Introduction
The disorder of cell-cell adhesion has a significant
influence on tumor occurrence and development (1). Four major cell adhesion molecules,
which are known as the integrins, cadherins, selectins and the
immunoglobulin superfamily (IgSF), are involved in this
physiological process (2). The
Nectin cell adhesion molecule (Nectin) family is comprised of
Nectins 1–4, which are immunoglobulin-semblable transmembrane
proteins involved in the Ca2+-independent adherens
junctions (AJs) of cell-cell interactions via
homophilic/heterophilic interplay (3–5).
Moreover, Nectin family members enhance cellular viability and
movement ability (6–8). Nectins 1–3 are commonly enriched in
normal adult tissues, in which Nectins 1–2 are frequently expressed
in immune organs (bone marrow, thymus, spleen and lymph nodes), and
Nectin-3 is principally expressed in the spermary and placenta
(3,4). However, several studies have revealed
that Nectin-4 is specifically overexpressed in various cancer
types, including breast cancer (BC), ovarian cancer (OC) and
pancreatic cancer (PC) (9–14). A large number of studies have shown
that Nectin-4 is closely related to tumor oncogenesis and the poor
prognosis of affected patients (9–14).
Fabre-Lafay et al (13)
reported that both membranous and soluble forms of Nectin-4 were
upregulated in the majority of BC tissue samples, and Nectin-4 was
also confirmed as a novel biomarker associated with poor prognosis.
In PC, upregulated Nectin-4 strongly stimulates cell growth and has
a vital impact on intratumoral angiogenesis (14). To the best of our knowledge, the
function and biological changes in Nectin-4 protein have not been
collected systematically, thereby necessitating the study of
clinical significance and molecular mechanisms in different cancer
types. The present review aimed to investigate the prognostic
values and functions of Nectin-4 in various cancer types.
Molecular structures of Nectin family
members
The Nectin family of proteins are
Ca2+-independent cell surface adhesion molecules,
closely related to the formation of AJs and tight junctions (TJs)
(15,16). All Nectins are members of the IgSF,
initially depicted as molecules homologous to the poliovirus
receptor (PVR/CD155), and can thus be described as poliovirus
receptor-related proteins (PRR) or PVR-like proteins (PVRL)
(3,17,18).
Except for Nectin-4, Nectins 1–3 have more than one splice variant,
including Nectin-1α, −1β, −1γ, −2α, −2δ, −3α, −3 and −3γ (19). Nectin-1α and −2α were firstly
discovered as PRR proteins, and are also known as PRR-1 and −2,
respectively (19). However, a
study later confirmed the lack of correlation between Nectin-1α and
Nectin-2α, and PVR (20). Moreover,
they were subsequently verified to act as receptors for α-herpes
virus, mediating the processes of the virus infection and diffusion
Hence, the old names for these Nectins used to be HveC and HveB,
respectively (20). As Nectin-4 is
homologous to PVR/CD155, Nectin-4 was also known as PVRL4 (21) (Table
I).
 | Table I.General characteristics and tissue
distribution of Nectin family members. |
Table I.
General characteristics and tissue
distribution of Nectin family members.
| Nomenclature | Old
nomenclature | Splice
variants | Distribution | (Refs.) |
|---|
| Nectin-1 | PRR1/HveC | Nectin-1α | Immune system
organs | (3,4,19,20) |
|
|
| Nectin-1β |
|
|
|
|
| Nectin-1γ |
|
|
| Nectin-2 | PRR2/HveB | Nectin-2α | Blood cells and
spermatids | (19,20) |
|
|
| Nectin-2δ |
|
|
| Nectin-3 | PRR3 | Nectin-3α | Testes and
placenta | (19) |
|
|
| Nectin-3β |
|
|
|
|
| Nectin-3γ |
|
|
| Nectin-4 | PVRL4 | NS | Embryonic and
placental tissues | (3,4,21) |
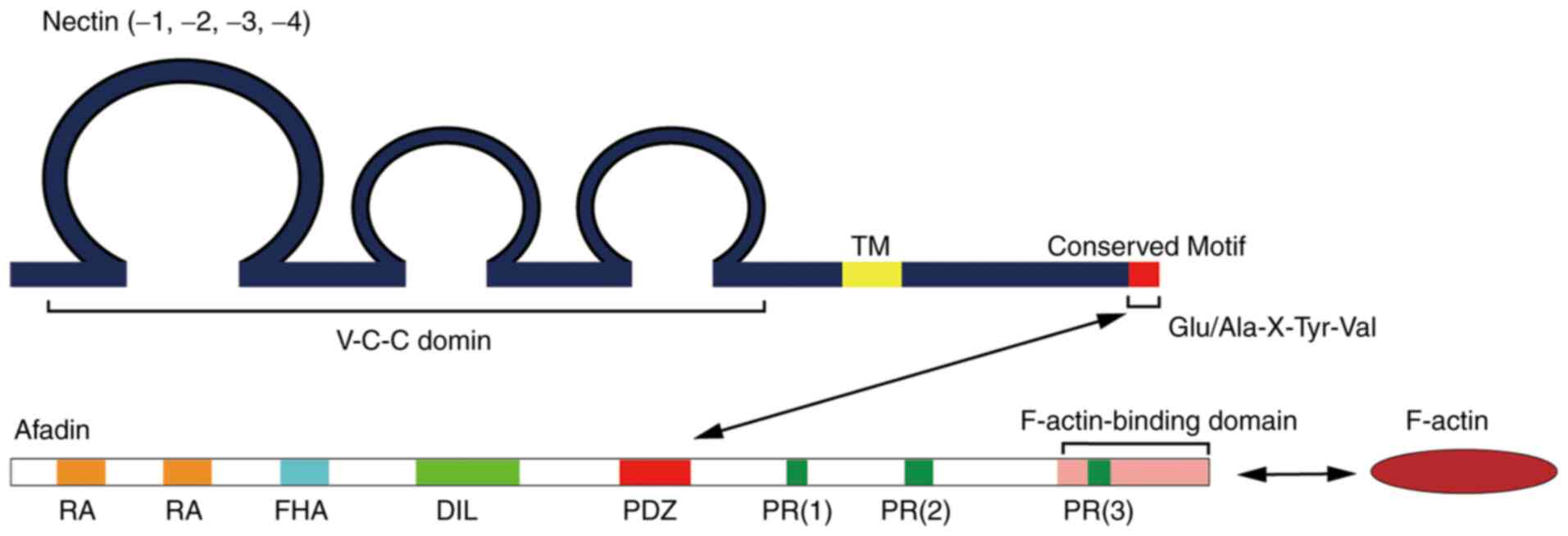
Except for Nectin-1γ, the other family members have
been found to have a semblable domain structure: Three conserved
immunoglobulin-like domains in the extracellular region (V-C-C
domain), one transmembrane region (TM) and one short tail protein
domain in the cytoplasm (Fig. 1)
(5,15). Nectin-1γ is considered as a secreted
protein due to the absence of a TM region (19). Furthermore, the V, C and C domains
bond with several growth factor receptors, including fibroblast
growth factor receptor and Erb-b2 receptor tyrosine kinase 3, which
might have a significant influence on cell growth, migration and
apoptosis (22–24).
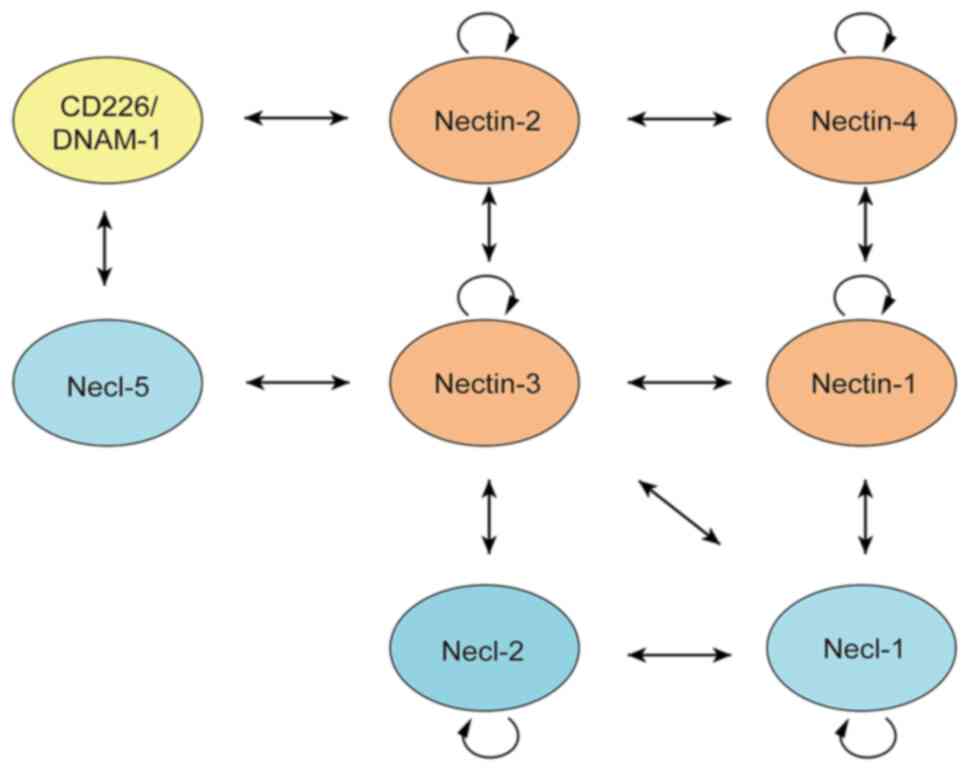
The Nectin family members interact with each other
via homo-cis-dimers on the surface of the cellular membrane or
hetero-trans-dimers among adjacent cells for homophilic and
heterophilic interactions through the extracellular region
(9). The binding specificity is
diverse among various Nectin family members. For example, Nectin-1
integrates with Nectin-3 and −4 to form hetero-trans-dimers. These
dimers are formed between Nectin-2 and −3, but not between Nectin-1
and −2 (Fig. 2). Moreover, these
hetero-trans-dimers are more tightly connected than the
homo-trans-dimers (9,25). Furthermore, Nectin-2 is combined
with CD226/DNAM-1 through trans-interaction. CD226/DNAM-1 contains
two Ig-like domains, and is mainly enriched in T and natural killer
(NK) cells, stimulating immune cells to enhance their ability of
differentiation and proliferation (26,27).
