Introduction
Cancer has become an increasingly global
life-threatening disease with the advancing age of the global
population. Based on estimations from the American Cancer Society
and the National Cancer Institute, >16.9 million cancer
survivors (individuals with a history of all types of cancer) were
alive in America on January 1, 2019 (1), among whom ~1.54 million patients
suffered from colon cancer (1).
The incidence of colon cancer varies up to 10-fold globally, being
associated with the human development index and different
lifestyles. In developed regions, the incidence rate of colon
cancer is >32.2 per 100,000 individuals (2). In 2018 in Europe, colon cancer was
the leading cause of new cancer diagnoses, accounting for 44.4 per
100,000 of estimated new cancer cases (3). Despite progress being made in the
development of mechanisms and treatment strategies, including
colectomy, radiotherapy and chemotherapy, 35% of patients with
colorectal cancer do not survive 5 years following the diagnosis
(1). Bladder dysfunction or
sexual dysfunction ascribed to colostomy (4-6)
and tumor chemoresistance induced by current therapies suggest that
novel therapeutic avenues and detailed mechanistic explorations are
required.
Numerous studies have explored the detailed
mechanisms responsible for tumorigenesis, including cell arrest or
death, tumor metabolism and enhancing the effect of traditional
anti- tumor agents, to combat cancer (7-11).
The present perspectives of cancer appear to promote the influence
of inflammation, which contributes to tumor initiation, progression
and metastasis (12). Interleukin
(IL)-6 is an inflammatory cytokine and has been documented to be
involved in cancer-related inflammation, cell proliferation, tumor
invasion, angiogenesis, metastasis and chemoresistance (13). The binding of IL-6 and its
membrane receptor (mIL-6R) allows the recruitment of glycoprotein
130 (gp130), resulting in a trimer comprised of IL-6, mIL-6R and
gp130 (14). The protein complex
promotes the close contact of Janus kinases and tyrosine kinase
with gp130, and facilitates a signaling cascade via phosphorylated
tyrosine residues of gp130 (IL-6 classical signaling) (14). In the context of cell signaling
without mIL-6R, IL-6 can alternatively bind to the soluble form of
IL-6R (sIL-6R) and then recruit gp130, inducing the formation of
the aforementioned protein complex to promote the downstream
signaling cascade (IL-6 trans-signaling) (15). Signal transducer and activator of
transcription 3 (STAT3) is the major downstream transcription
factor upon IL-6 induction in both pathways, which drives the
transcription of several survival-associated genes (16,17). The constitutive activation of
STAT3 plays a pivotal role in colon cancer (18-20). Previously, the authors' research
group demonstrated that STAT3 activation was necessary for tumor
survival and for the proliferation of cancer-initiating and stem
cell-like colon cancer cells (21,22). Agents targeting IL-6/STAT3
signaling exert potential inhibitory effects on colon cancer
(23-25). Moreover, in the current treatment
for colon cancer, agents targeting epidermal growth factor receptor
(EGFR) signaling have significantly contributed to the advancements
made in this field. However, a number of patients who receive
anti-EGFR therapy experience drug resistance, which has been found
to be associated with STAT3 activation. The combined inhibition of
EGFR and STAT3 may thus prove beneficial for this type of cancer
(26).
A disintegrin and metalloproteinases (ADAMs) are
transmembrane metalloproteinases capable of shedding membrane
anchoring proteins. ADAM17 was originally identified to contribute
to the cleavage of tumor necrosis factor-α (TNF-α) on the cell
membrane, allowing the release of soluble TNF-α and promoting the
inflammatory cascade by triggering TNF-α receptor signaling
(27,28). The expression of ADAM17 is under
strict orchestration by a series of factors. Rhomboid family membe
2 (RHBDF2, also known as iRhom2) and furin protein mediate the
trafficking and maturation of ADAM17 (29), while RHBDF2 combined with
phosphofurin acidic cluster sorting protein 2 (PACS-2) control the
degradation or preservation of ADAM17 (ADAM17 recycling) (30,31). When progress in the field of
elucidating the role of ADAM17 was made, additional substrates,
including ligands of EGFR, were described for ADAM17 (32), highlighting the involvement of
ADAM17 in various pathological processes, including cancer
(33). It has been reported that
ADAM17 promotes tumor development and may thus be a therapeutic
target in colon cancer (34). Of
note, considered not only a tumor promoter, ADAM17 cleaves mIL-6R
and EGF, promoting EGFR signaling and IL-6 trans-signaling,
enabling amplified cellular responses to EGF and IL-6 in a wide
range of cell types (33). Both
ADAM17 and STAT3 contribute to the progression of colon cancer and
may exert synergistic effects. Therefore, the discovery of agents
targeting these two proteins may benefit the development of colon
cancer therapeutics, particularly in overcoming the drug resistance
associated with anti-EGFR treatments.
Previously, natural product extracts have received
increasing attention for their antitumor properties via diverse
mechanisms, including autophagy, immune modulation and apoptosis
etc., in various types of cancer (35-37). In a previous study, the authors
demonstrated that ursolic acid, a natural triterpenoid compound,
inhibited IL-6/STAT3 signaling and suppressed tumor growth in
hepatocellular carcinoma (38).
Shikonin (SKN) is the major component of extracts from the roots of
Lithospermum erythrorhizon Sieb. Et Zucc. that belongs to
the Boraginaceae family (39).
The applications of this plant include several pathologies, such as
burns, carbuncles and measles (40). The antitumor properties of SKN
include the induction of apoptosis/necroptosis, promotion of
autophagy, induction of oxidative stress, etc. (41,42). The inhibition of phosphorylated
(p-)STAT3 by SKN in lung, breast, melanoma and skin cancer types
has been reported (43-46); however, the potential effects of
SKN on p-STAT3 and ADAM17 expression in colon cancer remain
unclear.
The present study used HCT116 and SW480 colon cancer
cell lines for in vitro experiments. As STAT3 contributes to
tumor initiation and progression, serving as a point of convergence
for numerous oncogenic signaling pathways, while ADAM17 controls
the cleavage of several tumor-associated ligands (21,47-49), cancer cell growth was assessed in
a series of experiments, including cell viability, colony
formation, wound healing and apoptosis assays. It was found that
SKN suppressed the growth of both cancer cell lines. Importantly,
SKN exerted an inhibitory effect on constitutive/IL-6-induced
p-STAT3 and ADAM17 expression in these two colon cancer cell lines.
Furthermore, it was found that the reactive oxygen species
(ROS)-mediated suppression of ADAM17 translation may contribute to
the decreased expression of ADAM17 induced by SKN.
Materials and methods
Reagents
SKN was purchased from MedChemExpress (cat. no.
HY-N0822) and was dissolved in dimethyl sulfoxide (DMSO) at a
concentration of 20 mM as a stock solution. IL-6 (cat. no. 200-06,
PeproTech China was dissolved in water at 25 µg/ml for
storage. Crystal violet (Thermo Fisher Scientific, Inc.) was
dissolved in methyl alcohol (0.5 g/ml) and prepared for staining.
N-acetylcysteine (NAC) was purchased from Sigma-Aldrich;
Merck KGaA (cat. no. A0737).
Cell culture and treatment
The HCT116 (cat. no. CCL-247) and SW480 (cat. no.
CCL-228) colon cancer cell lines were purchased from the American
Type Culture Collection (ATCC). All cell lines were authenticated
via STR profiling and mycoplasmas were tested monthly. The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Nanjing
KeyGen Biotech Co., Ltd.) supplemented with high glucose and fetal
bovine serum (10X; Gibco; Thermo Fisher Scientific, Inc.) and
penicillin/streptomycin (100X, Sigma-Aldrich; Merck KGaA). Prior to
treatment, the cells were cultured for 12 h in an incubator (37°C,
5% CO2). To explore STAT3 phosphorylation induced by
IL-6, serum-starved cells were pre-treated with SKN (10, 15 and 20
µM, 3 h) and then stimulated with IL-6 (25 ng/ml) for a
further 30 min. Various concentrations (10, 15 and 20 µM)
SKN (DMSO as a control) were added to the medium, and following 10
h of culture, the cells were collected. For experiments involving
NAC, cells were pre-treated with or without NAC (5 mM) for 2 h, and
shikonin (20 µM) was then added for a further 10 h.
Western blot analysis
Cell lysate (RIPA lysis buffer containing 1 mmol/l
protease inhibitor and 1 mmol/l phosphatase inhibitor, Shanghai
Sangon Biotech Co., Ltd.) preparation was performed as previously
described (50). The lysates were
centrifuged at 13,800 × g for 20 min at 4°C, and the supernatant
was collected. The concentration of protein was determined by
bicinchoninic acid (BCA) protein assay kit (Shanghai Sangon Biotech
Co., Ltd.). Equivalent amounts (15-25 µg) of protein
(pre-stained protein ladder; cat. no. 26617, Thermo Fisher
Scientific Inc.) were loaded on a 5% Bis-Tris SDS-PAGE gel and
separated using 10% gel electrophoresis, transferred to a PVDF
membrane, blocked with TBS-T (Tris-buffer saline containing 0.1%
Tween-20) containing 5% powdered milk for 60 min (at room
temperature), and consequently probed with the following primary
antibodies (1:1,000 dilution) overnight at 4°C: Anti-p-STAT3
tyrosine 705 (cat. no. 9145), anti-t-STAT3 (cat. no. 4904),
anti-cleaved caspase-3 (cat. no. 9661), anti-cleaved PARP (cat. no.
