Introduction
Traditional chemotherapy kills cancer cells using
agents that target actively dividing cells. However, haematological
cells, epithelial cells of the oral mucosa, intestinal mucosa,
nasal mucosa, vaginal mucosa, nails and hair also exhibit high
rates of division, and may thus be targeted by chemotherapy as well
(1). The side-effects of
chemotherapy are strictly related to the protocol used, the drug
doses, the period of treatment and the health status of the
patient. Thus, there is an increased focus on targeted therapies
for the management of cancer (2).
Targeted therapy aims to identify cancer-specific targets, thus
reducing the incidence of side-effects. It was originally hoped
that this type of therapy would exhibit considerably fewer issues,
representing the 'holy grail' of cancer therapy; however, research
has demonstrated that targeted therapeutic agents can in fact
induce severe side-effects (1).
The mammalian target of rapamycin (mTOR) is a
serine/threonine kinase that is involved in the PI3K/AKT/mTOR
pathway (2). This pathway is
important in the regulation of several cellular processes,
including proliferation, cell survival and angiogenesis (3). The discovery that this pathway is
dysregulated in several types of tumours has led to the development
of several mTOR inhibitors. The first generation of mTOR inhibitors
is represented by rapamycin and its analogues (2). These agents inhibit the action of
MTORC1 through the binding of FK506 binding protein-12 (FKB12),
which forms a ternary complex with mTOR (3). A total of four mTOR inhibitors are
currently available: Sirolimus, everolimus, temsirolimus and
ridaforolimus (4). These are
large molecules (molecular weight (MW) ~1,000 kDa) that bind to
FKBP-12 to generate a complex that blocks the mTOR protein kinase
complex. These molecules are characterised by various side-effects
compared with conventional chemotherapy (2,5,6).
Everolimus in particular, is clinically used for the treatment of
several solid tumours, such as advanced hormone receptor-positive
human epidermal growth factor receptor 2 (HER-2)-negative breast
cancer, renal cell carcinoma, neuroendocrine tumours of pancreatic
origin, and sub-ependymal giant cell astrocytoma (2,3).
The following systematic review was performed in
order to evaluate the most common side-effects of everolimus, and
the incidence of the reported side-effects.
Materials and methods
Literature search
PubMed and Scopus were searched using the following
combination of free words and MESH terms: 'everolimus' AND
'side-effects' OR 'toxicities' OR 'adverse events'. Only studies
fulfilling the following inclusion criteria were considered
eligible for inclusion in the present study: i) performed on human
subjects; ii) reporting on the use of everolimus; iii) written in
English and iv) reported the incidence of side-effects. Case
reports and studies on animal models were excluded. For each study,
the following information was recorded: Author, year of
publication, title, therapeutic protocol, number of patients
enrolled, number of events recorded for each toxicity, and grade of
the events recorded. Data were independently extracted by three
authors and assessed in a joint session.
Only the most numerically relevant toxicities and
data related to patients who completed treatment were included.
Data regarding patients that could not complete the treatment due
to dose delays or discontinuations were excluded.
Results
The titles and abstracts of 912 potentially relevant
studies were screened, of which, 731 were excluded as they did not
fulfil the inclusion criteria. Of the 181 studies included, all the
adverse events reported were recorded. The flow chart of the
selection process is presented in Fig. 1. The results of the present
meta-analysis revealed that the majority of adverse events reported
were of grade 1 or 2, as shown in Table I.
 | Table IMain systemic changes induced by
everolimus therapy: Summary table. |
Table I
Main systemic changes induced by
everolimus therapy: Summary table.
| Adverse effect | Adverse
effects/cases (%) | Cases with
grade/adverse effects (%) | Grade 1/2 %
(cases) | Grade 3/4 %
(cases) |
|---|
| Anaemia | 2,534/10,386
(24.4) | 2,470/9,922
(24.9) | 1,767 (17.8) | 703 (7.1) |
| Anorexia | 534/2,120
(25.2) | 534/2120
(25.2) | 463 (21.8) | 71 (3.3) |
| Asthenia | 1,415/6,847
(20.6) | 1,415/6,847
(20.6) | 1201 (17.5) | 214 (3.1) |
| Diarrhoea | 2,330/10,436
(22.3) | 2,029/8,818
(23) | 1,797 (20.4) | 232 (2.6) |
| Fatigue | 2,780/11,436
(23.7) | 2,709/10,923
(24.8) | 2,187 (20) | 522 (4.8) |
|
Hypercholesterolemia | 1,078/5,346
(20.2) | 1,074/5,213
(20.6) | 1,008 (19.3) | 66 (1.3) |
|
Hyperglycaemias | 1,853/10,878
(16.9) | 1,822/10135
(18) | 1,347 (13.3) | 475 (4.7) |
| Leukopenia | 495/1,672
(29.6) | 476/1,524
(31.2) | 283 (18.6) | 193 (12.6) |
| Pneumonitis | 628/6,201
(10.1) | 626/6,096
(10.3) | 429 (7) | 197 (3.3) |
| Pruritus | 386/3,187
(12.1) | 379/3,130
(12.1) | 365 (11.6) | 14 (0.5) |
| Pyrexia | 1,069/6,961
(15.4) | 1,036/6,692
(15.4) | 951 (14.2) | 85 (1.3) |
| Rash | 2,302/10,114
(22.7) | 2,220/9,273
(24) | 2,082 (22.5) | 138 (1.5) |
| Stomatitis | 3,568/8,259
(43.2) | 3,494/7,854
(44.5) | 2,959 (37.7) | 535 (6.8) |
|
Thrombocytopenia | 1,195/5,533
(21.8) | 1,163/5,095
(22.8) | 921 (18.1) | 242 (4.7) |
| Emesis | 883/5,913 (15) | 858/5,578
(15.4) | 781 (14) | 77 (1.4) |
For anaemia, 106 articles were read in full and 31
studies were excluded as they did not report the number of events.
The overall incidence of anaemia was 24.4% (2,534 cases out of
10,386 patients). A total of 70 out of 75 articles also reported
the grade: 2,470 cases out of 9,922 patients. The incidence of
grade 1 or 2 anaemia was 17.8% (1,767/9,922), whereas the incidence
of grade 3 or 4 anaemia was 7.1% (703/9,922) (Table SI).
For anorexia, 60 articles were read in full and 10
studies were excluded as they did not report the number of events.
The overall incidence of anorexia was 25.2% (534 cases out of 2,120
patients). All the articles reported the grade: The overall
incidence of grade 1 or 2 anorexia was 21.8% (463/2,120) and the
overall incidence of grade 3 or 4 anorexia was 3.3% (71/2,120)
(Table SII).
For asthenia, 38 articles were read in full text and
6 articles were excluded as they did not report the number of
events. The overall incidence of asthenia was 20.6% (1,415 cases
out of 6,847 patients). All the papers reported the grade: The
incidence of grade 1 or 2 asthenia was 17.5% (1,201/6.847), whereas
the incidence of grade 3 or 4 asthenia was 3.1% (214/6,847)
(Table SIII).
