Introduction
Chondroitin sulfate proteoglycan 4 (CSPG4), also
known as high molecular weight-melanoma associated antigen
(HMW-MAA) or melanoma chondroitin sulfate proteoglycan (MCSP) was
first characterized on human melanoma cells 40 years ago (1). CSPG4 is a single-pass type I
transmembrane protein expressed either as an 280-kDa N-linked
glycoprotein or as a 450-kDa chondroitin sulfate proteoglycan
(2). Although CSPG4 was
originally associated only with melanoma progression due to its
widespread expression in the majority (≥70%) of these tumors
(3), it was later also detected
in other hematological and solid neoplastic conditions, including
several types of leukemia (4),
head and neck squamous-cell carcinomas (5), triple-negative breast carcinoma
(TNBC) (6), gliomas (7), pancreatic tumors (8), soft-tissue sarcomas (9) and malignant mesothelioma (10). As a transmembrane proteoglycan,
CSPG4 functions as a key mediator molecule connecting the
extracellular matrix (ECM) with intracellular binding partners
(11). CSPG4 thus activates major
signaling pathways involved in melanoma cell survival,
proliferation, migration and invasion, in particular via the
integrin-regulated focal adhesion kinase (FAK) pathway and receptor
tyrosine kinase (RTK)-mediated mitogen-activated protein kinase
(MAPK) cascade (11-13).
Due to its ability to influence different functions
of tumor cells and to its restricted distribution in adult healthy
tissues, CSPG4 is perceived as an attractive target for anti-tumor
immunotherapy (13,14). To date, only a limited number of
anti-CSPG4 monoclonal antibodies (mAbs) targeting CSPG4-positive
tumors has been described, including 9.2.27 mAb, 225.28 mAb and
TP41.2 mAb (6,10,15).
The highly specific mAb 9.2.27 directed against the
core glycoprotein of the chondroitin sulfate proteoglycan has been
widely used for immunodiagnostic imaging of CSPG4 and as a basis
for immunotherapy. The majority of research on therapeutic
approaches involved CSPG4-specific 9.2.27 mAb coupled to a variety
of cell death-inducing agents (16). One interesting concept based on
the α-particle-emitting radioisotope 213bismuth
conjugated to the mAb (213Bi-9.2.27) was found to be
highly specific and cytotoxic to melanoma cells (17). Another treatment strategy involved
a chemical conjugate of 9.2.27 mAb with the pseudomonas exotoxin A
(PE), resulting in the melanoma-specific 9.2.27PE immunotoxin that
efficiently killed cells in vitro (18). A different approach was based on
the TNF-related apoptosis-inducing ligand (TRAIL) conjugated to an
anti-CSPG4 scFv based on the mAb 9.2.27. Treatment with this
anti-MCSP:TRAIL construct resulted in apoptotic melanoma cell death
in vitro and exerted no off-target effects on normal
melanocytes (19). In addition,
it caused a significant growth retardation of human melanoma
xenografts.
Apart from malignant melanoma, the 9.2.27 mAb was
employed to inhibit the growth of other CSPG4-positive tumor types,
including soft-tissue sarcoma (9), triple-negative breast carcinoma as a
9.2.27 mAb-based cytolytic fusion protein (αCSPG4(scFv)-MAP) with
pro-apoptotic activity (20) and
glioblastoma multiforme as a PEGylated mAb used in combination with
adoptive natural killer (NK) cell transfer (21).
Over the years, the use of BRAF inhibitors (BRAFi)
has become a valid anti-melanoma therapeutic strategy for patients
with confirmed BRAF mutations (22,23). However, even when combined with a
mitogen-activated protein kinase kinase (MEK) inhibitor, these
treatment modalities rarely lead to a complete clinical response
due to intrinsic or acquired resistance (24). Thus, additional treatment options
or alternative treatment combinations with the potential to
overcome resistance are required for the better management of
patients with metastatic melanoma.
Yu et al (25) indicated that the addition of the
anti-CSPG4 225.28 mAb to treatment with the BRAF inhibitor,
PLX4032, enhanced the response magnitude and the duration of
PLX4032 efficacy in CSPG4-positive melanoma cells. In addition, it
was previously revealed that CSPG4-specific polyclonal antibodies
enhanced the anti-proliferative effects of PLX4032 in melanoma cell
lines (26). However, the
beneficial effect of this combinational treatment was partially
blocked under hypoxic conditions (26).
On the ground of a number of promising studies
employing the CSPG4-specific 9.2.27 mAb, the authors wished to
determine whether it could synergize with the potent BRAF
inhibitor, PLX4032, in constraining various melanoma cellular
functions. Thus, the present study, validated the antitumor
efficacy of each agent individually, as well as the effects of a
combined treatment that may lead to better results than each agent
alone.
The present study investigated the behavior of
melanoma cells following treatments not only in two-dimensional
cell culture assays, but also in a more physiologically relevant
system utilizing 3D tumor spheroids. Spheroids are scaffold-free
spherical self-assembled aggregates of cancer cells (27). In living organisms, cells are
organized in 3D microenvironments with complex cell-cell and
cell-matrix interactions and intricate transport dynamics.
Therefore, 3D tumor spheroids better resemble a living tissue with
respect to the cellular communication and the development of an
extracellular matrix (28).
Moreover, 3D cell cultures provide more correct cell polarization,
since the cells in monolayers can be only partially polarized
(29). In addition, this type of
three-dimensional cell culture system has contributed to reduce the
use of laboratory animal models (27). Melanoma spheroids have been proven
to be a very useful model for studying novel therapeutics and their
anti-invasive and anti-metastatic effects (30-34). Such models are utilized in cancer
research as a more accurate representation of the in vivo
tumor microenvironment as compared to traditional two-dimensional
(2D) cell culture. A melanoma spheroid model is able to mimic the
effects of cell-cell interactions, hypoxia and nutrient
deprivation, and drug penetration. 3D tumor spheroids have been
established as tumor models for a number of years; however, over
the past decade, they have come into more common usage as an in
vitro model for solid tumors, e.g., melanoma (33). These models are increasingly being
used in high-throughput drug discovery screens as an intermediate
between complex, expensive and time-consuming in vivo models
and the simple, low cost 2D monolayer model (30).
In the present study, it was demonstrated that the
exposure of the CSPG4-positive WM164 melanoma cell line to the
CSPG4-specific 9.2.27 mAb decreased viability, colony formation
ability and invasion, which was not the case for the CSPG4-negative
cell line, M14. Notably, the 9.2.27 mAb contributed to an
additional inhibition of WM164 cell viability, as compared with the
use of PLX4032 alone. By contrast, combined treatment of the WM164
cells with 9.2.27 mAb and PLX4032 did not exert any significant
additional effect in clonogenic and invasion assays. Cell cycle
arrest in the S phase was observed upon exposure to the antibody.
These findings provide the basis for further investigation of
CSPG4-antibodies for the treatment of CSPG4-positive tumors.
Materials and methods
Cell lines and reagents
The human CSPG4-positive melanoma cell lines, WM9,
WM35, WM164, 451Lu, and the human CSPG4-negative melanoma cell
line, M14, all harboring the BRAF V600E mutation, were previously
described (35,36). The cells were maintained in
RPMI-1640 medium with 2 mM L-glutamine and 25 mM Hepes (Lonza
Group, Ltd.), supplemented with either 5% FBS (WM9, WM35, WM164 and
451Lu) or 10% FBS (M14) and 1% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). Cells were cultured in a
humidified atmosphere containing 5% CO2 and 95% ambient
air at 37°C. Prior to the experiments, all cell lines tested
negative for mycoplasma. PLX4032, a potent inhibitor of mutant BRAF
V600, was purchased from Selleck Chemicals. The mouse mAb clone
9.2.27 recognizing CSPG4 (#CUST04896) was obtained from
eBioscience™ (Thermo Fisher Scientific, Inc.). Control mouse IgG
(#I5381) was obtained from Sigma-Aldrich; Merck KGaA.
MTT assay
To investigate cell viability upon exposure to
increasing concentrations of PLX4032 and CSPG4-specific 9.2.27 mAb,
a CytoSelect™ MTT Cell Proliferation assay (Cell Biolabs, Inc.) was
performed according to the manufacturer's instructions. Briefly,
the melanoma cells were seeded in triplicates at a density of 6,000
cells per well in 96-well plates and subjected to the following
treatments for 24 and 72 h. The concentrations used for the
experiments were as follows: PLX4032 at 0, 0.01, 0.1 and 0.25
µM; anti-CSPG4 9.2.27 mAb or IgG control at 0, 0.2, 2, 5 and
10 µg/ml and their combinations thereof. The cells were then
incubated with MTT reagent for 3 h at 37°C and solubilized. The
absorbance was measured at 540 and 570 nm using a Spark®
multimode microplate reader (Tecan Group Ltd.). The presented data
are the results of three independent experiments performed in
triplicate and are shown as percentage of viable cells, compared
with the untreated control cells.