9544), anti-cyclin D1 (cat. no. 55506), anti-cyclin E1 (cat. no.
20808), anti-p-JAK1 (cat. no. 3331), anti-p-JAK2 (cat. no. 3776),
anti-p-eukaryotic initiation factor 2α (eIF2α; cat. no. 3398) (all
from Cell Signaling Technology, Inc.), anti-ADAM17 (cat. no.
ab2051; Abcam), anti-GAPDH (cat. no. 10494-1-AP), anti-β-tubulin
(cat. no. 66240-1-Ig), anti-PACS-2 (cat. no. 19508-1-AP) (all from
ProteinTech Group, Inc.), anti-JAK1 (cat. no. 29261), anti-JAK2
(cat. no. 3230) (both from Cell Signaling Technology, Inc.),
anti-eIF2α (cat. no. ET7111-34; HuaBio) and anti-RHBDF2 (cat. no.
AP13588A; Abcepta, Biotech Ltd., Co.). Horseradish
peroxidase-conjugated (HRP-conjugated) antibodies (1:5,000
dilution; cat. no. QSJ-005/QSJ-006, Promoter Co., Ltd.) and
Immobilon Western Chemiluminescent HRP Substrate (AntGene Co.,
Ltd.) were used for protein detection, operated on a ChemiDoc-It
510 Imager with VisionWorks software (version 8.17.16133.9147;
Ultra-Violet Products, Ltd.) according to the manufacturer's
instructions.
Wound healing assay
The cells were plated in six-well plates and
cultured until they reached 100% confluency. The serum-starved cell
layers were then scratched using a sterile 10 µl pipette tip
and washed with PBS to discard floating cells. Wounded cells were
imaged at baseline followed by 3 h of treatment with SKN (10 and 20
µM) or DMSO. The medium was replaced to remove the test
compound or DMSO and images were captured using a fluorescence
microscope (Guangzhou Micro-shot Technology Co., Ltd.) at the
indicated time-points. The wound surface area was assessed using
ImageJ software (version 1.53e), and the wound healing activity of
the colon cancer cells was determined by the quantification of
wound healing progression. Wound healing activity = 1-(wound
surface area at the indicated time-point/wound surface area at
baseline).
Cell viability assay
After adhering to a 96-well plate for 24 h, cells
(6,000 cells per well) were treated with or without various
concentrations (5, 10, 15, 20 and 25 µM) of SKN (DMSO as a
control) for 10 h, and cell viability was determined using a Cell
Counting Kit-8 (cat. no. HY-K0301MedChemExpress) according to the
manufacturer's instructions.
Colony formation assay
Following treatment with SKN (10 or 20 µM) or
DMSO for 3 h, the cells were collected, counted and seeded (4,000
cells per plate) in 10-cm plates in an incubator for 10 days (37°C,
5% CO2). Crystal violet (0.5 g/ml) was used for staining
as previously described (50).
Public database
The differential analysis of transcripts of human
IL-6 and ADAM17 in colon cancer ('colon adenocarcinoma', 'colon
mucinous adenocarcinoma' and 'colorectal cancer') compared to
normal counterparts was performed and downloaded from the public
Oncomine database (www.oncomine.org; access date: December 15, 2020,
threshold P-value: 0.05; fold change, 2; gene rank, top 10%). For
the comparison of genes of interest across different analyses,
studies that met the aforementioned criteria were excluded when the
total sample size was <40.
RNA interference and transfection
Negative control (NC) and small interfering RNA
targeting STAT3 (siSTAT3; sense, CCACUUUGGUGUUUCAUAATT; antisense,
UUAUGA AACACCAAAGUGGTT) were purchased from RiboBio (Guangzhou
RiboBio Co., Ltd.). Lipofectamine 2000® (cat. no.
11668030, Thermo Fisher Scientific, Inc.) and Opti-MEM®
I reduced serum medium (#31985070; Gibco; Thermo Fisher Scientific,
Inc.) were used for transfection. Seeded cells were cultured
overnight and then cultured in mixed Opti-MEM medium containing 50
nM siSTAT3 and Lipofectamine 2000® according to the
manufacturer's instructions. The medium was replaced with complete
medium after 6 h of transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
The cells were treated with SKN (20 µM) or
IL-6 (25 ng/ml) for 10 h and then prepared for RNA extraction using
a HiPure Total RNA Mini kit (Magen Biotechnology Co., Ltd.)
according to the manufacturer's instructions. A ReverTra Ace qPCR
RT kit (Toyobo Co., Ltd.) was used for cDNA production. qPCR was
performed as previously described (50) (initial denaturation at 95°C for 10
min, followed by 40 cycles of denaturation at 95°C for 15 sec,
annealing at 60°C for 4 sec and extension at 72°C for 45 sec.). The
primer sequences were as follows: Human ADAM17 forward,
ATCAAACCTTTCCTGCG and reverse, CAAACCCATCCTCGTCCA; and human
β-actin forward, CTGGAACGGTGAAGGTGACA and reverse, AAGGGACTT
CCTGTAACAATGCA.
Statistical analysis
Data are presented as the mean ± SEM. All data are
from three independent experiments. Statistical analysis was
performed using SPSS software (version 22.0; SPSS, Inc.).
Comparisons of multiple groups were analyzed using one-way analysis
of variance (ANOVA) with Bonferroni's post hoc test, and
comparisons of two groups were analyzed using paired t-tests. For
the comparison of the wound healing area, two-way ANOVA (time and
group) was used with Bonferroni's post hoc test. For the
quantification of the western blots, ImageJ software (version
1.53e) was used for assessment. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
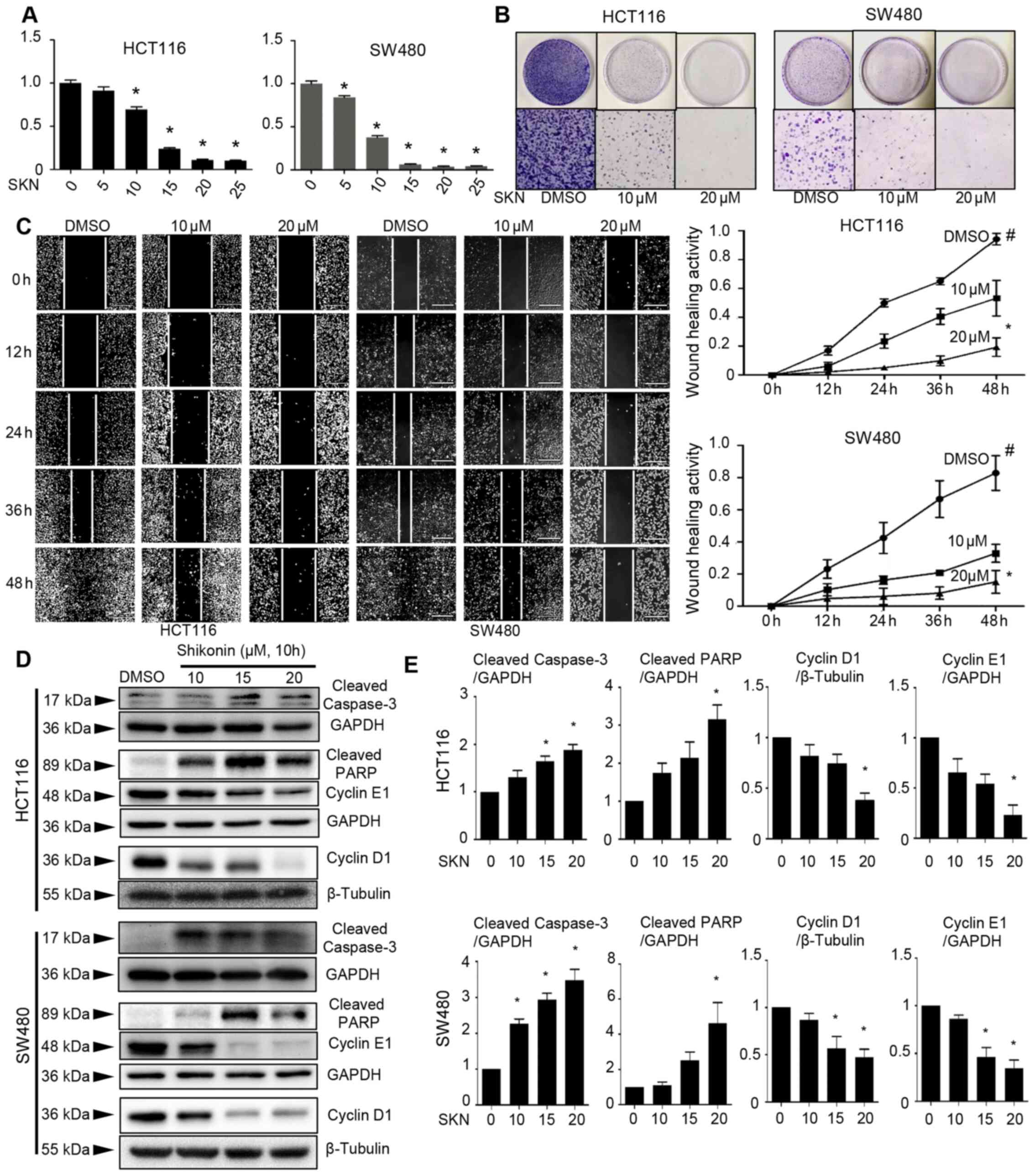
SKN decreases the viability, promotes the
apoptosis, disrupts the cell cycle, and suppresses the wound
healing and colony formation of colon cancer cells
ADAM17 and STAT3 play a key role in inflammation and
cancer, and have been found to be associated with colon cancer
initiation, progression and metastasis (47-49). Moreover, it has been shown that
the inhibition of IL-6/STAT3 signaling by small molecular compounds
results in a wide range of impeded cancer cell growth properties
(21,22,25). Herein, it was found that SKN
exerted inhibitory effects on the viability, colony formation and
wound healing ability of colon cancer cells; generally, a
suppression of cell growth was observed following treatment with
SKN. SKN induced the concentration-dependent suppression of cell
viability (Fig. 1A) and colony
formation (Fig. 1B) ability of
the HCT116 and SW480 cells. Moreover, it was found that treatment
with a higher concentration of SKN (20 µM) tended exert a
more prominent effect on the wound healing ability of the cells
than treatment with 10 µM SKN (Fig. 1C). Treatment with SKN promoted
cell apoptosis, as evidenced by increased expression levels of
cleaved caspase-3 and cleaved PARP in both cell lines (Fig. 1D and E), as well as by enhanced
staining-positive spots in the Annexin V/PI immunofluorescence
staining assay (Fig. S1).