For diarrhoea, 135 articles were read in full text
and 25 articles were excluded as they did not report the number of
events. The overall incidence was 22.3% (2,330 cases out of 10,436
patients). A total of 97 out of the 110 articles also reported the
grade (2,029 cases out of 8,818 patients); the incidence of grade 1
or 2 diarrhoea was 20.4%, whereas the incidence of grade 3 or 4
diarrhoea was 2.6% (Table
SIV).
For fatigue, 119 articles were read in full text and
18 studies were excluded as they did not report the number of
events. The overall incidence was 23.7% (2,780 cases out of 11,436
patients). A total of 93 out of 101 articles also reported the
grade (2,709 cases out of 10,923 patients). The incidence of grade
1 or 2 fatigue was 20% (2,187/10,923), whereas the incidence of
grade 3 or 4 fatigue was 4.8% (522/10,923) (Table SV).
For hypercholesterolaemia, 58 studies were read in
full text; 13 studies were excluded as they did not report the
number of events. The overall incidence was 20.2% (1,078 cases out
of 5,349 patients). A total of 44 out of 45 studies also reported
the grade (1,074 cases out of 5,213 patients). The incidence of
grade 1 or 2 hypercholesterolaemia was 19.3% (1,008/5,213), whereas
the incidence of grade 3 or 4 hypercholesterolemia was 1.3%
(66/5,213) (Table SVI).
For hyperglycaemia, 91 studies were read in full
text and 10 studies were excluded as they did not report the number
of events. The overall incidence was 16.9% (1,853 cases out of
10,878 patients). A total of 77 out of 81 studies also reported the
grade (1,822 cases out of 10,135 patients). The incidence of grade
1 or 2 hyperglycaemia was 13.3% (1,347/10,135), whereas the
incidence of grade 3 or 4 hyperglycaemia was 4.7% (475/10,135)
(Table SVII).
For leukopenia, 50 studies were read in full text
and 12 studies were excluded as they did not report the number of
events. The overall incidence was 29.6% (495 cases out of 1,672
patients). A total of 35 out of 38 papers also reported the grade
(476 cases out of 1,524 patients). The incidence of grade 1 or 2
leukopenia was 18.6% (283/1,524), whereas the incidence of grade 3
or 4 leukopenia was 12.6% (193/1,524) (Table SVIII).
For pneumonitis, 55 studies were read in full text
and 5 studies were excluded as they did not report the number of
events. The overall incidence was 10.1% (628 cases out of 6,201
patients). A total of 50 out of 55 studies also reported the grade
(626 cases out of 6,096 patients). The incidence of grade 1 or 2
pneumonitis was 7% (429/6,096), whereas the incidence of grade 3 or
4 pneumonitis was 3.3% (197/6,096) (Table SIX).
For pruritus, 34 studies were read in full text and
2 studies were excluded as they did not report the number of
events. The overall incidence was 12.1% (386 cases out of 3,187
patients). A total of 30 out of 32 studies also reported the grade
(379 cases out of 3,130 patients). The incidence of grade 1 or 2
pruritus was 11.6% (365/3,130), whereas the incidence of grade 3 or
4 pruritus was 0.5% (14/3,130) (Table SX).
For pyrexia, 42 studies were read in full text and 7
were excluded as they did not report the number of events. The
overall incidence was 15.4% (1,069 cases out of 6,961 patients). A
total of 30 out of 35 studies also reported the grade (1,036 cases
out of 6,692 patients). The incidence of grade 1 or 2 pyrexia was
14.2%, whereas the incidence of grade 3 or 4 pyrexia was 1.3%
(85/6,692) (Table SXI).
For rash, 112 studies were read in full text and 14
studies were excluded as they did not report the number of events.
The overall incidence was 22.7% (2,302 cases out of 10,114
patients). A total of 89 out of 98 studies also reported the grade
(2,220 cases out of 9,273 patients). The incidence of grade 1 or 2
rash was 22.5% (2,082/9,273), whereas the incidence of grade 3 or 4
rash was 1.5% (138/9,273) (Table
SXII).
For stomatitis, 181 studies were read in full text
and 111 studies were excluded as they did not report the number of
events. The overall incidence was 43.2% (3,568 cases out of 8,259
patients). A total of 62 out of 70 studies also reported the grade.
Of the cases of stomatitis, 37.7% (2,959/7,854) were grade 1 or 2,
whereas 6.8% (535/7,854) were grade 3 or 4 (Table SXIII).
For thrombocytopenia, 88 studies were read in full
text and 14 studies were excluded as they did not report the number
of events. The overall incidence was 21.8% (1,195 cases out of
5,533 patients). A total of 69 out of 74 studies also reported the
grade (1,163 cases out of 5,095 patients) The incidence of grade 1
or 2 thrombocytopenia was 18.1% (921/5,095), whereas the incidence
of grade 3 or 4 thrombocytopenia was 4.7% (242/5,095) (Table SXIV).
For emesis, 80 studies were read in full text and 8
studies were excluded as they did not report the number of adverse
events. The overall incidence was 15% (883 cases out of 5,913
patients). A total of 67 out of 72 studies also reported the grade
(858 cases out of 5,578 patients). The incidence of grade 1 or 2
emesis was 14% (781/5,578), whereas the incidence of grade 3 or 4
emesis was 1.4% (77/5,578) (Table
SXV).
The majority of studies used a dose of 10 mg/day;
only a few studies used a dosage of 2.5 mg/day and/or 5 mg/day.
However, the number of cases treated with 2.5/5 mg/day was not
sufficient to be used for statistical analysis for the evaluation
of adverse events compared with 10 mg/day (Table SXVI).