Flow cytometry
Melanoma cells were harvested by scraping, washed
with 1X PBS and dispensed into FACS tubes. The cells were then
incubated with Fixable Viability Dye eFluor® 506
(Affymetrix; Thermo Fisher Scientific, Inc.) for 30 min at 4°C in
dark according to the manufacturer's protocol. The cells were then
washed with FACS buffer (0.5% BSA and 0.05% sodium azide
(NaN3) in 1X PBS) and incubated with anti-CSPG4 antibody
9.2.27 (1:1,000; cat. no. 554275; BD Pharmingen; BD Biosciences)
for 10 min at 4°C, washed with FACS buffer and incubated with
donkey anti-mouse secondary IgG antibodies Alexa Fluor
488® (1:500) for 15 min at 4°C, protected from light. As
a control for the IgG antibodies, cells were incubated with the
Alexa Fluor 488® secondary antibody (1:500; cat. no.
A-21202, Thermo Fisher Scientific, Inc.) only also for 15 min at
4°C, protected from light. The cells were washed and resuspended in
FACS buffer. The samples were analyzed using a FACSCanto flow
cytometer (BD Biosciences). FlowJo software version 10.6.1
(TreeStar Inc.) was used for the analysis of the results.
Colony formation assay
Melanoma cells were seeded in duplicates into 6-well
plates at a density of 1,000 cells per well and subjected to the
following treatments: PLX4032 (0.1 µM), 9.2.27 mAb (2
µg/ml), PLX4032 (0.1 µM) plus 9.2.27 mAb (2
µg/ml), IgG control (2 µg/ml), PLX4032 (0.1
µM) plus IgG control (2 µg/ml). Untreated cells
served as a control. The cells were incubated until they formed
colonies at approximately after 12 days for the M14 cell line and
16 days for the WM164 cell line. The cells were then washed with
PBS, fixed with 4% paraformaldehyde solution in PBS for 30 min at
room temperature and stained using crystal violet solution (0.2%
crystal violet and 2% ethanol in ddH2O) for 30 min at
room temperature. The number of colonies including >50 cells was
quantified using the ImageJ software version 1.53 (National
Institutes of Health). The presented data are the results of three
independent experiments performed in duplicate.
Spheroid invasion assay
For creating spheroids, the hanging drop method was
used. The WM164 and M14 melanoma cells untreated or exposed to
specific treatments were harvested by scraping and resuspended in
culture medium containing 0.3% methylcellulose. Drops of 30
µl of the suspension (~1,000 cells) were distributed equally
over a 10-cm dish. The plates were incubated upside down for 2 days
at 37°C to allow the formation of stable spheroids. The hanging
drops were then collected into a 50 ml falcon tube and embedded
into 1.5% rat tail collagen gels (Corning™ 354236, Merck KGaA). To
prepare collagen gels, a 3% collagen solution was mixed with an
equal volume of 0.85% (w/v) methylcellulose with RPMI-1640 culture
medium supplemented with either 5% FBS (WM164) or 10% FBS (M14).
The spheroid suspension was pipetted into 24-well plates (350
µl/well) and placed into an incubator for 30 min at 37°C for
polymerization. For stimulation, collagen gels were overlaid with
medium containing 0.5% FBS and then incubated at 37°C. The
quantification of sprouting intensity after 24 h of incubation was
determined using a Nikon inverted phase-contrast microscope. The
area of spheroids was measured using ImageJ software version 1.53
(National Institutes of Health). For each treatment 3 spheroids
were quantified. The presented data are the results of three
independent experiments performed in triplicates.
Apoptosis assay
The Annexin V-CF Blue/7-AAD Apoptosis Detection kit
(Abcam) was used to estimate the percentage of intact
(Annexin−, 7-AAD−), apoptotic
(Annexin+, 7-AAD−) or necrotic
(Annexin+, 7-AAD+) cells following 72 h of
treatments. The analysis was performed according to the
manufacturer's instructions. In brief, cells were seeded in
triplicate in six-well plates and subjected to the following
treatments: PLX4032 (0.1 µM), 9.2.27 mAb or IgG control (2
µg/ml) and PLX4032 (0.1 µM) plus 9.2.27 mAb (2
µg/ml) or IgG control (2 µg/ml). Untreated cells
served as a control. The cells were then harvested, washed with PBS
and resuspended in Annexin-binding buffer. The cells were then
incubated in the dark with Annexin V-CF Blue Conjugate and 7-AAD
Staining Solution for 15 min at room temperature. The samples were
analyzed using a FACSCanto flow cytometer (BD Biosciences). FlowJo
software version 10.6.1 (TreeStar Inc.) was used for the analysis
of the results. The presented data are the results of three
independent experiments performed in triplicate.
Cell cycle analysis
The analysis of the cell cycle was performed using a
Propidium Iodide Flow Cytometry kit (Abcam) according to the
manufacturer's protocol. Briefly, melanoma cells were seeded in
triplicate in six-well plates and exposed to the following
treatments: PLX4032 (0.1 µM), 9.2.27 mAb (2 µg/ml),
PLX4032 (0.1 µM) plus 9.2.27 mAb (2 µg/ml), IgG
control (2 µg/ml), PLX4032 (0.1 µM) plus IgG control
(2 µg/ml) for 72 h. Untreated cells served as a control. The
cells were then harvested in a single cell suspension and fixed
with 66% ethanol for at least 2 h, at 4°C. The cells were then
washed with PBS and resuspended in 1X propidium iodide + RNase
staining solution. Following incubation for 30 min at 37°C, the
cells were analyzed for cell cycle distribution with a FACSCanto
flow cytometer (BD Biosciences). FlowJo software version 10.6.1
(TreeStar Inc.) was used for the analysis of the results. The
presented data are the results of three independent experiments
performed in triplicate.
Statistical analysis
The statistical analysis of differences between
treated groups was performed using one-way ANOVA with Tukey's
multiple comparisons test. P-values <0.05 were considered to
indicate statistically significant differences. All statistical
analyses of the experiments were carried out using GraphPad Prism
software version 4.03 and 9.01.151 (GraphPad Software, Inc.).
Results
Effects of CSPG4-specific 9.2.27 mAb and
PLX4032 alone or in combination on melanoma cell viability
The present study first verified the effects of the
potent BRAF V600 inhibitor, PLX4032, as well as those of the
CSPG4-specific 9.2.27 mAb, and the combination of both on the
viability of melanoma cell lines. To determine the most appropriate
concentrations for treatments, dose-titration experiments were
performed (Fig. S1). The
concentration of 0.1 µM PLX4032 was selected for the study
as it inhibited ~50% of the viability of the WM164 and M14 cells
(Fig. S1). For the
CSPG4-specific 9.2.27 mAb, the lowest concentration of the mAb (2
µg/ml) which exerted a statistically significant effect on
the viability of CSPG4-positive WM164 cells alone and in
combination with 0.1 µM PLX4032 was selected (Fig. S1A).
Exposure of the cells to PLX4032 resulted in a
decreased cell viability of CSPG4-negative M14 and CSPG4-positive
WM164 cells, as compared with the untreated cells (Fig. 1). The viability of the
PLX4032-exposed WM164 cells decreased by 18.3±1.7% following
incubation for 24 h and by 47.3±2.6% after 72 h (Fig. 1A). The viability of the M14 cells
exposed to PLX4032 decreased by 9±1.4% and by 39.3±3.9% following
24 and 72 h of incubation, respectively (Fig. 1B). The exposure of the WM164 cell
line to the CSPG4-specific 9.2.27 mAb significantly decreased cell
viability, which was not the case for the CSPG4-negative M14 cell
line (Fig. 1A and B). The
specificity of the effect of the 9.2.27 mAb was repeated in four
CSPG4-high expressing melanoma cells (Fig. S2). The IgG control antibody had
no detectable effect on both WM164 and M14 cell lines (Fig. 1A and 1B). When combined with PLX4032, the
anti-CSPG4 mAb contributed to a significant, additional inhibition
of WM164 cell viability, as compared with the cells treated with
BRAF inhibitor alone, decreasing the viability by 37.3±5.4% after
24 h and by 60.7±2.4% after 72 h (Fig. 1A). This decreased viability was
indeed CSPG4-dependent since the combined treatment of
CSPG4-negative M14 cells with PLX4032 plus 9.2.27 mAb did not exert
any additional effect, as compared to treatment with PLX4032 alone
(Fig. 1B). The combination of
PLX4032 with the IgG control resulted in the same effect as PLX4032
for both cell lines tested (Fig. 1A
and B).