Moreover, SKN decreased the expression of cyclin D1 and cyclin E1,
thus suggesting the disruption of the cell cycle and the
suppression of cell growth (Fig. 1D
and E).
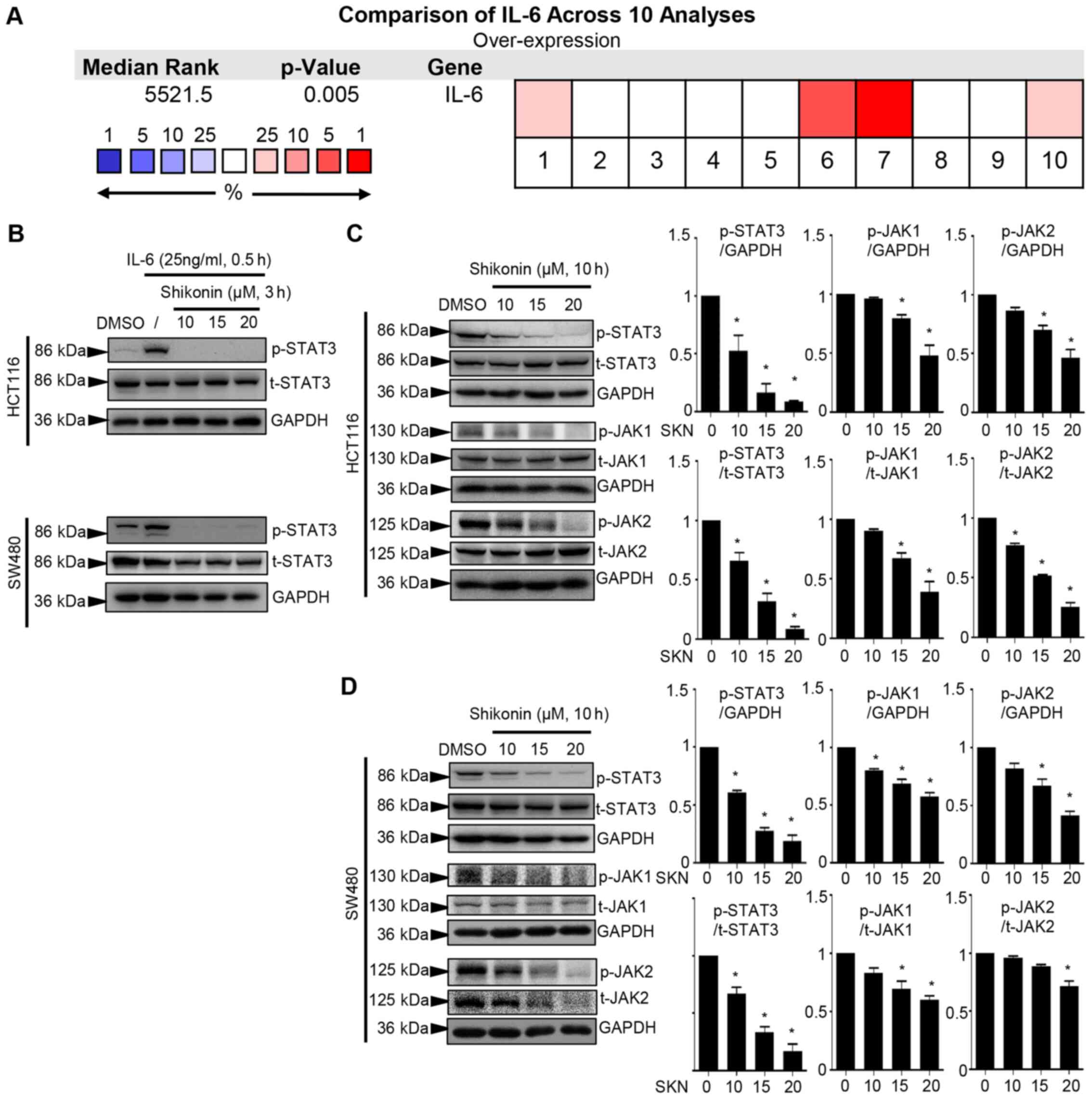
Shikonin inhibits IL-6-induced expression
and constitutive p-STAT3 activation in colon cancer cells
A comparison across 10 analyses performed using
Oncomine suggested that IL-6 transcripts were increased in colon
cancer (P=0.005, Fig. 2A). It has
been reported that IL-6 triggers the formation of the
IL-6/IL-6R/EGFR complex. The protein interaction involving EGFR
facilitates the phosphorylation of Y1068 of EGFR, which plays a
critical role in the biphasic pattern of STAT3 phosphorylation
(51). The present study examined
whether the concentration of IL-6 (25 ng/ml) used could activate
EGFR. As shown in Fig. S2, 25
ng/ml IL-6 did not significantly induce the phosphorylation of
Y1068 on EGFR (Fig. S2).
Moreover, in a previous study, the authors used this concentration
of IL-6 to induce the phosphorylation of STAT3, aiming to determine
the inhibitory effect on p-STAT3 of the compound of interest
(23,24). Herein, the concentration of 25
ng/ml IL-6 induced the robust phosphorylation of STAT3, and 3 h of
pre-treatment with SKN prominently suppressed the cellular response
to this trigger in the HCT116 and SW480 cancer cell lines (Fig. 2B). The persistent activation of
STAT3 plays a crucial role in colon cancer cells. It was
demonstrated that SKN affected the constitutive activation of STAT3
in two types of cancer cell lines. The downregulation of p-STAT3 in
response to 10 h of treatment with SKN tended to occur in a
concentration-dependent manner (10, 15 and 20 µM) in the
HCT116 and SW480 cancer cell lines (Fig. 2C and D). Moreover, JAK1 and JAK2
were activated in response to IL-6; these are potential upstream
regulators of STAT3 (52). The
inhibitory effects of SKN on the phosphorylation of both JAK1 and
JAK2 in the two cell lines were also observed (Fig. 2C and D).