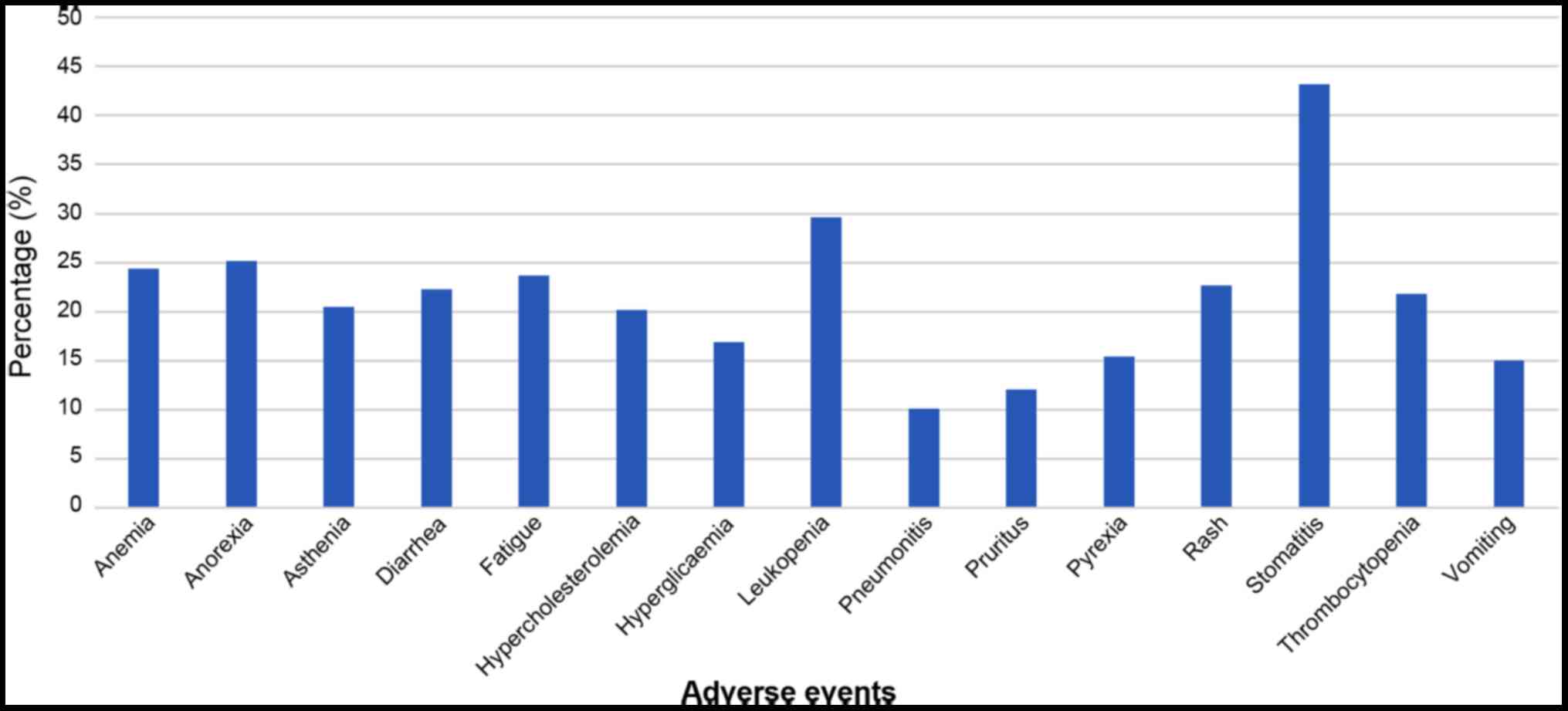
The primary adverse events reported in the studies
were stomatitis, leukopenia, anorexia, anaemia and fatigue
(Fig. 2). The analysis of
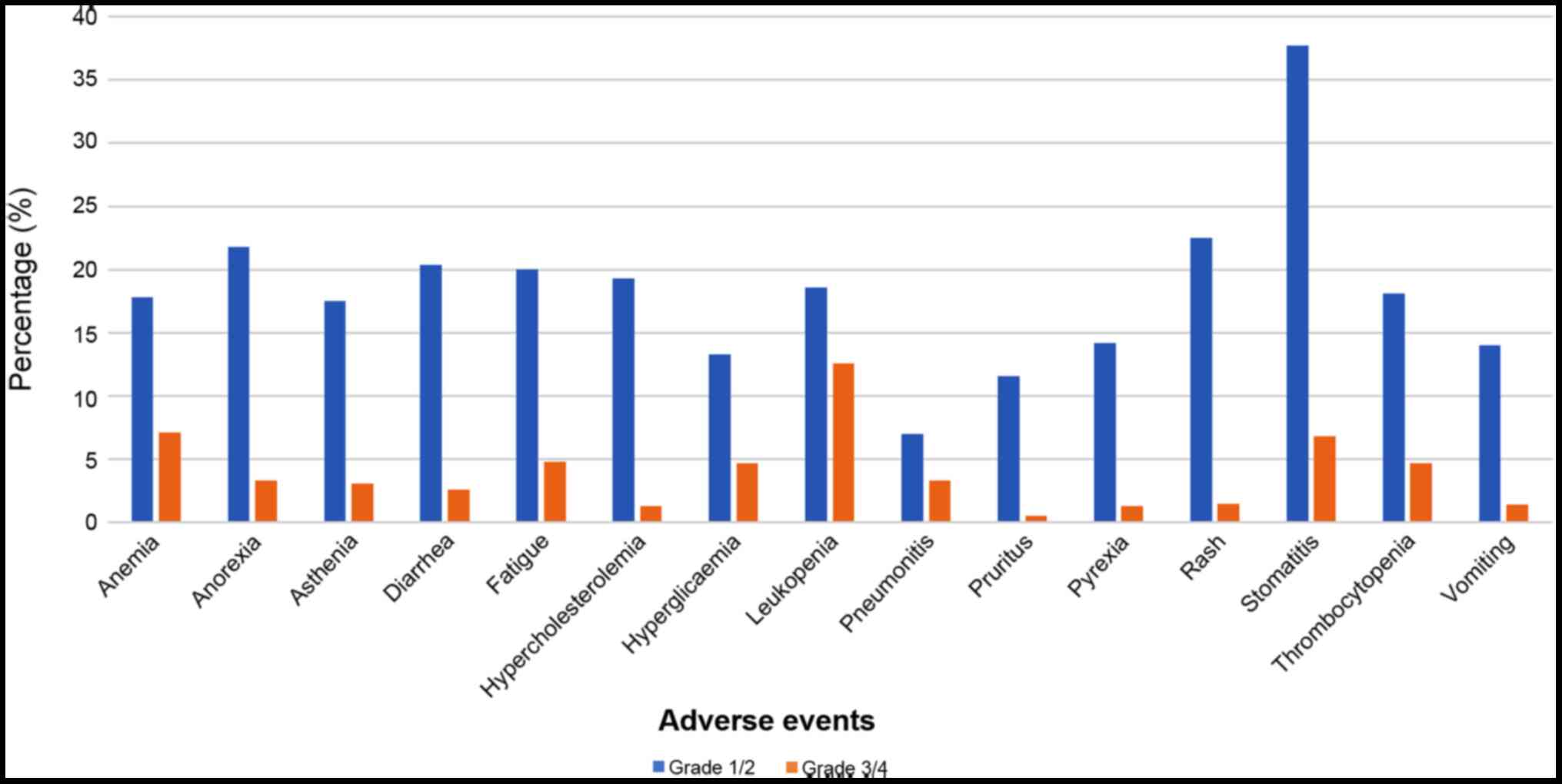
different grades revealed that for all adverse events, grade 1 and
2 side effects were more prevalent compared with grade 3 and 4 side
effects (Fig. 3).
Discussion
The results of the present meta-analysis revealed
that some of the most common adverse events reported were
stomatitis, leukopenia, anorexia, anaemia and fatigue. Fortunately,
the majority of events were classed as grade 1-2, which meant that
they could be easily managed by the clinicians.
Targeted therapy acts by blocking a specific target
in malignant cells. mTOR inhibitors belong to the class of signal
transduction inhibitors. mTOR is implicated in several cellular
processes that are essential for tumour progression, cell
proliferation and survival and, therefore if combined with other
anticancer drugs, mTOR inhibitors may function to sensitize the
tumour cells to the primary anticancer agent. Sirolimus was the
first mTOR inhibitor approved for clinical use (4). It is an antifungal agent already
known for its immunosuppressive properties. However, its poor
pharmacokinetic characteristics have led to the development of
analogues, including everolimus, temsirolimus and ridaforolimus.