The Nectin-like molecule (Necl) family is another group of Ig-like
cellular surface adhesion molecules consisting of five members:
Necl-1, −2, −3, −4 and −5. In addition, the protein domains of Necl
family members are similar to those of Nectin members. Also, Necls
interact with Nectins, which jointly promote cell growth and
differentiation, and inhibit cell apoptosis (20). The complicated and close interaction
between Nectins and Necls is shown in Fig. 2. Several studies have revealed that
the Nectin family members also serve as novel immune regulators
(28–30). Reportedly, Nectin-2 (CD112, PVRL2
and CD113), Nectin-3 (PVRL3) and Necl-5 (CD155/PVR) bind to T-cell
immunoreceptor via Ig and immunoreceptor tyrosine-based inhibitory
motif (ITIM) domains (TIGIT), among which, Necl-5 has the highest
binding affinity (28–30). Furthermore, TIGIT has emerged as a
significant molecule for immune checkpoint regulation, which
consists of a type I transmembrane protein with an Ig variable
extracellular domain solely enriched within a range of immune
cells, including NK cells, effector cells, memory T cells and
regulatory T cells (29,30). TIGIT negatively regulates the immune
response via multiple steps. Following ligand interaction, TIGIT
mediates the suppression of NK cell-mediated cytotoxicity and
interferon (IFN)-γ production through its cytosolic immunoglobulin
tail tyrosine-like phosphorylation motif and through ITIM in the
cytoplasmic region, which recruits Src kinases, Grb2 and SHIP-1
(28–32). The blockade of the interaction
between Nectin members and TIGIT markedly enhances the antitumor
immunity mediated by reinvigorated CD8+ T cells and NK
cells (30,32,33).
Nectin-induced signaling during the
formation of cell-cell junctions
Apart from Nectin-1β, −1γ, −3γ and −4, the remaining
Nectin members share the same conserved sequence at the carboxyl
terminus. In addition, there are four amino acid residues
(Glu/Ala-X-TyrVal) in this conserved sequence that interact with
the PDZ domain of Afadin (Fig. 1).
Despite the fact that Nectin-4 does not share the conserved
sequence, it can directly bind to the PDZ domain of Afadin via its
carboxyl terminus (34). Interplay
occurs between Nectins and the actin cytoskeleton protein via
Afadin, which can activate a series of intercellular communications
and signaling molecules, including AJs, TJs and inflammatory
cytokines (16). A previous study
reported that Nectin-4 firstly combines with Afadin and then
regulates actin cytoskeleton remodeling (35). Subsequently, it induces
epithelial-mesenchymal transition (EMT) and enhances the driving
force for pseudopod extension in tumor cell lines (36).
The cell-cell junction is strongly influenced by the
interactions among Nectins on adjacent cells. Once the interaction
is established, these cadherin-catenin complexes will be recruited
to the corresponding adhesion site. Subsequently, the
trans-interaction of cadherins forms the AJs on adjacent cells
(37–39). Synergetically, the Nectin/Afadin
complex and E-cadherin/catenin complex function through Afadin and
α-catenin, respectively, activating a signaling cascade (c-Src,
C3G, Crk, PI3K and Vav2), thus modulating molecules such as Rap1,
Cdc42 and Racs, and ultimately leading to actin cytoskeleton
realignment (40–42) (Fig.
3). In conclusion, the Nectin family in co-operation with
cadherin, have significant effects on the generation and
maintenance of AJs and TJs, which regulate several cellular
behaviors, including cell adhesion, growth, differentiation,
migration and apoptosis (43,44).
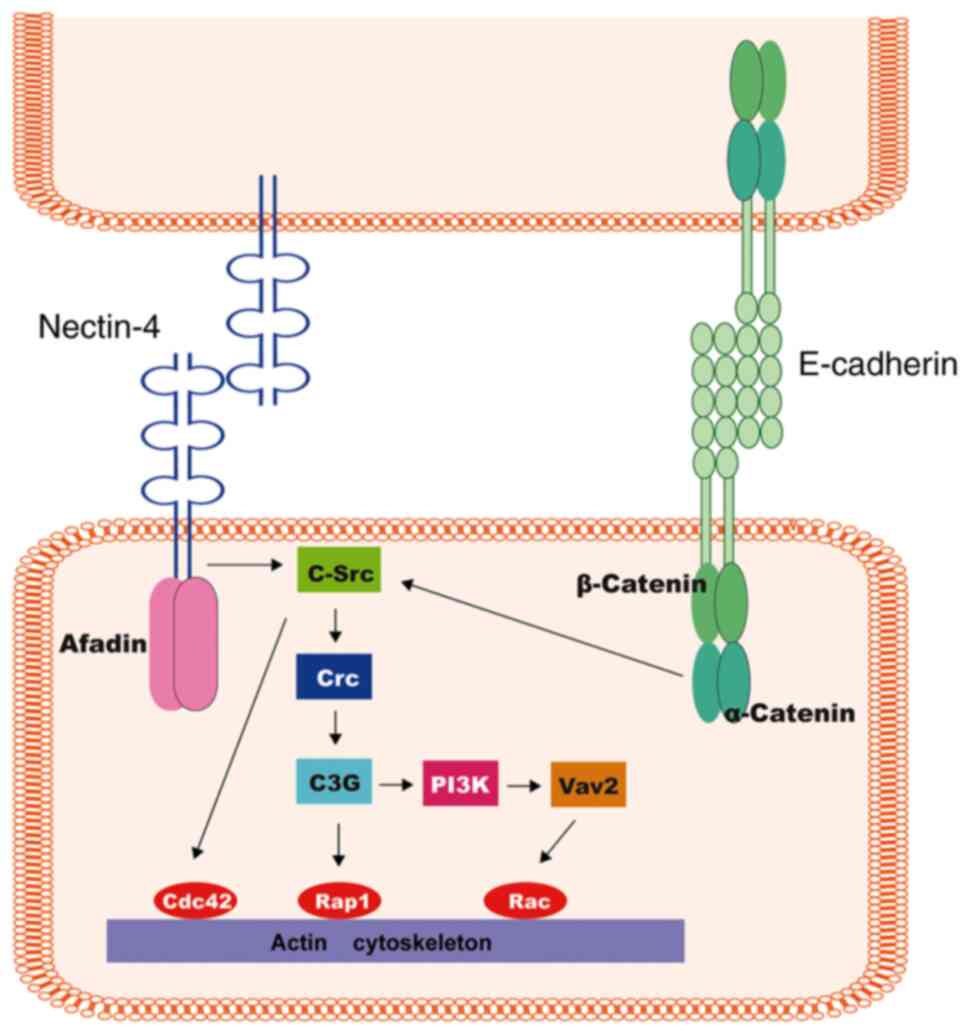 | Figure 3.Nectin-induced signaling during the
formation of cell-cell junctions. Nectins exert essential influence
on the primary step of cell-cell junction formation. Once the
interaction is established, these cadherin-catenin complexes will
be recruited to the corresponding adhesion site. Subsequently, the
Nectin/Afadin complex and the E-cadherin/catenin complex function
through Afadin and α-catenin, respectively, activating a signaling
cascade (c-Src, C3G, Crk, PI3K and Vav2), thus modulating molecules
such as Rap1, Cdc42 and Racs, and ultimately leading to actin
cytoskeleton realignment. |
Distribution and physiological function of
Nectins
Each Nectin family member has distinct effects
independently or interactively. In normal cellular conditions,
Nectins 1–3 are mainly located in neurons, fibroblasts and
epithelial cells (38), where
Nectin-2 and −3 are also enriched in hemocytes (B cells and
monocytes) and spermatids (Table I)
(19). In normal tissues, Nectin-1
and −2 are closely related to immune organs, while Nectin-4 is
widely enriched in embryonic and placental tissues, including the
skin, tonsils and tubular structure (trachea, esophagus and
nasopharynx) (3,4). Additionally, the abnormal expression
of Nectin is a cause for disease occurrence. For example, the
occurrence of human Zlotogora-Ogur syndrome is the result of
mutations in Nectin-1 (45). Also,
Nectin-4 significantly affects the development of ectodermal
organogenesis, and Nectin-4 mutations lead to a
dysplasia-syndactyly syndrome characterized by webbed hands and
feet (46).
Biological role of Nectin-4 proteins in
cancer
In contrast to the distribution of Nectins 1–3,
which are widely present in the tissues of a normal adult, Nectin-4
is specifically enriched in the embryonic and placental tissues,
but has significantly decreased levels in adult life (4). In recent years, Nectin-4 was found to
be overexpressed and served as an inducer in various malignant
tumors, including BC, OC, colorectal cancer (CRC), PC and lung
cancer (9–14). For example, Challita-Eid et
al (47) collected >2,000
tumor samples from head/neck, lung, bladder, breast, pancreatic,
ovarian and esophageal lesions, and approximately two-thirds were
positive for Nectin-4 according to immunohistochemical (IHC)
staining. In other studies, Nectin-4 was correlated with tumor
occurrence and development (12,14),
contributed to the occurrence of metastases in breast, lung and
gallbladder tumors (48,49), was associated with advanced
Tumor-Node-Metastasis (TNM) stage (III and IV) and decreased
survival rates (50), and promoted
cancer chemoresistance to 5-fluorouridine (5-FU) (51). Although the precise molecular
mechanisms in oncogenesis and progression have not been clarified,
numerous studies have reported that Nectin-4 promotes tumor
angiogenesis, proliferation and migration, and triggers EMT.
Nectin-4 promotes tumor
angiogenesis
Recent studies have revealed that Nectin-4 promotes
tumor angiogenesis via the activated PI3K/AKT signaling pathway
(52,53). Angiogenesis is the crucial
foundation for tumor growth, spread, invasion and expansion
(54,55). A considerable amount of vascular
endothelial growth factor (VEGF) and increased microvessel density
(IMD) were detected in the tumor microenvironment during
angiogenesis (56,57). Zhang et al (52) demonstrated that the high expression
of Nectin-4 protein is linked to integrin β1 (ITGB1) protein and
vasculogenic mimicry (VM) formation. In PC, upregulated Nectin-4
stimulates cell growth and has a vital impact on intratumoral
angiogenesis (14). The
downregulation of Nectin-4 inhibits the expression of VEGF and
tumor angiogenesis in lung cancer and CRC (53). Further studies have shown that the
interplay between Nectin-4 and endothelial ITGB4 modulates the
transcriptional activity of Src, PI3K, AKT and inducible nitric
oxide synthase, and ultimately induces angiogenesis (53).
Nectin-4 promotes tumor cell growth,
proliferation and migration
Nishiwada et al (14) reported that knockdown of Nectin-4
inhibited the proliferation of human PC cells. Similarly, Zhang
et al (48) demonstrated
that the low expression of Nectin-4 restrained gall bladder cancer
cell proliferation and migration in vivo and in
vitro. The potential mechanism by which Nectin-4 promotes tumor
cell growth, proliferation and migration is via Ras-related C3
botulinum toxin substrate 1 (Rac1) signaling activity (58,59).
Rac1 is one of the members of the Rho family of GTPases, which
exert a significant influence on tumor occurrence and development
(60,61). Rac1 GTPase switches Rac1-GDP (‘OFF’
state) to Rac1-GTP (‘ON’ state) (62,63).
Subsequently, it activates several protein kinases, including
p21-activated kinases and c-Jun N-terminal kinase, thereby
modulating downstream molecule signaling cascades, including the
regulation of cell growth, proliferation and microtubule
rearrangement (64–67). Several studies reported that
elevated levels of Rac1 could be attributed to the upstream
modulator of PI3K/AKT in gallbladder carcinoma, gastric cancer (GC)
and BC (48,50,54).