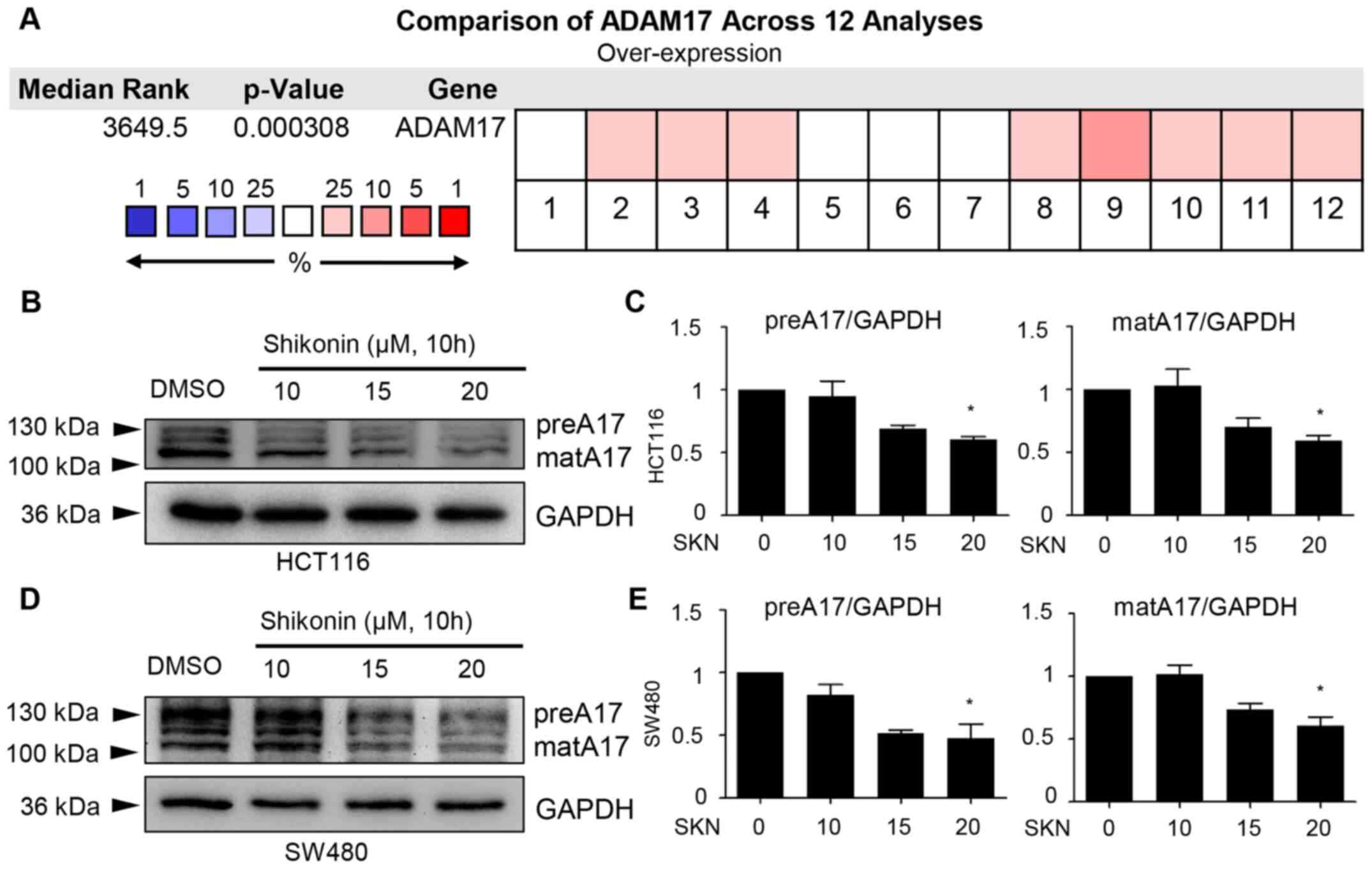
Decreased expression of ADAM17 by
SKN
Due to the specific role of ADAM17 in the regulation
of IL-6/STAT3 and EGFR signaling, the potential effects of SKN on
ADAM17 were further explored. First, a comparison of ADAM17 was
performed across 12 analyses, data of which were derived from the
Oncomine database. The results mined from this public database
revealed the overexpression of ADAM17 mRNA in colon cancer
(P=0.000308; Fig. 3A). Of note,
it was demonstrated that 10 h of treatment with 20 µM SKN
downregulated the expression of both the precursor form and mature
form of ADAM17 in the HCT116 and SW480 cancer cell lines (Fig. 3B-E).
IL-6/STAT3 signaling may not control
ADAM17 expression
By transfection using a negative control and
siSTAT3, it was found that the expression of both the precursor and
mature forms of ADAM17 was not markedly altered following STAT3
knockdown, despite a prominent decrease in t-STAT3 expression in
the two colon cancer cell lines (Fig.
4A). Additionally, IL-6 did not activate the transcripts of
ADAM17 (Fig. 4B, left panel).
However, SKN (20 µM, 10 h of treatment) significantly
increased the mRNA expression of ADAM17 in the two types of colon
cancer cells (Fig. 4B, right
panel).
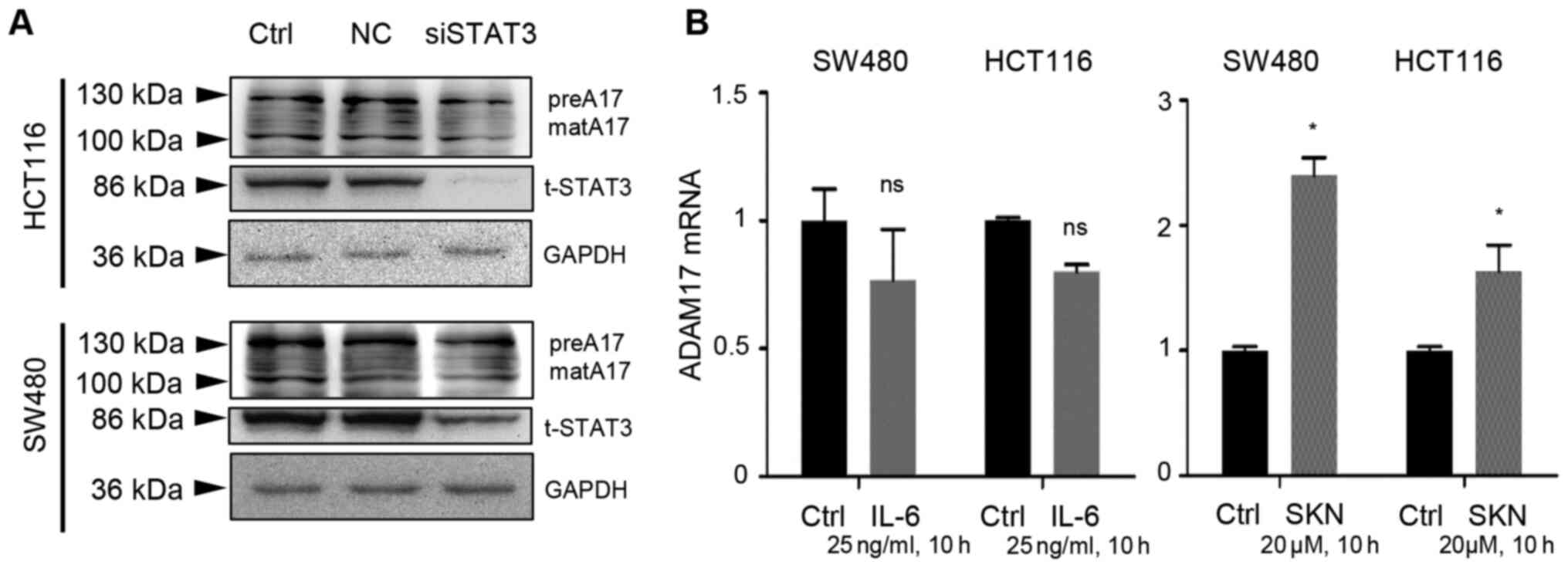 | Figure 4IL-6/STAT3 signaling does not
contribute to the SKN-induced increase in ADAM17 mRNA expression.
(A) Transfection with negative control (NC) did not exert an effect
on HCT116 or SW480 colon cancer cells. Transfection with siSTAT3
impeded STAT3 expression, but did not reduce the expression of the
precursor or mature form ○ ADAM17. (B) IL-6 (25 ng/ml, 10 h of
treatment) had no significant effect on ADAM17 mRNA expression in
HCT116 or SW480 cells; however, SKN (20 µM, 10 h of
treatment) significantly increased the mRNA expression of ADAM17 in
the two colon cancer cell lines. The transcripts of ADAM17 in the
control group was set as 1. All data represent the mean ± SEM.