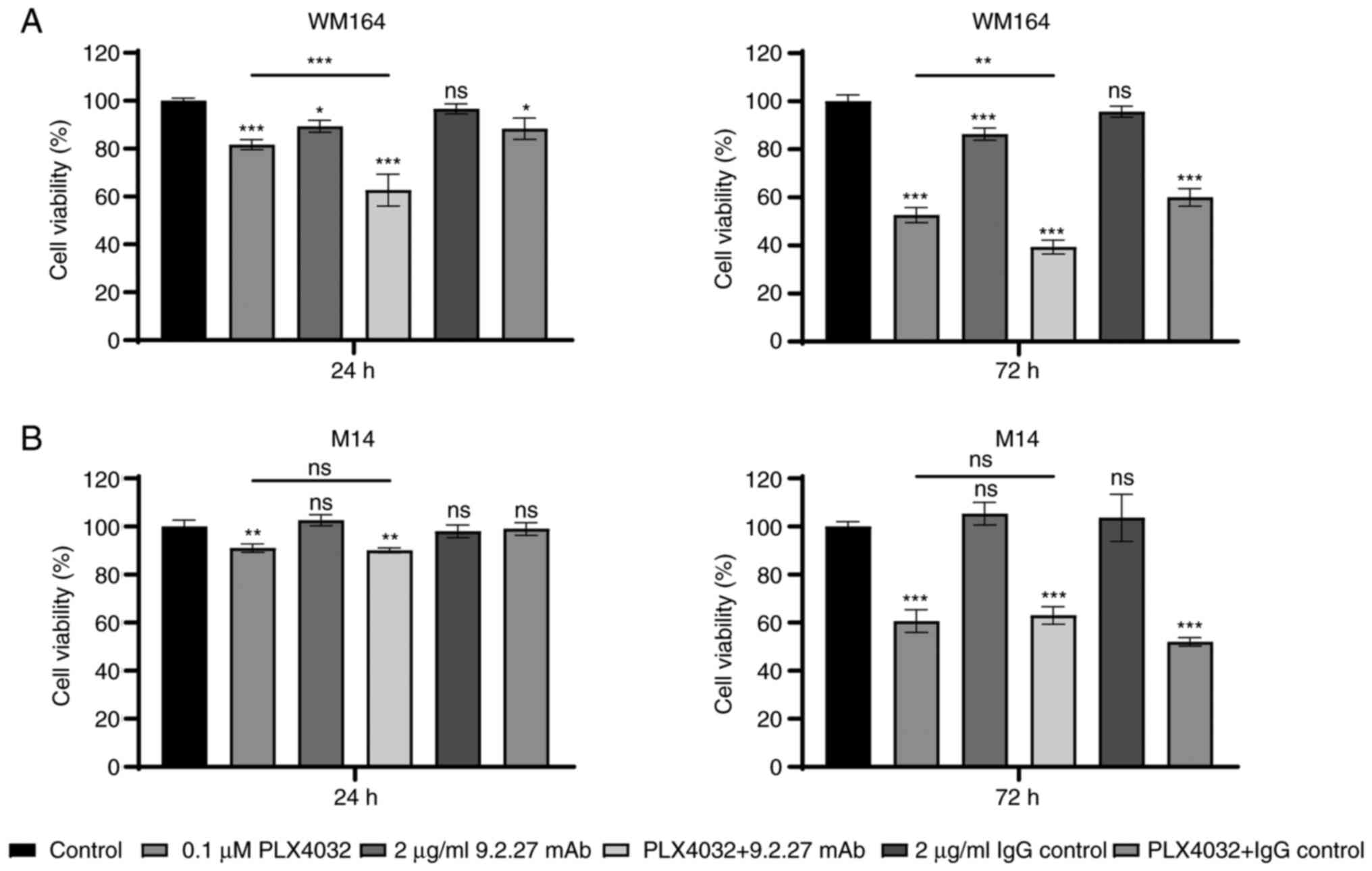 | Figure 1Viability of melanoma cells following
treatment with PLX4032, CSPG4-specific 9.2.27 mAb and the
combination thereof. (A) Viability of CSPG4-positive WM164 cells
and (B) viability of CSPG4-negative M14 cells following exposure of
the cells to the treatments [PLX4032 (0.1 µM), 9.2.27 mAb (2
µg/ml), PLX4032 (0.1 µM) plus 9.2.27 mAb (2
µg/ml), IgG control (2 µg/ml), PLX4032 (0.1
µM) plus IgG control (2 µg/ml)] was measured using
MTT assay after 24 h (left panels) and 72 h (right panels). The
results are presented as the percentage of viable cells, compared
with the untreated cells (control) and represent three independent
experiments performed in triplicate. Bars represent the mean ± SD.
Statistical analysis was carried out using one-way ANOVA with
Tukey's multiple comparisons test. P-values are represented by
asterisks (*): ***P<0.0001, **P<0.01,
*P<0.05; ns, not significant. Asterisks above each
bar indicate significance, as compared with the control bar. Line
with depicted asterisks above different bars indicates significance
between relevant bars. CSPG4, chondroitin sulfate proteoglycan 4;
mAb, monoclonal antibody. |
In addition, the present study evaluated using
bright-field microscopy, whether changes in cell viability
following the treatments were reflected in differences in the
density and morphology of the melanoma cells. Indeed, as
illustrated in the representative images, PLX4032 decreased the
number of both WM164 and M14 cells and it affected cell morphology,
as compared with the control (Fig.
S3). Exposure to the 9.2.27 mAb for 72 h resulted in a
decreased density of CSPG4-positive WM164 cells, whereas it had no
effect on CSPG4-negative M14 cells.
Taken together, the results of MTT assay proved that
the exposure to PLX4032 efficiently inhibited the viability of BRAF
V600E-mutant cell lines. Moreover, the anti-CSPG4 mAb specifically
decreased the viability of only CSPG4-positive cells and enhanced
the effects of PLX4032. The specific effect on cell viability was
observed at even after 24 h and continued after 72 h. Therefore,
these incubation times were used in the present study.
CSPG4-specific 9.2.27 mAb exerts similar
effect as PLX4032 on the colony-forming ability of CSPG4-positive
melanoma cells
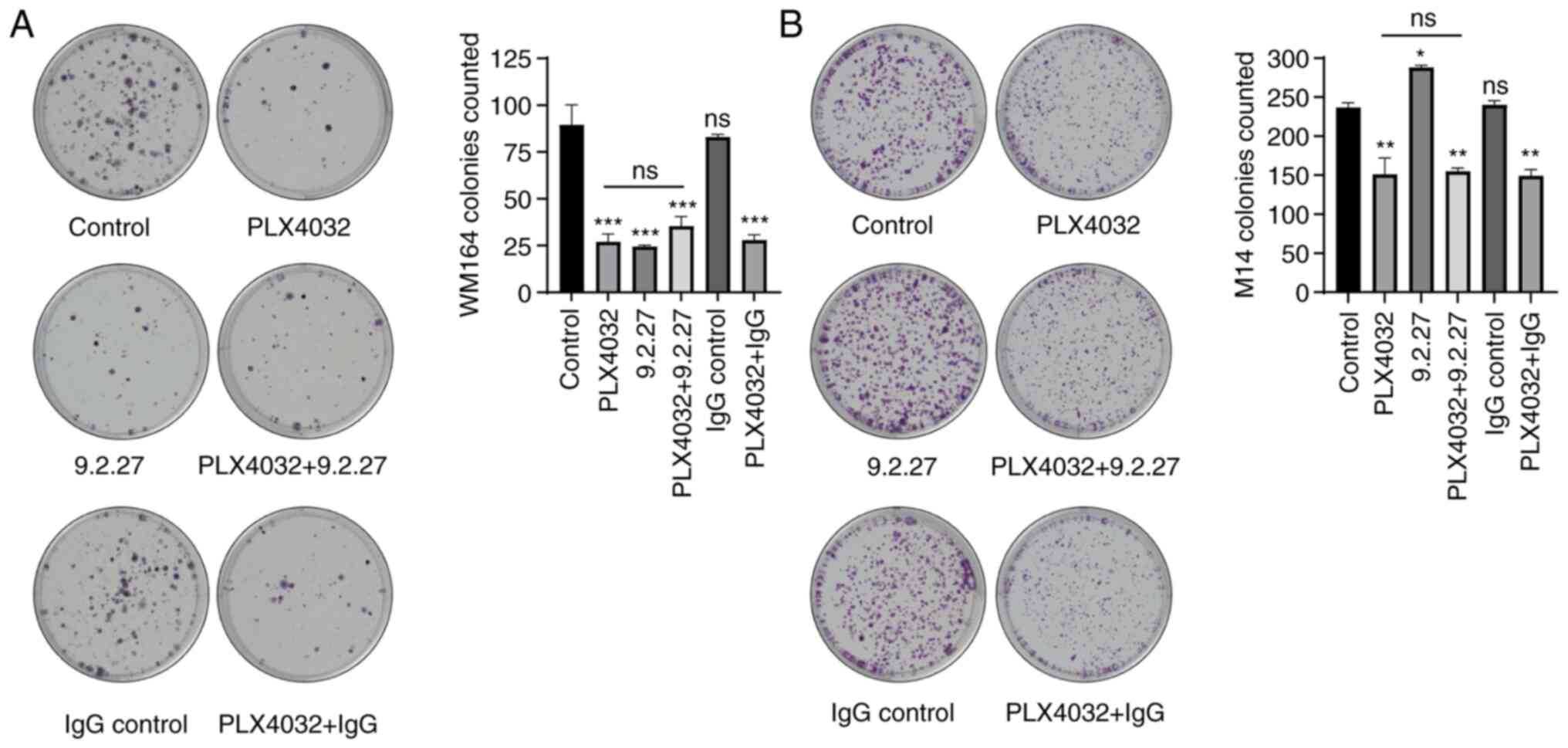
After demonstrating that the viability of both WM164
and M14 cells was reduced following exposure to PLX4032, and that
only CSPG4-positive cells were influenced by incubation with 9.2.27
mAb, the present study investigated whether these treatment
regimens had an effect on the colony-forming ability of melanoma
cells. For this purpose, cells were plated as single cells and
subjected to treatments as described in the Materials and methods
section for the time period required for cells to form colonies (12
days for the M14 cell line and 16 days for the WM164 cell line).