These molecules differ from sirolimus in their C-40-O positions,
with different pharmacokinetic and pharmacodynamic profiles, and
have now been approved for the treatment of solid tumours, such as
renal cell carcinoma, breast cancer and pancreatic neuroendocrine
tumours.
Even if these drugs target specific signalling
pathways that are upregulated in tumour cells, there is a
possibility that this signalling pathway serves a physiological
purpose in healthy cells, and thus the inhibition or activation of
these pathways may induce adverse events. Some of these adverse
events will be the same or similar to those observed in patients
treated with conventional chemotherapy, whereas others may be
unique to the targeted therapy.
Everolimus induces a wide range of side-effects that
may limit the clinical use of this drug. The results of the present
meta-analysis demonstrated that most of these events were grade 1
or 2, as shown in Table I.
The National Cancer Institute grades anaemia as
follows: Mild (grade 1), Hb between 10 g/dl and the lower
physiological level; moderate (grade 2), Hb 8.0-9.9 g/dl; severe
(grade 3), Hb <8 g/dl to 6.5 g/dl; and life-threatening (grade
4), Hb <6.5 g/dl (5). The
incidence of anaemia due to everolimus therapy varied between 3.31%
(6) and 100% (7). However, the mean value was 24.4%
(2,534 cases out of 10,386 patients). mTOR inhibitors are
immunosuppressive drugs that exert dose-dependent effects on
haematopoiesis, thus potentially inducing anaemia. The specific
mechanism by which everolimus induces anaemia is unclear; however,
a pathogenic link has recently been suggested between anaemia
induced by sirolimus and the appearance of an inflammatory state.
Sánchez Fructuoso et al (8) suggested that the anaemia induced by
everolimus, which is characterized by microcytosis, low serum iron
levels despite prominent ferritinaemia, and high levels of
C-reactive protein, was related to the induction of a chronic
inflammatory state. Anaemia is the most common haematological
side-effect in neoplastic patients (9). The anaemia induced by traditional
chemotherapy is due to the malignant invasion of normal tissues
with resultant blood loss and bone marrow infiltration, resulting
in interruption of erythropoiesis and functional iron deficiency
following inflammation (10). It
is estimated that 70% of patients undergoing chemotherapy develop
anaemia. The primary difference between everolimus-related anaemia
and chemotherapy-related anaemia is that the incidence of grade 3
and 4 anaemia due to everolimus treatment is only 7.1%, whereas the
incidence of mild or moderate anaemia (grade 1 and 2) in patients
with solid tumours is ~60% of patients following platinum-based
chemotherapy; severe (grade 3) anaemia in elderly patients with
haematological malignancies may occur in up to 74% of patients with
non-Hodgkin lymphoma following a standard
cyclophosphamide/doxorubicin/vincristine/prednisolone regimen
(11).
Everolimus-related anorexia (loss of appetite) is
observed in 25.2% of all patients (534/2,120); the values range
from 4.2% (12) to 93% (13). Anorexia is commonly associated
with cancer or chemotherapy (14), results in significant weight loss,
and may be the result of a decrease or a complete loss of appetite
with or without nausea, vomiting, oral pain, diarrhoea and
disturbances to taste (15). The
National Cancer Institute grades anorexia as follows: Mild (grade
1), loss of appetite without alterations in eating habits; moderate
(grade 2), oral intake altered without significant weight loss or
malnutrition; severe (grade 3), associated with significant weight
loss or malnutrition; life-threatening/disabling (grade 4), life
threatening consequences (16).
The primary difference between everolimus-related anorexia and
chemotherapy-related anorexia is that the incidence of grade 3 and
4 anorexia due to everolimus treatment is only 3.3%, whereas the
incidence of anorexia associated with traditional chemotherapy is
45% (17).
Cancer-related anorexia is often the result of an
increase in the levels of pro-inflammatory cytokines or an increase
in lactate levels. These two events can modulate central nervous
system neurotransmitter cascades.
Asthenia (weakness) is an adverse event that is
observed in 20.6% of patients treated with everolimus (1,415 cases
out of 6,847 patients); and the reported incidence in individual
studies varies between 2.4% (6)
and 49.8% (18). Asthenia is the
feeling of muscle tiredness; it is described as a lack of energy to
move certain muscles or even all the muscles in the body. In
oncological patients, asthenia is the most prevalent symptom; its
pathophysiology remains relatively unknown, despite the significant
impact it can have on quality of life (19). The incidence of asthenia
associated with traditional chemotherapy is 35.7% (20).
Diarrhoea is observed in 22.3% of all patients
treated with everolimus (2,330 cases out of 10,436 patients), with
the incidence in individual studies ranging from 2% (21) to 72.7% (22). Chemotherapy-related diarrhoea may
occur in 50-80% of patients, based on the specific chemotherapeutic
regimen (23). Everolimus-related
diarrhoea and chemotherapy-related diarrhoea have different
features. Everolimus-related diarrhoea is prevalently grade 1 and 2
(20.4%), and rarely grade 3 and 4 (2.6%); chemotherapy-related
diarrhoea is almost wholly grade 3-5 (30%), according to the Common
Toxicity Criteria (24),
particularly when treated with a bolus dose of 5-fluorouracil, or
combination therapies including irinotecan and fluoropyrimidines
(25). In patients with cancer,
diarrhoea can lead to a loss of fluids and electrolytes,
malnutrition followed by dehydration and hospitalization,
eventually leading to cardiovascular problems and potentially
death. Usually this adverse event is dose-related and may be
associated with other characteristics of toxicity (25). Several drugs can induce diarrhoea,
such as cyclophosphamide, daunorubicin, epirubicin, fluorouracil,
gemcitabine, methotrexate, paclitaxel and vincristine. However, the
pathophysiological mechanisms remain under investigation.
The analysis of the literature regarding fatigue
related to everolimus treatment revealed that the incidence ranged
from 5% (26) to 100% (26-28), with a mean of 23.7% (2,780 cases
out of 11,436 patients). Fatigue due to chemotherapy is one of the
most common problems amongst patients with cancer adversely
affecting their quality of life. It has been estimated that fatigue
affects up to 60% of patients treated with chemotherapy (27). The pathogenesis of cancer-related
fatigue is not clear. Physical fatigue (inactivity, laziness and
stress) and mental fatigue (reduced attention span, concentration,
learning and short-term memory loss) are amongst the most common
symptoms (28). A total of 10% of
patients with fatigue due to traditional chemotherapy exhibit grade
3-4 fatigue (17), whereas 4.8%
of everolimus-treated patients were reported to exhibit fatigue of
grade 1-2 (17).