*P<0.05 vs. control group; ns, no significance. SKN,
shikonin; ADAM17, A disintegrin and metalloproteinase 17; preA17,
precursor form of ADAM17; matA17, mature form of ADAM17; STAT3,
signal transducer and activator of transcription 3; IL,
interleukin. |
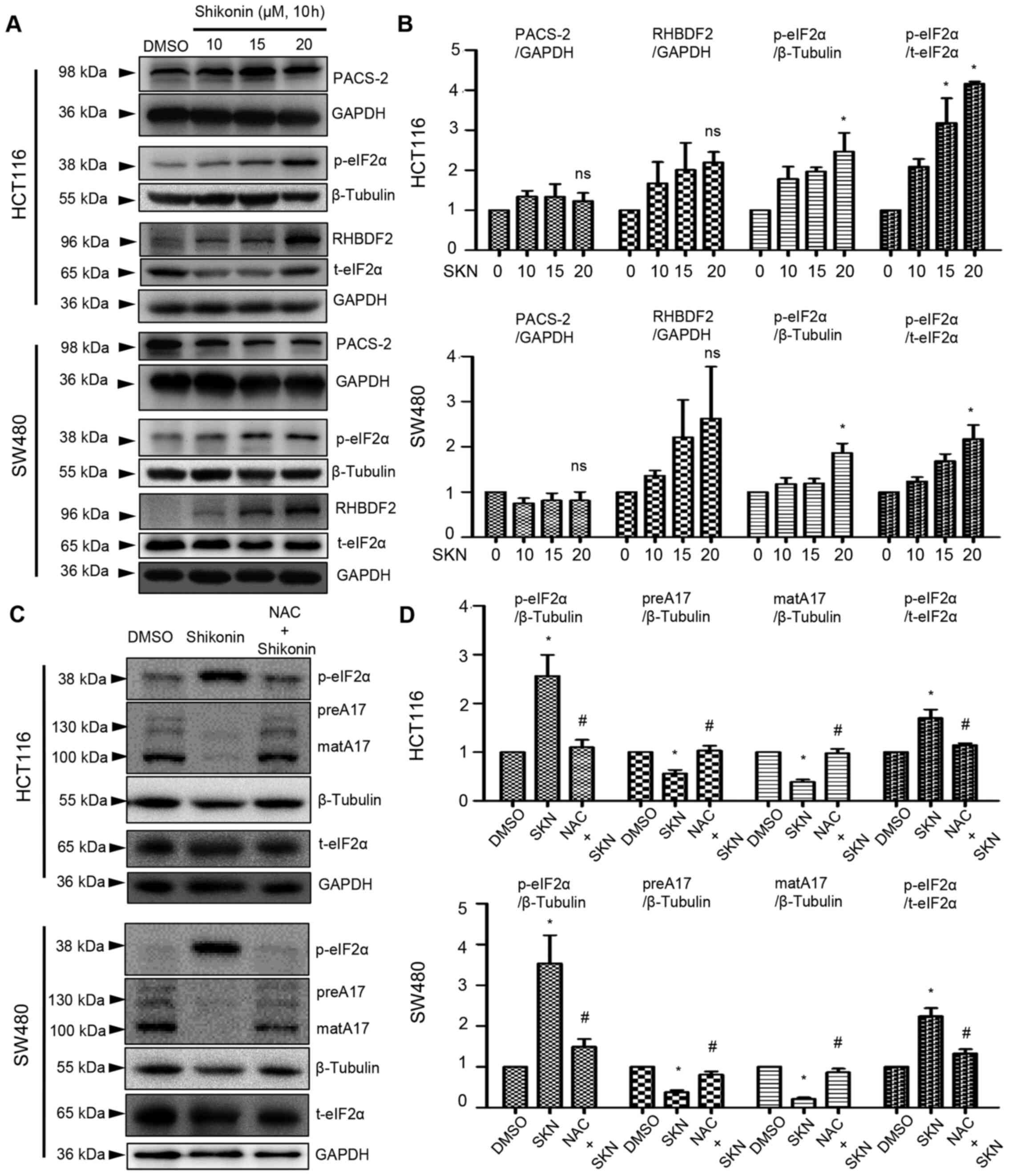
SKN decreases ADAM17 expression via
ROS-mediated post-transcriptional regulation
As demonstrated above (Fig. 4B, right panel), 20 µM SKN
increased the mRNA expression of ADAM17. However, ADAM17 protein
expression was decreased by SKN, as illustrated in Fig. 3B-E. Thus, further analysis was
performed involving critical steps in the life cycle of ADAM17. The
results of western blot analysis revealed that the expression of
PACS-2 was not significantly altered by SKN (Fig. 5A and B), while SKN tended to
increase RHBDF2 expression, although not significantly (Fig. 5A and B). The phosphorylation
levels of eIF2α were enhanced by SKN (20 µM) in the HCT116
and SW480 colon cancer cells (Fig. 5A
and B). ROS are known as inducers of eIF2α phosphorylation,
while NAC functions as a ROS scavenger. It was further found that
NAC decreased SKN-induced p-eIF2α expression and reversed the
SKN-mediated downregulation of ADAM17 protein expression (Fig. 5C and D).
On the whole, IL-6 classical and
trans-signaling contribute to cancer development, while
ADAM17 cleaves its substrates, including mIL-6R, which controls the
balance of two pathways of IL-6 and EGF, allowing EGFR signaling to
promote cancer. In the present study, SKN decreased the
phosphorylation of STAT3 and suppressed the expression of ADAM17
mediated by ROS-associated p-eIF2α expression in the HCT116 and
SW480 colon cancer cells (Fig.
6).
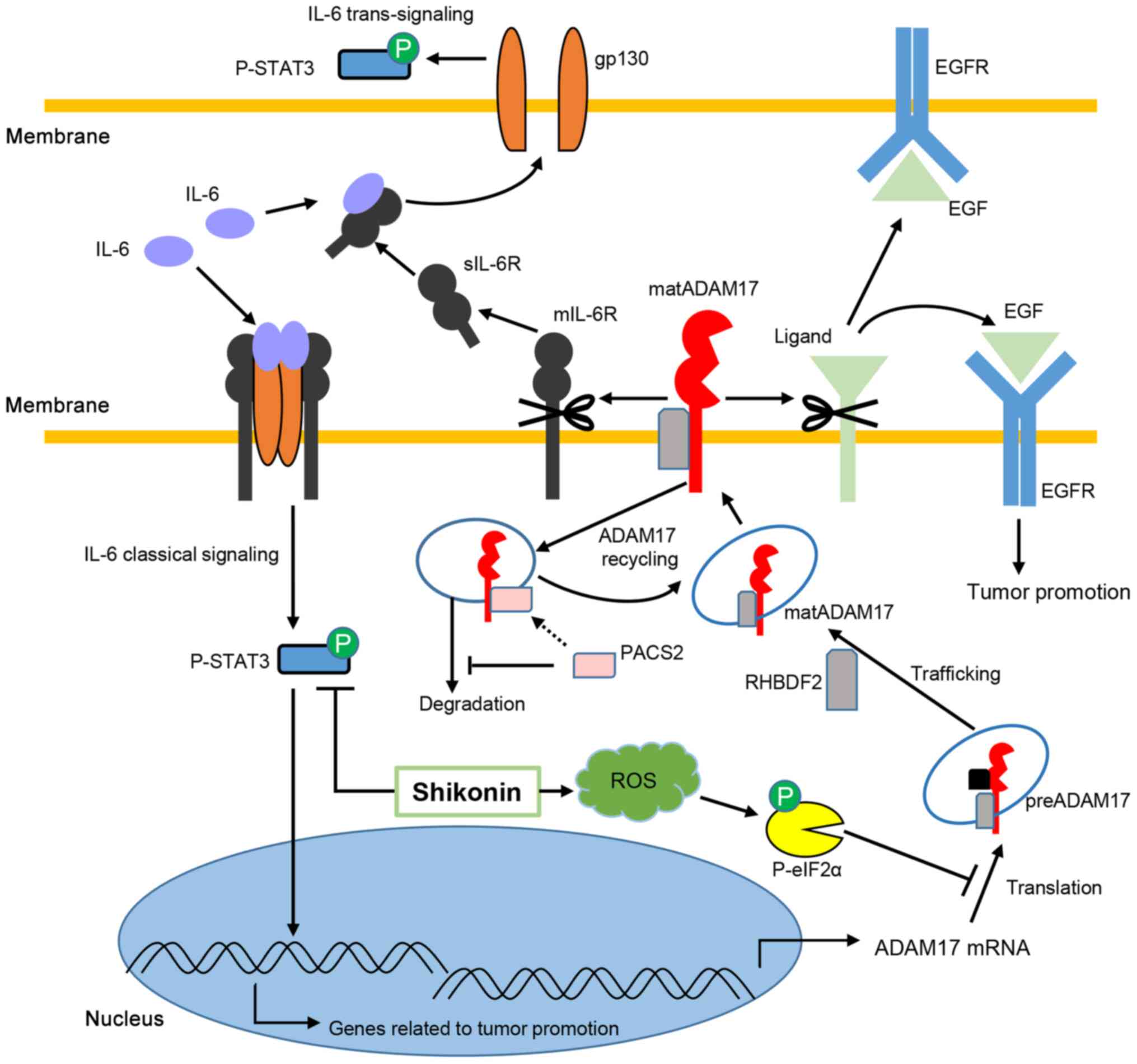 | Figure 6Potential mechanisms through which
shikonin regulates ADAM17 and IL-6/STAT3 signaling. IL-6 binds to
membrane IL-6R to activate classical signaling and binds to sIL-6R
to transduce trans-signaling, both of which result in STAT3
phosphorylation and contribute to cancer development. ADAM17 mRNA
is translated to the precursor form of ADAM17, which is modified to
mature ADAM17 mediated by RHBDF2. RHBDF2 also stabilizes mature
ADAM17, enabling substrate cleavage. PACS-2 plays an important role
in ADAM17 recycling, and PACS-2 deficiency leads to the degradation
of mature ADAM17 by endosomes. ADAM17 cleaves mIL-6R to enable IL-6
trans-signaling by releasing more sIL-6R and cleaves EGF,
allowing EGFR signaling, to promote cancer. Shikonin decreases
p-STAT3 and suppresses the expression of ADAM17 by inducing
ROS-mediated p-eIF2α. Translational inhibition by increased p-eIF2α
results in ADAM17 defects in HCT116 and SW480 colon cancer cells.
Concurrent inhibition of ADAM17 and IL-6/STAT3 signaling may
synergistically contribute to the suppression of colon cancer cells
in response to shikonin. STAT3, signal transducer and activator of
transcription 3; IL, interleukin; A disintegrin and
metalloproteinase 17; preA17, precursor form of ADAM17; matA17,
mature form of ADAM17; eIF2α, eukaryotic initiation factor 2α;
PACS-2, phosphofurin acidic cluster sorting protein 2; RHBDF2,
rhomboid family member 2. |
Discussion
Although significant advancements in the treatment
of colon cancer have been made, further efforts are still required.
IL-6/STAT3 signaling and ADAM17 play important roles in colon
cancer. Herein, it was demonstrated that the antitumor effects of
SKN on colon cancer cells were associated with its inhibition of
the IL-6/STAT3 signaling pathway. Moreover, the concomitant
suppression of ADAM17 expression may also contribute to the
inhibition of cancer cell growth.
