of
extracellular matrix is an essential step in cancer invasion and
metastasis (22,23). Previous findings have showed that
CTAB can selectively inhibit the proliferation of prostate cancer
cells and markedly decrease the invasion of DU-145 cells in the
collagen matrix (24). In
addition, CTAB has been used as an effective cytotoxic lavage
solution in breast cancer surgery (25). CTAB may also suppress the invasion
and metastasis of hepatocellular carcinoma cells by inhibiting TGF
signal transduction (26). As
bone metastasis is closely related to poor prognosis, controlling
the invasion and metastasis of osteosarcoma cells is one of the
difficulties in the treatment of osteosarcoma (22,27). Osteosarcoma cells have the ability
to migrate when cultured in vitro. The principle of the
scratch test is the assessment of cell wound healing to detect the
migration characteristics of cultured osteosarcoma cells. The
Transwell experiment was performed to assess the invasion of
osteosarcoma cells. We found that CTAB significantly inhibited the
invasion and migration of HOS cells even at a low concentration.
The ability of osteosarcoma cells to digest and penetrate Matrigel
matrix after treatment with different concentrations of CTAB
decreased significantly, and this was dependent on the
concentration of drug.
Apoptosis is an energy-dependent, genetically
programmed cell death mechanism that can be induced by either
external (death receptors) or internal (mitochondria) pathways and
is the main method for the elimination of tumors (28,29). Various cancer therapies, such as
chemotherapy, radiotherapy, immunotherapy and gene therapy, target
the activation of apoptotic signal transduction pathways (30). Ito et al identified CTAB as
a potential therapeutic agent for head and neck cancer due to its
cytotoxic effects on related cell lines through
mitochondria-mediated apoptosis pathways (31). In this study, CTAB intervention
resulted in an increase in cleaved caspase-3, cleaved caspase-8 and
cleaved caspase-9 expression in a dose-dependent manner, confirming
that caspase-dependent apoptosis is involved in the cytotoxic
effect of CTAB on osteosarcoma cells. The MMP was significantly
reduced after CTAB treatment, indicating that CTAB can induce
mitochondrial depolarization in OS cells. It is known that a
reduction in the Δψm triggers the release of cytochrome c
from mitochondria into the cytoplasm, thereby initiating
mitochondrial apoptosis signaling, and that the antiapoptotic Bcl-2
protein family participates in preventing the subsequent process
(32). CTAB can increase the
expression of proapoptotic Bax and downregulate the expression of
antiapoptotic Bcl-2, thereby increasing mitochondrial permeability.
The PI3K/AKT pathway is a widely studied intracellular signaling
pathway that plays an indispensable role in all malignant
phenotypes (such as tumorigenesis, cancer cell proliferation,
survival, migration, and chemoresistance) (33,34). According to reports, dysregulation
of the PI3K/AKT pathway also plays a vital role in the occurrence
and development of OS (35).
Based on these previous studies, we found that CTAB intervention
reduced the phosphorylation of PI3K/AKT in OS cells, indicating the
inhibitory effect of CTAB on the PI3K/AKT pathway. In addition,
rescue experiments revealed that activation of the PI3K/AKT pathway
by 740Y-P abolished the inhibitory effect of CTAB on OS cell
proliferation and apoptosis. These results indicated that CTAB
inhibits cell activity and induces OS cell apoptosis by inhibiting
the PI3K/AKT pathway. The key to exploring tumor cell radiotherapy
and chemotherapy and antitumor drug screening is the establishment
of tumor models. CTAB had no obvious toxic effects on and did not
induce side effects on BALB/C-NU/nu nude mice, and no abnormalities
in appetite, mental state or motor ability or obvious damage to the
liver, spleen, kidney or other organs were observed. The
aforementioned studies show that CTAB has a strong
anti-osteosarcoma effect in nude mice without inducing obvious
adverse drug reactions, which lays a good foundation for further
research and clinical application. It is noteworthy that the
antitumoral efficacy of CTAB has been studied for decades. Pan
et al (36) also found the
low toxicity of CTAB in vivo, which was consistent with the
present study. Therefore, the focus of future research is exploring
the drug delivery methods of CTAB in the treatment of osteosarcoma,
such as local lavage, nanoparticle delivery system and gel
sustained-release. Furthermore, future clinical studies need to be
performed to assess whether administration of CTAB to osteosarcoma
patients is beneficial.