The incidence of hypercholesterolemia was found to
be 20.2% following everolimus treatment, with the incidence in
individual studies ranging from 3% (29) to 89% (30). Grade 1 and 2 hypercholesterolaemia
are prevalent (present in 19.3% of patients), with grades 3 and 4
being reported in only 1.3% of the patients. Total cholesterol
levels were slightly increased prior to the final cycles of
chemotherapy compared with the prechemotherapy levels in several
treatment protocols (31). At 6
months post-chemotherapy, the levels returned to baseline, except
in taxane-treated patients (31).
Hyperglycaemia is observed in 16.9% (1,853 cases out
of 10,878 patients) of all patients treated with everolimus, with
the incidence in individual studies ranging from 1.7% (32) to 100% (33,34). The pathophysiology of
hyperglycaemia in association with mTOR develops via one of two
mechanisms: i) A direct effect of mTOR inhibitors on the β cells of
the pancreas causing a reduction in insulin secretion stimulated by
glucose, resulting in an increase in apoptosis and other effects on
cell viability and proliferation; ii) exaggeration of peripheral
insulin resistance via mTOR inhibitors. In the muscles, there is a
reduction in glucose absorption and a reduction in muscle mass.
mTOR inhibitors facilitate gluconeogenesis in the liver and reduce
the absorption of lipids in adipose tissue. Hyperglycaemia during
chemotherapy occurs in 10-30% of the patients (35). Patients with grade 1
hyperglycaemia have glucose levels ≤160 mg/dl; patients with grade
2 hyperglycaemia have glucose levels 160-250 mg/dl; grade 3
hyperglycaemia is characterized by a glucose level 250-500 mg/dl;
and grade 4 hyperglycaemia refers to a glucose level >500 mg/dl.
Grades 2, 3 and 4 hyperglycaemias should be treated according to
the consensus algorithm of the American Diabetes Association and
European Association for the Study of Diabetes (36,37).
In addition to anaemia, mTOR inhibitors can cause
other haematological toxicities, such as leukopenia and
thrombocytopenia, that require regimen modifications or treatment
suspension. Leukopenia and thrombocytopenia are caused by
inhibition of signal transduction via glycoprotein 130 (β) chain,
which is shared by certain cytokine receptors, granulocyte
colony-stimulating factor and erythropoietin, resulting in
stimulation of platelet, leukocyte and erythrocyte production
(38). The incidence of
leukopenia in patients subjected to everolimus treatment is 29.6%
(495 cases out of 1,672 patients), with the incidence in individual
studies ranging from 2.1% (12)
to 90% (39). Neutropenia is one
of the most serious haematological toxicities occurring during
chemotherapy, and increases the susceptibility of patients to
infection. Neutropenia generally occurs in 33.3% of patients
undergoing chemotherapy (40).
The incidence of thrombocytopenia in patients
treated with everolimus ranges from 0% (41) to 100% (33,42,43), with a mean of 21.8% (1,195 cases
out of 5,533 patients). During traditional chemotherapeutic
regimens, the incidence of chemotherapy-related thrombocytopenia
varies depending on the treatment used; patients treated with
gemcitabine and platinum-based regimens have the highest incidence
of thrombocytopenia (44). The
incidence of grade 3 and 4 thrombocytopenia was similar between
everolimus therapy (4.7%) and traditional chemotherapy (5%)
(17). The incidence of grade 1-2
thrombocytopenia undergoing traditional chemotherapy was higher
than in patients being treated with everolimus (20 and 18.1%,
respectively) (17).
Drug-related pneumonitis is one of the primary
toxicities observed during anticancer systemic therapy and presents
different radiographic manifestations on chest computed tomography
(45). Everolimus-related
pneumonitis has a mean incidence of 10.1% (628/6,201 patients),
with the incidence in individual studies ranging from 0% (33) to 48.6% (43). Conventional chemotherapy-related
toxicity can be dose-dependent, and may thus be observed at higher
cumulative doses (bleomycin and carmustine) or several years after
the end of therapy (cyclophosphamide, busulfan and carmustine)
(46). The incidence of
pneumonitis in patients treated with chemotherapy ranged between
1.5 and 50% (47).
Pruritus may be caused by standard chemotherapy,
radiation therapy and immunotherapy. The occurrence of pruritus
during conventional chemotherapy may be a sign of sensitivity to
the drugs used; drugs used in immunotherapy may also cause dryness
and itching. Everolimus can cause pruritus in 12.1% of all patients
treated (386 cases out of 3,187 patients); the lowest reported
incidence was 2.2% (6), whereas
the highest was 91% (48). It is
estimated that pruritus is observed in 10-25% of individuals
treated with traditional chemotherapy (49).
Pyrexia was observed in 15.4% of all patients
treated with everolimus (1,069 cases out of 6,961 patients); with
the incidence ranging from 2% (29) to 44.4% (50). A study on pyrexia as a result of
conventional chemotherapy reported an incidence of ~34% (51). The difference between
everolimus-related pyrexia and chemotherapy-related pyrexia is that
grade 3 and 4 pyrexia was only observed in 1.3% of patients treated
with everolimus, whereas it was observed in 5% of patients treated
with traditional chemotherapy (17).
The incidence of rash in patients undergoing
everolimus treatment ranges from 2.7% (52) to 100% (53), with a mean incidence of 22.7%;
instead, traditional chemotherapy caused rash in only 10% of all
patients (17). Rash due to mTOR
inhibitors can manifest as acneiform dermatitis that typically
affects the neck or the upper extremities and starts as an
inflammatory lesion (54).
Stomatitis is the most frequent adverse effect
observed in patients treated with mTOR inhibitors. Stomatitis was
observed in 43.2% of patients (3,568/8,259), with the incidence in
individual studies ranging from 5.26% (29) to 100% (55). The pathophysiology of this type of
stomatitis is not clear. Differences between mTOR inhibitor-related
oral mucositis and classical oral mucositis include the clinical
presentation and concomitant toxicities (56). mTOR inhibitor-associated
stomatitis are aphthous-like lesions that are notably different
from those related to chemotherapy or radiotherapy. The first
manifestations observed are typically single or multiple shallow,
well-circumscribed, round, painful ulcers localized in the
non-keratinized mucosa and sometimes surrounded by an erythematous
halo (57), whereas the second
lesions formed are characterized by painful inflammation, erythema,
swelling and ulcerations affecting the oral cavity, oropharynx and
hypopharynx (58,59). Oral mucositis can be classified as
grade 1 to 4. Oral pain during chemotherapy is quite common due to
inflammation of the oral mucosa; certain drug combinations are more
likely to cause mucositis compared with others. Oral mucositis
usually occurs a few days after the commencement of therapy and
subsides within a week. The degree of pain experienced may vary
according to the severity of mucositis. Patients affected by oral
mucositis are often neutropenic, and for this reason the pain can
be worsened by the occurrence of oral mycosis, such as candidosis,
which appears as whitish patches on the oral mucosa and on the
surface of the tongue. Oral pain can also affect the sense of
taste. The risk of developing oral mucositis is usually
dose-dependent. Cytotoxic drugs able to induce oral mucositis
include capecitabine, carboplatin, chlorambucil, cisplatin,
cyclophosphamide, dacarbazine, dactinomycin, daunorubicin,
doxorubicin, etoposide, fluorouracil, hydroxyurea, lomustine,
melphalan, mercaptopurine, methotrexate, mitomycin, paclitaxel,
raltitrexed, vinblastine and vincristine (60). Accordingly, at least 40% of
patients treated with conventional chemotherapy may present with
this condition (61,62). The frequency is higher (up to 80%)
in patients undergoing haematopoietic cell transplantation (HCT),
particularly myeloablative allogeneic HCT, and in those who are
conditioned with radiation-containing regimens, and with the use of
methotrexate for graft-versus-host disease prophylaxis. The
administration of 5-fluorouracil is often associated with grade 3-4
oral mucositis (>15%).