As shown using the Oncomine database, transcripts of
IL-6 and ADAM17 are increased in colon cancer. IL-6 activates
downstream signaling and promotes the transcription of
STAT3-related genes, which aids tumor growth. Of note, IL-6R is
expressed on specific cell types, such as hepatocytes, epithelial
cells and leukocytes, revealing that IL-6 classical signaling is
found only in cells expressing IL-6R (53). However, IL-6
trans-signaling may occur in almost all cells where gp130
exists. Moreover, the expression level of gp130 is higher than that
of IL-6R (54), allowing the
synergistic stimulation of both IL-6 signaling and amplifying the
inflammatory cascade in chronic inflammation, which critically
contributes to perturbed tissue homeostasis, including tumor
development and therapy (55).
Notably, ADAM17 not only cleaves proliferative ligands, such as
EGF, to trigger downstream signaling and controlling a wide range
of cell biological functions, but also modulates the balance
between IL-6 classical and trans-signaling pathways. The
enhanced activity of ADAM17 produces higher levels of sIL-6R,
enabling trans-signaling, which is considered to be
associated with the malignant proliferation of epithelial and colon
cancer cells (33,56). The benefit conferred by anti-human
IL-6 receptor monoclonal antibody has been documented with
downregulated colon stem markers and increased chemosensitivity
(57). Therefore, simultaneously
elevated levels of IL-6 signaling and ADAM17 may result in
increased p-STAT3 levels in a wide set of cell types.
On the one hand, STAT3 activation has been
documented to be associated with a poor prognosis of patients with
in stage II colon cancer (58).
On the other hand, IL-6/STAT3 signaling, regulated by secretory RAB
GTPase 3C (RAB3C), leads to a poor prognosis of patients with an
advanced pathological stage and distant metastasis (59). Moreover, the IL-6 rs2069837
genotype has been documented as a clinically relevant prognostic
factor in colon cancer patients treated with bevacizumab-based
chemotherapy (60), all of which
indicate that IL-6/STAT3 signaling activity may have clinical
significance. However, to date, studies on the prognostic role of
ADAM17 in cohorts appear to lack abundant evidence. Although ADAM17
functions as an oncogene and its expression is increased in
patients with colon cancer, as previously reported (61) and as shown in Oncomine, the
activity of ADAM17 is difficult to detect in patients. Further data
are still required to disclose the role of ADAM17 in cancer in
detail.
In previous studies, the authors found that
raloxifene targeted the interface of IL-6 and gp130 (62), inhibiting p-STAT3 and colon cancer
cell growth (23). SKN
prominently suppressed p-STAT3 expression in colon cancer cells.
The underlying mechanisms may include the predicted binding of SKN
with STAT3 in the pY-X and pY+0 sub-pockets located in the SH2
domains of STAT3, through which STAT3 dimers are formed to
facilitate subsequent phosphorylation and translocation into the
cell nucleus (63). As
aforementioned, ADAM17 is considered an oncogene, and targeting
ADAM17 represents another strategy for the treatment of cancer.
However, to date, different STAT3 inhibitors are being developed
(e.g., FLLL11, FLLL12 and LY5) (64,65), however, few agents to inhibit
ADAM17 expression are available. The majority of compounds
targeting ADAM17 involve catalytic sites and decrease the cleavage
activity. Li et al (66)
found that ZLDI-8 (ADAM17 inhibitor) downregulated the expression
of ADAM17 and synergistically promoted the antitumor effects of
5-fluorouracil on colon cancer. Regardless, the comprehensive
inhibitory effect on both constitutive p-STAT3, IL-6-induced
p-STAT3 and ADAM17 expression may indicate a potential broader
spectrum of the antitumor effects of SKN on colon cancer cells.
Moreover, SKN did not tend to alter the expression of β-catenin,
indicating that β-catenin-dependent tumorigenesis may not
significantly contribute to the inhibitory effects of SKN on STAT3
and ADAM17 (Fig. S3).
As illustrated in Fig.
4, the expression of ADAM17 was not significantly altered by
STAT3 knockdown in either colon cancer cell line examined.
Moreover, transcripts of ADAM17 were not associated with IL-6
administration in these two cell lines, suggesting that the basal
expression of ADAM17 may be independent of IL-6/STAT3 signaling in
the HCT116 and SW480 cells. As previously reported (33), ADAM17 enables IL-6
trans-signaling and EGF signaling by mIL-6R and EGF
cleavage, possibly promoting STAT3 activation in a wide range of
cell types, which may not increase the transcripts of ADAM17 in
turn; however, whether activated IL-6/STAT3 signaling enhances the
activity of this cleavage enzyme is unknown. In addition to the
other obscure potential mechanisms, the association between STAT3
and ADAM17 warrants further investigation in order to be fully
elucidated. Of note, in contrast to the downregulated protein
expression of ADAM17, SKN increased the mRNA expression of ADAM17.
Therefore, post-transcriptional regulation has attracted increasing
attention.
ADAM17 mRNA is driven to produce the precursor form
of ADAM17. RHBDF2 binds to the immature form of ADAM17 and promotes
its trafficking from the endoplasmic reticulum to the Golgi
apparatus. During this process, the precursor form of ADAM17 is
cleaved by furin to generate mature ADAM17. RHBDF2 contributes to
the post-translational regulation of ADAM17 in almost every step,
including trafficking, maturation, membrane stabilization and
degradation (31). Of note, the
amino terminus of RHBDF2 is critical in this reported biological
process (31); thus, herein, an
antibody targeting the N-terminal region of human RHBDF2 was used
to detect its expression level. On the other hand, PACS-2 has been
reported to divert endocytosed mature ADAM17 away from degradation
pathways, resulting in an increased ADAM17 membrane availability.
In the absence of PACS-2, the disrupted ADAM17 recycling leads to
decreased expression levels of membrane mature ADAM17 (30). In the present study, SKN treatment
did not significantly alter the expression of PACS-2 and appeared
to increase detectable RHBDF2 levels in the HCT116 and SW480 cells.
In fact, the expression levels of precursor and mature ADAM17 were
both downregulated by SKN, which revealed that SKN may not exert a
prominent inhibitory effect on the maturation and degradation
process in these two cancer cell lines.
Notably, SKN significantly increased the levels of
p-eIF2α. In brief, eIF2 delivers the initiator methionyl tRNA
(Met-tRNAiMet) to the 40S subunit of the ribosome to
form the 43S complex, permitting subsequent translation (67). The phosphorylation of the
α-subunit of eIF2 blocks translational initiation and attenuates
general translation, which is considered to help cells overcome
extracellular stress by easing the burden on protein folding and
amino acid consumption. Oxidative stress, viral infection, amino
acid deprivation and endoplasmic reticulum stress (ERS)-mediated
PERK activation are considered inducers of α-subunit
phosphorylation (68). Previous
studies have found that SKN is capable of inducing oxidative stress
in colon cancer cells (69) and
activates ERS, leading to PERK activation in SNU-407 cells (another
colon cancer cell line) (70). In
the present study, NAC, a ROS scavenger, reversed the expression of
ADAM17 and p-eIF2α in both cell lines, indicating that ROS-induced
p-eIF2α expression contributes to the decreased expression of
ADAM17 induced by SKN. However, eIF2α-associated general
translation inhibition is only the first step of translation, and
of note, the suppressive effect of SKN was observed at a
concentration of 10 µM (Fig.
1), while 10 µM SKN appeared to exert no overt effect on
p-eIF2α (Fig. 5A and B) or ADAM17
(Fig. 3B-E) expression. This
suggests that the inhibition of cancer cells may involve other
mechanisms where a low concentration of SKN was used. Overall, the
antitumor effect of shikonin is likely more intricate than what is
currently known.
Therefore, on the whole, IL-6 classical and
trans-signaling contribute to cancer development, while
ADAM17 cleaves its substrates, including mIL-6R, which controls the
balance of two pathways of IL-6 and EGF, allowing EGFR signaling to
promote cancer. In the present study, SKN decreased the
phosphorylation of STAT3 and suppressed the expression of ADAM17
mediated by ROS-associated p-eIF2α expression in the HCT116 and
SW480 colon cancer cells (Fig.
6). Shikonin shares the same backbone with LLL-12, which has
been previously described as an effective STAT3 inhibitor (21,63), indicating the promise of
developing SKN and its derivatives.
However, similar to other natural products in
Chinese herbal medicine, the potential toxicity and unknown
side-effects of SKN merit accelerated investigations. Indeed, SKN
can affect multiple biological processes, including protein
translation, folding, modification and translocation, as well as
exosome secretion and energy production (71). To date, the antitumor properties
of SKN have been demonstrated in several in vivo studies on
colon cancer (71-73). Moreover, it has been proven that
SKN functions as a complement to enhance the antitumor effect of
cisplatin in colon cancer in vivo (74). The present study also examined the
effects of SKN on non-cancer cells (NCM460 cells), as described in
Data S1 and as shown in Fig. S4.
Although 15 and 20 µM SKN decreased cell viability, SKN
appeared to exhibit a weaker toxicity in human normal colonic
epithelial cells (NCM460) than in colon cancer cells (please see
Fig. 1A). Importantly, SKN has
been reported to decrease tumor volume and exert antitumor effects
with minimal toxicity to non-cancer cells without liver or kidney
injury in xenograft tumor models (69,71). Although the present study did not
perform animal experiments, the authors aimed to determine the
potential effects of SKN on STAT3 and ADAM17 expression in
vitro, which may provide evidence of the use of SKN under
certain circumstances in which STAT3 and/or ADAM17 overactivation
occurs. In the era of modern medicine, combined therapy is the
current trend. Due to its pleiotropic effects on cancer, SKN may be
studied in further investigations where it is administered in
combination with other known chemo- therapies. Moreover, although
the present study demonstrated that SKN-induced p-eIF2α expression
was decreased by the ROS scavenger, NAC, which may disrupt general
translation, resulting in the decreased expression of ADAM17, the
alteration in transcripts of other oncogenes remains unknown in
response to SKN. Furthermore, RHBDF2 knockdown warrants further
investigations in order to elucidate the regulatory mechanisms
involved in protein processing. Further studies are also required
to determine the potential effects of SKN on the components
constituting transcription factor complexes, the stability of mRNA,
and the regulation of other types of RNAs.