The M14 cells exhibited a significantly reduced ability to form
colonies when exposed to PLX4032 (average of 236.5±4.5 colonies in
untreated cell line versus 149.5±16.5 colonies after BRAFi), while
no inhibitory effect of the CSPG4-specific mAb was observed
(Fig. 2B). Furthermore, the
combination of BRAF inhibitor with the mAb resulted in the same
effect as that observed with PLX4032 alone (Fig. 2B).
The colony-forming inhibitory effect of PLX4032 was
also observed in the WM164 cells (Fig. 2A). These CSPG4-expressing cells
exhibited a significantly reduced ability to form colonies also
when incubated with CSPG4-specific 9.2.27 mAb (Fig. 2A). Both treatments separately
reduced the number of colonies by ~70%. The combination of PLX4032
with the 9.2.27 mAb did not exert any additional inhibitory effect
on colony formation, as compared with either agent alone (Fig. 2A).
In summary, incubation with the 9.2.27 mAb exerted a
similar inhibitory effect on the colony-forming ability of
CSPG4-positive melanoma cells as that observed with PLX4032 alone.
It also did not contribute to an additional decrease in the number
of colonies when used in combination with the BRAF inhibitor.
CSPG4-specific 9.2.27 mAb decreases the
invasion of CSPG4-positive melanoma cells
CSPG4 is a multifunctional transmembrane
proteoglycan involved in the induction of melanoma cell invasion
(3). Hence, in the following
experiment, the present study investigated whether the
CSPG4-specfic 9.2.27 mAb alone or in combination with PLX4032
exerts an effect on melanoma cell invasion in a 3D spheroid model.
The ability to form stable spheroids has been previously reported
for WM164 cells (33).
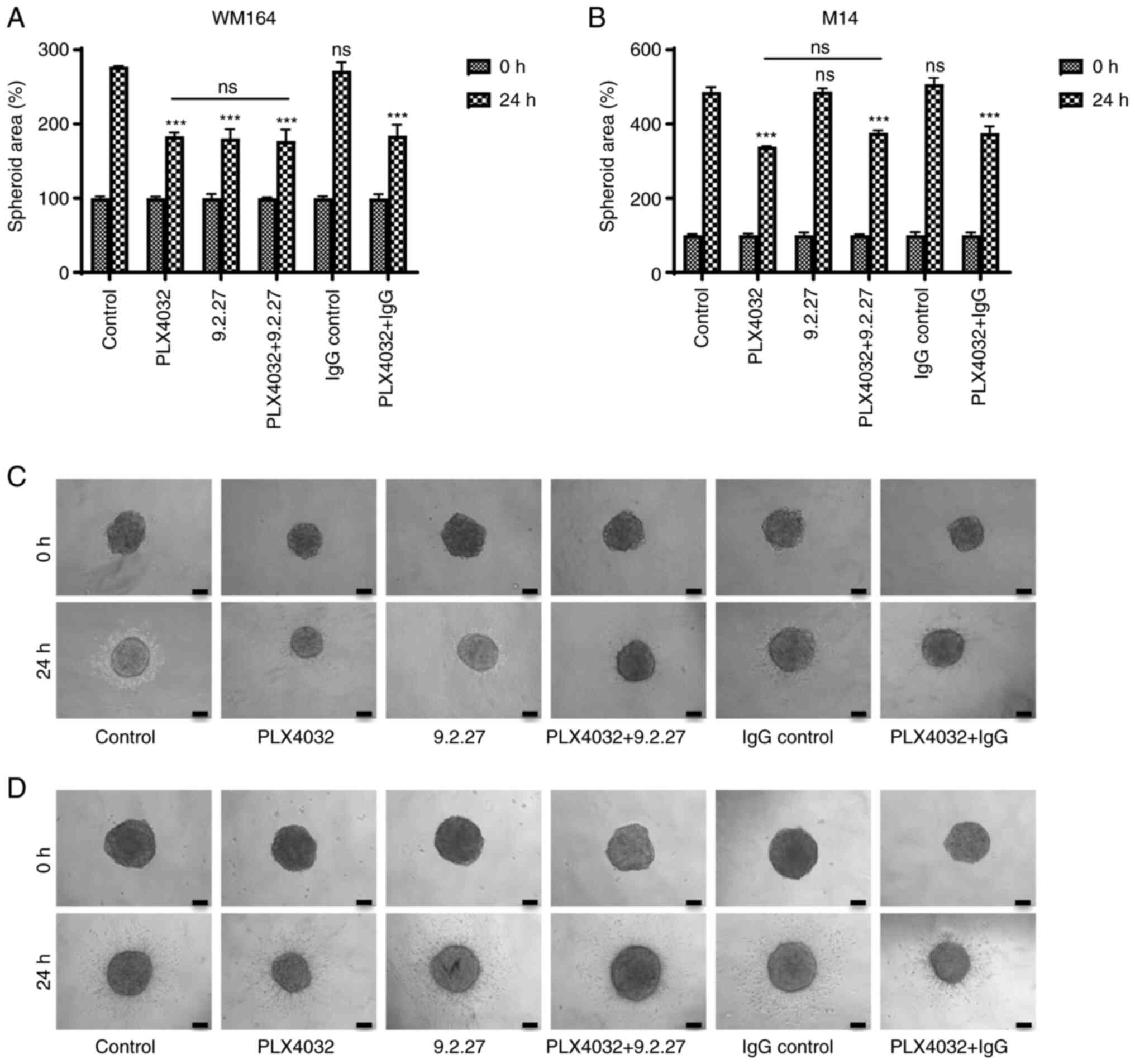
WM164 (CSPG4-positive) and M14 (CSPG4-negative)
cell-derived spheroids were exposed to different treatments and
their ability to invade into a collagen matrix was evaluated. In
line with the results of MTT assay, specific effects were observed
even after 24 h. Both the WM164 and M14 cell-derived spheroids
exposed to the BRAF inhibitor exhibited a significantly reduced
invasive ability as compared with the control (Fig. 3A and B). The size of the
PLX4032-exposed spheroids, as well as the area of invaded cells in
both cell lines was markedly smaller, when compared with the
control spheroids (Fig. 3C and
D). Incubation of WM164 spheroids with the CSPG4-specific
9.2.27 mAb significantly inhibited the invasion of CSPG4-positive
tumor cells (Fig. 3A). The
specificity of the effect of the 9.2.27 mAb was again confirmed by
the observation that the antibodies did not influence the invasion
of CSPG4-negative cells (Fig. 3B and
D). The area of invading cells was distinctly reduced in the
mAb-treated WM164 cell-derived spheroids, as compared with the
untreated or IgG control-treated spheroids (Fig. 3C). Notably, consistent with the
results of the colony formation assay, the combination of PLX4032
with the 9.2.27 mAb did not exert any additional effect, as
compared with the influence of each agent alone on spheroids
(Fig. 3A and C).
From these results, it can be concluded that the
9.2.27 mAb efficiently suppressed the CSPG4-mediated invasion of
CSPG4-positive, but not CSPG4-negative cells and that this effect
was comparable to the inhibitory effects of PLX4032.