Nectin-4 promotes EMT
EMT is the most critical cellular event before the
occurrence of tumor migration, invasion and metastasis (68). A previous study demonstrated that
Nectin-4 regulates cell-cell adhesion, remodels the actin
cytoskeleton, triggers EMT, enhances the driving force of pseudopod
extension in tumor cells, and eventually causes tumor development
and spread (35). In a recent study
by Hao et al (69), the
downregulation of Nectin-4 in papillary thyroid cancer (PTC) cells
suppressed EMT and inhibited PTC cell migration and invasion via
the PI3K/AKT signaling pathway. Zhang et al (48) also reported that the upregulation of
Nectin-4 regulated the formation of actin fibers by binding to
Afadin and activating the PI3K/AKT pathway, which in turn activated
Rac1 to regulate EMT and then control cell shape rearrangement and
metastasis.
Nectin-4 serves as a prognostic or
diagnostic marker for selected types of cancer
The biological role of Nectin-4 in promoting
proliferation, migration and triggering metastasis in
carcinogenesis is under intensive research focus (48–50,68,69).
Despite the fact that the expression level or the positive rate of
Nectin-4 are different among selected types of tumor specimens,
most studies have confirmed Nectin-4 as a prognostic and diagnostic
biomarker. The clinicopathological characteristics and prognostic
analysis based on tumor Nectin-4 expression are summarized in
Table II.
 | Table II.Clinicopathological characteristics
and prognostic analysis according tumor Nectin-4 expression. |
Table II.
Clinicopathological characteristics
and prognostic analysis according tumor Nectin-4 expression.
| First author | Year of
publication | Country | Cancer | Cases, n | Age (range),
years | Follow-up (range),
months | Nectin-4 protein
level | High Nectin-4
expression on IHC, % (n/total n) | OS, months | OS Univariate
analysis, HR (95% CI); P-value | OS Multivariate
analysis, HR (95% CI); P-value | Nectin-4- related
clinicopathological parameters | (Refs.) |
|---|
| Zeindler et
al | 2019 | Switzerland | TNBC | 168 | Mean, | 50.40 | ↑ | 58.00% | NA | 0.0271
(0.0077–0.0952); | 0.0220
(0.0055–0.0889); | Lower tumor
stage | (74) |
|
|
|
|
|
| 62 (47–77) |
|
| (86/148) |
| P<0.001 | P<0.001 | (P=0.025); pN0
lymph |
|
|
|
|
|
|
|
|
|
|
|
|
|
| node stage
(P=0.034) |
|
| Rajc et
al | 2017 | Croatia | Luminal-B BC | 147 | Mean, | 80.70 | ↑ | NA | NA | P<0.001 | 2.92 (1.8–4.75);
P<0.001 | Tumour size
(P<0.05) | (72) |
|
|
|
|
|
| 62 (53–70) | (35.70–103.50) |
|
|
|
|
|
|
|
| M-Rabet et
al | 2017 | France | TNBC | 61 | NA | 83 | ↑ | 62.00% | NA | 1.65
(1.1–2.47); | 1.53
(1.02–2.30); | TN (P<0.05);
Basal | (73) |
|
|
|
|
|
|
|
|
| (38/61) |
| P=0.0154 | P=0.039 | subtypes
(P<0.05) |
|
| Lattanzio et
al | 2014 | Italy | Luminal A-BC | 197 | Median, | 95 (6–298) | ↑ | m-Nectin-4: | NA | NA | m-Nectin-4: | m-Nectin-4: PR | (75) |
|
|
|
|
|
| 54.90 |
|
| 13.70%; |
|
| 4.0 (1.5–10.8) | (P=0.045);
c-Nectin-4: |
|
|
|
|
|
|
|
|
|
| c-Nectin-4: |
|
| P=0.007; | ER (P=0.038) |
|
|
|
|
|
|
|
|
|
| 61.90% |
|
| c-Nectin-4:
3.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1.1–11.6) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| P=0.038 |
|
|
| Athanassiadou et
al | 2011 | Greece | BC | 140 | Mean, | 60 | ↑ | 64.30% | Mean 36.71; | P>0.05 | P>0.05 | Grade (II and
III) | (71) |
|
|
|
|
|
| 55.72 |
|
| (90/140) | Median 37.5 |
|
| (P<0.0001);
tumor |
|
|
|
|
|
|
| (28–85) |
|
|
|
|
|
| size
(P<0.0001) |
|
| Fabre-Lafay et
al | 2007 | France | BC | 57 | NA | NA | ↑ | 61% | NA | NA | NA | Number of
metastases | (13) |
|
|
|
|
|
|
|
|
|
|
|
|
| (P=0.038) |
|
| Erturk et
al | 2019 | Turkey | LC | 74 | Median, | NA | ↑ | NA | NA | NA | P=0.758 | Disease stage;
history | (83) |
|
|
|
|
|
| 60 (28–78) |
|
|
|
|
|
| of surgery; tumor
size; |
|
|
|
|
|
|
|
|
|
|
|
|
|
| presence of
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (all,
P<0.05) |
|
| Takano et
al | 2009 | Japan | NSCLC | 422 | Median, | NA | ↑ | 58.10% | NA | 2.116
(1.551–2.887); | 2.145
(1.558–2.954); | Histological
type | (10) |
|
|
|
|
|
| 55 (31–83) |
|
| (245/422) |
| P<0.0001 | P<0.0001 | (P=0.0059) |
|
| Tomiyama et
al | 2020 | Japan | UTUC | 99 | NA | NA | ↑ | 65.70% | NA | 2.69
(0.90–6.50); | 2.10
(0.64–5.81); | Higher risk of | (85) |
|
|
|
|
|
|
|
|
| (65/99) |
| P=0.072 | P=0.179 | progression
(P=0.031); |
|
|
|
|
|
|
|
|
|
|
|
|
|
| CSM (P=0.036) |
|
| Zhang et
al | 2019 | China | CRC | 68 | Median, | NA | ↑ | 70.60% | Median, 25.0 | NA | NA | ITGB1
expression | (52) |
|
|
|
|
|
| 56 (26–81) |
|
| (48/68) |
|
|
| (P<0.01);
VM |
|
|
|
|
|
|
|
|
|
|
|
|
|
| formation
(P<0.05); |
|
|
|
|
|
|
|
|
|
|
|
|
|
| DMS (P=0.031); |
|
|
|
|
|
|
|
|
|
|
|
|
|
| TNM stage;
(P=0.033) |
|
| Deng et
al | 2019 | China | EC | NA | NA | NA | ↑ | NA | NA | 1.704
(1.027–2.825); | 1.795
(1.042–3.092); | Tumor size
(P=0.012); | (55) |
|
|
|
|
|
|
|
|
|
|
| P=0.039 | P=0.035 | tumor stage
(P=0.016) |
|
| Lin et
al | 2019 | China | EC | 94 | NA | NA | ↑ | 37.80% | NA | 1.747
(1.003–3.044); | P<0.05 | Tumor size
(P=0.012); | (12) |
|
|
|
|
|
|
|
|
| (31/82) |
| P<0.05 |
| depth of tumor
invasion |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (P=0.008) |
|
| Zhang et
al | 2018 | China | GC | 64 | NA | NA | ↑ | 70.30% | NA | NA | NA | LNMets
(P=0.025); | (50) |
|
|
|
|
|
|
|
|
| (45/64) |
|
|
| TNM stage
(P=0.006) |
|
| Zhang et
al | 2018 | China | GC | 212 | Median, | NA | ↑ | 60.40% | NA | 3.815
(2.243–6.490); | 2.402
(1.364–4.232); | Differentiation
(P=0.004); | (49) |
|
|
|
|
|
| 55.30 |
|
| (128/212) |
| P<0.001 | P=0.002 | primary tumor
(P=0.001); |
|
|
|
|
|
|
|
|
|
|
|
|
|
| LNMets
(P<0.001); |
|
|
|
|
|
|
|
|
|
|
|
|
|
| TNM stage
(P<0.001) |
|
| Nishiwada et
al | 2015 | Japan | PC | 123 | Median, | NA | ↑ | 51.4% | Median, | 1.628
(1.105–2.398); | 1.721
(1.085–2.730); | Ki-67
expression | (14) |
|
|
|
|
|
| 66 (33–82) |
|
|
| 14.20 | P=0.014 | P=0.021 | (P<0.001);
VEGF |
|
|
|
|
|
|
|
|
|
|
|
|
|
| expression
(P<0.001) |
|
| Izumi et
al | 2015 | Japan | PC | 49 | Median, | 27.20 | ↑ | NA | NA | NA | NA | Tumor size
(P=0.035) | (86) |
|
|
|
|
|
| 67 (50–87) | (2.40–117.20) |
|
|
|
|
|
|
|
| Zhang et
al | 2016 | China | GBC | 68 | NA | NA | ↑ | 63.20% (43/68) | Mean, 6.82 | 3.150
(1.788–5.552); | 2.704
(1.527–4.788); | Pathological T
stage | (48) |
|
|
|
|
|
|
|
|
|
|
| P<0.001 | P=0.001 | (P=0.029);
LNMets |
|
|
|
|
|
|
|
|
|
|
|
|
|
| P=0.041) |
|
| Ma et
al | 2016 | China | HCC | 87 | NA | 23 (2–60) | ↑ | 67.82% (59/87) | Median, | 2.054
(1.202–3.507); | 2.085
(1.216–3.574); | Tumor size
(P=0.029); | (87) |
|
|
|
|
|
|
|
|
|
| 21.92 | P=0.008 | P=0.008 | status of
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (P=0.023);
vascular |
|
|
|
|
|
|
|
|
|
|
|
|
|
| invasion
(P=0.018); |
|
|
|
|
|
|
|
|
|
|
|
|
|
| TNM stage
(P=0.003). |
|
BC
Mixed BC
BC is a complicated and molecularly heterogeneous
disease, presenting varied histological features (70). A total of 57 mixed BC samples were
collected in the study by Fabre-Lafay et al (13). According to the distinction of the
tumor histological type, the positive expression of Nectin-4
displayed a marked difference between the ductal carcinoma and
lobular carcinomas (~60 and 5%, respectively). However, a clear
difference between tumor histological types was not found in the
study by Athanassiadou et al (71). Approximately two-thirds of samples
were found to be positive for Nectin-4 protein expression and have
a correlation with tumor size, grade and lymph node infiltration.
Thus, the correlation between the expression of Nectin-4 and the
histological types is controversial. Perhaps, the definition of
Nectin-4 positive or negative expression may not be consistent, and
the expression might be influenced by the antibody titer.
Furthermore, the limited tumor specimens from each cohort might
affect the final results. Hence, additional studies with a large
number of samples are essential to verify the connection between
Nectin-4 and the histological type.