In brief, osteosarcoma metastasis, recurrence and
multidrug resistance (MDR) are the three major obstacles in the
clinic. Since identification of anti-osteosarcoma drugs is
imperative and because our study revealed the strong inhibitory
effect of CTAB in osteosarcoma, we have sufficient reason to
believe that CTAB is a potential therapeutic agent for
osteosarcoma.
Availability of data and materials
The datasets supporting the conclusions of this
article are available from the corresponding authors upon
reasonable request.
Authors' contributions
WD, LT and YZ contributed to the study conception
and design. Material preparation, data collection and analysis were
performed by WD and LT. The first draft of the manuscript was
written by WD. YZ was responsible for monitoring the progress of
the entire study. YZ and LT confirmed the authenticity of the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Research Committee of the First Affiliated Hospital of Chinese
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests..
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Takeuchi A, Yamamoto N, Hayashi K,
Matsubara H, Miwa S, Igarashi K and Tsuchiya H: Joint-preservation
surgery for pediatric osteosarcoma of the knee joint. Cancer
Metastasis Rev. 38:709–722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lilienthal I and Herold N: Targeting
molecular mechanisms underlying treatment efficacy and resistance
in osteosarcoma: A review of current and future strategies. Int J
Mol Sci. 21:68852020. View Article : Google Scholar :
|
|
3
|
Hattinger CM, Patrizio MP, Magagnoli F,
Luppi S and Serra M: An update on emerging drugs in osteosarcoma:
Towards tailored therapies? Expert Opin Emerg Drugs. 24:153–171.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers. 5:591–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li B, Zhou P, Xu K, Chen T, Jiao J, Wei H,
Yang X, Xu W, Wan W and Xiao J: Metformin induces cell cycle
arrest, apoptosis and autophagy through ROS/JNK signaling pathway
in human osteosarcoma. Int J Biol Sci. 16:74–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrari S and Serra M: An update on
chemotherapy for osteosarcoma. Expert Opin Pharmacother.
16:2727–2736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whelan JS, Jinks RC, McTiernan A, Sydes
MR, Hook JM, Trani L, Uscinska B, Bramwell V, Lewis IJ, Nooij MA,
et al: Survival from high-grade localised extremity osteosarcoma:
Combined results and prognostic factors from three European
Osteosarcoma Intergroup randomised controlled trials. Ann Oncol.
23:1607–1616. 2012. View Article : Google Scholar :
|
|
8
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar
|
|
10
|
Chen LB: Mitochondrial membrane potential
in living cells. Annu Rev Cell Biol. 4:155–181. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan Y, Wang Z, Shao D, Zheng H, Chen Y,
Zheng X, Zhang M, Li J, Li F and Chen L: CTAB induced mitochondrial
apoptosis by activating the AMPK-p53 pathway in hepatocarcinoma
cells. Toxicol Res. 4:1359–1365. 2015. View Article : Google Scholar
|
|
12
|
Umpleby HC and Williamson RC: The efficacy
of agents employed to prevent anastomotic recurrence in colorectal
carcinoma. Ann R Coll Surg Engl. 66:192–194. 1984.PubMed/NCBI
|
|
13
|
Sonişik M, Korkmaz A, Besim H, Karayalçin
K and Hamamci O: Efficacy of cetrimide-chlorhexidine combination in
surgery for hydatid cyst. Br J Surg. 85:12771998. View Article : Google Scholar
|
|
14
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kagan VE, Tyurin VA, Jiang J, Tyurina YY,
Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V,
et al: Cytochrome c acts as a cardiolipin oxygenase required for
release of proapoptotic factors. Nat Chem Biol. 1:223–232. 2005.
View Article : Google Scholar
|
|
16
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delaloge S and DeForceville L: Targeting
PI3K/AKT pathway in triple-negative breast cancer. Lancet Oncol.