Supplementary Data
Availability of data and materials
All the datasets generated and analyzed in the
present study are included in this article.
Authors' contributions
WS and LM designed the study and wrote the
manuscript. WS, LM, XP, DP, SH, PL and MW performed the biological
molecular experiments. TJ, LM and JG assisted with data analysis.
WS, YJ and LP interpreted the data and revised the manuscript. LL,
SL and JL were involved in the conception and design of the study,
and provided extensive support and reviewed the manuscript. SL and
JL confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82070396 and 81974032), the Hubei
Province Health and Family Planning Scientific Research Project
(grant no. WJ2019M120), the Science and Technology Project
Foundation of Wuhan (grant no. 2019020701011439) and the Natural
Science Foundation of Hubei Province (grant no. 2019CFB668).
References
|
1
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar
|
|
3
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Den Oudsten BL, Traa MJ, Thong MS, Martijn
H, De Hingh IH, Bosscha K and van de Poll-Franse LV: Higher
prevalence of sexual dysfunction in colon and rectal cancer
survivors compared with the normative population: A
population-based study. Eur J Cancer. 48:3161–3170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu L, Herrinton LJ, Hornbrook MC, Wendel
CS, Grant M and Krouse RS: Early and late complications among
long-term colorectal cancer survivors with ostomy or anastomosis.
Dis Colon Rectum. 53:200–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schover LR, van der Kaaij M, van Dorst E,
Creutzberg C, Huyghe E and Kiserud CE: Sexual dysfunction and
infertility as late effects of cancer treatment. EJC Suppl.
12:41–53. 2014. View Article : Google Scholar :
|
|
7
|
Mohammad N, Malvi P, Meena AS, Singh SV,
Chaube B, Vannuruswamy G, Kulkarni MJ and Bhat MK: Cholesterol
depletion by methyl-β-cyclodextrin augments tamoxifen induced cell
death by enhancing its uptake in melanoma. Mol Cancer. 13:2042014.
View Article : Google Scholar
|
|
8
|
Mohammad N, Singh SV, Malvi P, Chaube B,
Athavale D, Vanuopadath M, Nair SS, Nair B and Bhat MK: Strategy to
enhance efficacy of doxorubicin in solid tumor cells by
methyl-β-cyclodextrin: Involvement of p53 and Fas receptor ligand
complex. Sci Rep. 5:118532015. View Article : Google Scholar
|
|
9
|
Rashmi R, Jayachandran K, Zhang J, Menon
V, Muhammad N, Zahner M, Ruiz F, Zhang S, Cho K, Wang Y, et al:
Glutaminase inhibitors induce Thiol-mediated oxidative stress and
radiosensitization in treatment-resistant cervical cancers. Mol
Cancer Ther. 19:2465–2475. 2020.PubMed/NCBI
|
|
10
|
Singh S, Chouhan S, Mohammad N and Bhat
MK: Resistin causes G1 arrest in colon cancer cells through
upregulation of SOCS3. FEBS Lett. 591:1371–1382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar B, Chand V, Ram A, Usmani D and
Muhammad N: Oncogenic mutations in tumorigenesis and targeted
therapy in breast cancer. Curr Mol Biol Rep. 6:116–125. 2020.
View Article : Google Scholar
|
|
12
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Unver N and McAllister F: IL-6 family
cytokines: Key inflammatory mediators as biomarkers and potential
therapeutic targets. Cytokine Growth Factor Rev. 41:10–17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cron L, Allen T and Febbraio MA: The role
of gp130 receptor cytokines in the regulation of metabolic
homeostasis. J Exp Biol. 219:259–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rose-John S and Heinrich PC: Soluble
receptors for cytokines and growth factors: Generation and
biological function. Biochem J. 300:281–290. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma XT, Wang S, Ye YJ, Du RY, Cui ZR and
Somsouk M: Constitutive activation of Stat3 signaling pathway in
human colorectal carcinoma. World J Gastroenterol. 10:1569–1573.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Corvinus FM, Orth C, Moriggl R, Tsareva
SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal
K, et al: Persistent STAT3 activation in colon cancer is associated
with enhanced cell proliferation and tumor growth. Neoplasia.
7:545–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kusaba T, Nakayama T, Yamazumi K, Yakata
Y, Yoshizaki A, Nagayasu T and Sekine I: Expression of p-STAT3 in
human colorectal adenocarcinoma and adenoma; correlation with
clinicopathological factors. J Clin Pathol. 58:833–838. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C
and Lin J: STAT3 is necessary for proliferation and survival in
colon cancer-initiating cells. Cancer Res. 71:7226–7237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab
T and Lin J: STAT3 signaling pathway is necessary for cell survival
and tumorsphere forming capacity in
ALDH+/CD133+ stem cell-like human colon
cancer cells. Biochem Biophys Res Commun. 416:246–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi W, Yan D, Zhao C, Xiao M, Wang Y, Ma
H, Liu T, Qin H, Zhang C, Li C, et al: Inhibition of IL-6/STAT3
signaling in human cancer cells using Evista. Biochem Biophys Res
Commun. 491:159–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W, Zhao C, Jou D, Lü J, Zhang C, Lin
L and Lin J: Ursolic acid inhibits the growth of colon
cancer-initiating cells by targeting STAT3. Anticancer Res.
33:4279–4284. 2013.PubMed/NCBI
|
|
25
|
Zhao C, Wang W, Yu W, Jou D, Wang Y, Ma H,
Xiao H, Qin H, Zhang C, Lü J, et al: A novel small molecule STAT3
inhibitor, LY5, inhibits cell viability, colony formation, and
migration of colon and liver cancer cells. Oncotarget.
7:12917–12926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ung N, Putoczki TL, Stylli SS, Ng I,
Mariadason JM, Chan TA, Zhu HJ and Luwor RB: Anti-EGFR therapeutic
efficacy correlates directly with inhibition of STAT3 activity.
Cancer Biol Ther. 15:623–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Black RA, Rauch CT, Kozlosky CJ, Peschon
JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P,
Srinivasan S, et al: A metalloproteinase disintegrin that releases
tumour-necrosis factor-alpha from cells. Nature. 385:729–733. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moss ML, Jin SL, Milla ME, Bickett DM,
Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, et
al: Cloning of a disintegrin metalloproteinase that processes
precursor tumour-necrosis factor-alpha. Nature. 385:733–736. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khokha R, Murthy A and Weiss A:
Metalloproteinases and their natural inhibitors in inflammation and
immunity. Nat Rev Immunol. 13:649–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dombernowsky SL, Samsøe-Petersen J,
Petersen CH, Instrell R, Hedegaard AM, Thomas L, Atkins KM, Auclair
S, Albrechtsen R, Mygind KJ, et al: The sorting protein PACS-2
promotes ErbB signalling by regulating recycling of the
metalloproteinase ADAM17. Nat Commun. 6:75182015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grieve AG, Xu H, Künzel U, Bambrough P,
Sieber B and Freeman M: Phosphorylation of iRhom2 at the plasma
membrane controls mammalian TACE-dependent inflammatory and growth
factor signalling. eLife. 6:62017. View Article : Google Scholar
|
|
32
|
Pavlenko E, Cabron AS, Arnold P, Dobert
JP, Rose-John S and Zunke F: Functional characterization of colon
cancer-associated mutations in ADAM17: Modifications in the
pro-domain interfere with trafficking and maturation. Int J Mol
Sci. 20:202019. View Article : Google Scholar
|
|
33
|
Schumacher N and Rose-John S: ADAM17
activity and IL-6 trans-signaling in inflammation and cancer.
Cancers (Basel). 11:112019. View Article : Google Scholar
|
|
34
|
Mustafi R, Dougherty U, Mustafi D,
Ayaloglu-Butun F, Fletcher M, Adhikari S, Sadiq F, Meckel K, Haider
HI, Khalil A, et al: ADAM17 is a tumor promoter and therapeutic
target in western diet-associated colon cancer. Clin Cancer Res.
23:549–561. 2017. View Article : Google Scholar :
|
|
35
|
Muhammad N, Steele R, Isbell TS, Philips N
and Ray RB: Bitter melon extract inhibits breast cancer growth in
preclinical model by inducing autophagic cell death. Oncotarget.
8:66226–66236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bhattacharya S, Muhammad N, Steele R,
Kornbluth J and Ray RB: Bitter melon enhances natural
killer-mediated toxicity against head and neck cancer cells. Cancer
Prev Res (Phila). 10:337–344. 2017. View Article : Google Scholar
|
|
37
|
Bhattacharya S, Muhammad N, Steele R, Peng
G and Ray RB: Immunomodulatory role of bitter melon extract in
inhibition of head and neck squamous cell carcinoma growth.