Luminal BHER2 negative
BC
Rajc et al (72) analyzed results from 147 patients who
suffered from luminal BHER2 negative BC to determine the
correlation between Nectin-4 protein expression and
clinicopathological parameters. The results revealed that Nectin-4
expression was not correlated with Ki-67 and the hormone and growth
factor receptors. In addition, the downregulation of Nectin-4 may
improve the survival rate, including disease-free survival (DFS),
overall survival (OS) and distant relapse-free survival (RFS)
rates.
Triple-negative BC (TNBC)
A large retrospective study of ~6,000 patients with
BC was conducted by M-Rabet et al (73). The upregulation of Nectin-4 was
observed in the majority of specimens. Furthermore, the results
confirmed that Nectin-4 was a novel biomarker associated with the
poor prognosis for TNBC. Among the ~60 patients with TNBC, those
with upregulated Nectin-4 were more likely to have a shorter life
span compared to those with downregulated Nectin-4. In the
established animal models of TNBC, M-Rabet et al (73) used antibody-drug conjugates (ADCs)
targeting Nectin-4 to evaluate the curative effect, with
satisfactory results. The results revealed that this ADC induced
rapid, complete and durable responses in Nectin-4-positive
xenograft TNBC samples, including primary tumors, metastatic
lesions and local relapses. However, another study by Zeindler
et al (74) collected nearly
200 samples of TNBC, and the results showed that the elevated level
of Nectin-4 was the protective factor in TNBC. Moreover, the
results demonstrated that upregulated Nectin-4 expression was
correlated with low-grade malignancy, improved survival and no
lymph node involvement (LNI). The relationship between Nectin-4
overexpression and the prognosis of TNBC is controversial. It may
be that the final adjuvant treatment results were not unified and
that there was a lack of complete clinical information for the
aforementioned cohorts utilized. Therefore, high-quality evidence
from a large number of patients with TNBC is needed to clarify the
uncertainties.
Luminal-A BC
Nectin-4 exists in the cytoplasm and membrane of
malignant cells, which has been termed cytoplasmic-Nectin-4
(c-Nectin-4) and membranous-Nectin-4 (m-Nectin-4), respectively
(13). Approximately 200 luminal-A
patients were incorporated in the study by Lattanzio et al
(75). The distribution of high
Nectin-4 differed markedly between the cytoplasm and membrane (18
and 75%, respectively). Both m-Nectin-4 and c-Nectin-4 were shown
to be closely related to the DFS, as assessed by Cox proportional
hazards model. Furthermore, the upregulated level of the protein
could be considered as an adverse biomarker and therapeutic target
for luminal-A BC (75).
Nectin-4 occurs in soluble form in the plasma.
Soluble-Nectin-4 (s-Nectin-4) is formed from the ectodomain of
Nectin-4, which is cleaved by a disintegrin and metalloproteinase
17 (76). s-Nectin-4 could also be
regarded as a diagnostic indicator of BC. Fabre-Lafay et al
(13) demonstrated that s-Nectin-4
in serum increased the accuracy rate of clinal diagnosis for BC.
Compared to a single indicator (CEA/CA15-3), the diagnostic
accuracy was increased by 10% using a combination of
Nectin-4/CEA/CA15-3. Furthermore, s-Nectin-4 was significantly
connected with the number of metastases (Table II).
Reproductive system cancer
OC
Hibbs et al (77) and Derycke et al (11) reported that Nectin-4 is upregulated
in OC at both mRNA (OC cell lines) and protein (OC tissues) levels,
respectively. In the study by Nabih et al (78), 25 patients with OC were included.
The majority of patients presented with high expression of
Nectin-4. Furthermore, several studies demonstrated that Nectin-4
overexpression facilitates cell aggregation and formation of
spheroids in OC cell lines using functional assays and real-time
digital photographs (79–82). In addition, these multicellular
spheroids were resistant to chemotherapy drugs that lead to tumor
growth and metastasis (81).
Previous studies have shown that s-Nectin-4 might
serve as a marker of disease relapse and metastasis in breast
carcinoma (10,15). Derycke et al (11) also found that s-Nectin-4 was
upregulated in OC. Nabih et al (78) further revealed a close correlation
between s-Nectin-4 and tumor stages and disease progression. In
addition, the studies by Nabih et al (78) and Derycke et al (11) agreed that Nectin-4 is a valuable
diagnostic predictor to differentiate between benign and malignant
ovarian tumors. Furthermore, Nectin-4 combined with CA-125 had a
higher sensitivity and specificity compared with Nectin-4 or CA-125
alone. As a consequence, a Nectin-4 and CA-125 combination is able
to monitor the treatment effect and relapse of patients with
OC.
Respiratory system tumors
Lung cancer
Approximately 420 patients with non-small cell lung
cancer (NSCLC) were included in the study by Takano et al
(10). Nearly two-thirds of
patients presented with upregulation of Nectin-4 and poor survival.
The results also demonstrated that the upregulation of Nectin-4 was
one of the most crucial independent prognostic factors of OS for
NSCLC (Table II). The underlying
mechanism involved Nectin-4 acting on Rac1 and stimulating the
extension of lamellipodia, and improvement to the movement capacity
of lung cancer cells. In addition, s-Nectin-4 was upregulated in
patients with NSCLC. Notably, patients with high expression of
s-Nectin-4 had a short survival time and undesirable tumor
metastasis. In contrast to CEA and CYFRA21-1, Nectin-4 had the
advantages of high accuracy and specificity for lung cancer
diagnosis (10). In the recent
study of 77 lung cancer samples, Erturk et al (83) assessed the correlation between
Nectin-4 and clinicopathological parameters. The results showed
that Nectin-4 was involved in tumor size, tumor stage and distant
metastasis.
Urinary system tumors
Urothelial carcinoma (UC)
Recently, in a study investigating predominantly
bladder cancer cases, a study showed that more than half of UC
samples were positive for Nectin-4 protein expression (84). Another study showed that the
majority of patients with bladder cancer (83%) were
Nectin-4-positive, as assessed by IHC, and ~50% of specimens
exhibited moderate or high levels of staining of Nectin-4 (48). Similar results were found in the
study by Tomiyama et al (85), where ~66% of bladder cancer samples
tested were moderately or highly positive for Nectin-4.
Furthermore, upregulated Nectin-4 expression was correlated with
tumor progression.
In one study of UC, Nectin-4 was upregulated in 95%
of metastatic samples (48). In
addition, Tomiyama et al (85) reported that ~66% of patients
presented with high Nectin-4 levels in upper tract urothelial
carcinoma (UTUC). The upregulation of Nectin-4 was often
accompanied by poor prognostic markers, such as lymphovascular
invasion and high tumor grade. Moreover, UTUC with upregulated
Nectin-4 was associated with a risk of poor progression-free
survival (PFS) (Table II).
Digestive system cancer
CRC
A total of 370 CRC samples were obtained from The
Cancer Genome Atlas database (https://www.genome.gov/Funded-Programs-Projects/Cancer-Genome-Atlas).
The results demonstrated that Nectin-4 was connected with TNM stage
and LNI (52). To further
substantiate these findings, Zhang et al (52) collected a different cohort
encompassing 68 CRC samples. Upregulated Nectin-4 was observed in
>70% of patients, and its expression was strongly linked to
ITGB1 protein, VM formation and TNM stage (Table II). The study suggested that
Nectin-4 promoted angiogenesis and facilitated the progression of
CRC. It was also reported that Nectin-4 had a crucial impact on
colon cancer chemoresistance to 5-FU. The cell culture tests showed
that Nectin-4 overexpression in CRC cells facilitated the growth,
proliferation and movement of cells, and enhanced the resistance to
chemoradiotherapy via the PI3K/AKT signaling pathway. Nectin-4
silencing mediated by si-Nectin-4 reversed chemotherapeutic drug
resistance and improved the effect of treatment in the CRC cells,
thereby indicating that gene silencing could be considered as a
novel therapeutic strategy for CRC (51).
Esophageal cancer (EC)
In a recent study by Deng et al (55), results revealed that Nectin-4 was
upregulated in human EC samples. The study further confirmed a
close connection between Nectin-4 protein expression and tumor size
and stage. Moreover, patients with upregulated Nectin-4 had a worse
survival time than those with downregulated expression, as assessed
by the Cox model analysis [hazard ratio (HR), 1.795; P=0.035]
(Table II). Also, Nectin-4 was
shown to enhance cell viability and migration in EC cell lines, as
well as to facilitate tumor formation in vivo (55). These findings were consistent with a
recent study by Lin et al (12), wherein ~40% of patients presented
with increased Nectin-4 expression in EC. The study also revealed
that increased Nectin-4 was markedly involved in tumor size and
depth of tumor invasion. In addition, Nectin-4 expression was an
unfavorable risk factor for EC, as shown by the multivariate Cox
model (P<0.05; Table II).
GC
In the study by Zhang et al (50), over two-thirds of GC samples
presented upregulated Nectin-4. In addition, Nectin-4 upregulation
was closely correlated with LNI and TNM stage (Table II) and an increased risk of
decreased 5-year survival rate. The study also showed that the
overexpression of Nectin-4 specifically targets the downstream
molecule PI3K/AKT and then acts on Rac1 to facilitate cell
proliferation and movement. In another study (49), high expression of Nectin-4 was
detected in 60.4% (128/212) of GC tumors and was deemed to be an
adverse biomarker for survival in patients with GC (HR, 2.402;
P=0.002).
PC
Nishiwada et al (14) reported that >50% of PC tissues
are Nectin-4-positive, and upregulated Nectin-4 has been shown to
be associated with an unfavorable prognosis of PC. The patients
with upregulated Nectin-4 expression showed a significantly shorter
survival time compared with those in the low expression group
(P<0.01). The study also demonstrated a vital connection between
Nectin-4 and Ki-67 expression (P<0.001). Patients with
upregulated Nectin-4 were more likely to have a high expression
level of Ki-67. Hence, Nectin-4 could be considered as a novel
proliferation marker and was also confirmed as a crucial biomarker
associated with poor survival (P=0.021). Moreover, the findings
also revealed that Nectin-4 in PC was positively and prominently
associated with IMD and VEGF expression. In a similar study, Izumi
et al (86) reported that
upregulated Nectin-4 might be an undesirable risk factor for PC.
Also, patients with upregulated Nectin-4 expression exhibited a
larger tumor size compared with those with low expression.
Hepatocellular carcinoma (HCC)
In the study by Ma et al (87), a total of 87 HCC samples were
collected. Approximately 68% of HCC samples presented noticeably
higher expression of Nectin-4 protein than normal samples.
Moreover, upregulated Nectin-4 was correlated with TNM stage, tumor
size, spread and metastasis, and vascular involvement. Patients in
the Nectin-4-positive group exhibited a worse prognosis compared
with those in the negative expression group. Moreover, Nectin-4 was
a valuable biomarker for predicting RFS and OS. Also, Nectin-4
targeted PI3K/AKT via the Nectin-Afadin complex to regulate various
cellular processes, including increased cell growth, inhibited
apoptosis, and increased local infiltration and transfer in HCC
tumor cells (23).
Gallbladder cancer (GBC)
A total of 68 patients with GBC were enrolled in a
study by Zhang et al (48),
and positive Nectin-4 expression was observed in ~65% of samples.