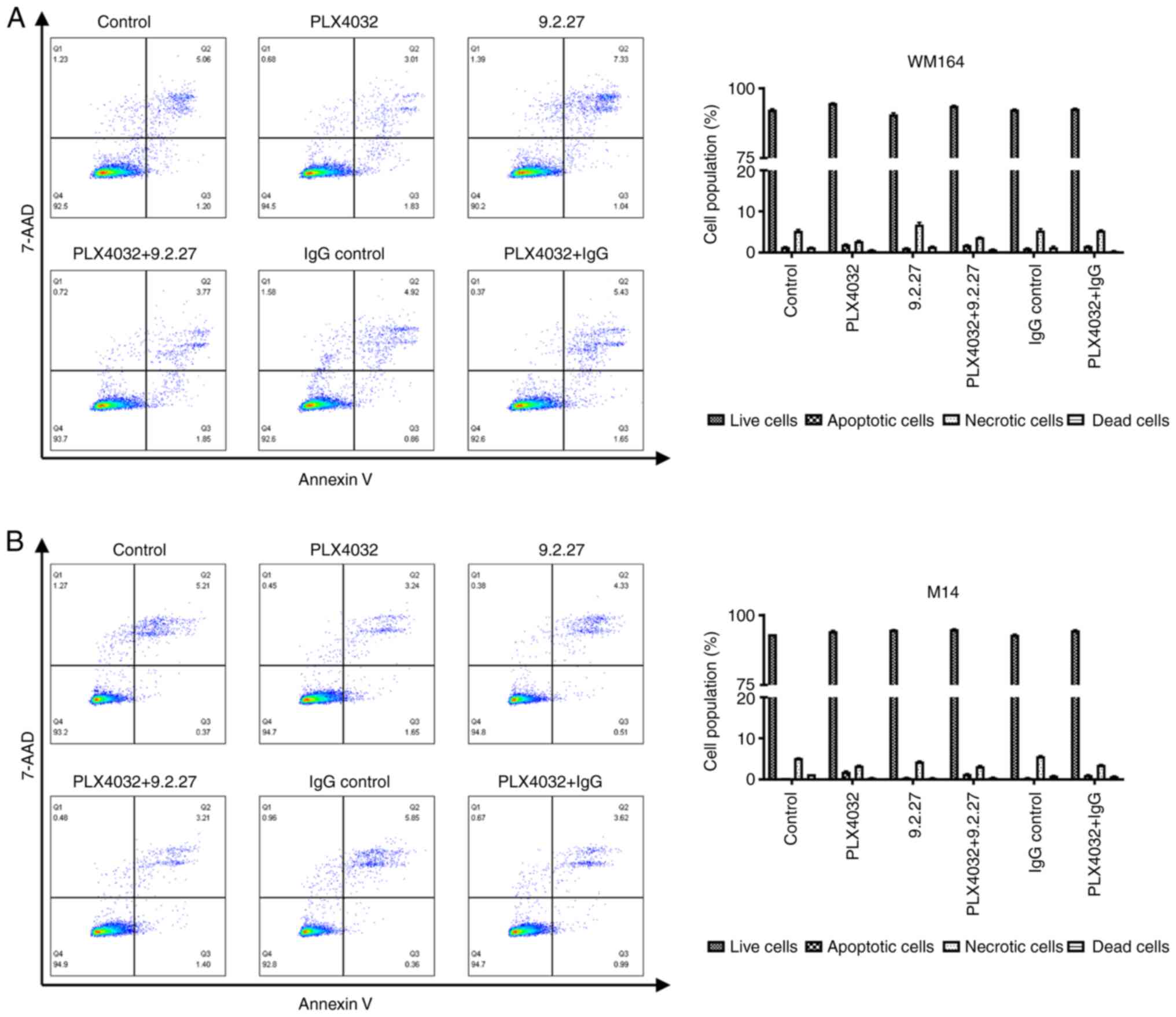
Characterization of the apoptosis of
melanoma cell lines
To gain insight into the potential mechanisms
through which these specific treatments (PLX4032, CSPG4-specific
mAb and the combination thereof) influenced the viability,
clonogenicity and invasiveness of melanoma cells, the flow
cytometric analysis of apoptosis was performed. Following exposure
to specific treatments, the WM164 and M14 cells were subjected to
Annexin V and 7-AAD staining in order to determine the proportions
of viable cells (Annexin−, 7-AAD−), early
apoptotic (Annexin+, 7-AAD−), late
apoptotic/necrotic (Annexin+, 7-AAD+), as
well as dead cells (Annexin−, 7-AAD+).
It was expected that the melanoma cells would
exhibit early signs of apoptosis following exposure to the
treatments at after 72 h if this process of programmed cell death
was induced. Exposure to PLX4032, the CSPG4-specific 9.2.27 mAb or
the combination thereof for 72 h did not induce the apoptosis of
either the WM164 nor M14 cells (Fig.
4). Incubation of the WM164 cells with PLX4032 induced early
apoptosis only in 1.96±0.12% of the cells, as compared with
1.32±0.14% of the control cells that were detected as apoptotic
(Fig. 4A, right panel). Exposure
of the WM164 cells to the CSPG4-specific 9.2.27 mAb increased the
percentage of necrotic cells to 6.81±0.94%, as compared with
5.11±0.38% of necrotic cells in the control; however, this effect
was not statistically significant (P=0.072; Fig. 4A, right panel). Moreover, the
increase in necrotic cells was not observed following treatment
with PLX4032 plus the 9.2.27 mAb. Exposure of the M14 cells to all
treatment variants resulted in 5.21±0.13% of cells that were
apoptotic, necrotic or dead, while 94.6±0.26% of the treated cells
were still detected as alive (Fig.
4B, right panel).
Taken together, these results indicated that the
incubation of melanoma cell lines during these treatments for 72 h
did not lead to the induction of apoptosis.
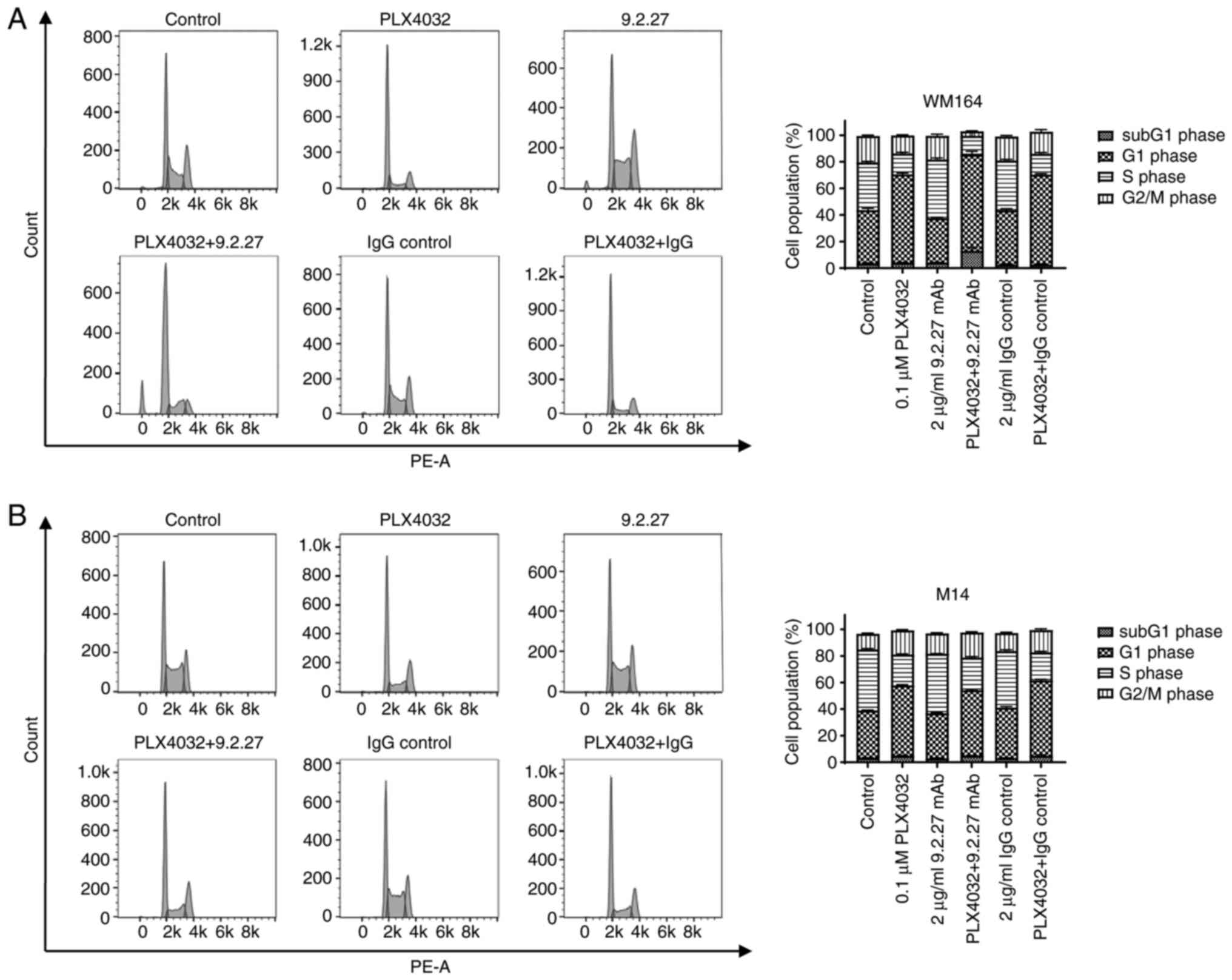
CSPG4-specific 9.2.27 mAb leads to WM164
cell cycle arrest in the S-phase
To determine whether the exposure of M14 and WM164
melanoma cells for 72 h to PLX4032, CSPG4-specific 9.2.27 mAb or
their combination in turn affects the cell cycle, the treated cells
were analyzed using flow cytometry after propidium iodide
staining.
Incubation of the CSPG4-positive WM164 cells with
the CSPG4-specific 9.2.27 mAb resulted in a significantly higher
number of cells arrested in the S phase (44.2±1.6%), as compared
with the control (35.9±0.96%) (Fig.
5A and Table SI). Moreover,
following the combined treatment, a significantly increased
accumulation of cells in the subG1 phase (16.63±5.1%), combined
with a decrease of cells in the G2/M phase (3.96±0.12%) was
observed, as compared with cell cycle distribution in the control
(3.44±0.66% of cells in the subG1 phase and 19.90±0.92% of cells in
the G2/M phase) (Fig. 5A and
Table SI). Exposure to PLX4032
led to a significant increase of cells in the G1 phase
(66.5±1.34%), as compared with the untreated cells (40.2% ±2.04)
(Fig. 5A and Table SI).