18:1293–1294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang F, Shi Y, Li GJ and Zhou F: A
meta-analysis of limb-salvage versus amputation in the treatment of
patients with Enneking‡U pathologic fracture osteosarcoma. Indian J
Cancer. 51(Suppl 2): e21–e24. 2015.
|
|
19
|
Yang C, Tian Y, Zhao F, Chen Z, Su P, Li Y
and Qian A: Bone microenvironment and osteosarcoma metastasis. Int
J Mol Sci. 21:69852020. View Article : Google Scholar :
|
|
20
|
Chindamo G, Sapino S, Peira E, Chirio D,
Gonzalez MC and Gallarate M: Bone diseases: Current approach and
future perspectives in drug delivery systems for bone targeted
therapeutics. Nanomaterials (Basel). 10:8752020. View Article : Google Scholar
|
|
21
|
Vos HI, Coenen MJ, Guchelaar HJ and Te Loo
DM: The role of pharmacogenetics in the treatment of osteosarcoma.
Drug Discov Today. 21:1775–1786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu Y, Yu W, Cai H and Lu A: Forecast of
actin-binding proteins as the oncotarget in osteosarcoma-a review
of mechanism, diagnosis and therapy. Onco Targets Ther.
11:1553–1561. 2018. View Article : Google Scholar :
|
|
23
|
Cui J, Dean D, Hornicek FJ, Chen Z and
Duan Z: The role of extracelluar matrix in osteosarcoma progression
and metastasis. J Exp Clin Cancer Res. 39:1782020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wissing MD, Mendonca J, Kim E, Kim E, Shim
JS, Kaelber NS, Kant H, Hammers H, Commes T, Van Diest PJ, et al:
Identification of cetrimonium bromide and irinotecan as compounds
with synthetic lethality against NDRG1 deficient prostate cancer
cells. Cancer Biol Ther. 14:401–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park K, Chetty U, Scott W and Miller W:
The activity of locally applied cytotoxics to breast cancer cells
in vitro. Ann R Coll Surg Engl. 73:96–99. 1991.PubMed/NCBI
|
|
26
|
Wu TK, Chen CH, Pan YR, Hu CW, Huang FM,
Liu JY and Lee CJ: Cetrimonium bromide inhibits cell migration and
invasion of human hepatic SK-HEP-1 cells through modulating the
canonical and Non-canonical TGF-β signaling pathways. Anticancer
Res. 39:3621–3631. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Liu T and Wang W: Prognostic
significance of matrix metalloproteinase 9 expression in
osteosarcoma: A meta-analysis of 16 studies. Medicine (Baltimore).
97:e130512018. View Article : Google Scholar
|
|
28
|
Wang G, Zhang T, Sun W, Wang H, Yin F,
Wang Z, Zuo D, Sun M, Zhou Z, Lin B, et al: Arsenic sulfide induces
apoptosis and autophagy through the activation of ROS/JNK and
suppression of Akt/mTOR signaling pathways in osteosarcoma. Free
Radic Biol Med. 106:24–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai L and Wang S: Targeting apoptosis
pathways for new cancer therapeutics. Annu Rev Med. 65:139–155.
2014. View Article : Google Scholar
|
|
30
|
Mohamed MS, Bishr MK, Almutairi FM and Ali
AG: Inhibitors of apoptosis: Clinical implications in cancer.
Apoptosis. 22:1487–1509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ito E, Yip KW, Katz D, Fonseca SB, Hedley
DW, Chow S, Xu GW, Wood TE, Bastianutto C, Schimmer AD, et al:
Potential use of cetrimonium bromide as an apoptosis-promoting
anticancer agent for head and neck cancer. Mol Pharmacol.
76:969–983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020. View Article : Google Scholar
|
|
33
|
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z,
Li W, Hu J, Lu C and Liu Y: PI3K/AKT pathway as a key link
modulates the multidrug resistance of cancers. Cell Death Dis.
11:7972020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fattahi S, Amjadi-Moheb F, Tabaripour R,
Ashrafi GH and Akhavan-Niaki H: PI3K/AKT/mTOR signaling in gastric
cancer: Epigenetics and beyond. Life Sci. 262:1185132020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang H, Jiang H, Zhang H, Liu J, Hu X and
Chen L: Anti-tumor efficacy of phellamurin in osteosarcoma cells:
Involvement of the PI3K/AKT/mTOR pathway. Eur J Pharmacol.
858:1724772019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan Y, Zhang Y, Chen Q, Tao X, Liu J and
Xiao GG: CTAB enhances chemo-sensitivity through activation of AMPK
signaling cascades in breast cancer. Front Pharmacol. 10:8432019.
View Article : Google Scholar : PubMed/NCBI
|