In contrast to normal tissues, GBC samples exhibited a higher
expression level of Nectin-4 (P<0.01). The study also showed
that Nectin-4 was closely associated with the pathological stage
and LNI (Table II). Notably,
patients with upregulated Nectin-4 were likely to have a short
survival time. Thus, upregulated Nectin-4 can be considered as a
novel biomarker associated with poor prognosis, as assessed using
Cox model analysis (HR, 2.704; P=0.001). Furthermore, Nectin-4 was
identified to regulate GBC cell growth, movement and spread by
stimulating the PI3K/AKT signaling cascade, and the process could
be suppressed by RNA interference in vitro and in
vivo (48).
Enfortumab vedotin (EV)
As aforementioned, Nectin-4 may be a prognostic
marker specifically upregulated in various cancer types, which
promotes tumorigenesis and progression (49,52,58,61).
Thus, it could be a promising novel molecular target for developing
therapeutic strategies for cancer. EV is a new type of ADC
targeting Nectin-4 in clinical practice (88,89).
ADCs are novel monoclonal antibodies coupled with robust biological
drugs via a labile crosslinker. Importantly, the antibody links
with a specific antigen only found on target tumor cells.
Therefore, ADCs have an advantage over traditional drugs in the
aspect of drug specificity (88).
When the monoclonal antibody binds to antigen receptors of tumor
cells, it triggers the internalization of the antibody and mediates
drug release that could be viewed as ‘targeted chemotherapy’
(89). The effectiveness of ADC
therapy depends on the specificity of the antibody (90). Typically, two classical ADCs,
Adcetris and Kadcyla, have been widely utilized in clinical
practice to treat Hodgkin's lymphomaCD30-positive and
BCHER2-positive, respectively (88). Moreover, as a novel ADC, EV
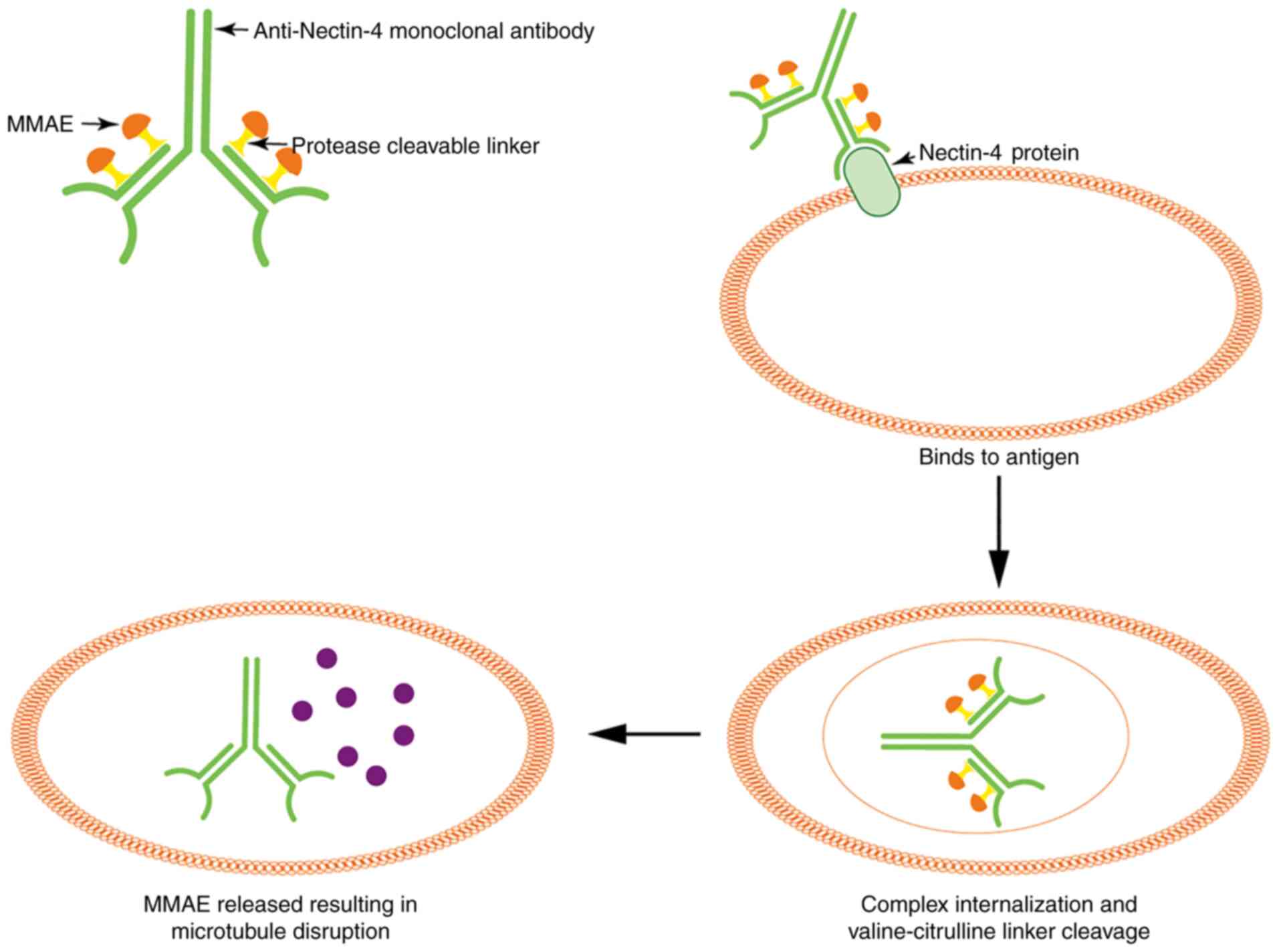
comprises the monoclonal antibody targeting Nectin-4 and is coupled
with a microtubule-disrupting agent, known as monomethyl auristatin
E (MMAE), via a protease-cleavable maleimidocaproyl
valine-citrulline linker (91).
After EV is linked to the V-C-C domain of Nectin-4 antigen, it
triggers complex internalization and translocates to the lysosome
to cleave the valine-citrulline linker and release MMAE in target
cells. Subsequently, MMAE combines with tubules and accelerates
microtubule disassembly, ultimately playing an efficient role
against cancer (88,90,91)
(Fig. 4). Clinically, EV was
approved by the US Food and Drug Administration (FDA) in 2019 for
treating locally advanced or metastatic UC (mUC) after the failure
of previous chemotherapy regimens and immune checkpoint inhibitors
(ICIs) (85).
In 1990, platinum-based chemotherapy was the first
choice for the treatment of mUC. Although the survival of patients
was prolonged, the tolerance to intensive chemotherapy was poor
(85). Since 2016, several ICIs,
including atezolizumab, nivolumab, pembrolizumab, durvalumab and
sacituzumab govitecan antibodies, targeting programmed cell death
protein-programmed death ligand 1 (PD-1/PD-L1) and tumor-associated
calcium signal transducer 2, achieved outstanding results. However,
despite better drug tolerance and fewer adverse drug reactions, the
response rates to PD-1/PD-L1 were low (91). In 2019, targeted EV therapeutics
showed promising results in terms of response rates for patients
who had undergone heavy treatment, including ICI and/or
platinum-containing chemotherapy (91,92).
The approval process timeline for treatments for mUC is shown in
Fig. 5. Owing to the encouraging
nature of existing data, several clinical trials based on EV
treatment are underway. The current review presents the clinical
efficacy data for patients treated with EV.
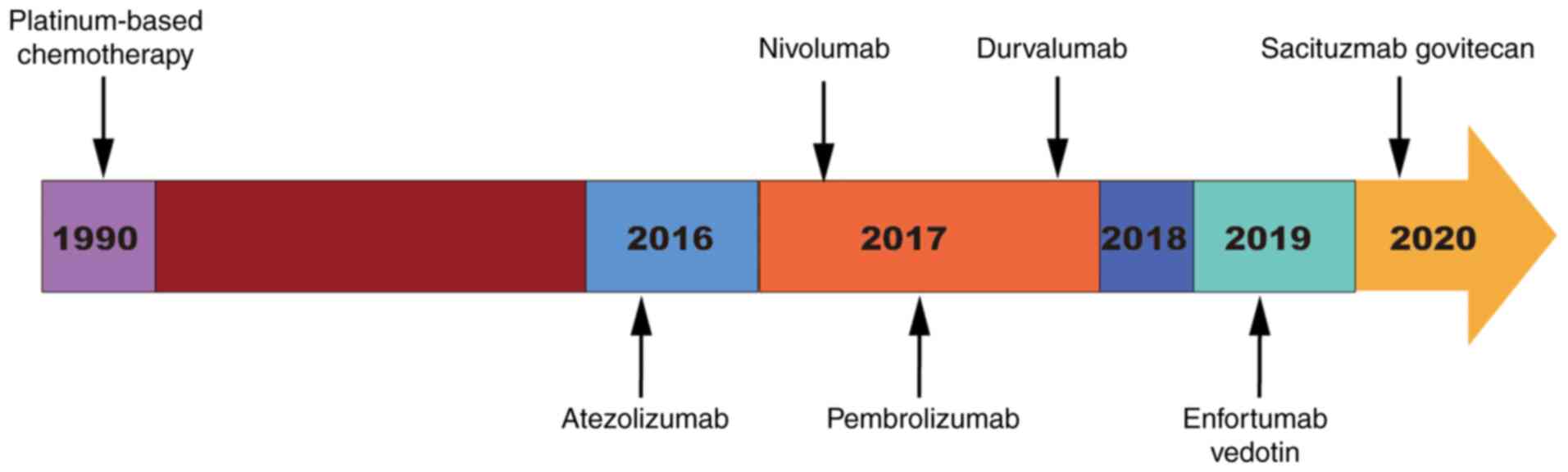 | Figure 5.US Food and Drug Administration
approval timeline for chemotherapy, ICIs and EV against mUC. In
1990, platinum-based chemotherapy was first used to treat mUC.
Since 2016, ICIs, including atezolizumab, nivolumab, pembrolizumab,
durvalumab and sacituzumab govitecan antibodies, which targeted
programmed cell death protein 1 and tumor-associated calcium signal
transducer 2, successively came on the market against mUC. In 2019,
EV was approved for treating patients with mUC. ICIs, immune
checkpoint inhibitors; mUC, metastatic urothelial carcinoma; EV,
enfortumab vedotin. |
EV-101 trial
A total of 155 patients with mUC who suffered from
drug (chemotherapy or ICI) failure or did not meet the requirements
of chemotherapy were recruited in the phase I trial (EV-101,
NCT02091999) between June 23, 2014, and October 25, 2018 (93). Subsequently, 112 patients were
treated with a recommended phase II dose (1.25 mg/kg EV once a week
over a 4-week cycle). Prior to EV treatment, ~96% of these patients
experienced chemotherapy failure, and 72% of patients accepted
anti-PD-1/PD-L1 treatment. The overall response rate (ORR),
complete response (CR) rate and partial response (PR) rate was 43,
5 and 38%, with a median PFS time of 5.4 months and a median
duration of response (DOR) of 7.4 months. In patients with a
history of liver metastasis and treatment with PD-1/PD-L1
inhibitor, the ORR was 42 and 36%, respectively (Table III). The median OS time with 1.25
mg/kg EV was 12.3 months (95% CI, 9.3–15.3), and the OS rate at 1
year was 51.8%, with a median follow-up time of 16.4 months
(94).
 | Table III.Clinical trials using EV in advanced
or metastatic urothelial carcinoma. |
Table III.