The cell cycle analysis of the CSPG4-negative M14
cell line revealed that the untreated control cells presented the
following cell cycle distribution: The subG1 phase, 3.43±0.17%; G1
phase, 35.6±0.21%; S phase, 45.87±0.82%; and G2/M phase,
11.73±0.78% cells (Fig. 5B).
Incubation with CSPG4-specific 9.2.27 mAb did not affect the cell
cycle distribution of M14 cells (Fig.
5B). The combination of PLX4032 and 9.2.27 mAb resulted in
similar cell cycle phases, as observed for PLX4032 alone (Fig. 5B). Upon exposure to PLX4032, a
significant increase of M14 cells in the G1 phase was observed
(52.9±0.78%) (Fig. 5B and
Table SII).
To verify whether the CSPG4-specific 9.2.27 mAb
affects CSPG4-positive WM164 cells in a concentration-dependent
manner, the cells were exposed to increasing concentrations of the
mAb (2-10 µg/ml) and cell cycle analysis was performed.
Indeed, higher antibody concentrations were associted with a higher
percentage of cells arrested in the S phase (Fig. S4).
Taken together, these results indicate that the
CSPG4-specific mAb can lead to cell cycle arrest in the S phase and
that the combination with the BRAF inhibitor PLX4032 may lead to an
increased cell death of CSPG4-postitive cells.
Discussion
Malignant melanoma is one of the most prevalent
forms of fatal skin cancer with a continuous increasing incidence
worldwide (37). Despite a
significant improvement of treatment options owing to the
introduction of BRAF and MEK inhibitors, a vast majority of
patients with melanoma cannot fully benefit from therapy due to the
intrinsic and acquired resistance to these drugs (22-24). mAbs constitute a rapidly expanding
class of agents for the treatment of different cancer types,
offering an alternative option to patients who have failed or
progressed on a standard therapy (38). One approach is the use of mAbs
that target the negative regulators of T-cell activation to yield
increased anti-tumor immunity, including antibodies directed
against programmed cell death-ligand 1 (PD-L1) and its receptor
programmed cell death protein 1 (PD-1). These immune checkpoint
inhibitor-based therapies have exhibited satisfactory clinical
results in the treatment of patients with metastatic melanoma and
other malignancies, such as lung cancer, colorectal cancer or renal
cell carcinoma (38). The
analysis of a phase 3 clinical trial revealed that the addition of
the anti-PD-L1 antibody atezolizumab to targeted therapy with BRAF
and MEK inhibitors significantly increased progression-free
survival in patients with advanced melanoma (39). However, there are currently no
clinically available antibodies that directly target membrane
associated melanoma-specific proteins, such as cell-adhesion
receptors. Some of the preclinical approaches focus on targeting
CSPG4, since this proteoglycan is overexpressed on melanomas with
only limited distribution on normal tissues and plays a central
role in oncogenic pathways required for malignant progression and
metastasis (13,14).
The use of an appropriate mAb against CSPG4, as well
as the validation of whether this specific anti-CSPG4 mAb can
synergize with kinase inhibitors in order to enhance the initial
response to the drug, may contribute to the design of more
effective treatments against melanoma. The present study analyzed
the antitumor effects of the CSPG4-specific mAb clone 9.2.27 alone
and in combination with the commonly used selective BRAF inhibitor,
PLX4032. To the best of our knowledge, this is the first study on
the combination of these two agents on different melanoma cellular
functions.
The results of the present study proved that
exposure to PLX4032 efficiently inhibited the viability of BRAF
V600E-mutant cells, both expressing (WM164) and not-expressing
(M14) CSPG4 (Figs. 1 and S1). Studies employing the
CSPG4-specific mAb clone 225.28 (25), as well as anti-CSPG4 polyclonal
Abs (26) demonstrated that these
antibodies reduced the viability of melanoma cells in vitro
by ~30%. In the present study, the mAb clone 9.2.27 also decreased
the viability of CSPG4-positive WM164 cells, whereas no reduction
of the CSPG4-negative M14 cell line could be observed (Fig. 1 and S1). This result confirmed the
specificity of the 9.2.27 mAb and proved that there was not even a
minimal off-target effect on CSPG4-negative M14 cells. The extent
of the decrease in viability following exposure to the 9.2.27 mAb
was lower than that in studies focusing on mAb conjugated to
radioisotopes or toxins (17,18). However, the effect of the antibody
alone on melanoma cell viability was not examined in these studies.
Nevertheless, the CSPG4-specific 9.2.27 mAb significantly enhanced
the effect of PLX4032 and reduced the viability of WM164 cells even
after 24 h by an additional 19% (Fig.
1A). This finding provided the rationale for further
experiments described in the present study, analyzing whether the
combination of a BRAF inhibitor with CSPG4-specific mAbs would be
more effective in inhibiting melanoma cell survival and
invasion.
CSPG4 is known to enhance cell survival through its
involvement in promoting high levels of integrin-related signals
and thus activating intracellular signaling cascades, particularly
the FAK and PI3K⁄AKT pathways (11,12). Indeed, incubation with the 9.2.27
mAb exerted a significant inhibitory effect on the colony-forming
ability of CSPG4-positive melanoma cells, to the same extent as to
treatment with PLX4032 alone (Fig.
2A). A combination of PLX4032 and 9.2.27 mAb, which
theoretically should influence different pathways and result in a
decreased capability of melanoma cells to form colonies, did not
contribute to any additional inhibitory effect (Fig. 2A).
It was hypothesized speculate that the
downregulation of CSPG4 expression in melanoma colonies may be
involved in this observation. As recently demonstrated by the
authors, exposure of CSPG4-positive melanoma cells to PLX4032 for
up to 14 days led to gradually reduced levels of the CSPG4 protein
and decreased levels of its mRNA (36). Therefore, the exposure of WM164
colonies to PLX4032 for a longer time period has probably led to a
downregulation of CSPG4 expression and -as a consequence-to a lower
binding of the CSPG4-specific mAb.
The progression of metastatic melanoma is a complex,
multi-step process of molecular events that eventually results in
an invasive phenotype. Since CSPG4 possesses the ability to
coordinate several melanoma pathways, it is involved in
tumorigenesis at multiple levels (3). Thus, the present study investigated
the invasive properties of cells following exposure to treatments
using 3D melanoma tumor spheroids.
Spheroids embedded in a collagen matrix reflect the
in vivo tumor architecture and microenvironment, as they
reconstruct the oxygen and nutrient gradient within the spheroid
with central necrosis and a hypoxic zone; features that may
influence the response to the treatment (30). The inhibitory effect of PLX4032 on
the growth and invasion of 3D melanoma spheroids was successfully
reflected in the regression of tumor growth in melanoma xenografts
(31,32). In line with these data, the
present study demonstrated that PLX4032 significantly inhibited the
invasion of M14 and WM164 spheroids (Fig. 3). Exposure to 9.2.27 mAb inhibited
the invasion of CSPG4-positive spheroids to the same extent as
PLX4032 (Fig. 3A and C). This
result may be attributed to the suppression of the CSPG4-mediated
invasion of cells, which involves the activation of MMP complexes
on the cell surface and binding to ECM components, including
collagen and fibronectin (40,41).
The use of spheroids in this experiment allows us to
hypothesize that this result could be projected to an in
vivo situation. Indeed, Hsu et al (9) demonstrated that a 9.2.27 mAb
immunotherapy inhibited the tumor growth of human sarcoma
xenografts. In addition, it has been demonstrated showed that
unconjugated 9.2.27 mAb is also able to suppress tumor growth in
athymic mice to the same extent as with antibody conjugated to
diphtheria toxin A chain (15).
The combination of anti-CSPG4 antibodies with BRAF inhibitors has
been studied to date only in two-dimensional cell culture assays
(25,26). The results presented herein
indicated that 9.2.27 mAb did not enhance the inhibitory effect of
PLX4032 on the invasiveness of CSPG4-positive spheroids (Fig. 3A and C). This may suggest that the
combination would have a similar effect on tumor growth in
vivo.
Hypoxia strongly influences the response to PLX432
treatment in melanoma cells which switch to a more invasive and
aggressive phenotype (26).
Therefore, therapeutic efforts have to take into account that the
microenvironment of melanoma cells has an impact on tumor
progression. Spheroids resemble the tumor hypoxic zone and CSPG4
expression has been shown to be upregulated both at the mRNA level
and the protein level under hypoxic conditions (8). The 9.2.27 mAb significantly
inhibited the invasion of CSPG4-positive spheroids, possibly
overcoming the CSPG4 overexpression by hypoxic conditions.
Therefore, it would be of importance to test
additional treatment variants before moving to in vivo
studies with the 9.2.27 mAb and BRAF inhibitors. One approach could
consist of first treating CSPG4-positive melanoma cells with the
9.2.27 mAb in order to restrict the CSPG4-dependendent growth,
motility and invasiveness of tumor and then adding PLX4032. The
intermittent dosing of this BRAF inhibitor alternating with
CSPG4-specfic mAb could increase the duration of the initial
response and delay or even prevent the development of resistance to
the BRAF inhibitor.