Emesis (vomiting) was reported in 15% of patients
treated with everolimus (883 cases out of 5,913 patients), with the
incidence in individual studies ranging from 0% (33) to 75% (42). The incidence of emesis due to
everolimus treatment was 15%, whereas emesis due to chemotherapy
was reported in ~30% of individuals. The severity of emesis differs
according to the specific chemotherapeutic drugs used, and it is
classified as high-risk (>90% of patients are likely to be
affected), moderate-risk (30-90% of patients affected), low-risk
(10-30%) and minimal-risk (<10% of patients affected).
Furthermore, females are more at risk than males, and younger
individuals are more at risk than older individuals.
Even if patients treated with everolimus appear to
exhibit fewer of the 'standard' toxicities usually associated with
chemotherapy (for example, emesis), there is an increase in a new
group of frequently occurring side-effects, including dermal,
vascular and gastrointestinal toxicities, which may be caused by
receptor cross-reactivity or the presence of receptors on or in
non-cancerous cells. Moreover, the incidence of side-effects may
vary depending on the tumour type, likely due to differences in the
complex tumour biology. Other features involved in the treatment
response may be age, sex and ethnicity of patients treated. Further
research is required for more accurate comparisons of side effects
provoked by targeted therapy and those caused by conventional
chemotherapy.
In conclusion, the role of the oncologists is not
limited to the treatment/therapy of the disease; instead, they must
aim to prolong survival, control symptoms and improve the quality
of life of the patients. Targeted therapy is a relatively novel
therapeutic approach to the management of several types of tumours.
Among these new drugs, everolimus expands the therapeutic
armamentarium available to fight cancer and other diseases. The aim
of the present study was to evaluate the quality of life of
patients undergoing everolimus therapy through the evaluation of
side effects related to everolimus, compared with conventional
chemotherapy. Despite a global reduction in side-effects with the
intake of everolimus (Table
SXVII), certain adverse effects, such as stomatitis and rash,
were more commonly related to this type of therapy compared with
traditional chemotherapy. However, the majority of adverse effects
reported in patients treated with everolimus were grade 1 and 2,
whereas those induced by conventional chemotherapy were primarily
grade 3 and 4.
Thus, it may be easier to manage
everolimus-associated side-effects compared with those of
traditional chemotherapy. However, it is necessary to perform
trials with larger cohorts to better evaluate the safety of
everolimus.
As regards limitations, the present meta-analysis
could not be registered on Prospero, and the study was based on
literature available from only two databases. Future prospective
studies are required to improve the accuracy of the results.
Furthermore, only studies written in the English language were
included. The online search retrieved ~1,100 duplicates that were
identified and excluded by EndNote X9. Only numerically relevant
adverse events were included, as this systematic review was
intended to be easily readable by clinicians, and the data are
based entirely on previous studies. These shortcomings are
acknowledged as limitations and further studies are required to
improve the quality of the results and to include all adverse
events that were reported in clinical trials.
Supplementary Data
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CA extracted and analysed the data, and contributed
to the conception of the hypothesis for the study. MEB extracted
and analysed the data, and contributed to the preparation of the
manuscript. VCAC analysed the data. GT contributed to data analysis
and revised the manuscript. KZ contributed to data extraction and
contributed to the preparation of the manuscript. SL contributed to
data analysis revised the manuscript. LLM extracted and analysed
the data, and approved the submitted version. All authors read and
approved the final manuscript. CA and LLM confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Keefe DM and Bateman EH: Tumor control
versus adverse events with targeted anticancer therapies. Nat Rev
Clin Oncol. 9:98–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gomez-Pinillos A and Ferrari AC: mTOR
signaling pathway and mTOR inhibitors in cancer therapy. Hematol
Oncol Clin North Am. 26:483–505. vii2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng LH and Zheng XF: Toward rapamycin
analog (rapalog)-based precision cancer therapy. Acta Pharmacol
Sin. 36:1163–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie J, Hao Y, Zhou ZY, Qi CZ, De G and
Glück S: Economic evaluations of everolimus versus other hormonal
therapies in the treatment of HR+/HER2- advanced breast cancer from
a US payer perspective. Clin Breast Cancer. 15:e263–e276. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madeddu C, Gramignano G, Astara G,
Demontis R, Sanna E, Atzeni V and Macciò A: Pathogenesis and
treatment options of cancer related anemia: perspective for a
targeted mechanism-based approach. Front Physiol. 9:12942018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar
|
|
7
|
Chocteau-Bouju D, Chakiba C, Mignot L,
Madranges N, Pierga JY, Beuzeboc P, Quenel-Tueux N, Dieras V,
Bonnefoi H, Debled M, et al: Efficacy and tolerance of everolimus
in 123 consecutive advanced ER positive, HER2 negative breast
cancer patients. A two center retrospective study. Breast.
24:718–722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez Fructuoso A, Ruiz San Millán JC,
Calvo N, Rodrigo E, Moreno MA, Cotorruelo J, Conesa J,
Gómez-Alamillo C, Arias M and Barrientos A: Evaluation of the
efficacy and safety of the conversion from a calcineurin inhibitor
to an everolimus-based therapy in maintenance renal transplant
patients. Transplant Proc. 39:2148–2150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Belle SJ and Cocquyt V: Impact of
haemoglobin levels on the outcome of cancers treated with
chemotherapy. Crit Rev Oncol Hematol. 47:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilreath JA, Stenehjem DD and Rodgers GM:
Diagnosis and treatment of cancer-related anemia. Am J Hematol.
89:203–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bohlius J, Weingart O, Trelle S and Engert
A: Cancer-related anemia and recombinant human erythropoietin - an
updated overview. Nat Clin Pract Oncol. 3:152–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nozawa M, Nonomura N, Ueda T, Nishimura K,
Kanayama HO, Miki T, Nakatani T, Tomita Y, Azuma H, Yoshioka T, et
al: Adverse event profile and dose modification of everolimus for
advanced renal cell carcinoma in real-world Japanese clinical
practice. Jpn J Clin Oncol. 43:1132–1138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gross ME, Dorff TB, Quinn DI, Diaz PM,
Castellanos OO and Agus DB: Safety and efficacy of docetaxel,
bevacizumab, and everolimus for castration-resistant prostate
cancer (CRPC). Clin Genitourin Cancer. Jul 14–2017.Epub ahead of
print. PubMed/NCBI
|
|
14
|
Amitani M, Asakawa A, Amitani H and Inui
A: Control of food intake and muscle wasting in cachexia. Int J
Biochem Cell Biol. 45:2179–2185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisen T, Sternberg CN, Robert C, Mulders
P, Pyle L, Zbinden S, Izzedine H and Escudier B: Targeted therapies
for renal cell carcinoma: Review of adverse event management
strategies. J Natl Cancer Inst. 104:93–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
U.S. Department of Health and Human
Services: Common Terminology Criteria for Adverse Events (CTCAE).
Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
Accessed November 27, 2017.
|
|
17
|
Niu Q, Wang W, Li Y, Qin S, Wang Y, Wan G,
Guan J and Zhu W: Cord blood-derived cytokine-induced killer cells
biotherapy combined with second-line chemotherapy in the treatment
of advanced solid malignancies. Int Immunopharmacol. 11:449–456.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moscetti L, Vici P, Gamucci T, Natoli C,
Cortesi E, Marchetti P, Santini D, Giuliani R, Sperduti I, Mauri M,
et al: Safety analysis, association with response and previous
treatments of everolimus and exemestane in 181 metastatic breast
cancer patients: A multicenter Italian experience. Breast.
29:96–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González Barón M, Feyjóo M, Carulla
Torrent J, Camps C, Escobar Y and Belda-Iniesta C: Study of the
prevalence of tumour-related asthenia in Spanish cancer patients.
Clin Transl Oncol. 10:351–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moioli M, Barra F, Maramai M, Valenzano
Menada M, Vellone VG, Costantini S and Ferrero S: Mucinous ovarian
cancer: Current therapeutic targets, preclinical progress, and
experimental drugs. Expert Opin Investig Drugs. 28:1025–1029. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bajetta E, Catena L, Fazio N, Pusceddu S,
Biondani P, Blanco G, Ricci S, Aieta M, Pucci F, Valente M, et al:
Everolimus in combination with octreotide long-acting repeatable in
a first-line setting for patients with neuroendocrine tumors: An
ITMO group study. Cancer. 120:2457–2463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Besse B, Leighl N, Bennouna J,
Papadimitrakopoulou VA, Blais N, Traynor AM, Soria JC, Gogov S,
Miller N, Jehl V, et al: Phase II study of everolimus-erlotinib in
previously treated patients with advanced non-small-cell lung
cancer. Ann Oncol. 25:409–415. 2014. View Article : Google Scholar
|
|
23
|
Benson AB III, Ajani JA, Catalano RB,
Engelking C, Kornblau SM, Martenson JA Jr, McCallum R, Mitchell EP,
O'Dorisio TM, Vokes EE, et al: Recommended guidelines for the
treatment of cancer treatment-induced diarrhea. J Clin Oncol.
22:2918–2926. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trotti A, Byhardt R, Stetz J, Gwede C,
Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T,
et al: Common toxicity criteria: version 2.0. an improved reference
for grading the acute effects of cancer treatment: impact on
radiotherapy. Int J Radiat Oncol Biol Phys. 47:13–47. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stein J and Mann J: Specialty pharmacy
services for patients receiving oral medications for solid tumors.
Am J Health Syst Pharm. 73:775–796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Franz DN, Belousova E, Sparagana S, Bebin
EM, Frost M, Kuperman R, Witt O, Kohrman MH, Flamini JR, Wu JY, et
al: Everolimus for subependymal giant cell astrocytoma in patients
with tuberous sclerosis complex: 2-year open-label extension of the
randomised EXIST-1 study. Lancet Oncol. 15:1513–1520. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Irvine D, Vincent L, Graydon JE, Bubela N
and Thompson L: The prevalence and correlates of fatigue in
patients receiving treatment with chemotherapy and radiotherapy. A
comparison with the fatigue experienced by healthy individuals.
Cancer Nurs. 17:367–378. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohandas H, Jaganathan SK, Mani MP, Ayyar
M and Rohini Thevi GV: Cancer-related fatigue treatment: An
overview. J Cancer Res Ther. 13:916–929. 2017.PubMed/NCBI
|
|
29
|
Motzer RJ, Alyasova A, Ye D, Karpenko A,
Li H, Alekseev B, Xie L, Kurteva G, Kowalyszyn R, Karyakin O, et
al: Phase II trial of second-line everolimus in patients with
metastatic renal cell carcinoma (RECORD-4). Ann Oncol. 27:441–448.
2016. View Article : Google Scholar :
|
|
30
|
Sarkaria JN, Galanis E, Wu W, Peller PJ,
Giannini C, Brown PD, Uhm JH, McGraw S, Jaeckle KA and Buckner JC:
North Central Cancer Treatment Group Phase I trial N057K of
everolimus (RAD001) and temozolomide in combination with radiation
therapy in patients with newly diagnosed glioblastoma multiforme.
Int J Radiat Oncol Biol Phys. 81:468–475. 2011. View Article : Google Scholar
|
|
31
|
Tian W, Yao Y, Fan G, Zhou Y, Wu M, Xu D
and Deng Y: Changes in lipid profiles during and after
(neo)adjuvant chemotherapy in women with early-stage breast cancer:
A retrospective study. PLoS One. 14:e02218662019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Capdevila J, Sevilla I, Alonso V, Antón
Aparicio L, Jiménez Fonseca P, Grande E, Reina JJ, Manzano JL,
Alonso Lájara JD, Barriuso J, et al: Evaluation of the efficacy and
safety of lanreotide in combination with targeted therapies in
patients with neuroendocrine tumours in clinical practice: A
retrospective cross-sectional analysis. BMC Cancer. 15:4952015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun JM, Kim JR, Do IG, Lee SY, Lee J, Choi
YL, Ahn JS, Ahn MJ and Park K: A phase-1b study of everolimus plus
paclitaxel in patients with small-cell lung cancer. Br J Cancer.
109:1482–1487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tobinai K, Ogura M, Maruyama D, Uchida T,
Uike N, Choi I, Ishizawa K, Itoh K, Ando K, Taniwaki M, et al:
Phase I study of the oral mammalian target of rapamycin inhibitor
everolimus (RAD001) in Japanese patients with relapsed or
refractory non-Hodgkin lymphoma. Int J Hematol. 92:563–570. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hwangbo Y and Lee EK: Acute hyperglycemia
associated with anti-cancer medication. Endocrinol Metab (Seoul).
32:23–29. 2017. View Article : Google Scholar
|
|
36
|
Nathan DM, Buse JB, Davidson MB,
Ferrannini E, Holman RR, Sherwin R and Zinman B; American Diabetes
Association; European Association for Study of Diabetes: Medical
management of hyperglycemia in type 2 diabetes: a consensus
algorithm for the initiation and adjustment of therapy: a consensus
statement of the American Diabetes Association and the European
Association for the Study of Diabetes. Diabetes Care. 32:193–203.