Clinical trials using EV in advanced
or metastatic urothelial carcinoma.
| Factor | EV-101 | EV-201 | EV-103 | EV-301 | EV-302 |
|---|
| NCT (clinicaltrials.gov) no. | NCT02091999 | NCT03219333 | NCT03288545 | NCT03474107 | NCT04223856 |
| (Refs.) | (93,94) | (95) | (96) | (97) | (95,97) |
| Phase | I | II | I | III | III |
| Line of
treatment | Later-line | Later-line | First-line | Later-line | First-line |
| Prior
treatment | CT | CT or ICI | NO | Both platinum-based
CT and ICI | NO |
| Comparison | NA | Once accepted,
platinum-based CT and ICI vs. ICI | Comparing EV in
combination with ICI(P) and/or CT | EV vs. CT (except
platinum) | Comparing EV in
combination with P with or without CT vs. CT |
| EV dose | 1.25 mg/kg on days
1, 8 and 15 of a 28-day cycle. | 1.25 mg/kg on days
1, 8 and 15 of a 28-day cycle. | 1.25 mg/kg on days
1 and 8 of a 21-day cycle. | 1.25 mg/kg on days
1, 8 and 15 of a 28-day cycle. | 1.25 mg/kg on days
1, 8 and 15 of a 28-day cycle. |
| Population | 112 | 125 | 45
(preliminary) | 301 | 1,095 (aim) |
| ORR (95% CI),
% | 43 (33.6–52.6) | 52 (41–62) | 73.3
(58.1–85.4) | 40.6
(34.9–46.5) | NA |
| CR, % | 5 | 20 | 15.6 | 4.9 | NA |
| PR, % | 38 | 31 | 58 | NA | NA |
EV-201 trial
A total of 125 patients undergoing chemotherapy or
PD-1/PD-L1 inhibitor treatment for advanced mUC were enrolled in
the phase II trial (EV-201 trial, NCT03219333) between October 8,
2017, and February 11, 2020. Prior to EV treatment, these enrolled
patients were categorized into two groups as follows: Group 1, once
received combination treatment of platinum-based chemotherapy and
PD-1/PD-L1 inhibitor; and group 2, only once received PD-1/PD-L1
inhibitor. In group 2 of EV-201, the enrolled 89 patients were
treated with EV at a dosage of 1.25 mg/kg once a week over a 4-week
cycle. At data cutoff (September 8, 2020), the ORR, CR rate and PR
rate were 52% (46/89 patients), 20% (18/89 patients) and 31% (28/89
patients), respectively, with a median follow-up time of 13.4
months (Table III). The median
PFS time was 5.8 months (95% CI, 5.03–8.28) (95).
EV-103 trial
The EV-103 trial (NCT03288545) is another phase I,
multicenter clinical trial in progress. All patients received a
combination treatment of EV plus PD-1 inhibitor (pembrolizumab)
and/or chemotherapy as the first choice for treating advanced UC or
mUC. Prior to combination treatment, the trial collected 45 mUC
patients unsuitable for chemotherapy. In addition, these patients
were treated with EV (at a dosage of 1.25 mg/kg once a week over a
3-week cycle) combined with a PD-1/PD-L1 inhibitor (at a dose of
200 mg on days 1, 8 and 15 over a 3-week cycle). At the recent 2020
American Society of Clinical Oncology (ASCO) virtual meeting,
Rosenberg reported that these combined therapies showed encouraging
and durable activity, with an ORR of 73.3%, a CR rate of 15.6%, a
PR rate of 58% and a median PFS time of 12.3 months, while 93% had
a decline in target lesions (96).
Due to these results, on February 18, 2020, the FDA granted a
breakthrough therapy designation for the combination of EV and
pembrolizumab for cisplatin-ineligible patients with locally
advanced or metastatic urothelial carcinoma as the first-line
treatment (96).
EV-301 trial
Patients with previous platinum-based chemotherapy
and PD-1/PD-L1 inhibitor treatment were enrolled in the phase III
trial of EV-301 (NCT03474107) between June 2018 and July 2020. A
total of 608 patients were randomly assigned to two groups in a 1:1
ratio. A total of 301 patients accepted EV alone (at a dosage of
1.25 mg/kg once a week over a 4-week cycle) and 307 patients
accepted a chemotherapy regimen, excluding platinum (given on days
1, 7 and 15 over a 3-week cycle) (Table III). The ORR and CR rate were
lower in the chemotherapy group than in the EV group (17.9 vs.
40.6% and 2.7 vs. 4.9%, respectively). In addition, the OS and PFS
times were longer in the EV group than those in the chemotherapy
group, with a median follow-up time of 11.1 months (HR, 0.70;
P=0.001; and HR, 0.62; P<0.001, respectively) (97).
EV-302 trial
The EV-302 trial (NCT04223856) is a phase III study
enrolling patients with mUC who have not received any prior
treatment. This trial aims to observe and compare the therapeutic
effect between chemotherapy alone and the combination of EV and
PD-1/PD-L1 inhibitors with or without chemotherapy. The study is
divided into three groups: Group A, pembrolizumab plus EV; group B,
cisplatin/carboplatin plus gemcitabine; and group C, pembrolizumab
plus EV plus cisplatin/carboplatin. The PFS and OS are the main
observation indexes. ORR, DOR and disease control rate are
secondary observation indexes. This trial is open for registration
and aims to enroll 1,095 patients by November 2023 (Table III) (95,97).
Oncolytic virus
As aforementioned, Nectin-4 is a tumor cell marker
highly expressed on the apical surface of a number of
adenocarcinoma cell lines and correlated with tumor progression and
worse prognosis (49–55,66,71).
Unexpectedly, Nectin-4 can also serve as another receptor for
measles virus (MV) oncolytic therapy (98). In the past decades, MV, as a member
of the Paramyxoviridae family, was found to be likely to infect the
respiratory system (99).
Importantly, MV can serve as an oncolytic virus, characterized by
the ability to attack and dissolve cancer cells, but to not hurt
normal cells. Previously, several studies have shown that MV could
act as a ‘natural cancer cell-killer’ for Burkitt's lymphoma and
Hodgkin's disease during natural virus infections. This phenomenon
could be attributed to the fact that MV recognizes and binds to
CD150/SLAM receptors that are explicitly expressed in these tumors,
inducing a cascade of immune responses (100,101). However, accumulating evidence over
the past decade has shown that MV infects several cell lines
independently of the CD150/SLAM receptors, leading to the discovery
of new receptors for MV therapy. Noyce et al (21) reported that cells that synthesized
Nectin-4 became susceptible to MV infection, identifying this
membrane protein as the elusive epithelial receptor. The
interaction between MV and Nectin-4-positive cancer cells triggers
MV internalization and exerts an oncolytic effect (21). In addition, some synthetic MVs carry
therapeutic substances, such as the sodium iodide symporter, which
trigger the internalization and aggregation of radioactive iodine
to enhance cell killing (102). In
conclusion, advantage could be taken of the natural killing effect
of MV for the treatment of Nectin-4-induced cancer.
Conclusion
The present review has shown that Nectin-4
expression has been altered in numerous cancer types, and that it
is crucial in regulating tumor occurrence and development. This
review also revealed that the overexpression of Nectin-4 is
correlated with the poor prognosis of patients. However, the exact
mechanism underlying increased levels of Nectin-4 and its role in
transcriptional and protein control during carcinogenesis is yet to
be elucidated. The putative association between Nectin-4 proteins
and cancer would open a novel avenue for identifying potential
therapeutic targets to improve patient outcomes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81872184, 81702161,
81773031 and 80191101553).
Availability of data and materials
Not applicable.
Authors' contributions
GW, YangZ, and XL conceptualized and designed this
present review. YantingZ, XH and GL performed the literature search
for this article. LL and MY collected and analyzed the relevant
data. YL and XH wrote the manuscript. CX and PZ designed and
finalized the tables and figures. FS and ZY reviewed and corrected
the manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boumahdi S, Driessens G, Lapouge G, Rorive
S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E,
et al: SOX2 controls tumour initiation and cancer stem-cell
functions in squamous-cell carcinoma. Nature. 511:246–250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chothia C and Jones EY: The molecular
structure of cell adhesion molecules. Annu Rev Biochem. 66:823–862.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reymond N, Fabre S, Lecocq E, Adelaïde J,
Dubreuil P and Lopez M: Nectin4/PRR4, a new Afadin-associated
member of the nectin family that trans-interacts with nectin1/PRR1
through V domain interaction. J Biol Chem. 276:43205–43215. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fabre S, Reymond N, Cocchi F, Menotti L,
Dubreuil P, Campadelli-Fiume G and Lopez M: Prominent role of the
Ig-like V domain in trans-interactions of nectins. Nectin3 and
nectin 4 bind to the predicted C′-C′-D beta-strands of the nectin1
V domain. J Biol Chem. 277:27006–27013. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yasumi M, Shimizu K, Honda T, Takeuchi M
and Takai Y: Role of each immunoglobulin-like loop of nectin for
its cell-cell adhesion activity. Biochem Biophys Res Commun.
302:61–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takai Y, Miyoshi J, Ikeda W and Ogita H:
Nectins and nectin-like molecules: Roles in contact inhibition of
cell movement and proliferation. Nat Rev Mol Cell Biol. 9:603–615.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakisaka T, Ikeda W, Ogita H, Fujita N and
Takai Y: The roles of nectins in cell adhesions: Cooperation with
other cell adhesion molecules and growth factor receptors. Curr
Opin Cell Biol. 19:593–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakanishi H and Takai Y: Roles of nectins
in cell adhesion, migration and polarization. Biol Chem.
385:885–892. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavlova NN, Pallasch C, Elia AE, Braun CJ,
Westbrook TF, Hemann M and Elledge SJ: A role for PVRL4-driven
cell-cell interactions in tumorigenesis. Elife. 2:e003582013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takano A, Ishikawa N, Nishino R, Masuda K,
Yasui W, Inai K, Nishimura H, Ito H, Nakayama H, Miyagi Y, et al:
Identification of Nectin-4 oncoprotein as a diagnostic and
therapeutic target for lung cancer. Cancer Res. 69:6694–6703. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Derycke MS, Pambuccian SE, Gilks CB,
Kalloger SE, Ghidouche A, Lopez M, Bliss RL, Geller MA, Argenta PA,
Harrington KM and Skubitz AP: Nectin 4 overexpression in ovarian
cancer tissues and serum: Potential role as a serum biomarker. Am J
Clin Pathol. 134:835–845. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin X, Hu H, Pan Y and Gao S: The
prognostic role of expression of Nectin-4 in esophageal cancer. Med
Sci Monit. 25:10089–10094. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fabre-Lafay S, Monville F, Garrido-Urbani
S, Berruyer-Pouyet C, Ginestier C, Reymond N, Finetti P, Sauvan R,
Adélaïde J, Geneix J, et al: Nectin-4 is a new histological and
serological tumor associated marker for breast cancer. BMC Cancer.