The present study focused on investigating the
underlying mechanisms of treatments on melanoma cells by
discriminating live, early apoptotic and late apoptotic or necrotic
cells along with assessing cell cycle distribution by flow
cytometry. Exposure to PLX4032, the CSPG4-specific 9.2.27 mAb or
the combination thereof for 72 h did not induce the apoptosis of
either the M14 or WM164 cells (Fig.
4). Thus, it was suspected that changes in cell cycle
distribution would be detected in cells exposed to treatments.
Indeed, exposure of both the M14 and WM164 cells to
PLX4032 resulted in a significant increase in the number of cells
in the G1 phase as compared with the control (Fig. 5). This is in line with the
findings of another study analyzing the effects of PLX4032 on
melanoma cell lines (42). Of
note, the present study revealed that the incubation of
CSPG4-positive cells with the CSPG4-specific 9.2.27 mAb resulted in
a significantly higher number of cells arrested in the S phase and
that this effect was concentration-dependent (Figs. 5A and S2). The effect was specific since the
incubation of the M14 cells with the anti-CSPG4 9.2.27 mAb did not
influence the cell cycle distribution among these cells, while the
combination of PLX4032 and the 9.2.27 mAb resulted in similar cell
cycle phases, as observed with PLX4032 alone (Fig. 5B).
Relatively little is known about the mechanisms that
control progression through the S phase in mammalian cells.
Antibody treatment acts presumably as a CSPG4-dependent exogenous
trigger that allows cells neither to progress in the cell cycle nor
to retreat to the G1 status. Further investigation of molecular
mediators of the S phase arrest in the context of CSPG4 inhibition
may shed light not only on the S phase arrest mechanisms, but also
on as yet unidentified CSPG4 functions.
Moreover, following the combined treatment of WM164
cells, a significantly increased accumulation of cells in the subG1
phase, which may indicate cell death, combined with a decrease in
the G2/M phase cells was observed (Fig. 5B). This result may explain the
decreased viability of WM164 cells exposed to PLX4032 and the
9.2.27 mAb (Fig. 1A).
In conclusion, the findings of the present study
indicate that the CSPG4-specific 9.2.27 mAb exerted an
anti-clonogenic and anti-invasive effect on CSPG4-expressing
melanoma cells. In addition, antibody treatment led to cell cycle
arrest in the S phase. Albeit the combination of the 9.2.27 mAb
with PLX4032 did not exert any additional effect on the colony
formation ability and invasiveness of CSPG4-positive cells, the
combined treatment may lead to increased cell death. The outcomes
of the present study provide the basis for further investigations
and emphasize the need for new considerations when designing
studies involving the combination of CSPG4-specific mAbs with
kinase inhibitors for the treatment of CSPG4-positive tumors.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KU and CH conceived and designed the study, and
analyzed and interpreted the data. MS, TK, MP and HB participated
in designing the study and in analyzing the data. KU and MS
performed all the experiments. CH and HB confirm the authenticity
of all the raw data. KU, CH and HB wrote the manuscript. All
authors have read and revised the manuscript, and approved the
final version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
MP is a clinical investigator for Bayer, BMS, Lilly
and Roche; MP received speaker honoraria from Bayer, BMS, Eisai,
Lilly, and MSD; MP is a consultant for Bayer, BMS, Ipsen, Eisai,
Lilly, MSD, and Roche outside the presented work; MP received
travel support from Bayer and BMS. KU, MS, TK, HB and CH declare
that they have no competing interests.
Authors' information
The ORCID IDs of all the authors are as follows: KU,
0000-0003-0093-6047; MS, 0000-0003-0842-6578; TK,
0000-0002-9641-0244; MP, 0000-0002-7260-532X; CH,
0000-0003-3745-1414; and HB, 0000-0003-2022-8689.
Acknowledgments
The authors would like to thank Dr Claudia
Kitzmüller (Department of Pathophysiology and Allergy Research,
Center for Pathophysiology, Infectiology and Immunology, Medical
University of Vienna, Austria) for providing technical assistance
with the flow cytometry experiments and Dr Helmut Schaider
(Dermatology Research Centre, The University of Queensland
Diamantina Institute, Translational Research Institute, The
University of Queensland, Brisbane, Australia) for providing the
WM164 melanoma cell line. The authors also wish to acknowledge the
NÖ Landesgesundheitsagentur, legal entity of University Hospitals
in Lower Austria, for providing the organizational framework for
conducting this research.
Abbreviations:
|
CSPG4
|
chondroitin sulfate proteoglycan 4
|
|
ECM
|
extracellular matrix
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FAK
|
focal adhesion kinase
|
|
mAb
|
monoclonal antibody
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MEK
|
mitogen-activated protein kinase
kinase
|
|
PBS
|
phosphate-buffered saline
|
|
RTK
|
receptor tyrosine kinase
|
References
|
1
|
Wilson BS, Imai K, Natali PG and Ferrone
S: Distribution and molecular characterization of a cell-surface
and a cytoplasmic antigen detectable in human melanoma cells with
monoclonal antibodies. Int J Cancer. 28:293–300. 1981. View Article : Google Scholar
|
|
2
|
Campoli MR, Chang CC, Kageshita T, Wang X,
McCarthy JB and Ferrone S: Human high molecular
weight-melanoma-associated antigen (HMW-MAA): A melanoma cell
surface chondroitin sulfate proteoglycan (MSCP) with biological and
clinical significance. Crit Rev Immunol. 24:267–296. 2004.
View Article : Google Scholar
|
|
3
|
Campoli M, Ferrone S and Wang X:
Functional and clinical relevance of chondroitin sulfate
proteoglycan 4. Adv Cancer Res. 109:73–121. 2010. View Article : Google Scholar
|
|
4
|
Fenton M, Whiteside TL, Ferrone S and
Boyiadzis M: Chondroitin sulfate proteoglycan-4 (CSPG4)-specific
monoclonal antibody 225.28 in detection of acute myeloid leukemia
blasts. Oncol Res. 22:117–121. 2015. View Article : Google Scholar
|
|
5
|
Warta R, Herold-Mende C, Chaisaingmongkol
J, Popanda O, Mock A, Mogler C, Osswald F, Herpel E, Küstner S,
Eckstein V, et al: Reduced promoter methylation and increased
expression of CSPG4 negatively influences survival of HNSCC
patients. Int J Cancer. 135:2727–2734. 2014. View Article : Google Scholar
|
|
6
|
Wang X, Osada T, Wang Y, Yu L, Sakakura K,
Katayama A, McCarthy JB, Brufsky A, Chivukula M, Khoury T, et al:
CSPG4 protein as a new target for the antibody-based immunotherapy
of triple-negative breast cancer. J Natl Cancer Inst.
102:1496–1512. 2010. View Article : Google Scholar
|
|
7
|
Svendsen A, Verhoeff JJ, Immervoll H,
Brøgger JC, Kmiecik J, Poli A, Netland IA, Prestegarden L,
Planagumà J, Torsvik A, et al: Expression of the progenitor marker
NG2/CSPG4 predicts poor survival and resistance to ionising
radiation in glioblastoma. Acta Neuropathol. 122:495–510. 2011.
View Article : Google Scholar
|
|
8
|
Keleg S, Titov A, Heller A, Giese T,
Tjaden C, Ahmad SS, Gaida MM, Bauer AS, Werner J and Giese NA:
Chondroitin sulfate proteoglycan CSPG4 as a novel hypoxia-sensitive
marker in pancreatic tumors. PLoS One. 9:e1001782014. View Article : Google Scholar
|
|
9
|
Hsu SC, Nadesan P, Puviindran V, Stallcup
WB, Kirsch DG and Alman BA: Effects of chondroitin sulfate
proteoglycan 4 (NG2/CSPG4) on soft-tissue sarcoma growth depend on
tumor developmental stage. J Biol Chem. 293:2466–2475. 2018.
View Article : Google Scholar
|
|
10
|
Rivera Z, Ferrone S, Wang X, Jube S, Yang
H, Pass HI, Kanodia S, Gaudino G and Carbone M: CSPG4 as a target
of antibody-based immunotherapy for malignant mesothelioma. Clin
Cancer Res. 18:5352–5363. 2012. View Article : Google Scholar
|
|
11
|
Price MA, Colvin Wanshura LE, Yang J,
Carlson J, Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA and
McCarthy JB: CSPG4, a potential therapeutic target, facilitates
malignant progression of melanoma. Pigment Cell Melanoma Res.
24:1148–1157. 2011. View Article : Google Scholar
|
|
12
|
Yang J, Price MA, Neudauer CL, Wilson C,
Ferrone S, Xia H, Iida J, Simpson MA and McCarthy JB: Melanoma
chondroitin sulfate proteoglycan enhances FAK and ERK activation by
distinct mechanisms. J Cell Biol. 165:881–891. 2004. View Article : Google Scholar
|
|
13
|
Ilieva KM, Cheung A, Mele S, Chiaruttini
G, Crescioli S, Griffin M, Nakamura M, Spicer JF, Tsoka S, Lacy KE,
et al: Chondroitin sulfate proteoglycan 4 and its potential as an
antibody immunotherapy target across different tumor types. Front
Immunol. 8:19112017. View Article : Google Scholar
|
|
14
|
Rolih V, Barutello G, Iussich S, De Maria
R, Quaglino E, Buracco P, Cavallo F and Riccardo F: CSPG4: A
prototype oncoantigen for translational immunotherapy studies. J
Transl Med. 15:1512017. View Article : Google Scholar
|
|
15
|
Bumol TF, Wang QC, Reisfeld RA and Kaplan
NO: Monoclonal antibody and an antibody-toxin conjugate to a cell
surface proteoglycan of melanoma cells suppress in vivo tumor
growth. Proc Natl Acad Sci USA. 80:529–533. 1983. View Article : Google Scholar
|
|
16
|
Jordaan S, Chetty S, Mungra N, Koopmans I,
van Bommel PE, Helfrich W and Barth S: CSPG4: A target for
selective delivery of human cytolytic fusion proteins and TRAIL.