2009. View Article : Google Scholar :
|
|
37
|
Nathan DM, Buse JB, Davidson MB,
Ferrannini E, Holman RR, Sherwin R and Zinman B; American Diabetes
Association; European Association for the Study of Diabetes:
Medical management of hyperglycaemia in type 2 diabetes mellitus: a
consensus algorithm for the initiation and adjustment of therapy: a
consensus statement from the American Diabetes Association and the
European Association for the Study of Diabetes. Diabetologia.
52:17–30. 2009. View Article : Google Scholar
|
|
38
|
Mita M, Mita A and Rowinsky EK: mTOR
inhibition for cancer therapy: past, present and future.
Springer-Verlag; Paris: 2015
|
|
39
|
Molina AM, Feldman DR, Voss MH, Ginsberg
MS, Baum MS, Brocks DR, Fischer PM, Trinos MJ, Patil S and Motzer
RJ: Phase 1 trial of everolimus plus sunitinib in patients with
metastatic renal cell carcinoma. Cancer. 118:1868–1876. 2012.
View Article : Google Scholar
|
|
40
|
Hassan B, Yusoff Z and Othman: A close
look at neutropenia among cancer patients - risk factor and
management. Updates on Cancer Treatment. IntechOpen.
2015.https://www.intechopen.com/books/updates-on-cancer-treatment/a-close-look-at-neutropenia-among-cancer-patients-risk-factor-and-management.
Accessed October 28, 2015. View
Article : Google Scholar
|
|
41
|
Ju Y, Hu Y, Sun S, Wang J and Jiao S:
Toxicity and adverse effects of everolimus in the treatment of
advanced nonsmall cell lung cancer pretreated with chemotherapy -
Chinese experiences. Indian J Cancer. 52(Suppl 1): e32–e36. 2015.
View Article : Google Scholar
|
|
42
|
Kanesvaran R, Watt K, Turnbull JD,
Armstrong AJ, Wolkowiez MC and George DJ: A single-arm phase 1b
study of everolimus and sunitinib in patients with advanced renal
cell carcinoma. Clin Genitourin Cancer. 13:319–327. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Amato RJ, Jac J, Giessinger S, Saxena S
and Willis JP: A phase 2 study with a daily regimen of the oral
mTOR inhibitor RAD001 (everolimus) in patients with metastatic
clear cell renal cell cancer. Cancer. 115:2438–2446. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kuter DJ: Managing thrombocytopenia
associated with cancer chemotherapy. Oncology (Williston Park).
29:282–284. 2015.
|
|
45
|
Nishino M, Brais LK, Brooks NV, Hatabu H,
Kulke MH and Ramaiya NH: Drug-related pneumonitis during mammalian
target of rapamycin inhibitor therapy in patients with
neuroendocrine tumors: A radiographic pattern-based approach. Eur J
Cancer. 53:163–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Torrisi JM, Schwartz LH, Gollub MJ,
Ginsberg MS, Bosl GJ and Hricak H: CT findings of
chemotherapy-induced toxicity: What radiologists need to know about
the clinical and radiologic manifestations of chemotherapy
toxicity. Radiology. 258:41–56. 2011. View Article : Google Scholar
|
|
47
|
Limper AH: Chemotherapy-induced lung
disease. Clin Chest Med. 25:53–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vlahovic G, Meadows KL, Uronis HE, Morse
MA, Blobe GC, Riedel RF, Zafar SY, Alvarez-Secord A, Gockerman J,
Starodub AN, et al: A phase I study of bevacizumab, everolimus and
panitumumab in advanced solid tumors. Cancer Chemother Pharmacol.
70:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weisshaar E, Fleischer AB Jr, Bernhard JD,
et al: Pruritus and dysesthesia. Dermatology. Bolognia JL, Jorizzo
JL and Schaffer JV: Elsevier Saunders; pp. 111–125. 2012
|
|
50
|
Jóźwiak S, Kotulska K, Berkowitz N,
Brechenmacher T and Franz DN: Safety of everolimus in patients
younger than 3 years of age: results from EXIST-1, a randomized,
controlled clinical trial. J Pediatr. 172:151–155.e1. 2016.
View Article : Google Scholar
|
|
51
|
Castagnola E, Fontana V, Caviglia I,
Caruso S, Faraci M, Fioredda F, Garrè ML, Moroni C, Conte M,
Losurdo G, et al: A prospective study on the epidemiology of
febrile episodes during chemotherapy-induced neutropenia in
children with cancer or after hemopoietic stem cell
transplantation. Clin Infect Dis. 45:1296–1304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tan P, Tiong IS, Fleming S, Pomilio G,
Cummings N, Droogleever M, McManus J, Schwarer A, Catalano J, Patil
S, et al: The mTOR inhibitor everolimus in combination with
azacitidine in patients with relapsed/refractory acute myeloid
leukemia: A phase Ib/II study. Oncotarget. 8:52269–52280. 2016.
View Article : Google Scholar
|
|
53
|
Amato RJ, Flaherty AL and Stepankiw M:
Phase I trial of everolimus plus sorafenib for patients with
advanced renal cell cancer. Clin Genitourin Cancer. 10:26–31. 2012.
View Article : Google Scholar
|
|
54
|
Aapro M, Andre F, Blackwell K, Calvo E,
Jahanzeb M, Papazisis K, Porta C, Pritchard K and Ravaud A: Adverse
event management in patients with advanced cancer receiving oral
everolimus: Focus on breast cancer. Ann Oncol. 25:763–773. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Quek R, Wang Q, Morgan JA, Shapiro GI,
Butrynski JE, Ramaiya N, Huftalen T, Jederlinic N, Manola J, Wagner
AJ, et al: Combination mTOR and IGF-1R inhibition: Phase I trial of
everolimus and figitumumab in patients with advanced sarcomas and
other solid tumors. Clin Cancer Res. 17:871–879. 2011. View Article : Google Scholar
|
|
56
|
Staves KL and Ramchandran KJ: Prevention
and treatment options for mTOR inhibitor-associated stomatitis.
JCSO. 15:74–81. 2017. View Article : Google Scholar
|
|
57
|
Lacouture M and Sibaud V: Toxic Side
Effects of Targeted Therapies and Immunotherapies Affecting the
Skin, Oral Mucosa, Hair, and Nails. Am J Clin Dermatol. 19(Suppl
1): 31–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Watters AL, Epstein JB and Agulnik M: Oral
complications of targeted cancer therapies: A narrative literature
review. Oral Oncol. 47:441–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Raber-Durlacher JE, Elad S and Barasch A:
Oral mucositis. Oral Oncol. 46:452–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Priestman T: Cancer chemotherapy in
clinical practice. Springer; New York, NY: 2012, View Article : Google Scholar
|
|
61
|
Razmara F and Khayamzadeh M: An
Investigation into the prevalence and treatment of oral mucositis
after cancer treatment. Int J Cancer Manag. 12:e884052019.
View Article : Google Scholar
|
|
62
|
Naidu MU, Ramana GV, Rani PU, Mohan IK,
Suman A and Roy P: Chemotherapy-induced and/or radiation
therapy-induced oral mucositis - complicating the treatment of
cancer. Neoplasia. 6:423–431. 2004. View Article : Google Scholar : PubMed/NCBI
|