7:732007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishiwada S, Sho M, Yasuda S, Shimada K,
Yamato I, Akahori T, Kinoshita S, Nagai M, Konishi N and Nakajima
Y: Nectin-4 expression contributes to tumor proliferation,
angiogenesis and patient prognosis in human pancreatic cancer. J
Exp Clin Cancer Res. 34:302015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakisaka T and Takai Y: Biology and
pathology of nectins and nectin-like molecules. Curr Opin Cell
Biol. 16:513–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamada A, Fujita N, Sato T, Okamoto R,
Ooshio T, Hirota T, Morimoto K, Irie K and Takai Y: Requirement of
nectin, but not cadherin, for formation of claudin-based tight
junctions in annexin II-knockdown MDCK cells. Oncogene.
25:5085–5102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lopez M, Aoubala M, Jordier F, Isnardon D,
Gomez S and Dubreuil P: The human poliovirus receptor related 2
protein is a new hematopoietic/endothelial homophilic adhesion
molecule. Blood. 92:4602–4611. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reymond N, Borg JP, Lecocq E, Adelaide J,
Campadelli-Fiume G, Dubreuil P and Lopez M: Human nectin3/PRR3: A
novel member of the PVR/PRR/nectin family that interacts with
Afadin. Gene. 255:347–355. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takai Y and Nakanishi H: Nectin and
Afadin: Novel organizers of intercellular junctions. J Cell Sci.
116:17–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takai Y, Irie K, Shimizu K, Sakisaka T and
Ikeda W: Nectins and nectin-like molecules: Roles in cell adhesion,
migration, and polarization. Cancer Sci. 94:655–667. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson
G, Tsao MS and Richardson CD: Tumor cell marker PVRL4 (nectin 4) is
an epithelial cell receptor for measles virus. PLoS Pathog.
7:e10022402011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bojesen KB, Clausen O, Rohde K,
Christensen C, Zhang L, Li S, Køhler L, Nielbo S, Nielsen J,
Gjørlund MD, et al: Nectin-1 binds and signals through the
fibroblast growth factor receptor. J Biol Chem. 287:37420–37433.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanzaki N, Ogita H, Komura H, Ozaki M,
Sakamoto Y, Majima T, Ijuin T, Takenawa T and Takai Y: Involvement
of the nectin-Afadin complex in PDGF-induced cell survival. J Cell
Sci. 121:2008–2017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogita H and Takai Y: Cross-talk among
integrin, cadherin, and growth factor receptor: Roles of nectin and
nectin-like molecule. Int Rev Cytol. 265:1–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinez-Rico C, Pincet F, Perez E, Thiery
JP, Shimizu K, Takai Y and Dufour S: Separation force measurements
reveal different types of modulation of E-cadherin-based adhesion
by nectin-1 and −3. J Biol Chem. 280:4753–4760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Xie X, Zhang X, Jia W, Jian J,
Song C and Jin B: The expression, regulation and adhesion function
of a novel CD molecule, CD226, on human endothelial cells. Life
Sci. 73:2373–2382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shibuya K, Shirakawa J, Kameyama T, Honda
S, Tahara-Hanaoka S, Miyamoto A, Onodera M, Sumida T, Nakauchi H,
Miyoshi H and Shibuya A: CD226 (DNAM-1) is involved in lymphocyte
function-associated antigen 1 costimulatory signal for naive T cell
differentiation and proliferation. J Exp Med. 198:1829–1839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stanietsky N, Simic H, Arapovic J, Toporik
A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al:
The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell
cytotoxicity. Proc Natl Acad Sci USA. 106:17858–17863. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu X, Harden K, Gonzalez LC, Francesco M,
Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al: The
surface protein TIGIT suppresses T cell activation by promoting the
generation of mature immunoregulatory dendritic cells. Nat Immunol.
10:48–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stanietsky N, Rovis TL, Glasner A, Seidel
E, Tsukerman P, Yamin R, Enk J, Jonjic S and Mandelboim O: Mouse
TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur
J Immunol. 43:2138–2150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li M, Xia P, Du Y, Liu S, Huang G, Chen J,
Zhang H, Hou N, Cheng X, Zhou L, et al: T-cell immunoglobulin and
ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand
engagement suppresses interferon-γ production of natural killer
cells via β-arrestin 2-mediated negative signaling. J Biol Chem.
289:17647–17657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu S, Zhang H, Li M, Hu D, Li C, Ge B,
Jin B and Fan Z: Recruitment of Grb2 and SHIP1 by the ITT-like
motif of TIGIT suppresses granule polarization and cytotoxicity of
NK cells. Cell Death Differ. 20:456–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnston RJ, Comps-Agrar L, Hackney J, Yu
X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al:
The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T
cell effector function. Cancer Cell. 26:923–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takahashi K, Nakanishi H, Miyahara M,
Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi
A and Takai Y: Nectin/PRR: An immunoglobulin-like cell adhesion
molecule recruited to cadherin-based adherens junctions through
interaction with Afadin, a PDZ domain-containing protein. J Cell
Biol. 145:539–549. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Samanta D and Almo SC: Nectin family of
cell-adhesion molecules: Structural and molecular aspects of
function and specificity. Cell Mol Life Sci. 72:645–658. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shankar J and Nabi IR: Correction: Actin
cytoskeleton regulation of epithelial mesenchymal transition in
metastatic cancer cells. PLoS One. 10:e01327592015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Irie K, Shimizu K, Sakisaka T, Ikeda W and
Takai Y: Roles and modes of action of nectins in cell-cell
adhesion. Semin Cell Dev Biol. 15:643–656. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimizu K and Takai Y: Roles of the
intercellular adhesion molecule nectin in intracellular signaling.
J Biochem. 134:631–636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ikeda W, Nakanishi H, Miyoshi J, Mandai K,
Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H,
et al: Afadin: A key molecule essential for structural organization
of cell-cell junctions of polarized epithelia during embryogenesis.
J Cell Biol. 146:1117–1132. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Letessier A, Garrido-Urbani S, Ginestier
C, Fournier G, Esterni B, Monville F, Adélaïde J, Geneix J, Xerri
L, Dubreuil P, et al: Correlated break at PARK2/FRA6E and loss of
AF-6/Afadin protein expression are associated with poor outcome in
breast cancer. Oncogene. 26:298–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fukuyama T, Ogita H, Kawakatsu T, Fukuhara
T, Yamada T, Sato T, Shimizu K, Nakamura T, Matsuda M and Takai Y:
Involvement of the c-Src-Crk-C3G-Rap1 signaling in the
nectin-induced activation of Cdc42 and formation of adherens
junctions. J Biol Chem. 280:815–825. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kawakatsu T, Ogita H, Fukuhara T, Fukuyama
T, Minami Y, Shimizu K and Takai Y: Vav2 as a Rac-GDP/GTP exchange
factor responsible for the nectin-induced, c-Src- and
Cdc42-mediated activation of Rac. J Biol Chem. 280:4940–4947. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takai Y, Ikeda W, Ogita H and Rikitake Y:
The immunoglobulin-like cell adhesion molecule nectin and its
associated protein Afadin. Annu Rev Cell Dev Biol. 24:309–342.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Okabe N, Shimizu K, Ozaki-Kuroda K,
Nakanishi H, Morimoto K, Takeuchi M, Katsumaru H, Murakami F and
Takai Y: Contacts between the commissural axons and the floor plate
cells are mediated by nectins. Dev Biol. 273:244–256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ahmad F, Nasir A, Thiele H, Umair M, Borck
G and Ahmad W: A novel homozygous missense variant in NECTIN4
(PVRL4) causing ectodermal dysplasia cutaneous syndactyly syndrome.
Ann Hum Genet. 82:232–238. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brancati F, Fortugno P, Bottillo I, Lopez
M, Josselin E, Boudghene-Stambouli O, Agolini E, Bernardini L,
Bellacchio E, Iannicelli M, et al: Mutations in PVRL4, encoding
cell adhesion molecule Nectin-4, cause ectodermal
dysplasia-syndactyly syndrome. Am J Hum Genet. 87:265–473. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Challita-Eid PM, Satpayev D, Yang P, An Z,
Morrison K, Shostak Y, Raitano A, Nadell R, Liu W, Lortie DR, et
al: Enfortumab vedotin antibody-drug conjugate targeting Nectin-4
is a highly potent therapeutic agent in multiple preclinical cancer
models. Cancer Res. 76:3003–3013. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Y, Liu S, Wang L, Wu Y, Hao J, Wang
Z, Lu W, Wang XA, Zhang F, Cao Y, et al: A novel PI3K/AKT signaling
axis mediates Nectin-4-induced gallbladder cancer cell
proliferation, metastasis and tumor growth. Cancer Lett.
375:179–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Zhang J, Shen Q, Yin W, Huang H,
Liu Y and Ni Q: High expression of Nectin-4 is associated with
unfavorable prognosis in gastric cancer. Oncol Lett. 15:8789–8795.
2018.PubMed/NCBI
|
|
50
|
Zhang Y, Chen P, Yin W, Ji Y, Shen Q and
Ni Q: Nectin-4 promotes gastric cancer progression via the PI3K/AKT
signaling pathway. Hum Pathol. 72:107–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Das D, Satapathy SR, Siddharth S, Nayak A
and Kundu CN: Nectin-4 increased the 5-FU resistance in colon
cancer cells by inducing the PI3K-AKT cascade. Cancer Chemother
Pharmacol. 76:471–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang J, Liu K, Peng P, Li S, Ye Z, Su Y,
Liu S, Qin M and Huang J: Upregulation of Nectin-4 is associated
with ITGB1 and vasculogenic mimicry and may serve as a predictor of
poor prognosis in colorectal cancer. Oncol Lett. 18:1163–1170.