Biomedicines. 5:372017. View Article : Google Scholar
|
|
17
|
Abbas Rizvi SM, Sarkar S, Goozee G and
Allen BJ: Radioimmunoconjugates for targeted alpha therapy of
malignant melanoma. Melanoma Res. 10:281–289. 2000. View Article : Google Scholar
|
|
18
|
Risberg K, Fodstad O and Andersson Y: The
melanoma specific 9227PE immunotoxin efficiently kills melanoma
cells in vitro. Int J Cancer. 125:23–33. 2009. View Article : Google Scholar
|
|
19
|
de Bruyn M, Rybczynska AA, Wei Y,
Schwenkert M, Fey GH, Dierckx RA, van Waarde A, Helfrich W and
Bremer E: Melanoma-associated Chondroitin Sulfate Proteoglycan
(MCSP)-targeted delivery of soluble TRAIL potently inhibits
melanoma outgrowth in vitro and in vivo. Mol Cancer. 9:3012010.
View Article : Google Scholar
|
|
20
|
Amoury M, Mladenov R, Nachreiner T, Pham
AT, Hristodorov D, Di Fiore S, Helfrich W, Pardo A, Fey G,
Schwenkert M, et al: A novel approach for targeted elimination of
CSPG4-positive triple-negative breast cancer cells using a MAP
tau-based fusion protein. Int J Cancer. 139:916–927. 2016.
View Article : Google Scholar
|
|
21
|
Poli A, Wang J, Domingues O, Planagumà J,
Yan T, Rygh CB, Skaftnesmo KO, Thorsen F, McCormack E, Hentges F,
et al: Targeting glioblastoma with NK cells and mAb against
NG2/CSPG4 prolongs animal survival. Oncotarget. 4:1527–1546. 2013.
View Article : Google Scholar
|
|
22
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: Improved survival with vemurafenib in melanoma with BRAF V600E
mutation. N Engl J Med. 364:2507–2516. 2011. View Article : Google Scholar
|
|
23
|
Proietti I, Skroza N, Michelini S, Mambrin
A, Balduzzi V, Bernardini N, Marchesiello A, Tolino E, Volpe S,
Maddalena P, et al: BRAF Inhibitors: Molecular targeting and
immunomodulatory actions. Cancers (Basel). 12:18232020. View Article : Google Scholar
|
|
24
|
Patel H, Yacoub N, Mishra R, White A, Long
Y, Alanazi S and Garrett JT: Current advances in the treatment of
BRAF-mutant melanoma. Cancers (Basel). 12:4822020. View Article : Google Scholar
|
|
25
|
Yu L, Favoino E, Wang Y, Ma Y, Deng X and
Wang X: The CSPG4-specific monoclonal antibody enhances and
prolongs the effects of the BRAF inhibitor in melanoma cells.
Immunol Res. 50:294–302. 2011. View Article : Google Scholar
|
|
26
|
Pucciarelli D, Lengger N, Takacova M,
Csaderova L, Bartosova M, Breiteneder H, Pastorekova S and Hafner
C: Anti-chondroitin sulfate proteoglycan 4-specific antibodies
modify the effects of vemurafenib on melanoma cells differentially
in normoxia and hypoxia. Int J Oncol. 47:81–90. 2015. View Article : Google Scholar
|
|
27
|
Costa EC, Moreira AF, de Melo-Diogo D,
Gaspar VM, Carvalho MP and Correia IJ: 3D tumor spheroids: An
overview on the tools and techniques used for their analysis.
Biotechnol Adv. 34:1427–1441. 2016. View Article : Google Scholar
|
|
28
|
Bialkowska K, Komorowski P, Bryszewska M
and Milowska K: Spheroids as a type of three-dimensional cell
cultures-examples of methods of preparation and the most important
application. Int J Mol Sci. 21:62252020. View Article : Google Scholar
|
|
29
|
Pampaloni F, Reynaud EG and Stelzer EH:
The third dimension bridges the gap between cell culture and live
tissue. Nat Rev Mol Cell Biol. 8:839–845. 2007. View Article : Google Scholar
|
|
30
|
Beaumont KA, Mohana-Kumaran N and Haass
NK: Modeling melanoma in vitro and in vivo. Healthcare (Basel).
2:27–46. 2013. View Article : Google Scholar
|
|
31
|
Tsai J, Lee JT, Wang W, Zhang J, Cho H,
Mamo S, Bremer R, Gillette S, Kong J, Haass NK, et al: Discovery of
a selective inhibitor of oncogenic B-Raf kinase with potent
antimelanoma activity. Proc Natl Acad Sci USA. 105:3041–3046. 2008.
View Article : Google Scholar
|
|
32
|
Lee JT, Li L, Brafford PA, van den Eijnden
M, Halloran MB, Sproesser K, Haass NK, Smalley KS, Tsai J, Bollag G
and Herlyn M: PLX4032, a potent inhibitor of the B-Raf V600E
oncogene, selectively inhibits V600E-positive melanomas. Pigment
Cell Melanoma Res. 23:820–827. 2010. View Article : Google Scholar
|
|
33
|
Smalley KS, Haass NK, Brafford PA, Lioni
M, Flaherty KT and Herlyn M: Multiple signaling pathways must be
targeted to overcome drug resistance in cell lines derived from
melanoma metastases. Mol Cancer Ther. 5:1136–1144. 2006. View Article : Google Scholar
|
|
34
|
Haass NK, Sproesser K, Nguyen TK,
Contractor R, Medina CA, Nathanson KL, Herlyn M and Smalley KS: The
mitogen-activated protein/extracellular signal-regulated kinase
kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in
melanoma cells and tumor regression when combined with docetaxel.
Clin Cancer Res. 14:230–239. 2008. View Article : Google Scholar
|
|
35
|
Hafner C, Breiteneder H, Ferrone S,
Thallinger C, Wagner S, Schmidt WM, Jasinska J, Kundi M, Wolff K,
Zielinski CC, et al: Suppression of human melanoma tumor growth in
SCID mice by a human high molecular weight-melanoma associated
antigen (HMW-MAA) specific monoclonal antibody. Int J Cancer.
114:426–432. 2005. View Article : Google Scholar
|
|
36
|
Uranowska K, Kalic T, Valtsanidis V,
Kitzwogerer M, Breiteneder H and Hafner C: Expression of
chondroitin sulfate proteoglycan 4 (CSPG4) in melanoma cells is
downregulated upon inhibition of BRAF. Oncol Rep. 45:142021.
View Article : Google Scholar
|
|
37
|
Schadendorf D, van Akkooi ACJ, Berking C,
Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A and Ugurel
S: Melanoma. Lancet. 392:971–984. 2018. View Article : Google Scholar
|
|
38
|
Kimiz-Gebologlu I, Gulce-Iz S and
Biray-Avci C: Monoclonal antibodies in cancer immunotherapy. Mol
Biol Rep. 45:2935–2940. 2018. View Article : Google Scholar
|
|
39
|
Gutzmer R, Stroyakovskiy D, Gogas H,
Robert C, Lewis K, Protsenko S, Pereira RP, Eigentler T, Rutkowski
P, Demidov L, et al: Atezolizumab, vemurafenib, and cobimetinib as
first-line treatment for unresectable advanced BRAFV600
mutation-positive melanoma (IMspire150): Primary analysis of the
randomised, double-blind, placebo-controlled, phase 3 trial.
Lancet. 395:1835–1844. 2020. View Article : Google Scholar
|
|
40
|
Iida J, Wilhelmson KL, Ng J, Lee P,
Morrison C, Tam E, Overall CM and McCarthy JB: Cell surface
chondroitin sulfate glycosaminoglycan in melanoma: Role in the
activation of pro-MMP-2 (pro-gelatinase A). Biochem J. 403:553–563.
2007. View Article : Google Scholar
|
|
41
|
Tang F, Lord MS, Stallcup WB and Whitelock
JM: Cell surface chondroitin sulphate proteoglycan 4 (CSPG4) binds
to the basement membrane heparan sulphate proteoglycan, perlecan,
and is involved in cell adhesion. J Biochem. 163:399–412. 2018.
View Article : Google Scholar
|
|
42
|
Sondergaard JN, Nazarian R, Wang Q, Guo D,
Hsueh T, Mok S, Sazegar H, MacConaill LE, Barretina JG, Kehoe SM,
et al: Differential sensitivity of melanoma cell lines with
BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Transl
Med. 8:392010. View Article : Google Scholar
|