Oncotarget. 7:33202–33209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu T, Ma H, Shi W, Duan J, Wang Y, Zhang
C, Li C, Lin J, Li S, Lv J, et al: Inhibition of STAT3 signaling
pathway by ursolic acid suppresses growth of hepatocellular
carcinoma. Int J Oncol. 51:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Cui JH, Meng QQ, Li SS, Zhou W
and Xiao S: Advance in anti-tumor mechanisms of shikonin, alkannin
and their derivatives. Mini Rev Med Chem. 18:164–172. 2018.
View Article : Google Scholar
|
|
40
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo C, He J, Song X, Tan L, Wang M, Jiang
P, Li Y, Cao Z and Peng C: Pharmacological properties and
derivatives of shikonin-A review in recent years. Pharmacol Res.
149:1044632019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boulos JC, Rahama M, Hegazy MF and Efferth
T: Shikonin derivatives for cancer prevention and therapy. Cancer
Lett. 459:248–267. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang JC, Ren YG, Zhao J, Long F, Chen JY
and Jiang Z: Shikonin enhances sensitization of gefitinib against
wild-type EGFR non-small cell lung cancer via inhibition
PKM2/stat3/cyclinD1 signal pathway. Life Sci. 204:71–77. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thakur R, Trivedi R, Rastogi N, Singh M
and Mishra DP: Inhibition of STAT3, FAK and Src mediated signaling
reduces cancer stem cell load, tumorigenic potential and metastasis
in breast cancer. Sci Rep. 5:101942015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cao HH, Liu DY, Lai YC, Chen YY, Yu LZ,
Shao M and Liu JS: Inhibition of the STAT3 signaling pathway
contributes to the anti-melanoma activities of shikonin. Front
Pharmacol. 11:7482020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tian R, Li Y and Gao M: Shikonin causes
cell-cycle arrest and induces apoptosis by regulating the
EGFR-NF-κB signalling pathway in human epidermoid carcinoma A431
cells. Biosci Rep. 35:352015. View Article : Google Scholar
|
|
47
|
Zou S, Tong Q, Liu B, Huang W, Tian Y and
Fu X: Targeting STAT3 in cancer immunotherapy. Mol Cancer.
19:1452020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chalikonda G, Lee H, Sheik A and Huh YS:
Targeting key transcriptional factor STAT3 in colorectal cancer.
Mol Cell Biochem. 476:3219–3228. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Scheller J, Chalaris A, Garbers C and
Rose-John S: ADAM17: A molecular switch to control inflammation and
tissue regeneration. Trends Immunol. 32:380–387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shi W, Ma H, Liu T, Yan D, Luo P, Zhai M,
Tao J, Huo S, Guo J, Li C, et al: Inhibition of
Interleukin-6/glycoprotein 130 signalling by Bazedoxifene
ameliorates cardiac remodelling in pressure overload mice. J Cell
Mol Med. 24:4748–4761. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang Y, van Boxel-Dezaire AH, Cheon H,
Yang J and Stark GR: STAT3 activation in response to IL-6 is
prolonged by the binding of IL-6 receptor to EGF receptor. Proc
Natl Acad Sci USA. 110:16975–16980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rose-John S: IL-6 trans-signaling via the
soluble IL-6 receptor: Importance for the pro-inflammatory
activities of IL-6. Int J Biol Sci. 8:1237–1247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peters M, Blinn G, Solem F, Fischer M,
Meyer zum Büschenfelde KH and Rose-John S: In vivo and in vitro
activities of the gp130-stimulating designer cytokine Hyper-IL-6. J
Immunol. 161:3575–3581. 1998.PubMed/NCBI
|
|
55
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chalaris A, Garbers C, Rabe B, Rose-John S
and Scheller J: The soluble Interleukin 6 receptor: Generation and
role in inflammation and cancer. Eur J Cell Biol. 90:484–494. 2011.
View Article : Google Scholar
|
|
57
|
Ying J, Tsujii M, Kondo J, Hayashi Y, Kato
M, Akasaka T, Inoue T, Shiraishi E, Inoue T, Hiyama S, et al: The
effectiveness of an anti-human IL-6 receptor monoclonal antibody
combined with chemotherapy to target colon cancer stem-like cells.
Int J Oncol. 46:1551–1559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cross-Knorr S, Lu S, Perez K, Guevara S,
Brilliant K, Pisano C, Quesenberry PJ, Resnick MB and Chatterjee D:
RKIP phosphorylation and STAT3 activation is inhibited by
oxaliplatin and camptothecin and are associated with poor prognosis
in stage II colon cancer patients. BMC Cancer. 13:4632013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chang YC, Su CY, Chen MH, Chen WS, Chen CL
and Hsiao M: Secretory RAB GTPase 3C modulates IL6-STAT3 pathway to
promote colon cancer metastasis and is associated with poor
prognosis. Mol Cancer. 16:1352017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Matsusaka S, Hanna DL, Cao S, Zhang W,
Yang D, Ning Y, Sunakawa Y, Okazaki S, Berger MD, Miyamato Y, et
al: Prognostic impact of IL6 genetic variants in patients with
metastatic colorectal cancer treated with bevacizumab-based
chemotherapy. Clin Cancer Res. 22:3218–3226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Walkiewicz K, Kozieł P, Bednarczyk M,
Błażelonis A, Mazurek U and Muc-Wierzgoń M: Expression of
migration-related genes in human colorectal cancer and activity of
a disintegrin and metalloproteinase 17. BioMed Res Int.
2016:82089042016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li H, Xiao H, Lin L, Jou D, Kumari V, Lin
J and Li C: Drug design targeting protein-protein interactions
(PPIs) using multiple ligand simultaneous docking (MLSD) and drug
repositioning: Discovery of raloxifene and bazedoxifene as novel
inhibitors of IL-6/GP130 interface. J Med Chem. 57:632–641. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qiu HY, Zhu X, Luo YL, Lin HY, Tang CY, Qi
JL, Pang YJ, Yang RW, Lu GH, Wang XM, et al: Identification of new
shikonin derivatives as antitumor agents targeting STAT3 SH2
domain. Sci Rep. 7:28632017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lin L, Hutzen B, Ball S, Foust E, Sobo M,
Deangelis S, Pandit B, Friedman L, Li C, Li PK, et al: New curcumin
analogues exhibit enhanced growth-suppressive activity and inhibit
AKT and signal transducer and activator of transcription 3
phosphorylation in breast and prostate cancer cells. Cancer Sci.
100:1719–1727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xiao H, Bid HK, Jou D, Wu X, Yu W, Li C,
Houghton PJ and Lin J: A novel small molecular STAT3 inhibitor,
LY5, inhibits cell viability, cell migration, and angiogenesis in
medulloblastoma cells. J Biol Chem. 290:3418–3429. 2015. View Article : Google Scholar :
|
|
66
|
Li DD, Zhao CH, Ding HW, Wu Q, Ren TS,
Wang J, Chen CQ and Zhao QC: A novel inhibitor of ADAM17 sensitizes
colorectal cancer cells to 5-fluorouracil by reversing Notch and
epithelial-mesenchymal transition in vitro and in vivo. Cell
Prolif. 51:e124802018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sonenberg N and Hinnebusch AG: Regulation
of translation initiation in eukaryotes: Mechanisms and biological
targets. Cell. 136:731–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ryoo HD and Vasudevan D: Two distinct
nodes of translational inhibition in the Integrated Stress
Response. BMB Rep. 50:539–545. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liang W, Cui J, Zhang K, Xi H, Cai A, Li
J, Gao Y, Hu C, Liu Y, Lu Y, et al: Shikonin induces ROS-based
mitochondria-mediated apoptosis in colon cancer. Oncotarget.
8:109094–109106. 2017. View Article : Google Scholar
|
|
70
|
Han X, Kang KA, Piao MJ, Zhen AX, Hyun YJ,
Kim HM, Ryu YS and Hyun JW: Shikonin exerts cytotoxic effects in
human colon cancers by inducing apoptotic cell death via the
endoplasmic reticulum and mitochondria-mediated pathways. Biomol
Ther (Seoul). 27:41–47. 2019. View Article : Google Scholar
|
|
71
|
Chen Y, Ni J, Gao Y, Zhang J, Liu X, Chen
Y, Chen Z and Wu Y: Integrated proteomics and metabolomics reveals
the comprehensive characterization of antitumor mechanism
underlying Shikonin on colon cancer patient-derived xenograft
model. Sci Rep. 10:140922020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen Y, Si L, Zhang J, Yu H, Liu X, Chen Y
and Wu Y: Uncovering the antitumor effects and mechanisms of
Shikonin against colon cancer on comprehensive analysis.
Phytomedicine. 82:1534602021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li MY, Mi C, Wang KS, Wang Z, Zuo HX, Piao
LX, Xu GH, Li X, Ma J and Jin X: Shikonin suppresses proliferation
and induces cell cycle arrest through the inhibition of
hypoxia-inducible factor-1α signaling. Chem Biol Interact.
274:58–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He G, He G, Zhou R, Pi Z, Zhu T, Jiang L
and Xie Y: Enhancement of cisplatin-induced colon cancer cells
apoptosis by shikonin, a natural inducer of ROS in vitro and in
vivo. Biochem Biophys Res Commun. 469:1075–1082. 2016. View Article : Google Scholar : PubMed/NCBI
|