2019.PubMed/NCBI
|
|
53
|
Siddharth S, Nayak A, Das S, Nayak D,
Panda J, Wyatt MD and Kundu CN: The soluble Nectin-4 ecto-domain
promotes breast cancer induced angiogenesis via endothelial
Integrin-β4. Int J Biochem Cell Biol. 102:151–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Siddharth S, Goutam K, Das S, Nayak A,
Nayak D, Sethy C, Wyatt MD and Kundu CN: Nectin-4 is a breast
cancer stem cell marker that induces WNT/β-catenin signaling via
Pi3k/Akt axis. Int J Biochem Cell Biol. 89:85–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Deng H, Shi H, Chen L, Zhou Y and Jiang J:
Over-expression of Nectin-4 promotes progression of esophageal
cancer and correlates with poor prognosis of the patients. Cancer
Cell Int. 19:1062019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yu X, Zhen Y, Yang H, Wang H, Zhou Y, Wang
E, Marincola FM, Mai C, Chen Y, Wei H, et al: Loss of connective
tissue growth factor as an unfavorable prognosis factor activates
miR-18b by PI3K/AKT/C-Jun and C-Myc and promotes cell growth in
nasopharyngeal carcinoma. Cell Death Dis. 4:e6342013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bousquet E, Calvayrac O, Mazières J,
Lajoie-Mazenc I, Boubekeur N, Favre G and Pradines A: RhoB loss
induces Rac1-dependent mesenchymal cell invasion in lung cells
through PP2A inhibition. Oncogene. 35:1760–1769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Karlsson R, Pedersen ED, Wang Z and
Brakebusch C: Rho GTPase function in tumorigenesis. Biochim Biophys
Acta. 1796:91–98. 2009.PubMed/NCBI
|
|
61
|
Schmidt A and Hall A: Guanine nucleotide
exchange factors for Rho GTPases: Turning on the switch. Genes Dev.
16:1587–1609. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guo Y, Kenney SR, Muller CY, Adams S,
Rutledge T, Romero E, Murray-Krezan C, Prekeris R, Sklar LA, Hudson
LG, et al: R-ketorolac targets Cdc42 and Rac1 GTPases and alters
ovarian tumor cell behaviors critical for invasion and metastasis.
Cancer Res. 75 (Suppl 15):S40442015.
|
|
63
|
Vial E, Sahai E and Marshall CJ: ERK-MAPK
signaling coordinately regulates activity of Rac1 and RhoA for
tumor cell motility. Cancer Cell. 4:67–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Coso OA, Chiariello M, Yu JC, Teramoto H,
Crespo P, Xu N, Miki T and Gutkind JS: The small GTP-binding
proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK
signaling pathway. Cell. 81:1137–1146. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Eswaran J, Li DQ, Shah A and Kumar R:
Molecular pathways: Targeting p21-activated kinase 1 signaling in
cancer-opportunities, challenges, and limitations. Clin Cancer Res.
18:3743–3749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Henderson V, Smith B, Burton LJ, Randle D,
Morris M and Odero-Marah VA: Snail promotes cell migration through
PI3K/AKT-dependent Rac1 activation as well as PI3K/AKT-independent
pathways during prostate cancer progression. Cell Adh Migr.
9:255–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ray RM, Vaidya RJ and Johnson LR: MEK/ERK
regulates adherens junctions and migration through Rac1. Cell Motil
Cytoskeleton. 64:143–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Slabáková E, Pernicová Z, Slavíčková E,
Staršíchová A, Kozubík A and Souček K: TGF-β1-induced EMT of
non-transformed prostate hyperplasia cells is characterized by
early induction of SNAI2/Slug. Prostate. 71:1332–1343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hao RT, Zheng C, Wu CY, Xia EJ, Zhou XF,
Quan RD and Zhang XH: NECTIN4 promotes papillary thyroid cancer
cell proliferation, migration, and invasion and triggers EMT by
activating AKT. Cancer Manag Res. 11:2565–2578. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the carolina
breast cancer study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Athanassiadou AM, Patsouris E, Tsipis A,
Gonidi M and Athanassiadou P: The significance of survivin and
Nectin-4 expression in the prognosis of breast carcinoma. Folia
Histochem Cytobiol. 49:26–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rajc J, Gugić D, Fröhlich I, Marjanović K
and Dumenčić B: Prognostic role of Nectin-4 expression in luminal B
(HER2 negative) breast cancer. Pathol Res Pract. 213:1102–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
M-Rabet M, Cabaud O, Josselin E, Finetti
P, Castellano R, Farina A, Agavnian-Couquiaud E, Saviane G,
Collette Y, Viens P, et al: Nectin-4: A new prognostic biomarker
for efficient therapeutic targeting of primary and metastatic
triple-negative breast cancer. Ann Oncol. 28:769–776. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zeindler J, Soysal SD, Piscuoglio S, Ng
CKY, Mechera R, Isaak A, Weber WP, Muenst S and Kurzeder C:
Nectin-4 expression is an independent prognostic biomarker and
associated with better survival in triple-negative breast cancer.
Front Med (Lausanne). 6:2002019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lattanzio R, Ghasemi R, Brancati F, Sorda
RL, Tinari N, Perracchio L, Iacobelli S, Mottolese M, Natali PG and
Piantelli M: Membranous Nectin-4 expression is a risk factor for
distant relapse of T1-T2, N0 luminal-A early breast cancer.
Oncogenesis. 3:e1182014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fabre-Lafay S, Garrido-Urbani S, Reymond
N, Goncalves A, Dubreuil P and Lopez M: Nectin-4, a new serological
breast cancer marker, is a substrate for tumor necrosis
factor-alpha-converting enzyme (TACE)/ADAM-17. J Biol Chem.
280:19543–19550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hibbs K, Skubitz KM, Pambuccian SE, Casey
RC, Burleson KM, Oegema TR Jr, Thiele JJ, Grindle SM, Bliss RL and
Skubitz AP: Differential gene expression in ovarian carcinoma:
Identification of potential biomarkers. Am J Pathol. 165:397–414.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nabih ES, Abdel Motaleb FI and Salama FA:
The diagnostic efficacy of nectin 4 expression in ovarian cancer
patients. Biomarkers. 19:498–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Boylan KL, Buchanan PC, Manion RD, Shukla
DM, Braumberger K, Bruggemeyer C and Skubitz AP: The expression of
Nectin-4 on the surface of ovarian cancer cells alters their
ability to adhere, migrate, aggregate, and proliferate. Oncotarget.
8:9717–9738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pradeep S, Kim SW, Wu SY, Nishimura M,
Chaluvally Raghavan P, Miyake T, Pecot CV, Kim SJ, Choi HJ,
Bischoff FZ, et al: Hematogenous metastasis of ovarian cancer:
Rethinking mode of spread. Cancer Cell. 26:77–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Desoize B and Jardillier J: Multicellular
resistance: A paradigm for clinical resistance? Crit Rev Oncol
Hematol. 36:193–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Boylan KLM, Manion RD, Shah H, Skubitz KM
and Skubitz APN: Inhibition of ovarian cancer cell spheroid
formation by synthetic peptides derived from Nectin-4. Int J Mol
Sci. 21:46372020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Erturk K, Karaman S, Dagoglu N, Serilmez
M, Duranyildiz D and Tas F: Serum Nectin-2 and Nectin-4 are
diagnostic in lung cancer: Which is superior? Wien Klin Wochenschr.
131:419–426. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Alhalabi O, Rafei H, Shah A,
Siefker-Radtke A, Campbell M and Gao J: Targeting advanced
urothelial carcinoma-developing strategies. Curr Opin Oncol.
31:207–215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tomiyama E, Fujita K, Rodriguez Pena MDC,
Taheri D, Banno E, Kato T, Hatano K, Kawashima A, Ujike T, Uemura
M, et al: Expression of Nectin-4 and PD-L1 in upper tract
urothelial carcinoma. Int J Mol Sci. 21:53902020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Izumi H, Hirabayashi K, Nakamura N and
Nakagohri T: Nectin expression in pancreatic adenocarcinoma:
Nectin-3 is associated with a poor prognosis. Surg Today.
45:487–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ma J, Sheng Z, Lv Y, Liu W, Yao Q, Pan T,
Xu Z, Zhang C and Xu G: Expression and clinical significance of
Nectin-4 in hepatocellular carcinoma. Onco Targets Ther. 9:183–190.
2016.PubMed/NCBI
|
|
88
|
Thomas A, Teicher BA and Hassan R:
Antibody-drug conjugates for cancer therapy. Lancet Oncol.
17:e254–e262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sarfaty M and Rosenberg JE: Antibody-drug
conjugates in urothelial carcinomas. Curr Oncol Rep. 22:132020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Leyton JV: Improving receptor-mediated
intracellular access and accumulation of antibody therapeutics-the
tale of HER2. Antibodies (Basel). 9:322020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Rosenberg JE, O'Donnell PH, Balar AV,
McGregor BA, Heath EI, Yu EY, Galsky MD, Hahn NM, Gartner EM,
Pinelli JM, et al: Pivotal trial of enfortumab vedotin in
urothelial carcinoma after platinum and anti-programmed death
1/programmed death ligand 1 therapy. J Clin Oncol. 37:2592–2600.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bednova O and Leyton JV: Targeted
molecular therapeutics for bladder cancer-a new option beyond the
mixed fortunes of immune checkpoint inhibitors? Int J Mol Sci.
21:72682020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
No authors listed. Targeting Nectin-4 in
bladder cancer. Cancer Discov. 7:OF32017. View Article : Google Scholar
|
|
94
|
Rosenberg J, Sridhar SS, Zhang J, Smith D,
Ruether D, Flaig TW, Baranda J, Lang J, Plimack ER, Sangha R, et
al: EV-101: A phase I study of single-agent enfortumab vedotin in
patients with Nectin-4-positive solid tumors, including metastatic
urothelial carcinoma. J Clin Oncol. 38:1041–1049. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yu EY, Petrylak DP, O'Donnell PH, Lee JL,
van der Heijden MS, Loriot Y, Stein MN, Necchi A, Kojima T,
Harrison MR, et al: Enfortumab vedotin after PD-1 or PD-L1
inhibitors in cisplatin-ineligible patients with advanced
urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2
trial. Lancet Oncol. 22:872–882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hoimes CJ, Rosenberg JE, Petrylak DP,
Carret AS, Sasse C, Chaney MF and Flaig TW: Study EV-103: New
cohorts testing enfortumab vedotin alone or in combination with
pembrolizumab in muscle invasive urothelial cancer. J Clin Oncol.
38 (Suppl 6):TPS5952020. View Article : Google Scholar
|
|
97
|
Powles T, Rosenberg JE, Sonpavde GP,
Loriot Y, Durán I, Lee JL, Matsubara N, Vulsteke C, Castellano D,
Wu C, et al: Enfortumab vedotin in previously treated advanced
urothelial carcinoma. N Engl J Med. 384:1125–1135. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Delpeut S, Sisson G, Black KM and
Richardson CD: Measles virus enters breast and colon cancer cell
lines through a PVRL4-mediated macropinocytosis pathway. J Virol.
91:e02191–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Griffin DE and Oldstone MB: Measles:
History and basic biology. Introduction. Curr Top Microbiol
Immunol. 329:12009.PubMed/NCBI
|
|
100
|
Bluming AZ and Ziegler JL: Regression of
Burkitt's lymphoma in association with measles infection. Lancet.
2:105–106. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Taqi AM, Abdurrahman MB, Yakubu AM and
Fleming AF: Regression of Hodgkin's disease after measles. Lancet.
1:11121981. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li H, Peng KW and Russell SJ: Oncolytic
measles virus encoding thyroidal sodium iodide symporter for
squamous cell cancer of the head and neck radiovirotherapy. Hum
Gene Ther. 23:295–301. 2012. View Article : Google Scholar : PubMed/NCBI
|